Abstract
Background:
There is a strong association between nutrition and long-term FEV1 in cystic fibrosis (CF), but studies have been driven by data from subjects with pancreatic insufficiency (PI-CF). We thus evaluated the association between body mass index (BMI) and FEV1 percent-predicted (FEV1pp) in children with pancreatic sufficiency (PS-CF) and contrasted it with the association in PI-CF.
Methods:
We utilized data from the CF Foundation Patient Registry. The cohort included children born 1995–2010, diagnosed <2 years of age, and who had annualized data on BMI percentile and FEV1pp at ages 6–16 years. Pancreatic status was defined based on pancreatic enzyme replacement therapy. The association between BMI and FEV1 was evaluated using linear and mixed-effects longitudinal regression.
Results:
There were 424 children with PS-CF and 7,849 with PI-CF. The association between BMI and FEV1 differed significantly by pancreatic status: each 10-pct higher BMI was associated with 2% [95%CI=1.9–2.1] higher FEV1pp in PI-CF, compared to just 0.9% [0.5–1.3] in PS-CF (PINTERACTION<0.001). Within the at-risk nutritional category (BMI <25pct), each 10-pct higher BMI was associated with 5% higher FEV1pp in PI-CF, but no significant increase in PS-CF. Moreover, in PS-CF, overweight/obesity (BMI ≥85pct) was associated with decreasing FEV1pp. In addition, FEV1pp decline through age 20 years in youth with PS-CF was small (−0.6% per year) and independent of BMI (BMI*age PINTERACTION=0.37).
Conclusions:
In children with PS-CF, BMI remains an important determinant of lung function. However, it may be less critical to attain a BMI >50th percentile; and BMI ≥85th percentile may be detrimental.
Keywords: Cystic fibrosis, nutrition, pancreatic insufficiency, BMI, FEV1, lung function
INTRODUCTION
Cystic fibrosis (CF) is an autosomal recessive genetic disorder caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. It is a multisystem disease, causing thickened secretions in the airways, intestine, pancreas, and liver ducts, which lead to frequent and recurrent pulmonary infections, nutrient malabsorption, growth retardation, delayed puberty, and male infertility(1, 2). Despite being a monogenic disorder, phenotypes and outcomes vary widely as a result of interactions between specific types of CFTR mutations, modifier genes, and environmental factors.
While recurrent lung infections are the most prominent manifestation in CF, the first descriptions of the disease were made in the 1930s by Guido Fanconi and Dorothy Andersen, who each reported cystic lesions and fibrosis in the pancreas of children with malnutrition and bronchiectasis(3, 4). In one of the earliest clinical manifestations of CF, inspissated secretions lead to obstruction of pancreatic ducts, causing inflammation, fibrosis, fatty infiltration, and ultimately injury and destruction(1, 5, 6). Pancreatic injury usually begins in utero and many children present after birth with exocrine pancreatic insufficiency. Approximately 85% of children with CF will be pancreatic insufficient (PI) at birth or become PI over the first few years of life, while 10%−15% will retain pancreatic sufficiency (PS)(7). Pancreatic insufficiency leads to malabsorption, malnutrition, and growth deficits, and nutritional status is correlated with pulmonary function and overall long-term survival(8–13).
The Cystic Fibrosis Foundation recommends aiming for a weight-for-length (WFL) or body mass index (BMI) ≥ 50th percentile for all children with CF(8, 13). While adequate nutrition is certainly essential for lung function and overall survival, existing studies have been performed in the overall population of individuals living with CF(8, 10–12). To date, there has been no large cohort study examining the association between nutritional status and lung function in the PS CF population. Furthermore, with the rising prevalence of overweight/obese patients it is pertinent to examine this relationship closely to determine whether it is as critical in this subset of patients to achieve the aforementioned nutritional goals(14–16). In the present study, we hypothesized that in children with PS-CF the association between growth trajectory and lung function at school age and adolescence would be less pronounced than among those with PI-CF.
METHODS
Study population
We obtained deidentified patient-level and encounter-level data from the CF Foundation Patient Registry (CFFPR), including demographics, mode of diagnosis, CFTR genotype, microbiology, fecal elastase, and FEV1. For the current analysis, the cohort included children with CF born between 1995 and 2010, diagnosed by newborn screen (NBS) or before 2 years of age, and who have data on exocrine pancreatic status, lung function, and clinical outcomes at ages 6–16 years. To prevent inclusion of individuals with CF-related metabolic disorder (CRMS)(17), we excluded records with sweat chloride <60 mmol/L. This study was approved by the Institutional Review Board of the University of Pittsburgh (PRO18010357).
Based on data from the CFFPR, children were classified as PS if they met any of the following criteria: 1) Never received pancreatic enzyme replacement therapy (PERT); 2) Received PERT prior to 6 years of age but it was discontinued at ≤6 years of age and was never reinstated afterwards; 3) Received PERT for ≤3 years but treatment discontinued for ≥5 years prior to the most recent visit; or 4) Received PERT for ≤3 years but had subsequent fecal elastase >200 μg/g. Patients who did not meet any of the aforementioned criteria were classified as PI and were used as controls. Growth and nutritional status were determined using annualized BMI percentiles for age and sex, calculated by the CFFPR using CDC charts(18). BMI was further analyzed by stratifying into four categories: “at risk” (BMI <25th percentile), “borderline” (BMI 25th–50th pct), “normal” (50th–85th pct) and “overweight/obese” (“ov/ob”, ≥85th pct).
Statistical analyses
Our primary outcome was FEV1, assessed as percent-of-predicted (%pred, or FEV1pp) using Global Lung Initiative (GLI) equations(19) at ages 6, 12, and 16 years, as well as a longitudinal analysis incorporating repeated measures for ages 6 through 16 years. Unadjusted analyses were performed using chi-squared and t-tests to compare proportions and means, respectively. Adjusted analyses were performed using linear multivariable regression adjusted for sex, race/ethnicity, insurance status, chronic inhaled medications (inhaled tobramycin, hypertonic saline, and DNAse), and age at first acquisition of Pseudomonas aeruginosa, Burkholderia sp., and Burkholderia cepacia. We evaluated effect modification by sex, F508del status (none, heterozygous, homozygous), and birth cohort (<2003 or ≥2003) using multiplicative interaction terms (e.g., BMI*sex). Longitudinal analysis was performed using a random effects model to account for repeated measures across all time points. Within this analysis, we looked at BMI both as a continuous variable as well as within the four pre-specified BMI categories.
