Abstract
Background
There were concerns about the psychological outcomes of coronavirus disease from the beginning of the pandemic. Parkinson’s disease (PD) patients seem to be more vulnerable to mental health disorders like stress, depression, anxiety, or worsening quality of life during COVID-19 lockdown. We aimed to conduct a systematic review to investigate the psychological outcomes of COVID-19 among the PD population.
Methods
A systematic search was conducted using PubMed, Scopus, and Web of Science. We included original studies which reported the psychological impact of COVID-19 in the PD population with a minimum of 10 cases.
Results
After the screening, 21 studies with a total of 5236 PD cases were included in our qualitative synthesis. Depression, anxiety, and to less extent sleep disorders and apathy are the most studied psychological outcomes. Most of the studies indicated that the severity or the prevalence of psychiatric disturbance increased due to the COVID-19 pandemic in PD patients. The prevalence of anxiety was 14% to 66.5%, while depression was reported in 0% to 50% of PD patients during and after the pandemic. Also, sleep problems were reported in 35.4% to 68.9% of PD patients.
Conclusion
Considering the overall trend of increment in the severity of the main psychological outcomes observed in the present systematic review, it is suggested that future studies conduct a more accurate analysis of the prevalence, severity, and associated pathology of psychological outcomes of COVID-19 in PD patients.
Keywords: COVID-19, Parkinson’s disease, Mental health, Psychological outcome, Lockdown
1. Introduction
There were concerns about the psychological outcomes of coronavirus disease from the beginning of the pandemic. Several studies have reported a probable association between COVID-19 infection and further psychiatric manifestations such as anxiety [1], insomnia [2], and depression [1], [3]. A cohort study in the US revealed that the risk of psychiatric disorders, dementia, and insomnia is considerably higher in COVID-19 survivors compared to the control group. This study showed that previous psychiatric disease is also associated with more severe COVID-19 infection [4]. Another study performed by Taquet et al. similarly demonstrated that there is a clear association between COVID-19 and neurological and psychiatric disorders and the incidence of these complications was higher in patients who required hospitalization and intensive care during the COVID-19 pandemic [5].
COVID-19 pandemic affected especially people with neurological health conditions not only due to the infection but also because of the general lockdown followed by social distancing [6]. Studies suggest that restrictions during the COVID-19 pandemic could potentially affect the quality of life in patients with multiple sclerosis (MS) [7] and Parkinson’s disease (PD) [8] mainly due to the lack of social interaction. A recent systematic review concluded that 60% of studies reported changes in cognition and 93% of studies reported worsening psychological symptoms in patients with dementia [9].
A recent study by Janiri et al. investigated the impact of the COVID-19 pandemic on the mental health of people with PD and demonstrated that PD patients with preexisting psychiatric disorders are more likely to experience worse psychiatric consequences of COVID-19 outbreak especially depression and insomnia [8]. Another study showed that the association between the COVID-19 pandemic and psychiatric symptoms such as anxiety and depression was more prominent in patients with parkinsonism [10]. PD patients seem to be more vulnerable to mental health disorders like stress, depression, anxiety, or worsening quality of life after and during COVID-19 quarantine [10]. Several studies addressed the psychiatric consequence of the COVID-19 pandemic on patients with neurological diseases including PD.
Several systematic review and meta-analysis studies investigated the psychological outcomes of the COVID-19 pandemic in the general population. However, it is necessary to investigate the impact of the COVID-19 pandemic on mental health outcomes in high-risk patients such as PD patients. Therefore, we aimed to conduct a systematic review to investigate the psychological outcomes of the COVID-19 pandemic among the PD population.
2. Methods
The preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement guidelines were used to perform this study [11].
2.1. Search strategy
A systematic search was conducted using PubMed, Scopus, and Web of Science in February 2022. The following retrieved search strategy was used: (Parkinson’s disease) AND (SARS-CoV-2 OR coronavirus OR COVID-19) AND (psychiatric OR mental health). Moreover, we identified additional studies using manual search and hand-searching of reference list of review studies.
2.2. Eligibility criteria
We included original studies (except case reports) which reported the psychological impact of the COVID-19 pandemic on the PD population with a minimum of 10 cases.
2.3. Study selection
Two independent reviewers (M.R, M.B) screened the papers in two steps. First, the title and abstract of the studies were checked for selecting relevant investigations. In the next step, the full text of selected articles was reviewed to identify papers meeting our inclusion criteria. Any disagreements were resolved by consultation with the third investigator (F.N).
2.4. Data extraction
The same reviewers extracted the following information using a pre-designed data form: Demographics of the studies, study design, sample size, number of PD patients, number of females, age, disease duration, number of patients with a history of psychiatric and mental disorders, time frame of studies respecting the COVID-19 pandemic and lockdown (before, during, and after), data collection, reported psychiatric and mental disorders, and psychometric scales. The extracted data were further evaluated by a third investigator (F.N) to ensure accuracy. The time frame of studies concerning lockdown was demonstrated in each study.
2.5. Quality assessments
Methodological quality assessments were carried out by two reviewers (M.R, M.B) using Newcastle-Ottawa Scale (NOS) for cross-sectional and cohort studies [12]. The quality of studies was evaluated in three domains including selection, outcome, and comparability.
