Abstract
Ror2 is a signaling receptor for Wnt ligands that is known to play important roles in limb development, but having no essential roles known in adult tissues. Recent evidence has implicated Ror2 in mediating both canonical and non-canonical signaling pathways. Ror2 was initially found to be highly expressed in osteosarcoma and renal cell carcinomas, and has recently been found in an increasingly long list of cancers currently including melanoma, colon cancer, melanoma, squamous cell carcinoma of the head and neck, and breast cancer. In the majority of these cancer types, Ror2 expression is associated with more aggressive disease states, consistent with a role mediating Wnt signaling regardless of the canonical or noncanonical signal. Because of the pattern of tissue distribution, the association with high-risk diseases, and the cell surface localization of this receptor, Ror2 has been identified as a potential high value target for therapeutic development. However, the recent discovery that Ror2 may function through non-kinase activities challenges this strategy and opens up opportunities to target this important molecule through alternative means.
Keywords: Ror2, Cancer, Wnt signaling, Receptor tyrosine kinase, Pseudokinase
1. Introduction
Ror2 is a member of the receptor tyrosine kinase orphan receptor (ROR) family, which belongs to the receptor tyrosine kinase (RTK) superfamily. RTKs are a large family of glycoproteins that regulate cell proliferation, polarity, differentiation, migration, metabolism and survival (Ullrich & Schlessinger, 1990; Blume-Jensen & Hunter, 2001). Aberrant RTK activation due to deregulated receptor expression and/or constitutive activation are major mechanisms by which tumor cells contribute to the development of various forms of cancer in humans (Forbes et al., 2001; Lemmon & Schlessinger, 2010).
Ror2 is a developmentally regulated protein that has significant expression and roles in a wide variety of tissues during early embryonic development. Expression is downregulated during midgestation and the protein is absent in adult tissues except for residual compartments of expression in the uterus and osteoblasts ((Saldanha et al., 1998; Yoda, Oishi, & Minami, 2003; Billiard et al., 2005; Cha et al., 2014). Roles in normal adult tissues are not understood. However, it is now established that Ror2 is upregulated in a large number of human tumors including osteosarcoma (Morioka et al., 2009), melanoma (O'Connell et al., 2010), renal cell carcinoma (Wright et al., 2009), prostate carcinoma (Yamamoto et al., 2010), colorectal cancer (Mei et al., 2014), squamous cell carcinomas of the head and neck (Kobayashi et al., 2009), and stromal tumors (Edris et al., 2012), and recently was identified as a feature of breast cancers (Henry et al., 2015). Ror2 has become a focus of attention in the cancer community due to its expression in cancers, its frequent association with higher risk disease, and competing roles as a tumor progressor, or some studies that suggest that it may act as a negative disease modifier in certain tissue specific scenarios (Ford et al., 2013).
Previously an orphan receptor, Ror2 is now known to interact with several of the Wnt ligands, which makes the name inapt (Green et al., 2008). Wnt5a and Wnt3a are now well known to act as ligands for Ror2 to activate a combination of noncanonical and canonical Wnt signaling activity, respectively (Yamamoto et al., 2007; Green et al., 2008; Rasmussen et al., 2013). Through these signaling cascades, Ror2 mediates polarized cell migration, invasion, and tumor growth. However, the mechanism by which Ror2 functions to promote cancer remains incompletely understood. Long taken as a kinase, the kinase activity of Ror2 is now also controversial (Artim et al., 2012; Mendrola et al., 2013).
Due to these interesting features and its association with cancer signaling, Ror2 has recently become a central focus for developing therapeutic intervention. Here, we review what is known about Ror2, its roles in cancer and the potential as a therapeutic target in cancer.
2. The Ror receptors
There are two Ror receptors in the Ror family, Ror1 and Ror2, and were first identified in a human neuroblastoma cell line by screens for tyrosine kinase-encoding genes (Masiakowski & Carroll, 1992). The Ror-encoding genes, Ror1 and Ror2, are located on chromosome 1 and 9, respectively, both encoding 104-kDa proteins (Reddy et al., 1997; Forrester, 2002). These receptors are highly homologous to one another, and structurally are very similar to Trk and Musk families of RTKs (Forrester, 2002). Similar to the other RTKs, the molecular architecture and key intracellular signaling pathways of Ror1 and Ror2 are highly conserved in evolution from the nematode Caenorhabditis elegans to humans (Yoda et al., 2003).
