Abstract
Background
To describe the clinical profile, risk factors, and outcomes that are associated with candida infection among critically ill children.
Patients and methods
A retrospective case-control study wherein 109 children admitted to the pediatric intensive care unit (PICU) in the years between 2015 and 2017 with the growth of candida from blood, urine, endotracheal (ET) aspirate, and pus swabs were included and compared to 97 age and sex-matched controls chosen from the same time period.
Results
Of the 124 candida isolates from 109 children, 37% were from blood, 24% from urine, and 14% in pus; 40% of the isolates were from ET aspirate. Candida non-albicans types (70%) predominated with Candida tropicalis causing 50% of the infections. Risk factors for candida infection were neutropenia [OR 20.01, 95% CI (0.94–422.32)], mechanical ventilation [OR 5.97, 95% CI (2.44–14.62)], peritoneal dialysis [OR 5.81, 95% CI (1.27–26.50)], institution of amino acids [OR 5.41, 95% CI (0.85–34.13)], presence of central venous catheter [OR 3.83, 95% CI (1.59–9.19)], antibiotic use >5 days [OR 3.58, 95% CI (1.38–9.29)]. Candida Cases (95.4%) had a septic shock with acute kidney injury in 34% and had significantly lower survival than controls [72 (66%) of 109 vs. 74 (80%) of 92] (p = 0.023).
Conclusions
The rate of candida infection in our PICU was 4.2% of PICU admissions. The most common species was C. tropicalis. The independent risk factors for candida infection were neutropenia, antibiotic duration >5 days, peritoneal dialysis, amino acid administration, mechanical ventilation, and presence of a central venous catheter (CVC).
How to cite this article
Rajeshwari R, Vyasam S, Chandran J, Porwal S, Ebenezer K, Thokchom M, et al. Risk Factors for Candida Infection among Children Admitted to a Pediatric Intensive Care Unit in a Tertiary Care Centre in Southern India. Indian J Crit Care Med 2022;26(6):717–722.
Keywords: Candida, Pediatric intensive care unit, Risk factors
Highlights
Any sick child in septic shock and ventilated needing central lines, parenteral nutrition, and antibiotic duration for more than 5 days should be screened for candida infection. Monitoring for candida colonization in critically ill children with the above risk factors could be a cost-effective strategy for the early detection of candida infections.
In a study done among 109 PICU children in South India who grew candida, C. non-albicans (70%) predominated. Risk factors for candida were: neutropenia, mechanical ventilation, peritoneal dialysis, amino acid infusion, and central venous catheter. Antibiotic use for over 5 days had three times the risk of developing candidiasis. Survival was significantly lower among those with candidiasis.
Introduction
Among critically ill infants and children, invasive fungal infection (IFI) has been of concern with candidiasis being more frequent than aspergillosis.1 Candida infections are the third most common cause of health care-associated bloodstream infections and the second most common cause of central catheter-associated bloodstream infections in children.2,3
Advancements in intensive care and the increased use of invasive procedures have led to a rise in invasive candida infections (ICI) in non-immunocompromised children that are considerably challenging to recognize and treat. As more families opt for chemotherapy and bone marrow transplants for childhood cancers, immunocompromised pediatric patients are a growing cohort in our setting.
Prolonged use of antibiotics, use of central venous catheters (CVCs), parenteral nutrition, renal replacement therapy, neutropenia, malignancy, and immunosuppressive therapies are the major risk factors for candida infections in ICUs.1
Among candida species, Candida albicans is the most common species in the pediatric population, However, in recent years the incidence of non-albicans types (C. glabrata, C. parapsilosis, C. krusei, and C. tropicalis) has increased.4,5
We attempted to identify predisposing factors in children who had candida in the PICU and their outcome in the Indian setting.
Materials and Methods
This study was conducted as a retrospective case-control study in the pediatric intensive care unit (PICU) of Christian Medical College Hospital, Vellore.
Cases were children aged 0 months to 15 years who were admitted to PICU from January 2015 to December 2017, and with the growth of candida in their blood, urine, endotracheal (ET) aspirate, or pus swabs. Controls were chosen during the same time period and were age and sex-matched.
