Abstract
The goal of this study was to develop an atlas of the metabolic, transcriptional, and proteomic changes that occur with pregnancy in the maternal heart. Timed pregnancy studies in FVB/NJ mice revealed a significant increase in heart size by day 8 of pregnancy (midpregnancy; MP), which was sustained throughout the rest of the term compared with nonpregnant control mice. Cardiac hypertrophy and myocyte cross-sectional area were highest 7 days after birth (postbirth; PB) and were associated with significant increases in end-diastolic and end-systolic left ventricular volumes and higher cardiac output. Metabolomics analyses revealed that by day 16 of pregnancy (late pregnancy; LP) metabolites associated with nitric oxide production as well as acylcholines, sphingomyelins, and fatty acid species were elevated, which coincided with a lower activation state of phosphofructokinase and higher levels of pyruvate dehydrogenase kinase 4 (Pdk4) and β-hydroxybutyrate dehydrogenase 1 (Bdh1). In the postpartum period, urea cycle metabolites, polyamines, and phospholipid levels were markedly elevated in the maternal heart. Cardiac transcriptomics in LP revealed significant increases in not only Pdk4 and Bdh1 but also genes that regulate glutamate and ketone body oxidation, which were preceded in MP by higher expression of transcripts controlling cell proliferation and angiogenesis. Proteomics analysis of the maternal heart in LP and PB revealed significant reductions in several contractile filament and mitochondrial subunit complex proteins. Collectively, these findings describe the coordinated molecular changes that occur in the maternal heart during and after pregnancy.
NEW & NOTEWORTHY Little is known of the underlying molecular and cellular mechanisms that contribute to pregnancy-induced cardiac growth. Several lines of evidence suggest that changes in cardiac metabolism may contribute. Here, we provide a comprehensive metabolic atlas of the metabolomic, proteomic, and transcriptomic changes occurring in the maternal heart. We show that pregnancy-induced cardiac growth is associated with changes in glycerophospholipid, nucleotide, and amino acid metabolism, with reductions in cardiac glucose catabolism. Collectively, these results suggest that substantial metabolic changes occur in the maternal heart during and after pregnancy.
Keywords: hypertrophy, metabolomics, pregnancy, proteomics, transcriptomics
INTRODUCTION
The second and third trimesters of pregnancy are associated with remarkable physiological adaptations and cardiac remodeling (1–3). To support fetal growth, plasma volume increases along with requirements for higher cardiac output. This adaptation involves a hypertrophic response in the heart that is associated with higher stroke volume (SV) and heart rate (HR) (4–6), with cardiac function being preserved (7) or slightly depressed during late pregnancy (1, 3, 8, 9). Pregnancy is also associated with reductions in total peripheral resistance and reductions in systolic and diastolic blood pressure (10). The maternal myocardium hypertrophies significantly, with as much as a 50% increase in left ventricular mass (7, 11–13). Moreover, there is higher capillary density (14, 15), lack of apparent fibrosis (3, 14, 16, 17), and lack of fetal gene induction (3, 13) in the maternal heart during pregnancy, which suggests that pregnancy-induced cardiac growth is a reversible, physiological form of hypertrophy that differs substantially from pathological hypertrophy.
Despite our extensive knowledge of cardiac remodeling during pregnancy, the molecular mechanisms that contribute to pregnancy-induced cardiac remodeling are not well understood. These mechanisms are important to understand because failure of the heart to adapt during pregnancy could promote pathology (e.g., peripartum cardiomyopathy, PPCM) or diminish the ability of the maternal circulation to meet the demands of the growing fetus. A metabolic mechanism is insinuated by studies suggesting that cardiac glucose catabolism decreases in the maternal heart (18, 19). Lower cardiac glucose catabolism during pregnancy (20–23) appears to be caused by upregulation of pyruvate dehydrogenase kinase 4 (Pdk4), as activation of glucose oxidation via treatment of pregnant mice with a Pdk4 inhibitor prevented pregnancy-induced myocardial growth (21). This is interesting given the role of metabolism in other forms of physiological cardiac growth, such as that which occurs with exercise (24, 25). These convergent findings suggest that changes in cardiac metabolism may play a causal role in pregnancy-induced cardiac remodeling.
Although these studies suggest a mechanism that involves metabolism, there is little knowledge of the distinct metabolic, transcriptional, and proteomic changes that occur in the maternal heart during and after pregnancy. Therefore, in this study, we used an integrated, multi-omics approach to identify how the metabolome, transcriptome, and proteome of the maternal heart are influenced by pregnancy and the postpartum period. The data suggest that pregnancy-induced cardiac growth is associated with not only a reduction in cardiac glucose metabolism but also significant changes in urea cycle and polyamine metabolism. Moreover, these findings suggest temporal regulation of angiogenesis, extracellular matrix (ECM) dynamics, and mitochondrial metabolism in the maternal heart.
MATERIALS AND METHODS
Materials
Unless otherwise stated, all reagents were obtained from VWR.
Timed Pregnancy Studies
All experimental protocols used in this study were approved by the University of Louisville Institutional Animal Care and Use Committee (IACUC) and adhered to the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals. All study animals received standard chow (Lab Diets no. 5010) and water on an ad libitum basis. Lighting was maintained on a 12-h:12-h light-dark cycle. Ten-week-old FVB/NJ female and male mice were purchased from The Jackson Laboratory and were allowed 2 wk of acclimatization in the animal facility before initiation of studies. During the final week of acclimatization, nulliparous females (2–4 per cage) were introduced to urine-soaked soiled bedding from age-matched males for synchronization of the estrus cycle [i.e., the Whitten effect (26)]. At 12 wk of age, once in estrus (determined visually as per Ref. 27), females were paired daily with males until the presence of a copulatory plug, which refers to day 0 of pregnancy. After the presence of the copulatory plug, female mice were weighed daily as a secondary measure of pregnancy confirmation based on findings documented in Ref. 28. Age-matched males were used only for initiation of pregnancy.
The following time points were examined during this study: nonpregnant, diestrus (NP); midpregnancy (day 8 of pregnancy, MP); late pregnancy (day 16 of pregnancy, LP); and 1 wk postbirth with lactation (PB). Mice that were not pregnant at euthanasia for the MP and LP time points were excluded from the analysis. NP mice were synchronized, as above, but were not paired with male mice. Day 8 and day 16 of pregnancy were selected to represent MP and LP, respectively, based on time points used in previous studies in C57BL/6 mice, which typically use a range between 7 and 11 days for early to midpregnancy and between 17 and 20 days for LP (3, 13, 14, 21, 29–33). Because FVB/NJ mice have shorter gestational times compared with C57 mice (34) and because in our hands several mice, irrespective of litter size, gave birth on gestational day 17 (unpublished observation), day 16 was selected to represent LP in this study. PB mice remained with their respective litters and were allowed to lactate until 1 wk after birth. Upon completion of mouse studies, mice were euthanized through the removal of the heart after anesthetization with pentobarbital sodium (150 mg/kg ip). All tissues were harvested at 10:00 AM, i.e., at Zeitgeber time 4 (ZT4). ARRIVE guidelines (35) were followed, and all euthanasia procedures used were consistent with the 2020 AVMA Guidelines on Euthanasia.
Tissue Harvest and Gravimetry
Before euthanasia, body weight (BW) measurements and tail snip blood to assess blood glucose, blood lactate, blood ketone, hemoglobin, and cholesterol were obtained for each study mouse. During euthanasia, cardiac puncture was performed on all mice. EDTA (0.2 M)-coated syringes and tubes were used to harvest blood for plasma extraction for future analyses. Hearts were collected either for histology and immunoblotting or for omics studies using extraction methods outlined below. Heart weight (HW) measurements were obtained from non-freeze-clamped hearts (i.e., those collected for non-omics end points) for determination of total HW (ventricles and atria) and tibias were collected for assessment of tibial length (TL). For all studies, only ventricular heart tissue [left ventricle (LV) and right ventricle (RV); powdered] was used and atria were stored at −80°C. Other organs (i.e., liver, kidney, lung, gastrocnemius muscle, and spleen) were collected, weighed, and snap frozen in liquid nitrogen for future analyses. For all pregnant and postpartum mice (i.e., MP, LP, and PB), the number of pups and pup weights were documented. All study mice and their respective downstream analyses are outlined in Supplemental Fig. S1 (all Supplemental Figures are available at https://doi.org/10.6084/m9.figshare.19651137).
Echocardiography
Transthoracic echocardiography of the left ventricle was performed with the Vevo VisualSonics 3100 echocardiography system as previously described (36, 37). To reduce maternal stress and the documented effects of repeated anesthesia on fetal growth and signaling (38–41), a separate group of female mice were used for echocardiographic analyses. Briefly, nonpregnant (NP), pregnant (MP and LP), and postpartum (PB; n = 5 per group) mice were anesthetized with 2% isoflurane and maintained under 1.5% isoflurane. Body temperature was maintained between 36.5°C and 37.5°C with a rectal thermometer and a heat lamp. Mice were placed in the supine position on an examination board associated with the echocardiography system. Depilatory cream was applied to each mouse’s chest and wiped clean to remove any residual hair. Respiration, heart rate, and ECG were recorded during echocardiography measurements. A high-frequency transducer was used to obtain two-dimensional (2-D) images of the parasternal long axis and short axis in 2-D brightness mode (B-mode) and motion mode (M-mode). B-mode imaging was used to calculate cardiac ejection fraction (EF%), cardiac output (CO), stroke volume (SV), and LV end-diastolic and end-systolic volumes (LVEDV and LVESV). CO was calculated as SV × HR. SV was calculated as diastolic volume – systolic volume. M-mode imaging was used to obtain measurements on corrected LV mass, heart rate (HR), LV internal diameter (LVID), LV anterior wall thickness (LVAW), and LV posterior wall thickness (LVPW) during both systole and diastole and fractional shortening (FS%). FS% was calculated as [(LVIDd − LVIDs)/LVIDd] × 100. After terminal echocardiographic measurements, mice were euthanized and hearts harvested for subsequent analyses. Mice were given a postanesthesia recovery time up to 1 day before tissue harvest. The sonographer was blinded to experimental group, where possible.
Histology
Hearts were perfused from the apex with ice-cold PBS containing 25 mM KCl to clear blood and arrest the heart in diastole. Hearts were then extracted and weighed, and the midline transverse section of the heart was removed (the apex and base sections of the heart were snap-frozen in liquid nitrogen) and fixed for up to 24 h in 10% neutral buffered formalin. After 24 h, hearts were moved to 70% ethanol until processing on a Sakura Tissue-Tek VIP processor and subsequent embedding in paraffin. Sections were cut at 4 µm and stained with hematoxylin-eosin, Sirius Red, and wheat germ agglutinin to assess cardiac morphology, cardiac fibrosis, and cardiomyocyte hypertrophy, respectively. Stained slides were imaged with a Keyence BZX-810 imaging system, and analysis of myocyte cross-sectional area and percent fibrosis was performed.
RNA Extraction
Heart tissue was pulverized in liquid nitrogen. Heart powder (∼10–20 mg) was added to TRIzol reagent (ThermoFisher 15596026) and hand homogenized using a BioMasher micro tissue homogenizer (VWR KT749625-0030). RNA was extracted as per the TRIzol manufacturer’s indications, with the addition of an overnight −20°C step to precipitate smaller RNA species. Extracted RNA was quantified with a NanoDrop where RNA with OD260/280 > 2.0 was used for downstream analyses [i.e., RNA sequencing (RNA-seq)]. RNA integrity number (RIN) was analyzed, and only RNA with a RIN score of >6.8 was used for downstream analyses.
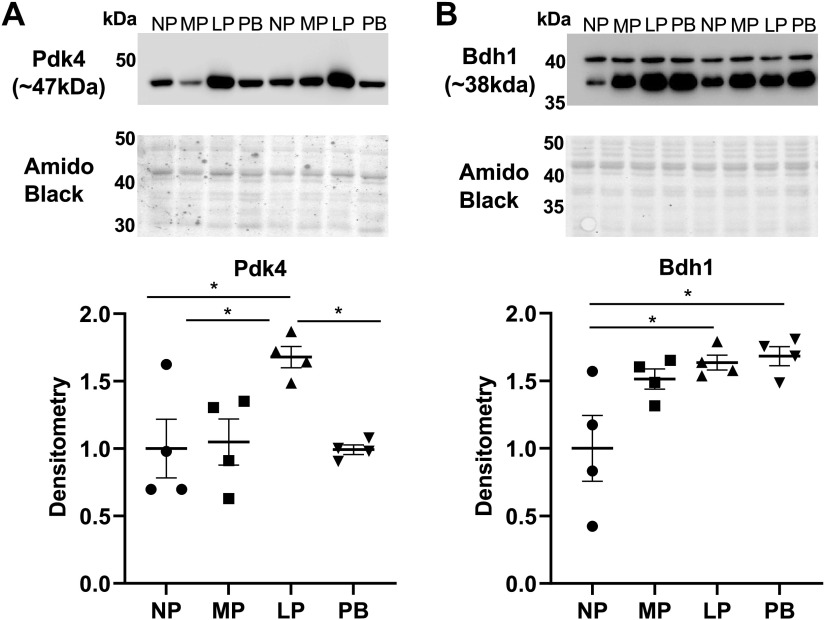
Immunoblotting
Heart tissue was pulverized in liquid nitrogen. Heart powder (∼20 mg) was added to lysis buffer (20 mM HEPES, 100 mM KCl, 1 mM EDTA, 1% IGEPAL, 0.1% SDS, pH 7.4) containing protease (protease inhibitor cocktail; Sigma P8340) and phosphatase (Halt phosphatase inhibitor; ThermoFisher 78426) and then vortexed and sonicated. Protein was quantified with a Bio-Rad DC assay, and lysates were prepared with a 5× sample buffer (125 mM Tris·HCl pH 6.8, 10% SDS, 50% glycerol, 0.05% bromophenol blue, and 250–500 mM DTT) and boiled for 5 min. Protein (25 µg) was separated on SDS-PAGE gels (7.5%, 10%, or 12% depending on protein of interest size) and then transferred to polyvinylidene difluoride (PVDF) membrane. PVDF membranes were dried, Ponceau stained, and imaged to assess total protein levels and adequate transfer. After Ponceau destaining and washing, membranes were blocked for 1 h at room temperature with 5% nonfat dry milk-1× Tris-buffered saline-Tween (TBST) and incubated overnight at 4°C with primary antibodies specific for PDK4 (no. Ab214938, Abcam; 1:5,000), p-PFKFB2 (no. 13064, Cell Signaling; 1:2,000; Ser483), and Bdh1 (no. Ab193156, Abcam; 1:2,000). After primary antibody, membranes were washed with 1× TBST and incubated with appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies (Cell Signaling goat anti rabbit no. 7074; between 1:2,000 and 1:10,000 depending on primary antibody used) for 2 h at 4°C, followed by additional washing in 1× TBST. Blots were exposed to ECL chemiluminescent reagent (34580; Supersignal West Pico Plus) and imaged with a Bio-Rad ChemiDoc imaging system. Protein expression was normalized to total protein levels assessed through Amido Black poststaining. Densitometry was performed with Bio-Rad Image Lab software.
