Abstract
Tyrosine phenol-lyase (Tpl), which can synthesize 3,4-dihydroxyphenylalanine from pyruvate, ammonia, and catechol, is a tyrosine-inducible enzyme. Previous studies demonstrated that the tpl promoter of Erwinia herbicola is activated by the TyrR protein of Escherichia coli. In an attempt to create a high-Tpl-expressing strain, we cloned the tyrR gene of E. herbicola and then randomly mutagenized it. Mutant TyrR proteins with enhanced ability to activate tpl were screened for by use of the lac reporter system in E. coli. The most increased transcription of tpl was observed for the strain with the mutant tyrR allele involving amino acid substitutions of alanine, cysteine, and glycine for valine-67, tyrosine-72, and glutamate-201, respectively. A tyrR-deficient derivative of E. herbicola was constructed and transformed with a plasmid carrying the mutant tyrR allele (V67A Y72C E201G substitutions). The resultant strain expressed Tpl without the addition of tyrosine to the medium and produced as much of it as was produced by the wild-type strain grown under tyrosine-induced conditions. The regulatory properties of the mutant TyrRV67A, TyrRY72C, TyrRE201G, and TyrRV67A Y72C E201G proteins were examined in vivo. Interestingly, as opposed to the wild-type TyrR protein, the mutant TyrRV67A protein had a repressive effect on the tyrP promoter in the presence of phenylalanine as the coeffector.
Tyrosine phenol-lyase (Tpl) (EC 4.1.99.2) normally catalyzes the degradation of tyrosine into pyruvate, ammonia, and phenol (26–28, 56). However, this reaction is reversible, and if catechol is substituted for phenol, l-dihydroxyphenylalanine (l-DOPA) is produced (24, 57). l-DOPA is used in the treatment of Parkinson's disease, which afflicts 1 out of every 1,700 individuals. About 250 tons of l-DOPA is now supplied per year, and more than half of it is produced by an enzymatic method involving Tpl (24, 57).
On an industrial scale, Erwinia herbicola cells with extremely high Tpl activity are prepared by cultivation in a medium containing l-tyrosine as an inducer of Tpl. The intact cells are then harvested by centrifugation and transferred to the reactor, as the catalyst, together with the substrate. This microbiological method is efficient; however, it actually has one serious drawback. Since Tpl is only synthesized under l-tyrosine-induced conditions (16, 49), the cells must be grown in medium supplemented with l-tyrosine. The extremely low solubility of l-tyrosine results in considerable carryover of it into the reactor, which severely complicates the separation of the final product, l-DOPA (hydroxyl derivative of l-tyrosine), from the remaining l-tyrosine. To avoid this drawback, the tpl genes of E. herbicola (17, 20, 50) and Citrobacter freundii (21) were cloned and expressed in Escherichia coli under the control of the tac promoter, respectively. In either case, Tpl was highly induced upon the addition of isopropyl-β-d-thiogalactopyranoside (IPTG); however, the l-DOPA productivity of the cells was inferior to that of E. herbicola cells. Some factors other than the level of Tpl expression should be considered in order to explain this observation, for example, the transmittance of substrates and l-DOPA through the cell membrane (Tpl is located in the cytoplasmic space) (50) and the tolerance of cells to catechol. It is noteworthy that E. herbicola possesses one copy of the tpl gene on its chromosome; nevertheless, it is the best source for l-DOPA production.
The regulatory mechanism underlying expression of tpl was investigated by means of the lac reporter system, and it was demonstrated that, at least in E. coli, both the TyrR protein and cyclic AMP receptor protein (CRP) participate in it (23, 47). The TyrR protein plays a major role in the regulation of genes that are essential for the biosynthesis, transport, and degradation of aromatic amino acids (1, 5, 8, 23, 34, 42, 47). TyrR contains a helix-turn-helix DNA-binding motif near its carboxyl end (60) and binds to DNA with a palindromic consensus sequence (TGTAAAN6TTTACA) (19, 42). The central domain of the TyrR protein exhibits significant similarity to those of other regulators such as NtrC (40) and NifA (4), although TyrR completely differs from them in the respect that it regulates transcription from ς70-dependent promoters, not ς54-dependent promoters (11, 30, 33, 42). The N-terminal domain is considered to be involved in the interaction with the α subunit of RNA polymerase as a class I transcriptional activator (33). Using tyrosine, phenylalanine, and tryptophan as coeffectors (2, 42, 54), TyrR regulates transcription from target promoters positively and/or negatively in various manners, which depends on the locations of its binding sites (designated as TyrR boxes) (42). In vitro studies have shown that the TyrR protein ordinarily exists as a dimer in solution (12, 54, 55); however, in the presence of ATP and tyrosine (or a high concentration of phenylalanine), it undergoes a reversible conformational change to a hexameric form (54, 55).
The regulatory region of the tpl gene contains three TyrR boxes that are separated from each other by 11 helical turns and two CRP-binding sites that are juxtaposed between the two upstream TyrR boxes (3, 23). Evidence has been obtained that the tyrosine induction of tpl is caused by tyrosine-mediated hexamerization of the TyrR protein bound to three distant boxes (3, 23). DNA bending of the intervening region triggered by the binding of CRP (13, 36, 43) facilitates the self-association of three TyrR dimers (3, 23).
To create a more efficient and available strain for l-DOPA production, we cloned the tyrR gene from an E. herbicola genomic library and randomly mutagenized it. Mutant forms of the TyrR protein resulting in high expression of tpl were screened for with the lac reporter system. The mutant tyrR allele obtained was then introduced into an E. herbicola tyrR-deficient strain, and the ability of its product to activate Tpl expression was evaluated.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains used in this study were derivatives of E. herbicola or E. coli K-12. The strains and plasmids are listed in Table 1 with their characteristics. All lac fusions were created by use of pRS552 to produce translational fusions (46). Construction of the Φ(tpl′-′lac) gene was described elsewhere (23). The DNA fragment containing the rrnB terminator (rrnBT1) and Φ(tpl′-′lac) gene in that order was cut off by HindIII and SalI digestion and then integrated into the E. coli chromosome as described previously (14, 23), or the fragment was blunt-ended and then subcloned into a mini-F plasmid, pMBO131 (41), at the SalI (end-filled) site. The Φ(aroF′-′lac) and Φ(tyrP′-′lac) genes were constructed as follows. DNA fragments containing the respective regions required for TyrR-mediated regulation and parts of the N-terminal region (1, 8, 19, 42) were amplified by PCR using the DNA polymerase from Pyrococcus kodakaraensis (KOD polymerase; Toyobo, Osaka, Japan) with the genomic DNA of E. coli strain MG1655 as a template and a pair of primers (primers 63 and 64 for tyrP, and primers 65 and 66 for aroF [Table 1]). The primers were designed to produce an EcoRI site at the upstream end and a BamHI site at the downstream end in order to facilitate the connection with pRS552 (46). After being confirmed by sequencing (45), these fragments were subcloned into pRS552. The SalI-HindIII 8-kb fragment was cut off as described above and then inserted into a low-copy-number plasmid, pMW118 (Nippon Gene, Tokyo, Japan). The construction of the other plasmids is described when they are first mentioned in the text.
TABLE 1.
