Abstract
Hirudo medicinalis, the medicinal leech, usually carries in its digestive tract a pure culture of Aeromonas veronii bv. sobria. Such specificity is unusual for digestive tracts that are normally colonized by a complex microbial consortium. Important questions for the symbiotic interaction and for the medical application after microvascular surgery are whether other bacteria can proliferate or at least persist in the digestive tract of H. medicinalis and what factors contribute to the reported specificity. Using a colonization assay, we were able to compare experimentally the ability of clinical isolates and of a symbiotic strain to colonize H. medicinalis. The symbiotic A. veronii bv. sobria strain proliferated well and persisted for at least 7 days inside the digestive tract. In contrast, the proliferation of Pseudomonas aeruginosa and Staphylococcus aureus was inhibited inside the animal compared to growth in the in vitro control, indicating that the ingested blood was modified within the digestive tract. However, both strains were able to persist in the digestive tract for at least 7 days. For an Escherichia coli strain, the viable counts decreased approximately 1,000-fold within 42 h. The decrease of viable E. coli could be prevented by interfering with the activation of the membrane-attack complex of the complement system that is present in blood. This suggests that the membrane-attack complex remained active inside H. medicinalis and prevented the proliferation of sensitive bacteria. Thus, antimicrobial properties of the ingested vertebrate blood contribute to the specificity of the A. veronii-H. medicinalis symbiosis, in addition to modifications of the blood inside the digestive tract of H. medicinalis.
The digestive tract of most animals is colonized by a complex microbial flora (9, 31, 38). For example, the human colon houses hundreds of different bacterial species (31), and certain blood-feeding animals, such as mosquitoes, carry a diverse microbial flora in their midgut (9). In contrast to this diversity, the digestive tract flora of Hirudo medicinalis, the medicinal leech, is very simple; most studies report the presence of a pure culture of an Aeromonas sp. (15). This simplicity suggests the presence of mechanisms that control the composition of digestive-tract flora and is of importance for the medical application of leeches.
The medicinal leech feeds on vertebrate blood and can consume in a single blood meal over six times its own body weight (23). The ingested blood is stored in the extensive crop of the digestive tract (39) that is colonized by Aeromonas veronii bv. sobria (16). The consistent isolation of a pure Aeromonas culture from the digestive tract prompted early investigators to consider these bacteria to be symbionts (6, 22). For the symbionts, several functions were proposed that would benefit the host, such as the digestion of the ingested blood (18), the provision of essential nutrients (39), and the prevention of other bacteria from colonizing the digestive tract (6, 44). While most studies reported only the presence of Aeromonas in the digestive tract (6, 7, 16, 21, 22), a few studies occasionally detected other bacteria (25, 33, 46). Wilde attributed these bacteria to feeding the animals contaminated blood prior to the dissections (46). Two more recent studies also reported the detection of other bacterial species and proposed that these bacteria may present an additional risk for the medical application of H. medicinalis (25, 33). While all of these studies concluded that the major isolate is Aeromonas, the degree of specificity was questioned on the basis of the isolation of non-aeromonads from the digestive tract. Due to the lack of quantitative data and the ease of contamination from the microbiota present on the skin, an experimental assessment of the specificity and the identification of the mechanisms involved is needed. Recently, we developed a colonization essay that allows us to determine the ability of individual strains to colonize the medicinal leech and that is sufficiently amendable to allow the identification of factors that may play a role in the apparent specificity (16). Two studies from the 1940s and 1950s provided some experimental evidence that the bacterial symbionts of H. medicinalis were able to suppress the growth of other bacteria in vitro (7) and to weaken the virulence of pathogens (44). Yet, to our knowledge, no factors that contribute to the specificity of this symbiosis have been reported.
Such specificity is not uncommon in symbioses where the bacteria are housed in specialized organs (4, 26) or are intracellular (1, 4, 40), but it is unusual for digestive tracts. In the medicinal leech, potential sources of bacteria, other than the symbionts, include the nutritious yolk that the juvenile leeches feed on and that has been shown to contain a mixture of bacteria (7), the skin of H. medicinalis which carries a consortium of bacteria (34), the substrate that the animals attach to, the skin of the animal that the leeches feed on, and possibly the ingested blood of a bacteremic host. Yet in the digestive tract, Aeromonas is the predominant bacterium.
The revival of the medical application of H. medicinalis (17, 32) and the associated risk of wound infections at the attachment site by the symbiotic digestive-tract flora (8, 11, 27, 45) have increased the need to assess the ability of pathogenic bacteria to colonize the leech's digestive tract (15, 16, 33). Medicinal leeches are used to relieve venous congestion after reconstructive or plastic surgery (17, 32). In cases with complications, the application of H. medicinalis greatly enhances the success rate of the recovery in a way that cannot be duplicated using pharmaceuticals (8, 17, 32, 39). However, without preemptive antibiotic treatment, up to 20% of the patients receiving leech treatment become infected with Aeromonas, the digestive-tract symbiont of H. medicinalis (8, 27). A potential problem that may be more difficult to treat than an Aeromonas infection is an infection by other, more pathogenic, bacteria that were transmitted during leech therapy. In order to be able to cause infections, these bacteria would need to be able to proliferate to a critical density or persist at a sufficient concentration inside the digestive tract for an extended period of time.
The recent development of a colonization assay allowed us to evaluate the ability of specific strains to colonize H. medicinalis (16). Using this assay, we demonstrated that Aeromonas hydrophila and A. veronii bv. sobria strains isolated from human feces could colonize H. medicinalis to a similar extent as a symbiotic strain (16). In the present study, we extended our investigation to non-aeromonads and tested three strains that had been isolated from the blood of patients (Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus). These three species are important causative agents of septicemia, a serious condition that could result from a wound infection. Our approach was to feed rifampin-resistant (Rifr) derivatives of these strains in a blood meal to H. medicinalis and to monitor the ability of these strains to colonize the leech. The growth of each strain inside the digestive tract of H. medicinalis (in vivo) was compared to the ability of the same strain to grow in a blood sample that was not fed to the animal (in vitro). We hypothesized that if a strain failed to proliferate inside the host there would be three sources of factors that could affect the bacterial growth and survival: the host, the symbiotic microbiota, and the ingested mammalian blood (15).
