Abstract
Dynein motor proteins, often considered to be missing in land plants, are found in plants that reproduce with flagellated sperm.
Dear Editor,
Cytoskeletal motor proteins are essential to eukaryotes by facilitating cell division, cytoplasmic organization, and locomotion. Although plant genomes encode numerous kinesin and myosin motors (Zhu and Dixit, 2012; Lee et al., 2015), the presence of dyneins in land plants has been unclear in the scientific literature. Here, we summarize the debate regarding plant dyneins and clarify the presence of plant dyneins through analysis of recent and updated genomes. Initially, dynein was identified by RT-PCR and in early genome annotations rice (Oryza sativa) and Nicotiana benthamiana (King, 2002; Moscatelli et al., 2003; Scali et al., 2003). Subsequently, those dynein sequences were removed from the NCBI and retracted from the rice genome. We determined that these initially reported “plant” sequences likely originated from fungal and algal contaminants. Dyneins were not found in seed plants Arabidopsis (Arabidopsis thaliana), rice, or cottonwood (Populus trichocarpa) (Lawrence et al., 2001; Wickstead and Gull, 2007; Kollmar, 2016). Dynein transport proteins were reported absent in the moss Physcomitrium patens (Rensing et al., 2008). Understandably, dyneins were considered absent in land plants and kinesins were hypothesized to compensate for that loss (Zhu and Dixit, 2012; Lee et al., 2015; Gicking et al., 2018). The absence of plant dyneins was corroborated by a recent genome analysis (Brawley et al., 2017), while other investigations identified dyneins in the gymnosperm Ginkgo biloba and other plants (Kollmar, 2016; Cheng et al., 2019, 2021; Liu et al., 2021). We sought to resolve these conflicting reports through analysis of key genomes representing major branches of plant evolutionary history. Our survey of Viridiplantae for conserved dynein components revealed a gradual loss of specific dynein subunits along the evolutionary path from the ancestor of green algae to land plants with flagellated sperm and the complete loss of dynein heavy chains (DHC) in plants without flagella. We conclude that a core set of dyneins is found in plants that depend upon flagellated sperm for reproduction and these data help clarify an important cytological aspect of evolutionary genomics.
Dyneins are multi-subunit protein complexes and each complex contains at least one conserved heavy chain responsible for motor activity (Wickstead and Gull, 2007; Kollmar, 2016). We surveyed Chlorophycean algae, Charaphycean algae, and all land plants with assembled genomes in JGI, NCBI, and 1KP databases using BLAST and HMM searches for the conserved Dynein Heavy Chain (DHC) PFAM model PF:3028 (Wickstead and Gull, 2007; Kollmar, 2016). Multiple DHCs were identified in bryophytes, lycophytes, ferns, and the gymnosperm G. biloba (Figures 1A and 2). DHCs were not found in any angiosperm or conifer, and we confirmed this absence through TBLASTN searches using Selaginella and Ginkgo DHCs as query sequences.
Figure 1.
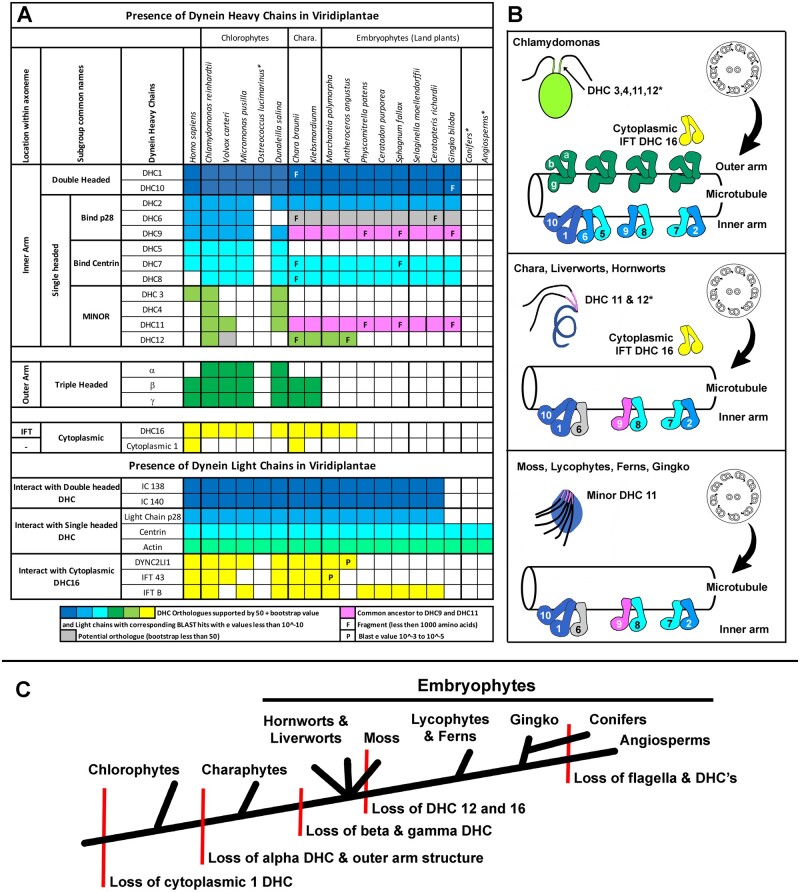
DHCs in select land plants and Chlorophycean and Charaphycean Algae. A, Presence of DHCs in key Viridiplantae species and Homo sapiens, for comparison. Table of DHCs represents data from maximum parsimony analysis (Figure 2 and Supplemental Figure S2). Fragments (F) may be an artifact of sequencing. Light chain presence was determined through BLAST searches and colored boxes signify e values less than 10^−10, and “P” represents larger e values. Asterisks indicate organisms that do not produce flagella or cilia. B, Comparison of DHCs within axonemes of key plant and algal groups. In each panel, swimming cells and sperm (upper left) and axonemes are diagrammed in cross section (upper right) and longitudinal section (bottom). Four monomeric DHCs are considered “minor” and localize close to cell body (colored regions of flagella indicated with small arrow), while the localization of DHC12 is undetermined (*) (Yagi et al., 2009). C, Viridiplantae phylogeny showing loss of DHCs and axonemal structures.
