Abstract
Heterotrimeric G-protein complexes comprising Gα-, Gβ-, and Gγ-subunits and the regulator of G-protein signaling (RGS) are conserved across most eukaryotic lineages. Signaling pathways mediated by these proteins influence overall growth, development, and physiology. In plants, this protein complex has been characterized primarily from angiosperms with the exception of spreading-leaved earth moss (Physcomitrium patens) and Chara braunii (charophytic algae). Even within angiosperms, specific G-protein components are missing in certain species, whereas unique plant-specific variants—the extra-large Gα (XLGα) and the cysteine-rich Gγ proteins—also exist. The distribution and evolutionary history of G-proteins and their function in nonangiosperm lineages remain mostly unknown. We explored this using the wealth of available sequence data spanning algae to angiosperms representing extant species that diverged approximately 1,500 million years ago, using BLAST, synteny analysis, and custom-built Hidden Markov Model profile searches. We show that a minimal set of components forming the XLGαβγ trimer exists in the entire land plant lineage, but their presence is sporadic in algae. Additionally, individual components have distinct evolutionary histories. The XLGα exhibits many lineage-specific gene duplications, whereas Gα and RGS show several instances of gene loss. Similarly, Gβ remained constant in both number and structure, but Gγ diverged before the emergence of land plants and underwent changes in protein domains, which led to three distinct subtypes. These results highlight the evolutionary oddities and summarize the phyletic patterns of this conserved signaling pathway in plants. They also provide a framework to formulate pertinent questions on plant G-protein signaling within an evolutionary context.
Heterotrimeric G-proteins show sporadic presence in algae, and specific components are frequently lost in land plants, suggesting distinct distribution and evolutionary histories of their constituents.
Introduction
Heterotrimeric G-proteins (G-proteins) are key signaling complexes in eukaryotes (Cabrera-Vera et al., 2003; Offermanns, 2003; Pandey, 2019). The core complex comprised one Gα and one nondissociable Gβγ dimer. The Gα protein can bind and hydrolyze guanine nucleotides. As per established paradigm, during resting phase, the Gα is GDP bound and remains associated with the Gβγ dimer. Upon activation by a G-protein-coupled receptor (GPCR), the GDP on Gα is exchanged for GTP, which causes dissociation of the complex into GTP-Gα and Gβγ dimer. Both these freed entities can interact with downstream effectors to transduce the signal. The inherent GTPase activity of Gα causes hydrolysis of bound GTP, regenerating its GDP-bound form. GDP-Gα associates with Gβγ restoring the trimeric complex, ready to be activated again (Cabrera-Vera et al., 2003; Offermanns, 2003; Pandey, 2019). The Regulator of G-protein Signaling (RGS) proteins interact with Gα and act as GTPase-activity Accelerating Proteins (GAPs) to increase the rate of GTP hydrolysis, and are key components of the G-protein signaling in metazoan (Siderovski and Willard, 2005).
The importance of G-protein components in influencing a multitude of cellular responses has led to their detailed characterization in multiple eukaryotes, including fungi (yeast), humans, and plants. In brewer’s yeast (Saccharomyces cerevisiae), G-proteins control pheromone perception and mating response (Dohlman, 2002). In humans, G-protein regulated pathways control key sensory and chemical perception and are a major target for the pharmaceutical industry (Hauser et al., 2017; Sun et al., 2019). In fruit fly (Drosophila melanogaster) and roundworms (Caenorhabditis elegans), G-proteins are involved in controlling several critical developmental and sensory pathways (Byrne et al., 2018; Koelle, 2018; Ma et al., 2019; Venkatesh and Singh, 2021). In plants, G-protein signaling controls almost all aspects of growth and development as well as biotic and abiotic stress responses (Pandey, 2019).
Because of the initial characterization of heterotrimeric G-proteins from metazoans and yeast, the first 20 years of plant G-protein signaling research remained focused on demonstrating the extent to which these are similar to the metazoan proteins. The key concepts were essentially borrowed from mammalian studies. These studies were, and remain, very Arabidopsis (Arabidopsis thaliana) centric and emphasize the roles of Gα and its regulation through RGS (Jones and Assmann, 2004; Johnston et al., 2007; Temple and Jones, 2007; Jones et al., 2011a, 2011b; Urano et al., 2012a, 2012b). However, the study of G-proteins in the last decade with a handful of other plant species showed that the situation is vastly different, not only in terms of signaling mechanisms but also in the context of key components themselves (Roy Choudhury et al., 2011, 2012, 2016, 2017; Hackenberg et al., 2017; Roy Choudhury et al., 2019).
Given their importance, the origin of G-protein components has been tracked in eukaryotic lineages (Anantharaman et al., 2011). Phylogenetic analysis suggests that the GTP-binding Gα originated from the ADP-ribosylation factor (Arf)-like monomeric GTPase family, early in eukaryotic evolution (Leipe et al., 2002). Structurally, Gα contain a myristoylated glycine and N-terminal helix followed by the core GTPase domain containing G1–G5 motifs (Cabrera-Vera et al., 2003). The GTPase domain of Gα has an α-helical insert before the G2 motif, a switch-III-insert between G3 and G4 motifs, and a bi-helical insert at the C-terminal of the G4 motif. While all monomeric GTPase families have the core GTPase domain, the N-terminal helix and myristoylation of glycine are found only in Arf GTPases and heterotrimeric Gα. It is the presence of multiple inserts within the core GTPase domain of Gα that distinguishes it from Arf and other monomeric GTPases. Typically, an arginine finger motif is present in the GAP proteins of monomeric GTPases and enhances their GTPase activity. In contrast, Gα has an in-built arginine finger and its GAP protein, RGS, lacks this motif. The RGS protein exerts its function by interacting with the core GTPase domain and hence it may not be derived from the GAP proteins of monomeric GTPases (Leipe et al., 2002; Cabrera-Vera et al., 2003). The other two subunits—Gβ and Gγ either co-occur or are absent in all eukaryotes analyzed, to date, and form obligate, functional dimers (Anantharaman et al., 2011). The Gβ subunit of the complex belongs to the larger group of seven WD40 (approximately 40-aa motifs often terminating in a Trp-Asp (W-D) dipeptide) repeat-containing proteins and has a characteristic N-terminal bi-helical extension to its seven-bladed β-propeller (Sondek et al., 1996). The Gγ subunit also has a bi-helical domain, which forms a coiled-coil structure with the bi-helical domain of Gβ. Because of the structural similarities between the bi-helical domains of Gβ and Gγ, they are hypothesized to have evolved from a common ancestral protein (Anantharaman et al., 2011). The entire Gγ protein participates in the interaction with Gβ except for the C-terminal CAAX motif (where C = cysteine, A = aliphatic amino acid, and X = any amino acid), which is posttranslationally modified for plasma membrane anchoring (Escribá et al., 2007; Wensel, 2008).
The degree of expansion in the number of genes encoding individual G-protein components varies greatly across eukaryotic lineages. Although phylogenetic analyses support the presence of single Gα- and Gβ-subunits in the last eukaryotic common ancestor (Anantharaman et al., 2011; De Mendoza et al., 2014), Gα has expanded into five families in metazoa, four of which are also present in fungi. The human, fruit fly (D. melanogaster), roundworm (C. elegans), and yeast (S. cerevisiae) genomes encode 23, 12, 23, and 3 Gα protein paralogs, respectively (Wilkie and Yokoyama, 1994; Wilkie, 1999; McCudden et al., 2005; Shpakov and Pertseva, 2008). Certain fungi such as Laccaria sp. have an unusually high number (up to 30) of Gα paralogs (Anantharaman et al., 2011). The Gβ proteins have expanded into two major families in metazoa, but only a single family is present in fungi (De Mendoza et al., 2014). The human, fruit fly, worm, and yeast genomes encode 5, 3, 2, and 1 Gβ proteins, respectively (Wilkie, 1999; Dohlman, 2002; Cabrera-Vera et al., 2003; Shpakov and Pertseva, 2008). The number of Gγ proteins is generally higher than the number of Gβ proteins across eukaryotic lineages (Anantharaman et al., 2011).
Studies of G-protein signaling in plants have identified multiple exceptions to the rules established based on the findings in metazoans and suggest that G-protein signaling is neither simple nor completely understood. For example, plants possess relatively fewer paralogs for each G-protein component. Arabidopsis and rice (Oryza sativa), the two widely studied representatives of eudicot and monocot plants, respectively, each contain only one Gα, one Gβ, and a few Gγ in their genomes (Pandey, 2019). Higher numbers of genes exist only in plants that have undergone recent whole-genome duplications (WGDs; Bisht et al., 2011; Roy Choudhury et al., 2011), implying the genes have undergone purifying selection after ancient WGD. Moreover, despite exhibiting similar biochemical activities and a core trimeric complex, the regulation of the G-protein cycle appears to be different in the plant lineage. Canonical GPCRs have not been identified yet; instead, G-proteins seem to interact with receptor-like kinases, which are prevalent in plants (Aranda-Sicilia et al., 2015; Liang et al., 2016, 2018; Pandey and Vijayakumar, 2018; Yu and Assmann, 2018; Pandey, 2019; Wu et al., 2019; Zhou et al., 2019; Pandey, 2020; Roy Choudhury and Pandey, 2022). Furthermore, plant-specific components, such as extra-large Gα (XLGα) and cysteine-rich Gγ proteins, are also an integral part of the trimeric core (Ding et al., 2008; Chakravorty et al., 2011; Roy Choudhury et al., 2011; Li et al., 2012a, 2012b; Thung et al., 2012; Wolfenstetter et al., 2014; Chakravorty et al., 2015; Hackenberg et al., 2016; Lou et al., 2020; Roy Choudhury et al., 2020) but their provenance is not clear yet.