MATERIALS AND METHODS
Strains, media, and growth conditions.
The symbiotic test strain, HM21R, is a spontaneous Rifr mutant derived from HM21 (16). The strains HM21 and HM221 were identified as A. veronii bv. sobria and isolated from the crop of H. medicinalis (16). The non-aeromonad strains were blood isolates and obtained from the culture collection of the Institute for Infectious Diseases of the University of Berne (IMM 637156, an E. coli strain isolated from 58-year-old man with acute sepsis after an aortic valve replacement; IMM 638585, a P. aeruginosa strain from a 22-year-old man with a retropubic abscess after polytrauma; and IMM 637577, an S. aureus strain from a 48-year-old woman with agranulocytosis).
The spontaneous Rifr mutants were derived from the clinical strains by growing cultures overnight in Luria-Bertani (LB) broth, spreading 100 μl of the broth on LB agar plates containing rifampin (RIF) (100 μg/ml) and incubating the plates at 30°C overnight (30, 37). Several Rifr strains were streaked for isolation and examined for normal growth. Representative strains were named as follows: EcR1 derived from IMM 637156, PaR1 derived from IMM 638585, and SaR1 derived from IMM 637577. The bacteria were stored at −80°C and grown at 30°C on blood agar plates (4.4% [vol/vol] whole sheep blood) that were made as described previously (16). For the colonization experiments, the bacteria were cultured as described previously in LB medium at 30°C (16, 37).
Animals.
The medicinal leeches used in this study were farm-raised animals obtained from Ricarimpex (Eysines, France). The animals were starved for 4 months prior to delivery by the supplier and maintained without feeding in leech tanks (Biopharm, Dyfed, United Kingdom) containing tap water at room temperature (23°C). The water was changed weekly.
Colonization assay.
The colonization assay used in this study was identical to the assay we described previously (16). For each time point, at least three animals were used. The limit of detection was 10 CFU/ml.
The animals were fed heparinized sheep blood (25 U/ml; Sigma Chemical Co.) that had been stored for between 18 and 24 h at room temperature. Different concentrations of bacteria, either a high inoculum (2 × 104 CFU/ml) or low inoculum (2 × 102 CFU/ml), were added to the blood that had been preheated to 37°C. The animals were fed 5 ml of blood, and individual animals were incubated for 18, 72, or 162 h at room temperature (23°C). After incubation, the intraluminal fluid (the ingested blood) was collected from the crop of the digestive tract. The serially diluted samples were plated on LB-RIF agar plates and blood agar plates. This allowed the comparison of counts on LB-RIF agar plates where only the introduced Rifr test strain proliferated and on blood agar plates where the native symbiotic microbiota and the test strain were recovered. The number of doublings (g) for the Rifr test strain was determined using the total numbers of bacteria ingested (CFUT0) and those present at 18 h (CFUT18): g = (log CFUT18 − log CFUT0)/log 2.
Growth of strains under in vitro conditions.
In addition to the in vivo colonization assay, an in vitro assay was done. An aliquot of the blood sample was inoculated with the test strain and incubated in 1.5-ml microcentrifuge tubes for the same length of time and at the same temperature as the animals to determine the growth potential of each strain in blood. Growth of the bacteria in the in vitro controls was determined by plating serial dilutions on blood agar plates. For the heat-inactivated blood, the sample was heated to 56°C for 40 min and allowed to cool to 37°C before inoculation (29). For the in vitro competition assay, the blood was inoculated with a 1:1 mixture (200 CFU of each strain per ml; low inoculum) of the Rifs symbiotic isolate HM221 and the Rifr test strain, i.e., either PaR1 or SaR1.
Inhibition of the activation of the membrane-attack complex.
The activation of the membrane-attack complex of the complement was inhibited by interfering with the classical pathway of activation, the alternative pathway of activation, or both pathways. For these experiments, we used heparinized human blood (25 U/ml; Sigma Chemical Co.). Immediately after the blood was obtained, it was aliquoted into 1-ml fractions and pretreated for 40 min as follows: heat inactivated (heating the blood to 56°C [29]), EGTA treatment (20 mM EGTA [Sigma Chemical Co.] and 2 mM MgCl2 [12, 29]), EDTA treated (20 mM EDTA Sigma Chemical Co.] [29]), and inulin treated (inulin at 2 mg/ml [Sigma Chemical Co.] [29]). To all of the samples an equal volume was added (made up to equal 82 μl with saline solution [145 mM NaCl]). The E. coli strain was added at a concentration of 2 × 104 CFU/ml, and the samples were incubated at 23°C for 18 h. The mean concentration was determined from four samples using blood from two different donors.
Statistical analysis.
The data were analyzed using the program GraphPad Prism 2.01. The data were log transformed, and the two-sided Mann-Whitney test was performed to compare the concentration that each test strain reached in the intraluminal fluid to the concentration in the in vitro blood control. For the competition experiments, the concentrations reached by the Rifr test strain in the in vitro competition were compared to its concentration in the intraluminal fluid. For the inhibition of the activation of the membrane-attack complex, the mean concentrations of the treated samples were compared to the nontreated control. Significant differences (P < 0.05) are reported in the figures and in Table 1.
TABLE 1.