3. Ror2 structure and function
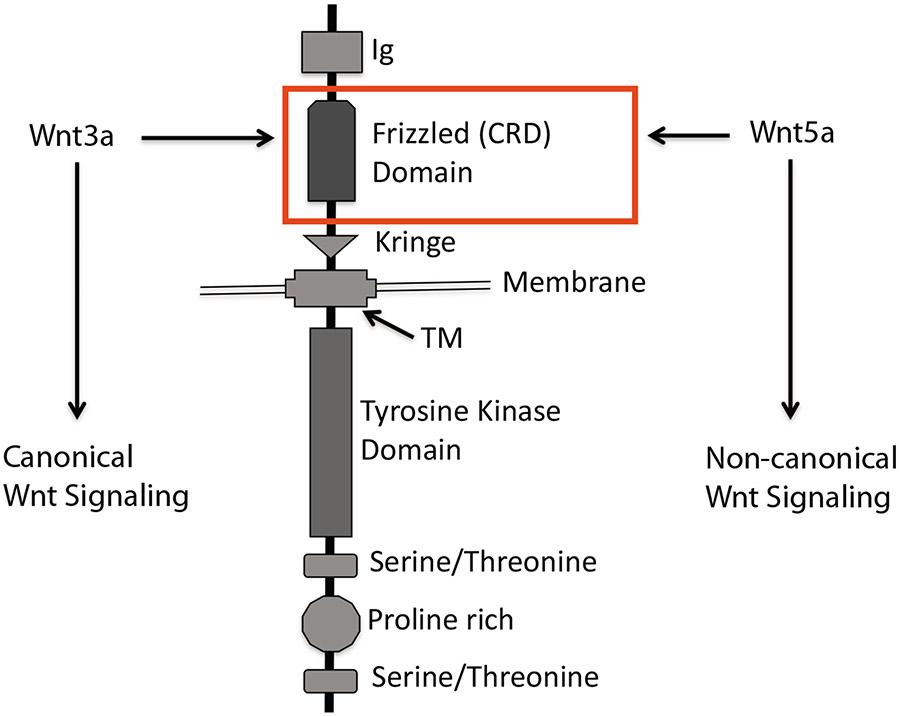
Ror2 is a Frizzled family protein which belongs to the 7 transmembrane class of receptors. As a member of the RTK family, the structure of Ror2 consists of three main parts (Fig. 1): extracellular, transmembrane and intracellular regions (Hojjat-Farsangi, 2014). The extracellular regions of Ror2 are divided into several domains, the immunoglobulin (Ig)-like domain, the cysteine rich domain (CRD), and the Kringle domain (Hojjat-Farsangi, 2014). Ror2 possesses a single CRD within the extracellular region that is defined by the presence of 10 conserved cysteines and by several additional conserved amino acids (Forrester, 2002). The CRDs are composed mainly of α-helices and act as the binding site for the Wnt ligand (Bhanot et al., 1996; Green et al., 2008). The Ig-like domains consist of ~100 amino acids residues, including a conserved disulfide bridge, and are thought to mediate protein–protein interactions and also modify the function of CRD and Kringle domains (Minami et al., 2010). The Kringle domain is a triple-disulfide-linked domain and is reported to function as the recognition module for binding to other proteins (Oishi et al., 1997).
Fig. 1.
Depiction of Ror2 domains and Wnt signaling: Extracellular part of Ror2 contains an Ig-like domain (Ig), a frizzled or cysteine-rich (CRD) domain and a kringle domain. The extracellular and intracellular domains are separated by a transmembrane (TM) domain across the cell membrane. Intracellularly, ROR2 contains a tyrosine kinase (TK) domain and a prolinerich domain (PR) flanked by serine/threonine (ST) rich domains. The CRD domain acts as a binding site for Wnt3a and Wnt5a to mediate canonical and non-canonical Wnt signaling respectively.
The cytoplasmic regions of Ror2 contain the putative tyrosine kinase (TK) domain. Both Ror family members contain the predominant RTK YXXDYY sequence (Feike et al., 2010). And the kinase domain contains a conserved tyrosine containing motif (YALM) that has the potential to bind the SH2 domains of shc, csk or the p85 subunit of phosphotidyl inositol 3 kinase upon phosphorylation (Songyang et al., 1994; Songyang & Cantley, 1995; Feike et al., 2010). Ror2 also has proline rich domains in the cytoplasmic tail, which may provide a docking structure for interactions with other cell signaling molecules. Finally, Ror2 contains sequence regions C-terminal to the kinase domain rich in serine and threonine residues, the function of which is not known (Kani et al., 2004).
There are only few Ror2 interacting proteins that have been reported, including Wnt5a, serine/threonine protein kinases GSK3α and GSK3β, frizzled proteins FZD2 and FZD5, and neurotrophin receptor-interacting MAGE homolog (MAGED1) (Minami et al., 2010; Stark et al., 2006). Oishi et al. (Oishi et al., 2003) were the first to show that Wnt5a binds to Ror2, and also Ror2 associates via its CRD domain to the FZDs. Another study showed that interaction of Wnt5a to Ror2 recruits GSKβ to Ror2 and induce Ror2 phsphorylation followed by activation of the Wnt-JNK pathway that mediates cell migration (Yamamoto et al., 2007).
4. Ror2 expression and roles in development
Ror2 is expressed in highly regulated developmental patterns (Yoda et al., 2003). Ror2 expression is indicated in neural and non-neural tissues, developing limbs, in primitive alveoli of the developing lung, and developing brain (Al-Shawi et al., 2001; Matsuda et al., 2001). It was established that Ror2 plays a key role in skeletal and neural organogenesis, as well as in midgut development in the mouse (Yamada et al., 2010), but then becomes repressed in adult tissues (Al-Shawi et al., 2001).
Mutations in Ror2 are associated with two human skeletal diseases: autosomal recessive Robinow syndrome (RRS) (Afzal et al., 2000) and autosomal dominant brachydactyly type B (BDB) as a result of inactivating mutations (Afzal et al., 2000; Afzal & Jeffery, 2003; Schwarzer et al., 2009; Huang et al., 2014). RRS predominately involves mutations that result in early termination or predict for nonfunctional proteins, whereas BBS occurs from distal truncations after the putative tyrosine kinase domain (van Bokhoven et al., 2000). The effect of this mutation to confer a heterozygous autosomal dominant effect is not well understood. Both congenital diseases are characterized by skeletal dysplasia with generalized limb bone shortening and brachydactyly (Afzal & Jeffery, 2003). Ror2 as well as Wnt5a knockout mice exhibit similar developmental abnormalities (Schwabe et al., 2004; Ford et al., 2013), suggesting that the execution of these processes requires the ligand–receptor interaction to mediate proper signaling events. The phenotypic discrepancies of the Ror2 gene mutation were explained by the Ror2 protein stability and distribution, whereby RRS mutant proteins were less abundant and retained intracellularly, and BDB mutants were stable and predominantly located at the cell membrane. This suggested that the RRS versus the BDB phenotype is determined by the relative degree of protein retention/degradation and the amount of mutant protein reaching the plasma membrane (Schwarzer et al., 2009).
