Abstract
Interleukin-6 (IL-6) is a highly pleiotropic glycoprotein factor that can modulate innate and adaptive immunity as well as various aspects of metabolism, including glycolysis, fatty acid oxidation and oxidative phosphorylation. Recently, the expression and release of IL-6 is shown to be significantly increased in numerous diseases related to virus infection, and this increase is positively correlated with the disease severity. Immunity and metabolism are two highly integrated and interdependent systems, the balance between them plays a pivotal role in maintaining body homeostasis. IL-6-elicited inflammatory response is found to be closely associated with metabolic disorder in patients with viral infection. This brief review summarizes the regulatory role of IL-6 in immunometabolic reprogramming among seven viral infection-associated diseases.
Keywords: Interleukin-6, Immunometabolic reprogramming, Viral infection, COVID-19
1. Introduction
Immunity is a physiological function that involves immune surveillance, immune defense and immune homeostasis. And it enables the human body to recognize self and non-self components, thereby destroying and excluding foreign antigenic substances such as viruses [1], [2], [3].
Metabolism is a fundamental biological process responsible for biomass and bioenergy production. While immunity and metabolism are two highly integrated and interdependent systems, the balance between them plays a pivotal role in maintaining body homeostasis [4], [5], [6]. It has been shown that the complex cellular microenvironment has a significant impact on the metabolism of immune cells. Among all studies involving the effects of immunometabolism, those on obesity, type 2 diabetes and cardiovascular diseases have been most widely conducted. In recent years, studies have also found that immunometabolism may play a significant role in the occurrence and progression of viral infection. Notably, immunometabolic reprogramming caused by viral infection not only contributes to viral replication, but also antagonizes the host antiviral immune response [7], [8].
Interleukin-6 (IL-6) is one of the most important members of the cytokine network that is involved in the immune defense mechanism. The IL-6 pathway has emerged as a key pathway implicated in health immune regulation and immune dysregulation [9]. The pleiotropic activity of IL-6 makes it both pro- and anti-inflammatory function [10]. Viral infection and inflammation usually lead to an acute increase in the level of IL-6, which serves as one of the reliable indicators for stress response [11], [12]. Recent studies have demonstrated that IL-6 regulates various aspects of metabolism, including glucose uptake, glycolysis, fatty acid oxidation and oxidative phosphorylation [13], [14], [15], [16]. Moreover, the expression and release of IL-6 have been found to be significantly increased in numerous diseases related to virus infection, and this increase is positively correlated with the disease severity [17], [18], [19]. In this review, we summarize the regulatory role of IL-6 in immunometabolism among some virus infection-associated diseases. The classification and transmission routes of these viruses have some representativeness and commonality. Among them, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), human immunodeficiency virus (HIV), and Enterovirus 71 (EV71) are RNA viruses, whereas hepatitis B virus (HBV), human cytomegalovirus (HCMV), adenovirus (AdV), and Dengue viruses (DV) are DNA viruses. HBV, HIV, and HCMV are all transmissible through the blood, from mother to child, and by sexual transmission. Conversely, SARS-CoV-2, AdV, and EV71 can be all transmitted through the digestive tract, respiratory tract, and direct contact, whereas DV is transmitted through mosquito bites. Analyzing and comparing the role of IL-6 in similar or different types of viral infections can provide new insights into the target role of IL-6 in linking different viruses, immunity, and metabolism.
2. The structure and function of IL-6
IL-6 belongs to the interleukin family and has been identified as a 26-kD secreted protein [20]. IL-6 is a single-chain glycoprotein mainly composed of four α-helices and a C-terminal (amino acids 175–181) receptor binding site [20]. It has a tertiary structure with four α helixes containing 184 amino acids, as well as a helical structure with the C-terminal involved in binding to the receptor [21], [22]. The gene encoding human IL-6 is located on chromosome 7 and consists of four introns and five exons [22]. The precursor of human IL-6 has 212 amino acids, and, after excision of the N-terminal with 28 amino acids, the mature IL-6 consisting of 184 amino acids is formed [22]. The human IL-6 gene is 65% homologous to mouse IL-6, whereas the human IL-6 amino acid sequence is 42% homologous to that of mouse IL-6. The amino acid sequence of rat IL-6 is 93% and 58% identical to those of mature mouse and human IL-6, respectively [22], [23]. IL-6 has three receptor binding sites, one site for IL-6 binding receptor protein (IL-6R) and two for signal-transducing protein (gp130) [24]. IL-6 binds to its receptor to establish cell–cell communication (Fig. 1 ). IL-6R consists of IL-6Rα (also known as CD126 or gp80) and IL-6Rβ (also known as CD130 or gp130). Of the two, IL-6Rα is a specific high-affinity ligand-binding chain with a molecular weight of 80 kDa and is mainly distributed on the cell surface of hepatocytes, neutrophils, macrophages, and some lymphocytes [22]. The molecular weight of gp130 can range from 100 to 130 kDa after glycosylation. It functions as a signal transduction chain and is expressed on the surface of almost all cells, including heart, kidney, spleen, liver, lung, placenta, and brain cells [24]. IL-6 initially binds to IL-6Rα and then to gp130 to form a trimer, and the two trimer complexes connect to homodimerize gp130 and initiate signal transduction [24], [25].
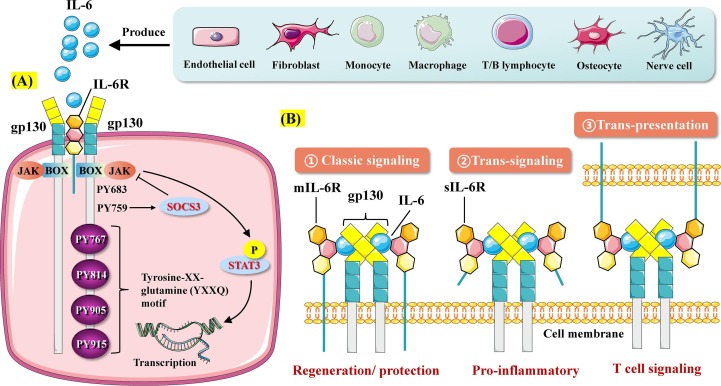
Fig. 1.
Three features of IL-6 action. A. IL-6 signaling. IL-6 produced by various cells such as lymphocytes binds to IL-6 receptor (IL-6R). IL-6 cannot transduce intracellular signals and needs the assistance of transmembrane protein gp130. The IL-6/IL-6R complex binds to gp130 to form a hexameric structure, which in turn induces Box/JAK activation. Activation of tyrosine 759 of gp130 mediates the negative feedback loop formed by SOCS3 and JAK. The terminal four tyrosine residues of gp130 (Y767, Y814, Y905 and Y915) form the YXXQ motif necessary for STAT3 activation. B. Three features of IL-6R signaling. IL-6R includes mIL-6R expressed on the cell membrane surface and sIL-6R present in the circulatory system. IL-6/mIL-6R signaling is called classic signaling and mainly mediates the regenerative and protective effects. IL-6/sIL-6R signaling, known as trans-signaling, primarily mediates the pro-inflammatory effect. The recently discovered trans-presentation signaling pathway occurs mainly in the antigen-specific interaction of dendritic cells involved in IL-6 signaling and T cells receptive to IL-6.
IL-6 signaling occurs mainly through three pathways, namely, classical signaling, trans-signaling, and trans-presentation signaling [19], [26], [27]. In classical signaling, the pro-inflammatory cytokine IL-6 binds to a complex composed of membrane IL-6R (mIL-6R) and gp130 and then transduces signals by activating the JAK-STAT3 pathway. mIL-6R is mainly expressed in hepatocytes, neutrophils, monocytes, and T cells and performs normal physiological functions mediated by IL-6 [28]. In trans-signaling, IL-6 binds to soluble IL-6 (sIL-6R), which is found in serum and tissue fluid. sIL-6R is generated from mIL-6R through proteolytic cleavage by metalloprotease gene family members (ADAM10 and ADAM17) or secreted directly after the translation of alternatively spliced mRNAs [29]. Elevated sIL-6R levels are associated with autoimmune diseases. Most of the pro-inflammatory effects of IL-6 have been attributed to trans-signaling pathways [29]. IL-6 trans-signaling has also been determined to play a key role in liver regeneration, hematopoietic stem cell expansion, and neuronal and smooth muscle cell stimulation [19]. In contrast, anti-inflammatory and regenerative signals, including the antibacterial acute-phase response of the liver, are mediated by IL-6 classical signaling [27]. Different from the first two signal features, trans-presentation signaling mainly involves the binding of IL-6 to mIL-6R expressed on immune cells, thereby forming a complex with gp130 on T helper 17 (TH17) cells, resulting in downstream T cell signaling [26]. For example, mIL-6R on dendritic cells combined with IL-6 is presented to T cells expressing gp130 on the surface, phosphorylating STAT3 in T cells, and subsequently triggering T cell activation [26].
Upon stimulation of specific inducers, many kinds of cells can secrete IL-6, including epithelial cells, endothelial cells, fibroblasts, nerve cells, monocyte-macrophages, activated T/B lymphocytes and osteocytes [20], [30]. IL-6 is a pleiotropic cytokine involved in the regulation of numerous physiopathological processes such as cell proliferation, survival, migration, invasion, metastasis, angiogenesis, inflammation and metabolism [20], [30], [31]. It can play an important role in adaptive immune response by stimulating B cells to produce antibodies and inducing the differentiation of naive CD4 + T cells into effector T cells [32]. Notably, IL-6 has a dual role in inflammation, exerting pro-inflammatory and anti-inflammatory effects [33]. On the one hand, IL-6 displays a similar pro-inflammatory effect with TNF and IL-1β. In this case, IL-6 activates the immune system, recruits monocytes, stimulates the expression of endothelial cells and smooth muscle cells, and inhibits the differentiation of regulatory T cells (Treg) and apoptosis of T cells [33], [34]. Furthermore, IL-6 can also induce the expression of intercellular adhesion factor 1 (ICAM-1), leukocyte integrin, lymphocyte function-associated antigen 1 (LFA-1, CD11a/CD18), and the ligand for Mac-1 (CD11b/CD18) [34], [35]. On the other hand, IL-6 can suppress bacterial infection, promote the proliferation of epithelial cells, and inhibit apoptosis of epithelial cells, thus exerting an anti-inflammatory effect [31], [34].
IL-6 levels have been significantly correlated with the occurrence and progression of various diseases, such as autoimmune disease, infections, and cancers [36], [37], [38]. Rheumatoid arthritis (RA) manifests as polyclonal plasmacytosis with elevated autoantibodies, C-reactive protein (CRP), and platelets. High levels of IL-6 can be detected in the serum and synovial fluid of patients with acute RA [37], [39]. Significant correlations exist between IL and 6 and IgG in synovial fluid and between IL and 6 and CRP in serum [37]. Morever, T cells, B cells, synoviocytes, and chondrocytes collected from patients with RA all produce high levels of IL-6 [40]. Furthermore, patients with inflammation or infection are noted to have markedly increased IL-6 levels, including burn patients, postoperative patients, and those who underwent organ transplantation or contracted SARS-CoV-2 infection [12], [28], [41]. In cancer, IL-6 is one of the pro-oncogenic cytokines, which can activate the JAK-STAT3, ERK, and PI3K-Akt signaling pathways [35], [36], [42], [43]. Among these targets, STAT3 is considered a primary downstream signal transducer of gp130 signaling and a critical oncogene mainly involved in the link between inflammation and cancer [33], [43], [44], [45]. Recent studies have confirmed that IL-6 enhances the ability of primary breast cancer cells to invade and metastasize [46]. Disseminated cancer cells (DCCs) detach from the primary tumor in the early stage of cancer formation and metastasize in the blood and lymph. IL-6 has a high copy number variation in DCCs, and it is only known to expresses the trans-signaling receptor gp130 [46]. Upon activation, the trans-signaling receptor enhances the invasive capacity of DCCs and induces the non-stem cells to transform into stem-like cells [46]. Morever, IL-6 also promotes DCCs growth, increases tumor angiogenesis, and degrades the extracellular matrix [46].
3. Role of IL-6 in immunity and inflammation
As one of the reliable indicators for stress response, IL-6 participates in innate and adaptive immunity and may affect the accumulation of specific sub-populations of immune cells in the body [28], [38]. IL-6 deficiency could prolong neutrophil infiltration in the infection sites [32]. IL-6 regulates the release of inflammatory mediators from stromal tissue cells including endothelial cells, smooth muscle cells, epithelial cells, mesothelial cells, and fibroblasts via trans-signal transduction, thereby triggering neutrophil-mediated phagocytosis and killing [32], [34]. In antigen presenting cells, IL-6 modulates the effector characteristics of various CD4 + T helper cell populations and drives tissue-specific pathological processes and fibrosis by stimulating the expansion of Th1 cells [34]. Interestingly, IL-6 shows no direct effect on promoting differentiation of primitive CD4 + T cells into Th1 or Th2 cells. Instead, it controls the cell survival by facilitating the action of other lymphokines [32], [34]. Morever, IL-6 has the ability to expand the effector T cell population including Th17 and Th22 cells [32]. Th17 cells play a critical role in maintaining integrity of mucosal barrier and immune homeostasis as well as preventing fungal infection and development of inflammation related tissue damage. Besides, IL-6 suppresses the function of Treg cells and prevents differentiation of Th17 cells into Treg cells, thus mediating pro-inflammatory and rejection-promoting responses [32] (Fig. 2 ).
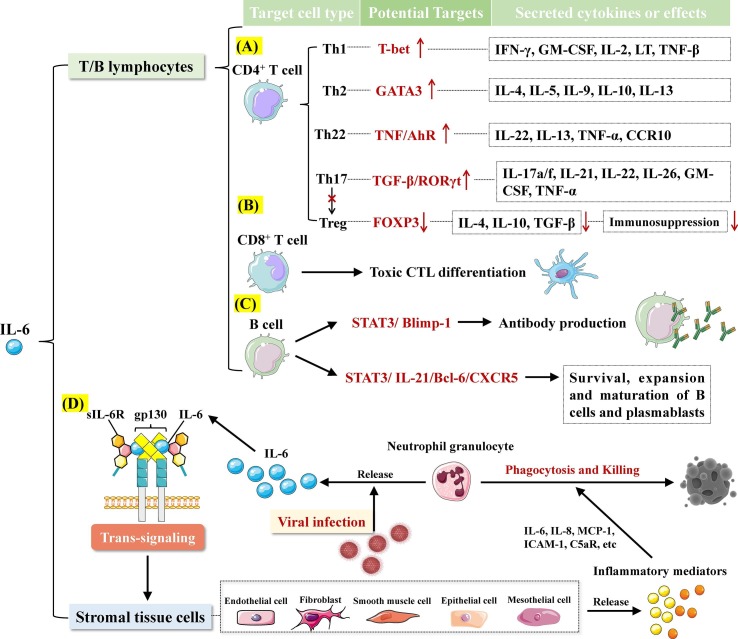
Fig. 2.