Inhibition of the activation of complement affects the growth of E. coli in vitro
| Parameter | Pretreatment of blooda with:
|
||||
|---|---|---|---|---|---|
| Heat | EGTA | EDTA | Inulin | None | |
| x̄ (CFU/ml)b | 320,000* | 12,000* | 150,000* | 35 | 42 |
| SD | 520,000 | 1,200 | 83,000 | 50 | 33 |
For 40 min, the blood was pretreated by heating the blood to 56°C (i), adding 20 mM EGTA and 2 mM MgCl2 (ii), adding 20 mM EDTA (iii), or adding 2 mg of inulin per ml (iv). An asterisk indicates there was a significant difference between the pretreated and untreated blood using the Mann-Whitney test (P < 0.05).
The mean concentration was determined after 18 h using four replicates.
RESULTS
Colonization of H. medicinalis by A. veronii bv. sobria.
In a previous study, the colonization dynamics of the symbiotic A. veronii bv. sobria strain HM21R were monitored for the initial 24 h after feeding (16). In the present study, we extended our observations to 162 h after feeding. Throughout this time, the symbiotic test strain was maintained at a similar mean concentration in the intraluminal fluid of the crop (x̄ = 5.8 × 107 to 1.5 × 108 CFU/ml). At 42 and 162 h, the concentrations that were reached inside the animal were 10-fold lower than the concentrations reached in the in vitro blood control (data not shown). The lower concentration may be due to the removal of water (30% of the ingested weight is lost within 12 h and up to 60% is lost within 6 days [23]), the removal of nutrients, the competition with the native microbiota for nutrients and space, the release of inhibitory substances (36), and/or the sampling error. At 162 h after feeding, the intraluminal concentration of the introduced test strain decreased in three out of four animals to a mean of 3.6 × 106 CFU/ml. Whether these changes are indicative of a downward trend will require sampling the animals at a later time point. These results demonstrate that A. veronii bv. sobria can survive for extended periods of time in blood and inside the crop of H. medicinalis.
Colonization of H. medicinalis by E. coli.
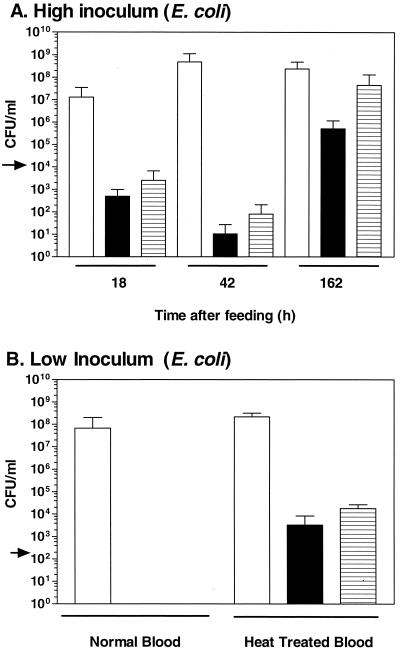
The concentration of colony-forming EcR1 decreased dramatically inside the animal and in the in vitro blood control (Fig. 1A). The animals consumed 105 CFU of EcR1 and by 42 h no EcR1 colonies could be detected in two out of three animals. By 162 h, EcR1 colonies were detectable in two out of four animals at a concentration of 106 CFU/ml, suggesting that the antimicrobial property that affected EcR1 had been inactivated over time or that EcR1 had become resistant to it. The native symbiotic microbiota reached concentrations of between 7.0 × 106 and 1.2 × 109 CFU/ml in the animals. In the in vitro blood control, a similar pattern was observed for EcR1. After an initial decrease in the concentration of colony-forming EcR1, a period of proliferation occurred (Fig. 1A). These results suggested that the antimicrobial property(ies) of the ingested blood affected the viability of EcR1 and remained active for some time within H. medicinalis.
FIG. 1.
Colonization of H. medicinalis by E. coli. (A) High inoculum. During the first 42 h after feeding, the concentration of EcR1 decreased inside the leech and in the in vitro control. The extent of the colonization by EcR1, an Rifr E. coli strain, was assessed 18, 42, or 162 h after inoculation. The blood was either fed to the animal or incubated in vitro. The arrow depicts the concentration of EcR1 in the inoculum. The concentration of the bacteria present in the intraluminal fluid of the digestive tract was determined (native flora and EcR1 recovered on blood agar [open bar] and EcR1 alone recovered on LB-RIF plates [solid bar]) and of EcR1 in the vitro blood control (hatched bar). (B) Low inoculum and heat treatment. Heat treatment of the blood permitted the proliferation of EcR1 inside the animal and in the in vitro blood control. The bacteria were either inoculated into untreated blood or heat-inactivated blood which was fed to the animal or incubated in vitro. The proliferation was assessed 18 h after inoculation. The error bars represent one standard deviation.
Interference with the activation of the membrane-attack complex.
Blood has numerous antimicrobial properties that act in concert to inhibit bacterial growth. An important property of blood that it is capable of inactivating high concentrations of bacteria is the membrane-attack complex of the complement system. The membrane-attack complex, C5b-9, inserts into the membrane of susceptible cells, renders the cells permeable, and thus inactivates them (42, 47). The formation of the membrane-attack complex can be achieved either by the classical pathway or by the alternative pathway (42, 47). Because compounds that inhibit the activation pathways have been better studied in humans, we used human blood for the following in vitro experiments. Heating blood to 56°C for at least 30 min and the addition of EDTA inhibit the activation of the membrane-attack complex by both the classical and alternative pathways (29). The addition of EGTA and MgCl2 inhibits only the activation by the classical pathway, whereas inulin inhibits the activation by the alternative pathway (12).
We determined the effect of these inhibitors on the proliferation of E. coli under in vitro conditions (Table 1). Prolonged heat treatment and the addition of either EDTA or EGTA plus MgCl2 inhibited the bacteriocidal properties of the blood that killed the E. coli strain. This suggests that the E. coli strain used in this study activated complement via the classical pathway and was killed by the membrane-attack complex.
