Abstract
Background/purpose
The temporomandibular joint (TMJ) is a bi-arthrodial joint that is composed of the temporal bone glenoid fossa and the condylar head of the mandible both having fibrocartilaginous articular surfaces. Functional overloading of the TMJ is the main cause of TMJ osteoarthritis (TMJ OA) disease. The aim of this study was to establish immortalized TMJ fibrocartilage cell clones to provide enough cells to adequately investigate the molecular mechanisms studies of TMJ OA.
Materials and methods
We have isolated temporomandibular condyle chondrocytes from adult Sprague Dawley rat. The cells were cultured and immortalized by treating with Y-27632, a well-characterized inhibitor of Rho-Associated Kinase (ROCK). Clones were characterized on the basis of cell morphology and analyses of marker gene expression through 45 passages.
Results
Cells from the condylar fibrocartilage of the TMJ were successfully immortalized by ROCK inhibitor, retaining a consistent cuboidal cell morphology and the expression of several cell markers of polymorphic cell fate. In addition, they retained phenotype features similar to the primary parental TMJ fibrocartilage cells when the cells were challenged with different cytokines and growth factors.
Conclusion
These studies establish a novel immortalized cell line through ROCK inhibitor Y-27632, that retains the polymorphic phenotype of primary cell lines from TMJ fibrocartilage chondrocyte cell through a high number of passages, serving as a valuable preclinical resource for mechanistic in vitro assessment of TMJ health, disease, and regeneration.
Keywords: Rho-associated kinase inhibitor, Immortalization, TMJ fibrocartilage cell
Introduction
The temporomandibular joint (TMJ) is a bi-arthrodial joint that is composed of the temporal bone glenoid fossa and the condylar head of the mandible both having fibrocartilaginous articular surfaces. The joint is divided into two synovial lined cavities by an articular disc and enclosed in a fibrous synovial lined capsule. It provides unique movements such as rotation and translation during mandibular function.1 TMJ chondrocytes play an important anatomical role in maintaining normal mandibular function.2 Structurally, TMJ condylar cartilage has characteristics common to both hyaline and fibro-cartilaginous which are separated by a thin proliferative zones. The deeper layer is rich in flattened mature chondrocytes with extracellular production of collagen and aggrecan.3 The main causes of TMJ osteoarthritis are mechanical functional overloading, micro-trauma, macro-trauma, and parafunctional oral habits involving masticatory muscle hyperfunction.4
TMJ osteoarthritis (TMJ OA) is characterized by variable degrees of cartilage destruction, inflammation, and sub-chondral bone sclerosis that can cause pain and limited jaw mobility. Condylar cartilage degeneration begins as chondrocyte cluster formation, cartilage erosion, vertical fissure formation and matrix loss, with end stage disease resulting in high levels of hypocellularity.5 During the progression of TMJ OA, pro-inflammatory cytokines such as IL-1β and TNFα damage the TMJ fibrocartilage, altering the metabolism of articular chondrocytes.6 The abnormal secretion of disintegrins and matrix metalloproteinases (MMPs) with thrombospondin motifs (Adamts) contribute to the proteolysis and degradation of the fibrocartilage extracellular matrix (ECM).7
Since TMJ OA is the most prevalent joint-related disease that causes suffering and distress to millions of patients, it is important to investigate and understand the molecular mechanisms associated with this disease in order to determine treatment.8 However, problems such as limited access to intact TMJ chondrocytes derived from mice or human, the loss of original phenotype during cell culture, and the fact that these cells cannot recapitulate the heterogeneity of the in vivo mandibular condylar cartilage, makes investigating the molecular mechanism of TMJ OA difficult.9,10 The aim of this study is to establish immortalized TMJ fibrocartilage cell clones to provide enough cells to adequately investigate the molecular mechanisms studies of TMJ OA as well as to provide a valuable resource to understanding progression of this disease, ultimately leading to prevention and future therapeutic strategies.
In previous studies of TMJ cell lines, disc cells from TMJ were immortalized by transfection of human telomerase reverse transcriptase DNA and characterized as chondrocyte-like and fibroblast-like clones with retained multi-differentiation potential up to 50 passages.11 Since genetic mice are most commonly used to study TMJ OA molecular mechanisms, rodent TMJ chondrocytes contribute to the tools used to study growth factor signaling pathways. Therefore, we established immortalized TMJ fibrocartilage chondrocytes cell lines from rats to provide more cell for study. In a previous study, we were successful in reliably and efficiently immortalizing NP and AF cells using inhibitor of Rho-associated kinase (ROCK) and proven ROCK.12 ROCK inhibitor, Trans-4-[(1R)-aminoethyl]-N-(4-pyridinyl) cyclohexanecarboxamide dihydrochloride (Y-27632), is the first small molecule ROCK inhibitor. It inhibits ROCK1 with 140 nM Ki selectively13 and has many cellular functions related to cell adhesion, cell motility, actin cytoskeleton organization and anti-apoptosis.13 The goal of this work was to investigate whether ROCK inhibitor can immortalize primary TMJ chondrocytes and characterize the immortalized TMJ fibrocartilage chondrocytes by analyzing cell shape, gene and protein expression changes.