Since prior-year BMI may impact current FEV1, or may have led to interventions that could also impact lung function, we performed a structural equation modeling (SEM) analysis to estimate the effect of BMI on FEV1 after accounting for the effect of BMI and FEV1 in prior years (e.g., the effect of BMI on FEV1 at 10 years of age [BMI10 → FEV110], considering that BMI at 9 years of age had an effect on FEV1 at that age [BMI9 → FEV19], and that each of them have an effect on measures at age 10 years [BMI9 → BMI10, FEV19 → FEV110, and BMI9 → FEV110]); the generalized sandwich estimator was used to account for within-subject effects.
Finally, to assess the robustness of our results and eliminate potential sources of bias derived from our definitions or other sources, we performed a series of sensitivity analyses: 1) Models adjusted for maternal education rather than insurance type as proxy for socioeconomic status; 2) Models additionally adjusted for CF comorbidities and complications including asthma, pancreatitis, CF-related diabetes (CFRD), and CF liver disease (CFLD); 3) Analysis stratified by newborn screen diagnosis (yes vs no); 4) Analysis stratified by birth cohort (1995–2002 vs 2003–2010); 5) PS defined exclusively as subjects never placed on PERT; 6) PS defined exclusively as patients with available fecal elastase >200 μg/g; and 7) PS defined as having at least one class IV–VI mutation.
RESULTS
Cohort Characteristics
Of 12,258 children in the CFFPR database, 3,987 were excluded because they did not meet the selection criteria (32 were diagnosed after age 2; 1,347 did not have follow-up until at least age 6 years; 503 had a sweat chloride value <60 mmol/L; and 2,103 had unclear pancreatic sufficiency status) (Figure 1). There were thus 8,273 eligible subjects included in the study, of whom 424 (5.1%) had PS-CF and 7,849 (94.9%) had PI-CF. Baseline demographic data for each cohort are shown in Table 1 and Supplementary Table S1. Within the PS cohort (424 patients with a total of 4,284 patient-years of follow-up), 49.8% of children were female; the majority were White (90.8%), born between 2003–2010 (66.0%), diagnosed via NBS (56.6%), F508del heterozygous (64.9%), and had at least one class IV–VI mutation (91.5% of those with genotype available). Within the three cross-sectional analyses, mean FEV1pp decreased with age (100.9%, 95.1%, and 94.7% respectively) while average BMI percentile remained stable (65.2th, 64.4th, and 66.8th percentiles). Compared to the PS cohort, children in the PI cohort were more likely to be diagnosed by methods other than NBS (70.6%), to be F508del homozygous (55.5%), and to have two class I–III mutations (98.4% of those with genotype available). Mean FEV1 and BMI were lower in the PI cohort, and both decreased with age: at 6, 12, and 16 years of age, respective mean FEV1 were 92.8%, 86.5%, and 80.8%; mean BMI percentiles were 55.7, 46.9, and 47.7.
Figure 1 – Overview of cohort selection.
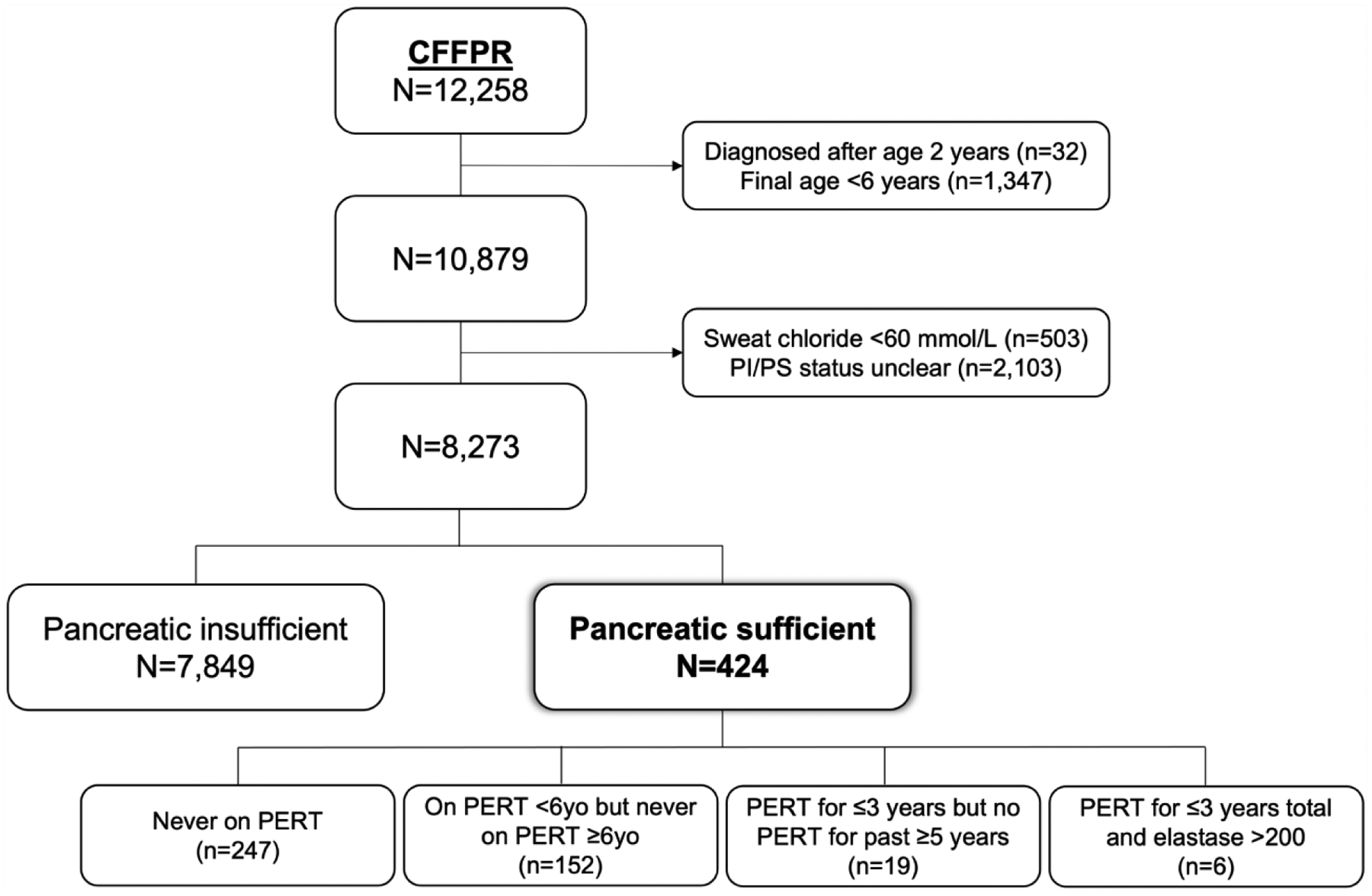
CFFPR: CF Foundation Patient Registry. PERT: Pancreatic enzyme replacement therapy. PI: Pancreatic insufficient. PS: Pancreatic sufficient.