Tissue Acquisition for Multi-omics Analyses
For omics studies, hearts from NP, MP, LP, and PB mice were freeze-clamped in situ with liquid nitrogen-precooled Wollenberger forceps. Hearts were pulverized under liquid nitrogen by means of a steel mortar and pestle and used as described below.
RNA-seq Analyses
RNA sequencing (RNA-seq) of cardiac RNA generated from heart tissue from NP, MP, LP, and PB mice (n = 3–5 per group) was performed at Novogene, resulting in 32 paired-end FASTQ files. Quality control of the raw sequence data was performed with FastQC (version 0.10.1) for each sequencing run. The sequences were of good quality, and no sequence trimming was necessary. The raw sequences were directly aligned to the Mus musculus reference genome (GRCm38.93) with STAR (version 2.6). Raw gene counts were generated with HTSeq (version 0.10.0) and normalized with relative log expression (RLE). Differential expression analysis was implanted in R with DESeq2, where a negative binomial regression model was used for analyses of pairwise comparisons. The R package ClusterProfiler was used to identify enriched Gene Ontology biological processes and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways for each set of differentially expressed genes. The Short Time Series Expression Miner was used to evaluate patterns of gene expression over time. Volcano plots were created for each comparison to examine the distribution of log2 fold change (FC) at different significance levels.
Proteomics
Untargeted proteomics of heart tissue from NP, MP, LP, and PB mice (n = 5 per group) was performed. Briefly, 20 mg of powdered heart tissue was added to 200 µL of RIPA buffer (no. 89901, Pierce RIPA buffer; Thermo Fisher) containing protease and phosphatase inhibitors (see above). Protein concentrations were calculated after a Lowry assay. Protein samples with a concentration of 100 µg were used in the following workflow: 1) chloroform/methanol extraction with a trypsin digestion, 2) Orbitrap Exploris 480 (DIA; 60 min gradient per samples and gas-phase fractionation), 3) chromatogram library construction using Prosit, 4) chromatogram library search using EncyclopeDIA, and 5) Bioinformatics for QC, normalization, and differential expression analysis.
Metabolomics
Untargeted metabolomics of heart tissue from NP, MP, LP, and PB mice (n = 7 or 8 per group) was performed at Metabolon (Durham, NC). Briefly, 60–80 mg of frozen heart powder was sent to Metabolon. Samples were prepared with an automated MicroLab STAR system. For quality control purposes, several standards were added before the first step in the extraction process. Proteins were precipitated with methanol. The extract was then divided into five fractions, as follows: two fractions for analysis by two separate reverse-phase (RP)/ultrahigh-performance liquid chromatography-tandem mass spectroscopy (UPLC-MS/MS) methods with positive ion-mode electrospray ionization (ESI), one for analysis by RP/UPLC-MS/MS with negative ion-mode ESI, one for analysis by hydrophilic interaction chromatography (HILIC)/UPLC-MS/MS with negative ion-mode ESI, and a spare fraction. Organic solvent was removed with a TurboVap, and samples were stored under nitrogen. For the purposes of analysis, unknown and unannotated biochemicals that were identified in the data set were removed before formal analysis. Downstream analysis of metabolite changes was performed with Metaboanalyst 5.0 software, where the following information and analyses were collected and performed: fold change analysis, volcano plot, statistical testing [analysis of variance (ANOVA)/t test], principal component analysis (PCA), partial least squares-discriminant analysis (PLS-DA), variable importance in projection (VIP) plot, heatmap, enrichment analysis, biomarker analysis, and pathway impact analysis.
Multi-omics Integration
Metaboanalyst 5.0 software was used for basic joint pathway impact analysis to integrate significantly changed proteins in our proteomics analysis with significantly changed metabolites and for the integration of transcriptional changes with metabolomic changes. Tri-omics pathway analysis was performed with OmicsNet (https://www.omicsnet.ca), where significantly changed transcripts, proteins, and metabolites were analyzed with a knowledge-based network approach.
Statistical Analyses
Statistical significance was calculated with an analysis of variance (ANOVA) followed by a Tukey post hoc test or Student’s t test with GraphPad Prism 9, as appropriate, whereby P values of <0.05 were deemed statistically significant. All data are represented as means ± SE. The present studies were performed with a minimum of three mice per experiment, typically between three and eight mice per manipulation. Densitometric analyses of Western blotting data were performed with Image Lab software. Proteomics and metabolomics analyses were performed with Metaboanalyst 5.0 software.
RESULTS
Structural and Functional Changes in the Heart during Pregnancy and Postpartum Period
To examine the physiological adaptations in the heart during pregnancy and the postpartum period, we used 12-wk-old female FVB/NJ mice in the following groups: nonpregnant diestrus (NP), midpregnant (MP; day 8 of pregnancy), late pregnancy (LP; day 16 of pregnancy), and 1-wk postbirth (PB), as outlined in Fig. 1A. During all stages of pregnancy and postpartum, we observed significantly higher body weight versus nonpregnant control mice, with body weight peaking at LP (Fig. 1B). We also observed significantly higher heart weight and heart weight-to-tibia length ratio during all stages of pregnancy (MP and LP) and the postpartum period (PB). The maternal heart was largest at the PB time point (Fig. 1, C and D), which was associated with larger myocyte cross-sectional area (Fig. 1E). Myocardial collagen content in the maternal heart was similar to NP control mice, as detected by Sirius Red staining (Fig. 1F). In a separate group of mice, we examined changes in cardiac structure and function during pregnancy and the postpartum period by serial echocardiographic analyses. We observed significantly higher end-diastolic (Fig. 1G) and end-systolic (Fig. 1H) volumes at PB with no change in ejection fraction (Fig. 1I). In addition, we observed significantly higher cardiac output in LP and PB (Fig. 1J), which was mediated by increases in stroke volume (Fig. 1K); heart rate remained unchanged during and after pregnancy (Fig. 1L). Collectively, these results demonstrate marked cardiac growth and remodeling of the mouse heart during pregnancy, which phenocopies the myocardial changes that occur in the human heart during pregnancy.
Figure 1.
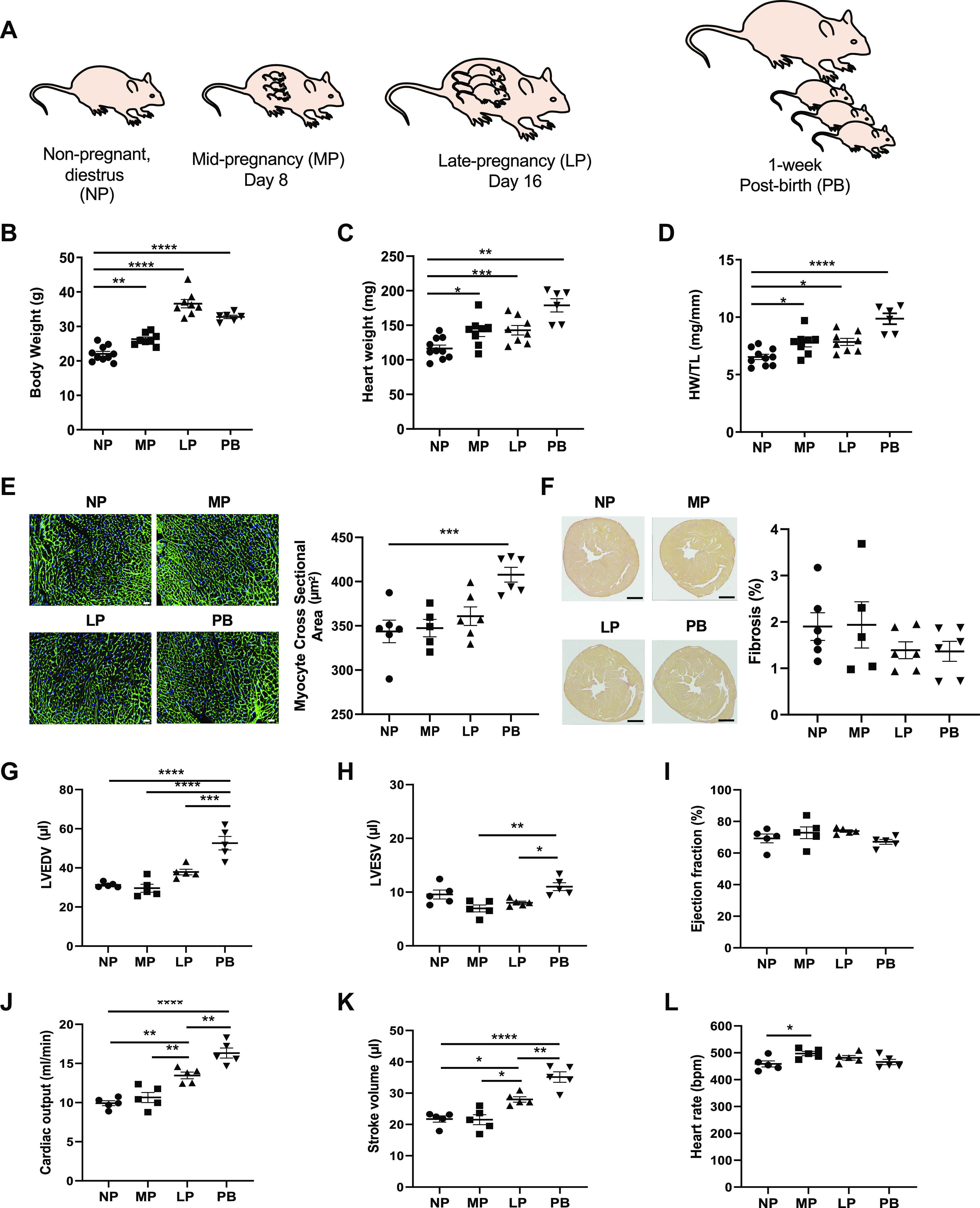
Pregnancy and postpartum period are associated with structural and functional remodeling of the maternal heart. A: schematic of study design showing the time points analyzed, which include nonpregnant, diestrus (NP), midpregnant (MP; day 8 of pregnancy), late pregnant (LP; day 16 of pregnancy), and 1-wk postbirth with lactation (PB). B–D: gravimetric measurements of body weight (B), heart weight (C), and heart weight-to-tibia length ratio (HW/TL; D) in NP, MP, LP, and PB female mice (n = 6–10 per group). E: representative images of wheat germ agglutinin and quantification of myocyte cross section. Scale bars, 200 μm. F: representative images of Sirius Red staining and quantification of % fibrosis from NP, MP, LP, and PB female mice (n = 6 per group). G–L: echocardiographic measurements of left ventricular end-diastolic volume (LVEDV; G), left ventricular end-systolic volume (LVESV; H), ejection fraction (I), cardiac output (J), stroke volume (K), and heart rate (L) from NP, MP, LP, and PB female mice (n = 5 per group). The respective n numbers in this figure correspond to 3 parallel groups of mice. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ANOVA with Tukey’s post hoc test.
