Abstract
Background
Head injury is a common event and can cause a spectrum of motor and cognition disabilities. A frequent complication is seizures. Antiepileptic drugs (AED) such as phenytoin are often used in clinical practice with the hopes of preventing post‐traumatic epilepsy. Whether immediate medical intervention following head trauma with either AEDs or neuroprotective drugs can alter the process of epileptogenesis and lead to a more favorable outcome is currently unknown. This review attempted to address the effectiveness of these treatment interventions. This review updates and expands on the earlier Cochrane review.
Objectives
To compare the efficacy of antiepileptic drugs and neuroprotective agents with placebo, usual care or other pharmacologic agents for the prevention of post‐traumatic epilepsy in people diagnosed with any severity of traumatic brain injury.
Search methods
We searched The Cochrane Epilepsy Group's specialized register, CENTRAL, MEDLINE, ClinicalTrials.gov and World Health Organization International Clinical Trials Registry Platform (ICTRP) in January 2015. We searched EMBASE, Biological Abstracts and National Research Register in September 2014 and SCOPUS in December 2013. The Cochrane Epilepsy Group performed handsearches of relevant journals.
Selection criteria
We included randomized controlled trials (RCTs) that include AEDs or neuroprotective agents compared with placebo, another pharmacologic agent or a usual care group. The outcomes measured included a seizure occurring within one week of trauma (early seizure), seizure occurring later than one week post‐trauma (late seizure), mortality and any adverse events.
Data collection and analysis
Two review authors independently assessed study quality and extracted the data. We calculated risk ratios (RR) and 95% confidence intervals (CI) for each outcome. We used random‐effects models in the meta‐analyses and performed pre‐defined subgroup and sensitivity analyses.
Main results
This review included 10 RCTs (reported in 12 articles) consisting of 2326 participants The methodological quality of the studies varied. The type of intervention was separated into three categories; AED versus placebo or standard care, alternative neuroprotective agent versus placebo or standard care and AED versus other AED. Treatment with an AED (phenytoin or carbamazepine) decreased the risk of early seizure compared with placebo or standard care (RR 0.42, 95% CI 0.23 to 0.73; very low quality evidence). There was no evidence of a difference in the risk of late seizure occurrence between AEDs and placebo or standard care (RR 0.91, 95% CI 0.57 to 1.46; very low quality evidence). There was no evidence of a significant difference in all‐cause mortality between AEDs and placebo or standard care (RR 1.08 95% CI 0.79 to 1.46,very low quality of evidence). Only one study looked at other potentially neuroprotective agents (magnesium sulfate) compared with placebo. The risk ratios were: late seizure 1.07 (95% CI 0.53 to 2.17) and all‐cause mortality 1.20 (95% CI 0.80 to 1.81). The risk ratio for occurrence of early seizure was not estimable.
Two studies looked at comparison of two AEDs (levetiracetam, valproate) with phenytoin used as the main comparator in each study. The risk ratio for all‐cause mortality was 0.53 (95% CI 0.30 to 0.94). There was no evidence of treatment benefit of phenytoin compared with another AED for early seizures (RR 0.66, 95% 0.20 to 2.12) or late seizures(RR 0.77, 95% CI 0.46 to 1.30).
Only two studies reported adverse events. The RR of any adverse event with AED compared with placebo was 1.65 (95% CI 0.73 to 3.66; low quality evidence). There were insufficient data on adverse events in the other treatment comparisons.
Authors' conclusions
This review found low‐quality evidence that early treatment with an AED compared with placebo or standard care reduced the risk of early post‐traumatic seizures. There was no evidence to support a reduction in the risk of late seizures or mortality. There was insufficient evidence to make any conclusions regarding the effectiveness or safety of other neuroprotective agents compared with placebo or for the comparison of phenytoin, a traditional AED, with another AED.
Plain language summary
Medicines for preventing epilepsy following traumatic head injury
Background
Traumatic head injury is a frequent event and can injure the brain. This severe injury is often followed by seizures (fits), which may worsen the damage and can lead to chronic epilepsy, a neurologic disorder characterized by frequent recurrent seizures. Antiepileptic drugs are usually given to suppress already diagnosed seizures. Their role in curing the disease and preventing the development of epilepsy in people who are considered at risk for seizures after any brain injury, including head trauma, is not well understood.
Study characteristics
We searched for studies evaluating the effect of early administration of antiepileptic drugs or other potentially neuroprotective agents (which act by protecting the structure or function of nerves) on post‐traumatic epilepsy. The primary outcomes of interest were early post‐traumatic seizures (within one week of trauma) and late seizures (later than one week post‐trauma). We also looked at death, time to late seizure and side effects. The evidence is current to January 2015.
Key results
We found 10 clinical trials involving 2326 people reported in 12 published articles. The evidence available indicated that early treatment with a traditional antiepileptic drug (phenytoin or carbamazepine) may reduce the risk of early post‐traumatic seizures. Traditional antiepileptic drugs are no more effective than placebo (a pretend pill) or standard care in reducing late seizures or mortality. Limited data were available for the comparison of an AED with another AED and for the comparison of other potentially neuroprotective agents with placebo. Most studies did not report serious side effects and other side effects.
Quality of the evidence
The overall quality of the evidence varied and findings should be interpreted with caution.
Summary of findings
Background
Description of the condition
Head injury is a common event and can cause a spectrum of motor and cognition disabilities. A frequent complication is seizures. While 'early seizures' are frequently considered to be nonspecific diffuse reactions as a result of an acute encephalopathy and are self limited, seizures following several weeks or months after head trauma seem to reflect an underlying process of post‐traumatic scar formation and epileptogenesis. However, there is evidence from epidemiologic studies that early seizures can be predictors for late seizures (Wyllie 2010). This suggests that these definitions reflect simplifications of the underlying ongoing tissue transformation over time. The risk for late 'unprovoked' seizure recurrence increases with the severity of the injury, involvement of the cerebral cortex, presence of dura penetration, skull fracture and intracerebral hematoma, and the occurrence of early seizures (Jennett 1981; Annegers 1998). Timing and the interplay of potentially involved factors in the development of this epileptogenic process are unclear.
Description of the intervention
Behind the concept of preventing post‐traumatic epilepsy stands the hope that the silent period of weeks and months after the trauma, before seizure occurrence, is a window of opportunity to stop the process using appropriate interventional treatment strategies (Temkin 2009). Antiepileptic drugs (AEDs) can suppress seizures; however, it is the subject of a controversial debate if they are also able to interfere positively with the process leading to epilepsy. Experimental studies looking at neuroprotective agents, such as antioxidants and free radicals, have also been promising but historically have not translated well into the clinical environment (Slemmer 2008). Therefore, this Cochrane review will carefully evaluate the impact of either early or late use of AEDs and neuroprotective agents on the occurrence of unprovoked seizures following the trauma.
How the intervention might work
Current experimental epilepsy research using animal models, such as kindling and post‐status epileptic condition, suggests that some new AEDs may have the potential to alter the underlying epileptogenic process and act as disease‐modifying agents (Löscher 2002; Brandt 2006). There is also some evidence that neuroprotective agents may alter the epileptogenic process. For example, antioxidants may be able to suppress this process by interfering with free radical reactions initiated by hemorrhage associated with brain injuries (Willmore 2009).
Why it is important to do this review
Post‐traumatic seizures are quite prevalent. Most of these people undergo a careful functional and structural diagnostic algorithm including electroencephalography (EEG) and magnetic resonance imaging (MRI) or at least computed tomography (CT). Therefore, post‐traumatic seizures can be considered an ideal model to study tissue changes and regional hyperexcitability as part of the evolving epileptogenic scar. It is not yet known whether immediate medical intervention following head trauma with either AEDs or neuroprotective drugs can alter the process of epileptogenesis and lead to a more favorable outcome. There are limited data on traditional AEDs such as phenytoin, phenobarbital, valproate and carbamazepine.
With the advent since the mid‐2000s of many new AEDs and research into alternative treatments such as neuroprotective agents, it seems critical and timely to review the human experience carefully and evaluate how these experimental findings might translate into the prevention of post‐traumatic epilepsy in clinical practice. Therefore, this review will conduct a systematic, up‐to‐date review of randomized controlled trials (RCTs) examining the effectiveness and safety of both AEDs and neuroprotective agents with special focus on recently licensed products.
Objectives
To compare the efficacy of antiepileptic drugs and neuroprotective agents with placebo, usual care or other pharmacologic agents for the prevention of post‐traumatic epilepsy in people diagnosed with any severity of traumatic brain injury.
Methods
Criteria for considering studies for this review
Types of studies
RCTs that included AEDs or neuroprotective agents compared with placebo, another pharmacologic agent or a usual care group. We included studies published in any languages. We excluded quasi‐randomized studies, dose‐finding studies and cluster randomized or cross‐over trials.
Types of participants
People of all ages diagnosed with acute traumatic brain injury (TBI) who received prophylactic treatment with AEDs or neuroprotective agents. Administration was post‐injury and prior to the occurrence of a first post‐traumatic seizure (FPS). We excluded people with previously documented unprovoked seizures.
Types of interventions
Treatment
Any conventional AED post‐injury and prior to the occurrence of an FPS. Traditional AEDs included, but were not limited to, carbamazepine, phenytoin and valproate, and examples of new AEDs included but were not limited to oxcarbazepine, lamotrigine, levetiracetam and topiramate.
Any alternative neuroprotective pharmacologic treatments, including administration of distinct neurotrophic factors, hormones or antioxidants post‐injury and prior to the occurrence of an FPS.
Comparison
Other pharmacologic agent, placebo or usual care.
Pharmacologic agents (AED) versus placebo or usual care.
Neuroprotective agent versus placebo or usual care.