Table 1 –
Characteristics of pancreatic-sufficient (PS-CF) study cohort
| Full dataset | Age 6 years | Age 12 years | Age 16 years | |
|---|---|---|---|---|
| Number of patients | 424 | 381 | 137 | 60 |
| Number of visits | 4,284 | 381 | 137 | 60 |
| Years of follow-up1 | 9 [7–13] | --- | --- | --- |
| Female sex | 211 (49.8%) | 188 (49.3%) | 69 (50.4%) | 34 (56.7%) |
| Mean age, years | 6.7 (4.5) | 6.5 (0.3) | 12.5 (0.3) | 16.5 (0.3) |
| - Hispanic | 66 (15.6%) | 53 (13.9%) | 18 (13.1%) | 8 (13.3%) |
| - 2003–2010 | 280 (66.0%) | 264 (69.3%) | 23 (16.8%) | N/A |
| - Other | 184 (43.4%) | 154 (40.4%) | 84 (61.3%) | 43 (71.7%) |
| - 1–2yrs | 49 (11.7%) | 41 (10.9%) | 18 (13.3%) | 8 (13.3%) |
| ≥1 F508del mutation | 277 (65.3%) | 255 (66.9%) | 88 (64.2%) | 36 (60.0%) |
| - Missing both | 24 (8.5%) | 16 (6.3%) | <5* | <5* |
| - Missing / no inurance | 185 (43.6%) | 15 (3.9%) | <5* | <5* |
| - Unknown/missing | 376 (88.7%) | 149 (39.6%) | 48 (35.0%) | 19 (31.7%) |
| Age at first Pseudomonas1 | 4.5 [2.3–7.6] | --- | --- | --- |
| - Not available | 258 (60.9%) | 226 (59.3%) | 104 (75.9%) | 47 (78.3%) |
| FEV1 (%pred) | 98.6 (13.9) | 100.9 (14.5) | 95.1 (12.8) | 94.7 (13.1) |
| BMI (percentile) | 63.4 (26.2) | 65.2 (24.9) | 64.4 (28.4) | 66.8 (25.0) |
| - Overweight/obese (≥85th) | 192 (45.4%) | 96 (25.6%) | 45 (34.1%) | 20 (33.3%) |
| - Tobramycin | 156 (36.8%) | 38 (10.0%) | 23 (16.8%) | 8 (13.3%) |
| - Asthma | 131 (31.1%) | 52 (15.7%) | 38 (28.6%) | 20 (33.3%) |
Categorical variables shown as n (%) and continuous variables as mean (SD), unless otherwise indicated. See Supplementary Table S1 for characteristics of pancreatic-insufficient (PI) cohort.
Median [IQR].
May add to more than the total number of patients and/or more than 100%, as categories are not mutually exclusive.
May add to more than the total number of patients and/or more than 100%, as categories are not mutually exclusive and/or patients may change insurance over time.
May add to more than the total number of patients and/or 100%, as they may have changed categories over time.
Lowest fecal elastase value recorded for each patient.
Per CF Foundation policy, exact values suppressed in cells with counts <5.
BMI: Body mass index. CFLD: CF liver disease. CFRD: CF-related diabetes. HTS: Hypertonic saline. IGT: Impaired glucose tolerance. NBS: Newborn screen.
Cross-Sectional and Longitudinal Analyses
In the cross-sectional analyses of PS-CF, BMI was significantly associated with FEV1 at ages 6 (β=0.13 higher FEV1pp [95%CI 0.07–0.19] per each 1-percentile higher BMI), 12 (β=0.13 [95%CI 0.05–0.22]), and 16 (β=0.22 [95%CI 0.07–0.37]) after adjusting for sex, race/ethnicity, insurance status, and relevant clinical covariates (Table 2). These effects were not modified by sex, F508 genotype, or birth cohort (see interaction analysis in Supplementary Table S2). In the adjusted longitudinal analysis, BMI was also significantly associated with FEV1 (β=0.08 higher FEV1pp [95%CI 0.05–0.11] per each 1-percentile higher BMI, Table 3 Model 1).
Table 2 –
BMI and FEV1 in children with PS-CF at 6, 12, and 16 years of age
| Age 6 years (n=325) | Age 12 years (n=129) | Age 16 years (n=57) | |
|---|---|---|---|
| BMI percentile | 0.13 (0.07, 0.20) ‡ | 0.13 (0.045–0.22)† | 0.22 (0.074–0.37)† |
| Female sex | 2.24 (−0.90, 5.40) | 1.80 (−2.82, 6.41) | 0.68 (−6.28, 7.65) |
| Non-white race | −0.22 (−6.55, 6.10) | −2.30 (−13.58, 9.00) | 4.36 (−7.41, 16.13) |
| Hispanic/Latino Data missing1 |
0.71 (−4.00, 5.42) 9.15 (1.34, 16.95)* |
−1.44 (−8.47, 5.59) 6.66 (−10.34, 23.66) |
−1.02 (−10.76, 8.73) −25.82 (−53.30, 1.66) |
| Non-private insurance Data missing1 |
−0.90 (−4.36, 2.56) −0.05 (−5.26, 5.17) |
−1.31 (−6.16, 3.53) −5.94 (−19.01, 7.14) |
−4.54 (−12.16, 3.08) −6.84 (−13.37, 27.05) |
|
Pseudomonas sp. Data missing1 |
0.55 (−2.74, 3.85) 49.31 (17.30, 81.32)† |
2.46 (−2.48, 7.41) n/a |
−3.44 (−10.86, 4.00) n/a |
|
Burkholderia sp. Data missing1 |
7.51 (−20.50, 35.52) −51.49 (−79.13, −23.86)‡ |
−3.37 (−16.65, 9.91) n/a |
−6.53 (−33.54, 20.48) n/a |
| Tobramycin | −4.38 (−10.00, 1.23) | −1.46 (−7.94, 5.01) | 10.71 (−1.17, 22.58) |
| HTS Data missing1 |
2.81 (−0.86, 6.48) 4.26 (−1.68, 10.20) |
−2.18 (−6.90, 2.53) n/a |
−0.40 (−8.02, 7.23) n/a |
| DNase | −1.21 (−4.52, 2.10) | 0.66 (−4.70, 6.02) | −5.11 (−13.78, 3.56) |
Results from cross-sectional adjusted linear regression at each age. Shown are effect estimates (β coefficients) for FEV1pp, with 95% confidence intervals (95%CI) in parentheses.