Another notable exception is the distribution of the G-protein components themselves in the plant lineage. G-proteins were considered to be ubiquitous among eukaryotes, but despite the rich genomic resources available, no G-protein sequences have been reported from chlorophycean algae such as Volvox carteri, Chlamydomonas reinhardtii, Micromonas pusilla, and Ostreococcus lucimarinus (Hackenberg and Pandey, 2014). A complete set of canonical G-protein components (Gα, Gβ, Gγ, and RGS) exists in the genome of a charophyte alga, Chara braunii (Hackenberg et al., 2013; Hackenberg and Pandey, 2014) and XLGα, Gα, and Gβ genes have been identified in Klebsormidium nitens (Hackenberg et al., 2016; Urano et al., 2016). Furthermore, specific proteins of the core complex are missing from various plant species. For example, despite the critical role of RGS proteins in regulating the G-protein cycle in eudicots, many monocot plants do not possess an RGS coding gene in their genome (Hackenberg et al., 2017), whereas all eudicot genomes analyzed to date have both Gα and RGS genes (Urano et al., 2012a, 2012b, 2016; Kumar and Bisht, 2018). The reference moss Physcomitrium (Physcomitrella) patens (a bryophyte) lacks both a canonical Gα and an RGS gene but possesses Gβ, Gγ, and an XLGα gene homologs (Hackenberg et al., 2016). It is not known if this is an isolated incident in P. patens or a representation of all mosses.
Similar to the nonuniform distribution of G-protein components, their biological function and importance also varies across land plants. Surprisingly for proteins with such a wide range of regulatory roles, G-proteins are not essential for survival in eudicots. Arabidopsis thaliana mutants in which the function of most of the G-protein components is disrupted, exhibit a range of different phenotypes but are able to survive and complete their life cycle (Urano et al., 2016; Roy Choudhury et al., 2020). In contrast, G-proteins are essential in the monocot lineage, as rice or maize (Zea mays) plants lacking a functional Gβ gene or all XLGα genes fail to grow past the early seedling stage (Utsunomiya et al., 2012; Wu et al., 2018, 2019). In P. patens, the loss of Gβ or XLGα causes defects in gametophyte development and a complete loss of sporophyte formation (Hackenberg et al., 2016). No information exists on the distribution or function of G-protein components in other land plant lineages such as hornworts and liverworts (bryophytes), lycophytes and monilophytes (pteridophytes), and gymnosperms. This lack of knowledge restricts our understanding of the relevance of findings from the flowering plants to the entire plant kingdom. With the availability of a substantial number of genomes and transcriptomes covering a wide range of plant species (e.g. 1,000 plant [1KP] transcriptomes; Matasci et al., 2014; Carpenter et al., 2019), we are in a position to address this knowledge gap.
In this study, we perform a comprehensive analysis of the heterotrimeric G-protein components to determine their distribution among the photosynthetic lineages spanning millions of years of evolution. We analyzed all currently publicly available genomes (70 genomes), and transcriptomes from the 1KP database and additional sources (Matasci et al., 2014; Carpenter et al., 2019). Construction of phylogenetic trees for each of these proteins allowed us to analyze their expansion or reduction in plant genomes. We performed synteny analysis and Hidden Markov Model (HMM)-based searches using six-frame translated chromosome sequences to show the loss of gene in specific plant lineages. We also highlight evolutionary oddities such as Gα retrogenes in hornworts, putative Chlorophyte Gα, and trace the conservation of intron–exon position between Gα and XLGα genes.
Because the provenance and evolutionary trajectories of all these proteins in the plant lineages remain mostly unexplored, the information presented in this manuscript provides an evolutionary framework for the presence/absence of these proteins leading to the synthesis of such information to generate functional hypotheses. This is critical for future research, as G-proteins subunits are key determinants of important yield traits, and the established metazoan or even the Arabidopsis model of G-protein signaling mechanisms does not fully explain thier function (Botella, 2012; Li et al., 2012a, 2012b; Roy Choudhury et al., 2014; Sun et al., 2014; Wendt et al., 2016; Xu et al., 2016; Kaur et al., 2018; Kan et al., 2022).
Results
Phyletic distribution patterns of G-protein complex in plant and algal lineages
Extant land plant lineages including the nonvascular plants (i.e. mosses, liverworts, and hornworts), spore-producing vascular plants (i.e. lycophytes and monilophytes), and vascular, seed plants (i.e. gymnosperms, basal angiosperms, monocots, and eudicots) form a monophyletic group and share common ancestry with charophytic and chlorophytic green algae. Brown algae, red algae, glaucophytes, and chromista constitute sister groups to land plants and green algae. All these lineages are included in our analyses. The genomes of Amborella trichopoda, stout camphor tree (Cinnamomum kanehirae), and water lily (Nymphaea colorata), which form successive sister clades to all other flowering plants and A. thaliana as a representative of eudicots, are also included (Supplemental Tables S1 and S2).
Homology searches in the sequenced genomes identified homologs of XLGα, Gβ, and Gγ in all land plant lineages (Figure 1;Supplemental Tables S3–S6, Supplemental Dataset 1). The only exception was the absence of genes coding Gγ in Selaginella spp., which is surprising because these do show the presence of Gβ coding sequences, which form functional dimers with Gγ. Analysis of transcriptome data (Supplemental Tables S7–S13) expanded these results to a wide range of species. We would emphasize that the absence of a transcript does not necessarily suggest a missing gene. Therefore, we denote a gene as being absent only when it is missing in the sequenced genomes. The homologs of XLGα were found in all land plant transcriptomes except for one species each in bryophytes (Monoclea gottschei), lycophytes (Huperzia myrsinites) and gymnosperms (Ephedra sinica) (Figure 1;Supplemental Figures S1–S4). Similarly, except for a few bryophytes (Frullania sp. and Climacium dendroides), one gymnosperm (E. sinica) and a few monocots (Yucca filamentosa, Triglochin maritimum, and Sagittaria latifolia) all land plant transcriptomes show the presence of Gβ homologs (Figure 1;Supplemental Figures S1–S4).
Figure 1.
Presence/absence of G-protein components among plants and algae. Presence/absence of G-protein components among all plant and algal species with publicly available genomic resources, excluding eudicots. Squares in different colors mark the presence of Gα, XLG, RGS, Gβ, and Gγ proteins, respectively. Boxes were skipped wherever these proteins are absent. Species names with sequenced genomes are highlighted.
The evolutionary history of canonical Gα and RGS proteins in angiosperms is complex. We did not detect any canonical Gα homolog, which are the founding members of the G-protein complex, in the genomes of the Bryopsida, which includes P. patens, Ceratodon purpureus, and Syntrichia caninervis (Figure 1) and represents ∼95% of all mosses. In contrast, both sequenced genomes from the class Sphagnopsida (i.e. Sphagnum fallax and S. magellanicum) possess Gα homologs. Homology searches of transcriptome data corroborated these results and revealed a clear distinction between Bryopsida and its sister groups (Supplemental Figure S4). All Bryopsida transcriptomes lack a Gα homolog, whereas its sister groups Polytrichopsida, Tetraphidopsida, and Sphagnopsida have Gα sequences in their transcriptomes. The loss of canonical Gα in Bryopsida represents a major deviation from the established dogma of G-protein signaling in eukaryotes.
The presence of RGS homologs is sporadic throughout the land plant lineage, which corroborates our previous study using a subset of monocots (Hackenberg et al., 2017; Figure 1). Among mosses, the distribution of RGS is identical to that of Gα (Supplemental Figure S4), that is, not present in Bryopsida but present in its sister groups. Many liverworts, including the genome sequenced Marchantia polymorpha, lack an RGS homolog. All hornwort transcriptomes have an RGS homolog (Supplemental Figure S4) whereas monilophyte genomes (Azolla filiculoides, Salvinia cucullata, and Ceratopteris richardii), and most transcriptomes lack an RGS homolog (Supplemental Figure S2). RGS homologs are also missing in many gymnosperm transcriptomes (Supplemental Figure S2). These data suggest that RGS, a functional partner of Gα, is under relaxed selection across plant lineages and is frequently lost, as was seen within the monocots. We did not identify any instance where an RGS coding gene was present in the absence of a canonical Gα, confirming their functional link at the evolutionary level. These patterns suggest that a minimal set of the G-protein trimer comprising an XLGα, a Gβ, and a Gγ, is present in all land plants.
To ascertain that the absence of RGS in monocots and Gα and RGS in Bryopsida mosses is not because of errors in the genome annotation (i.e. false negative), we performed synteny block analysis and HMM-based searches (Supplemental Figures S5–S8, Supplemental Tables S14). We used the genome of Setaria viridis, which has an RGS homolog in its genome, as a reference and identified its syntenic regions with other closely related Poaceae genomes such as Brachypodium distachyon, Panicum hallii, and O. sativa (Supplemental Figures S5A, S6A, and S7A). These analyses revealed a localized loss of synteny around the putative RGS locus in all three genomes where we could not detect an RGS gene (Supplemental Figures S5B, S6B, and S7B). To further confirm these observations, these genomic regions were translated to all six reading frames and queried against the HMM profile of RGS domain. No significant hits were identified except in the S. viridis genome, which was used as a positive control (Supplemental Table S14). These results indicate that a complex recombination event has led to the loss of RGS in many monocots. Similarly, the genome of S. fallax, which encodes a Gα and an RGS homolog, was used as a reference to identify its syntenic regions with other Bryopsida genomes. However, syntenic blocks were not detected even between S. fallax and P. patens, the species with reasonably well assembled and annotated genomes (Supplemental Figure S8). This is not surprising, as these genomes have diverged approximately 280 million years ago (Mya; Shaw et al., 2010). To address this, the Gα and RGS HMM profiles were queried against the six frame-translated chromosome sequences of Bryopsida genomes. Only XLGα genomic loci were identified in Gα HMM searches, while no significant hit was found for RGS (Supplemental Table S14). As an additional control for Gα and to test the power of this analysis, we performed HMM profile searches with XLGα, which identified the previously characterized XLGα gene on chromosome 1 of P. patens. Moreover, this analysis also identified a second XLGα locus on chromosome 2 (Supplemental Table S14). This locus showed the conservation of G2–G5 motifs of the Gα domain; however, four stop codons were present in the reading frame (Supplemental Figure S9), suggesting that it is likely a pseudogene. Similarly, a second truncated XLGα locus was also identified in S. fallax genome (Supplemental Table S14). These results highlight the sensitivity of this method in identifying even the truncated genes. Therefore, we conclude that the loss of Gα and RGS homologs in specific plant lineages is not because of annotation errors or our inability to detect them and corroborate the gene presence and absence patterns identified through transcriptome analysis.
