Keywords: autonomic nervous system, hindlimb unloading, IPSC, synaptic transmission
Abstract
The nucleus tractus solitarii (nTS) is the major integrative brainstem region for autonomic modulation and processing of cardiovascular reflexes. GABA and glutamate are the main inhibitory and excitatory neurotransmitters, respectively, within this nucleus. Alterations in the GABA-glutamate regulation in the nTS are related to numerous cardiovascular comorbidities. Bedridden individuals and people exposed to microgravity exhibit dysautonomia and cardiovascular deconditioning that are mimicked in the hindlimb unloading (HU) rat model. We have previously shown in the nTS that HU increases glutamatergic neurotransmission yet decreases neuronal excitability. In this study, we investigated the effects of HU on nTS GABAergic neurotransmission. We hypothesized that HU potentiates GABA signaling via increased GABAergic release and postsynaptic GABA receptor expression. Following HU or control postural exposure, GABAergic neurotransmission was assessed using whole cell patch clamp whereas the magnitude of GABA release was evaluated via an intensity-based GABA sensing fluorescence reporter (iGABASnFR). In response to GABA interneuron stimulation, the evoked inhibitory postsynaptic current (nTS-IPSC) amplitude and area, as well as iGABASnFR fluorescence, were greater in HU than in control. HU also elevated the frequency but not the amplitude of spontaneous miniature IPSCs. Picoapplication of GABA produced similar postsynaptic current responses in nTS neurons of HU and control. Moreover, HU did not alter GABAA receptor α1 subunit expression, indicating minimal alterations in postsynaptic membrane receptor expression. These results indicate that HU increases GABAergic signaling in the nTS likely via augmented release of GABA from presynaptic terminals. Altogether, our data indicate GABA plasticity contributes to the autonomic and cardiovascular alterations following cardiovascular deconditioning (CVD).
NEW & NOTEWORTHY Gravity influences distribution of blood volume and autonomic function. Microgravity and prolonged bed rest induce cardiovascular deconditioning (CVD). We used hindlimb unloading (HU), a rat analog for bed rest, to investigate CVD-induced neuroplasticity in the brainstem. Our data demonstrate that HU increases GABA modulation of nucleus tractus solitarii (nTS) neurons via presynaptic plasticity. Given the importance of nTS in integrating cardiovascular reflexes, this study provides new evidence on the central mechanisms behind CVD following HU.
INTRODUCTION
The nucleus tractus solitarii (nTS) is the primary brainstem region for peripheral autonomic and cardiovascular reflex integration (1–3). Although many neurotransmitters are involved in the processing of sensory inputs within the nTS, afferent signaling to this nucleus is primarily glutamatergic (4, 5). Glutamate signaling is counterbalanced by inhibitory γ-aminobutyric acid (GABA) neurotransmission, and the predominant source of GABA within the nTS arises from GABAergic interneurons (6). This inhibitory modulation plays a key role in the processing of the afferent signal in the nTS (7–9). The pharmacological activation of GABA receptors in the nTS inhibits afferent-driven glutamate signaling, ultimately leading to impaired cardiovascular reflexes and increased sympathetic outflow and blood pressure (8, 10, 11). Moreover, plasticity in GABA neurotransmission within the nTS has been associated with several experimental models of hypertension including DOCA-salt, renal-wrap, and spontaneous hypertension (12–16).
Cardiovascular deconditioning (CVD), as observed in prolonged bed rest or microgravity, induces physiological consequences including cephalad fluid shifts, postural muscle atrophy, reduced baroreflex-mediated sympathoexcitation to low blood pressure (BP), and increased resting heart rate (HR) (17–19). These changes also are observed in the hindlimb unloaded (HU) rat model of deconditioning (20–23). We have suggested that the physiological (mal)adaptations in HU are likely due to central nervous system plasticity as baroafferent discharge is not altered by HU (24). Furthermore, we have recently demonstrated that HU enhances nTS glutamatergic neurotransmission to nTS neurons. However, those nTS neurons were also hyperpolarized with decreased excitability that was associated with enhanced GABA tone (25). Consistent with increased GABA function shown in our studies, elevated GABAergic signaling in the hippocampus was identified as one of the mechanisms related to the impaired cognition after HU (26).
Given the importance of GABA in the coordination of autonomic function and our earlier study suggesting that GABA modulation is augmented in HU, in this study we sought to determine the electrophysiological and molecular mechanisms related to inhibitory signaling in the nTS following HU. We tested the hypothesis that HU potentiates GABA signaling within the nTS via increased GABA release and postsynaptic GABA receptor (GABAR) expression. Whole cell patch-clamp recordings and live-cell imaging demonstrated that, compared with controls, HU increased nTS-evoked inhibitory postsynaptic currents (nTS-IPSC) and the fluorescence response of the intensity-based GABA sensing reporter (iGABASnFR). HU also tended to elevate the frequency of spontaneous IPSCs (sIPSC), and significantly increased the frequency of miniature IPSCs (mIPSC), which together suggest enhanced presynaptic GABA release. The similar s/mIPSC amplitude, current response to brief exogenous GABA application, and GABAAR α1 subunit expression between HU and control indicate that postsynaptic receptors were not changed. Furthermore, molecular analysis revealed that HU and control rats have comparable expression of glutamic acid decarboxylase 67 (GAD67), a critical enzyme for GABA synthesis (27, 28), and vesicular GABA transporter (VGAT) that packages GABA in the presynaptic terminal (29). Taken together, our data support the concept that HU increases GABAergic signaling in the nTS likely via augmented presynaptic release. This GABAergic synaptic plasticity may contribute to the autonomic and cardiovascular alterations following CVD.
EXPERIMENTAL PROCEDURES
Ethical Approval and Animals
Experiments were performed on male Sprague–Dawley rats (ENVIGO, Indianapolis, IN). Rats were individually housed in the animal facility in a 12:12-h light-dark cycle, temperature ∼ 22°C, with free access to water and food. All the experiments were conducted following the National Institutes of Health guidelines, and protocols were approved by the Animal Care and Use Committee of the University of Missouri. Rats were randomly assigned to HU or control groups.
HU Protocol
As detailed previously (25, 30), 3-wk old rats were anesthetized with isoflurane (2%), two stainless steel wire rings were positioned in the tail, antibiotic (Enrofloxacin 10 mg/kg, Newry, Northern Ireland, UK) was administered subcutaneously, and animals were allowed 1 wk of recovery. Three days before the HU protocol was initiated, rats were trained to the unloading procedures in which the animal was suspended via the attached rings at an angle of 30°–35° for 1–3 h/day. After acclimation, the rats were tail suspended for 2 wk, with free access to food and water. Control animals were maintained in normal postural conditions for the comparable 2-wk period. The welfare of rats, food and water intake, hygiene, and signs of discomfort were monitored daily.
In Vitro nTS Slice Preparation, Electrophysiology, and Pharmaceuticals
Brainstem slices were prepared via our published methods (25, 31) following the HU or control protocol (6-wk-old rats). The brainstem was taken from rats anesthetized with isoflurane and placed in ice‐cold artificial cerebrospinal fluid (aCSF) cutting solution containing (in mM) 93 N-methyl-d-glucamine (NMDG), 2.5 KCl, 1.2 NaH2PO4, 10 MgSO4, 30 NaHCO3, 20 HEPES, 25 d‐glucose, 5 l‐ascorbic acid, 2 thiourea, 3 sodium pyruvate, and 0.5 CaCl2. The pH was adjusted to 7.4 with ∼93 mM HCl, osmolarity 295–310 mosmol/L and aerated with 95% O2 + 5% CO2. Horizontal nTS slices (∼280 μm) were generated using a vibratome (VT 1000S or 1200, Leica, Wetzlar, Germany). Sequentially, the slices were incubated at 35°C for 12 min in NMDG‐cutting solution, then transferred and maintained for 30 min at room temperature (∼22°C) in recording aCSF containing (in mM): 124 NaCl, 3 KCl, 1.2 NaH2PO4, 1.2 MgSO4, 25 NaHCO3, 11 d‐glucose, 0.4 l‐ascorbic acid, and 2 CaCl2, aerated with 95% O2 + 5% CO2, pH 7.4, 295–310 mosmol/L.
For whole cell patch‐clamp recordings, nTS slices were positioned in a heated recording chamber (TC‐344B, Warner Instruments, Hamden, CT) and placed on a fixed-stage upright microscope with DIC optics (BX51WI, Olympus, Japan). Slices were continuously superfused (∼2.5 mL/min) with recording aCSF at ∼32°C aerated with 95% O2 + 5% CO2. To record inhibitory postsynaptic currents (IPSCs), recording electrodes were filled with a high chloride-based solution containing (in mM: 140 CsCl, 5 NaCl, 10 EGTA, 10 HEPES, 1.2 MgSO4, 3 K-ATP, 0.2 Na-GTP, 5 QX314, pH 7.3, ∼280 mosmol/L) (32). With this solution, pipette resistance was 4–6 MΩ and the chloride reversal potential was 2.7 mV. Cells were held at −60 mV in voltage-clamp configuration and these currents were of the inward direction. Recordings were performed in the presence of pharmacological block of NMDA and AMPA glutamatergic receptors using antagonists AP5 (10 µM) and CNQX (10 µM), respectively, to isolate IPSCs from excitatory postsynaptic currents (EPSCs).