5. Wnt/Ror2 cell signaling
Wnt signaling is essential for embryonic development and cellular processes including differentiation, polarity, migration, invasion, adhesion and survival (Chien et al., 2009). Deregulation of the Wnt signaling is implicated in numerous cancers including colorectal, breast, ovarian, and prostate cancer (Ying & Tao, 2009), and has long been considered a target pathway for therapeutic intervention in cancer. The human Wnt family consists of 19 members, encoding evolutionarily conserved secreted glycoproteins that are hydrophobic and highly cysteine rich secreted molecules (Katoh & Katoh, 2007). Wnt signals are traditionally divided to act as ligands that transduce either the canonical pathway or one of several non-canonical pathways. Canonical signals are transduced through frizzled (FZD) family receptors with LRP5/LRP6 co-receptors to stabilize beta-catenin for translocation to the nucleus and transactivation of TCF/LEF regulated genes involved in dedifferentiation and proliferation (Bhanot et al., 1996; Pinson et al., 2000). Noncanonical signals encompass at least two different signaling pathways—a Ca2+ dependent pathway involving CaMKII, protein-kinase-C and NFAT, and the planar-cell polarity pathway thought to signal through RhoA, Rac and c-Jun N-terminal kinase.
Wnt1, 3a and 7 activate the canonical pathway, whereas Wnt5a, 5b, and 11 will activate non-canonical signaling (Weeraratna, 2005). However, recent studies show that significant crosstalk occurs, particularly via the prototypical non-canonical ligand Wnt5a (He et al., 1997; Nordstrom et al., 2006). Reports indicate that Wnt signals are transduced to the canonical or non-canonical pathways based on the profile of Wnt ligands, soluble inhibitors such as the secreted frizzled related protein, frizzled family receptors, coreceptors, and the activity of cytoplasmic Wnt signaling regulators (Katoh & Katoh, 2007). In melanocytes, for example, canonical Wnt signaling is important for tumor initiation, whereas non-canonical Wnt signaling is activated later in tumorigenesis, shifting away from a beta-catenin transcriptional proliferative phenotype to one marked more by de-differentiation, invasion, and metastasis (O'Connell & Weeraratna, 2009). The role of Ror2 in effecting these transitions is an active area of interest.
Although Ror2 is most closely linked to non-canonical Wnt signaling due to its close relationship to Wnt5a expression and activity, the mechanism by which Ror2 triggers the non-canonical pathway is not well understood. The Ror2/Wnt5a pathway has been shown to involve signaling through Wnt-c-Jun N-terminal kinase via activation of the actin binding protein, filamin A (Nomachi et al., 2008; Oishi et al., 2003). Through activation of this pathway, mice fibroblasts could be induced to undergo cellular migration and invasion, features closely linked to malignancy. Wnt5a is capable of binding to not only Ror2 but also numerous other receptors including Fzd3, Fzd5 and Fzd7, and these ligand–receptor pairings are likely responsible for the initiation of tissue and context-specific downstream signaling.
Ror2 has recently been clarified to play a dual role in Wnt signaling, with potential to influence on both canonical and non-canonical Wnt signaling pathways (Henry et al., 2015). Knockdown of Ror2 from expression in renal tumor cells decreased both beta-catenin-dependent and beta-catenin-independent target genes (Rasmussen et al., 2013), and further promoted a state of stabilized available beta-catenin that allowed for heightened responsiveness to Wnt3a as a result of Ror2 expression. The pleiotropic role of Ror2 might be due to the need for different additional co-receptors. Overall, the role appears to be complex, and likely context dependent, influenced by receptor and coreceptor availability as well as the mixture of Wnt ligands and inhibitors outside the cell.
6. Ror2 activation
The kinase activity of Ror2 has been controversial. Many groups have reported that Ror2 may be phosphorylated or autophosphorylated. Looking at the sequence similarity of Ror2 with known RTKs, it is reasonable to expect intrinsic kinase activity of Ror2. However, mammalian Ror2 exhibits alterations within the highly conserved amino acids within the kinase domains that possibly indicates that the kinase activity may have been evolutionarily lost (Forrester, 2002).
Much of the evidence for Ror2 activity as a kinase has been indirect. Although both Wnt3a and Wnt5a bind Ror2 well, in various contexts only Wnt5a was shown to induce Ror2 mediated activities (Billiard et al., 2005; Liu et al., 2008). Several earlier studies have suggested a catalytic activity of Ror2 (Manning et al., 2002). Mikels et al. showed that Ror2 acts as a classical ligand-dependent tyrosine kinase receptor, with Wnt5a and Ror2 interacting to block classical canonical signaling (Mikels et al., 2009). And, Kani et al. showed that Ror2 can be phosphorylated by casein kinase Iε, a key regulator of the canonical Wnt signaling (Maeda et al., 2012), followed by Ror2 autophosphorylation mediated by GSK-3α and β (Yamamoto et al., 2007). Further work suggested Ror2 phosphorylation of various substrates including G protein-coupled receptor kinase 2 (Liu et al., 2007). Ligand binding has long been shown to induce dimerization of RTKs resulting in autophosphorylation and activation (Cha et al., 2014) and subsequent phosphorylation of 14-3-3β (Liu et al., 2007). However, almost all the studies that suggested Ror2 intrinsic kinase activity were able to show only modest levels of phosphorylation, and remain inconclusive as to the direct mechanism of Ror2-mediated kinase action.
Although several studies have implicated for Ror2 to be active, and it's expression and interactions with Wnt ligands consistently suggest a functional role in promoting cellular signals, the structure of Ror2 kinase domain suggests that Ror2 may function principally as an RTK-like pseudokinase without typical catalytic activity (Mendrola et al., 2013; Bainbridge et al., 2014). About 10% of the protein kinases in humans lack the highly conserved residues thought to be required for catalytic activity, rendering these proteins kinase-dead pseudokinases. Structure studies of Ror2 confirmed binding to Wnt ligands through the cysteine-rich Wnt-binding domains found in the extracellular domain, but were not revealing in how ligand binding can drive dimerization (Lemmon & Schlessinger, 2010). The crystal structure of Ror2 TKD greatly resembles with the insulin receptor TKD (IRK insulin receptor kinase), suggesting a similar mode of autoinhibition (Hubbard et al., 1994). An aspartate residue to a glycine substitution was indicated to likely impair ATP binding. Also, the projection of a tyrosine residue into the ATP-binding pocket fills up the cavity, and the crystal structure of Ror2 displays a conformation of autoinhibition (Artim et al., 2012). The crystal structure showed that the activation loop conformation of Ror2 TKDs is such that it directly occludes the space in which the phosphate moieties of ATP would bind to an active kinase (Artim et al., 2012). Ror2 autoinhibition was implicated due to occlusion of the substrate and ATP binding sites from the activation loop, and also unique contribution from a tyrosine side chain close to the gatekeeper residue.