Role of IL-6 in immunity and inflammation. A. Potential targets of IL-6 to regulate CD4+ T cells to secrete cytokines. B. IL-6 promotes the differentiation of CD8+ T cells into cytotoxic T cells (CTL). C. IL-6 promotes the survival, expansion and maturation of B cell and plasmablast, as well as antibody production, by activating STAT3. D. IL-6 controls the release of inflammatory mediators from stromal tissue cells through trans-signaling, inducing the phagocytosis and killing of neutrophils.
In normal conditions, IL-6 concentration in human body is below 7 pg/mL. Infection or inflammation may lead to an acute rise in the level of IL-6. In the case of septic shock, the median serum level of IL-6 can increase to 189,000 pg/mL, which is 1,000 times higher than that in patients with meningitis (200 pg/ml) or bacteriaemia (200 pg/ml) [47], [48]. Recent study has demonstrated that there is an inverse correlation between high serum IL-6 with the density of T cells and lymphopenia in patients with SARS-CoV-2-induced disease [49]. Upon being produced in focal lesions at early stage of inflammation, IL-6 is released into liver via bloodstream and subsequently induces the expression of a number of acute phase proteins, such as activating c-reactive protein (CRP), serum amyloid A (SAA), fibrinogen gamma chain (FGG), haptoglobin (HP), and A1 antichymotrypsin [47]. The long-term presence of IL-6-induced high level of SAA can elicit serious complications of several chronic inflammatory diseases via the formation of amyloid A protein [47]. Moreover, IL-6 is involved in regulation of serum iron and zinc levels through regulating the serum transporters [50]. In bone marrow, IL-6 can promote the maturation of megakaryocytes, thereby resulting in the release of platelets [19]. For routine assays in the clinical laboratory, the severity of inflammation can be assessed based on changes in the level of acute phase proteins and counts of erythrocytes and platelets.
In response to inflammation, the increase of IL-6 not only occurs earlier than that of other cytokines, as well as CRP and procalcitonin (PCT), but also lasts for a long time to assist early diagnosis of acute infection [33], [47]. Upon bacterial infection, the level of IL-6 increases rapidly, reaching the peak at 2 h, and its elevated level is positively correlated with the infection severity [47]. Regardless of the status of liver function, IL-6 can be detected 12–24 h earlier than CRP after surgery and has more correlations with duration of surgery, amount of bleeding and stress level [47]. Thus, IL-6 has more advantages as an inflammatory marker. Currently, monoclonal antibodies against IL-6 or IL-6 receptor (IL-6R) have been successfully developed for the treatment of autoimmune diseases [51]. Tocilizumab is the first marketed humanized antibody against IL-6R, which can inhibit IL-6 signaling through preventing the binding of IL-6 to IL-6R. To date, tocilizumab has been approved for the treatment of various diseases, including rheumatoid arthritis, polyarticular juvenile idiopathic arthritis (pJIA), systemic juvenile idiopathic arthritis (sJIA), giant cell arteritis (GCA), cytokine release syndrome (CRS), castleman disease, and takayasu arteritis [19], [50], [52], [53].
4. Role of IL-6 in metabolism
IL-6 is a highly pleiotropic factor that can modulate inflammation as well as various aspects of metabolism, including glucose homeostasis and fat tissue metabolism [19], [54], [55] (Fig. 3 ). However, activation of gp130 signaling associated with IL-6 can be detrimental in some cases and contribute to the development of metabolic diseases such as obesity, chronic liver injury, inflammatory bowel disease (IBD) and cancer [56]. It has been demonstrated that IL-6 in skeletal muscle appears to affect the substance selection in the liver under the basal state, while exerting an indirect effect on the markers of glucose metabolism during long-duration exercise, thus acting as a negative regulator of hepatic glycose export [54], [57]. In addition, CD8 + T cells activated by liver sinusoidal endothelial cells (LSEC) display a transient burst of oxidative phosphorylation and glycolysis. Co-stimulation of IL-6 signals ensures a high expression of FOXO1 in LSEC-derived CD8 + T cells, which subsequently reduces metabolic activities related to T cell activation [54], [58]. Conversely, glucose transporter 4 (GLUT4) inhibitor WZB117 is capable of effectively blocking the stimulating effect of glucose on LPS-induced mRNA expression of IL-6, indicating that a high level of blood glucose could stimulate the expression of IL-6 [59]. Notably, the level of circulating IL-6 in patients with type 2 diabetes and/or obesity is 2–3 times as high as that in healthy individuals with normal glucose tolerance [13], [16]. Acute administration of IL-6 in mice can promote glucose uptake and fatty acid oxidation in skeletal muscle, probably leading to an improvement in insulin sensitivity and prevention of diet-caused obesity [60]. Meanwhile, IL-6 released from the contracted muscle can drive the production of glucagon-like peptide-1 (GLP-1) in the intestines and pancreas and subsequently control insulin secretion via GLP-1 to maintain glucose homeostasis [19], [61]. Thus, high levels of IL-6 appear to release energy through cachexia and lipolysis, and may distribute energy through regulation of insulin resistance. However, in chronic inflammatory conditions, whether IL-6 increases the energy uptake of immune cells by promoting metabolic transformation requires further exploration [62] .
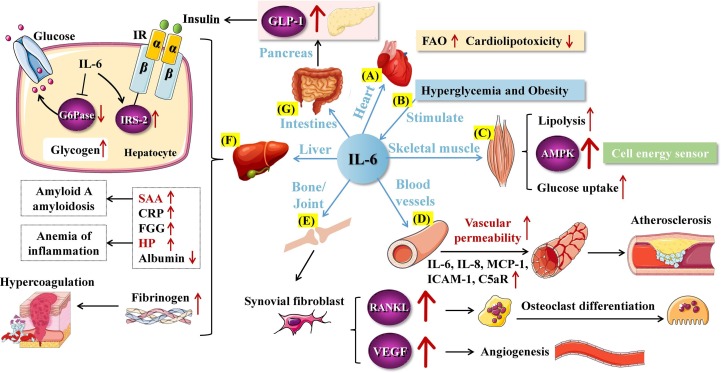
Fig. 3.
Role of IL-6 in metabolism. A. In cardiac tissue, IL-6 can promote FAO, inhibit lipid accumulation and reduce cardiolipotoxicity. B. Hyperglycemia and obesity promote the expression of IL-6. C. In skeletal muscle, IL-6 promotes glucose uptake, lipolysis, and activation of AMPK to produce ATP. D. The pro-inflammatory effect of IL-6 leads to increased vascular permeability and plaque instability. E. IL-6 can induce synovial fibroblast-mediated osteoclast differentiation and angiogenesis. F. In liver, IL-6 induces the expression of IRS-2 on hepatocytes, thereby enhancing insulin signaling. IL-6-dependent G6Pase inhibition reduces peripheral blood glucose levels and increases liver glycogen storage. Besides, IL-6 drives the increase of SAA, CRP, FGG, HP and the decrease of Albumin. IL-6 can promote the coagulation function of liver by regulating the production of fibrinogen. G. IL-6 maintains glucose homeostasis by controlling insulin secretion through GLP-1. GLP-1: glucagon-like peptide-1; FAO: fatty acid oxidation; AMPK: AMP-activated protein kinase; MCP-1: monocyte chemotactic protein-1; ICAM-1: intercellular cell adhesion molecule-1; C5aR: complement component 5a receptor 1; RANKL: receptor activator for nuclear factor-κB ligand; VEGF: vascular endothelial growth factor; IR: insulin receptor; IRS-2: insulin receptor substrate-2; SAA: serum amyloid A; CRP: c-reactive protein; FGG: fibrinogen gamma chain; HP: haptoglobin.
AMP-activated protein kinase (AMPK) is a pivotal cellular energy sensor that can facilitate ATP production to meet energy requirements [63]. AMPK activation may stimulate glucose uptake and glycolysis, as well as fatty acid oxidation [63], [64]. AMPK regulates the de novo synthesis pathway of fatty acid (FA) through activating downstream acetyl CoA carboxylase (ACC) [64]. IL-6 has been found to exert an indirect effect on stimulating phosphorylation of AMPK and ACC [65]. Hence, IL-6 deficiency may negatively regulate fatty acid oxidation (FAO) and mitochondrial biosynthesis, resulting in ATP depletion and AMP accumulation [61], [65].
In vivo assays have shown that IL-6 can promote steatolysis in skeletal muscle and facilitate FAO throughout the body [66]. Moreover, as a neutralizing antibody against IL-6, tocilizumab can induce body weight gain, hypertriglyceridemia and hypercholesterolemia [67]. Interestingly, the plasma level of IL-6 in individuals carrying polymorphic allele of IL-6 locus G174C tends to be elevated. This observation may be related to a rise in the levels of plasma triglyceride (TG), very low density lipoprotein (VLDL) and free fatty acid (FFA) [66], [67]. Notably, steatolysis is found to be enhanced in abdominal fatty tissues of individuals with overweight or obesity, which were incubated with IL-6 only [66]. On the contrary, mice with IL-6 deficiency exhibit obesity during the maturation as well as disrupted metabolism of carbohydrates and lipids [66]. Hence, IL-6 deficiency may lead to lipid accumulation and cardiolipotoxicity, which further elicit cardiac dysfunction.
However, obesity itself can also induce IL-6 production [68]. Obesity-mediated recruitment and activation of immune cell subsets in white adipose tissue systemically increase IL-6 levels. Massive secretion of inflammatory factors, macrophage infiltration, and adipose tissue remodeling have been identified as the main features of chronic inflammation in obesity [69]. Adipose tissue is composed of mature adipocytes, non-adipocyte cells, connective tissue matrix, blood vessels, and nerve cells and secretes various pro-inflammatory factors, including IL-6 [68], [70]. Adipose tissue in a long-term inflammatory state will exhibit increased adaptability in connective tissue, such as blood vessels and fibers, resulting in adipose tissue remodeling and even fibrosis [69]. Macrophages are mainly divided into M1 and M2 types [71]. Of these, M1-type macrophages are known to be involved in the induction of obesity and chronic inflammation by secreting IL-6 [68], [70]. Adipose tissue inflammation promotes apoptosis and recruits monocyte-macrophages in large numbers [71]. In this process, the expression of M1-type macrophages increases significantly, which further promotes IL-6 secretion and inhibits insulin signaling, resulting in glucose and lipid metabolism disorders [68]. The excessive release of IL-6 not only impairs insulin action in metabolic tissues, but also contributes to cancer development [33]. In addition, acute stress stimulation also induces IL-6 secretion in brown adipocytes, resulting in the inability of brown adipocytes to normally regulate the metabolism of glucose and break down fat, eventually causing obesity and obesity-related complications [33], [70].
The above studies indicate that normal IL-6 levels contribute to glucose metabolism regulation, lipid homeostasis, and anti-obesity treatment. Conversely, the excessive release of IL-6 can aggravate the inflammatory state, which, in turn, interferes with insulin signaling and causes lipid metabolism disorders.
5. Roles of IL-6-mediated immunometabolic reprogramming in viral infection-associated diseases
5.1. Immunometabolic reprogramming in viral infection-associated diseases
The term “Immunometabolic reprogramming” here refers specifically to the manipulation of the metabolism, immune function, and the relationship between the two by a virus in the host. Energy metabolism underlies an excessive immune-inflammatory response and plays a key role in controlling immune cell phenotype and function [72]. Immunity and metabolism are two highly integrated and interdependent systems, and balancing them is key to maintain the normal state of the body. The term “immunometabolism” has been used to explain the close relationship between metabolic regulation and immune function. Immunometabolic reprogramming is a mechanism by which immune cells promote cell proliferation and differentiation and exert immune effects by changing immune function and metabolic patterns to meet energy needs [72]. Immunometabolic reprogramming caused by a viral infection not only facilitates viral replication, but also antagonizes host antiviral responses associated with the immune system.
In viral infection-associated diseases, the phenomenon of immunometabolic reprogramming is common, including the mutual regulation of immune function and glucose, lipid, and amino acid metabolism [73], [74], [75]. Immunometabolic reprogramming has been closely related to disease occurrence and progression, and key proteins in related signaling pathways can also be the targets of drug action [74]. Our current understanding of how host metabolism is related to immune responses and affects cytokine release remains to be limited. However, several studies have uncovered specific immunometabolic reprogramming phenomena observed in some viral infectious diseases. For example, in coronavirus disease 2019 (COVID-19), metabolism can modulate the immune response to SARS-CoV-2 infection [73], [75]. Correlation analysis revealed strong associations between metabolites and pro-inflammatory cytokines/chemokines, such as IL-6, M−CSF, IL-1α, and IL-1β, and suggested a potential link between arginine, tryptophan, and purine metabolism and excessive inflammation [76]. Targeting metabolism may be a potential strategy for the treatment of fatal CRS in COVID-19 [77]. Small-molecule inhibitors targeting PI3 kinase VPS34 or fatty acid metabolism have been shown to inhibit SARS-CoV-2 activity [77]. HIV infection in adulthood is more likely to cause metabolic syndrome, which includes excess abdominal fat, abnormal blood sugar and lipid levels, and high blood pressure, as well as an increased risk of diabetes, heart disease, and stroke [78], [79]. All of these are attributable to HIV itself, inflammation, and side effects of antiretroviral therapy [79]. AIDS-related inflammation and immune activation also occur throughout the infection and treatment process, which is related to the persistence of HIV, translocation of intestinal flora, reduction of regulatory T cells, and combination or secondary infection with other viruses [80], [81]. People who were born with HIV or acquired HIV in early childhood were found to be significantly more likely to develop metabolic abnormalities, including insulin resistance, high triglyceride levels, and adverse weight gain [82].
The roles of IL-6 in inflammation and metabolism are complex. While IL-6 is generally considered a pro-inflammatory cytokine, other studies suggest that it exhibits anti-inflammatory effects [33]. Under viral infection conditions, a high IL-6 concentration is a reliable indicator of the immune response [83]. In addition, as an inflammatory marker, IL-6 is closely related to the occurrence and development of metabolic syndrome [56]. IL-6 may be a bridge molecule linking systemic inflammation and metabolic disorders. IL-6 upregulation induced under viral infection may trigger IL-6-dependent metabolic reprogramming in the host.
