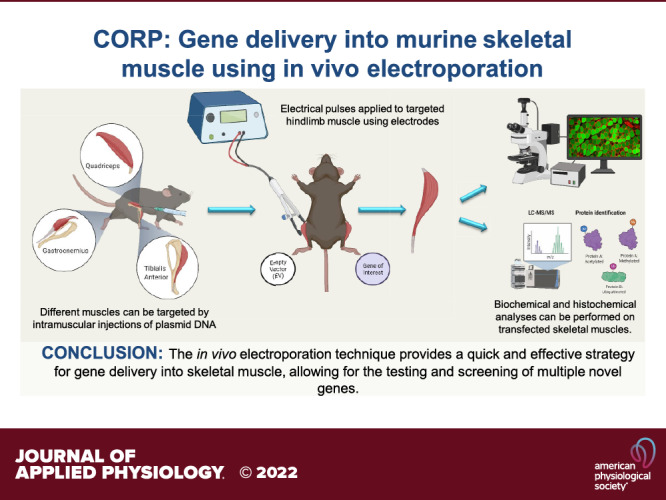
Keywords: atrophy, electroporation, gene transfer, hypertrophy, protein synthesis
Abstract
The strategy of gene delivery into skeletal muscles has provided exciting avenues in identifying new potential therapeutics toward muscular disorders and addressing basic research questions in muscle physiology through overexpression and knockdown studies. In vivo electroporation methodology offers a simple, rapidly effective technique for the delivery of plasmid DNA into postmitotic skeletal muscle fibers and the ability to easily explore the molecular mechanisms of skeletal muscle plasticity. The purpose of this review is to describe how to robustly electroporate plasmid DNA into different hindlimb muscles of rodent models. Furthermore, key parameters (e.g., voltage, hyaluronidase, and plasmid concentration) that contribute to the successful introduction of plasmid DNA into skeletal muscle fibers will be discussed. In addition, details on processing tissue for immunohistochemistry and fiber cross-sectional area (CSA) analysis will be outlined. The overall goal of this review is to provide the basic and necessary information needed for successful implementation of in vivo electroporation of plasmid DNA and thus open new avenues of discovery research in skeletal muscle physiology.
INTRODUCTION
Skeletal muscle is a highly adaptable tissue that can respond to various stimuli (e.g., inactivity, disease, and exercise) and has the ability to get bigger (i.e., hypertrophy) or smaller (i.e., atrophy) when required (1–3). Advancements in molecular techniques and tools have enhanced our understanding of cellular and molecular mechanisms that regulate skeletal muscle plasticity, especially protein synthesis and protein degradation (4–8). Over the past 20 years, genetically engineered mice have been used to yield insights into regulatory mechanisms of different tissues (including skeletal muscle), and to target tissue-specific mechanisms by way of loss- and gain-of-function studies through gene knockout and gene overexpressing transgenic mice (9). Another tool of discovery research, with potential therapeutic application, is tissue-specific gene transfer. Exogenous gene transfer into skeletal muscle fibers has been developed in two forms: 1) viral-mediated delivery using adeno-associated virus (AAV) vectors and 2) nonviral delivery of “naked” plasmid DNA vectors (10–15). The latter has been optimized over the past 20 years to efficiently introduce exogenous DNA using in vivo electroporation for targeted delivery into skeletal muscle myofibers (16–18). Other methods have been developed to allow for exogenous gene transfer into tissues such as lentiviral and adenoviral vectors (19–21). However, the focus of the review is to provide detailed insight and the necessary information on how to successfully implement in vivo electroporation in skeletal muscle physiology research.
BACKGROUND
Since the mid-1960s, the application of electrical fields to living cells has been under investigation with a focus on the delivery of macromolecules, including polynucleotides, the interior of the cell (22–24). Several models have been proposed in the field of electroporation to explain the potential mechanisms behind the movement of extracellular material (such as plasmid DNA) across the cell membrane (25, 26). These models are centered around two integral components, electropermeabilization of the cell membrane and electrophoresis of the DNA molecule (Fig. 1). In the proposed model by Neumann et al. (25), constant electrical pulses create “pores” in the cell membrane that are opened, allowing for extracellular molecules to gain entry into the cell and over time the pores close (25). By trapping the macromolecule within the cell, the biological effects of the molecule may result in an observable cellular phenotype. The cell membrane is proposed to act as a capacitor when subjected to an applied voltage (Fig. 1B). This is achieved through the bipolar lipid membrane being sandwiched between the conductive intracellular and extracellular fluids. In the presence of an applied electrical field, the lipid membrane is subjected to the buildup of a transmembrane potential until a permeation event occurs due to the dielectric strength of the membrane being exceeded (25, 26). This permeation event allows for the facilitation of molecules to be transported across the membrane barrier. It is at this step where hydrophilic pores of varying size are hypothesized to develop and allow for the successful movement of charged macromolecules, such as nucleic acids, into the cytoplasm (25). It is suggested that the resealing of the membrane may take up to 9 min in vivo, as observed in mouse and rat skeletal muscle (28, 29).
Figure 1.
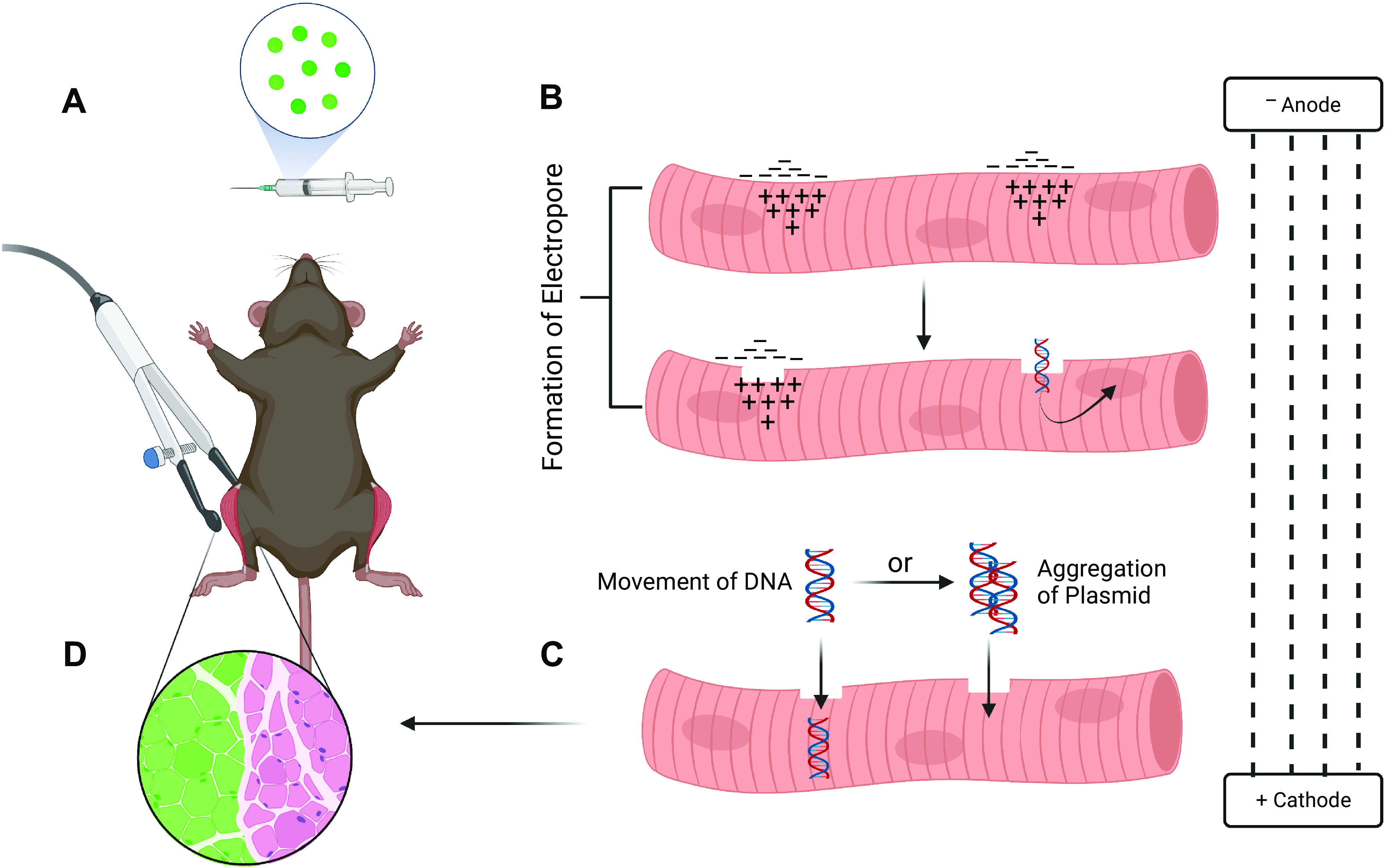
Proposed mechanisms for the movement of plasmid DNA into electroporated muscle fibers. Generally, in vivo electroporation experiments allow for a within-animal study design to be used where one side of the animal is electroporated with an expression plasmid containing a gene of interest and the contralateral leg serves as the empty vector control. A: after plasmid injection, each leg is exposed to electrical pulses through an electroporation generator via electrodes placed on the targeted hindlimb muscle. B: application of electrical pulses to the muscle generates positive and negative charge on either side of the membrane. In turn, this charge imbalance leads to the formation of hydrophobic pore defects enabling plasmid DNA to enter the myofiber. C: movement of plasmid DNA into the myofiber is proposed to occur either through movement toward the anode electrophoretically or by localizing with other DNA molecules at the cell membrane and moving together into the myofiber as a complex without electrophoretic forces being required. D: these proposed mechanisms contribute to the successful introduction of the plasmid DNA into the cell resulting in a transfected myofiber [depicted as green fluorescent protein (GFP) positive]. Adapted from McMahon and Wells (27). Created with Biorender.com.
The effects of electrophoretic forces on DNA molecules into the cytoplasm have been studied. In this instance, the applied electrical field acts on the nucleic acid by creating an “electrophoretic” effect that results in the DNA moving across the cell membrane after pore formation (Fig. 1C). Evidence for this model is supported by single cell and in vivo studies through manipulation of electrical pulse parameters and fluorescent microscope imaging (30–33). A study by Golzio et al. (31) showed fluorescent-labeled plasmids localize only to the side of the electropermeabilized single-cell membrane that faced the cathode (positive electrode). In addition, at this location point, the authors observed the formation of aggregates, suggesting the potential for DNA to form stable interactions with other DNA molecules, before movement into the cytoplasm. This step of the proposed formation of DNA aggregates before moving through the membrane may be beneficial as it may not rely on electrophoresis (27). Interestingly, it was only upon completion of the electrical pulses and up to 30 min after their application that the DNA was observed to be present in the cytoplasm. Sukharev et al. (32) investigated the effect of the electrical field on membrane electroporation and DNA electrophoresis using two-pulse experiments in vitro. Notably, the authors reported two observations: 1) high-voltage and short-duration pulses increased membrane pore formation and 2) a second lower amplitude pulse and longer duration lead to increased DNA movement into the cell. However, the understanding of the precise mechanism for the movement of macromolecules into the cytoplasm is incomplete, especially in vivo where the complexity of three-dimensional extracellular spaces within tissue may alter the mechanisms for DNA cellular entry during the electroporation process (26, 27).
In the next sections, transfection efficiency, electrical-pulse parameters, and hyaluronidase pretreatment, which are integral components of the electroporation process in vivo, will be discussed and the studies that were performed in skeletal muscle to optimize the electroporation technique for successful DNA delivery into myofibers will be summarized. These sections will provide key information on critical studies that were performed to understand the mechanisms of in vivo electroporation and how these components contribute to successful gene transfer in skeletal muscle. The attention and focus of optimization for the delivery of plasmid DNA into skeletal muscle tissue gained traction as a potential avenue for the correction of myopathies (such as Duchenne’s muscular dystrophy) and for promoting the release of angiogenic and neurotrophic factors (34–38). An additional application was the use of skeletal muscle’s role as an endocrine organ, and the use of electroporation to promote the expression and secretion of proteins, such as cytokines into the circulation (39–42). Thus, numerous methodological studies have been performed with the aim of increasing transfection efficiency, enhancing the longevity of gene expression observed in myofibers, and assessing the level of muscle damage/injury present after electroporation implementation (16–18, 43–45).
TRANSFECTION EFFICIENCY
The term transfection refers to the process of deliberately introducing naked or purified nucleic acids into cells. Subsequently, transfection efficiency conveys the proportion of cells that have successfully taken up the foreign DNA and is often described as a percentage value of the total number of cells. The transfected cells, or muscle fibers, can be identified by the introduction of an expression plasmid that contains either a reporter gene or a fluorescent or epitope tagged gene to visualize and distinguish transfected from nontransfected cells/fibers (Fig. 1D). In a seminal study by Wolff et al. (46) targeting skeletal muscle with gene transfer, the transfection efficiency was very low with 1%–2% of fibers deemed to display evidence (β-galactosidase positive) for successful plasmid DNA delivery. The caveat in the study by Wolff et al. was that the transfer of DNA into skeletal muscle fibers occurred without the use of electropermeabilization being applied. In the area of skeletal muscle disease and gene therapeutics, the low transfection efficiency led to multiple investigations being performed to enhance transfection efficiency in skeletal muscle (16–18, 30, 47–49). The goal in the application of gene transfer into skeletal muscle disease is to improve the overall muscle pathology of the disease and thus achieving high transfection efficiency levels is essential for testing, identifying novel gene therapeutics, and making the findings clinically relevant (50). In healthy skeletal muscle, a transfection efficiency as high as 80% of adult mouse myofibers has been reported (51). On the other hand, in diseased skeletal muscle, studies have reported transfection efficiencies ranging from 40% to 50% (50, 52). In a study by Gollins et al. (50) using the Duchenne muscular dystrophy (DMD) mouse model, mdx, a transfection efficiency of 40%–50% was observed irrespective of age or sex of wild-type and mdx mice. Similarly, a study by Vilquin et al. (52) utilized multiple animal models of muscular dystrophy and observed 40% transfection efficiency with electroporation. These studies highlight the ability for diseased skeletal muscles to be electroporated and take up plasmid DNA into the muscle fibers with modest transfection efficiency.
An important consideration that may contribute to the level of transfection efficiency that can be achieved in healthy and diseased skeletal muscle is the size of the plasmid DNA (e.g., >10 kb vs. <6 kb). This is evident in diseased models where the electroporation of a plasmid that contains the open reading frame of a large protein such as dystrophin, utrophin, and laminin α2, have been observed to display reduced numbers of muscle fibers expressing the protein of interest, compared with a smaller construct (52). The plasmid DNA size factor is best exemplified in a study performed by Molnar et al. (44). Here, the authors electroporated into tibialis anterior (TA) skeletal muscle fibers of dystrophic mdx mice, with a full-length dystrophin construct (19 kb), a microdystrophin construct (9 kb), and an empty vector construct (8 kb). The microdystrophin construct displayed a similar number of successfully transfected fibers (dystrophin positive) compared with the empty vector construct, whereas muscle fibers with the full-length dystrophin construct displayed a reduced uptake (44). However, there is limited evidence on the effect of large plasmid constructs on transfection efficiency in healthy muscle, with a study by Wolff et al. (46) observing no difference between 6- and 16-kb sized plasmids. Overall, it is important to be aware of the impact that a large plasmid DNA construct may have on the level of transfection efficiency in skeletal muscle fibers, yet further research is needed in this area.
In terms of the application for in vivo electroporation toward mechanistic studies, it may not be necessary to have a high level of transfection efficiency (>60%) in healthy skeletal muscle. For example, when assessing the hypertrophic/atrophic effect of a particular protein, a comparison of transfected versus nontransfected control muscle fibers in the same muscle would require a significant number of nontransfected fibers to adequately perform fiber cross-sectional area (CSA) analysis. Indeed, having a significant proportion of fibers that are not transfected allows one to compare the nontransfected fibers from the same region and depth as the transfected fibers. Furthermore, having a significant number of nontransfected fibers affords the researcher enough area to avoid analyzing fibers immediately adjacent to transfected fibers that may appear nontransfected but may, in fact, be weakly transfected. In other applications, such as types of “omics” analyses, high transfection efficiency is preferred to investigate target gene effects across the whole muscle (53, 54) to avoid a “dilution” effect whereby changes in gene/protein expression are diluted by a large proportion of nontransfected fibers, leading to an underestimation of the effect of the gene of interest.
As 100% transfection efficiency is not typically achieved with in vivo electroporation, it is also important to note that transfection efficiency does vary from muscle to muscle, and thus there are practices used to normalize these differences. One such example is the use of a reporter construct (discussed in detail later), such as a Renilla expression plasmid, that can be used to correct for differences in transfection efficiency. This is based on the premise that >90% of fibers that take up one plasmid DNA construct will also take up and express a second plasmid construct at the same time (55, 56). Another example is the use of a GST-tagged ribosomal protein p70 S6 kinase (GST-p70S6K) construct in combination with a construct containing the gene of interest, to determine whether the protein of interest (e.g., Raptor, Rheb) regulates mechanistic target of rapamycin complex I (mTORC1) signaling (57, 58). By cotransfecting a GST-p70S6K construct with the gene of interest construct, changes in the phosphorylation of GST-p70S6K represents altered mTORC1 signaling only in the transfected muscle fibers, allowing a more accurate measure of mTORC1 signaling than trying to examine whole muscle changes in the phosphorylation of endogenous p70S6K where the effect size may be markedly diluted and underestimated (57, 58). These examples highlight that, depending on the goal of the experiment (e.g., changes in fiber CSA, intracellular signaling, gene/protein expression, etc.), high levels of transfection efficiency might not be essential but some measures can be undertaken to control for this variation in transfection efficiency.
