Abstract
Mechanical forces collaborate across length scales to coordinate cell fate during development and the dynamic homeostasis of adult tissues. Similarly, steroid hormones interact with their nuclear and nonnuclear receptors to regulate diverse physiological processes necessary for the appropriate development and function of complex multicellular tissues. Aberrant steroid hormone action is associated with tumors originating in hormone-sensitive tissues and its disruption forms the basis of several therapeutic interventions. Prolonged perturbations to mechanical forces may further foster tumor initiation and the evolution of aggressive metastatic disease. Recent evidence suggests that steroid hormone and mechanical signaling intersect to direct cell fate during development and tumor progression. Potential mechanosensitive steroid hormone signaling pathways along with their molecular effectors will be discussed in this context.
Keywords: tissue mechanics, steroid hormones, cell fate, reproductive cancers, mechanosensitive, mechanotransduction
Mechanical forces are fundamental to the proper development, structure, and function of multicellular tissues. These forces include tension, compression, and shear stress and occur at the tissue, cellular, and subcellular levels (1). Cells respond to mechanical cues through localized or diffuse mechanosensors that transduce and integrate these signals together with biochemical pathways to direct cell fate determination (2). For example, more compliant extracellular matrix (ECM) mechanical properties can prime human embryonic stem cells for subsequent mesoderm specification by promoting Wnt/β-catenin activity in response to stimulation with mesoderm differentiation factors (3). Conversely, stiffer ECM substrates promote ubiquitination and degradation of β-catenin and suppression of Wnt signaling to inhibit conversion of human embryonic stem cells toward a mesoderm fate. In similar fashion, mesenchymal stem cells can be directed toward neurogenic, myogenic, or osteogenic cell lineages by mimicking the ECM substrate elasticity associated with these tissues (4).
Chronic aberrant mechanical stresses, or alternatively a defect in a cells ability to respond to mechanical cues, may not only lead to developmental abnormalities, but can also drive perturbations to the dynamic homeostasis required for mature tissue function (2). Indeed, tissue fibrosis is associated with heightened risk for cancer (2), and elevated ECM stiffness has been implicated in tumor initiation and progression to aggressive disease with poor patient outcomes (5-7). For example, lysyl oxidase (LOX)-mediated crosslinking of stromal collagen, and enhanced integrin signaling can drive breast cancer growth and metastasis (8-11). What’s more, stromal populations in the tumor microenvironment including immune cells and fibroblasts can induce matrix remodeling to promote cancer progression. For instance, the activity of collagen crosslinking enzymes expressed by tumor-associated macrophages were found to be more prevalent in aggressive breast cancers and were associated with greater propensity for metastasis (12). High tensile stress can also activate fibroblast transition to ECM-producing myofibroblast fates to further engineer remodeling and stiffening of the ECM (13).
Similarly, steroid hormone signaling is critical for development and the normal physiology associated with tissue-specific cell fate determination, growth, metabolism, and reproduction (14). Steroid hormones are derived from cholesterol and interact with nuclear- and membrane-associated receptors to regulate genomic and nongenomic signaling in cells (15). In mammalian reproductive tissues, they orchestrate significant dynamic tissue remodeling, such as that associated with menstruation and pregnancy. However, estrogenic and androgenic signaling are also critical regulators of osteoclast- and osteoblast-mediated bone homeostasis, muscle growth, cardiovascular system function, neuronal development, and adipose tissue metabolism (16-18). These multiple roles in diverse tissue contexts carry implications for exercise and muscle wasting and protection against neurodegenerative and cardiovascular disease (19, 20).
The majority of reproductive tissue cancers are linked to aberrant hormone signaling. For this reason, several first-line therapies for breast and prostate cancer, among others, target a reduction in steroid hormone signaling in hormone-sensitive tumor cells of epithelial origin. Although these cancers initially respond well to endocrine therapy, they frequently develop resistance and long-term dormancy that ultimately generates recurrent disease (21). Because of their pleiotropic effects on cell growth and metabolism, and complex interactions that control gene expression and paracrine signaling to direct tumor and stromal cell fates, much work remains to understand the hormone responsive molecular mechanisms that modulate tumor progression, dormancy and therapeutic resistance. Indeed, several expanding lines of investigation are exploring the roles of steroid hormone signaling in stromal fibroblasts and immune cell populations that often skew toward creating an immunosuppressive environment (22-25). These hormone-specific observations might help explain why immune checkpoint inhibitors have encountered limited success as a therapy for many breast and prostate cancer patients (26, 27). Moreover, emerging evidence points toward consequential interplay between mechanical stress induced cell responses and hormone signaling. Therefore, this review will highlight some of this accumulating evidence with a spotlight on mechanosignaling in hormone-sensitive cells in reproductive tissues and its implications for tumor progression.
Integrating Mechanical Forces Through Mechanosensing and Mechanotransduction
To discuss convergent factors mediating an interplay between mechanical forces and hormone signaling in governing cell fate, it is important to understand the manner in which cells integrate mechanical cues and transduce them through cytoskeletal- and membrane-mediated molecular responses to modify cell behavior. Several of these have been reviewed elsewhere (1, 2, 28); however, a brief summary of relevant mechanosensing and signaling pathways follows.
Much of mechanosensing in cells occurs at or associated with the cell membrane, where protein residents modify membrane tension and structure to influence responses to mechanical cues. For example, a bulky glycocalyx can alter membrane height to facilitate integrin clustering, epithelial cell growth, and survival (29). Membrane spanning integrin receptors mediate cell-ECM adhesion that are also critical for enabling cells to sense the physical properties of their microenvironment. For example, under mechanical stress, several ECM proteins including fibronectin can be extended or unfolded to reveal cryptic binding sites, thereby encouraging further integrin adhesion and fibronectin fibrillogenesis (30, 31). Integrins themselves undergo a stretched or unfolded conformational change in response to a stiff ECM that facilitates ligand binding (32). Integrin adhesions mature from small focal contacts on more compliant matrices, to larger integrin clusters needed for responses to increasing substrate rigidity. This process then triggers the recruitment of intracellular adhesion plaque proteins, several of which also exhibit force induced stretching. As an example, forces of 12 pN are required to unfold Talin and reveal cryptic binding sites for Vinculin at focal adhesions (33). Integrin adhesions link ECM binding to actin cytoskeleton remodeling and actomyosin mediated contractility which enable cells to generate reciprocal mechanoresponses to exogenously applied forces and enact cell behaviors such as migration and invasion (2). Additional mechanosensors and transducers existing at the cell membrane include mechanically gated ion channels such as Piezo1 and Piezo2 (34), G protein coupled receptors (35), and proteins participating in cell-cell adhesions. E-cadherin containing adherens junctions between cells are reinforced by the actin cytoskeleton and critical for force transmission between cells and maintenance of cell polarity (36).
All these pathways can impinge on growth factor signaling and intracellular kinase cascades to fine tune their activity and consequences (2), and many of these mechanosignaling pathways converge at the level of gene regulation. In this regard, the cell membrane can transmit mechanical forces to the nucleus through direct physical linkages mediated by the Linker of nucleoskeleton and cytoskeleton complex (37). Moreover, several transcription factors, including yes associated protein 1 (YAP) and WW domain-containing transcription regulator protein 1 (TAZ), are highly sensitive to mechanical cues (38). These direct physical links and DNA binding complexes then participate in whole-order chromatin reorganization to interpret and transduce the combined inputs of mechanical and biochemical signals into outputs of altered gene expression programs that direct cell fate determination (39).
