Abstract
Objectives
The prognosis of severe acute pancreatitis (SAP) patients is closely related to early nutritional support. It is well-established that changes in glutamine (Gln), an important amino acid and nutritional supplement, can reflect disease severity. However, no consensus has been reached on the role of Gln nutrition therapy for SAP patients. We conducted this systematic review and meta-analysis to summarize and evaluate the advantages of Gln supplementation in SAP.
Methods
PubMed, Web of Science, the Embase, Cochrane Library, and Chinese databases (CNKI, SinoMed, Wanfang, and VIP) were systematically searched for eligible studies that included glutamine supplementation in SAP patients from inception to October 31 2021, excluding non-SAP studies. Primary outcome measures included mortality, APACHE II score, complications, and length of hospital stay. The meta-analysis was registered with PROSPERO (CRD42021288371) and was conducted using Review Manager and Stata softwares.
Results
This meta-analysis included 30 randomized controlled trials (RCTs) with a total of 1,201 patients. Six primary outcomes and six secondary outcomes were analyzed. For the primary outcomes, Gln supplementation was associated with lower mortality (OR = 0.38, 95% CI: 0.21–0.69, P = 0.001), total hospital stay (MD = −3.41, 95% CI: −4.93 to −1.88, P < 0.0001) and complications (OR = 0.45, 95% CI: 0.31–0.66, P < 0.0001) compared with conventional nutrition. Further subgroup analysis found that parenteral glutamine was more effective in reducing mortality. In terms of secondary outcomes, Gln supplementation helped restore liver, kidney and immune function, with significantly increased serum albumin (SMD = 1.02, 95% CI: 0.74–1.31, P < 0.00001) and IgG levels (MD = 1.24, 95% CI: 0.82–1.67, P < 0.00001), and decreased serum creatinine (Scr) (MD = −12.60, 95% CI: −21.97 to −3.24, P = 0.008), and inflammatory indicators such as C-reaction protein (CRP) (SMD = −1.67, 95% CI: −2.43 to −0.90, P < 0.0001).
Conclusion
Although Gln supplementation is not routinely recommended, it is beneficial for SAP patients. Indeed, glutamine nutrition has little effect on some indicator outcomes but contributes to improving the prognosis of this patient population.
Systematic Review Registration: PROSPERO (york.ac.uk). Unique Identifier: CRD42021288371.
Keywords: glutamine, severe acute pancreatitis, treatment, prognosis, meta-analysis
Introduction
Acute pancreatitis (AP) is a predominant cause of acute abdomen during clinical practice and can be divided into mild, moderately severe, and severe AP according to its severity (1). Although most cases present with mild disease, more than 20% of patients progress to severe acute pancreatitis (SAP) (2, 3). SAP is characterized by hemorrhagic and necrotizing pancreatitis that frequently causes systemic complications and multiorgan failure, resulting in high mortality and poor prognosis (4, 5). As an important feature of SAP, high metabolism and increased protein decomposition rate can easily lead to malnutrition and subsequent immunosuppression, further aggravating SAP (6, 7). Early fasting is a crucial part of the conservative treatment of AP to reduce the secretion of pancreatic enzymes and the inflammatory response. However, it should be borne in mind that due to the high metabolic activity of the disease itself and the lack of exogenous nutritional supplements, early fasting can lead to the further progression of SAP, which ultimately increases the hospitalization time, medical costs and mortality (8, 9). Mounting evidence suggests that early enteral or parenteral nutrition support can help reduce multisystem organ failure (MOF), pancreatic infection complications and mortality, leading to a better prognosis (10).
As the most abundant amino acid in the human body, glutamine (Gln) is widely utilized by the liver, lung and intestine, and its supplementation may correct the negative nitrogen balance caused by SAP, reduce inflammation and improve prognosis (11, 12). However, much controversy surrounds the use of Gln in the treatment of SAP. Petrov et al. (13) found that Gln-supplemented enteral nutrition did not reduce total infectious complications relative to standard enteral nutrition (P = 0.53). This standpoint was confirmed in a meta-analysis by Jiang et al. (14), who demonstrated that Gln-containing enteral nutrition could significantly reduce the risk of multiple organ dysfunction syndromes (MODS) and death and shorten the length of hospital stay. Another study found that parenteral nutrition supplemented with Gln was more effective than enteral nutrition in reducing complications such as infection, pseudocyst and necrosis, but the difference was not statistically significant (15). This is significantly inconsistent with the study by Li et al., who believed that enteral nutrition was more meaningful than parenteral nutrition in reducing pancreatic infection and related complications in patients (OR, 0.41; 95% CI, 0.22–0.77; P = 0.006) (16). In a randomized controlled study that enrolled patients with SAP (n = 47), Gln-containing parenteral nutrition significantly increased serum albumin levels, decreased mortality and morbidity, shortened hospital stay, and improved patient nutritional status (17). The conclusion was consistent with Tina et al. (18). They confirmed that parenteral Gln supplementation significantly reduced the risk of infectious complications by enrolling 226 AP patients (RR = 0.59; 95% CI, 0.39–0.88; P ≤ 0.05) and mortality (RR = 0.26; 95% CI, 0.11–0.59; P ≤ 0.001) and shorter length of hospital stay (MD = −2.93 days; 95% CI, −4.70 to −1.15; P ≤ 0.001). However, the length of hospital stay was not consistent with Varsha et al. (MD = −1.35; 95% CI, −3.25 to 0.56, P = 0.17) (19). Therefore, much heterogeneity surrounds the application of Gln-containing nutritional support therapy in SAP. In addition, the 2019 Chinese Guidelines for the Diagnosis and Treatment of Acute Pancreatitis stated that the application of Gln preparations to SAP patients is beneficial to protect the intestinal mucosal barrier (20), but this was not mentioned in the American Gastroenterological Association Institute Guideline on Initial Management of Acute Pancreatitis (21). Although a meta-analysis by Li et al. (22) attempted to evaluate the efficacy of Gln-rich nutritional support for SAP patients, the small number of included studies limited robustness of the findings. To this end, we included updated studies and data for meta-analysis, including 30 randomized controlled trials (RCTs) and a large sample of more than 1,800 patients. In addition, we performed subgroup analyses of Gln supplementation patterns and even detailed supplementation doses, which were not addressed in previous studies. This will provide more reliable and robust evidence for the efficacy of Gln-containing nutritional therapy.
Methods
Our study was registered in the PROSPERO database (CRD42021288371). This study was conducted according to the Cochrane Handbook 6.0, and the results were presented according to the PRISMA statement (23, 24).
Search Strategy
Databases, including PubMed, Web of Science, the Embase, Cochrane Library, and Chinese databases (CNKI, SinoMed, Wanfang, and VIP), were searched until October 31, 2021. We used the following subject headings plus free words: “SAP (or severe acute pancreatitis, or acute necrotizing pancreatitis, or hemorrhagic necrotic pancreatitis)” and “Gln (or glutamine, or L-Glutamine, or D-Glutamine);” other quality conference papers and journals were also searched. The specific retrieval strategy for PubMed database is provided in Table 1.
Table 1.
Retrieval strategy of PubMed.
| Number | Search strategy |
|---|---|
| #1 | “Glutamine” [MeSH] |
| #2 | “L-Glutamine” [Title/Abstract] |
| #3 | “L Glutamine” [Title/Abstract] |
| #4 | “D-Glutamine” [Title/Abstract] |
| #5 | “D Glutamine” [Title/Abstract] |
| #6 | “Gln” [Title/Abstract] |
| #7 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 |
| #8 | “Pancreatitis, Acute Necrotizing” [MeSH] |
| #9 | “Necrotizing Pancreatitis, Acute” [Title/Abstract] |
| #10 | “Pancreatitis Necrotising” [Title/Abstract] |
| #11 | “Acute Necrotizing Pancreatitis” [Title/Abstract] |
| #12 | “Pancreatitis Necrotizing” [Title/Abstract] |
| #13 | “Necrosis, Pancreatic” [Title/Abstract] |
| #14 | “Hemorrhagic Necrotic Pancreatitis” [Title/Abstract] |
| #15 | “Hemorrhagic Necrotic Pancreatitides” [Title/Abstract] |
| #16 | “Necrotic Pancreatitis, Hemorrhagic” [Title/Abstract] |
| #17 | “Pancreatitis, Hemorrhagic Necrotic” [Title/Abstract] |
| #18 | “Severe Acute Pancreatitis” [Title/Abstract] |
| #19 | “SAP” [Title/Abstract] |
| #20 | #1 OR #2 OR #3 #8OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 |
| #21 | #7 AND #20 |
Inclusion and Exclusion Criteria
Study selection was based on the following criteria: (1) study population: SAP patients; (2) intervention and comparison: treatment with Gln (supplemented enterally or parenterally) or without Gln for SAP patients; (3) outcomes: primary outcomes such as mortality, APACHE II score, shortening intensive care unit (ICU) hospital stay, total length of hospital stay, bloating recovery time, complications and secondary outcomes such as liver function indicators [serum albumin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin (TBIL)], kidney function indicators [serum creatinine (Scr) and blood urea nitrogen (BUN)], inflammatory indicators [C-reaction protein (CRP), interleukin-6 (IL-6), IL-8 and tumor necrosis factor α (TNF-α)], immune indicators (IgA and IgG) and serum amylase recovery time; and (4) study design: randomized controlled trials (RCTs).
Exclusion criteria: (1) research type: review, guideline, systematic review, animal experiment, cell experiment; (2) research content inconsistent with the theme and the full text unavailable; (3) duplicate studies.
Definitions
The diagnosis of AP requires two of the following three criteria: (1) epigastric pain consistent with acute pancreatitis (acute, sudden, persistent and severe abdominal pain that can radiate to the back); (2) serum lipase activity; (3) Enhanced computed tomography (CT)/magnetic resonance imaging (MRI) showed typical AP imaging changes (pancreatic edema or peripancreatic effusion). Cases that met the dignostic criteria of AP with APACHE II score ≥ 8, Ranson score ≥ 3, CT grade D/E, accompanied by persistent (>48 h) organ dysfunction (especially shock, respiratory disorder, and renal insufficiency) and/or local complications (pancreatic necrosis, abscess, and pseudocyst) were diagnosed as SAP (1).
