Abstract
Maternal-child microbial seeding interventions expose Cesarean-section (C-section)-delivered infants to the maternal microbiome they bypass during Cesarean delivery. It is theorized such interventions restore the microbiome and normalize immune development to reduce the occurrence of C-section-associated inflammatory conditions. Here we discuss the rationale, evidence, and controversies surrounding such interventions.
Background
Cesarean section (C-section) delivery has been associated with many chronic inflammatory conditions in the offspring, which are in part thought to be gut microbiome mediated. Maternal-child microbial seeding interventions expose C-section-delivered infants to the maternal microbiome that they would have otherwise been exposed to during a vaginal delivery. Observational studies have shown that such interventions may partially restore the microbiome. It is theorized that they can also reduce the risk of C-section-associated diseases by normalizing microbiome-associated immune and metabolic development. Here, we discuss the rationale for such interventions, the current evidence of efficacy, the controversies surrounding maternal-child microbial seeding, and future directions.
C-section-associated diseases and microbiome-immune mechanisms
The microbes that first colonize our bodies from birth play a key role in immune system education and metabolic programming (Gensollen et al., 2016). Disruption to the order and timing of colonization during this critical period of microbiome development may have long-lasting immune and inflammatory health consequences (Cox et al., 2014).
Over 30% of infant deliveries in the United States are via C-section. C-section delivery, though usually necessary and lifesaving, represents a major disruption to early life microbial colonization. Infants delivered via C-section are not exposed to the vaginal canal and therefore bypass the first intended major microbiota exposure that they would have received during a vaginal delivery, thought to be critical for subsequent microbiome development. Indeed, more than 20 studies including our own work have found differences in microbiome acquisition and development in infants born by C-section compared with those born by vaginal delivery (Dominguez-Bello et al., 2010). Shortly after birth, vaginally delivered infants typically acquire bacterial communities resembling their own mother’s vaginal and perineal microbiota, including Lactobacillus spp., while C-section-delivered infants often harbor bacterial communities similar to those found on the skin surface, dominated by Staphylococcus, Corynebacterium, and Propionibacterium spp. (Dominguez-Bello et al., 2010). There is subsequent differential development of the gut microbiome between children delivered by C-section and vaginal delivery, notably with Bacteroides spp. and Bifidobacterium spp. being underrepresented and potential path-obionts overrepresented, in the gut microbiomes of C-section-delivered infants. While these differences diminish over time, some can still be detected into early childhood.
Moreover, there is significant epidemiological evidence that infants delivered by C-section are at increased risk for a range of chronic inflammatory and metabolic conditions, with the most evidence for obesity and atopy but also including systemic connective tissue disorders, juvenile arthritis, inflammatory bowel disease, immune deficiencies, and leukemia (Słabuszewska-Jóźwiak et al., 2020). It should be noted that while these findings have not always been consistent, several of the associations have been supported by either meta-analyses or very large observational studies (Słabuszewska-Jóźwiak et al., 2020).
The associations of C-section with these health outcomes are at least in part thought to be microbiome mediated, as evidenced by a recent large longitudinal cohort study with repeated measures and by transfer of these disease phenotypes, including obesity, with microbiota transplantation into germ-free murine models (Cox et al., 2014; Vu et al., 2021). However, mechanistic studies in humans are lacking, and some also argue that maternal and prenatal factors necessitating a C-section delivery may also play a role in the development of these C-section-associated diseases. Nevertheless, it is theorized that C-section-delivered infants who lack exposure to the maternal vaginal and perineal microbiome, and therefore have different pioneering gut microbes and subsequent colonization of the gastrointestinal tract, will have aberrant associated immune and metabolic development and therefore an increased risk of chronic inflammatory C-section-associated diseases compared with those born by vaginal delivery (Figure 1).
Figure 1.