Materials and methods
Cell culture of rat TMJ fibrocartilage cells
The fibrocartilage chondrocytes were isolated from TMJ condylar cartilage of 2-month-old Sprague Dawley rats weighing between 250 g and 300 g (n = 5). First, the chondrocytes were digested with 0.25% trypsin for 20 min, followed by 0.2% type II collagenase (Sigma–Aldrich, Inc. St. Louis, MO, USA) digestion for 2 h. The TMJ fibrocartilage chondrocytes were resuspended in Dulbecco's modified Eagle's medium (Thermofisher scientific, Waltham, MA, USA) containing 10% fetal bovine serum (Thermofisher scientific), 1% (vol/vol) penicillin, and streptomycin. Next, the TMJ chondrocytes were cultured in a humidified 37 °C constant temperature atmosphere containing 5% CO2 for 3 days. Media changes occurred every 72 h in the presence of 10 μM ROCK inhibitor Y-27632 (Sigma–Aldrich, Inc.) and when cells were subcultured by trypsinization and passaged 1:3 onto 60 mm culture dishes. The morphology of TMJ chondrocyte cells were observed microscopically. After 45 passages, cells were transferred to 96 well plate for single clone selection and several clones were analyzed to determine whether their characteristics were comparable with the early passage cell morphology and gene profile. All experiments in this study were carried out with the recommendation in the Guide for the Care and Use of Laboratory Animals of the National Institute of Health, USA. The procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Rush University Medical Center (Chicago).
Animal
Agc1-CreERT2 transgenic mice14 were obtained from Jackson Laboratories (Bar Harbor, ME, USA). β-catenin(ex3)flox/flox mice were originally reported by Harada et al.15 and we have used these mice in our previous studies.16 β-cat(ex3)AgcCreER mice and the Cre-negative littermates were generated. Twelve 2-week-old mice were divided into two groups, 6 mice per group. Tamoxifen (Sigma–Aldrich, Inc.) was administered by intraperitoneal (I.P.) injection (1 mg/10 g body weight for 5 consecutive days) for β-cat(ex3)AgcCreER mice group. Corn oil was injected intraperitoneally to the Cre-negative group, using the identical volume. We demonstrated in previous studies that this dosing regimen could efficiently induce Cre-mediated recombination and activate β-catenin signaling in condylar chondrocytes.17 The animal protocol of this study has been approved by the IACUC of the Rush University and all experimental methods and procedures were carried out in accordance with the approved guidelines.
Immunohistochemistry
The mouse skulls were dissected, and samples containing TMJ tissue from β-cat(ex3)Agc1CreER mice and Cre-negative control mice were fixed in 10% neutral-buffered formalin (VWR, Atlanta, GA, USA) for 3 days, followed by decalcification with formic acid (Decal Chemical Corp. Suffern, NY, USA) for 14 days. Samples were processed and embedded in paraffin, and 3-μm-thick mid-sagittal sections were used for immunostaining. The paraffin sections were baked at 65 °C overnight. Slides were then deparaffinized and rehydrated. Dako endogenous blocking reagent (Dako, Carpinteria, CA, USA) was then used to quench endogenous peroxidase for 15 min. Non-specific binding sites were blocked with 1:10 normal horse/goat serum (Vector Laboratories, Burlingame, CA, USA) for 30 min at room temperature. Primary antibodies: 1:500 dilution of SOX9 (Abcam, Waltham, MA, USA), 1:250 dilution of β-catenin (BD Biosciences, Franklin Lakes, NJ, USA) were added, and the slides were incubated at 4 °C overnight. For IHC assays, the secondary biotinylated goat anti-mouse or rabbit antibodies (Vector Laboratories) at the dilution of 1:200 was added for 30 min on the second day, followed by incubation with 1:250 streptavidin (Thermofisher Scientific) for 30 min. Results were analyzed using an Olympus BX43 upright microscope.
Western blotting
After washing with PBS twice, immortalized cells were lysed using RIPA buffer (10 mM Tri-HCl, pH 7.4, 0.01% sodium dodecyl sulfate (SDS), and 0.1% Nonidet P-40 with protease inhibitors). The lysate protein concentration was measured by BCA protein assay and standardized by total protein using 3× sample buffer (Bio-Rad, Hercules, CA, USA). The samples were separated by SDS-10% polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. Importantly, the protein on the membrane should be blocked with milk in PBS, treated with primary antibody, washed and incubated with horseradish peroxidase-conjugated secondary antibody. After following washes, the protein bands could be visualized by ECL detection kit (Thermofisher Scientific) under X-ray exposure. Afterwards, the membrane could be re-probed using internal reference primary antibody after incubation with stripping buffer (Abcam). The primary antibodies used were rabbit anti-SOX9 antibody (Abcam), anti-β-catenin antibody (BD Biosciences), anti-α-tubulin (Santa Cruz, Santa Cruz, CA, USA) and anti-β-actin antibody (Santa Cruz).
Measurement of mRNA expression
Total RNA extracted from articular TMJ fibrocartilage chondrocytes was prepared using RNeasy Plus Mini Kit (QIAGEN, Germantown, MD, USA) according to the manufacturer's protocol. cDNA was synthesized by iScripts cDNA synthesis kit (Bio-rad). The specific primers for each RNA, SYBR Master Mix and Bio-Rad CFX96 system for Quantitative polymerase chain reaction (qPCR) were performed. Delta Ct value of each sample and GAPDH was calculated and record.
Gene expression analysis
Genes relevant to metabolism and proliferation of TMJ fibrocartilage chondrocytes were analyzed by RT-PCR. Comparison mRNA expression of specific genes between early and late passage cell were performed. Specific PCR primer names and sequences for real-time PCR are listed in Table 1.
Table 1.