Literature Screening and Data Extraction
Literature screening and data extraction were carried out by two researchers (Dong and Zhao), and any disagreements were resolved through third-party consultation (Li). A table was used to extract the relevant data of the included literature, including: (1) basic information of the study: first author, publication year, sample size, age, and gender ratio; (2) intervention measures: with or without Gln; (3) outcome indicators: mortality, APACHE II score, ICU hospital stay, total length of hospital stay, complications, bloating recovery time, liver function indicators, kidney function indicators, inflammatory indicators, immune indicators, and serum amylase recovery time.
Literature Quality Assessment
The quality of each included study was assessed by the two investigators using tools in the Cochrane Handbook for the Systematic Review of Interventions (23). The evaluation content was divided into six aspects: (1) whether the random allocation method is correct; (2) whether the concealment of the allocation scheme is correct; (3) whether blinding is used; (4) whether the data results are complete; (5) whether there is selective reporting of research results; (6) whether there are other sources of bias.
Statistical Analysis
All studies were analyzed using Review Manager version 5.3 and Stata 12SE. The Odd Ratio (OR) was used as the effect index for dichotomous variables, and the mean difference (MD) or standard mean difference (SMD) for continuous variables. Its estimates and 95% confidence interval (CI) were provided. The chi-square (χ2) and I2 tests were used to evaluate the heterogeneity. If significant heterogeneity was present among studies (P < 0.10 and I2 > 50%), a random-effects model was used; otherwise, a fixed-effects model was used (25). Sensitivity analysis was used to analyze sources of heterogeneity. A P-value <0.05 was statistically significant. Potential publication bias was assessed with funnel plots and Egger's test (26).
Results
Literature Search Results
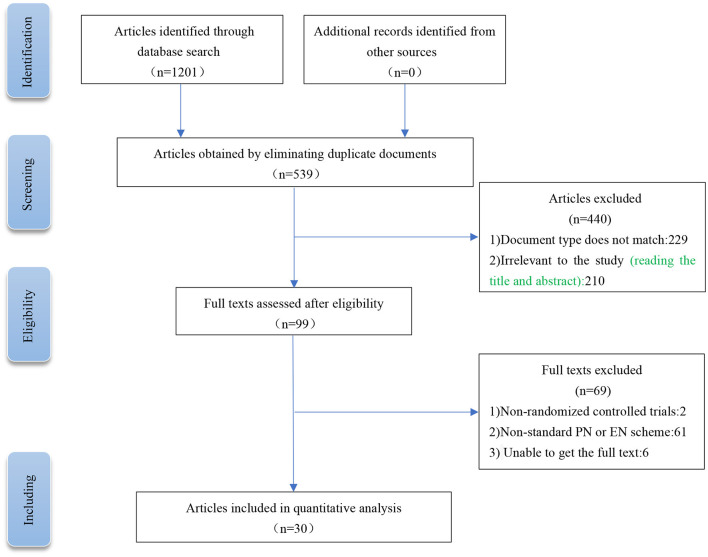
A total of 1,201 related literatures were initially retrieved from the above databases. After screening, browsing the titles and abstracts, and reading the full texts, we finally included 30 RCTs (6, 12, 17, 27–53). The experimental group (nutritional support containing Gln) included 915 patients, and the control group (conventional nutritional support) included 917 patients. The PRISMA flow chart for literature screening is shown in Figure 1. The basic characteristics of the included studies are shown in Table 2.
Figure 1.
Current meta-analysis article searching and screening strategies.
Table 2.
Summary of the basic characteristics of the included studies.
| Reference | Interventions |
No. of subjects enrolled |
Age (years, mean ± SD) |
Sex (M/F) |
APACHE II score |
Outcomes |
|---|---|---|---|---|---|---|
| He et al. (6) | TPN+Gln | 20 | 39.4 ± 8.6 | 11/9 | Unstates | A, D, E, F, G, K |
| TPN | 21 | 40.2 ± 7.8 | 11/10 | Unstates | ||
| Guo et al. (34) | TPN+Gln | 20 | Unstates | 12/8 | Unstates | A, G |
| TPN | 21 | Unstates | 14/7 | Unstates | ||
| Ding et al. (29) | TPN+Gln | 10 | Unstates | Unstates | Unstates | G, J |
| TPN | 10 | Unstates | Unstates | Unstates | ||
| Fuentes-Orozco (12) | TPN+Gln | 22 | 43.8 ± 14.4 | 12/10 | 10.3 ± 1.6 | A, C, D, G, I, J |
| TPN | 22 | 41.5 ± 14.2 | 12/10 | 10.7 ± 1.9 | ||
| Gu et al. (32) | TPN+Gln | 30 | Unstates | Unstates | Unstates | G |
| TPN | 30 | Unstates | Unstates | Unstates | ||
| Tong et al. (41) | EEN+Gln | 16 | 41.5 ± 10.8 | 10/6 | 12.1 ± 3.7 | B, I, J |
| EEN | 16 | 41.9 ± 11.4 | 9/7 | 12.1 ± 3.7 | ||
| Wang et al. (44) | TPN+Gln | 23 | Unstates | Unstates | Unstates | A, D, E, F, G, K |
| TPN | 25 | Unstates | Unstates | Unstates | ||
| Huang et al. (35) | TPN+Gln | 24 | 63.6 ± 6.2 | 18/6 | 20.8 ± 3.5 | B, G, I, J |
| TPN | 24 | 62.4 ± 4.8 | 16/8 | 21.0 ± 3.2 | ||
| Wu et al. (45) | EEN+Gln | 15 | 37.24 ± 3.56 | 9/6 | Unstates | A, D, F, G |
| EEN | 15 | 38.61 ± 2.51 | 10/5 | Unstates | ||
| Yang et al. (47) | EEN+Gln | 14 | 42.82 ± 9.02 | 7/7 | 9.14 ± 0.77 | B, C, D, I, J |
| EEN | 14 | 43.25 ± 8.73 | 7/7 | 9.21 ± 0.89 | ||
| Ran et al. (38) | TPN+Gln | 25 | 54.3 ± 16.2 | 18/7 | 9.9 ± 2.4 | B, C, D, G, H, I, J |
| TPN | 25 | 56.2 ± 15.9 | 20/5 | 9.5 ± 2.3 | ||
| Jin et al. (36) | EEN+Gln | 26 | Unstates | Unstates | 12.5 ± 4.1 | A, B, D, F |
| EEN | 23 | Unstates | Unstates | 12.0 ± 5.0 | ||
| Singh et al. (52) | EEN+Gln | 41 | 40.78 ± 15.5 | 24/27 | 8.7 ± 4.4 | A, F |
| EEN | 39 | 35.64 ± 13.0 | 25/14 | 7.1 ± 2.5 | ||
| Wa et al. (42) | EEN+Gln | 54 | 59.3 ± 3.9 | 28/26 | 10.4 ± 1.2 | B, G, H, I |
| EEN | 54 | 62.4 ± 4.1 | 31/23 | 10.5 ± 1.2 | ||
| Liu et al. (17) | TPN+Gln | 24 | 40 ± 3.96 | 15/9 | Unstates | A, C, D, F |
| TPN | 23 | 39.13 ± 4.46 | 14/9 | Unstates | ||
| Lei et al. (37) | EEN+Gln | 38 | Unstates | Unstates | 16.5 ± 1.7 | A, B, D, F, I, J, L |
| EEN | 38 | Unstates | Unstates | 15.9 ± 2.2 | ||
| Yin et al. (49) | TPN+Gln | 20 | 41 ± 3.2 | Unstates | Unstates | A, D, E, F, G, K |
| TPN | 20 | 41 ± 3.2 | Unstates | Unstates | ||
| Wang et al. (43) | EEN+Gln | 49 | 51.85 ± 3.49 | 29/20 | 9.59 ± 1.33 | G, I |
| EEN | 49 | 51.83 ± 3.52 | 28/21 | 9.63 ± 1.31 | ||
| Yang et al. (46) | EEN+Gln | 34 | Unstates | 22/12 | 11.98 ± 1.42 | I |
| EEN | 34 | Unstates | 21/13 | 12.08 ± 1.36 | ||
| Zhao et al. (51) | EEN+Gln | 48 | 58.6 ± 3.8 | Unstates | 10.6 ± 1.2 | B, G, I |
| EEN | 48 | 58.6 ± 3.8 | Unstates | 10.4 ± 1.2 | ||
| Cui et al. (28) | EEN+Gln | 47 | 52.7 ± 8.3 | 32/15 | 9.7 ± 2.5 | G, I, J |
| EEN | 47 | 53.5 ± 8.8 | 34/13 | 9.8 ± 2.7 | ||
| Gao et al. (31) | EEN+Gln | 45 | 47.93 ± 6.24 | 24/21 | Unstates | B, G, I |
| EEN | 45 | 48.56 ± 6.37 | 25/20 | Unstates | ||
| Yuan et al. (50) | EEN+Gln | 23 | 51.32 ± 11.65 | Unstates | Unstates | I, J |
| EEN | 24 | 51.32 ± 11.65 | Unstates | Unstates | ||
| Arutla et al. (53) | EEN+Gln | 18 | 38.11 ± 16.3 | 17/11 | 8.6 ± 4.5 | A, C, D, I |
| EEN | 22 | 39.77 ± 15.1 | 20/2 | 8.76 ± 3.7 | ||
| Ren et al. (39) | EEN+Gln | 30 | 47.95 ± 7.79 | 21/9 | 11.34 ± 2.37 | A, B, E, F, I, K |
| EEN | 30 | 51.71 ± 7.09 | 25/14 | 9.73 ± 1.02 | ||
| Sun et al. (40) | EEN+Gln | 39 | 51.71 ± 7.09 | 25/14 | 9.73 ± 1.02 | G, H |
| EEN | 39 | 51.68 ± 7.15 | 24/15 | 9.64 ± 1.07 | ||
| Chu et al. (27) | EEN+Gln | 42 | 50.62 ± 5.74 | 27/15 | 9.76 ± 1.12 | G, H, L |
| EEN | 42 | 49.48 ± 6.06 | 26/16 | 9.63 ± 1.44 | ||
| Guan et al. (33) | EEN+Gln | 40 | 58.15 ± 3.13 | 25/15 | Unstates | F, G, I |
| EEN | 40 | 58.33 ± 3.2 | 23/17 | Unstates | ||
| Fan et al. (30) | EEN+Gln | 46 | 51.6 ± 3.3 | 28/18 | Unstates | A, B, D, E, F, G |
| EEN | 46 | 52.2 ± 2.9 | 26/20 | Unstates | ||
| Yang et al. (48) | EEN+Gln | 33 | 45.51 ± 22.46 | 17/16 | 14.03 ± 1.97 | B, G, L |
| EEN | 32 | 45.52 ± 22.42 | 16/16 | 14.17 ± 2.03 |
M/F, Male/Female; TPN, Total Parenteral Nutrition; EEN, Enter Enteral Nutrition; Gln, Glutamine; A, Mortality; B, APACHE II score; C, ICU hospital stay; D, Total length of hospital stay; E, Bloating recovery time; F, Complications; G, Liver function index; H, Kidney function index; I, Inflammatory index; J, Immune index; K, Serum amylase recovery time; L, Response rate.