Having established that the antimicrobial property of the blood that killed EcR1 could be inactivated by heating the blood in vitro, we examined whether heating the blood would allow EcR1 to proliferate in the intraluminal fluid. The inoculum was lowered to 200 CFU/ml, and the concentration of EcR1 was determined after 18 h. Using the untreated sheep blood, no CFU could be detected in any of the four animals tested nor in the in vitro blood control (Fig. 1B). However, in the heat-treated sheep blood, EcR1 proliferated to a concentration of 3.3 × 103 CFU/ml inside H. medicinalis and to 1.8 × 104 CFU/ml in the in vitro control. This indicates that a heat-sensitive antimicrobial property of the blood was responsible for killing EcR1 in vitro and inside the medicinal leech, suggesting that the membrane-attack complex remained active and functional within the crop of H. medicinalis, thus contributing to the specificity of the symbiosis.
Colonization of H. medicinalis by P. aeruginosa.
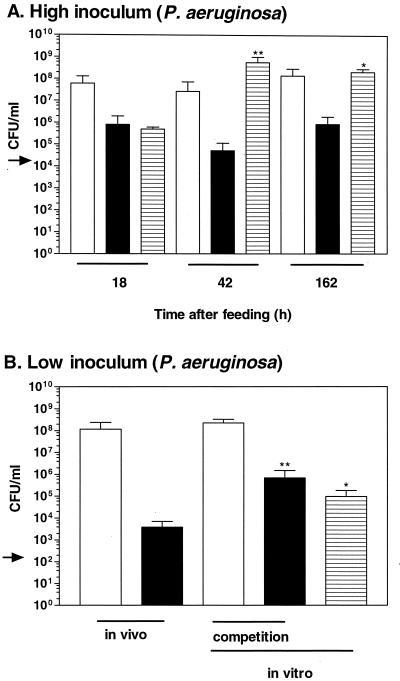
In contrast to the E. coli strain, the P. aeruginosa strain, PaR1, was able to proliferate in the crop of H. medicinalis (Fig. 2A). By 18 h, PaR1 had proliferated in the intraluminal fluid from the initial concentration (2 × 104 to 8.0 × 105 CFU/ml and remained at a similar concentration for at least 162 h (x̄ = 5.1 × 104 CFU/ml at 42 h and 8.6 × 105 CFU/ml at 162 h). The observed increase represented 3.4 doublings when calculated with the total number of bacteria. In the in vitro blood control, PaR1 reached a 10,000-fold-higher concentration (5.7 × 108 CFU/ml) at 42 h. For 42 and 162 h after feeding, the concentrations reached by PaR1 in the intraluminal fluid were significantly lower than those obtained in the in vitro blood control (Fig. 2A). These results indicate that PaR1 was unable to reach its growth potential inside the H. medicinalis and persisted at concentrations that were at least 100-fold lower than the concentration obtained by the native symbiotic flora.
FIG. 2.
Colonization of H. medicinalis by P. aeruginosa. (A) High inoculum. The P. aeruginosa strain was unable to replicate as well inside the animal as in the in vitro control. The extent of the colonization by PaR1, a Rifr P. aeruginosa strain, was assessed 18, 42, or 162 h after inoculation. The blood was fed to the animal or incubated in vitro. The arrow depicts the concentration of PaR1 in the inoculum. The concentration of the bacteria recovered from the intraluminal fluid of the digestive tract was determined (native flora and PaR1 recovered on blood agar [open bar] and PaR1 recovered on LB-RIF plates [solid bar]). The concentration of PaR1 recovered from the in vitro blood control is depicted with a hatched bar. (B) Low inoculum after 18 h of incubation. PaR1 was fed to the animal and incubated in vitro either alone or in competition with a symbiotic strain that was sensitive to RIF. For the competition experiment, the concentration of A. veronii strain and PaR1 recovered on blood agar is represented with an open bar and that of PaR1 recovered on LB-RIF plates is represented with a solid bar. ∗ and ∗∗, the mean concentrations differed significantly from the mean concentrations obtained in vivo at P < 0.05 and P < 0.005, respectively.
Under natural conditions, an inoculum of 2 × 104 CFU/ml is seldom encountered, and antimicrobial factors in blood can be overpowered by a large bacterial inoculum. Thus, we were interested in testing a lower inoculum (200 CFU/ml). By 18 h, PaR1 had doubled 2.7 times and reached a concentration of 3.8 × 103 CFU/ml in the intraluminal fluid. In the in vitro blood control, a significantly higher concentration of 9.5 × 104 CFU/ml was reached (Fig. 2B), indicating that the replication of PaR1 was inhibited inside the host. The number of doublings in vivo were similar to those achieved by PaR1 when inoculated at a higher initial concentration. PaR1 accounted for <0.002% of the total bacteria (native symbiont flora and PaR1) present in the intraluminal fluid of H. medicinalis.
Previous studies suggested that the symbiont of the medicinal leech could prevent other bacteria from proliferating when coinoculated in blood (7, 44). We evaluated this possibility by coinoculating the Rifr PaR1 with a symbiotic A. veronii bv. sobria strain HM221 that is sensitive to RIF. The two strains were inoculated at equal concentrations (200 CFU/ml each). In the competition experiment, PaR1 reached a similar concentration as when growing in the absence of the symbiotic strain, and this concentration was significantly higher than the concentration reached in vivo. In contrast to PaR1, HM221 reached a level similar to that obtained in the intraluminal fluid. No inhibition of PaR1 by the symbiotic strain was detected under these conditions.
Colonization of H. medicinalis by S. aureus.