2.6. Data synthesis
We conducted a narrative synthesis on several major psychological and mental health disorders including anxiety, depression, sleep problems, stress, Impulsive-Compulsive Disorders (ICD), apathy, and a problem with mood and motivation.
3. Results
3.1. Search results
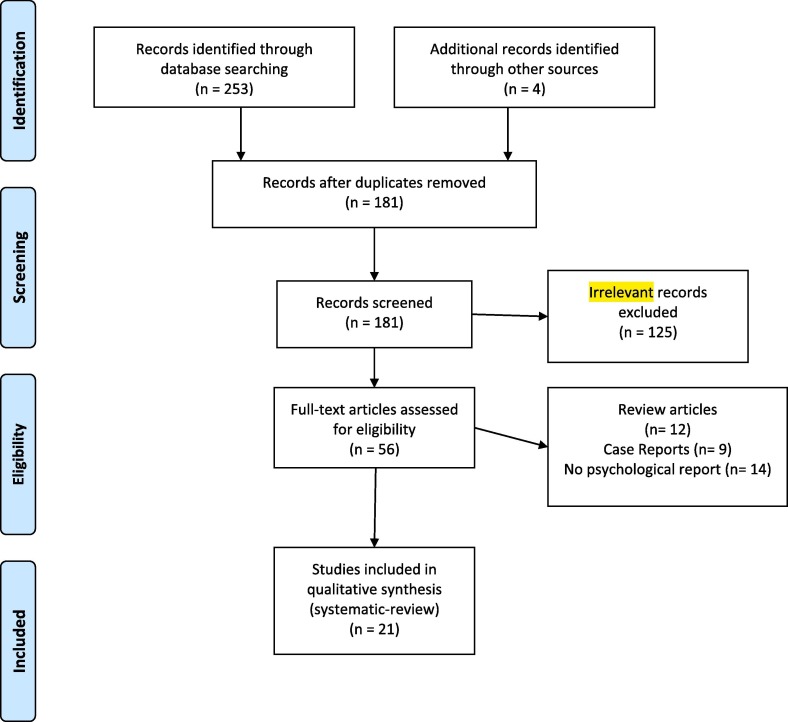
A total of 181 studies entered our screening process after duplicate removal (Fig. 1 ). After title and abstract review, 56 papers remained. Finally, 21 studies were included in our qualitative synthesis after the full-text screening.
Fig. 1.
PRISMA flow diagram depicting the flow of information through the different phases of a systematic review.
3.2. Characteristics of included studies and quality assessments
Of the 21 studies included, two were cohort and the others were cross-sectional (Table 1 ). The included studies assessed a total of 5236 PD cases. Seven studies were conducted in Italy and the remained were performed in Netherlands (n = 2), UK (n = 2), Iran (n = 1), Poland (n = 1), China (n = 1), India (n = 1), USA (n = 1), Israel (n = 1), Turkey (n = 1), Japan (n = 1), Morocco (n = 1), and Denmark and Sweden (n = 1). Six studies used online surveys for data collection while others utilized telephone surveys (n = 5), in-person surveys (n = 4), and datasets (n = 1).
Table 1.
Demographic, clinical characteristics, and findings of included studies.
| Author | Year | Country | Study design | Sample size | Number of PD patients | Number of females | Mean age years | PD disease duration | Number of patients with history of psychiatric and mental disorders | COVID-19 pandemic time frame of study | Lockdown time frame of study | Data collection | Reported psychiatric and mental disorders | Psychometric scales |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Janiri et al. | 2020 | Italy | Cross-sectional | 134 | 134 | 43 | 65< | NR | 101 | During | During | Interview, medical records, telephone survey, and in-person survey | Depression, apathy, sleep problems, and ICD | Diagnostic and Statistical Manual of Mental Disorders (DSM–5) |
| De Micco et al. | 2020 | Italy | Cohort | 94 | 94 | 32 | 66.4 | 3.2 | Excluded | During | After | Dataset | Psychological distress | Kessler Psychological Distress Scale (K-10) |
| Del Prete et al. | 2021 | Italy | Cross-sectional | 740 | 740 (7 with COVID-19) | 3 (COVID-19) | 75.7 (COVID-19) | 9.2 (COVID-19) | NR | During | During | Telephone survey | Anxiety, depression, and sleep problems | Depression Anxiety Stress Scale-21 item (DASS-21) |
| El Ottmani et al. | 2021 | Morocco | Cross-sectional | 50 | 50 | 26 | 60.4 | NR | NR | During | During | Telephone survey | Anxiety and depression | Hospital Anxiety and Depression Scale (HADS) |
| Oppo et al. | 2020 | Italy | Cross-sectional | 32 | 32 | 8 | 72.5 | NR | NR | During | During | NR | Stress, anxiety, and Impulsive-Compulsive Disorders (ICD) | Hospital Anxiety and Depression Scale (HADS), Non-Motor Symptoms Scale (NMSS), Impulsive-Compulsive Disorders in Parkinson’s Disease (QUIP) |
| Salari et al. | 2020 | Iran | Cross-sectional | 674 | 137 | 91 | 55 | NR | Excluded | During | During | In-person survey | Anxiety | Beck’s Depression Inventory (BDI-II) |
| Van Der Heide et al. | 2020 | Nethersland | Cross-sectional | 358 | 358 | 138 | 62.8 | 4 | NR | During | During | Online survey | Psychological distress, anxiety, depression | Perceived Stress Scale (PSS), Parkinson Anxiety Scale (PAS) |