Figure 2.
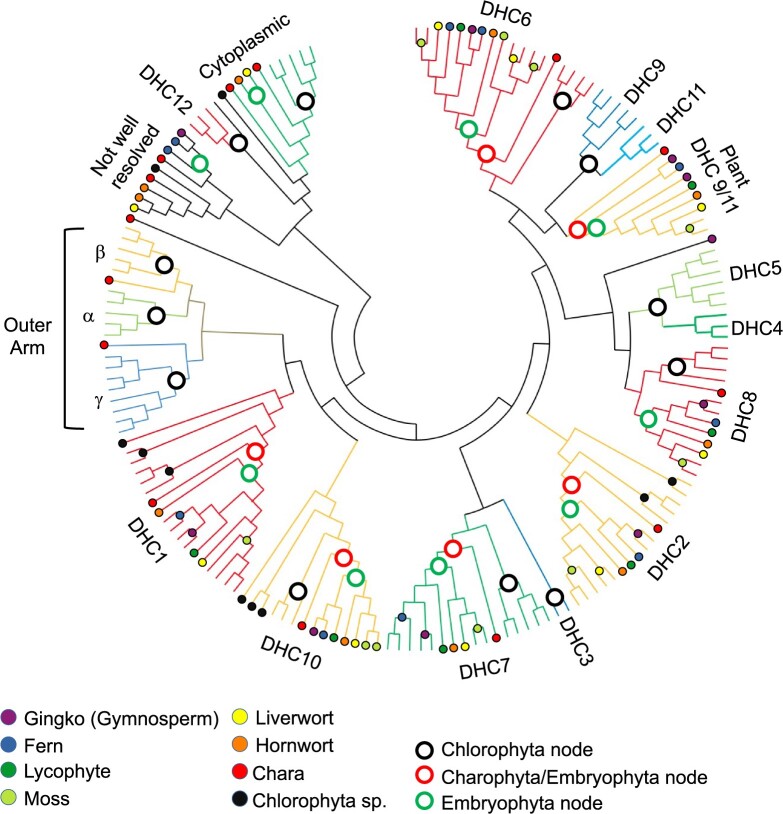
Topology of maximum parsimony tree showing DHC subcategories in Viridiplantae and humans. Amino acid sequences of 169 DHCs were sorted into classes by maximum-parsimony analysis of their evolutionary history. Groups in bold text are found in all plants with flagellated sperm and asterisks on DHC12 and DHC16 indicate these are found only in hornworts and liverwort but not other land plants. Three outer arm dynein sequences are found in C. reinhardtii: α, β, and γ. Additional moss (Ceratodon purpureus and Sphagnum magellanicum) genomes were searched to corroborate this loss. Proteins were classified based upon the identity of Homo sapiens and the C. reinhardtii protein within each color-coded clade (human sequences are noted with a black dot for comparison). Sequences were aligned in ALIVIEW and analyzed using MEGAX (Kumar et al., 2018). Sequence fragments less than 1.5 kb were removed prior to alignment. All sites with less than 50% coverage were eliminated leaving a total of 3,766 positions. The bootstrap consensus tree topology of 500 replicates is presented using circular format. A linear format version of this tree including individual sequence accessions and bootstrap replicate values is included as Supplemental Figure S1.