Changes in Cardiac Metabolites during Pregnancy and Postpartum Period
Because the metabolic profile of the maternal heart is not well characterized, we examined changes in the cardiac metabolome during pregnancy and the postpartum period with an unbiased, unsupervised metabolomics analysis. Of the 847 metabolites measured, 492 metabolites were significantly changed during pregnancy and postpartum [false discovery rate (FDR) < 0.05], which were composed mainly of changes in the lipid (47.0%) and amino acid (23.9%) superfamilies (Supplemental Fig. S2). The remaining 29% of the changed metabolites included the following superfamilies: nucleotide, carbohydrate, xenobiotics, cofactors and vitamins, peptides, and energy. To visualize the differences in cardiac metabolite abundance, the top 75 most changed metabolites among NP, MP, LP, and PB hearts are shown as a heatmap (Fig. 2A). The heatmap highlights major changes in metabolites at the LP and PB time points, which show substantial evidence of cardiac growth. Of note, the urea cycle-associated metabolite homoarginine was the most changed metabolite, peaking in abundance at LP alongside dimethylarginine [symmetric (SDMA) + asymmetric (ADMA)]. The LP heart also shows higher abundance of metabolites associated with other amino acid pathways, including metabolites associated with glycine, serine, and threonine (e.g., N-acetylthreonine, threonine), histidine metabolism (e.g., 1-methyl-4-imidazoleacetate), methionine, cysteine, S-adenosyl methionine (SAM), and taurine metabolism (e.g., taurocyamine, cysteine-S-sulfate), and tryptophan metabolism (e.g., kynurenine). In addition, LP was associated with increases in metabolites involved in fatty acid metabolism (e.g., long-chain fatty acids and sphingomyelins) and cofactors and vitamins. Similar to LP, metabolites associated with the urea cycle (e.g., urea, homocitrulline, N-delta-acetylornithine) and polyamine metabolism (e.g., spermine, spermidine) were significantly higher at PB, in addition to increases in amino acid (e.g., dimethylglycine, glycine, betaine, N6-methyllysine), phospholipid (e.g., phosphoethanolamines, phosphatidylcholines), purine (e.g., allantoin), pyrimidine (e.g., pseudouridine, orotidine), and cofactor and vitamin metabolites (e.g., pyridoxate). PB was also associated with several higher plant and food component metabolites and associated with significant reductions in the abundance of several fatty acid, sphingomyelin, and diacylglycerol metabolites.
Figure 2.
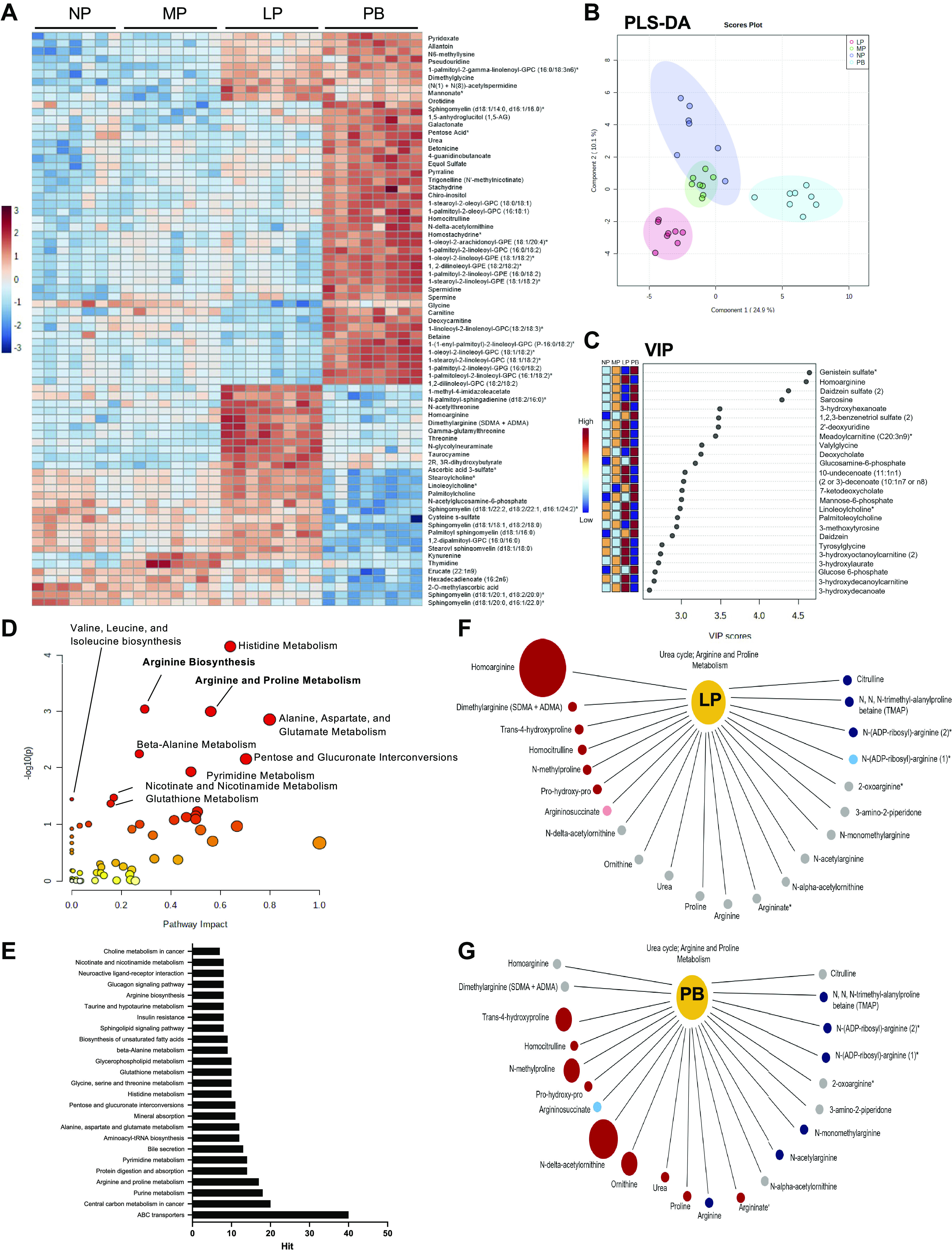
Distinct changes in the cardiac metabolome during pregnancy and postpartum period. Unsupervised and supervised metabolomics analyses from hearts extracted from nonpregnant, diestrus (NP), midpregnant (MP; day 8 of pregnancy), late pregnant (LP; day 16 of pregnancy), and 1 wk postbirth (PB) female mice (n = 7–8 per group). A: unsupervised analysis was performed and a heatmap generated showing the top 75 most changed metabolites in hearts from NP, MP, LP, and PB female mice. Red indicates increased abundance, and blue indicates reduced abundance. Intensity of color indicates increased significance. B: supervised analysis showing partial least squares discriminant analysis (PLS-DA) highlighting group separation. C: the variable importance in projection plot (VIP) of the top 25 metabolites contributing to the group separation observed in the PLS-DA plot. D: Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway impact analysis of the significantly changed cardiac metabolites between NP, MP, LP, and PB female mice. E: KEGG metabolite analysis of changed pathways during pregnancy and postpartum period. F and G: pathway analysis of urea cycle metabolites during LP (F) and PB (G). Red indicates significantly increased metabolite abundance, blue indicates reduced metabolite abundance, and gray indicates no change. The size of the circles indicates the degree of significance and importance of the increase in abundance (e.g., larger refers to greater significance and importance).
We next performed supervised metabolomics analysis. Partial least-squares discriminant analysis (PLS-DA) shows distinct separation between the NP, LP, and PB groups, with overlap between the NP and MP groups (Fig. 2B). To determine which metabolites contributed to group separation, we next performed variable importance in projection (VIP) analyses. The VIP plot (Fig. 2C) shows that the urea cycle-associated metabolite homoarginine was among the top 25 metabolites contributing to group separation in addition to metabolites associated with fatty acid metabolism (e.g., acylcarnitines, medium-chain fatty acids, and monohydroxy fatty acids), secondary bile acid metabolism (e.g., deoxycholate, 7-ketodeoxycholate), amino acid metabolism (e.g., sarcosine, 3-methoxytyrosine), dipeptides (e.g., valylglycine, tyrosylglycine), and aminosugar metabolism (e.g., glucosamine 6-phosphate). We again observed several plant/food component metabolites to be higher during PB (e.g., genistein sulfate, daidzein sulfate, daidzein). Pathway impact analysis (Fig. 2, D and E) confirmed that several amino acid pathways were significantly impacted during pregnancy, including arginine and proline metabolism and arginine biosynthesis, which are associated with the urea cycle and polyamine metabolism. Additional amino acid pathways impacted included branched-chain amino acid (BCAA) metabolism (i.e., valine, leucine, and isoleucine biosynthesis); alanine, aspartate, and glutamate metabolism; histidine metabolism; and beta-alanine metabolism. Pentose and glucuronate interconversions, pyrimidine metabolism, nicotinate and nicotinamide metabolism, and glutathione metabolism pathways were also significantly impacted between NP, MP, LP, and PB groups. Closer examination of the changes in urea cycle-associated metabolites at LP shows significantly higher homoarginine and dimethylarginine (SDMA + ADMA), trans-4-hydroxyproline, homocitrulline, N-methylproline, and prolylhydroxyproline (Fig. 2F). At LP, there were also significant reductions in citrulline, N,N,N-trimethyl-alanylproline betaine, and N-(ADP-ribosyl)-arginine, with no change in the remaining urea cycle metabolites (Fig. 2F). At PB, there were higher levels of urea, proline, N-delta-acetylornithine, and ornithine; however, homoarginine and dimethylarginine (SDMA + ADMA) abundance were not significantly different at the PB time point (Fig. 2G).
Comparisons of the cardiac metabolomes of NP and MP (Supplemental Fig. S3), NP and LP (Supplemental Fig. S4), and NP and PB (Supplemental Fig. S5) mice further indicate the involvement of urea cycle and polyamine metabolites at each stage of pregnancy and after birth and again show significant changes in metabolites associated with nucleotide metabolism, amino acid metabolism, and phospholipid metabolism. Pathway impact analysis between NP and MP revealed significant changes in pentose and glucuronate interconversions, glutathione metabolism, purine metabolism, beta-alanine metabolism, alanine, aspartate, and glutamate metabolism, and sphingolipid metabolism pathways (Supplemental Fig. S3D). Many of the pathways affected at MP were also significantly impacted at LP; however, additional pathways were impacted, including pyrimidine metabolism, arginine and proline metabolism, and glycerophospholipid metabolism (Supplemental Fig. S4D). Pathway impact analysis between NP and PB revealed changes in arginine biosynthesis, arginine and proline metabolism, and beta-alanine metabolism pathways (Supplemental Fig. S5D). Collectively, these data suggest that pregnancy and the postpartum period are associated with significant changes in cardiac glycerophospholipid metabolism, nucleotide metabolism, and amino acid metabolism, the last of which is associated with increases in urea cycle metabolites such as homoarginine and polyamine metabolites such as spermidine and spermine.
Transcriptional Changes during Pregnancy and Postpartum Period
To determine pregnancy-induced changes in cardiac transcripts, we performed RNA-seq analysis on RNA extracted from NP, MP, LP, and PB hearts. Unsupervised analysis of the RNA-seq was initially performed with principal component analysis (PCA) and heatmap analyses. PCA shows group separation between NP and PB and between MP and LP groups (Fig. 3A). The heatmap in Fig. 3B shows the top 50 significantly changed transcripts during pregnancy and the postpartum period and highlights distinct patterns of gene expression between all four groups (e.g., NP and MP, MP and LP, and LP and PB). The heatmap shows increases in transcripts associated with cell proliferation, cytokinesis, angiogenesis, and transcription in MP hearts. In addition, we observed higher levels of transcripts associated with metabolism, which peak during LP and include Pdk4, which corroborates previous findings (23). Furthermore, we observed significant increases in transcripts associated with extracellular matrix (ECM) remodeling, peaking in expression at PB.
Figure 3.
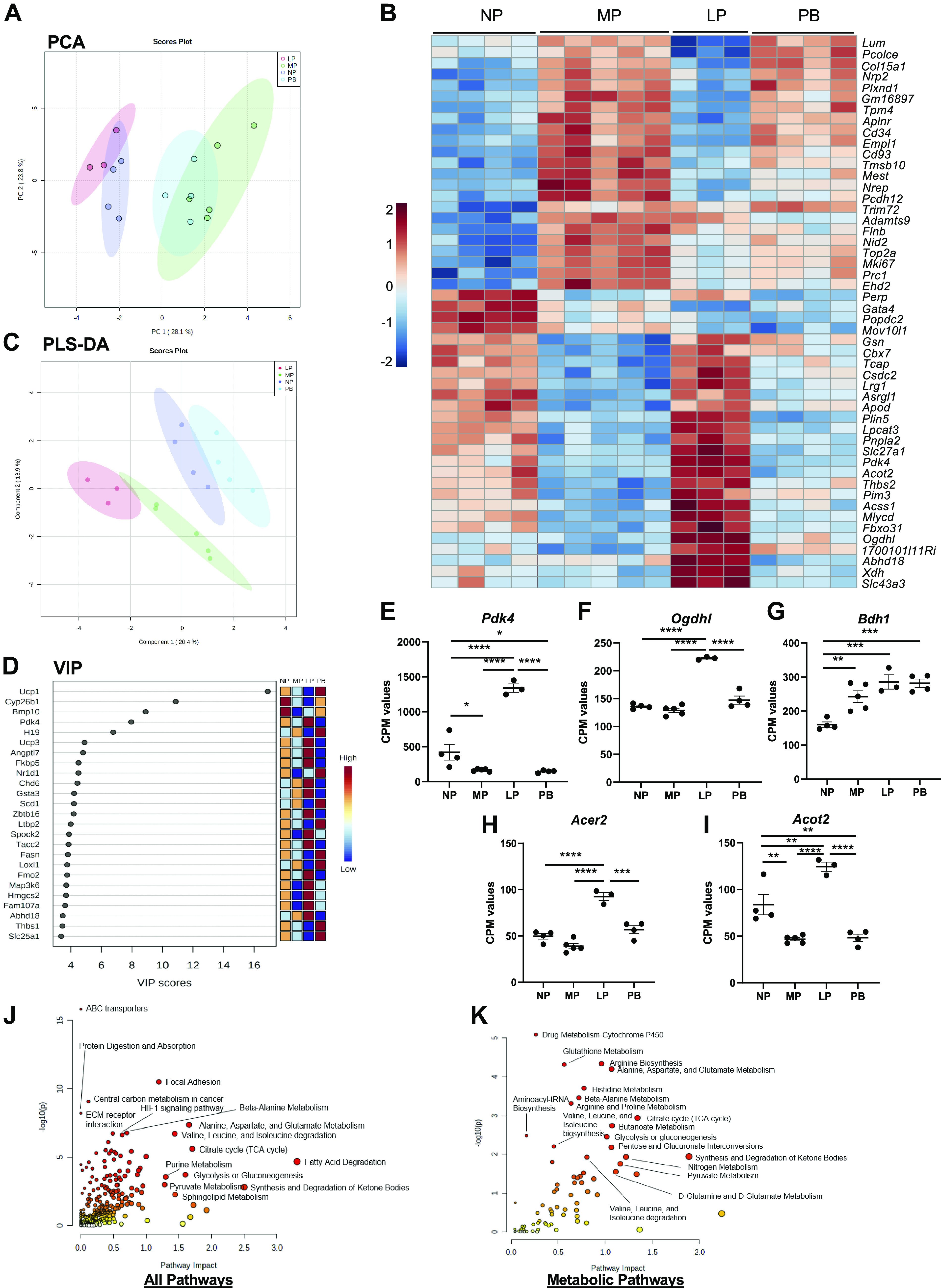
Transcriptomic analysis of the maternal heart during pregnancy and postpartum. Analyses of transcriptional changes in hearts extracted from nonpregnant, diestrus (NP), midpregnant (MP; day 8 of pregnancy), late pregnant (LP; day 16 of pregnancy), and 1 wk postbirth (PB) female mice (n = 3–5 per group). A: principal component analysis (PCA) was performed to examine group separation during analysis of differentially expressed genes. B: heatmap showing the top 75 differentially expressed transcripts in hearts from NP, MP, LP, and PB female mice. C: analysis showing partial least squares discriminant analysis (PLS-DA) highlighting group separation based on differentially expressed transcripts. D: the variable importance in projection plot (VIP) of the top 25 transcripts contributing to the group separation observed in the PLS-DA plot. E–I: counts per million (CPM) values for transcript expression of Pdk4 (E), Ogdhl1 (F), Bdh1 (G), Acer2 (H), and Acot2 (I). ANOVA analysis was performed to identify significantly expressed cardiac metabolite and transcript abundances [P <0.05; false discovery rate (FDR) 0.05] across all time points (NP, MP, LP, and PB). Human Metabolome Database (HMDB) and official gene names of those significantly expressed targets were added to the joint pathway analysis tool in Metaboanalyst 5.0. J and K: joint pathway analysis was performed with Fisher’s exact test with degree centrality topology applied and these data integrated based on pathway level combined P values for all pathways (J) and metabolic pathways (K). *P < 0.05, **P <0.01, ***P < 0.001, ****P < 0.0001, ANOVA with Tukey’s post hoc test.