The Interplay Between Mechanical and Hormonal Signaling in Cell Fate Determination
Osteocytes provide an excellent example of highly mechanosensitive cells critical for orchestrating the mechanical adaptation of bone (40). Composing 90% of cells in the bone matrix, they form a network of cell-cell contacts through slender processes that facilitate rapid signal transduction and enable acute discernment of mechanical loading frequency and subtle pressure changes to interstitial flow within bone matrix canaliculi (40). In responding to matrix strain, they produce signaling molecules (bone morphogenetic proteins, Wnts, prostaglandins, and nitric oxide) to inform the cell fate and activity of osteoclasts and osteoblasts to regulate bone resorption and formation (41). Molecular mediators of osteocyte mechanosensing include stretch activated ion channels, integrin-matrix adhesions, cell-cell interactions including gap junctions, and primary cilia (42, 43). Dynamic integrin complexes with links to the cell cytoskeleton are essential to the coordination of responses to mechanical stimuli, and clear differences in the distribution and architecture of the cytoskeleton between osteoblasts and osteocytes are associated with distinctive requirements for microtubules and actin filaments (40). Estrogens and estrogen receptors (ER) further modify how bone cells respond to mechanical strain. In general, estrogens suppress bone resorption and formation, and estrogen withdrawal leads to reduced bone mass and increased risk of fracture (44). ERα regulates several known factors that influence overall bone adaptation to mechanical loading (45, 46). For instance, ERα contributes to osteocyte and osteoblast mechanosensing by binding directly to IGF-1R to stimulate the expression of genes that control early responses to loading (47, 48). ERα can also regulate β1-integrin to alter ECM adhesion formation required for osteocytes and osteoblasts to effectively coordinate bone remodeling (40). Specifically in osteocytes, ERα induces expression of connexin 43 to facilitate gap junction intercellular communication and osteocyte mechanotransduction (49).
Taking such inspiration from bone, as well as muscle tissue, where the effects of mechanical loading clearly involve steroid hormone action on multiple unique cell types (16), examples of mechanosensitive hormone signaling pathways and their associated molecular mediators that play a role in directing cell fate will be discussed in the next section, with a particular focus on development and malignant progression in reproductive tissues.
Steroid Hormone Signaling and the ECM
ECM composition and architecture are reorganized by steroid hormone signaling in tissues. An obvious example can be taken from the mammary gland, where ovarian hormones influence the abundance of fibroglandular stroma that can be detected as opaque regions in radiographic images of breast tissue (6). This ECM-rich stromal content is diminished by treatment with the selective estrogen receptor modulator, tamoxifen, and expanded by hormone replacement therapy including estrogen with progestins in postmenopausal women (50, 51). In one extension of these clinical observations, it was demonstrated that specific ECM components, such as fibronectin, can induce tamoxifen resistance in breast cancer cells through β1-integrin adhesion, which has also been linked to tension-dependent mammary epithelial cell invasion (52, 53). Subsequent work demonstrated that estrogen stimulation promotes fibronectin bound β1-integrin colocalization with ERα at the cell membrane thereby favoring endocytic recycling of ERα to the cell surface in lieu of lysosome targeting for degradation (54). Moreover, membrane bound ERα, which can induce rapid lipid membrane raft initiated signaling responses in concert with other growth factors, appears to be necessary for efficient ECM remodeling associated with ductal outgrowth in the developing mammary gland. In this context, expression of a palmitoylation defective mutant of ERα (C451A-ERα) in mice resulted in impaired paracrine signaling of luminal mammary epithelial cells to basal mammary stem cells, thereby decreasing their expression of fibronectin and MMP9, which are required for cell migration and branching morphogenesis on transplantation (55). Cotransplantation of basal mammary epithelial cells derived from C451A-ERα-expressing mice with wild-type ERα expressing luminal cells restored normal ductal outgrowth (55). Another potential ECM-related mechanism linking hormone stimulation to mammary stem cell regulation that may complement these data, involves the secreted protease, a disintegrin and metalloproteinase with thrombospondin motifs 18 (Adamts18). Sensing of estrogen and progesterone by luminal epithelial cells induces Adamts18 expression in myoepithelial cells via Wnt4-stimulated canonical Wnt signaling, whereby Adamts18 can then remodel the matrix to support mammary stem cell regeneration. In contrast, loss of Adamts18 in mouse mammary epithelial cells causes increased collagen deposition, decreased hippo signaling and reduced Fibroblast growth factor receptor 2 expression to impair mammary stem cell function (56). This pathway puts forth an additional account of how hormone signaling in luminal cells might modify the matrix associated with a mammary stem cell niche to foster stem cell activation.
A remodeled and stiffened collagen ECM has been associated with increased tumor cell migration and invasion, accelerated tumor progression, and metastasis (5, 7-9, 12, 57). Several studies have made use of a mouse model of high collagen density (Col1a1tm1Jae/+) and 3-dimensional ECM or 2-dimensional polyacrylamide hydrogels to study the influence of matrix density/stiffness on ER-positive mammary tumor cell fate (57, 58). Specifically for prolactin-induced ER-positive tumor cells, a dense collagen matrix induces several signaling changes, including reduced Stat5 activation and enhanced focal adhesion kinase, Src family kinase, and extracellular signal-regulated kinase (ERK) activation (59-63), to ultimately increase numbers of circulating tumor cells and the incidence of metastases. In this context in vivo, estrogen stimulation resulted in more aligned peritumoral collagen and elevated expression of ECM components (fibronectin, tenascin C, maspin, periostin, LOX) associated with breast cancer progression (63). Last, a dense collagen matrix was found to promote the expansion of prolactin induced ERα-positive breast cancer stem cells through elevated Akt/mechanistic target of rapamycin kinase (mTOR)/YAP signaling, whereas metastatic lesions lost their dependence on mTOR (64). Importantly, this illustrates that the physical properties of the ECM intersect with hormone responses in a context-dependent manner to direct cell fate.
Further evidence supporting a role for matrix biophysical properties in dictating epithelial/tumor cell identity can be found in an elegant study that established a breast cancer and normal breast tissue explant culture platform (65). Using this system, the authors determined that ECM-rich Matrigel and collagen-I-based matrices promoted basal epithelial lineage identity, whereas more bioinert alginate and agarose based-matrices preserved luminal epithelial identity. Interestingly, only the stiffest agarose-based matrix was shown to maintain ERα expression over the long term in culture, a feat that has been notoriously difficult to achieve with primary mammary epithelial/breast cancer cell cultures. In addition, this method could be effectively enhanced for human tissue explant cultures by applying additional exogenous compressive stress upon the hydrogels (65). In this example, compression induced p38α mitogen-activated protein kinase (MAPK) activation, resulting in inhibition of ERα gene silencing by enhancer of Zeste 2 polycomb repressive complex 2 subunit-mediated histone methylation (65). This report is corroborated by separate findings that a calcium ion cross-linked alginate hydrogel encapsulation method for the culture of explanted breast cancer tissue microstructures was also effective at preserving ERα expression, in addition to overall tissue and fibrillar collagen architecture (66).
