Abstract
Bacterial infection and the ever-increasing bacterial resistance have imposed severe threat to human health. And bacterial contamination could significantly menace the wound healing process. Considering the sophisticated wound healing process, novel strategies for skin tissue engineering are focused on the integration of bioactive ingredients, antibacterial agents included, into biomaterials with different morphologies to improve cell behaviors and promote wound healing. However, a comprehensive review on anti-bacterial wound dressing to enhance wound healing has not been reported. In this review, various antibacterial biomaterials as wound dressings will be discussed. Different kinds of antibacterial agents, including antibiotics, nanoparticles (metal and metallic oxides, light-induced antibacterial agents), cationic organic agents, and others, and their recent advances are summarized. Biomaterial selection and fabrication of biomaterials with different structures and forms, including films, hydrogel, electrospun nanofibers, sponge, foam and three-dimension (3D) printed scaffold for skin regeneration, are elaborated discussed. Current challenges and the future perspectives are presented in this multidisciplinary field. We envision that this review will provide a general insight to the elegant design and further refinement of wound dressing.
Keywords: Antibacterial activity, Wound healing, Wound dressing, Skin tissue engineering, Biomaterials
Graphical Abstract
Graphical Abstract
1. Introduction
Skin, as a general defense system, is the largest organ in human body, accounting for 15% of the body weight. It plays vital roles in protective, thermoregulatory, sensory and immunologic functions, and body fluid equilibrium as well. Skin consists of three orderly arranged layers: epidermis, dermis, and hypodermis (subcutaneous tissue), and each of the layers has its own functions in body. The disruption of the integrity or malfunction of the skin tissue is generally referred to as a wound [1], [2], [3]. Damage to skin, caused by traumas or burn injury, dramatically reduces the quality of life for patients [4]. Meanwhile, the long period of management required for chronic wounds, such as diabetic foot ulcers, pressure and venous leg ulcers, significantly increased the financial burden of healthcare. According to the retrospective analysis of the Medicare 5% data set for 2014, the chronic nonhealing wounds impact about 8.2 million (15%) Medicare beneficiaries. The medicare cost projections for all wounds ranged from $28.1 to $96.8 billion, and the outpatient costs ($9.9–$35.8 billion) were higher than inpatient costs ($5.0–$24.3 billion) [5]. Moreover, the diabetic foot ulcers (30.5%) have a comparable 5-year mortality ratio to cancer (31%), which has remained unchanged since 2007 [6]. Naturally, the design and development of biomaterials to accelerate the contraction of wounds is urgently demanded.
Wound healing is a dynamic and interactive process involving four phases: coagulation and hemostasis, inflammation, proliferation, and wound remodeling with scar tissue formation. The four phases mediating the wound closure process involves the interaction between multiple cell population, soluble mediators, cytokines and so forth [7,8], and the healing process is initiated immediately after the injury occurred [9]. At the early phases of acute or chronic wound healing, degranulation of platelets induced the release of platelet basic protein. And after proteolytic cleavage, it could release neutrophil attractant NAP-2 family of proteins, which provides a bolus dose of neutrophil chemoattractant activity to recruit and activate neutrophil leukocytes to destroy planktonic bacteria [10]. Moreover, the neutrophil response of the wound site needs to be rapid and sustained in order to effectively prevent the formation of biofilm. And the initiated neutrophil response from platelets has to be maintained by resident cells. And fibroblasts express several neutrophil chemoattractants very highly and secrete them in substantial quantities, which is a major source for these cytokines in skin. However, the chronic inflammation in the wound microenvironment caused by oxidative stress, impaired angiogenesis, increased expression of proinflammatory cytokines and bacterial infection, which might induce fibroblasts becoming senescent. And the senescence-like fibroblasts cannot recruit enough neutrophils to prevent the formation of biofilm, which is a self-produced polysaccharide matrix by bacteria [11,12]. It is worthy note that the biofilm could protect bacteria from pharmacological therapies and neutrophils, and in this case, the wound becomes chronic due to the prolonged inflammatory phase [8,13]. And the process of infection caused by harmful bacteria has been reviewed[14,15]. Overall, bacterial infection might cause the wound come into chronic inflammatory state, and the wound will become chronic or nonhealing if no effective strategies were treated [14,16,17]. Besides the prolonged wound healing process caused by bacterial contamination, it has been reported that about 75% of the mortality following burn injuries stems from infection [1]. Therefore, the timely application of effective antibacterial agents is of great importance for the promoted wound healing.
Considering the sophisticated wound healing process, an optimal wound dressing should be capable to remove multifaceted obstacles in healing process of skin wound. Except anti-bacterial property, an ideal dressing should be biocompatible and biodegradable, maintain the moisture, be permeable to oxygen, enable the exudate removal, prevent the wound from pathogens and mechanical irritation, improve cell behaviors and promote wound healing [18,19]. Different kinds of antibacterial agents including antibiotics, nanoparticles (metal and metallic oxides and light-induced antibacterial agents), cationic organic agents and others [20,21], are of great importance in defense against infection. The integration of those antimicrobials into different forms of wound dressings, such as film [22], hydrogel [23], electrospun scaffold [24], sponge and foams [25,26], have been developed to enhance antibacterial effect with controlled anti-infection behavior. The integrated dressings with specific morphology, composed of natural/synthetic polymers and bioactive molecules, could not only achieve controlled antibacterial treatment, but also stimulate extracellular matrix (ECM) to improve cellular behavior and promote wound healing [27], [28], [29], [30].
Although painstaking effort has been devoted to the design and development of effective wound dressing, there is still no dressing that tends to meet all the criterions required for accelerated wound closure [9]. Moreover, a review paper that concerns different forms of wound dressings with antimicrobial behavior has not been reported. In this review, we will discuss various antibacterial agents (antibiotics, antibacterial nanoparticles, cationic organic agents and others) impregnated into different formulations of dressing materials (films, hydrogels, electrospun scaffolds, sponges and foams) to accelerate wound healing, and the advantages and disadvantages of biomaterials with different morphology served as wound dressing will be discussed as well. Therefore, it will provide a general insight to the elegant design and further refinement of wound dressing.
2. Antibacterial agents
Bacterial infections have been ever-growing threats to human health. With the improvement of public hygiene and biological technology, various anti-infectious agents have emerged to effectively suppress bacteria contamination. Antibiotics have been extensively applied to fight against bacteria, and conquered many infections. However, the development of drug resistance due to the improper use of antibiotics remains tough challenges to public health. To prevent human beings from infections, drug-resistant bacteria included, a new generation of advanced antibacterial materials have been extensively studied. And the existence antibacterial materials could be classified into the following types: (I) antibiotics, (II) antibacterial nanoparticles, (III) cationic organic agents, (IV) others. Herein, the possible action mechanisms are described as well.
2.1. Antibiotics
Antibiotics have been frequently used in the treatment of pathogenic strains since the 1940s [31]. Due to the effective antibacterial therapies, various new antibiotics have been discovered by the sophisticated semi-synthesis and modification of natural molecules, which sheds new lights on the treatment of infected diseases. However, among the thousands of antibiotics that have been known, less than 1% are currently prescribed to patients with chronic wounds in clinics because of their toxicity to tissues [13,32]. And so far only beta-lactams, quinolones, aminoglycosides, glycopeptides, tetracyclines and sulphonamides have been applied to fabricate antibacterial biomaterials [13]. Traditional antibiotics act on intracellular targets to disrupt the function of bacteria structure or their metabolic pathways. The more detailed antibacterial mechanisms of antibiotics have been extensively reported [13]. Among the various bactericidal agents, antibiotics are the main agents for the treatment of infected diseases in clinical practice. Nevertheless, the evolution of various pathogens with antibiotic resistance and systemic poisoning are promoted because of its over-prescription and improper use [13,33]. Thus, the development of appropriate local delivery systems for antibiotics attracts lots of attention. Topical delivery could maintain higher antibiotic concentration for a long period in wound bed and avoid systematic toxicity [1]. Meanwhile, the assembled platforms with various morphologies could improve the antibacterial effect and reduce the abuse/misuse of antibiotics, while regulating the bacteriostatic activities, which makes antibiotic-based biomaterials powerful weapons to fight against infected diseases.
2.2. Nanoparticles as antibacterial agents
2.2.1. Metal and metallic oxide
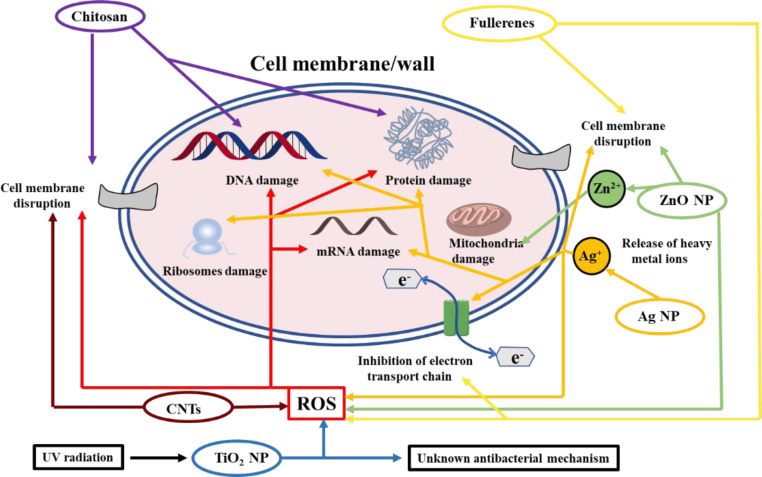
Silver, known as broad spectrum antimicrobial agent, exhibits strong activity against anaerobic, aerobic, Gram-positive and Gram-negative bacterial strains [34,35], and has been applied as a healing agent by civilizations worldwide for century. Its salt, silver nitrate and/or silver sulfadiazine, is frequently used in clinical practice. Nevertheless, the application of silver sulfadiazine might impair wound healing [36]. Silver nanoparticles (Ag NPs) as both bactericidal and antifungal agents have attracted much attention, and wound dressings based on Ag NPs, such as Actisorb and Acticoat, are commercialized [1]. One possible mechanism suggested for the bactericidal property of Ag NPs is as follows. Metallic silver is inactive and hardly absorbed by mammalian or bacterial cells, while the ionized silver could interact with the membrane proteins and increase the permeability of membrane. After penetration into cell, silver ions could inactivate vital enzymes and damage DNA, and the production of reactive oxygen species (ROS) impose oxidative stress on bacteria [1,34]. The antibacterial mechanism has been depicted in Fig. 1. However, the easy oxidation and the aggregation of Ag NPs in practical application could greatly reduce the bactericidal effect [37]. The combination of silver with functional biomaterials could address those issues and enhance the antibacterial effect. Moreover, Au nanoparticles (Au NPs), usually known as biologically inert, play vital roles in biomedical treatments, such as photothermal therapy (PTT) and antibacterial activities, which could attach onto the bacterial membrane and inactivate bacteria [38]. Au NPs with various size could be functionalized with desired polymer to form biocompatible biomaterials with multiple applications.
Fig. 1.
Antibacterial mechanism of chitosan, metal-based nanoparticles and carbon-based composites.
Metallic oxide nanoparticles, including zinc oxide (ZnO) and titanium dioxide (TiO2), have been applied as inorganic antibacterial materials, and the action mechanisms are presented in Fig. 1. Among various metallic oxides, ZnO nanoparticles have been widely applied as antimicrobial agents, which exhibits efficient bactericidal activity against Gram-positive and Gram-negative pathogens, as well as high-pressure resistant and heat-resistant bacteria [39]. Some reported antibacterial mechanisms are as follows: ZnO can bind to the cell membrane and induce increased membrane permeability and cell lysis. Meanwhile, it could produce ROS and inactivate pathogens [38]. Besides, the released zinc ions could promote the growth of fibroblast and the migration of keratinocytes, thus enhancing tissue repair during wound healing [40,41]. Moreover, there are several other metal and metallic oxide NPs (such as Ga, MgO, CuO, NiO, CeO2 and Sb2O3) have been demonstrated with bactericidal activity and have been well documented in the reported reviews[42], [43], [44], [45], including the extensively accepted antibacterial mechanism for mental and metallic oxide NPs. However, it is widely accepted that nanoparticles could penetrate biological membranes because of their small size, and concerns about the long-term safety of those nanoparticles, such as the toxicity caused by undesirable diffusion and degradation, and their influence on the immune system, during the treatment against infections have not been clarified [1,46]. Besides, the physical properties, including size, shape and nanostructure, also make a difference on the antibacterial effect [46]. Naturally, the constrained or topical application of those nanoparticles should be achieved with the integration of specific formulations.
2.2.2. Light-induced antibacterial nanoparticles
Nanotechnology and nanomaterials have obtained significant progress in providing innovative platforms to revolutionize antibiotic-free strategies [47], [48], [49], [50], [51], [52]. PPT is a safe and efficient strategy to fight against infections based on the heat generated by nanoagents under the irradiation of near-infrared (NIR) light (ranging from 700 to 1400 nm) [53]. NIR light exhibits deeper tissue penetration without obvious damage to normal tissues, which makes precise remote control of bactericidal treatment possible [54], [55], [56]. The antibacterial nanomaterials with the ability to convert NIR light to heat could be classified into the following four categories: carbon-based composites (i.e. graphene oxides, carbon nanotubes (CNTs) and fullerenes), noble metal nanomaterials (i.e. gold and silver), metal sulfide nanomaterials (i.e. molybdenum sulfide (MoS2) and copper sulfide (CuS)), and polymeric nanomaterials [46,57]. And several nanomaterials with special electronic structure including CNTs and fullerenes, show semi-conductivity and ROS generation properties, which could improve the antibacterial activity of those agents (Fig. 1) [58,59]. The photothermal effect of nanoagents (about 50 °C) could denature the protein in bacteria, which has been declared without bacterial resistance [31]. Although the feasible PTT has made great progress in clinical applications, the damage to normal tissues and relatively low efficiency remain tough challenges. Recent efforts have been devoted to the design of nanomaterials with localized or bacteria-specific PTT-based antibacterial applications [60], [61], [62].
Photodynamic therapy (PDT), as an alternative to antibiotics, is another promising precise remote strategy to treat persistent bacteria [63]. Photosensitizer (PS), light source, and oxygen are the three simultaneously present factors required in PDT [64]. The excited PS, under irradiation of light whose wavelength matches the maximum absorption of PS, could generate toxic ROS, including superoxides, singlet oxygen (1O2) and hydroxyl radicals. The generated ROS could destroy the cell membrane and DNA of pathogens [65]. The mechanism of ROS generated by PS has been reviewed extensively [31]. Most PS exhibit excellent bactericidal activity toward Gram-positive bacteria, but their activity is insufficient for Gram-negative bacteria due to the distinct membrane structure. Meanwhile, the application of PDT has been limited by the hydrophobicity of most PS and the side effects to healthy tissues. Therefore, various vehicles, such as polymer and nanomaterial, have been developed as PS delivery systems to improve the efficiency of PDT. Besides, there are several materials themselves, including inorganic and organic matrix, could produce ROS [66,67]. However, it is noteworthy that the concentration of ROS, which could be a contributing factor for cytotoxity, should be controlled in a therapeutic window for antibacterial treatment while ensuring the proliferation of cells.
2.3. Cationic organic agents
Cationic organic systems, including a series of natural and synthetic organic polymers that possess large amounts of positively charged groups, have the capacity to interact with and be imported into negatively charged bacterial cell membrane [31]. Moreover, some cationic organic chemicals are positively charged amphiphiles with cationic and hydrophobic moieties. The positively charged group could interact with the negatively charged lipid head groups in the outer surface of cell membrane, and the hydrophobic segments then promote the penetration and disruption of bacterial cell membrane, leading to the efflux of the cellular contents and bacterial death. Different from the antibacterial mechanism of antibiotics, the cationic organic amphiphiles exhibit broad-spectrum antibacterial activity and decreased pathogen resistance [33]. Due to the well-defined structure, biocompatibility and ease of synthesis, those cationic chemicals, including chitosan, antibacterial peptides, cationic polymers and others, have been extensively applied to design and develop intrinsic antimicrobial systems for wound recovery.
2.3.1. Chitosan
Chitosan, as a cationic polymer derived from chitin, is a liner polysaccharide composed of β (1–4) linked d-glucosamine with randomly located N -acetyl-d-glucosamine groups, which can significantly accelerate the re-epithelization [68]. Considering its biocompatibility and degradability, chitosan has attracted tremendous effort to design and develop various chitosan-based biomaterials with different structures for various applications [69,70]. The amino groups on chitosan are easily protonated and endows the polymer with intrinsic bactericidal effect (Fig. 1). Meanwhile, the FDA approved chitosan with hemostasis property has been widely reported as promising dressing materials for wound and burn treatments [71], and the commercial chitosan-based dressing has emerged. However, chitosan itself exhibits limited solubility and antibacterial property. After combined with other antibacterial agents, chitosan-based materials often exhibit synergistic antibacterial activities with improved specificity and enhanced efficiency [72]. Moreover, the facile modification of chitosan grants the materials with multiple functionalities [73], [74], [75], and quaternized chitosan has been extensively applied as biomedical material because of the improved solubility and antimicrobial activity [76], [77], [78]. Therefore, chitosan-based materials with elegant design show prosperous application in biomedical filed, especially in wound recovery.
2.3.2. Antibacterial peptides
Natural antimicrobial peptides (AMPs) play vital roles in the innate immune systems based on their anti-inflammatory and immunostimulatory activities. AMPs are mostly cationic amphiphilic compounds, and mainly target bacterial membranes or other targets instead of proteins as most antibiotics do, which makes AMPs promising candidates for treating resistance strains [64,[79], [80], [81], [82]. However, the application of natural AMPs is limited due to the high production costs and its environmental sensitivity. Inspired by natural AMPs, synthetic amphiphilic polymers, composed of cationic and hydrophobic residues, have been designed to assemble polymers as bioactive reagents delivery systems that exhibit inherent antibacterial property [83]. The well-designed synthetic AMPs with improved stability could achieve better targeting efficiency [84]. Nevertheless, more efforts are needed to achieve the controlled synthesis of AMPs with mass production to achieve the extensive application of AMPs.
2.3.3. Other cationic polymers
Cationic polymers consist of natural and synthetic organic polymers, whose networks possess numerous positively charged groups. The electrostatic interaction between the positively charged polymers and negatively charged bacterial cell membrane could effective inactivate the pathogens, and the cationic polymers have been applied to construct antibacterial biomaterials [85,86]. Cationic polyethyleneimine with linear or branched structures has been extensively applied as antimicrobial materials for wound healing [87], [88], [89]. Meanwhile, some positively charged monomers, such as 1-vinyl-3-butylimidazolium bromide [85,90] and sulfobetaine methacrylate [91], have been used to fabricate cationic polymers as dressings for wound healing. Due to the flexible synthesis of polymers, the emerging positively charged polymers will provide a great variety for the fabrication of antimicrobial materials as wound dressing. Although the cationic polymers exhibit high antibacterial efficiency without bacterial resistance, the complex fabrication and long-term toxicity of those materials should not be ignored.
2.4. Others
Materials with micro- or nanoscale structures commonly exhibit distinctive functions [92,93]. Among the various functionalities, the antimicrobial activity has magnified extensive interests in recent years. The unique physiochemical properties of patterned materials could induce the bursting of bacterial cell membrane through direct contact of materials and pathogens. It has been demonstrated that graphene oxide nanosheets could damage the lipid bilayer along the sheet edges [57]. Except for the unique morphology of nanomaterials, the cicada and dragonfly wings exhibit antimicrobial activity because of the nanoscale pillar structure on their surfaces. Inspired by the unique structure, some synthesized biomimetic surfaces with similar nanotopography have been applied for effective bacterial killing [94]. Moreover, the unique nano and micro-patterned morphology on lotus leaves endows the anti-biofouling surfaces with hydrophobicity and self-cleaning properties, which could repel bacterial adhesion and cell attachment. And the materials with anti-fouling surfaces have been applied in the treatment of wound healing [91,95]. The materials with patterned surfaces have shed new lights on the elegant design of novel wound dressings. However, the sophisticated preparation of the materials with patterned surface discourages their wide application as antibacterial agents.
Natural product extracts, containing of secondary metabolites, are precious medicinal sources for drug development in biomedical application [96]. Herbal extractions with integrative bioactive ingredients have been extensively applied in the treatment of wound healing since ancient times. Most of the extractions possess biocompatibility, anti-inflammatory, oxidation resistance, and could promote cell behaviors during wound contraction. Curcumin, a hydrophobic pigment obtained from the rhizome of the herb Curcuma longa, has powerful modulating effects on regeneration and functional reconstruction of skin tissue during wound healing [97]. Besides, many of other extracts, such as lawsone [98], berberine [99], ostholamide [96], aloe vera [100], gymnema sylvestre [101], tea tree oil [102] and so on, have been impregnated into different formulations as wound dressing to accelerate wound contraction. Meanwhile, the complex isolation and purification of those extractions still remains a challenge.
Inorganic biological materials, including bioactive glass (BG) and biological ceramics, with biocompatibility, biodegradability and tissue regeneration, has been extensively applied in bone tissue recovery. It has been reported that the monodispersed BG nanoparticles could accelerate chronic wound healing through promoting angiogenesis. Biological ceramics and BG with inherent antimicrobial properties show promising application in wound recovery [103,104]. Moreover, doped with copper or modified with AMPs could enhance the antibacterial activity [105,106].
Apart from the categories of antibacterial agents mentioned above, other antimicrobial agents such as honey [107], lysozyme [106], NO [108], elemental iodine [109], diclofenac [110], cationic betaine ester [111], chlorhexidine [112], bentonite [113], metal-organic frameworks [95], and other chemicals have been impregnated into different formulations for accelerated wound recovery. The healing properties of honey have been proved since time immemorial and it can promote wound healing while reducing scar formation, especially in burns wound management [114,115]. Many essential oils derived from natural products have been reported to show good antibacterial properties, such as eupatorium adenophorum essential oil [116], zataria multiflora essential oil [117] and salvia officinalis essential oil [118]. The possible antibacterial mechanism of essential oil has been reported in the existing review [13]. It has also been reported that eugenol [119], seaweed extract [120], grapefruit seed extract [121], thymol [122], cordycepin [123] and sanguinarine derived from Sanguinaria canadensis L. and Chelidonium majus L [124], exhibit good antibacterial activity after impregnated into proper platforms. NO has broad antibacterial properties by forming ROS that can interact with various bacterial proteins, DNA, and enzymes to cause bacterial cell death [125]. Moreover, iodine has been used in a variety of antibacterial detergents. Povidone iodine prepared with povidone as a polymer carrier [126,127] and iodine doped into titanium dioxide [128] have been reported with excellent antibacterial property.
3. Antibacterial biomaterial as wound dressing
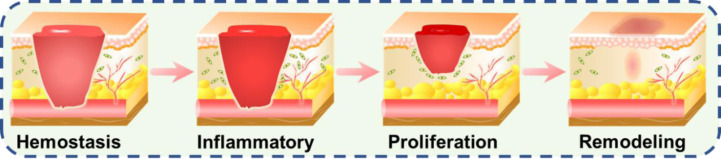
It is widely accepted that bacteria could inherently colonize on both biological and non-biological surfaces, an open wound included. At the beginning of chronic wound formation, Gram-positive bacteria, especially S. aureus, are dominant. Then Gram-negative species, such as E. coli and P. seudomonas, are detected and tend to invade deeper layers of skin causing significant tissue damage [13]. To mitigate the high risk of morbidity and mortality, labs around the world have devoted to developing antibacterial dressing to prevent contamination and accelerate wound healing [87,89,[129], [130], [131], [132], [133], [134]. Biomaterials with different forms, such as films, hydrogels, electrospun scaffolds, sponges and foams, made of synthetic or natural materials have been developed for the treatment of wounds, which could significantly promote the process of wound healing, including the four phases of coagulation and hemostasis, inflammation, proliferation, and wound remodeling with scar tissue formation (Fig. 2).