We next performed supervised analysis of the RNA-seq data set using PLS-DA and VIP analysis. The PLS-DA plot shows distinct separation patterns between all four of the groups (Fig. 3C). The VIP plot (Fig. 3D) shows the top 25 transcripts that are most important with respect to the PLS-DA model. Among the top 25 transcripts identified in the VIP plot, we again observed an increase in Pdk4 at LP and additional changes in several metabolic transcripts including Ucp1, Cyp26b1, Ucp3, Scd1, Fasn, Hmgsc2, and Slc25a1. The VIP plot also shows several transcripts that could play significant roles in mediating angiogenesis in MP, including Map3k6 and Angptl7. To further analyze the increase in metabolic transcripts at LP, we plotted cardiac expression of transcripts encoding Pdk4, Ogdhl1, Bdh1, Acer2, and Acot2. We found that Pdk4, Ogdhl, Acer2, and Acot2 were significantly higher at LP (Fig. 3, E, F, H, and I). Interestingly, Bdh1 was higher at all stages of pregnancy (MP and LP) and during the postpartum period (PB) (Fig. 3G). The effect sizes (reported as log2 FC as per Ref. 42) and corrected P values (corrected for multiple comparisons to reduce probability of false positives) corresponding to the metabolic transcripts shown in Fig. 3, E–I, are reported in Table 1. Integrated pathway analysis of the transcriptomic and metabolomic data (Fig. 3, J and K) show significant impact to ABC transporters, focal adhesion, ECM receptor interaction, and several metabolic pathways including Hif1 signaling pathway, citrate cycle, glycolysis and gluconeogenesis, pyruvate metabolism, sphingolipid metabolism, synthesis and degradation of ketone bodies, fatty acid degradation, purine metabolism, and amino acid metabolism between NP, MP, LP, and PB hearts.
Table 1.
Effect sizes and corrected P values for highlighted metabolic transcripts
| Transcript | NP vs. MP |
NP vs. LP |
NP vs. PB |
|||
|---|---|---|---|---|---|---|
| Log2 FC (MP/NP) | P Value | Log2 FC (LP/NP) | P Value | Log2 FC (PB/NP) | P Value | |
| Pdk4 | −0.85754 | 2.33E−05 | 1.00543 | 3.57E−06 | −0.8845 | 2.79E−05 |
| Ogdhl | −0.08441 | 0.472391 | 0.64227 | 1.73E−14 | 0.09613 | 0.447203 |
| Bdh1 | 0.52477 | 3.17E−06 | 0.76654 | 8.39E−10 | 0.74687 | 1.07E−10 |
| Acer2 | −0.31665 | 0.033756 | 0.75637 | 1.45E−07 | 0.16962 | 0.401973 |
| Acot2 | −0.72191 | 2.03E−08 | 0.50937 | 0.002308 | −0.67367 | 1.91E−06 |
Effect sizes are reported as log2 fold change (FC). LP, late pregnant (day 16 of pregnancy; n = 3 mice); MP, midpregnant (day 8 of pregnancy; n = 5 mice); NP nonpregnant, diestrus; n = 4 mice); PB, 1 wk postbirth with lactation (n = 4 mice).
To garner more information on the temporal changes in the maternal transcriptome, we next examined two-group comparisons. Examination of the NP and MP groups shows significant upregulation of transcripts involved in cell cycle, cell migration, and cell motility (Supplemental Fig. S6A). Volcano plot analysis between NP and MP highlighted 19 transcripts that are significantly increased, including Ccna2, Prc1, Nuf2, Aurkb, Knl1, Mki67, Anln, Foxm1, Ckap2l, Ckap2, Tpx2, and Mest—all of which are associated with the cell cycle, cell division, transcription, and cytoskeletal organization (Supplemental Fig. S6B). We also found one transcript (Apod) is significantly reduced between NP and MP. KEGG analysis identified pathways associated with cell cycle, cytoskeletal organization, and angiogenesis, and enrichment analysis of KEGG pathways shows significant changes in phosphatidylinositol 3-kinase (PI3K)-Akt signaling pathway, focal adhesion, cell cycle, and regulation of actin cytoskeleton (Supplemental Fig. S6, C and D). PLS-DA analysis shows significant group separation between NP and MP (Supplemental Fig. S6E), and the VIP plot shows that among the 25 transcripts identified Mki67, Prc1, Top2a, and Bmp10 are among the top transcripts contributing to group separation (Supplemental Fig. S6F).
Examination of the NP and LP groups shows significant upregulation of transcripts involved in metabolism, including Ogdhl1, Bdh1, and Acer2 (Supplemental Fig. S7A). Volcano plot analysis between NP and LP revealed 12 transcripts that increased significantly, including those transcripts associated with metabolism (e.g., Cyp2b10, Ogdhl, Bdh1, Rbp7, Gpihbp1, and Abhd18), innate immunity (e.g., Oas2 and Btnl9), and metalloproteinases (e.g., Adamsts9 and Lvrn; Supplemental Fig. S7B). We also identify eight transcripts downregulated between NP and LP, which include Alpl, Cacna1g, Fam78a, Scn5a, Gpr22, Gata4, Rcor2, and Lum. KEGG analysis shows that pathways associated with cardiac muscle contraction, blood circulation, and regulation of actin filament-based process are among those enriched, and enrichment analyses of KEGG pathways shows significant enrichment in pathways associated with metabolism, including PI3K-Akt signaling pathway, peroxisome proliferator-activated receptor (PPAR) signaling, Hif-1 signaling, FOXO signaling, and glycolysis/gluconeogenesis (Supplemental Fig. S7, C and D). The PLS-DA showed significant group separation between NP and LP (Supplemental Fig. S7E), and the VIP plot (Supplemental Fig. S7F) showed that several metabolic transcripts contributed to group separation, including Cyp26b1, Cyp2b10, Pdk4, and Pck1, many of which were highlighted as significant contributors to group separation between all four groups (as seen in Fig. 3).
Examination of the NP and PB groups shows significant upregulation of transcripts associated with ECM remodeling including the collagen transcripts for Col4a1, Col4a2, and Col15a1 (Supplemental Fig. S8A). Volcano plot analysis shows significant upregulation of 4 transcripts, including Lum, Alpl, Col15a1, and Pcolce, and 16 downregulated transcripts, many of which are associated with metabolism, including Plin5, Scd4, Pdk4, Cyp2b10, Acot1, Acot2, Xdh, Slc27a1, and Cyp4b1 (Supplemental Fig. 8B). KEGG analysis showed that pathways associated with circulatory system process, extracellular structure organization, extracellular matrix organization, and actin filament organization were among those enriched, and KEGG pathway enrichment analysis shows significant enrichment in pathways associated with focal adhesion, ECM-receptor interaction, and regulation of actin cytoskeleton (Supplemental Fig. S8, C and D). The PLS-DA shows significant group separation between NP and PB (Supplemental Fig. S8E), and the VIP scores showed that among the 25 transcripts identified a significant number of those contributing to group separation are involved in ECM remodeling, including Col3a1, Loxl2, Lgals4, Col5a3, Fbn1, Col15a1, Fgf12, and Postn (Supplemental Fig. S8F). Collectively, these data suggest that pregnancy and the postpartum period are associated with distinct, temporal changes in transcripts associated with angiogenesis, metabolism, and ECM remodeling—all of which have documented roles in cardiac growth (14, 16, 21, 43, 44).
Changes in the Cardiac Proteome during Pregnancy and Postpartum Period
To determine the extent to which pregnancy and the postpartum period impact the cardiac proteome, we next performed proteomics on hearts isolated from NP, MP, LP, and PB mice. The heatmap in Fig. 4A shows the top 75 changed proteins during pregnancy and the postpartum period. The heatmap shows significant increases at LP in several proteins that have been previously shown to be increased during pregnancy, such as coagulation factor V, ceruloplasmin, alpha fetoprotein, and prolactin (45–47). Changes were also observed in the protein expression of several metabolic proteins, including Pdk4, sterol carrier protein 2 (Scp2), enoyl-CoA hydratase 1 (Echs1), and acyl CoA oxidase 1 (Acox1), which correlated with reductions in their respective expression patterns in MP compared with NP. The greatest reduction in levels of Pdk4, Scp2, Echs1, and Acox1 were seen at PB. In addition, the heatmap highlights significantly lower levels of several mitochondrial complex subunit proteins (e.g., cytochrome-c oxidase subunits and NADH:ubiquinone oxidoreductase core subunits), histones (e.g., histone H3), and proteins associated with muscle contraction (e.g., actin, myosin) at the LP and PB time points compared with both MP and NP.
Figure 4.