Even though Ror2 may not be not catalytically active, there are two main mechanisms of the receptor signals (Mendrola et al., 2013). Primarily, the minimal catalytic phosphotransfer site may be sufficient to function despite their unusual sequences. Secondly, Ror2 may function as a scaffold protein by allosterically activating a functional kinase (Boudeau et al., 2006; Nishita et al., 2010; Zeqiraj & van Aalten, 2010). In support of this idea, Ror2 kinase activity was not required for stimulation of filopodia formation, cytoskeletal reorganization and cell migration in response to Wnt5a (Nishita et al., 2006). Using a Wnt reporter assay, the study also showed that the kinase domain of Ror2 is not essential for its role in inhibition of canonical Wnt activity. Src-family protein tyrosine kinases were shown to be activated through Wnt5a/Ror2 signaling which suggests that these members may actually mediate the phosphorylation signals. The non-receptor kinase Src is shown in several studies to act downstream of Ror2 (Akbarzadeh et al., 2008; Feike et al., 2010; Lai et al., 2012), as has been shown for another non-canonical Wnt receptor, Ryk (Wouda et al., 2008; Petrova et al., 2013). Finally, in other cellular contexts, Ror2 was shown to not require catalytic activity, but act to sequester specific Wnt ligands away from other Wnt receptors. Ultimately, the mechanism of action remains uncertain, although the influence of Ror2 on these critical pathways is clear.
7. Role of Ror2 in cancer
Ror2 is a highly pleiotropic receptor with a complex role in tumorigenesis. Many of the activities Ror2 has been associated with developmentally are also programs that are indicated in cancer promotion, such as cell migration and cell invasiveness (Morioka et al., 2009). For example, Ror2 is shown to play a role enhancing polarization, elongation, reorientation and directional migration of primordial germ cells (Laird et al., 2011), features usurped in the cancer invasion and metastasis process.
The up-regulation of Ror2 has been established in a multitude of tumor types. Our lab was among the first to identify Ror2 as a cancer associated protein using renal cell carcinoma cell lines and primary tumors and associated Ror2 expression in RCC mediated extracellular matrix remodeling (Wright et al., 2009). Although Ror2 is physiologically expressed in the normal embryonic kidney at low levels, expression in renal cell carcinoma is associated with expression of extracellular matrix remodeling (EMT) associated genes Twist and MMP2 indicating a role as a regulator of EMT and cell migration (Wright et al., 2009). The suppression of Ror2 not only inhibited cell migration, but also inhibited anchorage-independent growth in soft agar and growth in an orthotropic xenograft model. In RCC, Ror2 is associated with pVHL loss of function, and Ror2 expression is regulated in the VHL–HIF axis as a transcriptional target of HIF-1α or HIF-2α (Wright & Rathmell, 2010). However, other mechanisms may contribute to the upregulation of Ror2 in other tumor settings.
In osteosarcoma cell lines, Ror2 is overexpressed and treatment with siRNA targeted against Ror2 also significantly inhibited cell proliferation and migration (Forrester, 2002; Morioka et al., 2009). Ror2 expression is also higher in oral cancer than normal oral mucosa, and expression was associated with cell polarity, motility, and malignancy (Kobayashi et al., 2009). A recent report identified Ror2 expression in the majority of breast cancer patients (87%), but not in normal breast tissue, and showed that expression confers a poorer disease-specific survival for these patients (Henry et al., 2015). Ror2 is also a key mediator of Wnt5a-induced invasion in melanoma (O'Connell et al., 2010). Ror2 expression correlates with Wnt5a expression and to poorer survival in melanoma patients (Da Forno et al., 2008; O'Connell et al., 2010).
Seemingly contradictory, evidence indicates that Ror2 may be associated with less aggressive disease in some cancers, indicating a complex role of Ror2 in tumorigenesis. In medulloblastoma, Ror2 was found to be expressed in a subset of tumors, although in this tumor a high Ror2 expression had a better prognosis than those with tumors that had low Ror2 expression (Lee et al., 2013). In hepatocellular carcinoma, (HCC) Wnt5a overexpression is associated with decrease of cell proliferation and migration (Bi et al., 2014), suggesting that the Wnt5a signaling axis may have a tumor suppressive role in HCC. Wnt5a characteristically functions through a non-canonical Wnt signaling pathway by binding to the Ror2 and E-cadherin receptor (Bi et al., 2014). Tumor suppressive relationships of Ror2 have been suggested with colon cancer and hepatocellular carcinoma (Roman-Gomez et al., 2007; Lara et al., 2010; Geng et al., 2012), although these reports must be considered in context, as Ror2 is expressed in a subset of these tumors, and recent evidence in colon cancer considers that subset may have more aggressive disease (Bordonaro et al., 2011). Further work in this area is needed to understand the complex roles this protein plays in influencing disease phenotypes.
It might be possible that in cancers driven by canonical Wnt signaling such as colon or hepatocellular cancer, Ror2 is interacting with different members of canonical signaling pathway (Lara et al., 2010; Ford et al., 2013; Bi et al., 2014), giving rise to variable association with the severity of the disease. Alternatively, in cancers more driven by non-canonical Wnt signaling, such as melanoma, Ror2 expression may play a more critical and direct role in driving tumorigenesis (O'Connell et al., 2010; Ford et al., 2013).