In accordance with the CDC surveillance criteria,6 blood, urine, tracheal aspirate, and cultures were evaluated retrospectively. The presence of at least one type of candida in blood culture and the presence of concomitant fever, hypothermia, leukocytosis, elevation in acute phase reactants, tachycardia, and hypotension were evaluated as candidemia. In patients with new or progressive infiltrates on chest X-ray, the presence of two or more of the findings of fever >38°C or hypothermia, leukocytosis or leukopenia, purulent secretion, and candida isolation in tracheal aspirate culture was defined as pneumonia. Diagnosis of symptomatic urinary infection was made in the case of fever >38°C, and at least one of the signs and symptoms of urgency, frequency, dysuria or suprapubic tenderness, and isolation of candida species in the urine culture.6
Cases were identified from an electronic medical database maintained by the computerized hospital information processing services (CHIPS) of the institution. Details of demographic profile, clinical presentation, laboratory investigations, and outcome with regards to PICU admission were collected from the in-patient charts and discharge summaries. Chest X-rays and CT scans were reviewed by the primary investigator and findings were noted. Variables such as duration of mechanical ventilation, duration of antibiotics, presence of a central venous catheter, and other risk factors were collected from the ICU flow sheet. Controls were selected from the PICU database, and their details were collected similarly. The datasheet was filled in by using standard pro forma which was then entered using EpiData software. The data thus collected were used to process the results needed to achieve the objectives.
Statistical Analysis
For continuous data that were skewed, median, and interquartile range (IQR) were done and categorical data were presented using numbers and percentages. Based on the normality assumption, a nonparametric Mann Whitney U test was used to evaluate the difference between cases and controls. The Chi-square, Fisher's exact test (less cell count), and Yates continuity correction (zero count in the cell) were used to determine the association between categoric variables. All risk factors were assessed with binary logistic regression and penalized logistic regression analysis. Penalized logistic regression was used when the odds ratio could not be estimated due to empty cells or cells with low frequency, because of failure of the maximum likelihood estimate to converge. Point estimates were reported as odds ratio (OR) and 95% confidence interval (CI). All tests were two-sided at α = 0.05 level of significance. Analyses were done using SAS® (Ver. 9.2, SAS Institute, and the USA).
Ethical Considerations
This study was approved by the Institutional Review Board prior to the commencement of the study (IRB min no: 11416 dated June 3, 2018). Patient confidentiality was maintained using unique identifiers and by password-protected data entry software with restricted users.
Results
During the 3-year period, 109 children—47 (44%) in the year 2015, 34 (30%) in the year 2016, and 28 (26%) in the year 2017 were identified to have had candida infection from among 2,540 PICU admissions. In the age and sex-matched controls, 92 children had also been admitted during the same period. The median age among candida cases and controls was 7 (IQR 4–14.5) years and 6 (IQR 2–12) years, respectively. Infants were a major proportion of the study population in both groups (43 and 47%, respectively). Male predominance was also seen in both groups. Clinical characteristics and outcomes of candida cases and controls are compared and shown in Table 1.
Table 1.