In contrast to land plants, G-protein components are sparsely distributed in algal lineages (Figure 1;Supplemental Figure S10 and S11). In brown algae, the sequenced genomes (Ectocarpus siliculosus and Undaria pinnatifida) and many transcriptomes show the presence of Gα and Gβ homologs; however, RGS was present in only one genome (E. siliculosus) and a few transcriptomes. Similarly, many transcriptomes from Chromista possess Gα and Gβ but no RGS homologs. In red algae, all genomes (Chondrus crispus, Galdieria sulphuraria, and Cyanidioschyzon merolae) and many transcriptomes lack G-protein components. G-protein components are also absent in glaucophytes. Among green algae, G-protein components are scant in Chlorophyta but prevalent in Charophyta (Supplemental Figure S11). Among Chlorophyta, one sequenced genome (Chloropicon primus) contains Gα-like sequences; although the gene is missing from the genomes of widely studied chlorophytes such as Chlorella variabilis, C. reinhardtii, and V. carteri. Profile HMM analysis showed that the previously reported C. reinhardtii Gα (Lee et al., 2017) is related to the monomeric G-protein gene family (Arf) and not to the heterotrimeric G-protein component (Supplemental Figure S12). In addition, a few transcriptomes have a potential Gβ, but not an RGS coding transcript.
In Charophyta, the genomes of K. nitens and C. braunii and other transcriptomes of Charophyceae, Klebsormidiophyceae, and Coleochaetophyceae have Gα, Gβ, and RGS homologs (Supplemental Figure S11). Among the class Zygnematophyceae, only the algae in the order Zygnematales retain Gα and Gβ coding sequences. No evidence for G-protein homologs is found in the transcriptomes of any other Zygnematophyceae algae, or in the genome of Penium margaritaceum. Among all algal species analyzed, XLGα homologs are found only in Charophyta. The genomes of K. nitens and the transcriptomes of Entransia fimbriata and Coleochaete spp. have XLGα homologs. Gγ homologs are found only in the genomes of C. braunii and K. nitens and absent in all other algae, although it could be due to the small size and the fast-diverging nature of the Gγ proteins (Trusov et al., 2008, 2012; Thung et al., 2012).
Lineage-specific gene duplications are rare in Gα but prevalent in XLGα
Homology searches identify only a single Gα homolog in most land plant lineages except hornworts. Phylogenetic tree construction shows that the hornwort Gα gene duplication occurred in the common ancestor of all hornwort species analyzed (Figure 2). A similar lineage-specific Gα duplication is also observed among brown algae. Other Gα duplications in species such as A. filiculoides, Psilotum nudum, Triticum spp., and Setaria spp., are relatively recent and species or genus-specific (Figure 2), as has also been reported for some recently duplicated eudicot genomes (Bisht et al., 2011; Roy Choudhury et al., 2019). The Gα sequences from other algae and diatoms form a sister clade to the green plant Gα sequences.
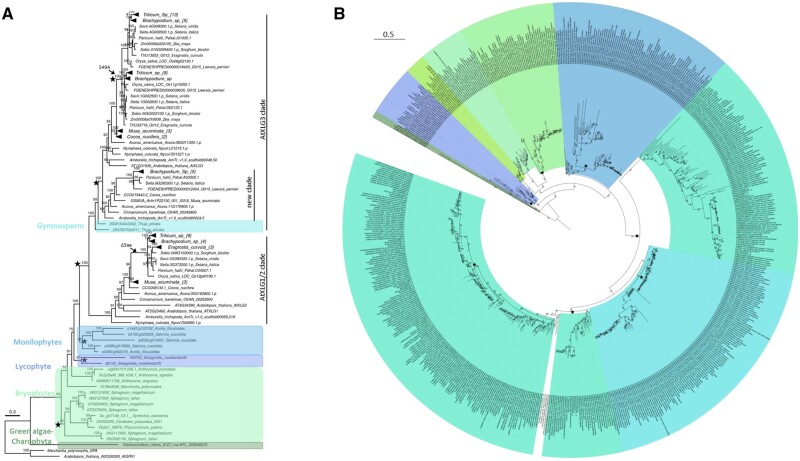
Figure 2.
Phylogenetic analysis of plant and algal Gα proteins. Maximum likelihood phylogenetic tree of Gα proteins from all available plant and algal sequences, excluding eudicots. A, Phylogenetic tree from publicly available genomes. Monomeric GTPase (ARF) sequence from Arabidopsis is used as outgroup. Many nodes are collapsed and labeled with triangles to improve readability. The total number of sequences in each collapsed node is mentioned in their respective tip label inside the curly bracket. Tree scale, 0.3 amino acid substitution per site. B, Phylogenetic tree constructed using the Gα sequences from publicly available genomes and transcriptomes. Human Gα sequences are used as outgroup. The branch length of jgi_Chloropicon_primus is plotted as 0.5 instead of the actual value 3.0526289 to improve the view of the tree. Tree scale 0.1 amino acid substitution per site. In both trees, each plant lineage is highlighted and labeled in different colors. Bootstrap support values >40% are shown at each node (1,000 bootstrap replicates). Star marks the gene duplication events inferred from the tree topology. Clades of Gα gene tree conflicting with the species tree are labeled in red.
Surprisingly, the only chlorophyte Gα sequences identified from the sequenced genome of C. primus do not follow the established species tree (Figure 2A). These sequences (A3770_8p51710; CpGα1 and A3770_2p14290; CpGα2) exhibit ∼20%–30% sequence similarity with A.thaliana G Protein Alpha subunit 1 (AtGPA1), but show overall conservation of the 3D structure. Homology modeling of CpGα1 and CpGα2 superimposed on AtGPA1 structure with root mean square deviation (RMSD) of 0.90 and 1.18 Å, respectively (Supplemental Figure S13), suggesting substantial overall similarity. However, due to their paucity among chlorophytes, the origin of CpGα needs to be experimentally validated to rule out contamination during collection of algal samples.
The XLGα phylogenetic tree shows several instances of lineage-specific gene duplication (Figure 3). In bryophytes, a lineage-specific duplication was found in mosses that suggests the loss of an XLGα homolog in Bryopsida model organisms such as P. patens and C. purpureus (Figure 3A) that have only a single homolog. The tree also suggests one lineage-specific gene duplication each in lycophytes and monilophytes. The tree also confirmed that the two XLGα homologs of Selaginella moellendorffii (lycophyte) and A. filiculoides (monilophyte) are the result of respective lineage-specific gene duplications (Figure 3B). An additional duplication is found prior to the emergence of seed plants (Figure 3). Importantly, this led to two distinct clades: XLGα1/2 (AtXLGα1/2) and XLGα3 (AtXLGα3), as identified in the model plant A. thaliana where these genes were first discovered (Lee and Assmann, 1999; Ding et al., 2008). Intriguingly, the two XLGα homologs of gymnosperm Thuja plicata both belong to the AtXLGα3 clade, suggesting the loss of an AtXLGα1/2 clade homolog in this lineage. The tree also supports the notion that the duplication to form the AtXLGα1 and 2 genes occurred after the divergence of eudicots because similar lineage-specific gene duplication is not observed in the monocot AtXLGα1/2 clade (Figure 3A). In contrast, two lineage-specific gene duplications exist within the AtXLGα3 clade. The first gene duplication appears to have occurred in the common ancestor of all angiosperms that led to the formation of a clade comprising the AtXLGα3, and a new clade whose members are lost in A. thaliana. The former clade underwent a second gene duplication in the common ancestor of all grasses (Poaceae). The tree confirms that the XLGα sequence identified from the K. nitens genome is sister to all plant XLGα. All these gene duplications interpreted from the genome sequenced species (Figure 3A) are also supported by the XLGα phylogenetic tree comprising sequences from genomes and transcriptomes (Figure 3B). This analysis of the two Gα domain-containing proteins in plants highlights that the lineage-specific gene duplications are rare in the canonical Gα but prevalent in XLGα evolutionary history.
Figure 3.
Phylogenetic analysis of plant and algal XLGα proteins. Maximum likelihood tree of XLGα proteins from all plant and algal species, excluding eudicots. A, Tree of XLGα sequences from publicly available genomes. Plant Gα sequences are used as outgroup. Many nodes are collapsed and labeled with triangles to improve readability. The total number of sequences in each collapsed node is mentioned in their respective tip label inside the curly bracket. Tree scale 0.3 amino acid substitution per site. B, Tree of XLGα sequences from publicly available genomes and transcriptomes. Tree scale 0.5 amino acid substitution per site. In both trees, each plant lineage is highlighted and labeled in different colors. Bootstrap support values above 40% are shown at each node (1,000 bootstrap replicates). Star marks the gene duplication events inferred from the tree topology.
Similar to canonical Gα, its regulatory protein RGS also did not expand in the plant lineages (Supplemental Figure S14). The RGS gene is absent in all Bryopsida and many liverworts, monilophytes, and monocot species analyzed. No lineage or species-specific gene duplications are found in the RGS phylogenetic tree. In algae, the charophyte RGS forms the sister clade to the land plant RGS proteins. Surprisingly, the E. siliculosus (brown algae) genome has an unusually high number of RGS proteins (eight sequences) that form the expected sister clade to charophyte RGS (Supplemental Figure S14A). Some of the E. siliculosus RGS proteins lack the seven-transmembrane domain, which is present in all plant RGS.
Gα and XLGα share highly conserved sequence features
Multiple sequence alignment (MSA) of Gα proteins revealed that the N-terminal myristoylation site of AtGPA1 (glycine at position 2) is conserved in all land plants and charophyte algae (Figure 4A). The N-terminal palmitoylation site of AtGPA1 (cysteine at position 5) is conserved among liverworts, hornworts, and all other vascular plants. In moss (Sphagnum spp.) and algae sequences, the cysteine at position 3 is expected to be palmitoylated, as in humans (Figure 4A). Both these modifications anchor the Gα protein in the plasma membrane. The XLGα proteins lack these features but contain nuclear localization signal at their N-terminal and a cysteine-rich motif of unknown function (Figure 4B). The P-loop phosphorylation site in the G1 motif is conserved in all plant and charophyte algae Gα and XLGα except in a few homologs of Setaria spp. and Triticum spp., which also possess additional homologs with the conserved G1 motif. The proteins that form one of the grass XLGα3 subclades have a serine to alanine substitution at this position (Figures 3, A and 4, B). This may have functional consequences as this residue has been identified as a key phosphosite with critical regulatory roles (Chakravorty and Assmann, 2018; Kalogriopoulos et al., 2020; Roy Choudhury and Pandey, 2022). Additionally, grass protein sequences from AtXLGα1/2 clade have a deletion of 3 amino acids (aa) inside the G1 motif (Figures 3, A and 4, B). A threonine in the G2 motif (the T sensor) is conserved in all canonical Gα but not in XLGα proteins. Similarly, the glutamine corresponding to position 222 in AtGPA1, which is required for GTP hydrolysis, is conserved in all canonical Gα (and monomeric Arf sequences) but is mostly replaced by either arginine or lysine in XLGα proteins (Figure 4). Moreover, the highly conserved arginine finger motif of canonical Gα that facilitates the GTPase activity is also replaced by either a glycine or glutamic acid in all XLGα sequences (Figure 4).