Fig. 2.
Schematic illustrations of wound healing phases consist of hemostasis, inflammatory, proliferation and wound remodeling with scar tissue formation. Reproduced from [135] with permission from American Chemical Society.
3.1. Films
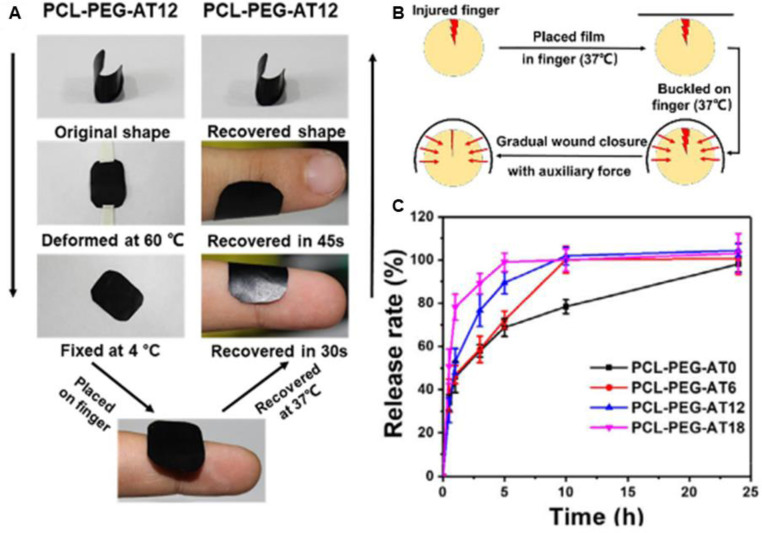
Films are thin and elastic polymers, which are often applied as semi-permeable dressings due to their ability to protect the wound from external environment, and to be shaped to fit different body configurations [136], [137], [138], [139]. The film dressings are commonly permeable to oxygen and moisture vapor while impermeable to bacterial. They have been developed to specifically control superficial lacerations and wounds that are mildly exudative, such as thin burn wounds, venous catheter sites, and donor sites for split thickness skin grafts, due to the limited absorptive capacity [140]. One added advantage is that the films made from functionalized biocompatible materials are excellent carriers for bioactive agents [65,112,[141], [142], [143], such as antibiotics [110,144,145], and nanoparticles [106,146]. A list of antibacterial films as wound dressing is presented in Table 1. Moreover, the loaded films afford easy sterilization and become pliable after hydration, without compromise to their mechanical strength [147]. Therefore, the design and development of novel film dressings show promising application in treatment of wound healing. Antibiotic, as potent bactericidal agents, has been incorporated into different formulas to promote wound healing through controlled release behavior. Our group has developed electroactive anti-oxidant polyurethane elastomers with shape memory property based on polycaprolactone (PCL), poly (ethylene glycol) (PEG), and aniline trimer (AT) (Fig. 3). The films, with a Tg close to human body temperature, show good shape memory capacity, which is beneficial for wound recovery through shape recovery-assisted closure of cracked wounds. The vancomycin loaded film exhibits a rapid release in the first hour and completely release in 24 h, which is beneficial for the anti-infection of wound, and it could significantly accelerate in vivo wound healing process [148]. Contardi et al. [149] fabricated a self-adherent transparent bilayer films, for the sequential delivery of antiseptic and ciprofloxacin, based on polyvinylpyrrolidone and hyaluronic acid. The in vitro release of antiseptic reached 100% within the first 24 h, while the release of ciprofloxacin sustained over a period of 5 d, and the sequential delivery of antimicrobials endows the films with effective antibacterial properties. The in vivo wound healing evaluation demonstrates that the bilayer films could be completely resorbed by the wound, most likely via integrating into the repaired tissue.
Table 1.
Antibacterial films for wound healing applications.
| Main component | Antibacterial agent | Bacteria | Wound model | Ref. |
|---|---|---|---|---|
| CS/ PVA | CS/gentamicin | E. coli, S. aureus | – | [150] |
| PVP/HA | Antiseptic/ciprofloxacin | E. coli, S. aureus | I | [149] |
| PCL/PEG/AT | Vancomycin | S. aureus | I | [148] |
| Carrageenan/ PEO | Streptomycin, diclofenac | E. coli, S. aureus, P. aeruginosa | – | [110] |
| Astaxanthin/ collagen | Gentamicin | – | I | [145] |
| CS/PEG/ZnO/Ag | CS/ZnO/Ag | E. coli | – | [151] |
| CNCs/CS/Cur | Ag/Cur/CS | – | I | [146] |
| LL37-SH peptide/collagen/Ag | LL37-SH / Ag | P. aeruginosa | – | [142] |
| PDMS/PNIPAM/Au | Au | E. coli, S. aureus, P. aeruginosa | II | [60] |
| PVA/ZnO/CuO/Au | Zn2+, Cu2+, Au | E. coli, S. aureus | III | [152] |
| Cu-BG/ESM | Cu−BG | E. coli | I | [106] |
| GO/CS/CaSiO3 | GO /CS | E. coli | III | [153] |
| CS/montmorillonite | Chlorhexidie/CS | S. aureus, P. aeruginosa | – | [112] |
| PVA/CS/ GO | GO/CS | E. coli, S. aureus | I | [143] |
| Cur/MPEG/CS | Cur/CS | – | I | [97] |
| CS/aloe/PLGA/Cur | CS/aloe/Cur | E. coli, S. aureus | I | [100] |
| CS/NO | CS/NO | S. aureus, P. aeruginosa | I | [108] |
| CS/PVP/BN | CS/BN | E. coli, S. aureus, P. aeruginosa | I | [113] |
| PU/gelatin/HA/bemiparin | AMPs | – | IV | [137] |
| Cornstarch/HA/EEP | EEP | E. coli, S. aureus, P. aeruginosa | I | [141] |
Fig. 3.
(A) The shape memory behavior displays in practical applications of PCL-PEG-AT12. (B) Illustration of wound recovery by applying PCL-PEG-AT12 film. (C) Release behavior of vancomycin from the copolymers at 37 °C in pH 7.4 PBS. PCL-PEG-ATx: PCL-PEG-AT with different weight ratio of AT. Reproduced from [148] with permission from Elsevier.
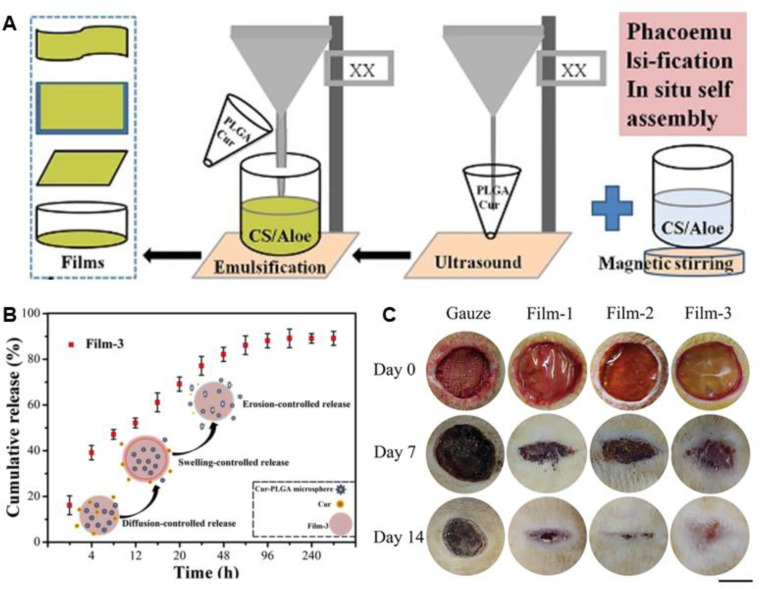
Chitosan (CS), as an antibacterial material, has been applied to prepare various dressings. Because of the insufficient bactericidal effect, CS is usually applied in combination with other agents [100,113,150,151,153]. Curcumin, as an antioxidant and anti-inflammatory agent, plays important roles in the regeneration and functional reconstruction of skin tissue. Li et al. [97] developed a curcumin loaded film based on CS and methoxy PEG, and the biocompatible film with antioxidation property could greatly increase the collagen synthesis and promote wound healing. Liu et al. [100] designed a CS/aloe film with curcumin-encapsulated poly(lactic-co-glycolic acid) (PLGA) microspheres incorporated for skin regeneration (Fig. 4). The encapsulation efficiency and loading efficiency of curcumin are 77.56% and 13.85%, respectively. Curcumin in the film shows a release profile with a moderated burst release followed by a sustained release (with a cumulative release amount of ∼89.20%). The bacterial colonies around the films were completely inhibited due to the sustained release of curcumin, the presence of CS and aloe. In vivo wound closure evaluation shows that the films could promote wound healing with a higher level of integrity and reduced inflammation as compared to other groups. As silver and its derivatives show broad spectrum of antimicrobial activity, Liu and Kim [151] prepared a genipin-crosslinked CS/PEG film, with various amounts of ZnO and Ag NPs loaded. The resulting film shows significantly enhanced antibacterial activity against Gram-negative and Gram-positive pathogens. Moreover, the promising therapeutic agent with antibacterial capacity, NO, could be incorporated into CS films to achieve controlled release of NO and potent bactericidal effect [108].
Fig. 4.
The design and application of curcumin-loaded PLGA microspheres incorporated CS/aloe films. (A) The illustration for the preparation of the films. (B) The release behavior of curcumin. (C) Images of full-thickness skin wound treated with different samples during the healing process. Film-1: CS/aloe films. Film-2: CS/aloe-PLGA microspheres films. Film-3: curcumin-loaded Film-2. Reproduced from [100] with permission from Elsevier.
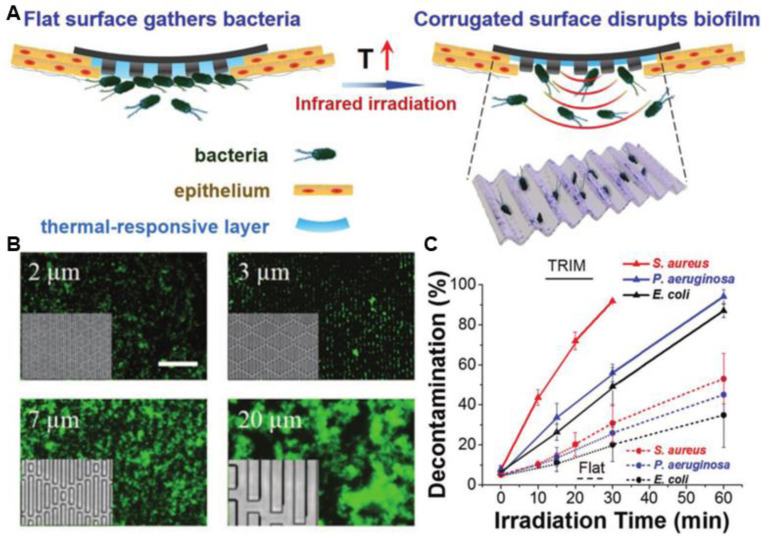
Photo-assisted bacteria killing has emerged as an effective strategy against pathogens even after drug resistance has been developed. Wang et al. [152] designed a PVA@ZnO@CuO@Au NPs film for combined antimicrobial phototherapies. Upon irradiation with 635 nm laser (2.0 W/cm2), Au NPs could induce efficient temperature increment, while enhancing the ROS generation by ZnO and CuO. The film has an antibacterial efficacy of 98.7% against E. coli and 97.5% against S. aureus after 10 min irradiation. The in vivo evaluation of diabetic wound healing turns out that the composite films could significantly accelerate the wound healing process. However, untargeted thermoablative approaches could cause injury to the surrounding normal tissues. To downscale the heat sources and reduce the side effects, Hu et al. [60] presented a thermal-disrupting interface induced mitigation (TRIM) bi-layer film based on a poly(dimethylsiloxane) (PDMS) substrate with patterned 3-µm wide microridges and microvalleys, and a thermal responsive poly(N-isopropylacryl-amide) (pNIPAM) hydrogel immobilized with gold nanostars (Au NS) (Fig. 5). Under moderate NIR irradiation (70 mW/cm2), pNIPAM shrinks and localizes the Au NS in the microvalleys, where planktonic bacteria preferentially attach to. The resulting film shows almost 100% decontamination efficiency with 30 min NIR irradiation, while minimizing the risk of host cells damage and reducing intercellular cohesion loss during PTT. The in vivo studies in S. aureus infected mice reveals that the films were effective at eliminating infection and promoting wound healing.
Fig. 5.
The topical thermal ablation of the TRIM films. (A) TRIM films with flat surface normally while reproducing corrugated microtopography during heating (via infrared-light irradiation). The corrugated surface would induce thermally activated disruption of the biofilm and facilitate the ablation of planktonic bacteria, while mitigating the thermal harm to host epithelium. (B) Fluorescence images show that S. aureus (green) attached within feature gaps when dispersed on films with different features. On surfaces with 5 µm and larger features, S. aureus formed aggregates. Inset: PDMS film with varied features. Scale bar: 50 µm. (C) Antibacterial efficiency of TRIM films or flat film. Reproduced from [60] with permission from WILEY-VCH. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. Hydrogels
Hydrogels, with three-dimensional porous structure, are commonly composed of natural/synthetic polymers through physical or chemical crosslinking. Much attention has been attracted to hydrogels as wound dressing because they exhibit excellent wound exudate absorptive property, moisture retention capacity, and oxygen permeability [154], [155], [156], [157], [158], [159], [160]. More importantly, the desired traits of hydrogels could be obtained through carefully selecting or modifying monomers and crosslinkers, and the highly inclusive hydrogel could be applied as carrier for various bioactive factors [161], [162], [163], [164], [165], [166], [167]. The design and development of intelligent responsive hydrogel with injectability further expanded the application of hydrogel dressing[12,135,[168], [169], [170], [171], [172], [173], [174], [175], [176], [177], [178], which is conducive for the treatment of wounds with an irregular shape. However, in addition to providing a moist environment during wound healing, the highly hydrated hydrogel matrix is also attractive for pathogens, and the risk of infection increased. Hydrogels with inherent antibacterial property [72,77,85,87,[179], [180], [181], [182], or loaded with bactericidal agent, such as antibiotics [168,[183], [184], [185], [186], [187], [188], metallic reagent [95,104,[189], [190], [191], [192], AMPs [83,193,194], photo-assisted antibacterial agents [195], [196], [197], [198], [199], or surfactant [200,201], have shown desired antimicrobial activity for promising application in chronic wound healing (Table 2). The rapid development of science and technology encourages the emerging of novel hydrogels as dressing to promote wound healing.
Table 2.
Antibacterial hydrogels for wound healing applications.
| Main component | Antibacterial agent | Bacteria | Wound model | Ref. |
|---|---|---|---|---|
| Alginate /bFGF | Rifamycin | S. aureus | I | [183] |
| PDMS/PEG/PEGDA | Gentamicin | S. aureus | II | [61] |
| GC/PDA | Ciprofloxacin/PDA (PTT) | E. coli, S. aureus | II | [202] |
| DLeu-Phe-Phe | Ciprofloxacin | E. coli, S. aureus | – | [184] |
| Cellulose/PDA | Tetracycline | E. coli, S. aureus | I | [185] |
| CEC/OHA-AT | Amoxicillin | E. coli, S. aureus | I | [168] |
| HA/PDA/chitin | Ag/gentamicin | E. coli, S. aureus, MRSA | II | [188] |
| GT-DA/CS/CNT-PDA | Doxycycline/CSCNT-PDA | E. coli, S. aureus | II | [186] |
| HA-DA/rGO/ PDA | Doxycycline/rGO/PDA (PTT) | S. aureus, P. aeruginosa | I | [187] |
| Gelatin/ODex | Chlorhexidine | E. coli, S. aureus | I | [201] |
| PAM/HPMC | Ag | E. coli, S. aureus | I | [131] |
| PDA/PVA/PANI | Ag | E. coli, S. aureus | III | [203] |
| CMC | Ag/Ag@AgCl/ZnO | E. coli, S. aureus | VI | [204] |
| PEP | Ag@rGO | E. coli, MRSA | II | [205] |
| SA/HS | HS (Zn2+) | E. coli | I | [104] |
| ZIF-8/PVA | Zn2+ | E. coli | II | [95] |
| FA/PDA | Quantum dots/ZnO/PDA (PTT) | E. coli, S. aureus | I | [40] |
| p(NIAAm/Am) | CuS | E. coli, S. aureus | I | [191] |
| PVA | Ag3PO4/MoS2 | S. aureus | II | [198] |
| PVA | CuS/MoS2 | E. coli, S. aureus | II | [199] |
| PVA/SA | Tb3+/rGO | S. aureus, P. aeruginosa | III | [206] |
| Agarose/TA/Fe (III) | TA/Fe (III) | E. coli | II | [196] |
| PLGA/bFGF/ CMCS | Sinoporphyrin sodium | S. aureus, MRSA | V | [197] |
| CS | BP (PDT) | E. coli, S. aureus | II | [195] |
| PEI-PEGMA/ PEGDMA | PEI-PEGMA | MRSA, CRPA, A. baumannii | I | [87] |
| CMMC/MCs | Quinone groups/ amino groups | E. coli, S. aureus,MRKO, ARSA, AREC | I | [182] |
| QCS/PF127CHO/Cur | QCS/Cur | E. coli, S. aureus | I | [72] |
| QCSP/PEGS-FA | QCSP/PEGS-FA | E. coli, S. aureus | I | [180] |
| CS/oxidized KGM | CS | E. coli, S. aureus | I | [181] |
| Modified HA | Halogenated HA | E. coli, S. aureus | II | [179] |
| PIL/PVA | PIL | E. coli, S. aureus, B. subtilis | I | [85] |
| PVP/ p(Am/VBIMBr) | VBIMBr | E. coli, S. aureus, C. albicans | I | [90] |
| PEA/AMPs | AMPs | E. coli, S. aureus | II | [83] |
| BCD/PDA/PAM | BCD | E. coli, S. aureus | II | [86] |
| QCS/PA@Fe | QCS/PA@Fe | E. coli, S. aureus, MRSA | II | [135] |
| SFMA-Ce6 | PDT(Ce6) | S. aureus | II | [174] |
| EGCG/PBA/PAM | EGCG | E. coli, S. aureus | III | [12] |
| PCEC-QAS | QAS | E. coli, MRSA | II | [173] |
| ODEX/HA-AMPs/PRP | AMPs | E. coli, S. aureus, P. aeruginosa | II | [82] |
| Arg-PEUU/ CS-GMA | Arg-PEUU/ CS-GMA | E. coli, S. aureus | – | [164] |
| BSA/KK/Ag+ | Ag+ | S. aureus | II | [166] |
| PGE/OHA/ MXene@PDA | PGE/ MXene@PDA | E. coli, S. aureus, MRSA | II | [165] |
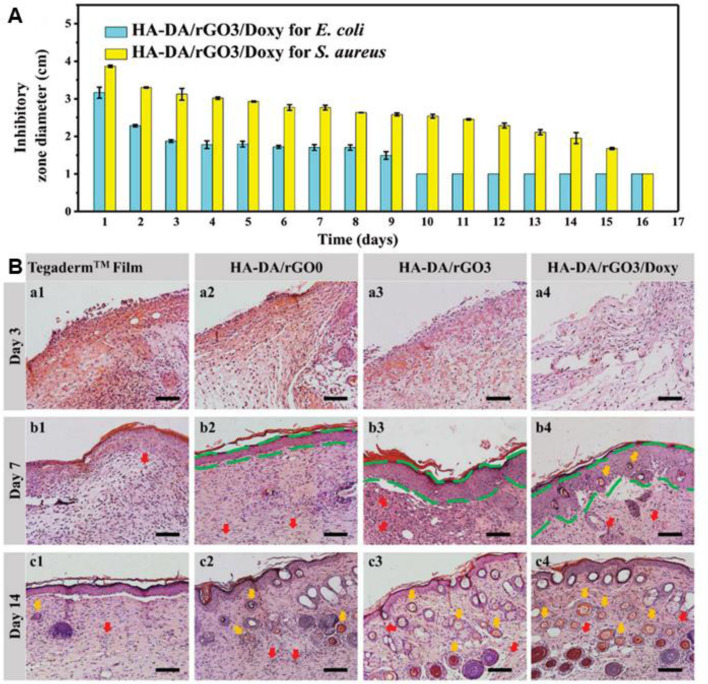
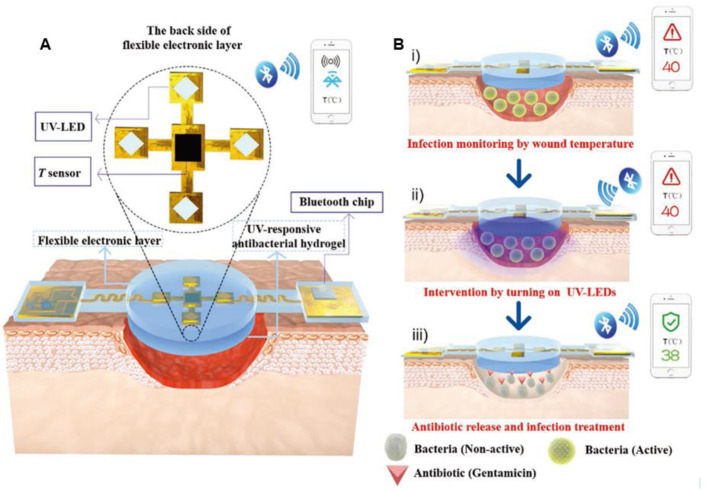
As commonly applied bactericidal agents, antibiotics play important roles in anti-infection. Topical delivery of antibiotics with a sustained release behavior is of significant importance in the treatment of chronic wound. Our lab has reported several conducting hydrogels to achieve the sustained release of antibiotics[161,186,187,207]. The antibacterial hydrogels based on hyaluronic acid-graft-dopamine and reduced graphene oxide achieved sustained release of doxycycline with zone of inhibition against E. coli and S. aureus over several days (Fig. 6). The in vivo evaluation demonstrated that the multifunctional hydrogel could enhance vascularization through upregulating the production of CD31, improve the granulation tissue thickness and collagen deposition. To achieve an on-demand therapy, Wu et al. [202] reported a NIR triggered on-demand drug delivery system based on ciprofloxacin (Cip) loaded polydopamine nanoparticles and glycol chitosan. The injectable hydrogel exhibits markedly enhanced release of Cip under NIR irradiation (0.5 W/cm2), and the composite hydrogel could effectively inactivate ∼99.88% S. aureus and ∼99.99% E. coli cells with NIR irradiation due to the synergistical effect of photothermal treatment and boosted antibiotics release, and the morphology of bacteria after different treatment was investigated. The in vivo wound healing was evaluated in the infected skin wound model of mice. The wound treated by the composite hydrogel with NIR irradiation almost disappeared on day 4, demonstrating the significantly accelerated wound healing process. To achieve real-time monitoring, point-of-care diagnosis and on-demand therapy, Pang et al. [61] designed a smart flexible electronics-integrated double-layer wound dressing. The upper layer is a PDMS network incorporated a thermal sensor and an ultraviolet light-emitting diode (UV-LED), and the lower layer is a gentamicin loaded hydrogel through an UV-cleavable linker. And the temperature of wound could be wirelessly transmitted to a portable terminal equipment by Bluetooth (Fig. 7). Once the temperature is higher than 40 °C for a certain period, the UV-LED would be turned on to trigger the release of antibiotics in situ to achieve on-demand dynamic intervention. The evaluation of the real-time monitoring and on-demand treatment of the integrated wound dressing in infected wound model reveals that the proof-of-concept study shed a new light on the diagnosing and treatment of infected wounds.