Pharmacologic agent A (AED) versus pharmacologic agent B (AED).
We analyzed each comparison separately.
Types of outcome measures
Primary outcomes
Proportion of participants who experience an early seizure post‐trauma, defined as occurring within one week of trauma.
Proportion of participants who experience a late seizure post‐trauma, defined as occurring later than one week post‐trauma.
Secondary outcomes
Mortality from any cause during follow‐up period.
Time to first seizure from randomization.
Proportion of participants experiencing serious treatment‐related adverse events.
Search methods for identification of studies
Electronic searches
We searched the following databases:
Cochrane Epilepsy Group Specialized Register (latest search date: 13 January 2015) using the search strategy given in Appendix 1;
Cochrane Central Register of Controlled Trials (CENTRAL) via Cochrane Register of Studies Online (CRSO), latest search date: 13 January 2015, using the search strategy given in Appendix 2;
MEDLINE (OVID; 1946 to 13 January 2015) using the search strategy given in Appendix 3;
EMBASE (Elsevier; latest search date: 5 September 2014) using the search strategy given in Appendix 4;
ClinicalTrials.gov and World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) searched on 13 January 2015 for 'traumatic head injury AND epilepsy';
SCOPUS (1823 to 17 December 2013) using the search strategy given in Appendix 5;
Biological Abstracts (latest search date: 5 September 2014) using the search strategy given in Appendix 6.
The electronic search strategy used in the review by Schierhout 2001 was expanded upon.
It is no longer necessary to search SCOPUS or EMBASE, because RCTs listed in EMBASE are now included in CENTRAL, and SCOPUS is a substitute for EMBASE.
Searching other resources
In addition to searching electronic databases, we consulted the following sources.
Bibliographies of related Cochrane reviews.
Reference sections of included papers and key systematic reviews (Temkin 2001; Beghi 2003).
Authors of relevant reports regarding any further published or unpublished work.
National Research Register.
Handsearching of content related journals as conducted by The Cochrane Epilepsy Group.
Data collection and analysis
Selection of studies
Two review authors independently reviewed titles and abstracts obtained from the literature search. Review authors then ranked each as follows.
Include: study met criteria.
Unclear: insufficient to determine if study met criteria.
Exclude: study did not meet criteria.
The review authors compared and discussed results; a third review author arbitrated any disagreements. We excluded papers where insufficient evidence was available in a study. If necessary, we contacted authors for further clarification. When multiple publications of the same study were found, we included the original study that met the inclusion criteria; we included the second study if it contained different outcomes. We placed no language or time restriction on included studies.
Data extraction and management
We used the full text to include or exclude studies where it was unclear from reading the abstracts. Two review authors independently extracted data from included RCTs in the following categories.
Participant characteristics:
inclusion and exclusion criteria;
number of participants per group;
age;
sex;
trauma characteristics such as severity, MRI or CT documented pattern of injury,
EEG findings.
Methods:
study design;
duration of study;
randomization method;
treatment allocation;
completeness of follow‐up;
presence of blinding;
intention‐to‐treat (ITT) analysis.
Intervention:
type of agent, treatment 1, treatment 2 or control;
method of administration;
dosage and duration of treatment;
time post‐trauma treatment was delivered;
control or usual care intervention.
Outcome measures and clinical findings:
seizure occurrence;
mortality;
number of seizures;
time to first seizure;
adverse events;
neurologic findings.
Possible sources of heterogeneity:
median age of participant at time of injury;
severity of trauma;
pharmacologic agent;
duration of treatment;
timing of treatment.
Other:
country and setting of study;
year of publication;
title;
authors.
We used a predefined form for this task (Appendix 7).
The form was developed and pilot tested on three trials prior to use on all studies.
Assessment of risk of bias in included studies
Two review authors independently assessed the quality of studies. Methods used for summary assessment are provided in the Cochrane Handbook for Systematic Reviews of Interventions 5.1, Section 8.4 (Higgins 2011). We scored each of the following domains as at 'high', 'low' or 'unclear' risk and reported the scores in the 'Risk of bias' table.
-
Selection bias:
sequence generation;
allocation of concealment.
-
Performance bias:
blinding of participants and personnel;
other potential threats to validity.
-
Detection bias:
blinding of outcome assessment;
other potential threats to validity.
-
Attrition bias:
incomplete outcome data.
-
Reporting bias:
selective outcome reporting.
Other bias
Measures of treatment effect
We performed statistical analyses and produced a summary of the data using Review Manager 5 (RevMan 2012). We presented dichotomous outcomes as risk ratios (RR) with corresponding 95% confidence intervals (CI). We planned to present time‐to‐event outcomes as hazard ratios with 95% CI. If hazard ratios were not given, we planned to use indirect estimation methods (Parmar 1998; Williamson 2002). None of the included studies presented time‐to‐event data; we therefore did not summarize results using hazard ratios. For individual listed adverse effects, we quoted 99% CIs, making an allowance for multiple testing. We performed separate analyses for each control group. We used an ITT analysis on outcomes from all randomized participants where possible for primary analyses.
Unit of analysis issues
The unit of analysis for this review was the individual participant
Dealing with missing data
We contacted authors where substantial outcomes of interest were not reported or to clarify uncertainty about study characteristics. We waited one month for a response from authors, after which time we formally considered data to be missing.
ITT analyses were performed.
Assessment of heterogeneity
We used forest plots to assess the statistical heterogeneity of studies visually and the Chi2 test to assess evidence of heterogeneity. We used a P value < 0.1 to determine statistical significance (Whitehead 1991). We calculated the I2 statistic with I2 values greater than 50% indicating substantial to considerable heterogeneity (Higgins 2011). If we found values of heterogeneity greater than 50%, we attempted to explain the heterogeneity based on the differences in study characteristics and participant profiles (such as severity of trauma, age).
Assessment of reporting biases
We planned to assess publication bias using funnel plots if more than 10 studies were included. Reasons for funnel plot asymmetry include publication bias, outcome reporting bias, language bias, citation bias, poor methodologic design and heterogeneity. We assessed these for each trial, where possible. We planned to include an ORBIT table to explore the impact of selective outcome reporting further (Kirkham 2010). We found few studies assessing each of the pre‐specified outcomes; we therefore did not prepare funnel plots or orbit tables
Data synthesis
To pool the data for each outcome, we used the random‐effects method (DerSimonian 1986) based on the inverse variance method , rather than using an fixed effects method. A random‐effects model meta‐analysis involves an assumption that the effects being estimated in the different studies are not identical but follow the same distribution (Higgins 2011). Summary intervention estimates are a weighted mean of the estimate from each individual study. A fixed‐effect model was considered as a sensitivity analysis.
'GRADEing' the evidence
We followed the recommended GRADE approach to assess the quality of the evidence for each outcome. We produced 'Summary of findings' tables for each treatment comparison for the primary outcomes (early seizure and late seizure) and secondary outcomes (all cause mortality, time to first seizure from randomisation and proportion of participants experiencing serious treatment‐related adverse events) based on established recommendations (Schünemann 2011).
Subgroup analysis and investigation of heterogeneity
We intended to evaluate clinical and methodologic heterogeneity across studies by comparing the characteristics of participants, interventions and study designs.
We performed the following clinically relevant subgroup analyses to investigate possible sources of clinical heterogeneity.
Mean age of participants in study at time of injury: adults (ages over 17 years), school age children (ages six to 17 years) and children (ages under six years).
Pharmacologic agent: recently licensed AEDs and traditional AEDs.
Severity of trauma: minor, moderate and severe TBI. This review followed the paper by Teasell 2007 for classification of head trauma (Appendix 8).
Duration of treatment: short‐term treatment (treatment less than three months post‐injury), mid‐term treatment (more than three months and less than 12 months post‐injury) and long‐term treatment (any duration longer than one year post‐injury).
Tests of Interaction (Cochran's Q and Higgins I2) for subgroup differences were performed. Data were not available for all the pre‐planned subgroup analysis (see Differences between protocol and review).
Sensitivity analysis
We performed sensitivity analysis to evaluate the robustness of decisions made in the review methodology.
Study quality: excluding studies which were high risk of bias
Age range of participants: analysis repeated excluding those studies where it was not possible to separate participants that did not meet the inclusion criteria for age.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
Of the 1929 initial citations identified, we screened 75 reports (See Figure 1).
1.
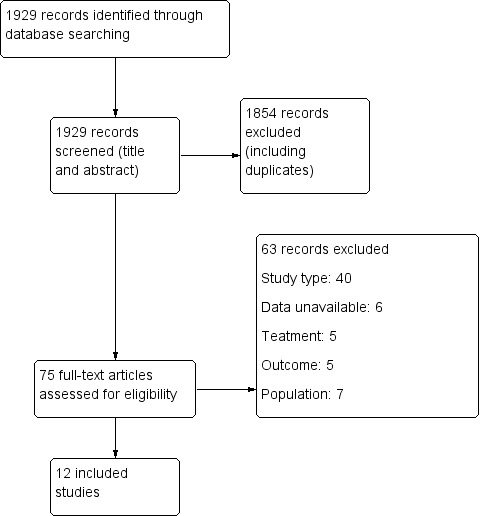
Study flow diagram.
Included studies
Ten RCTs described in 12 published articles met review inclusion criteria and included 2326 randomized participants ages five years and older. All trials included participants with moderate and severe TBI and excluded people with pre‐existing epilepsy.
Five trials included children (McQueen 1983; Young 1983; Pechadre 1991; Manaka 1992; Young 2004). Young 2004 included only children under the age of 10 years.
