Abstract
Recombinant human endostatin (Endostar) plus vinorelbine/cisplatin (NP) had been approved for the treatment of non-small cell lung cancers (NSCLC). But the real-world treatment pattern and effectiveness of Endostar plus other combination chemotherapy, namely docetaxel/platinum (DP), gemcitabine/platinum (GP), pemetrexed/platinum (PP), and paclitaxel/platinum (TP) in both treatment-naïve and re-treatment patients with advanced NSCLC were still unclear. A retrospective observational study was conducted based on the electronic medical record (EMR) system and advanced patients with NSCLC were identified from 7 cancer hospitals in China from 2012 to 2019. These patients were divided into five groups, Endostar plus NP, Endostar plus DP, Endostar plus GP, Endostar plus PP, and Endostar plus TP groups. The disease control rate (DCR), overall response rate (ORR), and the progression-free survival (PFS) were evaluated. Of the eligible 512 advanced patients with NSCLC, 10.35% were in Endostar plus NP group, while the numbers were 15.43%, 32.42%, 26.56%, 15.23% in Endostar plus DP group, Endostar plus GP group, Endostar plus PP group, and Endostar plus TP group, respectively. The ORRs were 31%, 28%, 22%, 41% and 27%, and the DCRs were 71%, 72%, 57%, 72% and 76%, respectively. The median of PFSs for the above groups were 7.9, 6.8, 5.6, 13.7, and 5.4 months. Compared with Endostar plus NP group, the hazard ratios (HRs) and 95%CIs of Endostar plus other chemotherapy were 1.86 (0.75–4.61), 2.15 (0.83–5.60), 1.33 (0.51–3.44), and 2.42 (0.86–6.81). This real-world study found the effectiveness of Endostar plus DP, Endostar plus GP, Endostar plus PP, and Endostar plus TP were of no statistically significant differences compared with Endostar plus NP and reflected the good effectiveness of Endostar plus different chemotherapy in advanced patients with NSCLC.
Subject terms: Lung cancer, Targeted therapies
Introduction
Lung cancer remains a great global health burden as the deadliest cancer with an estimated 2,206,771 new cases diagnosed and 1,796,144 deaths in 2020 worldwide, and 787,000 new cases diagnosed and 631,000 deaths in China since 20151,2. Non-small cell lung cancers (NSCLC) comprise about 85% of all lung cancer, and the majority are diagnosed in late stage3.
Platinum-based chemotherapy remains the primary first-line treatment for the advanced NSCLC patients, but the efficacy is unsatisfactory4. Thus, a new strategy, such as antiangiogenic therapy and immunotherapy, for NSCLC therapy is urgent. Recombinant human endostatin (Endostar), an angiogenesis inhibitor, has shown the effect of downregulating transplantation matrix metalloproteinases (MMP) and vascular endothelial growth factor (VEGF) to inhibit neovascularization and tumor growth5. Endostar has also shown clinical efficacy in the treatment of advanced NSCLC in China6–8.
Although clinical application of antiangiogenic therapy has brought promise for the treatment of NSCLC and Endostar plus vinorelbine/cisplatin (NP) were approved by the Chinese Food and Drug Administration (CFDA) in 2005, in real-world clinical practice, NSCLC patients are treated with different regimens according to their physical and economic status. Although studies were conducted focused on first-line treatment patients or early-stage patients, the real-world treatment pattern and evidence of the effectiveness of Endostar in combination with other chemotherapy regimens in advanced NSCLC patients remains unclear and need to be explored. Therefore, this study aimed to evaluate the treatment pattern and effectiveness of Endostar plus different combination chemotherapy, such as Endostar plus docetaxel/platinum (DP), Endostar plus gemcitabine/platinum (GP), Endostar plus pemetrexed/platinum (PP) and Endostar plus paclitaxel/platinum (TP) compared with Endostar plus NP in treatment-naïve patients and re-treatment NSCLC patients with advanced-stage in real-world settings.