Figure 4.
Important motifs traced on to the intron–exon structure of Gα and XLGα genes. Phylogenetic relationship of Gα domain-containing proteins, Gα (A) and XLGα (B), from model plant species representing major plant lineages (right). Schematics of the intron–exon structure are shown along with the G1–G5 motifs and other sequence features (middle). Exon and introns are drawn as boxes and lines, respectively, with sizes (base pairs) mentioned above and below each. Conserved introns between Gα and XLGα are labeled with asterisks and blue rectangles. Important polymorphic regions of the Gα domain-containing proteins are highlighted on the MSA (left), with residues corresponding to AtGPA1 glycine (G) 2 (myristoylation site) and cysteine (C) 5 (palmitoylation site) are highlighted. Serine (S) 49 and the T sensor of AtGPA1 which are regulated by phosphorylation are highlighted. The arginine finger (R) is also highlighted. The glutamine residue at position 222 (Q222) of AtGPA1 is labeled. C, Transcriptional activity of intron-less Gα genes from A. agrestis (hornwort) at different growth stages, from Li et al. (2020).
The intron–exon organization and sizes of the exons of canonical Gα genes are highly conserved from charophyte algae to land plants (Figure 4A). The notable exceptions are intron-less single Gα found in C. braunii and both Gα paralogs from all three sequenced hornworts (Anthoceros spp.). Available transcriptomes confirmed the expression of these Gα genes from C. braunii (Hackenberg et al., 2013) and hornworts (Figure 4C). The intron–exon organization and size of exons are also conserved among XLGα genes (Figure 4B). Since these are hypothesized to have arisen from the conventional Gα genes by gene fusion (Urano et al., 2016), we looked for conserved introns between them. Most of the introns of XLGα differ from those in the canonical Gα genes, except one conserved position (Figure 4, A and B) downstream of the G1 motif. Additionally, the intron upstream of the G2 motif is conserved between charophyte XLGα and all charophyte and plant Gα genes. These observations support the possibility of XLGα origin by gene fusion between a canonical Gα and a plant-specific protein but rules out transcript fusion mechanisms.
The evolutionary histories of the obligate dimers Gβ and Gγ are distinct
Analyzing the evolutionary histories of Gβ and Gγ is particularly challenging due to the presence of WD-40 repeats and small protein size, respectively. All known Gβ sequences, including those from metazoans have WD-40 repeats. WD-40 repeats usually assume a 7–8 bladed β-propeller structure, but proteins with 4–16 repeated units also exist and are prevalent in all eukaryotes. The Gβ sequences identified from transcriptomes were mostly truncated, lacking the nonrepeat sequences that is, N-terminal sequence. This scenario complicates the faithful alignment of the repeats according to their position. Therefore, we considered only complete sequences identified from the genomes for phylogenetic analysis of Gβ proteins. Gβ from charophyte algae forms the sister group for all the land plant Gβ (Figure 5A). The S. moellendorffii sequence did not fall in the expected position in the tree, which could be due to the small sample size among lycophytes. Bryophytes such as Sphagnum spp. and P. patens have species- and genus-specific gene duplications of Gβ; however, it is not clear whether the gene duplication observed in Anthoceros spp. is lineage-specific or genus-specific due to the lack of genome sequences for other hornworts (Figure 5A). No lineage-specific Gβ gene duplication is observed in the vascular plants, but several species- and genus-specific duplications can be identified throughout the Gβ phylogenetic tree. We also identify a few Gβ sequences with repeats more or less than the conventional seven WD-40 repeats (Figure 5A). These sequences formed a separate clade, likely due to challenges in repeat sequence alignment.
Figure 5.
Phylogenetic analysis of plant and algal Gβ and Gγ proteins. Maximum likelihood phylogenetic tree of (A) Gβ and (B) Gγ proteins from all plant and algal species. Human Gβ and Gγ sequences are used as outgroups. Many nodes are collapsed and labeled with triangles to improve readability. Total number of sequences in each collapsed node is mentioned in their respective tip label inside the curly bracket. In both trees, each plant lineage is highlighted and labeled in different colors. Bootstrap support values >40% are displayed at each node (1,000 bootstrap replicates). Star marks the gene duplication events inferred from the tree topology. Tree scales 0.1 and 0.3 amino acid substitution per site for Gβ and Gγ phylogenetic trees, respectively.
Canonical Gγ proteins are small (ca 100–120 aa) and exhibit substantial sequence variations (Trusov et al., 2012). The proteins are defined by the presence of the G gamma-like domain (GGL domain, ca 100 aa), which was used to construct the phylogenetic tree. We found several lineage-specific Gγ duplications (Figure 5B). The phylogenetic tree suggests an early gene duplication event in the common ancestor of land plants, which led to the formation of a clade containing archetypal and prenylation-less Gγ proteins and another clade containing cysteine-rich Gγ proteins (Figure 5B). However, the lack of complete Gγ sequence from lycophytes and the position of M. polymorpha Gγ with respect to other bryophyte sequences does not permit a straightforward conclusion. Hence, we propose that this gene duplication could have occurred anywhere between the common ancestor of land plants to the ancestor of monilophytes and seed plants (Figure 5B). Our analysis also reveals that the nonseed plants lack the cysteine-rich Gγ proteins. Consequently, all nonseed plant Gγ, except M. polymorpha, form sister clades to the archetypal and prenylation-less Gγ clade. Clades containing the archetypal and the prenylation-less Gγ proteins have diverged before the emergence of seed plants. However, T. plicata Gγ (Thupl_29380971s0015) from the prenylation-less clade has the sequence signature of the CaaX prenylation motif. In the cysteine-rich Gγ clade, we detect at least one gene duplication event specific for grasses (Figure 5B). In charophytes, the Gγ is coded by a single locus, and these form the expected sister clade to all land plant Gγ sequences. These results show that the Gβ and Gγ have undergone distinct evolutionary processes, despite being obligate dimers.
Discussion
We analyzed the phyletic patterns in the distribution of G-protein components across plant and algal lineages and outlined much of these components’ evolutionary histories such as origin, gene duplication and loss events (Figure 6). Our focus on relatively less-explored, nonflowering plant and algal lineages provides insights into the evolution of plant G-protein components and evaluates the applicability of the Arabidopsis and/or Angiosperm-centric hypotheses to the rest of the plant kingdom. In addition to corroborating some of the conclusions derived from previous studies based on analysis of a limited number of model plant genomes, we also expand their phylogenetic scope by including data from the 1KP transcriptomes (Matasci et al., 2014; Carpenter et al., 2019) and performing synteny-based and HMM profile-based searches.
Figure 6.
Schematics summarizing duplication and loss of G-protein signaling components in plants. Land plant lineages are listed on the left side of the figure. Bryophytes are further divided into mosses, liverworts and hornworts. The origin of each G-protein component is indicated at the bottom of the figure by circles. The origin of XLGα from canonical Gα by gene fusion is depicted at the bottom with a dashed line. Arabidopsis G-protein component gene names are given at the eudicot branch. A solid line with a terminal filled circle indicates the presence of the respective G-protein component in all species within the indicated lineage. A dotted line with a terminal circle indicates the presence of the respective component in only a subset of species within the indicated lineage. A dotted line with a terminal open circle indicates the absence of the respective component in all species within the indicated lineage. The triangle and the asterisk mark the emergence of the Cysteine-rich (C-rich) domain and loss of CAAX motif of Gγ proteins, respectively. The shaded box on Gγ indicates a region of low confidence in the branching pattern. The question marks denote a region of further uncertainty due to a lack of complete Gγ sequence availability. The three clades of seed-plant Gγ are labeled as C-rich, Archetypal, and Prenylation-less Gγ.
Our results illustrate several unique aspects of plant G-protein evolution, and evolutionary oddities, which challenge many previous interpretations. For example, we confirm that heterotrimeric G-protein signaling can be dispensable in some eukaryotic lineages, especially algae (Figure 1). This is not the case with any of the Opisthokonts (which include fungi and animals) and other basal clades such as Amoebozoa, which can possess highly expanded families of G-protein components (Shpakov and Pertseva, 2008; Anantharaman et al., 2011). The loss of G-protein subunits in rhodophyta, glaucophyta, and chlorophyta suggests a possible loss in the common ancestor of this group.
Compared to the loss of the entire G-protein complex, which is limited to specific algal lineages, the loss of individual components is observed all across major groups except in the eudicots (Figure 1; Supplemental Dataset 1). The gene most commonly lost is the one coding for the RGS protein; even though when present, it shows inter- or intra-species interactions with the Gα proteins (Hackenberg et al., 2017). Based on biochemical studies of Gα protein from a few model species, it has been proposed that RGS-mediated deactivation of the G-protein cycle is the critical regulatory step of plant G-protein signaling (Urano et al., 2012a, 2012b, 2013; Bradford et al., 2013; Phan et al., 2013). But, the absence of RGS in many species without any obvious effect on plant fitness argues against such a scenario and purpose. It is possible that additional proteins biochemically compensate for the loss of RGS function. Alternatively, deactivation of classical G-protein cycle may not be as crucial in planta as it appears based on in vitro biochemical experiments. Regardless, this study also shows that the presence of RGS is linked with the presence of a canonical Gα. There were no examples where an RGS gene is present without a Gα, although the reciprocal situation is widespread (Figure 1; Supplemental Dataset 1). The absence of Gα and RGS in P. patens (Hackenberg et al., 2016) and their presence in S. fallax also highlights the importance of including a wide range of species to understand the prevalence and phyletic patterns of specific proteins. Our results draw a clear distinction between the mosses with and without the Gα and RGS proteins. In this instant, the non-Bryopsida mosses (with Gα and RGS) are a promising organismal system to predict the effect of the loss of canonical Gα/RGS module and potential role of XLGα proteins in this context.