Six trials reported on short‐term treatments (five to seven days to one month) (Young 1983; Pechadre 1991; Temkin 1999; Young 2004; Temkin 2007; Szaflarski 2010), three reported on mid‐term treatments (six to 12 months) (McQueen 1983; Temkin 1990; Temkin 1999), and three trials reported on long‐term treatments (18 months to two years) (Glotzner 1983; Young 1983; Manaka 1992). Most studied traditional AEDs versus placebo or usual care: phenytoin (McQueen 1983; Young 1983; Temkin 1990; Pechadre 1991; Young 2004), phenobarbital (Manaka 1992), carbamazepine (Glotzner 1983), and valproate (Temkin 1999); one studied a newly licensed agent: levetiracetam versus phenytoin (Szaflarski 2010), and one studied an 'other' agent, magnesium sulfate (MgSO4) versus placebo (Temkin 2007).
Six trials were conducted in the USA (Young 1983; Temkin 1990; Temkin 1999; Young 2004; Temkin 2007; Szaflarski 2010), three in Europe (Glotzner 1983; McQueen 1983; Pechadre 1991), and one in Japan (Manaka 1992).
We included nine trials in the meta‐analysis and assessed the primary and secondary outcomes of early seizures, late seizures, all‐cause mortality and adverse events (Glotzner 1983; McQueen 1983; Young 1983; Temkin 1990; Pechadre 1991; Manaka 1992; Temkin 1999; Young 2004; Szaflarski 2010). All but two trials reported incidence of early and late seizures; Manaka 1992 and McQueen 1983 reported only late seizures. All trials but Manaka 1992 and Pechadre 1991 reported mortality. The majority of trials primarily investigated whether AEDs (traditional or newly licensed) prevented early or late (or both) seizure occurrence in people with TBI. One trial primarily investigated safety and reported on adverse events (Szaflarski 2010). McQueen 1983 and Temkin 1990 also reported the occurrence of skin rashes. Temkin 2007 was not included in a meta‐analysis as it was the only study included in the review that studied an 'other' agent. See Characteristics of included studies table for details.
Excluded studies
We excluded 63 studies from the review. Forty were not RCTs or quasi‐randomized trials, in seven the data were unavailable (i.e. trial cancelled due to lack of enrolment or unable to acquire details from author), four studies were secondary publications of studies already included, which contained no further relevant information. Five studies did not report treatment of interest, seven did not include the population of interest. See Characteristics of excluded studies table for details.
Risk of bias in included studies
Figure 2 and Figure 3 summarize the risk of bias of included studies. We deemed no study to be at low risk of bias in all bias types. The majority of studies had a mix of low and unclear bias to varying degrees. Five trials had a number of bias types classified as high risk of bias (Glotzner 1983; McQueen 1983; Young 1983; Pechadre 1991; Manaka 1992).
2.
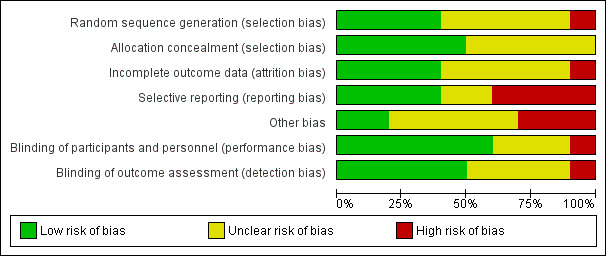
Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
3.
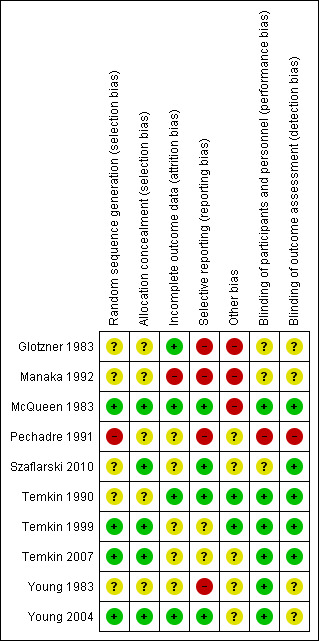
Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
Allocation
Only three trials adequately described the sequence generation and allocation process (Temkin 1999; Young 2004; Temkin 2007). Szaflarski 2010 indicated the participants were randomized by the pharmacy but did not describe the sequence generation and, therefore, the risk of selection bias was unclear. Risk of selection bias was unclear in McQueen 1983; Young 1983; Temkin 1990; Manaka 1992 , due to lack of clear description of the sequence generation and allocation process. The sequence generation in Glotzner 1983 and Pechadre 1991 was based on odd/even birthday or days of admission and, therefore, at high risk of predicting group allocation.
Blinding
Six of the 10 trials were low risk for performance bias as they adequately described blinding of participants and personnel (McQueen 1983; Young 1983; Temkin 1990; Temkin 1999; Young 2004; Temkin 2007). In Glotzner 1983; Manaka 1992; and Szaflarski 2010, risk of bias for performance bias was unclear as these trials did not report on blinding of participants and personnel. Risk of detection bias was low in five trials (McQueen 1983; Temkin 1990; Temkin 1999; Temkin 2007; Szaflarski 2010), and unclear in four publications as they did not describe blinding of the outcome assessment (Glotzner 1983; Young 1983; Manaka 1992; Young 2004). Pechadre 1991 was at high risk for performance and detection bias as it did not describe blinding of the participants, personnel or outcome assessment and the predictable randomization process suggested that assessors could easily determine which participants were allocated to treatment and control groups.
Incomplete outcome data
Three trials were low risk for attrition bias as they clearly described outcome data and attrition patterns (McQueen 1983; Temkin 1990; Young 2004). Six trials had unclear risk for attrition bias due to poor descriptions of reasons for attrition (Glotzner 1983; Young 1983; Pechadre 1991; Temkin 1999; Temkin 2007; Szaflarski 2010), and Manaka 1992 was high risk of bias for lack of description or details on 52 people who were excluded or dropped out from study.
Selective reporting
Only four of the 10 trials were low risk for reporting bias (McQueen 1983; Temkin 1990; Young 2004; Szaflarski 2010). Four trials were at high risk of selective reporting as they did not report adverse events (Glotzner 1983; Young 1983; Pechadre 1991; Manaka 1992). In addition, Manaka 1992 and Pechadre 1991 did not report mortality and Young 1983 reported mortality inconsistently across age groups. Young 1983 reported mortality as a count of events for all ages in the short‐term treatment; however, mortality in adults on long‐term treatment were not reported as a count of events and, therefore, overall deaths for the entire trial were underestimated. Manaka 1992 was high risk for reporting bias as the trial did not report baseline characteristics. The remaining two trials had unclear risk (Temkin 1999; Temkin 2007).
Other potential sources of bias
Three publications were at high risk for other types of bias (Glotzner 1983; McQueen 1983; Manaka 1992). Glotzner 1983 reported that the majority of participants received an AED in the first week regardless of allocation group resulting in potential contamination of controls. McQueen 1983 reported potential significant baseline differences between the control and treatment groups with more five to 15 year olds in the treatment group and below therapeutic levels of the phenytoin were reported. Manaka 1992 did not report baseline characteristics, so it was impossible to compare baseline characteristics between treatment groups. Several studies reported difficulties with compliance (McQueen 1983; Young 1983; Temkin 1990; Temkin 1999), and maintaining therapeutic levels particularly when evaluating late seizure outcome.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. Antiepileptic drugs compared with placebo or standard care for people at risk of epilepsy following traumatic head injury.
| Antiepileptic drugs compared with placebo or standard care for people at risk of epilepsy following traumatic head injury | ||||||
| Patient or population: people with traumatic head injuries Settings: Neurosurgery departments, ICU and trauma centers in North America, UK and Europe Intervention: antiepileptic drugs Comparison: placebo or standard care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or standard care | Antiepilepticdrugs | |||||
| Early seizures Count of events Follow‐up: 5‐7 days | 139 per 1000 | 59 per 1000 (32 to 102) | RR 0.42 (0.23 to 0.74) | 987 (5 studies) | ⊕⊕⊝⊝ low1,2, | Sensitivity analysis by quality of the study shows that RR for early seizures in low/unclear risk studies was no longer significant (RR 0.59, 95% CI 0.20 and 1.73) |
| Late seizures Count of events Follow‐up: 3‐24 months | 178 per 1000 | 162 per 1000 (100 to 260) | RR 0.91 (0.57 to 1.46) | 1029 (6 studies) | ⊕⊝⊝⊝ very low3,4,5 | RR of late seizures remained insignificant regardless of type of antiepileptic drug, treatment duration, age of population or quality of the study |
| All‐cause mortality Follow‐up: 5 days to 24 months | 174 per 1000 | 188 per 1000 (138 to 255) | RR 1.08 (0.79 to 1.46) | 1065 (5 studies) | ⊕⊝⊝⊝ very low1,4,5 | RR for all‐cause mortality remained insignificant regardless of treatment duration, age of population or quality of the study |
|
Any serious adverse event of treatment count of events Follow up: 12 months |
94 per 1000 |
154 per 1000 (69 to 345) |
RR 1.63 (0.73 to 3.66) |
568 (2 studies) |
⊕⊕⊝⊝ low5,6 | |
| Time to first seizure from randomization | See comment | See comment | Not estimable | 0 (0 studies) | See comment | No study reported time to first seizure in an interpretable way |
| *The basis for the assumed risk is the event rate in the control (placebo or standard care) group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded one level due to serious risk of bias: Two studies included in this outcome had instances of high risk of bias assessment. The remaining studies had a mix of low and unclear risk of bias. 2 Downgraded one level due to imprecision: RR for early seizures by study was inconsistent and ranged from 0.24 to 1.22. The difference in risk tends to be associated with differences in risk of bias between studies. 3 Downgraded one level due to serious risk of bias: Four studies included in this outcome had one to four instances of high risk in risk of bias assessment. The remaining two studies had a mix of low and unclear risk of bias. 4Downgraded one level due to inconsistency of results (I2=54%): Some heterogeneity may be explained by study design, population, intervention (dose) or follow‐up. However, there is wide variation in the results showing both considerable harm and considerable benefit.