P<0.05,
P<0.01,
P<0.001.
Variables with >10% missing data were assigned a “missing” category. HTS: Hypertonic saline.
Table 3 –
Longitudinal analysis of BMI and FEV1
| Model 1 (PS only) | Model 2 (PS+PI) | Model 3 (PS+PI) | |
|---|---|---|---|
| BMI | 0.079 (0.051, 0.11) ‡ | --- | --- |
|
BMI
Pancreatic status Interaction term |
--- |
0.20 (0.195, 0.207)‡ 13.19 (10.36, 16.01)‡ −0.11 (−0.15, −0.08)‡ |
--- |
|
BMI in PI-CF
BMI in PS-CF |
--- | --- |
0.20 (0.195, 0.207)
‡
0.09 (0.055, 0.13) ‡ |
| Female sex | 1.07 (−1.45, 3.60) | −1.21 (−1.88, −0.53)‡ | −1.21 (−1.88, −0.53)‡ |
| Non-white race | −2.94 (−7.60, 1.72) | 0.55 (−0.84, 1.95) | 0.55 (−0.84, 1.95) |
| Hispanic/Latino Data missing1 |
0.99 (−2.54, 4.52) 1.54 (−4.32, 7.40) |
−5.39 (−6.49, −4.30)‡ 0.25 (−1.42, 1.93) |
−5.39 (−6.49, −4.30)‡ 0.25 (−1.42, 1.93) |
| Non-private insurance Data missing1 |
0.12 (−1.07, 1.30) 0.34 (−1.44, 2.11) |
−0.84 (−1.12, −0.56)‡ −1.13 (−1.56, −0.70)‡ |
−0.84 (−1.12, −0.56)‡ −1.13 (−1.56, −0.70)‡ |
|
Pseudomonas Data missing1 |
−0.25 (−1.81, 1.30) 6.93 (−0.62, 14.47) |
−2.16 (−2.56, −1.76)‡ −0.70 (−2.48, 1.08) |
−2.16 (−2.56, −1.76)‡ −0.70 (−2.48, 1.08) |
|
Burkholderia Data missing1 |
−6.21 (−10.55, −1.86)† −7.63 (−14.28, −0.97)* |
−7.30 (−8.13, −6.47)‡ 1.62 (0.75, 2.50)‡ |
−7.30 (−8.13, −6.47)‡ 1.62 (0.75, 2.50)‡ |
| Tobramycin | −1.29 (−2.62, 0.03) | 0.80 (0.60, 1.01) ‡ | 0.80 (0.60, 1.01) ‡ |
| HTS Data missing1 |
−0.88 (−1.95, 0.18) 4.20 (2.46, 5.93)‡ |
−3.73 (−3.95, −3.51)‡ 4.04 (3.71, 4.36)‡ |
−3.73 (−3.95, −3.51)‡ 4.04 (3.72, 4.36)‡ |
| DNase | −1.57 (−2.74, −0.41)† | −1.00 (−1.33, −0.67)‡ | −1.00 (−1.33, −0.67)‡ |
Results from the adjusted longitudinal regression analysis. Shown are effect estimates (β coefficients) for FEV1pp, with 95% confidence intervals (95%CI) in parentheses. Model 1: Main effect of BMI in the PS-CF cohort. Model 2: Analysis of the whole cohort (PS and PS) showing the main effects of BMI, pancreatic status (PI or PS), and their interaction (BMI*pancreatic status). Model 3: Main effect of BMI in each of the pancreatic status groups, accounting for the effect modification.
Variables with >10% missing data were assigned a “missing” category.
P<0.05,
P<0.01,
P<0.001.
We then formally evaluated whether pancreatic status modifies the association between BMI and FEV1 by performing an interaction analysis including both PS and PI patients. In the adjusted longitudinal analysis, the interaction between pancreatic status and BMI was statistically significant (βINTERACTION = −0.11 [−0.15, −0.08]; Table 3 Model 2), indicating that the association between FEV1 and BMI differs between PS-CF and PI-CF. Therefore, we conducted an analysis stratified by pancreatic status, accounting for the interaction, which showed a significantly smaller effect of BMI on FEV1 among PS (β=0.09 higher FEV1pp [0.06–0.13] per each 1-percentile higher BMI) than among PI subjects (β=0.20 [0.20–0.21]) (Table 3 Model 3).
We then used structural equation modeling (SEM) analysis to estimate the effect of BMI on FEV1 after accounting for the effects of BMI and FEV1 in prior years (Supplementary Figure S1), which yielded similar results: in the PS cohort, for each 1-percentile higher BMI, current FEV1 was 0.09% higher (β=0.09 [0.05–0.13]); for instance, BMI at the 50th compared to the 10th percentile would be associated with ~3.6% [2.1%−5.1%] higher FEV1. In the PI cohort, the magnitude of the association was larger (β=0.14 [0.13–0.15]); a similar comparison (BMI 50th vs 10th percentile) was associated with ~5.6% (5.2-5-9%) higher FEV1.
To examine the association between BMI and FEV1 in further detail, BMI was stratified into four subgroups (at-risk: BMI <25th pct; borderline: BMI 25th–50th pct; normal: BMI 50th–85th pct; or overweight/obese: BMI >85th pct). Compared to PS children with normal BMI, those with borderline and at-risk BMI had mean FEV1pp ~3.4% and ~4.6% lower, respectively (Supplementary Table S3); among PI children, the respective differences were ~3.4% and ~9.1% lower. Next, we examined the linear association between BMI and FEV1 within each specific BMI category (Figure 2): among PS children, FEV1 increased with increasing BMI up to the 85th percentile, but then decreased among overweight/obese children. On the other hand, among PI children, the increase in FEV1 was markedly steeper as BMI increased up to the 25th percentile, but it continued to increase beyond that through the 99th percentile.
Figure 2 – Fitted values of FEV1 by BMI and pancreatic status.
