Abstract
Background
There is considerable disparity in institutional practices surrounding routine pathologic examination of femoral heads removed during total hip arthroplasty (THA). Multiple groups have studied the merits of routine femoral head pathology in THA, without clear consensus. We sought to further investigate the existing evidence on routine pathologic examination of femoral heads retrieved during THA to determine if this practice provides additional clinical value and is cost-effective.
Material and methods
To conduct a systematic review of the literature, a medical librarian was consulted to develop and perform comprehensive searches in PubMed (1809-present), Embase (embase.com 1974-present), CINAHL (EBSCO, 1937-present), and the Cochrane Central Register of Controlled Trials (Wiley). Final searches resulted in 727 references. Through multiple reviewer screenings and assessments of eligible full-text articles, we included 14 articles for review.
Results
Our systematic review yielded pathologic examination results from 17,388 femoral head specimens collected during THA. In 0.85% of cases, the pathologic diagnosis differed in a meaningful way from the preoperative clinical diagnosis. Routine pathology changed patient management in approximately 0.0058% of cases. The average cost for pathologic examination of each specimen was $126.38.
Conclusion
Routine pathologic examination of femoral heads retrieved during THA has limited impact on patient management. With an estimated 500,000 THAs performed in 2019, the economic feasibility of routine femoral head pathology is limited at an annual cost of up to $63,000,000 and cost per quality-adjusted life-year approaching infinity. However, surgeon discretion on a patient-specific or practice-specific basis should be used to make the final determination on the need for femoral head pathology.
Keywords: Femoral head, Hip, Arthroplasty, Pathology, Routine, Cost
Introduction
Approximately 500,000 total hip arthroplasties (THAs) were estimated to be performed in 2019 in the United States alone, and this number is increasing exponentially over time [1,2]. Intraoperative practices during THA are thus consequential in terms of quality of care provided to patients as well as cost incurred by health-care systems. While osteoarthritis, inflammatory arthritis, and avascular necrosis are the most common indications for THA, occult diagnoses such as malignancy or infection may have significant implications for postoperative management, patient outcomes, as well as cost [3].
To determine if routine pathologic examination of the femoral head retrieved during THA is indicated and/or cost-effective, it is necessary to determine the frequency with which the pathologic diagnosis differs from the preoperative diagnosis as well as the cost of pathologic examination of the femoral head. While multiple studies have strived to make this determination, the conclusions are widely disparate [[4], [5], [6], [7]].
Several reports have found that the routine pathologic examination of femoral head specimens retrieved during THA provides little additional diagnostic value and is an unnecessary and costly practice [4,5]. Others report routine femoral head pathology is critical to the diagnosis of occult disease, such as malignancy, and is cost-effective in terms of quality-adjusted life-years (QALY) [6,7]. As a result, it is not surprising that institutional policies and clinical practices surrounding pathologic examination of femoral head specimens are quite variable.
The purpose of the study was to further investigate the existing evidence on routine pathologic examination of femoral head specimens retrieved during THA through systematic review in order to determine if this practice provides additional diagnostic value and, if so, evaluate associated costs.
Material and methods
Literature search strategy
A medical librarian (L.W.) was consulted to develop and conduct comprehensive searches in PubMed (1809-present), Embase (embase.com 1974-present), CINAHL (EBSCO, 1937-present), and the Cochrane Central Register of Controlled Trials (Wiley). Search strategies were individually developed for each database utilizing database-appropriate controlled vocabulary (see below). Controlled vocabulary and text words encompassed hip arthroplasty, routine diagnostic test, and femur terms.