Fig. 6.
(A) Inhibitor zones from sterilizing effectiveness test against E. coli and S. aureus. (B) Histomorphological analysis for regeneration of wounds treated with different samples. Reproduced from [187] with permission from WILEY-VCH.
Fig. 7.
The design and working principles of the smart flexible electronics-integrated double-layer wound dressing. (A) The smart dressing consists of a PDMS-encapsulated flexible electronic layer and an UV-responsive antibacterial hydrogel. (B) Schematic illustration of the integrated dressing for infected-wound monitoring and on-demand treatment. Reproduced from [61] with permission from WILEY-VCH.
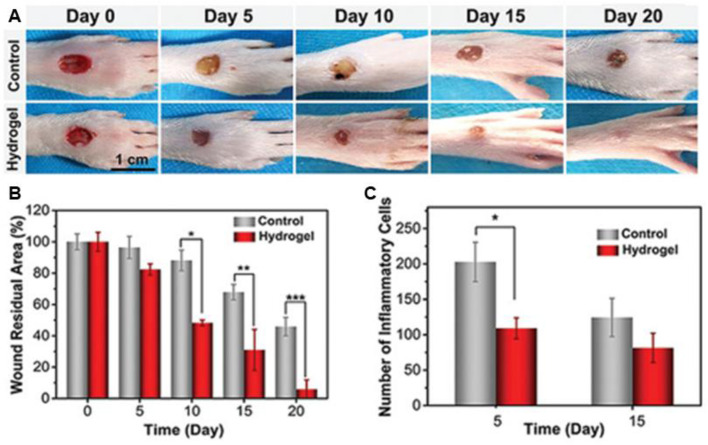
Nanocomposite hydrogel loaded with inorganic antibacterial materials, especially Ag NPs and ZnO NPs, could maintain potent antibacterial effect for a long period of time, which reduces the productions of superbugs [39]. Lin et al. [203] reported a conductive antibacterial hydrogel with polydopamine decorated Ag NPs (PDA@Ag NPs/CPHs) for epidermal sensors and diabetic foot wound dressing. The hydrogel exhibits broad antibacterial activity and significantly promotes the healing of diabetic foot wounds with an appropriate inflammatory environment conducive to wound recovery, compared with the control group (PBS) (Fig. 8). Block copolymer with determined constitutions could form micelle, and thermal-responsive irreversible gelation of the block polymers could be initiated through micelle packing induced by temperature enhancement, which is an effective strategy to prepare hydrogels in situ [173,205]. A sprayable in situ formed hydrogel based on reduced graphene oxide nanosheets decorated with Ag NPs is designed [205]. The dressing exhibits excellent antibacterial capacity and could obviously promote the healing of an infected skin defect. Moreover, a zeolitic imidazolate framework-8 contained composite hydrogel was fabricated based on PVA hydrogel membrane by microfluidic-emulsion-templating method [95]. The omniphobic dressings from intrinsic hydrophilic polymers with exquisite reentrant microstructures endows the material with antifouling property, and the hydrogel exhibits bactericidal and anti-inflammatory properties due to the released Zn2+ form the network. And the in vivo results demonstrated the enhanced healing efficacy for chronic wounds. Except for the inherent antibacterial property, the ROS generated in the photocatalytic activity of the nanomaterials has been reported as a potent bactericidal strategy. Wu et al. [204] designed a Ag/Ag@AgCl/ZnO nanostructure embedded carboxymethyl cellulose hydrogel in situ through a two-step process. Under visible light irradiation, the ZnO incorporated with Ag NPs increased the generation of ROS with highly effective antibacterial property due to the enhanced photocatalytic activity. And the released Ag+ and Zn2+ further promote the antibacterial effect. The hydrogel could inactivate 95.95% of E. coli and 98.49% of S. aureus after exposure to simulated sunlight for 20 min. The hydrogel could stimulate the immune function to produce a large number of white blood cells and neutrophils, and significantly accelerate the wound healing process.
Fig. 8.
The wound healing evaluation of PDA@Ag NPs/CPHs. (A) Photos of the diabetic feet treated with different samples (The control group is treated by pure carboxymethyl cellulose hydrogel). (B) Quantification of wound residual area. (C) Quantification of inflammatory cells. *P < 0.05, **P < 0.01, and ***P < 0.001. Reproduced from [203] with permission from WILEY-VCH.
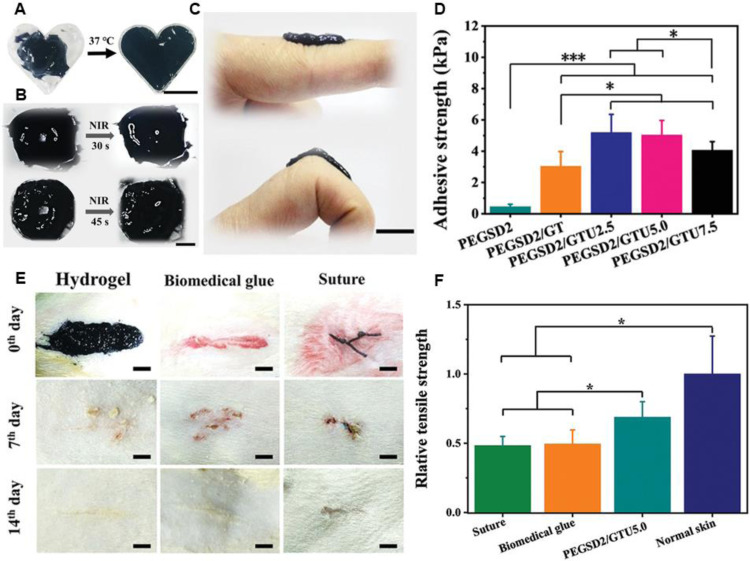
Phototherapy against infections is a minimally invasive strategy that could cause irreversible damage to pathogens through a photochemical reaction, which may not induce bacterial resistance. Our lab has reported an injectable and dissolvable antibacterial hydrogel dressings based on poly (glycerol sebacate)-co-poly (ethylene glycol)-g-catechol prepolymer (PEGSD) and UPy-hexamethylene diisocyanate (HDI) synthon modified gelatin (GTU). The catechol–Fe3+coordination between PEGSD and Fe3+ bestows the dressing with pH-responsiveness, photothermal capacity, tissue adhesiveness, and free radical scavenging property. Combined with the quadrupole hydrogen bonding of GTU, the physical double-network hydrogel possesses self-healing, shape adaptability and on demand acidic solution washing/NIR irradiation-assisted removal (Fig. 9). The hydrogel (PEGSD2/GTU5.0) could completely inactivate E. coli and S. aureus under NIR irradiation for more than 5 min (1.4 W/cm2). With adhesion strength of ∼5 KPa, The PEGSD2/GTU5.0 could seal the incisions and promote wound closure, and the healed skin exhibit higher mechanical strength compared with the surgical suture and medical glue groups [208]. Besides, hydrogel could be applied as an ideal delivery platform for PS. Wang et al. developed a smart hydrogel composed of porphyrin PS sinoporphyrin sodium and growth factor encapsulated PLGA nanospheres that embedded in the hybride network [197], which could achieve the controlled release of the bioactive ingredients. Under mild photoirradiation (30 J/cm2, 5 min), the resulting hydrogel could almost completely (higher than 99.9%) inactivate S. aureus and multidrug-resistant S. aureus in vitro. In an in vivo burn wound model, the hydrogel along with PDT accelerate the wound healing process with limited inflammation, higher collagen deposition, and rapid epithelialization. Due to the instability of organic PS, inorganic ingredients have attracted much attention. Wu et al. [195] fabricated a black phosphorus nanosheets embedded hybrid hydrogel via simple electrostatic interaction. Almost 95% pathogens could be killed by the hydrogel even after four times repeated illumination of visible light. Therefore, the inorganic PS loaded hydrogel could fulfill repeated antibacterial effect and promote wound healing.
Fig. 9.
Beneficial effects and application of the physical double-network hydrogel. (A) Shape-adaptive performance of PEGSD2/GTU5.0. Scale bar: 1 cm. (B) Photographs of hydrogel macroscopic self-healing performance when assisted with NIR irradiation (1.4 W cm−2). Scale bar: 1 cm. (C) The original state and withstanding joint bending of the soft and flexible hydrogel PEGSD2/GTU5.0. Scale bar: 1 cm. (D) Adhesive strength of the hydrogels on pig skin. (E) Representative photographs of rat full-thickness skin incision wounds at day 0, 7 and 14 post-surgery, scale bar: 5 mm. (F) Relative tensile strength of healed skins after treated for 14 d *P < 0.05 and ***P < 0.001. Reproduced from [208] with permission from WILEY-VCH.
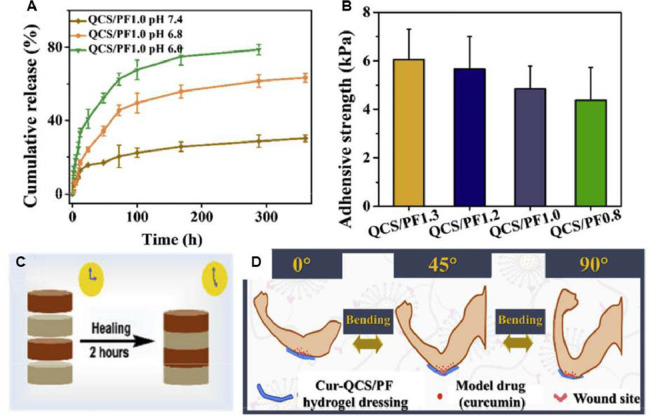
Hydrogels with inherent antibacterial activity have been developed as effective antibacterial reagents with minimal side effects [39]. Various ionic liquids and poly (ionic liquids) with excellent bactericidal activity against pathogens have been applied to prepare antibacterial dressing. Li et al. [90] fabricated antibacterial hydrogels through a facile approach by mixing PVA and the copolymer of 1-vinyl-3-butylimidazolium bromide and acrylamide, and the positive-charged poly(ionic liquid) endows the hydrogel with excellent antibacterial capacity. The in vivo results demonstrate that the hydrogel could effectively promote the wound healing process. Besides, antibacterial peptides, with strong antibacterial activities, are recognized as a possible strategy for the treatment of antibiotic-resistant pathogens. A biomimetic dopamine-modified-poly-l-lysine-PEG-based hydrogel was developed to prevent infection and promote wound healing [194]. Moreover, to further improve the antimicrobial activity and cell behavior during the healing process, our group loaded curcumin into quaternized chitosan and benzaldehyde-terminated Pluronic F127 [72]. The inherent antibacterial hydrogel from QCS exhibits pH-responsive release of curcumin, good wet adhesiveness on porcine skin (∼5 kPa) and fast self-healing ability to bear deformation of joints (Fig. 10). The formed injectable hydrogel exhibits robust bioadhesion performance and hemostatic property, and possesses antimicrobial activity against Gram-negative and Gram-positive bacteria, which shed a new light on the fabrication of antibacterial dressing for joints skin wound healing.
Fig. 10.
(A) In vitro release kinetics of curcumin from the hydrogels in PBS at pH values of 7.4, 6.8 and 6.0. (B) Adhesive strength of different hydrogels. (C) Scheme of the self-healing process. (D) Schematic diagram of model drug (curcumin) released from hydrogel when it was applied on the joints. Reproduced from [72] with permission from Elsevier.
3.3. Electrospun fibrous scaffolds
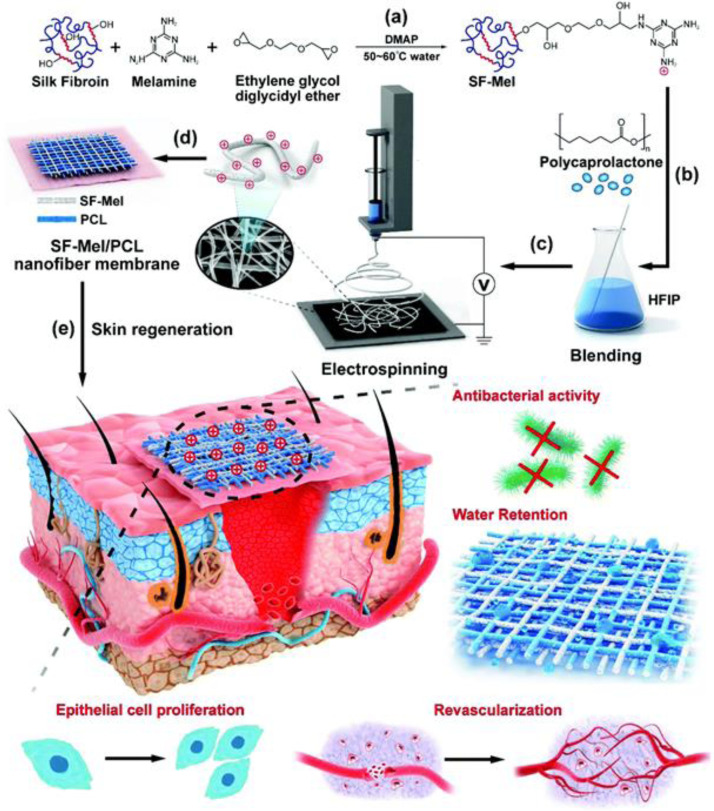
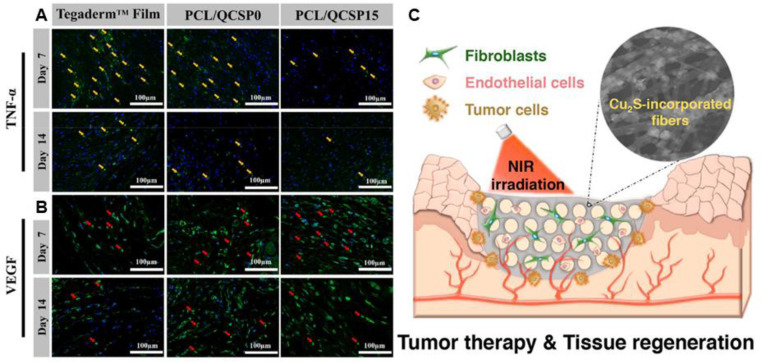
Wound healing scaffold with architecture similar to ECM can improve cell behavior and tissue regeneration [1,[210], [211], [212]. Electrospinning is known as a promising technique to produce fibers with diameters in the range of nanometers to micrometers. High voltages are applied to overcome the surface tension of polymer solution and generate aligned or random nanofibers mats [103,213,214]. Moreover, the morphology and properties of the fibers could be adjusted accompanied by the change of electrospinning parameters. The electrospun mats exhibit high specific surface area, high aspect ratio and high porosity, which endows the mats with enhanced exudate absorption and permeability for water and oxygen [101,215,216]. The inherent small pores enable the fibers to prevent the invasion of exogenous microorganisms, and several mats with inherent bactericidal activity have been reported. Nanofiber films composed of PCL and melamine-modified silk fibroin (SF–Mel/PCL) were fabricated via electrospinning for skin regeneration [209]. The films with a structure similar to that of ECM, exhibit broad-spectrum antibacterial activity against E. coli and S. aureus, and promoted epithelial cell proliferation and revascularization (Fig. 11). Guo et al. [217] prepared a series inherent antibacterial nanofibers through electrospinning poly(ε-caprolactone) and quaternized chitosan-graft-polyaniline polymer solutions. The fabricated mats with anti-oxidant and electroactive property, could reduce the production of TNF-α and upregulate expression of VEGF (Fig. 12A and 12B), and accelerate the healing of cutaneous skin wound. Meanwhile, antibacterial and bioactive agents can be facilely incorporated into the fibers in situ or through post-electrospinning modification to satisfy multiple biomedical application [218], [219], [220], and the methods have been discussed in previous reviews [221]. The release behavior of reagents could be controlled by the selection of polymer and electrospinning parameters. Antibacterial agents, such as antibiotics [91,[222], [223], [224], [225], [226], inorganic nanoparticles (Ag, ZnO, TiO2) [24,88,[227], [228], [229], [230], [231], herbs [96,98,99,105], AMPs [84,232,233], positively charged polymers [209,217,234], and PS [235,236], have been incorporated into nanofibers, which shows promising application in wound healing. Table 3 summarizes the reports on antibacterial electrospun scaffolds as wound dressing. The design and fabrication of various functional electrospun nanofibers inspired the innovation of wound dressing.
Fig. 11.
The electrospinning of SF–Mel/PCL nanofibers as wound dressing for skin repair and regeneration. Reproduced from [209] with permission from The Royal Society of Chemistry.
Fig. 12.
Pictures of the regenerated wound tissue on 7th, and 14th d by immunofluorescence staining labeling with (A) TNF-α (green) and (B) VEGF (green), yellow arrows show the expression of TNF-α and red arrows present VEGF. Reproduced from Ref. 217 with permission from Elsevier. (C) Schematic illustration of dressing with bifunctions of tumor therapy and skin tissue regeneration. Reproduced from [237] with permission from American Chemical Society. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 3.
Antibacterial electrospun scaffolds for wound healing applications.
| Main component | Antibacterial agent | Bacteria | Wound model | Ref. |
|---|---|---|---|---|
| p(NIPAAm-HMAAm) | Moxifloxacin hydrochloride | S. aureus | – | [222] |
| PCL/zwitterionic polymer | Tetracycline hydrochloride | E. coli, S. aureus | I | [91] |
| PVA/sodium alginate | Moxifloxacin hydrochloride | S. aureus, P. aeruginosa | I | [224] |
| PCL/gelatin | Ciprofloxacin hydrochloride | S. aureus | I | [226] |
| PCL/HMZS | Ciprofloxacin hydrochloride/Zn2+ | E. coli | II | [225] |
| PHB/gelatin/collagen | Ostholamide | E. coli, S. aureus, P. aeruginosa | I | [96] |
| Collagen/zein | Berberine | E. coli, S. aureus | I | [99] |
| PCL/gelatin | CaCuSi4O10/quercetin | S. aureus | VII | [105] |
| PCL/gelatin | lawsone | S. aureus, P. aeruginosa | I | [98] |
| PCL/gum tragacanth | Cur | MRSA | III | [238] |
| MADO | Ag NPs | E. coli, S. aureus, P. aeruginosa | VI | [227] |
| PCL/jellyfish protein | Ag NPs | E. coli, S. aureus | I | [228] |
| PCL/GO | Ag NPs/arginine/GO | E. coli, S. aureus | I | [230] |
| PCL/gelatin | Modified Au NPs | E. coli, S. aureus, P. aeruginosa | II | [239] |
| SA | ZnO | E. coli | – | [24] |
| CS/PVA | ZnO | E. coli, S. aureus, P. aeruginosa | III | [229] |
| HA/silk fibroin | ZnO | E. coli, S. aureus | VII | [231] |
| PVA/PF127/PEI | TiO2 NPs/PEI | E. coli, S. typhi, P. aeruginosa | – | [88] |
| PLA/PCL | Cu2S | – | I | [237] |
| PCL/QCSP | QCSP | E. coli, S. aureus | I | [217] |
| PCL/PMTA | PMTA | E. coli, S. aureus | – | [234] |
| PCL/melamine-modified fibroin | Melamine-modified fibroin | E. coli, S. aureus | I | [209] |
| PLA/PVP/PEG/Cur | AMPs/Cur | E. coli, S. aureus | I | [232] |
| Cellulose/PU | AMPs | S. aureus, P. aeruginosa | – | [84] |
| PEO/CS | Phthalocyanines | S. aureus | – | [235] |
| Zein/ethyl cellulose | Protoporphyrin | S. aureus, P. aeruginosa | I | [236] |
Through blend electrospinning, antibiotics could be facilely loaded into nanofibers [240]. Zhao et al. [91] fabricated a tetracycline hydrochloride loaded zwitterionic composite nanofibers mats through a gradational co-electrospinning method. The combination of hydrophobic PCL with the amino-modified hydrophilic zwitterionic polymer endows the membrane with anti-fouling performance, moisture retention, as well as the sustained release of the antibiotics with the intervention of halloysite (about 20 d). The diameters of the inhibition zone almost remained unchanged after 7 d of continuous incubation. The in vivo recovery of skin defects demonstrates that the prepared membrane could significantly promote the wound recovery. To grant the nanofibers with bactericidal effect against multidrug-resistant (MDR) pathogens, antibiotic intermediate capped Au NPs was embedded into the PCL/gelatin electrospun nanofibers [239], the scaffolds as wound dressing in MDR bacteria infected full-thickness wounds show promoted wound healing process. Besides enhanced bactericidal efficiency, bioactive reagents could also be incorporated into nanofibers to promote wound healing. The PCL nanofibers, impregnated with ciprofloxacin hydrochloride-loaded and zinc ions containing mesoporous silica nanospheres, show the ability to stimulate the behavior of cells, promoting angiogenesis and hair follicle regeneration. The small amount of zinc ions and antibiotics synergistically enhance the bactericidal efficiency [225]. The nanocomposite mats could significantly accelerate the process of infected wound healing. Moreover, on-demand infection therapy could be achieved by flexible electronic device, assembled from pNIPAM-based thermoresponsive electrospun mats with moxifloxacin hydrochloride-loaded, through a conductive pattern [222].
Herbal extracts have been extensively applied for the treatment of burns and wounds since ancient times [98,238]. Santhanakrishnan et al. [96] loaded ostholamide into polyhydroxybutyrate and gelatin nanofibers with a coating of collagen, which exhibits a structure similar to that of ECM, as well as excellent biocompatibility and biodegradability. The prepared nanofibers show high antibacterial efficiency, especially against E. coli and P. aeruginosa. Additionally, the scaffolds could promote the re-epithelialization of a full-thickness wound while reduce the inflammation. Curcumin, with anti-inflammatory, anti-oxidant and antibacterial properties, has been widely used to promote wound healing. The curcumin loaded nanofibers based on PCL and gum tragacanth were fabricated for wound healing in diabetic rats [238]. The resulting mats could inactivate 99.9% MRSA, increase collagen content, and effectively promote the wound recovery. In addition, inspired by Chinese herbal formulas, Chang et al. [105] prepared a PCL/gelatin electrospun fiber containing cuprorivaite and quercetin-copper chelates, which could stimulate hair follicle regeneration and promote wound healing.