Interneuron-mediated IPSCs were evoked by stimulating the nTS (nTS-IPSC). A concentric bipolar electrode (FHC, Bowdoin, ME) was placed within the medial nTS and stimulated at 0.5 Hz and 20 Hz. Spontaneous IPSCs (sIPSC) were recorded in the absence of any stimulus. Miniature IPSC (mIPSC) recordings included tetrodotoxin (TTX 1 µM, Tocris) in the aCSF. At the end of the protocol, GABAA receptor antagonists Gabazine (20 µM, Tocris) and Bicuculline (20 µM, Tocris) were used to block phasic and tonic GABAA receptor activity, respectively. GABA receptors were activated via brief picoapplication of GABA (100 µM, 15 ms) (33) via a pipette placed ∼50 µm away from the recorded cell.
Signals were acquired using a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA), connected to a data acquisition system (Digidata 1440, Molecular Devices, Sunnyvale, CA) and filtered at 2 kHz. Neurons were excluded from data analysis if holding currents were more negative than −50 pA, series resistance higher than 25 MΩ upon initial rupture or unstable (≥20% during recording). The capacitance and input resistance of the neurons were comparable between the groups (HU: 24 ± 10 pF, 832 ± 224 MΩ, n = 15 cells, N = 12 rats; control: 21 ± 10 pF, 790 ± 375 MΩ, n = 16 cells, N = 14 rats, P > 0.05).
Live Imaging of GABA Release
Rats at 3 wk of age were anesthetized with isoflurane and positioned prone in a stereotaxic frame (Kopf Instruments). A localized occipital craniotomy was performed to expose the brainstem. The intensity-based GABA sensing fluorescence reporter (iGABASnFR, pAAV.hSyn-FLEX.iGABASnFR, Addgene plasmid No. 112163) (34) was injected bilaterally into the nTS (100 nL) using a glass micropipette at the following coordinates relative to calamus scriptorius (in mm): 0.3 anteroposterior, 0.3 mediolateral, and −0.3 ventral to the dorsal surface of the medulla. Following injection, the pipette remained in place for at least 5 min to prevent effluent movement up the injection tract. The incision was closed with 4-0 vicryl. After the nanoinjection, stainless steel wire rings were positioned in the tail of the rats as aforementioned. Antibiotic (Enrofloxacin 10 mg/kg, Newry, Northern Ireland, UK) and analgesic (Buprenorphine 0.05 mg/kg, Columbus, OH) were administered subcutaneously. After recovery from anesthesia, the animals were placed in their home cage. Two weeks after iGABASnFR injection rats underwent either HU or control. Fluorescence imaging occurred ∼4 wk after virus injection, which was adequate for iGABASnFR expression in the nTS.
For the imaging protocol, coronal nTS slices (250 μm) were generated to increase the opportunity to obtain responses in an individual rat. The slices were transferred to the recording chamber and constantly superfused at a rate of 2.5 mL/min with aCSF (aerated with 95% O2 + 5% CO2) at a temperature of 33°C. A concentric bipolar electrode (FHC, Bowdoin, ME) was placed on the nTS and a train of stimuli was applied (10 stimuli at 50 Hz). Time-lapse confocal iGABASnFR images were acquired via a Yokagawa CSU-W1 confocal system (3i, Denver, CO) using ×40 water-immersion objective (Olympus ×40/0.8 W LUMPlanFL/IR), Prime 95B sCMOS camera (Photometrics), and a fast 488-nm excitation laser.
Immunoblot Analysis
Relative concentration of GAD67, VGAT or GABAAR α1 protein was quantified via immunoblot as previously reported by us (25). The animals were anesthetized with isoflurane, decapitated, the brainstem removed, and a horizontal brainstem slice was generated (∼280 μm). The nTS was identified under a dissection scope, quickly isolated from surrounding tissue using a microblade, flash-frozen in liquid nitrogen, and stored at −80°C until used. The samples were homogenized in RIPA buffer [150 mM NaCl, 50 mM Tris-HCl, 2.5 mM EDTA, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 1% Nonidet P40 Substitute, protease inhibitor cocktail (Complete Mini, EDTA-free; Cat. No. 1183617001, Roche Diagnostics, Indianapolis, IN)], centrifuged (10 min, 13,000 rpm, 4°C), and the supernatant was obtained. Protein concentration was determined using the Bio-Rad Protein Assay Dye Reagent (Bio-Rad, Hercules, CA), 20 µg of protein was separated in a 4%–20% stain-free gel and transferred to an Immun-Blot polyvinylidene difluoride membrane (Bio-Rad).
The membranes were incubated overnight at 4°C with primary antibodies against GAD67 (1:2,500, Chemicon No. MAB5406), VGAT (1:1,000, Synaptic Systems No. 131003), or GABAAR α1 (1:1,000, Synaptic Systems No. 224203). Following a Tris-buffered saline-Tween 20 (TBST) wash, membranes were incubated with horseradish peroxidase-linked secondary antibodies (1:10,000 Jackson Immunoresearch Laboratories). Immunoblot images were obtained with the ChemiDoc XRS+ Imager using Image Laboratory Software (v. 5.1; Bio-Rad). Relative amounts of GAD67, VGAT, or GABAAR α1 were normalized to total protein.
Immunofluorescence
Rats were anesthetized with isoflurane and transcardially perfused with ice-cold 0.01 M phosphate-buffered saline (PBS, 125 mL) followed by 4% paraformaldehyde (250 mL, pH = 7.4; Sigma). The brains were removed, postfixed for 2 h in 4% paraformaldehyde, and then stored in 0.01 M PBS. Coronal brainstem sections (30 µm) were obtained using a vibratome (VT1000S, Leica). Sections were washed in 0.01 M PBS (3 times for 5 min), incubated for 1 h with hydrogen peroxide (3%), then blocked with 10% Normal Donkey Serum (NDS; S30; Millipore) in 0.01 M PBS for 1 h. Tissue sections were then rinsed with 0.01 M PBS (3 times for 5 min) and incubated with the primary antibodies: mouse anti-GAD67 (1:5,000, Chemincon No. MAB5406) and rabbit anti-VGAT (1:6,000, Synaptic Systems, No. 131003) in 1% NDS in 0.01 M PBS. After 48 h of incubation, sections were rinsed and incubated for 2 h in donkey anti-mouse biotin (1:200, Jackson ImmunoResearch No. 715065150) and donkey anti-rabbit Cy3 (1:200, Jackson ImmunoResearch, No. 711165152) with 1% NDS, in PBS (0.01 M). GAD67 was visualized following tyramide signal amplification as directed by the manufacturer (Invitrogen TSA kit, Cat. No. B40931). In brief, sections were sequentially incubated in 1× horseradish peroxidase (HRP) conjugated streptavidin (60 min), TSA-working solution (10 min), and streptavidin Cy2 (1:200, 2 h) in 3% NDS, in PBS (0.01 M). Sections were washed (3 times for 10 min), mounted on gelatin-coated slides, coverslipped with ProLong Gold, and sealed with nail polish. One section for each rat was incubated without primary antibody and used as a negative control. Immunofluorescence images were acquired either using an upright fluorescence microscope (BX51, Olympus, Japan) equipped with an ORCA-ER CCD camera (Hamamatsu Photonics, K.K., Hamamatsu City, Japan) or a Nikon Eclipse Ti2 microscope equipped with a ×60 oil objective (NA 1.4), Yokakawa CSU-W1 SoRa confocal scanner unit, and Hamamatsu Orca-Fusion CMOS camera (C14440-20UP).
Data Analysis
Clampfit 10 (Molecular Devices) and Microsoft Excel software were used to analyze electrophysiological data. nTS-IPSC amplitude was evaluated from 20 replicates of the stimulus at 0.5 Hz. The average trace of nTS-IPSC was normalized to its peak for the analysis of the rise time, decay time, and half-width of the evoked events. Total area of inward currents was analyzed from five replicates of a train of stimulus (10 stim at 20 Hz). The total current derived from the first stimulus to 200 ms following last current was considered for the evoked area analyses. Clampfit templates were used to detect sIPSCs and mIPSCs (set at 2 times the root‐mean‐square noise level). mIPSC rise time was analyzed via Easy Electrophysiology (v. 2.4.0). The amplitude of GABA-currents generated by GABA puffer was analyzed from three replicates of the protocol. OriginPro software (OriginLab, Northampton, MA) was used to quantify iGABASnFR fluorescence. The peak and area of fluorescence change was obtained by taking the change in fluorescence intensity to nTS stimulation relative to the original intensity [(ΔF/F)] of iGABASnFR. The analyses were performed on up to three regions of interest (ROI) per slice and the fluorescent response to ipsilateral nTS stimulus was averaged (n = 3 control and n = 4 HU rats). Immunoblots for GAD67, VGAT, and GABAAR α1 were analyzed using BioRad software. Immunofluorescence images were analyzed via ImageJ/FIJI; images of three slices comprising the caudomedial nTS from each rat were acquired and the average grayscale values for each animal was used for the analysis. Statistical analysis was performed with GraphPad Prism (v. 9.0; GraphPad Software). Normality was evaluated using Shapiro–Wilk test and outliers were identified by Rout test. Differences between groups were determined via Student’s t test or Mann–Whitney when appropriate. Results are expressed as dispersion of individual values with means ± standard deviation (SD). “N” indicates number of rats whereas “n” indicates number of cells. P values <0.05 were considered significant.