We categorized plant DHCs by subclass using neighbor joining and maximum parsimony analyses (Figure 2 and Supplemental Figure S1). We rooted these analyses with Chlamydomonas reinhardtii, a close relative to land plants and model system with well-characterized dynein complexes. The C. reinhardtii genome contains 16 distinct DHCs, all involved in flagellar beating (King, 2016). Most DHCs form inner and outer axonemal arms that slide microtubule doublets to create movement. Chlamydomonas reinhardtti DHC1-12 form inner arms and three subunits (α, β, and γ) comprise the outer arms (Figure 1B). We found no outer arm sequences in any land plant, which aligns with studies showing plant sperm flagella lack outer arms (Hyams and Campbell, 1985). Interestingly, the Charophycean algae, Chara braunii and Klebsormidium flaccidum, contained sequences homologous to β and γ (Figure 1B), despite the lack of visible outer arms (Jin and Hasenstein, 2009).
All plants with flagella encode a core set of seven inner arm DHCs sequences. We identified full length orthologs for five of 12 C. reinhardtii DHC inner arms (Supplemental Table S1). Inner arm DHCs are subcategorized as double-headed heterodimers or single-headed monomers that bind either centrin or p28 (King, 2016; Figure 1A). All land plants genomes evaluated with flagellated sperm and charophytes encode orthologs for each inner arm DHC type: double-headed DHC1 and DHC10, two DHCs that bind centrin (DHC7 and DHC8), and one that associates with p28 (DHC2). Charophytes and land plants also contain a potential ortholog for DHC6 and an out-paralog of C. reinhardtii DHC9 and DHC11 (Figure 2 and Supplemental Figure S1). Our phylogenetic analysis suggests that C. reinhardtii DHC9 and DHC11 arose from duplication and are out-paralogs in the Chlorophycean algae, while charophytes and land plants contain DHC sequences derived from the common ancestor.
Two DHCs were found in a subset of flagellated plants. Orthologs of C. reinhardtii DHC12 and DHC16 were found in charophytes, liverwort Marchantia polymorpha, hornwort Antheroceros angustus, but no other land plant. DHC12 is considered a “minor” DHC with unknown function (Yagi et al., 2009). DHC16 is often called “cytoplasmic dynein 2” due to sequence similarity to cytoplasmic dynein 1 needed for mitosis (Kollmar, 2016; Braschi et al., 2022). Our study confirms that C. reinhardtii and land plants do not possess a true cytoplasmic dynein 1 (Figure 1; Wickstead and Gull, 2007). Chlamydomonas reinhardtii DHC16 is needed for flagellar synthesis, maintenance, and transport (Porter et al., 1999). DHC16 orthologs contain all six required AAA domains and sequence relationship was supported by maximum-likelihood phylogenetic analysis (Supplemental Figure S2). In this regard, hornworts and liverworts are more similar to algae than other land plants.
Multiple light and intermediate-light chains associate with DHCs to modulate motor activity. We surveyed Viridiplantae genomes for eight dynein light and intermediate light chains known to complex with C. reinhardtii DHCs (Figure 1). We found centrin and actin orthologs in all organisms. Other light chains, such as light intermediate chain 8, have been found in organisms without flagella and these light chains are speculated to participate in non-motor activities (Wickstead and Gull, 2007). The presence of six light chains reflected the patterns observed in the plant DHCs. Similar to DHC12 and DHC16, orthologs of C. reinhardtii intraflagellar transport 43 (IFT43) and dynein 2 light intermediate chain 1 (DYNC2LI1) were found in many green algae, charophytes, and only two bryophytes (Figure 1). The presence of intermediate chains 138 and 140, p28, and intraflagellar transport B (IFT B) orthologs nearly mimicked the core set of inner arm DHCs. These four chains were found in all land plants except G. biloba, conifers, and angiosperms.
In conclusion, we identified a core dynein set in major groups of land plants that reproduce with swimming sperm. Flagellated sperm is ancestral in land plants and was lost within gymnosperms and before angiosperms divergenced (Hodges et al., 2012). DHCs are completely absent from seed plants that reproduce without swimming sperm.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Table S1. Dynein sequences used for phylogenetic reconstruction.
Supplemental Figure S1. Topology of linear maximum parsimony tree of Viridiplantae and human DHCs.
Supplemental Figure S2. Maximum-likelihood tree of cytoplasmic DHCs.
Supplementary Material
Acknowledgments
We thank Erin McArthur MLIS for help with reference formatting.
Funding
This work was supported by funds to JL from the University of Wisconsin—Oshkosh College of Letters and Science and the University of Wisconin—Oshkosh Faculty Development Program.
Conflict of interest statement. None declared.
Contributor Information
Jessica Lucas, Department of Biology, University of Wisconsin—Oshkosh, Oshkosh, Wisconsin 54901, USA.
Matt Geisler, Plant Biology Program, School of Biological Sciences, Southern Illinois University—Carbondale, Carbondale, Illinois 62901, USA.