We further validated these results in monocots where a considerable number of species with high-quality genome assembly are available. Within grasses, we identified syntenic regions of S. viridis (PACMAD clade) with P. hallii (PACMAD clade), B. distachyon (BOP clade), and O. sativa (BOP clade). A similar analysis between S. fallax and P. patens failed to identify any syntenic blocks (Supplemental Figures S5–S8), which is possibly due to the evolutionary time scale between when these clades diverged. The BOP-PACMAD clades split 70–80 Mya (Christin et al., 2014), while Sphagnoposida and Bryopsida mosses have diverged ∼280 Mya (Shaw et al., 2010). The second approach based on HMM profiles tolerated accumulation of stop codons and identified a lost XLGα paralog in the lower arm of chromosome 2 in P. patens genome (Supplemental Figure S9). Since the chromosomal positions of PpXLGα (Chromosome 1 upper arm) and the newly identified XLGα pseudogene (chromosome 2 lower arm) are syntenic and derived from duplication of an ancestral chromosome during the WGD event, ∼38–50 Mya (Lang et al., 2018), we speculate that the pseudogene represents a duplicated XLGα, which has undergone purifying selection.
The phyletic pattern of Gα suggests that the expansion of Gα in plants is relatively limited, which is starkly different from the metazoan lineages (Anantharaman et al., 2011). The phylogenetic analysis also identifies a few previously unrecognized lineage-specific gene duplication events of Gα among nonflowering plants (Figures 1 and 2). Homologs identified from the 1KP transcriptomes are useful to distinguish whether the homologs in species with whole-genome sequences are a result of lineage- (e.g. Anthoceros spp. Gα) or species-specific gene duplications (e.g. A. filiculoides Gα and several other). On the other hand, XLGα, whose earliest known origin is in the common ancestor of Charophytes, has expanded and diversified (Figure 6). Furthermore, XLGα and Gβ are present consistently after their appearance, with no evidence of loss in land plants. This supports a theory that XLGα-Gβ-Gγ constitutes the minimal set of G-protein trimer in all land plants (Figure 1).
Our sequence analysis shows that the arginine finger motif, which distinguishes canonical Gα from the monomeric GTPases, is absent in XLGα (Figure 4, A and B). As a consequence, XLGα are expected to lack the inherent GAP function and thus will have substantially compromised GTPase activity. Reduced GTPase activity has been experimentally demonstrated for AtXLG2 (Heo et al., 2012; Lou et al., 2020; Maruta et al., 2021). The role of RGS proteins in regulating XLGα function is equivocal (Liang et al., 2016, 2017). Based on the phyletic patterns of XLGα and Gα, as well as the sporadic presence of RGS, we speculate that XLGα is the primary member of the G-protein heterotrimer in plants, along with Gβγ. Our speculation is supported by functional studies in P. patens, where both XLGα and Gβ are required for life cycle completion (Hackenberg et al., 2016). Likewise, in many monocots loss of either Gβ or XLGα results in seedling lethality but the loss of Gα has relatively minor effects (Utsunomiya et al., 2012; Wu et al., 2018, 2019; Bhatnagar and Pandey, 2020). In eudicots too, XLGα (and Gβ) play critical roles, but are nonessential for plant survival (Roy Choudhury et al., 2020). The functional diversification of these proteins in eudicots versus monocots needs further analysis.
The origin of XLGα has not been clearly established. It has been hypothesized that XLGα originated by gene fusion of canonical Gα with a plant-specific gene (Urano et al., 2016). Our analysis supports that XLGα originated either in or before the common ancestors of Klebsormidophyceae and land plants (Figures 1 and 6;Supplemental Figures S10 and S11). Conservation of the exon–intron organization between the Gα and XLGα (Figure 4, A and B), argues against the possibility of XLGα origin by transcript fusion and points toward gene fusion through other mechanisms.
Analysis of exon–intron organization also revealed the lack of any introns in the Gα homologs from C. braunii and hornworts (Figure 4A). Some primate Gα lack introns and is reported to be either retrogenes (G Protein subunit Alpha Q Pseudogene 1; GNAQP1) or derived from an ancestral retrogene (precursor G Protein Alpha subunit 12; preGNA12; Lokits et al., 2018). Among them, preGNA12 is conserved and functional. Our results highlight a parallel to preGNA12 in plants—and possibly the retro-origin of Gα homologs in C. braunii and hornworts. The protein sequences of intron-less Gα homologs of hornworts and C. braunii are similar to those of all other Gα and are transcriptionally active (Figure 4).
The Gγ phylogeny beyond seed plants has not been explored previously (Trusov et al., 2012). Our analysis shows that the archetypal and prenylation-less Gγ diverged in the common ancestor of seed plants (Figures 5, B and 6). In addition, the cysteine-rich Gγ has diverged from the other two somewhere between the emergence of land plants and vascular plants (Figures 5, B and 6). This ambiguity is due to the limited availability of full-length Gγ sequences from bryophytes and lycophytes and the small protein size of Gγ. The Gγ sequences identified from 1KP transcriptomes were truncated and too small to be useful for phylogenetic analysis. We expect that similar to the Gβ genes, the genes for Gγ proteins are also present widely. Overall, our analysis confirms this, with one exception. In the genomes and transcriptomes of Selaginella spp., we did not detect any Gγ homologs (Supplemental Figure S3). We speculate that our failure to detect the Gγ proteins is because of their small in size, overall poor sequence conservation, and rapidly diverging nature. However, as we could detect these proteins in other species using similar criteria, it cannot be ruled out that Gγ homologs are indeed lost in Selaginella spp. If this turns out to be the case, it will represent another deviation from the established norm of G-protein signaling. Regardless, the phyletic patterns of Gγ distribution and the phylogenetic analysis of Gβ and Gγ homologs can be improved with the availability of more genomic sequences and better quality transcriptome assemblies in future.
Our study offers the most comprehensive evolutionary framework of plant heterotrimeric G-protein components, to date, which will help initiate interesting questions for future research. A comparative analysis of the G-protein signaling of P. patens and Sphagnum spp., representing the minimal and complete set of G-protein components, respectively, would reveal the rewiring of the G-protein signaling network in the absence of Gα and RGS. In the case of XLGα, an in-depth analysis of how its structure and function are affected due to the loss of the arginine finger would help predict early evolutionary constraints faced by the XLGα precursor. The apparent absence of Gγ in the Selaginella genome supports the importance of searching for highly divergent Gγ homologs. Alternatively, Selaginella sp. can be used to understand the evolutionary consequences of the loss of Gγ on the function of its obligate dimer Gβ or the localization of the G-protein complex to the plasma membrane. Functional characterization of hornwort Gα homologs will potentially determine the contribution of retrotransposition events in shaping the evolution of this highly conserved signaling pathway.
Materials and methods
Sequence sources and analysis
All currently available land plant lineages (except eudicots) and all algal lineages (Charophyta and Chlorophyta, Rhodophyta, Glaucophyta, Phaeophyta, and Chromista) are included in this analysis. We include A. trichopoda, stout Camphor tree (C. kanehirae), and water lily (N. colorata) in this analysis because they are sister to all angiosperms. We do not include eudicots (except Arabidopsis [A. thaliana] used as a placeholder) because all species with well-annotated genomic sequence information have genes coding each of the G-protein components (Supplemental Dataset 1; Hackenberg et al., 2017). Phylogeny of G-protein components in eudicots has been explored well (Trusov et al., 2012; Urano et al., 2012a, 2012b, 2016; Kumar and Bisht, 2018). Publicly available plant and algal genome sequence data were used as primary data. Only a few genome sequences were available to represent the highly diverse nonvascular plants and charophycean algal species. Therefore, in addition to species with whole-genome sequences, we also analyzed transcriptome data from the 1KP database (Matasci et al., 2014; Carpenter et al., 2019) to cover a broad taxonomic range and to assess the generalizability of the presence or absence data inferred from genome sequences. A list of all the genomes and transcriptomes analyzed is presented in the Supplemental Tables S1 and S2.
Homology searches to identify the G-protein components
Protein models of all available plant and algal genome sequences were downloaded from Phytozome (www.phytozome-next.jgi.doe.gov), PhycoCosm (www.phycocosm.jgi.doe.gov), FernBase (www.fernbase.org), and EnsemblPlants (www.plants.ensembl.org; Supplemental Table S1; Supplemental Dataset 1). For the rest of the species, protein models predicted from their respective transcriptomes were downloaded from the 1KP database (Supplemental Table S2). Local protein blast databases were built from these protein models using the BLASTP 2.9.0+ toolkit and queried against Arabidopsis G-protein components: GPA1 (Gα); AT2G26300, XLGα3; AT1G31930, RGS1; AT3G26090, AGB1 (Gβ); AT4G34460 and AGG1 (Gγ); AT3G63420 (Camacho et al., 2009). BLAST hits from genome sequenced species with scores ˂150, 200, 100, 100, and 25 for Gα, XLGα, RGS, Gβ, and Gγ, respectively, were retrieved (Supplemental Tables S3–S13). These relaxed scores, which are sufficient to identify highly divergent homologs from human and E. siliculosus, were chosen to avoid any false-negative blast hits. To remove sequences from closely related protein families, preliminary phylogenetic trees were constructed.
To identify the G-protein components from 1KP transcriptomes, we built custom HMM profiles for each G-protein component from their verified plant homologs using hmmbuild from HMMER version 3.3 (http://hmmer.org/; Supplemental Datasets 2–6). The pfam website was used for generating HMM profiles, which were queried against all protein models using hmmsearch from HMMER version 3.3 (http://hmmer.org/). The protein sequences with scores >200, 250, 100, 300, and 50 for Gα, XLGα, RGS, Gβ, and Gγ, respectively, were retrieved. These scores were chosen to avoid any false negative HMM search hits, which are relaxed enough to identify highly divergent homologs from human and E. siliculosus. Since both Gα and XLGα have the Gα domain, their HMM search results often overlapped, but their scores differed. In such cases, these sequences were grouped based on their higher score among the two. These protein sequences were verified using a reciprocal BLAST against Arabidopsis protein models. A few transcriptomes from the 1KP database had transcript contamination from nonplants. Hence, a phylogenetic tree was constructed using sequences from both genome and transcriptome sources as described in the next section. A dendrogram containing all species analyzed in this study was constructed based a published phylogenetic tree (Gitzendanner et al., 2018). The dendrogram which shows the presence or absence of G-protein components, was plotted using the R packages ggtree, ggplot2, and treeio (Wickham, 2016; Wang et al., 2020; Yu, 2020).