Database-specific searches
PubMed (1809-present): 321 references retrieved
Single-line search run in the “New PubMed” interface:
((("arthroplasty, replacement, hip"[mh] OR (((hip joint∗[tiab]) AND (replacement∗[tiab] OR arthroplast∗[tiab])) OR ((hip[tiab]) AND (implantation∗[tiab] OR replacement∗[tiab] OR arthroplasty[tiab]))))) AND ("diagnostic tests, routine"[mh] OR "pathology, clinical"[mh] OR "pathology, surgical"[mh] OR "histology"[mh] OR (diagnostic test∗[tiab] OR ((histopatholog∗[tiab] OR histolog∗[tiab] OR pathologic∗[tiab]) AND (examin∗[tiab]))))) AND (("femur head"[mh] OR (femoral[tiab]OR femur∗[tiab] OR caput-femoris[tiab])))
Embase (Embase.com): 372 references retrieved
Single-line search run in “Results” tab of Embase.com interface:
(‘hip replacement’/exp OR (((‘hip joint’) NEAR/3 (replacement∗ OR arthroplast∗)) OR ((hip) AND (implantation∗ OR replacement∗ OR arthroplast∗))):ti,ab) AND (‘diagnostic test’/exp OR (((clinical OR surgical) NEAR/3 (pathology)) OR ‘diagnostic test∗’ OR ((histopatholog∗ OR histolog∗ OR pathologic∗) NEAR/3 (examin∗))):ti,ab) AND (‘femoral head’/exp OR (femoral OR femur∗ OR ‘caput femoris’):ti,ab)
CINAHL (EBSCOhost): 30 references retrieved
Search run in Advanced Search interface by entering each line into separate search box with each search box combined with AND:
((MH "Arthroplasty, Replacement, Hip”) OR ("hip replacement∗" OR "hip joint replacement∗" OR "hip joint arthroplast∗" OR "hip implantation∗" OR "hip replacement∗" OR "hip arthroplast∗")) AND ((MH “Diagnostic Tests, Routine” OR MH “Pathology, Clinical” OR MH “Histology”) OR ("diagnostic test∗" OR "histopatholog∗ examin∗" OR "histolog∗ examin∗" OR "pathologic∗ examin∗")) AND ((MH "Femur Head") OR (femoral OR femur∗ OR "caput femoris"))
Cochrane Library (WileyOnline; Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, Cochrane Methodology Register): 4 references retrieved
Using Search Manager in Advanced Search:
(hip NEAR/3 (arthroplasty OR replacement OR (joint NEXT arthroplasty) OR (joint NEXT replacement) OR implantation)) AND (diagnostic NEAR/3 test) OR (clinical pathology) OR (histopathology NEAR/3 examin∗) OR (pathologic NEXT examin∗) AND (femur NEXT head) OR femoral OR (caput femoris) OR femur
Article selection and data collection protocol
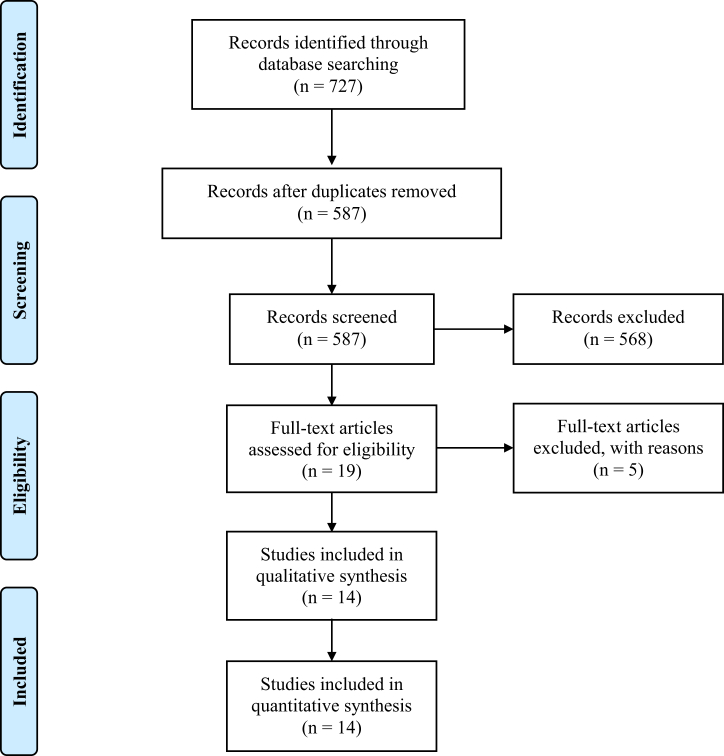
Final searches were run on November 24, 2020, resulting in 727 total references. After removing duplicates, 587 references remained. Two reviewers (S.N. and R.S.) independently performed title and abstract screening of the references. Conflicts were resolved by a third reviewer (A.C.) to arrive at 19 articles. Article screening was performed using Covidence (Melbourne, Australia) systematic review management software. After full-text review, 14 of these articles were confirmed to meet study inclusion and exclusion criteria (Table 1) and comprised our final cohort for data extraction [[4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17]]. Figure 1, illustrating the aforementioned process, is the Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram [18].
Table 1.
Article inclusion and exclusion criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
| English language manuscript | Review article |
| Routine pathology/histology of femoral head | Meeting abstract |
| Specimens obtained during total hip arthroplasty | Cadaveric study |
| Case report | |
| Repeat publication of same patient cohort | |
| Patients younger than 18 | |
| Specimens obtained during hemiarthroplasty |
Figure 1.
PRISMA flow diagram.
Data points collected from each article by a single reviewer (S.N.), if available, were as follows: title, authors, journal, year of publication, study design, country of origin, source population, number of femoral head specimens, pathologic diagnosis, discrepancies between clinical and pathologic diagnoses, cost analysis, and article conclusion regarding routine pathologic examination. A discordant diagnosis was defined as a pathologic diagnosis that both differed from the clinical diagnosis and altered patient management according to the majority of authors of the articles reviewed.
Statistical analysis
Descriptive statistics were utilized for data analysis, including the calculation of frequencies, percentages, and means (Microsoft Excel, Redmond, WA).
Results
A summary of data extracted from included articles is provided in Table 2.
Table 2.