Results
Real-world treatment pattern and baseline characteristics
Table 1 showed the real-world treatment pattern and baseline characteristics of Endostar plus different chemotherapy in all 512 patients with advanced NSCLC. Endostar plus NP group only accounted for 10.35%, while Endostar plus DP group accounted for 15.43%, Endostar plus GP group accounted for 32.42%, Endostar plus PP group accounted for 26.56%, and Endostar plus TP group accounted for 15.23%. The mean ages were 57.0, 57.9, 59.2, 56.4 and 59.3 years old, and male patients accounted for 66.0%, 69.6%, 88.0%, 63.2% and 80.8% respectively. The real-world treatment pattern and baseline characteristics of treatment-naïve patients and re-treatment patients were summarized in Table 2.
Table 1.
Real-world treatment pattern and baseline characteristics of Endostar plus different chemotherapy in all patients.
| Variables | All patients | Endostar plus NP | Endostar plus DP | Endostar plus GP | Endostar plus PP | Endostar plus TP |
|---|---|---|---|---|---|---|
| N (%) | 512 | 53 (10.35) | 79 (15.43) | 166 (32.42) | 136 (26.56) | 78 (15.23) |
| Age, years | ||||||
| Mean ± SD | 58.1 ± 10.0 | 57.0 ± 8.2 | 57.9 ± 9.6 | 59.2 ± 9.3 | 56.4 ± 11.7 | 59.3 ± 9.3 |
| Median (Q1-Q3) | 59.2 (51.3–65.0) | 56.1 (51.0–62.0) | 58.1 (50.3–65.6) | 60.1 (53.5–65.7) | 58.6 (48.0–65.0) | 59.6 (54.1–65.4) |
| Sex, n (%) | ||||||
| Unknown | 14 (2.7) | 2 (3.8) | 2 (2.5) | 5 (3.0) | 1 (0.7) | 4 (5.1) |
| Male | 385 (75.2) | 35 (66.0) | 55 (69.6) | 146 (88.0) | 86 (63.2) | 63 (80.8) |
| Female | 113 (22.1) | 16 (30.2) | 22 (27.8) | 15 (9.0) | 49 (36.0) | 11 (14.1) |
| Smoking history | ||||||
| No | 185 (36.1) | 24 (45.3) | 31 (39.2) | 44 (26.5) | 70 (51.5) | 16 (20.5) |
| Yes | 327 (63.9) | 29 (54.7) | 48 (60.8) | 122 (73.5) | 66 (48.5) | 62 (79.5) |
| Family history of lung cancer | ||||||
| No | 493 (96.3) | 51 (96.2) | 77 (97.5) | 163 (98.2) | 131 (96.3) | 71 (91.0) |
| Yes | 19 (3.7) | 2 (3.8) | 2 (2.5) | 3 (1.8) | 5 (3.7) | 7 (9.0) |
| Disease stage | ||||||
| III | 188 (36.7) | 21 (39.6) | 33 (41.8) | 83 (50.0) | 21 (15.4) | 30 (38.5) |
| IV | 324 (63.3) | 32 (60.4) | 46 (58.2) | 83 (50.0) | 115 (84.6) | 48 (61.5) |
| Pathological type | ||||||
| Squamous cell carcinoma | 181 (35.4) | 20 (37.7) | 36 (45.6) | 99 (59.6) | 6 (4.4) | 20 (25.6) |
| Adenocarcinoma | 205 (40.0) | 19 (35.8) | 37 (46.8) | 8 (4.8) | 116 (85.3) | 25 (32.1) |
| Unknown | 118 (23.0) | 12 (22.6) | 3 (3.8) | 59 (35.5) | 12 (8.8) | 32 (41.0) |
| Other | 8 (1.6) | 2 (3.8) | 3 (3.8) | 0 (0) | 2 (1.5) | 1 (1.3) |
| Patient type | ||||||
| Treatment-naïve | 417 (81.4) | 26 (49.1) | 54 (68.4) | 148 (89.2) | 122 (89.7) | 67 (85.9) |
| Re-treatment | 95 (18.6) | 27 (50.9) | 25 (31.6) | 18 (10.8) | 14 (10.3) | 11 (14.1) |
Table 2.