5.2. Roles of IL-6-mediated immunometabolic reprogramming in SARS-CoV-2 infection-associated COVID-19
Immune responses induced by SARS-CoV-2 infection contribute to virus clearance while probably causing cytokine release syndrome (CRS) in severe corona virus disease 2019 (COVID-19) patients [84], [85]. The level of IL-6 is elevated in critically ill COVID-19 patients. In this case, a large amount of pathogenic T cells and inflammatory monocytes secreting a high level of IL-6 may enter pulmonary circulation and trigger an inflammatory storm, leading to immune dysfunction and extensive injury, and eventually resulting in multiple system organ failure and even a high mortality [86], [87], [88], [89] (Fig. 4 ). Thus, IL-6 acts as a pivotal inflammatory factor triggering CRS in COVID-19 patients, which can be used to effectively assess the disease severity and predict prognosis of COVID-19 patients [90], [91], [92]. Recent clinical trials have shown that tocilizumab could suppress hyperactive immune response, improving the inflammatory symptoms of patients with or without mild organ damage at the early stage of COVID-19 [86], [88], [93]. All these data indicate that targeting IL-6 may play an important role in preventing and relieving CRS, while IL-6 could serve as a potential therapeutic target for COVID-19 (Table 1 ). Besides, IL-6R blockers in combination with glucocorticoid have been demonstrated to be capable of reducing all-cause mortality in COVID-19 patients undergoing respiratory support therapy [93], [94]. However, it is not recommended that IL-6R blockers be widely used among COVID-19 patients with mild symptoms or receiving long term invasive mechanical ventilation [89], [94], [95].
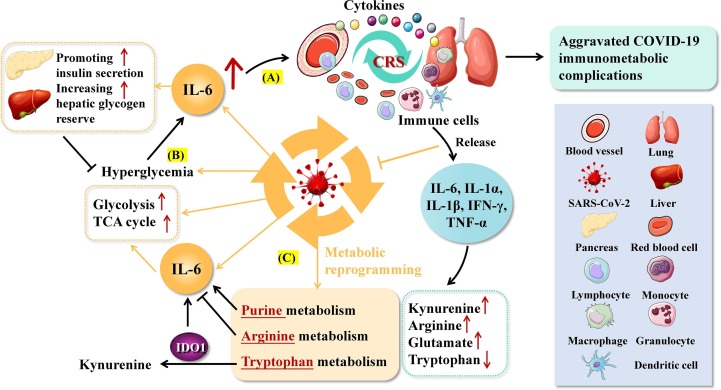
Fig. 4.
Roles of IL-6-mediated immunometabolic reprogramming in SARS-CoV-2-related COVID-19. A. High levels of IL-6 under SARS-CoV-2 infection promote massive entry of immune cells and cytokines into the pulmonary circulation, triggering cytokine storm (CRS) and extensive inflammatory responses. B. SARS-CoV-2 infection can lead to hyperglycemia and stimulate the expression of IL-6, which in turn promotes insulin secretion and hepatic glucose utilization, forming a negative feedback loop. Elevated IL-6 can also promote glycolysis and the tricarboxylic acid cycle (TCA) in infected cells. C. Inter-regulation of IL-6 and amino acid metabolism in patients with COVID-19.
Table 1.
Current status of research and development of COVID-19 therapeutic drugs targeting IL-6.
| Drug name | R&D code | The first R&D company | Targets (combined with simplified targets) | The world's highest R&D stage | Indications |
|---|---|---|---|---|---|
| Tocilizumab | MR16-1; R-1569; RG-1569; hPM-1; RO-4877533 | Roche | IL-6 receptor antagonist (IL-6R); IL-6 receptor modulator (IL-6R) | Listed stage | Adult Still's disease; autoimmune encephalomyelitis; giant lymphadenopathy; chronic lymphocytic leukemia; COVID-19; familial mediterranean fever; juvenile rheumatoid arthritis; motor neuron disease ; osteoarthritis; polymyalgia rheumatica; pulmonary hypertension; rheumatoid arthritis; systemic lupus erythematosus; toxicity; viral pneumonia |
| Siltuximab | CNTO-328 | Johnson&Johnson | Heat shock protein inhibitor (HSP); IL-6 receptor antagonist (IL-6R) | Listed stage | Megalymphadenopathy; chronic lymphocytic leukemia; COVID-19; pneumonia |
| Levilimab | BCD-089 | Biocad Ltd | IL-6 receptor antagonist (IL-6R) | Listed stage | COVID-19; rheumatoid arthritis |
| Olokizumab | CDP-6038 | UCB | IL-6 receptor antagonist (IL-6R) | Listing application | COVID-19; rheumatoid arthritis |
| Emiplacel | PLX-1; PLX-R18 | Pluristem Therapeutics Inc | IL-6 receptor agonist (IL-6R) | Phase III clinical stage | Aplastic anemia; autoimmune disease; bone injury; bone marrow transplantation; heart failure; COVID-19; diabetic foot ulcer; graft-versus-host disease; hematopoietic stem cell transplantation; inflammatory bowel disease; intermittent claudication; interstitial lung diseases; ischemia; lung injury; multiple sclerosis; muscle injury; muscular atrophy; muscular dystrophy; neurological disorders; neuropathic pain; peripheral vascular disease; preeclampsia; pulmonary fibrosis; radiation sickness; respiratory distress syndrome symptoms; skin burns; tendon injuries; thromboangiitis obliterans; wound healing |
| Emvododstat | PTC-299; PTC-VG; PTC-VH; PTC-VJ; PTC-VK; PTC-WS | PTC Therapeutics Inc | Dihydroorotate dehydrogenase inhibitor (DHODH); IL-6 receptor antagonist (IL-6R); VEGF receptor antagonist (VEGFR) | Phase III clinical stage | Acute myeloid leukemia; COVID-19; myelodysplastic syndromes |
| Clazakizumab | ALD-518;BMS-945429 | Alder | IL-6 receptor antagonist (IL-6R) | Phase III clinical stage | COVID-19; kidney transplant rejection; lung failure; organ transplant rejection; respiratory distress syndrome |
| Sirukumab | CNTO-136 | Johnson&Johnson | IL-6 receptor antagonist (IL-6R) | Phase III clinical stage | COVID-19; major depressive disorder |
| MP-1032 | MP-1000;MP-1000 program; MP-1031;MP-1032 | MetrioPharm AG | Catalase stimulator (CAT); Interleukin-1 ligand inhibitor (IL-1); Interleukin-6 ligand inhibitor (IL-6); Interleukin-8 ligand inhibitor (IL-8); Superoxide dismutase stimulator (SOD); TNF alpha ligand inhibitor (TNF-α) | Phase Ⅱ clinical stage | COVID-19; inflammatory diseases; multiple sclerosis; psoriasis |
| Amilo-5MER | —— | Galmed Pharmaceuticals Ltd | Apolipoprotein B modulator (APOB); IL-6 receptor antagonist (IL-6R); Serum amyloid A protein modulator (SAA); Transthyretin modulator (TTR) | Phase Ⅰ clinical stage | COVID-19; crohn's disease; inflammatory bowel disease; multiple sclerosis; psoriasis; respiratory distress syndrome; rheumatoid arthritis; ulcerative colitis |
| KSI-501 | KSI-501; KSI-501p;OG-2072 | Kodiak Sciences Inc | Interleukin-6 ligand inhibitor (IL-6); VEGF ligand inhibitor (VEGF) | Preclinical research stage | COVID-19; diabetic macular edema; diabetic retinopathy; uveitis; wet age-related macular degeneration |
| Tocilizumab biosimilar | BP-08 | Curateq Biologics Pvt Ltd | IL-6 receptor modulator (IL-6R) | Preclinical research stage | COVID-19 |
| ACE2-Fc-anti-IL-6R scfv fusion protein | —— | Shanghai Keqi Pharmaceutical Technology Co Ltd | COVID-19 spike glycoprotein inhibitor; IL-6 receptor antagonist (IL-6R) | Preclinical research stage | COVID-19 |
| rIFN-alpha 14 | —— | ILC Therapeutics Ltd | CXC10 chemokine ligand inhibitor (CXCL10); CXC5 chemokine ligand inhibitor (CXCL5); Growth regulated protein alpha ligand inhibitor (CXCL1); Interferon alpha 14 ligand (IFNA14); Interferon gamma receptor agonist (IFNGR); Interleukin 17A ligand inhibitor (IL-17A); Interleukin 17F ligand inhibitor (IL-17F); Interleukin-6 ligand inhibitor (IL-6); Interleukin-8 ligand inhibitor (IL-8); Monocyte chemotactic protein 1 ligand inhibitor (CCL2); NK cell receptor agonist (KIR2DL4); RANTES ligand (CCL5); T cell receptor agonist (TCR) | Preclinical research stage | COVID-19; respiratory distress syndrome |
*The source of the data is from the global drug research and development database of the one-stop retrieval platform of pharnexcloud (https://www.pharnexcloud.com).
While sMAdCAM is a critical marker for intestinal immune migration, the level of circulating sMAdCAM in COVID-19 patients appears to be lower than that in healthy individuals or convalescent COVID-19 patients [96]. There is a negative correlation between the level of IL-6 and that of sMAdCAM in COVID-19 patients. Among convalescent COVID-19 patients, the level of sMAdCAM is markedly elevated, while the level of IL-6 declines significantly [96]. Assimilation of IL-6 and sMAdCAM levels can be expressed as sMIL (the ratio of sMAdCAM to IL-6 level), a new comprehensive marker index, which shows the highest value in convalescent COVID-19 patients [96]. Collectively, IL-6 may potentially serve as a novel marker for assessing intestinal immune microenvironment and metabolic function in COVID-19 patients. Meanwhile, the integrated analysis of metabolic remodeling and inflammatory response in COVID-19 patients reveals that intervening in the metabolism of arginine, tryptophan or purine can significantly improve the excessive inflammatory response in COVID-19 patients. There is a close correlation between metabolic pathway dysregulation and high levels of IL-6 in critically ill COVID-19 patients [76]. Moreover, rise in blood sugar level among COVID-19 patients may facilitate the replication of SARS-CoV-2 and cytokine production in monocytes. SARS-CoV-2 infection-induced IL-6 regulates metabolism of glucoses and lipids. It demonstrates that IL-6 release is associated with metabolic reprogramming in COVID-19 patients, and IL-6 could be the key player that links over-activated immune response to metabolic reprogramming in those patients. Therefore, regulation of host metabolism via biological agents such as tocilizumab could become a new pathway for improving SARS-CoV-2-caused CRS [87].
The level of CRS-related cytokine IL-6 in COVID-19 patients is gradually increased with the increasing severity ranging from asymptomatic cases to mild and severe cases [76], [92]. Similarly, the correlation between the IL-6 level and metabolites increases with the increasing disease severity. Strikingly, IL-6 shows a correlation with 8 and 20 metabolites in mild and severe COVID-19 patients, respectively [76]. In these cases, the level of IL-6 is positively correlated with several key metabolites of arginine metabolism such as arginine, glutamine, aspartic acid, citrulline, urea and proline, multiple key metabolites of purine metabolism such as xanthine, guanosine, adenine, GMP, adenosine and guanine, as well as the metabolites of tryptophan and NAD + metabolism including kynurenine and NMN [76]. By contrast, niacin is inversely correlated with the level of IL-6. Overall, the disorder of these metabolic pathways is closely associated with IL-6-induced excessive inflammation.
Interestingly, arginine supplementation can significantly inhibit SARS-CoV-2-induced IL-6 release of peripheral blood mononuclear cells (PBMCs). Conversely, arginine supplements cause no changes in IL-6 release of PBMCs derived from healthy individuals [76]. In the meantime, serum metabolites involved in tryptophan and kynurenine metabolism are correlated with IL-6 in COVID-19 patients. While conversion of tryptophan to kynurenine in immune cells is precisely regulated by indoleamine2,3-dioxygenase1 (IDO1), excessive IDO1 can lead to the depletion of tryptophan in lymphocytes, and the toxicity of metabolite kynurenine can also cause lymphocyte apoptosis. Administration with IDO1 inhibitor epacadostat can suppress SARS-CoV-2-induced IL-6 release [76]. On the contrary, purine metabolism inhibitor mycophenolic acid (MPA) can increase the level of IL-6, indicating that interference of purine metabolism exacerbates excessive inflammatory response partly through promoting IL-6 release [76]. All these results indicate that targeting metabolic pathways could control the release of IL-6, further modulating inflammatory response in COVID-19 patients. Moreover, exogenous cytokine mixture including IL-6, IL-1α, IL-1β, IFN-γ and TNF-α can increase the release of urinary ammonia, arginine and glutamate into the culture medium of PBMCs while decreasing tryptophan release [76]. The consistence between changes in the above metabolites and metabolic changes in the serum of COVID-19 patients demonstrates that the metabolic reprogramming may be partly attributed to excessive inflammatory response induced by SARS-CoV-2 infection.
5.3. Roles of IL-6-mediated immunometabolic reprogramming in HBV infection-associated HCC
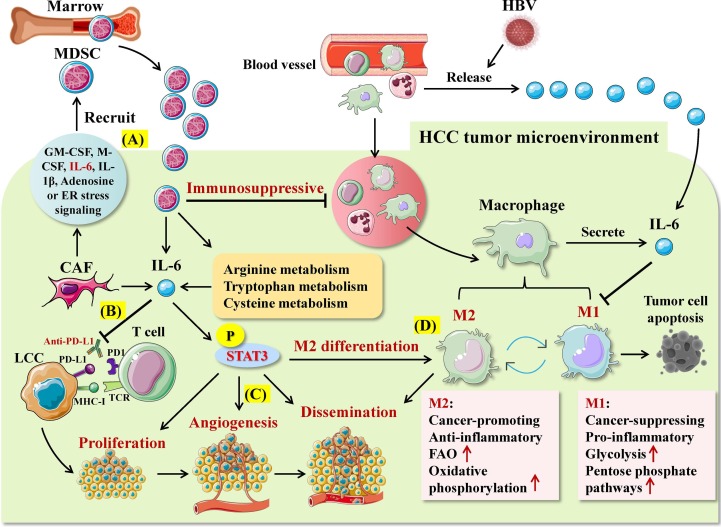
Compared with healthy individuals, the level of IL-6 in peripheral blood is significantly increased in both chronic hepatitis B virus (HBV) carriers and inactive HBsAg carriers [97]. The level of IL-6 in chronic HBV carriers is lower than that in inactive HBsAg carriers. Comparison of the cytokine levels in peripheral blood among individuals with different HBV DNA loads reveals that the average level of IL-6 in chronic HBV carriers with a medium HBV DNA load is higher than that in those with a high or low HBV DNA load [98]. It has been demonstrated that IL-6 initiates transcription of various downstream genes involved in liver development and metabolism. IL-6 can activate STAT3, promote proliferation, angiogenesis and anti-apoptotic genes, and inhibit pro-apoptotic genes, thus facilitating the proliferation and migration of HepG2 cells both in vivo and in vitro [99], [100], [101] (Fig. 5 ). While ATF3-mediated up-regulation of IL-6Rα promotes the drug resistance to sorafenib and regofinib in HCC, targeting IL-6R could increase the therapeutic efficacy of sorafenib/regofinib in advanced HCC [100], [102]. IL-6 can upregulate the inhibitor of differentiation 1 (Id1) from bone marrow-derived myeloid cells, which is contributed to shifting dendritic cell differentiation toward myeloid-derived suppressor cells [103]. Moreover, cancer-related fibroblasts (CAFs) highly expressing IL-6 exert a powerful immunosuppressive effect in HCC microenvironment by recruiting bone marrow derived immunosuppressive cells [100]. Furthermore, IL-6 disrupt the function of tumor infiltrating T cells through up-regulating inhibitory immune checkpoints. Notably, targeted inhibition of IL-6 could enhance the efficacy of anti-PD-L1 therapy in HCC and reverse the drug resistance [104]. Taken together, IL-6 could serve as a potential therapeutic target for HBV-related HCC. Combined use of IL-6-targeting therapy and immune checkpoint inhibitors may provide a new alternative for the treatment of HCC. The mechanism of the effectiveness of such combination regimen is the inducible effect of IL-6 on strengthening T cell infiltration into the tumor area [105]. Furthermore, due to the inducible effect of IL-6 on PD-L1 expression on tumor cells and non-tumor cells, it is presumable that targeting the IL-6/JAK/STAT3 axis will sensitize tumor to PD-1/PD-L1 inhibitor therapy [106].