| Yule et al. | 2021 | UK | Cross-sectional | 218 | 167 | 79 | 66 | NR | NR | During | During | Online survey | Impulsive-Compulsive Disorders (ICD), and anxiety | Impulsive-Compulsive Disorders in Parkinson’s Disease (QUIP) |
| Dommershuijsen et al. | 2021 | Netherlands | Cross-sectional | 844 | 844 | 321 | 70.3 | 6.4 | 70 | During | During | Self-administered questionnaires and telephone survey | Depression, anxiety, reduced quality of life | Parkinson’s Disease Quality of Life Questionnaire (PDQ-39), Beck’s Depression Inventory (BDI), and State-Trait Anxiety Inventory (STAI) |
| Baschi et al. | 2020 | Italy | Cross-sectional | 96 | 96 | 38 | 67.3 | 4.6 | Excluded | During | During | NR | Depression, sleep problems, and aberrant motor behavior | Neuropsychiatric Inventory (NPI) |
| Krzyszton et al. | 2022 | Poland | Cross-sectional | 47 | 47 | 17 | 72.1 | NR | NR | During | During | Online survey | Anxiety, and reduced quality of life | Questionnaire |
| Feeney et al. | 2021 | USA | Cross-sectional | 1342 | 1342 | 679 | 70.9 | 7 | NR | During | During | Online survey | Anxiety, depression, and sleep problems | NR |
| Balci et al. | 2021 | Turkey | Cross-sectional | 88 | 45 | 34 | 67 | 8 | NR | During | During | Telephone survey | Anxiety, depression | Hospital Anxiety and Depression Scale (HADS) |
| HØrmann Thomsen et al. | 2021 | Denmark and Sweden | Cohort | 67 | 67 | 32 | 70 | 6 | NR | Before/during | Before/during | NR | Depression, apathy, anxiety, and sleep problems | Parkinson’s Disease Questionnaire (PDQ-39), Beck’s Depression Inventory (BDI-II), and Lille Apathy Rating Scale (LARS) |
| Leta et al. | 2021 | UK | Cross-sectional | 27 | 27 | 11 | 59 | 9.2 | NR | During | After | NR | Anxiety, depression, and sleep problems | NR |
| Kumar et al. | 2020 | India | Cross-sectional | 736 | 537 | 166 | NR | NR | 48 | During | During | Online survey | Sleep problems | Questionnaire |
| Montanaro et al. | 2021 | Italy | Cross-sectional | 100 | 100 | 40 | 62.4 | 13.4 | NR | During | During | Telephone survey | Anxiety and depression | Hospital Anxiety and Depression Scale (HADS) |
| Palermo et al. | 2020 | Italy | Cross-sectional | 28 | 28 | NR | 70.2 | 5.4 | NR | During | After | NR | Anxiety, depression, and sleep problems | Questionnaire |
| Suzuki et al. | 2021 | Japan | Cross-sectional | 200 | 100 | 92 | 72.2 | 5.8 | NR | During | During | In-person survey | Anxiety and depression | Hospital Anxiety and Depression Scale (HADS) |
| Xia et al. | 2020 | China | Cross-sectional | 347 | 149 | 151 | 61.1 | 6.8 | NR | During | During | In-person survey | Anxiety, depression, and sleep problems | Pittsburgh Sleep Quality Index (PSQI), Hospital Anxiety and Depression Scale (HADS) |
| Yogev–Seligmann et al. | 2021 | Israel | Cross-sectional | 142 | 142 | 41% | 70.6 | 10.6 | NR | During | During | Online survey | Anxiety, depression, and loneliness | Questionnaire |
NR: Not Reported, PD: Parkinson’s disease.
All included studies were assessed for risk of bias using NOS. There were concerns about a high risk of bias in three studies in outcome and selection (Table 2 ). The overall score of NOS quality assessments of studies was 7.28.
Table 2.
Results of quality assessments.