ELECTRICAL-PULSE PARAMETERS
The delivery of plasmid DNA into skeletal muscle through intramuscular injection alone does allow for the transfer of DNA into muscle fibers (36, 46, 59, 60). However, this individual step is highly inefficient and leads to low levels of gene expression and high levels of interindividual variability in myofibers with successful exogenous gene expression (16). Electrical pulses have been used to aid in the transfer of DNA into eukaryotic cells in vitro and in vivo through the process of cell electropermeabilization (4, 51, 61–64). Key components of the electroporation procedure are the voltage to distance ratio (V/cm), pulse number, pulse frequency (Hz), and pulse duration (milliseconds, ms) (17). Two approaches have commonly been adopted for these parameters: 1) a small pulse number (4–5) with medium pulse duration (1–50 ms) at a low frequency (1–2 Hz) or 2) higher frequency (10–1,000 Hz) with trains of very short duration (200–500 ms) (27, 65). Typically, applied voltages range between 1 and 500 V/cm, even upward to 900 V/cm (48), but higher frequency trains allow for 80–180 V/cm to be used (27, 66–68). The use of a single high-voltage pulse (800–1,000 V/cm) is suggested to increase the permeabilization of the cell, with the low-voltage pulses acting on the DNA to move through the pores created in the cell membrane (depicted in Fig. 1, B and C) (69). The strategy of high-voltage and low-voltage pulses combined, termed HVLV, is often used within electroporation of rat skeletal muscles (70–80). Although, some studies have used increased pulse number with low voltage to successfully transfer exogenous genes into rat TA muscles (81–83), which is a common protocol applied in mouse electroporation studies. A summary of electrical pulse parameters that have been used in the electroporation of hindlimb mouse and rat muscles are presented in Tables 1 and 2, respectively. These parameters represent values that can be modified depending on the skeletal muscle targeted for electroporation and the investigator’s research question and/or application upon gene delivery (e.g., therapeutics, “omics,” etc.). To note, the electric field distribution has been observed to be influenced by the style of electrodes implemented, with plate electrodes (e.g., Tweezertrodes) providing a more homogeneous electric field compared with needle electrodes; thus, needle electrodes require a higher voltage to distance ratio to be applied (17). The use of plate electrodes allows for the electrical field to be applied externally to either side of the limb muscles [e.g., tibialis anterior (TA) and gastrocnemius] and the addition of a conducting gel means that good electrical contact is maintained throughout the procedure (27). On the other hand, the use of needle electrodes does allow for electrical pulses to be applied to exposed muscle tissue or inserted under the skin (27). There is limited information on there being an advantage for using plate versus needle-type electrodes in gene delivery to skeletal muscle.
Table 1.
A selection of mouse hindlimb muscles and a range of parameters used in electroporation studies for gene delivery
| Mouse Skeletal Muscle |
||||
|---|---|---|---|---|
| Tibialis Anterior | Gastrocnemius | Plantaris | Quadriceps | |
| Hyaluronidase (Optional) | 0.4 U/µL (30 µL) | 0.4 U/µL (40 µL × 2) for each lateral and medial head | ||
| Surgery | Optional—small incision or blind injection | No | Yes | Yes |
| Plasmid volume | 5–20 µg (30 µL) | 5–50 µg (40 µL × 2) for lateral and medial head | 12 µL (2 × 6 µL) | 50 µg (50 µL) |
| Injection number | 1 to 2 | 1 to 2 | 2 | 1 |
| Syringe needle | 28-G | 28-G | 27-G | 28-G |
| Pulses | 5–10 | 8–10 | 8 | 8 |
| Voltage, V/cm | 50–175 | 175–200 | 180–160 | 150–200 |
| Pulse width, ms | 10–40 | 20–100 | 20 | 20–50 |
| Electrode type | 7-mm plate or needle | 10-mm plate | Needle | 10-mm needle |
| Reference | (51, 54, 57, 58, 66–68, 84, 85) | (84, 86) | (87) | (88–93) |
Table 2.
A selection of rat hindlimb muscles and a range of parameters used in electroporation studies for gene delivery
| Rat Skeletal Muscle |
|||
|---|---|---|---|
| Tibialis Anterior | EDL | Soleus | |
| Hyaluronidase (Optional) | 0.4 U/µL (60 µL) 45–120 U | ||
| Surgery | Optional—small incision or blind injection | Yes | Small incision—lateral side |
| Plasmid volume | 20–200 µg (30–50 µL or 2 µg/µL) |
5 µg (10 µL) | 10–50 µg (50 µL) |
| Injection number | 1–6 | 1 | 1 |
| Syringe needle | 28-G, 29-G | 28-G | 28-G |
| Pulses | 5–10 | 5 | 5–6 |
| Voltage, V/cm |
|
HVLV pulse combination: 1,000 V/cm pulse (100 µs), 100 V/cm × 4 pulse | 125–209 V/cm |
| Pulse width, ms | 40–100 | 400 | 20 |
| Electrode type | 10-mm plate or needle | Plate | Plate |
| Reference | (70, 72–75, 78, 80–83, 94) | (95, 96) | (97–103) |
EDL, extensor digitorum longus; HVLV, high voltage and low voltage pulses combined; LV, low voltage.
Early studies have investigated the optimal electrical parameters required for successful DNA transfer into muscle fibers (16, 17, 51). By assessing successful electroporation of the luciferase gene, Mir et al. (16) observed no differences in measurable luciferase activity when using between 6 and 16 pulses in skeletal muscles. The authors did, however, observe increasing luciferase activity in TA muscle exposed to long-duration pulses (10–40 ms) and increased frequency (0.5–2 Hz). Donà et al. (51) reported increased transfection efficiency (∼80%) with only five pulses (50 V/cm) and noted the presence of muscle injury when performing seven pulses in the electroporation protocol that was accompanied by reduced transfection efficiency. The authors noted optimal transfection efficiency when using 50 V/cm, 5 pulses, and 20 ms pulse duration for their electroporation procedure in mouse TA muscle. This study indicates the possibility that muscle damage can be observed when a low voltage is applied to the skeletal muscle.
As electrical pulses are an integral step in the electroporation procedure, numerous studies have assessed the effect of voltage ranges on transfection efficiency and the level of damage caused with this technique in skeletal muscle (14, 17, 18, 49). McMahon et al. (18) reported an increased level of transfected muscle fibers at a voltage of 200 V/cm and 175 V/cm compared with TA muscles electroporated at voltages between 150 and 100 V/cm. Similarly, Schertzer et al. (49) observed increased numbers of positive green fluorescent protein (GFP) transfected fibers (70%–50%) in the 200 V/cm group up to 14 days postelectroporation, whereas lower voltage groups displayed only 30%–40% in positively transfected fibers. In both the McMahon and Schertzer studies, the high voltage used for electroporation resulted in significant muscle damage and this was most prevalent in the Schertzer study at 3- and 7 days postelectroporation. On the other hand, it has been observed that increased muscle damage can coincide with a reduction in DNA expression following electroporation (104). A study by Roche et al. (105) assessed the effect of electroporation on contractile function, histology, and the presence of necrotic myofibers in mouse TA muscles for a period of up to 1 year. The authors reported areas of necrosis, the presence of centralized nuclei in histological analysis, and significant reductions in contractile torque between 3- and 28 days postelectroporation procedure. Through in situ force measurements of electroporated TA muscles, Schertzer et al. (49) observed a 40% reduction in tetantic force after 3 days, but contractile force had returned to control levels by 7- and 14 days postelectroporation. It is important to note these reductions in force for the Schertzer study were using 75 V/cm versus the Roche study (105) that used a voltage of 180 V/cm for the electroporation procedure. These data indicate the possible detrimental effect of electroporation voltage alone on skeletal muscle. Overall, these observations allude to important considerations for implementing the electroporation procedure where the experimental design and analyses are time-dependent factors for physiological, biochemical, and molecular properties of skeletal muscle and thus could be confounded by the electroporation voltage.
HYALURONIDASE PRETREATMENT
An important factor for successful in vivo electroporation is the ability for plasmid DNA to enter the muscle fiber via the fiber surface. Each fiber is encapsulated by a plasma membrane known as the sarcolemma, and an extracellular matrix containing collagen and hyaluronan, which form barriers to the efficient delivery of plasmid DNA (18, 106). Early literature used a sucrose solution before injection of the plasmid, and it was observed to increase the spatial distance between muscle fibers and thus distribution of the plasmid within the whole muscle (59). However, specific enzymes such as collagenase and hyaluronidase offer potential for enhancing transfection efficiency as they are able to break down components of the extracellular matrix (107). In the case of hyaluronidase, the enzyme hydrolyses hyaluronic acid which is an integral part of the extracellular matrix (107). As a result, successful introduction of plasmid DNA into skeletal muscle fibers, by enhancing the largest possible distribution area for transfected fibers, has been observed to be dependent on the pretreatment of the targeted muscle with hyaluronidase (18, 44, 108).
An early study by McMahon et al. (18) assessed the effect of hyaluronidase pretreatment before the injection of plasmid DNA on transfection efficiency versus a saline control group. The authors noted a significant increase in the percentage of transfected muscle fibers (∼60%–70% efficiency at 175 V/cm vs. 10%–20% efficiency without hyaluronidase) with a 2-h pretreatment time point. Notably, the authors also observed reduced levels of muscle damage [indicated by neural cell adhesion molecule (NCAM) immunostaining]. Interestingly, evidence within the literature shows transgene expression to be elevated up to a year when using the hyaluronidase approach (44). A subsequent study by Mennuni et al. (108) assessed the effect of hyaluronidase on plasmid DNA transfection efficiency by considering different dosing regimens, timing of the hyaluronidase injection before electroporation, and the long-term effect of hyaluronidase on the expression of their gene of interest (mouse erythropoietin, Epo) over time (7-, 56-, and 120 days postinjection). The authors found that the timing of the hyaluronidase injection before electroporation did not impact gene expression, but hyaluronidase did increase plasmid DNA concentration within the muscle fibers and expression was maintained up to 120 days postelectroporation (108). Mennuni et al. (108) also observed that hyaluronidase more effectively enhances gene transfection efficiency versus using other enzymes, such as elastase and collagenase. As discussed earlier, the use of high electrotransfer voltage settings results in increased transfection efficiency but also causes elevated skeletal muscle damage and nontransfected regenerating fibers (18, 44, 49). Therefore, the benefit for the inclusion of a hyaluronidase injection before the electroporation protocol allows for enhanced transfection efficiency at lower voltage settings and a diminished likelihood for the presence of damaged muscle (18, 44, 49).
Regarding the timing of the hyaluronidase pretreatment, numerous studies to date have used either a 1- or 2-h hyaluronidase pretreatment in knockdown (e.g., siRNA) and overexpression studies using in vivo electroporation to better understand the molecular mechanisms of skeletal muscle mass regulation (53, 54, 66–68, 81, 84, 109–117). Importantly, the injection of hyaluronidase before delivery of plasmid DNA does not appear to be time-dependent (94, 108). For example, in rat TA skeletal muscle, Akerstrom et al. (94) assessed hyaluronidase delivery at either 1 or 10 min before electroporation. Through assessment of GFP protein expression, the authors reported increased GFP in the 1- and 10-min treatment groups compared with the vehicle pretreatment condition. Indeed, a study by Menniuni et al. (108) observed no difference between 10 min, 1- or 4-h preinjection with hyaluronidase to increase the expression of their gene of interest, and thus it appears the effect of hyaluronidase on skeletal muscle is long lasting. Overall, current evidence suggests that timing of hyaluronidase delivery is not critical in terms of transfection efficiency, but its incorporation into electroporation protocols significantly improves efficiency of plasmid DNA delivery across the whole skeletal muscle (18, 49, 108). Finally, it is important to note that the presence of hyaluronidase does not appear to affect skeletal muscle function at the whole or single fiber level (49).
APPLICATION OF PLASMID DNA IN SKELETAL MUSCLE PHYSIOLOGY RESEARCH
Plasmid DNA has been shown to remain stable in skeletal muscle for between 3 and 18 mo (35, 46, 47, 118) and evidence suggests that plasmid DNA does not stably integrate into chromosomes but remains episomal (47). The postmitotic nature of myofibers may contribute to the long-term gene expression observed in this tissue following electroporation. In addition, multiple plasmids can be successfully electroporated simultaneously into skeletal muscle that can allow for co-overexpression and analysis of several genes within a single muscle at the same time (54, 55, 84, 111). The inclusion of a trackable gene or epitope tag into a plasmid backbone (Fig. 2A) can allow an investigator to study either gene promoter activity by utilizing reporter gene plasmids (57, 58, 111, 119–122), protein localization (54, 123, 124), or protein complex interactions (53) (Fig. 2). In addition to the ability to study gene and protein function in skeletal muscle, fluorescent or epitope tags incorporated into expression plasmids can also be used to assess the level of transfection efficiency achieved when performing in vivo electroporation. In the following sections, several common plasmid systems that can be used as markers of electroporation efficiency and/or tools to explore gene and protein function are detailed.
Figure 2.
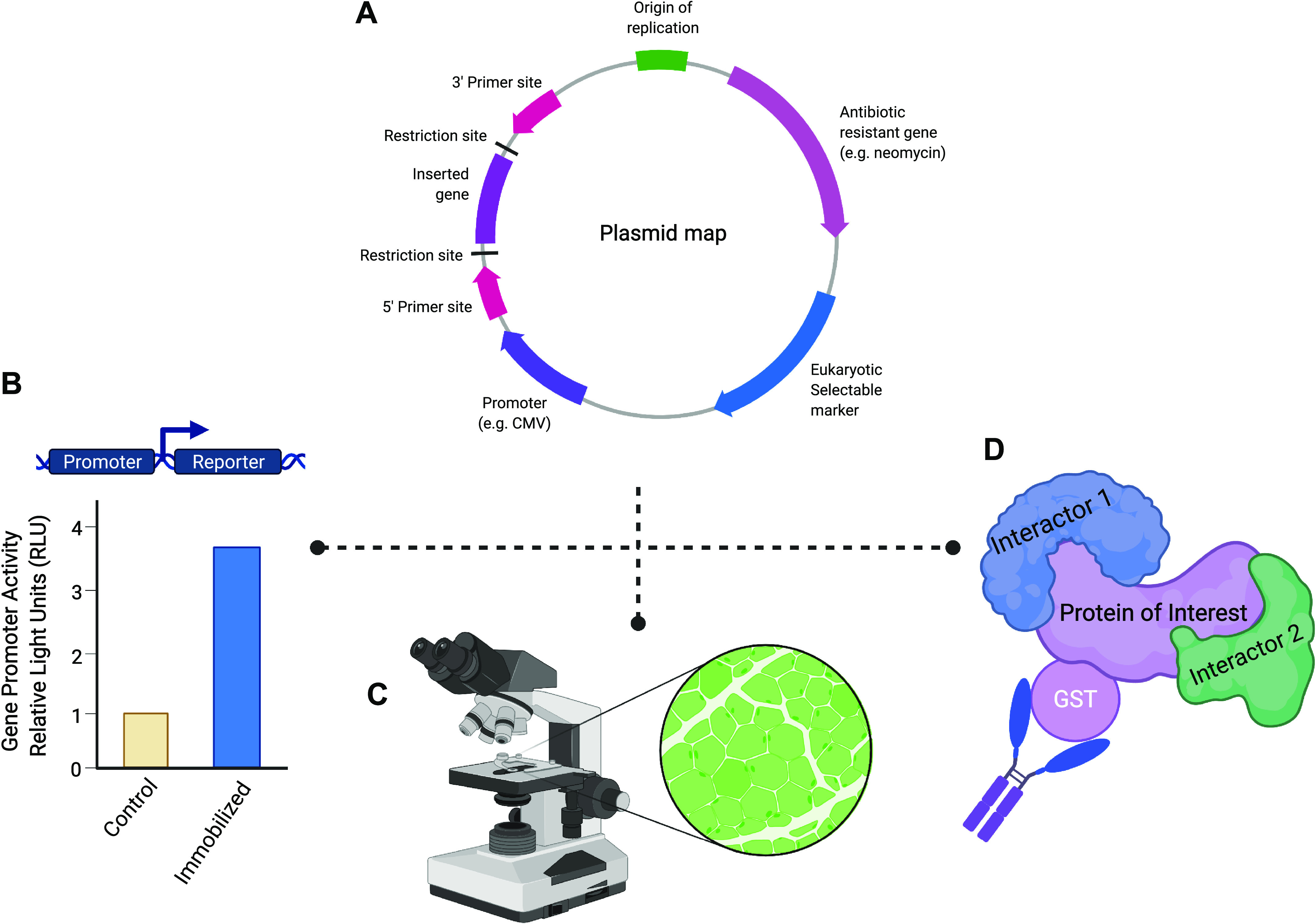
Applications of plasmid DNA into skeletal muscle physiology research. A: use of mammalian expression plasmids can aid in mechanistic experiments because they can be introduced by transfection (e.g., electroporation) into host cells. Expression plasmids possess a multiple cloning site (MCS) immediately downstream of a promoter sequence that allows for the amplified cDNA of a gene of interest to be cloned into the plasmid. The MCS of a plasmid consists of numerous unique restriction enzyme recognition sequences that simplify the cloning process and allow for the proper directional insertion of the cDNA of a gene of interest. Furthermore, the use of a viral promotor (e.g., CMV or SV40) allows for robust expression of the target gene within eukaryotic cells. Finally, the cDNA cloned into an expression plasmid can be easily sequenced using commonly used primer sites (e.g., T7, SP6, and T3) to confirm successful target gene insertion and to verify that no mutations have been introduced during the cloning process. B: transcriptional regulation of a gene in skeletal muscle under various stimuli can be ascertained by using a reporter plasmid construct containing the promoter of a gene of interest inserted upstream of a reporter gene (e.g., luciferase). For example, in immobilized/disuse muscle atrophy, the target gene promoter activity can be measured in control and immobilized tissues that have been transfected with the reporter plasmid construct. Under an atrophy stimulus, the gene of interest promoter activity will be detected through changes in the amount of luciferase activity. C: use of a fluorescent reporter, such as green fluorescent protein (GFP), allows for the identification of successfully transfected muscle fibers under the microscope. The incorporation of a GFP-tag into a target protein can aid in studying the localization of novel proteins in skeletal muscle fibers. D: to investigate protein-protein interactions and complexes, epitope tags [e.g., hemagglutinin (HA), FLAG, myc, and glutathione S-transferase (GST)] provide a useful tool that can be used in common molecular biology techniques such as coimmunoprecipitation to identify novel interactors of the protein of interest. Most commonly, the epitope tag is integrated into the plasmid sequence so that the tag will be easily added in frame with either the C- or N-terminus of the target protein. In addition, these tags can be used to identify proteins when a specific antibody against a protein of interest is not yet available. Created with Biorender.com.