8. Strategies to target Ror2 in cancer
If it can be confirmed that Ror2 is not expressed in most normal adult tissues, it represents an extremely attractive target in those cancers displaying increased protein expression. As a transmembrane receptor with putative kinase activity, we and others originally held great hope that Ror2 would provide a valuable target for small molecule inhibitor therapy. There are several drugs targeting activated RTKs for treating an increasingly wide variety of cancers, with particular success for the small-molecule inhibitors that target the ATP-binding site of the intracellular TKD (Shawver et al., 2002). The kinase domain of Ror2 is relatively unique amongst the RTK superfamily, with similarity only to the family of discoidin domain receptors and MUSK. Current research, however, as detailed above has suggested that the kinase domain in Ror2's cytoplasmic tail may be inactive, or minimally functional. In the present state of research into Ror2 kinase activity it unfortunately must be considered to be acting as a pseudokinase, or to have conditions of activation that have not yet been revealed.
However, this protein has clearly been linked to aggressive disease patterns and has been implicated in important tumor promoting signaling pathways. Therefore, it is worthwhile to consider alternative strategies for targeting tumors marked by Ror2. Monoclonal antibodies (MAb) have been developed that bind and/or neutralize cell surface proteins of a variety of types. Even with the widespread development of small molecule inhibitors, therapeutic strategies have been considered that both interfere with RTK activation and target RTK-expressing cells for destruction by the immune system (Reichert and Valge-Archer, 2007). A second option, therefore, would be to utilize the fact that Ror2 is a cell surface receptor with a distinct extracellular domain and develop a MAb for therapeutic use. Such antibodies can function to neutralize the receptor by shielding it from ligand stimulation, or in some cases antibodies with the highest affinity and slowest rate of internalization can mediate an antibody-dependent cell mediated cytotoxicity. It is important to determine the number of Ror2 molecules present on the cell surface in order for future antibody therapies to be effective. One possible caveat to the success of this approach could be the number of Ror2 receptors on the surface of a given cell. Attempts to develop MAbs against Ror1 have been promising, but have come across the difficulty of low antigen density, and it is possible that similar challenges may be present with Ror2 therapy (Hojjat-Farsangi et al., 2014).
As an alternative, antibodies can be conjugated to delivery effective payloads of highly potent toxins such as immunoconjugates, radioimmuno-conjugates, or antibody–drug conjugates to effectively kill target cells while limiting the exposure of normal cells to these agents. This method can have efficacy with lower surface levels of receptor, and has been applied with some success to cells expressing Ror1.
Finally, the expression of Ror2 on the surface of tumor cells may allow it to be considered as a target for emerging immune therapies. For example, bispecific antibodies are artificially engineered antibodies that can recruit cytotoxic cells of the immune system into close proximity with target antigen expressing tumor cells, creating a favorable setting for cell killing. Alternatively, Ror2 may present a realistic target for CAR-retargeted T-cells or NK cells, which rely on generating chimeric antigen receptors to proteins uniquely expressed on cancer cells. Harnessing the sensitivity and selectivity of the immune system could overcome antigen density limitations.
9. Conclusions
Ror2 can no longer be called an orphan receptor molecule. Tremendous evidence is mounting that Ror2 re-expression plays a substantial role in an increasingly wide array of malignant diseases. In the majority of tumors reported, Ror2 expression is associated with greater aggressive features. In a few select settings, expression of Ror2 has been associated with more favorable disease, but nonetheless displays expression in these cancers. As a protein normally not expressed in adult tissues, Ror2 has great potential as a target for therapeutic development. The direct path of characterizing and targeting Ror2 as a target kinase has revealed challenges and new considerations as to how Ror2 supports tumor cell growth. However, due to the cell surface localization of the receptor it remains a viable candidate for other strategies to target cancer cells expressing this protein.
Acknowledgments
We would like to especially thank Bill Janzen and his group at the Center for Integrated Chemical Biology and Drug Discovery for many helpful discussions and advice. We would also like to acknowledge National Cancer Institute (T32-CA009156) and the Merck Fellowship Program (ZD), and the American Cancer Society Research Scholar Grant (WKR).
Abbreviations:
- RTK
receptor tyrosine kinase
- Ror1 and Ror2
receptor tyrosine kinase like orphan receptor
- CRD
cysteine rich domain
- Wnt
wingless family gene
- SH2
src homology domain 2
- MMP
matrix metaloprotease
- EMT
epithelial to mesenchymal transition
- ATP
adenosine triphosphate
Footnotes
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Afzal AR, & Jeffery S. (2003). One gene, two phenotypes: ROR2 mutations in autosomal recessive Robinow syndrome and autosomal dominant brachydactyly type B. Hum Mutat 22, 1–11. [DOI] [PubMed] [Google Scholar]