| Author, year | Selection | Comparability | Outcome, or Exposure | Total score |
|---|---|---|---|---|
| Janir et al. 2020 | 4 | 1 | 3 | 8 |
| De Micco et al. 2020 | 4 | 1 | 2 | 7 |
| Del Prete et al. 2021 | 3 | 1 | 3 | 7 |
| El Ottmani et al. 2021 | 4 | 1 | 2 | 7 |
| Oppo et al. 2020 | 4 | 1 | 2 | 7 |
| Salari et al. 2020 | 3 | 1 | 2 | 6 |
| Van Der Heide et al. 2020 | 4 | 1 | 3 | 8 |
| Yule et al. 2021 | 4 | 1 | 2 | 7 |
| Dommershuijsen et al. 2021 | 4 | 1 | 3 | 8 |
| Baschi et al. 2020 | 3 | 1 | 2 | 6 |
| Krzyszton et al. 2022 | 4 | 1 | 3 | 8 |
| Feeney et al. 2021 | 4 | 1 | 2 | 7 |
| Balci et al. 2021 | 4 | 1 | 3 | 8 |
| Thomsen et al. 2021 | 4 | 1 | 3 | 8 |
| Leta et al. 2021 | 3 | 1 | 2 | 6 |
| Kumar et al. 2020 | 4 | 1 | 2 | 7 |
| Montanaro et al. 2021 | 4 | 1 | 3 | 8 |
| Palermo et al. 2020 | 3 | 1 | 3 | 7 |
| Suzuki et al. 2021 | 4 | 1 | 3 | 8 |
| Xia et al. 2020 | 4 | 1 | 3 | 8 |
| Yogev‑Seligmann et al. 2021 | 4 | 1 | 2 | 7 |
Newcastle–Ottawa scale (NOS).
3.3. Anxiety
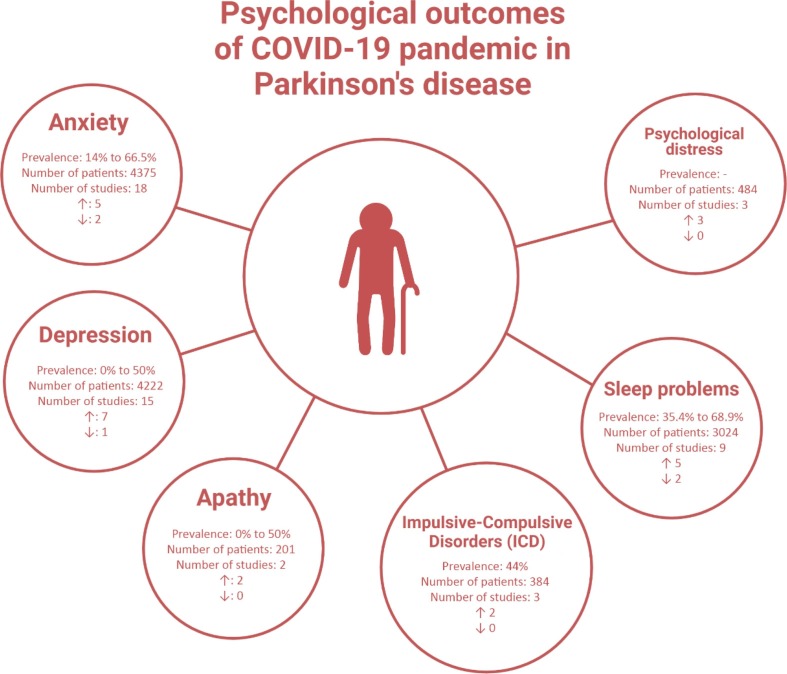
Anxiety was assessed in 18 studies of PD patients during the COVID-19 pandemic [10], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27] (Fig. 2 ). The measurements to assess anxiety were Depression Anxiety Stress Scale-21 item (DASS-21) [13], Hospital Anxiety and Depression Scale (HADS) [14], [15], [20], [23], [25], [26], Parkinson Anxiety Scale (PAS) [17], and State-Trait Anxiety Inventory (STAI) [10]. However, eight studies did not report their scales for anxiety assessments or used general questionnaires [16], [18], [19], [21], [22], [24], [27], [28].
Fig. 2.
Psychological outcomes of COVID-19 pandemic in Parkinson’s disease patients. The number of studies showed worsening and increase prevalence (↑) or improving or decrease in prevalence (↓) of each psychological outcome were represented.
In included studies, 14% to 66.5% of PD patients reported anxiety during and after the COVID-19 pandemic [16], [18], [19], [23], [24]. A study by Del Prete et al. demonstrated that 70% of PD cases did not experience a worsening of motor function or mood, anxiety, or sleep problems [13]. However, several other investigations showed that the level of anxiety increased in PD patients during the pandemic [16], [21], [27], [28]. Hørmann Thomsen et al. which consisted of two cohorts from Denmark and Sweden showed that the anxiety score measured by Beck’s Depression Inventory (BDI-II) was significantly increased during the pandemic compared to the pre-COVID-19 period (0.9 vs 1.6) [21]. A study by Montanaro et al. found that the presence of anxiety reduced after lockdown from 39% to 30% [23].
3.4. Depression
Fifteen studies examined depression among PD subjects [8], [10], [13], [14], [17], [19], [20], [21], [22], [23], [24], [25], [26], [27], [29]. The various scales were used to assess depression including DASS-21 [13], HADS [14], [15], [20], [23], [25], [26], BDI-II [16], and Neuropsychiatric Inventory (NPI)[29]. Six studies did not report their scales or used unknown questionnaires [17], [19], [22], [24], [27].
A study by Xia et al. demonstrated that the prevalence of depression was higher in PD patients compared to controls during the COVID-19 pandemic (34.5% vs 11.5%) which depression was defined as HADS-depression higher than 8 [26]. Other investigations reported the worsening of depression symptoms during the pandemic in these patients [8], [27]. Based on included studies the prevalence of depression among PD patients during the COVID-19 pandemic ranged from zero to nearly 50 percent [14], [17], [19], [20], [22], [24], [25], [26]. A study revealed that the depression scores were higher after the pandemic compared to the pre-COVID-19 period but it was not statistically significant [21]. Also, Montanaro et al. found a lower but not significant prevalence of depression after the pandemic in PD patients [23].