“Total length of hospital stay” refers to the total time spent in hospital for SAP patients, including ICU stay time. “Complications” refers to the number of people with complications from SAP. Regardless of Gln treatment, SAP patients have other diseases caused by disease progression, such as pancreatic pseudocyst, peripancreatic infection, abdominal infection, multiple organ failure, sepsis, and gastrointestinal bleeding. “Response rate” refers to whether it improves the overall treatment effect of severe acute pancreatitis and improves the severity of SAP patients (For example, after 2 weeks of treatment, the patient's symptoms such as abdominal pain and bloating were significantly relieved, and laboratory indicators such as blood/urine amylase, blood routine, liver function, and inflammatory factors were significantly improved).
Risk of Bias Assessment Results
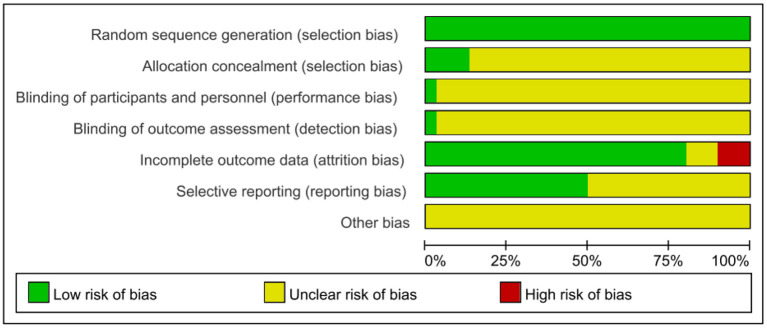
Given the risk of bias in the published literature, the included studies were analyzed separately for bias to determine the impact on the conclusions. Two authors independently assessed the included RCTs according to the tools of the Cochrane Systematic Review. The quality assessment of the included studies is shown in Figures 2, 3.
Figure 2.
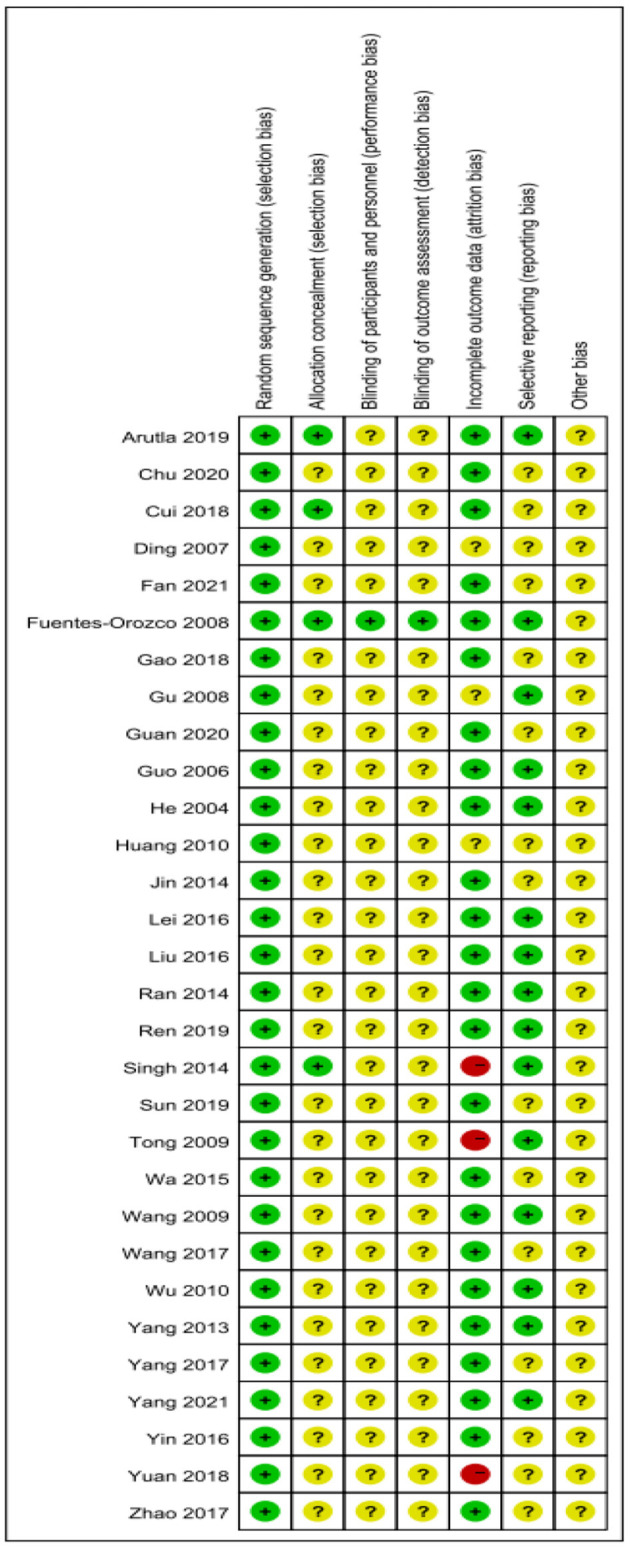
Risk of bias summary.
Figure 3.
Risk of bias graph.
Outcomes of the Intervention
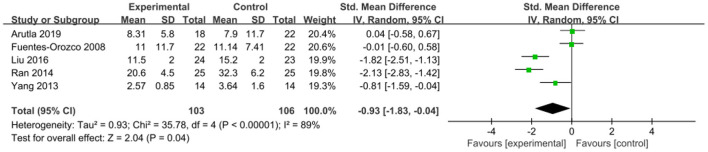
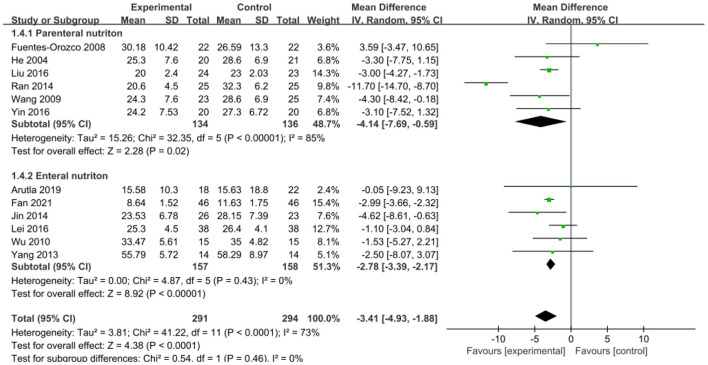
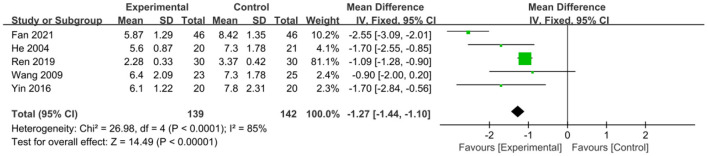
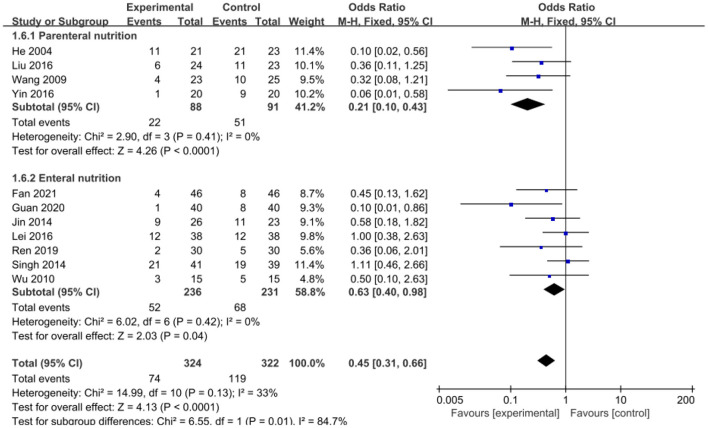
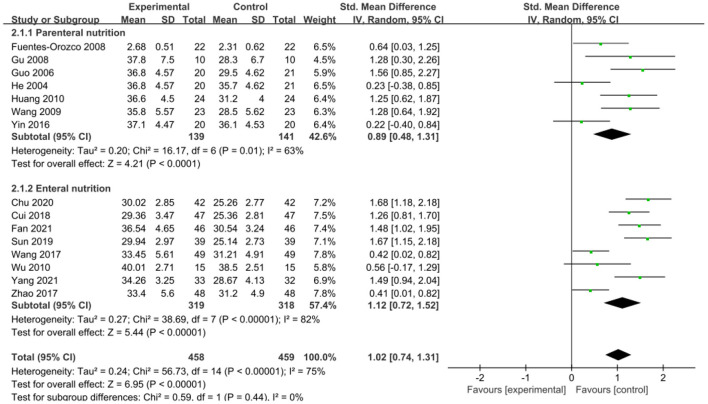
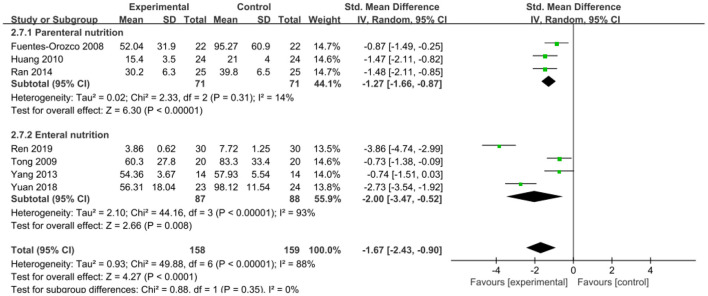
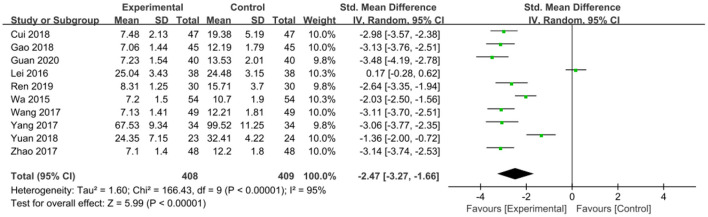
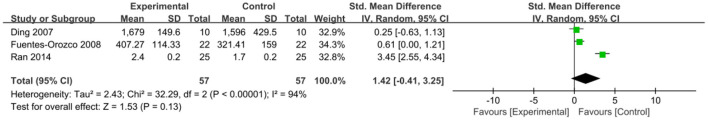
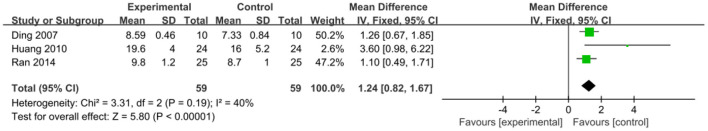
The intervention outcomes were divided into primary and secondary outcomes; the primary outcomes included mortality (Figure 4), APACHE II score (Figure 5), ICU hospital stay (Figure 6), total length of hospital stay (Figure 7), bloating recovery time (Figure 8) and complications (Figure 9), and secondary outcomes included liver function indicators (Figures 10–13), kidney function indicators (Figures 14, 15), inflammatory indicators (Figures 16–19), immune indicators (Figures 20, 21), serum amylase recovery time (Figure 22), and response rate (Figure 23). In Figures 4–23, “experimental” represents the parenteral or enteral nutrition group supplemented with Gln, and “control” means the conventional nutrition group. Rhombuses in the forest plot represent the results of the meta-analysis; the center of the rhombuses represents the point estimates of the effect size of the summary results, such as the OR value, MD value and SMD value, and the width of the rhombuses represents the 95% CIs of the effect size of the pooled results.