Clinical characteristics and outcome of the cases and controls
| Cases (n = 109) n (%) | Control (n = 92) n (%) | p value | |
|---|---|---|---|
| Age (years)ϕ | 7 (4–14.5) | 6 (2–12) | 0.041 |
| Age <1 year 1–5 years 5–15 years |
47 (43.1) 31 (28.4) 31 (28.4) |
44 (47.8) 28 (30.4) 20 (21.7) |
0.550 |
|
Sex Male Female |
63 (57.8) 46 (42.2) |
58 (63.0) 34 (37.0) |
0.449 |
|
Year 2015 2016 2017 |
47 (43.1) 34 (31.2) 28 (21.7) |
31 (33.7) 29 (31.5) 32 (34.8) |
0.283 |
|
Clinical diagnosis Septic shock Acute kidney injury |
104 (95.4) 37 (33.9) |
24 (26.08) 4 (4.34) |
0.177 |
|
Duration of hospitalization (days) <5 5–14 >14 |
30 (27.5) 37 (33.9) 42 (38.53) |
29 (31.52) 38 (41.3) 25 (27.17) |
0.231 |
|
Third line antibiotics Cephalosporin Meropenem Vancomycin Aminoglycoside Meropenem + Vancomycin |
44 (39.4) 100 (90.8) 25 (22.9) 34 (31.2) 22 (20.18) |
39 (42.39) 46 (50.0) 23 (25.0) 16 (17.39) 18 (19.56) |
0.063 |
|
System involvement Respiratory Cardiac Neurologic Renal Endocrine Chronic liver disease Post-operative Burns |
14 (12.8) 15 (13.8) 29 (26.6) 11 (10.3) 13 (11.9) 1 (0.9) 14 (12.8) 4 (3.7) |
6 (6.52) 14 (15.2) 10 (10.8) 4 (4.34) 4 (4.34) 1 (1.08) 11 (11.9) 2 (2.17) |
0.455 |
|
Outcome Alive Death |
72 (66.1) 37 (33.9) |
74 (80.4) 18 (19.6) |
0.023 |
ϕValues are presented as Median (IQR) and p value is obtained from the Mann Whitney test; Values are presented as n (%) and p value is obtained from Chi-square and Fishers exact test
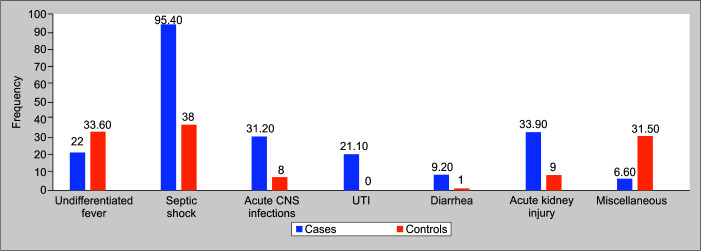
Septic shock was the commonest PICU diagnosis (95%) followed by acute kidney injury (33.9%) and acute CNS infections (31.2%) among those with a candida infection. The other clinical diagnostic group among all children is shown in Figure 1. Eleven (10%) among candida cases had absolute neutrophilic count (ANC) less than 1.0 × 109/L whereas none of the controls were neutropenic.
Fig. 1.
Clinical diagnosis of children with invasive candida infection
There were 124 isolates of candida from 109 children as follows: endotracheal (ET) aspirate—42 (39%), blood—40 (37%), urine—27 (24%), and pus—15 (14%). Ten children had candida species isolated from two different sites, and one each had grown from more than two sites. Of the 42 who showed candida in their ET aspirates, 8 (19.9%) grew candida from other sites also. Ninety-nine (91%) of the cases had chest X-ray findings such as ground-glass opacities and perihilar infiltrates. Seven underwent CT scans of the thorax of which five showed findings suggestive of fungal pneumonia.
Of the 124 isolates, 33 (30%) were C. albicans, 55 (50%) were C. tropicalis, others were: C. glabrata—6 (5.5%), C. parapsilosis—5 (4.5%), C. krusei—1 (1%), and other candida species—9 (9%).
Multivariate analyses showed neutropenia [OR 20.01, 95% CI (0.94–422.32)], mechanical ventilation [OR 5.97, 95% CI (2.44–14.62)], peritoneal dialysis [OR 5.81, 95% CI (1.27–26.50)], institution of amino acids [OR 5.41, 95% CI (0.85–34.13)], central venous catheter in-situ [OR 3.83, 95% CI (1.59–9.19)], antibiotic use >14 days [OR 3.58, 95% CI (1.38–9.29)] to be significant factors associated with candida infection. Other risk factors that were less significant were infancy, previous admission and immunosuppression, and the presence of comorbidities (Table 2).
Table 2.