Primer sequences for qPCR.
| Primers | Forward (5′→3′) | Reverse (5′→3′) |
|---|---|---|
| Aggrecan | AGGATGGCTTCCACCAGTGC | TGCGTAAAAGACCTCACCCTCC |
| Col2a1 | CCTGGACCCCGTGGCAGAGA | CAGCCATCTGGGCTGCAAAG |
| SOX9 | TAAATTCCCAGTGTGCATCC | GCACCAGGGTCCAGTCATA |
| β-catenin | GACAGAGTTGCTCCACTCCA | TGGCTTGTCCTCAGACATTC |
| Adamts5 | GGCTGTGGTGTGCTGTG | CTGGTCTTTGGCTTTGAAC |
| MMP13 | GCAGCTCCAAAGGCTACAA | CATCATCTGGGAGCATGAAA |
| TGF-β | CTTTGTACAACAGCACCCGC | TAGATTGCGTTGTTGCGGTC |
| IGF-1 | ATGAGCGCACCTCCAATAAAGA | ACGAACTGAAGAGCGTCCAC |
| GAPDH | CCACAGTCCATGCCATCAC | TCCACCACCCTGTTGCTGTA |
Abbreviations: Col2a1, Collagen, type II, alpha 1; SOX9, SRY-Box Transcription Factor 9; Adamts5, The abnormal secretion of disintegrins and matrix metalloproteinases (MMPs) with thrombospondin motifs 5; MMP13, Matrix metalloproteinase 13; TGF-β, Transforming growth factor beta; IGF-1, Insulin-like growth factor 1; GAPDH, Glyceraldehyde-3-Phosphate Dehydrogenase.
Results
Telomerase reverse transcriptase (TERT) expression and cell morphology in immortalized TMJ fibrocartilage cells
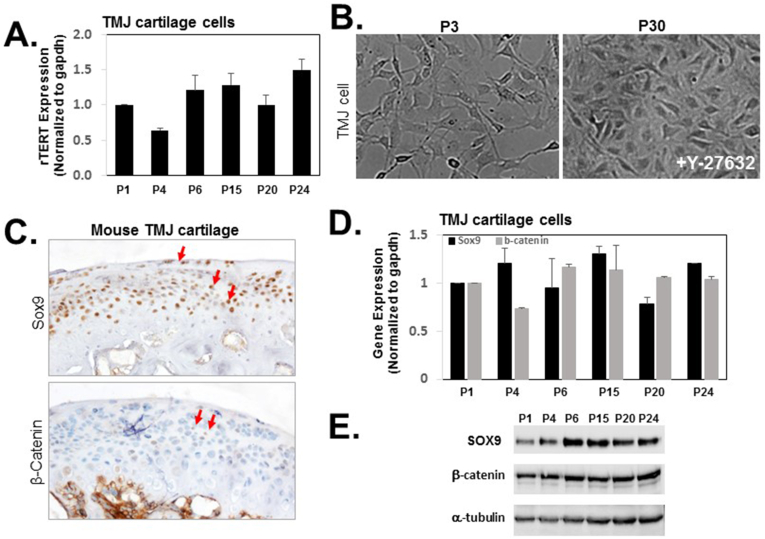
Telomerase reverse transcriptase (TERT) is the catalytic subunit of telomerase, which could limit telomerase activity in multiple rounds of replication. Therefore, overexpression of rTERT (rat TERT) could immortalize normal cells endowing self-renewal properties against replicative senescence.18 Quantitative RT-PCR analysis showed the level of rTERT mRNA increased with the passaging of TMJ fibrocartilage cells in the presence of Y-27632 (Fig. 1A). The fibrocartilage cells from TMJ were isolated from the TMJ condylar cartilage of 2-month-old adult rats. The cells were grown in the presence of 10 μM Y-27632 from day 3 forward. These cells continued to proliferate with more than 45 passages. In early passages, the cells were actively dividing. The shape of early passage cells were relatively small, separate and polygonal. After treatment of Y-27632 after several passages, the filapodia and lamellipodia were not longer and extended, cell density was higher, but the polygon shape was still highly similar to early passage (Fig. 1B P3 and P30).
Figure 1.
Characterization of Y-27632-immortalized TMJ fibrocartilage cells. Cells were expanded in monolayer culture in the presence of 10 μM Y-27632 at the passage indicated and TERT mRNA levels from TMJ fibrocartilage cells were quantitated by real-time qRT-PCR (A) and representative phase-contrast images of Y-27632-immortalized TMJ fibrocartilage cells (B). (C) Representative and comparable histological sections from TMJs were stained. Immunohistochemistry (brown) showed expression of SOX9 (upper panel) and β-catenin (lower panel). The mRNA level of SOX9 and β-catenin were analyzed by real-time qRT-PCR at the passage indicated (D) and the protein level of SOX9 and β-catenin were analyzed on cell lysates from the indicated passages by western blotting (E). Equal sample loading was also confirmed by detecting α-tubulin. Data for a typical experiment are presented and experiments were performed more than three times from different passages with similar results. TERT indicates telomerase reverse transcriptase. Each bar represents the mean of duplicated samples with standard deviation.
Passage mediated changes in SOX9 and β-catenin expression
The condylar fibrocartilage from TMJ consists of superficial, middle (polymorphic zone and flattened chondrocytes) and deep layers (hypertrophic zone).19,20 SOX9 protein plays a key role in rat skeleton development by binding to specific regions of DNA that control bone growth by regulating the extracellular matrix,21 while Sox9 is highly expressed in the middle layers of TMJ condylar cartilage.19 β-catenin is an essential part of Wnt/β-catenin pathway and is extremely important in development and maintenance of bone.22
In our previous report, β-catenin expression was significantly increased in the superficial, middle and deep layers of TMJ condylar cartilage by the conditional activation of β-catenin mice (β-cateninAgc1CreER), resulting in condylar cartilage defects compared to the Cre negative mice.17 We next investigated the expression of SOX9 and β-catenin in mouse condylar cartilage to determine whether immortalized cells retain their original characteristics. As shown in Fig. 1C, SOX9 was present throughout articular cartilage, but β-catenin was only detectable in deep layers, including in hypertrophic chondrocytes. To determine if immortalized TMJ fibrocartilage cells maintain the expression level of Sox9 and β-catenin, we have examined gene expression and protein expression. As expected, immortalized TMJ fibrocartilage cells exhibited sustained SOX9 and β-catenin expression at the level of mRNA and protein undergoing passages (Fig. 1D and E).