Strains, plasmids, and oligonucleotides used in this work
| Strain, plasmid, or oligonucleotide | Characteristic(s) | Source or reference |
|---|---|---|
| Strains | ||
| E. herbicola | ||
| AJ2985 | Wild type | Laboratory stock |
| YG17 | ΔtyrR::kan | This study |
| E. coli K-12 | ||
| CJ236 | pCJ105 [F′ cat+]/dut-1 ung-1 thi-1 relA1 | 22 |
| CSH26 | F−ara Δ(lac-pro) thi | 39 |
| JM107 | F′[traD36 proA+B+ lacIq Δ(lacZ)M15]/endA1 gyrA96 thi hsdR17 supE44 relA1 Δ(lac-proAB) | 61 |
| JP2144 | λ−tsx-84 trpA9605 tyrR366 his-85 ilv-632 | 5 |
| MG1655 | λ−rph | Laboratory stock |
| MV1184 | F′[traD36 proA+B+ lacIq Δ(lacZ)M15]/ara Δ(lac-proAB) rpsL thi φ80Δ(lac)M15 Δ(srl-recA)306::Tn10 | 52 |
| TE2680 | F− λ− IN(rrnD-rrnE)1 Δ(lac)X74 rpsL galK2 recD1903::Tn10d-tet+ trpDC700::putPA1303::[kan cat+ ′lac] | T. Elliott (14) |
| TK314 | TE2680 trpDC700::putPA1303::[kan+ Φ(tpl′-′lac)] | 23 |
| TK453 | JM107 Δ(srl-recA)306::Tn10 tyrR366 trpDC700::putPA1303::[kan+ Φ(tpl′-′lac)] | This study |
| TK481 | TK453 trpDC700::putPA1303::[kan+ Φ(tpl′-′lac) (containing only tplp)] | This study |
| TK596 | CSH26 ΔtyrR::kan+ Δ(srl-recA)306::Tn10 | This study |
| TK747 | CSH26 trpDC700::putPA1303::[kan+ Φ(tpl′-′lac)] ΔtyrR::cat+ Δ(srl-recA)306::Tn10 | This study |
| TK809 | CSH26 ΔtyrR::cat+ Δ(srl-recA)306::Tn10 | This study |
| Plasmids | ||
| pACYC177 | p15A replicon bla+ kan+ | 7 |
| pBR322 | ColE1 replicon bla+ tet+ | 48 |
| pMBO131 | Mini-F replicon cat+ | 41 |
| pMU400 | ColE1 replicon bla+ tyrR+E. coli | J. Pittard (10) |
| pMW118 | pSC101 replicon lacZα+ bla+ | Nippon Gene |
| pMW219 | pSC101 replicon lacZα+ kan+ | Nippon Gene |
| pRS552 | ColE1 replicon bla+ kan+ rrnBT1 ′lac | R. W. Simons (46) |
| pTK#-13 | ColE1 replicon bla+ tet::tyrR+E. herbicola | This study |
| pTK#-20 | ColE1 replicon bla+ tet::tyrR+E. herbicola | This study |
| pTK479 | p15A replicon bla+; the 0.2-kb SmaI-NruI fragment was removed from pACYC177 | This study |
| pTK588 | pSC101 replicon kan+ rrnBT1 Φ(aroF′-′lac) | This study |
| pTK589 | pSC101 replicon kan+ rrnBT1 Φ(tyrP′-′lac) | This study |
| pTK631 | pSC101 replicon bla::tet+; the tet gene of pBR322 was inserted into the ScaI site in the bla gene of pMW118 and the 0.3-kb PvuII fragment was removed to delete the lacZα gene | This study |
| pTK723 | p15A replicon bla+ tyrR+E. coli; the 2.4-kb NdeI(end-filled)-HindIII fragment of pMU400 was ligated with the 3.5-kb HindIII-NruI fragment of pACYC177 | This study |
| pTK766 | ColE1 replicon bla+ tet::ΔtyrRE. herbicola::kan+ | This study |
| pTK774 | p15A replicon bla+ Δkan::tyrRp | This study |
| pTK775 | p15A replicon bla+ tyrR+E. herbicola; the 2.4-kb SalI-SspI fragment of pTK#-20 was ligated with the 3.7-kb XhoI-SmaI fragment of pACYC177 | This study |
| pTK871 | Mini-F replicon cat+ rrnBT1 Φ(tpl′-′lac) | This study |
| pTK919 | pSC101 replicon bla::tet+ tyrR+E. herbicola | This study |
| pTK922 | pSC101 replicon bla::tet+ tyrR5E. herbicola (V67A Y72C E201G substitutions) | This study |
| pTZ19R | ColE1 replicon lacZα+ bla+ f1ori | Pharmacia |
| pUC4K | ColE1 replicon bla+ lacZα::kan+ | Pharmacia |
| Oligonucleotides | ||
| 63 | 5′-CCGAATTCCAGACTGGCATGCGTATATTGC-3′, for cloning the tyrP regulatory region (upstream end) | |
| 64 | 5′-CCGGATCCTTCACGCTTTCTTCTGTCCTGACGA-3′, for cloning the tyrP regulatory region (downstream end) | |
| 65 | 5′-CCGAATTCGCTAAATGCATCGTCATCTTTTATG-3′, for cloning the aroF regulatory region (upstream end) | |
| 66 | 5′-CCGGATCCTTTTGCATGATGGCGATCCTGTTTA-3′, for cloning the aroF regulatory region (downstream end) | |
| 75 | 5′-GATTAAGGCCCACCATATGCGTTTAGAAG-3′, for random mutagenesis of the tyrRE. herbicola gene | |
| 76 | 5′-TGAGCATGACAAAAAGCTTTACAGCCAG-3′, for random mutagenesis of the tyrRE. herbicola gene | |
| 91 | 5′-TCAGACGGCATACAGGGGACCGTGC-3′, for site-directed mutagenesis of the tyrRE. herbicola gene (Y72C substitution) |
Media and chemicals.
Bacto MacConkey agar base was purchased from Difco Laboratories (Detroit, USA) and d-lactose was added at a final concentration of 1% as a fermentable carbon source. For the cultivation of E. herbicola, basal medium consisting of 0.5% peptone, 0.5% yeast extract, 0.5% meat extract, and 0.2% KH2PO4 (pH 8.0) was used. l-Tyrosine was added as an inducer of tpl at a final concentration of 0.1%. M63-glucose (39) was used as the minimal medium (MM) for E. coli, and l-proline and thiamine-HCl were added as growth requirements at final concentrations of 30 and 1 μg/ml, respectively. Ampicillin, kanamycin, chloramphenicol, and tetracycline were used at final concentrations of 50, 30, 15, and 15 μg/ml, respectively. The chemicals were all obtained commercially and not purified further.
Genetic techniques.
Standard recombinant DNA procedures were used essentially as described by Sambrook et al. (44). The method for generalized transduction involving the P1 phage was that described by Miller (39). The tyrR transductant was selected based on resistance to 0.2 mM l-3-fluorotyrosine (5). To prevent gene conversion, strains were made recA with Tn10 as a marker (52). The transductants were examined for sensitivity to nitrofurantoin (1.5 μg/ml) (37).
Determination of DNA sequences.
DNA sequences were determined by the method of Sanger et al. (45) using a Thermo sequenase fluorescent labeled primer cycle sequencing kit with 7-deaza-dGTP (Amersham) and a DSQ-2000L sequencer (Shimadzu, Kyoto, Japan).
Construction of an E. herbicola genomic library.
Genomic DNA was extracted from E. herbicola AJ2985 (53), partially digested with Sau3AI, and then fractionated by low-melting-point agarose gel electrophoresis to obtain 4- to 8-kb fragments. A recovered DNA fragment was inserted into a compatible BamHI site of pBR322 (48). A ligation mixture was used to transform TK453 (a derivative of E. coli strain [see text and Table 1]), with about 20,000 transformants being obtained.
Random mutagenesis of the tyrR gene using error-prone PCR.
Localized random mutagenesis was carried out by the error-prone PCR amplification method (35) using pTK#-20 containing the tyrR gene of E. herbicola as a template, and synthetic oligonucleotides 75 and 76 (Table 1) as a pair of primers. Primer 75 was designed to introduce an NdeI site in the initiation codon and primer 76 was designed to introduce a HindIII site downstream of the putative transcription terminator of the tyrR gene. The amplified 1.6-kb DNA fragment was treated with NdeI and HindIII and then ligated with pTK774 (Table 1) that had been predigested similarly. pTK774 carries the wild-type promoter and 5′ untranslated region of the tyrR gene except that GCAATG of the translation initiation site was changed to CATATG (NdeI) so that the amplified fragment is placed downstream of the tyrR wild-type promoter.