3.5. Sleep problems
Nine studies investigated the impact of the COVID-19 pandemic on sleep problems [8], [13], [19], [21], [22], [24], [26], [29], [30]. One study used Pittsburgh Sleep Quality Index (PSQI) to measure sleep quality [30]. This study found that 35.4% of PD patients experienced sleep disturbance during home confinement. Also, 23.9% of subjects reported new-onset or worsening sleep disturbance [30]. Xia et al. study revealed that PD patients had a higher prevalence of sleep problems compared to healthy individuals [26].
Sleep quality was longitudinally investigated by a study and it was found that PD patients had fewer sleep problems during the pandemic (PDQ-39 = 1.5) compared to the pre-COVID-19 (PDQ-39 = 1.7) period using Parkinson’s disease questionnaire (PDQ-39) [21]. Also, Del Prete et al. study showed that PD patients with COVID-19 mostly did not experience worsening insomnia [13]. Based on a study by Janiri et al., 52% of PD patients experienced worsening insomnia during the COVID-19 outbreak [8]. Prevalence of sleep disturbance ranged from 35.4% to 68.9% of PD patients during COVID-19 according to included studies [8], [24], [26], [30].
3.6. iCD
Three studies examined ICD in PD subjects during the COVID-19 outbreak [8], [15], [28]. The Impulsive-Compulsive Disorders in Parkinson’s Disease (QUIP) [15], [28], and the diagnostic and Statistical Manual of Mental Disorders (DSM–5) were used to assess ICD [8]. Yule et al. demonstrated that the prevalence of ICD was higher during the lockdown period in PD subjects (37.01% vs 44.67%) [28]. Also, Janiri et al. study reported that 17.4 percent of PD patients experienced worsening of ICD during the COVID-19 outbreak [8].
3.7. Apathy
Two studies reported apathy in PD patients during the outbreak [8], [21]. The Lille Apathy Rating Scale (LARS) [21] and DSM–5 [8] were used to identify apathy. Both studies reported a worsening of apathy during the pandemic compared to the pre-pandemic period.
3.8. Psychological distress
Two cross-sectional and one cohort studies investigated psychological distress by using Kessler Psychological Distress Scale (K-10)[31], and the Perceived Stress Scale (PSS)[15], [17]. A study from Netherland reported that the presence of anxiety and depression before the pandemic can predict an increase in psychological distress during the COVID-19 pandemic [17]. Also, a study by De Micco et al. showed that the mean score of K-10 in PD patients during the pandemic was 14.73 (±6.17) [31].
4. Discussion
The present study systematically reviewed the psychological outcomes of the COVID-19 outbreak in PD patients. Depression, anxiety, and to less extent sleep disorders and apathy are the most studied psychological outcomes [10], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29].
4.1. Anxiety
Despite using different scales, most of the studies have shown a significant increase in the severity of anxiety in PD patients. Yet, the reported prevalence of anxiety in PD patients was variable, which can be due to the heterogeneous sample of PD subjects, considering the fact that depression and anxiety become more prevalent throughout the course of disease progression into more advanced stages [32]. A previous systematic review with more homogenous samples reported a pooled prevalence of 30.1% (95% confidence interval 26.1%–34.0%). Yet, due to the reasons discussed above, with the present available data, a systematic comparison of the prevalence of anxiety before and after the COVID-19 pandemic is not plausible. Meanwhile, one study reported that the prevalence of anxiety in advanced PD patients was significantly reduced during lockdown [23].
Compared with non-PD patients, two studies with a total sample size of 286 PD patients and 735 controls, using different psychometric scales, reported that the severity of anxiety symptoms was higher in PD patients [16], [26]. However, two other studies with smaller sample sizes, but using similar scales, reported no difference in the severity of those symptoms between the two groups [14], [20].
In addition to home confinement, a few other studies reported that other COVID-19 pandemic associated stressors (e.g., confronting coping, self-controlling, planful problem-solving, seeking social support, escape-avoidance, accepting responsibility, positive reappraisal, and some important governmental events) are also correlated with higher psychological pressure on PD patients, which in turn leads to worsening of the motor and non-motor symptoms (NMS) in PD patients. Importantly, models have shown that reducing this stressor could improve the psychological status [10], [17]. Putting it all together, while the present data is not completely conclusive, a general trend of increment in severity and to some degree prevalence of anxiety in PD patients was observed.
Unlike anxiety, while using more homogeneous psychometric scales (i.e., HADS), only a few studies reported a significant increase in the severity of depressive symptoms [26]. Of note, studies with a longer period of home confinement (i.e., 1–6 months) reported no increase in depressive symptoms compared either with healthy individuals or with PD patients’ baseline depression scores. Finally, as seen in anxiety, diminished frequency of depressive symptoms was reported in advanced PD patients [29], highlighting the fact that homogenizing study samples in terms of disease stage and progression can better elucidate the psychiatric outcomes in PD patients.