Response of the cells to growth factors
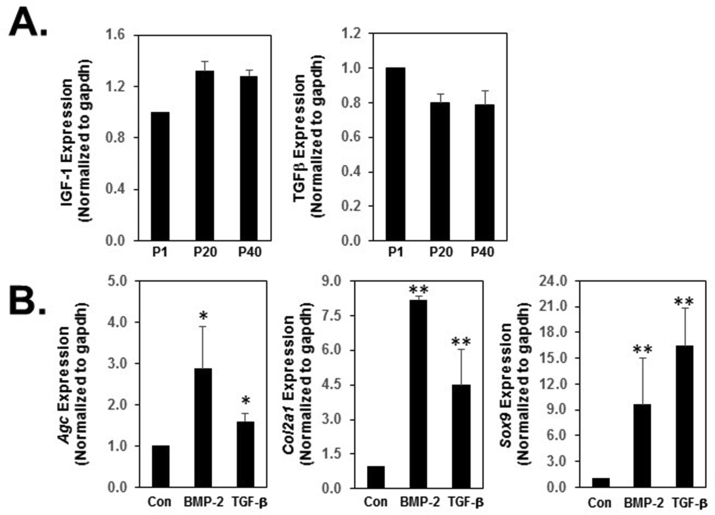
Transforming growth factor-β (TGF-β) and insulin-like growth factor 1 (IGF-1) have been shown to stimulate proliferation of chondrocyte and endotheliocyte and matrix synthesis in vitro.23 In addition, TGF-β1 and IGF-1 promote cellular proliferation and secretion of type I collagen and GAGs in vitro in a bioengineered mandibular condyle.24 To determine changes in cell proliferation in Y-27632 immortalized TMJ fibrocartilage cells, we examined the expression of TGF-β and IGF-1 between early and late passage TMJ fibrocartilage cells. We found that TMJ fibrocartilage cells continued to proliferate even at passage 40 in the presence of Y-27632. There was no significant changes in proliferation rate with Y-27632 treatment. TGF-β expression between early and late passages was not significantly changed, while the IGF-1 levels slightly increased in the late passage cells comparing to the early passage cells (Fig. 2A).
Figure 2.
IGF-1 and TGF-β mRNA expression and specific marker gene expression profile in immortalized TMJ fibrocartilage cells. (A) Analyses of gene expression levels for IGF-1 and TGF-β were performed by qRT-PCR. IGF-1 and TGF-β mRNA levels were compared between early passage and late passage cells with immortalized TMJ fibrocartilage cells. (B) The effect of growth factors on immortalized TMJ fibrocartilage cells. The immortalized TMJ fibrocartilage cells on late passage were cultured in the absence or presence of 100 ng/ml of BMP-2 or 10 ng/ml of TGF-β in normal medium for 24 h. Relative gene expression analysis of Aggrecan, Col2a1 and Sox9 was performed by quantitative qRT-PCR. Agc, Col2a1 and Sox9 mRNA were detected after treatment with TGF-β and the level of mRNA of Col2a1 and Sox9 were highly increased by TGF-β treatment. BMP-2 increased the level of Agc, Col2a1 and Sox9 mRNA in immortalized TMJ fibrocartilage cells. IGF indicates Insulin-like growth factor 1; TGF, transforming growth factor; BMP2, Bone morphogenetic protein 2; Agc, Aggrecan; Col2a1, type II collagen. All experiments were normalized to GAPDH and statistical significance was assessed by student t-test (∗P < 0.05, ∗∗P < 0.0001).