With the rapid development of nanotechnology, various functional nanoparticles could be uniformly incorporated into nanofibers during electrospinning to enhance the efficiency of wound healing. Richter et al. [228] reported a novel nanofibers composed of jellyfish biomass and PCL, then Ag NPs was incorporated into the mats through in situ bio-template synthesis. The biocompatible nanofibers with core-shell structure could completely inhibit the growth of pathogens, and the porcine wound healing evaluation reveals the promoted wound healing with higher degree of wound epithelialization. Moreover, ZnO was loaded into crosslinked alginate electrospun nanofibers with PEO as a template [24]. The strontium crosslinked nanofibers, with cell adhesion and growth performance similar to commercial collagen membrane, exhibit significantly lower protein absorption and almost no adhesion of pathogens. The prepared mats with considerable mechanical strength and wettability show promising application as wound dressing. Meanwhile, PTT as a minimally invasive treatment has attracted much attention. Wu et al. [237] presented a Cu2S nanoflowers incorporated micropatterned nanocomposite membrane comprising of poly(D,L-lactic acid) and PCL for skin tumor therapy and wound healing (Fig. 12C). The uniformly embedded Cu2S nanoflowers in the nanofibers exhibit excellent photothermal effect and could inactivate 90% skin tumor cells, and the in vivo evaluation demonstrates that the mats could significantly suppress tumor growth and promote wound healing. The black scars caused by PTT left at the original sites after 8 d and the wound even completely healed without tumor recurrence within 14 d With the fast development of biology nanotechnology, nanofibers embedded with functionalized nanoparticles are immensely intriguing to researchers.
3.4. Sponges
Sponges, with thermal insulation, are capable of absorbing huge amounts of wound exudate due to the interconnected porous structure, which endows the materials with moisture-retention capacity. And porous structure could be tuned by the concentration, type and the degree of crosslinking of the polymers [241]. It has been reported that sponge with pore sizes between tens to hundreds microns in diameter and interconnected structure could increase tissue in growth [132]. The sponge dressing, with hydrophilicity and cell interaction, are commonly used as hemostatic agents and healing materials for burn wounds [76,147,242]. However, hindrances still exist in the practical application of sponge substitutes owning to the weak mechanical strength, the insufficient antibacterial property, and the maceration of wound. Besides, sponge dressings are unsuitable for severe burn wound (such as three-degree burn) or wounds with dry eschar [13]. A variety of systems based on sponge dressing have been developed to accelerate the process of wound healing. And a majority of the reported sponges is fabricated through a freeze-drying method based on chitosan. To enhance the antimicrobial property of sponge dressings, various bactericidal agents, including antibiotics [243,244], Ag and its derivatives [36,[245], [246], [247], [248], [249], Au/Ag nanoclusters (PDT) [250], ZnO [251,252], cationic polymers [253], [254], [255], [256] and others [257], [258], [259], were loaded into the networks (Table 4), and those materials show promising application in wound healing.
Table 4.
Antibacterial sponges for wound healing applications.
| Main component | Antibacterial agent | Bacteria | Wound model | Ref. |
|---|---|---|---|---|
| BC | Amoxicillin | E. coli, S. aureus, C. albicans | I | [243] |
| SA/GO/PVA | Norfloxacin/GO | E. coli, S. aureus | I | [244] |
| CS/stearic acid | Ag NPs/CS | E. coli, S. aureus, P. aeruginosa MRSA, DREC, DRPA | VI | [25] |
| L-GA/CS/HA | Ag NPs/CS | E. coli, S. aureus | I | [249] |
| KGM | Ag NPs | E. coli, S. aureus | I | [247] |
| CS | Ag NPs/ZnO/CS | E. coli, S. aureus, P. aeruginosa MRSA, DREC, DRPA | VI | [251] |
| Gelatin/alginate | Ag SD | – | I | [245] |
| PLL/PEG/PLL | Ag SD | P. aeruginosa, S. aureus | VIII | [246] |
| Collagen/EGF | Ag SD | – | VII | [36] |
| CS | Ag SD/CS |
E. coli, C. albicans S. aureus B. subtilis |
– | [248] |
| Gelatin/Au/Ag | Au ((PDT)/Ag | P. aeruginosa | – | |
| CS/PVA | CS | – | I | [254] |
| HBC/CS | HBC/CS | E. coli, S. aureus | I | [255] |
| CS/HA/AND | CS/AND | – | VII | [258] |
| CS/SA | CS/Cur | – | I | [259] |
| QCS NPs/CS/ stearic acid | QCS NPs/CS | E. coli, S. aureus | III | [260] |
| CNFs/QC/OREC | QC/OREC | E. coli, S. aureus | I | [257] |
| QHEC/MCF | QHEC | E. coli, S. aureus | I | [253] |
| Pullulan/CMCS | CMCS | – | I | [256] |
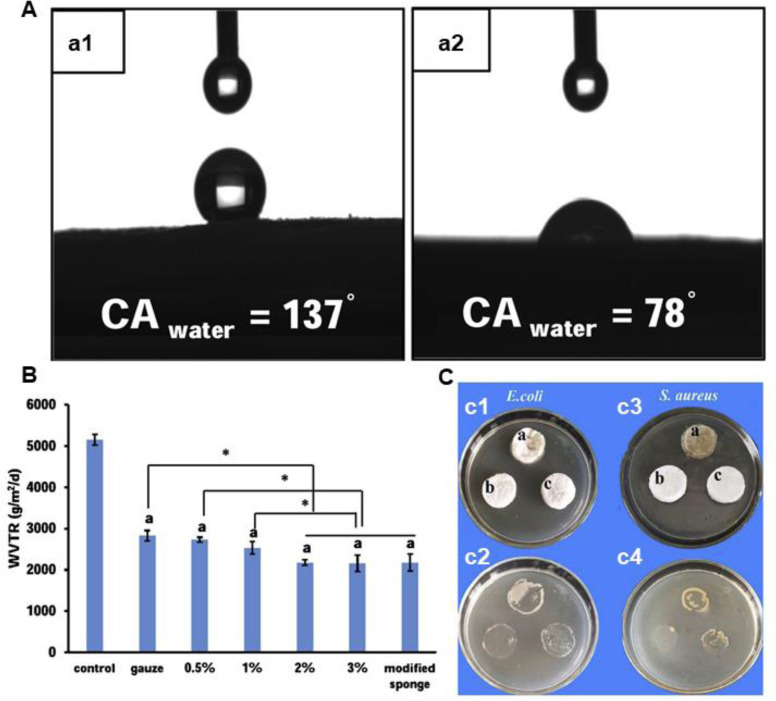
Polysaccharide, especially chitosan, has been commonly applied to fabricate sponge dressings through a freeze-drying method. Other antibacterial agents were often assembled into CS to improve bactericidal effect due to its limited anti-infective activity [261]. Our group reported a polydopamine crosslinked chitosan sponge with antioxidant activity and high hemostasis efficiency for lethal noncompressible/coagulopathy hemorrhage. The sponge exhibits outstanding photothermal antibacterial properties due to the existence of PDA. And the resulting sponge, showed much better wound closure effect than both chitosan sponge and Tegaderm™ dressing by reducing inflammatory infiltration, promoting vascularization and recruiting cells [262]. Moreover, a chitosan sponge containing ZnO/N-halamine hybrid nanoparticles was produced via a simple vacuum freeze-drying procedure (Fig. 13). The optimal sponge could inactivate 99.93% S. aureus and 88.01% E. coli, and exhibit much stronger hemostatic ability with a blood clotting index of 5% [252]. To improve the solubility and antibacterial property of chitosan, Xia et al. [260] presented a composite sponge based on quaternary ammonium chitosan nanoparticles (TMC NPs) and CS with hydrophobic stearic acid surfaces, and the hydrophobic surface endows the dressing with waterproof, infiltration and adhesion resistance for bacteria, and enhanced water retention time (Fig. 14). The CS sponge shows excellent antibacterial property (> 99.9%) and accelerated complete diabetic full-thickness wound healing. With broad spectrum of antibacterial activity, Ag NPs has been widely applied to fabricate antibacterial materials. Chen et al. [25] assembled Ag NPs into lyophilized CS sponge, then stearic acid was asymmetrically modified onto one side of the sponge. After soaked into 0.2 mmol/l Ag NPs solution, the sponge exhibits obvious toxicity toward both drug-sensitive and drug-resistant pathogens. The in vivo wound healing evaluation in mice with partial thickness wound indicates that the composite sponge could promote the wound healing, accelerate the re-epithelialization and collagen deposition with the lowest silver accumulation. Gelatin-based sponge with moisture-retention capacity could absorb wound exudate, improve the adhesion and proliferation of cells during wound healing process. A polydopamine crosslinked gelatin sponge, with excellent hemostatic property, achieved promoted wound healing [263]. The designed sponge with interconnected microporous structure could encapsulate antibiotics by reswelling, which endows the sponge with excellent antibacterial activity with the assistance of photothermal effect of PDA. Meanwhile, collagen, as the majority component of extra cellular matrix, has been widely applied in wound dressing. Lee et al. [36] prepared a collagen based sponge with epidermal growth factors and silver sulfadiazine (Ag SD) incorporated, the wound healing was evaluated in a partial thickness burn mouse model. It turns out that the composite sponge could reverse the impairment of wound closure caused by Ag SD. Due to the weak mechanical strength of natural polymers, synthetic polymers are applied to prepare sponge materials. Cho et al. [246] reported a triblock polymer sponge composed of poly(L-leucine) /PEG/poly(L-leucine), and Ag SD (50 µg/cm2) was incorporated into the sponge. The sustained release of Ag SD endows the sponge with effective antibacterial property, and the sponge could promote the healing of a full-thickness burn wound.
Fig. 13.
Schematic illustration of preparation and biomedical application of the chitosan dressing containing ZnO/N-halamine hybride nanoparticles. Reproduced from [252] with permission from American Chemical Society.
Fig. 14.
Beneficial effects of asymmetric CS sponge. (A) Water contact angles of hydrophobic surface (a1) and hydrophilic surface (a2) of CS sponge, respectively. (B) The water vapor transmission rate of different materials (including sponges with 0.5%, 1%, 2% and 3% CS (w/v)). *P< 0.05. (C) Bacteria infiltration activity of CS (a), modified TMC NPs/CS (b) and modified CS (c) sponges against E. coli ((c1) and (c2)) and S. aureus ((c3) and (c4)). Reproduced from [260] with permission from Elsevier. Elsevier.
3.5. Foams
Foams are protective, highly absorbent, insulating and could conform to body surfaces [276]. Foam dressings, composed of polyurethane or silicone-based materials, are semipermeable and either hydrophilic or hydrophobic with a bacterial barrier [277]. As dressings similar to sponges, foam dressings, as another type of moisture-retentive material fabricated to accommodate fluid while adding bulk and cushion to the wound bed [278], possess porous structure to enable the penetration of oxygen and water, allow the absorption for moderate to high levels of wound exudates, and prevent microorganism invasion. More important, foams, with large filling capabilities and rapid clotting of shape memory properties, are padded to protect wounds and maintain a moisture environment in treatment for deep wounds, while unsuitable for dry wounds as they might induce skin maceration [109]. Besides, foam dressings are frequently nonadherent and a secondary dressing as a bolster to prevent shifting are needed, and the regenerated tissue might grow into the foam due to the infrequent dressing change, which could cause second damage to the tissue [276]. Considering the advantages and application of foam dressings, more efforts are needed to improve the performance of it.
The most commonly used polyurethanes foam dressing is fabricated through foaming process. To accelerate the wound recovery, antimicrobial agents, such as antibiotics [264,265], silver [266], ZnO [268,269], cationic polymers [270,271], herbal extractions [272], BG [272] and others [273], [274], [275], as well as bioactive molecules [267], are incorporated into the porous foams to bestow the dressings with improved antibacterial property, cell proliferation and migration. Meanwhile, many methods of foam preparation, such as phase separation/emulsion freeze drying, gas foaming and solvent casting, have been developed to fabricate novel foam dressings to meet the criteria of wound closure [268]. A list of different antibacterial foams is presented in Table 5. Those functional foams show prosperous application in hemostasis and wound repair.
Table 5.
Antibacterial foams for wound healing applications.
| Main component | Antibacterial agent | Bacteria | Wound model | Ref. |
|---|---|---|---|---|
| CS/modified CS | Chloramphenicol/CS | E. coli, S. aureus, P. aeruginosa | – | [264] |
| PNIPAM/PAA/ PEGDA | Ciprofloxacin or tetracycline | S. aureus | – | [265] |
| AgHAP/PU | AgHAP | E. coli, S. aureus, P. aeruginosa | II | [266] |
| PU/rhEGF | Ag NPs | E. coli, S. aureus | II | [267] |
| BC | ZnO | E. coli | – | [268] |
| PU | ZnO | E. coli, S. aureus, P. aeruginosa | – | [269] |
| Cationic PU | Cationic PU | E. coli, S. aureus, P. aeruginosa | II | [270] |
| pDADMAC modified PU | pDADMAC | A. baumannii, S. aureus, P. aeruginosa | – | [271] |
| Silk fibroin protein | Herbal extraction | – | III | [102] |
| MC/MH/BG | MH/BG | E. coli, S. aureus | – | [272] |
| PU | Propolis | E. coli, S. aureus | I | [273] |
| UA/PANI/PU | UA | E. coli, S. aureus | – | [274] |
| PEGDA/NVP/PU | PVP-I2 | S. aureus | – | [109] |
| PAM-co-NaPA/PU | Elemental iodine | E. coli, S. aureus | – | [275] |
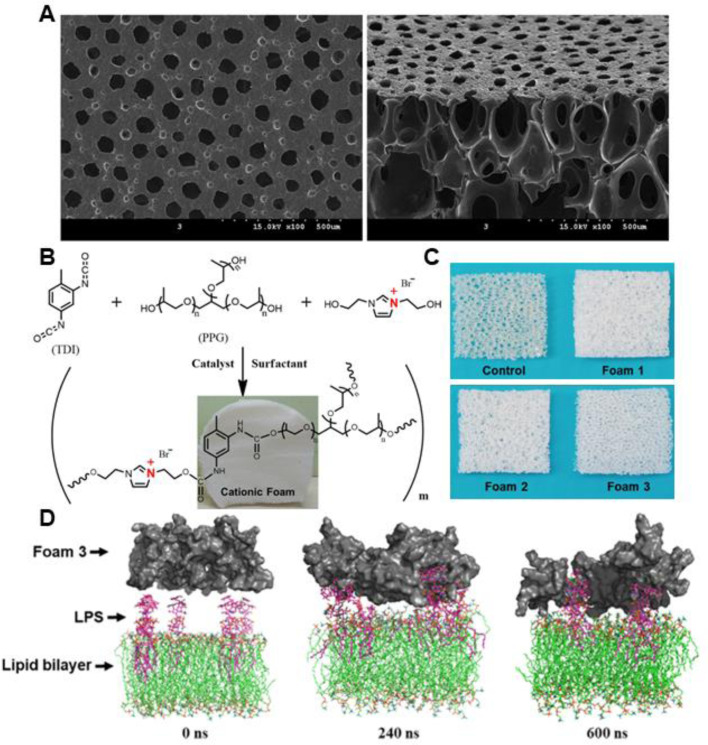
Polyurethanes (PU) are synthetic polymers consisting of urethane linkages in the main chains, formed between isocyanate and polyol through polyaddition polymerization. The properties of PU could be easily regulated by the chemical structure and molecular weight of polyol, the constitution of phase-separated hard and soft segments, and the synthetic methods. Due to the biocompatibility and good mechanical properties, much effort has been devoted to investigating the biomedical application of PU, and PU based wound dressings have been extensively studied. To enhance the antimicrobial activity of PU based foams, various bactericidal agents have been impregnated into the porous networks. Lee et al. [266] loaded silver-hydroxyapatite into PU foams formed by toluene diisocyanate and polyol mixture. The resulting foams, with high absorbency (0.66–0.70 g/cm2), fluid retention rates (0.32–0.35 g/cm2) and sustained Ag release behavior, could promote the re-epithelialization and collagen deposition of the infected full-thickness wound. To maximize diabetic wound healing, recombinant human epidermal growth factors was incorporated into the Ag NPs loaded porous PU foams, SEM images of the surface and cross-section of the foams are depicted in Fig. 15A [267]. The prepared foams exhibit good antibacterial activity against E. coli and S. aureus, and the in vivo evaluation demonstrated that the dressings could promote the healing of diabetic wound by stimulating re-epithelialization and regeneration of skin appendages. Besides, positively charged segments could be integrated into PU foams to grant the foams with inherent antimicrobial activity [271]. Yan et al. [270] prepared a series of imidazolium-type PU foams containing varied content of imidazolium ionic diol (2 mmol for foam 1, 4 mmol for foam 2, and 6 mmol for foam 3), and the resulting dressings with positive charge show excellent endotoxin adsorption and antibacterial properties (Fig. 15B-15D). The antibacterial efficiency of the foams increased with the content of cationic groups, and the positively charged imidazolium could improve the biocompatibility of the foams. The prepared cationic foam could significantly accelerate the closure of infected full-thickness wound in mice. Moreover, other antibacterial agents, such as ZnO [269], elemental iodine [109,275], usnic acid [274], and propolis [273], have been impregnated into PU based foams, which shows promising applications in wound recovery.
Fig. 15.
(A) SEM images of the surface and cross-section of PU foams with Ag NPs and human epidermal growth factors loaded. Reproduced from [267] with permission from The Royal Society of Chemistry. (B) Schematic illustration of the synthesis of PU foams with cationic imidazolium diol. (C) Photographs of three cationic PU foams and the control one. (D) Computer simulation of the endotoxin adsorption mechanism displaying that the snapshots for the interaction process of the P. aeruginosa outer membrane (LPS molecules and lipid bilayer) with foam 3 at different simulation times (0, 240, and 600 ns). Reproduced from [270] with permission from American Chemical Society.
3.6. 3D-printed scaffolds
Traditional scaffolds with submicron- or nano-sized structure or non-porous structure usually show confined nutrients transportation, which limits cell proliferation and restricts the tissue regeneration process [279]. The emerging 3D printing achieves the combination of material science and biomedical engineering, which has revolutionized the tissue engineering field. It could print sophisticated structure, with biological functions, layer by layer precise positioning of specific cells in a controlled manner [280,281]. Being able to effectively loading bioactive materials or living cells into biocompatible scaffolds are necessary for clinical translation. Artificial organs fabricated through 3D printing technology have achieved impressive progress. And several researchers have demonstrated the potential of bio-printed skin scaffolds based on hydrogel bioink [66,[282], [283], [284], [285], [286]. Gelatin methacryloyl (GelMA), a chemically modified and denatured collagen, has controllable chemical and physical properties. And the excellent biocompatibility and photo-induced gelation makes GelMA an ideal candidate for 3D printing hydrogel bioink [66]. Shin and Lee et al. [66] presented a 3D printing GelMA hydrogel patch incorporated with VEGF loaded tetrapodal zinc oxide (t-ZnO) microparticles (30–100 µm). Defects was introduced into t-ZnO by etching with hydrogen peroxide while reducing the bandgap of t-ZnO into the range of green light. Inconsistent polarity caused by the oxygen vacancy concentration enables the strong physical interactions between t-ZnO and VEGF. And controlled VEGF release could be achieved due to the formation of electrons and holes with the intervention of ultraviolet/visible light via electrostatic interaction. The angiogenic properties improved by the released VEGF and the antibacterial properties of t-ZnO of the 3D printing scaffold could effectively promote the process of wound healing. Marquette et al. [287] reported a 3D bio-printed fibroblasts loaded hydrogel scaffold, and then seeded with keratinocytes to simulate bilayer skin structure with dermal and epidermal layers (Fig. 16A), which demonstrates the potential application of 3D printing scaffolds in skin regeneration. To enhance mass transportation in skin-equivalent, Takeuchi et al. [288] fabricated perfusable vascular channels coated with endothelial cells. With both edges of the vascular channels fixed to the connectors of a culture device attached to a perfusion system composed of a peristaltic pump and silicone tubes, the perfusable vascular channels could achieve nutrition deliver to the skin-equivalent (Fig. 16B). Moreover, to incorporate interconnected channels in the printed 3D scaffolds, Hong et al. fabricated antibacterial superporous hydrogel dressings through the combination of silver−ethylene interaction and 3D printing technique (Fig. 16C). The interaction between silver and N,N′-methylenebisacrylamide provide the sites to fix Ag, after which polymerization of acrylamide occurred in a 3D printed template. The reduced AgNPs-loaded nanocomposite hydrogels with controlled release behavior show good antibacterial effect and decreased toxicity. The wound healing effect of the hydrogels on a full-thickness skin wound model indicates that the prepared hydrogel could accelerate the healing process of the infected wounds and restrain the formation of scar tissue. Except for hydrogel-based bioink, temperature-sensitive bioactive reagents loaded with functional nanoparticles, antibacterial agents included, could also be applied to print 3D scaffold [289,290].
Fig. 16.
(A) Schematic representation of the 3D bioprinting, consolidation, and maturation steps of the bilayer skin structure (DE: dermal–epidermal junction; SE: surface–epidermal junction). Reproduced from [287] with permission from WILEY-VCH. (B) Scheme of skin-equivalent integrated with perfusable vascular channels and culture device. Reproduced from [288] with permission from Elsevier. (C) The schematic presentation for the preparation of Ag NPs-cross-linked hydrogel dressing. Reproduced from Ref. [131] with permission from American Chemical Society.
Although 3D printing has shown promising application in tissue engineering, the biosafety, structural integrity, complicated culture equipment and practice are bond to take into consideration prior to the implantation of the printed scaffolds. To remove those obstacles, in situ 3D printing has been inspired to achieve direct printing customized tissues at the site of injury or defects [291]. And the in situ delivery of cells and bioactive molecules in 3D scaffolds similar to ECM could impose favorable effect on the interaction between cells, which makes a big difference on wound recovery [292,293]. 3D printing technology, with superiorities in time saving, facile processing and high resolution, has made a significant breakthrough in constructing sophisticated structure. However, there are still some challenges, such as the selection and preparation of bioink, the optimization of printing process, printing of skin appendages, and the biofunction of the printed scaffolds, need to be overcome in skin-equivalent printing. Meanwhile, it is no doubt that 3D printing is a prosperous technology in tissue engineering, skin regeneration included.
4. Conclusions and perspectives
The healing of wound is an incredibly sophisticated process depending on the complex interplay between multiple cell population, soluble mediators, cytokines and so forth. The defects in the skin's structures or functions could significantly reduce the quality of patient's life because of its irreplaceable multifunction in human body. It is commonly accepted that the contamination of bacterial menaces the wound recovery process, which is also one of the main obstacles in wound closure. Therefore, the design and development of wound dressings to reduce the risk of infection and further enhance the healing quality remains urgently demanded. Herein, we have discussed antibacterial wound dressings with different morphologies and advantages, in which various antimicrobial agents have been incorporated to accelerate wound recovery. And those functional dressings have shown promising application in wound closure. Moreover, with the rapid development of the emerging fields, such as bioelectronic devices, 3D printing and smart materials, it will achieve seamless monitor and on-demand treatment of wound healing in the near future.
Although the existing agents exhibit excellent antimicrobial activity, which shows promising application in the treatment of wound closure, some obstacles still occurred during the process. Among the various antibacterial materials, antibiotics still are the predominant medicines in clinical in spite of the drug resistance, and the controlled release of antibiotics has attracted much attention. However, the abuse of antibiotics stills exists, and there is no uniform standard to evaluate the controlled release of those drugs to ensure the optimal therapy efficiency. Metal and metallic oxide, have been proved to possess excellent bactericidal activity with reduced bacterial resistance, and the bioactive properties are dependent on their shape, size, and nanostructures [67]. Nevertheless, their synthesis, especially the control of morphology, the removal and the potential toxicity of those nanoparticles remain challenges to overcome. Besides, some of the photo-assisted antibacterial agents (such as carbon-based material, metal and metal sulfide) faced with similar obstacles. Meanwhile, the inconvenience caused by the intervention of light during therapy, the damage to normal tissues and relatively low efficiency make the application of precise local light-assisted therapy a big challenge. The limited bactericidal activity or complicated synthesis makes cationic organic agents applied synergistically with other molecules, and whether the metabolic product is harmful to human body remains unclear. The complex isolation and purification of herbal extractions, and the sophisticated preparation of materials with micro-structures discourages the wide application of themselves. Therefore, more efforts will be needed to circumvent those issues, and the development of more effective bactericidal materials seems to be desperately needed. Moreover, the on-demand treatment and removal of materials will be an irresistible trend.