Integrin-mediated Mechanosignaling
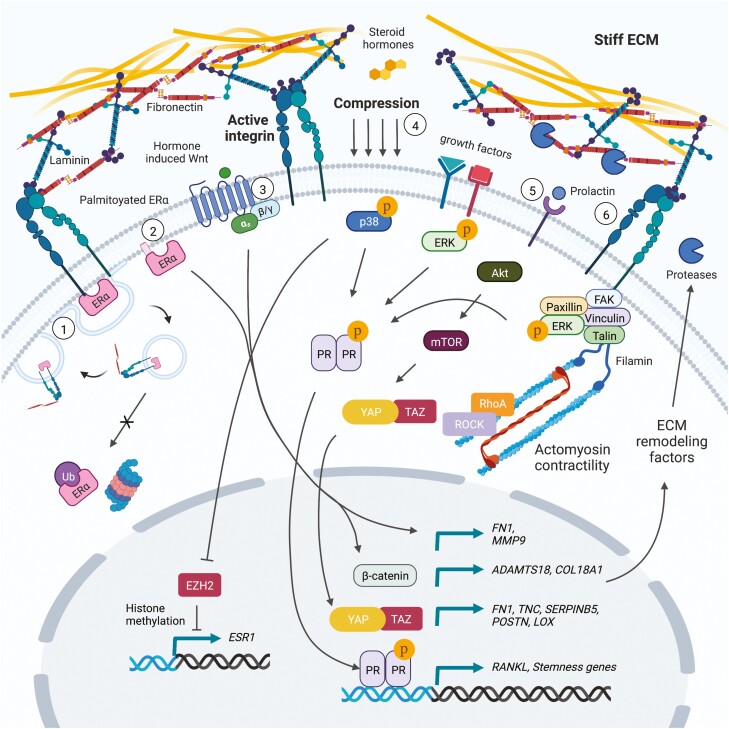
A principal response to elevated matrix rigidity is increased integrin ligation and activation of intracellular signaling kinase cascades that may intersect with hormone signaling. For instance, uterine fibroids are abnormal expansions of cells that exhibit an abundant and remodeled ECM, and uterine fibroid cells cultured on rigid substrates exhibit increased progesterone receptor-B activation in response to progesterone, in a mitogen-activated protein kinase kinase and Ras homolog family member A (RhoA)/rho associated coiled-coil containing protein kinase (ROCK)-dependent manner (67). Given evidence that Akt, ERK, and other MAPKs can all be activated in response to integrin mechanosignaling and that several residues on steroid hormone receptors undergo kinase-dependent posttranslational modification (5, 6, 10, 68, 69), future efforts should be directed toward understanding the crosstalk between these signaling pathways and how they regulate cell plasticity and tumor progression. That phosphorylation of progesterone receptor (PR) at a specific serine residue (294) is associated with stem-like gene expression in breast cancer cells underscores the potential importance for mechanosignaling within a hormone-responsive framework (70, 71). Moreover, a prior study found that cells from early human epidermal growth factor receptor 2/Neu-induced mammary tumor lesions expressed high levels of PR, RANKL, and Wnt4, which enhanced the early dissemination of breast cancer cells (72). Interestingly, when primary tumor cells were cultured as well-dispersed single cells with rich cell-substrate adhesion, as compared with high cell density conditions, PR levels were increased and cells became like highly migratory early lesion cells, further suggesting that adhesion mediated mechanosignaling influences PR activity and tumor cell fate (72). Interestingly, in this example, elevated human epidermal growth factor receptor 2 expression in tumor cells was related to the production of exosomes containing PR-gene (Pgr) targeting microRNAs (72). In further support of a role for mechanosignaling in this process, our laboratory and others have demonstrated that several microRNAs are regulated in an ECM stiffness-dependent manner (5, 6, 73, 74). See Fig. 1 for a summary of the pathways discussed in the current and preceding section.
Figure 1.
Mechanosensitive hormone signaling associated with ECM remodeling and integrin activity. (1) Estrogen promotes fibronectin bound β1-integrin colocalization with ERα at the cell membrane and endocytic recycling of ERα to the cell surface. (2) Palmitoylated membrane-bound ERα is critical for paracrine signaling to mammary epithelial stem cells, and the expression of fibronectin and MMP9 required for efficient epithelial cell migration and branching morphogenesis. (3) Steroid hormone-induced paracrine Wnt signaling promotes the expression of Adamts18 in myoepithelial cells, which is required for genetic interaction with the basement membrane proteoglycan, Col18a1, and matrix remodeling to generate a mammary stem cell niche. (4) Compressive forces induce p38α MAPK activation in mammary epithelial cells, resulting in inhibition of ERα gene silencing by EZH2-mediated histone methylation. (5) A dense collagen matrix was found to promote the expansion of prolactin induced ERα-positive breast cancer stem cells through elevated Akt/mTOR/YAP signaling. Moreover, estrogen stimulation of prolactin induced ER-positive tumor cells results in more aligned peritumoral collagen and elevated expression of ECM components (fibronectin, tenascin C, maspin, periostin, LOX) associated with breast cancer progression. (6) Elevated ECM stiffness and integrin mechanosignaling may intersect with growth factor signaling to potentiate ERK/MAPK-mediated phosphorylation of PR.
Membrane Dynamics
Dynamic cell membrane organization is one manner in which cells coordinate the transduction of mechanical cues. Notably, physical alterations to cell membrane architecture and tension could impact the conformation, oligomerization, and activity of any membrane-bound or associated proteins (75). Likewise, changes in lipid and protein composition can modify the physical properties of the cell membrane giving rise to additional considerations for membrane-imitated signaling cascades and their potential to crosstalk with steroid receptor signaling in cells (75). For example, membrane-integrated mechanosensitive ion channels, such as transient receptor potential cation channel subfamily V member 4 (TRPV4), likely require forces derived from both membrane stretch and integrin-matrix adhesions for their activation, and progesterone can further enhance TRPV4 activity and calcium (Ca2+) influx to regulate ciliated oviductal cells (76, 77).
The glycocalyx is a membrane-associated, cell-surface coating of glycoproteins and proteoglycans that is also integral to cell mechanosensing (78). A bulky glycocalyx is defined as a dense, thick sugar coating that includes increased numbers of glycoconjugates and glycoconjugate sites, and extensive elaboration of glycan structures. Human breast tumor cells exhibit a bulkier glycocalyx with an increased expression of sialoglycoproteins, which are higher in fibrotic regions in all breast tumor subtypes, as well as in more aggressive breast cancer subtypes that are generally more fibrotic (79). Sialoglycoproteins may drive tumor progression through immunosuppression by binding to siglec receptors on immune cells (79). A bulky tumor cell glycocalyx also functions to enhance integrin clustering and downstream phosphatidylinositol 3-kinase/Akt signaling to promote cell-cycle progression and increase metastatic potential (29, 80). The cell surface glycoprotein mucin 1 (MUC1), which is a major contributor to a bulky glycocalyx and overexpressed in multiple cancers, directly interacts with ERα to inhibit its ubiquitination and degradation and binds with ERα at estrogen-responsive gene promoters to stimulate ERα-mediated transcription (81). Moreover, estrogen and progesterone stimulate MUC1 expression, and MUC1 is associated with tamoxifen resistance in ER-positive breast cancers (82-84). Estrogen is also mechanically linked to the glycocalyx through protective effects on endothelial cells exposed to hypoxic insult (85). Under such culture conditions with microfluidic flow, estrogen treatment prevents glycocalyx degradation in endothelial cells (85). However, in opposition to these observations in breast cancer, elevated MUC1 expression is associated with androgen independence, lineage plasticity, and neuroendocrine differentiation in prostate cancer cells (86). Still, overall, these data suggest that steroid hormone receptor complexes can influence glycocalyx properties and vice versa to tune cell mechanosignaling and tumor promoting effects such as tumor cell survival and immunosuppression. Moreover, given the presence of numerous membrane steroid hormone receptors (87), future studies should be aimed at understanding how these receptors may be similarly regulated through altered membrane mechanical properties and architecture.