Luciferase Reporter Gene Systems
Commonly used in electroporation studies are luciferase reporter gene systems (Fig. 2B), which provide easy quantification and sensitive detection for analysis of myofiber transfection efficiency. The most well-established reporter gene expression system uses the Firefly luciferase gene (from Photinis nyralis) and its substrate d-luciferin to generate bioluminescence within the muscle tissue and thus luciferase activity can be analyzed via in vivo imaging or ex vivo homogenates (125). Another luciferase enzyme utilized in reporter systems is Renilla which is responsible for the green bioluminescence of the sea coral Renilla reinformis and uses the substrate coelenterazine to produce light (126).
Besides being useful as an internal control for assessing transfection efficiency, luciferase reporter gene systems can also be used to study gene expression. Gene expression studies utilize recombinant reporter gene plasmids that have the regulatory promoter region of a gene of interest cloned upstream of the luciferase reporter gene. Electroporation of a recombinant reporter gene plasmid into myofibers allows an investigator to assess the activation or repression of a gene of interest’s promoter via an increase or decrease in recombinant reporter gene activity, respectively, which is assumed to reflect regulation of the endogenous gene (125, 127). Early studies in the development of the in vivo electroporation technique utilized luciferase reporter systems to assess transfection efficiency by measuring the activity of the reporter gene being utilized (16, 51, 59, 128). Furthermore, luciferase-based constructs can be used as a proxy for cap-dependent and cap-independent translation using a bicistronic construct that encodes a single mRNA from which Firefly luciferase is translated via normal cap-dependent mechanisms, while Renilla is translated via an internal ribosome entry site (IRES), which is indicative of the numbers of ribosomes or translational capacity (58). An example electroporation protocol for the introduction of luciferase promoter constructs into rat soleus muscle fibers is presented by Senf and Judge (129).
Fluorescent Tag Systems
The green fluorescent protein (GFP; 27 kDa) is the most commonly used indicator for successful gene transfection and expression in tissues such as skeletal muscle. The gene encoding GFP was identified in the jellyfish Aequorea victoria and produces a protein with fluorescent excitation and emission that can be viewed under the microscope or through in vitro and in vivo live imaging (130, 131). One of the advantages of using GFP is its production of a fluorescent signal in the intracellular space allowing for direct observation and identification of successfully transfected cells over time (Fig. 2C). The advancement of fluorescent bioimaging in intact organelles, live cells, and whole organisms has led to the development of GFP-like proteins. These GFP-like proteins from Anthozoa species have fluorescent spectra positions for blue, orange-red, far-red, cyan, and yellow (130). In skeletal muscle, proteins that are conjugated (tagged) with GFP or GFP-like proteins can be used in localization studies performed using fusion proteins to determine if they are targeted to subcellular structures such as neuromuscular junctions or mitochondria (54, 123, 124, 132). The MitoTimer reporter gene has a GFP localized to the mitochondria (mitochondrial targeting sequence of the cytochrome c oxidase subunit VIII gene) and that shifts to red fluorescence over time (133). In skeletal muscle, this fluorescence-based reporter has been used to report mitochondrial content, structure, stress, and damage in vivo under physiological and pathological conditions such as exercise, high-fat diet, cancer cachexia, and hindlimb unloading (133–136).
Epitope Tag Systems
The use of expression plasmids encoding for recombinant proteins that are tagged with a detectable epitope allows for previously uncharacterized proteins to be more easily studied, which is especially useful when no protein-specific antibodies are available (137, 138). An epitope tag added to a protein of interest allows an investigator to analyze function, localization, and identification of putative interactors of the tagged protein (Fig. 2D) using bioanalytical techniques such as immunoblotting, immunohistochemistry, and immunoprecipitation (137). Typical tags that have been used in cellular and molecular biology are hexahistidine (6× His-tag; 0.8 kDa), FLAG (1.0 kDa), glutathione S-transferase (GST; 26 kDa), myc (1.2 kDa), and hemagglutinin (HA-tag; 1.1 kDa) epitopes (139). In a recent electroporation study that utilized an epitope tag system, Bullard et al. (53) used TA muscles transfected with a tandem affinity purification (TAP) construct, containing FLAG and S-tag (2.2 kDa) sequences, to identify proteins that interact with Gadd45a in skeletal muscle atrophy. Through tandem affinity purification, the authors identified 75 proteins, 13 of which were protein kinases, that interacted with Gadd45a in transfected mouse TA muscles (53). Subsequently, the authors targeted one protein, Mekk4, in immobilization-induced atrophy and observed skeletal muscle sparing with MEKK4 knockdown (53). Other studies in the field of muscle physiology have utilized epitope tags to explore proteins and their interactors to gain greater understanding of the molecular mechanisms of atrophy and hypertrophy (140–144).
Gene Knockdown Systems
Inducible tissue-specific and whole body knockout mice have been used in skeletal muscle to investigate gene regulation and gene product function in areas of physiology and metabolism (9, 145, 146). However, the generation of these animal model lines can be costly, time consuming, and labor intensive. Gene knockdown plasmids have been successfully used to express antisense RNA molecules, such as small interfering or short hairpin RNA molecules (i.e., siRNA and shRNA), that target and knockdown gene expression by inducing mRNA degradation resulting in reduced overall expression of a targeted gene in a given tissue (147). An important consideration when using this approach is the potential for off-target knockdown if target gene specificity is lacking (147). Gene knockdown plasmids can be designed to have a biphasic fluorescent component where transfection efficiency and successful introduction of the siRNA or shRNA molecule can easily be identified in vitro and in vivo (67, 84). The biphasic design aids in transfected muscle fiber identification and there is a strong correlation between fluorophore expression and knockdown activity. In vivo electroporation studies have successfully utilized transient RNA interference strategies to identify and study genes that positively and negatively influence skeletal muscle mass and homeostasis (67, 84, 111, 148, 149).
Site-Directed Mutagenesis
A powerful molecular biology technique for further assessing and exploring the function of proteins and protein domains is site-directed mutagenesis (SDM). The SDM technique allows an investigator to change, delete, or insert one or more nucleotides in a template DNA molecule. These mutations can be performed using multiple strategies such as polymerase chain reaction (PCR), cassette, or whole plasmid mutagenesis (150, 151). In the Bodine and Waddell laboratories, we recently used SDM to inhibit RING domain activity in the E3 ubiquitin ligase, MuRF1, by mutating two conserved cysteine residues (54). Overexpression of the RING domain mutant led to no observed skeletal muscle atrophy and a reduction in ubiquitinated proteins in the myofibrillar protein fractions of transfected muscles versus muscle overexpressing the wild-type MuRF1 construct (54). Similar observations for the importance of the RING domain in MuRF1 function have been made in transgenic mice expressing a MuRF1 RING domain deletion mutant (152). Other studies in skeletal muscle have also reported the use of SDM as a tool to study the function of genes of interest (143, 153, 154).
Measuring Relative Changes in Protein Synthesis Rates
Cell size is largely determined by the net balance in rates of protein degradation and protein synthesis (155). For experiments examining the effect of a protein of interest on muscle fiber size, the experimenter can choose to combine plasmid DNA electroporation with the puromycin-based SUnSET (SUrface SEnsing of Translation) method for determining differences in the rate of protein synthesis between transfected and nontransfected fibers from the same muscle using standard immunohistochemical/immunofluorescence techniques, or via Western blot, if the transfection efficiency is sufficiently high (142, 156). This in vivo method was developed by the Hornberger Lab and has been used successfully to detect increases and decreases in protein synthesis rates when combined with electroporation of plasmid DNA (67, 85, 142, 154, 157).
TARGETING SPECIFIC SKELETAL MUSCLES WITH IN VIVO ELECTROPORATION
In the published literature, an array of murine hindlimb muscles have been targeted for gene transfer by in vivo electroporation including the TA, plantaris (PLN), and the gastrocnemius (GA) under basal conditions (5, 54, 67, 140, 141, 157, 158) (Fig. 3). The targeting of multiple hindlimb muscles has allowed for successful gene transfer to occur in conjunction with atrophy and hypertrophy experimental models (4, 84, 87, 97–99, 110, 159), nutritional and exercise interventions (68, 114, 116), aging muscle (81, 160), and advanced analytical technologies such as proteomics (53, 54, 111). All these approaches have opened up insights and avenues for discovery into skeletal muscle plasticity such as protein synthesis, protein degradation, and metabolism. It is important to note that multiple research groups have successfully used in vivo electroporation over the years, with many modifications and adjustments made to achieve successful gene transfer in skeletal muscle, depending on the investigators research question. In Tables 1 and 2, a summary and range of parameters for in vivo electroporation are presented based on previously published studies in mouse and rat muscles, respectively. In Table 3, an example step-by-step overview for in vivo electroporation of the mouse TA muscle is presented. In addition, important considerations for several hindlimb muscles are discussed in the following sections.
Figure 3.
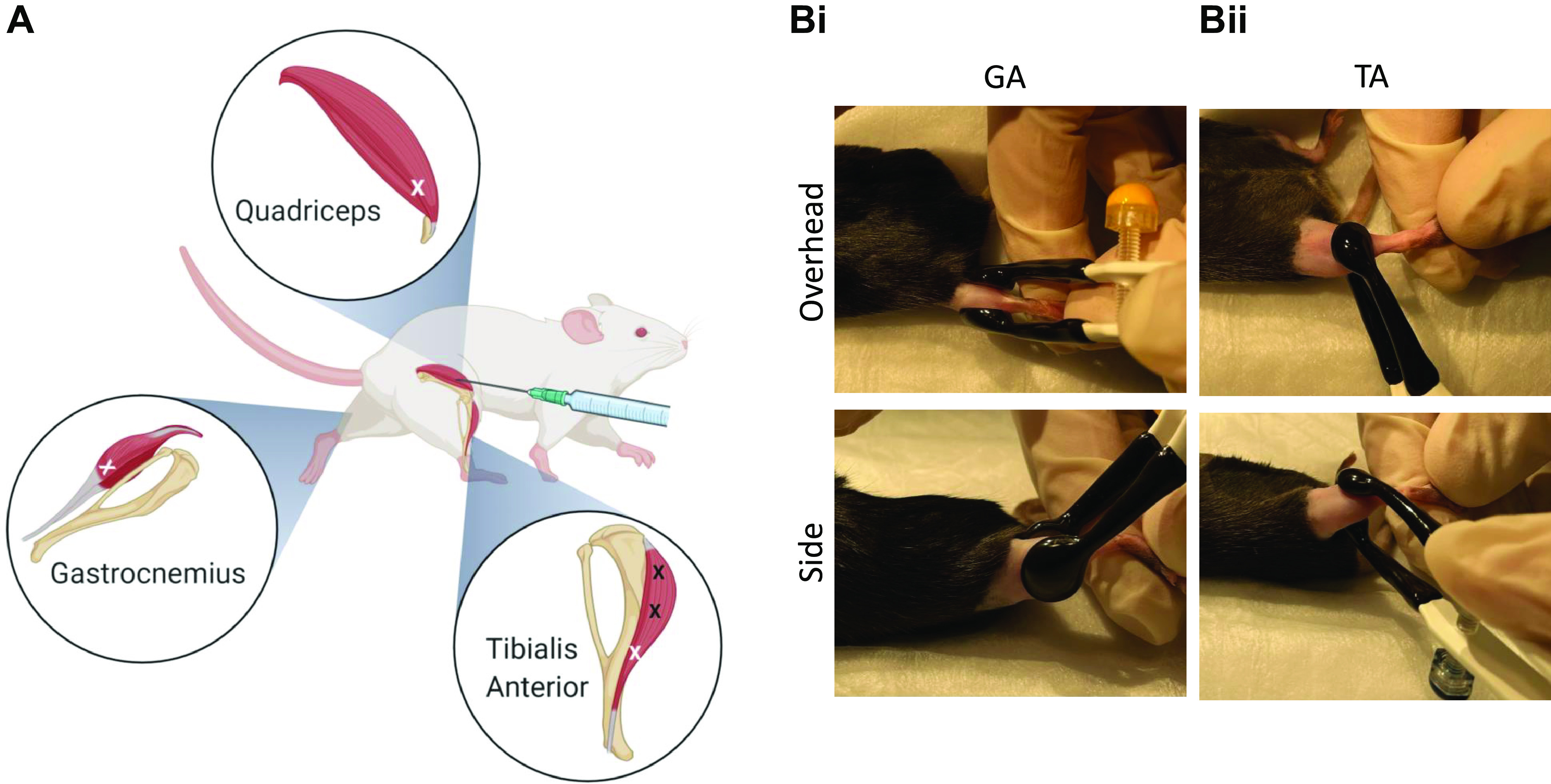
Injection site, number, and electrode placement in mouse hindlimb muscles for in vivo electroporation. In mouse quadriceps, gastrocnemius (GA) and tibialis anterior (TA) muscles, single injections can be applied using 0.5-mL insulin syringe (28-gauge) to the distal area of the muscles, as depicted by the white X mark (A). In the GA muscle, the lateral and medial GA muscle require an injection in each head of the muscle. Additional injections can be applied to the TA muscle at the proximal end and mid belly of the muscle (depicted as black X mark) and this can be beneficial if utilized within the rat TA muscle. In B, electrode placement is illustrated for the GA and TA muscles from an overhead and side view. For the GA muscles (Bi), plate electrodes (∼10 mm size) are placed on each side of the leg, one plate covering the lateral and the other plate covering medial GA muscle area. For the TA muscle (Bii), electrode plates (∼7 mm size) are placed, with one plate on top of the mid belly of the muscle between the distal and proximal end. The other plate is located at the back of the leg over the GA muscles. Good contact between the electrode and skin is enhanced with removal of the hair and use of a conductive gel. Created with Biorender.com.
Table 3.
An example of the in vivo electroporation procedure for the tibialis anterior muscle of mice
|
|
|
|
|
|
|
|
TA, tibialis anterior.