Risk factors for candida infection
| Risk factors | Cases n (%) | Controls n (%) | p value * | OR (95% CI) | p value ϕ | aOR (95% CI) | p value ‡ |
|---|---|---|---|---|---|---|---|
| Age (years) <1 1–5 5–15 |
47 (43.1) 31 (28.4) 31 (28.4) |
44 (47.8) 28 (30.4) 20 (21.7) |
0.550 |
0.69 (0.34, 1.38) 0.71 (0.33, 1.53) |
0.295 0.385 |
0.62 (0.25, 1.57) 0.35 (0.14, 0.89) |
0.901 0.034 |
| Presence of co-morbidities | 72 (66.1) | 48 (52.2) | 0.046 | 1.78 (1.01, 3.15) | 0.046 | 1.58 (0.79, 3.20) | 0.195 |
| Neutropenia (ANC <1000) | 11 (10.1) | 0 (0.0) | 0.005 | 21.63 (1.11, 422.92) | 0.043 | 20.01 (0.94, 422.32) | 0.054 |
| Immunosuppressants | 13 (11.9) | 7 (7.6) | 0.308 | 1.64 (0.63, 4.31) | 0.312 | 1.09 (0.30, 3.97) | 0.889 |
| Duration of antibiotics (>5 days) | 46 (42.2) | 29 (31.5) | 0.002 | 3.89 (1.68, 9.03) | 0.002 | 3.58 (1.38, 9.29) | 0.022 |
| Peritoneal dialysis | 13 (11.9) | 2 (2.2) | 0.009 | 6.09 (1.34, 27.76) | 0.019 | 5.81 (1.27, 26.50) | 0.023 |
| Institution of amino acids | 11 (10.1) | 1 (1.1) | 0.007 | 10.21 (1.29, 80.69) | 0.028 | 5.41 (0.85, 34.13) | 0.073 |
| Mechanical ventilation | 96 (88.1) | 57 (62.0) | <0.0001 | 4.53 (2.22, 9.29) | <0.0001 | 5.97 (2.44, 14.62) | <0.0001 |
| Presence of central venous line | 37 (33.9) | 11 (12.0) | <0.0001 | 3.78 (1.79, 7.96) | <0.0001 | 3.83 (1.59, 9.19) | 0.003 |
*p value is obtained from Chi-square test
ϕp value is obtained from univariate logistic regression and penalized logistic regression
‡p value is obtained from the multiple penalized logistic regression
While 56 (51%) children were treated with fluconazole 59 (54%) had received amphotericin. Six had succumbed to the illness before the culture results became available. Among 109 candida cases 72 (66%) had survived whereas among controls there were 74 (80%) survivors; the difference achieved statistical significance (p = 0.023) (Table 1). There was no significant difference in the survival between albicans and non-albicans groups [24 (72%) vs 48 (63%), p = 0.33] (Fig. 2).
Fig. 2.
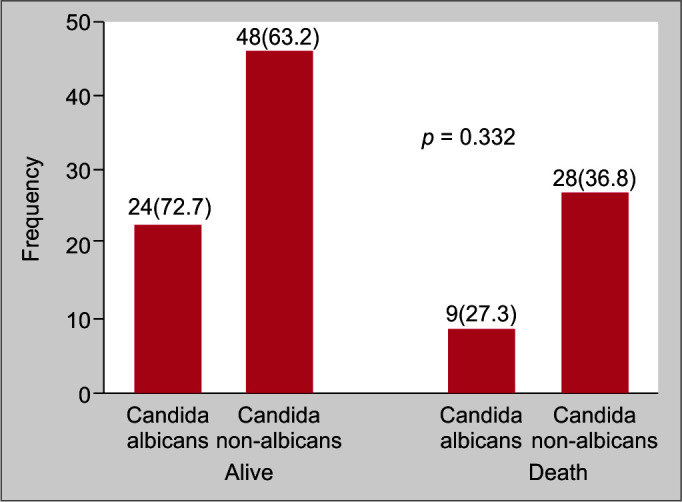
Comparison of outcomes of Candida albicans and non albicans infection
Discussion
In this retrospective study, we found six factors—neutropenia, mechanical ventilation, peritoneal dialysis, the institution of amino acids, CVC in-situ, antibiotic use for more than days to be significantly associated with candida infections.7 Candida non-albicans group contributed to 70% of the isolates with C. tropicalis (50%) being the most common. C. albicans accounted for 30%. Previously, CVC,7–13 mechanical ventilation,9,11,12,14,15 and underlying malignancy7,9 have been demonstrated to be risk factors for invasive candidiasis; prematurity and ICU stay are the main risk factors in new-borns.7 Among PICUs broad-spectrum antibiotic treatment has been identified as one of the foremost risk factors for candida infection.8,11,16 We have shown that receiving antibiotics for over 5 days itself to be associated with an odds of 3.5 for developing candidiasis.