5 Downgraded one level due to imprecision of results: wide 95% CI that includes both considerable harm and benefit.
6 Downgraded one level due to serious risk of bias: selection bias was likely in both trials
Summary of findings 2. Neuroprotective agent versus placebo for people at risk of epilepsy following traumatic head injury.
| Neuroprotective agents compared with placebo for people at risk of epilepsy following traumatic head injury | ||||||
| Patient or population: people with traumatic head injuries Settings: Neurosurgery departments, ICU and trauma centers in North America, UK and Europe Intervention: Neuroprotective agents Comparison: Placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Neuroprotective agents | |||||
|
Early seizure Count of events Follow‐up: 7 days |
0 per 1000 | 0 per 1000 (0 to 0) |
RR 2.99 (0.12 to 73.00) |
499 (1 study) | ⊕⊕⊝⊝ low1,2 | No events occurred in the control group therefore corresponding risk is also zero |
|
Late seizure Count of events Follow‐up: 6 months |
56 per 1000 | 60 per 1000 (30 to 122) |
RR 1.07 (0.53 to 2.17) |
498 (1 study) | ⊕⊕⊕⊕ high | |
|
All‐cause mortality Follow‐up: 6 months |
150 per 1000 | 180 per 1000 (120 to 272) |
RR 1.20 (0.80 to 1.81) |
466 (1 study) | ⊕⊕⊕⊕ high | |
| Any serious adverse event of treatment | See comment | See comment | Not estimable | 0 (0 studies) | See comment | No study reported adverse event data |
| Time to first seizure from randomization | See comment | See comment | Not estimable | 0 (0 studies) | See comment | No study reported time to first seizure in an interpretable way |
| *The basis for the assumed risk is the event rate in the control (placebo or standard care) group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded one level due to risk of bias: As reported in the study paper, 96% of participants received phenytoin for the first week in both treatment groups. This may have resulted in a very low early seizure rate
2 Downgraded one level due to imprecision of results: wide 95% CI that includes both considerable harm and benefit.
Summary of findings 3. Anti‐epileptic drugs compared to other anti‐epileptic drugs for people at risk of epilepsy following traumatic head injury.
| Anti‐epileptic drugs compared to other anti‐epileptic drugs for people at risk of epilepsy following traumatic head injury | ||||||
| Patient or population: people with traumatic head injuries Settings: Neurosurgery departments, ICU and trauma centers in North America, UK and Europe Intervention: Phenytoin Comparison: Other anti‐epileptic drugs (AEDs) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Other AEDs | Phenytoin | |||||
|
Early seizure Counts of events Follow up: 7 days |
57 per 1000 | 38 per 1000 (11 to 121) |
RR 0.66 (0.20 to 2.12) |
431 (2 studies) | ⊕⊕⊝⊝ low1,2 | |
|
Late seizure Counts of events Follow up: 6 months to 2 years |
166 per 1000 | 128 per 1000 (76 to 216) |
RR 0.77 (0.46 to 1.30) |
378 (2 studies) | ⊕⊕⊕⊝ moderate1 | |
|
All‐cause mortality Follow up: 6 months to 2 years |
164 per 1000 | 87 per 1000 (49 to 154) |
RR 0.53 (0.30 to 94) |
431 (2 studies) | ⊕⊕⊕⊝ moderate1 | |
| Any serious adverse event of treatment | See comment | See comment | Not estimable | 0 (0 studies) | See comment | No study reported adverse event data |
| Time to first seizure from randomization | See comment | See comment | Not estimable | 0 (0 studies) | See comment | No study reported time to first seizure in an interpretable way |
| *The basis for the assumed risk is the event rate in the control (placebo or standard care) group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded one level due to risk of bias; unclear information reported in one study regarding study design (randomisation and blinding) and loss to follow up from the study
2 Downgraded one level due to imprecision of results: wide 95% CI that includes both considerable harm and benefit.
1. Antiepileptic drug versus placebo or usual care
1.1 Occurrence of early seizure
Five trials involving 987 participants examined the occurrence of early seizures (Glotzner 1983; Pechadre 1991; Temkin 1990; Young 2004; Young 1983). All trials compared a traditional AED (carbamazepine or phenytoin) with a placebo or usual care. The trials included a range of ages from children to adult. Duration of treatment for this outcome varied from five to seven days. The proportion of participants experiencing an early seizure in the treatment group was 5.0% (25/499) compared with 13.9% (68/488) in the placebo group/usual care group. The pooled results favored the traditional AED treatment compared with the control group (RR 0.42, 95% CI 0.23 to 0.73; Analysis 1.1). Heterogeneity was low among the studies (I2 = 29%). We reanalyzed the outcome using fixed‐effect methods; results were consistent with those obtained using random‐effects models. We rated the quality of the evidence as low due to high selective reporting bias in 3 of the studies and inconsistency in RR results in the two studies with low risk of bias (Analysis 1.7).
1.1. Analysis.
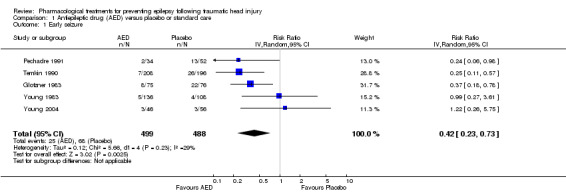
Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 1 Early seizure.
1.7. Analysis.
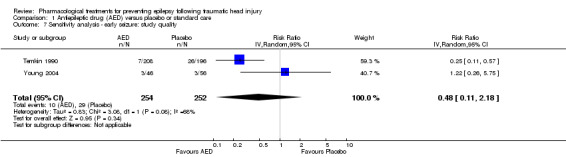
Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 7 Sensitivity analysis ‐ early seizure: study quality.
1.2 Occurrence of late seizure
Six trials reported on late seizures in 1029 participants (Glotzner 1983; McQueen 1983; Young 1983; Temkin 1990; Pechadre 1991; Manaka 1992). Manaka 1992 compared phenobarbital with usual care, Glotzner 1983 compared carbamazepine with placebo, while the other four trials compared phenytoin with placebo. Duration of treatment varied from three months to two years. Five of the six trials included adults and children; Temkin 1999 was the only trial to assess adults exclusively. About 15.6% (81/518) of participants receiving AED treatment experienced a late seizure compared with 17.8% (91/511) receiving placebo/usual care. The six‐pooled studies showed no statistically significant effect for traditional AEDs compared with placebo or usual care on late seizure occurrence (RR 0.91, 95% CI 0.57 to 1.467; Analysis 1.2). There was evidence of heterogeneity among the studies (I2 = 54%). This result was rated as very low quality due to high risk of bias in 2 or more categories for several studies, imprecision of pooled RR estimate and moderate level of heterogeneity.
1.2. Analysis.
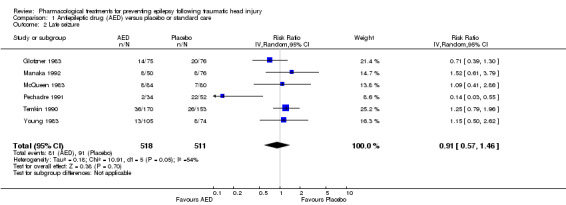
Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 2 Late seizure.
1.3 All‐cause mortality
Five trials reported mortality in 1065 participants (Glotzner 1983; McQueen 1983; Young 1983; Temkin 1990; Young 2004). They compared a traditional AED (carbamazepine or phenytoin) with placebo. Duration of treatments varied from five days to 24 months. About 18.4% (101/549) of participants in the AED group died compared with 17.4% (90/516) in the placebo group. The five pooled trials showed no statistically significant difference in the RR of death between participants treated with traditional AEDs compared with placebo (RR 1.08, 95% CI 0.79 to 1.46; Analysis 1.3). Heterogeneity was low among the studies (I2 = 19%). This result was rated as very low quality due to high risk of bias in 2 or more categories for several studies, imprecision of pooled RR estimate with confidence interval ranging from benefit to harm.
1.3. Analysis.
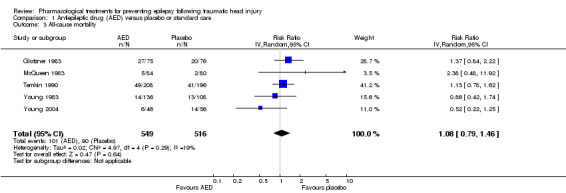
Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 3 All‐cause mortality.
1.4 Any serious event
McQueen 1983 and Temkin 1990 looked at any serious events comparing phenytoin with placebo. About 14.4% (42/292) of participants in treatment group experienced an adverse event compared with 9.4% (26/276) in the placebo group. The pooled RR of an adverse event in treatment group compared with placebo was 1.63 (95% CI 0.73 to 3.66; 568 participants; Analysis 1.4). Heterogeneity was low among the studies (I2 = 18%). We performed no subgroup analysis due to too few studies.The result was rated as low based on imprecision of RR estimate with confidence intervals covering both benefit and harm and serious risk of bias in one study.
1.4. Analysis.
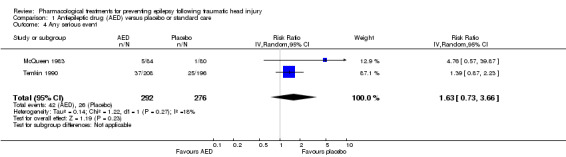
Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 4 Any serious event.