Site-directed mutagenesis.
Site-directed mutagenesis was carried out by the method of Kunkel et al. (29). To replace the tyrosine residue at amino acid position 72 with cysteine, the oligonucleotide 91 (Table 1) and single-stranded pTK852, which was generated by inserting the 0.4-kb EcoRI-PstI fragment of pTK#-20 into pTZ19R, were used. The entire fragment used for later manipulation was sequenced to ensure that no base change other than those planned had occurred.
β-Galactosidase assay.
To study TyrR-mediated regulation, M63-glucose (39) was used as the minimal medium. Either tyrosine or phenylalanine was added as an effector of the TyrR protein (2, 42, 54) at a final concentration of 1 mM. Cultures were grown at 37°C to the mid-exponential phase and then subjected to β-galactosidase assaying according to the method of Miller (39). Assays were performed in duplicate for three separate cultures, and the values obtained showed less than 10% error.
Preparation of anti-Tpl antibodies and immunoblotting.
Tpl was purified from an E. herbicola cell extract as described previously (27, 50). One milligram of the protein emulsified in Freund's complete adjuvant was used to immunize a female New Zealand White rabbit. Booster immunizations with 1 mg of the protein in Freund's incomplete adjuvant were administered twice with an interval of 2 weeks. A small amount of blood was taken to test for anti-Tpl activity after each booster immunization. Whole blood was collected and kept at 37°C for 1 h, and then the clot was removed by centrifugation to obtain crude antiserum. The immunoglobulin G fraction was purified from the crude antiserum by protein-A Sepharose CL-6B column chromatography as recommended by the supplier (Amersham).
Immunoblotting was performed as described previously (18) with slight modifications. In brief, an overnight liquid culture was diluted with the same medium to give an optical density of 1.0 at 600 nm. Cells were collected from 1 ml of dilution by centrifugation and suspended in 100 μl of cracking buffer (60 mM Tris-HCl [pH 6.8], 1% 2-mercaptoethanol, 1% sodium dodecyl sulfate [SDS], 10% glycerol, 0.01% bromophenol blue), and then boiled for 5 min. The whole-cell extract was separated on an SDS–12.5% polyacrylamide gel (32) and then electroblotted onto a polyvinylidene difluoride membrane (Millipore). Anti-Tpl antibodies and anti-rabbit immunoglobulin, horseradish peroxidase-linked whole antibodies (from donkey) (Amersham), were used in 4,000-fold dilution and 3,000-fold dilution, respectively. Specific cross-reactions were visualized with ECL chemiluminescent detection agent (Amersham). The image on X-ray film was analyzed with a Fuji Film ImageGauge program, and the values were estimated within the linear range.
Construction of the E. herbicola ΔtyrR::kan strain.
The chromosomal region in E. herbicola corresponding to the tyrR gene was deleted and replaced with the kanamycin resistance gene (kan) through a homologous recombination event. A plasmid carrying the ΔtyrRE. herbicola::kan gene was constructed as follows. pTK#-13, which contains long flanking regions on both the upstream and downstream sides of the E. herbicola tyrR gene, was digested with EcoRI, resulting in the production of 5.5-, 6.5-, 1.6-, and 0.3-kb DNA fragments. The kan gene was isolated from pUC4K (Amersham) by EcoRI digestion and then ligated, in the proper orientation, with the 5.5- and 6.5-kb fragments of pTK#-13 obtained above. As a result, a plasmid in which almost all the E. herbicola tyrR gene (1.6 kb) and the proximal downstream 0.3-kb fragment were replaced with the kan gene was constructed (pTK766). An 8.0-kb FspI fragment containing 4.0- and 2.2-kb DNA regions corresponding to either side of the chromosomal tyrR locus was recovered from pTK766, and then introduced into E. herbicola by electroporation. Transformants in which the correct replacement event had occurred were screened for by genomic Southern hybridization analysis with the kan and tyrR genes as specific probes.
Nucleotide sequence accession number.
The GenBank accession number of the E. herbicola tyrR gene is AF035010.
RESULTS AND DISCUSSION
Cloning of the tyrR gene from E. herbicola.
In E. herbicola, expression of Tpl is induced by tyrosine and is subject to cyclic AMP-dependent catabolite repression (49). In a previous study, the lac reporter system of E. coli was employed to elucidate the regulatory mechanism of tpl (23). Although the tpl gene is not normally found in E. coli (15, 25), both induction and repression of this gene were observed in the same manner as observed in E. herbicola (23). Consequently, the TyrR protein and CRP of E. coli were identified as regulators of tpl that are responsible for tyrosine induction and carbon catabolite repression, respectively (23). Somerville and his colleagues have also shown that the expression of C. freundii tpl is regulated by the TyrR protein, integration host factor, and CRP in E. coli (3, 47). They carried out precise in vitro experiments; however, their studies were demonstrated with the noncognate (E. coli) TyrR protein. In this study, therefore, as part of an effort to elucidate the regulatory mechanism of tpl and to find a suitable means of constructing a Tpl high expression strain, we attempted to clone the tyrR gene of E. herbicola.
A derivative of E. coli strain JM107 (61), TK453, carrying the Φ(tpl′-′lac) gene, tyrR mutation, and recA mutation was constructed by P1 transduction using TK314 (23), JP2144 (5), and MV1184 (52) as donors, respectively. An E. herbicola genomic library was constructed using TK453 as a host, spread on MacConkey agar-lactose plates containing 0.1% tyrosine as an inducer, and then screened for red color formation. At this time, three possible reasons were considered for this phenotype change; (i) the gene for a positive regulator of tpl was cloned, the product of which triggered expression of the fusion; (ii) the gene for β-galactosidase was cloned, the activity of which was expressed; and (iii) an unknown factor(s) was involved, such as one causing a pH decrease. In order to exclude the second and third possibilities, a plasmid extracted from a red color-forming colony was subsequently introduced into another E. coli strain (TK481). In strain TK481, the upstream regulatory region of the fusion was deleted, leaving only the tpl promoter. Therefore, when a gene encoding an activator of tpl was introduced into TK481, expression of the fusion would remain basal (forming a white colony) since the activator did not have its target region. On the other hand, in the second and third cases, transformants would show red color again.
In this way, we obtained 20 positive clones. Every plasmid produced the same DNA fragment (1.6 kb) on EcoRI digestion and conferred the TyrR+ phenotype (5) on the host strain. One of these plasmids, pTK#-20, with the shortest insert (6 kb), was studied further. By means of the TyrR phenotypic check (5), it was confirmed that a 3.5-kb SalI fragment certainly contained the gene of interest. The nucleotide sequence was determined and deposited in GenBank (accession number AF035010). The sequence analysis proved that the cloned gene was tyrR.
Multiple amino acid sequence alignment of TyrR proteins.
Analysis of the E. herbicola tyrR gene revealed two potential translation initiation codons separated by 46 frames. Since removal of the upstream ATG codon did not affect the ability of the protein to activate tpl (data not shown) and the amino acid sequence deduced from the downstream ATG codon showed good agreement with those of other TyrR proteins (Fig. 1), we concluded that the downstream ATG is the actual translation initiation codon.