To avoid any false negatives in genome annotation, HMM searches were also performed on genomic sequences of a few monocots and all the moss genomes available. Initially, the genome assembly of each species was downloaded from Phytozome version 13. The genome assembly was translated in six reading frames (three forward and three reverse) using the transeq tool (Rice et al., 2000). The Gα, RGS, and XLGα HMM profiles were queried against the translated reading frames using hmm search from HMMER version 3.3 (http://hmmer.org/). The forward filter of HMM search was set to a relaxed value of 10.
Synteny analysis was performed among monocots (Poaceae) and mosses, separately, using QUOTA-ALIGN algorithm (Tang et al., 2011). For monocots, S. viridis (version 2.1) sequences were subjected to blast search against the sequences of B. distachyon (version 3.2), P. hallii (version 3.2), and O. sativa (Kitaake_v3.1) using the BLASTP version 2.9.0+ toolkit (Camacho et al., 2009). Similarly, S. fallax (version 1.1) protein sequences were searched against P. patens (version 3.3) sequences. The BLAST results were processed and syntenic blocks were inferred. The syntenic blocks were plotted in dot plot using the Python package Matplotlib version 3.5.1 (Hunter, 2007).
Phylogenetic tree construction
All Gα, XLGα, and RGS homologs identified were analyzed individually. Protein sequences were aligned using MAFFT version 7.271 (Katoh and Standley, 2013). Since many sequences from transcriptomes were truncated, we chose a sequence range based on conserved domains and number of sequences spanning that range (Supplemental Table S15). Sequences that do not span this range are not included in the analysis. Amino acids flanking this range were trimmed using MEGA X software (Kumar et al., 2018). In the remaining MSA, columns with gaps greater than a specific threshold were removed using the program trimAL (Capella-Gutiérrez et al., 2009; Supplemental Table S15). We used RaxML to prepare an initial tree and removed duplicate sequences originating from the same species transcriptome (Stamatakis, 2014). The ModelFinder algorithm from IQ-TREE was used to find the best-fit model for each phylogenetic tree (Kalyaanamoorthy et al., 2017). Maximum-likelihood phylogenetic trees were computed with 1,000 bootstrap replicates using IQ-TREE (Nguyen et al., 2015). Phylogenetic trees were plotted and annotated using iTOL software (Letunic and Bork, 2019). All the parameters and thresholds used in phylogenetic tree construction are summarized in Supplemental Table S15.
Intron–exon structure mapping
Intron–exon structure data for Gα and XLGα genes from representative species were gathered from the gene models available in Phytozome (www.phytozome-next.jgi.doe.gov), PhycoCosm (www.phycocosm.jgi.doe.gov), and FernBase (www.fernbase.org). Publicly available RNAseq data for Anthoceros agrestis was used to confirm transcriptional activity of the hornwort Gα genes (PRJEB34743, 2020). Actin (Sc2ySwM_228_4670) was used as a reference gene to infer the expression pattern of retrogenes.
3D structure analysis
The 3D structure of the chlorophyte Gα sequences Chloropicon_primus_A3770_08p51710 (CpGα1) and Chloropicon_primus_A3770_02p14290 (CpGα2) were generated by homology modeling using the Arabidopsis Gα 3D structure model 2XTZ.pdb as the template structure. CpGα1 (1–359 aa) and CpGα2 show 21.9 and 30.2% sequence identity with 2XTZ.pdb, respectively. Homology modeling was performed using Modeller version 9.24 (Webb and Sali, 2016). The model with the lowest discrete optimized protein energy value was chosen for further validation. Constructed structures were validated by the inspection of phi/psi distributions of Ramachandran plot obtained through PROCHECK (Laskowski et al., 1993), and the significance of consistency between template and models was evaluated using ProSA server. In addition, the RMSD was analyzed by Chimera (match-maker) (Pettersen et al., 2004) on superimposition of template (2XTZ.pdb) with predicted structures to check the reliability of models.
Accession numbers
Sequence data from this article can be found in the GenBank/EMBL/Phytozome/1KP data libraries under the accession numbers listed in Supplemental Tables S3–S14.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. Presence/absence of G-protein components among monocots.
Supplemental Figure S2. Presence/absence of G-protein components among gymnosperms and monilophytes.
Supplemental Figure S3. Presence/absence of G-protein components among lycophytes.
Supplemental Figure S4. Presence/absence of G-protein components among bryophytes.
Supplemental Figure S5. Synteny between S. viridis and B. distachyon genomes.
Supplemental Figure S6. Synteny between S. viridis and Panicum halli genomes.
Supplemental Figure S7. Synteny between S. viridis and O. sativa genomes.
Supplemental Figure S8. Synteny between S. fallax and Physcomitrium patens genomes.
Supplemental Figure S9. The additional truncated PpXLGα locus identified by HMM searches on translated chromosomal sequences.
Supplemental Figure S10. Presence/absence of G-protein components among brown algae, chromista, red algae and glaucophytes.
Supplemental Figure S11. Presence/absence of G-protein components among green algae.
Supplemental Figure S12. Previously reported CGA1 (XP_001691481) does not qualify as Gα.
Supplemental Figure S13. Sequence and 3D structure similarities between Gα homologs identified from C. primus genome and AtGPA1.
Supplemental Figure S14. Phylogenetic analysis of plant and algal RGS proteins.
Supplemental Table S1. List of all genome sequences used in this study.
Supplemental Table S2. List of transcriptomes from 1KP (Matasci et al., 2014) used in this study.
Supplemental Table S3. Gα-XLGα BLAST results for genomes.
Supplemental Table S4. Gβ BLAST results for genomes.
Supplemental Table S5. Gγ BLAST results for genomes.
Supplemental Table S6. RGS BLAST results for genomes
Supplemental Table S7. Gα-XLGα BLAST results for transcriptomes.
Supplemental Table S8. Gα-XLGα HMM search results for transcriptomes.
Supplemental Table S9. Gβ BLAST search results for transcriptomes.
Supplemental Table S10. Gβ HMM search results for transcriptomes.
Supplemental Table S11. Gγ HMM search results for transcriptomes.
Supplemental Table S12. RGS BLAST search results for transcriptomes.
Supplemental Table S13. RGS HMM search results for transcriptomes.
Supplemental Table S14 . HMM search results for translated chromosome sequences.
Supplemental Table S15. Parameters and thresholds used in phylogenetic tree construction.
Supplemental Dataset 1. List of eudicot G-protein components using BLAST analysis.
Supplemental Dataset 2. HMM profile of Gα.
Supplemental Dataset 3. HMM profile of XLGα.
Supplemental Dataset 4. HMM profile of RGS.
Supplemental Dataset 5. HMM profile of Gβ.
Supplemental Dataset 6. HMM profile of Gγ.
Supplementary Material
Acknowledgments
The authors sincerely thank Dr Elizabeth (Toby) Kellogg (DDPSC), and Prof Kenneth Olsen (Washington University in St Louis) for helpful discussion and critical comments on the manuscript.
Funding
This research in Pandey lab is supported by National Science Foundation (IOS-1557942, MCB-1714693, and UroL-1921724) grants.
Data availability statement
The data that supports the findings of this study are available in the supplementary material of this article.
Conflict of interest statement. None declared.
Contributor Information
Boominathan Mohanasundaram, Donald Danforth Plant Science Center, St Louis, Missouri 63132, USA.
Audrey Dodds, Donald Danforth Plant Science Center, St Louis, Missouri 63132, USA.
Vandna Kukshal, Department of Biology, Washington University, St Louis, Missouri 63130, USA.
Joseph M Jez, Department of Biology, Washington University, St Louis, Missouri 63130, USA.
Sona Pandey, Donald Danforth Plant Science Center, St Louis, Missouri 63132, USA.
B.M. and S.P. conceived the project. B.M. and A.D. retrieved all sequence information, performed all analysis, and phylogenetic tree construction. V.K. and J.M.Z. performed 3D modeling of various Gα sequences. B.M., A.D., and S.P. wrote the manuscript with input from V.K. and J.M.Z.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/general-instructions) is: Sona Pandey (spandey@danforthcenter.org).