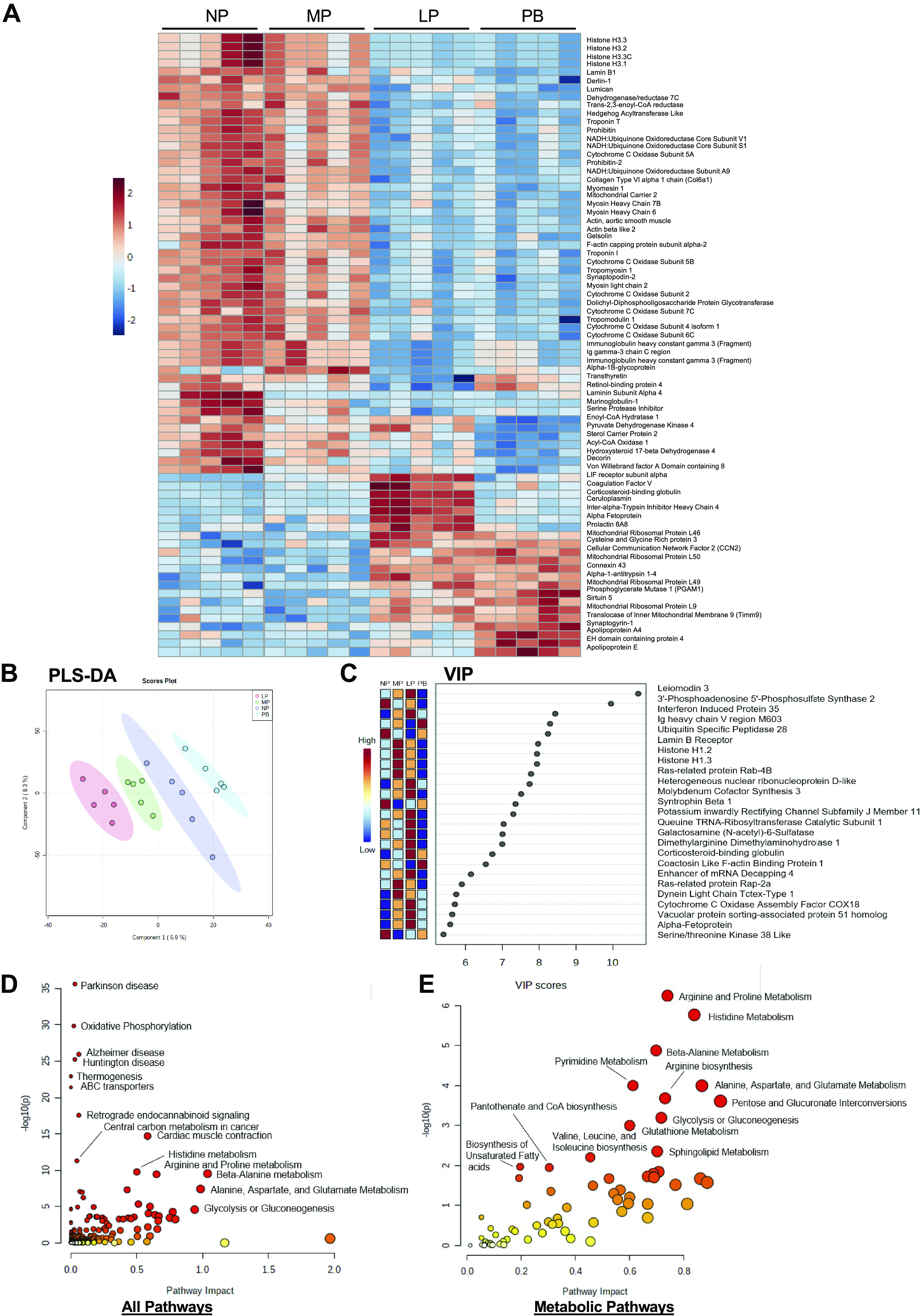
Changes in the cardiac proteome during pregnancy and postpartum period. Proteomics analyses from hearts extracted from nonpregnant, diestrus (NP), midpregnant (MP; day 8 of pregnancy), late pregnant (LP; day 16 of pregnancy), and 1 wk postbirth (PB) female mice (n = 5 per group). A: unsupervised analysis was performed and a heatmap generated showing the top 75 differentially expressed proteins in hearts from NP, MP, LP, and PB female mice. Red indicates increased abundance, and blue indicates reduced abundance. Intensity of color indicates increased significance. B: supervised analysis showing partial least squares discriminant analysis (PLS-DA) highlighting group separation. C: the variable importance in projection plot (VIP) of the top 25 proteins contributing to the group separation observed in the PLS-DA plot. ANOVA analysis was performed to identify significantly expressed cardiac metabolite and protein abundances [P < 0.05; false discovery rate (FDR) 0.05] across all time points (NP, MP, LP, and PB). Human Metabolome Database (HMDB) and Uniprot IDs of those significantly expressed targets were added to the joint pathway analysis tool in Metaboanalyst 5.0., which corresponded to 493 changed metabolites and 350 changed proteins across all 4 time points. D and E: joint pathway analysis was performed with Fisher’s exact test with degree centrality topology applied and these data integrated based on pathway level combined P values for all pathways (D) and metabolic pathways (E).
We next performed supervised analysis of the proteomics data. The PLS-DA plot shows distinct separation between all four of the groups (Fig. 4B). Among the top 25 proteins identified in the VIP plot (Fig. 4C), we observed changes in several histones (e.g., histone H1.2 and histone H1.3) and mitochondrial complex subunit proteins. In support of our metabolomics data showing significant enrichment in the arginine and proline metabolism pathways and increased homoarginine levels, we found that dimethylarginine dimethylaminohydrolase 1 (Ddah1), the enzyme responsible for ADMA degradation, also contributed to group separation of the cardiac proteome and was significantly higher at LP. Integrated KEGG pathway analysis of cardiac proteomic and metabolomic data showed significant impact to ABC transporters, oxidative phosphorylation, glycolysis and gluconeogenesis, cardiac muscle contraction, thermogenesis, and amino acid metabolism between NP, MP, LP, and PB hearts (Fig. 4D). Upon integration of both proteomic and metabolomic data, arginine and proline metabolism was found to be the most impacted metabolic pathway between NP, MP, LP, and PB hearts (Fig. 4E). In addition, metabolic pathways associated with the production of building blocks for cardiac growth, such as nucleotide and amino acid metabolism, were also significantly impacted. Comparisons of the cardiac proteomes of NP/MP (Supplemental Fig. S9), NP/LP (Supplemental Fig. S10), and NP/PB (Supplemental Fig. S11) mice further support these findings. Together, these data indicate that the abundance of proteins associated with glucose, lipid, and amino acid metabolism changes during pregnancy and that several mitochondrial complex subunits appear largely reduced in expression, which could influence mitochondrial metabolism in the maternal heart.
Validation of Changes in Metabolic Proteins during Pregnancy and Postpartum Period
To validate changes in the expression of some key metabolic transcripts and proteins during LP, we next performed immunoblot analysis. We observed a significant upregulation of Pdk4 protein expression at LP (Fig. 5A) and significant upregulation of Bdh1 at LP and PB (Fig. 5B), both of which reflected changes observed in the transcript levels. We also observed a significant reduction in the phosphorylation at Ser483 and thereby activity of Pfkfb2 at LP (Supplemental Fig. S12A). These data are suggestive of a reduction in glucose catabolism during the time points associated with pregnancy-induced cardiac growth (i.e., LP/PB). The reduction of glucose catabolism at the level of both phosphofructokinase and pyruvate dehydrogenase could mediate coordination of ancillary biosynthetic pathway activity to spare glucose-derived carbon for nucleotide and phospholipid synthesis (Supplemental Fig. S12B).
Figure 5.
Validation of transcriptomic and proteomic changes in the cardiac expression of metabolism markers during pregnancy and postpartum. A: immunoblot and densitometric analysis of Pdk4 protein expression levels in hearts from nonpregnant, diestrus (NP), midpregnant (MP; day 8 of pregnancy), late pregnant (LP; day 16 of pregnancy), and 1 wk postbirth (PB) female mice. B: immunoblot and densitometric analysis of Bdh1 protein expression levels in hearts from NP, MP, LP, and PB female mice. Protein expression levels of target proteins were normalized to total protein levels with Amido Black staining. *P <0.05 vs. age-matched control (ANOVA with Tukey’s post hoc test); n = 4 per group.
Integrative Analysis of the Cardiac Transcriptome, Proteome, and Metabolome
We next integrated our omics data with a tri-omic approach utilizing OmicsNet. Pathway analysis revealed that nucleotide, amino acid, and phospholipid metabolism were all changed in the maternal heart, which included purine metabolism, glycerophospholipid metabolism, and arginine and proline metabolism among the top 25 impacted pathways (Fig. 6).
Figure 6.
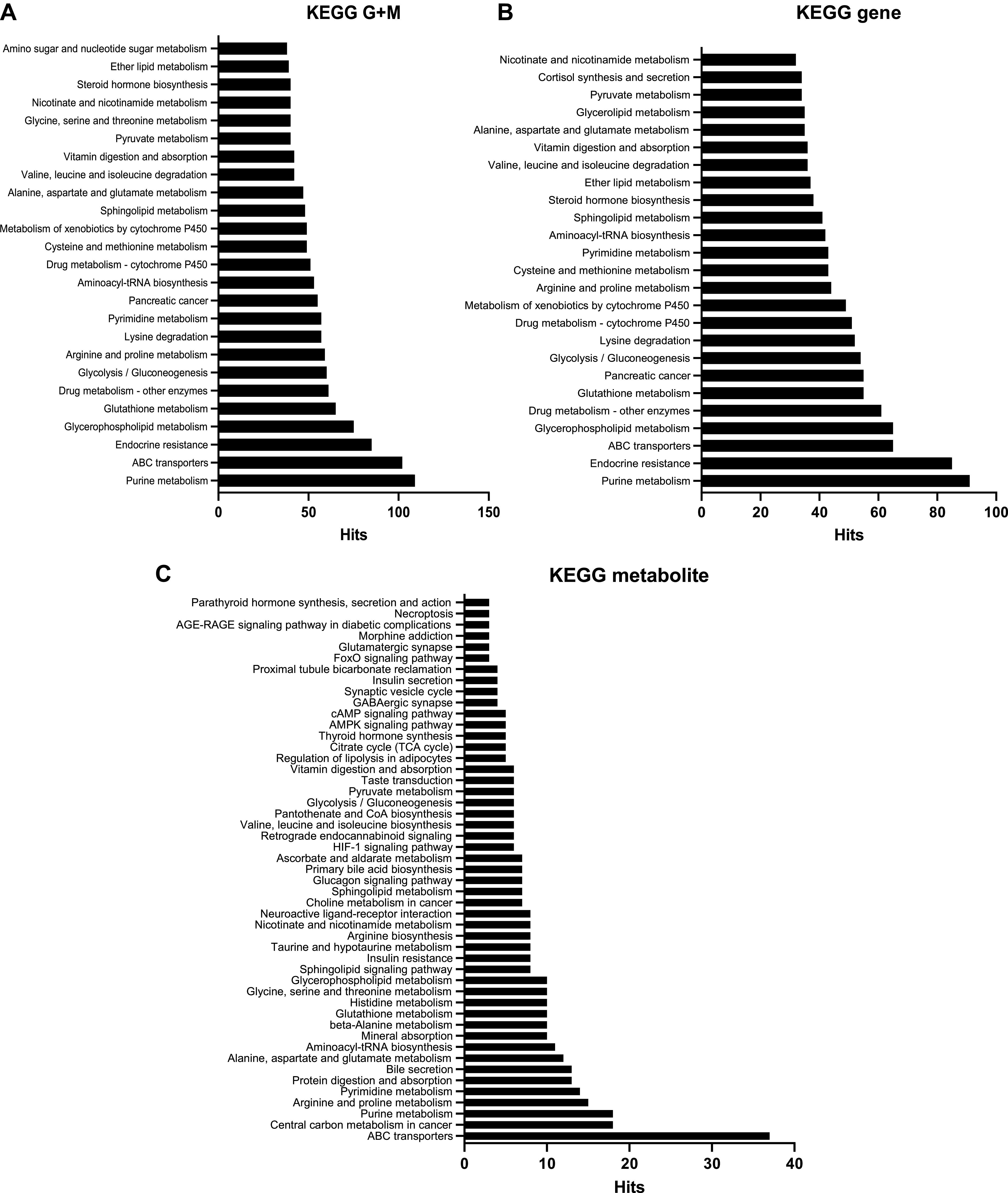
Tri-omics analysis of significant changes in the cardiac transcriptome, metabolome and proteome changes in during pregnancy. ANOVA analysis was performed to identify significantly expressed cardiac transcripts, metabolites and protein abundances [P <0.05; false discovery rate (FDR) 0.05] across all time points [nonpregnant, diestrus (NP), midpregnant (MP; day 8 of pregnancy), late pregnant (LP; day 16 of pregnancy), and 1 wk postbirth with lactation (PB)]. Official gene names and Human Metabolome Database (HMDB) and Uniprot IDs of those significantly expressed targets were added to the tri-omics analysis software OmicsNet. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis was performed to analyze significantly impacted pathways using KEGG gene + metabolite (G+M; A), KEGG gene (B), and KEGG metabolite (C).
DISCUSSION
Changes in cardiac metabolism are thought to play a key role in pregnancy-induced cardiac remodeling (23). Nevertheless, a full appreciation of the molecular changes occurring in the heart during pregnancy is lacking. Therefore, we sought to determine the coordinated changes in the metabolome, transcriptome, and proteome in the heart during pregnancy and the postpartum period. Our results are consistent with previously published findings suggesting that pregnancy is a physiological form of cardiac hypertrophy associated with a lack of ventricular fibrosis, minimal induction of the fetal gene program, increases in ventricular chamber dimensions, increases in cardiac output, and minimal changes in ejection fraction (3, 13, 14, 16, 20, 29, 48–51). Cardiac metabolomics analyses revealed significant increases in metabolites associated with amino acid metabolism, nucleotide, and phospholipid synthesis during LP and PB, along with increases in glutathione metabolism and cofactors, which are essential for intermediary metabolism. Of note, urea cycle and polyamine metabolites were among the most changed metabolites during pregnancy. Cardiac proteomics and transcriptomics revealed not only significant increases in urea cycle enzymes at LP and PB, consistent with increases in urea cycle metabolites, but also significant reductions in several structural proteins and mitochondrial complex subunit proteins at LP and PB, which may also impact the remodeling and metabolism of the maternal heart. In addition, transcriptomics revealed significant upregulation of transcripts associated with cell cycle and angiogenesis at MP, transcripts associated with metabolism at LP, and transcripts associated with ECM remodeling at PB, indicating a temporal regulation of growth-related transcripts in the maternal heart.
Pregnancy results in significant changes in systemic metabolism including reductions in glucose metabolism, increases in fatty acid oxidation (FAO), and increases in circulating ketone bodies (KBs) and triglycerides (18, 19). Few studies have examined changes in cardiac metabolites in the maternal heart. We show that pregnancy increases the abundance of cardiac metabolites associated with progrowth pathways including amino acid metabolism, glycerophospholipid synthesis, and nucleotide synthesis. The most profound changes in amino acid metabolism were in polyamine and urea cycle-associated metabolites, the latter of which is supported by increased expression of urea cycle enzymes. The most conspicuous metabolite in the pregnant heart was homoarginine. Increased homoarginine and arginine levels are associated with reduced cardiovascular mortality (52, 53). In addition, homoarginine and arginine levels are associated with improvements in blood glucose in diet-induced obese mice (54) and are increased in response to high estrogen levels (55). Several studies have noted that plasma levels of homoarginine are increased during pregnancy, peaking at LP (56, 57), where it mediates changes in flow-mediated vasodilation (57). Reductions in homoarginine and increases in the NOS inhibitor asymmetric dimethylarginine (ADMA) have been associated with preeclampsia (58). It is not known whether these changes contribute to cardiac growth during pregnancy or whether reductions in homoarginine could contribute to PPCM pathology, similar to that observed during preeclampsia. Urea cycle disorders are associated with adverse pregnancy outcomes (59) and type 2 diabetes (60), so it is possible that urea cycle metabolites may have significant roles not only in the maternal heart but also in other maternal organ systems. Homoarginine has been shown to protect the heart from dilatation and preserve cardiac systolic function (61), which could be its function in the maternal heart. Homoarginine could also help to facilitate increases in endothelial nitric oxide synthase (eNOS) expression and activity and subsequent increases in nitric oxide (NO) observed during pregnancy (62–64). This is intriguing since coronary NO has been shown to regulate cardiac substrate metabolism during pregnancy and contribute to an inhibition of glucose oxidation and an increase of FAO (65). Despite this, little is known of the importance of the urea cycle in the heart. Additional studies are required to determine the precise role of homoarginine and its impact on bioavailable NO in the maternal heart.