Summary of data extraction from articles selected for review.
| Authors | Year of publication | Study design | Country of origin | Source population | Femoral head specimens | Discordant diagnoses | Cost analysis | Conclusion on routine pathology |
|---|---|---|---|---|---|---|---|---|
| Campbell et al. [8] | 1997 | Multicenter, retrospective | US | THA for any indication | 283 | 1.06% | $140-$200 per specimen; $17.5-25 million in savings without routine pathology | Against |
| Dermawan et al. [9] | 2021 | Multicenter, retrospective | US | Elective THA | 1722 | 0% | None | For |
| DiCarlo et al. [7] | 2014 | Single center, retrospective | US | THA for any indication | 7968 | 1.46% | Less than 0.5% of total costs saved without routine pathology | For |
| Kocher et al. [5] | 2000 | Single center, retrospective | US | THA for OA | 471 | 0.21% | $89.08 per specimen; $122,728 per discordant case | Against |
| Lawrence et al. [10] | 1999 | Single center, retrospective | US | THA for any indication | 562 | 0% | $102.59 per specimen | Against |
| Layfield et al. [11] | 2020 | Single center, retrospective | US | THA for OA | 953 | 0.52% | None | For |
| Lin et al. [4] | 2012 | Single center, retrospective | US | Elective THA | 457 | 0.22% | $102.37 per specimen; no gain in QALY | Against |
| Liow et al. [6] | 2017 | Single center, retrospective | US | Elective THA | 3200 | 0.16% | $185.14 per specimen; $122,932.96 per discordant case; $49,569.74/QALY | For |
| Meding et al. [12] | 2000 | Single center, retrospective | US | THA for any indication | 313 | 0% | $60-$283 per specimen | Against |
| Niggemeyer et al. [13] | 2011 | Single center, prospective | Germany | THA for inflammatory arthritis or OA | 100 | 0% | None | Against |
| O'Connell et al. [14] | 1999 | Single center, retrospective | US | THA for any indication | 164 | 1.22% | None | None |
| Raab et al. [15] | 1998 | Single center, retrospective | US | Elective THA | 79 | 0% | $64 per specimen | Against |
| Sissons et al. [16] | 1992 | Single center, retrospective | US | THA for idiopathic osteonecrosis | 264 | 0% | None | None |
| Zwitser et al. [17] | 2009 | Single center, prospective | Netherlands | Elective THA | 852 | 1.64% | None | For |
OA, osteoarthritis.
OA, osteoarthritis. Our systematic review yielded pathologic examination results from a total of 17,388 femoral head specimens collected during primary THA performed for any indication. All specimens were fixed, decalcified, sectioned, and stained with hematoxylin and eosin for histologic evaluation. Immunohistochemistry and flow cytometry were performed as needed. Only in 147 of these cases, or 0.85% of the time, did the authors believe that the pathologic diagnosis differed from the preoperative clinical diagnosis in a manner that affected patient management (discordant diagnosis). The frequency of each discordant diagnosis is listed in Table 3. Inflammatory arthritis and B-cell lymphoma were the 2 most common discordant diagnoses.
Table 3.
Frequency table of discordant diagnoses.
| Diagnosis | Frequency |
|---|---|
| Inflammatory arthritis | 82 |
| B-cell lymphoma | 22 |
| Pigmented villonodular synovitis (PVNS) | 19 |
| Unenumerated malignancies (most commonly B-cell lymphoma, includes metastatic carcinoma) | 14 |
| Septic arthritis | 5 |
| Osteomyelitis | 3 |
| Metastatic carcinoma | 1 |
| Granulomatous inflammation | 1 |
| Total = 147 |
We re-evaluated all femoral head pathologic diagnoses labeled as discordant in the literature. Final pathologic diagnoses are only available postoperatively. As a result, the value of each discordant pathologic diagnosis following THA was considered. For most discordant diagnoses in Table 3, THA is a definitive treatment, routine postoperative surveillance is sufficient for the condition, or management is dictated by clinical symptoms alone. As a result, patient care or prognosis did not benefit from these pathologic data. Previously undiagnosed metastatic carcinoma was the only truly discordant diagnosis. As a result, routine pathology changed patient management in approximately 0.0058% (1 out of 17,388) of cases.
Eight studies conducted in the United States provided cost-analyses of routine pathologic examination of femoral head specimens obtained from THA [[4], [5], [6], [7], [8], [10], [12], [15]]. The mean cost per specimen was $126.38 (range $60 to $283), based on available data from 7 of these articles [[4], [5], [6], [8], [10], [12], [15]]. If 500,000 THAs are performed annually [1,2], the cost of routine femoral head pathology is $126.38/femoral head × 500,000 femoral heads/year = $63,190,000/y. While DiCarlo and Klein reported routine pathologic examination of femoral heads accounted for just 0.5% of total costs at a single musculoskeletal specialty hospital, Campbell et al. reported the absolute annual cost of routine pathology examination of femoral heads in 1997 was $17.5 to $25 million [7,8]. In order to arrive at 1 discordant case, 2 of the articles found pathology costs approached $123,000 [5,6]. The cost per QALY for routine pathologic examination of femoral heads was highly disparate in the articles that reported this metric. Liow et al. calculated a cost of $49,569.74 per QALY after assuming a benefit in QALY with routine femoral head pathology [6]. Lin et al. arrived at essentially infinite cost per QALY by not finding any gain in QALY (QALY = 0) with routine pathology [4,6].