Since Bone Morphogenetic Protein-2 (BMP-2) signaling has been associated with destructive TMJ arthritis25 and conditionally deleted BMP2 from aggrecan expressing TMJ cartilage cells, both resulting in accelerated degeneration of the TMJ,26 we investigated whether TGF-β or BMP-2 regulates extracellular matrix production in immortalized TMJ cells. TMJ cells were treated with 10 ng/ml of BMP-2 or 10 ng/ml of TGF-β for 24 h to examine the response of immortalized TMJ fibrocartilage cells to cytokines. We found either BMP2 or TGF-β increased aggrecan and type II collagen expression, which was predominant in hypertrophic zone, as well as increased expression of Sox9 compared to control (Fig. 2B). These results suggest that the TGF-β or BMP-2 successfully regulates extracellular matrix in immortalized TMJ fibrocartilage cells, indicating that those effect are consistent with in vivo reported data.24,26
Response of the cells to cytokines
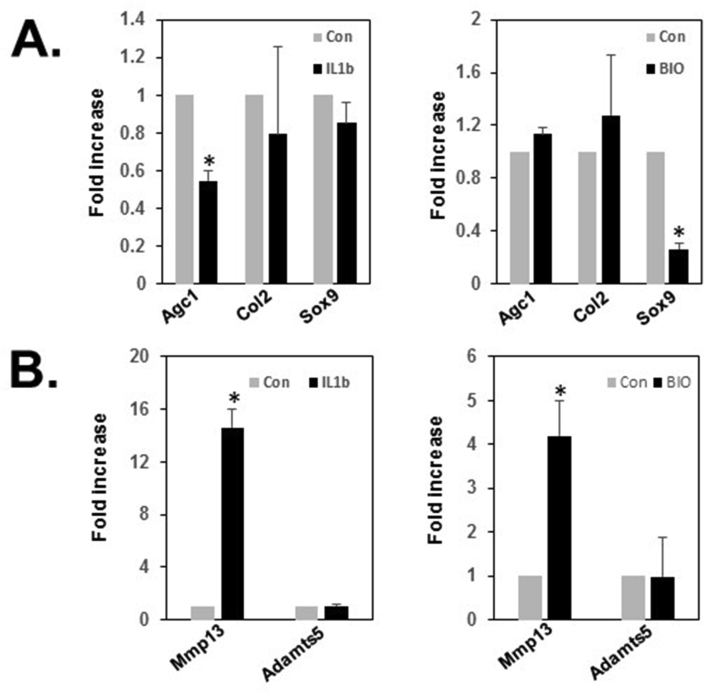
To examine the responses of immortalized cells to cytokines, TMJ cells were treated for 24 h with 10 ng/ml of IL-1β, a cytokine highly expressed in arthritic joints. IL-1β induces TMJ condylar cartilage damage by enhancing MMP-13 production and mechanical loading reduced IL-1β−induced MMP-13 gene expression,27 suggesting that IL-1β possibly modifies expression or degradation of matrix proteins. As expected, 24 h of IL-1β stimulation in immortalized TMJ fibrocartilage cells reduced aggrecan, type II collagen and Sox9 gene expression, but enhanced MMP13 expression by 15-fold, but only in MMP13 expression not Adamts5 expression (Fig. 3A and B).
Figure 3.
Expression of cartilage markers and degradation enzymes. (A) The TMJ fibrocartilage cells on late passage were cultured in the absence or presence of 10 ng/ml IL-1β or 1 μM BIO, GSK3β inhibitor for 24 h. IL1β highly decreased the level of expression of Agc but BIO decreased Sox9 mRNA. (B) The mRNA level of Mmp13 and Adamts5, degradation enzymes that are related to the onset of articular cartilage degeneration, was determined using qRT-PCR after 24 h in the absence or presence of 10 ng/ml IL-1β or 1 μM BIO. Mmp13 expression was increased by IL1β or BIO treatment but Adamts5 was not changed after treatment. Mmp13 indicates Matrix Metalloproteinase 13; Adamts5, A disintegrin and metalloproteinase with thrombospondin motifs 5. Experiments were performed more than three times from different passages and normalized to GAPDH and statistical significance was assessed by student t-test (∗P < 0.0001).
In previous studies, we demonstrated that activation of β-catenin in aggrecan expressing TMJ cartilage showed TMJ OA-like phenotype accompanied by cartilage damage and enhanced expression of MMP13 and Adamts5.17 Next, we examined the expression of matrix protein and degradation enzyme in the presence or absence of BIO, a β-catenin-mediated Wnt signaling activator in immortalized TMJ fibrocartilage cells. In cartilage marker analysis, the expression of matrix proteins including aggrecan and type II collagen was not altered by BIO treatment, but Sox9, a regulator of aggrecan and type II collagen, was significantly decreased by BIO treatment in immortalized fibrocartilage cells. However, after 24 h of 1 μM of BIO stimulation, MMP13 gene expression was upregulated by 4.2-fold (p < 0.001), but Adamts5 gene expression was not changed in the immortalized fibrocartilage cells (Fig. 3B). These data suggest that immortalized TMJ fibrocartilage cells may harbor features of primary TMJ fibrocartilage cells in the expression of matrix proteins and degradation enzymes, MMP13, However, Adamts5 expression should be confirmed with more specific layer-dependent cell type analysis.
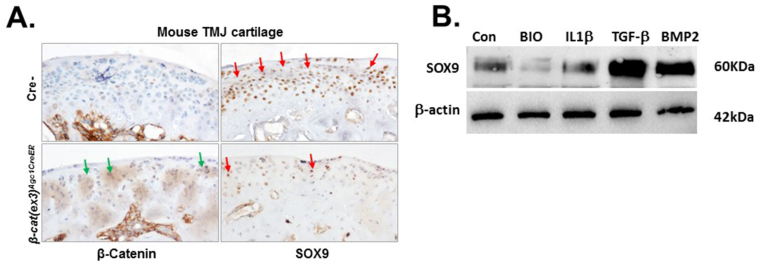
Validating β-catenin and Sox9 expressing in the immortalized cell line with in vivo data
To confirm that the immortalized TMJ fibrocartilage cells maintain the characteristics of primary cells, the protein level of β-catenin and SOX9 was discerned by use of immunohistochemistry and western blot analysis. Up-regulation of β-catenin protein in aggrecan expressing TMJ cartilage resulted in a reduction in the number of SOX9 positive cells (Fig. 4A lower), consistent with the mRNA expression of Sox9 following BIO treatment (Fig. 3A). As expected, BIO treatment significantly reduced the protein level of SOX9 in immortalized fibrocartilage cells (Fig. 4B). Further, the protein and gene expression profile of SOX9 by treatment of IL-1β, TGF-β, and BMP2 in immortalized TMJ fibrocartilage cells illustrates that immortalized TMJ fibrocartilage cells had similar expression pattern (Figure 3, Figure 3 and 4B). These results indicated that the regulatory mechanism of Sox9 expression in the level of gene and protein was similar between the primary cells and immortalized cells.