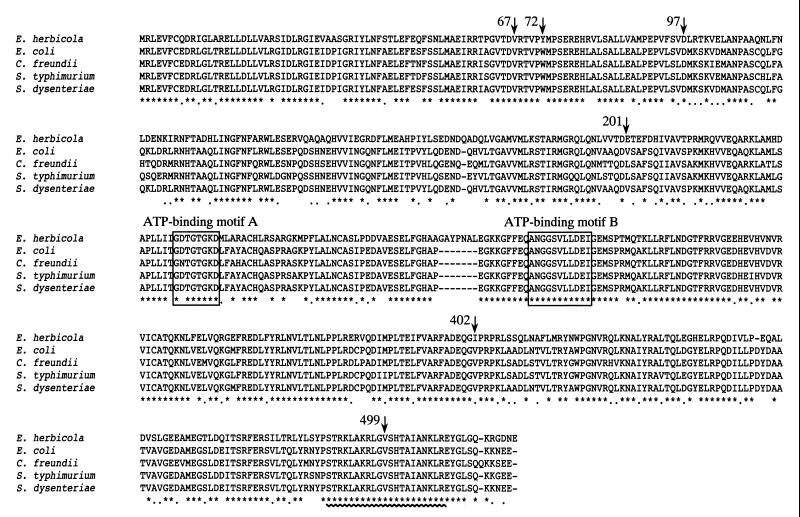
FIG. 1.
Multiple amino acid sequence alignment of TyrR proteins. The deduced amino acid sequence of the E. herbicola TyrR protein was aligned with those of the TyrR proteins from E. coli (GenBank accession number M12114) (9), S. enterica serovar Typhimurium (S. typhimurium) (GenBank accession number U90141) and C. freundii (GenBank accession number U90140) (Bai and Somerville, unpublished data), and S. dysenteriae (GenBank accession number AF153317) (38) by use of the Clustal W 1.6 program (51). The asterisks indicate residues that are conserved in all five TyrR proteins, and the dots indicate positions at which only conservative changes have occurred. Only the E. herbicola TyrR protein possessed seven extra amino acid residues between the two ATP-binding motifs (A and B), which are enclosed by boxes. The sequences in the HTH motifs are identical in all five proteins and are underlined with a wavy line. Mutations mapped in this experiment are indicated by arrows labeled with their amino acid positions.
The primary structure of the E. herbicola TyrR protein was aligned with and compared to those of other bacterial TyrR proteins by use of the Clustal W 1.6 program (51) (Fig. 1). The E. coli tyrR gene has been sequenced by Cornish et al. (9), and Bai and Somerville have cloned and sequenced the tyrR genes of Salmonella enterica serovar Typhimurium and C. freundii (GenBank accession numbers U90141 and U90140, respectively) (Q. Bai and R. L. Somerville, unpublished data). Recently, McDonough and Butterton determined the DNA sequence of the tyrR gene of Shigella dysenteriae (GenBank accession number AF153317) (38). While the TyrR proteins of E. coli, S. enterica serovar Typhimurium, C. freundii, and S. dysenteriae exhibited marked resemblance (more than 90% identity) to each other, the TyrR protein of E. herbicola showed relatively low (less than 72% identity) similarity to the others. As shown in Fig. 1, all five TyrR proteins had identical helix-turn-helix (HTH) motifs in their C-terminal domains (60); therefore, they could recognize the common DNA sequence (TGTAAAN6TTTACA) (19, 42). Only the E. herbicola TyrR protein possessed seven extra amino acid residues within the central domain; however, deletion of this region did not alter its regulatory properties at least in E. coli (data not shown).
Screening for mutant TyrR proteins with enhanced ability to activate tpl.
As mentioned above, in the case of l-DOPA production with E. herbicola cells, the presence of tyrosine in the medium is absolutely required but is troublesome. To date, various mutant forms of the TyrR protein have been isolated and analyzed in vivo and in vitro (19, 31, 58–60); however, these studies were mainly focused on proteins with impaired capacity to activate or repress the gene expression. In order to obtain a constitutive activator form of TyrR, localized random mutagenesis was carried out. The DNA region containing the open reading frame and putative transcription terminator of the tyrRE. herbicola gene was amplified by the error-prone PCR method (35). The amplified fragments were placed under the control of the tyrR wild-type promoter (6). A derivative of E. coli strain CSH26, TK747, carrying the Φ(tpl′-′lac) gene and ΔtyrR::cat+ gene was transformed with two independently derived plasmid libraries and then spread on basal medium plates containing 2 mM X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside), and about 50,000 transformants were obtained. The mutagenized tyrR genes were then screened for the ability of their products to activate the tpl promoter without additional tyrosine in the medium. Colonies were visually screened for enhanced blue color formation. One hundred highly blue-colored colonies were selected from the bulk of the population and then streaked on the same plate again. Finally, five colonies that exhibited the deepest blue were selected as candidates. The β-galactosidase activities of these strains grown in the basal medium are shown in Table 2. The tyrR2, tyrR3, and tyrR4 alleles were obtained with 25 cycles of error-prone PCR, and tyrR5 and tyrR6 were obtained with 30 cycles. The highest activity was attained by the strain carrying the tyrR5 allele, and it was eight times as high as that of the strain carrying the wild-type tyrR gene.
TABLE 2.
Mutant TyrR proteins leading to high expression of the Φ(tpl′-′lac) gene in E. coli grown in basal mediuma
| tyrR allele present on pACYC177b | Sp. act. of β-galactosidase (Miller units) | Mutation(s) (putative amino acid replacement[s]) |
|---|---|---|
| None | 27.4 | |
| tyrR+ | 100 | |
| tyrR2c | 389 | GTC for V-67→GCC for A (V67A), GTT for V-499→ATT for I (V499I) |
| tyrR3 | 393 | GTC for V-67→GCC for A (V67A) |
| tyrR4 | 231 | GAT for D-97→GGT for G (D97G), ATT for I-402→GTT for V (I402V) |
| tyrR5d | 863 | GTC for V-67→GCC for A (V67A), TAT for Y-72→TGT for C (Y72C), GAA for E-201→GGA for G (E201G) |
| tyrR6 | 850 | GTC for V-67→GCC for A (V67A), TAT for Y-72→TGT for C (Y72C), GAA for E-201→GGA for G (E201G) |
Basal medium consists of 0.5% peptone, 0.5% meat extract, 0.5% yeast extract, and 0.2% KH2PO4.
Four tyrR alleles (tyrR5 and tyrR6 were identical) were obtained through two different cycles of error-prone PCR. The randomly mutagenized tyrR gene was expressed under the control of its wild-type promoter on the pACYC-derived plasmid (pTK774 [see text]).
25 PCR cycles.
30 PCR cycles.
Mapping of mutations by DNA sequencing.
The DNA sequences of the above five tyrR alleles were determined. The tyrR5 and tyrR6 alleles were found to be identical. Although these tyrR alleles were isolated in two independent experiments, the substitution of alanine for valine at position 67 (V67A) was seen in both cases (tyrR2, tyrR3, and tyrR5), suggesting a significant effect of this mutation on the ability of the TyrR protein to activate tpl. The tyrR2 allele contained mutations leading to substitutions of alanine and isoleucine for valine-67 (V67A) and valine-499 (V499I), respectively. Valine-499 of the E. herbicola TyrR protein corresponds to valine-492 of the E. coli TyrR protein (Fig. 1) (9). Replacement of valine-499 with isoleucine (V499I) caused discordance within the conserved HTH motif of the TyrR protein; however, the effect of this substitution was thought to be negligible, at least as to the activation of tpl, because the β-galactosidase level of the strain carrying the tyrR2 allele was almost equal to that of the strain carrying the tyrR3 allele. Mutations in the tyrR4 allele resulted in amino acid substitutions of glycine and valine for aspartate-97 (D97G) and isoleucine-402 (I402V), respectively. It seems likely that the replacement of isoleucine at position 402 with valine (I402V) has no or a little, if any, effect on the function of the TyrR protein because, as can be seen in Fig. 1, all the other TyrR proteins have valine residues at the corresponding position. The change of aspartate-97 to glycine (D97G) seemed to have a moderate effect on the ability of the protein to activate the tpl promoter. This substitution (D97G) has already been demonstrated in a study on the E. coli TyrR protein to cause a twofold increase in transcription from the mtr and tyrP+4 promoters (59). Our results exactly agree with the case of the E. coli TyrR protein, provided that the I402V substitution has no effect on the function of the E. herbicola TyrR protein.
