Abstract
Background
The purpose of this study is to evaluate and redefine patients at high risk for increased resource utilization and complications after total joint arthroplasty (TJA), so interventions may focus on patients standing to receive the most benefit.
Material and methods
This is a retrospective study of 787 patients undergoing primary unilateral TJA from September 1, 2020, to September 31, 2021. Patients were deemed to be at “high risk” based on criteria derived from published literature and triaged to an enhanced preoperative education program. Patients that were discharged to a skilled nursing facility, had a length of stay ≥ 2 days, returned to the emergency department, or readmitted within 30 days were classified as having a composite outcome. A univariate analysis compared patients who did and did not experience the composite outcome, and multivariate regression was performed to evaluate predictors of this endpoint.
Results
Differences in rates of 5 of the 28 risk factors were present between patients who did and did not experience composite outcomes. After controlling for other factors, African American race, planned discharge to skilled nursing facility, mental health conditions or drug use, cardiac, and neurologic conditions were predictive of the composite outcome. Patients who were reclassified as “high risk” with 1 or more of these characteristics, experienced longer length of stay and lower rates of home discharge than the rest of the population.
Conclusion
This study presents a profile of high-risk TJA patients that can be incorporated into clinical practice for risk stratification and targeted intervention.
Keywords: Length of stay, Skilled nursing facility, Readmissions, Disparities, High risk
Introduction
As the use of total joint arthroplasty (TJA) continues to expand, increasing effort has been placed on identifying patients at risk for increased resource utilization and postoperative complications. A variety of models for identifying patients at increased risk for prolonged length of stay (LOS), nonhome discharge, complications, and readmissions based on preoperative characteristics have been presented with variable predictive accuracy [1,2]. The majority of models use preoperative demographics and comorbidities to stratify risk for a single postoperative outcome [1,2]. Commonly reported risk factors for these outcomes include increased American Society of Anesthesiologists scores, obesity, and cardiac conditions [1,2].
Multiple studies have identified preventative measures leading to lower length of hospital stays, decreased emergency department (ED) or readmission rates, and complications such as more aggressive rapid recovery and rehabilitation protocols and multimodal pain management [[3], [4], [5]]. In addition, increased preoperative education and care coordination has been associated with improved postoperative outcomes including reduced LOS and cost [6,7]. However, even with such advancements, health disparities are still present. Studies have described patients with lower socioeconomic status and African American (AA) race have a greater risk for increased LOS, nonhome discharge, 30-day readmissions, and postoperative complications [[8], [9], [10], [11]].
Given the lack of a consensus definition for the “high-risk” TJA patient, few protocols are in place to improve the surgical outcomes of these patients. At our institution, we have implemented an enhanced preoperative education pathway (EPrEP) for high-risk TJA patients. In the program, AA patients or those with 2 or more risk factors derived from previously published research are triaged to a nurse navigator (NN) for individualized education prior to surgery. The early results of the program are forthcoming. The purpose of this study is to assess the leading risk factors in high-risk patients undergoing TJA and to further analyze groups based on a number of risk factors. We then aim to redefine the definition of a “high-risk” patient to create more effective predictive models for postoperative outcomes and better target patients who may derive the most benefit from the EPrEP.
Material and methods
This study was deemed exempt from institutional review board approval by the institutional clinical research committee. A retrospective review of all patients undergoing primary unilateral total knee arthroplasty (TKA) or total hip arthroplasty (THA) by 10 board-certified surgeons at a single institution between September 1, 2020, and September 30, 2021, was performed. Demographics, comorbidities, and hospital outcomes were extracted from the electronic medical record. Comorbidities were classified based on International Classification for Disease 10th Edition codes, and comorbidity burden was calculated using the Charlson Comorbidity Index (CCI). For all patients, a single NN collected data on additional risk factors.
Study population
Patients that had same-day discharge (n = 384), did not meet the criteria for a high-risk patient (described below [n = 1055]), had simultaneous bilateral TJA (n = 6), or revision TJA (n = 160) were excluded from this study. A total of 787 patients met the inclusion criteria. All patients included in the study were deemed to be at high risk as assessed using the instrument presented in Appendix A. The definition of each risk factor is also included in this Appendix A. At the time of decision for surgery, surgeons documented risk factors and placed NN consults for enhanced education in appropriate patients. Patients scheduled for surgery were sent a survey for self-reporting risk factors. The NN then classified patients as high risk based on the surgeon and self-reported risk factors or presence of risk factors documented in the electronic medical record. Patients of non-white race or those with 2 or more risk factors identified were classified as high risk. All patients included in the study received individualized NN counseling either in person or by telemedicine. Patients who did not complete the self-reported survey in advance of the NN counseling session verbally completed the survey with the NN to ensure a comprehensive risk assessment was completed. Counseling included discussions of preoperative management of medical comorbidities and triage to outside resources including smoking cessation, weight management, transportation services, and in-home care as deemed necessary by the NN. The NN then provided additional education regarding expectations of surgery, pain management techniques, and appropriate resources for postoperative questions and concerns.