Cell Contractility
Hormone signaling may also modify cell cytoskeleton contractility to alter mechanoresponses in cells and dictate cell fate and tissue function (88-91). For example, estrogen induces rapid actin cytoskeleton and membrane remodeling through the stimulation of ERα-Gα13 interactions, which in turn induce actin filament-based cell migration in vascular endothelial cells via a RhoA/ROCK/Moesin axis (90). In subsequent work, a 7 transmembrane-domain G-protein coupled estrogen receptor (GPER), was found to link estrogen stimulation to phospholipase C beta-protein kinase C and RhoA/ROCK induced actin assembly to drive mechanosensitive TAZ activation and cell migration (88, 91). These observations suggest that combined membrane hormone receptor activity and mechanosignaling in cells might foster resistance to endocrine therapies, such as tamoxifen. In contrast, GPER activity appears to protect against cartilage degeneration by dampening mechanical stress induced RhoA activity and actin polymerization, resulting in inhibition of apoptosis induced by the mechanosensitive ion channel, Piezo1 (92). Again, this emphasizes the importance of avoiding assumptions based on seemingly similar mechanoresponsive hormone signaling pathways, which might evoke entirely different responses depending on cell context.
Alternatively, cell contractility may be required for efficient synthesis of steroid hormones. For example, deletion of RhoA in PR-expressing cells in mice results in disrupted cell cytoskeletal architecture in the luteal cells of the corpus luteum, as well as reduced mitochondrial density, impaired cholesterol transport and progesterone synthesis (89). Ultimately, these defects produced infertility in female mice because of progesterone insufficiency despite normal ovulation (89). In another example, mechanical stretch and testosterone stimulation of skeletal muscle cells resulted in the increased expression of estrogen related synthases and estrogen production, which acts as a protective factor against exercise induced muscle cell damage (93). These data indicate that reciprocal feedback is intimately involved in the hormone-sensitive mechanosignaling pathways controlling cell fate.
Mechanosensitive Transcription Factors
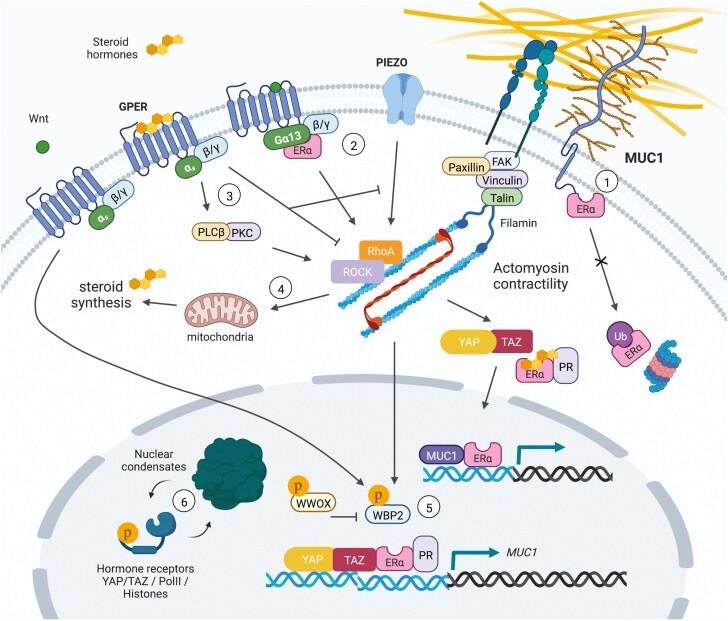
As previously alluded to, mechanosensitive steroid hormone signaling can converge at the level of gene expression through the interactions of nuclear hormone receptors with different transcriptional coactivators and co-repressors. Among mechanosensitive transcription factors, the Hippo signaling mediators, YAP and TAZ, are perhaps the most widely known and are strongly associated with stem-like character in epithelial cells and their associated cancers (38). In this regard, a combination of mechanical and hormonal stimuli in myometrium activates and enhances YAP/TAZ activity in uterine fibroids (94). In breast cancer, glucocorticoid stimulated glucocorticoid receptor signaling can potentiate YAP levels and activity through increasing fibronectin production, cell adhesion, and contractility (95). YAP is also present at ERα bound enhancers in vivo and was shown to augment ERα transcriptional activity and tumor growth in response to estrogens (96). Moreover, WW domain-binding protein 2 (WBP2) was identified as a cofactor that can bridge ERα/PR and YAP/TAZ to enhance hormone-induced transcriptional activity (97). Further studies have shown that Wnt signaling can suppress ubiquitin-mediated degradation of WBP2 to promote breast cancer progression, whereas phosphorylation of the tumor suppressor, WW domain containing oxidoreductase, triggers its binding to WBP2 to inhibit ER-mediated transcriptional activity (97-99). Lastly, mechanosensitive tyrosine kinase signaling can phosphorylate WBP2 to enhance ER-mediated transcription (100, 101). Together, these data suggest that WBP2 represents a node of convergence for Wnt, YAP/TAZ, WW domain containing oxidoreductase, and hormone receptor (ER/PR)-regulated gene promoters that regulates tumor cell mechanoresponses and behaviors. Elucidation of similar transcriptional nodes will enable the design of novel interventions capable of manipulating mechanical stress induced hormone signaling. See Fig. 2 for a summary of the pathways discussed in the current and preceding 2 sections.
Figure 2.
Mechanosensitive hormone signaling associated with membrane/cytoskeletal dynamics and transcription factor activity. (1) The glycoprotein MUC1 directly interacts with ERα to inhibit its ubiquitination and degradation, and binds with ERα at estrogen-responsive gene promoters to stimulate ERα-mediated transcription. Steroid hormones can also stimulate MUC1 expression. (2) Estrogen induces rapid actin cytoskeleton and membrane remodeling through ERα-Gα13 interactions upstream of RhoA/ROCK/moesin signaling. (3) GPER links estrogen stimulation to PLCβ-PKC and RhoA/ROCK induced actin assembly to drive mechanosensitive TAZ activation and cell migration. GPER can alternatively dampen mechanical stress induced RhoA activity and actin polymerization, resulting in inhibition of apoptosis induced by the mechanosensitive ion channel, Piezo1, as a protective mechanism against cartilage degeneration. (4) RhoA activity is required in PR-expressing luteal cells of the corpus luteum to maintain mitochondrial density, efficient cholesterol transport, and progesterone synthesis. In skeletal muscle cells, mechanical stretch and testosterone stimulation results in increased expression of estrogen-related synthases and estrogen production. (5) WBP2 is a cofactor for ERα/PR and YAP/TAZ transcriptional activity. Wnt signaling suppresses ubiquitin mediated degradation of WBP2 to promote breast cancer progression, whereas phosphorylation of the tumor suppressor, WWOX, triggers its binding to WBP2 to inhibit ERα-mediated transcription. Alternatively, mechanosensitive tyrosine kinase signaling can phosphorylate WBP2 to enhance ERα-mediated transcription. (6) As observed for YAP/TAZ, the intrinsically disordered regions of hormone receptors (AR/PR) could allow for mechanosensitive phase separation and condensate formation to govern hormone-induced transcriptional complex activity.