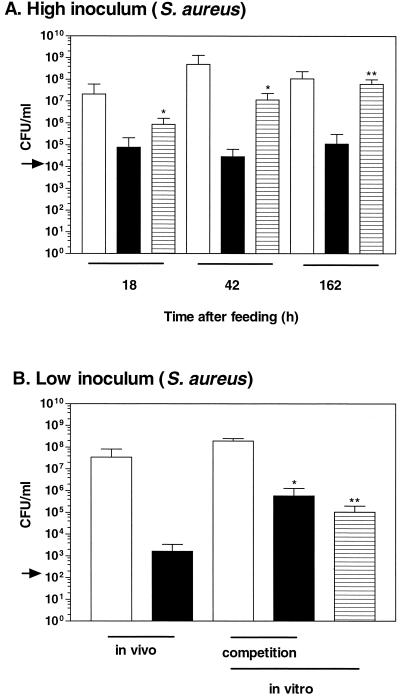
The S. aureus strain, SaR1, was unable to replicate well inside the leech but was able to persist in the crop of H. medicinalis (Fig. 3A). At 18 h after feeding, SaR1 reached a concentration of 105 CFU/ml in the intraluminal fluid (inoculum = 2 × 104 CFU/ml; Fig. 3A). However, in three of the four animals no increase in viable counts was detected. These results suggest that SaR1 was not able to proliferate well inside the intraluminal fluid, but SaR1 was able to persist at a similar concentration for at least 162 h. In contrast, in the in vitro blood control, the strain proliferated and reached significant higher concentrations at all three time points. These results show that the growth of S. aureus was restricted inside the crop of H. medicinalis. The concentrations reached by SaR1 inside H. medicinalis are 1,000-fold lower than those obtained by the symbiotic test strain.
FIG. 3.
Colonization of H. medicinalis by S. aureus. (A) High inoculum. The S. aureus strain was unable to replicate as well inside the animal as in the in vitro control. The extent of the colonization by SaR1, an Rifr S. aureus strain, was assessed 18, 42, or 162 h after inoculation. The blood was fed to the animal or incubated in vitro. The arrow depicts the concentration of SaR1 in the inoculum. The concentration of the bacteria recovered from the intraluminal fluid of the digestive tract was determined (native flora and SaR1 recovered on blood agar [open bar] and SaR1 recovered on LB-RIF plates [solid bar]). The concentration of SaR1 recovered from the in vitro blood control is depicted with a hatched bar. (B) Low Inoculum after 18 h of incubation. SaR1 was fed to the animal and incubated in vitro either alone or in competition with a symbiotic strain that was sensitive to RIF. For the competition experiment, the concentration of A. veronii strain and SaR1 recovered on blood agar is represented with an open bar and that of SaR1 recovered on LB-RIF plates is represented with a solid bar. ∗ and ∗∗, the mean concentrations differed significantly from the mean concentrations obtained in vivo at P < 0.05 and P < 0.005, respectively.
In the experiment using the lower inoculum, SaR1 doubled three times and reached an in vivo concentration (1.6 × 103 CFU/ml) that was significantly lower than the concentration reached in the in vitro blood control (Fig. 3B). This result further suggested that the growth of SaR1 is inhibited inside H. medicinalis.
In the in vitro competition assay, SaR1 reached a similar concentration in the presence or absence of HM221, indicating that under these conditions no inhibitory effect of HM221 could be detected (Fig. 3B). The concentration reached in the competition experiment was significantly higher than in the intraluminal fluid.
DISCUSSION
Previous studies that characterized the bacterial flora from the digestive tract of H. medicinalis suggested that this association was unusual because it consisted essentially of an Aeromonas monoculture (15). A few studies reported the occasional presence of other bacteria that were considered by some authors to be contaminants from the ingested blood (46) and suggested by others to be potential infectious agents during the medical application of leeches (33). The frequent detection of pure cultures of Aeromonas suggested a capability of H. medicinalis to control the composition of the microbial flora in its digestive tract, which is important for the understanding of the symbiosis (15, 43) and for the clinical application of leeches in modern medicine (8, 11, 27, 41, 45). We addressed this issue experimentally by comparing three clinical strains (E. coli, P. aeruginosa, and S. aureus) to a symbiotic strain (HM21R) that proliferated well and persisted at a concentration of ca. 108 CFU/ml for 162 h.
The three clinical strains revealed different colonization dynamics. For the E. coli strain, EcR1, the viable counts dropped dramatically both in the intraluminal fluid of the crop and in the in vitro blood control (Fig. 1A). The decrease in viable EcR1 could be prevented by heat treatment of the blood, the addition of EDTA, or EGTA with MgCl2, which interfere with the activation of the membrane-attack complex of the complement. This indicates that EcR1 was sensitive to the membrane-attack complex, and the in vivo data suggest that this complex remained active inside the animal for some time before becoming inactivated and permitting the growth of any surviving EcR1 cells. It remains possible that, in addition to the membrane-attack complex, cell-mediated processes, such as phagocytosis by macrophages, contribute to the decrease of EcR1. A previous study reported the long-term survival of E. coli in H. medicinalis (33). In that study, the crop of H. medicinalis was rinsed with saline prior to injecting LB medium containing 109 CFU of an E. coli strain (33); thus, no protective effect due to blood that would normally be ingested by the animal could have been detected. The membrane-attack complex appears to provide potent protection against sensitive bacteria inside the medicinal leech because when the animals were fed blood containing 105 CFU of EcR1 in 50% of the animals, no culturable EcR1 were detected by 162 h.
These results imply that the symbiotic strains need a mechanism to protect themselves against the membrane-attack complex. Previous studies have investigated the sensitivity of virulent A. hydrophila and A. veronii bv. sobria strains to complement-mediated killing (28, 29). The Aeromonas strains used in the studies by Merino et al. (28, 29) were shown to be resistant to complement-mediated killing due to the presence of an S layer that covers the lipopolysaccharides and apparently prevents the activation of complement. It will be important to determine whether the symbiotic Aeromonas strains also possess an S layer and whether the serum-sensitive mutants will be unable to colonize the medicinal leech.