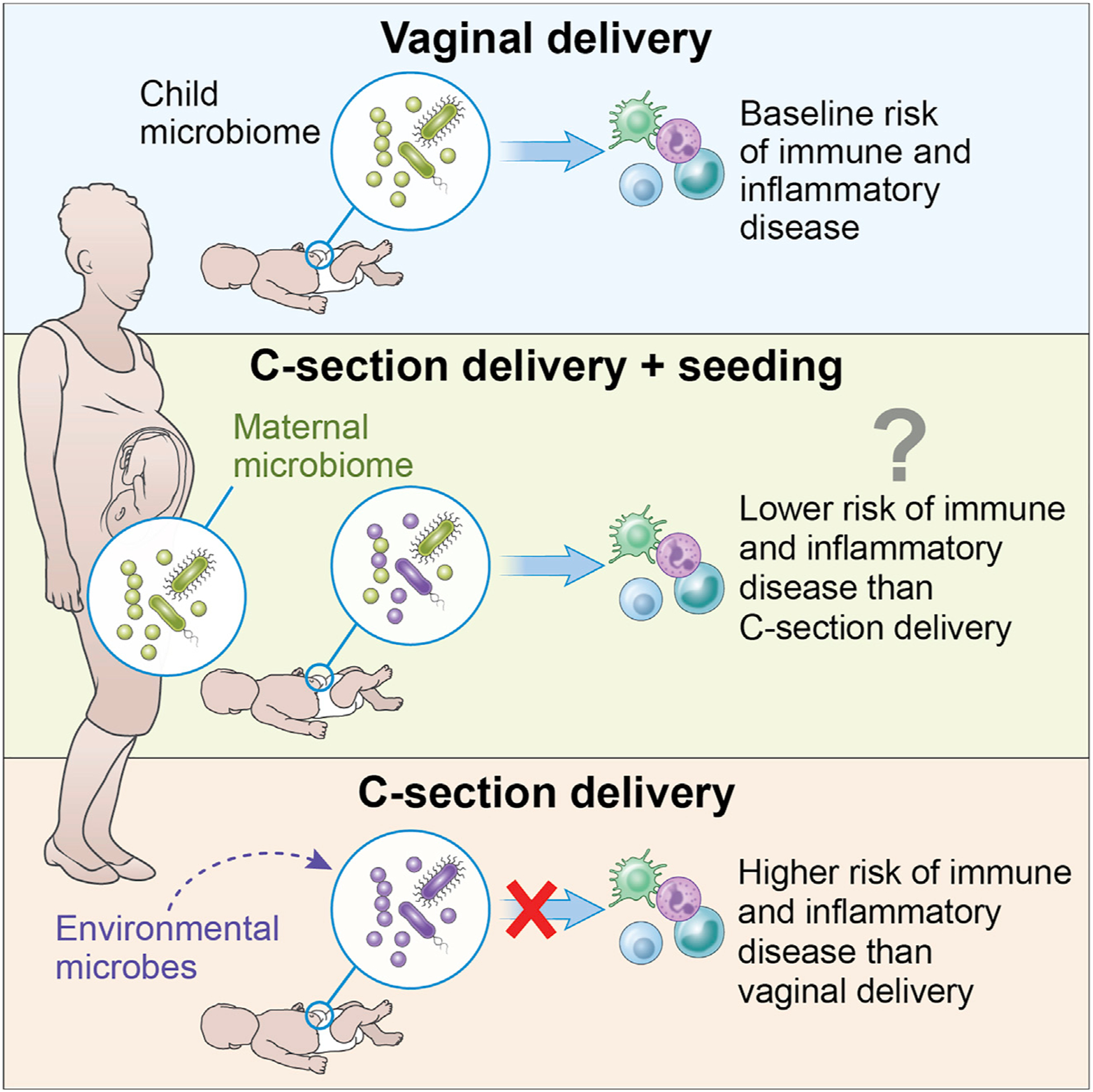
Schematic of rationale for maternal-child microbial seeding interventions
Rationale for maternal-child microbial seeding interventions
Maternal-child microbial seeding is a process by which a C-section-delivered newborn receives exposure to the maternal microbes which they would have been exposed to if not for the C-section. The most recognized form of this process is also known as “vaginal seeding” (exposure to their own mother’s vaginal fluid) (Dominguez-Bello et al., 2016). During this process, a sterile gauze is inserted into the mother’s vagina prior to C-section delivery and then wiped over the newborn’s mouth, face, and rest of the body, in an attempt to replicate exposure in a vaginal birth. This process is compelling, as in theory this simple intervention could “restore” the pioneering colonizing microbes to a C-section-delivered infant and reduce their risk for C-section-associated chronic inflammatory diseases (Figure 1).