- Afzal AR, Rajab A, Fenske CD, Oldridge M, Elanko N, Ternes-Pereira E, et al. (2000). Recessive Robinow syndrome, allelic to dominant brachydactyly type B, is caused by mutation of ROR2. Nat Genet 25, 419–422. [DOI] [PubMed] [Google Scholar]
- Akbarzadeh S, Wheldon LM, Sweet SM, Talma S, Mardakheh FK, & Heath JK (2008). The deleted in brachydactyly B domain of ROR2 is required for receptor activation by recruitment of Src. PLoS One 3, e1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shawi R, Ashton SV, Underwood C, & Simons JP (2001). Expression of the Ror1 and Ror2 receptor tyrosine kinase genes during mouse development. Dev Genes Evol 211, 161–171. [DOI] [PubMed] [Google Scholar]
- Artim SC, Mendrola JM, & Lemmon MA (2012). Assessing the range of kinase autoinhibition mechanisms in the insulin receptor family. Biochem J 448, 213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge TW, DeAlmeida VI, Izrael-Tomasevic A, Chalouni C, Pan B, Goldsmith J, et al. (2014). Evolutionary divergence in the catalytic activity of the CAM-1, ROR1 and ROR2 kinase domains. PLoS One 9, e102695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, et al. (1996). A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature 382, 225–230. [DOI] [PubMed] [Google Scholar]
- Bi L, Liu X, Wang C, Cao Y, Mao R, Li P, et al. (2014). Wnt5a involved in regulation of the biological behavior of hepatocellular carcinoma. Int J Clin Exp Pathol 7, 987–995. [PMC free article] [PubMed] [Google Scholar]
- Billiard J, Way DS, Seestaller-Wehr LM, Moran RA, Mangine A, & Bodine PV (2005). The orphan receptor tyrosine kinase Ror2 modulates canonical Wnt signaling in osteoblastic cells. Mol Endocrinol 19, 90–101. [DOI] [PubMed] [Google Scholar]
- Blume-Jensen P, & Hunter T (2001). Oncogenic kinase signalling. Nature 411, 355–365. [DOI] [PubMed] [Google Scholar]
- Bordonaro M, Tewari S, Cicco CE, Atamna W, & Lazarova DL (2001). A switch from canonical to noncanonical Wnt signaling mediates drug resistance in colon cancer cells. PLoS One 6, e27308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudeau J, Miranda-Saavedra D, Barton GJ, & Alessi DR (2006). Emerging roles of pseudokinases. Trends Cell Biol 16, 443–452. [DOI] [PubMed] [Google Scholar]
- Cha J, Bartos A, Park C, Sun X, Li Y, Cha SW, et al. (2014). Appropriate crypt formation in the uterus for embryo homing and implantation requires Wnt5a-ROR signaling. Cell Rep 8, 382–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien AJ, Conrad WH, & Moon RT (2009). A Wnt survival guide: From flies to human disease. J Invest Dermatol 129, 1614–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Forno PD, Pringle JH, Hutchinson P, Osborn J, Huang Q, Potter L, et al. (2008). WNT5A expression increases during melanoma progression and correlates with outcome. Clin Res 14, 5825–5832. [DOI] [PubMed] [Google Scholar]
- Edris B, Espinosa I, Muhlenberg T, Mikels A, Lee CH, Steigen SE, et al. (2012). ROR2 is a novel prognostic biomarker and a potential therapeutic target in leiomyosarcoma and gastrointestinal stromal tumour. J Pathol 227, 223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feike AC, Rachor K, Gentzel M, & Schambony A (2010). Wnt5a/Ror2-induced upregulation of xPAPC requires xShcA. Biochem Biophys Res Commun 400, 500–506. [DOI] [PubMed] [Google Scholar]
- Forbes SA, Bhamra G, Bamford S, Dawson E, Kok C, et al. (2001). The Catalogue of Somatic Mutations in Cancer (COSMIC). Current Protocols in Human Genetics. John Wiley & Sons, Inc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford CE, Qian Ma SS, Quadir A, & Ward RL (2013). The dual role of the novel Wnt receptor tyrosine kinase, ROR2, in human carcinogenesis. Int J Cancer 133, 779–787. [DOI] [PubMed] [Google Scholar]
- Forrester WC (2002). The Ror receptor tyrosine kinase family. Cell Mol Life Sci 59, 83–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng M, Cao YC, Chen YJ, Jiang H, Bi LQ, & Liu XH (2012). Loss ofWnt5a and Ror2 protein in hepatocellular carcinoma associated with poor prognosis. World J Gastroenterol 18, 1328–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JL, Kuntz SG, & Sternberg PW (2008). Ror receptor tyrosine kinases: Orphans no more. Trends Cell Biol 18, 536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Saint-Jeannet JP, Wang Y, Nathans J, Dawid I, & Varmus H (1997). A member of the Frizzled protein family mediating axis induction by Wnt-5A. Science 275, 1652–1654. [DOI] [PubMed] [Google Scholar]
- Henry C, Quadir A, Hawkins NJ, Jary E, Llamosas E, Kumar D, et al. (2015). Expression of the novel Wnt receptor ROR2 is increased in breast cancer and may regulate both beta-catenin dependent and independent Wnt signalling. J Cancer Res Clin Oncol 141, 243–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojjat-Farsangi M (2014). Small-molecule inhibitors of the receptor tyrosine kinases: Promising tools for targeted cancer therapies. Int J Mol Sci 15, 13768–13801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojjat-Farsangi M, Moshfegh A, Daneshmanesh AH, Khan AS, Mikaelsson E, Osterborg A, et al. (2014). The receptor tyrosine kinase ROR1—An oncofetal antigen for targeted cancer therapy. Semin Cancer Biol 29, 21–31. [DOI] [PubMed] [Google Scholar]
- Huang D, Jiang S, Zhang Y, Liu X, Zhang J, & He R (2014).A new mutation in the gene ROR2 causes brachydactyly type B1. Gene 547, 106–110. [DOI] [PubMed] [Google Scholar]
- Hubbard SR, Wei L, Ellis L, & Hendrickson WA (1994). Crystal structure of the tyrosine kinase domain of the human insulin receptor. Nature 372, 746–754. [DOI] [PubMed] [Google Scholar]
- Kani S, Oishi I, Yamamoto H, Yoda A, Suzuki H, Nomachi A, et al. (2004). The receptor tyrosine kinase Ror2 associates with and is activated by casein kinase Iepsilon. J Biol Chem 279, 50102–50109. [DOI] [PubMed] [Google Scholar]