Tibialis Anterior
The TA muscle in rodent models is the most commonly selected muscle for in vivo electroporation. Due to its anatomical location, the TA is easily accessible for intramuscular injections and the placement of electrodes for the delivery of electrical pulses to the hindlimb muscle (highlighted in Fig. 3, A and B). The TA muscle can be prepared for intramuscular injections and electroporation by performing a close shave and applying hair removal cream to the area between the knee and ankle. If the goal is to maximize transfection efficiency, then 2 h before electroporation, 31 µL of 0.4 U/µL hyaluronidase in 0.9% sterile saline can be slowly injected into the mouse TA muscle. Otherwise, 30 µL of plasmid DNA diluted in sterile saline (0.9%) is injected into the muscle. Plasmid amounts between 2 µg and 50 µg have been delivered successfully into the TA muscle in previous studies (18, 54, 84, 111, 116). However, these volumes and plasmid amounts may differ between the sex and age of the mice being used (e.g., 2 mo old vs. 9 mo old) and thus it is important to optimize parameters before performing critical experiments. In the final steps, electrodes are placed onto the hindlimb leg with one electrode plate placed directly onto the TA muscle, with the other plate positioned behind the location of the gastrocnemius muscles (Fig. 3Bii). In addition, a small amount of conductive gel is applied to the electrode plates directly and the areas where the plates will be placed before the application of electrical pulses. To note, previous studies have also utilized needle electrodes combined with 10 electrical pulses lasting 20 ms at 86 V (175 V/cm field strength) with 480 ms intervals between pulses (54, 67, 84, 109). A range of pulse parameters have been successfully applied to the mouse TA muscle for enhancing gene expression or gene knockdown, as detailed in Table 1. It is important to note that delivery of the plasmid through intramuscular injection can occur by performing a blind injection or by making a small incision through the skin covering the TA muscle and using surgical glue upon completion of the electroporation procedure to close the incision (87, 154, 157). Opening the skin above the TA muscle will allow for more precise observation of the injection position and to see how the injected volume is distributed. Several studies have successfully utilized this approach, without hyaluronidase pretreatment or conductive gel, combined with eight 20-ms pulses (1 Hz frequency) and a field strength of 180–160 V/cm (57, 58, 87, 140–142, 154, 157, 159, 161–163).
Gastrocnemius
As the gastrocnemius muscle comprises the lateral (LGA) and medial (MGA) head at is origin, it is optimal that the in vivo electroporation procedure targets the lateral and medial heads simultaneously. Before electroporation, 41 µL of 0.4 U/µL hyaluronidase is slowly injected into each head (LGA and MGA) of the gastrocnemius muscle (84). After 2 h, 80 µL of total (40 µL per muscle) plasmid DNA diluted in sterile saline (0.9%) is injected into the LGA and MGA muscles. The suggested sites for the intramuscular injection of the hyaluronidase and the plasmid are illustrated in Fig. 3. Next, with a small amount of conductive gel, electrodes are placed onto the hindlimb leg with one electrode plate placed directly onto the LGA muscle and the other plate positioned onto the MGA muscle without compression of the two muscles (Fig. 3Bi). Then, using an electroporator, 10 electrical pulses lasting 20 ms at 86 V (175 V/cm field strength) with 480 ms intervals between pulses are applied to the LGA and MGA muscles (84). Previous literature has assessed varying field strength (50–200 V/cm) in the electroporation of the gastrocnemius muscles with upper field strength parameters (140–200 V/cm) showing enhanced gene expression (86).
Plantaris
Unlike the TA and gastrocnemius muscles, the plantaris, soleus, and quadriceps muscles require minor surgery to be performed before the injection of plasmid DNA. In mice, the proximal and distal ends of the plantaris muscle belly are injected with plasmid DNA solution through a 27-gauge needle. Then, through stainless steel pin electrodes placed on the proximal and distal myotendinous junctions of the gastrocnemius muscles, eight 20 ms electrical pulses with a field strength of 160 V/cm at 1 Hz are delivered onto the muscle (87). After completion of the electroporation procedure, the incision can be closed with a wound clip, surgical glue, or polysorb suture. In comparison, rat plantaris muscle can be electroporated using four pulses of 200 V/cm for 50 ms at 1 Hz with an additional four pulses of opposing polarity being applied to the muscle (164, 165). Furthermore, a plasmid solution (e.g., phosphate-buffered saline, PBS) of 20 µL can be delivered into the rat plantaris muscle using a 29-gauge needle (164).
Soleus
There are a limited number of published studies using in vivo electroporation of the soleus muscle in mice which, in part, may be due to the relatively small size (∼9–10 mg) and accessibility of the muscle compared with the TA, quadriceps, and GA muscles (100, 166,167). In a study by Judge et al. (100), the mouse soleus muscle was exposed and injected with 10 µg of reporter plasmid in a 5 µL volume. Electrical pulses were delivered by placing paddle-like electrodes onto the muscle. The electrical parameters used by Judge et al. (100) consisted of five electrical pulses at 125 V/cm with each pulse duration of 20 ms, and intervals of 200 ms. In rat soleus muscle, studies by Vitadello et al. (97, 98) electroporated muscles before the implementation of an atrophy model (hindlimb suspension). Specifically, the soleus muscle is exposed and bilaterally injected with 50 µg of plasmid DNA. Upon closure of the wound, six electrical pulses at 20 ms/pulse with a field strength of 209 V/cm at 1 Hz and 200 ms intervals is delivered via an electroporator (97, 98, 101). The electrodes themselves are placed upon the anterior and posterior side of the hindlimb skin (98). Other studies that have incorporated electroporating the rat soleus muscle have used either 8 or 9 electrical pulses at 20 ms/pulse and field strength of 200 V/cm (166, 168). In studies from the Judge Lab (102, 103), the authors electroporated rat soleus muscle using 200 ms interpulse intervals with 20 ms durations for five pulses at either 125 V/cm or 75 V/cm.
Quadriceps
The quadriceps muscle is another option for in vivo electroporation but is also a muscle where, due to its anatomical location, a small incision is made above the quadriceps to allow for intramuscular injections to be performed (88–91). Following the incision, up to 50 µg of plasmid can be injected into the muscle in a 50 µL volume of 0.9% sterile phosphate-buffered saline (PBS). The location of the suggested site for intramuscular injection of the plasmid is illustrated in Fig. 3A. Subsequently a series of eight 50 ms electrical pulses at 100 V are applied to the medial and lateral quadriceps muscle through needle electrodes (169). Upon completion of the electroporation protocol, the incision can be closed with a wound clip/staple (89). Numerous studies have utilized the quadriceps muscle to increase the concentration of circulating factors, such as cytokines and growth hormones (88, 89, 91, 169–171). The physiological and pathophysiological consequences of these systemic factors can then be examined in distal tissues (e.g., liver, epidydimal fat, and brain) and muscles. Notably, the Carson laboratory has routinely used this approach to examine mechanisms related to systemic IL-6-induced wasting with cancer (89, 92, 93, 172). Importantly, this approach offers a low-cost way to modulate systemic levels of secreted factors, such as IL-6, compared with infusion or injection of recombinant proteins and growth factors into skeletal muscle.
Other Muscles
Studies in the field of skeletal muscle physiology have also deployed the electroporation technique in the extensor digitorum longus (EDL) and flexor digitorum brevis (FDB) muscles of mice (55, 128, 135, 136, 173–178). In the literature, there have been protocols published that highlighted how these skeletal muscles are electroporated by providing a visual resource (175, 179). The skeletal muscle targeted for in vivo electroporation might be chosen based on muscle mass size, ability to assess contractile function, and the array of muscle fiber types present (167, 180). These factors are important considerations for gene manipulation studies where the skeletal muscle biochemistry may respond differently under various conditions such as atrophy and aging (181–185).
FACTORS AFFECTING ELECTROPORATION IN MOUSE VERSUS RAT SKELETAL MUSCLE
The incorporation of in vivo electroporation into a skeletal muscle physiology research program requires optimization of the technique in the selected muscle. The difference in mass between mouse and rat skeletal muscle means that different approaches for gene delivery may be required. Commonly, a single or double injection to deliver plasmid DNA has been applied to the mouse TA muscle, as highlighted in Table 1 (58, 67, 84, 141). However, in the rat TA muscle, injection numbers have ranged from one to seven spaced sites being used to deliver the plasmid intramuscularly (70–73, 81, 94). Noticeably, between the various strategies used in rat TA muscle is that those protocols which used increasing numbers of injection sites had electrical-pulse parameters set with one high voltage (HV) pulse (800–900 V/cm) and four low voltage (LV) pulses (80–90 V/cm) without the use of hyaluronidase pretreatment. Highlighted in Table 2 is the HVLV pulse combination which has been utilized in the rat TA, EDL, and soleus muscles. A single injection site plus hyaluronidase has also been used to electroporate the rat TA muscle, with electrical parameters (8–10 pulses, 160–175 V/cm) being performed similar to those used in mice (81). The added benefit of multiple injection sites allows for the increased probability that more plasmid DNA will be introduced into the muscle fibers (94). However, to caution, increased amounts of concentrated plasmid DNA (>50 µg) delivered to muscle fibers have been observed to contribute toward the early presence of increased injury in rat muscles (186). The studies in rat hindlimb muscles are summarized in Table 2 and highlight the different parameters used in rat muscle to successfully perform gene transfer in this rodent model.
Notable studies have postulated the importance of optimal injection volumes on fluid capacity in skeletal muscle and how this relates to intramuscular hydrostatic pressure created by the injection volume (187, 188). A study by Dupuis et al. (188) observed detrimental impact of large injection volumes (50 µL) on a small muscle mass (mouse) leading to increased muscle swelling and damage. In contrast, the combination of a small injection volume (5 µL) with electrical pulses displayed reduced muscle swelling and increased DNA uptake in muscle myofibers. Thus, in terms of rat skeletal muscle, injection parameters for larger species need to be optimized to account for fluid capacity and intramuscular hydrostatic pressure. Indeed, a 50 µL injection volume into the quadriceps mouse muscle has also been reported to redistribute plasmid DNA to the area of the myotendinous junction (188, 189). These two factors, volume and electrical pulses, can influence the distribution of plasmid DNA upon delivery into skeletal muscle and alter the efficiency of gene transfer (187).
With regard to the electroporation protocols used in rat skeletal muscle, the adoption of high-voltage, low-voltage pulse combinations are commonly used. Studies have been performed to address the impact of this strategy on biochemical and physiological measures for muscle function and force (95, 96). A study by Hojman et al. (96) assessed muscle force recovery up to 240 min postelectroporation and observed early force recovery in the HVLV combination versus high-voltage only pulses. A follow-up study by Gissel (95) assessed the effects of HVLV pulses and high-voltage only pulses on ion homeostasis (Ca2+, Na+, and K+ content) and cellular integrity. The author observed that the HVLV pulse combination only caused acute changes (>4 h) in ion content and reductions in force. However, to note, in the Gissel study, the HVLV consisted of only one high and one low pulse. Numerous subsequent studies in rat TA muscle have been published using the HVLV combination to successfully manipulate gene expression (71–76, 78–80). However, future research is warranted to provide a temporal assessment (hours and days) of the HVLV strategy on physiological, histological, and biochemical changes in rat skeletal muscle. Similar studies have been performed in mouse muscle (69, 105) and subsequently these findings have been extrapolated to rat electroporation studies, even though different electroporation parameters were used. Finally, there is a lack of published literature in the implementation of the HVLV pulse combination in female and aged skeletal muscle, where muscle mass size compared with adult male rats would be an important consideration.
CONSIDERATIONS FOR IMMUNOHISTOCHEMISTRY PROCESSING AND FIBER CROSS-SECTIONAL AREA ANALYSIS OF TRANSFECTED SKELETAL MUSCLES
As discussed earlier in the transfection efficiency, the identification of muscle fibers that have successfully taken up the plasmid DNA can provide pivotal information for determining the effectiveness of the electroporation protocol. From a mechanistic perspective, positively transfected fibers can be analyzed for fiber cross-sectional area (CSA) providing key data in exploring for any effect of the target gene on skeletal muscle homeostasis. Other factors that are important considerations in fiber CSA analysis when utilizing the electroporation procedure are the inclusion of nontransfected fiber CSA, the possible impact of the empty vector/control on fiber CSA size alone, and the processing of tissue for microscopy depending on the use of fluorescent versus epitope-tagged constructs. These factors are important as understanding each element will aid in accurate, robust data acquisition, and analysis when investigating newly identified genes that may be involved in skeletal muscle hypertrophy or atrophy.
In the Bodine laboratory when using GFP-tagged constructs, we perform an immunohistochemistry processing protocol that uses a 4% paraformaldehyde fixative followed by a sucrose gradient (10%, 20%, and 30%) to enhance the image quality of transfected skeletal muscles under the fluorescent microscope (detailed in Table 4). Furthermore, we process the transfected muscles by pinning the tissue onto cork and placing pins at the proximal and distal ends of the muscle, followed by an additional pin in the mid belly (Table 4). This allows the muscle to be cut in half with a razor blade so that tissue can be used for both histological and biochemical analyses for the experiment. Alternatively, the length of the muscle can be measured before freezing the whole muscle in embedding compound [e.g., optimal cutting temperature (OCT) compound]. Then, based on the length measurement, the muscle can be cut using the cryostat to the exact mid-belly point before taking the first sections and/or serial sections can be taken at specific intervals across the length of the muscle (58, 142). Overall, these steps can be performed on mouse and rat skeletal muscles that have been electroporated with a plasmid that possess a fluorescent tag (67, 81, 84). Processing the tissue in this manner allows for hematoxylin and eosin staining (H&E) and laminin staining (detailed in Table 5) to be performed on the transfected muscles and prepare the tissues for fiber cross-sectional area analysis (54, 67, 84).
Table 4.
Example protocol of processing transfected skeletal muscles for immunohistochemistry
|
|
TA, tibialis anterior.
Table 5.
Example protocols for hematoxylin/eosin and laminin staining on electroporated skeletal muscles
| Hematoxylin and Eosin | Laminin |
|---|---|
|
|
In studies assessing the molecular mechanisms of skeletal muscle hypertrophy and atrophy, the measuring of fiber CSA provides important data, in relation to change in muscle mass or force, for any possible effect of the intervention. A fluorescent or epitope tag marker should be used to identify transfected versus nontransfected muscles fibers (e.g., GFP-positive vs. GFP-negative) in the assessment of fiber CSA with in vivo electroporation studies (Fig. 4). This can be achieved by cotransfections of two individual DNA plasmids or transfecting a single expression plasmid containing a GFP/epitope fusion gene of interest (54, 58, 67, 140, 154, 157). In the Bodine laboratory, when studying a new gene of interest, we utilize an approach for coexpression of two DNA plasmids; 1) the untagged target gene and 2) a GFP construct into skeletal muscle fibers, as the addition of a GFP-tag on the protein may interfere with its localization and/or function in transfected muscles. In Fig. 4A, the nontransfected muscle fibers are identified with a black center and are most identifiable in the images with a laminin stain. The analysis of transfected and nontransfected muscle fibers allows for the effect of the gene of interest to be studied in transfected fibers while also accounting for any possible effect of the electroporation procedure on neighboring nontransfected fibers to be factored into the overall analysis.
Figure 4.
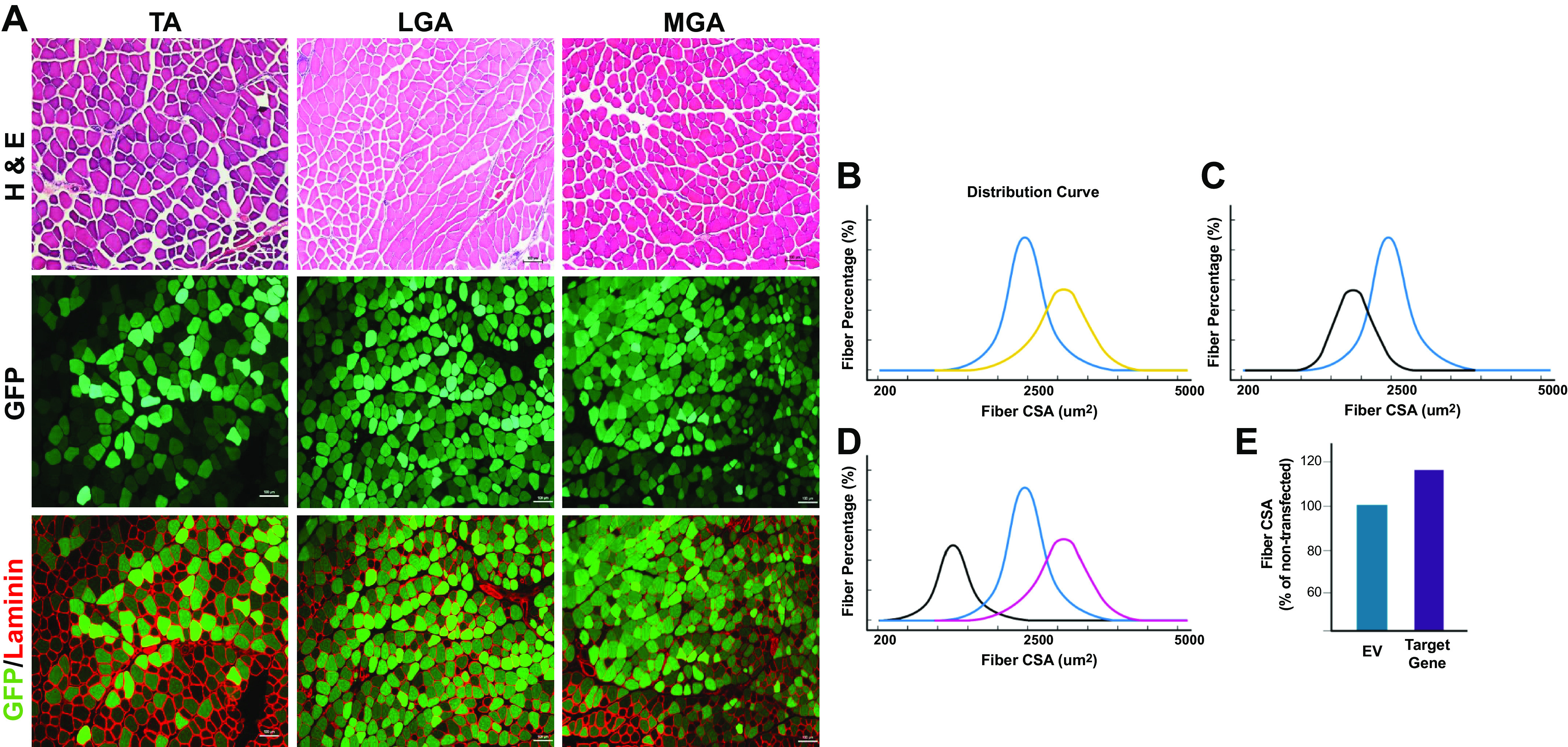
Identification and quantification of transfected skeletal muscle fibers in various hindlimb muscles. A: representative images of hindlimb skeletal muscles from 12- to 16-wk-old C57/BL6 male mice electroporated with an emerald GFP (emGFP) plasmid for 7 days. Separately, tibialis anterior (TA) and gastrocnemius (GA) muscles are given an intramuscular injection with 0.4 U/μL of hyaluronidase before electroporation. After 2 h, the TA and GA muscles are then injected with 2 µg and 5 µg of emGFP respectively and electrical pulses (175 V/cm, 10 pulses, 20 ms) are applied using two-paddle plate electrodes as detailed in published protocols (54, 67, 84). The TA and gastrocnemius muscles were stained for hematoxylin and eosin (H&E), and laminin using protocols detailed in Table 5. ×10 magnification, scale bar = 100 µm. In the analysis of transfected muscle fiber cross-sectional area (CSA), comparisons can be made between empty vector and gene of interest transfected fibers in unchallenged skeletal muscle to assess for hypertrophy (B; yellow line) and atrophy (C; black line). The blue line represents normal fiber CSA distribution of nontransfected or empty vector fibers in B and C. The nontransfected fibers can be assessed for fiber CSA to confirm that the plasmids are not impacting surrounding local muscle fibers. D: in transfected muscle fibers undergoing an additional intervention (e.g., denervation), fiber CSA comparisons between empty vector (black line), gene of interest (purple line), and nontransfected/innervated (blue line) fibers can be analyzed. E: an alternative strategy to incorporate nontransfected muscle fibers in the CSA quantification is to represent the fiber CSA as a percentage of the nontransfected fibers and compare the empty vector to the gene of interest expression plasmid, as used in previously published studies (57,58, 157). Created with Biorender.com.