Real-world treatment pattern and baseline characteristics of Endostar plus different chemotherapy in treatment-naïve and re-treatment patients.
| Variables | Treatment-naï ve patients (N = 417) | Re-treatment patients (N = 95) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Endostar plus NP | Endostar plus DP | Endostar plus GP | Endostar plus PP | Endostar plus TP | Endostar plus NP | Endostar plus DP | Endostar plus GP | Endostar plus PP | Endostar plus TP | |
| N (%) | 26 (6.24) | 54 (12.95) | 148 (35.49) | 122 (29.26) | 67 (16.07) | 27 (28.42) | 25 (26.32) | 18 (18.95) | 14 (14.74) | 11 (11.58) |
| Age, years | ||||||||||
| Mean ± SD | 57.2 ± 7.0 | 57.8 ± 10.2 | 59.5 ± 9.5 | 56.4 ± 11.6 | 59.8 ± 9.2 | 56.8 ± 9.3 | 58.0 ± 8.4 | 57.5 ± 7.0 | 56.9 ± 12.2 | 57.4 ± 9.6 |
| Median (Q1-Q3) | 57.2 (52.4–61.4) | 58.9 (49.4–66.5) | 50.7 (53.6–66.7) | 56.4 (49.4–66.5) | 58.9 (49.4–66.5) | 55.0 (48.8–52.6) | 58.0 (52.4–64.3) | 57.6 (52.8–59.1) | 57.8 (45.4–63.1) | 56.5 (49.8–55.4) |
| Sex, n (%) | ||||||||||
| Unknown | 2 (7.7) | 2 (3.7) | 5 (3.4) | 1 (0.8) | 4 (6.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Male | 17 (65.4) | 38 (70.4) | 131 (88.5) | 76 (62.3) | 54 (80.6) | 18 (66.7) | 17 (68.0) | 15 (83.3) | 10 (71.4) | 9 (81.8) |
| Female | 7 (26.9) | 14 (25.9) | 12 (8.1) | 45 (36.9) | 9 (13.4) | 9 (33.3) | 8 (32.0) | 3 (16.7) | 4 (28.6) | 2 (18.2) |
| Smoking history | ||||||||||
| No | 10 (38.5) | 22 (40.7) | 38 (25.7) | 63 (51.6) | 12 (17.9) | 14 (51.9) | 9 (36.0) | 6 (33.3) | 7 (50.0) | 4 (36.4) |
| Yes | 16 (61.5) | 32 (59.3) | 110 (74.3) | 59 (48.4) | 55 (82.1) | 13 (48.2) | 16 (64.0) | 12 (66.7) | 7 (50.0) | 7 (63.6) |
| Family history of lung cancer | ||||||||||
| No | 26 (100.0) | 54 (100.0) | 145 (98.0) | 117 (95.9) | 61 (91.0) | 25 (92.6) | 23 (92.0) | 18 (100.0) | 14 (100.0) | 10 (90.9) |
| Yes | 0 | 0 | 3 (2.0) | 5 (4.1) | 6 (9.0) | 2 (7.4) | 2 (8.0) | 0 | 0 | 1 (9.1) |
| Disease stage | ||||||||||
| III | 12 (46.2) | 21 (38.9) | 75 (50.7) | 17 (13.9) | 28 (41.8) | 9 (33.3) | 12 (48.0) | 8 (44.4) | 4 (28.6) | 2 (18.2) |
| IV | 14 (53.9) | 33 (61.1) | 73 (49.3) | 105 (86.1) | 39 (58.2) | 18 (66.7) | 13 (52.0) | 10 (55.6) | 10 (71.4) | 9 (81.8) |
| Pathological type | ||||||||||
| Squamous cell carcinoma | 8 (30.8) | 23 (42.6) | 88 (59.5) | 3 (2.5) | 18 (26.9) | 12 (44.4) | 13 (52.0) | 11 (61.1) | 3 (21.4) | 2 (18.2) |
| Adenocarcinoma | 5 (19.2) | 27 (50.0) | 5 (3.4) | 107 (87.7) | 18 (26.9) | 14 (51.9) | 10 (40.0) | 3 (16.7) | 9 (64.3) | 7 (63.6) |
| Unknown | 11 (42.3) | 1 (1.9) | 55 (37.2) | 11 (9.0) | 31 (46.3) | 1 (3.7) | 2 (8.0) | 4 (22.2) | 1 (7.1) | 1 (9.1) |
| Other | 2 (7.7) | 3 (5.6) | 0 (0) | 1 (0.8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (7.1) | 1 (9.1) |
Effectiveness of Endostar plus different chemotherapy
292 patients who had at least one dosage of Endostar and at least one time of response evaluation record before and after baseline were included in the effectiveness analysis set with 25 in Endostar plus NP group, 49 in Endostar plus DP group, 90 in Endostar plus GP group, 89 in Endostar plus PP group and 39 in Endostar plus TP group.