Future Frontiers in Mechanosensitive Hormone Signaling
Compressive forces and dense cell packing impact molecular crowding in cells; a feature that has been associated with macromolecular condensate formation or liquid-liquid phase separation (102). For example, condensate formation has been observed in the nucleus for the transcription factors YAP and TAZ as a mechanism to regulate and reprogram their transcriptional output (103, 104). Given that intrinsically disordered regions of hormone receptors such as the androgen receptor and PR form droplets in vitro (105, 106), the potential for mechanosensitive phase separation and condensate formation in regulating hormone-induced transcriptional complex activity represents an intriguing line of investigation. Together with condensate facilitating histone tail and RNA polymerase II C-terminal domain posttranslational modifications (107, 108), broad epigenetic programs related to hormone stimulation and transcription under the high mechanical stresses especially relevant to tumor progression could begin to be deciphered.
Although hormone-related tumors generally respond well to endocrine therapy, both primary and disseminated tumor cells frequently go dormant for several years before awakening as recurrent treatment refractory disease (109). The mechanical properties of the ECM, which can be remodeled by both tumor and stromal cells, can have a significant impact on escape from dormancy and disease progression (110-112). For instance, sustained lung inflammation was recently shown to promote neutrophil extracellular trap formation, cleavage of laminin matrix, and exposure of integrin complexes (αβ1) that stimulate focal adhesion kinase/ERK/myosin light chain kinase/YAP signaling and the awakening of dormant tumor cells (113). Likewise, hormone levels and hormone receptor signaling can restrain or reactivate the growth of dormant tumor cells, as has been shown for glucocorticoid or estrogen/progesterone signaling respectively (114, 115), and hormone deprivation therapies can prolong dormant states (116, 117). Furthermore, the ratio between phospho-p38 and phospho-ERK MAPKs has been implicated in the control of tumor dormancy (118, 119), and, as described, there may be significant interplay between mechanosensitive hormone signaling and MAPK activity. Therefore, future investigation should be aimed at improving our understanding of how the combination of different mechanical cues and hormone signals interact to control the maintenance or exit of tumor cells from dormant states.
Acknowledgments
Figures were adapted from “Blank Pathway (Round Landscape)”, by BioRender.com (2022). Retrieved from https://app.biorender.com/biorender-templates.
Glossary
Abbreviations
- Adamts 18
a disintegrin and metalloproteinase with thrombospondin motifs 18
- ECM
extracellular matrix
- ER
estrogen receptor
- ERK
extracellular signal-regulated kinase
- GPER
G-protein coupled estrogen receptor
- LOX
lysyl oxidase
- MAPK
mitogen-activated protein kinase
- MUC1
mucin 1
- PR
progesterone receptor
- RhoA
Ras homolog family member A
- ROCK
rho associated coiled-coil containing protein kinase
- TAZ
WW domain-containing transcription regulator protein 1
- WBP2
WW domain-binding protein 2
- YAP
yes associated protein 1
Contributor Information
Jason J Northey, Department of Surgery, University of California, San Francisco, CA 94143, USA; Center for Bioengineering and Tissue Regeneration, University of California, San Francisco, San Francisco, CA 94143, USA.
Valerie M Weaver, Department of Surgery, University of California, San Francisco, CA 94143, USA; Center for Bioengineering and Tissue Regeneration, University of California, San Francisco, San Francisco, CA 94143, USA; UCSF Helen Diller Comprehensive Cancer Center, University of California, San Francisco, San Francisco, CA 94143, USA; Department of Bioengineering and Therapeutic Sciences, University of California, San Francisco, San Francisco, CA 94143, USA; Department of Radiation Oncology, Eli and Edythe Broad Center of Regeneration Medicine and Stem Cell Research, University of California, San Francisco, San Francisco, CA 94143, USA.
Funding
National Institutes of Health and National Cancer Institute (R01CA192914, 1R01CA222508-01, and 1R35CA242447-01A1) to V.M.N. American Association for Cancer Research (AACR) Basic Cancer Research Fellowship (15-40-01-NORT) and AACR-Janssen Fellowship in Cancer Interception Research (17-40-48-NORT) to J.J.N.
Disclosures
The authors have nothing to disclose.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Hayward MK, Muncie JM, Weaver VM. Tissue mechanics in stem cell fate, development, and cancer. Dev Cell. 2021;56(13):1833-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Northey JJ, Przybyla L, Weaver VM. Tissue force programs cell fate and tumor aggression. Cancer Discov. 2017;7(11):1224-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Przybyla L, Lakins JN, Weaver VM. Tissue mechanics orchestrate Wnt-dependent human embryonic stem cell differentiation. Cell Stem Cell. 2016;19(4):462-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677-689. [DOI] [PubMed] [Google Scholar]
- 5. Mouw JK, Yui Y, Damiano L, et al. Tissue mechanics modulate microRNA-dependent PTEN expression to regulate malignant progression. Nat Med. 2014;20(4):360-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Northey JJ, Barrett AS, Acerbi I, et al. Stiff stroma increases breast cancer risk by inducing the oncogene ZNF217. J Clin Invest. 2020;130(11):5721-5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Laklai H, Miroshnikova YA, Pickup MW, et al. Genotype tunes pancreatic ductal adenocarcinoma tissue tension to induce matricellular fibrosis and tumor progression. Nat Med. 2016;22(5):497-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pickup MW, Laklai H, Acerbi I, et al. Stromally derived lysyl oxidase promotes metastasis of transforming growth factor-beta-deficient mouse mammary carcinomas. Cancer Res. 2013;73(17):5336-5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Levental KR, Yu H, Kass L, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139(5):891-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paszek MJ, Zahir N, Johnson KR, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8(3):241-254. [DOI] [PubMed] [Google Scholar]
- 11. Erler JT, Bennewith KL, Cox TR, et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15(1):35-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maller O, Drain AP, Barrett AS, et al. Tumour-associated macrophages drive stromal cell-dependent collagen crosslinking and stiffening to promote breast cancer aggression. Nat Mater. 2021;20(4):548-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Piersma B, Hayward MK, Weaver VM. Fibrosis and cancer: a strained relationship. Biochim Biophys Acta Rev Cancer. 2020;1873(2):188356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Singh RR, Kumar R. Steroid hormone receptor signaling in tumorigenesis. J Cell Biochem. 2005;96(3):490-505. [DOI] [PubMed] [Google Scholar]
- 15. Wilkenfeld SR, Lin C, Frigo DE. Communication between genomic and non-genomic signaling events coordinate steroid hormone actions. Steroids. 2018;133:2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carson JA, Manolagas SC. Effects of sex steroids on bones and muscles: similarities, parallels, and putative interactions in health and disease. Bone. 2015;80:67-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Diotel N, Charlier TD, Lefebvre d’Hellencourt C, et al. Steroid transport, local synthesis, and signaling within the brain: roles in neurogenesis, neuroprotection, and sexual behaviors. Front Neurosci. 2018;12:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mayes JS, Watson GH. Direct effects of sex steroid hormones on adipose tissues and obesity. Obes Rev. 2004;5(4):197-216. [DOI] [PubMed] [Google Scholar]