References
- Anantharaman V, Abhiman S, de Souza RF, Aravind L (2011) Comparative genomics uncovers novel structural and functional features of the heterotrimeric GTPase signaling system. Gene 475: 63–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranda-Sicilia MN, Trusov Y, Maruta N, Chakravorty D, Zhang Y, Botella JR (2015) Heterotrimeric G proteins interact with defense-related receptor-like kinases in Arabidopsis. J Plant Physiol 188: 44–48 [DOI] [PubMed] [Google Scholar]
- Bhatnagar N, Pandey S (2020) Heterotrimeric G-Protein interactions are conserved despite regulatory element loss in some plants. Plant Physiol 184: 1941–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisht NC, Jez JM, Pandey S (2011) An elaborate heterotrimeric G-protein family from soybean expands the diversity of plant G-protein networks. New Phytol 190: 35–48 [DOI] [PubMed] [Google Scholar]
- Botella JR (2012) Can heterotrimeric G proteins help to feed the world? Trends Plant Sci 17: 563–568 [DOI] [PubMed] [Google Scholar]
- Bradford W, Buckholz A, Morton J, Price C, Jones AM, Urano D (2013) Eukaryotic G protein signaling evolved to require G protein-coupled receptors for activation. Sci Signal 6: ra37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne EF, Luchetti G, Rohatgi R, Siebold C (2018) Multiple ligand binding sites regulate the Hedgehog signal transducer Smoothened in vertebrates. Curr Opin Cell Biol 51: 81–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera-Vera TM, Vanhauwe J, Thomas TO, Medkova M, Preininger A, Mazzoni MR, Hamm HE (2003) Insights into G protein structure, function, and regulation. Endocr Rev 24: 765–781 [DOI] [PubMed] [Google Scholar]
- Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL (2009) BLAST+: architecture and applications. BMC Bioinformatics 10: 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capella-Gutiérrez S, Silla-Martínez JM, Gabaldón T (2009) trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25: 1972–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter EJ, Matasci N, Ayyampalayam S, Wu S, Sun J, Yu J, Jimenez Vieira FR, Bowler C, Dorrell RG, Gitzendanner MA, et al. (2019) Access to RNA-sequencing data from 1,173 plant species: The 1000 Plant transcriptomes initiative (1KP). Gigascience 8: giz126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravorty D, Assmann SM (2018) G protein subunit phosphorylation as a regulatory mechanism in heterotrimeric G protein signaling in mammals, yeast, and plants. Biochem J 475: 3331–3357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravorty D, Gookin TE, Milner M, Yu Y, Assmann SM (2015) Extra-Large G proteins (XLGs) expand the repertoire of subunits in Arabidopsis heterotrimeric G protein signaling. Plant Physiol 169: 512–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravorty D, Trusov Y, Zhang W, Acharya BR, Sheahan MB, McCurdy DW, Assmann SM, Botella JR (2011) An atypical heterotrimeric G-protein g-subunit is involved in guard cell K+-channel regulation and morphological development in Arabidopsis thaliana. Plant J 67: 840–851 [DOI] [PubMed] [Google Scholar]
- Christin PA, Spriggs E, Osborne CP, Stromberg CA, Salamin N, Edwards EJ (2014) Molecular dating, evolutionary rates, and the age of the grasses. Syst Biol 63: 153–165 [DOI] [PubMed] [Google Scholar]
- De Mendoza A, Sebé-Pedrós A, Ruiz-Trillo I (2014) The evolution of the GPCR signaling system in eukaryotes: modularity, conservation, and the transition to metazoan multicellularity. Genome Biol Evo 6: 606–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Pandey S, Assmann SM (2008) Arabidopsis extra-large G proteins (XLGs) regulate root morphogenesis. Plant J 53: 248–263 [DOI] [PubMed] [Google Scholar]
- Dohlman HG (2002) G proteins and pheromone signaling. Annu Rev Physiol 64: 129–152 [DOI] [PubMed] [Google Scholar]
- Escribá PV, Wedegaertner PB, Goñi FM, Vögler O (2007) Lipid–protein interactions in GPCR-associated signaling. Biochim Biophys Acta 1768: 836–852 [DOI] [PubMed] [Google Scholar]
- Gitzendanner MA, Soltis PS, Wong GK, Ruhfel BR, Soltis DE (2018) Plastid phylogenomic analysis of green plants: a billion years of evolutionary history. Am J Bot 105: 291–301 [DOI] [PubMed] [Google Scholar]
- Hackenberg D, McKain MR, Lee SG, Roy Choudhury S, McCann T, Schreier S, Harkess A, Pires JC, Wong GKS, Jez JM (2017) Gα and regulator of G‐protein signaling (RGS) protein pairs maintain functional compatibility and conserved interaction interfaces throughout evolution despite frequent loss of RGS proteins in plants. New Phytol 216: 562–575 [DOI] [PubMed] [Google Scholar]
- Hackenberg D, Pandey S (2014) Heterotrimeric G-proteins in green algae: an early innovation in the evolution of the plant lineage. Plant Signal Behav 9: e28457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackenberg D, Perroud P-F, Quatrano R, Pandey S (2016) Sporophyte formation and life cycle completion in moss requires heterotrimeric G-proteins. Plant Physiol 172: 1154–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackenberg D, Sakayama H, Nishiyama T, Pandey S (2013) Characterization of the heterotrimeric G-protein complex and its regulator from the green alga Chara braunii expands the evolutionary breadth of plant G-protein signaling. Plant Physiol 163: 1510–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser AS, Attwood MM, Rask-Andersen M, Schiöth HB, Gloriam DE (2017) Trends in GPCR drug discovery: new agents, targets and indications. Nat Rev Drug Discov 16: 829–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo JB, Sung S, Assmann SM (2012) Ca2+-dependent GTPase, extra-large G protein 2 (XLG2), promotes activation of DNA-binding protein related to vernalization 1 (RTV1), leading to activation of floral integrator genes and early flowering in Arabidopsis. J Biol Chem 287: 8242–8253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter JD (2007) Matplotlib: a 2D graphics environment. Comput Sci Eng 9: 90–95 [Google Scholar]
- Johnston CA, Taylor JP, Gao Y, Kimple AJ, Grigston JC, Chen JG, Siderovski DP, Jones AM, Willard FS (2007) GTPase acceleration as the rate-limiting step in Arabidopsis G protein-coupled sugar signaling. Proc Natl Acad Sci USA 104: 17317–17322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AM, Assmann SM (2004) Plants: the latest model system for G-protein research. EMBO Rep 5: 572–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JC, Duffy JW, Machius M, Temple BR, Dohlman HG, Jones AM (2011a) The crystal structure of a self-activating G protein alpha subunit reveals its distinct mechanism of signal initiation. Sci Signal 4: ra8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JC, Temple BR, Jones AM, Dohlman HG (2011b) Functional reconstitution of an atypical G protein heterotrimer and regulator of G protein signaling protein (RGS1) from Arabidopsis thaliana. J Biol Chem 286: 13143–13150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalogriopoulos NA, Lopez-Sanchez I, Lin C, Ngo T, Midde KK, Roy S, Aznar N, Murray F, Garcia-Marcos M, Kufareva I, et al. (2020) Receptor tyrosine kinases activate heterotrimeric G proteins via phosphorylation within the interdomain cleft of Galphai. Proc Natl Acad Sci USA 117: 28763–28774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyaanamoorthy S, Minh BQ, Wong TK, von Haeseler A, Jermiin LS (2017) ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods 14: 587–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan Y, Mu XR, Zhang H, Gao J, Shan JX, Ye WW, Lin HX (2022) TT2 controls rice thermotolerance through SCT1-dependent alteration of wax biosynthesis. Nat Plants 8: 53–67 [DOI] [PubMed] [Google Scholar]
- Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30: 772–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur J, Roy Choudhury S, Vijayakumar A, Hovis L, Rhodes Z, Polzin R, Blumenthal D, Pandey S (2018) Arabidopsis type III ggamma protein AGG3 is a positive regulator of yield and stress responses in the model monocot Setaria viridis. Front Plant Sci 9: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelle MR (2018) Neurotransmitter signaling through heterotrimeric G proteins: insights from studies in C. elegans. WormBook 2018: 1–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Bisht NC (2018) Duplicated RGS (Regulator of G-protein signaling) proteins exhibit conserved biochemical but differential transcriptional regulation of heterotrimeric G-protein signaling in Brassica species. Sci Rep 8: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35: 1547–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D, Ullrich KK, Murat F, Fuchs J, Jenkins J, Haas FB, Piednoel M, Gundlach H, Van Bel M, Meyberg R, et al. (2018) The Physcomitrella patens chromosome-scale assembly reveals moss genome structure and evolution. Plant J 93: 515–533 [DOI] [PubMed] [Google Scholar]
- Laskowski RA, MacArthur MW, Moss DS, Thornton JM (1993) PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Crystallogr 26: 283–291 [Google Scholar]
- Lee CS, Ahn W, Choi YE (2017) The G-protein alpha-subunit gene CGA1 is involved in regulation of resistance to heat and osmotic stress in Chlamydomonas reinhardtii. Cell Mol Biol 63: 29–39 [DOI] [PubMed] [Google Scholar]
- Lee YR, Assmann SM (1999) Arabidopsis thaliana ‘extra-large GTP-binding protein’ (AtXLG1): a new class of G-protein. Plant Mol Biol 40: 55–64 [DOI] [PubMed] [Google Scholar]
- Leipe DD, Wolf YI, Koonin EV, Aravind L (2002) Classification and evolution of P-loop GTPases and related ATPases. J Mol Biol 317: 41–72 [DOI] [PubMed] [Google Scholar]
- Letunic I, Bork P (2019) Interactive Tree Of Life (iTOL) v4: recent updates and new developments. Nucleic Acids Res 47: W256–W259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li FW, Nishiyama T, Waller M, Frangedakis E, Keller J, Li Z, Fernandez-Pozo N, Barker MS, Bennett T, Blázquez MA (2020) Anthoceros genomes illuminate the origin of land plants and the unique biology of hornworts. Nat Plants 6: 259–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Liu W, Zhang X, Liu Y, Li N, Li Y (2012a) Roles of the Arabidopsis G protein g subunit AGG3 and its rice homologs GS3 and DEP1 in seed and organ size control. Plant Signal Behav 7: 1357–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Liu Y, Zheng L, Chen L, Li N, Corke F, Lu Y, Fu X, Zhu Z, Bevan MW, et al. (2012b) The plant-specific G protein gamma subunit AGG3 influences organ size and shape in Arabidopsis thaliana. New Phytol 194: 690–703 [DOI] [PubMed] [Google Scholar]
- Liang X, Ding P, Lian K, Wang J, Ma M, Li L, Li M, Zhang X, Chen S, Zhang Y, et al. (2016) Arabidopsis heterotrimeric G proteins regulate immunity by directly coupling to the FLS2 receptor. Elife 5: e13568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Ma M, Zhou Z, Wang J, Yang X, Rao S, Bi G, Li L, Zhang X, Chai J, et al. (2018) Ligand-triggered de-repression of Arabidopsis heterotrimeric G proteins coupled to immune receptor kinases. Cell Res 28: 529–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Gao Y, Jones AM (2017) Extra large G-protein interactome reveals multiple stress response function and partner-dependent XLG subcellular localization. Front Plant Sci 8: 1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokits AD, Indrischek H, Meiler J, Hamm HE, Stadler PF (2018) Tracing the evolution of the heterotrimeric G protein α subunit in Metazoa. BMC Evol Biol 18: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou F, Abramyan TM, Jia H, Tropsha A, Jones AM (2020) An atypical heterotrimeric Ga protein has substantially reduced nucleotide binding but retains nucleotide-independent interactions with its cognate RGS protein and Gbg dimer. J Biomol Struct Dyn 38: 5204–5218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Meng Z, Chen R, Guan KL (2019) The hippo pathway: biology and pathophysiology. Annu Rev Biochem 88: 577–604 [DOI] [PubMed] [Google Scholar]