In unchallenged and challenged skeletal muscle (i.e., basal vs. atrophy stimulus, Fig. 4, B–D), separating the analyses between GFP-positive and GFP-negative fibers can tease out the effect of the target gene being manipulated especially in muscle fibers exposed to an intervention stimulus (84, 141). Both the empty vector and target gene expression plasmid should be analyzed in this manner to validate any effect observed in the target gene transfected muscles. To note, it is important to consider the use of an empty vector that gives no transcriptional or translational stress compared with the experimental plasmid or to use a “control” expression plasmid that encodes for a protein like GFP or LacZ, which could aggregate and cause proteotoxic stress. It has been observed that GFP and LacZ can induced 5%–7% increase in muscle fiber size compared with nontransfected fibers (140, 142). In unchallenged basal skeletal muscle only, the GFP-positive fibers for the empty vector and gene of interest expression plasmid groups could be presented as the whole muscle has not been exposed to a challenging stimulus (Fig. 4, B and C) and thus no effect on fiber CSA in the nontransfected fibers should be observed (54, 67). Furthermore, an alternative approach for fiber CSA analysis is to present the data for GFP-positive fibers relative to the mean of the nontransfected fibers in the same muscle (87, 140, 157) (Fig. 4E). This approach helps to take into account intermouse differences in absolute fiber size and intermuscle differences in the region transfected, and therefore where transfected and nontransfected fibers were selected for analysis. The various strategies to present fiber CSA data on transfected versus nontransfected muscles is illustrated in Fig. 4, B–E.
Another important consideration in the analysis of electroporated muscle fiber CSA is the use of a threshold for the number of GFP-positive fibers analyzed. As not all fibers are successfully transfected with the electroporation process, a threshold for the number of GFP-positive fibers to be analyzed will enable the detection of any effect of the overexpression of the gene of interest being studied. Commonly, studies have assessed ∼300–400 GFP-positive fibers for CSA analysis per muscle, with ≥200 GFP-positive fibers being stipulated (54, 68, 84); however, this will also be influenced by the transfection efficiency. The total number of transfected fibers analyzed may be determined by the field of magnification selected when performing image capture on the fluorescent microscope. Ideally, a similar number of nontransfected fibers will be analyzed that can allow for the comparison between transfected and nontransfected control fibers in the same muscle (e.g., assessing the hypertrophic/atrophic effect of a particular protein). The difference between a ×10 and ×20 magnification will present a different field size for imaging of transfected muscle fibers but can also distort the overall level of transfection efficiency achieved. Indeed, an important consideration is the occurrence of regional transfection and how this influences the percentage of total transfected fibers across the whole muscle section. The use of a threshold for the number of transfected and nontransfected fibers that need to be quantified can account the percentage of transfected fibers across the whole muscle versus only specific regions. With all these factors considered, images of transfected GFP-positive and GFP-negative fibers can be successfully quantified (54, 67, 84) using semiautomated software for fiber analysis such as MyoVision (190, 191) and Imaris (192).
One of the most notable considerations when using in vivo electroporation for exploring molecular mechanisms of skeletal muscle plasticity is that plasmid transfection does not occur in all fibers within the muscle being targeted. Thus, data obtained by analyzing the whole muscle at the biochemical level will likely underestimate the overall magnitude of the effect observed. Another consideration is the variability between intermuscular expression as injections for plasmid delivery are unlikely to be identical and the same number of fibers are unlikely to be transfected. However, the within-animal experimental design will often increase the experimental power for observing any potential statistical significance between empty vector (control) and gene of interest transfected muscles. Finally, compared with transgenic mice and AAV technology, in vivo electroporation only allows for the targeting of one individual skeletal muscle per experiment/group (i.e., TA or GA muscles only), which reduces the ability to perform cross muscle comparisons under experimental conditions.
CONCLUSIONS
The in vivo electroporation technique provides a quick and effective strategy for gene delivery into skeletal muscle allowing for knockdown and overexpression studies to be performed. In addition, the technique provides the researcher the ability to rapidly and effectively screen the function of multiple novel genes in skeletal muscle before the development of a genetically engineered rodent model. This method can also be used to manipulate circulating cytokine and growth factor concentrations at a lower cost alternative compared with the use of recombinant protein infusion and/or injections. This review describes critical components and protocols needed for the successful incorporation of electroporation to study molecular mechanisms of skeletal muscle plasticity. This review should serve as a valuable resource for researchers and laboratories that currently perform electroporation studies, while also giving those researchers looking to implement this technique into their research program every possible opportunity for success.
GRANTS
D. C. Hughes was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number K01AR077684. J. P. Hardee was supported by a McKenzie Research Fellowship from The University of Melbourne.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.C.H., J.P.H., D.S.W., and C.A.G. conceived and designed research; D.C.H., J.P.H., D.S.W., and C.A.G. prepared figures; D.C.H., J.P.H., D.S.W., and C.A.G. drafted manuscript; D.C.H., J.P.H., D.S.W., and C.A.G. edited and revised manuscript; D.C.H., J.P.H., D.S.W., and C.A.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank our previous mentors Dr. Troy Hornberger, Dr. Sue Bodine, Dr. James Carson, Dr. Keith Baar, and Dr. Christopher Adams for all training and guidance in the application of the in vivo electroporation technique. Figures and Graphical Abstract were created with BioRender and published with permission.
REFERENCES
- 1.Sharples AP, Hughes DC, Deane CS, Saini A, Selman C, Stewart CE. Longevity and skeletal muscle mass: the role of IGF signalling, the sirtuins, dietary restriction and protein intake. Aging cell 14: 511–523, 2015. doi: 10.1111/acel.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ebert SM, Al-Zougbi A, Bodine SC, Adams CM. Skeletal muscle atrophy: discovery of mechanisms and potential therapies. Physiology (Bethesda) 34: 232–239, 2019. doi: 10.1152/physiol.00003.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hughes DC, Ellefsen S, Baar K. Adaptations to endurance and strength training. Cold Spring Harb Perspect Med 8: a029769, 2018. doi: 10.1101/cshperspect.a029769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3: 1014–1019, 2001. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 5.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294: 1704–1708, 2001. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 6.Sartori R, Romanello V, Sandri M. Mechanisms of muscle atrophy and hypertrophy: Implications in health and disease. Nature Communications 12: 12, 2021. doi: 10.1038/s41467-020-20123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lang F, Aravamudhan S, Nolte H, Türk C, Hölper S, Müller S, Günther S, Blaauw B, Braun T, Krüger M. Dynamic changes in the mouse skeletal muscle proteome during denervation-induced atrophy. Dis Model Mech 10: 881–896, 2017. doi: 10.1242/dmm.028910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunt LC, Graca FA, Pagala V, Wang Y-D, Li Y, Yuan Z-F, Fan Y, Labelle M, Peng J, Demontis F. Integrated genomic and proteomic analyses identify stimulus-dependent molecular changes associated with distinct modes of skeletal muscle atrophy. Cell Rep 37: 109971, 2021. doi: 10.1016/j.celrep.2021.109971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mobley CB, Vechetti IJ, Valentino TR, McCarthy JJ. CORP: Using transgenic mice to study skeletal muscle physiology. J Appl Physiol (1985) 128: 1227–1239, 2020. doi: 10.1152/japplphysiol.00021.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naso MF, Tomkowicz B, Perry WL, Strohl WR. Adeno-associated virus (AAV) as a vector for gene therapy. BioDrugs 31: 317–334, 2017. doi: 10.1007/s40259-017-0234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher KJ, Jooss K, Alston J, Yang Y, Haecker SE, High K, Pathak R, Raper SE, Wilson JM. Recombinant adeno-associated virus for muscle directed gene therapy. Nat Med 3: 306–312, 1997. doi: 10.1038/nm0397-306. [DOI] [PubMed] [Google Scholar]
- 12.Davis HL, Demeneix BA, Quantin B, Coulombe J, Whalen RG. Plasmid DNA is superior to viral vectors for direct gene transfer into adult mouse skeletal muscle. Hum Gene Ther 4: 733–740, 1993. doi: 10.1089/hum.1993.4.6-733. [DOI] [PubMed] [Google Scholar]
- 13.Jiao S, Williams P, Berg RK, Hodgeman BA, Liu L, Repetto G, Wolff JA. Direct gene transfer into nonhuman primate myofibers in vivo. Hum Gene Ther 3: 21–33, 1992. doi: 10.1089/hum.1992.3.1-21. [DOI] [PubMed] [Google Scholar]
- 14.Aihara H, Miyazaki J-I. Gene transfer into muscle by electroporation in vivo. Nat Biotechnol 16: 867–870, 1998. doi: 10.1038/nbt0998-867. [DOI] [PubMed] [Google Scholar]
- 15.Gregorevic P, Blankinship MJ, Allen JM, Crawford RW, Meuse L, Miller DG, Russell DW, Chamberlain JS. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat Med 10: 828–834, 2004. doi: 10.1038/nm1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mir LM, Bureau MF, Gehl J, Rangara R, Rouy D, Caillaud J-M, Delaere P, Branellec D, Schwartz B, Scherman D. High-efficiency gene transfer into skeletal muscle mediated by electric pulses. Proc Natl Acad Sci USA 96: 4262–4267, 1999. doi: 10.1073/pnas.96.8.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gehl J, Sørensen TH, Nielsen K, Raskmark P, Nielsen SL, Skovsgaard T, Mir LM. In vivo electroporation of skeletal muscle: threshold, efficacy and relation to electric field distribution. Biochim Biophys Acta 1428: 233–240, 1999. doi: 10.1016/s0304-4165(99)00094-x. [DOI] [PubMed] [Google Scholar]
- 18.McMahon JM, Signori E, Wells KE, Fazio VM, Wells DJ. Optimisation of electrotransfer of plasmid into skeletal muscle by pretreatment with hyaluronidase – increased expression with reduced muscle damage. Gene Ther 8: 1264–1270, 2001. doi: 10.1038/sj.gt.3301522. [DOI] [PubMed] [Google Scholar]
- 19.Talbot GE, Waddington SN, Bales O, Tchen RC, Antoniou MN. Desmin-regulated lentiviral vectors for skeletal muscle gene transfer. Mol Ther 18: 601–608, 2010. doi: 10.1038/mt.2009.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kimura E, Li S, Gregorevic P, Fall BM, Chamberlain JS. Dystrophin delivery to muscles of mdx mice using lentiviral vectors leads to myogenic progenitor targeting and stable gene expression. Mol Ther 18: 206–213, 2010. doi: 10.1038/mt.2009.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Konieczny P, Swiderski K, Chamberlain JS. Gene and cell‐mediated therapies for muscular dystrophy. Muscle Nerve 47: 649–663, 2013. doi: 10.1002/mus.23738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coster H. A quantitative analysis of the voltage-current relationships of fixed charge membranes and the associated property of “punch-through. Biophys J 5: 669–686, 1965. doi: 10.1016/S0006-3495(65)86745-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coster H, George E, Simons R. The electrical characteristics of fixed charge membranes: solution of the field equations. Biophys J 9: 666–684, 1969. doi: 10.1016/S0006-3495(69)86411-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sale A, Hamilton W. Effects of high electric fields on micro-organisms. III. Lysis of erythrocytes and protoplasts. Biochim Biophys Acta 163: 37–43, 1968. doi: 10.1016/0005-2736(68)90030-8. [DOI] [PubMed] [Google Scholar]
- 25.Neumann E, Kakorin S, Toensing K. Fundamentals of electroporative delivery of drugs and genes. Bioelectrochem Bioenerg 48: 3–16, 1999. doi: 10.1016/s0302-4598(99)00008-2. [DOI] [PubMed] [Google Scholar]
- 26.Weaver JC, Chizmadzhev YA. Theory of electroporation: a review. Bioelectrochem Bioenerg 41: 135–160, 1996. doi: 10.1016/S0302-4598(96)05062-3. [DOI] [Google Scholar]
- 27.McMahon JM, Wells DJ. Electroporation for gene transfer to skeletal muscles. BioDrugs 18: 155–165, 2004. doi: 10.2165/00063030-200418030-00002. [DOI] [PubMed] [Google Scholar]
- 28.Gehl J, Skovsgaard T, Mir LM. Vascular reactions to in vivo electroporation: characterization and consequences for drug and gene delivery. Biochim Biophys 1569: 51–58, 2002. doi: 10.1016/s0304-4165(01)00233-1. [DOI] [PubMed] [Google Scholar]
- 29.Bier M, Hammer SM, Canaday DJ, Lee RC. Kinetics of sealing for transient electropores in isolated mammalian skeletal muscle cells. Bioelectromagnetics 20: 194–201, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 30.Bureau M, Gehl J, Deleuze V, Mir L, Scherman D. Importance of association between permeabilization and electrophoretic forces for intramuscular DNA electrotransfer. Biochim Biophys Acta 1474: 353–359, 2000. doi: 10.1016/S0304-4165(00)00028-3. [DOI] [PubMed] [Google Scholar]
- 31.Golzio M, Teissié J, Rols M-P. Direct visualization at the single-cell level of electrically mediated gene delivery. Proc Natl Acad Sci USA 99: 1292–1297, 2002. doi: 10.1073/pnas.022646499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sukharev S, Klenchin V, Serov S, Chernomordik L, YuA C. Electroporation and electrophoretic DNA transfer into cells. The effect of DNA interaction with electropores. Biophys J 63: 1320–1327, 1992. doi: 10.1016/S0006-3495(92)81709-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klenchin V, Sukharev S, Serov S, Chernomordik L, YuA C. Electrically induced DNA uptake by cells is a fast process involving DNA electrophoresis. Biophys J 60: 804–811, 1991. doi: 10.1016/S0006-3495(91)82115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chapdelaine P, Moisset P-A, Campeau P, Asselin I, Vilquin J-T, Tremblay JP. Functional EGFP–dystrophin fusion proteins for gene therapy vector development. Protein Eng 13: 611–615, 2000. doi: 10.1093/protein/13.9.611. [DOI] [PubMed] [Google Scholar]
- 35.Danko I, Fritz JD, Latendresse JS, Herweijer H, Schultz E, Wolff JA. Dystrophin expression improves myofiber survival in mdx muscle following intramuscular plasmid DNA injection. Hum Mol Genet 2: 2055–2061, 1993. doi: 10.1093/hmg/2.12.2055. [DOI] [PubMed] [Google Scholar]