- 19. Ueda K, Fukuma N, Adachi Y, et al. Sex differences and regulatory actions of estrogen in cardiovascular system. Front Physiol. 2021;12:738218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pike CJ, Carroll JC, Rosario ER, Barron AM. Protective actions of sex steroid hormones in Alzheimer’s disease. Front Neuroendocrinol. 2009;30(2):239-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hanker AB, Sudhan DR, Arteaga CL. Overcoming endocrine resistance in breast cancer. Cancer Cell. 2020;37(4):496-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chakraborty S, Pramanik J, Mahata B. Revisiting steroidogenesis and its role in immune regulation with the advanced tools and technologies. Genes Immun. 2021;22(3):125-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Werner LR, Gibson KA, Goodman ML, et al. Progesterone promotes immunomodulation and tumor development in the murine mammary gland. J ImmunoTher Cancer. 2021;9(5):1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xavier AM, Anunciato AK, Rosenstock TR, Glezer I. Gene expression control by glucocorticoid receptors during innate immune responses. Front Endocrinol (Lausanne). 2016;7:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schuler LA, Murdoch FE. Endogenous and therapeutic estrogens: maestro conductors of the microenvironment of ER+ breast cancers. Cancers (Basel). 2021;13(15):1-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vonderheide RH, Domchek SM, Clark AS. Immunotherapy for breast cancer: what are we missing? Clin Cancer Res. 2017;23(11):2640-2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bilusic M, Madan RA, Gulley JL. Immunotherapy of prostate cancer: facts and hopes. Clin Cancer Res. 2017;23(22):6764-6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006;20(7):811-827. [DOI] [PubMed] [Google Scholar]
- 29. Paszek MJ, DuFort CC, Rossier O, et al. The cancer glycocalyx mechanically primes integrin-mediated growth and survival. Nature. 2014;511(7509):319-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oberhauser AF, Badilla-Fernandez C, Carrion-Vazquez M, Fernandez JM. The mechanical hierarchies of fibronectin observed with single-molecule AFM. J Mol Biol. 2002;319(2):433-447. [DOI] [PubMed] [Google Scholar]
- 31. Sechler JL, Rao H, Cumiskey AM, et al. A novel fibronectin binding site required for fibronectin fibril growth during matrix assembly. J Cell Biol. 2001;154(5):1081-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Case LB, Waterman CM. Integration of actin dynamics and cell adhesion by a three-dimensional, mechanosensitive molecular clutch. Nat Cell Biol. 2015;17(8):955-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP. Stretching single talin rod molecules activates vinculin binding. Science. 2009;323(5914):638-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Douguet D, Honore E. Mammalian mechanoelectrical transduction: structure and function of force-gated ion channels. Cell. 2019;179(2):340-354. [DOI] [PubMed] [Google Scholar]
- 35. Chachisvilis M, Zhang YL, Frangos JA. G protein-coupled receptors sense fluid shear stress in endothelial cells. Proc Natl Acad Sci USA. 2006;103(42):15463-15468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Charras G, Yap AS. Tensile forces and mechanotransduction at cell-cell junctions. Curr Biol. 2018;28(8):R445-R457. [DOI] [PubMed] [Google Scholar]
- 37. Alam SG, Zhang Q, Prasad N, et al. The mammalian LINC complex regulates genome transcriptional responses to substrate rigidity. Sci Rep. 2016;6:38063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Piccolo S, Cordenonsi M, Dupont S. Molecular pathways: YAP and TAZ take center stage in organ growth and tumorigenesis. Clin Cancer Res. 2013;19(18):4925-4930. [DOI] [PubMed] [Google Scholar]
- 39. Nava MM, Miroshnikova YA, Biggs LC, et al. Heterochromatin-driven nuclear softening protects the genome against mechanical stress-induced damage. Cell. 2020;181(4):800-817 e822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Klein-Nulend J, van Oers RF, Bakker AD, Bacabac RG. Bone cell mechanosensitivity, estrogen deficiency, and osteoporosis. J Biomech. 2015;48(5):855-865. [DOI] [PubMed] [Google Scholar]
- 41. Klein-Nulend J, van Oers RF, Bakker AD, Bacabac RG. Nitric oxide signaling in mechanical adaptation of bone. Osteoporos Int. 2014;25(5):1427-1437. [DOI] [PubMed] [Google Scholar]
- 42. Ingber DE. Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol. 1997;59:575-599. [DOI] [PubMed] [Google Scholar]
- 43. Zhang Y, Paul EM, Sathyendra V, et al. Enhanced osteoclastic resorption and responsiveness to mechanical load in gap junction deficient bone. PLoS One. 2011;6(8):e23516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bakker AD, Klein-Nulend J, Tanck E, Albers GH, Lips P, Burger EH. Additive effects of estrogen and mechanical stress on nitric oxide and prostaglandin E2 production by bone cells from osteoporotic donors. Osteoporos Int. 2005;16(8):983-989. [DOI] [PubMed] [Google Scholar]
- 45. Lee K, Jessop H, Suswillo R, Zaman G, Lanyon L. Endocrinology: bone adaptation requires oestrogen receptor-alpha. Nature. 2003;424(6947):389. [DOI] [PubMed] [Google Scholar]
- 46. Windahl SH, Saxon L, Borjesson AE, et al. Estrogen receptor-alpha is required for the osteogenic response to mechanical loading in a ligand-independent manner involving its activation function 1 but not 2. J Bone Miner Res. 2013;28(2):291-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sunters A, Armstrong VJ, Zaman G, et al. Mechano-transduction in osteoblastic cells involves strain-regulated estrogen receptor alpha-mediated control of insulin-like growth factor (IGF) I receptor sensitivity to ambient IGF, leading to phosphatidylinositol 3-kinase/AKT-dependent Wnt/LRP5 receptor-independent activation of beta-catenin signaling. J Biol Chem. 2010;285(12): 8743-8758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liedert A, Wagner L, Seefried L, Ebert R, Jakob F, Ignatius A. Estrogen receptor and Wnt signaling interact to regulate early gene expression in response to mechanical strain in osteoblastic cells. Biochem Biophys Res Commun. 2010;394(3):755-759. [DOI] [PubMed] [Google Scholar]
- 49. Ren J, Wang XH, Wang GC, Wu JH. 17beta estradiol regulation of connexin 43-based gap junction and mechanosensitivity through classical estrogen receptor pathway in osteocyte-like MLO-Y4 cells. Bone. 2013;53(2):587-596. [DOI] [PubMed] [Google Scholar]
- 50. Chlebowski RT, Hendrix SL, Langer RD, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative Randomized Trial. JAMA. 2003;289(24):3243-3253. [DOI] [PubMed] [Google Scholar]