Antibacterial dressings with different morphologies have been widely investigated to promote wound recovery. However, it is difficult for us to compare those dressings from the aspect of wound recovery. A main reason is that those dressings with different structures could not meet the requirements of different wounds at the same time, which requires researchers to investigate wound healing effect of dressings more specifically, instead of one wound model for all dressings. Besides, it is inevitable to use organic solvents and crosslinking agents during the fabrication of dressings with specific morphology. The biocompatibility of those materials requires strict evaluation before clinical application. Finally, the development of large-volume production facilities is of great importance for their practical application, which needs researchers to adopt cost-effective materials and relatively simple synthesis process to fabricate dressings.
In summary, the integration of various antibacterial agents into dressings with specific morphologies and the explorations or evaluations of effects of bactericidal agents, as well as the wound healing promotion were discussed. And we envision that this review will provide in-depth fundamental insights for the design of the next-generation dressings with broad-spectrum antibacterial activities, especially for pathogens with drug resistance. Continued development of material science and the expanding arsenal of antibacterial agents will further inspire novel dressings. Combined with the emerging technology in biology and artificial intelligence, we envision that the expanding investigation of the antibacterial biomaterial with enhanced therapeutic efficiency will achieve advanced health care.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
This work was jointly supported by the National Natural Science Foundation of China (grant numbers: 51973172), Natural Science Foundation of Shaanxi Province (No. 2020JC-03 and 2019TD-020), State Key Laboratory for Mechanical Behavior of Materials, and Opening Project of Key Laboratory of Shaanxi Province for Craniofacial Precision Medicine Research, College of Stomatology, Xi'an Jiaotong University (No. 2019LHM-KFKT008) and the World-Class Universities (Disciplines) and the Characteristic Development Guidance Funds for the Central Universities.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajps.2022.01.001.
Appendix. Supplementary materials
Reference
- 1.Norouzi M., Boroujeni S.M., Omidvarkordshouli N., Soleimani M. Advances in skin regeneration: application of electrospun scaffolds. Adv Healthcare Mater. 2015;4(8):1114–1133. doi: 10.1002/adhm.201500001. [DOI] [PubMed] [Google Scholar]
- 2.Yu R., Zhang H., Guo B. Conductive biomaterials as bioactive wound dressing for wound healing and skin tissue engineering. Nano-Micro Lett. 2022;14(1):1. doi: 10.1007/s40820-021-00751-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo B., Dong R., Bang Y., Li M. Haemostatic materials for wound healing applications. Nat Rev Chem. 2021;5(11):773–791. doi: 10.1038/s41570-021-00323-z. [DOI] [PubMed] [Google Scholar]
- 4.Modaresifar K., Azizian S., Ganjian M., Fratila-Apachitei L.E., Zadpoor A.A. Bactericidal effects of nanopatterns: a systematic review. Acta Biomater. 2019;83:29–36. doi: 10.1016/j.actbio.2018.09.059. [DOI] [PubMed] [Google Scholar]
- 5.Nussbaum S.R., Carter M.J., Fife C.E., DaVanzo J., Haught R., Nusgart M., et al. An economic evaluation of the impact, cost, and medicare policy implications of chronic nonhealing wounds. Value in Health. 2018;21(1):27–32. doi: 10.1016/j.jval.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong D.G., Swerdlow M.A., Armstrong A.A., Conte M.S., Padula W.V., Bus S.A. Five year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J Foot Ankle Res. 2020;13(1):1–4. doi: 10.1186/s13047-020-00383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nour S., Baheiraei N., Imani R., Khodaei M., Alizadeh A., Rabiee N., et al. A review of accelerated wound healing approaches: biomaterial-assisted tissue remodeling. J Mater Sci: Mater Med. 2019;30(10):120. doi: 10.1007/s10856-019-6319-6. [DOI] [PubMed] [Google Scholar]
- 8.Mandla S., Huyer L.D., Radisic M. Review: multimodal bioactive material approaches for wound healing. APL Bioeng. 2018;2(2) doi: 10.1063/1.5026773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mir M., Ali M.N., Barakullah A., Gulzar A., Arshad M., Fatima S., et al. Synthetic polymeric biomaterials for wound healing: a review. Prog Biomater. 2018;7(1):1–21. doi: 10.1007/s40204-018-0083-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mansbridge J. Skin tissue engineering. J Biomater Sci, Polym Ed. 2008;19(8):955–968. doi: 10.1163/156856208784909417. [DOI] [PubMed] [Google Scholar]
- 11.Jaggessar A., Shahali H., Mathew A., Yarlagadda P.K.D.V. Bio-mimicking nano and micro-structured surface fabrication for antibacterial properties in medical implants. J Nanobiotechnol. 2017;15(1):64. doi: 10.1186/s12951-017-0306-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao X., Pei D., Yang Y., Xu K., Yu J., Zhang Y., et al. Green tea derivative driven smart hydrogels with desired functions for chronic diabetic wound treatment. Adv Funct Mater. 2021;31(18) [Google Scholar]
- 13.Simoes D., Miguel S.P., Ribeiro M.P., Coutinho P., Mendonca A.G., Correia I.J. Recent advances on antimicrobial wound dressing: a review. Eur J Pharm Biopharm. 2018;127:130–141. doi: 10.1016/j.ejpb.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 14.Siddiqui A.R., Bernstein J.M. Chronic wound infection: facts and controversies. Clin. Dermatol. 2010;28(5):519–526. doi: 10.1016/j.clindermatol.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Bowler P.G., Davies B.J. The microbiology of infected and noninfected leg ulcers. Int J Dermatol. 1999;38(8):573–578. doi: 10.1046/j.1365-4362.1999.00738.x. [DOI] [PubMed] [Google Scholar]
- 16.Mustoe T.A., O'shaughnessy K., Kloeters O.J.P. surgery r. Chronic wound pathogenesis and current treatment strategies: a unifying hypothesis. Plast Reconstr Surg. 2006;117(7S) doi: 10.1097/01.prs.0000225431.63010.1b. 35S–41S. [DOI] [PubMed] [Google Scholar]
- 17.Fougère B., Boulanger E., Nourhashémi F., Guyonnet S., Cesari M. Retracted: chronic inflammation: accelerator of biological aging. J Gerontol: Ser A. 2016;72(9):1218–1225. doi: 10.1093/gerona/glw240. [DOI] [PubMed] [Google Scholar]
- 18.Zhang W., Chen L., Chen J., Wang L., Gui X., Ran J., et al. Silk fibroin biomaterial shows safe and effective wound healing in animal models and a randomized controlled clinical trial. Adv Healthcare Mater. 2017;6(10) doi: 10.1002/adhm.201700121. [DOI] [PubMed] [Google Scholar]
- 19.Gil E.S., Panilaitis B., Bellas E., Kaplan D.L. Functionalized silk biomaterials for wound healing. Adv Healthcare Mater. 2013;2(1):206–217. doi: 10.1002/adhm.201200192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaya S., Cresswell M., Boccaccini A.R. Mesoporous silica-based bioactive glasses for antibiotic-free antibacterial applications. Mater Sci Eng C. 2018;83:99–107. doi: 10.1016/j.msec.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Martinotti S., Ranzato E. Honey, wound repair and regenerative medicine. J Funct Biomater. 2018;9(2):34. doi: 10.3390/jfb9020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim H., Kong W.H., Seong K.-.Y., Sung D.K., Jeong H., Kim J.K., et al. Hyaluronate-epidermal growth factor conjugate for skin wound healing and regeneration. Biomacromolecules. 2016;17(11):3694–3705. doi: 10.1021/acs.biomac.6b01216. [DOI] [PubMed] [Google Scholar]
- 23.Liu G., Bao Z., Wu J. Injectable baicalin/F127 hydrogel with antioxidant activity for enhanced wound healing. Chin Chem Lett. 2020;31(7):1817–1821. [Google Scholar]
- 24.Dodero A., Scarfi S., Pozzolini M., Vicini S., Alloisio M., Castellano M. Alginate-based electrospun membranes containing ZnO nanoparticles as potential wound healing patches: biological, mechanical, and physicochemical characterization. ACS Appl Mater Interfaces. 2020;12(3):3371–3381. doi: 10.1021/acsami.9b17597. [DOI] [PubMed] [Google Scholar]
- 25.Liang D., Lu Z., Yang H., Gao J., Chen R. Novel asymmetric wettable AgNPs/chitosan wound dressing: in vitro and in vivo evaluation. ACS Appl Mater Interfaces. 2016;8(6):3958–3968. doi: 10.1021/acsami.5b11160. [DOI] [PubMed] [Google Scholar]
- 26.Namuiriyachote N., Lipipun V., Althhatuattananglzul Y., Charoonrut P., Ritthidej G.C. Development of polyurethane foam dressing containing silver and asiaticoside for healing of dermal wound. Asian J Pharm Sci. 2019;14(1):63–77. doi: 10.1016/j.ajps.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qu J., Liang Y.P., Shi M.T., Guo B.L., Gao Y.Z., Yin Z.H. Biocompatible conductive hydrogels based on dextran and aniline trimer as electro-responsive drug delivery system for localized drug release. Int J Biol Macromol. 2019;140:255–264. doi: 10.1016/j.ijbiomac.2019.08.120. [DOI] [PubMed] [Google Scholar]
- 28.Zhang M.Y., Wu Y.B., Zhao X., Gao K., Ma P.X., Guo B.L. Biocompatible degradable injectable hydrogels from methacrylated poly(ethylene glycol)-co-poly(xylitol sebacate) and cyclodextrins for release of hydrophilic and hydrophobic drugs. RSC Adv. 2015;5(82):66965–66974. [Google Scholar]
- 29.Li L.C., Ge J., Ma P.X., Guo B.L. Injectable conducting interpenetrating polymer network hydrogels from gelatin-graft-polyaniline and oxidized dextran with enhanced mechanical properties. RSC Adv. 2015;5(112):92490–92498. [Google Scholar]
- 30.Joseph B., SV K., Sabu C., Kalarikkal N., Thomas S. Cellulose nanocomposites: fabrication and biomedical applications. J Bioresour Bioprod. 2020;5(4):223–237. [Google Scholar]
- 31.Li X., Bai H., Yang Y., Yoon J., Wang S., Zhang X. Supramolecular antibacterial materials for combatting antibiotic resistance. Adv Mater. 2019;31(5) doi: 10.1002/adma.201805092. [DOI] [PubMed] [Google Scholar]
- 32.Howell-Jones R.S., Wilson M.J., Hill K.E., Howard A.J., Price P.E., Thomas D.W. A review of the microbiology, antibiotic usage and resistance in chronic skin wounds. J Antimicrob Chemother. 2005;55(2):143–149. doi: 10.1093/jac/dkh513. [DOI] [PubMed] [Google Scholar]
- 33.Hu B., Owh C., Chee P.L., Leow W.R., Liu X., Wu Y.L., et al. Supramolecular hydrogels for antimicrobial therapy. Chem Soc Rev. 2018;47(18):6917–6929. doi: 10.1039/c8cs00128f. [DOI] [PubMed] [Google Scholar]
- 34.Konop M., Damps T., Misicka A., Rudnicka L. Certain aspects of silver and silver nanoparticles in wound care: a minireview. J Nanomater. 2016;2016:47. [Google Scholar]
- 35.Bonda Rama R., Rajnish K., Shagufta H., Jerald Mahesh K., Rao T.N., Raju V.S.N.K., et al. Ag2[Fe(CN)5NO]-fabricated hydrophobic cotton as a potential wound healing dressing: an in vivo approach. ACS Appl Mater Interfaces. 2021;13(9):10689–10704. doi: 10.1021/acsami.0c19904. [DOI] [PubMed] [Google Scholar]
- 36.Lee A.R.C., Leem H., Lee J., Park K.C. Reversal of silver sulfadiazine-impaired wound healing by epidermal growth factor. Biomaterials. 2005;26(22):4670–4676. doi: 10.1016/j.biomaterials.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 37.Tong C., Zhong X., Yang Y., Liu X., Zhong G., Xiao C., et al. PB@PDA@Ag nanosystem for synergistically eradicating MRSA and accelerating diabetic wound healing assisted with laser irradiation. Biomaterials. 2020;243 doi: 10.1016/j.biomaterials.2020.119936. [DOI] [PubMed] [Google Scholar]
- 38.Yang K., Han Q., Chen B., Zheng Y., Zhang K., Li Q., et al. Antimicrobial hydrogels: promising materials for medical application. Int J Nanomed. 2018;13:2217–2263. doi: 10.2147/IJN.S154748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li S., Dong S., Xu W., Tu S., Yan L., Zhao C., et al. Antibacterial hydrogels. Adv Sci. 2018;5(5) doi: 10.1002/advs.201700527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiang Y., Mao C., Liu X., Cui Z., Jing D., Yang X., et al. Rapid and superior bacteria killing of carbon quantum dots/ZnO decorated injectable folic acid-conjugated PDA hydrogel through dual-light triggered ROS and membrane permeability. Small. 2019;15(22) doi: 10.1002/smll.201900322. [DOI] [PubMed] [Google Scholar]
- 41.Raguvaran R., Manuja B.K., Chopra M., Thakur R., Anand T., Kalia A., et al. Sodium alginate and gum acacia hydrogels of ZnO nanoparticles show wound healing effect on fibroblast cells. Int J Biol Macromol. 2017;96:185–191. doi: 10.1016/j.ijbiomac.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 42.Gold K., Slay B., Knackstedt M., Gaharwar A.K. Antimicrobial activity of metal and metal-oxide based nanoparticles. Adv Ther. 2018;1(3) [Google Scholar]
- 43.Raghunath A., Perumal E. Metal oxide nanoparticles as antimicrobial agents: a promise for the future. Int J Antimicrob Agents. 2017;49(2):137–152. doi: 10.1016/j.ijantimicag.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 44.Dizaj S.M., Lotfipour F., Barzegar-Jalali M., Zarrintan M.H., Adibkia K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater Sci Eng C. 2014;44:278–284. doi: 10.1016/j.msec.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 45.Hajipour M.J., Fromm K.M., A Akbar Ashkarran, D Jimenez de Aberasturi, IRd Larramendi, Rojo T., et al. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012;30(10):499–511. doi: 10.1016/j.tibtech.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 46.Xu J.-.W., Yao K., Xu Z.-.K. Nanomaterials with a photothermal effect for antibacterial activities: an overview. Nanoscale. 2019;11(18):8680–8691. doi: 10.1039/c9nr01833f. [DOI] [PubMed] [Google Scholar]
- 47.Qiao Y., He J., Chen W., Yu Y., Li W., Du Z., et al. Light-activatable synergistic therapy of drug-resistant bacteria-infected cutaneous chronic wounds and nonhealing keratitis by cupriferous hollow nanoshells. ACS Nano. 2020;14(3):3299–3315. doi: 10.1021/acsnano.9b08930. [DOI] [PubMed] [Google Scholar]
- 48.Su K., Tan L., Liu X., Cui Z., Zheng Y., Li B., et al. Rapid photo-sonotherapy for clinical treatment of bacterial infected bone implants by creating oxygen deficiency using sulfur doping. ACS Nano. 2020;14(2):2077–2089. doi: 10.1021/acsnano.9b08686. [DOI] [PubMed] [Google Scholar]
- 49.Ji H., Dong K., Yan Z., Ding C., Chen Z., Ren J., et al. Bacterial hyaluronidase self-triggered prodrug release for chemo-photothermal synergistic treatment of bacterial infection. Small. 2016;12(45):6200–6206. doi: 10.1002/smll.201601729. [DOI] [PubMed] [Google Scholar]
- 50.Chen M., Long Z., Dong R., Wang L., Zhang J., Li S., et al. Titanium incorporation into Zr-porphyrinic metal-organic frameworks with enhanced antibacterial activity against multidrug-resistant pathogens. Small. 2020;16(7) doi: 10.1002/smll.201906240. [DOI] [PubMed] [Google Scholar]
- 51.Yu X., Wang S., Zhang X., Qi A., Qiao X., Liu Z., et al. Heterostructured nanorod array with piezophototronic and plasmonic effect for photodynamic bacteria killing and wound healing. Nano Energy. 2018;46:29–38. [Google Scholar]
- 52.Maleki A., He J., Bochani S., Nosrati V., Shahbazi M.-.A., Guo B. Multifunctional photoactive hydrogels for wound healing acceleration. ACS Nano. 2021;15(12):18895–18930. doi: 10.1021/acsnano.1c08334. [DOI] [PubMed] [Google Scholar]
- 53.Zhang B., He J., Shi M., Liang Y., Guo B. Injectable self-healing supramolecular hydrogels with conductivity and photo-thermal antibacterial activity to enhance complete skin regeneration. Chem Eng J. 2020;400 [Google Scholar]
- 54.Yu S., Li G., Zhao P., Cheng Q., He Q., Ma D., et al. NIR-laser-controlled hydrogen-releasing PdH nanohydride for synergistic hydrogen-photothermal antibacterial and wound-healing therapies. Adv Funct Mater. 2019;29(50) [Google Scholar]
- 55.Yu Y., Li P., Zhu C., Ning N., Zhang S., Vancso G.J. Multifunctional and recyclable photothermally responsive cryogels as efficient platforms for wound healing. Adv Funct Mater. 2019;29(35) [Google Scholar]
- 56.Deng T., Zhao H., Shi M., Qiu Y., Jiang S., Yang X., et al. Photoactivated trifunctional platinum nanobiotics for precise synergism of multiple antibacterial modes. Small. 2019;15(46) doi: 10.1002/smll.201902647. [DOI] [PubMed] [Google Scholar]
- 57.Wang Y., Yang Y., Shi Y., Song H., Yu C. Antibiotic-free antibacterial strategies enabled by nanomaterials: progress and perspectives. Adv Mater. 2019;32(18) doi: 10.1002/adma.201904106. [DOI] [PubMed] [Google Scholar]
- 58.Zhang J., Xu J., Ma H., Bai H., Liu L., Shu C., et al. Designing an amino-fullerene derivative C-70-(EDA)(8) to fight superbacteria. ACS Appl Mater Interfaces. 2019;11(16):14597–14607. doi: 10.1021/acsami.9b01483. [DOI] [PubMed] [Google Scholar]
- 59.Maas M. Carbon nanomaterials as antibacterial colloids. Materials (Basel) 2016;9(8):617. doi: 10.3390/ma9080617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu B., Berkey C., Feliciano T., Chen X., Li Z., Chen C., et al. Thermal-disrupting interface mitigates intercellular cohesion loss for accurate topical antibacterial therapy. Adv Mater. 2020;32(12) doi: 10.1002/adma.201907030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pang Q., Lou D., Li S., Wang G., Qiao B., Dong S., et al. Smart flexible electronics-integrated wound dressing for real-time monitoring and on-demand treatment of infected wounds. Adv Sci. 2020;7(6) doi: 10.1002/advs.201902673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mao C., Xiang Y., Liu X., Zheng Y., Yeung K.W.K., Cui Z., et al. Local photothermal/photodynamic synergistic therapy by disrupting bacterial membrane to accelerate reactive oxygen species permeation and protein leakage. ACS Appl Mater Interfaces. 2019;11(19):17902–17914. doi: 10.1021/acsami.9b05787. [DOI] [PubMed] [Google Scholar]
- 63.Yu Y., Mei L., Shi Y., Zhang X., Cheng K., Cao F., et al. Ag-Conjugated graphene quantum dots with blue light-enhanced singlet oxygen generation for ternary-mode highly-efficient antimicrobial therapy. J Mater Chem B. 2020;8(7):1371–1382. doi: 10.1039/c9tb02300c. [DOI] [PubMed] [Google Scholar]
- 64.Xiao F., Cao B., Wang C., Guo X., Li M., Xing D., et al. Pathogen-specific polymeric antimicrobials with significant membrane disruption and enhanced photodynamic damage to inhibit highly opportunistic bacteria. ACS Nano. 2019;13(2):1511–1525. doi: 10.1021/acsnano.8b07251. [DOI] [PubMed] [Google Scholar]
- 65.Ding L.G., Wang S., Yao B.J., Li F., Li Y.A., Zhao G.Y., et al. Synergistic antibacterial and anti-inflammatory effects of a drug-loaded self-standing porphyrin-COF membrane for efficient skin wound healing. Adv Healthcare Mater. 2021;10(8) doi: 10.1002/adhm.202001821. [DOI] [PubMed] [Google Scholar]
- 66.Leonard S., Eder L.C., Luis Enrique G.R., Junsung O., JunHwee J., Diego A.R.G., et al. Light-controlled growth factors release on tetrapodal ZnO-incorporated 3D-printed hydrogels for developing smart wound scaffold. Adv Funct Mater. 2021;31(22) doi: 10.1002/adfm.202007555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen C.Y., Yin H., Chen X., Chen T.H., Liu H.M., Rao S.S., et al. Ångstrom-scale silver particle–embedded carbomer gel promotes wound healing by inhibiting bacterial colonization and inflammation. Sci Adv. 2020;6(43):eaba0942. doi: 10.1126/sciadv.aba0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen Z., Yao X., Liu L., Guan J., Liu M., Li Z., et al. Blood coagulation evaluation of N-alkylated chitosan. Carbohydr Polym. 2017;173:259–268. doi: 10.1016/j.carbpol.2017.05.085. [DOI] [PubMed] [Google Scholar]
- 69.Zhang L., Wang L., Guo B.L., Ma P.X. Cytocompatible injectable carboxymethyl chitosan/N-isopropylacrylamide hydrogels for localized drug delivery. Carbohydr Polym. 2014;103:110–118. doi: 10.1016/j.carbpol.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 70.Xia Q., Jiamin Z., Lilong C., Qin J., Junhao Z., Lijun Y., et al. Antifouling antioxidant zwitterionic dextran hydrogels as wound dressing materials with excellent healing activities. ACS Appl Mater Interfaces. 2021;13(6):7060–7069. doi: 10.1021/acsami.0c17744. [DOI] [PubMed] [Google Scholar]