Peritoneal dialysis was an unique risk factor present in our study population. Peritoneal dialysis was the primary mode of renal support in our ICU for acute kidney injury (AKI) which was seen in 34% of cases. Furthermore, in our PICU we predominantly offered partial parenteral nutrition with an intravenous amino acid infusion which increased the odds of acquiring candida by five times. We observed a predominance of ventilator-associated pneumonia (VAP) with candida among our cases; possibly related to the preponderance of infant population with high rates of ventilatory support in a large case volume PICU. The inclusion of clinically suspect VAP (new-onset fever, purulent secretions, or increasing ventilatory requirements) rather than those with routine surveillance cultures is in favor of an infection rather than incidental colonization. The X-ray chest findings seen in all of them further support this. Differentiating candida colonization of the respiratory tract from VAP can be challenging. Candida score has been proposed and all our cases had a score >2.5 cut off. In children, candidal colonization and biofilm formation can worsen pulmonary inflammation resulting in prolonged ventilation with higher morbidity and mortality.17,18
C. albicans remains the most common species of candida in Australia, France, South Africa and North American PICUs7,12,19,20 as well as in the 5-year retrospective pediatric study from Saudi Arabia.13 Other studies, however, report more non-albican types of candida.8,11,14,21,22 Even those studies that reported a preponderance of C. albicans observe a strong trend towards the emergence of candida non-albicans.12,20 Indian studies from PICUs10,23 identified C. tropicalis, C. parapsilosis, and C. albicans as common isolates similar to ours. In the non-albicans candida (NAC) study24 from UK NAC was isolated more frequently in those with hematological malignancies and bone marrow transplant (BMT) recipients but was less common among ICU and surgical children.
Management guidelines for ICI in pediatric patients suggest the use of fluconazole, echinocandins, and amphotericin B.25,26 We had treated our patients equally with intravenous amphotericin or fluconazole. The growing incidence of non-albicans infections poses a challenge in resource-limited settings as they are often resistant to azoles necessitating treatment with either amphotericin or echinocandins. Echinocandins and liposomal amphotericin are prohibitively expensive. The side effects of conventional amphotericin such as fever, hypersensitivity reaction, and hypokalemia warranting large doses of potassium replacement are a concern. Recently new orally effective drugs are available like posaconazole, and isavuconazole but cost is a limiting factor.
The mortality rates of invasive candidiasis vary from 22 to 55%7,9,11–13,15 with the overall attributable mortality of C. tropicalis and C. glabrata being the highest.24 In our study, those children with candida infection had significantly lower survival than controls as in other studies.9,11,14,20,24,27,28 We however did not find any significant difference in the survival between C. albicans and non-albicans groups of our population. Our study besides being a single-center study was also of retrospective design and hence clinical data on each patient was dependent on what was documented in the charts, which is a limitation.
Close monitoring of critically ill children with the above risk factors could be a cost-effective strategy for the early detection of candida infections.
Conclusion
The rate of candida infection is 4.2% of PICU admissions. The most common species was C. tropicalis. The independent risk factors for candida infection were neutropenia, antibiotic duration >5 days, peritoneal dialysis, amino acid administration, mechanical ventilation, and presence of CVC.
Research Quality and Ethics Statement
The authors of this manuscript declare that this scientific work complies with reporting is IRB Min no: 11416 dated June 3, 2018. We also certify that we have not plagiarized the quality, formatting, and reproducibility guidelines set forth by the EQUATOR network. The authors also attest that this clinical investigation was determined to require an Institutional Review Board/Ethics Committee review, and the corresponding protocol/approval number the contents in this submission and have done a Plagiarism Check.