Fig. 5.
Roles of IL-6-mediated immunometabolic reprogramming in HBV-related hepatocellular carcinoma (HCC). A. Cancer-associated fibroblasts (CAFs) can recruit marrow-derived immunosuppressive cells (MDSCs) by secreting cytokines such as IL-6, thereby exerting strong immunosuppressive effects in the HCC microenvironment. B. IL-6 can inhibit the efficacy of anti-PD-L1 in HCC and induce tumor resistance. C. IL-6/STAT3 signaling promotes the proliferation, angiogenesis and dissemination of liver cancer cell (LCC). D. IL-6/STAT3 signaling can promote the differentiation and metabolic transformation of tumor-associated macrophages to the M2 phenotype.
It has been demonstrated that IL-6 produced by tumor-associated macrophages (TAMs) can facilitate the sphere formation of CD44 + cells in HCC tumor tissue and increase the growth capability of mouse xenografted tumors [107]. Variation of the metabolism among TAMs affects their polarization, thus leading to varied functional properties of TAMs. Among the TAMs, M1 macrophages mainly secrete pro-inflammatory cytokines and exert an anti-cancer effect presumably through supplying energy via glycolysis and pentose phosphate pathway [108]. By contrast, M2 macrophages secrete anti-inflammatory cytokines and exert a tumor-promoting effect mainly by supplying energy via oxidative phosphorylation and promoting FAO [108]. Strikingly, inhibition of IL-6/STAT3 signaling pathway via antagonizing IL-6 could promote polarization of TAMs into M1 macrophages, markedly enhancing the inhibitory effects of M1 macrophages on HCC. Co-culture of HCC cells with M1 macrophages leads to a reduction in the viability, proliferation, invasion, migration and drug resistance of HCC cells, but an increase in the tumor apoptosis [101], [107]. Thus, IL-6 appears to facilitate metabolic transformation of TAMs into M2 phenotypes in the HCC microenvironment, thereby exerting a cancer-promoting effect.
5.4. Roles of IL-6-mediated immunometabolic reprogramming in HIV infection-associated AIDS
IL-6 is closely associated with the morbidity and mortality of human immunodeficiency virus (HIV) + individuals [109]. While the serum level of IL-6 increases with the occurrence of immunosuppression during HIV infection, the level decreases dramatically after the antiretroviral therapy (ART), but remains significantly higher than that in the non-infection group [110]. HIV expresses protein Vpr to counteract host antiviral restriction factors. In this case, Vpr degrades TET2 to maintain the expression of IL-6, promoting HIV viral replication and AIDS progression [110], [111]. It has been shown that the elevated level of IL-6 in HIV-infected individuals is related to older age, nonblack race, higher body mass index, reduced level of blood lipids, HIV replication, lowest level of CD4 + cell counts, use of protease inhibitors, complications and reduced level of eGFR [111], [112], [113]. Compared with the general population, HIV-positive people undergoing treatment were noted to have 50%–100% higher IL-6 levels [113]. Demographic factors independently associated with higher IL-6 levels include older age, non-Black race, and higher BMI [112]. In a multivariate analysis of older patients, older age was associated with higher IL-6 levels (FD [95% CI]: 1.09 [1.08–1.11] per 10 years), considering that the aging process is closely related to inflammation [112]. During the aging process, long-term IL-6 stimulation in the body can lead to a chronic, low-grade, microinflammatory aging state, thereby causing or increasing the occurrence of age-related degenerative diseases [112]. A multivariate analysis revealed lower IL-6 levels in Black participants compared with White participants (FD [95% CI]: 0.96 [0.93–0.99]), which partly suggests that IL-6 levels are genetically determined [112]. In addition, similar to studies involving HIV-infected individuals and the general population, BMI was positively associated with IL-6 (FD [95% CI]: 1.02 [1.01–1.04] per 5 kg/m2) [113]. Subcutaneous adipose tissue can produce up to 25% of circulating IL-6 under stressful conditions [112], [114].
The level of inflammatory cytokine IL-6 is significantly increased in HIV patients complicated with obesity as compared to non-obese/non-HIV-infected individuals [115]. In particular, a higher level of circulating IL-6 is correlated with a lower level of serum lipids, implying that IL-6 may exert a promoting effect on lipid metabolism and utilization among HIV-infected individuals. The elevated level of IL-6 is associated with both obesity in the absence of HIV infection and HIV infection in the absence of obesity [115]. Notably, IL-6 can be produced by either active macrophages derived from blood vessels, or adipocytes, and increases with the increasing diameter of adipocytes. Among obese patients, IL-6 is mainly derived from adipocyte [116]. By contrast, IL-6 produced in chronic HIV-infected patients may be derived from activated immune cells [116]. Therefore, the disorder of the metabolic function in obesity may aggravate the excessive release of IL-6 in HIV patients.
IL-6 also plays a certain role in the regulation of immune metabolism in AIDS-related complications. HIV-1 Tat is an auxiliary viral protein that affects the integrity of blood–brain barrier by disrupting tight junction proteins in cerebral microvascular endothelial cells [117], [118]. It induces the expression of IL-6 and IL-8 at both mRNA and protein levels in cerebral astrocytes in a time-dependent manner, which subsequently triggers neuropathy and inflammation of the central nervous system, causing HIV-associated neurocognitive disorder (HAND) [117]. Moreover, HIV protease inhibitors (PIs) are involved in the occurrence of severe metabolism syndrome, and HIV PIs have been demonstrated to promote the expression of TNF-α and IL-6, the main mediators of inflammatory response in macrophages [119]. The chronic inflammatory state caused by inflammatory factors could lead to the occurrence of metabolism syndromes and emerges as a main risk factor for cardiovascular diseases including atherosclerosis. Human herpes virus 8 (HHV-8) is considered the pathogen of Kaposi sarcoma (KS), one of the most common cancers among HIV/AIDS patients. Compared with HIV or HIV/HHV-8 individuals, the serum level of IL-6 is significantly elevated in patients suffering from both AIDS and KS [120]. And the IL-6 level in visceral AIDS-KS patients is higher than that in cutaneous AIDS-KS patients, indicating that IL-6 may be a critical marker for identifying and distinguishing the type of AIDS-KS [120]. Besides, hepatic fibrosis is independently correlated with the elevated level of IL-6 among individuals infected with both HCV and HIV, indicating that hepatic fibrosis mainly mediates enhanced pro-inflammatory state related to co-infection with HCV and HIV [120].
5.5. Roles of IL-6-mediated immunometabolic reprogramming in HCMV infection-associated diseases
Human cytomegalovirus (HCMV) has been found to be associated with accelerated development of atherosclerosis, restenosis after angioplasty and chronic graft arteriosclerosis [121]. One of the hallmarks of these metabolic diseases is angiogenesis (AG) and neovascularization. Persistence of HCMV within endothelial cells (ECs) is an important mechanism of neovascularization [122]. IL-6 is an effective angiogenic cytokine that inhibits apoptosis of ECs and participates in neovascularization during tumor progression, wound healing and development of the cerebrovascular system [123], [124]. Moreover, IL-6 accumulation may contribute to the growth and instability of atherosclerotic plaques [124]. HCMV induces angiogenesis and lymphangiogenesis indirectly through stimulating the release of IL-6 and GM-CSF by lymphatic endothelial cells [125]. In the course of inflammation or acute rejection of organ transplants, elevated IL-6 levels accompany HCMV replication in transplanted lung and bone marrow, elevate survivin expression and activate effector caspases [15]. Strikingly, HCMV can promote angiogenesis and long-term survival of endothelial cells by up-regulating the expression of anti-apoptotic factor survivin via stimulation of IL-6, thus facilitating HCMV invasion and progression of metabolic diseases in the inflammatory state [15]. Survivin potentially serves as a unique marker for dedifferentiated cells involved in neointima formation. It suggests that targeting IL-6 could potentially exert positive effects on correcting HCMV-caused inflammation and metabolic disorder.
US28 is a HCMV-encoded viral G protein (heterotrimeric guanosine triphosphate binding protein) coupled receptor that can induce the proliferation of glioblastoma cells infected with HCMV through forming a positive feedback loop via activated IL-6-STAT3 signaling axis [126]. HCMV has also been found to be the main cause of vision loss in children with congenital infection. Meanwhile, HCMV infection of retinal pericytes can induce the expression of IL-6, resulting in increased retinal neurovascular permeability and further triggering retinal inflammation and neovascularization [127]. Moreover, HCMV infection is considered a critical independent risk factor for gestational diabetes mellitus (GDM). HCMV activation could aggravate the chronic inflammatory state, thus promoting the occurrence and development of GDM and type 2 diabetes [128]. Compared with individuals without GDM or HCMV infection, the level of IL-6 is significantly increased in GDM women infected with HCMV at 24–28 weeks of gestation [128]. IL-6 has been shown to maintain glucose homeostasis by controlling insulin secretion. Hence, persistent infection of HCMV and a high level of blood sugar in pregnancy exacerbate IL-6-mediated pro-inflammatory state, possibly leading to insulin resistance and glucose intolerance in women infected with HCMV.
5.6. Roles of IL-6-mediated immunometabolic reprogramming in AdV infection-associated diseases
Human adenovirus (AdV) infection is prevalent and leads to severe community acquired pneumonia. And the inflammatory storm caused by AdV infection shares the similar symptoms with adult COVID-19 infection [129]. AdV pneumonia accounts for approximately 10% of all childhood pneumonia cases and is mainly caused by infection with human AdV type 3/7 [130]. It is considered that AdV-induced inflammation mainly underlies serious symptoms of AdV pneumonia. Among all inflammatory cytokines, IL-6 displays the most significantly increasing trend in nasal lavage fluids of children with AdV 7 infection [130]. While markedly elevated levels of IL-6 are identified as a warning indicator for poor prognosis of AdV pneumonia, the high level of IL-6 (>100 ng/L) serves as an independent risk factor for death in children with critical AdV pneumonia [131]. Moreover, the level of IL-6 in children with AdV pneumonia and kawasaki disease is significantly higher than that in those with other type of viral pneumonia [132]. Overall, IL-6 could be an independent predictive factor for childhood AdV pneumonia.
IL-6 overexpression has been found to be associated with pulmonary immune injury [133], [134]. IL-6-caused monopenia may lead to an inflammatory cell imbalance and serves as an independent predictive factor for respiratory failure. Hypoxemia is among the critical clinical manifestations of respiratory failure, and the level of IL-6 is markedly correlated with acute hypoxemia among patients with AdV pneumonia [135]. Notably, persistent hypoxemia can cause insufficient oxygen supply to tissues. In this case, cells fail to make full use of oxygen for metabolism and energy production, resulting in a series of physiological dysfunction and metabolic disorders.
It has been demonstrated that while AdV 7 increases the mRNA and protein expression of IL-6 in bronchial epithelial cell lines and primary airway epithelial cells in a dose-dependent manner, the underlying mechanism involves enhanced IL-6 signaling via p38/NF-κB-mediated trans-activation of IL-6 promoter [136], [137]. NF-kB and IL-6 act as the main regulators of inflammation, which modulate various aspects of innate and adaptive immune responses. The activation of NF-kB/IL-6 is associated with chronic inflammation, autoimmune diseases and cancer [138]. In particular, NF-kB emerges as one of the key factor linking metabolism, inflammation and insulin function, which regulates energy homeostasis and metabolic adaptation by modulating the balance between glycolysis and oxidative phosphorylation [139], [140]. Ad-36 infection in children has been shown to be correlated with obesity, and the level of IL-6 is elevated in obese children with Ad-36 seropositivity [141]. These observations suggest that NF-kB and IL-6 signaling may exert a coordinating effect in AdV-induced inflammation and metabolic disorders. Moreover, AdV vector-mediated transduction induces hepatotoxicity at 2 and 10 days after administration [130]. In this circumstance, IL-6 acts as the main AdV vector-induced inflammatory cytokine which can activate JAK and STAT3 to promote leaked expression of AdV genes, thereby eliciting acute hepatotoxicity [130].
5.7. Roles of IL-6-mediated immunometabolic reprogramming in EV71 infection-associated diseases
Enterovirus 71 (EV71) is the most recently identified member of the genus enterovirus, with high infectivity and pathogenicity [142]. EV71 is one of the main pathogens causing hand-foot-mouth disease (HFMD) in infants, which may cause various neurological diseases such as aseptic meningitis, brainstem encephalitis and polio like paralysis [143], [144]. Notably, EV71 infection leads to a marked increase in the release of circulating IL-6. And the level of IL-6 gradually rises with the progression of the disease and is associated with poor prognosis of patients infected with EV71 [145], [146]. Among severe and critical cases infected with EV71 undergoing intravenous immunoglobulin (IVIG) and glucocorticoid treatment, convalescent patients have a significantly lower level of IL-6 in cerebrospinal fluids than acute stage patients [147]. Aseptic meningitis is considered the most common neurological complications of HFMD caused by EV 71 infection. Among all cytokines related to HFMD complicated with aseptic meningitis, IL-6 shows the strongest correlation with aseptic meningitis, with the highest sensitivity and specificity [148]. It indicates that IL-6 could serve as a potential marker for predicting the occurrence of aseptic meningitis in children with EV71-caused HFMD.
It has been shown that EV71-induced autophagy regulates the production and release of IL-6 via p38/MAPK and ERK signaling pathway in human gastric epithelial cells (GES-1) infected with EV71 [149]. Autophagy contributes to metabolism and decomposition of nutrients while playing a pivotal role in maintenance of cellular balance [150]. Upon the uptake of sufficient glucose and lipids, cells undergo autophagy to degrade cellular energy-storing substances. By contrast, nutrition deficiency and infection stress enable cells to mobilize intracellular glycogen and lipids for energy generation via autophagy [151]. Given the critical role of IL-6 in glucose and lipid metabolism, it is proposed that EV71-caused autophagy regulates metabolic reprogramming in the host cells presumably through targeting IL-6.