RESULTS
Spontaneous IPSCs in HU
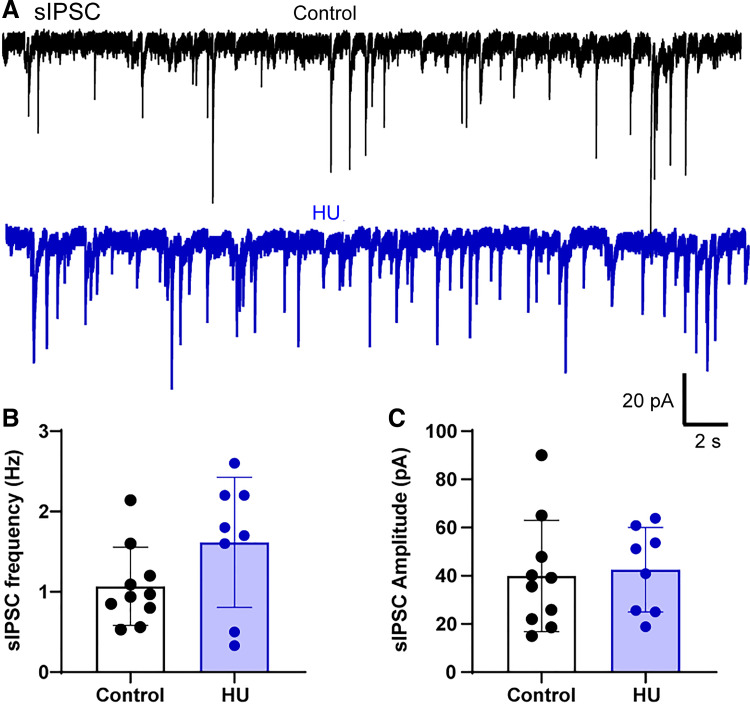
Our previous studies have suggested that following HU, increased nTS glutamate signaling is counter-balanced by elevated GABA tone (25). To begin to examine the locus of this increased GABA signaling, we examined spontaneous inhibitory postsynaptic currents (sIPSCs) in nTS neurons. As shown in the representative examples from a control and HU rat (Fig. 1A), sIPSCs appeared increased following HU, although the elevated sIPSC frequency did not reach statistical significance (control vs. HU, P = 0.0921, unpaired t test, control: n = 10, N = 10; HU: n = 8, N = 8, Fig. 1B). HU and control sIPSC amplitudes were similar (Fig. 1C). The addition of the GABAAR antagonists gabazine and bicuculline to the bath completely abolished sIPSCs indicating that these currents were mediated by GABAARs.
Figure 1.
Hindlimb unloading (HU) does not affect spontaneous inhibitory postsynaptic currents (sIPSCs) in nucleus tractus solitarii (nTS). A: example traces of sIPSC recordings in nTS neurons from a control and HU rat. Average data showing frequency (B) and amplitude (C) of sIPSCs. HU tended to increase sIPSC frequency, but it was not statistically significant (P = 0.0921, control: n = 10, N = 10; HU: n = 8, N = 8, unpaired t test).
HU Increases GABAergic Currents in Response to nTS Stimulation
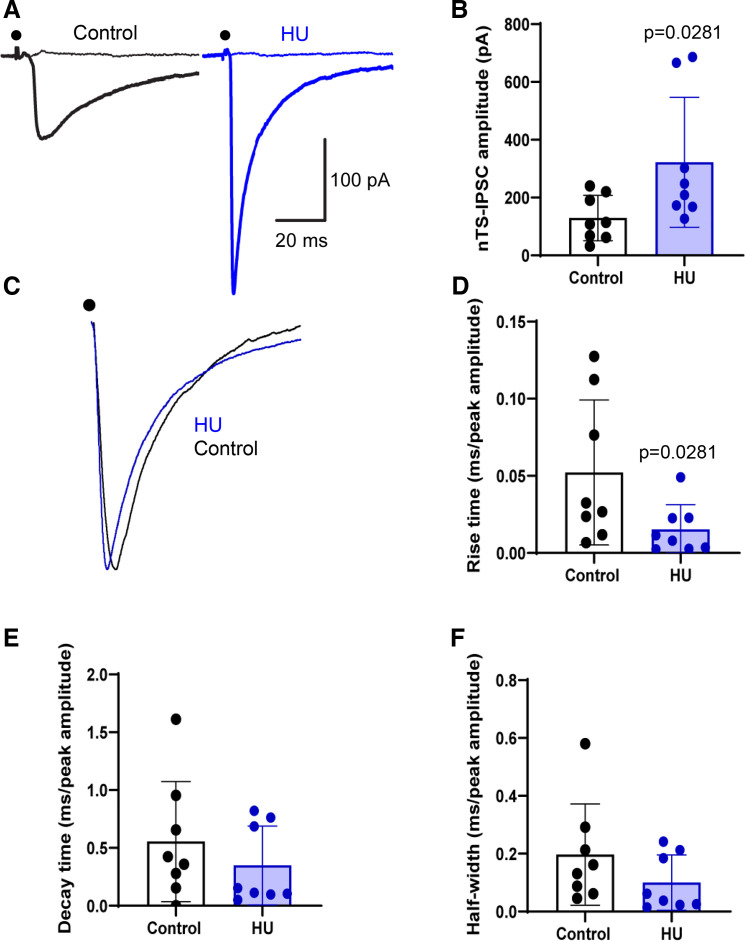
Given that sIPSCs depend upon the tonic activity of a multitude of synapses that impinge on the recorded cell, we focused on evoked GABA signaling. GABAergic interneurons interspersed throughout the nTS critically participate in the modulation of cardiovascular function (35, 36). To assess the direct impact of HU on inhibitory neurotransmission predominantly from such interneurons, we stimulated the nTS and recorded IPSCs evoked at 0.5 and 20 Hz stimulation that encompasses the range of sensory input to the nTS (1, 37). As shown in the example (Fig. 2A), nTS stimulation (0.5 Hz) produced IPSCs in both groups but currents were larger in amplitude in HU compared with control, as quantified in Fig. 2B (control vs. HU, P = 0.0281, Mann–Whitney test, control: n = 8, N = 8; HU: n = 8, N = 8). Augmented nTS-IPSC amplitude was also observed during increased stimulation frequency (see Fig. 3). Similar to sIPSCs, the GABAAR antagonists gabazine and bicuculline completely abolished nTS-IPSCs. Examining the kinetics of normalized IPSCs demonstrated that HU also decreased IPSC rise-time (control vs. HU, P = 0.0281, Mann–Whitney test, Fig. 2, C and D) whereas the decay time and half-width were comparable between groups (Fig. 2, E and F).
Figure 2.
Hindlimb unloading (HU) enhances evoked GABAergic neurotransmission in the nucleus tractus solitarii (nTS). A: representative data showing the increased nTS-evoked inhibitory postsynaptic currents (IPSCs) amplitude in HU compared with control. Dot indicates the point of stimulus. Flat lines indicate the absence of currents after GABAR blockade with gabazine and bicuculline. B: mean data revealing the significant increase in nTS-IPSC amplitude in HU group (control: n = 8, N = 8; HU: n = 8, N = 8). C and D: re-scaled current traces showing that HU reduced the rise time of nTS-IPSCs but had no effect on their decay time (E) and half-width (F). Rise time, decay time, and half-width were normalized to the nTS-IPSC amplitude. B and D, Mann–Whitney test.
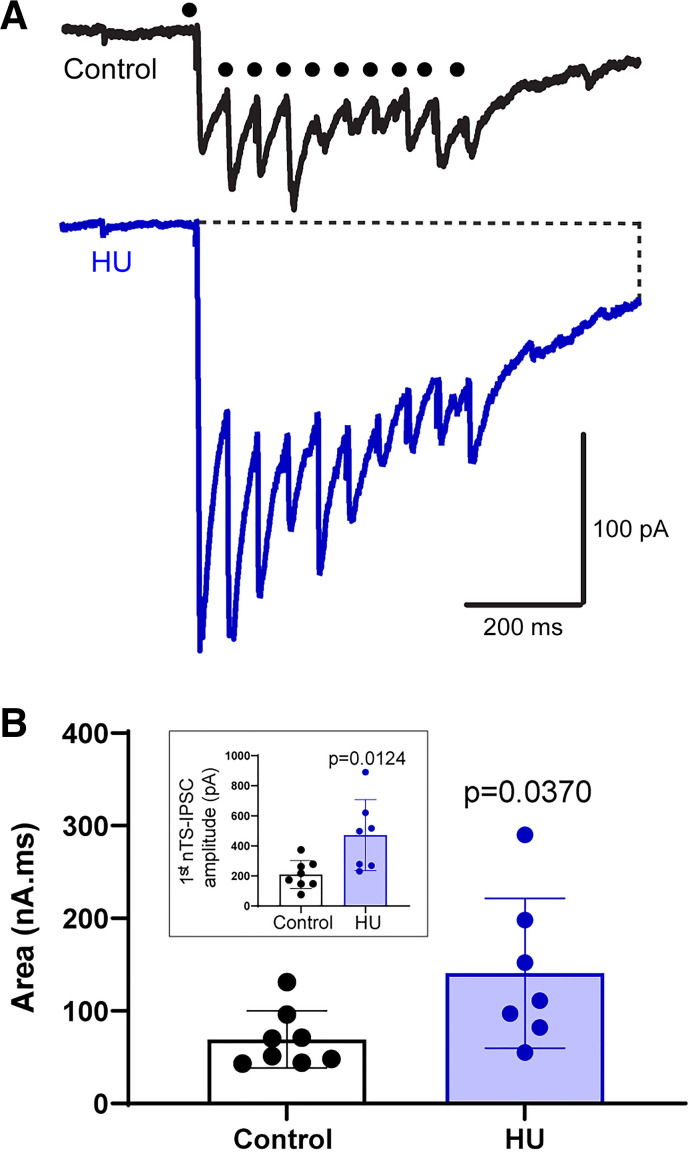
Figure 3.