Polyamines, which are products derived from the urea cycle intermediate ornithine, have been shown to contribute to cell growth and differentiation, promote angiogenesis, and contribute to hypertrophy (66–68). Polyamine depletion has also been shown to inhibit hypertrophy (69), suggesting that polyamines could be important regulators of cardiac growth during pregnancy. Betaine aldehyde dehydrogenase has been shown to be increased in the maternal heart during MP and LP (70) and is associated with polyamine catabolism, adding additional support for the importance of these metabolites in the maternal heart. Polyamines have been shown to contribute to hypertrophy in the liver and kidney during pregnancy (71); however, this is the first time that changes in polyamine metabolites have been documented in the heart during pregnancy and have been linked to pregnancy-induced cardiac growth. Future studies will be required to elucidate the significance and role of polyamine metabolism in the maternal heart.
Many of the metabolomics changes we observe in the maternal heart are similar to those seen in pressure-overloaded hearts, suggestive of overlapping metabolic mechanisms contributing to both types of growth. Consistent with our findings in the maternal heart at LP/PB, 1 wk of pressure overload was associated with higher levels of BCAAs and increases in metabolites associated with polyamine metabolism and the urea cycle (i.e., arginine and proline metabolism) (72). Our data also indicate changes in one-carbon metabolism. One-carbon metabolism plays a role in nucleotide and amino acid metabolism, epigenetics, and redox metabolism (73). Metabolites associated with one-carbon metabolism have been shown to change during pregnancy (74). The one-carbon pool is maybe responsible for the control of mitochondrial energy metabolism via complex I (75), suggesting that one-carbon metabolism could have a significant role in the maternal heart.
Pregnancy-induced decreases in cardiac glucose utilization are partially mediated by increases in Pdk4 (21–23) and may contribute to cardiac growth. Consistent with a reduction in glucose catabolism, we have shown that Pdk4 expression is increased at LP alongside reductions in the phosphorylation of Pfkfb2. In these studies, we did not examine the mechanism of increased Pdk4 in the maternal heart; however, progesterone is likely responsible for the increase in Pdk4 expression during LP (21). Other studies suggest that increases in fibroblast growth factor 21 (Fgf21) during pregnancy contribute to Pdk4 upregulation and increases in FAO (76). Of note, Fgf21, like Pdk4, may be essential for pregnancy-induced cardiac remodeling because cardiac-specific Fgf21−/− mice do not undergo pregnancy-induced cardiac remodeling (76).
Like pregnancy, exercise also induces physiological cardiac growth, potentially via common mechanisms. The decrease in Pfkfb2 phosphorylation observed during LP resembles the transient decrease in Pfkfb2 phosphorylation that occurs during exercise. Because this post-translational modification influences phosphofructokinase activity, it could contribute to lower glucose catabolism in the maternal heart (24). Metabolomics studies in Pfkfb2-mutant hearts also showed evidence of altered phospholipid metabolism, and transcriptomic changes in hearts of mice with low glycolytic activity were associated with increases in transcripts involved in intermediary metabolism, cell proliferation, transcription, and changes in serine biosynthesis and one-carbon metabolism (24), all of which are similar to what we observe in the maternal heart. Pfkfb2 could indirectly contribute to changes in the pentose phosphate pathway, the polyol pathway, and the glycerophospholipid pathway through allosteric modulation of Pfk1 (18, 77). Thus, an overall reduction in Pfk1 activity may redirect glucose to these pathways (78). It is possible that the reduction of glucose catabolism at the level of both phosphofructokinase and pyruvate dehydrogenase mediates a coordination of ancillary biosynthetic pathway activity to allocate glucose-derived carbon for nucleotide and glycerophospholipid synthesis; however, additional studies are required to elucidate how biosynthetic pathways in the maternal heart are affected by pregnancy. Nevertheless, it has been shown that genes associated with the glycerophospholipid pathway, important for production of phospholipids, are upregulated during pregnancy (79). Interestingly, the glycerolipid synthesis pathway is regulated by both phosphofructokinase and Hif1α, both of which are higher in the heart during pregnancy (79).
Our proteomic analyses identified significant reductions in the expression of several mitochondrial complex I (e.g., NADH:ubiquione oxidoreductase) and IV (e.g., cytochrome-c oxidase) subunits during both LP and PB. Steroid sex hormones can inhibit complex IV activity (80), which could contribute to changes in the maternal heart. Because complex I is responsible for the generation of reactive oxygen species (ROS), it could be that the lower levels of complex I subunits influence ROS generation during pregnancy. Complex I deficiency contributes to hypertrophic cardiomyopathy (81). Interestingly, NO can reversibly inhibit cytochrome-c oxidase activity (82), which could occur in the maternal heart, especially given the higher levels of homoarginine, a substrate for nitric oxide synthesis. Because we find that not all complex I and IV subunits are reduced during pregnancy and the postpartum period, it could be that subunits are switched during pregnancy and the postpartum period to fine-tune mitochondrial metabolism. Additional studies are required to address how mitochondrial metabolism changes during pregnancy.
Our transcriptomics data suggest temporal changes in gene expression during pregnancy and the postpartum period, with MP being associated with increases in cell cycle, cell proliferation, and angiogenic transcripts; LP being associated with increases in metabolic transcripts; and PB being associated with ECM remodeling transcripts. In support of these changes, a recent study (51) showed that cell cycle, cell proliferation, and ECM reorganization were the most significant gene ontology terms identified in pregnant mice. Others (16) showed similar changes, with MP being associated with cytoskeleton, ubiquitin conjugation, vasculature development, and transcription regulation and LP with circadian rhythm, metabolism, and ECM. Despite the differences in mouse strain and time points examined, our data support these changes and highlight significant temporal regulation of angiogenic, metabolic, and extracellular matrix remodeling in the maternal heart.
There are a few limitations of our study. The omics data were generated from whole heart tissue lysates and do not provide an assessment of cell-specific contributions to the metabolome, transcriptome, and proteome. This is important, as our transcriptomic analysis alludes to coordinated changes between cardiomyocytes and vascular endothelial cells at MP and extracellular matrix and fibroblast-mediated changes at PB. In addition, our metabolomics data provide only snapshots of metabolite changes and do not provide information on metabolic pathway flux and carbon allocation into ancillary pathways of glucose metabolism. Determining the precise nature of these changes will likely shed light on the underlying processes that contribute to cardiac growth. Few studies document omics changes in the rodent or human heart during pregnancy and studies that exist focus on omics changes in the maternal circulation, the assessment of noncardiac samples (e.g., plasma, serum, urine, placenta, cord blood) (83), or the impact of the maternal metabolome on the cardiovascular physiology of the offspring (84), making comparisons with these studies difficult. The inclusion of plasma metabolomics in the present study would vastly increase the power of our study, which would allow for additional comparisons between rodent and human pregnancy studies.
The selection of time points for examination during pregnancy and the postpartum period is largely inconsistent in the field. We used day 8 and day 16 of pregnancy as mid- and late pregnancy, respectively, and 1-wk postbirth as our major investigative time points; however, many studies using C57BL/6 mice include days 6–11 for midpregnancy and days 17–20 for late pregnancy. Time points examined postpartum include 12 h after parturition for up to several weeks after birth (3, 13, 14, 16, 29, 50, 51). It is possible that the gestational time points selected and fetal size could contribute to the differences we observe; however, this is unlikely in the present study, because FVB/NJ mice have shorter gestational lengths than those of C57BL/6 mice (34). Regardless, studies that interrogate additional time points during pregnancy and the postpartum period and account for possible strain differences could reveal additional changes not detected in our study.
Another important consideration is the time frame in which pregnancy-induced cardiac growth resolves. Although the present study shows that pregnancy is associated with cardiac growth, we do not know how long these changes persist in the postpartum period. Studies in humans suggest that cardiac size decreases by 12 wk after birth and occurs in tandem with decreases in cardiac output and restoration of hemodynamic parameters (8, 85); however, other studies suggest that pregnancy-induced cardiac hypertrophy can persist for up to a year after birth (86). It has also been shown that pregnancy-induced hypertrophy and increases in cardiac output are resolved in the first 1–3 wk after parturition in mouse models (13, 14, 16); however, it is unclear in these studies whether the reported measurements of heart size were taken after a period of lactation or not, which could impact reversal of pregnancy-induced cardiac growth. The full impact of circulating hormones, the placenta, maternal organ-organ interactions, and maternal-fetal interactions on pregnancy-induced cardiac growth also requires additional study.
Although we did not examine levels of circulating hormones in the present study, lactation and lactogenic hormones, such as prolactin, could contribute to changes in not only cardiac metabolism but also cardiac growth. In addition, because prolactin signaling is associated with PPCM (15, 87, 88), this would be important to examine further. Furthermore, pregnancy elicits changes in the physiological responses of other maternal organ systems, such as the liver and kidney, and significant changes in systemic metabolism; therefore, changes in organ-organ communication and cooperation cannot be ruled out as contributing factors to pregnancy-induced cardiac growth. This is particularly important since several suspected liver-derived metabolites, such as homoarginine and ketone bodies, are increased at LP. We also observed increased expression of cardiac Bdh1, which may indicate changes in cardiac ketone body metabolism during pregnancy. Increases in Bdh1 and ketone bodies preserve cardiac function and protect the failing heart from adverse remodeling (89, 90), which may also be the case in the maternal heart. Although changes in metabolism occur during pregnancy, we have not established a causal link between changes in metabolism and cardiac growth. Addressing these unanswered questions will be an important next step to determine the underlying mechanisms that contribute to pregnancy-induced cardiac growth.
In summary, the findings of this study show substantial metabolic changes that occur in the maternal heart during pregnancy which may facilitate pregnancy-induced cardiac growth. In particular, we find evidence of reductions in glucose catabolism and altered nucleotide, glycerophospholipid, and amino acid metabolism, with the last comprising significant changes in urea cycle and polyamine metabolites. The findings of this study also insinuate that pregnancy is associated with temporal, coordinated changes in angiogenesis, metabolism, and ECM remodeling in the maternal heart. Understanding how metabolism changes in the maternal heart and how this may contribute to pathology associated with maternal cardiovascular diseases, such as PPCM, is a future goal arising from these studies.
SUPPLEMENTAL DATA
Supplemental Figs. S1–S12: https://doi.org/10.6084/m9.figshare.19651137.
GRANTS
This work has been supported by National Institutes of Health (NIH) Grants GM127607 (to H.E.C. and S.P.J.), HL147844 (to B.G.H. and S.P.J.), HL154663 (to K.L.F.), and S10 OD025178 (to S.P.J.); a Jewish Heritage Fund for Excellence faculty support grant (to H.E.C.); the Jewish Heritage Fund for Excellence Research Enhancement Award (to H.E.C.); an NIGMS IDeA state proteomics voucher (to H.E.C.); and a University of Louisville School of Medicine Basic Science grant (to H.E.C.). Sequencing and Bioinformatics support for this work was provided by NIH Grants GM103436 (Martha Bickford) and GM106396 (Donald Miller). Proteomics support for this work was provided by IDeA National Resource for Quantitative Proteomics Grant R24GM137786.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.P.J., B.G.H., and H.E.C. conceived and designed research; K.L.F., J.B.S., L.F.G., K.R.B., L.A.M., and H.E.C. performed experiments; K.L.F., J.B.S., J.C., L.F.G., K.R.B., P.L., L.A.M., S.U., and H.E.C., analyzed data; P.L., S.P.J., B.G.H., and H.E.C. interpreted results of experiments; H.E.C. prepared figures; H.E.C. drafted manuscript; K.L.F., J.B.S., J.C., K.R.B., P.L., L.A.M., S.U., S.P.J., B.G.H., and H.E.C. edited and revised manuscript; K.L.F., J.B.S., J.C., L.F.G., K.R.B., P.L., L.A.M., S.U., S.P.J., B.G.H., and H.E.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank all members of the Hill, Jones, and Collins laboratories for technical assistance and valued discussions, the animal care staff for excellent care and animal husbandry, and the DOC Imaging and Physiology core for assistance with echocardiography and histology studies. We thank Dr. Samuel McIntosh and Heather Douglas at the IDeA National Resource for Quantitative Proteomics for discussions on study design and sample preparation for proteomics studies.