Orcid
Raja Rajeshwari https://orcid.org/0000-0001-6680-6611
Siva Vyasam https://orcid.org/0000-0002-0887-1535
Jolly Chandran https://orcid.org/0000-0003-0021-8897
Sanketh Porwal https://orcid.org/0000-0003-2841-0055
Muniya Thokchom https://orcid.org/0000-0003-3932-9025
Ebor J James https://orcid.org/0000-0001-6302-6572
Reka Karuppusami https://orcid.org/0000-0001-9913-2713
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Delaloye J, Calandra T. Invasive candidiasis as a cause of sepsis in the critically ill patient. Virulence. 2014;5(1):161–169. doi: 10.4161/viru.26187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ElGendy FM, Hassan FM, Khatab AA, El-Hendawy GR, Saleh NY. Study of fungal infections in pediatric intensive care unit in Menoufiya University Hospital. Menoufia Med J. 2014;27(1):55. doi: 10.4103/1110-2098.132742. [DOI] [Google Scholar]
- 3.Mesini A, Bandettini R, Caviglia I, Fioredda F, Amoroso L, Faraci M, et al. Candida infections in paediatrics: results from a prospective single-centre study in a tertiary care children's hospital. Mycoses. 2017;60(2):118–123. doi: 10.1111/myc.12570. [DOI] [PubMed] [Google Scholar]
- 4.Orasch C, Mertz D, Garbino J, van Delden C, Emonet S, Schrenzel J, et al. Fungal Infection Network of Switzerland (FUNGINOS). Fluconazole non-susceptible breakthrough candidemia after prolonged low-dose prophylaxis: a prospective FUNGINOS study. J Infect. 2018;76(05):489–495. doi: 10.1016/j.jinf.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 5.Harrington R, Kindermann SL, Hou Q, Taylor RJ, Azie N, Horn DL. Candidemia and invasive candidiasis among hospitalized neonates and pediatric patients. Curr Med Res Opin. 2017;33(10):1803–1812. doi: 10.1080/03007995.2017.1354824. [DOI] [PubMed] [Google Scholar]
- 6.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Blyth CC, Chen SC, Slavin MA, Serena C, Nguyen Q, Marriott D, et al. Australian Candidemia Study. Not just little adults: candidemia epidemiology, molecular characterization and antifungal susceptibility in neonatal and pediatric patients. Pediatrics. 2009;123(5):1360–1368. doi: 10.1542/peds.2008-2055. [DOI] [PubMed] [Google Scholar]
- 8.Devrim İ, Demirağ B, Yaman Y, Bayram N, Özdemir F, Kara A, et al. A 7-year study of the distribution of nosocomial candidemia in children with cancer. Turk J Pediatr. 2015;57(3):225–229. 26701939 [PubMed] [Google Scholar]
- 9.Almooosa Z, Ahmed GY, Omran A, AlSarheed A, Alturki A, Alaqeel A, et al. Invasive candidiasis in pediatric patients at King Fahad Medical City in Central Saudi Arabia. A 5-year retrospective study. Saudi Med J. 2017;38(11):1118–1124. doi: 10.15537/smj.2017.11.21116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singhi S, Rao DSVR, Chakrabarti A. Candida colonization and candidemia in a pediatric intensive care unit. Pediatr Crit Care Med. 2008;9(1):91–95. doi: 10.1097/01.PCC.0000298643.48547.83. [DOI] [PubMed] [Google Scholar]
- 11.Conde-Rosa A, Amador R, Pérez-Torres D, Colón E, Sánchez-Rivera C, Nieves-Plaza M, et al. Candidemia distribution, associated risk factors, and attributed mortality at a university-based medical center. P R Health Sci J. 2010;29(1):26–29. 20222330 [PMC free article] [PubMed] [Google Scholar]
- 12.Brissaud O, Guichoux J, Harambat J, Tandonnet O, Zaoutis T. Invasive fungal disease in PICU: epidemiology and risk factors. Ann Intensive Care. 2012;2(1):6. doi: 10.1186/2110-5820-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaoutis TE, Prasad PA, Localio AR, Coffin SE, Bell LM, Walsh TJ, et al. Risk factors and predictors for candidemia in pediatric intensive care unit patients: implications for prevention. Clin Infect Dis. 2010;51(5):e38–e45. doi: 10.1086/655698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aslan N, Yildizdas D, Alabaz D, Horoz OO, Yontem A, Kocabas E. Invasive candida infections in a pediatric intensive care unit in Turkey: evaluation of an 11-year period. J Pediatr Intensive Care. 2020;9(1):21. doi: 10.1055/s-0039-1695061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Acar A, Oncul O, Kucukardali Y, Ozyurt M, Haznedaroglu T, Cavujlu S. Epidemiological features of candida infections detected in intensive care units and risk factors affecting mortality. Mikrobiyol Bul. 2008;42(3):451–461. 18822889 [PubMed] [Google Scholar]