Hindlimb unloading (HU) increases overall nucleus tractus solitarii-evoked inhibitory postsynaptic currents (nTS-IPSC) signaling. A: example traces of nTS-IPSCs produced by consecutive stimulation of nTS neuropil at higher frequency (20 Hz). Note the enhanced inward current in HU compared to control (control: n = 8, N = 10; HU: n = 7, N = 7). Dot indicates time of nTS stimulation. B: the area of IPSCs produced by nTS stimulation at 20 Hz was greater in HU vs. control. Inset: the amplitude of the initial nTS-IPSCs was greater in HU compared with control. B, Mann–Whitney test’ inset, unpaired t test.
GABAergic neurons within the nTS can be directly activated by cardiovascular afferents or via polysynaptic pathways from the solitary tract (35). We stimulated the nTS at 20 Hz (10 stimuli), which is within the range of baroafferent discharge that influences nTS neuronal activity (1, 37). As illustrated in Fig. 3A, repetitive nTS stimulation generated an inward current as well as phasic nTS-IPSCs. As the effects of lower frequency stimulation, the amplitude of the initial nTS-IPSC that was not influenced by the inward current was larger in HU than control (control vs. HU, P = 0.0124, unpaired t test, control: n = 8, N = 10; HU: n = 7, N = 7, Fig. 3B, inset). To estimate the magnitude of GABA signaling in response to repetitive stimulation, we measured the total inward current from the initial stimulation to 200 ms following the last phasic current. The area of total inward current produced by nTS repetitive stimulation was greater in HU versus control (Fig. 3B, P = 0.0370, Mann–Whitney test). Altogether, our data indicate augmented-evoked GABA signaling in the nTS following HU.
HU Enhances Evoked GABA Release from Interneurons
To determine if the enhanced GABA neurotransmission in the HU nTS was due to increased presynaptic release of GABA, we used nTS slices from rats previously transfected with the genetically encoded GABA sensor, iGABASnFR (34). Repetitive stimulation of the nTS transiently elevated iGABASnFR fluorescence in both control and HU rats (Fig. 4A). As seen in Fig. 4B in response to nTS stimulation and quantified in Fig. 4, C and D, the peak fluorescence increase from baseline [(ΔF/F)] as well as the total fluorescence area was greater in slices from HU compared with control (peak ΔF/F: control vs. HU, P = 0.0452; area: P = 0.0361, unpaired t test, control: 10 averaged ROIs, N = 3; HU: 10 averaged ROIs, N = 4). These data indicate that increased presynaptic release of GABA contributes to the enhanced inhibitory modulation in the nTS of HU rats.
Figure 4.
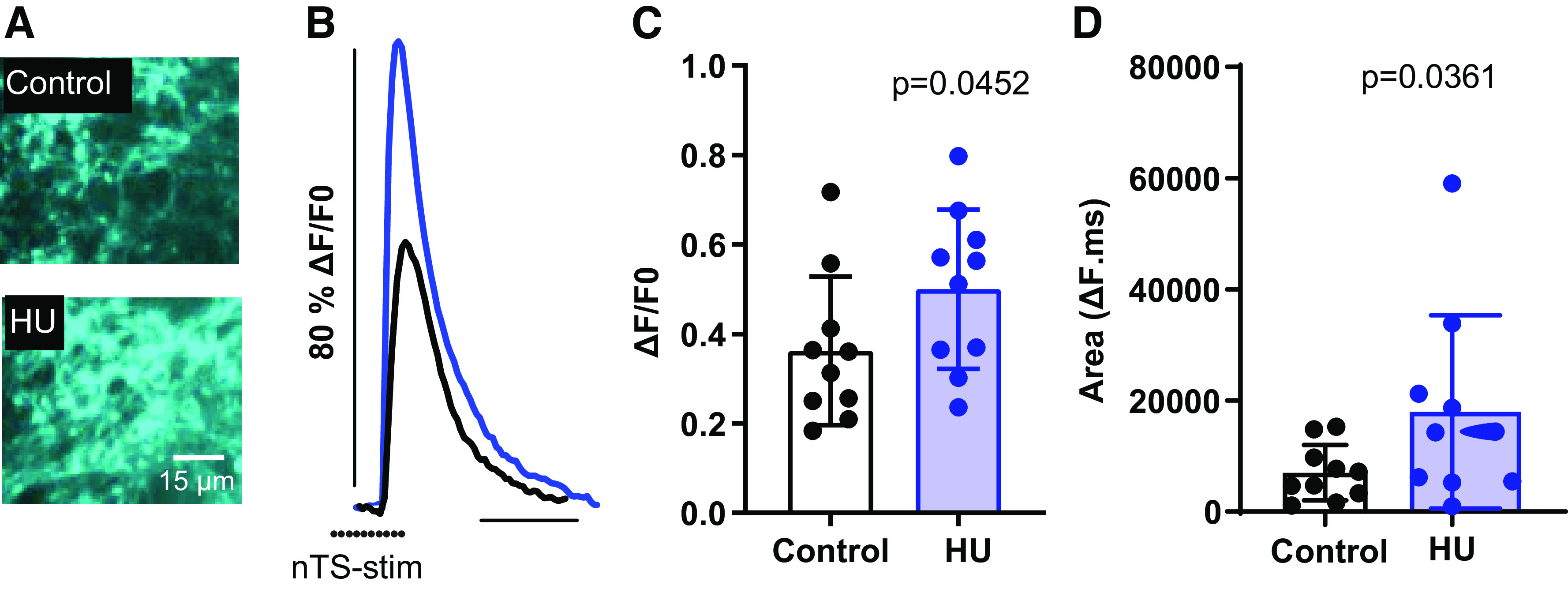
Hindlimb unloading (HU) enhances presynaptic release of GABA in the nucleus tractus solitarii (nTS). A: example of intensity-based GABA sensing fluorescence reporter (iGABASnFR) after nTS stimulation. B: iGABASnFR fluorescence response was greater in HU than control. C: quantitative data of the peak iGABASnFR fluorescence reported as change relative to pre-stimulation baseline (ΔF/F0). D: average fluorescence area showing the increased response in HU compared with control [control: 10 averaged regions of interests (ROIs), N = 3; HU: 10 averaged ROIs, N = 4]. Increased iGABASnFR fluorescence indicates greater presynaptic release of GABA. Dots indicate the point of stimulus. C and D, unpaired t test.
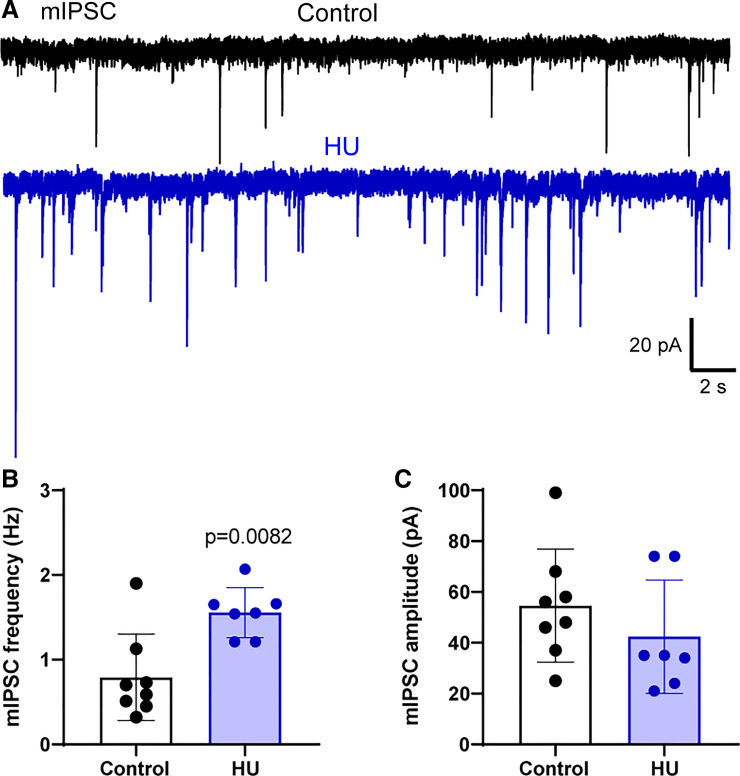
The Frequency of Miniature IPSCs Is Greater following HU
To further identify the loci of increased GABA release in HU rats, we examined sIPSCs following inhibition of neuronal activity. The inclusion of the Na+ channel blocker TTX in the aCSF isolated miniature IPSCs (mIPSC), the currents generated by the spontaneous action potential-independent release of GABA. Bath solution also contained AP5 and CNQX to block NMDARs and AMPARs. As shown in our representative examples (Fig. 5A) and quantified in Fig. 5B, HU increased the frequency of mIPSCs (control vs. HU, P = 0.0082, Mann–Whitney test, control; n = 9, N = 4; HU: n = 7, N = 4). mIPSC amplitudes were comparable in HU and control (Fig. 5C) as were their rise time (control: 0.034 ± 0.022, HU: 0.053 ± 0.030, P > 0.05, unpaired t test). The data indicate presynaptic alterations in HU with minimal changes in postsynaptic receptor function.
Figure 5.