Of the articles that commented on the need for routine pathologic examination of femoral heads retrieved during THA, 58% (7 of 12 articles) advised against this practice as it was not found to change patient management [4,5,8,10,12,13,15]. It is our opinion that the remaining 5 articles that favored routine pathologic examination did so after mischaracterizing many pathologic diagnoses that did not impact patients’ clinical course as discordant [6,7,9,11,17]. This resulted in an overvaluation of routine femoral head pathology, which skewed analyses of cost-effectiveness and ultimately these articles’ conclusions.
Discussion
There is a disparity in institutional practices surrounding routine pathologic examination of femoral heads obtained during THA. Multiple groups have studied the merits of routine pathology in THA, but the conclusions are conflicting [[4], [5], [6], [7]]. We conducted a systematic review of existing evidence to determine if routine pathologic examination of femoral head specimens obtained during THA is indicated and, if so, cost-effective. The results of our study demonstrate that routine femoral head pathology seldom impacts patient management and is a significant economic burden.
When femoral head specimens from THA were routinely sent for pathologic examination, we found that the pathologic diagnosis meaningfully differed from the preoperative clinical diagnosis 0.85% of the time. Cases in which the pathologic diagnosis differed from the clinical diagnosis, resulting in a change in patient management, were described as discordant. While less than 1% of cases were labeled discordant in the papers reviewed, patient management was rarely, if ever, changed by routine pathology in these cases.
The most common discordant diagnosis was inflammatory arthritis. A patient with a single joint affected by inflammatory arthritis that has undergone joint replacement requires little further intervention. Without involvement of any other joints, initiation of disease-modifying antirheumatic drugs would be undesirable due to the increased risk of infection and malignancy [19]. Thus, a diagnosis of inflammatory arthritis on routine pathology does not significantly change patient management.
B-cell lymphoma was the second most common discordant diagnosis. Many of the included studies assumed an improvement in clinical outcome with early diagnosis of B-cell lymphoma in patients who were otherwise asymptomatic. However, even with early diagnosis, indolent subtypes of this condition are often left untreated unless patients are symptomatic or there are aberrations on physical examination, radiographs, or laboratory studies such as complete blood count [[20], [21], [22], [23], [24], [25], [26], [27]]. As there is no clinical benefit to early diagnosis of asymptomatic indolent B-cell lymphoma , there is little utility in its detection on routine pathologic examination of femoral heads. While high-grade B-cell lymphomas do benefit from early diagnosis, patients with bone marrow involvement detectable on femoral head pathology would likely present with significant constitutional symptoms, rapidly growing mass, laboratory abnormalities, and/or radiographic findings [28].
The third most common discordant diagnosis was pigmented villonodular synovitis (PVNS). The endpoint of this aggressive, benign condition that causes the destruction of primarily a single joint is arthroplasty [29]. As a result, a finding of PVNS on routine pathology after THA does not alter a patient’s treatment course. The definitive treatment has already been performed, and monitoring occurs through routine postoperative surveillance even without the diagnosis of PVNS.
Infection (septic arthritis, osteomyelitis) is another discordant diagnosis made with permanent pathology of femoral head specimens in THA. One would assume if THA is performed in the setting of an active infection, then management with debridement, antibiotics, irrigation, and retention of implants or one- or two-stage revision will likely be required whether infection is diagnosed clinically or through pathologic examination. Thus, routine pathology does not likely impact patient management. There is also evidence that femoral head pathology is insufficiently specific for diagnosing acute infection. O’Connell et al. histologically identified sterile subchondral acute inflammation in femoral head specimens with severe arthritis that could easily be mistaken for infection [14]. Furthermore, Raab et al. noted that a pathologic diagnosis of chronic osteomyelitis in a femoral head specimen resulted in unnecessary administration of antibiotics for 6 weeks; revisiting the histologic findings demonstrated that they were in fact consistent with degenerative joint disease, and there was no evidence of infection in clinical follow-up [15]. Others have also found initial femoral head histology to yield false-positive diagnoses of infection [13].
Out of the 17,388 femoral head pathologic examinations reported in the included studies, approximately 1 was a discordant diagnosis of metastatic carcinoma. If a primary malignancy is unknown at the time of pathologic diagnosis of metastatic disease, then routine pathology proves beneficial in 0.0058% of cases. Nonetheless, even metastatic disease detected by routine femoral head pathology may have limited impact on management or prognosis if the presence of a primary malignancy was known preoperatively. Patients with a history of malignancy often undergo routine oncologic monitoring to evaluate for metastases, which are unlikely to be limited to the femoral head and have often already occurred by the time of pathologic examination.