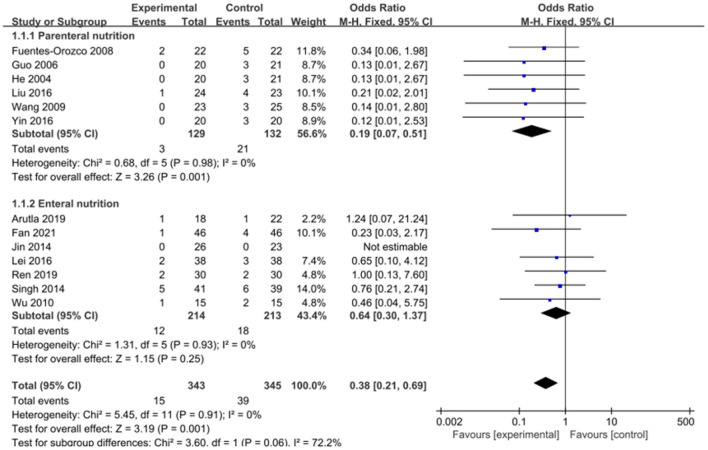
Figure 4.
Forest plots of mortality associated with experimental group (parenteral or enteral nutrition group supplemented with Gln) vs. control group (conventional nutrition group). I2 tests and P are the criteria for the heterogeneity test, ♦, pooled odds ratio; —■—, odds ratio, and the edges of ♦, 95% CI.
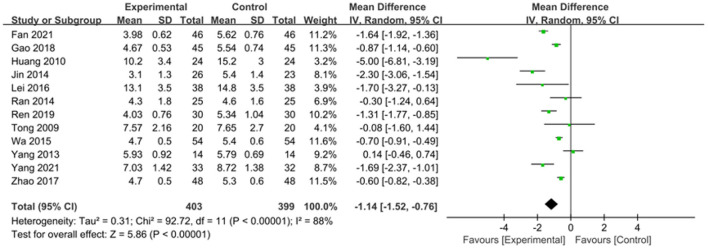
Figure 5.
Forest plots of APACHE II score associated with experimental group (parenteral or enteral nutrition group supplemented with Gln) vs. control group (conventional nutrition group). I2 tests and P are the criteria for the heterogeneity test, ♦, pooled mean difference; —■—, mean difference, and the edges of ♦, 95% CI.
Figure 6.
Forest plots of ICU hospital stay associated with experimental group (parenteral or enteral nutrition group supplemented with Gln) vs. control group (conventional nutrition group). I2 tests and P are the criteria for the heterogeneity test, ♦, pooled standard mean difference; —■—, standard mean difference, and the edges of ♦, 95% CI.
Figure 7.
Forest plots of total length of hospital stay associated with experimental group (parenteral or enteral nutrition group supplemented with Gln) vs. control group (conventional nutrition group). I2 tests and P are the criteria for the heterogeneity test, ♦, pooled mean difference; —■—, mean difference, and the edges of ♦, 95% CI.
Figure 8.
Forest plots of bloating recovery time associated with experimental group (parenteral or enteral nutrition group supplemented with Gln) vs. control group (conventional nutrition group). I2 tests and P are the criteria for the heterogeneity test, ♦, pooled mean difference; —■—, mean difference, and the edges of ♦, 95% CI.
Figure 9.
Forest plots of complications associated with experimental group (parenteral or enteral nutrition group supplemented with Gln) vs. control group (conventional nutrition group). I2 tests and P are the criteria for the heterogeneity test, ♦, pooled odds ratio; —■—, odds ratio, and the edges of ♦, 95% CI.
Figure 10.
Forest plots of serum albumin associated with experimental group (parenteral or enteral nutrition group supplemented with Gln) vs. control group (conventional nutrition group). I2 tests and P are the criteria for the heterogeneity test, ♦, pooled standard mean difference; —■—, standard mean difference, and the edges of ♦, 95% CI.
Figure 13.
Forest plots of TBIL associated with experimental group (parenteral or enteral nutrition group supplemented with Gln) vs. control group (conventional nutrition group). I2 tests and P are the criteria for the heterogeneity test, ♦, pooled mean difference; —■—, mean difference, and the edges of ♦, 95% CI.
Figure 14.
Forest plots of Scr associated with experimental group (parenteral or enteral nutrition group supplemented with Gln) vs. control group (conventional nutrition group). I2 tests and P are the criteria for the heterogeneity test, ♦, pooled mean difference; —■—, mean difference, and the edges of ♦, 95% CI.
Figure 15.
Forest plots of BUN associated with experimental group (parenteral or enteral nutrition group supplemented with Gln) vs. control group (conventional nutrition group). I2 tests and P are the criteria for the heterogeneity test, ♦, pooled mean difference; —■—, mean difference, and the edges of ♦, 95% CI.
Figure 16.
Forest plots of CRP associated with experimental group (parenteral or enteral nutrition group supplemented with Gln) vs. control group (conventional nutrition group). I2 tests and P are the criteria for the heterogeneity test, ♦, pooled standard mean difference; —■—, standard mean difference, and the edges of ♦, 95% CI.
Figure 19.
Forest plots of TNF-α associated with experimental group (parenteral or enteral nutrition group supplemented with Gln) vs. control group (conventional nutrition group). I2 tests and P are the criteria for the heterogeneity test, ♦, pooled standard mean difference; —■—, standard mean difference, and the edges of ♦, 95% CI.
Figure 20.
Forest plots of IgA associated with experimental group (parenteral or enteral nutrition group supplemented with Gln) vs. control group (conventional nutrition group). I2 tests and P are the criteria for the heterogeneity test, ♦, pooled standard mean difference; —■—, standard mean difference, and the edges of ♦, 95% CI.
Figure 21.
Forest plots of IgG associated with experimental group (parenteral or enteral nutrition group supplemented with Gln) vs. control group (conventional nutrition group). I2 tests and P are the criteria for the heterogeneity test, ♦, pooled mean difference; —■—, mean difference, and the edges of ♦, 95% CI.
Figure 22.
Forest plots of serum amylase recovery time associated with experimental group (parenteral or enteral nutrition group supplemented with Gln) vs. control group (conventional nutrition group). I2 tests and P are the criteria for the heterogeneity test, ♦, pooled mean difference; —■—, mean difference, and the edges of ♦, 95% CI.
Figure 23.
Forest plots of response rate associated with experimental group (parenteral or enteral nutrition group supplemented with Gln) vs. control group (conventional nutrition group). I2 tests and P are the criteria for the heterogeneity test, ♦, pooled odds ratio; —■—, odds ratio, and the edges of ♦, 95% CI.
Figure 12.
Forest plots of AST associated with experimental group (parenteral or enteral nutrition group supplemented with Gln) vs. control group (conventional nutrition group). I2 tests and P are the criteria for the heterogeneity test, ♦, pooled mean difference; —■—, mean difference, and the edges of ♦, 95% CI.