Study outcomes
Postoperative outcomes of interest included LOS (measured in hours and days), home-discharge status, 30-day ED returns, and 30-day readmissions. ED returns and readmissions were manually collected and included all returns to hospitals participating in the Epic Care-Everywhere program. Patients that were discharged to a skilled nursing facility (SNF), had a LOS greater than 2 days, returned to the ED within 30 days, or were readmitted within 30 days were classified as having a composite outcome.
Statistical analysis
The prevalence of risk factors for the entire population was calculated, and demographics, comorbidities, and outcomes were assessed for each number of risk factors; 1, 2, 3, 4, and 5+ risk factors. One-way analysis of variance was used to determine differences between groups.
Univariate analysis including chi-square tests and two-sided independent samples t-tests were used to determine demographic, comorbidity, and risk factor differences between those with and without the composite outcome. The Fisher’s Exact test was performed when the assumptions of chi-square testing were not met, and the Mann-Whitney U test was used for nonparametric continuous data. The data were randomly split into a training set (80% for building a predictive model) and a test set (20% for evaluating the model). Stepwise multiple logistic regression was used to evaluate predictors of the composite outcome in the training set. A receiver operator curve was generated on the test set, and the area under the curve was calculated to measure the accuracy of the model in predicting the composite outcome. Using the significant predictors found from the logistic model, a new criterion was used to classify high-risk patients. Any patient of AA race, planned SNF, mental health or drug-use issues, and cardiac or neurologic conditions were redefined as a high-risk patient. Univariate analysis was used to determine demographic, comorbidity, and postoperative outcome differences between redefined high-risk patients and those who were not. The Fisher’s Exact test was performed when the assumptions of chi-square testing were not met, and the Mann-Whitney U-test was used for nonparametric continuous data. All statistical analyses were performed using R Studio (Version 1.4.1717 2009-2021 RStudio, PBC). Statistical significance was assessed at P < .05.
Results
The average number of risk factors for the overall population was 3.13. The top 3 risk factors were living outside the county of the hospital, being a woman over the age of 65 years, and having a Body Mass Index (BMI) greater than 35 (Table 1). Patient demographics were assessed by a number of risk factors. Patients with 4 and 5+ risk factors had significantly higher BMIs (P = .032), were more likely to be female (P = .029), and AA (P < .001) than those with 1, 2, or 3 risk factors. Patients with 5+ risk factors had a greater overall comorbidity burden as measured by the CCI (P < .001) (Table 2). Postoperatively, patients with 5+ risk factors had a significantly longer LOS in hours (P = .012) and days (P = .021) and a higher rate of 30-day ED returns (P = .005) (Table 3).
Table 1.
Prevalence of risk factors.
| Risk factors | Patients with ≥1 risks (n = 787) |
|---|---|
| Average number of risk factors | 3.13 ± 1.52 |
| Pain management | 79 (10.0) |
| Sleep apnea | 155 (19.7) |
| Diabetes with A1c > 7 | 84 (10.7) |
| Insulin dependent diabetes | 23 (2.9) |
| Current smoker | 58 (7.4) |
| African American race | 134 (17.0) |
| Hispanic | 6 (0.8) |
| Other ethnicity | 10 (1.3) |
| BMI >35 | 208 (26.4) |
| Lives alone | 188 (23.9) |
| Wants/Planned discharge to SNF | 66 (8.4) |
| Socioeconomic concerns | 43 (5.5) |
| Worker’s comp | 5 (0.6) |
| Homeless | 3 (0.4) |
| Woman >65 | 365 (46.4) |
| Lives outside primary service area | 381 (48.4) |
| Alcohol dependence | 10 (1.3) |
| Mental health/drug use | 20 (2.5) |
| Cardiac | 155 (19.7) |
| Pulmonary | 11 (1.4) |
| Vascular | 20 (2.5) |
| Neurologic | 21 (2.7) |
| Family concerns | 7 (0.9) |
| Spine | 14 (1.8) |
| Autoimmune | 17 (2.2) |
| Diabetes | 45 (5.7) |
| Other | 180 (22.9) |
Data are expressed as mean ± SD or n (%).
Table 2.
Patient demographics.
| Demographics and comorbidities | 1 Risk factor (n = 95) | 2 Risk factors (n = 208) | 3 Risk factors (n = 205) | 4 Risk factors (n = 143) | 5+ Risk factors (n = 132) | P value |
|---|---|---|---|---|---|---|
| Age | 68.34 ± 9.52 | 70.17 ± 8.60 | 69.19 ± 9.24 | 69.32 ± 10.63 | 67.61 ± 8.74 | .537 |
| BMI | 29.76 ± 4.54 | 29.39 ± 5.17 | 30.11 ± 5.43 | 32.15 ± 5.48 | 34.04 ± 5.53 | .032 |
| Sex | .029 | |||||
| Female | 37(38.9) | 122 (58.7) | 132 (64.4) | 105 (73.4) | 95 (72.0) | |
| Male | 58 (61.1) | 86(41.3) | 73 (35.6) | 38 (26.6) | 37 (28.0) | |
| AA race | 1 (1.1) | 6 (2.9) | 52 (25.4) | 54 (37.8) | 81 (61.4) | <.001 |
| CCI | 3.17 ± 1.64 | 3.35 ± 1.49 | 3.56 ± 1.81 | 3.63 ± 1.78 | 4.07 ± 1.74 | <.001 |
P values <0.05 are given in bold.