REFERENCES
- 1.Schannwell CM, Zimmermann T, Schneppenheim M, Plehn G, Marx R, Strauer BE. Left ventricular hypertrophy and diastolic dysfunction in healthy pregnant women. Cardiology 97: 73–78, 2002. doi: 10.1159/000057675. [DOI] [PubMed] [Google Scholar]
- 2.Hunter S, Robson SC. Adaptation of the maternal heart in pregnancy. Br Heart J 68: 540–543, 1992. doi: 10.1136/hrt.68.12.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung E, Yeung F, Leinwand LA. Akt and MAPK signaling mediate pregnancy-induced cardiac adaptation. J Appl Physiol (1985) 112: 1564–1575, 2012. doi: 10.1152/japplphysiol.00027.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burwell CS. Observations on the output of the heart and the pressure in the veins of pregnant women. Trans Am Clin Climatol Assoc 50: 46–49, 1934. [PMC free article] [PubMed] [Google Scholar]
- 5.Hamilton HF. The cardiac output in normal pregnancy; as determined by the Cournand right catheterization technique. J Obstet Gynaecol Br Emp 56: 548–552, 1949. doi: 10.1111/j.1471-0528.1949.tb07124.x. [DOI] [PubMed] [Google Scholar]
- 6.Laird-Meeter K, van de Ley G, Bom TH, Wladimiroff JW, Roelandt J. Cardiocirculatory adjustments during pregnancy— an echocardiographic study. Clin Cardiol 2: 328–332, 1979. doi: 10.1002/clc.4960020503. [DOI] [PubMed] [Google Scholar]
- 7.Savu O, Jurcuţ R, Giuşcă S, van Mieghem T, Gussi I, Popescu BA, Ginghină C, Rademakers F, Deprest J, Voigt JU. Morphological and functional adaptation of the maternal heart during pregnancy. Circ Cardiovasc Imaging 5: 289–297, 2012. doi: 10.1161/CIRCIMAGING.111.970012. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez AM, Osorio JC, Manlhiot C, Gruber D, Homma S, Mital S. Hypertrophy signaling during peripartum cardiac remodeling. Am J Physiol Heart Circ Physiol 293: H3008–H3013, 2007. doi: 10.1152/ajpheart.00401.2007. [DOI] [PubMed] [Google Scholar]
- 9.Mone SM, Sanders SP, Colan SD. Control mechanisms for physiological hypertrophy of pregnancy. Circulation 94: 667–672, 1996. doi: 10.1161/01.CIR.94.4.667. [DOI] [PubMed] [Google Scholar]
- 10.Karamermer Y, Roos-Hesselink JW. Pregnancy and adult congenital heart disease. Expert Rev Cardiovasc Ther 5: 859–869, 2007. doi: 10.1586/14779072.5.5.859. [DOI] [PubMed] [Google Scholar]
- 11.Zentner D, Du Plessis M, Brennecke S, Wong J, Grigg L, Harrap SB. Deterioration in cardiac systolic and diastolic function late in normal human pregnancy. Clin Sci (Lond) 116: 599–606, 2009. doi: 10.1042/CS20080142. [DOI] [PubMed] [Google Scholar]
- 12.Pandey AK, Banerjee AK, Das A, Bhawani G, Kumar A, Majumadar B, Bhattacharya AK. Evaluation of maternal myocardial performance during normal pregnancy and post partum. Indian Heart J 62: 64–67, 2010. [PubMed] [Google Scholar]
- 13.Eghbali M, Deva R, Alioua A, Minosyan TY, Ruan H, Wang Y, Toro L, Stefani E. Molecular and functional signature of heart hypertrophy during pregnancy. Circ Res 96: 1208–1216, 2005. doi: 10.1161/01.RES.0000170652.71414.16. [DOI] [PubMed] [Google Scholar]
- 14.Umar S, Nadadur R, Iorga A, Amjedi M, Matori H, Eghbali M. Cardiac structural and hemodynamic changes associated with physiological heart hypertrophy of pregnancy are reversed postpartum. J Appl Physiol (1985) 113: 1253–1259, 2012. doi: 10.1152/japplphysiol.00549.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hilfiker-Kleiner D, Kaminski K, Podewski E, Bonda T, Schaefer A, Sliwa K, Forster O, Quint A, Landmesser U, Doerries C, Luchtefeld M, Poli V, Schneider MD, Balligand JL, Desjardins F, Ansari A, Struman I, Nguyen NQ, Zschemisch NH, Klein G, Heusch G, Schulz R, Hilfiker A, Drexler H. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell 128: 589–600, 2007. doi: 10.1016/j.cell.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 16.Chung E, Heimiller J, Leinwand LA. Distinct cardiac transcriptional profiles defining pregnancy and exercise. PLoS One 7: e42297, 2012. doi: 10.1371/journal.pone.0042297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lemmens K, Doggen K, De Keulenaer GW. Activation of the neuregulin/ErbB system during physiological ventricular remodeling in pregnancy. Am J Physiol Heart Circ Physiol 300: H931–H942, 2011. doi: 10.1152/ajpheart.00385.2010. [DOI] [PubMed] [Google Scholar]
- 18.Gibb AA, Hill BG. Metabolic coordination of physiological and pathological cardiac remodeling. Circ Res 123: 107–128, 2018. doi: 10.1161/CIRCRESAHA.118.312017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu LX, Arany Z. Maternal cardiac metabolism in pregnancy. Cardiovasc Res 101: 545–553, 2014. doi: 10.1093/cvr/cvu009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung E, Leinwand LA. Pregnancy as a cardiac stress model. Cardiovasc Res 101: 561–570, 2014. doi: 10.1093/cvr/cvu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu LX, Rowe GC, Yang S, Li J, Damilano F, Chan MC, Lu W, Jang C, Wada S, Morley M, Hesse M, Fleischmann BK, Rabinowitz JD, Das S, Rosenzweig A, Arany Z. PDK4 inhibits cardiac pyruvate oxidation in late pregnancy. Circ Res 121: 1370–1378, 2017. doi: 10.1161/CIRCRESAHA.117.311456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sugden MC, Holness MJ. Cardiac carbohydrate and lipid utilization during late pregnancy. Biochem Soc Trans 21: 312S–312S, 1993. doi: 10.1042/bst021312s. [DOI] [PubMed] [Google Scholar]
- 23.Sugden MC, Holness MJ. Control of muscle pyruvate oxidation during late pregnancy. FEBS Lett 321: 121–126, 1993. doi: 10.1016/0014-5793(93)80091-8. [DOI] [PubMed] [Google Scholar]
- 24.Gibb AA, Epstein PN, Uchida S, Zheng Y, McNally LA, Obal D, Katragadda K, Trainor P, Conklin DJ, Brittian KR, Tseng MT, Wang J, Jones SP, Bhatnagar A, Hill BG. Exercise-induced changes in glucose metabolism promote physiological cardiac growth. Circulation 136: 2144–2157, 2017. doi: 10.1161/CIRCULATIONAHA.117.028274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fulghum K, Hill BG. Metabolic mechanisms of exercise-induced cardiac remodeling. Front Cardiovasc Med 5: 127, 2018. doi: 10.3389/fcvm.2018.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitten WK. Modification of the oestrous cycle of the mouse by external stimuli associated with the male. J Endocrinol 13: 399–404, 1956. doi: 10.1677/joe.0.0130399. [DOI] [PubMed] [Google Scholar]
- 27.Byers SL, Wiles MV, Dunn SL, Taft RA. Mouse estrous cycle identification tool and images. PLoS One 7: e35538, 2012. doi: 10.1371/journal.pone.0035538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heyne GW, Plisch EH, Melberg CG, Sandgren EP, Peter JA, Lipinski RJ. A simple and reliable method for early pregnancy detection in inbred mice. J Am Assoc Lab Anim Sci 54: 368–371, 2015. [PMC free article] [PubMed] [Google Scholar]
- 29.Chung E, Yeung F, Leinwand LA. Calcineurin activity is required for cardiac remodelling in pregnancy. Cardiovasc Res 100: 402–410, 2013. doi: 10.1093/cvr/cvt208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong AY, Kulandavelu S, Whiteley KJ, Qu D, Langille BL, Adamson SL. Maternal cardiovascular changes during pregnancy and postpartum in mice. Am J Physiol Heart Circ Physiol 282: H918–H925, 2002. doi: 10.1152/ajpheart.00641.2001. [DOI] [PubMed] [Google Scholar]
- 31.Amano T, Ripperger JA, Albrecht U. Changing the light schedule in late pregnancy alters birth timing in mice. Theriogenology 154: 212–222, 2020. doi: 10.1016/j.theriogenology.2020.05.032. [DOI] [PubMed] [Google Scholar]
- 32.Cooke CL, Davidge ST. Pregnancy-induced alterations of vascular function in mouse mesenteric and uterine arteries. Biol Reprod 68: 1072–1077, 2003. doi: 10.1095/biolreprod.102.009886. [DOI] [PubMed] [Google Scholar]
- 33.Chlodzinska N, Gajerska M, Bartkowska K, Turlejski K, Djavadian RL. Lipopolysaccharide injected to pregnant mice affects behavior of their offspring in adulthood. Acta Neurobiol Exp (Wars) 71: 519–527, 2011. [DOI] [PubMed] [Google Scholar]
- 34.Murray SA, Morgan JL, Kane C, Sharma Y, Heffner CS, Lake J, Donahue LR. Mouse gestation length is genetically determined. PLoS One 5: e12418, 2010. doi: 10.1371/journal.pone.0012418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8: e1000412, 2010. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Audam TN, Howard CM, Garrett LF, Zheng YW, Bradley JA, Brittian KR, Frank MW, Fulghum KL, Pólos M, Herczeg S, Merkely B, Radovits T, Uchida S, Hill BG, Dassanayaka S, Jackowski S, Jones SP. Cardiac PANK1 deletion exacerbates ventricular dysfunction during pressure overload. Am J Physiol Heart Circ Physiol 321: H784–H797, 2021. doi: 10.1152/ajpheart.00411.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zelko IN, Dassanayaka S, Malovichko MV, Howard CM, Garrett LF, Uchida S, Brittian KR, Conklin DJ, Jones SP, Srivastava S. Chronic benzene exposure aggravates pressure overload-induced cardiac dysfunction. Toxicol Sci 185: 64–76, 2021. doi: 10.1093/toxsci/kfab125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olutoye OA, Sheikh F, Zamora IJ, Yu L, Akinkuotu AC, Adesina AM, Olutoye OO. Repeated isoflurane exposure and neuroapoptosis in the midgestation fetal sheep brain. Am J Obstet Gynecol 214: 542.e1–542.e8, 2016. doi: 10.1016/j.ajog.2015.10.927. [DOI] [PubMed] [Google Scholar]
- 39.Schubert H, Eiselt M, Walter B, Fritz H, Brodhun M, Bauer R. Isoflurane/nitrous oxide anesthesia and stress-induced procedures enhance neuroapoptosis in intrauterine growth-restricted piglets. Intensive Care Med 38: 1205–1214, 2012. doi: 10.1007/s00134-012-2576-2. [DOI] [PubMed] [Google Scholar]
- 40.Kong FJ, Tang YW, Lou AF, Chen H, Xu LH, Zhang XM, Lu HS. Effects of isoflurane exposure during pregnancy on postnatal memory and learning in offspring rats. Mol Biol Rep 39: 4849–4855, 2012. doi: 10.1007/s11033-011-1279-z. [DOI] [PubMed] [Google Scholar]
- 41.Palanisamy A, Crosby G, Culley DJ. Early gestational exposure to isoflurane causes persistent cell loss in the dentate gyrus of adult male rats. Behav Brain Funct 13: 14, 2017. doi: 10.1186/s12993-017-0132-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15: 550, 2014. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parrott ME, Aljrbi E, Biederman DL, Montalvo RN, Barth JL, LaVoie HA. Maternal cardiac messenger RNA expression of extracellular matrix proteins in mice during pregnancy and the postpartum period. Exp Biol Med (Maywood) 243: 1220–1232, 2018. doi: 10.1177/1535370218818457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Virgen-Ortiz A, Limon-Miranda S, Salazar-Enriquez DG, Melnikov V, Sanchez-Pastor EA, Castro-Rodriguez EM. Matrix metalloproteinases system and types of fibrosis in rat heart during late pregnancy and postpartum. Medicina (Kaunas) 55: 199, 2019. doi: 10.3390/medicina55050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mizejewski GJ. Levels of alpha-fetoprotein during pregnancy and early infancy in normal and disease states. Obstet Gynecol Surv 58: 804–826, 2003. doi: 10.1097/01.OGX.0000099770.97668.18. [DOI] [PubMed] [Google Scholar]
- 46.Biswas S, Rodeck CH. Plasma prolactin levels during pregnancy. Br J Obstet Gynaecol 83: 683–687, 1976. doi: 10.1111/j.1471-0528.1976.tb00913.x. [DOI] [PubMed] [Google Scholar]
- 47.Burrows S, Pekala B. Serum copper and ceruloplasmin in pregnancy. Am J Obstet Gynecol 109: 907–909, 1971. doi: 10.1016/0002-9378(71)90805-2. [DOI] [PubMed] [Google Scholar]
- 48.Eghbali M, Wang Y, Toro L, Stefani E. Heart hypertrophy during pregnancy: a better functioning heart? Trends Cardiovasc Med 16: 285–291, 2006. doi: 10.1016/j.tcm.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 49.Li J, Umar S, Amjedi M, Iorga A, Sharma S, Nadadur RD, Regitz-Zagrosek V, Eghbali M. New frontiers in heart hypertrophy during pregnancy. Am J Cardiovasc Dis 2: 192–207, 2012. [PMC free article] [PubMed] [Google Scholar]
- 50.Stefani E, Eghbali M, Minosyan T, Alioua A, Toro L. Molecular studies in heart hypertrophy during pregnancy. J Muscle Res Cell Motil 25: 607, 2004. [PubMed] [Google Scholar]