In the cerebral cortex of EV71 infected mice, blocking IL-6 can restore the weight loss caused by EV71 infection, reduce clinical score, improve survival rate, reduce neurofilament integrity, reduce caspase-3 cutting level, and reduce the induction of apoptosis in cerebral cortex [152]. Likewise, treatment of EV71 infecting human astrocytoma U251 cells with IL-6-Ab blocks IL-6-mediated autocrine and paracrine signal transduction of proximal astrocytes, mitigating cell apoptosis [152]. As a class of nerve cells derived from ectoderm and neuroepithelium, astrocytes function in maintaining homeostasis and protecting the central nervous system, while serving as a key regulator of inflammatory response. In the meantime, apoptotic cells could recruit macrophages by releasing metabolic molecules such as spermidine, guanosine monophosphate (GMP) and inosine monophosphate, thus altering the immune environment [153]. These indicate that the elevated level of IL-6 caused by EV71 infection can disrupt cerebral metabolic homeostasis, resulting in systematic development of neuroinflammation and injury in the brain.
5.8. Roles of IL-6-mediated immunometabolic reprogramming in DV infection-associated dengue fever
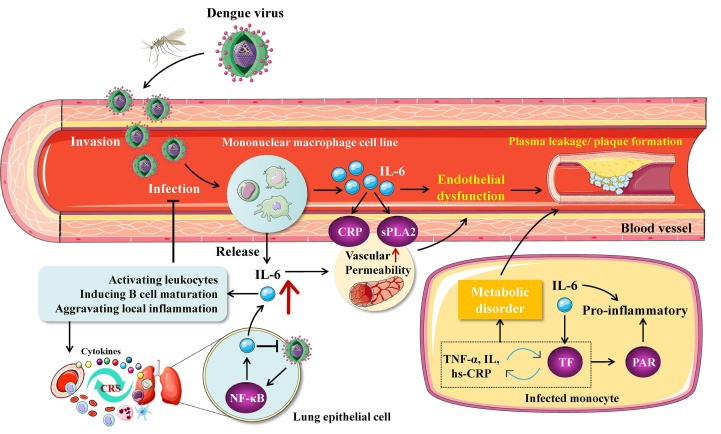
Dengue viruses (DV) are a serotype subgroup of flavivirus in flaviviridae that are transmitted by vector insects such as aedes aegypti and aedes albopictus [154]. Upon infection, DV first replicate in capillary endothelial cells, and amplified DV are then released into blood stream to form viremia and further infect monocyte-macrophages in blood and tissues, causing dengue fever [155]. DV-infected cells can produce protective pro-inflammatory cytokine IL-6, which attracts and activates leukocytes in the infection sites, promotes inflammatory response of macrophages, and induces B cell maturation [156], [157] (Fig. 6 ). While activation of IL-6 could inhibit DV replication, excessive IL-6 may aggravate inflammation and the disease progression [157]. Hence, IL-6 can potentially be used as a marker for predicting the aggravation of dengue fever. In the case of severe DV infection, IL-6 could trigger the cytokine storm and increase vascular permeability by up-regulating CRP and secretory phospholipase A2 (sPLA2), leading to functional instability of endothelial cells and plasma leakage [156]. In addition, pyrogenic properties of IL-6 can exacerbate the fever symptom of dengue fever patients.
Fig. 6.
Roles of IL-6-mediated immunometabolic reprogramming in DV-related dengue fever. Dengue virus (DV) infects monocyte-macrophage cells after invading blood vessels and stimulates their release of IL-6. On the one hand, IL-6 can aggravate the inflammatory response by activating c-reactive protein (CRP), secretory phospholipase A2 (sPLA2) and tissue factor (TF) signaling, leading to increased vascular permeability, endothelial instability and DV spread; on the other hand, DV infection can be inhibited by the IL-6-activated immune system. Overactivated immune response can trigger cytokine storm (CRS) and widespread immune metabolic dysregulation.
It has been shown that DV infection leads to production of large quantities of IL-6 in human umbilical vein endothelial cells (HUVEC) and can be inhibited by ribavirin, an antiviral synthetic guanosine analogue [158]. IL-6 released by HUVECs can recruit a large amount of leukocytes, aggravating local inflammatory response. Likewise, the serum level of IL-6 is elevated in patients with dengue hemorrhagic fever [158]. It indicate that IL-6 is implicated in the progression of dengue hemorrhagic fever and dengue shock syndrome. Pulmonary epithelial cells have also been identified as a potential target for DV. DV infection of lung cancer cells can significantly increase the expression of IL-6 through activating NF-κB [159]. Notably, there is a positive correlation between IL and 6 and D-dimer levels in the plasma of patients with dengue fever [160]. Recent studies have revealed a close correlation of IL-6 and D-dimer with severe COVID-19 [161], [162]. Thus, IL-6 and D-dimer may exert a synergetic pro-inflammatory effect in COVID-19 patients complicated with DV infection, thereby serving as a predictive factor for the combined viral infection.
Interestingly, IL-6 can activate tissue factor (TF) pathway in monocytes without affecting fibrinolysis, eliciting coagulation cascade [160]. In the meantime, TF exerts a pro-inflammatory effect through activating protease activated receptors (PARs), while it forms a positive feedback loop with IL-6 [160]. TF, also known as coagulation factor III, acts as an inducer of exogenous coagulation cascade [163]. In this case, it interacts with cytokines including TNF-α, hs-CRP and IL, participates in the occurrence and development of various metabolic syndromes such as insulin resistance, type 2 diabetes, central obesity, hypertension, and dyslipidemia, and accelerates the atherosclerosis process [163], [164], [165]. Signal transduction elicited by IL-6 and TF could regulate various aspects of immunometabolic disorder such as obesity, inflammation and thrombogenesis. Collectively, these observations suggest that IL-6/TF signaling may play an important role in immunometabolic reprogramming of host cells caused by DV infection.
6. Conclusion
The level of IL-6 is increased in diseases related to infection with SARS-CoV-2, HBV, HIV, HCMV, AdV, EV71 or DV, while being correlated with the disease severity. Furthermore, the overexpression and pro-inflammatory property of IL-6 are closely associated with immune response and cytokine storm elicited by the viral infection. It has also been demonstrated that IL-6 is involved in various physiopathological processes such as glucose metabolism, lipid metabolism, amino acid metabolism, and tumor angiogenesis during the pathogenesis of diseases related to infection with those 7 viruses. IL-6-elicited inflammatory response is found to be closely associated with metabolic disorder in patients with viral infection, implying that IL-6 exerts a significant regulatory effect on virus-induced inflammation and metabolic disorder. Overall, IL-6 signaling pathway may play a crucial role in viral infection-caused immunometabolic reprogramming of host cells.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
This work was supported by Department of Pharmacy, The Third People's Hospital of Qingdao for research. In addition, Ying-Shuang Li especially wishes to thank Hao-Wei Deng, who provided her with strong spiritual support during the writing of the article.
Funding
No funding was received.
Availability of data and materials
Data sharing is not applicable to this article, as no data sets were generated or analyzed during the current study.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Data availability
No data was used for the research described in the article.
References
- 1.Chowdhury M.A., Hossain N., Kashem M.A., Shahid M.A., Alam A. Immune response in COVID-19: A review. J Infect Public Health. 2020;13:1619–1629. doi: 10.1016/j.jiph.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castro D.X., Ols S., Loré K., Karlsson H.G. Immunity to SARS-CoV-2 induced by infection or vaccination. J INTERN MED. 2022;291:32–50. doi: 10.1111/joim.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fanucchi S., Domínguez-Andrés J., Joosten L., Netea M.G., Mhlanga M.M. The Intersection of Epigenetics and Metabolism in Trained Immunity. IMMUNITY. 2021;54:32–43. doi: 10.1016/j.immuni.2020.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Silvano A., Seravalli V., Strambi N., Cecchi M., Tartarotti E., Parenti A., Di Tommaso M. Tryptophan metabolism and immune regulation in the human placenta. J REPROD IMMUNOL. 2021;147 doi: 10.1016/j.jri.2021.103361. [DOI] [PubMed] [Google Scholar]
- 5.Pearce E.L., Pearce E.J. Metabolic pathways in immune cell activation and quiescence. IMMUNITY. 2013;38:633–643. doi: 10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xia L., Oyang L., Lin J., Tan S., Han Y., Wu N., Yi P., Tang L., Pan Q., Rao S., Liang J., Tang Y., Su M., Luo X., Yang Y., Shi Y., Wang H., Zhou Y., Liao Q. The cancer metabolic reprogramming and immune response. MOL CANCER. 2021;20:28. doi: 10.1186/s12943-021-01316-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pence B.D. Aging and monocyte immunometabolism in COVID-19. Aging (Albany NY) 2021;13:9154–9155. doi: 10.18632/aging.202918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sáez-Cirión A., Sereti I. Immunometabolism and HIV-1 pathogenesis: food for thought. NAT REV IMMUNOL. 2021;21:5–19. doi: 10.1038/s41577-020-0381-7. [DOI] [PubMed] [Google Scholar]
- 9.Choy E.H., De Benedetti F., Takeuchi T., Hashizume M., John M.R., Kishimoto T. Translating IL-6 biology into effective treatments. NAT REV RHEUMATOL. 2020;16:335–345. doi: 10.1038/s41584-020-0419-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Majidpoor J., Mortezaee K. Interleukin-6 in SARS-CoV-2 induced disease: Interactions and therapeutic applications. BIOMED PHARMACOTHER. 2022;145 doi: 10.1016/j.biopha.2021.112419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slaats J., Ten O.J., van de Veerdonk F.L., Netea M.G. IL-1β/IL-6/CRP and IL-18/ferritin: Distinct Inflammatory Programs in Infections. PLOS PATHOG. 2016;12 doi: 10.1371/journal.ppat.1005973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka T., Narazaki M., Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6 doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Timper K., Denson J.L., Steculorum S.M., Heilinger C., Engström-Ruud L., Wunderlich C.M., Rose-John S., Wunderlich F.T., Brüning J.C. IL-6 Improves Energy and Glucose Homeostasis in Obesity via Enhanced Central IL-6 trans-Signaling. CELL REP. 2017;19:267–280. doi: 10.1016/j.celrep.2017.03.043. [DOI] [PubMed] [Google Scholar]
- 14.Bertholdt L., Gudiksen A., Ringholm S., Pilegaard H. Impact of skeletal muscle IL-6 on subcutaneous and visceral adipose tissue metabolism immediately after high- and moderate-intensity exercises. Pflugers Arch. 2020;472:217–233. doi: 10.1007/s00424-019-02332-w. [DOI] [PubMed] [Google Scholar]
- 15.Botto S., Streblow D.N., DeFilippis V., White L., Kreklywich C.N., Smith P.P., Caposio P. IL-6 in human cytomegalovirus secretome promotes angiogenesis and survival of endothelial cells through the stimulation of survivin. BLOOD. 2011;117:352–361. doi: 10.1182/blood-2010-06-291245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krook A. IL-6 and metabolism-new evidence and new questions. DIABETOLOGIA. 2008;51:1097–1099. doi: 10.1007/s00125-008-1019-7. [DOI] [PubMed] [Google Scholar]
- 17.Guzmán-Beltrán S., Herrera M.T., Torres M., Gonzalez Y. CD33 is downregulated by influenza virus H1N1pdm09 and induces ROS and the TNF-α, IL-1β, and IL-6 cytokines in human mononuclear cells. BRAZ J MICROBIOL. 2022;53:89–97. doi: 10.1007/s42770-021-00663-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rose-John S. Interleukin-6 Family Cytokines. Cold Spring Harb Perspect Biol. 2018;10 doi: 10.1101/cshperspect.a028415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rose-John S. Therapeutic targeting of IL-6 trans-signaling. CYTOKINE. 2021;144 doi: 10.1016/j.cyto.2021.155577. [DOI] [PubMed] [Google Scholar]
- 20.Kaur S., Bansal Y., Kumar R., Bansal G. A panoramic review of IL-6: Structure, pathophysiological roles and inhibitors. Bioorg Med Chem. 2020;28 doi: 10.1016/j.bmc.2020.115327. [DOI] [PubMed] [Google Scholar]
- 21.Trovato M., Sciacchitano S., Facciolà A., Valenti A., Visalli G., Di Pietro A. Interleukin-6 signalling as a valuable cornerstone for molecular medicine (Review) INT J MOL MED. 2021;47 doi: 10.3892/ijmm.2021.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murakami M., Kamimura D., Hirano T. Pleiotropy and Specificity: Insights from the Interleukin 6 Family of Cytokines. IMMUNITY. 2019;50:812–831. doi: 10.1016/j.immuni.2019.03.027. [DOI] [PubMed] [Google Scholar]
- 23.Van Snick J., Cayphas S., Szikora J.P., Renauld J.C., Van Roost E., Boon T., Simpson R.J. cDNA cloning of murine interleukin-HP1: homology with human interleukin 6. EUR J IMMUNOL. 1988;18:193–197. doi: 10.1002/eji.1830180202. [DOI] [PubMed] [Google Scholar]
- 24.Ren Y., Feng J., Qu H., Li S., Shen B. Three-dimensional structure and function study on the active region in the extracellular ligand-binding domain of human IL-6 receptor. Sci China C Life Sci. 2000;43:425–432. doi: 10.1007/BF02879308. [DOI] [PubMed] [Google Scholar]
- 25.Tu P.C., Li C.T., Lin W.C., Chen M.H., Su T.P., Bai Y.M. Structural and functional correlates of serum soluble IL-6 receptor level in patients with bipolar disorder. J Affect Disord. 2017;219:172–177. doi: 10.1016/j.jad.2017.04.036. [DOI] [PubMed] [Google Scholar]