- 16.Sütçü M, Acar M, Genç GE, Kökçü İ, Aktürk H, Atay G, et al. Evaluation of Candida species and antifungal susceptibilities among children with invasive candidiasis. Turk Pediatr Ars. 2017;52(3):145–153. doi: 10.5152/TurkPediatriArs.2017.5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delisle MS, Williamson DR, Albert M, Perreault MM, Jiang X, Day AG, et al. Impact of Candida species on clinical outcomes in patients with suspected ventilator-associated pneumonia. Can Respir J. 2011;18(3):131–136. doi: 10.1155/2011/827692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Pascale G, Antonelli M. Candida colonization of respiratory tract: to treat or not to treat, will we ever get an answer? Intensive Care Med. 2014;40(9):1381–1384. doi: 10.1007/s00134-014-3364-y. [DOI] [PubMed] [Google Scholar]
- 19.Dramowski A, Cotton MF, Whitelaw A. Surveillance of healthcare-associated infection in hospitalised South African children: which method performs best? South Afr Med J. 2017;107(1):56–63. doi: 10.7196/SAMJ.2016.v107.i1.11431. [DOI] [PubMed] [Google Scholar]
- 20.Palazzi DL, Arrieta A, Castagnola E, Halasa N, Hubbard S, Brozovich AA, et al. Candida speciation, antifungal treatment and adverse events in pediatric invasive candidiasis: results from 441 infections in a prospective, multinational study. Pediatr Infect Dis J. 2014;33(12):1294–1296. doi: 10.1097/INF.0000000000000431. [DOI] [PubMed] [Google Scholar]
- 21.Çekin Y, Pekintürk N, Çekin AH. Evaluation of species distribution and antifungal resistance of Candida isolates from hospitalized patients. J Clin Anal Med. 2015;6(97):8–11. doi: 10.4328/JCAM.1638. [DOI] [Google Scholar]
- 22.Hazırolan G, Yıldıran D, Baran I, Mumcuoğlu İ, Aksu N. Yatan hasta örneklerinden izole edilen Candida izolatlarının tür dağılımlarının ve antifungal duyarlılık profillerinin değerlendirilmesi. Turk Hij Den Biyol Derg. 2015;72(1):17–26. doi: 10.5505/TurkHijyen.2015.75010. [DOI] [Google Scholar]
- 23.Mishra R, Behera C, Jena P, Mishra SN, Sahoo B, Patnaik S, et al. Candidemia in the pediatric intensive care unit in Eastern India. J Pediatr Crit Care. 2020;7(5):237. doi: 10.4103/JPCC.JPCC_38_20. [DOI] [Google Scholar]
- 24.Krcmery V, Barnes AJ. Non-albicans Candida spp. causing fungaemia: pathogenicity and antifungal resistance. J Hosp Infect. 2002;50(4):243–260. doi: 10.1053/jhin.2001.1151. [DOI] [PubMed] [Google Scholar]
- 25.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:1–50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hope WW, Castagnola E, Groll AH, Roilides E, Akova M, Arendrup MC, et al. ESCMID Fungal Infection Study Group. ESCMID guideline for the diagnosis and management of Candida diseases 2012: prevention and management of invasive infections in neonates and children caused by Candida spp. Clin Microbiol Infect. 2012;18(Suppl 7):38–52. doi: 10.1111/1469-0691.12040. [DOI] [PubMed] [Google Scholar]
- 27.Hlophe ST, Govender NP, Masekela R. Invasive fungal infections among critically ill children: epidemiology, risk factors and outcomes. Afr J Thorac Crit Care Med. 2018;24(1):11–14. doi: 10.7196/AJTCCM.2018.v24i1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arendrup MC, Sulim S, Holm A, Nielsen L, Nielsen SD, Knudsen JD, et al. Diagnostic issues, clinical characteristics, and outcomes for patients with fungemia. J Clin Microbiol. 2011;49(9):3300–3308. doi: 10.1128/JCM.00179-11. [DOI] [PMC free article] [PubMed] [Google Scholar]