Data are expressed as mean ± SD or n (%).
Table 3.
Outcomes.
| Outcomes | 1 Risk factor (n = 95) | 2 Risk factors (n = 208) | 3 Risk factors (n = 205) | 4 Risk factors (n = 143) | 5+ Risk factors (n = 132) | P value |
|---|---|---|---|---|---|---|
| LOS hours | 31.47 ± 13.34 | 34.59 ± 16.05 | 36.73 ± 26.28 | 36.42 ± 20.43 | 40.64 ± 25.49 | .012a |
| LOS days | 1.09 ± 0.54 | 1.22 ± 0.65 | 1.32 ± 1.08 | 1.28 ± 0.82 | 1.44 ± 1.07 | .021a |
| Discharge home | 94 (98.9) | 203 (97.6) | 197 (96.1) | 137 (95.8) | 116 (87.9) | .052 |
| 30-d ED return | 2 (2.1) | 8 (3.8) | 9 (4.4) | 7 (4.9) | 9 (6.8) | .005 |
| 30-d Readmission | 2 (2.1) | 6 (2.9) | 6 (3.0) | 3 (2.1) | 2 (1.5) | .385 |
P values <0.05 are given in bold.
Data are expressed as mean ± SD or n (%).
Mann-Whitney U-test.
Demographically, those that had the composite outcome (discharge to SNF, LOS ≥2 days, or 30-day ED return or readmission) were nearly 4 years older than those without a composite outcome (72.17 vs 68.37; P < .001) and had a significantly higher average CCI (4.24 vs 3.40; P < .001). There were no significant differences in BMI, sex, or race between those who had a composite outcome and those who did not (Table 4). When compared with no-composite-outcome patients, those with a composite outcome had, on average, a higher number of risk factors (3.50 vs 3.04; P = .002), a higher proportion of patients that lived alone (31.3% vs 22.2%; P = .029), a higher proportion of patients with planned discharge to SNF (24.3% vs 4.8%; P < .001), and a higher proportion of patients with mental health/drug use issues (5.6% vs 1.7%; P = .012) and cardiac issues (27.1% vs 17.9%; P = .017) (Table 5).
Table 4.
Patients with composite outcome (SNF or 2+ LOS or 30-day ED or 30-day readmit).
| Demographics and comorbidities | No composite outcome (n = 642) | Composite outcome (n = 144) | P value |
|---|---|---|---|
| Age | 68.37 ± 9.07 | 72.17 ± 9.82 | <.001 |
| BMI | 30.98 ± 5.50 | 30.64 ± 5.71 | .508 |
| Sex | .080 | ||
| Female | 393 (61.2) | 100 (69.4) | |
| Male | 249 (38.8) | 44 (30.6) | |
| AA race | 107 (16.7) | 30 (20.8) | .285 |
| CCI | 3.40 ± 1.61 | 4.24 ± 1.96 | <.001 |
P values <0.05 are given in bold.
Data are expressed as mean ± SD or n (%).
Table 5.
Risk factors by composite outcome.
| Risk factors | No composite outcome (n = 642) | Composite outcome (n = 144) | P value |
|---|---|---|---|
| Average number of risk factors | 3.04 ± 1.48 | 3.50 ± 1.63 | .002 |
| Pain management | 61 (9.5) | 18 (12.5) | .353 |
| Sleep apnea | 124 (19.3) | 31 (21.5) | .626 |
| Diabetes with A1c > 7 | 70 (10.9) | 14 (9.7) | .791 |
| Insulin dependent diabetes | 19 (3.0) | 4 (2.8) | 1a |
| Current smoker | 47 (7.3) | 11 (7.6) | 1 |
| African American race | 104 (16.2) | 30 (20.8) | .225 |
| Hispanic | 5 (0.8) | 1 (0.7) | 1a |
| Other ethnicity | 8 (1.2) | 2 (1.4) | 1a |
| BMI >35 | 175 (27.3) | 33 (22.9) | .336 |
| Lives alone | 143(22.2) | 45 (31.3) | .029 |
| Wants/Planned discharge to SNF | 31 (4.8) | 35 (24.3) | <.001 |
| Socioeconomic concerns | 30 (4.7) | 13 (9.0) | .061 |
| Worker’s comp | 4 (0.6) | 1 (0.7) | 1a |
| Homeless | 1 (0.02) | 2 (1.4) | .088 |
| Woman >65 | 287 (44.7) | 78 (54.2) | .049 |
| Lives outside primary service area | 320 (49.8) | 61 (42.4) | .126 |
| Alcohol dependence | 6 (0.09) | 3 (2.1) | .218a |
| Mental health/drug use | 11 (1.7) | 8 (5.6) | .012a |
| Cardiac | 115 (17.9) | 39 (27.1) | .017 |
| Pulmonary | 7 (1.1) | 3 (2.1) | .402a |
| Vascular | 16 (2.5) | 3 (2.1) | 1a |
| Neurologic | 15 (2.3) | 5 (3.5) | .748a |
| Family concerns | 5 (0.8) | 1 (0.7) | .589a |
| Spine | 10 (1.6) | 3 (2.1) | .932a |
| Autoimmune | 13 (2.0) | 3 (2.1) | 1a |
| Diabetes | 43 (6.7) | 5 (3.5) | .205 |
| Other | 126 (19.6) | 21 (14.6) | .199 |
Data are expressed as mean ± SD or n (%).