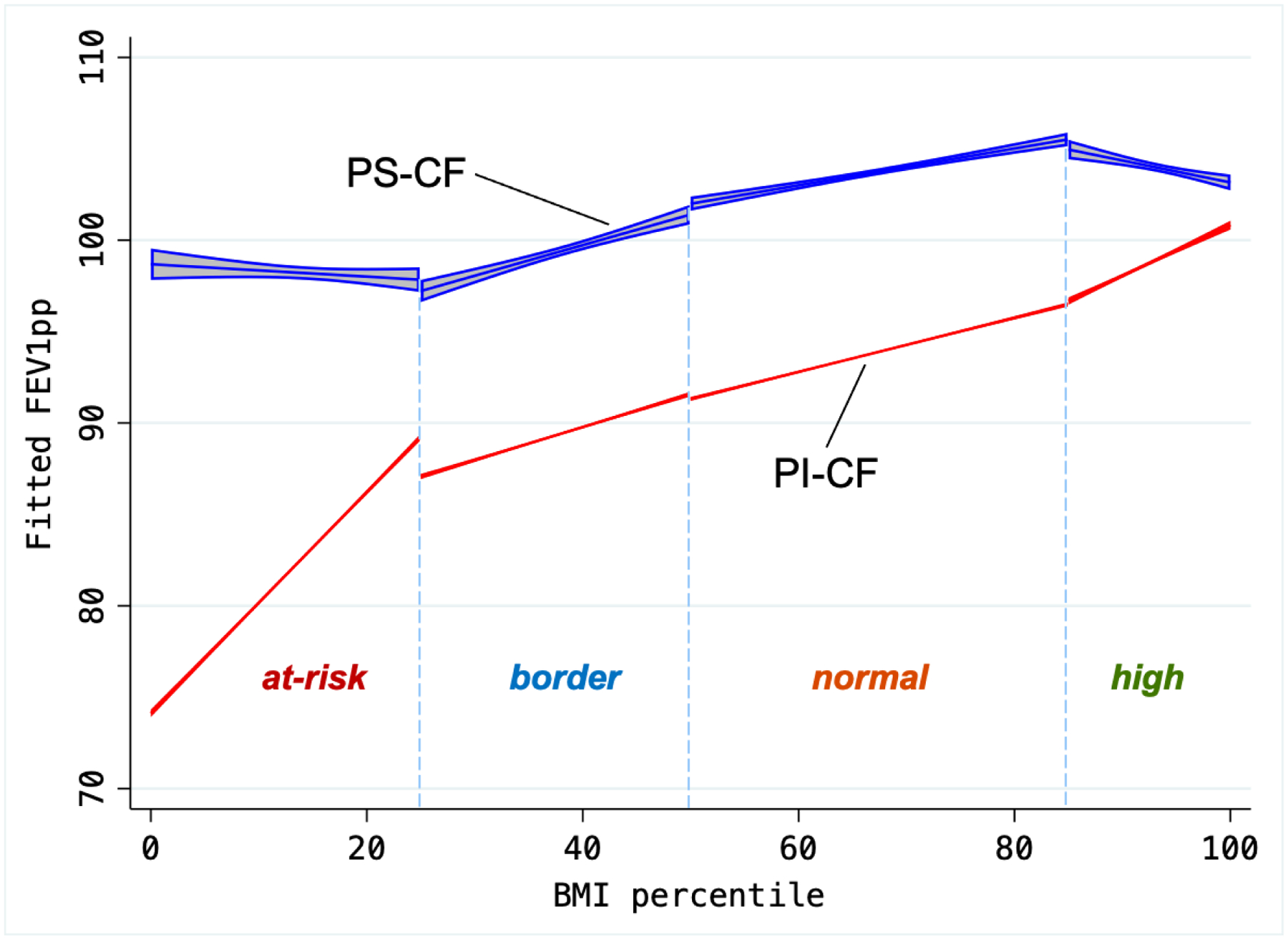
Predicted FEV1pp, based on adjusted linear model including the main effects of BMI and pancreatic status, as well as their interaction term, within each separate BMI category. FEV1 increases at relatively similar rates in PS across categories as BMI increases through the 85th percentile, but then it decreases among overweight/obese PS subjects. In contrast, FEV1 increases at a markedly higher rate in PI subjects as BMI increases in the at-risk category (through the 25th percentile), then keeps a steady rate as BMI increases through the 99th percentile.
FEV1 decline with age
Finally, given the important effect of nutritional status on lung function decline over time, we evaluated changes in FEV1 with age. In the longitudinal analysis, pancreatic status modified the association between age and FEV1 (pancreatic status * age interaction P<0.0001) and thus we stratified our models by PI/PS and BMI category (Figure 3 and Supplementary Table S4). Overall, the rate of FEV1 decline rate was −1.34% per year in PI-CF and −0.48% in PS-CF. In the PI cohort, all BMI groups showed a significant decline in FEV1 with age, and the decline was steeper the lower the BMI (BMI*age interaction P<0.0001): by age 20, for instance, FEV1pp had declined to a mean of 68.5% in the at-risk BMI category, compared to 81.6% in the normal BMI group. In the PS cohort, on the other hand, FEV1 decline with age was much less steep, and mean FEV1pp remained >90% by age 20 years regardless of BMI group (BMI*age interaction P=0.37).
Figure 3 – FEV1 over age by BMI category and pancreatic sufficiency status.
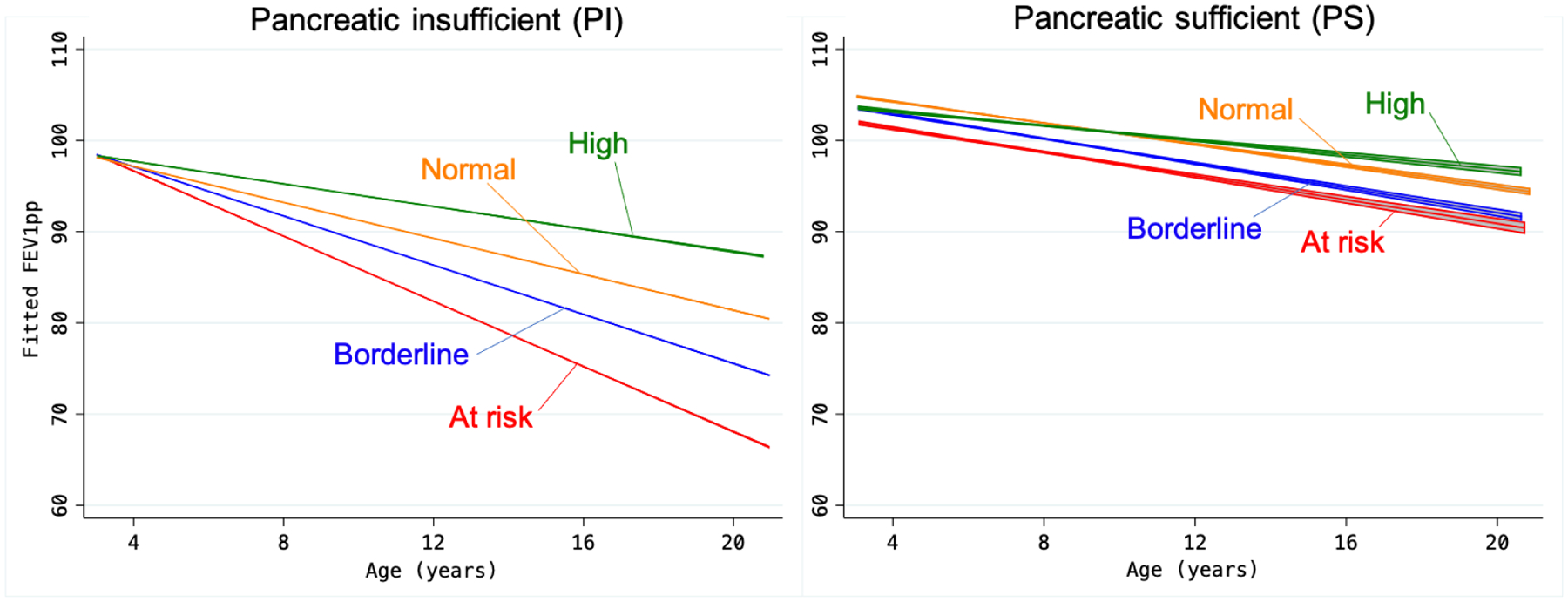
Predicted FEV1 by age, based on adjusted linear models, separately for PI-CF and PS-CF. BMI categories: At-risk (BMI <25th percentile); borderline (25th to <50th percentile); normal (50th to <85th percentile); and high (>85th percentile). See Supplementary Table S4 for predicted values at 12, 16, and 20 years of age.