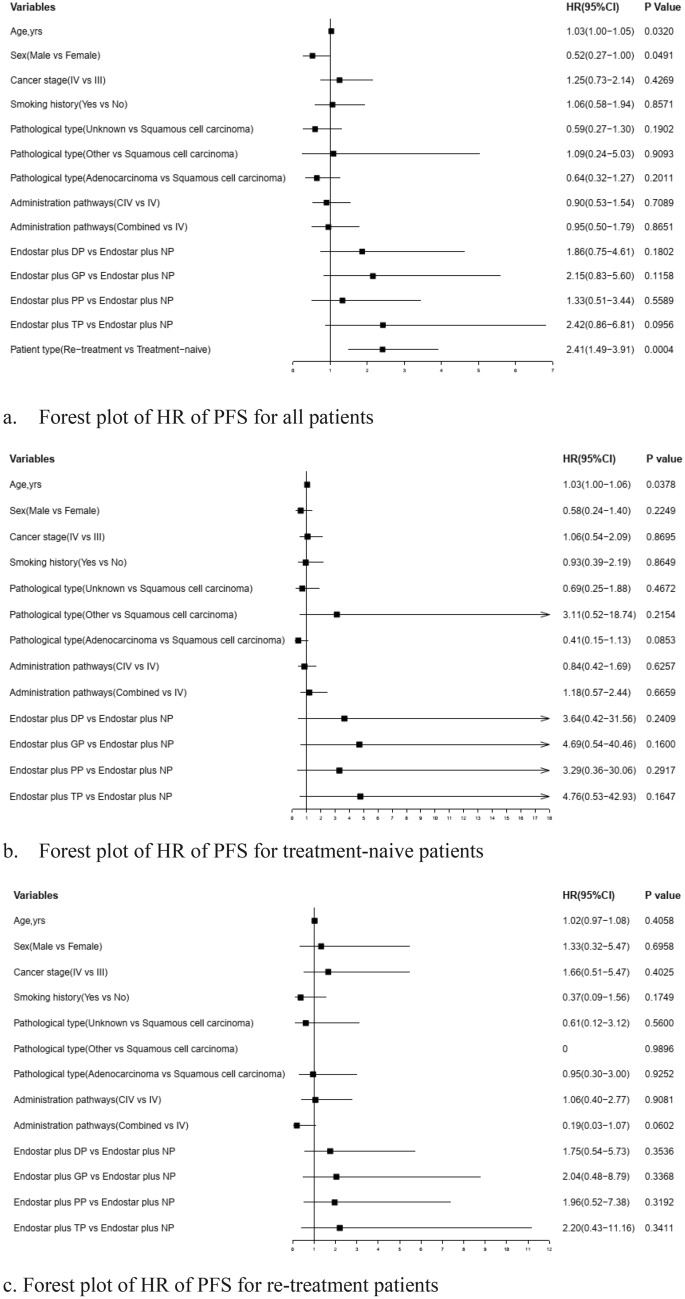
Table 3 showed the overall response rates (ORRs) for the above five groups were 28%, 22%, 41%, 27% and 31%, and the disease control rates (DCRs) were 72%, 57%, 72%, 76% and 74%, and the median of progression-free survivals (PFSs) were 7.9, 6.8, 5.6, 13.7 and 5.4 months, respectively. These differences of DCRs, ORRs and PFSs in Endostar plus different chemotherapy regimens groups were not statistically significant. Table 4 reported the DCRs, ORRs and PFSs of Endostar plus different chemotherapy in treatment-naïve patients and in re-treatment patients, and there were also no statistically significant differences. The Cox regression model revealed there were also no statistically significant differences in PFSs of Endostar plus other chemotherapy compared with Endostar plus NP group after adjusting age, sex, smoking history, family history of lung cancer, disease stage, pathological type and administration pathways. Figure 1 showed that the hazard ratios (HRs) (95%CI) of Endostar plus other chemotherapy were 1.86 (0.75–4.61), 2.15 (0.83–5.60), 1.33 (0.51–3.44) and 2.42 (0.86–6.81) as compared with Endostar plus NP group, and the re-treatment patients had decreased PFS as compared with treatment-naïve patients (HR = 0.84 [95% CI 0.75–0.95]). The exploratory subgroup analysis also demonstrated the PFSs of Endostar plus different chemotherapy groups were of no statistically significant differences, no matter in treatment-naïve patients or in re-treatment patients (Fig. 1).