Hindlimb unloading (HU) enhances the action potential-independent release of GABA. A: representative recordings of miniature inhibitory postsynaptic currents (mIPSCs) in the presence of tetrodotoxin (TTX, 1 µM) in nTS neurons from a control and HU rat. B: mean data showing the increased frequency of mIPSCs in HU compared with control. C: quantitative data demonstrating that the amplitude of mIPSCs was comparable between the groups (control; n = 9, N = 4; HU: n = 7, N = 4). B, Mann–Whitney test.
Postsynaptic Responses to GABA and GABARs Are Not Altered in HU
As demonstrated by the elimination of s- and nTS-IPSCs by gabazine and bicuculline, fast GABA neurotransmission in the nTS is mediated by GABAA receptors (GABAAR) on the postsynaptic membrane. The elevated nTS-IPSC amplitude and inward current may be due to increased conductance or number of GABARs to affect inhibitory signaling. To analyze the latter possibility as well as to confirm the effects of pre- versus postsynaptic GABA signaling after HU, GABA was locally applied (picospritzed) to the recorded neuron from an adjacent pipette located ∼50 µm upstream. Picoapplication of GABA (100 µM, 15 ms) (33) produced a large inward current in both groups (IGABA, Fig. 6A, control: n = 6, N = 4; HU: n = 7, N = 4). The amplitude and area of the GABA-generated current were comparable between HU and control (Fig. 6, B and C). The capacitance and input resistance of these neurons were similar between the groups (control: 21 ± 11 pF, 758 ± 196 MΩ, n = 6, N = 4; HU: 23.8 ± 9 pF, 894 ± 368 MΩ, n = 7, N = 4; P > 0.05, unpaired t test). These data further suggest postsynaptic GABAR expression and/or conductance were unaltered following HU.
Figure 6.
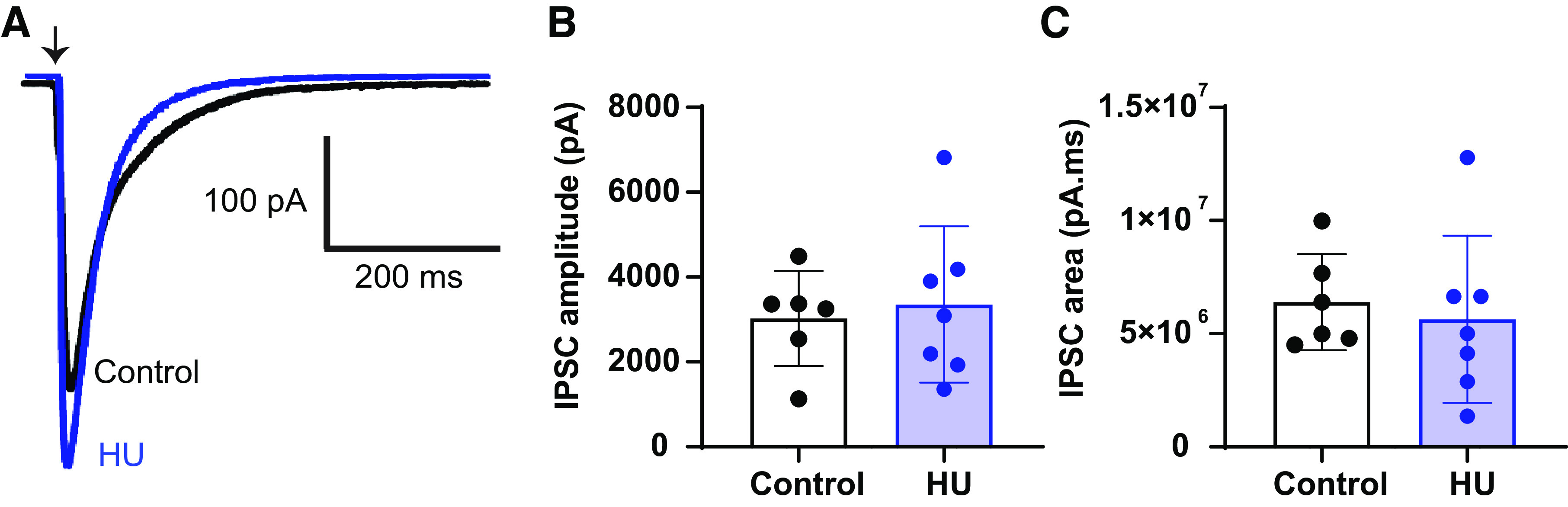
Hindlimb unloading (HU) has no effect on postsynaptic responses to GABA in nucleus tractus solitarii (nTS) neurons. A: puffer application of GABA (100 µM, 15 ms) produces similar postsynaptic responses in nTS neurons of HU and control (control: n = 6, N = 4; HU: n = 7, N = 4). B and C: average data demonstrating the amplitude and area of inward current generated by puffer application of GABA in HU and control. Arrow represents the point of puffer application of GABA. IPSC, inhibitory postsynaptic currents.
To further assess postsynaptic activation, we examined the holding current (Ihold) of neurons during our recording of sIPSCs and nTS-IPSCs. Ihold and its changes generally imply the activation of extrasynaptic GABARs on the postsynaptic cell (38). Overall, initial Ihold was not different between control versus HU (control: −38 ± 21 pA, n = 9, N = 9; HU: −28 ± 11 pA, n = 6, N = 6, P > 0.05, unpaired t test). The addition of gabazine and bicuculline did not change this current in either group (Delta: control, −4.2 ± 5.6 pA, n = 9; HU, −3.5 ± 7 pA, n = 6, P > 0.05, unpaired t test).
Finally, immunoblot analyses of whole tissue extracts from the nTS showed that HU did not alter the expression of GABAAR α1 subunit relative to that of control tissue (HU 0.95 ± 0.79 expression relative to control). Taken together, the lack of alteration of the IGABA, s- and mIPSC amplitude, and GABAAR α1 expression suggests that HU did not specifically alter GABAAR function in the nTS.
HU Does Not Affect the Expression of GAD67 and VGAT
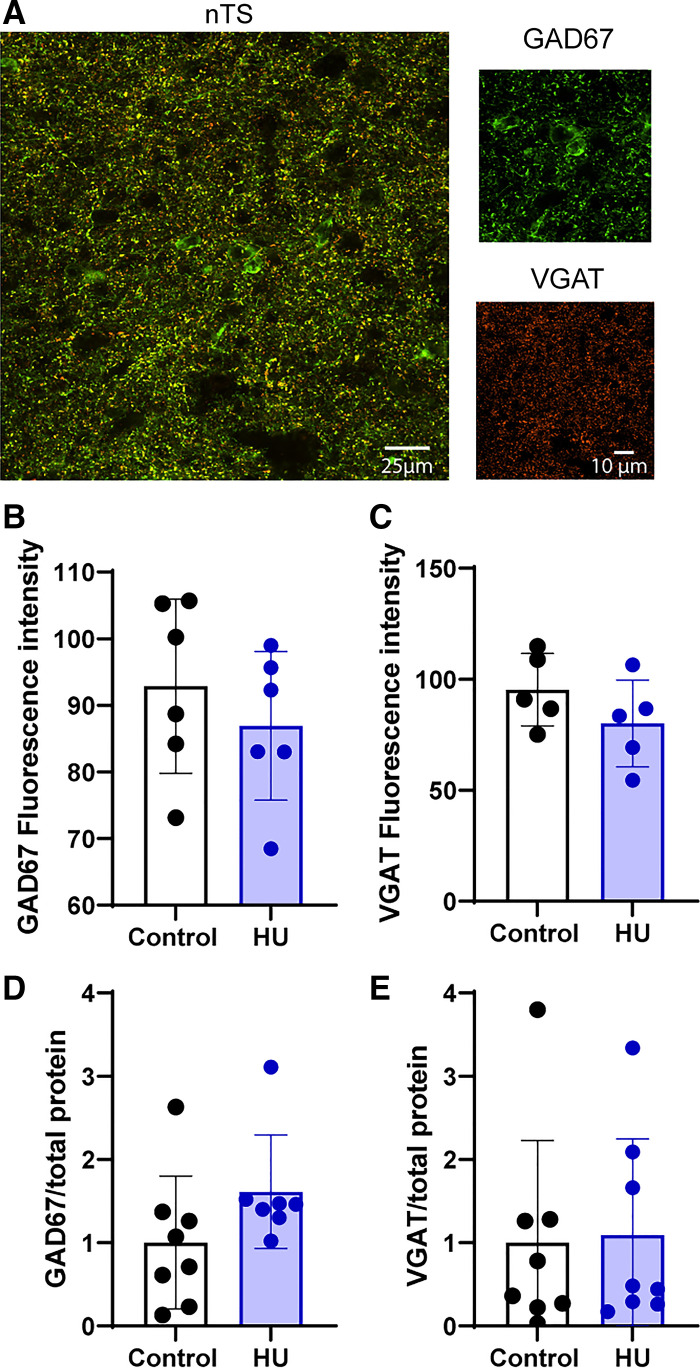
Considering the critical role of GAD67 in producing GABA, as well as VGAT proteins for GABA packaging into vesicles for release (27, 29), we evaluated whether an increase in the expression of these proteins in the nTS is related to the enhanced GABA signaling following HU. Figure 7A shows the immunofluorescence labeling of GAD67 and VGAT in the caudomedial nTS of a representative control rat. Analysis of fluorescence intensity showed that HU had no effect on either GAD67 (Fig. 7B, control: N = 6; HU: N = 6) or VGAT (Fig. 7C, control: N = 5; HU: N = 5) expression. These findings were confirmed by protein evaluation via immunoblots of the nTS region (Fig. 7, D and E, GAD67 control: N = 8; HU: N = 7; VGAT control: N = 8, HU: N = 8).