Primary Outcomes
Mortality
The effect of adding Gln on the mortality of SAP patients was repored in 13 included studies (n = 688 patients). A fixed-effects model was selected since no significant heterogeneity was present among the studies (P = 0.91, I2 = 0%). The pooled results showed that Gln supplementation significantly reduced patient' mortality (OR = 0.38, 95% CI: 0.21–0.69, P = 0.001; Figure 4). We performed an additional subgroup analysis by stratifying parenteral nutrition vs. enteral nutrition. Compared with the conventional nutrition group, enteral nutrition with Gln failed to effectively reduce the mortality of SAP patients (OR = 0.64, 95% CI: 0.30–1.37, P = 0.25), while parenteral Gln supplementation could significantly reduce patient mortality (OR = 0.19, 95% CI: 0.07–0.51, P = 0.001).
APACHE II Score
Twelve studies involving 802 patients reported the APACHE II score outcome. Given that significant heterogeneity was present among these studies (P < 0.00001, I2 = 88%), a random-effects model was used. The pooled results indicated that Gln supplementation effectively reduced the APACHE II score compared to the conventional nutrition group (SMD = −1.14, 95% CI: −1.52 to −0.76, P < 0.00001; Figure 5).
ICU Hospital Stay
ICU hospital stay was reported in five included studies involving 209 patients. There was significant heterogeneity among these studies (P < 0.00001, I2 = 89%), which was not reduced after removing one study at a time for sensitivity analysis; accordingly, a random-effects model was selected. The pooled results indicated that Gln supplementation was associated with a significantly shorter ICU hospital stay (SMD = −0.93, 95% CI: −1.83 to −0.04, P = 0.04; Figure 6).
Total Length of Hospital Stay
An association between the total length of hospital stay and Gln supplementation was reported in twelve studies involving 585 patients. There was significant heterogeneity between these studies (P < 0.0001, I2 = 73%), and, a random-effects model was applied. The pooled results showed that Gln supplementation significantly reduced the total length of hospital stay (MD = −3.41, 95% CI: −4.93 to −1.88, P < 0.0001; Figure 7). After subgroup analysis by stratifying enteral vs. parenteral supplementation, the modalities of Gln supplementation and the inclusion criteria of the Ran et al. study (32) were found to be the main source of heterogeneity. Furthermore, subgroup analysis showed both parenteral (MD = −4.14, 95% CI: −7.69 to −0.59, P = 0.02) and enteral supplementation (MD = −2.78, 95% CI: −3.39 to −2.17, P < 0.00001) can effectively reduce the total hospitalization time of patients compared with the conventionally nutrition group.
Bloating Recovery Time
To compare the effect of Gln on bloating recovery time in patients, five studies involving a total of 281 patients were included. There was significant heterogeneity among these studies (P < 0.0001, I2 = 85%), and a random-effects model was used. The pooled results showed that Gln supplementation significantly shortened the bloating recovery time in patients (MD = −1.27, 95% CI: −1.44 to −1.10, P < 0.00001; Figure 8).
Complications
Eleven included studies involving 646 patients reported complications as an outcome, involving 646 patients. No significant heterogeneity was found among these studies (P = 0.13, I2 = 33%). The pooled rates showed that Gln supplementation significantly reduced the incidence of complications in patients (OR = 0.45, 95% CI: 0.31–0.66, P < 0.0001; Figure 9). A subgroup analysis showed that compared with the conventional nutrition group, Gln parenteral supplementation (OR = 0.21, 95% CI: 0.10–0.43, P < 0.0001), and enteral nutrition could significantly reduce complications in SAP patients (OR = 0.63, 95% CI: 0.40–0.98, P = 0.04), and no significant heterogeneity was found among the included studies (I2 = 0).
Secondary Outcomes
Liver Function Indicators
Serum albumin. An association between serum albumin and Gln supplementation was reported in 15 included studies (n = 917). The pooled estimates showed that Gln supplementation significantly increased serum albumin level (SMD = 1.02, 95% CI: 0.74–1.31, P < 0.00001; Figure 10). However, there was significant heterogeneity among these studies (P < 0.00001, I2 = 75%). Substratification into parenteral and enteral subgroups failed to reduce the heterogeneity, suggesting that Gln supplementation was not a source of heterogeneity. Subgroup analysis showed that parenteral (SMD = 0.89, 95% CI: 0.48–1.31, P < 0.0001) and enteral Gln supplementation significantly increased serum albumin level (SMD = 1.12, 95% CI: 0.72–1.52, P < 0.00001) compared with the conventional nutrition group.
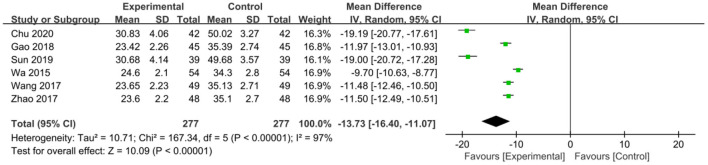
ALT. Six studies reported ALT for the two groups, involving 554 patients with severe pancreatitis, and there was significant heterogeneity among these studies (P < 0.00001, I2 = 97%). After conducting a sensitivity analysis, the heterogeneity was not reduced. Accordingly, the heterogeneity was attributed to differences in detection instruments (Supplementary Material 1). We selected a random-effects model, and the pooled estimates suggested that Gln supplementation significantly reduced ALT level (MD = −13.73, 95% CI: −16.40 to −11.07, P < 0.00001; Figure 11).
Figure 11.
Forest plots of ALT associated with experimental group (parenteral or enteral nutrition group supplemented with Gln) vs. control group (conventional nutrition group). I2 tests and P are the criteria for the heterogeneity test, ♦, pooled mean difference; —■—, mean difference, and the edges of ♦, 95% CI.
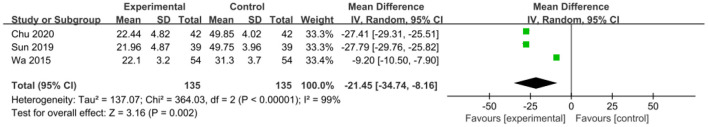
AST. AST was reported in 3 included studies involving a total of 270 patients. A random-effects model was used since significant heterogeneity was observed among these studies (P < 0.00001, I2 = 99%). The meta-analysis results showed that Gln supplementation was more effective in reducing AST level (MD = −21.45, 95% CI: −34.74 to −8.16, P = 0.002; Figure 7).
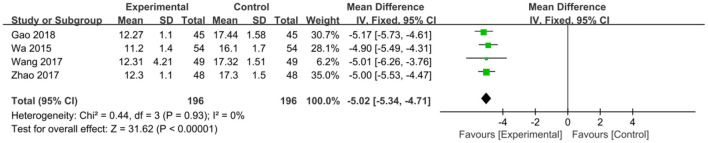
TBIL. Four studies with a total of 392 patients reported TBIL. There was little heterogeneity among these studies (P = 0.93, I2 = 0%), and a fixed-effects model was used. The pooled results suggested that Gln supplementation significantly reduced TBIL level (MD = −5.02, 95% CI: −5.34 to −4.71, P < 0.00001; Figure 13).
Kidney Function Indicators
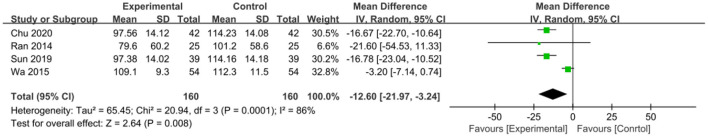
Scr. Four studies involving 320 patients were included to assess the effect of Gln supplementation on Scr levels. Significant heterogeneity was observed among these studies (P = 0.0001, I2 = 86%) and a random-effects model was selected. The pooled results suggested that Gln addition was effective in reducing Scr levels (MD = −12.60, 95% CI: −21.97 to −3.24, P = 0.008; Figure 14).
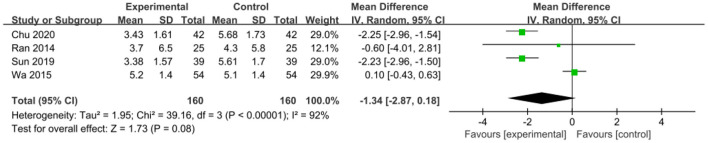
BUN. Four studies, including 320 patients, reported BUN results. There was significant heterogeneity among these studies (P < 0.00001, I2 = 92%) and a random-effects model was used. The combined results indicated that the addition of Gln was not significantly correlated with BUN levels (MD = −1.34, 95% CI: −2.87 to 0.18, P = 0.08; Figure 15).
Inflammatory Indicators
CRP. Seven studies involving a total of 317 patients reported this outcome. There was large heterogeneity among these studies (P < 0.00001, I2 = 88%), and the pooled estimates suggested that Gln supplementation effectively reduced CRP levels in SAP patients (SMD = −1.67, 95% CI: −2.43 to −0.90, P < 0.0001; Figure 16). After substratification into parenteral and enteral group, we found that the feeding route was a source of heterogeneity. Our subgroup analysis showed that compared with the conventional nutrition group, both parenteral (SMD = −1.27, 95% CI: −1.66 to −0.87, P < 0.00001) and enteral Gln significantly reduced CRP levels (SMD = −2.00, 95% CI: −3.47 to −0.52, P = 0.008).
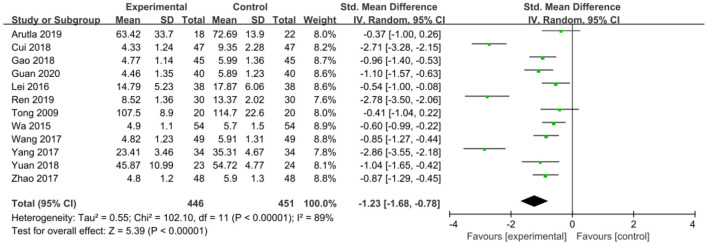
IL-6. IL-6 was reported in 12 studies involving 897 patients. There was significant heterogeneity among these studies (P < 0.00001, I2 = 89%) and a random-effects model was used. The results indicated that Gln supplementation effectively reduced IL-6 levels (SMD = −1.23, 95% CI: −1.68 to −0.78, P < 0.00001; Figure 17).
Figure 17.
Forest plots of IL-6 associated with experimental group (parenteral or enteral nutrition group supplemented with Gln) vs. control group (conventional nutrition group). I2 tests and P are the criteria for the heterogeneity test, ♦, pooled standard mean difference; —■—, standard mean difference, and the edges of ♦, 95% CI.