Maternal-child microbial seeding intervention studies
In the first study of vaginal seeding, published in 2016, Dominguez-Bello et al. showed that this simple intervention could partially restore the microbiome development over the first month of life (Dominguez-Bello et al., 2016). In this small observational study, the gut, oral, and skin bacterial communities of four C-section-delivered infants who underwent vaginal seeding was enriched in vaginal bacteria, similarly to vaginally delivered babies; vaginal bacteria was underrepresented in infants born by C-section without vaginal seeding (Dominguez-Bello et al., 2016). More recently, a larger, longitudinal observational study was conducted following 177 babies over their first year of life, 79 of whom were born by C-section and of which 30 received vaginal seeding (Song et al., 2021). Microbiota trajectories of C-section-born babies who received vaginal seeding aligned more closely with vaginally born babies than with C-section babies who did not receive vaginal seeding. Of note, in the infant gut, Bacteroides was identified as being consistently enriched with vaginal seeding over the first year of life. This is highly relevant as a higher Enterobacteriaceae/Bacteroidaceae ratio at 3 months after a C-section birth has been identified as a key microbial imbalance on the pathway to being overweight later in childhood (Vu et al., 2021).
Other approaches to maternal-child microbial seeding have also been proposed. A recent trial investigated the effect of giving oral administration of mother’s vaginal fluid to her infant shortly after C-section delivery on the infant gut microbiome (Wilson et al., 2021). Oral administration of maternal vaginal microbes was not found to alter the gut microbiome in 12 C-section-delivered infants at 1 and 3 months compared with 13 C-section-delivered infants who did not receive maternal vaginal microbes (Wilson et al., 2021). In this study, dilution of the vaginal microbiome during preparation may have led to a low vaginal bacterial load, and there was no exposure to other areas of an infant’s body as received during a vaginal delivery, which may have contributed to the lack of difference seen. Another recent proof-of-concept study reported on maternal fecal microbiota transplantation to their infants, given that during a vaginal delivery there is also exposure to the mother’s fecal microbes (Korpela et al., 2020). The authors postulated that because the infant gut is normally colonized by maternal gut microbes, the most natural inoculum to seed the microbiota of the C-section-delivered infant may be maternal fecal matter. Maternal fecal matter mixed with breast milk was given orally to infants within 2 h of birth (Korpela et al., 2020). The temporal development of the gut microbiome composition of the seven seeded infants more closely resembled that of vaginally born infants than C-section infants who were not treated, with most seeded showing enrichment of Bacteroides and Bifidobacterium spp. (Korpela et al., 2020). This suggests that exposure to maternal gut bacteria during a vaginal birth may also be important for initial microbial colonization. Consistent with these results, the intrapartum vaginal microbiota shows presence of bacteria from other maternal body sites, including feces (Song et al., 2021).
Murine models of vaginal seeding with human vaginal microbial communities also suggest that the composition of the transferred seeding material is important in subsequent infant immune and metabolic development, and additionally the response to vaginal seeding may be affected by prenatal maternal health status (Jašarević et al., 2021). In a recent study, mice were delivered by C-section and gavaged with human vaginal microbial communities. This inoculation showed lasting effects on offspring metabolism and immunity in a community-specific manner. However, the microbial effects were modified by prior gestation in a maternal obesogenic or vaginal dysbiotic environment where placental and fetal ileum development were altered, and an amplified immune response increased mortality rates in offspring (Jašarević et al., 2021). This suggests that not only does the composition of the inoculate matter, but also the prenatal environment shapes the post-natal response to inoculation. Translationally this may be of importance, as not all C-section-delivered infants may benefit from maternal-child seeding equally, and maternal prenatal history may be important to collect in clinical trials to provide insight into post-natal responses to seeding.
Taken together, there are data from observational human studies that maternal-child microbial seeding of C-section infants could change the trajectory of early life microbiome development to more closely resemble that of an infant delivered vaginally (Dominguez-Bello et al., 2016; Korpela et al., 2020; Song et al., 2021). Furthermore, murine models suggest seeding may affect later metabolomic and immune outcomes (Jašarević et al., 2021). However, there have been no randomized placebo-controlled trials in humans to date to demonstrate a benefit of seeding interventions on microbiome or health outcomes in offspring. Such randomized trials are currently being conducted with at least two underway in the US, including our own (clinicaltrials.gov identifiers NCT03298334 and NCT03567707) and one active but not yet recruiting trial in China (clinicaltrials.gov identifier NCT03809390), with primary outcomes including obesity and atopy. Given the growing interest of pregnant women in maternal-child microbial seeding, there is a critical need for such randomized controlled trials determine if there are any health benefits to offspring and if the process is safe.
Controversies over maternal-child microbial seeding
Despite the growing interest over maternal-child microbial seeding, there are still many controversies surrounding the process, including safety concerns, regarding the rationale and regulatory issues (Mueller et al., 2019).