As mentioned above, the highest expression of tpl was exhibited by the strain carrying the tyrR5 allele (TyrRV67A Y72C E201G). Therefore, to create a practical strain for l-DOPA production, we then tried to introduce the tyrR5 allele into E. herbicola.
Expression of Tpl in E. herbicola carrying a mutant tyrR allele.
Before introducing the tyrR5 allele into E. herbicola, the chromosomal locus corresponding to the tyrR gene was replaced with the kanamycin resistance gene, as described under Materials and Methods. Although the DNA fragment used for this recombination event contained a small N-terminal part of the tyrR gene, this ΔtyrR::kan allele did not exhibit negative dominance (data not shown). Following confirmation of the genetic cross by Southern hybridization analysis, the tyrR allele was introduced into the E. herbicola ΔtyrR::kan strain by use of the pSC101-derived vector (pMW118; Nippon Gene), which was shown to be stably maintained for more than 100 generations in the absence of selective pressure (data not shown). Since E. herbicola showed resistance to ampicillin for an unknown reason, the tetracycline resistance gene (tet) was substituted for the bla gene on pMW118. Also, in order to prevent read-through transcription into a subcloned gene (tyrR) from the lac promoter present in pMW118, the lacZα gene was removed by PvuII digestion, followed by self-ligation of the remaining large fragment. The wild-type E. herbicola tyrR gene was cloned into the PvuII site to give pTK919, and the 1.5-kb SacII-MscI internal region was replaced with the corresponding region of the tyrR5 allele to give pTK922.
The wild-type E. herbicola strain and the E. herbicola ΔtyrR::kan strain transformed with one of the following three plasmids—pTK631 (pSC101 replicon bla::tet+), pTK919 (pSC101 replicon bla::tet+ tyrR+), or pTK922 [pSC101 replicon bla::tet+ tyrR5 (TyrRV67A Y72C E201G)]—were cultured in the basal medium with and without additional tyrosine, and then expression of Tpl in these strains was assessed. Since Tpl easily loses its activity once cells are broken, we monitored the expression by immunoblotting instead of measuring the catalytic activity. Whole-cell extracts were obtained by disrupting cells and then subjected to SDS-polyacrylamide gel electrophoresis. The result of immunoblotting with anti-Tpl antibodies is presented in Fig. 2. The level of Tpl expression was expressed as a percentage relative to the amount of Tpl in the wild-type E. herbicola cells grown under tyrosine-induced conditions. Some smaller cross-reactants that appeared in lane 8 of Fig. 2 might result from degradation of Tpl.
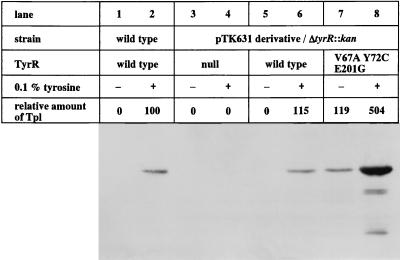
FIG. 2.
Immunoblot analysis of Tpl expression in various E. herbicola strains. Wild-type cells (lanes 1 and 2) and ΔtyrR::kan cells transformed with pTK631 (pSC101replicon bla::tet+, lanes 3 and 4) or pTK631 carrying the tyrR allele [tyrR+, lanes 5 and 6; tyrR5 (TyrRV67A Y72C E201G), lanes 7 and 8] were grown in basal medium in the presence (+) or absence (−) of additional (0.1%) tyrosine for 13 h at 30°C. Whole-cell extracts were obtained by disrupting cells and separated by SDS–12.5% polyacrylamide gel electrophoresis (32). Following electroblotting onto a polyvinylidene difluoride membrane, immunodetection with anti-Tpl antibodies was carried out as described in the text. The level of Tpl expression was expressed as a percentage relative to the amount of Tpl in the wild-type E. herbicola cells grown under tyrosine-induced conditions.
While the tyrR null mutant did not express detectable amounts of Tpl, the strain carrying the wild-type tyrR gene on the plasmid induced Tpl, as with the wild-type E. herbicola strain, when tyrosine was added to the medium. In the case where the tyrR5 allele was substituted for the wild-type tyrR gene, the cells expressed Tpl even in the absence of additional tyrosine in the medium and produced as much of it as was produced by wild-type cells grown under tyrosine-induced conditions. Furthermore, when tyrosine was added, expression of Tpl increased more than fivefold compared to that in the wild-type cells grown in the medium supplemented with tyrosine. These observations indicate that we had obtained a very powerful strain for l-DOPA production.
Effects of V67A, Y72C, and E201G substitutions on regulatory properties of TyrR protein.
To obtain a better understanding of the TyrRV67A Y72C E201G protein, three amino acid substitutions were singly introduced into the protein by means of genetic arrangement or site-directed mutagenesis, and then the effects of these amino acid replacements on the regulatory properties of the TyrR protein were investigated in vivo. The tyrR- and lac-deficient derivative of E. coli (TK596 or TK809) was transformed with two compatible plasmids. One was a pACYC-derived plasmid (7) containing one of the tyrR alleles (encoding the mutant TyrRV67A, TyrRY72C, TyrRE201G, and TyrRV67A Y72C E201G proteins), and the other was a low-copy-number plasmid carrying the Φ(aroF′-′lac), Φ(tyrP′-′lac), or Φ(tpl′-′lac) gene, whose promoter represents a major type of TyrR regulon (23, 42, 47). A parallel set of strains in which the wild-type tyrR gene of either E. coli or E. herbicola was present instead of the above tyrR alleles was also constructed. The aroF and tyrP genes of E. coli encode tyrosine-repressible 3-deoxy-arabinoheptulosonate 7-phosphate synthase and tyrosine-specific permease, respectively. The expression of aroF is repressed by tyrosine or phenylalanine (1, 5, 8, 42), while the expression of tyrP is activated by phenylalanine and repressed by tyrosine (19, 33, 42). The regulatory region of aroF encompasses one weak and two strong TyrR boxes. The weak box lies inside the RNA polymerase binding region (−35 sequence), and the strong boxes lie upstream of the weak box. Ligand-induced self-association of the TyrR protein (54, 55) causes cooperative binding of TyrR molecules to the strong and weak boxes in the aroF regulatory region, which results in elimination of RNA polymerase from the promoter and consequently causes repression of transcription of the aroF gene (1, 5, 8, 42). In the case of tyrP, the strong and weak TyrR boxes are juxtaposed. The strong box lies just upstream of the RNA polymerase binding site, while the weak one overlaps the −35 promoter. Repression by tyrosine was also caused by the cooperative binding of the TyrR protein to two adjacent boxes, whereas phenylalanine-mediated activation was brought about by the single TyrR dimer, which binds to the strong box upstream of the promoter (19, 33, 42).
Strains were grown in MM or in MM containing either tyrosine or phenylalanine and then were subjected to β-galactosidase assay. The results are shown in Table 3. When the wild-type E. herbicola tyrR gene was introduced into a tyrR-deficient background, transcription from the aroF promoter remarkably decreased (2,500 to 530 Miller units). Expression of aroF was moderately repressed by phenylalanine (1.9-fold) and severely repressed by tyrosine (17-fold) in the presence of TyrR. These results indicate that the TyrR protein acts as a repressor on the aroF promoter. On the tyrP promoter, the TyrR protein also had a repressive effect (39 to 14 Miller units for MM, 38 to 19 Miller units for MM plus F, and 39 to 0.5 Miller units for MM plus Y). The TyrR protein slightly activated tyrP transcription in the presence of phenylalanine (1.4-fold) and severely repressed it in the presence of tyrosine (28-fold). The presence of TyrR hardly affected the basal transcription of tpl (85 against 98 Miller units). Expression of tpl was activated 2.6-fold and 30-fold upon the addition of phenylalanine and tyrosine, respectively. It is easily speculated that the ligand-mediated conformational change of the TyrR protein is necessary to activate tpl.