Figure 7.
Hindlimb unloading (HU) does not change the expression of glutamic acid decarboxylase 67 (GAD67) and vesicular GABA transporter (VGAT). A: immunofluorescence image of GAD67 (green) and VGAT (orange) in the nucleus tractus solitarii (nTS) region of a representative rat from control group. B and C: mean data showing comparable values of fluorescent intensity of GAD67 (control: N = 6, HU: N = 6) and VGAT (control: N = 5, HU: N = 5) in HU and control. Immunoblot quantification of GAD67/total protein ratio (D, control: N = 8, HU: N = 7) and VGAT/total protein ratio (E, control: N = 8, HU: N = 8) confirmed that expression of these proteins was not altered by HU.
DISCUSSION
nTS neurons are crucial to cardiorespiratory and autonomic regulation (39). The wide variety of neurotransmitters and neuromodulators released in this nucleus determines not only nTS neuronal activity but ultimately influences downstream brain regions that are imperative to homeostasis and proper reflex response (1, 40). As a primary source of inhibition within the nTS, GABA offsets excitatory glutamate signaling including that initiated from visceral afferents (10, 35, 37, 41). In the present study, we demonstrate that GABA signaling is enhanced in the nTS of rats exposed to HU, an animal model of cardiovascular deconditioning. We further provide evidence that this plasticity is likely due to increased presynaptic release of GABA onto nTS neurons.
We first examined the magnitude by which HU alters overall GABA signaling and specifically interneuron GABA release by analyzing the effects of nTS stimulation at low and higher frequencies that encompass the rate of discharge of cardiorespiratory afferents projecting to the nTS (1, 37). Our results demonstrate that electrical stimulation of nTS GABAergic interneurons at both frequencies produced greater GABA currents in HU compared with control. This increased inhibitory signaling likely tempers neuronal activity within nTS. These results corroborate our previous findings showing cardiovascular deconditioning hyperpolarizes nTS neurons, increases their rheobase, and decreases their excitability (25). We also showed that HU increased glutamatergic neurotransmission to second-order nTS neurons. Considering that GABA interneurons may receive monosynaptic projections from the solitary tract (35), the increased GABA signaling following HU in vivo might be secondary to increased glutamate excitation of these interneurons and this increased GABA activity may then be maintained in vitro. One could speculate that the increased GABA signaling counterbalances glutamate effects to avoid the overactivation of nTS following cardiovascular deconditioning.
GABA and glutamate influence each other’s function and such cross talk represents an important regulatory mechanism influencing neurotransmission within the nTS. GABA modulates glutamate release through the activation of ionotropic (GABAAR) and metabotropic (GABABR) GABA receptors located on glutamatergic terminals (10, 42) and alters the functionality of ionotropic NMDA and AMPA glutamate receptors (43). Conversely, glutamate activation of metabotropic receptors for glutamate (mGluRs) may increase (mGluR I) or decrease (mGluR II + III) GABA release (10). We previously showed that HU enhances the contribution of postsynaptic NMDA receptors to glutamatergic neurotransmission in the nTS (25), but increased NMDA function on GABAergic interneurons is not likely to contribute to increased in vitro GABA signaling following HU since the IPSC recordings in this study were performed under NMDA receptor blockade.
Nevertheless, alterations in the heterosynaptic modulation of GABA/glutamate could contribute to GABA synaptic plasticity and altered autonomic function observed in HU. For example, HU blunts baroreflex increases in sympathetic nerve activity in response to depressor stimuli (30), yet sympathoinhibition is unaltered following pressor stimuli. The comparable baroreflex-evoked sympathoinhibition in HU rats may be due to the recruitment of GABA signaling that then balances increased baroafferent-mediated glutamate effects. Although the mechanisms are not clear, altered GABA/glutamate interactions may attenuate the reduction in nTS neuronal activity in response to depressor stimuli, leading to a blunted reflex sympathoexcitation. Future studies are needed to address the effects of HU on the cross regulation of GABA and glutamate in the nTS.
Our findings strongly suggest that HU-induced GABA plasticity is likely related to enhanced presynaptic release rather than postsynaptic alterations in receptor expression or conductance. This interpretation is supported by several sets of data, including our live cell imaging, electrophysiological and molecular expression findings. More specifically, we show that the fluorescence response of iGABASnFR to nTS stimuli was larger in HU compared with control, which indicates greater release of GABA by synaptic terminals. HU also significantly increased the frequency of miniature IPSCs (and tended to enhance the frequency of sIPSCs), further indicating a greater presynaptic release of GABA (44). Such greater release likely contributes to the enhanced nTS-evoked IPSC amplitude and the overall current (area) response. Several mechanisms may be responsible for the augmented GABA release. For instance, the enhanced evoked and spontaneous release of GABA following HU may be a result of upregulation of molecular machinery that is common between the two modes of neurotransmission, including voltage-activated Ca2+ channels and the canonical Ca2+ sensor synaptotagmin (45–47). Another possibility may include enhanced GABA synthesis or its packaging in the presynaptic terminals. However, our immunoblot and immunofluorescent analyses demonstrate HU does not alter the expression of GAD67 nor VGAT, indicating GABA synthesis and storage, respectively, are likely not increased to contribute to enhanced GABA signaling. Moreover, HU could increase the number of GABAergic active synapses (without altering the total number) from interneurons or from upstream areas projecting to the nTS (48, 49). The decreased rise time of evoked IPSCs, in contrast to mIPSCs, may also suggest a reorganization of GABAergic synaptic contacts on nTS neurons or increased priming of vesicles or calcium influx following action potential depolarization (47, 50). Finally, although the greater total inward current (area) following repetitive stimulation in HU is likely the result of elevated release, it may also be due to reduced GABA uptake by endogenous GABA transporters (GATs) that we have recently shown to contribute to nTS signaling (51). Future studies are needed to further differentiate these potential mechanisms.
Activation of GABAAR in the nTS inhibits neuronal activity and results in autonomic and cardiovascular alterations, including increased sympathetic nervous system activity, arterial pressure, and heart rate through baroreflex pathways (8, 15). Changes in the expression, subunit composition, phosphorylation state, and affinity of the binding sites are mechanisms by which altered GABAAR conductance may affect physiological function (52–54). Contrary to what we hypothesized, HU does not alter postsynaptic GABAAR expression or function. This conclusion stemmed from our results demonstrating, compared with controls, HU did not alter IGABA following picoapplication of GABA; the amplitude of sIPSC and mIPSC; nTS-IPSC decay time, or the expression of the α1 subunit, one of the predominant α subunit isoforms in GABAARs that is directly related to GABA signaling (55). In addition, in response to gabazine and bicuculline, changes in Ihold that reflect activation of extracellular GABA receptors (38) were comparable between groups. Therefore, our functional and molecular data suggest that HU does not affect postsynaptic GABAARs in the nTS.
Altered GABAergic neurotransmission in the nTS strongly impacts cardiovascular and autonomic function (56, 57). nTS activity can be modulated by GABAergic circuits within the nTS as well as from other brain regions, including amygdala and periaqueductal gray (48, 49). The nTS integrates multiple distinct cardiovascular reflexes, and the physiological consequences of changes in GABA signaling likely depend on the neuronal subpopulation affected. Although in this study we did not identify the nTS neuronal subgroup recorded, our present and recent findings (25) are consistent with an increase in the inhibitory modulation of neurons involved in cardiovascular function after HU. For example, inhibition of the nTS leads to increased basal activity of presympathetic neurons in the rostral ventrolateral medulla (RVLM), ultimately increasing heart rate and blood pressure (58, 59). In addition, HU blunts baroreflex control of sympathetic nervous system activity and heart rate (30), enhances reflex control of vasopressin release (60), and augments cardiopulmonary reflex-mediated sympathoinhibition (61). Since GABA also influences the nTS circuits controlling the parasympathetic (62), respiratory (39), gustatory (63), and gastrointestinal (64) systems it is possible that altered GABA signaling in the nTS, along with other central changes (65), could contribute to HU-induced alterations in reflex function. The influence of HU, and specifically altered GABA, in these circuits requires additional study.
In summary, the present study demonstrates that cardiovascular deconditioning increases the inhibitory modulation of nTS neurons and provides insight into the mechanisms involved in these alterations. HU enhances GABA signaling onto nTS neurons primarily due to presynaptic mechanisms. Functional and molecular alterations of GABAA receptors do not seem to be involved in this plasticity. Future studies are needed to determine the effects of HU on other GABAARs subunits, GABABRs, and GABA transporters. Considering that the fine balance between excitatory and inhibitory neurotransmitters in the nTS is crucial for cardiovascular and autonomic function, our results provide potential brainstem mechanisms related to cardiovascular deconditioning.
GRANTS
This study was supported by National Institutes of Health (NIH) HL132836 (to E.M.H.), HL128454 (to D.D.K.), AHA 836140 (to LL.-S.) and American Autonomic Society-Lundbeck Fellowship (L.L.-S.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.L.S., E.M.H., and D.D.K. conceived and designed research; L.L.S. performed experiments; L.L.S. analyzed data; L.L.S., E.M.H., and D.D.K. interpreted results of experiments; L.L.S. and D.D.K. prepared figures; L.L.S. and D.D.K. drafted manuscript; L.L.S., E.M.H., and D.D.K. edited and revised manuscript; L.L.S., E.M.H., and D.D.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Sarah Friskey for the technical support in Hindlimb Unloading protocol and nanoinjections, Britton Stamps for help with immunohistochemistry, and Heather Dantzler for technical expertise in immunoblots.