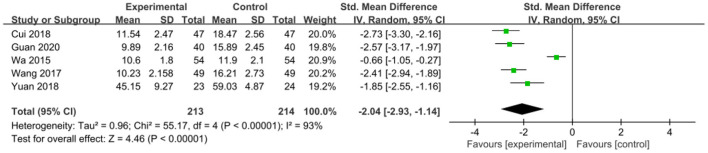
IL-8. To compare the effects on IL-8 levels in patients, five studies involving 427 patients were included. There was significant heterogeneity among these studies (P < 0.00001, I2 = 93%), and a random-effects model was used. The pooled results suggested that Gln supplementation effectively reduced IL-8 level (SMD = −2.04, 95% CI: −2.93 to −1.14, P < 0.00001; Figure 18).
Figure 18.
Forest plots of IL-8 associated with experimental group (parenteral or enteral nutrition group supplemented with Gln) vs. control group (conventional nutrition group). I2 tests and P are the criteria for the heterogeneity test, ♦, pooled standard mean difference; —■—, standard mean difference, and the edges of ♦, 95% CI.
TNF-α. To compare the effect of Gln supplementation on TNF-α level in SAP patients, 10 studies involving 817 patients were included. Given that significant heterogeneity was observed among these studies (P < 0.00001, I2 = 95%), we applied a random-effects model. The combined results showed that nutritional supplementation with Gln was effective in reducing TNF-α levels (SMD = −2.47, 95% CI: −3.27 to −1.66, P < 0.00001; Figure 19).
Immune Indicators
IgA. IgA was reported in three studies involving 114 patients. Given that there was significant heterogeneity among these studies (P < 0.00001, I2 = 94%), a random-effects model was selected. The results indicated no statistically significant difference in IgA levels between the Gln supplementation group and the conventional nutrition group (SMD = 1.42, 95% CI: −0.41 to 3.25, P = 0.13; Figure 20).
IgG. This outcome was reported in 3 included studies involving 118 patients. A fixed-effects model was used since no significant heterogeneity was observed among these studies (P = 0.19, I2 = 40%). The pooled estimates suggested that Gln supplementation can significantly increase IgG levels (MD = 1.24, 95% CI: 0.82–1.67, P < 0.00001; Figure 21).
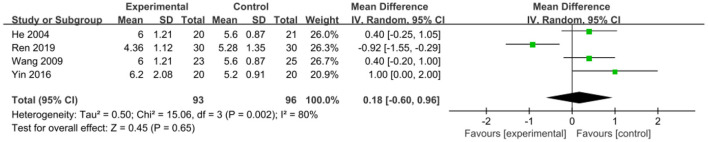
Serum Amylase Recovery Time
Serum amylase recovery time was included in four studies, involving 118 patients. There was significant heterogeneity among these studies (P = 0.002, I2 = 80%) and a random-effects model was used. The pooled results indicated no significant difference in serum amylase recovery time between treatment with or without Gln supplementation (MD = 0.18, 95% CI: −0.60 to 0.96, P = 0.65; Figure 22).
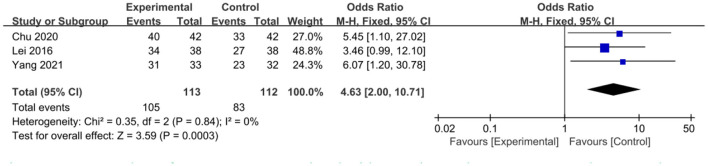
Response Rate
Three included studies involving 225 patients reported the response rate. Little heterogeneity was observed among these studies (P = 0.84, I2 = 0%), and, a fixed-effects model was used. The pooled results showed that the response rate between Gln-supplementation and the control group was statistically significant (OR = 4.63, 95% CI: 2.00–10.71, P = 0.0003; Figure 23).
Sensitivity Analysis
Sensitivity analyses were performed by omitting one study at a time. During analysis of kidney function indicators, when the study of Wa et al. (36) was excluded, the pooled estimates showed that Gln supplementation was more effective in reducing BUN levels than the control group (MD: −2.20, 95%CI, −2.71 to −1.70, P < 0.00001), which was inconsistent with the preliminary results and significantly reduced heterogeneity (I2 = 0%). In terms of serum amylase recovery time, when we excluded the study by Ren et al. (33), the pooled results showed that Gln supplementation failed to effectively reduce the serum amylase recovery time (MD: 0.50; 95% CI, 0.10–0.90; P < 0.00001), but the heterogeneity (I2 = 0%) was significantly reduced.
Publication Bias
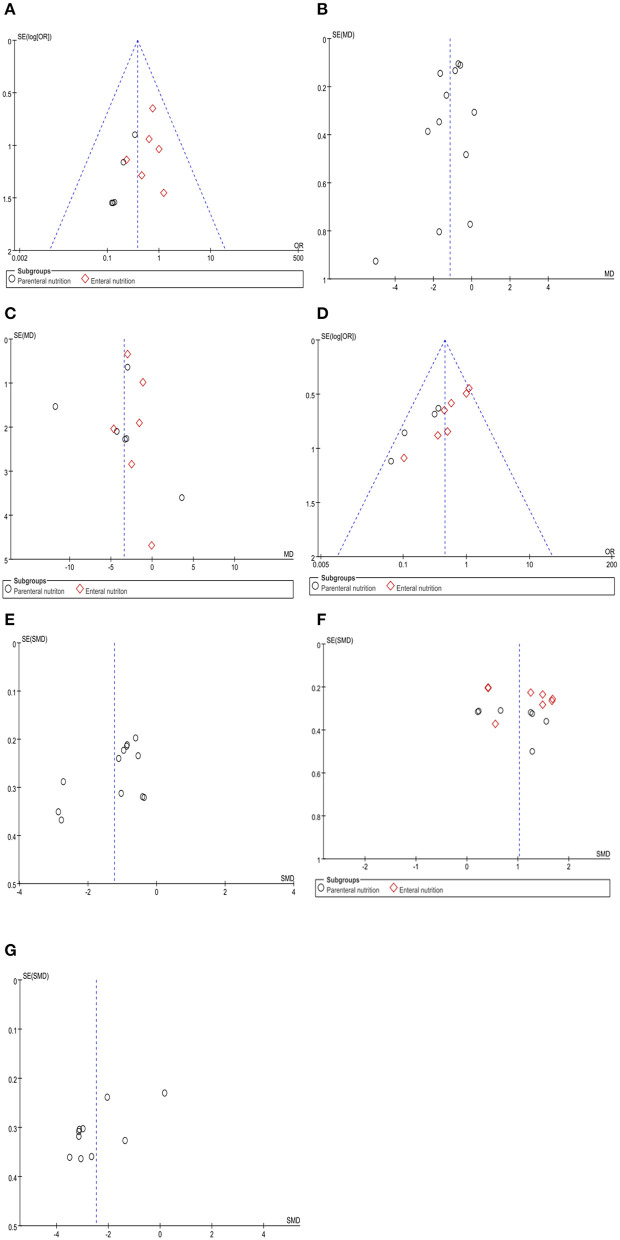
Funnel plot and Egger's test were used to evaluate publication bias of mortality, APACHE II score, complications, total length of hospital stay, serum albumin, IL-6, and TNF-α. The results showed significant publication bias for mortality (Egger's test, P = 0.025), complications (Egger's test, P = 0.000), and TNF-α (Egger's test, P = 0.020). However, no significant publication bias was observed for in the APACHE II score (Egger's test, P = 0.262), total length of hospital stay (Egger's test, P = 0.892), serum albumin (Egger's test, P = 0.545), and IL-6 (Egger's test, P = 0.059) (Figure 24).
Figure 24.
Funnel plots of the included studies for mortality (A), APACHE II score (B), total length of hospital stay (C), complications (D), serum albumin (F), IL-6 (E), and TNF-α (G).
Discussion
The meta-analysis aimed to evaluate the efficacy of Gln parenteral or enteral nutrition vs. conventional nutrition in patients with SAP. Outcomes, such as mortality, complications, the hospital stay of SAP patients, and other test results, were evaluated.
Consistent with the literature, we found that nutritional support with Gln may be an effective therapeutic approach for SAP. Importantly, unlike enteral Gln supplementation (OR = 0.64, 95% CI: 0.30–1.37, P = 0.25), parenteral nutrition with Gln effectively reduced mortality (OR = 0.19, 95% CI: 0.07–0.51, P = 0.001), which was inconsistent with the study by Jiang et al. (14) who reported that enteral Gln supplementation could significantly reduce the mortality risk (risk ratio= 0.38; 95% CI, 0.16–0.90; p = 0.03). Accordingly, future clinical studies are warranted to validate this finding. Regarding the total length of hospital stay and complications, both parenteral and enteral Gln supplementation were more effective than conventional nutrition (P < 0.05). Interestingly, we found that when a Gln concentration of 0.4 g/kg used in 17 included studies (6, 12, 27, 28, 30, 31, 33–35, 37, 40, 42–45, 48, 49, 51) for parenteral supplementation did not reduce the total hospital stay (P > 0.05), compared to enteral supplementation (P < 0.05; Supplementary Material 3). However, parenteral Gln supplementation was more effective than enteral supplementation in reducing the incidence of complications (Supplementary Material 3). In terms of APACHE II score, ICU hospital stay and bloating recovery time, Gln supplementation was more effective than conventional nutrition (P < 0.05). Nonetheless, given the limited number of included studies, subgroup analysis could not be performed. 0.4 g/kg Gln yield results, emphasizing the need for future research with large sample data. As for secondary outcomes, in terms of liver function indicators, nutritional support therapy with Gln effectively improved liver function (P < 0.05). Moreover, we found that Gln supplementation (enteral or parenteral nutrition) increased serum albumin levels. We further analyzed the pros and cons of different methods of 0.4 g/kg Gln supplementation and found that parenteral supplementation was inferior to as enteral supplementation (Supplementary Material 3). Interestingly, compared with the conventional nutrition group, Gln supplementation could effectively reduce Scr levels (MD = −12.60, 95% CI: −21.97 to −3.24, P = 0.008). However, BUN results were not consistent with the above findings (MD = −1.34, 95% CI: −2.87 to 0.18, P = 0.08), and this discrepancy may be attributed to the quality of the included literature. Gln supplementation was more effective than conventional nutrition in reducing the inflammatory indicators, including CRP, IL-6, IL-8, and TNF-α, and could effectively alleviate the inflammatory state in SAP. However, in terms of immune indicators, Gln therapy significantly improved IgG levels (MD = 1.24, 95% CI: 0.82–1.67, P < 0.00001), but no statistically significant differences in IgA were observed (SMD = 1.42, 95% CI: −0.41 to 3.25, P = 0.13). In addition, no significant difference in serum amylase recovery time was observed between the two groups (P > 0.05).