- 71.Miguel S.P., Moreira A.F., Correia I.J. Chitosan based-asymmetric membranes for wound healing: a review. Int J Biol Macromol. 2019;127:460–475. doi: 10.1016/j.ijbiomac.2019.01.072. [DOI] [PubMed] [Google Scholar]
- 72.Qu J., Zhao X., Liang Y., Zhang T., Ma P.X., Guo B. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials. 2018;183:185–199. doi: 10.1016/j.biomaterials.2018.08.044. [DOI] [PubMed] [Google Scholar]
- 73.Qu J., Zhao X., Ma P.X., Guo B.L. pH-responsive self-healing injectable hydrogel based on N-carboxyethyl chitosan for hepatocellular carcinoma therapy. Acta Biomater. 2017;58:168–180. doi: 10.1016/j.actbio.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 74.Zhao X., Li P., Guo B.L., Ma P.X. Antibacterial and conductive injectable hydrogels based on quaternized chitosan-graft-polyaniline/oxidized dextran for tissue engineering. Acta Biomater. 2015;26:236–248. doi: 10.1016/j.actbio.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 75.Zhang L., Li Y., Li L.C., Guo B.L., Ma P.X. Non-cytotoxic conductive carboxymethyl-chitosan/aniline pentamer hydrogels. React Funct Polym. 2014;82:81–88. [Google Scholar]
- 76.Zhao X., Guo B., Wu H., Liang Y., Ma P.X. Injectable antibacterial conductive nanocomposite cryogels with rapid shape recovery for noncompressible hemorrhage and wound healing. Nat Commun. 2018;9(1):2784. doi: 10.1038/s41467-018-04998-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gan D., Xu T., Xing W., Ge X., Fang L., Wang K., et al. Mussel-inspired contact-active antibacterial hydrogel with high cell affinity, toughness, and recoverability. Adv Funct Mater. 2019;29(1) [Google Scholar]
- 78.Ren Y.Z., Zhao X., Liang X.F., Ma P.X., Guo B.L. Injectable hydrogel based on quaternized chitosan, gelatin and dopamine as localized drug delivery system to treat Parkinson's disease. Int J Biol Macromol. 2017;105(Pt 1):1079–1087. doi: 10.1016/j.ijbiomac.2017.07.130. [DOI] [PubMed] [Google Scholar]
- 79.Stern D., Cui H. Crafting polymeric and peptidic hydrogels for improved wound healing. Adv Healthcare Mater. 2019;8(9) doi: 10.1002/adhm.201900104. [DOI] [PubMed] [Google Scholar]
- 80.Wang C., Shao C., Fang Y., Wang J., Dong N., Shan A. Binding loop of sunflower trypsin inhibitor 1 serves as a design motif for proteolysis-resistant antimicrobial peptides. Acta Biomater. 2021;124:254–269. doi: 10.1016/j.actbio.2021.01.036. [DOI] [PubMed] [Google Scholar]
- 81.Cai L., Liu S., Guo J., Jia Y.G. Polypeptide-based self-healing hydrogels: design and biomedical applications. Acta Biomater. 2020;113:84–100. doi: 10.1016/j.actbio.2020.07.001. [DOI] [PubMed] [Google Scholar]
- 82.Wei S., Xu P., Yao Z., Cui X., Lei X., Li L., et al. A composite hydrogel with co-delivery of antimicrobial peptides and platelet-rich plasma to enhance healing of infected wounds in diabetes. Acta Biomater. 2021;124:205–218. doi: 10.1016/j.actbio.2021.01.046. [DOI] [PubMed] [Google Scholar]
- 83.Zhu J., Han H., Li F., Wang X., Yu J., Qin X., et al. Peptide-functionalized amino acid-derived pseudoprotein-based hydrogel with hemorrhage control and antibacterial activity for wound healing. Chem Mater. 2019;31(12):4436–4450. [Google Scholar]
- 84.Weishaupt R., Zund J.N., Heuberger L., Zuber F., Faccio G., Robotti F., et al. Antibacterial, cytocompatible, sustainably sourced: cellulose membranes with bifunctional peptides for advanced wound dressings. Adv Healthcare Mater. 2020;9(7) doi: 10.1002/adhm.201901850. [DOI] [PubMed] [Google Scholar]
- 85.Fang H., Wang J., Li L., Xu L., Wu Y., Wang Y., et al. A novel high-strength poly(ionic liquid)/PVA hydrogel dressing for antibacterial applications. Chem Eng J. 2019;365:153–164. [Google Scholar]
- 86.Yang Z., Huang R., Zheng B., Guo W., Li C., He W., et al. Highly stretchable, adhesive, biocompatible, and antibacterial hydrogel dressings for wound healing. Adv Sci. 2021;8(8) doi: 10.1002/advs.202003627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yeo C.K., Vikhe Y.S., Li P., Guo Z., Greenberg P., Duan H., et al. Hydrogel effects rapid biofilm debridement with ex situ contact-kill to eliminate multidrug resistant bacteria in vivo. ACS Appl Mater Interfaces. 2018;10(24):20356–20367. doi: 10.1021/acsami.8b06262. [DOI] [PubMed] [Google Scholar]
- 88.El-Aassar M.R., El Fawal G.F., El-Deeb N.M., Hassan H.S., Mo X. Electrospun polyvinyl alcohol/Pluronic F127 blended nanofibers containing titanium dioxide for antibacterial wound dressing. Appl Biochem Biotechnol. 2016;178(8):1488–1502. doi: 10.1007/s12010-015-1962-y. [DOI] [PubMed] [Google Scholar]
- 89.Giano M.C., Ibrahim Z., Medina S.H., Sarhane K.A., Christensen J.M., Yamada Y., et al. Injectable bioadhesive hydrogels with innate antibacterial properties. Nat Commun. 2014;5:4095. doi: 10.1038/ncomms5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang K., Wang J., Li L., Xu L., Feng N., Wang Y., et al. Synthesis of a novel anti-freezing, non-drying antibacterial hydrogel dressing by one-pot method. Chem Eng J. 2019;372:216–225. [Google Scholar]
- 91.Feng Y., Wang Q., He M., Zhang X., Liu X., Zhao C. Antibiofouling zwitterionic gradational membranes with moisture retention capability and sustained antimicrobial property for chronic wound infection and skin regeneration. Biomacromolecules. 2019;20(8):3057–3069. doi: 10.1021/acs.biomac.9b00629. [DOI] [PubMed] [Google Scholar]
- 92.Wu Y.B., Wang L., Hu T.L., Ma P.X., Guo B.L. Conductive micropatterned polyurethane films as tissue engineering scaffolds for Schwann cells and PC12 cells. J Colloid Interface Sci. 2018;518:252–262. doi: 10.1016/j.jcis.2018.02.036. [DOI] [PubMed] [Google Scholar]
- 93.Hu T.L., Wu Y.B., Zhao X., Wang L., Bi L.Y., Ma P.X., et al. Micropatterned, electroactive, and biodegradable poly(glycerol sebacate)-aniline trimer elastomer for cardiac tissue engineering. Chem Eng J. 2019;366:208–222. [Google Scholar]
- 94.Ivanova E.P., Hasan J., Webb H.K., Gervinskas G., Juodkazis S., Vi Khanh T., et al. Bactericidal activity of black silicon. Nat Commun. 2013;4:2838. doi: 10.1038/ncomms3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yao X., Zhu G., Zhu P., Ma J., Chen W., Liu Z., et al. Omniphobic ZIF-8@hydrogel membrane by microfluidic-emulsion-templating method for wound healing. Adv Funct Mater. 2020;30(13) [Google Scholar]
- 96.Kandhasamy S., Perumal S., Madhan B., Umamaheswari N., Banday J.A., Perumal P.T., et al. Synthesis and fabrication of collagen-coated ostholamide electrospun nanofiber scaffold for wound healing. ACS Appl Mater Interfaces. 2017;9(10):8556–8568. doi: 10.1021/acsami.6b16488. [DOI] [PubMed] [Google Scholar]
- 97.Li X., Nan K., Li L., Zhang Z., Chen H. In vivo evaluation of curcumin nanoformulation loaded methoxy poly(ethylene glycol)-graft-chitosan composite film for wound healing application. Carbohydr Polym. 2012;88(1):84–90. [Google Scholar]
- 98.Adeli-Sardou M., Yaghoobi M.M., Torkzadeh-Mahani M., Dodel M. Controlled release of lawsone from polycaprolactone/gelatin electrospun nano fibers for skin tissue regeneration. Int J Biol Macromol. 2019;124:478–491. doi: 10.1016/j.ijbiomac.2018.11.237. [DOI] [PubMed] [Google Scholar]
- 99.Lin J., Li C., Zhao Y., Hu J., Zhang Ll-M. Co-electrospun nanofibrous membranes of collagen and zein for wound healing. ACS Appl Mater Interfaces. 2012;4(2):1050–1057. doi: 10.1021/am201669z. [DOI] [PubMed] [Google Scholar]
- 100.Liu X., You L., Tarafder S., Zou L., Fang Z., Chen J., et al. Curcumin-releasing chitosan/aloe membrane for skin regeneration. Chem Eng J. 2019;359:1111–1119. [Google Scholar]
- 101.Ramalingam R., Dhand C., Leung C.M., Ezhilarasu H., Prasannan P., Ong S.T., et al. Poly-epsilon-caprolactone/gelatin hybrid electrospun composite nanofibrous mats containing ultrasound assisted herbal extract: antimicrobial and cell proliferation study. Nanomaterials. 2019;9(3):462. doi: 10.3390/nano9030462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bai M.Y., Chen M.C., Yu W.C., Lin J.Y. Foam dressing incorporating herbal extract: an all-natural dressing for potential use in wound healing. J Bioact Compat Polym. 2017;32(3):293–308. [Google Scholar]
- 103.Zhou T., Sui B., Mo X., Sun J. Multifunctional and biomimetic fish collagen/bioactive glass nanofibers: fabrication, antibacterial activity and inducing skin regeneration in vitro and in vivo. Int J Nanomed. 2017;12:3495–3507. doi: 10.2147/IJN.S132459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li Y., Han Y., Wang X., Peng J., Xu Y., Chang J. Multifunctional hydrogels prepared by dual ion cross-linking for chronic wound healing. ACS Appl Mater Interfaces. 2017;9(19):16054–16062. doi: 10.1021/acsami.7b04801. [DOI] [PubMed] [Google Scholar]
- 105.Zhang Z., Dai Q., Zhang Y., Zhuang H., Wang E., Xu Q., et al. Design of a multifunctional biomaterial inspired by ancient chinese medicine for hair regeneration in burned skin. ACS Appl Mater Interfaces. 2020;12(11):12489–12499. doi: 10.1021/acsami.9b22769. [DOI] [PubMed] [Google Scholar]
- 106.Li J., Zhai D., Lv F., Yu Q., Ma H., Yin J., et al. Preparation of copper-containing bioactive glass/eggshell membrane nanocomposites for improving angiogenesis, antibacterial activity and wound healing. Acta Biomater. 2016;36:254–266. doi: 10.1016/j.actbio.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 107.Sarhan W.A., Azzazy H.M.E., El-Sherbiny I.M. Honey/chitosan nanofiber wound dressing enriched with allium sativum and cleome droserifolia: enhanced antimicrobial and wound healing activity. ACS Appl Mater Interfaces. 2016;8(10):6379–6390. doi: 10.1021/acsami.6b00739. [DOI] [PubMed] [Google Scholar]
- 108.Kim J.O., Noh J.-.K., Thapa R.K., Hasan N., Choi M., Kim J.H., et al. Nitric oxide-releasing chitosan film for enhanced antibacterial and in vivo wound-healing efficacy. Int J Biol Macromol. 2015;79:217–225. doi: 10.1016/j.ijbiomac.2015.04.073. [DOI] [PubMed] [Google Scholar]
- 109.Landsman T.L., Touchet T., Hasan S.M., Smith C., Russell B., Rivera J., et al. A shape memory foam composite with enhanced fluid uptake and bactericidal properties as a hemostatic agent. Acta Biomater. 2017;47:91–99. doi: 10.1016/j.actbio.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Boateng J.S., Pawar H.V., Tetteh J. Polyox and carrageenan based composite film dressing containing anti-microbial and anti-inflammatory drugs for effective wound healing. Int J Pharm. 2013;441(1–2):181–191. doi: 10.1016/j.ijpharm.2012.11.045. [DOI] [PubMed] [Google Scholar]
- 111.Mi L., Xue H., Li Y., Jiang S. A thermoresponsive antimicrobial wound dressing hydrogel based on a cationic betaine ester. Adv Funct Mater. 2011;21(21):4028–4034. [Google Scholar]
- 112.Ambrogi V., Pietrella D., Nocchetti M., Casagrande S., Moretti V., De Marco S., et al. Montmorillonite-chitosan-chlorhexidine composite films with antibiofilm activity and improved cytotoxicity for wound dressing. J Colloid Interface Sci. 2017;491:265–272. doi: 10.1016/j.jcis.2016.12.058. [DOI] [PubMed] [Google Scholar]
- 113.Shanmugapriya K., Kim H., Saravana P.S., Chun B.-.S., Kang H.W. Fabrication of multifunctional chitosan-based nanocomposite film with rapid healing and antibacterial effect for wound management. Int J Biol Macromol. 2018;118(Pt B):1713–1725. doi: 10.1016/j.ijbiomac.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 114.Yusof N., Hafiza A.H.A., Zohdi R.M., Bakar M.Z.A. Development of honey hydrogel dressing for enhanced wound healing. Radiat Phys Chem. 2007;76(11–12):1767–1770. [Google Scholar]
- 115.El-Kased R.F., Amer R.I., Attia D., Elmazar M.M. Honey-based hydrogel: in vitro and comparative in vivo evaluation for burn wound healing. Sci Rep. 2017;7(1):9692. doi: 10.1038/s41598-017-08771-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chuysinuan P., Chimnoi N., Reuk-Ngam N., Khlaychan P., Makarasen A., Wetprasit N., et al. Development of gelatin hydrogel pads incorporated with Eupatorium adenophorum essential oil as antibacterial wound dressing. Polym Bull. 2019;76(2):701–724. [Google Scholar]
- 117.Kavoosi G., Bordbar Z., Dadfar S.M., Dadfar S.M.M. Preparation and characterization of a novel gelatin-poly(vinyl alcohol) hydrogel film loaded with Zataria multiflora essential oil for antibacterial-antioxidant wound-dressing applications. J Appl Polym Sci. 2017;134(39):45351. [Google Scholar]
- 118.Gherman T., Popescu V., Carpa R., Gavril G.L., Rapa M., Oprescu E.E. Salvia officinalis essential oil loaded gelatin hydrogel as potential antibacterial wound dressing materials. Rev Chim. 2018;69(2):410–414. [Google Scholar]
- 119.Li M., Li F., Wang T., Zhao L., Shi Y. Fabrication of carboxymethylcellulose hydrogel containing beta-cyclodextrin-eugenol inclusion complexes for promoting diabetic wound healing. J Biomater Appl. 2020;34(6):851–863. doi: 10.1177/0885328219873254. [DOI] [PubMed] [Google Scholar]
- 120.Tan S.P., McLoughlin P., O'Sullivan L., Prieto M.L., Gardiner G.E., Lawlor P.G., et al. Development of a novel antimicrobial seaweed extract-based hydrogel wound dressing. Int J Pharm. 2013;456(1):10–20. doi: 10.1016/j.ijpharm.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 121.Jaiswal L., Shankar S., Rhim J.W. Carrageenan-based functional hydrogel film reinforced with sulfur nanoparticles and grapefruit seed extract for wound healing application. Carbohydr Polym. 2019;224 doi: 10.1016/j.carbpol.2019.115191. [DOI] [PubMed] [Google Scholar]
- 122.Koosehgol S., Ebrahimian-Hosseinabadi M., Alizadeh M., Zamanian A. Preparation and characterization of in situ chitosan/polyethylene glycol fumarate/thymol hydrogel as an effective wound dressing. Mater Sci Eng C. 2017;79:66–75. doi: 10.1016/j.msec.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 123.Song R., Zheng J., Liu Y., Tan Y., Yang Z., Song X., et al. A natural cordycepin/chitosan complex hydrogel with outstanding self-healable and wound healing properties. Int J Biol Macromol. 2019;134:91–99. doi: 10.1016/j.ijbiomac.2019.04.195. [DOI] [PubMed] [Google Scholar]
- 124.Zhu Q., Jiang M., Liu Q., Yan S., Feng L., Lan Y., et al. Enhanced healing activity of burn wound infection by a dextran-HA hydrogel enriched with sanguinarine. Biomater Sci. 2018;6(9):2472–2486. doi: 10.1039/c8bm00478a. [DOI] [PubMed] [Google Scholar]
- 125.Lee J., Hlaing S.P., Cao J., Hasan N., Ahn H.-.J., Song K.-.W., et al. In situ hydrogel-forming/nitric oxide-releasing wound dressing for enhanced antibacterial activity and healing in mice with infected wounds. Pharmaceutics. 2019;11(10):496. doi: 10.3390/pharmaceutics11100496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Reimer K., Vogt P.M., Broegmann B., Hauser J., Rossbach O., Kramer A., et al. An innovative topical drug formulation for wound healing and infection treatment: in vitro and in vivo investigations of a povidone-iodine liposome hydrogel. Dermatology. 2000;201(3):235–241. doi: 10.1159/000018494. [DOI] [PubMed] [Google Scholar]
- 127.Du L., Tong L., Jin Y., Jia J., Liu Y., Su C., et al. A multifunctional in situ-forming hydrogel for wound healing. Wound Repair Regen. 2012;20(6):904–910. doi: 10.1111/j.1524-475X.2012.00848.x. [DOI] [PubMed] [Google Scholar]
- 128.Wang Z., Wang Y., Peng X., He Y., Wei L., Su W., et al. Photocatalytic antibacterial agent incorporated double-network hydrogel for wound healing. Colloids Surf, B. 2019;180:237–244. doi: 10.1016/j.colsurfb.2019.04.043. [DOI] [PubMed] [Google Scholar]
- 129.Wang B.L., Ren K.F., Chao H., Wang J.L., Ji J. Construction of degradable multilayer films for enhanced antibacterial properties. ACS Appl Mater Interfaces. 2013;5(10):4136–4143. doi: 10.1021/am4000547. [DOI] [PubMed] [Google Scholar]
- 130.Hsu B.B., Conway W., Tschabrunn C.M., Mehta M., Perez-Cuevas M.B., Zhang S., et al. Clotting mimicry from robust hemostatic bandages based on self-assembling peptides. ACS Nano. 2015;9(9):9394–9406. doi: 10.1021/acsnano.5b02374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wu Z., Hong Y. Combination of the silver-ethylene interaction and 3D printing to develop antibacterial superporous hydrogels for wound management. ACS Appl Mater Interfaces. 2019;11(37):33734–33747. doi: 10.1021/acsami.9b14090. [DOI] [PubMed] [Google Scholar]
- 132.Feng Y., Li X., Zhang Q., Yan S., Guo Y., Li M., et al. Mechanically robust and flexible silk protein/polysaccharide composite sponges for wound dressing. Carbohydr Polym. 2019;216:17–24. doi: 10.1016/j.carbpol.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 133.Pei Y., Ye D., Zhao Q., Wang X., Zhang C., Huang W., et al. Effectively promoting wound healing with cellulose/gelatin sponges constructed directly from a cellulose solution. J Mater Chem B. 2015;3(38):7518–7528. doi: 10.1039/c5tb00477b. [DOI] [PubMed] [Google Scholar]
- 134.Tra Thanh N., Ho Hieu M., Tran Minh Phuong N., Do Bui Thuan T., Nguyen Thi Thu H., Thai Van P., et al. Optimization and characterization of electrospun polycaprolactone coated with gelatin-silver nanoparticles for wound healing application. Mater Sci Eng C. 2018;91:318–329. doi: 10.1016/j.msec.2018.05.039. [DOI] [PubMed] [Google Scholar]
- 135.Liang Y., Li Z., Huang Y., Yu R., Guo B. Dual-dynamic-bond cross-linked antibacterial adhesive hydrogel sealants with on-demand removability for post-wound-closure and infected wound healing. ACS Nano. 2021;15(4):7078–7093. doi: 10.1021/acsnano.1c00204. [DOI] [PubMed] [Google Scholar]
- 136.Wang X., Wang Y., Bi S., Wang Y., Chen X., Qiu L., et al. Optically transparent antibacterial films capable of healing multiple scratches. Adv Funct Mater. 2014;24(3):403–411. [Google Scholar]
- 137.Reyes-Ortega F., Cifuentes A., Rodriguez G., Rosa Aguilar M., Gonzalez-Gomez A., Solis R., et al. Bioactive bilayered dressing for compromised epidermal tissue regeneration with sequential activity of complementary agents. Acta Biomater. 2015;23:103–115. doi: 10.1016/j.actbio.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 138.Zhao X., Dong R.N., Guo B.L., Ma P.X. Dopamine-incorporated dual bioactive electroactive shape memory polyurethane elastomers with physiological shape recovery temperature, high stretchability, and enhanced C2C12 myogenic differentiation. ACS Appl Mater Interfaces. 2017;9(35):29595–29611. doi: 10.1021/acsami.7b10583. [DOI] [PubMed] [Google Scholar]
- 139.Jing Q., Zhi L., Shuolin C., Tamas N., May P.X. Synthesis and evaluation of an amphiphilic deferoxamine: gallium-conjugated cationic random copolymer against a murine wound healing infection model of pseudomonas aeruginosa. Acta Biomater. 2021;126:384–393. doi: 10.1016/j.actbio.2021.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Powers J.G., Morton L.M., Phillips T.J. Dressings for chronic wounds. Dermatol Ther. 2013;26(3):197–206. doi: 10.1111/dth.12055. [DOI] [PubMed] [Google Scholar]
- 141.Eskandarinia A., Kefayat A., Rafienia M., Agheb M., Navid S., Ebrahimpour K. Cornstarch-based wound dressing incorporated with hyaluronic acid and propolis: in vitro and in vivo studies. Carbohydr Polym. 2019;216:25–35. doi: 10.1016/j.carbpol.2019.03.091. [DOI] [PubMed] [Google Scholar]
- 142.McLaughlin S., Ahumada M., Franco W., Mah T.F., Seymour R., Suuronen E.J., et al. Sprayable peptide-modified silver nanoparticles as a barrier against bacterial colonization. Nanoscale. 2016;8(46):19200–19203. doi: 10.1039/c6nr07976h. [DOI] [PubMed] [Google Scholar]
- 143.Chen S., Wang H., Jian Z., Fei G., Qian W., Luo G., et al. Novel poly(vinyl alcohol)/chitosan/modified graphene oxide biocomposite for wound dressing application. Macromol Biosci. 2020;20(3) doi: 10.1002/mabi.201900385. [DOI] [PubMed] [Google Scholar]
- 144.Lin S.Y., Chen K.S., Liang R.C. Design and evaluation of drug-loaded wound dressing having thermo responsive, adhesive, absorptive and easy peeling properties. Biomaterials. 2001;22(22):2999–3004. doi: 10.1016/s0142-9612(01)00046-1. [DOI] [PubMed] [Google Scholar]
- 145.Veeruraj A., Liu L., Zheng J., Wu J., Arumugam M. Evaluation of astaxanthin incorporated collagen film developed from the outer skin waste of squid Doryteuthis singhalensis for wound healing and tissue regenerative applications. Mater Sci Eng C. 2019;95:29–42. doi: 10.1016/j.msec.2018.10.055. [DOI] [PubMed] [Google Scholar]