- 51. Boyd NF, Martin LJ, Yaffe MJ, Minkin S. Mammographic density and breast cancer risk: current understanding and future prospects. Breast Cancer Res. 2011;13(6):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Miroshnikova YA, Rozenberg GI, Cassereau L, et al. alpha5beta1-Integrin promotes tension-dependent mammary epithelial cell invasion by engaging the fibronectin synergy site. Mol Biol Cell. 2017;28(22):2958-2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sampayo RG, Toscani AM, Rubashkin MG, et al. Fibronectin rescues estrogen receptor alpha from lysosomal degradation in breast cancer cells. J Cell Biol. 2018;217(8):2777-2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pontiggia O, Sampayo R, Raffo D, et al. The tumor microenvironment modulates tamoxifen resistance in breast cancer: a role for soluble stromal factors and fibronectin through beta1 integrin. Breast Cancer Res Treat. 2012;133(2):459-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gagniac L, Rusidze M, Boudou F, et al. Membrane expression of the estrogen receptor ERalpha is required for intercellular communications in the mammary epithelium. Development. 2020;147(5):1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ataca D, Aouad P, Constantin C, et al. The secreted protease Adamts18 links hormone action to activation of the mammary stem cell niche. Nat Commun. 2020;11(1):1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Provenzano PP, Inman DR, Eliceiri KW, Keely PJ. Matrix density-induced mechanoregulation of breast cell phenotype, signaling and gene expression through a FAK-ERK linkage. Oncogene. 2009;28(49):4326-4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Provenzano PP, Inman DR, Eliceiri KW, et al. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008;6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Barcus CE, Holt EC, Keely PJ, Eliceiri KW, Schuler LA. Dense collagen-I matrices enhance pro-tumorigenic estrogen-prolactin crosstalk in MCF-7 and T47D breast cancer cells. PLoS One. 2015;10(1):e0116891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Barcus CE, Keely PJ, Eliceiri KW, Schuler LA. Stiff collagen matrices increase tumorigenic prolactin signaling in breast cancer cells. J Biol Chem. 2013;288(18):12722-12732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Barcus CE, O’Leary KA, Brockman JL, et al. Elevated collagen-I augments tumor progressive signals, intravasation and metastasis of prolactin-induced estrogen receptor alpha positive mammary tumor cells. Breast Cancer Res. 2017;19(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Barcus CE, Keely PJ, Eliceiri KW, Schuler LA. Prolactin signaling through focal adhesion complexes is amplified by stiff extracellular matrices in breast cancer cells. Oncotarget. 2016;7(30):48093-48106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Arendt LM, Evans LC, Rugowski DE, Garcia-Barchino MJ, Rui H, Schuler LA. Ovarian hormones are not required for PRL-induced mammary tumorigenesis, but estrogen enhances neoplastic processes. J Endocrinol. 2009;203(1):99-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Shea MP, O’Leary KA, Wegner KA, Vezina CM, Schuler LA. High collagen density augments mTOR-dependent cancer stem cells in ERalpha+ mammary carcinomas, and increases mTOR-independent lung metastases. Cancer Lett. 2018;433:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Munne PM, Martikainen L, Raty I, et al. Compressive stress-mediated p38 activation required for ERalpha + phenotype in breast cancer. Nat Commun. 2021;12(1):6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cartaxo AL, Estrada MF, Domenici G, et al. A novel culture method that sustains ERalpha signaling in human breast cancer tissue microstructures. J Exp Clin Cancer Res. 2020;39(1):161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cordeiro Mitchell CN, Islam MS, Afrin S, Brennan J, Psoter KJ, Segars JH. Mechanical stiffness augments ligand-dependent progesterone receptor B activation via MEK 1/2 and Rho/ROCK-dependent signaling pathways in uterine fibroid cells. Fertil Steril. 2021;116(1):255-265. [DOI] [PubMed] [Google Scholar]
- 68. Dwyer AR, Truong TH, Ostrander JH, Lange CA. 90 YEARS OF PROGESTERONE: Steroid receptors as MAPK signaling sensors in breast cancer: let the fates decide. J Mol Endocrinol. 2020;65(1):T35-T48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Drain AP, Zahir N, Northey JJ, et al. Matrix compliance permits NF-kappaB activation to drive therapy resistance in breast cancer. J Exp Med. 2021;218(5):1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Knutson TP, Truong TH, Ma S, et al. Posttranslationally modified progesterone receptors direct ligand-specific expression of breast cancer stem cell-associated gene programs. J Hematol Oncol. 2017;10(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Truong TH, Hu H, Temiz NA, et al. Cancer stem cell phenotypes in ER(+) breast cancer models are promoted by PELP1/AIB1 complexes. Mol Cancer Res. 2018;16(4):707-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hosseini H, Obradovic MMS, Hoffmann M, et al. Early dissemination seeds metastasis in breast cancer. Nature. 2016;540(7634):552-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Herault S, Naser J, Carassiti D, et al. Mechanosensitive pathways are regulated by mechanosensitive miRNA clusters in endothelial cells. Biophys Rev. 2021;13(5):787-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mohamed JS, Hajira A, Lopez MA, Boriek AM. Genome-wide mechanosensitive MicroRNA (MechanomiR) screen uncovers dysregulation of their regulatory networks in the mdm mouse model of muscular dystrophy. J Biol Chem. 2015;290(41):24986-25011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Cheng X, Smith JC. Biological membrane organization and cellular signaling. Chem Rev. 2019;119(9):5849-5880. [DOI] [PubMed] [Google Scholar]
- 76. Mendez-Resendiz KA, Enciso-Pablo O, Gonzalez-Ramirez R, Juarez-Contreras R, Rosenbaum T, Morales-Lazaro SL. Steroids and TRP channels: a close relationship. Int J Mol Sci. 2020;21(11):1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ji C, McCulloch CA. TRPV4 integrates matrix mechanosensing with Ca(2+) signaling to regulate extracellular matrix remodeling. FEBS J. 2021;288(20):5867-5887. [DOI] [PubMed] [Google Scholar]
- 78. Buffone A, Weaver VM. Don’t sugarcoat it: how glycocalyx composition influences cancer progression. J Cell Biol. 2020;219(1):1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Metcalf KJ, Hayward MK, Berens E, et al. Immunosuppressive glycoproteins associate with breast tumor fibrosis and aggression. Matrix Biol Plus. 2022;14:100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Woods EC, Kai F, Barnes JM, et al. A bulky glycocalyx fosters metastasis formation by promoting G1 cell cycle progression. Elife. 2017;6:1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wei X, Xu H, Kufe D. MUC1 oncoprotein stabilizes and activates estrogen receptor alpha. Mol Cell. 2006;21(2):295-305. [DOI] [PubMed] [Google Scholar]
- 82. Zaretsky JZ, Barnea I, Aylon Y, Gorivodsky M, Wreschner DH, Keydar I. MUC1 gene overexpressed in breast cancer: structure and transcriptional activity of the MUC1 promoter and role of estrogen receptor alpha (ERalpha) in regulation of the MUC1 gene expression. Mol Cancer. 2006;5:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Merikhian P, Ghadirian R, Farahmand L, Mansouri S, Majidzadeh AK. MUC1 induces tamoxifen resistance in estrogen receptor-positive breast cancer. Expert Rev Anticancer Ther. 2017;17(7):607-613. [DOI] [PubMed] [Google Scholar]
- 84. Pitroda SP, Khodarev NN, Beckett MA, Kufe DW, Weichselbaum RR. MUC1-induced alterations in a lipid metabolic gene network predict response of human breast cancers to tamoxifen treatment. Proc Natl Acad Sci USA. 2009;106(14):5837-5841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Diebel LN, Wheaton M, Liberati DM. The protective role of estrogen on endothelial and glycocalyx barriers after shock conditions: a microfluidic study. Surgery. 2021;169(3):678-685. [DOI] [PubMed] [Google Scholar]