In this meta-analysis, Gln supplementation was effective for all parameters except BUN, IgA and serum amylase recovery time. During the subgroup analysis, except for mortality, the nutrition route did not affect the outcome of corresponding indicators. At the similar Gln concentrations (0.4 g/kg), the two supplementation methods did not change mortality, suggesting that parenteral supplementation was more effective for SAP patients. Indeed, notwithstanding that parenteral Gln supplementation was inferior to enteral supplementation in terms of total hospital stay and serum albumin levels, parenteral supplementation was associated with a lower incidence of complications in SAP patients. Although some biochemical parameters, such as CRP, serum albumin and Scr, improved with Gln use, there were no significant changes in primary outcomes, such as mortality (with enteral supplementation). It has been established that the systemic inflammatory response in AP causes an increase in calorie and protein metabolism, which leads to systemic organ damage and substantial nutrient loss, and long-term fasting aggravates the negative nitrogen balance (54). Inflammatory storms and inadequate nutrient intake can exacerbate organ damage and intestinal dysfunction. Moreover, peripancreatic infection and necrosis cause severe sepsis, further disrupting the intestinal barrier and leading to high patient mortality. Therefore, protecting the integrity of the intestinal barrier is critical for reducing patient mortality and improving primary clinical outcomes. In the present study, we found that enteral Gln supplementation did not improve the mortality of SAP patients, which may be attributed to the non-recovery of the intestinal function, and intestinal absorption of Gln was significantly lower than that of intravenous infusion. For patients with very severe symptoms, Gln yielded no significant effects in the short term. In addition, although the effect of enteral supplementation of Gln can be effective, it is still inferior to parenteral nutrition, which is mainly related to whether Gln is effectively utilized during early stages. The current study found that parenteral supplementation was more effective than enteral supplementation on primary and secondary outcomes. However, a limited number of studies were included, and the severity of SAP patients and the timing, dose, and duration of Gln use remain primarily understudied, warranting further studies.
Our study findings are consistent with previous meta-analyses (14, 22). Seven RCTs were included in the meta-analysis by Jiang et al. (14), with a relatively smaller number of patients and outcomes involved. A meta-analysis by Li et al. (22), which included 10 RCTs showed that compared with enteral nutrition (SMD = 0.36, 95% CI: −0.08 to 0.80, P > 0.05), intravenous infusion Gln was more effective in reducing plasma albumin levels (SMD = 1.19, 95% CI: 0.62–1.77, P < 0.05), which was inconsistent with our study, and different results were also observed with CRP In the present meta-analysis, 30 RCTs were included based on strict inclusion and exclusion criteria with increased sample size, primary and secondary outcomes. We did not analyze indicators with <3 included studies to reduce the risk of bias and ensure the robustness and accuracy of our findings. Morever, we searched eight major databases to ensure that relevant articles were not missed. Finally, we performed a literature quality assessment, sensitivity analysis, and publication bias detection on the included literature and presented the results in forest and funnel plots for a comprehensive meta-analysis.
One strength of this study is that it summarizes and analyzes the latest related literature, which makes up for the knowledge gap in this research field. In addition, we included a relatively large number of RCTs and a large sample size, providing robust evidence for our findings. However, some limitations of our study warrant attention. First of all, some indicators such as BUN were reported in few studies which may be a source of publication bias. Moreover, the reliability of our findings was affected due to differences in detection method. Given the presence of high heterogeneity among the included studies, a random-effects model was used to improve the stability of these outcomes. Finally, since most of the included studies originated from China, the present study's findings cannot be generalized to other regions, emphasizing the need for more studies worldwide.
Conclusion
The findings of this study provided compelling evidence that nutritional support therapy with Gln is an effective therapy for SAP, especially for the recovery of relevant biochemical indicators of patients during hospitalization and the reduction of hospitalization time. Subgroup analysis found that parenteral nutrition supplementation with Gln was more likely to reduce mortality and complications in patients, so parenteral nutrition seemed to be a better choice for patients with severe symptoms. However, there are few studies on the timing and dose of Gln supplementation in SAP patients, and prospective trials are needed to prove it. In addition, the outcome of Gln nutrition therapy for some indicators is largely unclear, such as serum amylase and intestinal function recovery time, whether to transfer to surgery, etc. Safety is also the focus of research, including gastrointestinal reactions, metabolic disorders, but it has not been paid attention to in detail in previous studies. In the implementation of medical decisions, clinicians should weigh the patient's condition based on the above factors, so as to facilitate a good prognosis of patients. Further research with larger sample size is needed to improve the current understanding of these clinical outcomes and accurately evaluate the efficacy of nutritional therapy with Gln for SAP patients.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
SD: writing—original draft preparation, supervision, and project administration. ZZ: writing—original draft preparation and visualization. XL, ZC, and WJ: writing—review and editing and supervision. WZ: writing—original draft preparation, supervision, project administration, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Science and Technology Projects of Chengguan District in Lanzhou [grant number 2020-2-11-4] and Traditional Chinese Medicine Scientific Research Project of Gansu Province, China [grant number GZKP-2020-28].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We sincerely appreciate all the participants in our work.
Glossary
Abbreviations
- SAP
severe acute pancreatitis
- Gln
glutamine
- RCTs
randomized controlled trials
- Scr
serum creatinine
- CRP
C-reaction protein
- AP
acute pancreatitis
- MOF
multisystem organ failure
- MODS
multiple organ dysfunction syndromes
- RR
relative risk
- MD
mean difference
- SMD
standard mean difference
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- TBIL
total bilirubin
- BUN
blood urea nitrogen
- IL-6
interleukin- 6
- TNF-α
tumor necrosis factor α.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.865102/full#supplementary-material
References
- 1.Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis−2012: revision of the Atlanta classification and definitions by international consensus. Gut. (2013) 62:102–11. 10.1136/gutjnl-2012-302779 [DOI] [PubMed] [Google Scholar]
- 2.Pagliari D, Brizi MG, Saviano A, Mancarella FA, Dal Lago AA, Serricchio ML, et al. Clinical assessment and management of severe acute pancreatitis: a multi-disciplinary approach in the XXI century. Eur Rev Med Pharmacol Sci. (2019) 23:771–87. 10.26355/eurrev_201901_16892 [DOI] [PubMed] [Google Scholar]
- 3.Heckler M, Hackert T, Hu K, Halloran CM, Büchler MW, Neoptolemos JP. Severe acute pancreatitis: surgical indications and treatment. Langenbecks Arch Surg. (2021) 406:521–35. 10.1007/s00423-020-01944-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mofidi R, Duff MD, Wigmore SJ, Madhavan KK, Garden OJ, Parks RW. Association between early systemic inflammatory response, severity of multiorgan dysfunction and death in acute pancreatitis. Br J Surg. (2006) 93:738–44. 10.1002/bjs.5290 [DOI] [PubMed] [Google Scholar]
- 5.Garg PK, Singh VP. Organ failure due to systemic injury in acute pancreatitis. Gastroenterology. (2019) 156:2008–23. 10.1053/j.gastro.2018.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He XL, Ma QJ, Lu JG, Chu YK, Du XL. Effect of total parenteral nutrition (TPN) with and without glutamine dipeptide supplementation on outcome in severe acute pancreatitis (SAP). Clin Nutr Suppl. (2004) 1:43–7. 10.1016/j.clnu.2004.07.011 [DOI] [Google Scholar]
- 7.Biczo G, Vegh ET, Shalbueva N, Mareninova OA, Elperin J, Lotshaw E, et al. Mitochondrial dysfunction, through impaired autophagy, leads to endoplasmic reticulum stress, deregulated lipid metabolism, and pancreatitis in animal models. Gastroenterology. (2018) 154:689–703. 10.1053/j.gastro.2017.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wada K, Takada T, Hirata K, Mayumi T, Yoshida M, Yokoe M, et al. Treatment strategy for acute pancreatitis. J Hepatobiliary Pancreat Sci. (2010) 17:79–86. 10.1007/s00534-009-0218-z [DOI] [PubMed] [Google Scholar]
- 9.Rinninella E, Annetta MG, Serricchio ML, Dal Lago AA, Miggiano GA, Mele MC, et al. Nutritional support in acute pancreatitis: from physiopathology to practice. An evidence-based approach. Eur Rev Med Pharmacol Sci. (2017) 21:421–32. [PubMed] [Google Scholar]