- 146.Bajpai S.K., Ahuja S., Chand N., Bajpai M. Nano cellulose dispersed chitosan film with Ag NPs/curcumin: an in vivo study on Albino Rats for wound dressing. Int J Biol Macromol. 2017;104(Pt A):1012–1019. doi: 10.1016/j.ijbiomac.2017.06.096. [DOI] [PubMed] [Google Scholar]
- 147.Chattopadhyay S., Raines R.T. Collagen-based biomaterials for wound healing. Biopolymers. 2014;101(8):821–833. doi: 10.1002/bip.22486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Li M., Chen J., Shi M., Zhang H., Ma P.X., Guo B. Electroactive anti-oxidant polyurethane elastomers with shape memory property as non-adherent wound dressing to enhance wound healing. Chem Eng J. 2019;375 [Google Scholar]
- 149.Contardi M., Russo D., Suarato G., Heredia-Guerrero J.A., Ceseracciu L., Penna I., et al. Polyvinylpyrrolidone/hyaluronic acid-based bilayer constructs for sequential delivery of cutaneous antiseptic and antibiotic. Chem Eng J. 2019;358:912–923. [Google Scholar]
- 150.Zhang D., Zhou W., Wei B., Wang X., Tang R., Nie J., et al. Carboxyl-modified poly(vinyl alcohol)-crosslinked chitosan hydrogel films for potential wound dressing. Carbohydr Polym. 2015;125:189–199. doi: 10.1016/j.carbpol.2015.02.034. [DOI] [PubMed] [Google Scholar]
- 151.Liu Y., Kim H.I. Characterization and antibacterial properties of genipin-crosslinked chitosan/poly(ethylene glycol)/ZnO/Ag nanocomposites. Carbohydr Polym. 2012;89(1):111–116. doi: 10.1016/j.carbpol.2012.02.058. [DOI] [PubMed] [Google Scholar]
- 152.Wang W., Zheng T., Sheng B., Zhou T., Zhang Q., Wu F., et al. Functionalization of polyvinyl alcohol composite film wrapped in A(m)-ZnO@CuO@Au nanoparticles for antibacterial application and wound healing. Appl Mater Today. 2019;17:36–44. [Google Scholar]
- 153.Xue J., Wang X., Wang E., Li T., Chang J., Wu C. Bioinspired multifunctional biomaterials with hierarchical microstructure for wound dressing. Acta Biomater. 2019;100:270–279. doi: 10.1016/j.actbio.2019.10.012. [DOI] [PubMed] [Google Scholar]
- 154.Liang Y., He J., Guo B. Functional hydrogels as wound dressing to enhance wound healing. ACS Nano. 2021;15(8):12687–12722. doi: 10.1021/acsnano.1c04206. [DOI] [PubMed] [Google Scholar]
- 155.Ng V.W.L., Chan J.M.W., Sardon H., Ono R.J., Garcia J.M., Yang Y.Y., et al. Antimicrobial hydrogels: a new weapon in the arsenal against multidrug-resistant infections. Adv Drug Deliv Rev. 2014;78:46–62. doi: 10.1016/j.addr.2014.10.028. [DOI] [PubMed] [Google Scholar]
- 156.Xu W., Dong S., Han Y., Li S., Liu Y. Hydrogels as antibacterial biomaterials. Curr Pharm Des. 2018;24(8):843–854. doi: 10.2174/1381612824666180213122953. [DOI] [PubMed] [Google Scholar]
- 157.Liang Y., Wang M., Zhang Z., Ren G., Liu Y., Wu S., et al. Facile synthesis of ZnO QDs@GO-CS hydrogel for synergetic antibacterial applications and enhanced wound healing. Chem Eng J. 2019;378 [Google Scholar]
- 158.Liu Y., Li F., Guo Z., Xiao Y., Zhang Y., Sun X., et al. Silver nanoparticle-embedded hydrogel as a photothermal platform for combating bacterial infections. Chem Eng J. 2020;382 [Google Scholar]
- 159.Li M., Liang Y., He J., Zhang H., Guo B. Two-pronged strategy of biomechanically active and biochemically multifunctional hydrogel wound dressing to accelerate wound closure and wound healing. Chem Mater. 2020;32(23):9937–9953. [Google Scholar]
- 160.Yang Y., Liang Y., Chen J., Duan X., Guo B. Mussel-inspired adhesive antioxidant antibacterial hemostatic composite hydrogel wound dressing via photo-polymerization for infected skin wound healing. Bioactive Mater. 2022;8:341–354. doi: 10.1016/j.bioactmat.2021.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Qu J., Zhao X., Ma P.X., Guo B.L. Injectable antibacterial conductive hydrogels with dual response to an electric field and pH for localized "smart" drug release. Acta Biomater. 2018;72:55–69. doi: 10.1016/j.actbio.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 162.Zhao X., Guo B.L., Ma P.X. Single component thermo-gelling electroactive hydrogels from poly(caprolactone)-poly(ethylene glycol)-poly(caprolactone)-graft-aniline tetramer amphiphilic copolymers. J Mater Chem B. 2015;3(43):8459–8468. doi: 10.1039/c5tb01658d. [DOI] [PubMed] [Google Scholar]
- 163.Zhao J., Guo B.L., Ma P.X. Injectable alginate microsphere/PLGA-PEG-PLGA composite hydrogels for sustained drug release. RSC Adv. 2014;4(34):17736–17742. [Google Scholar]
- 164.Yin M., Wan S., Ren X., Chu C.C. Development of inherently antibacterial, biodegradable, and biologically active chitosan/pseudo-protein hybrid hydrogels as biofunctional wound dressings. ACS Appl Mater Interfaces. 2021;13(12):14688–14699. doi: 10.1021/acsami.0c21680. [DOI] [PubMed] [Google Scholar]
- 165.Zhou L., zheng H., Liu Z., Wang S., Liu Z., Chen F., et al. Conductive antibacterial hemostatic multifunctional scaffolds based on Ti3C2Tx MXene nanosheets for promoting multidrug-resistant bacteria-infected wound healing. ACS Nano. 2021;15(2):2468–2480. doi: 10.1021/acsnano.0c06287. [DOI] [PubMed] [Google Scholar]
- 166.Liang C., Zhengwei C., Tingjun Y., Xiaohua Y., Zhijie C., Yufei Y., et al. Injectable polypeptide-protein hydrogels for promoting infected wound healing. Adv Funct Mater. 2020;30(25) [Google Scholar]
- 167.Cheng H., Linyu L., Juan C., Shumang Z., Yunbing W. Dual-crosslinked mussel-inspired smart hydrogels with enhanced antibacterial and angiogenic properties for chronic infected diabetic wound treatment via pH-responsive quick cargo release. Chem Eng J. 2021;411 [Google Scholar]
- 168.Qu J., Zhao X., Liang Y., Xu Y., Ma P.X., Guo B. Degradable conductive injectable hydrogels as novel antibacterial, anti-oxidant wound dressings for wound healing. Chem Eng J. 2019;362:548–560. [Google Scholar]
- 169.Liang Y.P., Zhao X., Ma P.X., Guo B.L., Du Y.P., Han X.Z. pH-responsive injectable hydrogels with mucosal adhesiveness based on chitosan-grafted-dihydrocaffeic acid and oxidized pullulan for localized drug delivery. J Colloid Interface Sci. 2019;536:224–234. doi: 10.1016/j.jcis.2018.10.056. [DOI] [PubMed] [Google Scholar]
- 170.Zhao X., Zhang M.Y., Guo B.L., Ma P.X. Mussel-inspired injectable supramolecular and covalent bond crosslinked hydrogels with rapid self-healing and recovery properties via a facile approach under metal-free conditions. J Mater Chem B. 2016;4(41):6644–6651. doi: 10.1039/c6tb01776b. [DOI] [PubMed] [Google Scholar]
- 171.Wu Y.B., Wang L., Zhao X., Hou S., Guo B.L., Ma P.X. Self-healing supramolecular bioelastomers with shape memory property as a multifunctional platform for biomedical applications via modular assembly. Biomaterials. 2016;104:18–31. doi: 10.1016/j.biomaterials.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 172.Cheng H., Shi Z., Yue K., Huang X., Xu Y., Gao C., et al. Sprayable hydrogel dressing accelerates wound healing with combined reactive oxygen species-scavenging and antibacterial abilities. Acta Biomater. 2021;124:219–232. doi: 10.1016/j.actbio.2021.02.002. [DOI] [PubMed] [Google Scholar]
- 173.Liu W., Ou-Yang W., Zhang C., Wang Q., Pan X., Huang P., et al. Synthetic polymeric antibacterial hydrogel for methicillin-resistant staphylococcus aureus-infected wound healing: nanoantimicrobial self-assembly, drug and cytokine-free strategy. ACS Nano. 2020;14(10):12905–12917. doi: 10.1021/acsnano.0c03855. [DOI] [PubMed] [Google Scholar]
- 174.Tang X., Chen X., Zhang S., Gu X., Wu R., Huang T., et al. Silk-inspired in situ hydrogel with anti-tumor immunity enhanced photodynamic therapy for melanoma and infected wound healing. Adv Funct Mater. 2021;31(17) [Google Scholar]
- 175.Chen C., Yang X., Li S.J., Zhang C., Ma Y.N., Ma Y.-.X., et al. Tannic acid–thioctic acid hydrogel: a novel injectable supramolecular adhesive gel for wound healing. Green Chem. 2021;23(4):1794–1804. [Google Scholar]
- 176.Dong R., Guo B. Smart wound dressings for wound healing. Nano Today. 2021;41 [Google Scholar]
- 177.Li M., Liang Y., Liang Y., Pan G., Guo B. Injectable stretchable self-healing dual dynamic network hydrogel as adhesive anti-oxidant wound dressing for photothermal clearance of bacteria and promoting wound healing of MRSA infected motion wounds. Chem Eng J. 2022;427 [Google Scholar]
- 178.Deng Z., Yu R., Guo B. Stimuli-responsive conductive hydrogels: design, properties, and applications. Mater Chem Front. 2021;5(5):2092–2123. [Google Scholar]
- 179.Chen W., Zhu Y., Zhang Z., Gao Y., Liu W., Borjihan Q., et al. Engineering a multifunctional N-halamine-based antibacterial hydrogel using a super-convenient strategy for infected skin defect therapy. Chem Eng J. 2020;379 [Google Scholar]
- 180.Zhao X., Wu H., Guo B., Dong R., Qiu Y., Ma P.X. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials. 2017;122:34–47. doi: 10.1016/j.biomaterials.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 181.Chen H., Cheng J., Ran L., Yu K., Lu B., Lan G., et al. An injectable self-healing hydrogel with adhesive and antibacterial properties effectively promotes wound healing. Carbohydr Polym. 2018;201:522–531. doi: 10.1016/j.carbpol.2018.08.090. [DOI] [PubMed] [Google Scholar]
- 182.Wang L., Zhang X., Yang K., Fu Y.V., Xu T., Li S., et al. A novel double-crosslinking-double-network design for injectable hydrogels with enhanced tissue adhesion and antibacterial capability for wound treatment. Adv Funct Mater. 2020;30(1) [Google Scholar]
- 183.Shi M., Zhang H., Song T., Liu X., Gao Y., Zhou J., et al. Sustainable dual release of antibiotic and growth factor from pH-responsive uniform alginate composite microparticles to enhance wound healing. ACS Appl Mater Interfaces. 2019;11(25):22730–22744. doi: 10.1021/acsami.9b04750. [DOI] [PubMed] [Google Scholar]
- 184.Marchesan S., Qu Y., Waddington L.J., Easton C.D., Glattauer V., Lithgow T.J., et al. Self-assembly of ciprofloxacin and a tripeptide into an antimicrobial nanostructured hydrogel. Biomaterials. 2013;34(14):3678–3687. doi: 10.1016/j.biomaterials.2013.01.096. [DOI] [PubMed] [Google Scholar]
- 185.Liu Y., Sui Y., Liu C., Liu C., Wu M., Li B., et al. A physically crosslinked polydopamine/nanocellulose hydrogel as potential versatile vehicles for drug delivery and wound healing. Carbohydr Polym. 2018;188:27–36. doi: 10.1016/j.carbpol.2018.01.093. [DOI] [PubMed] [Google Scholar]
- 186.Liang Y., Zhao X., Hu T., Han Y., Guo B. Mussel-inspired, antibacterial, conductive, antioxidant, injectable composite hydrogel wound dressing to promote the regeneration of infected skin. J Colloid Interface Sci. 2019;556:514–528. doi: 10.1016/j.jcis.2019.08.083. [DOI] [PubMed] [Google Scholar]
- 187.Liang Y., Zhao X., Hu T., Chen B., Yin Z., Ma P.X., et al. Adhesive hemostatic conducting injectable composite hydrogels with sustained drug release and photothermal antibacterial activity to promote full-thickness skin regeneration during wound healing. Small. 2019;15(12) doi: 10.1002/smll.201900046. [DOI] [PubMed] [Google Scholar]
- 188.Yu N., Wang X., Qiu L., Cai T., Jiang C., Sun Y., et al. Bacteria-triggered hyaluronan/AgNPs/gentamicin nanocarrier for synergistic bacteria disinfection and wound healing application. Chem Eng J. 2020;380 [Google Scholar]
- 189.McMahon S., Kennedy R., Duffy P., Vasquez J.M., Wall J.G., Tai H., et al. Poly(ethylene glycol)-based hyperbranched polymer from RAFT and its application as a silver-sulfadiazine-loaded antibacterial hydrogel in wound care. ACS Appl Mater Interfaces. 2016;8(40):26648–26656. doi: 10.1021/acsami.6b11371. [DOI] [PubMed] [Google Scholar]
- 190.Ninan N., Forget A., Shastri V.P., Voelcker N.H., Blencowe A. Antibacterial and anti-inflammatory pH-responsive tannic acid-carboxylated agarose composite hydrogels for wound healing. ACS Appl Mater Interfaces. 2016;8(42):28511–28521. doi: 10.1021/acsami.6b10491. [DOI] [PubMed] [Google Scholar]
- 191.Li M., Liu X., Tan L., Cui Z., Yang X., Li Z., et al. Noninvasive rapid bacteria-killing and acceleration of wound healing through photothermal/photodynamic/copper ion synergistic action of a hybrid hydrogel. Biomater Sci. 2018;6(8):2110–2121. doi: 10.1039/c8bm00499d. [DOI] [PubMed] [Google Scholar]
- 192.Fan Z., Liu B., Wang J., Zhang S., Lin Q., Gong P., et al. A novel wound dressing based on Ag/graphene polymer hydrogel: effectively kill bacteria and accelerate wound healing. Adv Funct Mater. 2014;24(25):3933–3943. [Google Scholar]
- 193.Song A., Rane A.A., Christman K.L. Antibacterial and cell-adhesive polypeptide and poly(ethylene glycol) hydrogel as a potential scaffold for wound healing. Acta Biomater. 2012;8(1):41–50. doi: 10.1016/j.actbio.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wang R., Li J., Chen W., Xu T., Yun S., Xu Z., et al. A biomimetic mussel-inspired epsilon-poly-L-lysine hydrogel with robust tissue-anchor and anti-infection capacity. Adv Funct Mater. 2017;27(8) [Google Scholar]
- 195.Mao C., Xiang Y., Liu X., Cui Z., Yang X., Li Z., et al. Repeatable photodynamic therapy with triggered signaling pathways of fibroblast cell proliferation and differentiation to promote bacteria-accompanied wound healing. ACS Nano. 2018;12(2):1747–1759. doi: 10.1021/acsnano.7b08500. [DOI] [PubMed] [Google Scholar]
- 196.Deng H., Yu Z., Chen S., Fei L., Sha Q., Zhou N., et al. Facile and eco-friendly fabrication of polysaccharides-based nanocomposite hydrogel for photothermal treatment of wound infection. Carbohydr Polym. 2020;230 doi: 10.1016/j.carbpol.2019.115565. [DOI] [PubMed] [Google Scholar]
- 197.Mai B., Jia M., Liu S., Sheng Z., Li M., Gao Y., et al. Smart hydrogel-based DVDMS/bFGF nanohybrids for antibacterial phototherapy with multiple damaging sites and accelerated wound healing. ACS Appl Mater Interfaces. 2020;12(9):10156–10169. doi: 10.1021/acsami.0c00298. [DOI] [PubMed] [Google Scholar]
- 198.Zhang X., Zhang C., Yang Y., Zhang H., Huang X., Hang R., et al. Light-assisted rapid sterilization by a hydrogel incorporated with Ag3PO4/MoS2 composites for efficient wound disinfection. Chem Eng J. 2019;374:596–604. [Google Scholar]
- 199.Zhang X., Zhang G., Zhang H., Liu X., Shi J., Shi H., et al. A bifunctional hydrogel incorporated with CuS@MoS2 microspheres for disinfection and improved wound healing. Chem Eng J. 2020;382 [Google Scholar]
- 200.Wu D.Q., Zhu J., Han H., Zhang J.Z., Wu F.F., Qin X.H., et al. Synthesis and characterization of arginine-NIPAAm hybrid hydrogel as wound dressing: in vitro and in vivo study. Acta Biomater. 2018;65:305–316. doi: 10.1016/j.actbio.2017.08.048. [DOI] [PubMed] [Google Scholar]
- 201.Chen M., Tian J., Liu Y., Cao H., Li R., Wang J., et al. Dynamic covalent constructed self-healing hydrogel for sequential delivery of antibacterial agent and growth factor in wound healing. Chem Eng J. 2019;373:413–424. [Google Scholar]
- 202.Gao G., Jiang Y.W., Jia H.R., Wu F.G. Near-infrared light-controllable on-demand antibiotics release using thermo-sensitive hydrogel-based drug reservoir for combating bacterial infection. Biomaterials. 2019;188:83–95. doi: 10.1016/j.biomaterials.2018.09.045. [DOI] [PubMed] [Google Scholar]
- 203.Zhao Y., Li Z., Song S., Yang K., Liu H., Yang Z., et al. Skin-inspired antibacterial conductive hydrogels for epidermal sensors and diabetic foot wound dressings. Adv Funct Mater. 2019;29(31) [Google Scholar]
- 204.Mao C., Xiang Y., Liu X., Cui Z., Yang X., Yeung K.W.K., et al. Photo-inspired antibacterial activity and wound healing acceleration by hydrogel embedded with Ag/Ag@AgCl/ZnO nanostructures. ACS Nano. 2017;11(9):9010–9021. doi: 10.1021/acsnano.7b03513. [DOI] [PubMed] [Google Scholar]
- 205.Yan X., Fang W.W., Xue J., Sun T.C., Dong L., Zha Z., et al. Thermoresponsive in situ forming hydrogel with sol-gel irreversibility for effective methicillin-resistant staphylococcus aureus infected wound healing. ACS Nano. 2019;13(9):10074–10084. doi: 10.1021/acsnano.9b02845. [DOI] [PubMed] [Google Scholar]
- 206.Wang Y., Lu Y., Zhang J., Hu X., Yang Z., Guo Y., et al. A synergistic antibacterial effect between terbium ions and reduced graphene oxide in a poly(vinyl alcohol)-alginate hydrogel for treating infected chronic wounds. J Mater Chem B. 2019;7(4):538–547. doi: 10.1039/c8tb02679c. [DOI] [PubMed] [Google Scholar]
- 207.Guo B.L., Yuan J.F., Gao Q.Y. pH and ionic sensitive chitosan/carboxymethyl chitosan IPN complex films for the controlled release of coenzyme A. Colloid Polym Sci. 2008;286(2):175–181. [Google Scholar]
- 208.Zhao X., Liang Y., Huang Y., He J., Han Y., Guo B. Physical double-network hydrogel adhesives with rapid shape adaptability, fast self-healing, antioxidant and NIR/pH stimulus-responsiveness for multidrug-resistant bacterial infection and removable wound dressing. Adv Funct Mater. 2020;30(17) [Google Scholar]
- 209.Hu W., Wang Z., Xu Y., Wang X., Xiao Y., Zhang S., et al. Remodeling of inherent antimicrobial nanofiber dressings with melamine-modified fibroin into neoskin. J Mater Chem B. 2019;7(21):3412–3423. [Google Scholar]
- 210.Cheng R., Liu L., Xiang Y., Lu Y., Deng L., Zhang H., et al. Advanced liposome-loaded scaffolds for therapeutic and tissue engineering applications. Biomaterials. 2020;232 doi: 10.1016/j.biomaterials.2019.119706. [DOI] [PubMed] [Google Scholar]
- 211.Augustine R., Kalarikkal N., Thomas S. Electrospun PCL membranes incorporated with biosynthesized silver nanoparticles as antibacterial wound dressings. Appl Nanosci. 2016;6(3):337–344. [Google Scholar]
- 212.Cai Z.X., Mo X.M., Zhang K.H., Fan L.P., Yin A.L., He C.L., et al. Fabrication of chitosan/silk fibroin composite nanofibers for wound-dressing applications. Int J Mol Sci. 2010;11(9):3529–3539. doi: 10.3390/ijms11093529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Chen Y., Lu W., Guo Y., Zhu Y., Song Y. Electrospun gelatin fibers surface loaded ZnO particles as a potential biodegradable antibacterial wound dressing. Nanomaterials. 2019;9(4):525. doi: 10.3390/nano9040525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Faraz C., Tahereh M., Ali Hossein R., Ali Mohammad S., Aziz G., Jhamak N., et al. Design, fabrication, and optimization of a dual function three-layer scaffold for controlled release of metformin hydrochloride to alleviate fibrosis and accelerate wound healing. Acta Biomater. 2020;113:144–163. doi: 10.1016/j.actbio.2020.06.031. [DOI] [PubMed] [Google Scholar]
- 215.Bui H.T., Chung O.H., Dela Cruz J., Park J.S. Fabrication and characterization of electrospun curcumin-loaded polycaprolactone-polyethylene glycol nanofibers for enhanced wound healing. Macromol Res. 2014;22(12):1288–1296. [Google Scholar]
- 216.Jian X., Hao Z., Faquan Y., Ying P., Luo X. Superclear, porous cellulose membranes with chitosan-coated nanofibers for visualized cutaneous wound healing dressing. ACS Appl Mater Interfaces. 2020;12(21):24370–24379. doi: 10.1021/acsami.0c05604. [DOI] [PubMed] [Google Scholar]
- 217.He J., Liang Y., Shi M., Guo B. Anti-oxidant electroactive and antibacterial nanofibrous wound dressings based on poly(epsilon-caprolactone)/quaternized chitosan-graft-polyaniline for full-thickness skin wound healing. Chem Eng J. 2020;385 [Google Scholar]
- 218.Wang L., Wu Y.B., Hu T.L., Ma P.X., Guo B.L. Aligned conductive core-shell biomimetic scaffolds based on nanofiber yarns/hydrogel for enhanced 3D neurite outgrowth alignment and elongation. Acta Biomater. 2019;96:175–187. doi: 10.1016/j.actbio.2019.06.035. [DOI] [PubMed] [Google Scholar]
- 219.Wu Y.B., Wang L., Guo B.L., Ma P.X. Interwoven aligned conductive nanofiber yarn/hydrogel composite scaffolds for engineered 3D cardiac anisotropy. ACS Nano. 2017;11(6):5646–5659. doi: 10.1021/acsnano.7b01062. [DOI] [PubMed] [Google Scholar]
- 220.Chen J., Ge J., Guo B.L., Gao K., Ma P.X. Nanofibrous polylactide composite scaffolds with electroactivity and sustained release capacity for tissue engineering. J Mater Chem B. 2016;4(14):2477–2485. doi: 10.1039/c5tb02703a. [DOI] [PubMed] [Google Scholar]
- 221.Rieger K.A., Birch N.P., Schiffman J.D. Designing electrospun nanofiber mats to promote wound healing - a review. J Mater Chem B. 2013;1(36):4531–4541. doi: 10.1039/c3tb20795a. [DOI] [PubMed] [Google Scholar]