Interestingly, by ingesting mammalian blood a parasitic invertebrate not only obtains nutrition but also antimicrobial compounds, such as the membrane-attack complex, that prevent sensitive bacteria from proliferating. The results from the high-inoculum experiment suggest that antimicrobial properties of the ingested blood play an important role in determining which bacteria can proliferate in the digestive tract of H. medicinalis. A similar protection by mammalian antimicrobial compounds has been shown in Ixodes ticks. When Ixodes ticks feed on mice that have been immunized with OspA or OspC from Borrelia burgdorferi, the causative agent of the lyme disease, the anti-OspA and anti-OspC antibodies of the mouse remain active inside the digestive tract of the tick, bind the bacteria, and inhibit their proliferation and migration to the salivary glands (10, 14). Thus, the utilization of antimicrobial properties present in vertebrate blood by blood-sucking parasites occurs in two very different phylogenetic groups.
In contrast to the E. coli strain, the P. aeruginosa strain, PaR1, and the S. aureus strain, SaR1, were able to persist in the intraluminal fluid of H. medicinalis for at least 162 h (Fig. 2A and 3A). However, both strains were significantly inhibited in their ability to proliferate in the intraluminal fluid compared to the in vitro blood control. Because the in vitro blood control and the ectothermic animals were incubated at the same temperature, the observed difference was not due to different incubation temperatures. Instead, the inability of these strains to reach their growth potential had to be due either to the presence of the symbionts or to a modification of the ingested blood by the host.
We were interested in testing in vitro whether the symbionts could inhibit PaR1 and SaR1. In competition experiments, the clinical isolates reached a similar concentration as when inoculated alone, and the concentrations were significantly higher than those obtained in the intraluminal fluid of H. medicinalis (Fig. 2B and 3B). At least under these laboratory conditions, the symbionts did not exhibit a negative effect on the two clinical isolates. A previous study from 1953 by Büsing et al. (7) reported a similar experiment in which S. aureus proliferated poorly when coinoculated with the symbionts in blood at 37°C. Temperature is an important factor in controlling gene expression (5, 20), and it remains possible that at 37°C an inhibitory effect could occur, but this would not be relevant to the symbiosis. It is also possible that the symbionts only express certain genes inside the host and not under laboratory conditions. Induction of some virulence genes is thought to occur only upon contact with host cells (2, 13, 19) and symbiotic Vibrio fischeri strains, isolated from the light organ of the Hawaiian squid Euprymna scolopes, are 1,000-fold more luminous inside the light organ than under culture conditions (3). Our study provides no direct evidence that the symbionts are responsible for suppressing the growth of PaR1 and SaR1. An additional control mechanism could be a modification of the ingested blood by the host in a manner that would prevent the proliferation of the clinical isolates but not of the symbiont.
Already during the process of ingesting the blood, the medicinal leech secretes a number of different compounds that dilate the blood vessels and prevent the coagulation of blood (35, 39). In blood recovered from the crop, two protease inhibitors have been detected, bdellin and eglin, and it has been proposed that they prevent the proliferation of the bacterial symbionts (36). While our results do not support the inhibition of the native symbiont population (16), it is possible that these protease inhibitors reduce the ability of the clinical isolates to proliferate. The medicinal leech concentrates the erythrocytes and possibly the antimicrobial factors present in the ingested blood. Most of the water is secreted, and the osmolyte concentration is reduced and changed in its composition (48). While these examples show various modifications of the ingested blood by the host, an effect of any of these modifications on bacterial proliferation remains to be demonstrated, and additional compounds are likely to be secreted by the host. The mechanisms that contribute to the species specificity in this symbiosis, as well as in other symbioses, are likely to be multilayered (43).
An important finding of the present study is that, while bacteria other than Aeromonas cannot proliferate well, they can persist in the crop of H. medicinalis, even when fed to the animal at concentrations of as low as 200 CFU/ml. This suggests that if an animal ingests bacteria that survive the antimicrobial properties of the ingested blood, these bacteria may persist for at least 1 week in the crop. The bacteria we tested did not replace the native symbiotic flora. As the intraluminal fluid is moved from the crop into the intestinum, digested, and defecated, bacteria that failed to proliferate will eventually be lost. If such contamination occurs under natural conditions, in leech farms, or in the laboratory this could explain the occasional isolation of other bacteria besides the symbionts. The inability of the clinical strains to proliferate to high levels and an extended starvation period prior to applying leeches to patients suggest that the risk of contracting infections by other bacterial pathogens besides the native symbiont is low.
In conclusion, we have shown that the membrane-attack complex of the complement system remains active inside the medicinal leech and can prevent sensitive bacteria from proliferating. Thus, in H. medicinalis, a protective mechanism of vertebrates contributes to the specificity of the digestive-tract symbiosis of the leech. While other nonsymbiotic bacteria remained viable in the crop of H. medicinalis for extended periods of time, the native symbiotic bacteria accounted for the vast majority of the bacterial flora and persisted at high concentrations. For the maintenance of the symbiotic relationship, the long-term presence of the symbionts is important because H. medicinalis feeds infrequently and can survive on a single blood meal for more than 6 months (39). In addition, the ingested blood was modified in a manner that restricted the growth of P. aeruginosa and S. aureus. Our future studies will aim to identify what modifications (by symbionts and/or the host) prevent the introduced bacteria from proliferating in the crop of H. medicinalis.
ACKNOWLEDGMENTS
We thank K. Schopfer and M. Täuber for supporting this project; K. Mühlemann, S. Leib, C. Aebi, and T. Bodmer for helpful discussions; R. Troller for excellent technical assistance; and E. Mirkin, S. Leib, and M. Täuber for helpful comments on the manuscript. We are grateful to T. Bodmer for the clinical isolates used in this study and members of the lab for donating blood.
The Institute for Infectious Diseases of the University of Berne provided financial support for this work.