Denotes Fisher's exact test.
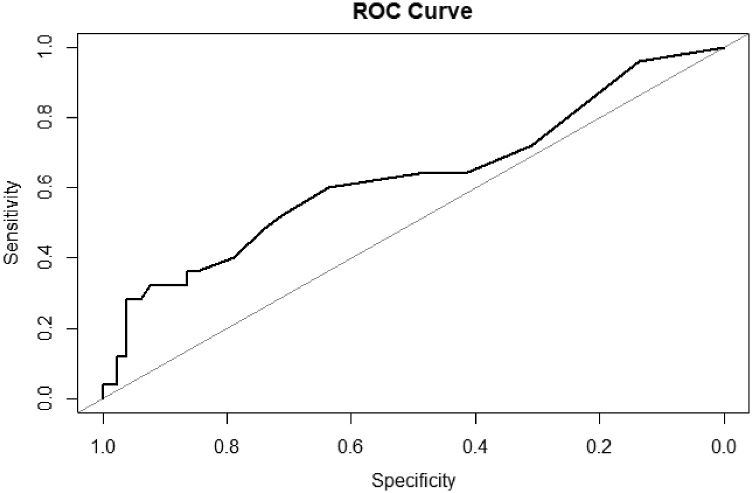
A stepwise multivariate logistic regression model on the training set found AA race, planned SNF discharge, mental health and drug use issues, cardiac issues, and neurologic issues to be predictors of the composite outcome. Those of AA race were 2.03 (95% confidence interval [CI]: 1.12 to 3.63; P = .018) times more likely to experience a composite outcome. Planned SNF patients were 5.20 (95% CI: 2.84 to 9.58; P < .001) times more likely to experience a composite outcome. Those with mental health and drug use issues, cardiac issues, and neurologic issues were 3.94 (95% CI: 1.14 to 12.24; P < .021), 2.55 (95% CI: 1.51 to 4.27; P < .001), and 3.39 (95% CI: 0.99 to 910.31; P = .037) times more likely to experience a composite outcome, respectively (Table 6). When applied to the test set, the model generated an area under the receiver operator curve of 0.63 indicating fair predictive accuracy (Fig. 1).
Table 6.
Multivariate logisitic regression: predictors of composite outcome (SNF or 2+ LOS or 30-day ED or 30-day readmit).
| Predictors | Odds ratio | 95% Confidence interval | P value |
|---|---|---|---|
| AA race | 2.03 | 1.12-3.63 | .018 |
| Planned SNF | 5.20 | 2.84-9.58 | <.001 |
| Woman >65 | 1.50 | 0.98-2.31 | .064 |
| Lives outside primary service area | 0.71 | 0.46-1.10 | .127 |
| Alcohol dependence | 3.67 | 0.72-15.09 | .082 |
| Mental health/drug use | 3.94 | 1.14-12.24 | .021 |
| Cardiac | 2.55 | 1.51-4.27 | <.001 |
| Neurologic | 3.39 | 0.99-10.31 | .037 |
P values < 0.05 are given in bold.
Figure 1.
Receiver operator curve of multivariate logisitic model of composite outcome, area under the curve = 0.6289, specificity = 0.924.
Using significant predictors found from the logistic model, a new criterion was used to classify high-risk patients. Any patient of AA race, planned SNF, mental health/drug use issues, cardiac issues, or neurologic issues were categorized as a redefined high-risk patient. Demographically, the high-risk patients were on average 2.5 years older (70.43 vs 67.80; P < .001) with a higher comorbidity burden (3.97 vs 3.19; P < .001) (Table 7). Postoperatively, high-risk patients had a longer LOS in hours (39.40 vs 32.76; P < .001) and days (1.41 vs 1.15; P < .001) and a significantly lower rate of home discharge (91.5% vs 98.8%, P < .001) (Table 8). When broken into number of risk factors, there were no significant differences in LOS, rate of discharge home, 30-day ED return rate, or 30-day readmission rates (Appendix B).
Table 7.