Safety of maternal-child microbial seeding interventions
As vaginal seeding grew in popularity, the American College of Obstetrics and Gynecology released a committee opinion in 2017 stating “At this time, vaginal seeding should not be performed outside the context of an institutional review board-approved research protocol until adequate data regarding the safety and benefit of the process become available.” Transmission of infections can occur from mothers to infants during a vaginal birth with adverse consequences for the offspring. Examples of such infections include Group B Streptococcus, HIV, hepatitis B virus, hepatitis C virus, Chlamydia trachomatis, Syphilis (Treponema pallidum), Neisseria gonorrhoeae, herpes simplex virus (HSV), and human papillomavirus. The risk of transmission for some of these infections can be decreased by a C-section delivery. Therefore, vaginal seeding in an infant scheduled for C-section may possibly increase the risk of transmission of infection that they may not otherwise have been exposed to during C-section delivery without vaginal seeding.
In reported studies of vaginal seeding to date, there has been no reported transmission of infection. Screening for certain infections were performed in the reported observational studies including “negative results for standard-of-care tests for Group B Streptococcus” and “STDs (including HIV and Chlamydia) [and] no signs of vaginosis or viral infections as determined by their obstetrician” (Song et al., 2021). More recent Food and Drug Administration (FDA)-regulated randomized controlled trials in vaginal seeding report more rigorous screening for infection above standard of care in pregnancy—for example, additional screening for sexually transmitted infections after 35 weeks gestation (clinicaltrials.gov identifiers NCT03298334 and NCT03567707). A potential transmission of HSV has been reported with vaginal seeding outside of an institutional review board-approved research protocol (Huynh et al., 2018). The report describes an infant delivered by C-section and received vaginal seeding, who developed HSV vesicular lesions on both eyelids at 10 days of age. The timing of the lesions and the symmetry on both eyelids, which were swabbed during vaginal seeding, indicate this may have been potentially transmitted via the vaginal seeding procedure. However, no testing of the swab materials were performed, and the mother reported only a history of occasional oral “cold sores” but no history of genital herpes (Huynh et al., 2018). This however emphasizes that for now vaginal seeding should only performed in an approved research trial where mothers are carefully screened for infections and safety data closely followed.
More recently with the COVID-19 pandemic, there has been question of whether SARS-CoV-2 may be transferred from mother to infant via vaginal seeding. However, detection of SARS-CoV-2 in the vaginal secretions of patients diagnosed with COVID-19 is rare, and at present it is unknown whether vaginal colonization can cause infection. Moreover, C-section deliveries are not currently clinically indicated to avoid transmission of COVID-19 from positive mothers.
Challenges to the rationale for maternal-child microbial seeding interventions
Early life antibiotic exposure has been associated with inflammatory diseases in offspring that are also positively associated with C-section delivery, including obesity and atopy (Cox et al., 2014). This is hypothesized to be through a similar microbiome-mediated mechanism, i.e., disruption of early life microbial colonization (Cox et al., 2014). It is standard practice to give maternal antibiotics at the time of C-section. Therefore, in epidemiological studies associating C-section with increased risk of inflammatory diseases, antibiotic exposure is an important confounder. However, importantly, an experimental study in mice indicated that C-section delivery caused excessive weight gain in the absence of antibiotics, supporting the involvement of maternal vaginal bacteria in metabolic programming (Martinez et al., 2017). It is likely that the effect on outcomes in humans is compounded by both C-section and antibiotic exposure together. Our own randomized controlled trial in vaginal seeding (clinicaltrials.gov identifier NCT03298334) attempts to decrease the effects of peripartum antibiotics by removing the vaginal seeding gauze from the mother’s vagina prior to receiving peripartum antibiotics, and therefore the microbes transferred to the infant on the gauze have not been exposed to antibiotics; this differs from other observational seeding trials (Dominguez-Bello et al., 2016; Song et al., 2021).
Some investigators also question the dogma of the “sterile womb” in healthy pregnancies and propose the first exposure of infants to the maternal microbiota occurs before birth, making the concept of vaginal seeding (i.e., giving the pioneering microbial exposure in C-section-delivered infants) possibly less important. While some early studies identified bacterial DNA in some placentas, these results have not been consistent throughout studies, and in a more recent study with ample technical controls, no resident microbiota were identified in placentas of full-term pregnancies without labor (Theis et al., 2019). The ability to raise germ-free animals, who are delivered by C-section from mothers with a normal microbiome, also argues against microbiota colonizing the human placenta and subsequently the fetus. Regardless, if there are prenatal exposures of the infant to maternal microbiota, the microbial abundance is incredibly low, and it is well accepted that the first major exposure to a significant microbial load is at birth.