- 26.Heink S., Yogev N., Garbers C., Herwerth M., Aly L., Gasperi C., Husterer V., Croxford A.L., Möller-Hackbarth K., Bartsch H.S., Sotlar K., Krebs S., Regen T., Blum H., Hemmer B., Misgeld T., Wunderlich T.F., Hidalgo J., Oukka M., Rose-John S., Schmidt-Supprian M., Waisman A., Korn T. Trans-presentation of IL-6 by dendritic cells is required for the priming of pathogenic T(H)17 cells. NAT IMMUNOL. 2017;18:74–85. doi: 10.1038/ni.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J., Sun Q., Zhang J., Wang H., Liu H. Classical Signaling and Trans-Signaling Pathways Stimulated by Megalobrama amblycephala IL-6 and IL-6R. INT J MOL SCI. 2022;23 doi: 10.3390/ijms23042019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang S., Kishimoto T. Interplay between interleukin-6 signaling and the vascular endothelium in cytokine storms. EXP MOL MED. 2021;53:1116–1123. doi: 10.1038/s12276-021-00649-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jarlborg M., Gabay C. Systemic effects of IL-6 blockade in rheumatoid arthritis beyond the joints. CYTOKINE. 2022;149 doi: 10.1016/j.cyto.2021.155742. [DOI] [PubMed] [Google Scholar]
- 30.Kang S., Narazaki M., Metwally H., Kishimoto T. Historical overview of the interleukin-6 family cytokine. J EXP MED. 2020;217 doi: 10.1084/jem.20190347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rose-John S. Blocking only the bad side of IL-6 in inflammation and cancer. CYTOKINE. 2021;148 doi: 10.1016/j.cyto.2021.155690. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka T., Narazaki M., Kishimoto T. Interleukin (IL-6) Immunotherapy. Cold Spring Harb Perspect Biol. 2018;10 doi: 10.1101/cshperspect.a028456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirano T. IL-6 in inflammation, autoimmunity and cancer. INT IMMUNOL. 2021;33:127–148. doi: 10.1093/intimm/dxaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanaka T., Narazaki M., Masuda K., Kishimoto T. Regulation of IL-6 in Immunity and Diseases. ADV EXP MED BIOL. 2016;941:79–88. doi: 10.1007/978-94-024-0921-5_4. [DOI] [PubMed] [Google Scholar]
- 35.Uciechowski P., Dempke W. Interleukin-6: A Masterplayer in the Cytokine Network. Oncology. 2020;98:131–137. doi: 10.1159/000505099. [DOI] [PubMed] [Google Scholar]
- 36.Yao X., Huang J., Zhong H., Shen N., Faggioni R., Fung M., Yao Y. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol Ther. 2014;141:125–139. doi: 10.1016/j.pharmthera.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Pandolfi F., Franza L., Carusi V., Altamura S., Andriollo G., Nucera E. Interleukin-6 in Rheumatoid Arthritis. INT J MOL SCI. 2020;21 doi: 10.3390/ijms21155238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones B.E., Maerz M.D., Buckner J.H. IL-6: a cytokine at the crossroads of autoimmunity. CURR OPIN IMMUNOL. 2018;55:9–14. doi: 10.1016/j.coi.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kishimoto T., Kang S. IL-6 Revisited: From Rheumatoid Arthritis to CAR T Cell Therapy and COVID-19. ANNU REV IMMUNOL. 2022;40:323–348. doi: 10.1146/annurev-immunol-101220-023458. [DOI] [PubMed] [Google Scholar]
- 40.Atzeni F., Nucera V., Masala I.F., Sarzi-Puttini P., Bonitta G. Il-6 Involvement in pain, fatigue and mood disorders in rheumatoid arthritis and the effects of Il-6 inhibitor sarilumab. PHARMACOL RES. 2019;149 doi: 10.1016/j.phrs.2019.104402. [DOI] [PubMed] [Google Scholar]
- 41.McConnell M.J., Kawaguchi N., Kondo R., Sonzogni A., Licini L., Valle C., Bonaffini P.A., Sironi S., Alessio M.G., Previtali G., Seghezzi M., Zhang X., Lee A.I., Pine A.B., Chun H.J., Zhang X., Fernandez-Hernando C., Qing H., Wang A., Price C., Sun Z., Utsumi T., Hwa J., Strazzabosco M., Iwakiri Y. Liver injury in COVID-19 and IL-6 trans-signaling-induced endotheliopathy. J HEPATOL. 2021;75:647–658. doi: 10.1016/j.jhep.2021.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weber R., Groth C., Lasser S., Arkhypov I., Petrova V., Altevogt P., Utikal J., Umansky V. IL-6 as a major regulator of MDSC activity and possible target for cancer immunotherapy. CELL IMMUNOL. 2021;359 doi: 10.1016/j.cellimm.2020.104254. [DOI] [PubMed] [Google Scholar]
- 43.Johnson D.E., O'Keefe R.A., Grandis J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. NAT REV CLIN ONCOL. 2018;15:234–248. doi: 10.1038/nrclinonc.2018.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumari N., Dwarakanath B.S., Das A., Bhatt A.N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. 2016;37:11553–11572. doi: 10.1007/s13277-016-5098-7. [DOI] [PubMed] [Google Scholar]
- 45.Zou S., Tong Q., Liu B., Huang W., Tian Y., Fu X. Targeting STAT3 in Cancer Immunotherapy. MOL CANCER. 2020;19:145. doi: 10.1186/s12943-020-01258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Werner-Klein M., Grujovic A., Irlbeck C., Obradović M., Hoffmann M., Koerkel-Qu H., Lu X., Treitschke S., Köstler C., Botteron C., Weidele K., Werno C., Polzer B., Kirsch S., Gužvić M., Warfsmann J., Honarnejad K., Czyz Z., Feliciello G., Blochberger I., Grunewald S., Schneider E., Haunschild G., Patwary N., Guetter S., Huber S., Rack B., Harbeck N., Buchholz S., Rümmele P., Heine N., Rose-John S., Klein C.A. Interleukin-6 trans-signaling is a candidate mechanism to drive progression of human DCCs during clinical latency. NAT COMMUN. 2020;11:4977. doi: 10.1038/s41467-020-18701-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jones S.A., Jenkins B.J. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. NAT REV IMMUNOL. 2018;18:773–789. doi: 10.1038/s41577-018-0066-7. [DOI] [PubMed] [Google Scholar]
- 48.Waage A., Brandtzaeg P., Halstensen A., Kierulf P., Espevik T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J EXP MED. 1989;169:333–338. doi: 10.1084/jem.169.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mortezaee K., Majidpoor J. CD8+ T Cells in SARS-CoV-2 Induced Disease and Cancer-Clinical Perspectives. FRONT IMMUNOL. 2022;13 doi: 10.3389/fimmu.2022.864298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.MacDonell S.O., Miller J.C., Harper M.J., Reid M.R., Haszard J.J., Gibson R.S., Houghton L.A. A comparison of methods for adjusting biomarkers of iron, zinc, and selenium status for the effect of inflammation in an older population: a case for interleukin 6. AM J CLIN NUTR. 2018;107:932–940. doi: 10.1093/ajcn/nqy052. [DOI] [PubMed] [Google Scholar]
- 51.Garbers C., Heink S., Korn T., Rose-John S. Interleukin-6: designing specific therapeutics for a complex cytokine. NAT REV DRUG DISCOV. 2018;17:395–412. doi: 10.1038/nrd.2018.45. [DOI] [PubMed] [Google Scholar]
- 52.Rosas I.O., Bräu N., Waters M., Go R.C., Hunter B.D., Bhagani S., Skiest D., Aziz M.S., Cooper N., Douglas I.S., Savic S., Youngstein T., Del S.L., Cubillo G.A., De La Zerda D.J., Ustianowski A., Bao M., Dimonaco S., Graham E., Matharu B., Spotswood H., Tsai L., Malhotra A. Tocilizumab in Hospitalized Patients with Severe Covid-19 Pneumonia. N Engl J Med. 2021;384:1503–1516. doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sheppard M., Laskou F., Stapleton P.P., Hadavi S., Dasgupta B. Tocilizumab. ActemraHum Vaccin Immunother. 2017;13:1972–1988. doi: 10.1080/21645515.2017.1316909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lehrskov L.L., Christensen R.H. The role of interleukin-6 in glucose homeostasis and lipid metabolism. SEMIN IMMUNOPATHOL. 2019;41:491–499. doi: 10.1007/s00281-019-00747-2. [DOI] [PubMed] [Google Scholar]
- 55.Norooznezhad A.H., Mansouri K. Endothelial cell dysfunction, coagulation, and angiogenesis in coronavirus disease 2019 (COVID-19) MICROVASC RES. 2021;137 doi: 10.1016/j.mvr.2021.104188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Giraldez M.D., Carneros D., Garbers C., Rose-John S., Bustos M. New insights into IL-6 family cytokines in metabolism, hepatology and gastroenterology. Nat Rev Gastroenterol Hepatol. 2021;18:787–803. doi: 10.1038/s41575-021-00473-x. [DOI] [PubMed] [Google Scholar]
- 57.Bertholdt L., Gudiksen A., Schwartz C.L., Knudsen J.G., Pilegaard H. Lack of skeletal muscle IL-6 influences hepatic glucose metabolism in mice during prolonged exercise. Am J Physiol Regul Integr Comp Physiol. 2017;312:R626–R636. doi: 10.1152/ajpregu.00373.2016. [DOI] [PubMed] [Google Scholar]
- 58.Dudek M., Lohr K., Donakonda S., Baumann T., Lüdemann M., Hegenbarth S., Dübbel L., Eberhagen C., Michailidou S., Yassin A., Prinz M., Popper B., Rose-John S., Zischka H., Knolle P.A. IL-6-induced FOXO1 activity determines the dynamics of metabolism in CD8 T cells cross-primed by liver sinusoidal endothelial cells. CELL REP. 2022;38 doi: 10.1016/j.celrep.2022.110389. [DOI] [PubMed] [Google Scholar]
- 59.Kato S., Tanabe N., Nagao M., Sekino J., Tomita K., Sakai M., Abe K., Suzuki N., Ueda K. Glucose transporter 4 mediates LPS-induced IL-6 production in osteoblasts under high glucose conditions. J ORAL SCI. 2020;62:423–426. doi: 10.2334/josnusd.20-0010. [DOI] [PubMed] [Google Scholar]
- 60.Molinero A., Fernandez-Perez A., Mogas A., Giralt M., Comes G., Fernandez-Gayol O., Vallejo M., Hidalgo J. Role of muscle IL-6 in gender-specific metabolism in mice. PLOS ONE. 2017;12 doi: 10.1371/journal.pone.0173675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.von Loeffelholz C., Lieske S., Neuschäfer-Rube F., Willmes D.M., Raschzok N., Sauer I.M., König J., Fromm M.F., Horn P., Chatzigeorgiou A., Pathe-Neuschäfer-Rube A., Jordan J., Pfeiffer A., Mingrone G., Bornstein S.R., Stroehle P., Harms C., Wunderlich F.T., Helfand S.L., Bernier M., de Cabo R., Shulman G.I., Chavakis T., Püschel G.P., Birkenfeld A.L. The human longevity gene homolog INDY and interleukin-6 interact in hepatic lipid metabolism. HEPATOLOGY. 2017;66:616–630. doi: 10.1002/hep.29089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kistner T.M., Pedersen B.K., Lieberman D.E. Interleukin 6 as an energy allocator in muscle tissue. Nat Metab. 2022;4:170–179. doi: 10.1038/s42255-022-00538-4. [DOI] [PubMed] [Google Scholar]
- 63.Herzig S., Shaw R.J. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19:121–135. doi: 10.1038/nrm.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rodríguez C., Muñoz M., Contreras C., Prieto D. AMPK, metabolism, and vascular function. FEBS J. 2021;288:3746–3771. doi: 10.1111/febs.15863. [DOI] [PubMed] [Google Scholar]
- 65.Mishra D., Richard J.E., Maric I., Porteiro B., Häring M., Kooijman S., Musovic S., Eerola K., López-Ferreras L., Peris E., Grycel K., Shevchouk O.T., Micallef P., Olofsson C.S., Wernstedt A.I., Grill H.J., Nogueiras R., Skibicka K.P. Parabrachial Interleukin-6 Reduces Body Weight and Food Intake and Increases Thermogenesis to Regulate Energy Metabolism. CELL REP. 2019;26:3011–3026. doi: 10.1016/j.celrep.2019.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu Y., Zhang Y., Ye J. IL-6: A Potential Role in Cardiac Metabolic Homeostasis. INT J MOL SCI. 2018;19 doi: 10.3390/ijms19092474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nakamura H., Miyagi K., Otsuki M., Higure Y., Nishiyama N., Kinjo T., Nakamatsu M., Haranaga S., Tateyama M., Fujita J. Acute Hypertriglyceridaemia Caused by Tocilizumab in a Patient with Severe COVID-19. Intern Med. 2020;59:2945–2949. doi: 10.2169/internalmedicine.5244-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kern L., Mittenbühler M.J., Vesting A.J., Ostermann A.L., Wunderlich C.M., Wunderlich F.T. Obesity-Induced TNFα and IL-6 Signaling: The Missing Link between Obesity and Inflammation-Driven Liver and Colorectal Cancers. Cancers (Basel) 2018;11 doi: 10.3390/cancers11010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rohm T.V., Meier D.T., Olefsky J.M., Donath M.Y. Inflammation in obesity, diabetes, and related disorders. IMMUNITY. 2022;55:31–55. doi: 10.1016/j.immuni.2021.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Henning R.J. Obesity and obesity-induced inflammatory disease contribute to atherosclerosis: a review of the pathophysiology and treatment of obesity. Am J Cardiovasc Dis. 2021;11:504–529. [PMC free article] [PubMed] [Google Scholar]
- 71.Noh J.W., Yang H.K., Jun M.S., Lee B.C. Puerarin Attenuates Obesity-Induced Inflammation and Dyslipidemia by Regulating Macrophages and TNF-Alpha in Obese Mice. Biomedicines. 2022;10 doi: 10.3390/biomedicines10010175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu Z., Le Y., Chen H., Zhu J., Lu D. Role of PKM2-Mediated Immunometabolic Reprogramming on Development of Cytokine Storm. FRONT IMMUNOL. 2021;12 doi: 10.3389/fimmu.2021.748573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Palasiewicz K., Umar S., Romay B., Zomorrodi R.K., Shahrara S. Tofacitinib therapy intercepts macrophage metabolic reprogramming instigated by SARS-CoV-2 Spike protein. EUR J IMMUNOL. 2021;51:2330–2340. doi: 10.1002/eji.202049159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Viurcos-Sanabria R., Escobedo G. Immunometabolic bases of type 2 diabetes in the severity of COVID-19, World. J Diabetes. 2021;12:1026–1041. doi: 10.4239/wjd.v12.i7.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Khwatenge C.N., Pate M., Miller L.C., Sang Y. Immunometabolic Dysregulation at the Intersection of Obesity and COVID-19. FRONT IMMUNOL. 2021;12 doi: 10.3389/fimmu.2021.732913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xiao N., Nie M., Pang H., Wang B., Hu J., Meng X., Li K., Ran X., Long Q., Deng H., Chen N., Li S., Tang N., Huang A., Hu Z. Integrated cytokine and metabolite analysis reveals immunometabolic reprogramming in COVID-19 patients with therapeutic implications. NAT COMMUN. 2021;12:1618. doi: 10.1038/s41467-021-21907-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Williams C.G., Jureka A.S., Silvas J.A., Nicolini A.M., Chvatal S.A., Carlson-Stevermer J., Oki J., Holden K., Basler C.F. Inhibitors of VPS34 and fatty-acid metabolism suppress SARS-CoV-2 replication. CELL REP. 2021;36 doi: 10.1016/j.celrep.2021.109479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Calvet-Mirabent M., Sánchez-Cerrillo I., Martín-Cófreces N., Martínez-Fleta P., de la Fuente H., Tsukalov I., Delgado-Arévalo C., Calzada M.J., de Los S.I., Sanz J., García-Fraile L., Sánchez-Madrid F., Alfranca A., Muñoz-Fernández M.Á., Buzón M.J., Martín-Gayo E. Antiretroviral therapy duration and immunometabolic state determine efficacy of ex vivo dendritic cell-based treatment restoring functional HIV-specific CD8+ T cells in people living with HIV. EBIOMEDICINE. 2022;81 doi: 10.1016/j.ebiom.2022.104090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mohan J., Ghazi T., Chuturgoon A.A. A Critical Review of the Biochemical Mechanisms and Epigenetic Modifications in HIV- and Antiretroviral-Induced Metabolic Syndrome. INT J MOL SCI. 2021;22 doi: 10.3390/ijms222112020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aouam A., Marmouch H., Kooli I., Marrakchi W., Hellara I., Neffati F., Najjar F., Chakroun M. Metabolic syndrome among people with HIV in central Tunisia: Prevalence and associated factors. Ann Pharm Fr. 2021;79:465–472. doi: 10.1016/j.pharma.2021.01.005. [DOI] [PubMed] [Google Scholar]