1.5 Skin rash
McQueen 1983 and Temkin 1990 reported skin rash comparing phenytoin with placebo. About 10.3% (30/292) of participants in the phenytoin group experienced skin rash compared with 6.5% (18/276) in the placebo group. The RR of skin rash in the phenytoin group compared with placebo was 1.65 (99% CI 0.54 to 5.04; 568 participants; Analysis 1.5). Heterogeneity was low among the studies (I2 = 17%). We performed no subgroup analysis due to too few studies. The result was rated as low based on imprecision of RR estimate with confidence intervals covering both benefit and harm and serious risk of bias in one study.
1.5. Analysis.
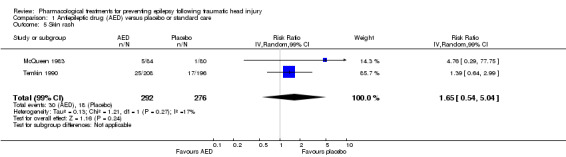
Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 5 Skin rash.
1.6 Sensitivity analysis
Occurrence of early seizure: age of population
Four of the five trials that reported on early seizures had a mean or median age that was greater than 18 years (Glotzner 1983; Young 1983; Temkin 1990; Pechadre 1991). Young 2004 consisted solely of children. We ran the analysis excluding Young 2004. About 4.9% (22/453) of participants treated with an AED experienced an early seizure compared with 15% (65/432) receiving placebo. The result still favored AED treatments compared with placebo; producing a marginally lower RR compared with the original analysis in Section 1.1 (RR 0.36, 95% CI 0.21 to 0.60, I2 = 12%; Analysis 1.6) (compare with Analysis 1.1).
1.6. Analysis.
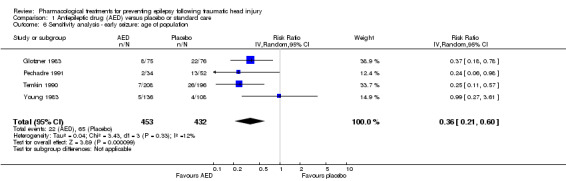
Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 6 Sensitivity analysis ‐ early seizure: age of population.
1.7 Sensitivity analysis
Occurrence of early seizure: study quality
Three of the five trials that examined early seizures had high risk of bias in one or more category (Glotzner 1983; Young 1983; Pechadre 1991). We reran the analysis excluding Glotzner 1983; Young 1983; and Pechadre 1991. The pooled results of the two remaining studies no longer showed a benefit of AED treatment compared with placebo (RR 0.48, 95% CI 0.11 to 2.18, 506 participants; Analysis 1.7) (Temkin 1990; Young 2004). Heterogeneity (I2 = 68%) increased compared with the original analysis (see Analysis 1.1). Differences in participant populations likely contributed to the increase in heterogeneity; participants in the Temkin 1990 trial were adults, whereas Young 2004 studied exclusively children.
1.8 Subgroup analysis
Occurrence of late seizure: type of antiepileptic drug
Four of the six trials compared a traditional AED treatment, phenytoin, with placebo for late seizures (McQueen 1983; Young 1983; Temkin 1990; Pechadre 1991). The two remaining studies treated participants with other AEDs; carbamazepine compared with placebo (Glotzner 1983), and phenobarbital compared with usual care (Manaka 1992). In subgroup analysis, 15% (59/393) of participants treated with phenytoin experienced a late seizure compared with 17.5% (63/359) receiving placebo (RR 0.83, 95% CI 0.40 to 1.70; 752 participants; Analysis 1.8). About 17.6% (22/125) of participants receiving carbamazepine or phenobarbital experienced a late seizure compared with 18.4% (28/152) in the placebo or usual care group (RR 0.96, 95% CI 0.46 to 1.99, 277 participants; Analysis 1.8). There was no statistically significant subgroup difference between the types of antiepileptic drug (P=0.78, I2 =0.0%). The subgroup RRs did not differ substantially from the primary analysis results (see Analysis 1.2).
1.8. Analysis.
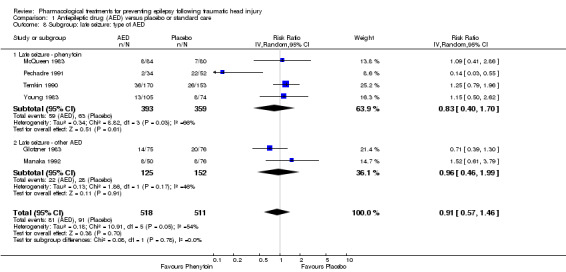
Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 8 Subgroup: late seizure: type of AED.
1.9 Subgroup analysis
Occurrence of late seizure: treatment duration
Five of the six trials that examined the occurrence of late seizures had a treatment duration ranging from 12 to 24 months (Glotzner 1983; McQueen 1983; Young 1983; Temkin 1990; Manaka 1992). One trial had a treatment duration less than one year (Pechadre 1991). In subgroup analysis, for treatment duration of 12 to 24 months, 16.3% (79/484) of participants in the AED group experienced late seizures compared with 15.1% (69/459) of participants in the control groups. In the Pechadre 1991 trial, 5.88% (2/34) of participants in the AED treatment group experienced late seizures compared with 42.3% (22/52) in the control group. Although there was no statistically significant subgroup difference between different treatment durations (P=0.87, I2 =0.004%) the results show greater risk in the AED treatment group for studies with longer treatment duration (12 to 24 months) (RR 1.08, 95% CI 0.81 to 1.46, 943 participants; Analysis 1.9) while the one study with treatment duration of less than one year showed reduced risk (RR 0.14, 95% CI 0.03 to 0.55, 86 participants; Analysis 1.9) (Pechadre 1991).
1.9. Analysis.
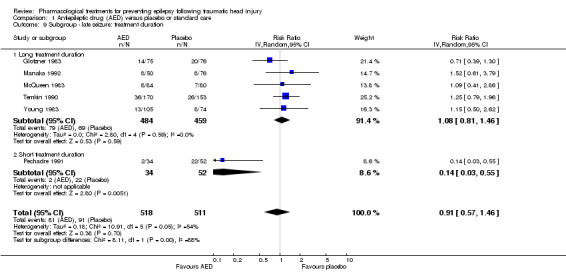
Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 9 Subgroup ‐ late seizure: treatment duration.
1.10 Sensitivity analysis
Occurrence of late seizure: age of population
Five of the six trials that examined occurrence of late seizures included both adults and children (Glotzner 1983; McQueen 1983; Young 1983; Pechadre 1991; Manaka 1992). Removing Temkin 1990, the only study that excluded children, from the analysis did not alter the results substantially. The pooled effect remained statistically non‐significant as per the original results (RR 0.81, 95% CI 0.44 to 1.48, 706 participants; Analysis 1.10) as per the original results (see Analysis 1.2).
1.10. Analysis.
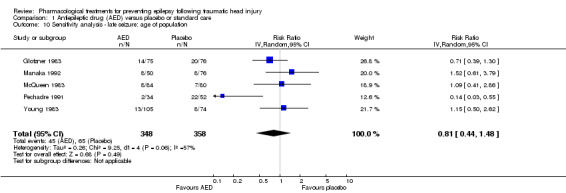
Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 10 Sensitivity analysis ‐ late seizure: age of population.
1.11 Sensitivity analysis
Occurrence of late seizure: comparison group
Five trials of the six trials that examined the occurrence of late seizures compared an AED with placebo (Glotzner 1983; McQueen 1983; Young 1983; Temkin 1990; Pechadre 1991). Manaka 1992 compared an AED treatment with usual care. The pooled RR, excluding Manaka 1992, remained not statistically significant (RR 0.83, 95% CI 0.48 to 1.41, 903 participants; Analysis 1.11) (see Analysis 1.2).
1.11. Analysis.
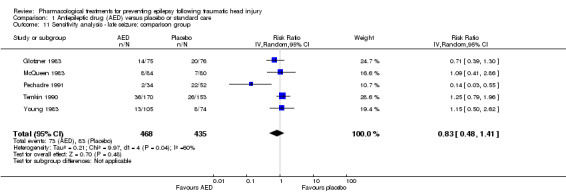
Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 11 Sensitivity analysis ‐ late seizure: comparison group.
1.12 Sensitivity analysis
Occurrence of late seizure: study quality
Five of the six trials that examined the occurrence of late seizures had a high risk of bias in one or more bias categories (Glotzner 1983; McQueen 1983; Young 1983; Pechadre 1991; Manaka 1992). Temkin 1990 was the only trial that did not have a high risk of bias in any category. The Temkin 1990 trial favored the placebo group (RR 1.25, 95% CI 0.79 to 1.96, 323 participants; Analysis 1.18), which differs from the original analysis, which favored the AED treatment (see Analysis 1.2). However, neither comparison was statistically significant.
1.13 Subgroup analysis
All‐cause mortality: age of population
Two studies examined all‐cause mortality in children only (ages under 17 years) (Young 1983; Young 2004). The pooled proportion of children that died in the AED treatment group was 8.5% (8/71) and 22.2% (16/72) died in the placebo group (RR 0.54, 95% CI 0.25 to 1.19, 143 participants; Analysis 1.13). Temkin 1990 was the only study that exclusively enrolled participants over 17 years of age. About 23.5% (49/208) of adults treated with AED died compared with 20.1% (41/196) of adults receiving placebo. The pooled RR was 1.13 (95% CI 0.78 to 1.62, 404 participants; Analysis 1.13). The remaining two studies examined all‐cause mortality in a predominantly adult population; although children were included (Glotzner 1983;McQueen 1983). The pooled proportion that died in the AED treatment group was 20.1% (32/159) and 14.1% (22/156) died in the placebo group (RR 1.43, 95% CI 0.90 to 2.27, 315 participants; Analysis 1.13). The studies including exclusively or predominately adults showed an increased risk of mortality in the AED group compared with placebo (Glotzner 1983; McQueen 1983; Temkin 1990), while the studies including only children showed a decreased risk with treatment. The test for subgroup differences showed moderate heterogeneity (P=0.11, I2 =53.9%).