TABLE 3.
Regulation of expression of the Φ(aroF′-′lac), Φ(tyrP′-′lac), and Φ(tpl′-′lac) genes by mutant TyrR proteins
| lac fusiona | Growth mediumb | Sp. act. (Miller units) of β-galactosidase (mode of expression) of various lac fusions in the presence of mutant TyrR proteinsc
|
||||||
|---|---|---|---|---|---|---|---|---|
| E. coli (wild type) |
E. herbicola
|
None | ||||||
| Wild type | TyrRV67A | TyrRY72C | TyrRE201G | TyrRV67A Y72C E201G | ||||
| Φ(aroF′-′lac) | MM | 550 | 530 | 650 | 790 | 560 | 900 | 2,500 |
| MM+F | 230 (R2.4)d | 280 (R1.9) | 280 (R2.3) | 330 (R2.4) | 360 (R1.6) | 390 (R2.3) | 2,500 | |
| MM+Y | 29 (R19) | 31 (R17) | 43 (R15) | 42 (R19) | 33 (R17) | 62 (R15) | 2,600 | |
| Φ(tyrP′-′lac) | MM | 10 | 14 | 43 | 23 | 14 | 64 | 39 |
| MM+F | 30 (A3.0) | 19 (A1.4) | 26 (A0.6) | 26 (A1.1) | 26 (A1.9) | 42 (A0.7) | 38 | |
| MM+Y | 0.3 (R33) | 0.5 (R28) | 1.7 (R25) | 0.9 (R26) | 0.5 (R28) | 3.4 (R19) | 39 | |
| Φ(tpl′-′lac) | MM | 99 | 98 | 160 | 140 | 100 | 400 | 85 |
| MM+F | 410 (A4.1) | 260 (A2.6) | 1,000 (A6.3) | 340 (A2.4) | 260 (A2.6) | 1,200 (A3.0) | 85 | |
| MM+Y | 3,200 (A32) | 2,900 (A30) | 9,300 (A58) | 5,100 (A36) | 3,200 (A32) | 13,000 (A33) | 84 | |
The β-galactosidase activities of the Φ(aroF′-′lac) and Φ(tyrP′-′lac) genes were assayed in E. coli TK809, and the β-galactosidase activity of the Φ(tpl′-′lac) gene was assayed in E. coli TK596. The Φ(aroF′-′lac) and Φ(tyrP′-′lac) genes were on the pSC101-derived plasmid, and the Φ(tpl′-′lac) gene was on the mini-F plasmid.
E. coli strains were grown in M63-glucose MM or MM containing phenylalanine (MM+F) or tyrosine (MM+Y) as the coeffector of the TyrR protein at a final concentration of 1 mM.
E. coli cells with an appropriate lac fusion were transformed with the pACYC-derived plasmid (pTK479) (None) or pTK479 containing the tyrR allele of either E. coli or E. herbicola.
The values in parentheses represent the ratios of effector-mediated regulation. R and A indicate ratios of repression and activation, respectively. The ratio of repression was determined as the level of β-galactosidase in the cells grown in MM divided by that in the cells grown in the medium supplemented with a coeffector. The ratio of activation was determined as the level of β-galactosidase in the cells grown in the medium supplemented with a coeffector divided by that in the cells grown in MM.
The β-galactosidase activities of the strains carrying the wild-type E. herbicola tyrR gene were, in any case, almost equal to those of the strains carrying the E. coli tyrR gene, indicative of equivalent properties of the two TyrR proteins. However, on close examination of the ligand-mediated regulation, a slight difference was recognized with respect to the magnitude of phenylalanine-mediated activation of the tyrP and tpl promoters. When the cells carrying the E. coli tyrR gene were grown in the medium supplemented with phenylalanine, transcription from the tyrP and tpl promoters increased threefold (10 to 30 Miller units) and fourfold (99 to 410 Miller units), respectively, compared to that in cells grown in MM. On the other hand, the E. herbicola TyrR protein activated these promoters 1.4-fold (14 to 19 Miller units) and 2.6-fold (98 to 260 Miller units), respectively, in the presence of phenylalanine as the coeffector. A minor disparity in the phenylalanine-mediated regulation was also observed in the aroF expression. In the presence of phenylalanine as a supplement, the E. coli TyrR protein repressed the aroF transcription more than the E. herbicola TyrR did (2.4- versus 1.9-fold). These results reveal a small but certain difference between the TyrR proteins of E. coli and E. herbicola concerning either the affinity to phenylalanine or the eventual structural change upon the binding of phenylalanine.
As compared to the strains carrying the wild-type tyrR gene of E. herbicola, the strains carrying the mutant tyrR allele involving the V67A substitution or Y72C substitution exhibited increased levels of transcription from all promoters when the cells were grown in MM. One might explain the increased transcription from the aroF and tyrP promoters as the results of the instability or impaired capacity of the TyrR protein (the presence of the TyrR protein decreased the transcription from these promoters; compare β-galactosidase values of the tyrR-deficient strain with those of the tyrR+ strain in Table 3); however, if so, how can one explain the activation of tpl [see line MM for the Φ(tpl′-′lac) gene in Table 3]? As mentioned previously, self-association of the TyrR dimers bound to three distant TyrR boxes is required to activate the transcription of tpl (3, 23). Considering that the TyrR protein routinely acts as a repressor on the aroF promoter regardless of the presence or absence of a ligand (1, 5, 8, 42), it is likely that the V67A and Y72C substitutions changed the structure of the TyrR protein to an attractive form for RNA polymerase to interact with rather than altering the affinity of the protein to coeffectors. It is probable that atypical recruiting of RNA polymerase occurs on the aroF promoter.
Interestingly, as opposed to the wild-type TyrR protein, the mutant TyrRV67A protein had a repressive effect on the tyrP promoter when phenylalanine was added as the coeffector (compare MM to MM plus F with regard to tyrP). Since repression of tyrP is caused by the cooperative binding of the TyrR protein to the promoter (19, 42), it was suggested that the V67A substitution stimulated the self-association of the TyrR protein in the presence of phenylalanine. The fact that the extents of phenylalanine- and tyrosine-mediated activation of tpl increased 2.3-fold (activation ratio [A], A2.6 to A6.3) and 1.9-fold (A30 to A58), respectively, upon the replacement of valine-67 with alanine also implies the efficient hexamerization of this mutant protein. At present, however, it is quite difficult to figure out the effect of the V67A substitution on the regulatory properties of the TyrR protein. Studies so far on the E. coli TyrR protein have distinguished the activation function of the protein from its ligand-mediated self-association function. But, if so, how does the mutant TyrRV67A protein with the ability of facilitated self-association concomitantly activate transcription from the aroF and tyrP promoters in cells grown in MM? In vitro studies on the TyrRV67A protein are necessary to clarify this problem.
As mentioned above, substituting cysteine for tyrosine-72 (Y72C) also increased transcription from the adopted three promoters; however, the mode of ligand-mediated regulation was not significantly different from that in the case of the wild-type TyrR protein of E. herbicola. Needless to say, the most-elevated level of transcription was seen in cells carrying the tyrR5 allele (the mutant TyrRV67A Y72C E201G protein). As expected, repression of tyrP by phenylalanine was observed in this strain as much as in the strain carrying the tyrR3 allele (TyrRV67A).