- Katoh M, & Katoh M (2007). WNT signaling pathway and stem cell signaling network. Clin Res 13, 4042–4045. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Shibuya Y, Takeuchi J, Murata M, Suzuki H, Yokoo S, et al. (2009). Ror2 expression in squamous cell carcinoma and epithelial dysplasia ofthe oral cavity. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 107, 398–406. [DOI] [PubMed] [Google Scholar]
- Lai SS, Xue B, Yang Y, Zhao L, Chu CS, Hao JY, et al. (2012). Ror2-Src signaling in metastasis of mouse melanoma cells is inhibited by NRAGE. Cancer Genet 205, 552–562. [DOI] [PubMed] [Google Scholar]
- Laird DJ, Altshuler-Keylin S, Kissner MD, Zhou X, & Anderson KV (2011). Ror2 enhances polarity and directional migration of primordial germ cells. PLoS Genet 7, e1002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara E, Calvanese V, Huidobro C, Fernandez AF, Moncada-Pazos A, Obaya AJ, et al. (2010). Epigenetic repression of ROR2 has a Wnt-mediated, pro-tumourigenic role in colon cancer. Mol Cancer 9, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SE, Lim SD, Kang SY, Suh SB, & Suh YL (2013). Prognostic significance of Ror2 and Wnt5a expression in medulloblastoma. Brain Pathol 23, 445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon MA, & Schlessinger J (2010). Cell signaling by receptor tyrosine kinases. Cell 141, 1117–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Ross JF, Bodine PV, & Billiard J (2007). Homodimerization of Ror2 tyrosine kinase receptor induces 14-3-3(beta) phosphorylation and promotes osteoblast differentiation and bone formation. Mol Endocrinol 21, 3050–3061. [DOI] [PubMed] [Google Scholar]
- Liu Y, Rubin B, Bodine PV, & Billiard J (2008). Wnt5a induces homodimerization and activation of Ror2 receptor tyrosine kinase. J Cell Biochem 105, 497–502. [DOI] [PubMed] [Google Scholar]
- Maeda K, Kobayashi Y, Udagawa N, Uehara S, Ishihara A, Mizoguchi T, et al. (2012). Wnt5a-Ror2 signaling between osteoblast-lineage cells and osteoclast precursors enhances osteoclastogenesis. Nat Med 18, 405–412. [DOI] [PubMed] [Google Scholar]
- Manning G, Whyte DB, Martinez R, Hunter T, & Sudarsanam S (2002). The protein kinase complement of the human genome. Science 298, 1912–1934. [DOI] [PubMed] [Google Scholar]
- Masiakowski P, & Carroll RD (1992). A novel family of cell surface receptors with tyrosine kinase-like domain. J Biol Chem 267, 26181–26190. [PubMed] [Google Scholar]
- Matsuda T, Nomi M, Ikeya M, Kani S, Oishi I, Terashima T, et al. (2001). Expression of the receptor tyrosine kinase genes, Ror1 and Ror2, during mouse development. Mech Dev 105, 153–156. [DOI] [PubMed] [Google Scholar]
- Mei H, Lian S, Zhang S, Wang W, Mao Q, & Wang H (2014). High expression of ROR2 in cancer cell correlates with unfavorable prognosis in colorectal cancer. Biochem Biophys Res Commun 453, 703–709. [DOI] [PubMed] [Google Scholar]
- Mendrola JM, Shi F, Park JH, & Lemmon MA (2013). Receptor tyrosine kinases with intracellular pseudokinase domains. Biochem Soc Trans 41, 1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikels A, Minami Y, & Nusse R (2009). Ror2 receptor requires tyrosine kinase activity to mediate Wnt5A signaling. J Biol Chem 284, 30167–30176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami Y, Oishi I, Endo M, & Nishita M (2010). Ror-family receptor tyrosine kinases in noncanonical Wnt signaling: Their implications in developmental morphogenesis and human diseases. Dev Dyn 239, 1–15. [DOI] [PubMed] [Google Scholar]
- Morioka K, Tanikawa C, Ochi K, Daigo Y, Katagiri T, Kawano H, et al. (2009). Orphan receptor tyrosine kinase ROR2 as a potential therapeutic target for osteosarcoma. Cancer Sci 100, 1227–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishita M, Itsukushima S, Nomachi A, Endo M, Wang Z, Inaba D, et al. (2010). Ror2/Frizzled complex mediates Wnt5a-induced AP-1 activation by regulating Dishevelled polymerization. Mol Cell Biol 30, 3610–3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishita M, Yoo SK, Nomachi A, Kani S, Sougawa N, Ohta Y, et al. (2006). Filopodia formation mediated by receptor tyrosine kinase Ror2 is required for Wnt5a-induced cell migration. J Cell Biol 175, 555–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomachi A, Nishita M, Inaba D, Enomoto M, Hamasaki M, & Minami Y (2008). Receptor tyrosine kinase Ror2 mediates Wnt5a-induced polarized cell migration by activating c-Jun N-terminal kinase via actin-binding protein filamin A. J Biol Chem 283, 27973–27981. [DOI] [PubMed] [Google Scholar]
- Nordstrom U, Maier E, Jessell TM, & Edlund T (2006). An early role for WNT signaling in specifying neural patterns of Cdx and Hox gene expression and motor neuron subtype identity. PLoS Biol 4, e252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell MP, Fiori JL, Xu M, Carter AD, Frank BP, Camilli TC, et al. (2010). The orphan tyrosine kinase receptor, ROR2, mediates Wnt5A signaling in metastatic melanoma. Oncogene 29, 34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell MP, & Weeraratna AT (2009). Hear the Wnt Ror: How melanoma cells adjust to changes in Wnt. Pigment Cell Melanoma Res 22, 724–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi I, Sugiyama S, Liu ZJ, Yamamura H, Nishida Y, & Minami Y (1997). A novel Drosophila receptor tyrosine kinase expressed specifically in the nervous system. Unique structural features and implication in developmental signaling. J Biol Chem 272, 11916–11923. [DOI] [PubMed] [Google Scholar]
- Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, et al. (2003).The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells 8, 645–654. [DOI] [PubMed] [Google Scholar]
- Petrova IM, Lahaye LL, Martianez T, de Jong AW, Malessy MJ, Verhaagen J, et al. (2013). Homodimerization of the Wnt receptor DERAILED recruits the Src family kinase SRC64B. Mol Cell Biol 33, 4116–4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinson KI, Brennan J, Monkley S, Avery BJ, & Skarnes WC (2000). An LDL-receptor-related protein mediates Wnt signalling in mice. Nature 407, 535–538. [DOI] [PubMed] [Google Scholar]
- Rasmussen NR, Wright TM, Brooks SA, Hacker KE, Debebe Z, Sendor AB, et al. (2013). Receptor tyrosine kinase-like orphan receptor 2 (Ror2) expression creates a poised state of Wnt signaling in renal cancer. J Biol Chem 288, 26301–26310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy UR, Phatak S, Allen C, Nycum LM, Sulman EP, White PS, et al. (1997). Localization of the human Ror1 gene (NTRKR1) to chromosome 1p31–p32 by fluorescence in situ hybridization and somatic cell hybrid analysis. Genomics 41, 283–285. [DOI] [PubMed] [Google Scholar]
- Reichert JM, & Valge-Archer VE (2007). Development trends for monoclonal antibody cancer therapeutics. Nat Rev Drug Discov. 6, 349–356. [DOI] [PubMed] [Google Scholar]
- Roman-Gomez J, Jimenez-Velasco A, Cordeu L, Vilas-Zornoza A, San Jose-Eneriz E, Garate L, et al. (2007). WNT5A, a putative tumour suppressor of lymphoid malignancies, is inactivated by aberrant methylation in acute lymphoblastic leukaemia. Eur J Cancer 43, 2736–2746. [DOI] [PubMed] [Google Scholar]
- Saldanha J, Singh J, & Mahadevan D (1998). Identification of a Frizzled-like cysteine rich domain in the extracellular region of developmental receptor tyrosine kinases. Protein Sci 7, 1632–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe GC, Trepczik B, Suring K, Brieske N, Tucker AS, Sharpe PT, et al. (2004). Ror2 knockout mouse as a model for the developmental pathology of autosomal recessive Robinow syndrome. Dev Dyn 229, 400–410. [DOI] [PubMed] [Google Scholar]
- Schwarzer W, Witte F, Rajab A, Mundlos S, & Stricker S (2009). A gradient of ROR2 protein stability and membrane localization confers brachydactyly type B or Robinow syndrome phenotypes. Hum Mol Genet 18, 4013–4021. [DOI] [PubMed] [Google Scholar]
- Shawver LK, Slamon D, & Ullrich A (2002). Smart drugs: Tyrosine kinase inhibitors in cancer therapy. Cancer Cell 1, 117–123. [DOI] [PubMed] [Google Scholar]
- Songyang Z, & Cantley LC (1995). Recognition and specificity in protein tyrosine kinase-mediated signalling. Trends Biochem Sci 20, 470–475. [DOI] [PubMed] [Google Scholar]
- Songyang Z, Shoelson SE, McGlade J, Olivier P, Pawson T, Bustelo XR, et al. (1994). Specific motifs recognized by the SH2 domains of Csk, 3BP2, fps/fes, GRB-2, HCP, SHC, Syk, and Vav. Mol Cell Biol 14, 2777–2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CBB, Reguly T, Boucher L, Breitkreutz A, & Tyers M (2006). Biogrid: a general repository for interaction datasets. Nucleic Acids Res 34, D535–D539 (Database issue). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A, & Schlessinger J (1990). Signal transduction by receptors with tyrosine kinase activity. Cell 61, 203–212. [DOI] [PubMed] [Google Scholar]
- van Bokhoven H, Celli J, Kayserili H, van Beusekom E, Balci S, Brussel W, et al. (2000). Mutation of the gene encoding the ROR2 tyrosine kinase causes autosomal recessive Robinow syndrome. Nat Genet 25, 423–426. [DOI] [PubMed] [Google Scholar]
- Weeraratna AT (2005). A Wnt-er wonderland—The complexity ofWnt signaling in melanoma. Cancer Metastasis Rev 24, 237–250. [DOI] [PubMed] [Google Scholar]
- Wouda RR, Bansraj MR, de Jong AW, Noordermeer JN, & Fradkin LG (2008). Src family kinases are required for WNT5 signaling through the Derailed/RYK receptor in the Drosophila embryonic central nervous system. Development 135, 2277–2287. [DOI] [PubMed] [Google Scholar]
- Wright TM, Brannon AR, Gordan JD, Mikels AJ, Mitchell C, Chen S, et al. (2009). Ror2, a developmentally regulated kinase, promotes tumor growth potential in renal cell carcinoma. Oncogene 28, 2513–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright TM, & Rathmell WK (2010). Identification of Ror2 as a hypoxia-inducible factor target in von Hippel–Lindau-associated renal cell carcinoma. J Biol Chem 285, 12916–12924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Udagawa J, Matsumoto A, Hashimoto R, Hatta T, Nishita M, et al. (2010). Ror2 is required for midgut elongation during mouse development. Dev Dyn 239, 941–953. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Oue N, Sato A, Hasegawa Y, Yamamoto H, Matsubara A, et al. (2010). Wnt5a signaling is involved in the aggressiveness of prostate cancer and expression of metalloproteinase. Oncogene 29, 2036–2046. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Yoo SK, Nishita M, Kikuchi A, & Minami Y (2007). Wnt5a modulates glycogen synthase kinase 3 to induce phosphorylation of receptor tyrosine kinase Ror2. Genes Cells 12, 1215–1223. [DOI] [PubMed] [Google Scholar]
- Ying Y, & Tao Q (2009). Epigenetic disruption of the WNT/beta-catenin signaling pathway in human cancers. Epigenetics 4, 307–312. [DOI] [PubMed] [Google Scholar]
- Yoda A, Oishi I, & Minami Y (2003). Expression and function of the Ror-family receptor tyrosine kinases during development: Lessons from genetic analyses of nematodes, mice, and humans. J Recept Signal Transduct Res 23, 1–15. [DOI] [PubMed] [Google Scholar]
- Zeqiraj E, & van Aalten DM (2010). Pseudokinases—Remnants of evolution or key allosteric regulators? Curr Opin Struct Biol 20, 772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]