1.13. Analysis.
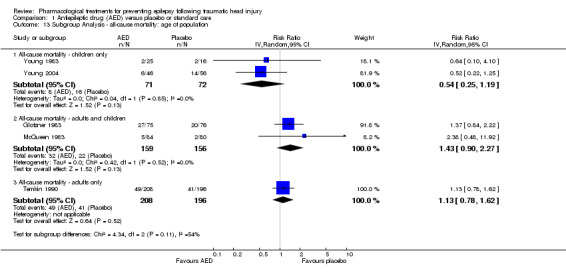
Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 13 Subgroup Analysis ‐ all‐cause mortality: age of population.
1.14 Subgroup analysis
All‐cause mortality: treatment duration
Two of the five trials examining all‐cause mortality had a short‐term treatment duration of less than one week (Young 1983; Young 2004). 9.9% (18/182) of participants treated with an AED for one week or less died compared with 15.2% (25/164) of participants in the control groups. The pooled RR for these short‐term treatments was non‐significant and favored AED treatment (RR 0.69, 95% CI 0.39 to 1.24, 346 participants; Analysis 1.14). In comparison, the three trials that used a treatment duration of a 12 months or longer had a non‐significant pooled RR that favored the control group; 22.1% (81/367) of participants in the AED group died compared with 17.9% (63/352) of participants in the control groups (RR 1.24, (95% CI 0.93 to 1.65, 719 participants; Analysis 1.14) (Glotzner 1983; McQueen 1983; Temkin 1990). The test for subgroup differences between studies of different treatment duration was statistically significant and suggested moderate heterogeneity (P=0.08, I2 =67.4%). Duration of treatment was not further divided into mid‐term and long‐term duration due to low number of studies.
1.14. Analysis.
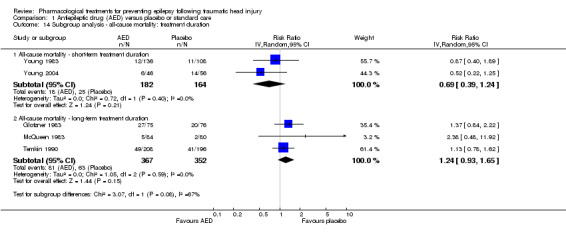
Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 14 Subgroup analysis ‐ all‐cause mortality: treatment duration.
1.15 Sensitivity analysis
All‐cause mortality: type of antiepileptic drug
In four of the five trials that examined mortality, participants received phenytoin in the AED group (McQueen 1983; Young 1983; Temkin 1990; Young 2004). Glotzner 1983 compared carbamazepine with placebo. Excluding Glotzner 1983, 15.6% (74/474) of participants treated with phenytoin died compared with 15.9% (70/440) of participants who received placebo (RR 0.97, 95% CI 0.65 to 1.43, 914 participants; Analysis 1.15). The results remain consistent with the original analysis (see Analysis 1.3).
1.15. Analysis.
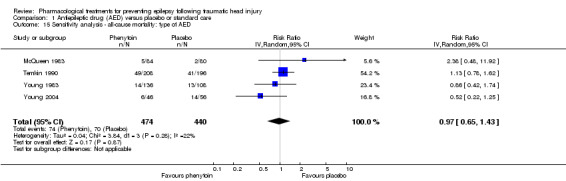
Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 15 Sensitivity analysis ‐ all‐cause mortality: type of AED.
1.16 Sensitivity analysis
All‐cause mortality: study quality
Three of the five trials that examined mortality had a high risk of bias in one or more bias categories (Glotzner 1983; McQueen 1983; Young 1983). We reran the analysis excluding these studies. The pooled results for the remaining studies with low/unclear risk of bias showed no statistically significant difference between treatment groups ((RR 1.00, 95% CI 0.72 to 1.41) 506 participants; Analysis 1.16) (Temkin 1990; Young 2004). The original results were also statistically non‐significant, but favored the placebo group (see Analysis 1.3).
1.16. Analysis.
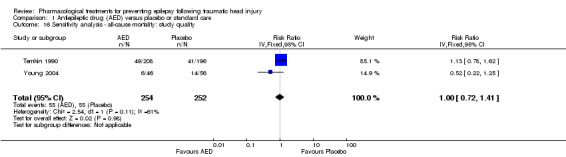
Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 16 Sensitivity analysis ‐ all‐cause mortality: study quality.
2. Neuroprotective agent versus placebo
Only one study compared a pharmacologic agent (magnesium sulfate; MgSO4) other than an AED with a placebo (Temkin 2007).
2.1 Occurrence of early seizure
Temkin 2007 reported on the occurrence of early seizures. About 0.4% (1/250) of participants in the neuroprotective agent group experienced an early seizure compared with 0% (0/249) in the placebo group (Analysis 2.1). However, as reported in the results section of their paper, 96% of participants received phenytoin for the first week in both treatment groups. This may have resulted in a very low early seizure rate.
2.1. Analysis.
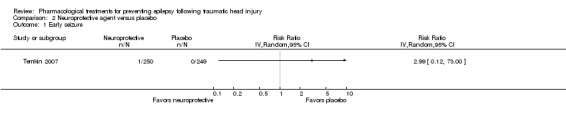
Comparison 2 Neuroprotective agent versus placebo, Outcome 1 Early seizure.
2.2 Occurrence of late seizure
Temkin 2007 reported on the occurrence of late seizures. About 6% (15/250) of participants treated with neuroprotective agent experienced late seizures compared with 5.6% (14/249) of participants treated with a placebo (RR 1.07, 95% CI 0.53 to 2.17, 498 participants; Analysis 2.2). There was no evidence of effect of neuroprotective agents compared with placebo on late seizures.
2.2. Analysis.
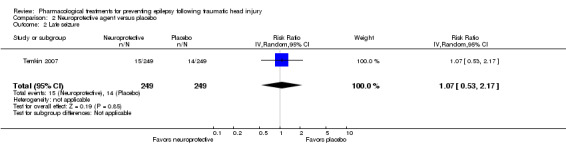
Comparison 2 Neuroprotective agent versus placebo, Outcome 2 Late seizure.
2.3 All‐cause mortality
Only Temkin 2007 reported mortality. About 21% (52/250) of participants died in the neuroprotective agent group compared with 14% (35/240) of participants in the control group (RR 1.20, 95% CI 0.80 to 1.81, 466 participants; Analysis 2.3).
2.3. Analysis.
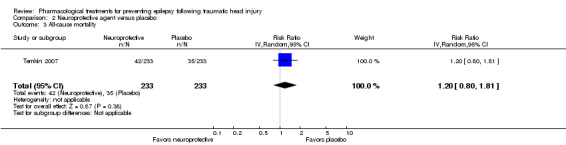
Comparison 2 Neuroprotective agent versus placebo, Outcome 3 All‐cause mortality.
3. Antiepileptic drugs versus other antiepileptic drugs
Two trials compared phenytoin with another AED (Temkin 1999; Szaflarski 2010). Szaflarski 2010 compared phenytoin with levetiracetam, a newly licensed AED while Temkin 1999 compared phenytoin with valproate. Treatment duration was one week in the Szaflarski 2010 trial compared with up to six months in the valproate arm of the Temkin 1999 study. The age ranges were similar in both studies and neither study showed high bias in any category. We performed no subgroup analysis due to too few studies and low evidence of heterogeneity between the two studies.
3.1 Occurrence of early seizure
Szaflarski 2010 and Temkin 1999 reported on the occurrence of early seizures and compared phenytoin with another AED. About 3.3% (5/150) of participants treated with phenytoin had an early seizure compared with 5.7% (16/281) of participants treated with another AED. The pooled results of Szaflarski 2010 and Temkin 1999 showed no statistically significant effect of phenytoin compared with another AED (RR 0.66, 95% CI 0.20 to 2.12, 558 participants; Analysis 3.1). Heterogeneity between the two studies was low (I2= 29%).
3.1. Analysis.
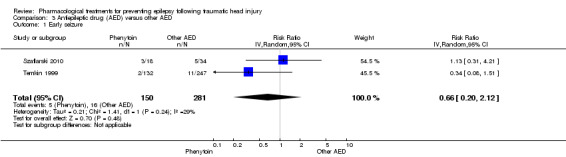
Comparison 3 Antiepileptic drug (AED) versus other AED, Outcome 1 Early seizure.
3.2 Occurrence of late seizure
Szaflarski 2010 and Temkin 1999 reported on the occurrence of late seizures and compared phenytoin with another AED drug. About 12.4% (17/137) of participants treated with phenytoin experienced late seizures compared with 16.6% (40/241) of participants treated with another AED. The pooled RR of experiencing late seizures on phenytoin compared with another AED was not statistically significant (RR 0.77, 95% CI 0.46 to 1.30, 378 participants; Analysis 3.2). Heterogeneity between the two studies was low (I2 = 0%).
3.2. Analysis.
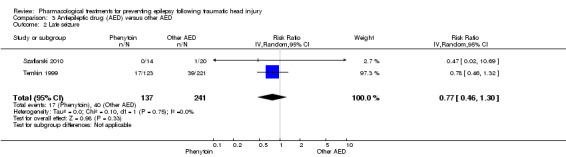
Comparison 3 Antiepileptic drug (AED) versus other AED, Outcome 2 Late seizure.
3.3 All‐cause mortality
Szaflarski 2010 and Temkin 1999 reported all‐cause mortality. About 8.7% (13/150) of participants in the phenytoin group died compared with 16.4% (46/281) of participants in the other AED group. The pooled RR for mortality in the phenytoin group compared with the other AED group was 0.53 (95% CI 0.30 to 0.94; Analysis 3.3). Heterogeneity between the two studies was low (I2 = 0%). We performed no subgroup analysis due to too few studies and low evidence of heterogeneity.