In order to construct a Tpl high expression strain, we attempted to obtain a mutant TyrR protein with enhanced ability to form a hexamer with a lower amount of tyrosine. The error-prone PCR method was employed for this purpose, and as a result, the tyrR5 allele (the mutant TyrRV67A Y72C E201G protein) was obtained. E. herbicola cells carrying this tyrR5 allele expressed as much Tpl without the addition of tyrosine to the basal medium as that produced by the tyrosine-induced wild-type cells. It should be mentioned, however, that the hexameric form of the TyrR protein causes repression of the genes that are required for the biosynthesis and transport of aromatic amino acids. Therefore, there is a possibility that ligand-irresponsive hexamerization of TyrR may result in a growth defect of cells. The regulatory properties of the mutant TyrRV67A Y72C E201G protein were investigated in vivo, and it was shown that not only the tpl promoter but also the aroF (biosynthesis) and tyrP (transport) promoters were activated, which might alleviate the growth deficiency.
ACKNOWLEDGMENTS
We are very grateful to A. J. Pittard for providing pMU400, R. W. Simons for providing pRS552, T. Elliott for providing TE2680, and S. N. Cohen for providing pMW118.
This work was partly supported by a Grant-in-Aid for Scientific Research (A), no. 10306007, from the Ministry of Education, Science and Culture, Japan, and by a Grant-in-Aid for Fine Enzymatic Synthesis of Useful Compounds from Research for the Future (RFTF) of the Japan Society for the Promotion of Science.
REFERENCES
- 1.Andrews A E, Dickson B, Lawley B, Cobbett C, Pittard A J. Importance of the position of TYR R boxes for repression and activation of the tyrP and aroF genes in Escherichia coli. J Bacteriol. 1991;173:5079–5085. doi: 10.1128/jb.173.16.5079-5085.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Argaet V P, Wilson T J, Davidson B E. Purification of the Escherichia coli regulatory protein TyrR and analysis of its interactions with ATP, tyrosine, phenylalanine, and tryptophan. J Biol Chem. 1994;269:5171–5178. [PubMed] [Google Scholar]
- 3.Bai Q, Somerville R L. Integration host factor and cyclic AMP receptor protein are required for TyrR-mediated activation of tpl in Citrobacter freundii. J Bacteriol. 1998;180:6173–6186. doi: 10.1128/jb.180.23.6173-6186.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buikema W J, Szeto W W, Lemley P V, Orme-Johnson W H, Ausubel F M. Nitrogen fixation specific regulatory genes of Klebsiella pneumoniae and Rhizobium meliloti share homology with the general nitrogen regulatory gene ntrC of K. pneumoniae. Nucleic Acids Res. 1985;13:4539–4555. doi: 10.1093/nar/13.12.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camakaris H, Pittard J. Regulation of tyrosine and phenylalanine biosynthesis in Escherichia coli K-12: properties of the tyrR gene product. J Bacteriol. 1973;115:1135–1144. doi: 10.1128/jb.115.3.1135-1144.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camakaris H, Pittard J. Autoregulation of the tyrR gene. J Bacteriol. 1982;150:70–75. doi: 10.1128/jb.150.1.70-75.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang A C Y, Cohen S N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the p15A cryptic miniplasmid. J Bacteriol. 1978;134:1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cobbett C S. Repression of the aroF promoter by the TyrR repressor in Escherichia coli K-12: role of the ‘upstream’ operator site. Mol Microbiol. 1988;2:377–383. doi: 10.1111/j.1365-2958.1988.tb00042.x. [DOI] [PubMed] [Google Scholar]
- 9.Cornish E C, Argyropoulos V P, Pittard J, Davidson B E. Structure of the Escherichia coli K12 regulatory gene tyrR: nucleotide sequence and sites of initiation of transcription and translation. J Biol Chem. 1986;261:403–410. [PubMed] [Google Scholar]
- 10.Cornish E C, Davidson B E, Pittard J. Cloning and characterization of Escherichia coli K-12 regulator gene tyrR. J Bacteriol. 1982;152:1276–1279. doi: 10.1128/jb.152.3.1276-1279.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui J, Ni L, Somerville R L. ATPase activity of TyrR, a transcriptional regulatory protein for ς70 RNA polymerase. J Biol Chem. 1993;268:13023–13025. [PubMed] [Google Scholar]
- 12.Cui J, Somerville R L. A mutational analysis of the structural basis for transcriptional activation and monomer-monomer interaction in the TyrR system of Escherichia coli K-12. J Bacteriol. 1993;175:1777–1784. doi: 10.1128/jb.175.6.1777-1784.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dripps D, Wartell R M. DNA bending induced by the catabolite activator protein allows ring formation of a 144 bp DNA. J Biomol Struct Dyn. 1987;5:1–13. doi: 10.1080/07391102.1987.10506370. [DOI] [PubMed] [Google Scholar]
- 14.Elliott T. A method for constructing single-copy lac fusions in Salmonella typhimurium and its application to the hemA-prfA operon. J Bacteriol. 1992;174:245–253. doi: 10.1128/jb.174.1.245-253.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Enei H, Matsui H, Yamashita K, Okumura S, Yamada H. Distribution of tyrosine phenol-lyase in microorganisms. Agric Biol Chem. 1972;36:1861–1868. [Google Scholar]
- 16.Enei H, Yamashita K, Okumura S, Yamada H. Culture conditions for preparation of cells containing high tyrosine phenol-lyase activity. Agric Biol Chem. 1973;37:485–492. [Google Scholar]
- 17.Foor F, Morin N, Bostian K A. Production of l-dihydroxy-phenylalanine in Escherichia coli with the tyrosine phenol-lyase gene cloned from Erwinia herbicola. Appl Environ Microbiol. 1993;59:3070–3075. doi: 10.1128/aem.59.9.3070-3075.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hashimoto W, Suzuki H, Yamamoto K, Kumagai H. Effects of site-directed mutations on processing and activity of γ-glutamyltranspeptidase of Escherichia coli K-12. J Biochem (Tokyo) 1995;118:75–80. doi: 10.1093/oxfordjournals.jbchem.a124894. [DOI] [PubMed] [Google Scholar]
- 19.Hwang J S, Yang J, Pittard A J. Critical base pairs and amino acid residues for protein-DNA interaction between the TyrR protein and tyrP operator of Escherichia coli. J Bacteriol. 1997;179:1051–1058. doi: 10.1128/jb.179.4.1051-1058.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwamori S, Oikawa T, Ishiwata K, Makiguchi N. Cloning and expression of the Erwinia herbicola tyrosine phenol-lyase gene in Escherichia coli. Biotechnol Appl Biochem. 1992;16:77–85. [PubMed] [Google Scholar]
- 21.Iwamori S, Yoshino S, Ishiwata K, Makiguchi N. Structure of tyrosine phenol-lyase gene from Citrobacter freundii and structural comparison with tryptophanase from Escherichia coli. J Ferment Bioeng. 1991;72:147–151. [Google Scholar]
- 22.Joyce C M, Grindley N D F. Method for determining whether a gene of Escherichia coli is essential: application to the polA gene. J Bacteriol. 1984;158:636–643. doi: 10.1128/jb.158.2.636-643.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katayama T, Suzuki H, Yamamoto K, Kumagai H. Transcriptional regulation of tyrosine phenol-lyase gene mediated through TyrR and cAMP receptor protein. Biosci Biotechnol Biochem. 1999;63:1823–1827. doi: 10.1271/bbb.63.1823. [DOI] [PubMed] [Google Scholar]