Sensitivity Analyses
Results from the sensitivity analyses are shown in Supplementary Table S2. Overall, the magnitude and direction of the association between BMI and FEV1 remained when we 1) stratified by mode of diagnosis (NBS or not); 2) stratified by birth cohort (1995–2002 or 2003–2010); 3) used maternal education as a proxy for socioeconomic status; 4) adjusted for CFRD, CLFD, asthma, and pancreatitis; 5) defined PS solely as never having been on PERT; 6) defined PS solely as having a fecal elastase >200 μg/g recorded in the CFFPR; or 7) defined PS based on CFTR mutation class. While some sensitivity analyses with small sample sizes did not achieve statistical significance, the direction and magnitude of the effect estimates was overall unchanged.
DISCUSSION
In this study we report that the relationship between nutritional status and lung function in CF differs significantly by pancreatic status. Higher BMI was associated with a much smaller slope of FEV1 increase among people with pancreatic-sufficient CF compared to those with pancreatic-insufficient CF. The largest differences were seen in the extremes of BMI: among PI-CF, the “at risk” BMI group had FEV1 ~9% lower than subjects with normal BMI, whereas among PS-CF the difference was only ~4.5%. Furthermore, in PS-CF the positive association between BMI and FEV1 was lost beyond the 85th BMI percentile, at which point FEV1 decreased with higher BMI. On the contrary, PI-exhibited a markedly steeper increase in FEV1 in the “at-risk” BMI group, followed by a smaller but steady increase through the 99th BMI percentile. Perhaps most importantly, we show that the decline in FEV1 with age is minimal in PS-CF and does not differ by BMI, in marked contrast to what happens in PI-CF.
Previous studies have shown a significant, positive association between nutritional status and lung function in people living with CF, as well as the positive impact of correcting malnutrition early in life(8, 10–12, 20, 21). However, most such studies examined CF cohorts without stratifying by pancreatic status, resulting in estimates that were heavily driven by the majority of patients with CF who are PI. This study reiterates the importance of nutrition in CF, but also overcomes these limitations by delineating the differences in subjects with PS-CF vs PI-CF. Our results support the goal of maintaining BMI >50th percentile throughout childhood, as children in both pancreatic subgroups who achieved this goal had better lung function. However, the benefits from achieving this goal in PS-CF are less pronounced, particularly over time, and providers should be cautious because overweight/obesity may in fact be detrimental to lung function.
Together with a decrease in the proportion of patients with CF who are underweight or malnourished, there has been an increase in the prevalence of overweight/obesity, from ~10% in a UK study in 2002(22) to ~23% in a study at our center in 2013(15). We found that, from 6 to 16 years of age, the prevalence of overweight/obesity increased from 26% to 34% in children with PS-CF –compared with 9% to 13% of those with PI-CF. To our knowledge, this is the first report evaluating the prevalence of overweight/obesity separately by pancreatic status in a large longitudinal pediatric CF cohort. Few studies in CF have evaluated BMI trends over time or the clinical characteristics associated with overweight/obesity(14–16, 22). A study in adults with CF followed from 1985 to 2011 reported that the proportion of overweight/obese subjects (>25 kg/m2) increased from 7% to 18.4% during that 26-year period(14). Similar to our findings, the authors reported the largest increases in lung function with improved nutrition were seen in the underweight group (<18.5 kg/m2), who were more likely to be PI, while overweight/obese patients were more likely to be PS and had the smallest increase in FEV1 with BMI(14). That study also reported a significant interaction between BMI and pancreatic status on FEV1, but they did not stratify the behavior of FEV1 by pancreatic status within the overweight/obese group, likely because of the smaller sample size. Our findings become even more relevant in the era of highly effective modulator therapies; both original trials of elexacaftor / tezacaftor / ivacaftor (ETI) reported significant weight gain(23, 24), and at our center we have seen an average weight gain of ~4 Kg in the first few months after starting ETI (unpublished data). In the absence of adequate nutritional counseling, patients on ETI may be at higher risk of developing overweight or obesity.
Another key finding of our study is that patients with PS-CF have a markedly slower rate of FEV1 decline with age, regardless of the BMI category. Even among youth in the at-risk nutritional category (BMI <25th percentile), FEV1 only declined to 90th percentile by age 20, whereas by that same age children with PI-CF in the at-risk and borderline BMI categories (BMI <50th pct) had their FEV1pp decline to <70% and ~75%, respectively. These findings are consistent with prior studies showing a baseline decline in FEV1 over time and an independent association with pancreatic status(25, 26). The overall rates of FEV1 decline we found (−1.34% per year in PI-CF and −0.48% in PS-CF) are similar to those in a recent report from the UK CF Registry (−1.52% and −0.55%, respectively)(27). However, no studies have previously analyzed the combined effects of pancreatic status and BMI categories on lung function over age, and our results show that higher BMI results in no benefit to patients with PS-CF, at least through 20 years of age. Given the overall negative health effects of obesity and the fact that obese adults with CF have a higher prevalence of hyperlipidemia than their counterparts with healthy BMI(14), our findings strongly suggest that BMI ≥85th percentile in these children is not beneficial and may in fact be detrimental, both to their lung function and their overall health.