3.3. Analysis.
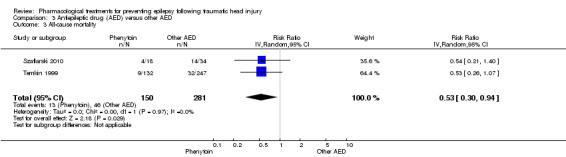
Comparison 3 Antiepileptic drug (AED) versus other AED, Outcome 3 All‐cause mortality.
Discussion
Summary of main results
The review included 10 RCTs described in 12 reports, involving 2326 participants. Interventions were reported in three categories; traditional AED versus placebo or usual care, phenytoin versus other AED treatment, and alternative neuroprotective agent versus placebo or usual care.
Five studies with 987 participants studied early seizure in participants treated with a traditional AED compared with placebo or usual care. There was low quality evidence that treatment with a traditional AED (phenytoin or carbamazepine) decreased the risk of early seizure compared with placebo or usual care (RR 0.42, 95% CI 0.23 to 0.73, P value = 0.003).
The risk of late seizure occurrence was reduced by AED treatment compared with placebo or usual care, although the benefit was not statistically significant (RR 0.91, 95% CI 0.57 to 1.46, 1029 participants). The risk of late seizure favored placebo in the only trial that did not have a high risk of bias in any category (RR 1.25, 95% 0.79 to 1.96, 323 participants), although evidence of effect remained non‐significant (Temkin 1990). Caution should be taking when considering this sensitivity analysis as it was based on only one study.
There was no significant difference in mortality between participants in the AED drug and participants in the placebo or usual care group (RR 1.08, 95% CI 0.79 to 1.46, P value = 0.64) although the results were based on very low quality of evidence due to imprecision and inconsistency in results.
The review included only one study that examined other potentially neuroprotective agents compared with placebo or usual care (Temkin 2007). There was no evidence of treatment effect on late seizures (RR 1.07, 95% CI 0.53 to 2.17) or all‐cause mortality (RR 1.20, 95% CI 0.80 to 1.81) for this comparison. There were no events in the placebo arm for the outcome of early seizure and a rate of 0.4% (1/250) early seizures in the treatment group. However, almost all participants (96%) in this study also received phenytoin for the first week following injury. No doses or details on phenytoin treatment levels were provided in the paper.
There was evidence of treatment benefit of phenytoin in comparison to another AED (levetiracetam or valproate). Phenytoin significantly reduced the risk of mortality compared with another AED (RR 0.53, 95% CI 0.30 to 0.94). Caution must be taken as this result was based on only two studies. No treatment benefit of phenytoin was observed compared with another AED for early seizure (RR 0.66, 95% CI 0.20 to 2.12) or late seizure (RR 0.77, 95% CI 0.46 to 1.3).
Only two of the included trials reported any serious treatment‐related adverse event. Both trials compared a traditional AED with placebo. There was no evidence of increased risk of adverse effects for the AED group (RR 1.63, 95% CI 0.73 to 3.66). Similarly there was no evidence of increased risk of skin rash (RR 1.65, 99% CI 0.54 to 5.04).
Overall completeness and applicability of evidence
All participants included in the review had a diagnosis of moderate‐to‐severe TBI. The methods of measurement of severity of TBI varied between studies. The majority of participants were admitted to trauma centers or emergency departments. Participants were randomized and received treatment within 24 hours of admission; however, one study reported that the majority of participants were treated within 14 days post‐injury. Three studies allowed the inclusion of participants with an immediate post‐injury seizure, while other studies listed this as an exclusion criteria. One study included participants if they had a pre‐injury seizure; however, these participants were excluded from outcomes of early and late seizure outcomes. Reporting of outcomes was not consistent across the studies with only two studies reporting any serious adverse event and skin rashes. Two studies also did not document mortality and the majority of studies did not consider time to first seizure or time to second seizure from first seizure. Maintaining therapeutic levels of AED was a challenge in many of the trials with reports of only 40% to 80% of participants maintaining therapeutic levels at follow‐up visits. Several studies did not follow the participants beyond the treatment duration, thereby limiting the ability to determine if the treatment effect is sustained once mediation is stopped.
Quality of the evidence
Overall quality of the evidence was varied. All included trials were RCTs yet the majority did not adequately describe the randomization and allocation processes clearly. The majority adequately blinded participants and personnel, but blinding of outcome assessment was considered either unclear or high in five of the 10 trials. Risk of bias of selective reporting was also unclear or high in six trials. Potential sources of bias in this review were: the inability to assess if treatment effects differed between children and adults adequately as children were included in many of the trials and not analyzed separately; and differing treatment protocols regarding timing and duration of treatment, AED dose, maintaining therapeutic levels of AEDs, differences in severity of trauma and different methods of evaluating seizure occurrence. Due to the high proportion of studies in this review that we categorized as unclear and high risk of bias, the findings in this review should be interpreted with caution.The evidence was graded as low to very low for all outcome comparisons based on the high risk of bias previously discussed as wells uncertainty in the estimates with many confidence intervals showing both harm and benefit.
Potential biases in the review process
The Cochrane Epilepsy Review Group conducted a comprehensive search of all published data as well as handsearching the bibliography of selected studies and reviews. We reviewed the full‐text reports and two review authors (KT and HA) extracted data and resolved disagreements by discussion to minimize bias. We were unable to obtain further information from some of the trials because they were published many years ago or the authors could not be contacted. We are unable to comment on the potential for publication bias in the review due to the insufficient number of studies to analyze publication bias in funnel plots.
Agreements and disagreements with other studies or reviews
This review differed from the original review by Schierhout 2001 in that we included a study looking at other potentially neuroprotective agents and studies with dual treatment groups. The comparison of traditional AEDs with placebo now includes a study carried out exclusively in children (Young 2004), which was not in the previous review. The results were consistent in both reviews, treatment with traditional AED reduced the risk of early seizure compared with placebo or usual care. When those studies with high bias in at least one category were removed, the evidence of treatment effect was no longer significant. However, heterogeneity among this subset of studies increased, potentially due to differences in treatment duration and median age of participants.
There was no evidence of treatment effect on late seizure occurrence or mortality. This result was consistent with the results for late seizure occurrence and mortality published in the previous review (Schierhout 2001).
Authors' conclusions
Implications for practice.
This review found low quality of evidence that early treatment with an antiepileptic drug (AED) compared with a placebo or usual care reduced the risk of early post‐traumatic seizures. There was no evidence to support a reduction in the risk of late seizures or mortality.However, these results must be interpreted with caution due to potential bias and high level of heterogeneity among studies and were graded as very low quality. The risk of serious or other adverse events was not greater among treatment versus placebo groups, but this may be due to limited number of trials included in the comparison and small sample size. There was insufficient evidence to make any conclusions regarding the effectiveness and safety of other neuroprotective agents compared with placebo or phenytoin, a traditional AED, compared with another AED.
Implications for research.
There have been very few studies in the area, with wide variability in the age of the target population, definition of early seizures, assessment of the extent of brain injury, timing of the intervention, AED dosage and duration of treatment. Further high‐quality randomized controlled trials are warranted, particularly for the newly licensed products. Only one published study looking at alternative neuroprotective agents was eligible for inclusion in the review; therefore, further investigation is needed.
Acknowledgements
The review authors would like to thank Tracey Daley, Research Assistant for her valued assistance in writing the protocol. We would also like to thank the Nova Scotia Health Research Foundation (NSHRF) for their funding support though the Systematic Review Grant.
Appendices
Appendix 1. Cochrane Epilepsy Group Specialized Register search strategy
#1 MeSH DESCRIPTOR Craniocerebral Trauma Explode All
#2 craniocerebral next injur*
#3 craniocerebral next trauma*
#4 brain next injur*
#5 brain next trauma*
#6 head next injur*
#7 head next trauma*
#8 "post‐trauma" or "post trauma" or posttrauma
#9 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8
#10 MeSH DESCRIPTOR Phenytoin Explode All
#11 MeSH DESCRIPTOR Phenobarbital Explode All
#12 MeSH DESCRIPTOR Carbamazepine Explode All
#13 phenytoin or phenobarb* or carbamazepine
#14 levetiracetam or etiracetam or lamotrigine or oxcarbazepine
#15 topiramate or gabapentin or lacosamide or harkeroside
#16 MeSH DESCRIPTOR Magnesium Sulfate Explode All
#17 "magnesium sulphate" or "magnesium sulfate"
#18 MeSH DESCRIPTOR Neuroprotective Agents Explode All
#19 MeSH DESCRIPTOR Nerve Growth Factors Explode All
#20 neurotrophic next factor*
#21 MeSH DESCRIPTOR Hormones Explode All
#22 MeSH DESCRIPTOR Antioxidants Explode All
#23 antioxida*
#24 MeSH DESCRIPTOR Anticonvulsants Explode All
#25 antiepilep* or "anti‐epilep*"
#26 #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25
#27 #9 AND #26
#28 #27 AND >16/12/2013:CRSCREATED AND INREGISTER
Appendix 2. CENTRAL (via CRSO) search strategy
#1 MESH DESCRIPTOR Craniocerebral Trauma EXPLODE ALL TREES
#2 (craniocerebral next injur*):TI,AB,KY
#3 (craniocerebral next trauma*):TI,AB,KY
#4 (brain next injur*):TI,AB,KY
#5 (brain next trauma*):TI,AB,KY
#6 (head next injur*):TI,AB,KY
#7 (head next trauma*):TI,AB,KY
#8 ((post‐trauma) or (post trauma) or (posttrauma)):TI,AB,KY
#9 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8
#10 MESH DESCRIPTOR Phenytoin EXPLODE ALL TREES
#11 MESH DESCRIPTOR Phenobarbital EXPLODE ALL TREES
#12 MESH DESCRIPTOR Carbamazepine EXPLODE ALL TREES
#13 (phenytoin or phenobarb* or carbamazepine):TI,AB,KY
#14 (levetiracetam or etiracetam or lamotrigine or oxcarbazepine):TI,AB,KY
#15 (topiramate or gabapentin or lacosamide or harkeroside):TI,AB,KY
#16 MESH DESCRIPTOR Magnesium Sulfate EXPLODE ALL TREES
#17 ("magnesium sulphate" or "magnesium sulfate"):TI,AB,KY
#18 MESH DESCRIPTOR Neuroprotective Agents EXPLODE ALL TREES
#19 MESH DESCRIPTOR Nerve Growth Factors EXPLODE ALL TREES
#20 (neurotrophic next factor*):TI,AB,KY
#21 MESH DESCRIPTOR Hormones EXPLODE ALL TREES
#22 MESH DESCRIPTOR Antioxidants EXPLODE ALL TREES
#23 antioxida*:TI,AB,KY
#24 MESH DESCRIPTOR Anticonvulsants EXPLODE ALL TREES
#25 (antiepilep* or anti‐epilep*):TI,AB,KY
#26 #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25
#27 (epilep* or convuls* or seizure*):TI,AB,KY
#28 MESH DESCRIPTOR Epilepsy, Post‐Traumatic EXPLODE ALL TREES
#29 #27 OR #28
#30 #9 AND #26 AND #29
#31 * NOT INMEDLINE AND 30/11/2013 TO 28/02/2015:DL
#32 #30 AND #31
Appendix 3. MEDLINE search strategy
This strategy is based on the Cochrane Highly Sensitive Search Strategy for identifying randomised trials published in Lefebvre 2011.