- 36.Acsadi G, Dickson G, Love DR, Jani A, Walsh FS, Gurusinghe A, Wolff JA, Davies KE. Human dystrophin expression in mdx mice after intramuscular injection of DNA constructs. Nature 352: 815–818, 1991. doi: 10.1038/352815a0. [DOI] [PubMed] [Google Scholar]
- 37.Jang H-S, Kim H-J, Kim J-M, Lee Y-S, Kim KL, Kim J-A, Lee J-Y, Suh W, Choi J-H, Jeon E-S, Byun J, Kim D-K. A novel ex vivo angiogenesis assay based on electroporation-mediated delivery of naked plasmid DNA to skeletal muscle. Mol Ther 9: 464–474, 2004. doi: 10.1016/j.ymthe.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 38.Lesbordes J-C, Bordet T, Haase G, Castelnau-Ptakhine L, Rouhani S, Gilgenkrantz H, Kahn A. In vivo electrotransfer of the cardiotrophin-1 gene into skeletal muscle slows down progression of motor neuron degeneration in pmn mice. Hum Mol Genet 11: 1615–1625, 2002. doi: 10.1093/hmg/11.14.1615. [DOI] [PubMed] [Google Scholar]
- 39.Levy M, Barron L, Meyer K, Szoka F Jr.. Characterization of plasmid DNA transfer into mouse skeletal muscle: evaluation of uptake mechanism, expression and secretion of gene products into blood. Gene Ther 3: 201–211, 1996. [PubMed] [Google Scholar]
- 40.Naffakh N, Pinset C, Montarras D, Li Z, Paulin D, Danos O, Heard JM. Long-term secretion of therapeutic proteins from genetically modified skeletal muscles. Hum Gene Ther 7: 11–21, 1996. doi: 10.1089/hum.1996.7.1-11. [DOI] [PubMed] [Google Scholar]
- 41.Rosa Lima E, Regina Cecchi C, Higuti E, Protasio Pacheco de Jesus G, Moura Gomes A, Aparecido Zacarias E, Bartolini P, Nunes Peroni C. Optimization of mouse growth hormone plasmid DNA electrotransfer into tibialis cranialis muscle of “little” mice. Molecules 25: 5034, 2020. doi: 10.3390/molecules25215034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trollet C, Bloquel C, Scherman D, Bigey P. Electrotransfer into skeletal muscle for protein expression. Curr Gene Ther 6: 561–578, 2006. doi: 10.2174/156652306778520656. [DOI] [PubMed] [Google Scholar]
- 43.Munroe M, Dvoretskiy S, Lopez A, Leong J, Dyle MC, Kong H, Adams CM, Boppart MD. Pericyte transplantation improves skeletal muscle recovery following hindlimb immobilization. FASEB J 33: 7694–7706, 2019. doi: 10.1096/fj.201802580R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Molnar MJ, Gilbert R, Lu Y, Liu A-B, Guo A, Larochelle N, Orlopp K, Lochmuller H, Petrof BJ, Nalbantoglu J, Karpati G. Factors influencing the efficacy, longevity, and safety of electroporation-assisted plasmid-based gene transfer into mouse muscles. Mol Ther 10: 447–455, 2004. doi: 10.1016/j.ymthe.2004.06.642. [DOI] [PubMed] [Google Scholar]
- 45.Schertzer JD, Lynch GS. Plasmid-based gene transfer in mouse skeletal muscle by electroporation. Methods Mol Biol 433: 115–125, 2008. doi: 10.1007/978-1-59745-237-3_7. [DOI] [PubMed] [Google Scholar]
- 46.Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, Felgner PL. Direct gene transfer into mouse muscle in vivo. Science 247: 1465–1468, 1990. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 47.Wolff JA, Ludtke JJ, Acsadi G, Williams P, Jani A. Long-term persistence of plasmid DNA and foreign gone expression in mouse muscle. Hum Mol Genet 1: 363–369, 1992. doi: 10.1093/hmg/1.6.363. [DOI] [PubMed] [Google Scholar]
- 48.Vicat JM, Boisseau S, Jourdes P, Lainé M, Wion D, Bouali-Benazzouz R, Benabid AL, Berger F. Muscle transfection by electroporation with high-voltage and short-pulse currents provides high-level and long-lasting gene expression. Hum Gene Ther 11: 909–916, 2000. doi: 10.1089/10430340050015518. [DOI] [PubMed] [Google Scholar]
- 49.Schertzer JD, Plant DR, Lynch GS. Optimizing plasmid-based gene transfer for investigating skeletal muscle structure and function. Mol Ther 13: 795–803, 2006. doi: 10.1016/j.ymthe.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 50.Gollins H, McMahon J, Wells KE, Wells DJ. High-efficiency plasmid gene transfer into dystrophic muscle. Gene Ther 10: 504–512, 2003. doi: 10.1038/sj.gt.3301927. [DOI] [PubMed] [Google Scholar]
- 51.Donà M, Sandri M, Rossini K, Dell'Aica I, Podhorska-Okolow M, Carraro U. Functional in vivo gene transfer into the myofibers of adult skeletal muscle. Biochem Biophys Res Commun 312: 1132–1138, 2003. doi: 10.1016/j.bbrc.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 52.Vilquin J, Kennel P, Paturneau-Jouas M, Chapdelaine P, Boissel N, Delaere P, Tremblay J, Scherman D, Fiszman M, Schwartz K. Electrotransfer of naked DNA in the skeletal muscles of animal models of muscular dystrophies. Gene Ther 8: 1097–1107, 2001. doi: 10.1038/sj.gt.3301484. [DOI] [PubMed] [Google Scholar]
- 53.Bullard SA, Seo S, Schilling B, Dyle MC, Dierdorff JM, Ebert SM, DeLau AD, Gibson BW, Adams CM. Gadd45a protein promotes skeletal muscle atrophy by forming a complex with the protein kinase MEKK4. J Biol Chem 291: 17496–17509, 2016. doi: 10.1074/jbc.M116.740308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baehr LM, Hughes DC, Lynch SA, Van Haver D, Maia TM, Marshall AG, Radoshevich L, Impens F, Waddell DS, Bodine SC. Identification of the MuRF1 skeletal muscle ubiquitylome through quantitative proteomics. Function (Oxf) 2: zqab029, 2021. doi: 10.1093/function/zqab029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rana Z, Ekmark M, Gundersen K. Coexpression after electroporation of plasmid mixtures into muscle in vivo. Acta Physiol Scand 181: 233–238, 2004. doi: 10.1111/j.1365-201X.2004.01282.x. [DOI] [PubMed] [Google Scholar]
- 56.Alzghoul MB, Gerrard D, Watkins BA, Hannon K. Ectopic expression of IGF‐I and Shh by skeletal muscle inhibits disuse‐mediated skeletal muscle atrophy and bone osteopenia in vivo. FASEB J 18: 221–223, 2004. doi: 10.1096/fj.03-0293fje. [DOI] [PubMed] [Google Scholar]
- 57.Frey JW, Jacobs BL, Goodman CA, Hornberger TA. A role for Raptor phosphorylation in the mechanical activation of mTOR signaling. Cell Signal 26: 313–322, 2014. doi: 10.1016/j.cellsig.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goodman CA, Miu MH, Frey JW, Mabrey DM, Lincoln HC, Ge Y, Chen J, Hornberger TA. A phosphatidylinositol 3-kinase/protein kinase B-independent activation of mammalian target of rapamycin signaling is sufficient to induce skeletal muscle hypertrophy. Mol Biol Cell 21: 3258–3268, 2010. doi: 10.1091/mbc.E10-05-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davis HL, Whalen RG, Demeneix BA. Direct gene transfer into skeletal muscle in vivo: factors affecting efficiency of transfer and stability of expression. Hum Gene Ther 4: 151–159, 1993. doi: 10.1089/hum.1993.4.2-151. [DOI] [PubMed] [Google Scholar]
- 60.Manthorpe M, Cornefert-Jensen F, Hartikka J, Felgner J, Rundell A, Margalith M, Dwarki V. Gene therapy by intramuscular injection of plasmid DNA: studies on firefly luciferase gene expression in mice. Hum Gene Ther 4: 419–431, 1993. doi: 10.1089/hum.1993.4.4-419. [DOI] [PubMed] [Google Scholar]
- 61.Neumann E, Schaefer‐Ridder M, Wang Y, Hofschneider P. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J 1: 841–845, 1982. doi: 10.1002/j.1460-2075.1982.tb01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Orlowski S, Mir LM. Cell electropermeabilization: a new tool for biochemical and pharmacological studies. Biochim Biophys Acta 1154: 51–63, 1993. doi: 10.1016/0304-4157(93)90016-h. [DOI] [PubMed] [Google Scholar]
- 63.Suzuki T, Shin B-C, Fujikura K, Matsuzaki T, Takata K. Direct gene transfer into rat liver cells by in vivo electroporation. FEBS Lett 425: 436–440, 1998. doi: 10.1016/s0014-5793(98)00284-1. [DOI] [PubMed] [Google Scholar]
- 64.Titomirov AV, Sukharev S, Kistanova E. In vivo electroporation and stable transformation of skin cells of newborn mice by plasmid DNA. Biochim Biophys Acta 1088: 131–134, 1991. doi: 10.1016/0167-4781(91)90162-F. [DOI] [PubMed] [Google Scholar]
- 65.Somiari S, Glasspool-Malone J, Drabick JJ, Gilbert RA, Heller R, Jaroszeski MJ, Malone RW. Theory and in vivo application of electroporative gene delivery. Mol Ther 2: 178–187, 2000. doi: 10.1006/mthe.2000.0124. [DOI] [PubMed] [Google Scholar]
- 66.Fuqua JD, Mere CP, Kronemberger A, Blomme J, Bae D, Turner KD, Harris MP, Scudese E, Edwards M, Ebert SM, de Sousa LGO, Bodine SC, Yang L, Adams CM, Lira VA. ULK2 is essential for degradation of ubiquitinated protein aggregates and homeostasis in skeletal muscle. FASEB J 33: 11735–12745, 2019. doi: 10.1096/fj.201900766R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hughes DC, Turner DC, Baehr LM, Seaborne RA, Viggars M, Jarvis JC, Gorski PP, Stewart CE, Owens DJ, Bodine SC, Sharples AP. Knockdown of the E3 Ubiquitin ligase UBR5 and its role in skeletal muscle anabolism. Am J Physiol Cell Physiol 320: C45–C56, 2021. doi: 10.1152/ajpcell.00432.2020. [DOI] [PubMed] [Google Scholar]
- 68.Ebert SM, Monteys AM, Fox DK, Bongers KS, Shields BE, Malmberg SE, Davidson BL, Suneja M, Adams CM. The transcription factor ATF4 promotes skeletal myofiber atrophy during fasting. Mol Endocrinol 24: 790–799, 2010. doi: 10.1210/me.2009-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Satkauskas S, Bureau MF, Puc M, Mahfoudi A, Scherman D, Miklavcic D, Mir LM. Mechanisms of in vivo DNA electrotransfer: respective contributions of cell electropermeabilization and DNA electrophoresis. Mol Ther 5: 133–140, 2002. doi: 10.1006/mthe.2002.0526. [DOI] [PubMed] [Google Scholar]
- 70.Cleasby ME, Reinten TA, Cooney GJ, James DE, Kraegen EW. Functional studies of akt isoform specificity in skeletal muscle in vivo; maintained insulin sensitivity despite reduced insulin receptor substrate-1 expression. Mol Endocrinol 21: 215–228, 2007. doi: 10.1210/me.2006-0154. [DOI] [PubMed] [Google Scholar]
- 71.Bruce CR, Brolin C, Turner N, Cleasby ME, Leij FRvd, Cooney GJ, Kraegen EW. Overexpression of carnitine palmitoyltransferase I in skeletal muscle in vivo increases fatty acid oxidation and reduces triacylglycerol esterification. Am J Physiol Endocrinol Metab 292: E1231–E1237, 2007. doi: 10.1152/ajpendo.00561.2006. [DOI] [PubMed] [Google Scholar]
- 72.Bass JJ, Nakhuda A, Deane CS, Brook MS, Wilkinson DJ, Phillips BE, Philp A, Tarum J, Kadi F, Andersen D, Garcia AM, Smith K, Gallagher IJ, Szewczyk NJ, Cleasby ME, Atherton PJ. Overexpression of the vitamin D receptor (VDR) induces skeletal muscle hypertrophy. Mol Metab 42: 101059, 2020. doi: 10.1016/j.molmet.2020.101059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bass JJ, Kazi AA, Deane CS, Nakhuda A, Ashcroft SP, Brook MS, Wilkinson DJ, Phillips BE, Philp A, Tarum J, Kadi F, Andersen D, Garcia AM, Smith K, Gallagher IJ, Szewczyk NJ, Cleasby ME, Atherton PJ. The mechanisms of skeletal muscle atrophy in response to transient knockdown of the vitamin D receptor in vivo. J Physiol 599: 963–979, 2021. doi: 10.1113/JP280652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Timmers S, de Vogel-van den Bosch J, Hesselink MKC, van Beurden D, Schaart G, Ferraz MJ, Losen M, Martinez-Martinez P, De Baets MH, Aerts JMFG, Schrauwen P. Paradoxical increase in TAG and DAG content parallel the insulin sensitizing effect of unilateral DGAT1 overexpression in rat skeletal muscle. PLoS One 6: e14503, 2011. doi: 10.1371/journal.pone.0014503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brandon AE, Tid-Ang J, Wright LE, Stuart E, Suryana E, Bentley N, Turner N, Cooney GJ, Ruderman NB, Kraegen EW. Overexpression of SIRT1 in rat skeletal muscle does not alter glucose induced insulin resistance. PLoS One 10: e0121959, 2015. doi: 10.1371/journal.pone.0121959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Watt MJ, Van Denderen BJ, Castelli LA, Bruce CR, Hoy AJ, Kraegen EW, Macaulay L, Kemp BE. Adipose triglyceride lipase regulation of skeletal muscle lipid metabolism and insulin responsiveness. Mol Endocrinol 22: 1200–1212, 2008. doi: 10.1210/me.2007-0485. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 77.Patel SA, Hoehn KL, Lawrence RT, Sawbridge L, Talbot NA, Tomsig JL, Turner N, Cooney GJ, Whitehead JP, Kraegen EW, Cleasby ME. Overexpression of the adiponectin receptor AdipoR1 in rat skeletal muscle amplifies local insulin sensitivity. Endocrinology 153: 5231–5246, 2012. doi: 10.1210/en.2012-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cleasby M, Lau Q, Polkinghorne E, Patel S, Leslie S, Turner N, Cooney G, Xu A, Kraegen E. The adaptor protein APPL1 increases glycogen accumulation in rat skeletal muscle through activation of the PI3-kinase signalling pathway. J Endocrinol 210: 81–92, 2011. doi: 10.1530/JOE-11-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bosma M, Sparks L, Hooiveld G, Jorgensen J, Houten S, Schrauwen P, Kersten S, Hesselink M. Overexpression of PLIN5 in skeletal muscle promotes oxidative gene expression and intramyocellular lipid content without compromising insulin sensitivity. Biochim Biophys Acta 1831: 844–852, 2013. doi: 10.1016/j.bbalip.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 80.Boden MJ, Brandon AE, Tid-Ang JD, Preston E, Wilks D, Stuart E, Cleasby ME, Turner N, Cooney GJ, Kraegen EW. Overexpression of manganese superoxide dismutase ameliorates high-fat diet-induced insulin resistance in rat skeletal muscle. Am J Physiol Endocrinol Metab 303: E798–E805, 2012. doi: 10.1152/ajpendo.00577.2011.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hughes DC, Marcotte GR, Baehr LM, West DW, Marshall AG, Ebert SM, Davidyan A, Adams CM, Bodine SC, Baar K. Alterations in the muscle force transfer apparatus in aged rats during unloading and reloading: impact of microRNA‐31. J Physiol 596: 2883–2900, 2018. doi: 10.1113/JP275833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tuckow AP, Vary TC, Kimball SR, Jefferson LS. Ectopic expression of eIF2Bε in rat skeletal muscle rescues the sepsis-induced reduction in guanine nucleotide exchange activity and protein synthesis. Am J Physiol Endocrinol Metab 299: E241–E248, 2010. doi: 10.1152/ajpendo.00151.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xu D, Shimkus KL, Lacko HA, Kutzler L, Jefferson LS, Kimball SR. Evidence for a role for Sestrin1 in mediating leucine-induced activation of mTORC1 in skeletal muscle. Am J Physiol Endocrinol Metab 316: E817–E828, 2019. doi: 10.1152/ajpendo.00522.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hughes DC, Baehr LM, Driscoll JR, Lynch SA, Waddell DS, Bodine SC. Identification and characterization of Fbxl22, a novel skeletal muscle atrophy-promoting E3 ubiquitin ligase. Am J Physiol Cell Physiol 319: C700–C719, 2020. doi: 10.1152/ajpcell.00253.2020. [DOI] [PubMed] [Google Scholar]