REFERENCES
- 1.Baumann P, Baumann L, Lai C Y, Rouhbakhsh D, Moran N A, Clark M A. Genetics, physiology, and evolutionary relationships of the genus Buchnera: intracellular symbionts of aphids. Annu Rev Microbiol. 1995;49:55–94. doi: 10.1146/annurev.mi.49.100195.000415. [DOI] [PubMed] [Google Scholar]
- 2.Beltrametti F, Kresse A U, Guzman C A. Transcriptional regulation of the esp genes of enterohemorrhagic Escherichia coli. J Bacteriol. 1999;181:3409–3418. doi: 10.1128/jb.181.11.3409-3418.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boettcher K J, Ruby E G. Depressed light emission by symbiotic Vibrio fischeri of the sepiolid squid Euprymna scolopes. J Bacteriol. 1990;172:3701–3706. doi: 10.1128/jb.172.7.3701-3706.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchner P. Endosymbiosis of animals with plant microorganisms. New York, N.Y: Wiley Interscience; 1965. [Google Scholar]
- 5.Busch E M, Domann E, Chakraborty T. Molecular, cell biological, and ecological aspects of infection by Listeria monocytogenes. In: Rosenberg E, editor. Microbial ecology and infectious disease. Washington, D.C.: American Society for Microbiology; 1999. pp. 187–192. [Google Scholar]
- 6.Büsing K-H. Pseudomonas hirudinis, ein bakterieller Darmsymbiont des Blutegels (Hirudo officinalis) Zentbl Bakteriol. 1951;157:478–485. [PubMed] [Google Scholar]
- 7.Büsing K-H, Döll W, Freytag K. Die Bakterienflora der medizinischen Blutegel. Arch Mikrobiol. 1953;19:52–86. [PubMed] [Google Scholar]
- 8.de Chalain T M. Exploring the use of the medicinal leech: a clinical risk-benefit analysis. J Reconstr Microsurg. 1996;12:165–172. doi: 10.1055/s-2007-1006471. [DOI] [PubMed] [Google Scholar]
- 9.Demaio J, Pumpuni C B, Kent M, Beier J C. The midgut bacterial flora of wild Aedes triseriatus, Culex pipiens, and Psorophora columbiae mosquitoes. Am J Trop Med Hyg. 1996;54:219–223. doi: 10.4269/ajtmh.1996.54.219. [DOI] [PubMed] [Google Scholar]
- 10.de Silva A M, Telford III S R, Brunet L R, Barthold S W, Fikrig E. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J Exp Med. 1996;183:271–275. doi: 10.1084/jem.183.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fenollar F, Fournier P E, Legre R. Unusual case of Aeromonas sobria cellulitis associated with the use of leeches. Eur J Clin Microbiol Infect Dis. 1999;18:72–73. doi: 10.1007/s100960050232. [DOI] [PubMed] [Google Scholar]
- 12.Fine D P, Marney S R, Colley D G, Sergent J S, DesPrez R M. C3 shunt activation in human serum chelated with EGTA. J Immun. 1972;20:807–809. [PubMed] [Google Scholar]
- 13.Galan J E, Collmer A. Type III secretion machines: bacterial devices for protein delivery into host cells. Science. 1999;284:1322–1328. doi: 10.1126/science.284.5418.1322. [DOI] [PubMed] [Google Scholar]
- 14.Gilmore R D, Piesman J. Inhibition of Borrelia burgdorferi migration from the midgut to the salivary glands following feeding by ticks on OspC-immunized mice. Infect Immun. 2000;68:411–414. doi: 10.1128/iai.68.1.411-414.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graf J. The symbiosis of Aeromonas and Hirudo medicinalis, the medicinal leech. ASM News. 2000;66:147–153. [Google Scholar]
- 16.Graf J. The symbiosis of Aeromonas veronii biovar sobria and H. medicinalis, the medicinal leech: a novel model for digestive tract associations. Infect Immun. 1999;67:1–7. doi: 10.1128/iai.67.1.1-7.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henderson H P, Matti B, Laing A G, Morelli S, Sully L. Avulsion of the scalp treated by microvascular repair: the use of leeches for post-operative decongestion. Br J Plast Surg. 1983;36:235–239. doi: 10.1016/0007-1226(83)90099-1. [DOI] [PubMed] [Google Scholar]
- 18.Hornbostel H. Ueber die bakteriologischen Eigenschaften des Darmsymbionten beim medizinischen Blutegel (Hirudo officinalis) nebst Bemerkungen zur Symbiosefrage. Zentbl Bakteriol. 1942;148:36–47. [Google Scholar]
- 19.Hueck C J. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev. 1998;62:379–433. doi: 10.1128/mmbr.62.2.379-433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurme R, Rhen M. Temperature sensing in bacterial gene regulation—what it all boils down to. Mol Microbiol. 1998;30:1–6. doi: 10.1046/j.1365-2958.1998.01049.x. [DOI] [PubMed] [Google Scholar]
- 21.Jennings J B, van der Lande V M. Histochemical and bacteriological studies on digestion in nine species of leeches (Annelidia: Hirudinea) Biol Bull. 1967;33:166–183. [Google Scholar]
- 22.Lehmensick R. Ueber einen neuen bakteriellen Symbionten im Darm von Hirudo officinalis L. Zentbl Bakteriol. 1941;147:317–321. [Google Scholar]
- 23.Lent C M, Fliegner K H, Freedman E, Dickinson M H. Ingestive behavior and physiology of the medicinal leech. J Exp Biol. 1988;137:513–527. doi: 10.1242/jeb.137.1.513. [DOI] [PubMed] [Google Scholar]
- 24.Lopez A L, Pineda E, Garakian A, Cherry J D. Effect of heat inactivation of serum on Bordetella pertussis antibody determination by enzyme-linked immunosorbent assay. Diagn Microbiol Infect Dis. 1998;30:21–24. doi: 10.1016/s0732-8893(97)00189-2. [DOI] [PubMed] [Google Scholar]