- 10.Wu P, Li L, Sun W. Efficacy comparisons of enteral nutrition and parenteral nutrition in patients with severe acute pancreatitis: a meta-analysis from randomized controlled trials. Biosci Rep. (2018) 38:BSR20181515. 10.1042/BSR20181515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cruzat V, Macedo Rogero M, Noel Keane K, Curi R, Newsholme P. Glutamine: metabolism and immune function, supplementation and clinical translation. Nutrients. (2018) 10:1564. 10.3390/nu10111564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuentes-Orozco C, Anaya-Prado R, González-Ojeda A, Arenas-Márquez H, Cabrera-Pivaral C, Cervantes-Guevara G, et al. L-alanyl-L-glutamine-supplemented parenteral nutrition improves infectious morbidity in secondary peritonitis. Clin Nutr. (2004) 23:13–21. 10.1016/S0261-5614(03)00055-4 [DOI] [PubMed] [Google Scholar]
- 13.Petrov MS, Atduev VA, Zagainov VE. Advanced enteral therapy in acute pancreatitis: is there a room for immunonutrition? A meta-analysis. Int J Surg. (2008) 6:119–24. 10.1016/j.ijsu.2008.01.003 [DOI] [PubMed] [Google Scholar]
- 14.Jiang X, Pei LY, Guo WX, Qi X, Lu XG. Glutamine supported early enteral therapy for severe acute pancreatitis: a systematic review and meta-analysis. Asia Pac J Clin Nutr. (2020) 29:253–61. 10.6133/apjcn.202007_29(2).0007 [DOI] [PubMed] [Google Scholar]
- 15.Hajdú N, Belágyi T, Issekutz A, Bartek P, Gartner B, Oláh A. Intravénás glutamin és korai nasojejunalis táplálás együttes alkalmazása súlyos acut pancreatitisben – prospektív randomizált kettos vak kontrollált klinikai vizsgálat [Intravenous glutamine and early nasojejunal nutrition in severe acute pancreatitis – a prospective randomized clinical study]. Magy Seb. (2012) 65:44–51. 10.1556/maseb.65.2012.2.2 [DOI] [PubMed] [Google Scholar]
- 16.Li W, Liu J, Zhao S, Li J. Safety and efficacy of total parenteral nutrition versus total enteral nutrition for patients with severe acute pancreatitis: a meta-analysis. J Int Med Res. (2018) 46:3948–58. 10.1177/0300060518782070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu X, Sun XF, Ge QX. The role of glutamine supplemented total parenteral nutrition (TPN) in severe acute pancreatitis. Eur Rev Med Pharmacol Sci. (2016) 20:4176–80. [PubMed] [Google Scholar]
- 18.Jafari T, Feizi A, Askari G, Fallah AA. Parenteral immunonutrition in patients with acute pancreatitis: a systematic review and meta-analysis. Clin Nutr. (2015) 34:35–43. 10.1016/j.clnu.2014.05.008 [DOI] [PubMed] [Google Scholar]
- 19.Asrani V, Chang WK, Dong Z, Hardy G, Windsor JA, Petrov MS. Glutamine supplementation in acute pancreatitis: a meta-analysis of randomized controlled trials. Pancreatology. (2013) 13:468–74. 10.1016/j.pan.2013.07.282 [DOI] [PubMed] [Google Scholar]
- 20.Chinese Pancreatic Surgery Association Chinese Chinese Society of Surgery Chinese Medical Association . Guidelines for the diagnosis and treatment of acute pancreatitis in China (2019, Shenyang). Chinese J Digest. (2019) 2019:721–730. [Google Scholar]
- 21.Crockett SD, Wani S, Gardner TB, Falck-Ytter Y, Barkun AN. American gastroenterological association institute guideline on initial management of acute pancreatitis. Gastroenterology. (2018) 154:1096–101. 10.1053/j.gastro.2018.01.032 [DOI] [PubMed] [Google Scholar]
- 22.Yong L, Lu QP, Liu SH, Fan H. Efficacy of glutamine-enriched nutrition support for patients with severe acute pancreatitis: a meta-analysis. J Parenter Enteral Nutr. (2016) 40:83–94. 10.1177/0148607115570391 [DOI] [PubMed] [Google Scholar]
- 23.Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP. Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (Updated July 2019). Cochrane. (2019). Available online at: www.training.cochrane.org/handbook (accessed September 7, 2020).
- 24.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. (2015) 350:g7647. 10.1136/bmj.g7647 [DOI] [PubMed] [Google Scholar]
- 25.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 26.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chu BX. Application of glutamine combined with early enteral nutrition in patients with severe acute pancreatitis effectiveness analysis. World Latest Med Inform. (2020) 20:84–6. [Google Scholar]
- 28.Cui HT, Zhao HM. Effects of glutamine combined with early enteral nutrition on bacterial shift and inflammatory response in patients with severe acute pancreatitis. Modern J Integr Tradition Chinese Western Med. (2018) 27:1334–7. [Google Scholar]
- 29.Ding YD, Zhu HX. Effects of glutamine on nutritional status and immune function in patients with acute severe pancreatitis. Chin J Hemorh. (2007) 2007:272–6. [Google Scholar]
- 30.Fan P. Effects of drugs and early enteral nutrition on severe acute pancreatitis. Chinese Urban Rural Enterprise Hygiene. (2021) 36:187–8. [Google Scholar]
- 31.Gao ZL. Clinical effect of early enteral nutrition combined with glutamine in patients with severe acute pancreatitis. J Clin Med. (2018) 5:24–5. [Google Scholar]
- 32.Gu X. Application of glutamine in severe pancreatitis. China Med Herald. (2008) 2008:65–6. [Google Scholar]
- 33.Guan YC, Zhang ZH. Effects of glutamine combined with EENs on inflammatory factors and nutritional metabolism in patients with severe acute pancreatitis. Clin Med Eng. (2020) 27:1359–60. [Google Scholar]
- 34.Guo YL, Xia M. Effects of glutamine on nutritional status of patients with severe pancreatitis. J Clin Military Med. (2006) 2006:12–4.19464771 [Google Scholar]
- 35.Huang XD, Huang M. Application of alanyl-glutamine dipeptide in severe acute pancreatitis. Modern Diagn Treat. (2010) 21:342–3. [Google Scholar]
- 36.Jin SL, Li LP. Efficacy of early enteral compound glutamine in the treatment of severe pancreatitis. Chinese J Practical Med. (2014) 41:122–3. [Google Scholar]
- 37.Lei W, Wang YL. Glutamine combined with early jejunal nutrition in the treatment of severe acute pancreatitis clinical research. Lab Med Clin. (2016) 13:819–22. [Google Scholar]
- 38.Ran J, Zhu Y. Effect of glutamine on disease course in severe pancreatitis patients. World Chinese J Digestol. (2014) 22:5159–63. 10.11569/wcjd.v22.i33.5159 [DOI] [Google Scholar]
- 39.Ren WS, Guo YL, Zhang T. Clinical efficacy analysis of glutamine combined with early enteral nutrition in the treatment of severe acute pancreatitis. Guizhou Med. (2019) 43:1887–9. [Google Scholar]
- 40.Sun YB. Effects of glutamine and early enteral nutrition on patients with severe acute pancreatitis. Practical Clin J Integr Tradition Chinese Western Med. (2019) 19:17–9. [Google Scholar]
- 41.Tong ZH, Li WQ, Yu WK, Wang XY, Ye XH, Li N, et al. Effects of early enteral nutrition supplementation with glutamine on inflammatory response and immune function in severe acute pancreatitis. Parenteral Enteral Nutr. (2009) 16:264–8. [Google Scholar]
- 42.Wa YL. Curative effect of early enteral compound glutamine in treatment of severe pancreatitis. World Chinese J Digestol. (2015) 23:1484–8. 10.11569/wcjd.v23.i9.1484 [DOI] [Google Scholar]
- 43.Wang LF. Glutamine combined with early jejunal nutrition in the treatment of 98 cases of severe acute pancreatitis. Medical J Chinese People's Health. (2017) 29:36–7. [Google Scholar]
- 44.Wang S, Huang SW, Liu P, Liu SJ, Wang Q. Significance of total parenteral nutrition combined with glutamine dipeptide in non-surgical treatment of patients with severe pancreatitis. Practical Prev Med. (2009) 16:844–6. [Google Scholar]
- 45.Wu D, Li Y. Influence of early enteral administration of glutamine on clinical prognosis in patients with severe acute pancreatitis. J Jiujiang Univ. (2010) 25:110–6. [Google Scholar]
- 46.Yang C, Wen J, Xia M, Wang SR. Glutamine nutritional support on intestinal mucosal barrier function and inflammatory response in patients with acute severe pancreatitis degree of influence. J Hainan Medical College. (2017) 23:18961899. [Google Scholar]
- 47.Yang TY, Zhang XY, Jiang MX. Clinical study of early enteral immunonutrition in severe acute pancreatitis. Clin Med China. (2013) 29:922–5.19087716 [Google Scholar]
- 48.Yang WH, Wan X, Dai WC, Liu C. Efficacy analysis of glutamine combined with early enteral nutrition in the treatment of severe acute pancreatitis. Smart Healthcare. (2021) 7:100–2. [Google Scholar]
- 49.Yin HY. The value of TPN and alanyl-glutamine dipeptide in the non-surgical treatment of severe acute pancreatitis. J Clin Medical. (2016) 3:7521–2. [Google Scholar]
- 50.Yuan XX, Zan JB, Xu AZ, He CL, Miao X. Effects of early enteral nutrition combined with Gln on systemic inflammatory response and immune function in patients with SAP. J Hepatopancreatobil Surg. (2018) 30:22–5. [Google Scholar]
- 51.Zhao CJ, Chen Q, Chen J, Cui H. Application of early enteral nutrition combined with glutamine in severe acute pancreatitis. China Continuing Medical Educ. (2017) 9:135–7. [Google Scholar]
- 52.Singh N, Mishra SK, Sachdev V, Sharma H, Upadhyay AD, Arora I, et al. Effect of oral glutamine supplementation on gut permeability and endotoxemia in patients with severe acute pancreatitis: a randomized controlled trial. Pancreas. (2014) 43:867–73. 10.1097/MPA.0000000000000124 [DOI] [PubMed] [Google Scholar]
- 53.Arutla M, Raghunath M, Deepika G, Jakkampudi A, Murthy HVV, Rao GV, et al. Efficacy of enteral glutamine supplementation in patients with severe and predicted severe acute pancreatitis- a randomized controlled trial. Indian J Gastroenterol. (2019) 38:338–47. 10.1007/s12664-019-00962-7 [DOI] [PubMed] [Google Scholar]
- 54.Al-Omran M, Albalawi ZH, Tashkandi MF, Al-Ansary LA. Enteral versus parenteral nutrition for acute pancreatitis. Cochrane Database Syst Rev. (2010) 2010:CD002837. 10.1002/14651858.CD002837.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.