Patient demographics of redefined high-risk patients.
| Demographics and comorbidities | Non-high risk (n = 422) | High risk (n = 364) | P value |
|---|---|---|---|
| Age | 67.89 ± 9.25 | 70.43 ± 9.22 | <.001 |
| BMI | 30.77 ± 5.53 | 31.09 ± 5.54 | .411 |
| Sex | .992 | ||
| Female | 264 (62.6) | 229 (62.9) | |
| Male | 158 (37.4) | 135 (37.1) | |
| AA race | 0 (0) | 137 (37.6) | <.001 |
| CCI | 3.19 ± 1.53 | 3.97 ± 1.81 | <.001 |
P values < 0.05 are given in bold.
Data are expressed as mean ± SD or n (%).
Table 8.
Outcomes of redefined high-risk patients.
| Outcomes | Non-high risk (n = 422) | High risk (n = 364) | P value |
|---|---|---|---|
| LOS hours | 32.76 ± 15.87 | 39.40 ± 27.14 | <.001 |
| LOS days | 1.15 ± 0.64 | 1.41 ± 1.11 | <.001 |
| Discharge home | 417 (98.8) | 333 (91.5) | <.001 |
| 30-d ED return | 9 (2.1) | 10 (2.7) | .645 |
| 30-d Readmission | 13 (3.1) | 22 (6.0) | .056 |
P values < 0.05 are given in bold.
Data are expressed as mean ± SD or n (%).
Discussion
The results of the current study demonstrate that those with 5 or more risk factors are more likely to have a greater BMI, be female, of AA race, and an increased comorbidity burden. With an increasing number of risk factors, trends toward longer length of hospital stay and higher 30-day ED return rates were observed. When examining risk factors for an endpoint of composite outcomes, AA race, planned discharge to SNF, mental health conditions/drug use, and cardiac or neurologic comorbidities were independent predictors of this endpoint. Using these factors to redefine high-risk patients, we confirmed that patients with 1 or more of these 5 characteristics experienced longer LOS and higher rates of nonhome discharge. Similar rates of ED returns and readmission were found in those with or without these characteristics. Based on these results, we suggest preoperative interventions, such as EPrEPs, for this subset of patients to improve their surgical success.
The current study’s finding that AA race was a significant predictor of composite outcomes highlights the continued need to refine interventions aimed at reducing racial disparities in TJA patients. Recent literature has found similar results encompassing the racial disparities that exist in health care, specifically in TJA. Inneh et al. conducted a study of 7924 lower extremity joint procedure cases and found an increase in nonhome discharge rate with AA race/ethnicity [8]. Another study by Amen et al. used the National Inpatient Sample Database to gather data on TJA patients and race [10]. AA patients were more likely to have longer LOS and increased complication rates (P < .001 for both) [10]. AA patients were also more likely to be discharged to a facility rather than home [10]. Stone et al. too found a greater LOS for AA TKA patients (P < .001) and THA patients (P = .065) than that for the white population [11]. This study was conducted from 2013 to 2017 and found that although discharge rates to a SNF decreased overtime, AA patients were still twice as likely to be discharged compared with white patients [11]. The current study adds to our understanding of the increased risk present in AA patients undergoing TJA in multiple ways. First, our data demonstrate that AA patients do present with an increased number of risk factors encompassing both medical comorbidities and psychosocial concerns. It is notable that within this cohort of high-risk patients, AAs made up only 1.8% of patients with 1 or 2 risk factors, but 61.4% of patients had 5 or more risk factors. Second, the results of the multivariate regression found AA patients to be at over twice the odds of experiencing discharge to SNF, 2+ days of LOS, or a 30-day ED return or readmission than non-AA patients. Third, AA race was both included in the final model and independently a significant predictor, demonstrating the impact of race on outcomes after TJA. With all patients considered high risk and managed through the EPrEP, collectively our findings suggest additional intervention may be required to improve the value of TJA for this selected subset of high-risk patients.
Other comorbidities such as mental health conditions/drug use, cardiac, and neurologic conditions play an important role as predictive measures for composite outcomes. A study by Ali et al. found those with anxiety or depression were 6 times more likely to be dissatisfied with their TKA than those without a diagnosis [12]. Depression and anxiety have also been associated with increased pain and lower postoperative Western Ontario and McMaster Universities Arthritis Index scores [13,14]. Current literature has shown investigations of drug use in surgical outcomes, most commonly preoperative opioid use [[15], [16], [17], [18]]. A study conducted by Best et al. investigated TJA patients and found those with drug misuse had longer lengths of hospital stays and higher nonroutine discharge rates than those without drug misuse [15].
Additional studies have found increased postoperative opioid consumption for those with preoperative opioid use [16,17]. Although drug users only accounted for 13% of patients in the mental health conditions/drug use group within the current study, further research is needed to evaluate the impact of other illicit drug use on TJA outcomes. Cardiac disease has also been associated with increased readmission rates after TJA [9]. Our study found 19.7% of the population had cardiac comorbidities such as congestive heart failure, atrial fibrillation, and coronary artery disease. A study conducted by Curtis et al. investigated heart failure in TKA and found an association with greater LOS and readmission [19]. Neurologic conditions such as dementia, cerebrovascular accident, and Parkinson’s disease were recorded in our study population and found to be a predictor of the composite outcome. Furthermore, Pomeroy et al. found an association between neurologic disorders and increased complication rates for TKA [20]. The data presented in the current study support the literature in concluding mental health/drug use, cardiac, and neurologic conditions are independent predictors of increased LOS, discharge to SNF, and 30-day ED returns or readmissions in high-risk TJA patients.