- Maruta N, Trusov Y, Urano D, Chakravorty D, Assmann SM, Jones AM, Botella JR (2021) GTP binding by Arabidopsis extra-large G protein 2 is not essential for its functions. Plant Physiol 186: 124–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matasci N, Hung LH, Yan Z, Carpenter EJ, Wickett NJ, Mirarab S, Nguyen N, Warnow T, Ayyampalayam S, Barker M, et al. (2014) Data access for the 1,000 plants (1KP) project. Gigascience 3: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCudden CR, Hains MD, Kimple RJ, Siderovski DP, Willard FS (2005) G-protein signaling: back to the future. Cell Mol Life Sci 62: 551–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LT, Schmidt HA, Von Haeseler A, Minh BQ (2015) IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol 32: 268–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offermanns S (2003) G-proteins as transducers in transmembrane signalling. Prog Biophys Mol Biol 83: 101–130 [DOI] [PubMed] [Google Scholar]
- Pandey S (2019) Heterotrimeric G-protein signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 70: 213–238 [DOI] [PubMed] [Google Scholar]
- Pandey S (2020) Plant receptor-like kinase signaling through heterotrimeric G-proteins. J Exp Bot 71: 1742–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey S, Vijayakumar A (2018) Emerging themes in heterotrimeric G-protein signaling in plants. Plant sci 270: 292–300 [DOI] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera—A visualization system for exploratory research and analysis. J Comput Chem 25: 1605–1612 [DOI] [PubMed] [Google Scholar]
- Phan N, Urano D, Srba M, Fischer L, Jones AM (2013) Sugar-induced endocytosis of plant 7TM-RGS proteins. Plant Signal Behav 8: e22814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRJEB34743 A (2020) RNA-seq on gametophyte and sporophyte samples of Anthoceros agrestis. NCBI ID: 614701
- Rice P, Longden I, Bleasby A (2000) EMBOSS: the European molecular biology open software suite. Trends Genet 16: 276–277 [DOI] [PubMed] [Google Scholar]
- Roy Choudhury S, Bisht NC, Thompson R, Todorov O, Pandey S (2011) Conventional and novel Ggamma protein families constitute the heterotrimeric G-protein signaling network in soybean. PLoS One 6: e23361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy Choudhury S, Li M, Lee V, Nandety RS, Mysore KS, Pandey S (2020) Flexible functional interactions between G-protein subunits contribute to the specificity of plant responses. Plant J 102: 207–221 [DOI] [PubMed] [Google Scholar]
- Roy Choudhury S, Marlin MA, Pandey S (2019) The role of gbeta protein in controlling cell expansion via potential interaction with lipid metabolic pathways. Plant Physiol 179: 1159–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy Choudhury S, Pandey S (2016) The role of PLDalpha1 in providing specificity to signal-response coupling by heterotrimeric G-protein components in Arabidopsis. Plant J 86: 50–61 [DOI] [PubMed] [Google Scholar]
- Roy Choudhury S, Pandey S (2017) Recently duplicated plant heterotrimeric Galpha proteins with subtle biochemical differences influence specific outcomes of signal-response coupling. J Biol Chem 292: 16188–16198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy Choudhury S, Pandey S (2022) SymRK-dependent phosphorylation of Galpha protein and its role in signaling during soybean (Glycine max) nodulation. Plant J 110: 277–291 [DOI] [PubMed] [Google Scholar]
- Roy Choudhury S, Riesselman AJ, Pandey S (2014) Constitutive or seed-specific overexpression of Arabidopsis G-protein gamma subunit 3 (AGG3) results in increased seed and oil production and improved stress tolerance in Camelina sativa. Plant Biotechnol J 12: 49–59 [DOI] [PubMed] [Google Scholar]
- Roy Choudhury S, Westfall CS, Laborde JP, Bisht NC, Jez JM, Pandey S (2012) Two chimeric regulators of G-protein signaling (RGS) proteins differentially modulate soybean heterotrimeric G-protein cycle. J Biol Chem 287: 17870–17881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw AJ, Devos N, Cox CJ, Boles SB, Shaw B, Buchanan AM, Cave L, Seppelt R (2010) Peatmoss (Sphagnum) diversification associated with Miocene Northern Hemisphere climatic cooling? Mol Phylogenet Evol 55: 1139–1145 [DOI] [PubMed] [Google Scholar]
- Shpakov AO, Pertseva MN (2008) Signaling systems of lower eukaryotes and their evolution. Int Rev Cell Mol Biol 269: 151–282 [DOI] [PubMed] [Google Scholar]
- Siderovski DP, Willard FS (2005) The GAPs, GEFs, and GDIs of heterotrimeric G-protein alpha subunits. Int J Biol Sci 1: 51–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondek J, Bohm A, Lambright DG, Hamm HE, Sigler PB (1996) Crystal structure of a GA protein βγdimer at 2.1 Å resolution. Nature 379: 369–374 [DOI] [PubMed] [Google Scholar]
- Stamatakis A (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Qian Q, Wu K, Luo J, Wang S, Zhang C, Ma Y, Liu Q, Huang X, Yuan Q, et al. (2014) Heterotrimeric G proteins regulate nitrogen-use efficiency in rice. Nat Genet 46: 652–656 [DOI] [PubMed] [Google Scholar]
- Sun Q, He Q, Xu J, Liu Q, Lu Y, Zhang Z, Xu X, Sun B (2019) Guanine nucleotide-binding protein G(i)alpha2 aggravates hepatic ischemia-reperfusion injury in mice by regulating MLK3 signaling. FASEB J 33: 7049–7060 [DOI] [PubMed] [Google Scholar]
- Tang H, Lyons E, Pedersen B, Schnable JC, Paterson AH, Freeling M (2011) Screening synteny blocks in pairwise genome comparisons through integer programming. BMC Bioinformatics 12: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple BR, Jones AM (2007) The plant heterotrimeric G-protein complex. Annu Rev Plant Biol 58: 249–266 [DOI] [PubMed] [Google Scholar]
- Thung L, Trusov Y, Chakravorty D, Botella JR (2012) Ggamma1+Ggamma2+Ggamma3=Gbeta: the search for heterotrimeric G-protein gamma subunits in Arabidopsis is over. J Plant Physiol 169: 542–545 [DOI] [PubMed] [Google Scholar]
- Trusov Y, Chakravorty D, Botella JR (2012) Diversity of heterotrimeric G-protein gamma subunits in plants. BMC Res Notes 5: 608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trusov Y, Zhang W, Assmann SM, Botella JR (2008) Ggamma1 + Ggamma2 not equal to Gbeta: heterotrimeric G protein Ggamma-deficient mutants do not recapitulate all phenotypes of Gbeta-deficient mutants. Plant Physiol 147: 636–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urano D, Fu Y, Jones AM (2013) Activation of an unusual G-protein in the simple protist Trichomonas vaginalis. Cell Cycle 12: 3127–3128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urano D, Jones JC, Wang H, Matthews M, Bradford W, Bennetzen JL, Jones AM (2012a) G protein activation without a GEF in the plant kingdom. PLoS Genet 8: e1002756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urano D, Maruta N, Trusov Y, Stoian R, Wu Q, Liang Y, Jaiswal DK, Thung L, Jackson D, Botella JR (2016) Saltational evolution of the heterotrimeric G protein signaling mechanisms in the plant kingdom. Sci Signal 9: ra93–ra93 [DOI] [PubMed] [Google Scholar]
- Urano D, Phan N, Jones JC, Yang J, Huang J, Grigston J, Taylor JP, Jones AM (2012b) Endocytosis of the seven-transmembrane RGS1 protein activates G-protein-coupled signalling in Arabidopsis. Nat Cell Biol 14: 1079–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsunomiya Y, Samejima C, Fujisawa Y, Kato H, Iwasaki Y (2012) Rice transgenic plants with suppressed expression of the β subunit of the heterotrimeric G protein. Plant Signal Behav 7: 443–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh SR, Singh V (2021) G protein-coupled receptors: the choreographers of innate immunity in Caenorhabditis elegans. PLoS Pathog 17: e1009151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LG, Lam TTY, Xu S, Dai Z, Zhou L, Feng T, Guo P, Dunn CW, Jones BR, Bradley T (2020) Treeio: an R package for phylogenetic tree input and output with richly annotated and associated data. Mol Biol Evol 37: 599–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb B, Sali A (2016) Comparative protein structure modeling using MODELLER. Curr Protoc Protein Sci 86: 2.9.1–2.9.37. [DOI] [PubMed] [Google Scholar]
- Wendt T, Holme I, Dockter C, Preuss A, Thomas W, Druka A, Waugh R, Hansson M, Braumann I (2016) HvDep1 is a positive regulator of culm elongation and grain size in barley and impacts yield in an environment-dependent manner. PLoS One 11: e0168924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensel TG (2008) Signal transducing membrane complexes of photoreceptor outer segments. Vision research 48: 2052–2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H (2016) Ggplot2: Elegant Graphics for Data Analysis. Springer, Berlin, Germany [Google Scholar]
- Wilkie TM (1999) G proteins, chemosensory perception, and the C. elegans genome project: an attractive story. Bioessays 21: 713–717 [DOI] [PubMed] [Google Scholar]
- Wilkie TM, Yokoyama S (1994) Evolution of the G protein alpha subunit multigene family. Soc Gen Physiol Ser 49: 249–270 [PubMed] [Google Scholar]
- Wolfenstetter S, Chakravorty D, Kula R, Urano D, Trusov Y, Sheahan MB, McCurdy DW, Assmann SM, Jones AM, Botella JR (2014) Evidence for an unusual transmembrane configuration of AGG3, a class C Ggamma subunit of Arabidopsis. Plant J 81: 388–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Regan M, Furukawa H, Jackson D (2018) Role of heterotrimeric Galpha proteins in maize development and enhancement of agronomic traits. PLoS Genet 14: e1007374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Xu F, Liu L, Char SN, Ding Y, Je BI, Schmelz E, Yang B, Jackson D (2019) The maize heterotrimeric G protein b subunit controls shoot meristem development and immune responses. Proc Natl Acad Sci USA 117: 1799–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Zhao M, Zhang Q, Xu Z, Xu Q (2016) The DENSE AND ERECT PANICLE 1 (DEP1) gene offering the potential in the breeding of high-yielding rice. Breed Sci 66: 659–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G (2020) Using ggtree to visualize data on tree‐like structures. Curr Protoc Bioinform 69: e96. [DOI] [PubMed] [Google Scholar]
- Yu Y, Assmann SM (2018) Inter-relationships between the heterotrimeric Gbeta subunit AGB1, the receptor-like kinase FERONIA, and RALF1 in salinity response. Plant Cell Environ 41: 2475–2489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Zhao Y, Bi G, Liang X, Zhou JM (2019) Early signalling mechanisms underlying receptor kinase-mediated immunity in plants. Philos Trans R Soc Lond B Biol Sci 374: 20180310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.
Conflict of interest statement. None declared.