- 85.Ebert SM, Dyle MC, Bullard SA, Dierdorff JM, Murry DJ, Fox DK, Bongers KS, Lira VA, Meyerholz DK, Talley JJ, Adams CM. Identification and small molecule inhibition of an activating transcription factor 4 (ATF4)-dependent pathway to age-related skeletal muscle weakness and atrophy. J Biol Chem 290: 25497–25511, 2015. doi: 10.1074/jbc.M115.681445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lefesvre P, Attema J, van Bekkum D. A comparison of efficacy and toxicity between electroporation and adenoviral gene transfer. BMC Mol Biol 3: 12, 2002. doi: 10.1186/1471-2199-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.You J-S, Dooley MS, Kim C-R, Kim E-J, Xu W, Goodman CA, Hornberger TA. A DGKζ-FoxO-ubiquitin proteolytic axis controls fiber size during skeletal muscle remodeling. Sci Signal 11: eaao6847, 2018. doi: 10.1126/scisignal.aao6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hetzler KL, Hardee JP, Puppa MJ, Narsale AA, Sato S, Davis JM, Carson JA. Sex differences in the relationship of IL-6 signaling to cancer cachexia progression. Biochim Biophys Acta 1852: 816–825, 2015. doi: 10.1016/j.bbadis.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hardee JP, Fix DK, Wang X, Goldsmith EC, Koh H-J, Carson JA. Systemic IL-6 regulation of eccentric contraction-induced muscle protein synthesis. Am J Physiol Cell Physiol 315: C91–C103, 2018. doi: 10.1152/ajpcell.00063.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.VanderVeen BN, Fix DK, Montalvo RN, Counts BR, Smuder AJ, Murphy EA, Koh H, Carson JA. The regulation of skeletal muscle fatigability and mitochondrial function by chronically elevated interleukin‐6. Exp Physiol 104: 385–397, 2019. doi: 10.1113/EP087429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.White JP, Puppa MJ, Sato S, Gao S, Price RL, Baynes JW, Kostek MC, Matesic LE, Carson JA. IL-6 regulation on skeletal muscle mitochondrial remodeling during cancer cachexia in the ApcMin/+mouse. Skelet Muscle 2: 14, 2012. doi: 10.1186/2044-5040-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Baltgalvis KA, Berger FG, Peña MM, Davis JM, White JP, Carson JA. Muscle wasting and interleukin-6-induced atrogin-I expression in the cachectic Apc (Min/+) mouse. Pflugers Arch 457: 989–1001, 2009. doi: 10.1007/s00424-008-0574-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Baltgalvis KA, Berger FG, Pena MM, Davis JM, Muga SJ, Carson JA. Interleukin-6 and cachexia in ApcMin/+ mice. Am J Physiol Regul Integr Comp Physiol 294: R393–R401, 2008. doi: 10.1152/ajpregu.00716.2007. [DOI] [PubMed] [Google Scholar]
- 94.Akerstrom T, Vedel K, Needham J, Hojman P, Kontou E, Hellsten Y, Wojtaszewski JF. Optimizing hyaluronidase dose and plasmid DNA delivery greatly improves gene electrotransfer efficiency in rat skeletal muscle. Biochem Biophys Rep 4: 342–350, 2015. doi: 10.1016/j.bbrep.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gissel H. Effects of varying pulse parameters on ion homeostasis, cellular integrity, and force following electroporation of rat muscle in vivo. Am J Physiol Regul Integr Comp Physiol 298: R918–R929, 2010. doi: 10.1152/ajpregu.00692.2009. [DOI] [PubMed] [Google Scholar]
- 96.Hojman P, Gissel H, Andre FM, Cournil-Henrionnet C, Eriksen J, Gehl J, Mir LM. Physiological effects of high-and low-voltage pulse combinations for gene electrotransfer in muscle. Hum Gene Ther 19: 1249–1260, 2008. doi: 10.1089/hum.2008.059. [DOI] [PubMed] [Google Scholar]
- 97.Vitadello M, Sorge M, Percivalle E, Germinario E, Danieli-Betto D, Turco E, Tarone G, Brancaccio M, Gorza L. Loss of melusin is a novel, neuronal NO synthase/FoxO3-independent master switch of unloading-induced muscle atrophy. J Cachexia Sarcopenia Muscle 11: 802–819, 2020. doi: 10.1002/jcsm.12546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vitadello M, Gherardini J, Gorza L. The stress protein/chaperone Grp94 counteracts muscle disuse atrophy by stabilizing subsarcolemmal neuronal nitric oxide synthase. Antioxid Redox Signal 20: 2479–2496, 2014. doi: 10.1089/ars.2012.4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Van Pelt DW, Confides AL, Judge AR, Vanderklish PW, Dupont-Versteegden EE. Cold shock protein RBM3 attenuates atrophy and induces hypertrophy in skeletal muscle. J Muscle Res Cell Motil 39: 35–40, 2018. doi: 10.1007/s10974-018-9496-x. [DOI] [PubMed] [Google Scholar]
- 100.Judge AR, Koncarevic A, Hunter RB, Liou H-C, Jackman RW, Kandarian SC. Role for IκBα, but not c-Rel, in skeletal muscle atrophy. Am J Physiol Cell Physiol 292: C372–C382, 2007. doi: 10.1152/ajpcell.00293.2006. [DOI] [PubMed] [Google Scholar]
- 101.Serrano AL, Murgia M, Pallafacchina G, Calabria E, Coniglio P, Lømo T, Schiaffino S. Calcineurin controls nerve activity-dependent specification of slow skeletal muscle fibers but not muscle growth. Proc Natl Acad Sci USA 98: 13108–13113, 2001. doi: 10.1073/pnas.231148598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Beharry AW, Sandesara PB, Roberts BM, Ferreira LF, Senf SM, Judge AR. HDAC1 activates FoxO and is both sufficient and required for skeletal muscle atrophy. J Cell Sci 127: 1441–1453, 2014. doi: 10.1242/jcs.136390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Senf SM, Dodd SL, McClung JM, Judge AR. Hsp70 overexpression inhibits NF-κB and Foxo3a transcriptional activities and prevents skeletal muscle atrophy. FASEB J 22: 3836–3845, 2008. doi: 10.1096/fj.08-110163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Taylor J, Babbs CF, Alzghoul MB, Olsen A, Latour M, Pond AL, Hannon K. Optimization of ectopic gene expression in skeletal muscle through DNA transfer by electroporation. BMC Biotechnol 4: 11–18, 2004. doi: 10.1186/1472-6750-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Roche JA, Ford-Speelman DL, Ru LW, Densmore AL, Roche R, Reed PW, Bloch RJ. Physiological and histological changes in skeletal muscle following in vivo gene transfer by electroporation. Am J Physiol Cell Physiol 301: C1239–C1250, 2011. doi: 10.1152/ajpcell.00431.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hughes DC, Wallace MA, Baar K. Effects of aging, exercise, and disease on force transfer in skeletal muscle. Am J Physiol Endocrinol Metab 309: E1–E10, 2015. doi: 10.1152/ajpendo.00095.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Favre D, Cherel Y, Provost N, Blouin V, Ferry N, Moullier P, Salvetti A. Hyaluronidase enhances recombinant adeno-associated virus (rAAV)-mediated gene transfer in the rat skeletal muscle. Gene Ther 7: 1417–1420, 2000. doi: 10.1038/sj.gt.3301256. [DOI] [PubMed] [Google Scholar]
- 108.Mennuni C, Calvaruso F, Zampaglione I, Rizzuto G, Rinaudo D, Dammassa E, Ciliberto G, Fattori E, La Monica N. Hyaluronidase increases electrogene transfer efficiency in skeletal muscle. Hum Gene Ther 13: 355–365, 2002. doi: 10.1089/10430340252792495. [DOI] [PubMed] [Google Scholar]
- 109.Seaborne RA, Hughes DC, Turner DC, Owens DJ, Baehr LM, Gorski P, Semenova EA, Borisov OV, Larin AK, Popov DV, Generozov EV, Sutherland H, Ahmetov II, Jarvis JC, Bodine SC, Sharples AP. UBR5 is a novel E3 ubiquitin ligase involved in skeletal muscle hypertrophy and recovery from atrophy. J Physiol 597: 3727–3749, 2019. doi: 10.1113/JP278073. [DOI] [PubMed] [Google Scholar]
- 110.Ebert SM, Dyle MC, Kunkel SD, Bullard SA, Bongers KS, Fox DK, Dierdorff JM, Foster ED, Adams CM. Stress-induced skeletal muscle Gadd45a expression reprograms myonuclei and causes muscle atrophy. J Biol Chem 287: 27290–27301, 2012. doi: 10.1074/jbc.M112.374777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ebert SM, Bullard SA, Basisty N, Marcotte GR, Skopec ZP, Dierdorff JM, Al-Zougbi A, Tomcheck KC, DeLau AD, Rathmacher JA, Bodine SC, Schilling B, Adams CM. Activating transcription factor 4 (ATF4) promotes skeletal muscle atrophy by forming a heterodimer with the transcriptional regulator C/EBPβ. J Biol Chem 295: 2787–2803, 2020. doi: 10.1074/jbc.RA119.012095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lagirand-Cantaloube J, Cornille K, Csibi A, Batonnet-Pichon S, Leibovitch MP, Leibovitch SA. Inhibition of atrogin-1/MAFbx mediated MyoD proteolysis prevents skeletal muscle atrophy in vivo. PloS one 4: e4973, 2009. doi: 10.1371/journal.pone.0004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Penna F, Bonetto A, Muscaritoli M, Costamagna D, Minero VG, Bonelli G, Fanelli FR, Baccino FM, Costelli P. Muscle atrophy in experimental cancer cachexia: is the IGF‐1 signaling pathway involved? Int J Cancer 127: 1706–1717, 2010. doi: 10.1002/ijc.25146. [DOI] [PubMed] [Google Scholar]
- 114.Knudsen JR, Henriquez‐Olguin C, Li Z, Jensen TE. Electroporated GLUT4‐7myc‐GFP detects in vivo glucose transporter 4 translocation in skeletal muscle without discernible changes in GFP patterns. Exp Physiol 104: 704–714, 2019. doi: 10.1113/EP087545. [DOI] [PubMed] [Google Scholar]
- 115.Liu Z, Chaillou T, Santos Alves E, Mader T, Jude B, Ferreira DMS, Hynynen H, Cheng AJ, Jonsson WO, Pironti G, Andersson DC, Kenne E, Ruas JL, Tavi P, Lanner JT. Mitochondrial NDUFA4L2 is a novel regulator of skeletal muscle mass and force. FASEB J 35: e22010, 2021. doi: 10.1096/fj.202100066R. [DOI] [PubMed] [Google Scholar]
- 116.Bruno NE, Nwachukwu JC, Hughes DC, Srinivasan S, Hawkins R, Sturgill D, Hager GL, Hurst S, Sheu S-S, Bodine SC, Conkright MD, Nettles KW. Activation of Crtc2/Creb1 in skeletal muscle enhances weight loss during intermittent fasting. FASEB J 35: e21999, 2021. doi: 10.1096/fj.202100171R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bruno NE, Kelly KA, Hawkins R, Bramah-Lawani M, Amelio AL, Nwachukwu JC, Nettles KW, Conkright MD. Creb coactivators direct anabolic responses and enhance performance of skeletal muscle. EMBO J 33: 1027–1043, 2014. doi: 10.1002/embj.201386145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tevz G, Pavlin D, Kamensek U, Kranjc S, Mesojednik S, Coer A, Sersa G, Cemazar M. Gene electrotransfer into murine skeletal muscle: a systematic analysis of parameters for long-term gene expression. Technol Cancer Res Treat 7: 91–101, 2008. doi: 10.1177/153303460800700201. [DOI] [PubMed] [Google Scholar]
- 119.Li S, MacLaughlin FC, Fewell JG, Li Y, Mehta V, French MF, Nordstrom JL, Coleman M, Belagali NS, Schwartz RJ, Smith LC. Increased level and duration of expression in muscle by co-expression of a transactivator using plasmid systems. Gene Ther 6: 2005–2011, 1999. doi: 10.1038/sj.gt.3301032. [DOI] [PubMed] [Google Scholar]
- 120.Akimoto T, Sorg BS, Yan Z. Real-time imaging of peroxisome proliferator-activated receptor-γ coactivator-1α promoter activity in skeletal muscles of living mice. Am J Physiol Cell Physiol 287: C790–C796, 2004. doi: 10.1152/ajpcell.00425.2003. [DOI] [PubMed] [Google Scholar]
- 121.Erlich AT, Brownlee DM, Beyfuss K, Hood DA. Exercise induces TFEB expression and activity in skeletal muscle in a PGC-1α-dependent manner. Am J Physiol Cell Physiol 314: C62–C72, 2018. doi: 10.1152/ajpcell.00162.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sandri M, Lin J, Handschin C, Yang W, Arany ZP, Lecker SH, Goldberg AL, Spiegelman BM. PGC-1α protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci USA 103: 16260–16265, 2006. doi: 10.1073/pnas.0607795103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Khan MM, Strack S, Wild F, Hanashima A, Gasch A, Brohm K, Reischl M, Carnio S, Labeit D, Sandri M, Labeit S, Rudolf R. Role of autophagy, SQSTM1, SH3GLB1, and TRIM63 in the turnover of nicotinic acetylcholine receptors. Autophagy 10: 123–136, 2014. doi: 10.4161/auto.26841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rudolf R, Bogomolovas J, Strack S, Choi K-R, Khan MM, Wagner A, Brohm K, Hanashima A, Gasch A, Labeit D, Labeit S. Regulation of nicotinic acetylcholine receptor turnover by MuRF1 connects muscle activity to endo/lysosomal and atrophy pathways. Age (Dordr) 35: 1663–1674, 2013. doi: 10.1007/s11357-012-9468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.De Wet JR, Wood K, DeLuca M, Helinski DR, Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol 7: 725–737, 1987. doi: 10.1128/mcb.7.2.725-737.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shifera AS, Hardin JA. Factors modulating expression of Renilla luciferase from control plasmids used in luciferase reporter gene assays. Anal Biochem 396: 167–172, 2010. doi: 10.1016/j.ab.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Prescher JA, Contag CH. Guided by the light: visualizing biomolecular processes in living animals with bioluminescence. Curr Opin Chem Biol 14: 80–89, 2010. doi: 10.1016/j.cbpa.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 128.Mathiesen I. Electropermeabilization of skeletal muscle enhances gene transfer in vivo. Gene Ther 6: 508–514, 1999. doi: 10.1038/sj.gt.3300847. [DOI] [PubMed] [Google Scholar]
- 129.Senf SM, Judge AR. Determination of gene promoter activity in skeletal muscles in vivo. Methods Mol Biol 798: 461–472, 2012. doi: 10.1007/978-1-61779-343-1_27. [DOI] [PubMed] [Google Scholar]
- 130.Stepanenko OV, Verkhusha VV, Kuznetsova IM, Uversky VN, Turoverov KK. Fluorescent proteins as biomarkers and biosensors: throwing color lights on molecular and cellular processes. Curr Protein Pept Sci 9: 338–369, 2008. doi: 10.2174/138920308785132668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Inouye S, Tsuji FI. Aequorea green fluorescent protein. Expression of the gene and fluorescence characteristics of the recombinant protein. FEBS Lett 341: 277–280, 1994. doi: 10.1016/0014-5793(94)80472-9. [DOI] [PubMed] [Google Scholar]
- 132.Romanello V, Guadagnin E, Gomes L, Roder I, Sandri C, Petersen Y, Milan G, Masiero E, Del Piccolo P, Foretz M, Scorrano L, Rudolf R, Sandri M. Mitochondrial fission and remodelling contributes to muscle atrophy. EMBO J 29: 1774–1785, 2010. doi: 10.1038/emboj.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Laker RC, Xu P, Ryall KA, Sujkowski A, Kenwood BM, Chain KH, Zhang M, Royal MA, Hoehn KL, Driscoll M, Adler PN, Wessells RJ, Saucerman JJ, Yan Z. A novel MitoTimer reporter gene for mitochondrial content, structure, stress, and damage in vivo. J Biol Chem 289: 12005–12015, 2014. doi: 10.1074/jbc.M113.530527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lim S, Deaver JW, Rosa-Caldwell ME, Haynie WS, Morena da Silva F, Cabrera AR, Schrems ER, Saling LW, Jansen LT, Dunlap KR, Wiggs MP, Washington TA, Greene NP. Development of metabolic and contractile alterations in development of cancer cachexia in female tumor-bearing mice. J Appl Physiol (1985) 132: 58–72, 2022. doi: 10.1152/japplphysiol.00660.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rosa-Caldwell ME, Lim S, Haynie WS, Brown JL, Lee DE, Dunlap KR, Jansen LT, Washington TA, Wiggs MP, Greene NP. Mitochondrial aberrations during the progression of disuse atrophy differentially affect male and female mice. J Cachexia Sarcopenia Muscle 12: 2056–2068, 2021. doi: 10.1002/jcsm.12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Brown JL, Rosa-Caldwell ME, Lee DE, Blackwell TA, Brown LA, Perry RA, Haynie WS, Hardee JP, Carson JA, Wiggs MP, Washington TA, Greene NP. Mitochondrial degeneration precedes the development of muscle atrophy in progression of cancer cachexia in tumour-bearing mice. J Cachexia Sarcopenia Muscle 8: 926–938, 2017. doi: 10.1002/jcsm.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Brizzard B. Epitope tagging. Biotechniques 44: 693–695, 2008. doi: 10.2144/000112841. [DOI] [PubMed] [Google Scholar]