Of the only two studies assessing apathy, both reported increased severity of apathy after lockdown. This is in line with the previous findings suggesting anxiety, depression, and apathy are the behavioral “non-motor triad” of PD [33]. However, the available data on depression and apathy is less conclusive than that on anxiety and more high-quality research is still needed to shed better light on this hypothesis.
Various sleep disorders including REM-sleep disorders, restless leg syndrome and periodic limb movement disorder, insomnia, nocturia, excessive daytime sleepiness, sleep-related breathing disorders, and circadian rhythm disruption have been reported in PD patients by pre-COVID studies [34], [35]. Yet, most of the included studies in this review have assessed “sleep disturbances” without further specification of the disorders. Hence, a comparison of the epidemiology of sleep disorders before and after the COVID-19 outbreak is not feasible with the present studies. Sleep disturbances in PD patients are important in two aspects. First, they largely contribute to the quality of life in PD patients. Second, sleep problem complicates other NMSs of PD [36]. Indeed, further studies are needed to better understand the sleep complications caused by COVID-19 lockdown in PD patients.
Psychologic disorders constitute an important part of PD-associated NMSs. Although the relationship between motor and non-motor symptoms of PD is complex [37], previous studies have suggested that the most important NMSs of PD (i.e., depression and anxiety)[38] are mainly caused by the main pathology of PD (i.e., dopaminergic system degeneration), so that the NMSs can be a clue for PD onset and severity [39]. In addition, NMSs can aggravate the motor symptoms and affect response to the medications [40], [41]. Furthermore, even before the COVID-19 pandemic emergence, NMSs have been causing a greater impact on the quality of life in PD patients than motor symptoms [42].
5. Limitations
We had several limitations. First, the COVID-19 pandemic mediator affecting psychological outcomes in most of the included studies was home confinement. However, this mediator interacts with other factors and results in psychological symptoms changes.
Second, important moderators of the COVID-19 pandemic psychological outcomes in PD patients were not considered in some of the included studies. As mentioned before, considering information about the type of treatments, cognitive status, comorbidities, stage, and progression of PD may help make a more detailed picture of psychological outcomes of COVID-19 outbreak in PD subjects.
Third, surveys used in most of the studies were based on the subjective sensation of the psychological outcomes, which may suffer from recall bias and not be an accurate assessment. In addition, the magnitude of the increment could not be captured by most of the surveys. Importantly, further investigation is needed to discover the mechanisms underlying the psychological disturbance and find out whether they are a normal physiological response to an external threat (i.e., COVID-19) or indicate an accelerated non-reversible degeneration of the corresponding brain circuits compared to previous pre-COVID studies.
Forth, the included studies came from a limited number of countries, and studies from low and middle-income countries were limited. This can reduce the generalizability of the findings of the present studies. Thereafter, further studies in other countries especially the before-mentioned ones are encouraged.
Fifth, most of the included studies have used questionnaires that consider the subjective reports of the PD patients. It would have been more accurate if the symptoms were assessed by a specialist using specific criteria.
Sixth, we did not include anxiety, depression, and sleep terms in our search strategy, and maybe some papers were not captured.
6. Conclusions
Considering the overall trend of increment in the severity of the main psychological outcomes observed in the present systematic review, and the briefly discussed key role of psychological status in pathology and burden of PD, it is suggested that future studies conduct more accurate (and quantitative if possible) analysis on the prevalence, severity and associated pathology of psychological outcomes of COVID-19 pandemic in PD patients. However, clinicians should consider the psychological complications of COVID-19 lockdown in this group of patients.
7. Availability of data and material
The datasets analyzed during the current study are available upon request with no restriction.