- 25.Mackay D R, Manders E K, Saggers G C, Banducci D R, Prinsloo J, Klugman K. Aeromonas species isolated from medicinal leeches. Ann Plast Surg. 1999;42:275–279. doi: 10.1097/00000637-199903000-00008. [DOI] [PubMed] [Google Scholar]
- 26.McFall-Ngai M J, Ruby E G. Symbiont recognition and subsequent morphogenesis as early events in an animal-bacterial mutualism. Science. 1991;254:1491–1494. doi: 10.1126/science.1962208. [DOI] [PubMed] [Google Scholar]
- 27.Mercer N S, Beere D M, Bornemisza A J, Thomas P. Medical leeches as sources of wound infection. Br Med J. 1987;294:937. doi: 10.1136/bmj.294.6577.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merino S, Camprubi S, Tomas J M. The role of lipopolysaccharide in complement-killing of Aeromonas hydrophila strains of serotype O:34. J Gen Microbiol. 1991;137:1583–1590. doi: 10.1099/00221287-137-7-1583. [DOI] [PubMed] [Google Scholar]
- 29.Merino S, Rubires X, Aguilar A, Alberti S, Hernandez-Alles S, Benedi V J, Tomas J M. Mesophilic Aeromonas sp. serogroup O:11 resistance to complement-mediated killing. Infect Immun. 1996;64:5302–5309. doi: 10.1128/iai.64.12.5302-5309.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 31.Moore W E, Moore L H. Intestinal floras of populations that have a high risk of colon cancer. Appl Environ Microbiol. 1995;61:3202–3207. doi: 10.1128/aem.61.9.3202-3207.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mutimer K L, Banis J C, Upton J. Microsurgical reattachment of totally amputated ears. Plast Reconstr Surg. 1987;79:535–541. doi: 10.1097/00006534-198704000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Nehili M, Ilk C, Mehlhorn H, Ruhnau K, Dick W, Njayou M. Experiments on the possible role of leeches as vectors of animal and human pathogens: a light and electron microscopy study. Parasitol Res. 1994;80:277–290. doi: 10.1007/BF02351867. [DOI] [PubMed] [Google Scholar]
- 34.Nonomura H, Kato N, Ohno Y, Itokazu M, Matsunaga T, Watanabe K. Indigenous bacterial flora of medicinal leeches and their susceptibilities to 15 antimicrobial agents. J Med Microbiol. 1996;45:490–493. doi: 10.1099/00222615-45-6-490. [DOI] [PubMed] [Google Scholar]
- 35.Rigbi M, Levy H, Iraqi F, Teitelbaum M, Orevi M, Alajoutsijarvi A, Horovitz A, Galun R. The saliva of the medicinal leech Hirudo medicinalis. I. Biochemical characterization of the high molecular weight fraction. Comp Biochem Physiol B. 1987;87:567–573. doi: 10.1016/0305-0491(87)90053-8. [DOI] [PubMed] [Google Scholar]
- 36.Roters F-J, Zebe E. Protease inhibitors in the alimentary tract of the medicinal leech Hirudo medicinalis: in vivo and in vitro studies. J Comp Physiol. 1992;B:85–92. doi: 10.1007/BF00257940. [DOI] [PubMed] [Google Scholar]
- 37.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 38.Savage D C. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- 39.Sawyer R T. Leech biology and behavior. Oxford, England: Clarendon Press; 1986. [Google Scholar]
- 40.Schroder D, Deppisch H, Obermayer M, Krohne G, Stackebrandt E, Holldobler B, Goebel W, Gross R. Intracellular endosymbiotic bacteria of Camponotus species (carpenter ants): systematics, evolution and ultrastructural characterization. Mol Microbiol. 1996;21:479–489. doi: 10.1111/j.1365-2958.1996.tb02557.x. [DOI] [PubMed] [Google Scholar]
- 41.Snower D P, Ruef C, Kuritza A P, Edberg S C. Aeromonas hydrophila infection associated with the use of medicinal leeches. J Clin Microbiol. 1989;27:1421–1422. doi: 10.1128/jcm.27.6.1421-1422.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor P W. Bacterial resistance to complement. In: Roth J A, editor. Virulence mechanisms of bacterial pathogens. Washington, D.C.: American Society for Microbiology; 1988. pp. 107–120. [Google Scholar]
- 43.Visick K L, McFall-Ngai M J. An exclusive contract: specificity in the Vibrio fischeri-Euprymna scolopes partnership. J Bacteriol. 2000;182:1779–1787. doi: 10.1128/jb.182.7.1779-1787.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weiler P. Untersuchungen über antibiotische Wirkungen an Blutegeln, Blutegelbakterien und deren keimfreien Filtrat. Experientia. 1949;5:446–447. doi: 10.1007/BF02165257. [DOI] [PubMed] [Google Scholar]
- 45.Whitlock M R, PM O H, Sanders R, Morrow N C. The medicinal leech and its use in plastic surgery: a possible cause for infection. Br J Plast Surg. 1983;36:240–244. doi: 10.1016/0007-1226(83)90100-5. [DOI] [PubMed] [Google Scholar]
- 46.Wilde V. Untersuchungen zum Symbioseverhältnis zwischen Hirudo officinalis und Bakterien. Zool Anz. 1975;195:289–306. [Google Scholar]
- 47.Yancey M K. Host defenses and bacterial resistance. Antibiot Obstet Gynecol. 1992;19:413–434. [PubMed] [Google Scholar]
- 48.Zebe E, Roters F-J, Kaiping B. Metabolic changes in the medical leech Hirudo medicinalis following feeding. Comp Biochem Physiol. 1986;84A:49–55. [Google Scholar]