Table 3.
The ORRs, DCRs and PFSs of Endostar plus different chemotherapy in all patients.
| Variables | All patients | Endostar plus NP | Endostar plus DP | Endostar plus GP | Endostar plus PP | Endostar plus TP | P value |
|---|---|---|---|---|---|---|---|
| N | 292 | 25 | 49 | 90 | 89 | 39 | |
| ORR (%) | 31 | 28 | 22 | 41 | 27 | 31 | 0.3667 |
| DCR (%) | 71 | 72 | 57 | 72 | 76 | 74 | 0.4104 |
| Median PFS (95%CI), months | 8.2 (6.0–13.7) | 7.9* | 6.8 (4.1–14.9) | 5.6 (3.7–8.3) | 13.7* | 5.4* | 0.1198 |
*Too few people to estimate confidence intervals.
Table 4.
The ORRs, DCRs and PFSs of Endostar plus different chemotherapy in treatment-naïve patients and in re-treatment patients.
| Variables | Subgroups in total | Endostar plus NP | Endostar plus DP | Endostar plus GP | Endostar plus PP | Endostar plus TP | P value |
|---|---|---|---|---|---|---|---|
| Treatment-naïve patients | |||||||
| N | 233 | 10 | 33 | 80 | 80 | 30 | |
| ORR (%) | 35 | 35 | 60 | 27 | 44 | 28 | 0.0813 |
| DCR (%) | 77 | 76 | 90 | 64 | 76 | 80 | 0.3045 |
| Median PFS (95%CI), months | 12.9 (7.2–14.9) | –* | 14.9 (4.1–22.4) | 5.6 (4.3–8.3) | 16.0* | 5.4* | 0.2025 |
| Re-treatment patients | |||||||
| N | 59 | 15 | 16 | 10 | 9 | 9 | |
| ORR (%) | 15 | 7 | 13 | 20 | 22 | 22 | 0.7725 |
| DCR (%) | 49 | 60 | 44 | 40 | 44 | 56 | 0.1886 |
| Median PFS (95%CI), months | 4.5 (2.6–9.0) | 5.6 (2.6–7.9) | 4.5 (1.8–9.7) | 3.4 (0.0–17.4) | 1.5* | 5.8 (0.0–5.8) | 0.8230 |
*Too few people to estimate PFS and confidence intervals.
Figure 1.
Forest plot of HR of PFS for all patients and subgroup patients.
Discussion
Endostar is an effective angiogenesis inhibitor which can directly target new capillary endothelial cells around the tumor9,10. Study showed that median PFS in the Endostar plus NP regimen was significantly increased compared with NP alone (6.3 and 3.6 months, P < 0.001), as well as the ORR (35.4% and 19.5%, P < 0.01)8. Endostar was approved in combination with NP for the treatment of NSCLC by the Chinese Food and Drug Administration in 2005, but the treatment pattern and real-world evidence of different combined regimens were still unknown for both treatment-naïve and re-treatment NSCLC patients with advanced stage. This study found that Endostar plus GP, instead of Endostar plus NP which had been approved by the CFDA, was the domain combination regimens, especially in treatment-naïve patients, and the effectiveness of Endostar plus DP, Endostar plus GP, Endostar plus PP and Endostar plus TP weren’t statistically significant different compared with that of Endostar plus NP.
Recently, several studies had been performed to evaluate the effectiveness of Endostar combined with docetaxel chemotherapy. For patients with stage Ib–IIIa postoperative NSCLC, QI Daliang etc. found that the PFS of Endostar plus docetaxel and carboplatin was not statistically significant than that of docetaxel and carboplatin alone11. But our study found, in the advanced patients with NSCLC, there was also no statistically significant effectiveness difference in Endostar plus NP group and Endostar plus DP group. The different result may be due to different TNM stages, and this study focused on advanced-stage patients represented patients with systematic treatment.