Figure 4.
SOX9 expression was significantly decreased in TMJ cartilage of 3-month old β-cat(ex3)Agc1CreER and immortalized TMJ fibrocartilage cells with BIO treatment. (A)β-cat(ex3)Agc1CreER mice were generated by crossing Agc1-CreERT2 mice with β-catenin(ex3)floxflox mice. TMJ sample were harvested from 3-month old mice after they were injected with tamoxifen at the age of 2 weeks for 5 consecutive days. Immunohistochemical (IHC) analysis showed that β-catenin was highly expressed in TMJ cartilage of β-cat(ex3)Agc1CreER mice compared with Cre negative mice (green arrows) but SOX9 expression was reduced in TMJ cartilage of β-cat(ex3)Agc1CreER mice in TMJ cartilage of β-cat(ex3)Agc1CreER mice (red arrows). (B) The protein level of SOX9 were analyzed on cell lysates from immortalized TMJ fibrocartilage cells with the indicated treatment for 24 h by western blotting. Data illustrate that activation of canonical WNT-signaling by BIO significantly downregulated the expression of SOX9 but TGF-β or BMP2 highly upregulated the expression of SOX9. Equal sample loading was also confirmed by detecting β-actin. Data for a typical experiment are presented and experiments were performed more than three times from different passages with similar results.
Discussion
TMJ functional overloading and masticatory muscle hyperactivity are the main causes of TMJ OA.4 TMJ fibrocartilage differs biologically and histologically from hyaline cartilage. TMJ fibrocartilage is considered one of the most difficult tissues to regenerate. In cases of end-stage TMJ OA, treatments include arthroscopy, arthrocentesis, and alloplastic total joint replacement.28 TMJ OA along with reflex masticatory muscle responses can contribute to signs and symptoms of tinnitus, headache, vertigo and neck pain.29 However, the relationship of the cellular mechanisms of TMJ OA are still not well understood.
TMJ OA mechanism research is challenging because it is difficult to obtain a sufficient quantity of the appropriate cell lines that have similar properties to the primary disease cells. In this study, we successfully established TMJ cell lines using ROCK inhibitor, Y-27632. Application of Y-27632 markedly reduced cell apoptosis and increased clone efficiency in dissociated human embryonic stem cells.30
First, we determined changes in rTERT in immortalized TMJ fibrocartilage cells and found that rTERT mRNA expression was upregulated in immortalized TMJ cells to maintain the telomere caps throughout the multiple cell divisions. Since it is possible that immortalized cells may change their properties and functions compared to the primary cells, we examined their morphological characteristics. We found that immortalized TMJ fibrocartilage cells could be passed over 50 passages without losing their proliferation and phenotypic properties. The morphology of TMJ cells remained polygonal in shape and there was no significant phenotypic differences when comparing early with late passage.
Furthermore, we detected the level of transcription factors Sox9 and β-catenin, which are important for Extracellular Matrix (ECM) expression and degradation in cartilage, in immortalized TMJ cells, and did not find significant differences in Sox9 and β-catenin expression between early and late passages of TMJ cells. The results suggest that immortalized TMJ fibrocartilage cells retain similar level of Sox9 and β-catenin to regulate ECM homeostasis in TMJ cartilage.
Growth factors can promote the differentiation and proliferation of cells and support extracellular matrix synthesis and mineralization.31 IGF-1 and TGF-β are known to promote cellular proliferation as well as collagen synthesis and GAGs in vitro in a bioengineered mandibular condyle.24 Using quantitative RT-PCR assay, we found that IGF-1 and TGF-β expression was not altered in both the early and late passages of TMJ fibrocartilage cells, but IGF-1 was slightly upregulated in late passage cells, and TGF-β remain high after 40 passages. These results suggest that TMJ chondrocytes could respond to both growth factor signaling and stimulation, while additionally continuing their proliferation, differentiation and apoptosis functions. We have evaluated cell growth rate in both the early and late passages of TMJ fibrocartilage cells by examining the confluency. Both cells reached full confluency with similar time period after splitting cells, suggesting that the proliferation rate of this cell line was not significantly changed by immortalization. TGF-β is known to promote collagen and fiber synthesis by increasing PRG4 mRNA, protein expression and MAPK-ERK respectively in condylar chondrocytes.32,33 In addition, BMP2 regulates endochondral bone formation and chondrogenesis.25,34 Since ECM proteins, which in cartilage contain mostly collagen fibers and proteoglycans, have a well-established role in TMJ function, we examined the effect on expression of ECM such as aggrecan and col2a1 by treatment of BMP2 or TGF-β. In our study, the immortalized TMJ fibrocartilage treated with BMP2 or TGF-β upregulated the expression of aggrecan and col2a1 and the transcription factor Sox9, as master regulator of aggrecan and col2a1, also increased by treatment of BMP2 or TGF-β suggesting that immortalized TMJ fibrocartilage cells could maintain the similar stimulating effect by growth factor in the production of proteoglycans and collagen fibers.