With an estimated 500,000 THAs performed in 2019 [1,2], the cost of routine pathologic examination of femoral heads for the year was up to $63,000,000. The cost per QALY is a measure of cost-effectiveness of an intervention. QALY for an intervention is calculated using the following formula: years of life gained × utility value gained. Utility value is 0 in death and 1 in perfect health. Based on our systematic review, routine pathology in THA infrequently impacts patient management and as a result, does little to increase lifespan or quality of life. Cost per QALY thus approaches infinity for routine pathologic examination of femoral heads given the high cost (numerator) and low QALY (denominator), as Lin et al. also reported [4]. Liow et al. calculated a low cost per QALY of $49,569.74 for routine pathology based on the flawed assumption that presymptomatic diagnosis of B-cell lymphoma benefitted prognosis [6].
We understand that our work has limitations. First, the quality of our data and conclusions is limited by that of the papers we included for review, particularly given that 12 of 14 papers were retrospective. For example, while DiCarlo and Klein reported approximately 14 malignancies that were discordant diagnoses, the text only noted B-cell lymphoma as the most common diagnosis without individually enumerating each malignancy type [7]. Additional potential limitations of the retrospective studies in our review include inconsistent criteria for pathologic diagnoses and incomplete past medical histories, both of which hamper the accurate identification of discordant diagnoses. Second, it is possible our search terms did not capture all relevant articles although a medical librarian performed our comprehensive search of multiple databases. Third, our calculated costs may vary with geographic location, practice setting, and year. Finally, our conclusions are not generalizable to routine pathologic examination in all orthopedic surgeries, only in THA which was the subject of our study.
Conclusions
Our review of the existing literature, although largely retrospective with inherent limitations, demonstrates routine pathologic examination of femoral head specimens collected during THA results in a change in patient management in approximately 0.0058% of cases. Articles advocating for routine femoral head pathology label several pathologic diagnoses as discordant, which, upon closer examination, affect neither treatment nor prognosis. At a cost of up to $63,000,000 per year and a cost per QALY approaching infinity, the economic feasibility of routine pathology of femoral heads in THA is limited. However, surgeon discretion on a patient-specific or practice-specific basis is essential in making the final determination on need for femoral head pathology. Future studies are needed to evaluate the cost-effectiveness of selective femoral head pathologic examination in THA.
Conflicts of interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: S. Nandi is in the editorial or governing board of Journal of Arthroplasty and is a board member in AAHKS and AAOS. R. Schwarzkopf receives royalties from Smith & Nephew; is a paid consultant for Smith & Nephew and Intellijoint; has stock or stock options in Intellijoint, Gauss Surgical, and PSI; receives research support as a principal investigator from Smith & Nephew and Intellijoint; receives financial or material support from Smith & Nephew; is in the editorial or governing board of JOA and Arthroplasty Today; and is a board member in AAHKS and AAOS. A. Chen receives royalties from Stryker; is a paid consultant for 3M, Avanos, BICMD, bOne, Convatec, Ethicon, GLG, Guidepoint, Heraeus, IrriMax, Pfizer, PhagoMed, and Stryker; has stock or stock options in bOne, Graftworx, Hyalex, IrriMax, Joint Purification Systems, and Sonoran; receives financial or material support from SLACK Inc. and UpToDate; is in the editorial or governing board of Journal of Arthroplasty, Clinical Orthopaedics and Related Research, Journal of Bone and Joint Surgery, and Journal of Orthopaedic Research; and is a board member in AAOS, AJRR, AAHKS, and European Knee Association. T. Seyler receives royalties from Total Joint Orthopaedics, Pattern Health, MiCare Path, and Restor3D; is a paid consultant for Total Joint Orthopaedics, Smith & Nephew, and Heraeus Medical; is an unpaid consultant for Next Science; has stock or stock options in Extrel Therapeutics and MiCare Path; receives research support as a principal investigator from Zimmer Biomet; receives financial or material support from Lippincott Williams and Wilkins; and is a board member in Musculoskeletal Infection Society and the American Association of Hip and Knee Surgeons. J. Parvizi receives royalties from Corentec; is a paid consultant for Zimmer Biomet, Corentec, Ethicon, Tenor, KCI/3M (Acelity), Heraeus, MicroGenDx, Jointstem, Peptilogics, and Fidia Pharm; has stock or stock options in Parvizi Surgical Innovations and Subsidiaries, Hip Innovation Technologies, Corentec, Alphaeon/Strathsby Crown, Joint Purification Systems, Ceribell, Acumed, PRN-Veterinary, MD-valuate, Intellijoint, MicroGenDx, Nanooxygenic, Sonata, Moleculae Surface Technologies; and receives financial or material support from Data Trace, Elsevier, Jaypee Publishers, SLACK Inc., Wolters Kluwer, and Becton Dickenson.
For full disclosure statements refer to https://doi.org/10.1016/j.artd.2022.03.016.
Footnotes
PROSPERO registration application #293599.
Contributor Information
Sumon Nandi, Email: sumon.nandi@gmail.com.