- 138.Vandemoortele G, Eyckerman S, Gevaert K. Pick a tag and explore the functions of your pet protein. Trends Biotechnol 37: 1078–1090, 2019. doi: 10.1016/j.tibtech.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 139.Zhao X, Li G, Liang S. Several affinity tags commonly used in chromatographic purification. J Anal Methods Chem 2013: 581093, 2013. doi: 10.1155/2013/581093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Goodman CA, Dietz JM, Jacobs BL, McNally RM, You JS, Hornberger TA. Yes-Associated Protein is up-regulated by mechanical overload and is sufficient to induce skeletal muscle hypertrophy. FEBS Lett 589: 1491–1497, 2015. doi: 10.1016/j.febslet.2015.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.You JS, Lincoln HC, Kim CR, Frey JW, Goodman CA, Zhong XP, Hornberger TA. The role of diacylglycerol kinase ζ and phosphatidic acid in the mechanical activation of mammalian target of rapamycin (mTOR) signaling and skeletal muscle hypertrophy. J Biol Chem 289: 1551–1563, 2014. doi: 10.1074/jbc.M113.531392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Goodman CA, Mabrey DM, Frey JW, Miu MH, Schmidt EK, Pierre P, Hornberger TA. Novel insights into the regulation of skeletal muscle protein synthesis as revealed by a new nonradioactive in vivo technique. FASEB J 25: 1028–1039, 2011. doi: 10.1096/fj.10-168799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Csibi A, Leibovitch MP, Cornille K, Tintignac LA, Leibovitch SA. MAFbx/Atrogin-1 controls the activity of the initiation factor eIF3-f in skeletal muscle atrophy by targeting multiple C-terminal lysines. J Biol Chem 284: 4413–4421, 2009. doi: 10.1074/jbc.M807641200. [DOI] [PubMed] [Google Scholar]
- 144.Lee D, Goldberg AL. SIRT1 protein, by blocking the activities of transcription factors FoxO1 and FoxO3, inhibits muscle atrophy and promotes muscle growth. J Biol Chem 288: 30515–30526, 2013. doi: 10.1074/jbc.M113.489716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.McCarthy JJ, Srikuea R, Kirby TJ, Peterson CA, Esser KA. Inducible Cre transgenic mouse strain for skeletal muscle-specific gene targeting. Skelet Muscle 2: 8, 2012. [Erratum in Skelet Muscle 2: 22, 2012]. doi: 10.1186/2044-5040-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rao P, Monks DA. A tetracycline-inducible and skeletal muscle-specific Cre recombinase transgenic mouse. Dev Neurobiol 69: 401–406, 2009. doi: 10.1002/dneu.20714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shan G. RNA interference as a gene knockdown technique. Int J Biochem Cell Biol 42: 1243–1251, 2010. doi: 10.1016/j.biocel.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 148.Magee TR, Artaza JN, Ferrini MG, Vernet D, Zuniga FI, Cantini L, Reisz‐Porszasz S, Rajfer J, Gonzalez‐Cadavid NF. Myostatin short interfering hairpin RNA gene transfer increases skeletal muscle mass. J Gene Med 8: 1171–1181, 2006. doi: 10.1002/jgm.946. [DOI] [PubMed] [Google Scholar]
- 149.Hetzler K, Collins B, Shanely R, Sue H, Kostek M. The homoeobox gene SIX1 alters myosin heavy chain isoform expression in mouse skeletal muscle. Acta Physiol 210: 415–428, 2014. doi: 10.1111/apha.12168. [DOI] [PubMed] [Google Scholar]
- 150.Papworth C. Site-directed mutagenesis in one day with> 80% efficiency. Strategies 8: 3–4, 1996. doi: 10.1385/0-89603-332-5:31. [DOI] [Google Scholar]
- 151.Weiner MP, Costa GL, Schoettlin W, Cline J, Mathur E, Bauer JC. Site-directed mutagenesis of double-stranded DNA by the polymerase chain reaction. Gene 151: 119–123, 1994. doi: 10.1016/0378-1119(94)90641-6. [DOI] [PubMed] [Google Scholar]
- 152.Cohen S, Brault JJ, Gygi SP, Glass DJ, Valenzuela DM, Gartner C, Latres E, Goldberg AL. During muscle atrophy, thick, but not thin, filament components are degraded by MuRF1-dependent ubiquitylation. J Cell Biol 185: 1083–1095, 2009. doi: 10.1083/jcb.200901052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hodge BA, Zhang X, Gutierrez-Monreal MA, Cao Y, Hammers DW, Yao Z, Wolff CA, Du P, Kemler D, Judge AR, Esser KA. MYOD1 functions as a clock amplifier as well as a critical co-factor for downstream circadian gene expression in muscle. Elife 8: e43017, 2019. doi: 10.7554/eLife.43017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Goodman CA, McNally RM, Hoffmann FM, Hornberger TA. Smad3 induces atrogin-1, inhibits mTOR and protein synthesis, and promotes muscle atrophy in vivo. Mol Endocrinol 27: 1946–1957, 2013. doi: 10.1210/me.2013-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lloyd AC. The regulation of cell size. Cell 154: 1194–1205, 2013. doi: 10.1016/j.cell.2013.08.053. [DOI] [PubMed] [Google Scholar]
- 156.Goodman CA, Hornberger TA. Measuring protein synthesis with SUnSET: a valid alternative to traditional techniques? Exerc Sport Sci Rev 41: 107–115, 2013. doi: 10.1097/JES.0b013e3182798a95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Goodman CA, Coenen AM, Frey JW, You JS, Barker RG, Frankish BP, Murphy RM, Hornberger TA. Insights into the role and regulation of TCTP in skeletal muscle. Oncotarget 8: 18754–18772, 2017. doi: 10.18632/oncotarget.13009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lundell LS, Massart J, Altıntaş A, Krook A, Zierath JR. Regulation of glucose uptake and inflammation markers by FOXO1 and FOXO3 in skeletal muscle. Mol Metab 20: 79–88, 2019. doi: 10.1016/j.molmet.2018.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kang C, Goodman CA, Hornberger TA, Ji LL. PGC‐1α overexpression by in vivo transfection attenuates mitochondrial deterioration of skeletal muscle caused by immobilization. FASEB J 29: 4092–4106, 2015. doi: 10.1096/fj.14-266619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yeo D, Kang C, Gomez-Cabrera MC, Vina J, Ji LL. Intensified mitophagy in skeletal muscle with aging is downregulated by PGC-1alpha overexpression in vivo. Free Radic Biol Med 130: 361–368, 2019. doi: 10.1016/j.freeradbiomed.2018.10.456. [DOI] [PubMed] [Google Scholar]
- 161.You JS, Anderson GB, Dooley MS, Hornberger TA. The role of mTOR signaling in the regulation of protein synthesis and muscle mass during immobilization in mice. Dis Model Mech 8: 1059–1069, 2015. doi: 10.1242/dmm.019414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Steinert ND, Potts GK, Wilson GM, Klamen AM, Lin KH, Hermanson JB, McNally RM, Coon JJ, Hornberger TA. Mapping of the contraction-induced phosphoproteome identifies TRIM28 as a significant regulator of skeletal muscle size and function. Cell Rep 34: 108796, 2021. doi: 10.1016/j.celrep.2021.108796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jacobs BL, McNally RM, Kim KJ, Blanco R, Privett RE, You JS, Hornberger TA. Identification of mechanically regulated phosphorylation sites on tuberin (TSC2) that control mechanistic target of rapamycin (mTOR) signaling. J Biol Chem 292: 6987–6997, 2017. doi: 10.1074/jbc.M117.777805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pandorf CE, Haddad F, Qin AX, Baldwin KM. IIx myosin heavy chain promoter regulation cannot be characterized in vivo by direct gene transfer. Am J Physiol Cell Physiol 293: C1338–C1346, 2007. doi: 10.1152/ajpcell.00221.2007. [DOI] [PubMed] [Google Scholar]
- 165.Giger JM, Haddad F, Qin AX, Baldwin KM. Functional overload increases β-MHC promoter activity in rodent fast muscle via the proximal MCAT (βe3) site. Am J Physiol Cell Physiol 282: C518–C527, 2002. doi: 10.1152/ajpcell.00444.2001. [DOI] [PubMed] [Google Scholar]
- 166.Eash J, Olsen A, Breur G, Gerrard D, Hannon K. FGFR1 inhibits skeletal muscle atrophy associated with hindlimb suspension. BMC Musculoskelet Disord 8: 32, 2007. doi: 10.1186/1471-2474-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sokołowska E, Błachnio-Zabielska AU. A critical review of electroporation as a plasmid delivery system in mouse skeletal muscle. Int J Mol Sci 20: 2776, 2019. doi: 10.3390/ijms20112776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tupling AR, Asahi M, MacLennan DH. Sarcolipin overexpression in rat slow twitch muscle inhibits sarcoplasmic reticulum Ca2+ uptake and impairs contractile function. J Biol Chem 277: 44740–44746, 2002. doi: 10.1074/jbc.M206171200. [DOI] [PubMed] [Google Scholar]
- 169.Umeda Y, Marui T, Matsuno Y, Shirahashi K, Iwata H, Takagi H, Matsumoto K, Nakamura T, Kosugi A, Mori Y, Takemura H. Skeletal muscle targeting in vivo electroporation-mediated HGF gene therapy of bleomycin-induced pulmonary fibrosis in mice. Lab Invest 84: 836–844, 2004. doi: 10.1038/labinvest.3700098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tjelle TE, Corthay A, Lunde E, Sandlie I, Michaelsen TE, Mathiesen I, Bogen B. Monoclonal antibodies produced by muscle after plasmid injection and electroporation. Mol Ther 9: 328–336, 2004. doi: 10.1016/j.ymthe.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 171.Rizzuto G, Cappelletti M, Maione D, Savino R, Lazzaro D, Costa P, Mathiesen I, Cortese R, Ciliberto G, Laufer R, La Monica N, Fattori E. Efficient and regulated erythropoietin production by naked DNA injection and muscle electroporation. Proc Natl Acad Sci USA 96: 6417–6422, 1999. doi: 10.1073/pnas.96.11.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Puppa MJ, White JP, Velázquez KT, Baltgalvis KA, Sato S, Baynes JW, Carson JA. The effect of exercise on IL-6-induced cachexia in the Apc (Min/+) mouse. J Cachexia Sarcopenia Muscle 3: 117–137, 2012. doi: 10.1007/s13539-011-0047-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hoover F, Kalhovde JM. A double-injection DNA electroporation protocol to enhance in vivo gene delivery in skeletal muscle. Anal Biochem 285: 175–178, 2000. doi: 10.1006/abio.2000.4730. [DOI] [PubMed] [Google Scholar]
- 174.Hunter RB, Stevenson EJ, Koncarevic A, Mitchell-Felton H, Essig DA, Kandarian SC. Activation of an alternative NF‐κB pathway in skeletal muscle during disuse atrophy. FASEB J 16: 529–538, 2002. doi: 10.1096/fj.01-0866com. [DOI] [PubMed] [Google Scholar]
- 175.DiFranco M, Quinonez M, Capote J, Vergara J. DNA transfection of mammalian skeletal muscles using in vivo electroporation. J Vis Exp 32: e1520, 2009. doi: 10.3791/1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhao X, Min CK, Ko J-K, Parness J, Kim DH, Weisleder N, Ma J. Increased store-operated Ca2+ entry in skeletal muscle with reduced calsequestrin-1 expression. Biophys J 99: 1556–1564, 2010. doi: 10.1016/j.bpj.2010.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lauritzen HP, Reynet C, Schjerling P, Ralston E, Thomas S, Galbo H, Ploug T. Gene gun bombardment-mediated expression and translocation of EGFP-tagged GLUT4 in skeletal muscle fibres in vivo. Pflugers Arch 444: 710–721, 2002. doi: 10.1007/s00424-002-0862-5. [DOI] [PubMed] [Google Scholar]
- 178.Ferey JLA, Brault JJ, Smith CAS, Witczak CA. Constitutive activation of CaMKKα signaling is sufficient but not necessary for mTORC1 activation and growth in mouse skeletal muscle. Am J Physiol Endocrinol Metab 307: E686–E694, 2014. doi: 10.1152/ajpendo.00322.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hain BA, Waning DL. Electroporation of plasmid DNA into mouse skeletal muscle. J Vis Exp e63916, 2022. doi: 10.3791/63916. [DOI] [PubMed] [Google Scholar]
- 180.Bloemberg D, Quadrilatero J. Rapid determination of myosin heavy chain expression in rat, mouse, and human skeletal muscle using multicolor immunofluorescence analysis. PLoS One 7: e35273, 2012. doi: 10.1371/journal.pone.0035273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Miller BF, Baehr LM, Musci RV, Reid JJ, Peelor FF 3rd, Hamilton KL, Bodine SC. Muscle-specific changes in protein synthesis with aging and reloading after disuse atrophy. J Cachexia Sarcopenia Muscle 10: 1195–1209, 2019. doi: 10.1002/jcsm.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Baehr LM, West DWD, Marshall AG, Marcotte GR, Baar K, Bodine SC. Muscle-specific and age-related changes in protein synthesis and protein degradation in response to hindlimb unloading in rats. J Appl Physiol (1985) 122: 1336–1350, 2017. doi: 10.1152/japplphysiol.00703.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wallace MA, Aguirre NW, Marcotte GR, Marshall AG, Baehr LM, Hughes DC, Hamilton KL, Roberts MN, Lopez-Dominguez JA, Miller BF, Ramsey JJ, Baar K. The ketogenic diet preserves skeletal muscle with aging in mice. Aging Cell 20: e13322, 2021. doi: 10.1111/acel.13322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Baehr LM, West DWD, Marcotte G, Marshall AG, De Sousa LG, Baar K, Bodine SC. Age-related deficits in skeletal muscle recovery following disuse are associated with neuromuscular junction instability and ER stress, not impaired protein synthesis. Aging (Albany NY) 8: 127–146, 2016. doi: 10.18632/aging.100879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hughes DC, Marcotte GR, Marshall AG, West DW, Baehr LM, Wallace MA, Saleh PM, Bodine SC, Baar K. Age-related differences in dystrophin: impact on force transfer proteins, membrane integrity, and neuromuscular junction stability. J Gerontol A Biol Sci Med Sci 72: 640–648, 2017. doi: 10.1093/gerona/glw109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Durieux AC, Bonnefoy R, Busso T, Freyssenet D. In vivo gene electrotransfer into skeletal muscle: effects of plasmid DNA on the occurrence and extent of muscle damage. J Gene Med 6: 809–816, 2004. doi: 10.1002/jgm.534. [DOI] [PubMed] [Google Scholar]
- 187.Hartikka J, Sukhu L, Buchner C, Hazard D, Bozoukova V, Margalith M, Nishioka WK, Wheeler CJ, Manthorp M, Sawdey M. Electroporation-facilitated delivery of plasmid DNA in skeletal muscle: plasmid dependence of muscle damage and effect of poloxamer 188. Mol Ther 4: 407–415, 2001. doi: 10.1006/mthe.2001.0483. [DOI] [PubMed] [Google Scholar]
- 188.Dupuis M, Denis-Mize K, Woo C, Goldbeck C, Selby MJ, Chen M, Otten GR, Ulmer JB, Donnelly JJ, Ott G, McDonald DM. Distribution of DNA vaccines determines their immunogenicity after intramuscular injection in mice. J Immunol 165: 2850–2858, 2000. doi: 10.4049/jimmunol.165.5.2850. [DOI] [PubMed] [Google Scholar]
- 189.Doh S, Vahlsing H, Hartikka J, Liang X, Manthorpe M. Spatial–temporal patterns of gene expression in mouse skeletal muscle after injection of lacZ plasmid DNA. Gene Ther 4: 648–663, 1997. doi: 10.1038/sj.gt.3300460. [DOI] [PubMed] [Google Scholar]
- 190.Wen Y, Murach KA, Vechetti IJ Jr, Fry CS, Vickery C, Peterson CA, McCarthy JJ, Campbell KS. MyoVision: software for automated high-content analysis of skeletal muscle immunohistochemistry. J Appl Physiol (1985) 124: 40–51, 2018. doi: 10.1152/japplphysiol.00762.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Viggars MR, Wen Y, Peterson CA, Jarvis JC. Automated cross-sectional analysis of trained, severely atrophied, and recovering rat skeletal muscles using MyoVision 2.0. J Appl Physiol (1985) 132: 593–610, 2022. doi: 10.1152/japplphysiol.00491.2021. [DOI] [PubMed] [Google Scholar]
- 192.Gilda JE, Ko JH, Elfassy AY, Tropp N, Parnis A, Ayalon B, Jhe W, Cohen S. A semiautomated measurement of muscle fiber size using the Imaris software. Am J Physiol Cell Physiol 321: C615–C631, 2021. doi: 10.1152/ajpcell.00206.2021. [DOI] [PubMed] [Google Scholar]