The better effectiveness of Endostar plus GP had been certified by several studies compared with GP alone in small samples of patients with NSCLC12,13. For example, in a study including 49 patients with advanced NSCLC, Yu Xun etc. found that the PFS in Endostar combined with gemcitabine and carboplatin regimen group was longer than that in gemcitabine and carboplatin regimen group with a statistically significant difference (P < 0.05)12. This study included 512 NSCLC patients with advanced stage and showed the PFS of Endostar plus GP was not significantly different from Endostar plus NP group. To some extent it can be said that this study demonstrated the good effectiveness of Endostar plus GP in a large sample patient.
For the efficacy of pemetrexed/cisplatin (PC), a trend of prolonged PFS was found in 56 previously untreated patients with lung adenocarcinoma, although the difference was lack of statistical significance in Endostar plus PC group compared with PC alone group. While this real-world study revealed the effectiveness in 136 patients treated with Endostar plus PP was no statistically significant difference compared with Endostar plus NP group. The different results may be due to different pathological tissue typing. For non-squamous advanced NSCLC, pemetrexed/cisplatin still was a relatively modern regimen which needs to be further studied14.
Paclitaxel-carboplatin (TC) approved by US Food and Drug administration was the first-line treatment for NSCLC. In this real-world study, the PFS of Endostar plus TP group had no statistically significant difference compared with that of Endostar plus NP group. Similarly, Ma Huifang etc. also revealed the Endostar plus TP had better curative effect than TP alone in the lung adenocarcinoma patients with stage IIIb and IV15. While Han Baohui etc. found in previously untreated, advanced NSCLC patients, first-line treatment with Endostar plus TC seemed to have an increased ORR compared with TC alone, but the differences in PFS or OS between the two groups were not statistically significant16. The contradictory findings about Endostar plus TP might be due to different patient types such as treatment-naïve patients and re-treatment patients.
Besides, the study has several advantages. Above all, this study firstly explored the real-world treatment pattern both in treatment-naïve patients and re-treatment patients and found that Endostar plus NP approved by the CFDA wasn’t the main combination regimens. Secondly, the patients in this study were more representative, including advanced-stage NSCLC patients with different pathological tissue typing and re-treatment patients. Lastly, this study was conducted in a larger sample than previous studies and to some extent, the effectiveness analysis result without statistically significance provided real-world evidence for the expansion of the combination regimen.
We acknowledge this study still has two limitations. Firstly, just as all the real-world studies, the retrospectively collected EMR information might lead to bias, but in some extent, the bias was reduced because this study identified patients from 7 cancer centers in China and the confounding factors were adjusted by Cox regression model. Secondly, the sample size of re-treatment patients was smaller than that of treatment-naïve patients, although this study was conducted in a larger sample patient compared with previous study.
In conclusion, this retrospective multi-center study showed in real-world practice, the Endostar plus GP was the domain combination regimens in advanced-stage NSCLC patients and revealed that Endostar plus other chemotherapy regimens also had good clinical benefits, with relatively high ORRs and DCRs.
Methods
Patients
A retrospective multi-center observational study was conducted based on electronical medical records (EMR) from 7 cancer hospitals in China, registered on Chinese Clinical Trial Registry (ChiCTR2000035129). This study was approved and waived informed constent by all the institutional and licensing committee [Ethics committee of National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Shenzhen, China (14/01/2020), Ethics committee of The Third Clinical College of Xinjiang Medical University, Urumqi, China (22/06/2020), Ethics committee of Yunnan Cancer Hospital, Kunming, China (29/03/2021), Ethics committee of Anhui Provincial Hospital, Hefei, China (10/06/2020), Ethics committee of Hunan Cancer Hospital, Changsha, China (10/03/2021), Ethics committee of Chongqing Cancer Hospital, Chongqing, China (22/05/2020), and Ethics committee of Shaanxi Provincial Cancer Hospital, Xi’an, China (25/03/2021)]. All research was performed in accordance with the the Declaration of Helsinki. Patients aged older than 18 years with clinical diagnosis of NSCLC, pathological stage III or IV [defined by American Joint Committee on Cancer tumor, node, metastasis (TNM) staging system version 7.0], and treated with Endostar combined with chemotherapy between 2012 and 2019 were included. According to the combination chemotherapy, the patients were furtherly divided into Endostar plus NP group, Endostar plus DP group, Endostar plus GP group, Endostar plus PP group and Endostar plus TP group. Treatment-naïve patients and re-treatment patients were separately defined as those who had Endostar administration during the 1st line therapy and those who had Endostar administration during 2nd line therapy or after, and exploratory subgroup analysis was furtherly performed.