- 51.Yang Y, Kurian J, Schena G, Johnson J, Kubo H, Travers JG, Kang C, Lucchese AM, Eaton DM, Lv M, Li N, Leynes LG, Yu D, Yang F, McKinsey TA, Kishore R, Khan M, Mohsin S, Houser SR. Cardiac remodeling during pregnancy with metabolic syndrome: prologue of pathological remodeling. Circulation 143: 699–712, 2021. doi: 10.1161/CIRCULATIONAHA.120.051264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pilz S, Meinitzer A, Tomaschitz A, Drechsler C, Ritz E, Krane V, Wanner C, Boehm BO, März W. Low homoarginine concentration is a novel risk factor for heart disease. Heart 97: 1222–1227, 2011. doi: 10.1136/hrt.2010.220731. [DOI] [PubMed] [Google Scholar]
- 53.Schwedhelm E, Song RJ, Vasan RS, van den Heuvel ER, Hannemann J, Xanthakis V, Böger R. Association of lower plasma homoarginine concentrations with greater risk of all-cause mortality in the community: the Framingham Offspring Study. J Clin Med 9: 2016, 2020. doi: 10.3390/jcm9062016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stockebrand M, Hornig S, Neu A, Atzler D, Cordts K, Böger RH, Isbrandt D, Schwedhelm E, Choe CU. Homoarginine supplementation improves blood glucose in diet-induced obese mice. Amino Acids 47: 1921–1929, 2015. doi: 10.1007/s00726-015-2022-1. [DOI] [PubMed] [Google Scholar]
- 55.Helm T, Varsi K, Fløtre CH, Lund A, Svingen GF, Ueland PM, Bjørke-Monsen AL. Plasma homoarginine concentrations according to use of hormonal contraception. Sci Rep 8: 12217, 2018. doi: 10.1038/s41598-018-30708-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sotgia S, Berlinguer F, Porcu C, Pasciu V, Molle G, Dattena M, Gallus M, Bassu S, Mangoni AA, Carru C, Zinellu A. Plasma homoarginine concentrations in ewe’s pregnancy and association with the number of fetuses. Res Vet Sci 144: 175–180, 2022. doi: 10.1016/j.rvsc.2021.11.012. [DOI] [PubMed] [Google Scholar]
- 57.Valtonen P, Laitinen T, Lyyra-Laitinen T, Raitakari OT, Juonala M, Viikari JS, Heiskanen N, Vanninen E, Punnonen K, Heinonen S. Serum L-homoarginine concentration is elevated during normal pregnancy and is related to flow-mediated vasodilatation. Circ J 72: 1879–1884, 2008. doi: 10.1253/circj.CJ-08-0240. [DOI] [PubMed] [Google Scholar]
- 58.Khalil AA, Tsikas D, Akolekar R, Jordan J, Nicolaides KH. Asymmetric dimethylarginine, arginine and homoarginine at 11-13 weeks’ gestation and preeclampsia: a case-control study. J Hum Hypertens 27: 38–43, 2013. doi: 10.1038/jhh.2011.109. [DOI] [PubMed] [Google Scholar]
- 59.Hsu CN, Tain YL. Impact of arginine nutrition and metabolism during pregnancy on offspring outcomes. Nutrients 11: 1452, 2019. doi: 10.3390/nu11071452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cao YF, Li J, Zhang Z, Liu J, Sun XY, Feng XF, Luo HH, Yang W, Li SN, Yang X, Fang Z-Z. Plasma levels of amino acids related to urea cycle and risk of type 2 diabetes mellitus in Chinese adults. Front Endocrinol (Lausanne) 10: 50, 2019. doi: 10.3389/fendo.2019.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rodionov RN, Begmatov H, Jarzebska N, Patel K, Mills MT, Ghani Z, Khakshour D, Tamboli P, Patel MN, Abdalla M, Assaf M, Bornstein SR, Millan JL, Bode‐Böger SM, Martens‐Lobenhoffer J, Weiss N, Savinova OV. Homoarginine supplementation prevents left ventricular dilatation and preserves systolic function in a model of coronary artery disease. J Am Heart Assoc 8: e012486, 2019. doi: 10.1161/JAHA.119.012486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Linke A, Li W, Huang H, Wang Z, Hintze TH. Role of cardiac eNOS expression during pregnancy in the coupling of myocardial oxygen consumption to cardiac work. Am J Physiol Heart Circ Physiol 283: H1208–H1214, 2002. doi: 10.1152/ajpheart.00108.2002. [DOI] [PubMed] [Google Scholar]
- 63.Khalil A, Hardman L, O Brien P. The role of arginine, homoarginine and nitric oxide in pregnancy. Amino Acids 47: 1715–1727, 2015. doi: 10.1007/s00726-015-2014-1. [DOI] [PubMed] [Google Scholar]
- 64.Owusu Darkwa E, Djagbletey R, Sottie D, Owoo C, Vanderpuye NM, Essuman R, Aryee G. Serum nitric oxide levels in healthy pregnant women: a case-control study in a tertiary facility in Ghana. Matern Health Neonatol Perinatol 4: 3, 2018. doi: 10.1186/s40748-017-0072-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Williams JG, Ojaimi C, Qanud K, Zhang S, Xu X, Recchia FA, Hintze TH. Coronary nitric oxide production controls cardiac substrate metabolism during pregnancy in the dog. Am J Physiol Heart Circ Physiol 294: H2516–H2523, 2008. doi: 10.1152/ajpheart.01196.2007. [DOI] [PubMed] [Google Scholar]
- 66.Flamigni F, Rossoni C, Stefanelli C, Caldarera CM. Polyamine metabolism and function in the heart. J Mol Cell Cardiol 18: 3–11, 1986. doi: 10.1016/s0022-2828(86)80977-4. [DOI] [PubMed] [Google Scholar]
- 67.Moruzzi G, Caldarera CM, Casti A. The biological effect of polyamines on heart RNA and histone metabolism. Mol Cell Biochem 3: 153–161, 1974. doi: 10.1007/BF01659187. [DOI] [PubMed] [Google Scholar]
- 68.Sagar NA, Tarafdar S, Agarwal S, Tarafdar A, Sharma S. Polyamines: functions, metabolism, and role in human disease management. Med Sci (Basel) 9: 44, 2021. doi: 10.3390/medsci9020044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lin Y, Zhang X, Wang L, Zhao Y, Li H, Xiao W, Xu C, Liu J. Polyamine depletion attenuates isoproterenol-induced hypertrophy and endoplasmic reticulum stress in cardiomyocytes. Cell Physiol Biochem 34: 1455–1465, 2014. doi: 10.1159/000366350. [DOI] [PubMed] [Google Scholar]
- 70.Rosas-Rodriguez JA, Soñanez-Organis JG, Godoy-Lugo JA, Espinoza-Salazar JA, López-Jacobo CJ, Stephens-Camacho NA, González-Ochoa G. Betaine aldehyde dehydrogenase expression during physiological cardiac hypertrophy induced by pregnancy. Biochem Biophys Res Commun 490: 623–628, 2017. doi: 10.1016/j.bbrc.2017.06.087. [DOI] [PubMed] [Google Scholar]
- 71.Piacentini M, Sartori C, Beninati S, Bargagli AM, Cerù-Argento MP. Ornithine decarboxylase, transglutaminase, diamine oxidase and total diamines and polyamines in maternal liver and kidney throughout rat pregnancy. Biochem J 234: 435–440, 1986. doi: 10.1042/bj2340435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sansbury BE, DeMartino AM, Xie Z, Brooks AC, Brainard RE, Watson LJ, DeFilippis AP, Cummins TD, Harbeson MA, Brittian KR, Prabhu SD, Bhatnagar A, Jones SP, Hill BG. Metabolomic analysis of pressure-overloaded and infarcted mouse hearts. Circ Heart Fail 7: 634–642, 2014. doi: 10.1161/CIRCHEARTFAILURE.114.001151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ducker GS, Rabinowitz JD. One-carbon metabolism in health and disease. Cell Metab 25: 27–42, 2017. doi: 10.1016/j.cmet.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gilley SP, Weaver NE, Sticca EL, Jambal P, Palacios A, Kerns ME, Anand P, Kemp JF, Westcott JE, Figueroa L, Garcés AL, Ali SA, Pasha O, Saleem S, Hambidge KM, Hendricks AE, Krebs NF, Borengasser SJ. Longitudinal changes of one-carbon metabolites and amino acid concentrations during pregnancy in the Women First Maternal Nutrition Trial. Curr Dev Nutr 4: nzz132, 2020. doi: 10.1093/cdn/nzz132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schober FA, Moore D, Atanassov I, Moedas MF, Clemente P, Végvári Á, Fissi NE, Filograna R, Bucher AL, Hinze Y, The M, Hedman E, Chernogubova E, Begzati A, Wibom R, Jain M, Nilsson R, Käll L, Wedell A, Freyer C, Wredenberg A. The one-carbon pool controls mitochondrial energy metabolism via complex I and iron-sulfur clusters. Sci Adv 7: eabf0717, 2021. doi: 10.1126/sciadv.abf0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Redondo-Angulo I, Mas-Stachurska A, Sitges M, Tinahones FJ, Giralt M, Villarroya F, Planavila A. Fgf21 is required for cardiac remodeling in pregnancy. Cardiovasc Res 113: 1574–1584, 2017. doi: 10.1093/cvr/cvx088. [DOI] [PubMed] [Google Scholar]
- 77.Tran DH, Wang ZV. Glucose metabolism in cardiac hypertrophy and heart failure. J Am Heart Assoc 8: e012673, 2019. doi: 10.1161/JAHA.119.012673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cortassa S, Caceres V, Bell LN, O’Rourke B, Paolocci N, Aon MA. From metabolomics to fluxomics: a computational procedure to translate metabolite profiles into metabolic fluxes. Biophys J 108: 163–172, 2015. doi: 10.1016/j.bpj.2014.11.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sonanez-Organis JG, Godoy-Lugo JA, Hernández-Palomares ML, Rodríguez-Martínez D, Rosas-Rodríguez JA, González-Ochoa G, Virgen-Ortiz A, Ortiz RM. HIF-1alpha and PPARgamma during physiological cardiac hypertrophy induced by pregnancy: transcriptional activities and effects on target genes. Gene 591: 376–381, 2016. doi: 10.1016/j.gene.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 80.Oleynikov IP, Azarkina NV, Vygodina TV, Konstantinov AA. Interaction of cytochrome c oxidase with steroid hormones. Cells 9: 2211, 2020. doi: 10.3390/cells9102211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chouchani ET, Methner C, Buonincontri G, Hu CH, Logan A, Sawiak SJ, Murphy MP, Krieg T. Complex I deficiency due to selective loss of Ndufs4 in the mouse heart results in severe hypertrophic cardiomyopathy. PLoS One 9: e94157, 2014. doi: 10.1371/journal.pone.0094157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mason MG, Nicholls P, Wilson MT, Cooper CE. Nitric oxide inhibition of respiration involves both competitive (heme) and noncompetitive (copper) binding to cytochrome c oxidase. Proc Natl Acad Sci USA 103: 708–713, 2006. doi: 10.1073/pnas.0506562103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Monni G, Atzori L, Corda V, Dessolis F, Iuculano A, Hurt KJ, Murgia F. Metabolomics in prenatal medicine: a review. Front Med (Lausanne) 8: 645118, 2021. doi: 10.3389/fmed.2021.645118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Walejko JM, Koelmel JP, Garrett TJ, Edison AS, Keller-Wood M. Multiomics approach reveals metabolic changes in the heart at birth. Am J Physiol Endocrinol Metab 315: E1212–E1223, 2018. doi: 10.1152/ajpendo.00297.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Clapp JF 3rd, Capeless E. Cardiovascular function before, during, and after the first and subsequent pregnancies. Am J Cardiol 80: 1469–1473, 1997. doi: 10.1016/S0002-9149(97)00738-8. [DOI] [PubMed] [Google Scholar]
- 86.Simmons LA, Gillin AG, Jeremy RW. Structural and functional changes in left ventricle during normotensive and preeclamptic pregnancy. Am J Physiol Heart Circ Physiol 283: H1627–H1633, 2002. doi: 10.1152/ajpheart.00966.2001. [DOI] [PubMed] [Google Scholar]
- 87.Hilfiker-Kleiner D, Struman I, Hoch M, Podewski E, Sliwa K. 16-kDa prolactin and bromocriptine in postpartum cardiomyopathy. Curr Heart Fail Rep 9: 174–182, 2012. doi: 10.1007/s11897-012-0095-7. [DOI] [PubMed] [Google Scholar]
- 88.Murata K, Ishida J, Ishimaru T, Mizukami H, Hamada J, Saito C, Fukamizu A. Lactation is a risk factor of postpartum heart failure in mice with cardiomyocyte-specific apelin receptor (APJ) overexpression. J Biol Chem 291: 11241–11251, 2016. doi: 10.1074/jbc.M115.699009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ho KL, Zhang L, Wagg C, Al Batran R, Gopal K, Levasseur J, Leone T, Dyck JR, Ussher JR, Muoio DM, Kelly DP, Lopaschuk GD. Increased ketone body oxidation provides additional energy for the failing heart without improving cardiac efficiency. Cardiovasc Res 115: 1606–1616, 2019. doi: 10.1093/cvr/cvz045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Horton JL, Davidson MT, Kurishima C, Vega RB, Powers JC, Matsuura TR, Petucci C, Lewandowski ED, Crawford PA, Muoio DM, Recchia FA, Kelly DP. The failing heart utilizes 3-hydroxybutyrate as a metabolic stress defense. JCI Insight 4: e124079, 2019. doi: 10.1172/jci.insight.124079. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figs. S1–S12: https://doi.org/10.6084/m9.figshare.19651137.