Lastly, postnatal environmental and nutritional factors associated with C-section, such as delayed early breast feeding initiation, may also impact the developing microbiome and may change the risk for subsequent C-section-associated diseases (Yan et al., 2014). While associations of C-section with microbiome development and health outcomes persist after controlling for confounding factors, including breastfeeding, the possibility of residual or unmeasured confounding factors cannot be completely ruled out in observational study designs. Therefore, to truly understand the impact of maternal-child microbial seeding on health outcomes, rigorous randomized controlled trials, where other prognostic factors are balanced between arms, are crucial to demonstrate causal relationships.
Regulatory and ethical issues surrounding maternal-child microbial seeding interventions
Currently, both randomized controlled trials of vaginal seeding in the US (clinicaltrials.gov identifiers NCT03298334 and NCT03567707) are being conducted under an Investigational New Drug (IND) application from the US FDA. Similar to fecal matter for fecal microbiota transplantation (FMT) studies, the FDA considers vaginal secretions containing microbiota a “biologic.” The FDA advises an IND application when a study “utilizes administration of live organisms to affect structure/function of the body” and falls within IND requirements as per Section VI.B of the FDA’s 2013 “Guidance for Clinical Investigators, Sponsors and IRBs — Investigational New Drug Applications (INDs) — Determining Whether Human Research Studies Can Be Conducted Without an IND” (Mueller et al., 2019).
It could be argued that this strict regulation should not be applied to vaginal seeding studies (Mueller et al., 2019). Maternal vaginal secretions are a heterogeneous substance and vary from mother to mother, so they are inherently difficult to characterize unlike many other drugs under the FDA purview, with the exception of fecal material for FMT. However, vaginal seeding differs significantly from FMT, so some might argue that the same regulations are not needed. With FMT, feces and its microbiota and microbial products are transferred from a donor, who is often unknown to the recipient, to a different individual. Outside the context of FMT, the subject would not have otherwise been exposed to another donor’s fecal matter. Vaginal seeding, however, differs from FMT in that if a mother delivered her infant vaginally, her own neonate would have naturally been exposed to her vaginal fluids and vaginal microbiota. C-section delivery constitutes a medical intervention that interrupts that process, and there is an argument that vaginal seeding is an attempt to replicate an exposure that occurs during the “intended” birthing process of a vaginal delivery (Mueller et al., 2019). Nevertheless, the additional layer of oversight over vaginal seeding trials allows for the rigorous exploration of the safety and efficacy of the process, which is greatly needed.
Conclusions and future directions
Although observational studies suggest that maternal-child microbial seeding interventions of C-section-delivered infants partially restores microbiome development closer to that of vaginally delivered infants, no randomized controlled trials have shown that vaginal seeding changes the infant microbiome, let alone improves health outcomes. Large-scale, well-designed, rigorous randomized controlled trials of vaginal seeding are needed to see if maternal-child microbial seeding improves health outcomes by reducing the incidence of C-section-associated inflammatory diseases in children and, critically, to demonstrate that it is safe. These trials are currently underway. If maternal-child microbial seeding improves health outcomes, it may be a simple, cost-effective public health strategy that could reduce the prevalence of C-section-associated diseases. In addition to measuring the overall effect of vaginal seeding, with larger sample sizes we will also learn whether there is heterogeneity in the health effects of maternal microbiota and whether there is an optimal composition. As some infants born by C-section may not be able to receive maternal-child microbial seeding for reasons such as maternal infection or comorbidities, knowing the most effective combination of microbes and their products may eventually allow for the development of targeted probiotics or a “live biotherapeutic” that could be used for these infants and are more standardized. However, before such endeavors we eagerly await the results of randomized controlled trials to assess the safety and efficacy of maternal-child microbial seeding.
ACKNOWLEDGMENTS
The authors would like to thank Mr. Alan Hoofring from the National Institutes of Health Medical Arts department for his contribution to the schematic figure. This work was supported in part by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under the Intramural Research Program (S.K.H.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
DECLARATION OF INTERESTS
S.K.H. declares no competing interests. M.G.D.B. has intellectual property on NYU patent US Patent 10357521. N.T.M. is on the scientific advisory board of Tiny Health Inc.