- 222.Gong M., Wan P., Ma D., Zhong M., Liao M., Ye J., et al. Flexible breathable nanomesh electronic devices for on-demand therapy. Adv Funct Mater. 2019;29(26) [Google Scholar]
- 223.Unnithan A.R., Gnanasekaran G., Sathishkumar Y., Lee Y.S., Kim C.S. Electrospun antibacterial polyurethane-cellulose acetate-zein composite mats for wound dressing. Carbohydr Polym. 2014;102:884–892. doi: 10.1016/j.carbpol.2013.10.070. [DOI] [PubMed] [Google Scholar]
- 224.Fu R., Li C., Yu C., Xie H., Shi S., Li Z., et al. A novel electrospun membrane based on moxifloxacin hydrochloride/poly(vinyl alcohol)/sodium alginate for antibacterial wound dressings in practical application. Drug Deliv. 2016;23(3):828–839. doi: 10.3109/10717544.2014.918676. [DOI] [PubMed] [Google Scholar]
- 225.Zhang Y., Chang M., Bao F., Xing M., Wang E., Xu Q., et al. Multifunctional Zn doped hollow mesoporous silica/polycaprolactone electrospun membranes with enhanced hair follicle regeneration and antibacterial activity for wound healing. Nanoscale. 2019;11(13):6315–6333. doi: 10.1039/c8nr09818b. [DOI] [PubMed] [Google Scholar]
- 226.Ajma G., Bonde G.V., Mittal P., Khan G., Pandey V.K., Bakade B.V., et al. Biomimetic PCL-gelatin based nanofibers loaded with ciprofloxacin hydrochloride and quercetin: a potential antibacterial and anti-oxidant dressing material for accelerated healing of a full thickness wound. Int J Pharm. 2019;567 doi: 10.1016/j.ijpharm.2019.118480. [DOI] [PubMed] [Google Scholar]
- 227.GhayamiNejad A., Unnithan A.R., Ramachandra A., Sasikala K., Samarikhalaj M., Thomas R.G., et al. Mussel-inspired electrospun nanofibers functionalized with size-controlled silver nanoparticles for wound dressing application. ACS Appl Mater Interfaces. 2015;7(22):12176–12183. doi: 10.1021/acsami.5b02542. [DOI] [PubMed] [Google Scholar]
- 228.Nudelman R., Alhmoud H., Delalat B., Fleicher S., Fine E., Guliakhmedova T., et al. Jellyfish-based smart wound dressing devices containing in situ synthesized antibacterial nanoparticles. Adv Funct Mater. 2019;29(38) [Google Scholar]
- 229.Ahmed R., Tariq M., Ali I., Asghar R., Khanam P.N., Augustine R., et al. Novel electrospun chitosan/polyvinyl alcohol/zinc oxide nanofibrous mats with antibacterial and antioxidant properties for diabetic wound healing. Int J Biol Macromol. 2018;120(Pt A):385–393. doi: 10.1016/j.ijbiomac.2018.08.057. [DOI] [PubMed] [Google Scholar]
- 230.Shahmoradi S., Golzar H., Hashemi M., Mansouri V., Omidi M., Yazdian F., et al. Optimizing the nanostructure of graphene oxide/silver/arginine for effective wound healing. Nanotechnology. 2018;29(47) doi: 10.1088/1361-6528/aadedc. [DOI] [PubMed] [Google Scholar]
- 231.Hadisi Z., Farokhi M., Bakhsheshi-Rad H.R., Jahanshahi M., Hasanpour S., Pagan E., et al. Hyaluronic acid (HA)-based silk fibroin/zinc oxide core-shell electrospun dressing for burn wound management. Macromol Biosci. 2020;20(4) doi: 10.1002/mabi.201900328. [DOI] [PubMed] [Google Scholar]
- 232.Li W., Yu Q., Yao H., Zhu Y., Topham P.D., Yue K., et al. Superhydrophobic hierarchical fiber/bead composite membranes for efficient treatment of burns. Acta Biomater. 2019;92:60–70. doi: 10.1016/j.actbio.2019.05.025. [DOI] [PubMed] [Google Scholar]
- 233.Liu B., Yao T., Ren L., Zhao Y., Yuan X. Antibacterial PCL electrospun membranes containing synthetic polypeptides for biomedical purposes. Colloids Surf, B. 2018;172:330–337. doi: 10.1016/j.colsurfb.2018.08.055. [DOI] [PubMed] [Google Scholar]
- 234.Huang Y., Dan N., Dan W., Zhao W., Bai Z., Chen Y., et al. Bilayered antimicrobial nanofiber membranes for wound dressings via in situ cross-linking polymerization and electrospinning. Ind Eng Chem Res. 2018;57(50):17048–17057. [Google Scholar]
- 235.Severyukhina A.N., Petrova N.V., Yashchenok A.M., Bratashov D.N., Smuda K., Mamonova I.A., et al. Light-induced antibacterial activity of electrospun chitosan-based material containing photosensitizer. Mater Sci Eng C. 2017;70(Pt 1):311–316. doi: 10.1016/j.msec.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 236.Qiu Y., Wang Q., Chen Y., Xia S., Huang W., Wei Q. A novel multilayer composite membrane for wound healing in mice skin defect model. Polymers (Basel) 2020;12(3):573. doi: 10.3390/polym12030573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Wang X., Lv F., Li T., Han Y., Yi Z., Liu M., et al. Electrospun micropatterned nanocomposites incorporated with Cu2S nanoflowers for skin tumor therapy and wound healing. ACS Nano. 2017;11(11):11337–11349. doi: 10.1021/acsnano.7b05858. [DOI] [PubMed] [Google Scholar]
- 238.Ranjbar-Mohammadi M., Rabbani S., Bahrami S.H., Joghataei M.T. Moayer F. Antibacterial performance and in vivo diabetic wound healing of curcumin loaded gum tragacanth/poly(epsilon-caprolactone) electrospun nanofibers. Mater Sci Eng C. 2016;69:1183–1191. doi: 10.1016/j.msec.2016.08.032. [DOI] [PubMed] [Google Scholar]
- 239.Yang X., Yang J., Wang L., Ran B., Jia Y., Zhang L., et al. Pharmaceutical intermediate-modified gold nanoparticles: against multidrug-resistant bacteria and wound-healing application via an electrospun scaffold. ACS Nano. 2017;11(6):5737–5745. doi: 10.1021/acsnano.7b01240. [DOI] [PubMed] [Google Scholar]
- 240.Unnithan A.R., Barakat N.A.M., Pichiah P.B.T., Gnanasekaran G., Nirmala R., Cha Y.-.S., et al. Wound-dressing materials with antibacterial activity from electrospun polyurethane-dextran nanofiber mats containing ciprofloxacin HCl. Carbohydr Polym. 2012;90(4):1786–1793. doi: 10.1016/j.carbpol.2012.07.071. [DOI] [PubMed] [Google Scholar]
- 241.Zhao X., Liu L., An T., Xian M., Luckanagul J.A., Su Z., et al. A hydrogen sulfide-releasing alginate dressing for effective wound healing. Acta Biomater. 2020;104:85–94. doi: 10.1016/j.actbio.2019.12.032. [DOI] [PubMed] [Google Scholar]
- 242.Jayakumar R., Prabaharan M., Kumar P.T.S., Nair S.V., Tamura H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol Adv. 2011;29(3):322–337. doi: 10.1016/j.biotechadv.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 243.Ye S., Jiang L., Wu J., Su C., Huang C., Liu X., et al. Flexible amoxicillin-grafted bacterial cellulose sponges for wound dressing: in vitro and in vivo evaluation. ACS Appl Mater Interfaces. 2018;10(6):5862–5870. doi: 10.1021/acsami.7b16680. [DOI] [PubMed] [Google Scholar]
- 244.Ma R., Wang Y., Qi H., Shi C., Wei G., Xiao L., et al. Nanocomposite sponges of sodium alginate/graphene oxide/polyvinyl alcohol as potential wound dressing: in vitro and in vivo evaluation. Composites Part B. 2019;167:396–405. [Google Scholar]
- 245.Choi Y.S., Hong S.R., Lee Y.M., Song K.W., Park M.H., Nam Y.S. Study on gelatin-containing artificial skin: I. preparation and characteristics of novel gelatin-alginate sponge. Biomaterials. 1999;20(5):409–417. doi: 10.1016/s0142-9612(98)00180-x. [DOI] [PubMed] [Google Scholar]
- 246.Kim H.J., Choi E.Y., Oh J.S., Lee H.C., Park S.S., Cho C.S. Possibility of wound dressing using poly(L-leucine)/poly(ethylene glycol)/poly(L-leucine) triblock copolymer. Biomaterials. 2000;21(2):131–141. doi: 10.1016/s0142-9612(99)00140-4. [DOI] [PubMed] [Google Scholar]
- 247.Chen H., Lan G., Ran L., Xiao Y., Yu K., Lu B., et al. A novel wound dressing based on a Konjac glucomannan/silver nanoparticle composite sponge effectively kills bacteria and accelerates wound healing. Carbohydr Polym. 2018;183:70–80. doi: 10.1016/j.carbpol.2017.11.029. [DOI] [PubMed] [Google Scholar]
- 248.Shao W., Wu J., Wang S., Huang M., Liu X., Zhang R. Construction of silver sulfadiazine loaded chitosan composite sponges as potential wound dressings. Carbohydr Polym. 2017;157:1963–1970. doi: 10.1016/j.carbpol.2016.11.087. [DOI] [PubMed] [Google Scholar]
- 249.Lu B., Lu F., Zou Y., Liu J., Rong B., Li Z., et al. In situ reduction of silver nanoparticles by chitosan-L-glutamic acid/hyaluronic acid: enhancing antimicrobial and wound-healing activity. Carbohydr Polym. 2017;173:556–565. doi: 10.1016/j.carbpol.2017.06.035. [DOI] [PubMed] [Google Scholar]
- 250.Wang X., Guo J., Zhang Q., Zhu S., Liu L., Jiang X., et al. Gelatin sponge functionalized with gold/silver clusters for antibacterial application. Nanotechnology. 2020;31(13) doi: 10.1088/1361-6528/ab59eb. [DOI] [PubMed] [Google Scholar]
- 251.Lu Z., Gao J., He Q., Wu J., Liang D., Yang H., et al. Enhanced antibacterial and wound healing activities of microporous chitosan-Ag/ZnO composite dressing. Carbohydr Polym. 2017;156:460–469. doi: 10.1016/j.carbpol.2016.09.051. [DOI] [PubMed] [Google Scholar]
- 252.Ma W., Li L., Lin X., Wang Y., Ren X., Huang T.-.S. Novel ZnO/N-halamine-mediated multifunctional dressings as quick antibacterial agent for biomedical applications. ACS Appl Mater Interfaces. 2019;11(34):31411–31420. doi: 10.1021/acsami.9b10857. [DOI] [PubMed] [Google Scholar]
- 253.Wang C., Niu H., Ma X., Hong H., Yuan Y., Liu C. Bioinspired, injectable, quaternized hydroxyethyl cellulose composite hydrogel coordinated by mesocellular silica foam for rapid, noncompressible hemostasis and wound healing. ACS Appl Mater Interfaces. 2019;11(38):34595–34608. doi: 10.1021/acsami.9b08799. [DOI] [PubMed] [Google Scholar]
- 254.Zhang K., Bai X., Yuan Z., Cao X., Jiao X., Li Y., et al. Layered nanofiber sponge with an improved capacity for promoting blood coagulation and wound healing. Biomaterials. 2019;204:70–79. doi: 10.1016/j.biomaterials.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 255.Hu S., Bi S., Yan D., Zhou Z., Sun G., Cheng X., et al. Preparation of composite hydroxybutyl chitosan sponge and its role in promoting wound healing. Carbohydr Polym. 2018;184:154–163. doi: 10.1016/j.carbpol.2017.12.033. [DOI] [PubMed] [Google Scholar]
- 256.Wang X., Zhang D., Wang J., Tang R., Wei B., Jiang Q. Succinyl pullulan-crosslinked carboxymethyl chitosan sponges for potential wound dressing. Int J Polym Mater Polym Biomater. 2017;66(2):61–70. [Google Scholar]
- 257.Gao H., Zhong Z., Xia H., Hu Q., Ye Q., Wang Y., et al. Construction of cellulose nanofibers/quaternized chitin/organic rectorite composites and their application as wound dressing materials. Biomater Sci. 2019;7(6):2571–2581. doi: 10.1039/c9bm00288j. [DOI] [PubMed] [Google Scholar]
- 258.Sanad R.A.-.B., HM Abdel-Bar. Chitosan-hyaluronic acid composite sponge scaffold enriched with Andrographolide-loaded lipid nanoparticles for enhanced wound healing. Carbohydr Polym. 2017;173:441–450. doi: 10.1016/j.carbpol.2017.05.098. [DOI] [PubMed] [Google Scholar]
- 259.Dai M., Zheng X., Xu X., Kong X., Li X., Guo G., et al. Chitosan-alginate sponge: preparation and application in curcumin delivery for dermal wound healing in rat. J Biomed Biotechnol. 2009;2009 doi: 10.1155/2009/595126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Xia G., Zhai D., Sun Y., Hou L., Guo X., Wang L., et al. Preparation of a novel asymmetric wettable chitosan-based sponge and its role in promoting chronic wound healing. Carbohydr Polym. 2020;227 doi: 10.1016/j.carbpol.2019.115296. [DOI] [PubMed] [Google Scholar]
- 261.Li M., Zhang Z., Liang Y., He J., Guo B. Multifunctional tissue-adhesive cryogel wound dressing for rapid nonpressing surface hemorrhage and wound repair. ACS Appl Mater Interfaces. 2020;12(32):35856–35872. doi: 10.1021/acsami.0c08285. [DOI] [PubMed] [Google Scholar]
- 262.Zhao X., Liang Y., Guo B., Yin Z., Han Y. Injectable dry cryogels with excellent blood-sucking expansion and blood clotting to cease hemorrhage for lethal deep-wounds, coagulopathy and tissue regeneration. Chem Eng J. 2021;403 [Google Scholar]
- 263.Huang Y., Zhao X., Zhang Z., Liang Y., Yin Z., Chen B., et al. Degradable gelatin-based IPN cryogel hemostat for rapidly stopping deep noncompressible hemorrhage and simultaneously improving wound healing. Chem Mater. 2020;32(15):6595–6610. [Google Scholar]
- 264.Michailidou G., Christodoulou E., Nanaki S., Barmpalexis P., Karavas E., Vergkizi-Nikolakaki S., et al. Super-hydrophilic and high strength polymeric foam dressings of modified chitosan blends for topical wound delivery of chloramphenicol. Carbohydr Polym. 2019;208:1–13. doi: 10.1016/j.carbpol.2018.12.050. [DOI] [PubMed] [Google Scholar]
- 265.McGann C.L., Streifel B.C., Lundin J.G., Wynne J.H. Multifunctional polyHIPE wound dressings for the treatment of severe limb trauma. Polymer (Guildf) 2017;126:408–418. [Google Scholar]
- 266.Pyun D.G., Yoon H.S., Chung H.Y., Choi H.J., Thambi T., Kimd B.S., et al. Evaluation of AgHAP-containing polyurethane foam dressing for wound healing: synthesis, characterization, in vitro and in vivo studies. J Mater Chem B. 2015;3(39):7752–7763. doi: 10.1039/c5tb00995b. [DOI] [PubMed] [Google Scholar]
- 267.Choi H.J., Thambi T., Yang Y.H., Bang S.I., Kim B.S., Pyun D.G., et al. AgNP and rhEGF-incorporating synergistic polyurethane foam as a dressing material for scar-free healing of diabetic wounds. RSC Adv. 2017;7(23):13714–13725. [Google Scholar]
- 268.Wang P., Zhao J., Xuan R., Wang Y., Zou C., Zhang Z., et al. Flexible and monolithic zinc oxide bionanocomposite foams by a bacterial cellulose mediated approach for antibacterial applications. Dalton Trans. 2014;43(18):6762–6768. doi: 10.1039/c3dt52858h. [DOI] [PubMed] [Google Scholar]
- 269.Buzarovska A., Dinescu S., Lazar A.D., Serban M., Pircalabioru G.G., Costache M., et al. Nanocomposite foams based on flexible biobased thermoplastic polyurethane and ZnO nanoparticles as potential wound dressing materials. Mater Sci Eng C. 2019;104 doi: 10.1016/j.msec.2019.109893. [DOI] [PubMed] [Google Scholar]
- 270.Ding Y., Sun Z., Shi R., Cui H., Liu Y., Mao H., et al. Integrated endotoxin adsorption and antibacterial properties of cationic polyurethane foams for wound healing. ACS Appl Mater Interfaces. 2019;11(3):2860–2869. doi: 10.1021/acsami.8b19746. [DOI] [PubMed] [Google Scholar]
- 271.Tran P.L., Hamood A.N., de Souza A., Schultz G., Liesenfeld B., Mehta D., et al. A study on the ability of quaternary ammonium groups attached to a polyurethane foam wound dressing to inhibit bacterial attachment and biofilm formation. Wound Repair Regen. 2015;23(1):74–81. doi: 10.1111/wrr.12244. [DOI] [PubMed] [Google Scholar]
- 272.Schuhladen K., Mukoo P., Liverani L., Nescakova Z., Boccaccini A.R. Manuka honey and bioactive glass impart methylcellulose foams antibacterial effects for wound healing applications. Biomed Mater. 2020;15(6) doi: 10.1088/1748-605X/ab87e5. [DOI] [PubMed] [Google Scholar]
- 273.Khodabakhshi D., Eskandarinia A., Kefayat A., Rafienia M., Navid S., Karbasi S., et al. In vitro and in vivo performance of a propolis-coated polyurethane wound dressing with high porosity and antibacterial efficacy. Colloids Surf, B. 2019;178:177–184. doi: 10.1016/j.colsurfb.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 274.dos Santos M.R., Alcaraz-Espinoza J.J., da Costa M.M., de Oliveira H.P. Usnic acid-loaded polyaniline/polyurethane foam wound dressing: preparation and bactericidal activity. Mater Sci Eng C. 2018;89:33–40. doi: 10.1016/j.msec.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 275.Kaur A., Chattopadhyay S., Jain S., Tyagi A., Singh H. Preparation of hydrogel impregnated antimicrobial polyurethane foam for absorption of radionuclide contaminated blood and biological fluids. J Appl Polym Sci. 2016;133(30):43625. [Google Scholar]
- 276.Weller C.D., Team V., Sussman G. First-line interactive wound dressing update: a comprehensive review of the evidence. Front Pharmacol. 2020;11(155):155. doi: 10.3389/fphar.2020.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Shi C., Wang C., Liu H., Li Q., Li R., Zhang Y., et al. Selection of appropriate wound dressing for various wounds. Front Bioeng Biotechnol. 2020;8(182):182. doi: 10.3389/fbioe.2020.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Dabiri G., Damstetter E., Phillips T. Choosing a wound dressing based on common wound characteristics. Adv Wound Care. 2016;5(1):32–41. doi: 10.1089/wound.2014.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Zhou F., Hong Y., Liang R., Zhang X., Liao Y., Jiang D., et al. Rapid printing of bio-inspired 3D tissue constructs for skin regeneration. Biomaterials. 2020;258 doi: 10.1016/j.biomaterials.2020.120287. [DOI] [PubMed] [Google Scholar]
- 280.Chouhan D., Dey N., Bhardwaj N., Mandal B.B. Emerging and innovative approaches for wound healing and skin regeneration: current status and advances. Biomaterials. 2019;216 doi: 10.1016/j.biomaterials.2019.119267. [DOI] [PubMed] [Google Scholar]
- 281.Hadi R., Mahnaz R., Kamrul H., Arash M., Tran Thanh T., Sarah V., et al. 3D bioprinting of a cell-laden antibacterial polysaccharide hydrogel composite. Carbohydr Polym. 2021;264 doi: 10.1016/j.carbpol.2021.117989. [DOI] [PubMed] [Google Scholar]
- 282.Xu C., Molino B.Z., Wang X., Cheng F., Xu W., Molino P., et al. 3D printing of nanocellulose hydrogel scaffolds with tunable mechanical strength towards wound healing application. J Mater Chem B. 2018;6(43):7066–7075. doi: 10.1039/c8tb01757c. [DOI] [PubMed] [Google Scholar]
- 283.Xu W., Molino B.Z., Cheng F., Molino P.J., Yue Z., Su D., et al. On low-concentration inks formulated by nanocellulose assisted with gelatin methacrylate (GelMA) for 3D printing toward wound healing application. ACS Appl Mater Interfaces. 2019;11(9):8838–8848. doi: 10.1021/acsami.8b21268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Chen C.S., Zeng F., Xiao X., Wang Z., Li X.L., Tan R.W., et al. Three-dimensionally printed silk-sericin-based hydrogel scaffold: a promising visualized dressing material for real-time monitoring of wounds. ACS Appl Mater Interfaces. 2018;10(40):33879–33890. doi: 10.1021/acsami.8b10072. [DOI] [PubMed] [Google Scholar]
- 285.Do A.V., Khorsand B., Geary S.M., Salem A.K. 3D printing of scaffolds for tissue regeneration applications. Adv Healthcare Mater. 2015;4(12):1742–1762. doi: 10.1002/adhm.201500168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Leppiniemi J., Lahtinen P., Paajanen A., Mahlberg R., Metsa-Kortelainen S., Pinornaa T., et al. 3D-printable bioactivated nanocellulose-alginate hydrogels. ACS Appl Mater Interfaces. 2017;9(26):21959–21970. doi: 10.1021/acsami.7b02756. [DOI] [PubMed] [Google Scholar]
- 287.Pourchet L.J., Thepot A., Albouy M., Courtial E.J., Boher A., Blum L.J., et al. Human skin 3D bioprinting using scaffold-free approach. Adv Healthcare Mater. 2017;6(4) doi: 10.1002/adhm.201601101. [DOI] [PubMed] [Google Scholar]
- 288.Mori N., Morimoto Y., Takeuchi S. Skin integrated with perfusable vascular channels on a chip. Biomaterials. 2017;116:48–56. doi: 10.1016/j.biomaterials.2016.11.031. [DOI] [PubMed] [Google Scholar]
- 289.Wu F., Zheng J., Li Z., Liu M. Halloysite nanotubes coated 3D printed PLA pattern for guiding human mesenchymal stem cells (hMSCs) orientation. Chem Eng J. 2019;359:672–683. [Google Scholar]
- 290.Afghah F., Ullah M., Zanjani J.S.M., Sut P.A., Sen O., Emanet M., et al. 3D printing of silver-doped polycaprolactone-poly(propylene succinate) composite scaffolds for skin tissue engineering. Biomed Mater. 2020;15(3) doi: 10.1088/1748-605X/ab7417. [DOI] [PubMed] [Google Scholar]
- 291.Ashammakhi N., Ahadian S., Pountos I., Hu S.K., Tellisi N., Bandaru P., et al. In situ three-dimensional printing for reparative and regenerative therapy. Biomed Microdevices. 2019;21(2):42. doi: 10.1007/s10544-019-0372-2. [DOI] [PubMed] [Google Scholar]
- 292.Skardal A., Murphy S.V., Crowell K., Mack D., Atala A., Soker S. A tunable hydrogel system for long-term release of cell-secreted cytokines and bioprinted in situ wound cell delivery. J Biomed Mater Res. 2017;105(7):1986–2000. doi: 10.1002/jbm.b.33736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Diogo G.S., Marques C.F., Sotelo C.G., Perez-Martin R.I., Pirraco R.P., Reis R.L., et al. Cell-laden biomimetically mineralized shark-skin-collagen-based 3D printed hydrogels for the engineering of hard tissues. ACS Biomater Sci Eng. 2020;6(6):3664–3672. doi: 10.1021/acsbiomaterials.0c00436. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.