REFERENCES
- 1.Andresen MC, Kunze DL. Nucleus tractus solitarius—gateway to neural circulatory control. Annu Rev Physiol 56: 93–116, 1994. doi: 10.1146/annurev.ph.56.030194.000521. [DOI] [PubMed] [Google Scholar]
- 2.Andresen MC, Doyle MW, Bailey TW, Jin YH. Differentiation of autonomic reflex control begins with cellular mechanisms at the first synapse within the nucleus tractus solitarius. Braz J Med Biol Res 37: 549–558, 2004. doi: 10.1590/s0100-879x2004000400012. [DOI] [PubMed] [Google Scholar]
- 3.Mifflin SW, Felder RB. Synaptic mechanisms regulating cardiovascular afferent inputs to solitary tract nucleus. Am J Physiol Heart Circ Physiol 259: H653–H661, 1990. doi: 10.1152/ajpheart.1990.259.3.H653. [DOI] [PubMed] [Google Scholar]
- 4.Talman WT, Perrone MH, Reis DJ. Evidence for l-glutamate as the neurotransmitter of baroreceptor afferent nerve fibers. Science 209: 813–815, 1980. doi: 10.1126/science.6105709. [DOI] [PubMed] [Google Scholar]
- 5.Andresen MC, Yang MY. Non-NMDA receptors mediate sensory afferent synaptic transmission in medial nucleus tractus solitarius. Am J Physiol Heart Circ Physiol 259: H1307–H1311, 1990. doi: 10.1152/ajpheart.1990.259.4.H1307. [DOI] [PubMed] [Google Scholar]
- 6.Izzo PN, Sykes RM, Spyer KM. γ-Aminobutyric acid immunoreactive structures in the nucleus tractus solitarius: a light and electron microscopic study. Brain Res 591: 69–78, 1992. doi: 10.1016/0006-8993(92)90979-j. [DOI] [PubMed] [Google Scholar]
- 7.Catelli JM, Giakas WJ, Sved AF. GABAergic mechanisms in nucleus tractus solitarius alter blood pressure and vasopressin release. Brain Res 403: 279–289, 1987. doi: 10.1016/0006-8993(87)90065-5. [DOI] [PubMed] [Google Scholar]
- 8.Callera JC, Bonagamba LG, Nosjean A, Laguzzi R, Machado BH. Activation of GABA receptors in the NTS of awake rats reduces the gain of baroreflex bradycardia. Auton Neurosci 84: 58–67, 2000. doi: 10.1016/S1566-0702(00)00184-3. [DOI] [PubMed] [Google Scholar]
- 9.Sved JC, Sved AF. Cardiovascular responses elicited by γ-aminobutyric acid in the nucleus tractus solitarius: evidence for action at the GABAB receptor. Neuropharmacology 28: 515–520, 1989. doi: 10.1016/0028-3908(89)90088-9. [DOI] [PubMed] [Google Scholar]
- 10.Fernandes LG, Jin YH, Andresen MC. Heterosynaptic crosstalk: GABA-glutamate metabotropic receptors interactively control glutamate release in solitary tract nucleus. Neuroscience 174: 1–9, 2011. doi: 10.1016/j.neuroscience.2010.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vitela M, Mifflin SW. γ-Aminobutyric acid(B) receptor-mediated responses in the nucleus tractus solitarius are altered in acute and chronic hypertension. Hypertension 37: 619–622, 2001. doi: 10.1161/01.HYP.37.2.619. [DOI] [PubMed] [Google Scholar]
- 12.Durgam VR, Vitela M, Mifflin SW. Enhanced γ-aminobutyric acid-B receptor agonist responses and mRNA within the nucleus of the solitary tract in hypertension. Hypertension 33: 530–536, 1999. doi: 10.1161/01.hyp.33.1.530. [DOI] [PubMed] [Google Scholar]
- 13.Moreira TS, Takakura AC, Colombari E. Important GABAergic mechanism within the NTS and the control of sympathetic baroreflex in SHR. Auton Neurosci 159: 62–70, 2011. doi: 10.1016/j.autneu.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Tsukamoto K, Sved AF. Enhanced γ-aminobutyric acid-mediated responses in nucleus tractus solitarius of hypertensive rats. Hypertension 22: 819–825, 1993. doi: 10.1161/01.HYP.22.6.819. [DOI] [PubMed] [Google Scholar]
- 15.Tolstykh G, Belugin S, Tolstykh O, Mifflin S. Responses to GABA(A) receptor activation are altered in NTS neurons isolated from renal-wrap hypertensive rats. Hypertension 42: 732–736, 2003. doi: 10.1161/01.HYP.0000084371.17927.02. [DOI] [PubMed] [Google Scholar]
- 16.Mei L, Zhang J, Mifflin S. Hypertension alters GABA receptor-mediated inhibition of neurons in the nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol 285: R1276–R1286, 2003. doi: 10.1152/ajpregu.00255.2003. [DOI] [PubMed] [Google Scholar]
- 17.Hasser EM, Moffitt JA. Regulation of sympathetic nervous system function after cardiovascular deconditioning. Ann NY Acad Sci 940: 454–468, 2001. doi: 10.1111/j.1749-6632.2001.tb03698.x. [DOI] [PubMed] [Google Scholar]
- 18.Taylor HL, Henschel A. and Effects of bed rest on cardiovascular function and work performance. J Appl Physiol 2: 223–239, 1949. doi: 10.1152/jappl.1949.2.5.223. [DOI] [PubMed] [Google Scholar]
- 19.Chobanian AV, Lille RD, Tercyak A, Blevins P. The metabolic and hemodynamic effects of prolonged bed rest in normal subjects. Circulation 49: 551–559, 1974. doi: 10.1161/01.cir.49.3.551. [DOI] [PubMed] [Google Scholar]
- 20.Shellock FG, Swan HJ, Rubin SA. Early central venous pressure changes in the rat during two different levels of head-down suspension. Aviat Space Environ Med 56: 791–795, 1985. [PubMed] [Google Scholar]
- 21.Morey-Holton E, Globus RK, Kaplansky A, Durnova G. The hindlimb unloading rat model: literature overview, technique update and comparison with space flight data. Adv Space Biol Med 10: 7–40, 2005. doi: 10.1016/s1569-2574(05)10002-1. [DOI] [PubMed] [Google Scholar]
- 22.Moffitt JA, Henry MK, Welliver KC, Jepson AJ, Garnett ER. Hindlimb unloading results in increased predisposition to cardiac arrhythmias and alters left ventricular connexin 43 expression. Am J Physiol Regul Integr Comp Physiol 304: R362–R373, 2013. doi: 10.1152/ajpregu.00391.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moffitt JA, Grippo AJ, Beltz TG, Johnson AK. Hindlimb unloading elicits anhedonia and sympathovagal imbalance. J Appl Physiol (1985) 105: 1049–1059, 2008. doi: 10.1152/japplphysiol.90535.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moffitt JA, Schadt JC, Hasser EM. Altered central nervous system processing of baroreceptor input following hindlimb unloading in rats. Am J Physiol Heart Circ Physiol 277: H2272–H2279, 1999. doi: 10.1152/ajpheart.1999.277.6.h2272. [DOI] [PubMed] [Google Scholar]
- 25.Lima-Silveira L, Martinez D, Hasser EM, Kline DD. Mechanisms underlying neuroplasticity in the nucleus tractus solitarii following hindlimb unloading in rats. Neuroscience 449: 214–227, 2020. doi: 10.1016/j.neuroscience.2020.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhai B, Fu J, Xiang S, Shang Y, Yan Y, Yin T, Zhang T. Repetitive transcranial magnetic stimulation ameliorates recognition memory impairment induced by hindlimb unloading in mice associated with BDNF/TrkB signaling. Neurosci Res 153: 40–47, 2020. doi: 10.1016/j.neures.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Fong AY, Stornetta RL, Foley CM, Potts JT. Immunohistochemical localization of GAD67-expressing neurons and processes in the rat brainstem: subregional distribution in the nucleus tractus solitarius. J Comp Neurol 493: 274–290, 2005. doi: 10.1002/cne.20758. [DOI] [PubMed] [Google Scholar]
- 28.Asada H, Kawamura Y, Maruyama K, Kume H, Ding RG, Kanbara N, Kuzume H, Sanbo M, Yagi T, Obata K. Cleft palate and decreased brain γ-aminobutyric acid in mice lacking the 67-kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci USA 94: 6496–6499, 1997. doi: 10.1073/pnas.94.12.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McIntire SL, Reimer RJ, Schuske K, Edwards RH, Jorgensen EM. Identification and characterization of the vesicular GABA transporter. Nature 389: 870–876, 1997. doi: 10.1038/39908. [DOI] [PubMed] [Google Scholar]
- 30.Moffitt JA, Foley CM, Schadt JC, Laughlin MH, Hasser EM. Attenuated baroreflex control of sympathetic nerve activity after cardiovascular deconditioning in rats. Am J Physiol Regul Integr Comp Physiol 274: R1397–R1405, 1998. doi: 10.1152/ajpregu.1998.274.5.r1397. [DOI] [PubMed] [Google Scholar]
- 31.Lima-Silveira L, Accorsi-Mendonca D, Bonagamba LGH, Almado CEL, da Silva MP, Nedoboy PE, Pilowsky PM, Machado BH. Enhancement of excitatory transmission in NTS neurons projecting to ventral medulla of rats exposed to sustained hypoxia is blunted by minocycline. J Physiol 597: 2903–2923, 2019. doi: 10.1113/JP277532. [DOI] [PubMed] [Google Scholar]