Mandibular condylar cartilage is a heterogeneous population of fibrochondrocytes within an ECM of collagens, proteoglycans and water. The ECM plays a key role in protecting the integrity of cartilage tissue. A characteristic of early-stage OA is the alteration of distribution or composition of the ECM, while in the late-stage of OA there is loss of ECM components.35 In support of this view, we examined the effect of several cytokines on immortalized TMJ cells. IL-1β is a destructive cytokines that affected TMJ extracellular matrix through regulation of MMPs. Upregulation of IL-1β contributes to TMJ cartilage breakdown.36 Our results showed IL-1β decreased expression of aggrecan and increased expression of MMP13. Phenotype changes in OA are always accompanied by ECM degradation, which finally results in cartilage destruction. β-catenin is a crucial role in cartilage formation and development which when excessively expressed could lead to TMJ OA phenotype.17 Our results showed that β-catenin was highly expressed in condylar cartilage from β-cat(ex3)Agc1CreER mice induced by Tamoxifen at 2-week-old mice compared with Cre negative mice, but Sox9 was decreased. The mechanism of Sox9 regulation by β-catenin is not fully understood. However, in vitro experiments with our immortalized cells, mRNA and protein data reveal that activation of the Wnt pathway through BIO treatment decreased Sox9 expression and upregulated Mmp13 expression suggesting that it was similar results to in vivo data. Our results further support the previous report that overactive Wnt signals disrupt fibrocartilage homeostasis causing degeneration and suppression of canonical Wnt signals promoting fibrocartilage stem cells in the TMJ condyle to differentiate into chondrocytes.37 In the present studies, we have not tested the transfection efficiency with this cell line. For future application to further determine the cell responsiveness to specific factors involved in TMJ pathogenesis, more detail investigations still remain to further characterize these immortalized cells. In addition, since the essential activity of the hypothalamic–pituitary–adrenal (HPA) axis is required in TMJ pathogenesis with stress and other etiological factors,38 we could possibly apply this cell line in studying the TMJ cell responsiveness to HPA axis related hormone.
Declaration of competing interest
All authors have no conflicts of interest relevant to this article.
Acknowledgements
This work was supported by the National Institutes of Health Grants (AG061460) and Searle Innovators Award. We would like to express our gratitude to Ms. Lily Yu for her help in processing and staining histological samples.
References
- 1.Chang C.L., Wang D.H., Yang M.C., et al. Functional disorders of the temporomandibular joints: internal derangement of the temporomandibular joint. Kaohsiung J Med Sci. 2018;34:223–230. doi: 10.1016/j.kjms.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Zhou Y., Shu B., Xie R., et al. Deletion of axin1 in condylar chondrocytes leads to osteoarthritis-like phenotype in temporomandibular joint via activation of beta-catenin and fgf signaling. J Cell Physiol. 2019;234:1720–1729. doi: 10.1002/jcp.27043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robert J., Hinton D.S.C. Regulation of growth in mandibular condylar cartilage. Semin Orthod. 2005;11:209–218. [Google Scholar]
- 4.Krisjane Z., Urtane I., Krumina G., et al. The prevalence of tmj osteoarthritis in asymptomatic patients with dentofacial deformities: a cone-beam ct study. Int J Oral Maxillofac Surg. 2012;41:690–695. doi: 10.1016/j.ijom.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Zhou S., Xie Y., Li W., et al. Conditional deletion of fgfr3 in chondrocytes leads to osteoarthritis-like defects in temporomandibular joint of adult mice. Sci Rep. 2016;6:24039. doi: 10.1038/srep24039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X.D., Zhang J.N., Gan Y.H., et al. Current understanding of pathogenesis and treatment of tmj osteoarthritis. J Dent Res. 2015;94:666–673. doi: 10.1177/0022034515574770. [DOI] [PubMed] [Google Scholar]
- 7.Chen D., Shen J., Zhao W., et al. Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Res. 2017;5:16044. doi: 10.1038/boneres.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Y.P., Zhang Z.Y., Wu Y.T., et al. Investigation of the clinical and radiographic features of osteoarthrosis of the temporomandibular joints in adolescents and young adults. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:e27–e34. doi: 10.1016/j.tripleo.2010.09.076. [DOI] [PubMed] [Google Scholar]
- 9.Yanoshita M., Hirose N., Okamoto Y., et al. Cyclic tensile strain upregulates pro-inflammatory cytokine expression via fak-mapk signaling in chondrocytes. Inflammation. 2018;41:1621–1630. doi: 10.1007/s10753-018-0805-8. [DOI] [PubMed] [Google Scholar]
- 10.Santoro A., Conde J., Scotece M., et al. Choosing the right chondrocyte cell line: focus on nitric oxide. J Orthop Res. 2015;33:1784–1788. doi: 10.1002/jor.22954. [DOI] [PubMed] [Google Scholar]
- 11.Park Y., Hosomichi J., Ge C., et al. Immortalization and characterization of mouse temporomandibular joint disc cell clones with capacity for multi-lineage differentiation. Osteoarthritis Cartilage. 2015;23:1532–1542. doi: 10.1016/j.joca.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oh C.D., Im H.J., Suh J., et al. Rho-associated kinase inhibitor immortalizes rat nucleus pulposus and annulus fibrosus cells: establishment of intervertebral disc cell lines with novel approaches. Spine (Phila Pa 1976) 2016;41:E255–E261. doi: 10.1097/BRS.0000000000001235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang T., Kang W., Du L., et al. Rho-kinase inhibitor y-27632 facilitates the proliferation, migration and pluripotency of human periodontal ligament stem cells. J Cell Mol Med. 2017;21:3100–3112. doi: 10.1111/jcmm.13222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henry S.P., Jang C.W., Deng J.M., et al. Generation of aggrecan-creert2 knockin mice for inducible cre activity in adult cartilage. Genesis. 