The Centers for Medicare and Medicaid Services decision to remove TKA and THA from the inpatient-only list is a potential confounding factor influencing the decreased LOS seen in our study. The inpatient-only list entails a list of procedures that Medicare would cover if taken place in a hospital inpatient setting as opposed to outpatient surgical centers [21]. In reference to our study period, TKA was removed from the inpatient-only list prior, in 2018, while THA was removed in 2020 [22,23]. Therefore, outside factors such as 90-day ED return risks and medical care coverage may be driving forces for deciding if a patient should undergo inpatient or outpatient surgical care. However, we suggest this potentially confounding factor likely had limited impact on our results, as the study period was after removal of both procedures from the inpatient-only list.
The current study does contain multiple limitations. First, as a single-institution study, the population may not be representative of the broader population of patients undergoing TJA. An opportunity exists for additional research validating the presented definition of high-risk patients at other institutions. Second, as a retrospective observational study, risk for selection bias exists. This risk is particularly present in our methodology as the population of “high-risk” patients were identified by surgeons and the NN. Although a standardized criterion was used, the potential for subjective interpretation of risk factors, particularly socioeconomic factors, exists. Third, limitations may exist in our collection of ED returns, and readmission rates for only hospitals participating in the Epic Care-Everywhere program were included. Therefore, 30-day ED returns or readmissions at non-Epic hospitals would not be included. Fourth, although we were able to identify independent predictors of the composite endpoint used in this study, the overall predictive accuracy of the model generated was limited. This highlights the difficulty of generating a single model for predicting multiple outcomes that may be influenced by different risk factors. Finally, this study is limited by a short follow-up period of 30 days and does not examine risk factors for long-term complications or lack of improvement in patient-reported outcomes.
Conclusions
Within a cohort of high-risk patients, AAs and those with mental health, cardiac, and neurologic conditions were at increased risk for composite outcomes after TJA. Interventions aimed at improving early postoperative outcomes and reducing disparities should be tailored to focus on this subset of the TJA population.
Conflicts of interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: P. J. King received research support from DePuy and Smith & Nephew and consulting fees from Smith & Nephew and is in the Journal of Arthroplasty Editorial Board.
For full disclosure statements refer to https://doi.org/10.1016/j.artd.2022.02.031.
Footnotes
Funding: This study was funded by a grant from the Orthopaedic Research and Education Foundation (OREF), United States with funding made possible by the J. Robert Gladden Orthopaedic Society (JRGOS).
Appendix
Appendix A.
Risk factor assessment used to assess patients for “high-risk” criteria.
| Risk factor | Point 1 per positive result | Definition details |
|---|---|---|
| High risk if positive | ||
| Minority | None | |
| African American | ||
| Hispanic | ||
| other | ||
| Same day discharge | None | |
| ASC patient | None | |
| High risk if 2 or more positive | ||
| Insulin dependent diabetes | None | |
| Diabetic with A1C > 7 | None | |
| Diabetic with unknown A1C | Patient is unaware of current A1C, with no recent A1C documented in the medical record | |
| Sleep apnea | Diagnosis of obstructive sleep apnea in the medical record or patient reports having sleep apnea requiring CPAP or BIPAP | |
| BMI >35 | None | |
| Current smoker | None | |
| Pain management | Patient is currently seeing a pain management specialist | |
| Female >65 | None | |
| Lives alone | Patient does not have a caregiver present in the home | |
| Special needs: (any) | ||
| Learning disability | Diagnosis of a learning disability (dyslexia, nonverbal, reading comprehension deficit) documented in the medical record or reported by the patient | |
| Dialysis patient | Patient is actively receiving hemodialysis | |
| Venous insufficiency | Diagnosis of chronic venous insufficiency in the medical record or reported by the patient | |
| Prosthetic limb | None | |
| Socioeconomic | ||
| Homeless | Patient does not maintain a permanent residence. | |
| Lives in a RV, camper, boat, or other nontraditional home | None | |
| Wants to go to SNF | Patient has requested to be discharged to a SNF | |