- 32.Ostrowski TD, Ostrowski D, Hasser EM, Kline DD. Depressed GABA and glutamate synaptic signaling by 5-HT1A receptors in the nucleus tractus solitarii and their role in cardiorespiratory function. J Neurophysiol 111: 2493–2504, 2014. doi: 10.1152/jn.00764.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forman CJ, Tomes H, Mbobo B, Burman RJ, Jacobs M, Baden T, Raimondo JV. Openspritzer: an open hardware pressure ejection system for reliably delivering picolitre volumes. Sci Rep 7: 2188, 2017. doi: 10.1038/s41598-017-02301-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marvin JS, Shimoda Y, Magloire V, Leite M, Kawashima T, Jensen TP, Kolb I, Knott EL, Novak O, Podgorski K, Leidenheimer NJ, Rusakov DA, Ahrens MB, Kullmann DM, Looger LL. A genetically encoded fluorescent sensor for in vivo imaging of GABA. Nat Methods 16: 763–770, 2019. doi: 10.1038/s41592-019-0471-2. [DOI] [PubMed] [Google Scholar]
- 35.Bailey TW, Appleyard SM, Jin YH, Andresen MC. Organization and properties of GABAergic neurons in solitary tract nucleus (NTS). J Neurophysiol 99: 1712–1722, 2008. doi: 10.1152/jn.00038.2008. [DOI] [PubMed] [Google Scholar]
- 36.Zhang W, Mifflin S. Plasticity of GABAergic mechanisms within the nucleus of the solitary tract in hypertension. Hypertension 55: 201–206, 2010. doi: 10.1161/HYPERTENSIONAHA.109.146407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andresen MC, Yang M. Dynamics of sensory afferent synaptic transmission in aortic baroreceptor regions on nucleus tractus solitarius. J Neurophysiol 74: 1518–1528, 1995. doi: 10.1152/jn.1995.74.4.1518. [DOI] [PubMed] [Google Scholar]
- 38.Park JB, Skalska S, Son S, Stern JE. Dual GABAA receptor-mediated inhibition in rat presympathetic paraventricular nucleus neurons. J Physiol 582: 539–551, 2007. doi: 10.1113/jphysiol.2007.133223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zoccal DB, Furuya WI, Bassi M, Colombari DS, Colombari E. The nucleus of the solitary tract and the coordination of respiratory and sympathetic activities. Front Physiol 5: 238, 2014. doi: 10.3389/fphys.2014.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Giersbergen PL, Palkovits M, De Jong W. Involvement of neurotransmitters in the nucleus tractus solitarii in cardiovascular regulation. Physiol Rev 72: 789–824, 1992. doi: 10.1152/physrev.1992.72.3.789. [DOI] [PubMed] [Google Scholar]
- 41.Kawai Y, Senba E. Organization of excitatory and inhibitory local networks in the caudal nucleus of tractus solitarius of rats revealed in in vitro slice preparation. J Comp Neurol 373: 309–321, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 42.Kang YH, Sun B, Park YS, Park CS, Jin YH. GABAA and GABAB receptors have opposite effects on synaptic glutamate release on the nucleus tractus solitarii neurons. Neuroscience 209: 39–46, 2012. doi: 10.1016/j.neuroscience.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 43.Kantamneni S. Cross-talk and regulation between glutamate and GABAB receptors. Front Cell Neurosci 9: 135, 2015. doi: 10.3389/fncel.2015.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Katz B. Elementary components of synaptic transmission. Naturwissenschaften 66: 606–610, 1979. doi: 10.1007/BF00405119. [DOI] [PubMed] [Google Scholar]
- 45.Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, Südhof TC. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell 79: 717–727, 1994. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- 46.Courtney NA, Briguglio JS, Bradberry MM, Greer C, Chapman ER. Excitatory and inhibitory neurons utilize different Ca2+ sensors and sources to regulate spontaneous release. Neuron 98: 977–991.e5, 2018. doi: 10.1016/j.neuron.2018.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Horvath PM, Piazza MK, Monteggia LM, Kavalali ET. Spontaneous and evoked neurotransmission are partially segregated at inhibitory synapses. eLife 9: e52852, 2020. doi: 10.7554/eLife.52852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saha S. Role of the central nucleus of the amygdala in the control of blood pressure: descending pathways to medullary cardiovascular nuclei. Clin Exp Pharmacol Physiol 32: 450–456, 2005. doi: 10.1111/j.1440-1681.2005.04210.x. [DOI] [PubMed] [Google Scholar]
- 49.Netzer F, Mandjee N, Verberne AJ, Bernard JF, Hamon M, Laguzzi R, Sévoz-Couche C. Inhibition of the bradycardic component of the von Bezold-Jarisch reflex and carotid chemoreceptor reflex by periaqueductal gray stimulation: involvement of medullary receptors. Eur J Neurosci 29: 2017–2028, 2009. doi: 10.1111/j.1460-9568.2009.06758.x. [DOI] [PubMed] [Google Scholar]
- 50.Magee JC, Cook EP. Somatic EPSP amplitude is independent of synapse location in hippocampal pyramidal neurons. Nat Neurosci 3: 895–903, 2000. doi: 10.1038/78800. [DOI] [PubMed] [Google Scholar]
- 51.Martinez D, Lima-Silveira L, Matott MP, Hasser EM, Kline DD. γ-Aminobutyric acid transporters in the nucleus tractus solitarii regulate inhibitory and excitatory synaptic currents that influence cardiorespiratory function. Front Physiol 12: 821110, 2021. doi: 10.3389/fphys.2021.821110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mehta AK, Ticku MK. An update on GABAA receptors. Brain Res Rev 29: 196–217, 1999. doi: 10.1016/s0165-0173(98)00052-6. [DOI] [PubMed] [Google Scholar]
- 53.Mody I. Aspects of the homeostaic plasticity of GABAA receptor-mediated inhibition. J Physiol 562: 37–46, 2005. doi: 10.1113/jphysiol.2004.077362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gaiarsa JL, Caillard O, Ben-Ari Y. Long-term plasticity at GABAergic and glycinergic synapses: mechanisms and functional significance. Trends Neurosci 25: 564–570, 2002. doi: 10.1016/s0166-2236(02)02269-5. [DOI] [PubMed] [Google Scholar]
- 55.Saha S, Sieghart W, Fritschy JM, McWilliam PN, Batten TF. γ-Aminobutyric acid receptor (GABAA) subunits in rat nucleus tractus solitarii (NTS) revealed by polymerase chain reaction (PCR) and immunohistochemistry. Mol Cell Neurosci 17: 241–257, 2001. doi: 10.1006/mcne.2000.0919. [DOI] [PubMed] [Google Scholar]
- 56.Andresen MC, Mendelowitz D. Sensory afferent neurotransmission in caudal nucleus tractus solitarius—common denominators. Chem Senses 21: 387–395, 1996. doi: 10.1093/chemse/21.3.387. [DOI] [PubMed] [Google Scholar]
- 57.Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR. Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol (1985) 101: 618–627, 2006. doi: 10.1152/japplphysiol.00252.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ito S, Sved AF. Tonic glutamate-mediated control of rostral ventrolateral medulla and sympathetic vasomotor tone. Am J Physiol Regul Integr Comp Physiol 273: R487–R494, 1997. doi: 10.1152/ajpregu.1997.273.2.R487. [DOI] [PubMed] [Google Scholar]
- 59.Moreira TS, Sato MA, Takakura AC, Menani JV, Colombari E. Role of pressor mechanisms from the NTS and CVLM in control of arterial pressure. Am J Physiol Regul Integr Comp Physiol 289: R1416–R1425, 2005. doi: 10.1152/ajpregu.00053.2005. [DOI] [PubMed] [Google Scholar]
- 60.Mueller PJ, Sullivan MJ, Grindstaff RR, Cunningham JT, Hasser EM. Regulation of plasma vasopressin and renin activity in conscious hindlimb-unloaded rats. Am J Physiol Regul Integr Comp Physiol 291: R46–R52, 2006. doi: 10.1152/ajpregu.00622.2005. [DOI] [PubMed] [Google Scholar]
- 61.Mueller PJ, Hasser EM. Enhanced sympathoinhibitory response to volume expansion in conscious hindlimb-unloaded rats. J Appl Physiol (1985) 94: 1806–1812, 2003. doi: 10.1152/japplphysiol.00979.2002. [DOI] [PubMed] [Google Scholar]
- 62.Frank JG, Jameson HS, Gorini C, Mendelowitz D. Mapping and identification of GABAergic neurons in transgenic mice projecting to cardiac vagal neurons in the nucleus ambiguus using photo-uncaging. J Neurophysiol 101: 1755–1760, 2009. doi: 10.1152/jn.91134.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Travers JB. Efferent projections from the anterior nucleus of the solitary tract of the hamster. Brain Res 457: 1–11, 1988. doi: 10.1016/0006-8993(88)90051-0. [DOI] [PubMed] [Google Scholar]
- 64.Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol 68: 279–305, 2006. doi: 10.1146/annurev.physiol.68.040504.094635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moffitt JA, Heesch CM, Hasser EM. Increased GABAA inhibition of the RVLM after hindlimb unloading in rats. Am J Physiol Regul Integr Comp Physiol 283: R604–R614, 2002. doi: 10.1152/ajpregu.00341.2001. [DOI] [PubMed] [Google Scholar]