2009;47:805–814. doi: 10.1002/dvg.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harada N., Tamai Y., Ishikawa T., et al. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J., Ma K., Yi D., et al. Nociceptive behavioural assessments in mouse models of temporomandibular joint disorders. Int J Oral Sci. 2020;12:26. doi: 10.1038/s41368-020-00095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hui T., Zhou Y., Wang T., et al. Activation of beta-catenin signaling in aggrecan-expressing cells in temporomandibular joint causes osteoarthritis-like defects. Int J Oral Sci. 2018;10:13. doi: 10.1038/s41368-018-0016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cukusic A., Skrobot Vidacek N., Sopta M., et al. Telomerase regulation at the crossroads of cell fate. Cytogenet Genome Res. 2008;122:263–272. doi: 10.1159/000167812. [DOI] [PubMed] [Google Scholar]
- 19.Ochiai T., Shibukawa Y., Nagayama M., et al. Indian hedgehog roles in post-natal tmj development and organization. J Dent Res. 2010;89:349–354. doi: 10.1177/0022034510363078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizoguchi I., Takahashi I., Nakamura M., et al. An immunohistochemical study of regional differences in the distribution of type I and type II collagens in rat mandibular condylar cartilage. Arch Oral Biol. 1996;41:863–869. doi: 10.1016/s0003-9969(96)00021-0. [DOI] [PubMed] [Google Scholar]
- 21.Symon A., Harley V. SOX9: a genomic view of tissue specific expression and action. Int J Biochem Cell Biol. 2017;87:18–22. doi: 10.1016/j.biocel.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Duan P., Bonewald L.F. The role of the wnt/β-catenin signaling pathway in formation and maintenance of bone and teeth. Int J Biochem Cell Biol. 2016;77:23–29. doi: 10.1016/j.biocel.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delatte M.L., Von den Hoff J.W., Nottet S.J., et al. Growth regulation of the rat mandibular condyle and femoral head by transforming growth factor-{beta}1, fibroblast growth factor-2 and insulin-like growth factor-i. Eur J Orthod. 2005;27:17–26. doi: 10.1093/ejo/cjh068. [DOI] [PubMed] [Google Scholar]
- 24.Kang H., Bi Y.D., Li Z.Q., et al. [Effect of transforming growth factor beta(1) and insulin-like growth factor-i on extracelluar matrix synthesis of self-assembled constructs of goat temporomandibular joint disc] Zhonghua Kou Qiang Yi Xue Za Zhi. 2011;46:541–546. doi: 10.3760/cma.j.issn.1002-0098.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Shirakura M., Kram V., Robinson J., et al. Extracellular matrix mediates bmp-2 in a model of temporomandibular joint osteoarthritis. Cells Tissues Organs. 2017;204:84–92. doi: 10.1159/000464102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Brien M.H., Dutra E.H., Mehta S., et al. Bmp2 is required for postnatal maintenance of osteochondral tissues of the temporomandibular joint. Cartilage. 2020 doi: 10.1177/1947603520980158. 1947603520980158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tabeian H., Betti B.F., Dos Santos Cirqueira C., et al. Il-1beta damages fibrocartilage and upregulates mmp-13 expression in fibrochondrocytes in the condyle of the temporomandibular joint. Int J Mol Sci. 2019;20:2260. doi: 10.3390/ijms20092260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dimitroulis G. Condylar morphology after temporomandibular joint discectomy with interpositional abdominal dermis-fat graft. J Oral Maxillofac Surg. 2011;69:439–446. doi: 10.1016/j.joms.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 29.Edvall N.K., Gunan E., Genitsaridi E., et al. Impact of temporomandibular joint complaints on tinnitus-related distress. Front Neurosci. 2019;13:879. doi: 10.3389/fnins.2019.00879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanabe K., Ueno M., Kamiya D., et al. A rock inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 31.Detamore M.S., Athanasiou K.A. Motivation, characterization, and strategy for tissue engineering the temporomandibular joint disc. Tissue Eng. 2003;9:1065–1087. doi: 10.1089/10763270360727991. [DOI] [PubMed] [Google Scholar]
- 32.Cheng J., Wang Y., Wang Z., et al. Differential regulation of proteoglycan-4 expression by il-1alpha and tgf-beta1 in rat condylar chondrocytes. Tohoku J Exp Med. 2010;222:211–218. doi: 10.1620/tjem.222.211. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y., Ke J., Long X., et al. Insulin-like growth factor-1 boosts the developing process of condylar hyperplasia by stimulating chondrocytes proliferation. Osteoarthritis Cartilage. 2012;20:279–287. doi: 10.1016/j.joca.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 34.Shi S., Wang C., Acton A.J., et al. Role of sox9 in growth factor regulation of articular chondrocytes. J Cell Biochem. 2015;116:1391–1400. doi: 10.1002/jcb.25099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Embree M.C., Kilts T.M., Ono M., et al. Biglycan and fibromodulin have essential roles in regulating chondrogenesis and extracellular matrix turnover in temporomandibular joint osteoarthritis. Am J Pathol. 2010;176:812–826. doi: 10.2353/ajpath.2010.090450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morel M., Ruscitto A., Pylawka S., et al. Extracellular matrix turnover and inflammation in chemically-induced tmj arthritis mouse models. PLoS One. 2019;14 doi: 10.1371/journal.pone.0223244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Embree M.C., Chen M., Pylawka S., et al. Exploiting endogenous fibrocartilage stem cells to regenerate cartilage and repair joint injury. Nat Commun. 2016;7:13073. doi: 10.1038/ncomms13073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salameh E., Alshaarani F., Hamed H.A., et al. Investigation of the relationship between psychosocial stress and temporomandibular disorder in adults by measuring salivary cortisol concentration: a case-control study. J Indian Prosthodont Soc. 2015;15:148–152. doi: 10.4103/0972-4052.158075. [DOI] [PMC free article] [PubMed] [Google Scholar]