The current study has several limitations. Despite the large dataset from the CFFPR, we had limited sample sizes for certain subgroups, particularly in the later teenage years. We were limited to data in the registry, and thus not all potential confounders may have been available or fully documented. For instance, more sensitive measures of lung function such as lung clearance index may detect more pronounced differences in lung function. Likewise, our evaluation of nutritional status was based on BMI since we were unable to evaluate body composition(28–30). BMI is not the best measure to comprehensively assess nutritional status; evaluating diet, body composition, and relevant biomarkers of nutritional health will become even more important in the era of CFTR modulators. Finally, we used documentation of PERT as a surrogate for pancreatic insufficiency, which may have introduced bias by excluding PS patients with poor growth that were empirically placed on PERT. However, our sensitivity analysis using mutation classes to assign pancreatic status yielded very similar results, as did the analysis including only the subgroup of subjects with documented normal fecal elastase yielded the same overall results –despite the markedly smaller sample size.
At the same time, our study has several significant strengths. It was based on the US CFFPR and evaluated a large sample of youth with CF both cross-sectionally –at 6, 12, and 16 years of age– and longitudinally over the course of their childhood and adolescence. Given the large sample size, we were able to formally evaluate pancreatic status as an effect modifier of the relationship between nutrition and lung function in CF, and we were then able to both stratify by pancreatic status and to analyze BMI within each nutritional category. As a result, this is the first study exposing the stark differences between PS- and PI-CF both across nutrition categories and over time. We were also able to perform sensitivity analyses, and our findings were robust to the definition of PS, birth cohort, means of diagnosis (NBS vs not), and additional adjustment for comorbidities like CFRD, CLFD, and asthma. Finally, using SEM we were able to analyze the longitudinal effect of BMI on a given year on FEV1 the following year, after accounting for prior FEV1.
In summary, we report that adequate nutritional status is an important determinant of lung function in all children with CF, but our analysis shows that in pancreatic-sufficient CF there is a smaller gain in FEV1 with increasing BMI, and that BMI in the overweight/obese range may be associated with lower lung function. Finally, youth with PS-CF had a markedly smaller decline in FEV1 over time, regardless of their nutritional category. Given these results and the overall detrimental consequences of obesity, further studies are needed to better determine the management and nutritional goals for patients with PS-CF.
Supplementary Material
Highlights.
The association between BMI and FEV1 in CF differs significantly by pancreatic status (PS vs PI).
Higher BMI was associated with a smaller slope of FEV1 increase in PS-CF.
In PS-CF, BMI about the 85th percentile may be associated with decreasing FEV1.
The decline in FEV1 with age is minimal in PS-CF and does not differ by BMI.
Funding:
Dr. Forno’s contribution was funded in part by grant HL149693 from the U.S. National Institutes of Health (NIH). The funding agencies had no role in the study or the preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Castellani C, Assael BM. Cystic fibrosis: a clinical view. Cell Mol Life Sci. 2017;74(1):129–40. Epub 2016/10/07. doi: 10.1007/s00018-016-2393-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245(4922):1066–73. Epub 1989/09/08. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 3.Andersen DH. Cystic fibrosis of the pancrease and its relation to celiac disease: a clinical and pathologic study. American Journal of Diseases of Children. 1938;56(2):344–99. doi: 10.1001/archpedi.1938.01980140114013. [DOI] [Google Scholar]
- 4.Fanconi G, Uehlinger E, Knauer C. Das Coeliakiesyndrom bei angeborener zystischer Pankreasfibromatose und Bronchiektasien. Wien Med Wochenschr. 1936;86:753–6. [Google Scholar]
- 5.Gibson-Corley KN, Meyerholz DK, Engelhardt JF. Pancreatic pathophysiology in cystic fibrosis. The Journal of pathology. 2016;238(2):311–20. Epub 2015/09/15. doi: 10.1002/path.4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turcios NL. Cystic Fibrosis Lung Disease: An Overview. Respir Care. 2020;65(2):233–51. Epub 2019/11/28. doi: 10.4187/respcare.06697. [DOI] [PubMed] [Google Scholar]
- 7.Sergeev V, Chou FY, Lam GY, Hamilton CM, Wilcox PG, Quon BS. The Extrapulmonary Effects of Cystic Fibrosis Transmembrane Conductance Regulator Modulators in Cystic Fibrosis. Annals of the American Thoracic Society. 2020;17(2):147–54. Epub 2019/10/30. doi: 10.1513/AnnalsATS.201909-671CME. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stallings VA, Stark LJ, Robinson KA, Feranchak AP, Quinton H. Evidence-based practice recommendations for nutrition-related management of children and adults with cystic fibrosis and pancreatic insufficiency: results of a systematic review. J Am Diet Assoc. 2008;108(5):832–9. Epub 2008/04/30. doi: 10.1016/j.jada.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 9.Lai HJ, Shoff SM, Farrell PM, Wisconsin Cystic Fibrosis Neonatal Screening G. Recovery of birth weight z score within 2 years of diagnosis is positively associated with pulmonary status at 6 years of age in children with cystic fibrosis. Pediatrics. 2009;123(2):714–22. Epub 2009/01/28. doi: 10.1542/peds.2007-3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yen EH, Quinton H, Borowitz D. Better nutritional status in early childhood is associated with improved clinical outcomes and survival in patients with cystic fibrosis. J Pediatr. 2013;162(3):530–5 e1. Epub 2012/10/16. doi: 10.1016/j.jpeds.2012.08.040. [DOI] [PubMed] [Google Scholar]