AAHKS Research Committee:
Muyibat A. Adelani, Timothy S. Brown, John C. Clohisy, Maxwell Courtney, Matthew J. Dietz, Brett R. Levine, Simon C. Mears, Jesse E. Otero, and Scott M. Sporer
Appendix
PRISMA 2020 for abstracts checklist.
| Section and topic | Item # | Checklist item | Reported (yes/no) |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a systematic review. | Line 2 |
| Background | |||
| Objectives | 2 | Provide an explicit statement of the main objective(s) or question(s) the review addresses. | Lines 7-10 |
| Methods | |||
| Eligibility criteria | 3 | Specify the inclusion and exclusion criteria for the review. | Table 1 |
| Information sources | 4 | Specify the information sources (eg, databases, registers) used to identify studies and the date when each was last searched. | Lines 11-14 |
| Risk of bias | 5 | Specify the methods used to assess risk of bias in the included studies. | Lines 14-15 |
| Synthesis of results | 6 | Specify the methods used to present and synthesise results. | Lines 14-15 |
| Results | |||
| Included studies | 7 | Give the total number of included studies and participants and summarise relevant characteristics of studies. | Line 15 |
| Synthesis of results | 8 | Present results for main outcomes, preferably indicating the number of included studies and participants for each. If meta-analysis was done, report the summary estimate and confidence/credible interval. If comparing groups, indicate the direction of the effect (ie, which group is favored). | Lines 16-20 |
| Discussion | |||
| Limitations of evidence | 9 | Provide a brief summary of the limitations of the evidence included in the review (eg, study risk of bias, inconsistency and imprecision). | Lines 24-26 |
| Interpretation | 10 | Provide a general interpretation of the results and important implications. | Lines 21-24 |
| Other | |||
| Funding | 11 | Specify the primary source of funding for the review. | None |
| Registration | 12 | Provide the register name and registration number. | Title Page |
From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi:10.1136/bmj.n71, For more information, visit: http://www.prisma-statement.org/
PRISMA 2020 checklist.
| Section and topic | Item # | Checklist item | Location where item is reported |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a systematic review. | Line 2 |
| Abstract | |||
| Abstract | 2 | See the PRISMA 2020 for Abstracts checklist. | See abstract checklist |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of existing knowledge. | Lines 45-50 |
| Objectives | 4 | Provide an explicit statement of the objective(s) or question(s) the review addresses. | Lines 52-55 |
| Methods | |||
| Eligibility criteria | 5 | Specify the inclusion and exclusion criteria for the review and how studies were grouped for the syntheses. | Table 1 |
| Information sources | 6 | Specify all databases, registers, websites, organisations, reference lists and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted. | Lines 59-61, 100 |
| Search strategy | 7 | Present the full search strategies for all databases, registers and websites, including any filters and limits used. | Lines 66-97 |
| Selection process | 8 | Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and if applicable, details of automation tools used in the process. | Lines 101-106, Table 1 |
| Data collection process | 9 | Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and if applicable, details of automation tools used in the process. | Lines 110-115 |
| Data items | 10a | List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought (eg, for all measures, time points, analyses), and if not, the methods used to decide which results to collect. | Lines 110-115 |
| 10b | List and define all other variables for which data were sought (eg, participant and intervention characteristics, funding sources). Describe any assumptions made about any missing or unclear information. | NA | |
| Study risk of bias assessment | 11 | Specify the methods used to assess risk of bias in the included studies, including details of the tool(s) used, how many reviewers assessed each study and whether they worked independently, and if applicable, details of automation tools used in the process. | Line 111 |
| Effect measures | 12 | Specify for each outcome the effect measure(s) (eg, risk ratio, mean difference) used in the synthesis or presentation of results. | Lines 118-119 |
| Synthesis methods | 13a | Describe the processes used to decide which studies were eligible for each synthesis (eg, tabulating the study intervention characteristics and comparing against the planned groups for each synthesis (item #5)). | NA |
| 13b | Describe any methods required to prepare the data for presentation or synthesis, such as handling of missing summary statistics, or data conversions. | NA | |
| 13c | Describe any methods used to tabulate or visually display results of individual studies and syntheses. | Lines 110-115, Table | |
| 13d | Describe any methods used to synthesize results and provide a rationale for the choice(s). If meta-analysis was performed, describe the model(s), method(s) to identify the presence and extent of statistical heterogeneity, and software package(s) used. | NA | |
| 13e | Describe any methods used to explore possible causes of heterogeneity among study results (eg, subgroup analysis, meta-regression). | NA | |