| Workers’ compensation | Patient is seeking care related to a workers’ compensation case | |
| Other | ||
| Alcohol dependence | Current diagnosis of alcoholism or alcohol dependence in the medical record or patient self-reports as an alcoholic of alcohol dependent | |
| Mental health condition/Illicit drug use | Current diagnosis of a mental health condition (depression, anxiety, bipolar, etc.) in the medical record of reported by the patient. Recent or ongoing use of an illicit drug (marijuana, cocaine, heroin, etc.) documented in the medical record or reported by the patient | |
| Cardiac condition | Current diagnosis of AFIB, CHF, CAD, aortic disease, or history of MI documented in the medical record or reported by the patient | |
| Pulmonary condition | Current diagnosis of COPD, asthma, emphysema, or cystic fibrosis documented in the medical record or reported by the patient | |
| Vascular condition | Current diagnosis of PVD, or history of PE/DVT documented in the medical record or reported by the patient | |
| Neurologic condition | Current diagnosis or history of Alzheimer’s disease, ALS, Guillain-Barre syndrome, Bell’s palsy, dementia, brain tumor, or cerebrovascular accident recorded in the medical record or reported by the patient | |
| Spine condition | History of spine surgery or current diagnosis of a spine condition being managed by a provider documented in the medical record or reported by the patient | |
| Autoimmune condition | Current diagnosis or history of rheumatoid arthritis, psoriatic arthritis, multiple sclerosis, systemic lupus erythematosus, IBD, Addison’s disease, Graves’ disease, or myasthenia gravis recorded in the medical record or reported by the patient | |
| Diabetes mellitus | Current diagnosis of diabetes mellitus without A1C > 7, unknown A1C, or NIDDM (controlled diabetic) | |
| Other family concern | Other family concerns including abusive relationship or domestic issues reported by the patient | |
| Other | Comorbidities or psychosocial/socioeconomic factors not otherwise classified | |
| Geographic | ||
| Resides outside of hospital county | None | |
| Total number of risk factors |
ASC, ambulatory surgery center; CPAP, continuous positive airway pressure; BIPAP, bilevel positive airway pressure; AFIB, atrial fibrillation; CAD, coronary artery disease; CHF, congestive heart failure; RV, recreational vehicle; MI, myocardial infarction; COPD, chronic obstructive pulmonary disease; PVD, peripheral vascular disease; PE, pulmonary embolism; DVT, deep vein thrombosis; ALS, amyotrophic lateral sclerosis; IBD, inflammatory bowel disease; NIDDM, non-insulin dependent diabetes mellitus.
Appendix B.
Outcomes by number of risk factors in redefined high-risk patients.
| Outcomes | 1 Risk factor (n = 21) | 2 Risk factors (n = 67) | 3 Risk factors (n = 94) | 4 Risk factors (n = 79) | 5+ Risk factors (n = 103) | P value |
|---|---|---|---|---|---|---|
| LOS hours | 36.60 ± 27.21 | 35.63 ± 16.98 | 42.30 ± 37.63 | 39.72 ± 23.60 | 42.19 ± 27.53 | .119a |
| LOS days | 1.30 ± 1.13 | 1.29 ± 0.68 | 1.53 ± 1.55 | 1.41 ± 0.96 | 1.51 ± 1.13 | .138a |
| Discharge home | 20 (95.2) | 64 (95.5) | 88 (93.6) | 74 (93.7) | 87 (84.5) | .099 |
| 30-d ED return | 0 (0) | 4 (6.0) | 6 (6.4) | 5 (6.3) | 7 (6.8) | .137 |
| 30-d Readmission | 0 (0) | 2 (3.0) | 4 (4.3) | 2 (2.5) | 2 (1.9) | .587 |
Data are expressed as mean ± SD or n (%).
Mann-Whitney U-test.
Appendix A. Supplementary data
References
- 1.Gronbeck C., Cote M.P., Lieberman J.R., Halawi M.J. Risk stratification in primary total joint arthroplasty: the current state of knowledge. Arthroplasty Today. 2019;5(1):126. doi: 10.1016/j.artd.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu S., Garvin K.L., Healy W.L., Pellegrini V.D., Iorio R. Preventing hospital readmissions and limiting the complications associated with total joint arthroplasty. J Am Acad Orthopaedic Surgeons. 2015;23(11):e60. doi: 10.5435/JAAOS-D-15-00044. [DOI] [PubMed] [Google Scholar]
- 3.Schwenk E.S., Kasper V.P., Smoker J.D., et al. Mepivacaine versus bupivacaine spinal anesthesia for early postoperative ambulation. Anesthesiology. 2020;133(4):801. doi: 10.1097/ALN.0000000000003480. [DOI] [PubMed] [Google Scholar]
- 4.Li C., Qu J., Pan S., Qu Y. Local infiltration anesthesia versus epidural analgesia for postoperative pain control in total knee arthroplasty: a systematic review and meta-analysis. J Orthop Surg Res. 2018;13(1):112. doi: 10.1186/s13018-018-0770-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarpong N.O., Lakra A., Jennings E., et al. Same-day physical therapy following total knee arthroplasty leads to improved inpatient physical therapy performance and decreased inpatient opioid consumption. J Arthroplasty. 2019;34(12):2931. doi: 10.1016/j.arth.2019.07.029. [DOI] [PubMed] [Google Scholar]