Funding
We do not have any financial support for this study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Paz C., Mascialino G., Adana-Díaz L., Rodríguez-Lorenzana A., Simbaña-Rivera K., Gómez-Barreno L., et al. Anxiety and depression in patients with confirmed and suspected COVID-19 in Ecuador. Psychiatry Clin Neurosci. 2020;74(10):554–555. doi: 10.1111/pcn.13106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mazza M.G., De Lorenzo R., Conte C., Poletti S., Vai B., Bollettini I., et al. Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. Brain Behav Immunity. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang J., Lu H., Zeng H., Zhang S., Du Q., Jiang T., et al. Brain Behav Immunity. 2020:1–2. doi: 10.1016/j.bbi.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taquet M., Luciano S., Geddes J.R., Harrison P.J. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry. 2021;8(2):130–140. doi: 10.1016/S2215-0366(20)30462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taquet M., Geddes J.R., Husain M., Luciano S., Harrison P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8(5):416–427. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carbone E.A., de Filippis R., Roberti R., Rania M., Destefano L., Russo E., et al. The mental health of caregivers and their patients with dementia during the COVID-19 pandemic: a systematic review. Front Psychol. 2021;12 doi: 10.3389/fpsyg.2021.782833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koc E.R., Demir A.B., Topaloglu E., Turan O.F., Ozkaya G. Effects of quarantine applied during the COVID-19 pandemic on mental health and quality of life in patients with multiple sclerosis and healthy controls. Neurol Sci. 2022;43(4):2263–2269. doi: 10.1007/s10072-022-05901-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janiri D., Petracca M., Moccia L., Tricoli L., Piano C., Bove F., et al. COVID-19 pandemic and psychiatric symptoms: the impact on Parkinson’s disease in the elderly. Front Psychiatry. 2020;11 doi: 10.3389/fpsyt.2020.581144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suárez-González A., Rajagopalan J., Livingston G., Alladi S. The effect of COVID-19 isolation measures on the cognition and mental health of people living with dementia: a rapid systematic review of one year of quantitative evidence. EClinicalMedicine. 2021;39:101047. doi: 10.1016/j.eclinm.2021.101047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dommershuijsen L.J., Van der Heide A., Van den Berg E.M., Labrecque J.A., Ikram M.K., Ikram M.A., et al. Mental health in people with Parkinson’s disease during the COVID-19 pandemic: potential for targeted interventions? NPJ Parkinson’s Dis. 2021;7(1) doi: 10.1038/s41531-021-00238-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed]
- 12.Lo C.-L., Mertz D., Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Method. 2014;14(1) doi: 10.1186/1471-2288-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Del Prete E., Francesconi A., Palermo G., Mazzucchi S., Frosini D., Morganti R., et al. Prevalence and impact of COVID-19 in Parkinson’s disease: evidence from a multi-center survey in Tuscany region. J Neurol. 2021;268(4):1179–1187. doi: 10.1007/s00415-020-10002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El Otmani H., El Bidaoui Z., Amzil R., Bellakhdar S., El Moutawakil B., Abdoh R.M. No impact of confinement during COVID-19 pandemic on anxiety and depression in Parkinsonian patients. Revue Neurologique. 2021;177(3):272–274. doi: 10.1016/j.neurol.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oppo V., Serra G., Fenu G., Murgia D., Ricciardi L., Melis M., et al. Parkinson’s disease symptoms have a distinct impact on caregivers’ and patients’ stress: a study assessing the consequences of the COVID-19 lockdown. Movement Disorders Clinical Practice. 2020;7(7):865–867. doi: 10.1002/mdc3.13030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salari M., Zali A., Ashrafi F., Etemadifar M., Sharma S., Hajizadeh N., et al. Incidence of anxiety in Parkinson’s disease during the coronavirus disease (COVID-19) pandemic. Mov Disord. 2020;35(7):1095–1096. doi: 10.1002/mds.28116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Heide A., Meinders M.J., Bloem B.R., Helmich R.C. The Impact of the COVID-19 Pandemic on Psychological Distress, Physical Activity, and Symptom Severity in Parkinson’s Disease. Journal of Parkinson’s Disease. 2020;10(4):1355–1364. doi: 10.3233/JPD-202251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krzysztoń K., Mielańczuk-Lubecka B., Stolarski J., Poznańska A., Kępczyńska K., Zdrowowicz A., et al. Secondary impact of COVID-19 pandemic on people with Parkinson’s disease-results of a polish online survey. Brain Sci. 2021;12(1):26. doi: 10.3390/brainsci12010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feeney M.P., Xu Y., Surface M., Shah H., Vanegas-Arroyave N., Chan A.K., et al. The impact of COVID-19 and social distancing on people with Parkinson’s disease: a survey study. Npj Parkinsons Disease. 2021;7(1) doi: 10.1038/s41531-020-00153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balci B., Aktar B., Buran S., Tas M., Donmez Colakoglu B. Impact of the COVID-19 pandemic on physical activity, anxiety, and depression in patients with Parkinson’s disease. Int J Rehabil Res. 2021;44(2):173–176. doi: 10.1097/MRR.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.HØrmann Thomsen T., Wallerstedt S.M., Winge K., Bergquist F. Life with Parkinson’s disease during the COVID-19 pandemic: the pressure is ‘OFF’. J Parkinson’s Disease. 2021;11(2):491–495. doi: 10.3233/JPD-202342. [DOI] [PubMed] [Google Scholar]