| 13f | Describe any sensitivity analyses conducted to assess robustness of the synthesized results. | NA | |
| Reporting bias assessment | 14 | Describe any methods used to assess risk of bias due to missing results in a synthesis (arising from reporting biases). | NA |
| Certainty assessment | 15 | Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome. | Line 111 |
| Results | |||
| Study selection | 16a | Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram. | Figure 1 |
| 16b | Cite studies that might appear to meet the inclusion criteria, but which were excluded, and explain why they were excluded. | Lines 100-108 | |
| Study characteristics | 17 | Cite each included study and present its characteristics. | Table 2 |
| Risk of bias in studies | 18 | Present assessments of risk of bias for each included study. | Table 2 |
| Results of individual studies | 19 | For all outcomes, present, for each study: (a) summary statistics for each group (where appropriate) and (b) an effect estimate and its precision (eg, confidence/credible interval), ideally using structured tables or plots. | Lines 124-161 |
| Results of syntheses | 20a | For each synthesis, briefly summarize the characteristics and risk of bias among contributing studies. | NA |
| 20b | Present results of all statistical syntheses conducted. If meta-analysis was done, present for each the summary estimate and its precision (eg, confidence/credible interval) and measures of statistical heterogeneity. If comparing groups, describe the direction of the effect. | NA | |
| 20c | Present results of all investigations of possible causes of heterogeneity among study results. | NA | |
| 20d | Present results of all sensitivity analyses conducted to assess the robustness of the synthesized results. | NA | |
| Reporting biases | 21 | Present assessments of risk of bias due to missing results (arising from reporting biases) for each synthesis assessed. | NA |
| Certainty of evidence | 22 | Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed. | Table 2 |
| Discussion | |||
| Discussion | 23a | Provide a general interpretation of the results in the context of other evidence. | Lines 179-226 |
| 23b | Discuss any limitations of the evidence included in the review. | Lines 239-250 | |
| 23c | Discuss any limitations of the review processes used. | Line 239-250 | |
| 23d | Discuss implications of the results for practice, policy, and future research. | Line 253-261 | |
| Other information | |||
| Registration and protocol | 24a | Provide registration information for the review, including register name and registration number, or state that the review was not registered. | Title page |
| 24b | Indicate where the review protocol can be accessed, or state that a protocol was not prepared. | Lines 103-104 | |
| 24c | Describe and explain any amendments to information provided at registration or in the protocol. | None | |
| Support | 25 | Describe sources of financial or non-financial support for the review, and the role of the funders or sponsors in the review. | None |
| Competing interests | 26 | Declare any competing interests of review authors. | None |
| Availability of data, code and other materials | 27 | Report which of the following are publicly available and where they can be found: template data collection forms; data extracted from included studies; data used for all analyses; analytic code; any other materials used in the review. | With corresponding author |
From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71, For more information, visit: http://www.prisma-statement.org/.
Appendix A. Supplementary data
References
- 1.Singh J.A., Yu S., Chen L., Cleveland J.D. Rates of total joint replacement in the United States: future projections to 2020-2040 using the National Inpatient Sample. J Rheumatol. 2019;46:1134–1140. doi: 10.3899/jrheum.170990. [DOI] [PubMed] [Google Scholar]
- 2.Sloan M., Premkumar A., Sheth N.P. Projected volume of primary total joint arthroplasty in the U.S., 2014 to 2030. J Bone Joint Surg Am. 2018;100:1455–1460. doi: 10.2106/JBJS.17.01617. [DOI] [PubMed] [Google Scholar]
- 3.Pivec R., Johnson A.J., Mears S.C., et al. Hip arthroplasty. Lancet. 2012;380:1768–1777. doi: 10.1016/S0140-6736(12)60607-2. [DOI] [PubMed] [Google Scholar]
- 4.Lin M.M., Goldsmith J.D., Resch S.C., et al. Histologic examinations of arthroplasty specimens are not cost-effective: a retrospective cohort study. Clin Orthop Relat Res. 2012;470:1452–1460. doi: 10.1007/s11999-011-2149-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kocher M.S., Erens G., Thornhill T.S., et al. Cost and effectiveness of routine pathological examination of operative specimens obtained during primary total hip and knee replacement in patients with osteoarthritis. J Bone Joint Surg Am. 2000;82:1531–1535. doi: 10.2106/00004623-200011000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Liow M.H.L., Agrawal K., Anderson D.W., et al. Unsuspected malignancies in routine femoral head histopathologic examination during primary total hip arthroplasty: cost-effectiveness analysis. J Arthroplasty. 2017;32:735–742. doi: 10.1016/j.arth.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 7.DiCarlo E.F., Klein M.J. Comparison of clinical and histologic diagnoses in 16,587 total joint arthroplasties: implications for orthopedic and pathologic practices. Am J Clin Pathol. 2014;141:111–118. doi: 10.1309/AJCPDMFQK6QZK9NN. [DOI] [PubMed] [Google Scholar]