Baseline covariates
Index date was defined as the date of first treatment with Endostar, and the baseline covariates data were collected, including birth date, sex, smoking history, family history of lung cancer, admission date and discharge date, tumor stage, pathological feature and administration pathways. The smoking history, family history of lung cancer, tumor stage and pathological feature were extracted by Natural Language Processing (NLP) from admission records and discharge note in EMR.
Effectiveness evaluation
The Response Evaluation Criteria in Solid Tumors (RECIST) Version 1.1 is used to evaluate the tumor response. ORR was defined as the percentage of patients who had complete response (CR) and/or partial response (PR). DCR was defined as the percentage of patients who had CR and/or PR and/or stable disease (SD). The PFS was defined as the interval(months) between first date of Endostar administration to the date of disease progression or death. In patients with progressive disease or death, the date of imaging progression or death was used as the endpoint date, and if PD and death were not observed, the date of the last evaluation was considered as the censored date.
Statistical methods
SAS 9.4 (SAS Institute INC., Cary, NC) was used to describe patient characteristics and compare the effectiveness among different treatment regimens. Quantitative variables, age, was described using Mean ± SD and Median(P25-P75), and qualitative variables, sex, tumor stage, family history of lung cancer, smoking history, administration pathways, and treatment regimen were described as count and component ratio. The Chi-squared test or Fisher exact test was used to compare ORRs and DCRs. The Kaplan–Meier method was used to describe PFSs and log-rank test was used to compare the differences in different groups. The HR (95%CI) of different chemotherapy groups were estimated by Cox regression model. A two-sided P < 0.05 was considered statistically significant.
Ethics declarations and consent to participate
This study is a retrospective, non-interventional study, which does not interfere with routine diagnosis and treatment, does not affect any medical rights of patients, does not increase the medical risk of patients. At the same time, the study did not identify individual patients. In addition, most of the patients to be included in this study have died or lost to follow-up and their informed consents could not be obtained. For the above reasons, we applied for exempting informed consents of all patients in this study and was approved by ethics committees from all centers. All research was performed in accordance with the Declaration of Helsinki.
The full name of all ethics committee that approved this study:
Ethics committee of National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Shenzhen, China (14/01/2020).
Ethics committee of The Third Clinical College of Xinjiang Medical University, Urumqi, China (22/06/2020).
Ethics committee of Yunnan Cancer Hospital, Kunming, China (29/03/2021).
Ethics committee of Anhui Provincial Hospital, Hefei, China (10/06/2020).
Ethics committee of Hunan Cancer Hospital, Changsha, China (10/03/2021).
Ethics committee of Chongqing Cancer Hospital, Chongqing, China (22/05/2020).
Ethics committee of Shaanxi Provincial Cancer Hospital, Xi’an, China (25/03/2021).
Author contributions
All authors have read and approved the manuscript. W.J. and W.S. equally contributed to the study conception, assessed the risk of bias of the studies, performed the data extraction and cleaning, conducted the statistical analyses, and drafted the manuscript. L.W. contributed to the study conception, supervised the statistical analyses, and reviewed the manuscript for important intellectual content. W.L., J.G., H.W., W.Z., and J.L. assisted in performing the search and reviewed the manuscript for important intellectual content. L.A. assisted in study conception and assessing the risk of bias of the studies.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Data availability
The data of this study are available on request from the corresponding author [LW]. The data are not publicly available due to [state restrictions, “Where the information on human genetic resources of China is provided or opened for use to organizations, individuals or institutions established or actually controlled outside the country, it shall report in advance to the competent department of science and technology under The State Council and submit a copy of the information”. Adopted at the 22nd Session of the Standing Committee of the 13th National People’s Congress of the People’s Republic of China on October 17, 2020 and effective as of April 15, 2021].