J.L. conceived the project, analyzed the data, and wrote the manuscript. M.G. performed the data analysis and edited the manuscript.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/General-Instructions) is: Jessica Lucas (lucasjr@uwosh.edu).
References
- Braschi B, Omran H, Witman GB, Pazour GJ, Pfister KK, Bruford EA, King SM (2022) Consensus nomenclature for dyneins and associated assembly factors. J Cell Biol 221: e202109014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawley SH, Blouin NA, Ficko-Blean E, Wheeler GL, Lohr M, Goodson HV, Jenkins JW, Blaby-Haas CE, Helliwell KE, Chan CX, et al. (2017) Insights into the red algae and eukaryotic evolution from the genome of Porphyra umbilicalis (Bangiophyceae, Rhodophyta). Proc Natl Acad Sci USA 114: E6361–E6370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S, Xian W, Fu Y, Marin B, Keller J, Wu T, Sun W, Li X, Xu Y, Zhang Y, et al. (2019) Genomes of subaerial Zygnematophyceae provide insights into land plant evolution. Cell 179: 1057–1067.e14 [DOI] [PubMed] [Google Scholar]
- Gicking AM, Swentowsky KW, Dawe RK, Qiu W (2018) Functional diversification of the kinesin-14 family in land plants. FEBS Lett 592: 1918–1928 [DOI] [PubMed] [Google Scholar]
- Hodges ME, Wickstead B, Gull K, Langdale JA (2012) The evolution of land plant cilia. New Phytol 195: 526–540 [DOI] [PubMed] [Google Scholar]
- Hyams JS, Campbell CJ (1985) Widespread absence of outer dynein arms in the spermatozoids of lower plants. Cell Biol Int Rep 9: 841–848 [DOI] [PubMed] [Google Scholar]
- Jin QJ, Hasenstein KH (2009) Cytoskeletal control of sperm release in Chara contraria. Bot Mar 52: 162–169 [Google Scholar]
- King SM (2002) Dyneins motor on in plants. Traffic 3: 930–931 [DOI] [PubMed] [Google Scholar]
- King SM (2016) Axonemal dynein arms. Cold Spring Harb Perspect Biol 8: a028100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollmar M (2016) Fine-tuning motile cilia and flagella: Evolution of the dynein motor proteins from plants to humans at high resolution. Mol Biol Evol 33: 3249–3267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35: 1547–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence CJ, Morris NR, Meagher RB, Dawe RK (2001) Dyneins have run their course in plant lineage. Traffic 2: 362–363 [DOI] [PubMed] [Google Scholar]
- Lee YR, Qiu W, Liu B (2015) Kinesin motors in plants: from subcellular dynamics to motility regulation. Curr Opin Plant Biol 28: 120–126 [DOI] [PubMed] [Google Scholar]
- Moscatelli A, Scali M, Vignani R, Onelli E, Cresti M (2003) Dynein heavy chain (DHC)-related polypeptides during pollen tube growth. Cell Biol Int 27: 237–238. 10.1016/s1065-6995(02)00318-9 [DOI] [PubMed] [Google Scholar]
- Liu H, Wang X, Wang G, Cui P, Wu S, Ai C, Hu N, Li A, He B, Shao X, et al. (2021) The nearly complete genome of Ginkgo biloba illuminates gymnosperm evolution. Nat Plants 7: 748–756 [DOI] [PubMed] [Google Scholar]
- Porter ME, Bower R, Knott JA, Byrd P, Dentler W (1999) Cytoplasmic dynein heavy chain 1b is required for flagellar assembly in Chlamydomonas. Mol Biol Cell 10: 693–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensing SA, Lang D, Zimmer AD, Terry A, Salamov A, Shapiro H, Nishiyama T, Perroud PF, Lindquist EA, Kamisugi Y, et al. (2008) The Physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science (New York, NY) 319: 64–69 [DOI] [PubMed] [Google Scholar]
- Scali M, Vignani R, Moscatelli A, Jellbauer S, Cresti M (2003) Molecular evidence for a cytoplasmic dynein heavy chain from Nicotiana tabacum L. Cell Biol Int 27: 261–262 [DOI] [PubMed] [Google Scholar]
- Wickstead B, Gull K (2007). Dyneins across eukaryotes: a comparative genomic analysis. Traffic (Copenhagen, Denmark) 8: 1708–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi T, Uematsu K, Liu Z, Kamiya R (2009) Identification of dyneins that localize exclusively to the proximal portion of Chlamydomonas flagella. J Cell Sci 122: 1306–1314 [DOI] [PubMed] [Google Scholar]
- Zhu C, Dixit R (2012) Functions of the Arabidopsis kinesin superfamily of microtubule-based motor proteins. Protoplasma 249: 887–899 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.