- 81.Shiau S., Yu W., Jacobson D.L., Nichols S., McFarland E.J., Chen J.S., Dirajlal-Fargo S., Surowiec K., Geffner M.E., Jao J. Components of metabolic syndrome associated with lower neurocognitive performance in youth with perinatally acquired HIV and youth who are HIV-exposed uninfected. J NEUROVIROL. 2021;27:702–715. doi: 10.1007/s13365-021-01005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sahagun S.J., Yeramosu T., Purdy J.B., Reynolds J.C., Hadigan C.M. Associations Between Central Obesity and Lifelong Antiviral Therapy in Adults Living With HIV Acquired From Early Childhood. J Acquir Immune Defic Syndr. 2022;89:208–214. doi: 10.1097/QAI.0000000000002841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rose-John S. Local and systemic effects of interleukin-6 (IL-6) in inflammation and cancer. FEBS LETT. 2022;596:557–566. doi: 10.1002/1873-3468.14220. [DOI] [PubMed] [Google Scholar]
- 84.Cui X., Zhao Z., Zhang T., Guo W., Guo W., Zheng J., Zhang J., Dong C., Na R., Zheng L., Li W., Liu Z., Ma J., Wang J., He S., Xu Y., Si P., Shen Y., Cai C. A systematic review and meta-analysis of children with coronavirus disease 2019 (COVID-19) J MED VIROL. 2021;93:1057–1069. doi: 10.1002/jmv.26398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lim Z.J., Subramaniam A., Ponnapa R.M., Blecher G., Kadam U., Afroz A., Billah B., Ashwin S., Kubicki M., Bilotta F., Curtis J.R., Rubulotta F. Case Fatality Rates for Patients with COVID-19 Requiring Invasive Mechanical Ventilation. A Meta-analysis, Am J Respir Crit Care Med. 2021;203:54–66. doi: 10.1164/rccm.202006-2405OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Potere N., Batticciotto A., Vecchié A., Porreca E., Cappelli A., Abbate A., Dentali F., Bonaventura A. The role of IL-6 and IL-6 blockade in COVID-19. Expert Rev Clin Immunol. 2021;17:601–618. doi: 10.1080/1744666X.2021.1919086. [DOI] [PubMed] [Google Scholar]
- 87.Liu B., Li M., Zhou Z., Guan X., Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J AUTOIMMUN. 2020;111 doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fu B., Xu X., Wei H. Why tocilizumab could be an effective treatment for severe COVID-19? J TRANSL MED. 2020;18:164. doi: 10.1186/s12967-020-02339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shankar-Hari M., Vale C.L., Godolphin P.J., Fisher D., Higgins J., Spiga F., Savovic J., Tierney J., Baron G., Benbenishty J.S., Berry L.R., Broman N., Cavalcanti A.B., Colman R., De Buyser S.L., Derde L., Domingo P., Omar S.F., Fernandez-Cruz A., Feuth T., Garcia F., Garcia-Vicuna R., Gonzalez-Alvaro I., Gordon A.C., Haynes R., Hermine O., Horby P.W., Horick N.K., Kumar K., Lambrecht B.N., Landray M.J., Leal L., Lederer D.J., Lorenzi E., Mariette X., Merchante N., Misnan N.A., Mohan S.V., Nivens M.C., Oksi J., Perez-Molina J.A., Pizov R., Porcher R., Postma S., Rajasuriar R., Ramanan A.V., Ravaud P., Reid P.D., Rutgers A., Sancho-Lopez A., Seto T.B., Sivapalasingam S., Soin A.S., Staplin N., Stone J.H., Strohbehn G.W., Sunden-Cullberg J., Torre-Cisneros J., Tsai L.W., van Hoogstraten H., van Meerten T., Veiga V.C., Westerweel P.E., Murthy S., Diaz J.V., Marshall J.C., Sterne J. Association Between Administration of IL-6 Antagonists and Mortality Among Patients Hospitalized for COVID-19: A Meta-analysis. JAMA. 2021;326:499–518. doi: 10.1001/jama.2021.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu F., Li L., Xu M., Wu J., Luo D., Zhu Y., Li B., Song X., Zhou X. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J CLIN VIROL. 2020;127 doi: 10.1016/j.jcv.2020.104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dhall A., Patiyal S., Sharma N., Usmani S.S., Raghava G. Computer-aided prediction and design of IL-6 inducing peptides: IL-6 plays a crucial role in COVID-19. BRIEF BIOINFORM. 2021;22:936–945. doi: 10.1093/bib/bbaa259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fujino M., Ishii M., Taniguchi T., Chiba H., Kimata M., Hitosugi M. The Value of Interleukin-6 among Several Inflammatory Markers as a Predictor of Respiratory Failure in COVID-19 Patients. Diagnostics (Basel) 2021;11 doi: 10.3390/diagnostics11081327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Atal S., Fatima Z. IL-6 Inhibitors in the Treatment of Serious COVID-19: A Promising Therapy? Pharmaceut Med. 2020;34:223–231. doi: 10.1007/s40290-020-00342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Matthay M.A., Luetkemeyer A.F. IL-6 Receptor Antagonist Therapy for Patients Hospitalized for COVID-19: Who, When, and How? JAMA. 2021;326:483–485. doi: 10.1001/jama.2021.11121. [DOI] [PubMed] [Google Scholar]
- 95.Herold T., Jurinovic V., Arnreich C., Lipworth B.J., Hellmuth J.C., von Bergwelt-Baildon M., Klein M., Weinberger T. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol. 2020;146:128–136. doi: 10.1016/j.jaci.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jagtap D., Bhor V.M., Bhowmick S., Kasarpalkar N., Sagvekar P., Kulkarni B., Pathak M., Chatterjee N., Dolas P., Palav H., Kaginkar S., Bhagat S., Munshi I., Parikh S., Agrawal S., Pawar C., Kaneria M., Mahale S.D., Shastri J., Patel V. sMAdCAM: IL-6 Ratio Influences Disease Progression and Anti-Viral Responses in SARS-CoV-2 Infection. FRONT IMMUNOL. 2021;12 doi: 10.3389/fimmu.2021.619906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wu Z.B., Zheng Y.B., Wang K., Mo Z.S., Zhen X., Yan Y., Gao Z.L. Plasma Interleukin-6 Level: A Potential Prognostic Indicator of Emergent HBV-Associated ACLF. Can J Gastroenterol Hepatol. 2021;2021:5545181. doi: 10.1155/2021/5545181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhou X., Yang F., Yang Y., Hu Y., Liu W., Huang C., Li S., Chen Z. HBV Facilitated Hepatocellular Carcinoma Cells Proliferation by Up-Regulating Angiogenin Expression Through IL-6. CELL PHYSIOL BIOCHEM. 2018;46:461–470. doi: 10.1159/000488614. [DOI] [PubMed] [Google Scholar]
- 99.Hu Z., Luo D., Wang D., Ma L., Zhao Y., Li L. IL-17 Activates the IL-6/STAT3 Signal Pathway in the Proliferation of Hepatitis B Virus-Related Hepatocellular Carcinoma. CELL PHYSIOL BIOCHEM. 2017;43:2379–2390. doi: 10.1159/000484390. [DOI] [PubMed] [Google Scholar]
- 100.Xu J., Lin H., Wu G., Zhu M., Li M. IL-6/STAT3 Is a Promising Therapeutic Target for Hepatocellular Carcinoma. FRONT ONCOL. 2021;11 doi: 10.3389/fonc.2021.760971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yin Z., Ma T., Lin Y., Lu X., Zhang C., Chen S., Jian Z. IL-6/STAT3 pathway intermediates M1/M2 macrophage polarization during the development of hepatocellular carcinoma. J CELL BIOCHEM. 2018;119:9419–9432. doi: 10.1002/jcb.27259. [DOI] [PubMed] [Google Scholar]
- 102.Pu X.Y., Zheng D.F., Shen A., Gu H.T., Wei X.F., Mou T., Zhang J.B., Liu R. IL-37b suppresses epithelial mesenchymal transition in hepatocellular carcinoma by inhibiting IL-6/STAT3 signaling. Hepatobiliary Pancreat Dis Int. 2018;17:408–415. doi: 10.1016/j.hbpd.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 103.Mortezaee K., Majidpoor J. (Im)maturity in Tumor Ecosystem. FRONT ONCOL. 2022;11 doi: 10.3389/fonc.2021.813897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu H., Shen J., Lu K. IL-6 and PD-L1 blockade combination inhibits hepatocellular carcinoma cancer development in mouse model. Biochem Biophys Res Commun. 2017;486:239–244. doi: 10.1016/j.bbrc.2017.02.128. [DOI] [PubMed] [Google Scholar]
- 105.Mortezaee K., Majidpoor J. Checkpoint inhibitor/interleukin-based combination therapy of cancer. Cancer Med. 2022 doi: 10.1002/cam4.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mortezaee K., Majidpoor J. Roles for macrophage-polarizing interleukins in cancer immunity and immunotherapy. Cell Oncol (Dordr) 2022 doi: 10.1007/s13402-022-00667-8. [DOI] [PubMed] [Google Scholar]
- 107.Wan S., Zhao E., Kryczek I., Vatan L., Sadovskaya A., Ludema G., Simeone D.M., Zou W., Welling T.H. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. GASTROENTEROLOGY. 2014;147:1393–1404. doi: 10.1053/j.gastro.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen D., Zhang X., Li Z., Zhu B. Metabolic regulatory crosstalk between tumor microenvironment and tumor-associated macrophages. THERANOSTICS. 2021;11:1016–1030. doi: 10.7150/thno.51777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hamlyn E., Fidler S., Stöhr W., Cooper D.A., Tambussi G., Schechter M., Miro J.M., Mcclure M., Weber J., Babiker A., Porter K. Interleukin-6 and D-dimer levels at seroconversion as predictors of HIV-1 disease progression. AIDS. 2014;28:869–874. doi: 10.1097/QAD.0000000000000155. [DOI] [PubMed] [Google Scholar]
- 110.Okay G., Koc M.M., Guler E.M., Yabaci A., Kocyigit A., Akkoyunlu Y. The Effect of Antiretroviral Therapy on IL-6, IL-1β, TNF-α, IFN-γ Levels and their Relationship with HIV-RNA and CD4+ T Cells in HIV Patients. CURR HIV RES. 2020;18:354–361. doi: 10.2174/1570162X18666200712174642. [DOI] [PubMed] [Google Scholar]
- 111.Lv L., Wang Q., Xu Y., Tsao L.C., Nakagawa T., Guo H., Su L., Xiong Y. Vpr Targets TET2 for Degradation by CRL4(VprBP) E3 Ligase to Sustain IL-6 Expression and Enhance HIV-1 Replication. MOL CELL. 2018;70:961–970. doi: 10.1016/j.molcel.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Borges Á.H., O'Connor J.L., Phillips A.N., Rönsholt F.F., Pett S., Vjecha M.J., French M.A., Lundgren J.D. Factors Associated With Plasma IL-6 Levels During HIV Infection. J INFECT DIS. 2015;212:585–595. doi: 10.1093/infdis/jiv123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Borges A.H., O'Connor J.L., Phillips A.N., Rönsholt F.F., Pett S., Vjecha M.J., French M.A., Lundgren J.D. Determinants of IL-6 levels during HIV infection. J INT AIDS SOC. 2014;17:19482. doi: 10.7448/IAS.17.4.19482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sindhu S., Thomas R., Shihab P., Sriraman D., Behbehani K., Ahmad R. Obesity Is a Positive Modulator of IL-6R and IL-6 Expression in the Subcutaneous Adipose Tissue: Significance for Metabolic Inflammation. PLOS ONE. 2015;10 doi: 10.1371/journal.pone.0133494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Taylor B.S., So-Armah K., Tate J.P., Marconi V.C., Koethe J.R., Bedimo R.J., Butt A.A., Gibert C.L., Goetz M.B., Rodriguez-Barradas M.C., Womack J.A., Gerschenson M., Lo R.V.R., Rimland D., Yin M.T., Leaf D., Tracy R.P., Justice A.C., Freiberg M.S. HIV and Obesity Comorbidity Increase Interleukin 6 but Not Soluble CD14 or D-Dimer. J Acquir Immune Defic Syndr. 2017;75:500–508. doi: 10.1097/QAI.0000000000001444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McDonald B., Moyo S., Gabaitiri L., Gaseitsiwe S., Bussmann H., Koethe J.R., Musonda R., Makhema J., Novitsky V., Marlink R.G., Wester C.W., Essex M. Persistently elevated serum interleukin-6 predicts mortality among adults receiving combination antiretroviral therapy in Botswana: results from a clinical trial. AIDS Res Hum Retroviruses. 2013;29:993–999. doi: 10.1089/aid.2012.0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ali A., Mishra R., Kaur H., Chandra B.A. HIV-1 Tat: An update on transcriptional and non-transcriptional functions. BIOCHIMIE. 2021;190:24–35. doi: 10.1016/j.biochi.2021.07.001. [DOI] [PubMed] [Google Scholar]
- 118.Nookala A.R., Kumar A. Molecular mechanisms involved in HIV-1 Tat-mediated induction of IL-6 and IL-8 in astrocytes. J Neuroinflammation. 2014;11:214. doi: 10.1186/s12974-014-0214-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhou H., Jarujaron S., Gurley E.C., Chen L., Ding H., Studer E., Pandak W.J., Hu W., Zou T., Wang J.Y., Hylemon P.B. HIV protease inhibitors increase TNF-alpha and IL-6 expression in macrophages: involvement of the RNA-binding protein HuR. ATHEROSCLEROSIS. 2007;195:e134–e143. doi: 10.1016/j.atherosclerosis.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 120.Lopes T., Gonçales J.P., Silva J.J., Lorena V., Toscano A., Akamatsu S.M., Salles A.C., Tozetto-Mendoza T.R., Morais V., Coêlho M. Association of IL-6, IL-10 and CXCL10 serum concentrations with visceral Kaposi's sarcoma in people living with HIV/AIDS. HUM IMMUNOL. 2020;81:26–31. doi: 10.1016/j.humimm.2019.11.007. [DOI] [PubMed] [Google Scholar]