- 86. Yasumizu Y, Rajabi H, Jin C, et al. MUC1-C regulates lineage plasticity driving progression to neuroendocrine prostate cancer. Nat Commun. 2020;11(1):338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Trevino LS, Gorelick DA. The interface of nuclear and membrane steroid signaling. Endocrinology. 2021;162(8):1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wang Z, Sun L, Liang S, et al. GPER stabilizes F-actin cytoskeleton and activates TAZ via PLCbeta-PKC and Rho/ROCK-LIMK-Cofilin pathway. Biochem Biophys Res Commun. 2019;516(3):976-982. [DOI] [PubMed] [Google Scholar]
- 89. El Zowalaty AE, Li R, Zheng Y, Lydon JP, DeMayo FJ, Ye X. Deletion of RhoA in progesterone receptor-expressing cells leads to luteal insufficiency and infertility in female mice. Endocrinology. 2017;158(7):2168-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Simoncini T, Scorticati C, Mannella P, et al. Estrogen receptor alpha interacts with Galpha13 to drive actin remodeling and endothelial cell migration via the RhoA/Rho kinase/moesin pathway. Mol Endocrinol. 2006;20(8):1756-1771. [DOI] [PubMed] [Google Scholar]
- 91. Zhou X, Wang S, Wang Z, et al. Estrogen regulates Hippo signaling via GPER in breast cancer. J Clin Invest. 2015;125(5):2123-2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sun Y, Leng P, Guo P, et al. G protein coupled estrogen receptor attenuates mechanical stress-mediated apoptosis of chondrocyte in osteoarthritis via suppression of Piezo1. Mol Med. 2021;27(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Feng Y, Wu J, Cheng Z, Zhang J, Lu J, Shi R. Mechanical stretch enhances sex steroidogenesis in C2C12 skeletal muscle cells. Steroids. 2019;150:108434. [DOI] [PubMed] [Google Scholar]
- 94. Purdy MP, Ducharme M, Haak AJ, et al. YAP/TAZ are activated by mechanical and hormonal stimuli in myometrium and exhibit increased baseline activation in uterine fibroids. Reprod Sci. 2020;27(4):1074-1085. [DOI] [PubMed] [Google Scholar]
- 95. Sorrentino G, Ruggeri N, Zannini A, et al. Glucocorticoid receptor signalling activates YAP in breast cancer. Nat Commun. 2017;8:14073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zhu C, Li L, Zhang Z, et al. A non-canonical role of YAP/TEAD is required for activation of estrogen-regulated enhancers in breast cancer. Mol Cell. 2019;75(4):791-806 e798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Dhananjayan SC, Ramamoorthy S, Khan OY, et al. WW domain binding protein-2, an E6-associated protein interacting protein, acts as a coactivator of estrogen and progesterone receptors. Mol Endocrinol. 2006;20(10):2343-2354. [DOI] [PubMed] [Google Scholar]
- 98. McDonald CB, Buffa L, Bar-Mag T, et al. Biophysical basis of the binding of WWOX tumor suppressor to WBP1 and WBP2 adaptors. J Mol Biol. 2012;422(1):58-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Lim SK, Lu SY, Kang SA, et al. Wnt signaling promotes breast cancer by blocking ITCH-mediated degradation of YAP/TAZ transcriptional coactivator WBP2. Cancer Res. 2016;76(21):6278-6289. [DOI] [PubMed] [Google Scholar]
- 100. Chen Y, Choong LY, Lin Q, et al. Differential expression of novel tyrosine kinase substrates during breast cancer development. Mol Cell Proteomics. 2007;6(12):2072-2087. [DOI] [PubMed] [Google Scholar]
- 101. Lim SK, Orhant-Prioux M, Toy W, Tan KY, Lim YP. Tyrosine phosphorylation of transcriptional coactivator WW-domain binding protein 2 regulates estrogen receptor alpha function in breast cancer via the Wnt pathway. FASEB J. 2011;25(9):3004-3018. [DOI] [PubMed] [Google Scholar]
- 102. A P, Weber SC. Evidence for and against liquid-liquid phase separation in the nucleus. Noncoding RNA. 2019;5(4):1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Cai D, Liu Z, Lippincott-Schwartz J. Biomolecular condensates and their links to cancer progression. Trends Biochem Sci. 2021;46(7):535-549. [DOI] [PubMed] [Google Scholar]
- 104. Cai D, Feliciano D, Dong P, et al. Phase separation of YAP reorganizes genome topology for long-term YAP target gene expression. Nat Cell Biol. 2019;21(12):1578-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. De Mol E, Szulc E, Di Sanza C, et al. Regulation of androgen receptor activity by transient interactions of its transactivation domain with general transcription regulators. Structure. 2018;26(1):145-152 e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Beato M, Wright RHG, Dily FL. 90 years of progesterone: molecular mechanisms of progesterone receptor action on the breast cancer genome. J Mol Endocrinol. 2020;65(1):T65-T79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Gibson BA, Doolittle LK, Schneider MWG, et al. Organization of chromatin by intrinsic and regulated phase separation. Cell. 2019;179(2):470-484 e421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Guo YE, Manteiga JC, Henninger JE, et al. Pol II phosphorylation regulates a switch between transcriptional and splicing condensates. Nature. 2019;572(7770):543-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Risson E, Nobre AR, Maguer-Satta V, Aguirre-Ghiso JA. The current paradigm and challenges ahead for the dormancy of disseminated tumor cells. Nat Cancer. 2020;1(7):672-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Barkan D, El Touny LH, Michalowski AM, et al. Metastatic growth from dormant cells induced by a col-I-enriched fibrotic environment. Cancer Res. 2010;70(14):5706-5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Shibue T, Weinberg RA. Integrin beta1-focal adhesion kinase signaling directs the proliferation of metastatic cancer cells disseminated in the lungs. Proc Natl Acad Sci USA. 2009;106(25):10290-10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Di Martino JS, Nobre AR, Mondal C, et al. A tumor-derived type III collagen-rich ECM niche regulates tumor cell dormancy. Nat Cancer. 2022;3(1):90-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Albrengues J, Shields MA, Ng D, et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science. 2018;361(6409):1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ogba N, Manning NG, Bliesner BS, et al. Luminal breast cancer metastases and tumor arousal from dormancy are promoted by direct actions of estradiol and progesterone on the malignant cells. Breast Cancer Res. 2014;16(6):489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Prekovic S, Schuurman K, Mayayo-Peralta I, et al. Glucocorticoid receptor triggers a reversible drug-tolerant dormancy state with acquired therapeutic vulnerabilities in lung cancer. Nat Commun. 2021;12(1):4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Cackowski FC, Heath EI. Prostate cancer dormancy and recurrence. Cancer Lett. 2022;524:103-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Zhang XH, Giuliano M, Trivedi MV, Schiff R, Osborne CK. Metastasis dormancy in estrogen receptor-positive breast cancer. Clin Cancer Res. 2013;19(23):6389-6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Bragado P, Estrada Y, Parikh F, et al. TGF-beta2 dictates disseminated tumour cell fate in target organs through TGF-beta-RIII and p38alpha/beta signalling. Nat Cell Biol. 2013;15(11):1351-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Aguirre-Ghiso JA, Ossowski L, Rosenbaum SK. Green fluorescent protein tagging of extracellular signal-regulated kinase and p38 pathways reveals novel dynamics of pathway activation during primary and metastatic growth. Cancer Res. 2004;64(20):7336-7345. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.