- 11.Heltshe SL, Borowitz DS, Leung DH, Ramsey B, Mayer-Hamblett N. Early attained weight and length predict growth faltering better than velocity measures in infants with CF. J Cyst Fibros. 2014;13(6):723–9. Epub 2014/06/12. doi: 10.1016/j.jcf.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanders DB, Fink A, Mayer-Hamblett N, Schechter MS, Sawicki GS, Rosenfeld M, Flume PA, Morgan WJ. Early Life Growth Trajectories in Cystic Fibrosis are Associated with Pulmonary Function at Age 6 Years. J Pediatr. 2015;167(5):1081–8 e1. Epub 2015/09/06. doi: 10.1016/j.jpeds.2015.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lahiri T, Hempstead SE, Brady C, Cannon CL, Clark K, Condren ME, Guill MF, Guillerman RP, Leone CG, Maguiness K, Monchil L, Powers SW, Rosenfeld M, Schwarzenberg SJ, Tompkins CL, Zemanick ET, Davis SD. Clinical Practice Guidelines From the Cystic Fibrosis Foundation for Preschoolers With Cystic Fibrosis. Pediatrics. 2016;137(4). Epub 2016/03/25. doi: 10.1542/peds.2015-1784. [DOI] [PubMed] [Google Scholar]
- 14.Stephenson AL, Mannik LA, Walsh S, Brotherwood M, Robert R, Darling PB, Nisenbaum R, Moerman J, Stanojevic S. Longitudinal trends in nutritional status and the relation between lung function and BMI in cystic fibrosis: a population-based cohort study. Am J Clin Nutr. 2013;97(4):872–7. Epub 2013/02/08. doi: 10.3945/ajcn.112.051409. [DOI] [PubMed] [Google Scholar]
- 15.Hanna RM, Weiner DJ. Overweight and obesity in patients with cystic fibrosis: a center-based analysis. Pediatr Pulmonol. 2015;50(1):35–41. Epub 2014/04/24. doi: 10.1002/ppul.23033. [DOI] [PubMed] [Google Scholar]
- 16.Harindhanavudhi T, Wang Q, Dunitz J, Moran A, Moheet A. Prevalence and factors associated with overweight and obesity in adults with cystic fibrosis: A single-center analysis. J Cyst Fibros. 2020;19(1):139–45. Epub 2019/11/16. doi: 10.1016/j.jcf.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren CL, Fink AK, Petren K, Borowitz DS, McColley SA, Sanders DB, Rosenfeld M, Marshall BC. Outcomes of infants with indeterminate diagnosis detected by cystic fibrosis newborn screening. Pediatrics. 2015;135(6):e1386–92. Epub 2015/05/13. doi: 10.1542/peds.2014-3698. [DOI] [PubMed] [Google Scholar]
- 18.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. CDC growth charts: United States. Adv Data. 2000;2001/02/24(314):1–27. [PubMed] [Google Scholar]
- 19.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, Enright PL, Hankinson JL, Ip MS, Zheng J, Stocks J, Initiative ERSGLF. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–43. Epub 2012/06/30. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konstan MW, Butler SM, Wohl ME, Stoddard M, Matousek R, Wagener JS, Johnson CA, Morgan WJ, Investigators, Coordinators of the Epidemiologic Study of Cystic F. Growth and nutritional indexes in early life predict pulmonary function in cystic fibrosis. J Pediatr. 2003;142(6):624–30. Epub 2003/07/03. doi: 10.1067/mpd.2003.152. [DOI] [PubMed] [Google Scholar]
- 21.Ashkenazi M, Nathan N, Sarouk I, Aluma BEB, Dagan A, Bezalel Y, Keler S, Vilozni D, Efrati O. Nutritional Status in Childhood as a Prognostic Factor in Patients with Cystic Fibrosis. Lung. 2019;197(3):371–6. Epub 2019/03/20. doi: 10.1007/s00408-019-00218-3. [DOI] [PubMed] [Google Scholar]
- 22.Kastner-Cole D, Palmer CN, Ogston SA, Mehta A, Mukhopadhyay S. Overweight and obesity in deltaF508 homozygous cystic fibrosis. J Pediatr. 2005;147(3):402–4. Epub 2005/09/27. doi: 10.1016/j.jpeds.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Middleton PG, Mall MA, Drevinek P, Lands LC, McKone EF, Polineni D, Ramsey BW, Taylor-Cousar JL, Tullis E, Vermeulen F, Marigowda G, McKee CM, Moskowitz SM, Nair N, Savage J, Simard C, Tian S, Waltz D, Xuan F, Rowe SM, Jain R, Group VXS. Elexacaftor-Tezacaftor-Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele. N Engl J Med. 2019;381(19):1809–19. Epub 2019/11/08. doi: 10.1056/NEJMoa1908639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heijerman HGM, McKone EF, Downey DG, Van Braeckel E, Rowe SM, Tullis E, Mall MA, Welter JJ, Ramsey BW, McKee CM, Marigowda G, Moskowitz SM, Waltz D, Sosnay PR, Simard C, Ahluwalia N, Xuan F, Zhang Y, Taylor-Cousar JL, McCoy KS, Group VXT. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet. 2019;394(10212):1940–8. Epub 2019/11/05. doi: 10.1016/S0140-6736(19)32597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konstan MW, Morgan WJ, Butler SM, Pasta DJ, Craib ML, Silva SJ, Stokes DC, Wohl ME, Wagener JS, Regelmann WE, Johnson CA, Scientific Advisory G, the I, Coordinators of the Epidemiologic Study of Cystic F. Risk factors for rate of decline in forced expiratory volume in one second in children and adolescents with cystic fibrosis. J Pediatr. 2007;151(2):134–9, 9 e1. Epub 2007/07/24. doi: 10.1016/j.jpeds.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 26.Taylor-Robinson D, Whitehead M, Diderichsen F, Olesen HV, Pressler T, Smyth RL, Diggle P. Understanding the natural progression in %FEV1 decline in patients with cystic fibrosis: a longitudinal study. Thorax. 2012;67(10):860–6. Epub 2012/05/05. doi: 10.1136/thoraxjnl-2011-200953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caley L, Smith L, White H, Peckham DG. Average rate of lung function decline in adults with cystic fibrosis in the United Kingdom: Data from the UK CF registry. J Cyst Fibros. 2021;20(1):86–90. Epub 2020/05/10. doi: 10.1016/j.jcf.2020.04.008. [DOI] [PubMed] [Google Scholar]
- 28.Pedreira CC, Robert RG, Dalton V, Oliver MR, Carlin JB, Robinson P, Cameron FJ. Association of body composition and lung function in children with cystic fibrosis. Pediatr Pulmonol. 2005;39(3):276–80. Epub 2005/01/26. doi: 10.1002/ppul.20162. [DOI] [PubMed] [Google Scholar]
- 29.Alvarez JA, Ziegler TR, Millson EC, Stecenko AA. Body composition and lung function in cystic fibrosis and their association with adiposity and normal-weight obesity. Nutrition. 2016;32(4):447–52. Epub 2016/01/08. doi: 10.1016/j.nut.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peralta GP, Fuertes E, Granell R, Mahmoud O, Roda C, Serra I, Jarvis D, Henderson J, Garcia-Aymerich J. Childhood Body Composition Trajectories and Adolescent Lung Function. Findings from the ALSPAC study. Am J Respir Crit Care Med. 2019;200(1):75–83. Epub 2019/01/12. doi: 10.1164/rccm.201806-1168OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