- 121.Butsabong T., Felippe M., Campagnolo P., Maringer K. The emerging role of perivascular cells (pericytes) in viral pathogenesis. J GEN VIROL. 2021;102 doi: 10.1099/jgv.0.001634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yaiw K.C., Mohammad A.A., Taher C., Cui H.L., Costa H., Kostopoulou O.N., Jung M., Assinger A., Wilhelmi V., Yang J., Strååt K., Rahbar A., Pernow J., Söderberg-Nauclér C. Human Cytomegalovirus Reduces Endothelin-1 Expression in Both Endothelial and Vascular Smooth Muscle Cells. Microorganisms. 2021;9 doi: 10.3390/microorganisms9061137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Prochnau D., Straube E., Figulla H.R., Rödel J. Supra-additive expression of interleukin-6, interleukin-8 and basic fibroblast growth factor in vascular smooth muscle cells following coinfection with Chlamydia pneumoniae and cytomegalovirus as a novel link between infection and atherosclerosis. Can J Infect Dis Med Microbiol. 2012;23:e26–e30. doi: 10.1155/2012/987476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Droho S., Cuda C.M., Perlman H., Lavine J.A. Macrophage-derived interleukin-6 is necessary and sufficient for choroidal angiogenesis. Sci Rep. 2021;11:18084. doi: 10.1038/s41598-021-97522-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fiorentini S., Luganini A., Dell'Oste V., Lorusso B., Cervi E., Caccuri F., Bonardelli S., Landolfo S., Caruso A., Gribaudo G. Human cytomegalovirus productively infects lymphatic endothelial cells and induces a secretome that promotes angiogenesis and lymphangiogenesis through interleukin-6 and granulocyte-macrophage colony-stimulating factor. J GEN VIROL. 2011;92:650–660. doi: 10.1099/vir.0.025395-0. [DOI] [PubMed] [Google Scholar]
- 126.Slinger E., Maussang D., Schreiber A., Siderius M., Rahbar A., Fraile-Ramos A., Lira S.A., Söderberg-Nauclér C., Smit M.J. HCMV-encoded chemokine receptor US28 mediates proliferative signaling through the IL-6-STAT3 axis. SCI SIGNAL. 2010;3 doi: 10.1126/scisignal.2001180. [DOI] [PubMed] [Google Scholar]
- 127.Wilkerson I., Laban J., Mitchell J.M., Sheibani N., Alcendor D.J. Retinal pericytes and cytomegalovirus infectivity: implications for HCMV-induced retinopathy and congenital ocular disease. J Neuroinflammation. 2015;12:2. doi: 10.1186/s12974-014-0219-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang Y., Zhang X., Zheng X., Song G., Fang L., Wang Y., Wang B. Human cytomegalovirus infection and its association with gestational diabetes mellitus during pregnancy. PEERJ. 2022;10 doi: 10.7717/peerj.12934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Greber U.F., Flatt J.W. Adenovirus Entry: From Infection to Immunity. ANNU REV VIROL. 2019;6:177–197. doi: 10.1146/annurev-virology-092818-015550. [DOI] [PubMed] [Google Scholar]
- 130.Shimizu K., Sakurai F., Iizuka S., Ono R., Tsukamoto T., Nishimae F., Nakamura S.I., Nishinaka T., Terada T., Fujio Y., Mizuguchi H. Adenovirus Vector-Induced IL-6 Promotes Leaky Adenoviral Gene Expression. Leading to Acute Hepatotoxicity, J IMMUNOL. 2021;206:410–421. doi: 10.4049/jimmunol.2000830. [DOI] [PubMed] [Google Scholar]
- 131.Huang H., Chen Y., Ma L.Y., Yan M.M., Deng Y., Zhang W.D., Yuan Y., Xiong P., Fang F., Liu T.L. Analysis of the clinical features and the risk factors of severe adenovirus pneumonia in children. Zhonghua Er Ke Za Zhi. 2021;59:14–19. doi: 10.3760/cma.j.cn112140-20200704-00687. [DOI] [PubMed] [Google Scholar]
- 132.Shen Y., Zhou Y., Ma C., Liu Y., Wei B. Establishment of a predictive nomogram and its validation for severe adenovirus pneumonia in children. Ann Palliat Med. 2021;10:7194–7204. doi: 10.21037/apm-21-675. [DOI] [PubMed] [Google Scholar]
- 133.Chen I.C., Wang S.C., Chen Y.T., Tseng H.H., Liu P.L., Lin T.C., Wu H.E., Chen Y.R., Tseng Y.H., Hsu J.H., Dai Z.K., Suen J.L., Li C.Y. Corylin Ameliorates LPS-Induced Acute Lung Injury via Suppressing the MAPKs and IL-6/STAT3 Signaling Pathways. Pharmaceuticals (Basel) 2021;14 doi: 10.3390/ph14101046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Conti P., Ronconi G., Caraffa A., Gallenga C.E., Ross R., Frydas I., Kritas S.K. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34:327–331. doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 135.Lim J.U., Choi J.Y., Jeong H.J., Ko J.H., Lee J.E., Rhee C.K. Comparison of clinical characteristics and inflammatory cytokines between hypoxemic and non-hypoxemic human adenovirus 55 pneumonia. J THORAC DIS. 2020;12:4044–4056. doi: 10.21037/jtd-19-4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chen Q., Liu J., Liang W., Chen Y., Dou M., Liu Z., Chen Y., Zheng Z., Zhu B., Lin Y. Clinical Features, Replication Competence, and Innate Immune Responses of Human Adenovirus Type 7 Infection. J INFECT DIS. 2021;223:1390–1399. doi: 10.1093/infdis/jiaa524. [DOI] [PubMed] [Google Scholar]
- 137.Qi L., Wang Y., Wang H., Deng J. Adenovirus 7 Induces Interlukin-6 Expression in Human Airway Epithelial Cells via p38/NF-κB Signaling Pathway. FRONT IMMUNOL. 2020;11 doi: 10.3389/fimmu.2020.551413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ren X., Wang L., Chen Z., Hou D., Xue Y., Diao X., Shen Q. Foxtail Millet Improves Blood Glucose Metabolism in Diabetic Rats through PI3K/AKT and NF-κB Signaling Pathways Mediated by Gut Microbiota. NUTRIENTS. 2021;13 doi: 10.3390/nu13061837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Barnabei L., Laplantine E., Mbongo W., Rieux-Laucat F., Weil R. NF-κB: At the Borders of Autoimmunity and Inflammation. FRONT IMMUNOL. 2021;12 doi: 10.3389/fimmu.2021.716469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pan L., Liu C., Liu Q., Li Y., Du C., Kang X., Dong S., Zhou Z., Chen H., Liang X., Chu J., Xu Y., Zhang Q. Human Wharton's jelly-derived mesenchymal stem cells alleviate concanavalin A-induced fulminant hepatitis by repressing NF-κB signaling and glycolysis. STEM CELL RES THER. 2021;12:496. doi: 10.1186/s13287-021-02560-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kocazeybek B., Dinc H.O., Ergin S., Saribas S., Ozcabi B.T., Cizmecigil U., Altan E., Atalik K., Yüksel P., Taner Z., Karakullukcu A., Sirekbasan S., Turan N., Cagatay P., Imamova N., Evliyaoglu O., Yilmaz H. Evaluation of Adenovirus-36 (Ad-36) antibody seropositivity and adipokine levels in obese children. Microb Pathog. 2017;108:27–31. doi: 10.1016/j.micpath.2017.04.034. [DOI] [PubMed] [Google Scholar]
- 142.Swain S.K., Gadnayak A., Mohanty J.N., Sarangi R., Das J. REV MED VIROL; Challenges and current opinion: 2022. Does enterovirus 71 urge for effective vaccine control strategies? p. e2322. [DOI] [PubMed] [Google Scholar]
- 143.Dong Y., Liu J., Lu N., Zhang C. Enterovirus 71 Antagonizes Antiviral Effects of Type III Interferon and Evades the Clearance of Intestinal Intraepithelial Lymphocytes. FRONT MICROBIOL. 2021;12 doi: 10.3389/fmicb.2021.806084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liao Y.T., Tsai H.P., Wang S.M., Chen S.H. Clinical and Immune Responses of Peripheral Chemical Sympathectomy in Enterovirus 71 Infection. FRONT IMMUNOL. 2021;12 doi: 10.3389/fimmu.2021.700903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chen Z., Li R., Xie Z., Huang G., Yuan Q., Zeng J. IL-6, IL-10 and IL-13 are associated with pathogenesis in children with Enterovirus 71 infection. INT J CLIN EXP MED. 2014;7:2718–2723. [PMC free article] [PubMed] [Google Scholar]
- 146.Liu J., Lu X.C., Zhou W.D. Elevated circulating miR-494 in plasma of children with enterovirus 71 induced hand, foot, and mouth disease and its potential diagnostic value. ACTA VIROL. 2020;64:338–343. doi: 10.4149/av_2020_311. [DOI] [PubMed] [Google Scholar]
- 147.Ye N., Gong X., Pang L.L., Gao W.J., Zhang Y.T., Li X.L., Liu N., Li D.D., Jin Y., Duan Z.J. Cytokine responses and correlations thereof with clinical profiles in children with enterovirus 71 infections. BMC INFECT DIS. 2015;15:225. doi: 10.1186/s12879-015-0965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lee J.Y., Son M., Kang J.H., Choi U.Y. Serum interleukin-6 levels as an indicator of aseptic meningitis among children with enterovirus 71-induced hand, foot and mouth disease. POSTGRAD MED. 2018;130:258–263. doi: 10.1080/00325481.2018.1416257. [DOI] [PubMed] [Google Scholar]
- 149.Cao L., Zhang X., Yuan S., Cheng K., Zhang X. Autophagy induced by enterovirus 71 regulates the production of IL-6 through the p38MAPK and ERK signaling pathways. Microb Pathog. 2019;131:120–127. doi: 10.1016/j.micpath.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 150.Lin P.W., Chu M.L., Liu H.S. Autophagy and metabolism. KAOHSIUNG J MED SCI. 2021;37:12–19. doi: 10.1002/kjm2.12299. [DOI] [PubMed] [Google Scholar]
- 151.White E., Lattime E.C., Guo J.Y. Autophagy Regulates Stress Responses, Metabolism, and Anticancer Immunity, Trends. Cancer. 2021;7:778–789. doi: 10.1016/j.trecan.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Luo Z., Su R., Wang W., Liang Y., Zeng X., Shereen M.A., Bashir N., Zhang Q., Zhao L., Wu K., Liu Y., Wu J. EV71 infection induces neurodegeneration via activating TLR7 signaling and IL-6 production. PLOS PATHOG. 2019;15 doi: 10.1371/journal.ppat.1008142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Green D.R. Ghostly metabolic messages from dying cells. NATURE. 2020;580:36–37. doi: 10.1038/d41586-020-00641-0. [DOI] [PubMed] [Google Scholar]
- 154.Roy S.K., Bhattacharjee S. Dengue virus: epidemiology, biology, and disease aetiology. CAN J MICROBIOL. 2021;67:687–702. doi: 10.1139/cjm-2020-0572. [DOI] [PubMed] [Google Scholar]
- 155.Norshidah H., Vignesh R., Lai N.S. Updates on Dengue Vaccine and Antiviral: Where Are We Heading? MOLECULES. 2021;26 doi: 10.3390/molecules26226768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rattanaburee T., Junking M., Panya A., Sawasdee N., Songprakhon P., Suttitheptumrong A., Limjindaporn T., Haegeman G., Yenchitsomanus P.T. Inhibition of dengue virus production and cytokine/chemokine expression by ribavirin and compound A. Antiviral Res. 2015;124:83–92. doi: 10.1016/j.antiviral.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 157.Masood K.I., Jamil B., Rahim M., Islam M., Farhan M., Hasan Z. Role of TNF α, IL-6 and CXCL10 in Dengue disease severity, Iran. J Microbiol. 2018;10:202–207. [PMC free article] [PubMed] [Google Scholar]
- 158.Huang Y.H., Lei H.Y., Liu H.S., Lin Y.S., Liu C.C., Yeh T.M. Dengue virus infects human endothelial cells and induces IL-6 and IL-8 production. AM J TROP MED HYG. 2000;63:71–75. doi: 10.4269/ajtmh.2000.63.71. [DOI] [PubMed] [Google Scholar]
- 159.Lee Y.R., Su C.Y., Chow N.H., Lai W.W., Lei H.Y., Chang C.L., Chang T.Y., Chen S.H., Lin Y.S., Yeh T.M., Liu H.S. Dengue viruses can infect human primary lung epithelia as well as lung carcinoma cells, and can also induce the secretion of IL-6 and RANTES. VIRUS RES. 2007;126:216–225. doi: 10.1016/j.virusres.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 160.Fiestas S.V., Da C.F.N., Dos S.C., Corrêa G., Cipitelli M., Dornelas R.M., de Souza L.J., Damasco P.V., Da C.R., Dos S.F., de Oliveira P.L., de Azeredo E.L. Different Profiles of Cytokines, Chemokines and Coagulation Mediators Associated with Severity in Brazilian Patients Infected with Dengue Virus. Viruses. 2021;13 doi: 10.3390/v13091789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Eljilany I., Elzouki A.N. D-Dimer, Fibrinogen, and IL-6 in COVID-19 Patients with Suspected Venous Thromboembolism: A Narrative Review. Vasc Health Risk Manag. 2020;16:455–462. doi: 10.2147/VHRM.S280962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Qi X., Keith K.A., Huang J.H. COVID-19 and stroke: A review. Brain Hemorrhages. 2021;2:76–83. doi: 10.1016/j.hest.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kobayashi S., Koizume S., Takahashi T., Ueno M., Oishi R., Nagashima S., Sano Y., Fukushima T., Tezuka S., Morimoto M., Nakamura S., Narimatsu H., Ruf W., Miyagi Y. Tissue factor and its procoagulant activity on cancer-associated thromboembolism in pancreatic cancer. CANCER SCI. 2021;112:4679–4691. doi: 10.1111/cas.15106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Samad F., Ruf W. Inflammation, obesity, and thrombosis. BLOOD. 2013;122:3415–3422. doi: 10.1182/blood-2013-05-427708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Edén D., Panagiotou G., Mokhtari D., Eriksson J.W., Åberg M., Siegbahn A. Adipocytes express tissue factor and FVII and are procoagulant in a TF/FVIIa-dependent manner. Ups J Med Sci. 2019;124:158–167. doi: 10.1080/03009734.2019.1645248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.
Data sharing is not applicable to this article, as no data sets were generated or analyzed during the current study.