- 8.Campbell M.L., Gregory A.M., Mauerhan D.R. Collection of surgical specimens in total joint arthroplasty. Is routine pathology cost effective? J Arthroplasty. 1997;12:60–63. doi: 10.1016/s0883-5403(97)90048-6. [DOI] [PubMed] [Google Scholar]
- 9.Dermawan J.K., Goldblum A., Reith J.D., et al. Accurate and reliable diagnosis of avascular necrosis of the femoral head from total hip arthroplasty specimens requires pathologic examination. Am J Clin Pathol. 2021;155:565–574. doi: 10.1093/ajcp/aqaa153. [DOI] [PubMed] [Google Scholar]
- 10.Lawrence T., Moskal J.T., Diduch D.R. Analysis of routine histological evaluation of tissues removed during primary hip and knee arthroplasty. J Bone Joint Surg Am. 1999;81:926–931. doi: 10.2106/00004623-199907000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Layfield L.J., Crim J.R., Oserowsky A., et al. Pathology assessment of femoral head resection specimens: an important quality assurance procedure. Arch Pathol Lab Med. 2020;144:580–585. doi: 10.5858/arpa.2019-0128-OA. [DOI] [PubMed] [Google Scholar]
- 12.Meding J.B., Ritter M.A., Jones N.L., et al. Determining the necessity for routine pathologic examinations in uncomplicated total hip and total knee arthroplasties. J Arthroplasty. 2000;15:69–71. doi: 10.1016/s0883-5403(00)91233-6. [DOI] [PubMed] [Google Scholar]
- 13.Niggemeyer O., Steinhagen J., Zustin J., et al. The value of routine histopathology during hip arthroplasty in patients with degenerative and inflammatory arthritis. Hip Int. 2011;21:98–106. doi: 10.5301/hip.2011.6300. [DOI] [PubMed] [Google Scholar]
- 14.O’Connell J.X., Nielsen G.P., Rosenberg A.E. Subchondral acute inflammation in severe arthritis: a sterile osteomyelitis? Am J Surg Pathol. 1999;23:192–197. doi: 10.1097/00000478-199902000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Raab S.S., Slagel D.D., Robinson R.A. The utility of histological examination of tissue removed during elective joint replacement. A preliminary assessment. J Bone Joint Surg Am. 1998;80:331–335. doi: 10.2106/00004623-199803000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Sissons H.A., Nuovo M.A., Steiner G.C. Pathology of osteonecrosis of the femoral head. A review of experience at the Hospital for Joint Diseases, New York. Skeletal Radiol. 1992;21:229–238. doi: 10.1007/BF00243063. [DOI] [PubMed] [Google Scholar]
- 17.Zwitser E.W., de Gast A., Basie M.J., et al. B-cell lymphoma in retrieved femoral heads: a long term follow up. BMC Musculoskelet Disord. 2009;10:53. doi: 10.1186/1471-2474-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moher D., Liberati A., Tetzlaff J., et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sepriano A., Kerschbaumer A., Smolen J.S., et al. Safety of synthetic and biological DMARDs: a systematic literature review informing the 2019 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis. 2020;79:760–770. doi: 10.1136/annrheumdis-2019-216653. [DOI] [PubMed] [Google Scholar]
- 20.Freedman A., Jacobsen E. Follicular lymphoma: 2020 update on diagnosis and management. Am J Hematol. 2020;95:316–327. doi: 10.1002/ajh.25696. [DOI] [PubMed] [Google Scholar]
- 21.Lumish M., Falchi L., Imber B.S., et al. How we treat mature B-cell neoplasms (indolent B-cell lymphomas) J Hematol Oncol. 2021;14(1):5. doi: 10.1186/s13045-020-01018-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Advani R., Rosenberg S.A., Horning S.J. Stage I and II follicular non-Hodgkin’s lymphoma: long-term follow-up of no initial therapy. J Clin Oncol. 2004;22:1454–1459. doi: 10.1200/JCO.2004.10.086. [DOI] [PubMed] [Google Scholar]
- 23.Brice P., Bastion Y., Lepage E., et al. Comparison in low-tumor-burden follicular lymphomas between an initial no-treatment policy, prednimustine, or interferon alfa: a randomized study from the Groupe d’Etude des Lymphomes Folliculaires. Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol. 1997;15:1110–1117. doi: 10.1200/JCO.1997.15.3.1110. [DOI] [PubMed] [Google Scholar]
- 24.Ardeshna K.M., Smith P., Norton A., et al. Long-term effect of a watch and wait policy versus immediate systemic treatment for asymptomatic advanced-stage non-Hodgkin lymphoma: a randomised controlled trial. Lancet. 2003;362:516–522. doi: 10.1016/s0140-6736(03)14110-4. [DOI] [PubMed] [Google Scholar]
- 25.Rosenberg S.A. Karnofsky memorial lecture. The low-grade non-Hodgkin’s lymphomas: challenges and opportunities. J Clin Oncol. 1985;3:299–310. doi: 10.1200/JCO.1985.3.3.299. [DOI] [PubMed] [Google Scholar]
- 26.Armitage J.O., Longo D.L. Is watch and wait still acceptable for patients with low-grade follicular lymphoma? Blood. 2016;127:2804–2808. doi: 10.1182/blood-2015-11-632745. [DOI] [PubMed] [Google Scholar]
- 27.Zelenetz A.D., Gordon L.I., Wierda W.G., et al. Non-Hodgkin’s lymphomas, version 4.2014. J Natl Compr Canc Netw. 2014;12:1282–1303. doi: 10.6004/jnccn.2014.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Padala S.A., Kallam A. StatPearls. StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC; Treasure Island, FL: 2021. Diffuse large B cell lymphoma. [Google Scholar]
- 29.Yoo J.J., Kwon Y.S., Koo K.H., et al. Cementless total hip arthroplasty performed in patients with pigmented villonodular synovitis. J Arthroplasty. 2010;25:552–557. doi: 10.1016/j.arth.2009.02.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.