- 24.Kumagai H, Matsui H, Ohkishi H, Ogata K, Yamada H, Ueno T, Fukami H. Synthesis of 3,4-dihydroxyphenyl-l-alanine from l-tyrosine and pyrocatechol by cystalline β-tyrosinase. Biochem Biophys Res Commun. 1969;34:266–270. doi: 10.1016/0006-291x(69)90826-2. [DOI] [PubMed] [Google Scholar]
- 25.Kumagai H, Matsui H, Yamada H. Formation of tyrosine phenol-lyase by bacteria. Agric Biol Chem. 1970;34:1259–1261. [Google Scholar]
- 26.Kumagai H, Kashima N, Yamada H. Racemization of d- or l-alanine by crystalline tyrosine phenol-lyase from Escherichia intermedia. Biochem Biophys Res Commun. 1970;39:796–801. doi: 10.1016/0006-291x(70)90393-1. [DOI] [PubMed] [Google Scholar]
- 27.Kumagai H, Yamada H, Matsui H, Ohkishi H, Ogata K. Tyrosine phenol lyase. I. Purification, crystallization, and properties. J Biol Chem. 1970;245:1767–1772. [PubMed] [Google Scholar]
- 28.Kumagai H, Yamada H, Matsui H, Ohkishi H, Ogata K. Tyrosine phenol lyase. II. Cofactor requirements. J Biol Chem. 1970;245:1773–1777. [PubMed] [Google Scholar]
- 29.Kunkel T A, Roberts J D, Zakour R A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- 30.Kustu S, North A K, Weiss D S. Prokaryotic transcriptional enhancers and enhancer-binding proteins. Trends Biochem Sci. 1991;16:397–402. doi: 10.1016/0968-0004(91)90163-p. [DOI] [PubMed] [Google Scholar]
- 31.Kwok T, Yang J, Pittard A J, Wilson T J, Davidson B E. Analysis of an Escherichia coli mutant TyrR protein with impaired capacity for tyrosine-mediated repression, but still able to activate at ς70 promoters. Mol Microbiol. 1995;17:471–481. doi: 10.1111/j.1365-2958.1995.mmi_17030471.x. [DOI] [PubMed] [Google Scholar]
- 32.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 33.Lawley B, Fujita N, Ishihama A, Pittard A J. The TyrR protein of Escherichia coli is a class I transcription activator. J Bacteriol. 1995;177:238–241. doi: 10.1128/jb.177.1.238-241.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lawley B, Pittard A J. Regulation of aroL expression by TyrR protein and Trp repressor in Escherichia coli K-12. J Bacteriol. 1994;176:6921–6930. doi: 10.1128/jb.176.22.6921-6930.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lerner C G, Gulati P S, Inouye M. Cold-sensitive conditional mutations in Era, an essential Escherichia coli GTPase, isolated by localized random polymerase chain reaction mutagenesis. FEMS Microbiol Lett. 1995;126:291–298. doi: 10.1111/j.1574-6968.1995.tb07432.x. [DOI] [PubMed] [Google Scholar]
- 36.Liu-Johnson H N, Gartenberg M R, Crothers D M. The DNA binding domain and bending angle of E. coli CAP protein. Cell. 1986;47:995–1005. doi: 10.1016/0092-8674(86)90814-7. [DOI] [PubMed] [Google Scholar]
- 37.Lu C, McCalla D R, Bryant D W. Action of nitrofurans on E. coli: mutation and induction and repair of daughter-strand gaps in DNA. Mutat Res. 1979;67:133–144. doi: 10.1016/0165-1218(79)90124-1. [DOI] [PubMed] [Google Scholar]
- 38.McDonough M A, Butterton J R. Spontaneous tandem amplification and deletion of shiga toxin operon in Shigella dysenteriae 1. Mol Microbiol. 1999;34:1058–1069. doi: 10.1046/j.1365-2958.1999.01669.x. [DOI] [PubMed] [Google Scholar]
- 39.Miller J H. A short course in bacterial genetics. A laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1992. [Google Scholar]
- 40.Miranda-Rios J, Sanchez-Pescador R, Urdea M, Covarrubias A A. The complete nucleotide sequence of the glnALG operon of Escherichia coli K-12. Nucleic Acids Res. 1987;15:2757–2770. doi: 10.1093/nar/15.6.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Connor M, Peifer M, Bender W. Construction of large DNA segments in Escherichia coli. Science. 1989;244:1307–1312. doi: 10.1126/science.2660262. [DOI] [PubMed] [Google Scholar]
- 42.Pittard A J, Davidson B E. TyrR protein of Escherichia coli and its role as repressor and activator. Mol Microbiol. 1991;5:1585–1592. doi: 10.1111/j.1365-2958.1991.tb01904.x. [DOI] [PubMed] [Google Scholar]
- 43.Saier M H, Jr, Ramseier T M, Reizer J. Regulation of carbon utilization. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella. 2nd ed. Washington, D.C.: American Society for Microbiology; 1996. pp. 1325–1343. [Google Scholar]
- 44.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 45.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simons R W, Houman F, Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene. 1987;53:85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- 47.Smith H Q, Somerville R L. The tpl promoter of Citrobacter freundii is activated by the TyrR protein. J Bacteriol. 1997;179:5914–5921. doi: 10.1128/jb.179.18.5914-5921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sutcliffe J G. Nucleotide sequence of the ampicillin resistance gene of Escherichia coli plasmid pBR322. Proc Natl Acad Sci USA. 1978;75:3737–3741. doi: 10.1073/pnas.75.8.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suzuki H, Katayama T, Yamamoto K, Kumagai H. Transcriptional regulation of tyrosine phenol-lyase gene of Erwinia herbicola AJ2985. Biosci Biotechnol Biochem. 1995;59:2339–2341. doi: 10.1271/bbb.59.2339. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki H, Nishihara K, Usui N, Matsui H, Kumagai H. Cloning and nucleotide sequence of Erwinia herbicola AJ2982 tyrosine phenol-lyase. J Ferment Bioeng. 1993;75:145–148. [Google Scholar]
- 51.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vieira J, Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- 53.Wilson K. Preparation of genomic DNA from bacteria. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1998. pp. 2.4.1–2.4.5. [Google Scholar]
- 54.Wilson T J, Argaet V P, Howlett G J, Davidson B E. Evidence for two aromatic amino acid-binding sites, one ATP-dependent and the other ATP-independent, in the Escherichia coli regulatory protein TyrR. Mol Microbiol. 1995;17:483–492. doi: 10.1111/j.1365-2958.1995.mmi_17030483.x. [DOI] [PubMed] [Google Scholar]
- 55.Wilson T J, Maroudas P, Howlett G J, Davidson B E. Ligand-induced self-association of the Escherichia coli regulatory protein TyrR. J Mol Biol. 1994;238:309–318. doi: 10.1006/jmbi.1994.1294. [DOI] [PubMed] [Google Scholar]
- 56.Yamada H, Kumagai H. Synthesis of l-tyrosine-related amino acids by β-tyrosinase. Adv Appl Microbiol. 1975;19:249–288. doi: 10.1016/s0065-2164(08)70431-3. [DOI] [PubMed] [Google Scholar]
- 57.Yamada H, Kumagai H, Kashima N, Torii H, Enei H, Okumura S. Synthesis of l-tyrosine from pyruvate, ammonia, and phenol by crystalline tyrosine phenol-lyase. Biochem Biophys Res Commun. 1972;46:370–374. doi: 10.1016/s0006-291x(72)80148-7. [DOI] [PubMed] [Google Scholar]
- 58.Yang J, Camakaris H, Pittard A J. Mutations in the tyrR gene of Escherichia coli which affect TyrR-mediated activation but not TyrR-mediated repression. J Bacteriol. 1993;175:6372–6375. doi: 10.1128/jb.175.19.6372-6375.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang J, Camakaris H, Pittard A J. Further genetic analysis of the activation function of the TyrR regulatory protein of Escherichia coli. J Bacteriol. 1996;178:1120–1125. doi: 10.1128/jb.178.4.1120-1125.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang J, Ganesan S, Sarsero J, Pittard A J. A genetic analysis of various functions of the TyrR protein of Escherichia coli. J Bacteriol. 1993;175:1767–1776. doi: 10.1128/jb.175.6.1767-1776.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]