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Zheng RS, et al. Report of cancer epidemiology in China, 2015. Chin. J. Oncol. 2019;41:19–28. doi: 10.3760/cma.j.issn.0253-3766.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J. Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 4.Soon YY, Stockler MR, Askie LM, Boyer MJ. Duration of chemotherapy for advanced non-small-cell lung cancer: A systematic review and meta-analysis of randomized trials. J. Clin. Oncol. 2009;27:3277–3283. doi: 10.1200/JCO.2008.19.4522. [DOI] [PubMed] [Google Scholar]
- 5.Ling Y, et al. Endostar, a novel recombinant human endostatin, exerts antiangiogenic effect via blocking VEGF-induced tyrosine phosphorylation of KDR/Flk-1 of endothelial cells. Biochem. Biophys. Res. Commun. 2007;361:79–84. doi: 10.1016/j.bbrc.2007.06.155. [DOI] [PubMed] [Google Scholar]
- 6.An J, Lv W. Endostar (rh-endostatin) versus placebo in combination with vinorelbine plus cisplatin chemotherapy regimen in treatment of advanced non-small cell lung cancer: A meta-analysis. Thorac. Cancer. 2018;9:606–612. doi: 10.1111/1759-7714.12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin Y, et al. Comparison of efficacy and toxicity of bevacizumab, endostar and apatinib in transgenic and human lung cancer xenograftzebrafish model. Sci. Rep. 2018;8:15837. doi: 10.1038/s41598-018-34030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J, et al. Results of randomized, multicenter, double-blind phase III trial of rh-endostatin (YH-16) in treatment of advanced non-small cell lung cancer patients. Chin. J. Lung Cancer. 2005;8:283–290. doi: 10.3779/j.issn.1009-3419.2005.04.07. [DOI] [PubMed] [Google Scholar]
- 9.Zhuang HQ, Yuan ZY. Process in the mechanisms of endostatin combined with radiotherapy. Cancer Lett. 2009;282:9–13. doi: 10.1016/j.canlet.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Folkman J. Antiangiogenesis in cancer therapy–endostatin and its mechanisms of action. Exp. Cell Res. 2006;312:594–607. doi: 10.1016/j.yexcr.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 11.Qi DL, et al. rh-endostatin in combination with docetaxel and carboplatin as adjuvant treatment for non-small lung cancer. Zhonghua Yi Xue Za Zhi. 2009;89:1057–1059. [PubMed] [Google Scholar]
- 12.Yu X, Zhang L, Chen J. Effectiveness of treatment with endostatin in combination with emcitabine, carboplatin, and gemcitabine in patients with advanced non-small cell lung cancer: A retrospective study. Open Med. (Wars.) 2018;13:142–147. doi: 10.1515/med-2018-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao X, et al. A randomized phase II study of recombinant human endostatin plus gemcitabine/cisplatin compared with gemcitabine/cisplatin alone as first-line therapy in advanced non-small-cell lung cancer. Investig. New Drugs. 2012;30:1144–1149. doi: 10.1007/s10637-011-9631-7. [DOI] [PubMed] [Google Scholar]
- 14.Zhou S, et al. Efficacy and safety of rh-endostatin (Endostar) combined with pemetrexed/cisplatin followed by rh-endostatin plus pemetrexed maintenance in non-small cell lung cancer: A retrospective comparison with standard chemotherapy. Thorac. Cancer. 2018;9:1354–1360. doi: 10.1111/1759-7714.12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huifang MA, Shixin LU, Zhang W, Wang L. Short-term effect of endostar/bevacizumab in combination with paclitaxel and platinum for advanced lung adenocarcinoma. J. Cancer Res. Prev. Treat. 2015;42:62–64. [Google Scholar]
- 16.Han B, et al. A multicenter, randomized, double-blind, placebo-controlled study to evaluate the efficacy of paclitaxel-carboplatin alone or with endostar for advanced non-small cell lung cancer. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer. 2011;6:1104–1109. doi: 10.1097/JTO.0b013e3182166b6b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data of this study are available on request from the corresponding author [LW]. The data are not publicly available due to [state restrictions, “Where the information on human genetic resources of China is provided or opened for use to organizations, individuals or institutions established or actually controlled outside the country, it shall report in advance to the competent department of science and technology under The State Council and submit a copy of the information”. Adopted at the 22nd Session of the Standing Committee of the 13th National People’s Congress of the People’s Republic of China on October 17, 2020 and effective as of April 15, 2021].