REFERENCES
- Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, Kim SG, Li H, Gao Z, Mahana D, et al. (2014). Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 158, 705–721. 10.1016/j.cell.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, and Knight R (2010). Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 107, 11971–11975. 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Bello MG, De Jesus-Laboy KM, Shen N, Cox LM, Amir A, Gonzalez A, Bokulich NA, Song SJ, Hoashi M, Rivera-Vinas JI, et al. (2016). Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat. Med 22, 250–253. 10.1038/nm.4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensollen T, Iyer SS, Kasper DL, and Blumberg RS (2016). How colonization by microbiota in early life shapes the immune system. Science 352, 539–544. 10.1126/science.aad9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh J, Palasanthiran P, and McMullan B (2018). Potential Transmission of Herpes Simplex Virus Via Vaginal Seeding. Pediatr. Infect. Dis. J 37, e278. 10.1097/inf.0000000000001965. [DOI] [PubMed] [Google Scholar]
- Jašarević E, Hill EM, Kane PJ, Rutt L, Gyles T, Folts L, Rock KD, Howard CD, Morrison KE, Ravel J, and Bale TL (2021). The composition of human vaginal microbiota transferred at birth affects offspring health in a mouse model. Nat. Commun 12, 6289. 10.1038/s41467-021-26634-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpela K, Helve O, Kolho KL, Saisto T, Skogberg K, Dikareva E, Stefanovic V, Salonen A, Andersson S, and de Vos WM (2020). Maternal Fecal Microbiota Transplantation in Cesarean-Born Infants Rapidly Restores Normal Gut Microbial Development: A Proof-of-Concept Study. Cell 183, 324–334.e5. 10.1016/j.cell.2020.08.047. [DOI] [PubMed] [Google Scholar]
- Martinez KA 2nd, Devlin JC, Lacher CR, Yin Y, Cai Y, Wang J, and Dominguez-Bello MG (2017). Increased weight gain by C-section: Functional significance of the primordial microbiome. Sci. Adv 3, eaao1874. 10.1126/sciadv.aao1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller NT, Hourigan SK, Hoffmann DE, Levy L, von Rosenvinge EC, Chou B, and Dominguez-Bello MG (2019). Bacterial Baptism: Scientific, Medical, and Regulatory Issues Raised by Vaginal Seeding of C-Section-Born Babies. J. Law Med. Ethics 47, 568–578. 10.1177/1073110519897732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Słabuszewska-Jóžwiak A, Szymański JK, Ciebiera M, Sarecka-Hujar B, and Jakiel G (2020). Pediatrics Consequences of Caesarean Section-A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 17, E8031. 10.3390/ijerph17218031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SJ, Wang JC, Martino C, Jiang LJ, Thompson WK, Shenhav L, McDonald D, Marotz C, Harris PR, Hernandez CD, et al. (2021). Naturalization of the microbiota developmental trajectory of Cesarean-born neonates after vaginal seeding. Med 2, 951–964.e5. 10.1016/j.medj.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis KR, Romero R, Winters AD, Greenberg JM, Gomez-Lopez N, Alhousseini A, Bieda J, Maymon E, Pacora P, Fettweis JM, et al. (2019). Does the human placenta delivered at term have a microbiota? Results of cultivation, quantitative real-time PCR, 16S rRNA gene sequencing, and metagenomics. Am. J. Obstet. Gynecol 220, 267.e1–267.e39. 10.1016/j.ajog.2018.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu K, Lou W, Tun HM, Konya TB, Morales-Lizcano N, Chari RS, Field CJ, Guttman DS, Mandal R, Wishart DS, et al. (2021). From Birth to Overweight and Atopic Disease: Multiple and Common Pathways of the Infant Gut Microbiome. Gastroenterology 160, 128–144.e10. 10.1053/j.gastro.2020.08.053. [DOI] [PubMed] [Google Scholar]
- Wilson BC, Butler ÉM, Grigg CP, Derraik JGB, Chiavaroli V, Walker N, Thampi S, Creagh C, Reynolds AJ, Vatanen T, et al. (2021). Oral administration of maternal vaginal microbes at birth to restore gut microbiome development in infants born by caesarean section: A pilot randomised placebo-controlled trial. EBioMedicine 69, 103443. 10.1016/j.ebiom.2021.103443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Liu L, Zhu Y, Huang G, and Wang PP (2014). The association between breastfeeding and childhood obesity: a meta-analysis. BMC Public Health 14, 1267. 10.1186/1471-2458-14-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]