- 22.Leta V., Rodríguez-Violante M., Abundes A., Rukavina K., Teo J.T., Falup-Pecurariu C., et al. Parkinson’s disease and post-COVID-19 syndrome: the Parkinson’s long-COVID spectrum. Mov Disord. 2021;36(6):1287–1289. doi: 10.1002/mds.28622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montanaro E., Artusi C.A., Rosano C., Boschetto C., Imbalzano G., Romagnolo A., et al. Anxiety, depression, and worries in advanced Parkinson disease during COVID-19 pandemic. Neurol Sci. 2022;43(1):341–348. doi: 10.1007/s10072-021-05286-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palermo G., Tommasini L., Baldacci F., Del Prete E., Siciliano G., Ceravolo R. Impact of coronavirus disease 2019 pandemic on cognition in Parkinson’s disease. Mov Disord. 2020;35(10):1717–1718. doi: 10.1002/mds.28254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki K., Numao A., Komagamine T., Haruyama Y., Kawasaki A., Funakoshi K., et al. Impact of the COVID-19 pandemic on the quality of life of patients with Parkinson’s disease and their caregivers: a single-center survey in Tochigi prefecture. J Parkinson’s Disease. 2021;11(3):1047–1056. doi: 10.3233/JPD-212560. [DOI] [PubMed] [Google Scholar]
- 26.Xia Y., Kou L., Zhang G., Han C., Hu J., Wan F., et al. Investigation on sleep and mental health of patients with Parkinson’s disease during the Coronavirus disease 2019 pandemic. Sleep Med. 2020;75:428–433. doi: 10.1016/j.sleep.2020.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yogev-Seligmann G., Kafri M. COVID-19 social distancing: negative effects on people with Parkinson disease and their associations with confidence for self-management. BMC Neurol. 2021;21(1) doi: 10.1186/s12883-021-02313-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yule E., Pickering J.S., McBride J., Poliakoff E. People with Parkinson’s report increased impulse control behaviours during the COVID-19 UK lockdown. Parkinson Relat Disord. 2021;86:38–39. doi: 10.1016/j.parkreldis.2021.03.024. [DOI] [PubMed] [Google Scholar]
- 29.Baschi R., Luca A., Nicoletti A., Caccamo M., Cicero C.E., D'Agate C., et al. Changes in motor, cognitive, and behavioral symptoms in Parkinson’s disease and mild cognitive impairment during the COVID-19 lockdown. Front Psychiatry. 2020;11 doi: 10.3389/fpsyt.2020.590134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar N., Gupta R., Kumar H., Mehta S., Rajan R., Kumar D., et al. Impact of home confinement during COVID-19 pandemic on sleep parameters in Parkinson’s disease. Sleep Med. 2021;77:15–22. doi: 10.1016/j.sleep.2020.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Micco R., Siciliano M., Sant'Elia V., Giordano A., Russo A., Tedeschi G., et al. Correlates of psychological distress in patients with Parkinson’s disease during the COVID-19 outbreak. Move Disord Clin Pract. 2021;8(1):60–68. doi: 10.1002/mdc3.13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weintraub D., Caspell‐Garcia C., Simuni T., Cho H.R., Coffey C.S., Aarsland D., et al. Neuropsychiatric symptoms and cognitive abilities over the initial quinquennium of Parkinson disease. Ann Clin Transl Neurol. 2020;7(4):449–461. doi: 10.1002/acn3.51022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maillet A., Krack P., Lhommée E., Météreau E., Klinger H., Favre E., et al. The prominent role of serotonergic degeneration in apathy, anxiety and depression in de novo Parkinson’s disease. Brain. 2016;139(9):2486–2502. doi: 10.1093/brain/aww162. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Ren R, Sanford LD, Yang L, Zhou J, Tan L, et al. Sleep in Parkinson’s disease: a systematic review and meta-analysis of polysomnographic findings. Sleep Med Rev. 2020;51:101281. [DOI] [PubMed]
- 35.Chahine L.M., Amara A.W., Videnovic A. A systematic review of the literature on disorders of sleep and wakefulness in Parkinson’s disease from 2005 to 2015. Sleep Med Rev. 2017;35:33–50. doi: 10.1016/j.smrv.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie D., Shen Q., Zhou J., Xu Y. Non-motor symptoms are associated with REM sleep behavior disorder in Parkinson’s disease: a systematic review and meta-analysis. Neurol Sci. 2021;42(1):47–60. doi: 10.1007/s10072-020-04769-9. [DOI] [PubMed] [Google Scholar]
- 37.Weintraub D., Aarsland D., Chaudhuri K.R., Dobkin R.D., Leentjens A.FG., Rodriguez-Violante M., et al. The neuropsychiatry of Parkinson’s disease: advances and challenges. Lancet Neurol. 2022;21(1):89–102. doi: 10.1016/S1474-4422(21)00330-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schrag A., Taddei R.N. Depression and anxiety in Parkinson’s disease. Int Rev Neurobiol. 2017;133:623–655. doi: 10.1016/bs.irn.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 39.Kano O., Ikeda K., Cridebring D., Takazawa T., Yoshii Y., Iwasaki Y. Neurobiology of depression and anxiety in Parkinson’s disease. Parkinson’s Disease. 2011;2011 doi: 10.4061/2011/143547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Velden R.M.J., Broen M.P.G., Kuijf M.L., Leentjens A.F.G. Frequency of mood and anxiety fluctuations in Parkinson’s disease patients with motor fluctuations: a systematic review. Mov Disord. 2018;33(10):1521–1527. doi: 10.1002/mds.27465. [DOI] [PubMed] [Google Scholar]
- 41.Avanzino L., Lagravinese G., Abbruzzese G., Pelosin E. Relationships between gait and emotion in Parkinson’s disease: a narrative review. Gait Posture. 2018;65:57–64. doi: 10.1016/j.gaitpost.2018.06.171. [DOI] [PubMed] [Google Scholar]
- 42.Rodríguez-Violante M., Ospina-García N., Dávila-Avila N.M., Cruz-Fino D., Cruz-Landero A., Cervantes-Arriaga A. Motor and non-motor wearing-off and its impact in the quality of life of patients with Parkinson’s disease. Arq Neuropsiquiatr. 2018;76(8):517–521. doi: 10.1590/0004-282X20180074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are available upon request with no restriction.