- 6.Moulton L.S., Evans P.A., Starks I., Smith T. Pre-operative education prior to elective hip arthroplasty surgery improves postoperative outcome. Int Orthop. 2015;39(8):1483. doi: 10.1007/s00264-015-2754-2. [DOI] [PubMed] [Google Scholar]
- 7.Kelmer G.C., Turcotte J.J., Dolle S.S., et al. Preoperative education for total joint arthroplasty: does reimbursement reduction threaten improved outcomes? J Arthroplasty. 2021;36(8):2651. doi: 10.1016/j.arth.2021.03.016. [DOI] [PubMed] [Google Scholar]
- 8.Inneh I.A., Clair A.J., Slover J.D., Iorio R. Disparities in discharge destination after lower extremity joint Arthroplasty: analysis of 7924 patients in an urban setting. J Arthroplasty. 2016;31(12):2700. doi: 10.1016/j.arth.2016.05.027. [DOI] [PubMed] [Google Scholar]
- 9.Keswani A., Lovy A.J., Robinson J., et al. Risk factors predict increased length of stay and readmission rates in Revision Joint arthroplasty. J Arthroplasty. 2016;31(3):603. doi: 10.1016/j.arth.2015.09.050. [DOI] [PubMed] [Google Scholar]
- 10.Amen T.B., Varady N.H., Rajaee S., Chen A.F. Persistent racial disparities in utilization rates and perioperative metrics in total joint arthroplasty in the U.S. J Bone Joint Surg Am. 2020;102(9):811. doi: 10.2106/JBJS.19.01194. [DOI] [PubMed] [Google Scholar]
- 11.Stone A.H., MacDonald J.H., Joshi M.S., King P.J. Differences in perioperative outcomes and complications between African American and white patients after total joint arthroplasty. J Arthroplasty. 2019;34(4):656. doi: 10.1016/j.arth.2018.12.032. [DOI] [PubMed] [Google Scholar]
- 12.Ali A., Lindstrand A., Sundberg M., Flivik G. Preoperative anxiety and depression correlate with dissatisfaction after total knee arthroplasty: a prospective longitudinal cohort study of 186 patients, with 4-year follow-up. J Arthroplasty. 2017;32(3):767. doi: 10.1016/j.arth.2016.08.033. [DOI] [PubMed] [Google Scholar]
- 13.Judge A., Arden N.K., Cooper C., et al. Predictors of outcomes of total knee replacement surgery. Rheumatology. 2012;51(10):1804. doi: 10.1093/rheumatology/kes075. [DOI] [PubMed] [Google Scholar]
- 14.Powell J.N., Jaiswal P.K., Khong H., Railton P., Smith C. Mental Health Status predicts outcome following total hip arthroplasty. Osteoarthritis Cartilage. 2017;25:S342. [Google Scholar]
- 15.Best M.J., Buller L.T., Klika A.K., Barsoum W.K. Outcomes following primary total hip or knee arthroplasty in substance misusers. J Arthroplasty. 2015;30(7):1137. doi: 10.1016/j.arth.2015.01.052. [DOI] [PubMed] [Google Scholar]
- 16.Pivec R., Issa K., Naziri Q., et al. Opioid use prior to total hip arthroplasty leads to worse clinical outcomes. Int Orthop. 2014;38(6):1159. doi: 10.1007/s00264-014-2298-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sing D.C., Barry J.J., Cheah J.W., Vail T.P., Hansen E.N. Long-acting opioid use independently predicts perioperative complication in total joint Arthroplasty. J Arthroplasty. 2016;31(9):170. doi: 10.1016/j.arth.2016.02.068. [DOI] [PubMed] [Google Scholar]
- 18.Hernandez N.M., Parry J.A., Mabry T.M., Taunton M.J. Patients at risk: preoperative opioid use affects opioid prescribing, refills, and outcomes after total Knee Arthroplasty. J Arthroplasty. 2018;33(7):S142. doi: 10.1016/j.arth.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Curtis G.L., Newman J.M., George J., et al. Perioperative outcomes and complications in patients with heart failure following total Knee Arthroplasty. J Arthroplasty. 2018;33(1):36. doi: 10.1016/j.arth.2017.07.043. [DOI] [PubMed] [Google Scholar]
- 20.Pomeroy E., Fenelon C., Murphy E.P., et al. A systematic review of total knee arthroplasty in neurologic conditions: survivorship, complications, and surgical considerations. J Arthroplasty. 2020;35(11):3383. doi: 10.1016/j.arth.2020.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Esch J. Medicare inpatient only list for 2022. MedicareFAQ. 2021. https://www.medicarefaq.com/blog/inpatient-only-list/ [accessed 24.01.22]
- 22.Howden C., Tross J. Fact sheet CMS issues hospital outpatient prospective payment system and Ambulatory Surgical Center Payment System and quality reporting programs changes for 2018 (CMS-1678-FC). CMS. 2017. https://www.cms.gov/newsroom/fact-sheets/cms-issues-hospital-outpatient-prospective-payment-system-and-ambulatory-surgical-center-payment [accessed 24.01.22]
- 23.Howden C., Tross J. Fact sheet CY 2020 Medicare Hospital outpatient prospective payment system and ambulatory surgical center payment system final rule (CMS-1717-FC). CMS. 2019. https://www.cms.gov/newsroom/fact-sheets/cy-2020-medicare-hospital-outpatient-prospective-payment-system-and-ambulatory-surgical-center-0 [accessed 24.01.22]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.