1. (randomized controlled trial or controlled clinical trial).pt. or (randomi?ed or placebo or randomly).ab.
2. clinical trials as topic.sh.
3. trial.ti.
4. 1 or 2 or 3
5. exp animals/ not humans.sh.
6. 4 not 5
7. exp Craniocerebral Trauma/
8. ((craniocerebral or brain or head) adj (injur* or trauma*)).tw.
9. (post‐trauma or post trauma or posttrauma).tw.
10. 7 or 8 or 9
11. exp Phenytoin/
12. exp Phenobarbital/
13. exp Carbamazepine/
14. (phenytoin or phenobarb* or carbamazepine).tw.
15. (levetiracetam or etiracetam or lamotrigine or oxcarbazepine).tw.
16. (topiramate or gabapentin or lacosamide or harkeroside).tw.
17. exp Magnesium Sulfate/
18. (magnesium sulphate or magnesium sulfate).tw.
19. exp Neuroprotective Agents/
20. exp Nerve Growth Factors/
21. neurotrophic factor*.tw.
22. exp Hormones/
23. exp Antioxidants/
24. antioxida*.tw.
25. exp Anticonvulsants/
26. (antiepilep* or anti‐epilep*).tw.
27. 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26
28. (epilep* or convuls* or seizure*).tw.
29. exp Epilepsy, Post‐Traumatic/
30. 28 or 29
31. 6 and 10 and 27 and 30
32. limit 31 to ed=20131216‐20150113
Appendix 4. EMBASE search strategy
#1 random*
#2 placebo*
#3doubl* NEAR/3 blind*
#4 assign*
#5 singl* NEAR/3 blind*
#6 allocat*
#7 volunteer*
#8 'double blind procedure'/exp
#9 'randomized controlled trial'/exp
#10 single AND 'blind'/exp
#11 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9
#12 #10 OR #11
#13 'animal'/exp NOT 'human'/exp
#14 #12 NOT #13
#15 'head injury'/exp
#16 (craniocerebral OR brain OR head) NEAR/3 (injur* OR trauma*)
#17 posttrauma*
#18 post NEAR/3 trauma*
#19 #15 OR #16 OR #17 OR #18
#20 'phenytoin'/exp
#21 'phenobarbital' /exp
#22 'phenobarbital'/exp
#23 'carbamazepine'/exp
#24 'etiracetam' /exp
#25 'gabapentin'/exp
#26 'harkoseride'/exp
#27 'lamotrigine'/exp
#28 'topiramate'exp
#29 'oxcarbazepine'exp
#30 lamotrigine OR topiramate OR oxcarbazepine
#31 levetiracetam OR etiracetam OR lacosamide OR harkoseride
#32 phenytoin OR phenobarb* OR carbamazepine OR gabapentin
#33 'magnesium sulfate'/exp
#34 'magnesium sulphate' OR 'magnesium sulfate'
#35 'neuroprotective agent'/exp
#36 'nerve growth factor'/exp
#37 'hormone'/exp
#38 'anticonvulsive agent'/exp OR 'anticonvulsant activity'/exp
#39 'antioxidant'/exp OR 'antioxidant activity'/exp
#40 neuro* NEAR/3 factor*
#41 antioxida*
#42 anti*epilep*
#43 hormon*
#44 'nerve growth' NEAR/3 factor*
#45 #20 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44
#46 epilep* OR convuls* OR seizure*
#47 'seizure'/exp OR 'epilepsy'/exp OR 'convulsion'/exp
#48 #46 OR #47
#49 #14 AND #19 AND #45 AND #48
#50 #14 AND #19 AND #45 AND #48 AND [15‐8‐2011]/sd NOT [5‐9‐2014]/sd
Appendix 5. SCOPUS search strategy
(TITLE‐ABS‐KEY(randomly OR "clinical trial" OR "controlled trial" OR randomised OR randomized OR placebo*)) AND (TITLE‐ABS‐KEY("craniocerebral trauma*" OR "craniocerebral injur*" OR "brain trauma*" OR "brain injur*" OR "head trauma*" OR "head injur*" OR posttrauma* OR post‐trauma* OR "post trauma*")) AND (TITLE‐ABS‐KEY(phenytoin OR phenobarb* OR carbamazepine OR levetiracetam OR etiracetam OR lamotrigine OR oxcarbazepine OR topiramate OR gabapentin OR lacosamide OR harkeroside OR "magnesium sulphate" OR "magnesium sulfate" OR "neurotrophic factor*" OR antioxida* OR antiepilep* OR anti‐epilep*)) AND (TITLE‐ABS‐KEY(epilep* OR convuls* OR seizure)) AND ( LIMIT‐TO(PUBYEAR,2013) OR LIMIT‐TO(PUBYEAR,2012) ) AND ( LIMIT‐TO(EXACTKEYWORD,"Human" ) OR LIMIT‐TO(EXACTKEYWORD,"Humans" ) )
Appendix 6. Biological Abstracts search strategy
#1 TS=(((craniocerebral or brain or head) NEAR/1 (injur* or trauma*))) Indexes=Biological Abstracts Timespan=All years
#2 TS=("post‐trauma" OR "posttrauma") Indexes=Biological Abstracts Timespan=All years
#3 TS=(phenytoin OR phenobarb* OR carbamazepine OR levetiracetam OR etiracetam OR lamotrigine OR oxcarbazepine OR topiramate OR gabapentin OR lacosamide OR harkeroside OR "magnesium sulphate" OR "magnesium sulfate" OR "neurotrophic factor*" OR antioxida* OR antiepilep* OR anti‐epilep*) Indexes=Biological Abstracts Timespan=All years
#4 TS=(epilep* OR convuls* OR seizure*) Indexes=Biological Abstracts Timespan=All years
#5 #1 OR #2 Indexes=Biological Abstracts Timespan=All years
#6 #3 AND #4 AND #5 Indexes=Biological Abstracts Timespan=All years
#7 TS=(randomly OR "clinical trial" OR "controlled trial" OR randomised OR randomized OR placebo*) Indexes=Biological Abstracts Timespan=All years
#8 #6 and #7 Indexes=Biological Abstracts Timespan=All years
#9 #7 AND #6 Indexes=Biological Abstracts Timespan=2011‐2015
Appendix 7. Data extraction form
| Reviewer: | Date of review: |
1. Study Description
| Study ID number: | RefWorksID number: |
|
Corresponding author’s name and institution: |
Corresponding author’s email: |
|
Full citation, including all author names: Only abstract was published. |
Author contacted: Yes No If Yes: Indicate reason: _____________________________ ___________________________________________ Date message sent: _____________ _____ Response summary: _________________________ ___________________________________________ Date of response: __________________ |
|
Setting of study: |
Language: English Other ___________________________________ |
|
Check off inclusion criteria: Study is an RCT or a quasi‐randomized trial. Patients were diagnosed with TBI. Study involves administration of pharmacologic agents for the prevention of post‐traumatic epilepsy. Study excluded patients that had a previous documented unprovoked seizure. Study reports outcomes of interest. Additional notes: | |
Part I: Data extraction form
2. General study design questions
| Was this a multicenter study? |
Not reported Yes No |
| Duration of enrolment: | |
| Duration of follow‐up: | |
| Study excluded patients six years and under? |
Not reported Yes No (indicate details in part 3: Participants) |
3. Participants
| Overall | Control | Treatment 1 | Treatment 2 | |
| N number randomized | ||||
| N number followed up | ||||
|
Age classification: |
All patients are < 17 yrs All patients are >= 17 Neither of the above. |
All patients are < 17 yrs All patients are >= 17 Neither of the above. |
All patients are < 17 yrs All patients are >= 17 Neither of the above. |
All patients are < 17 yrs All patients are >= 17 Neither of the above. |
|
Indicate age: range: or Mean/SD: or Median: Additional details: |
|
|
|
|
| Gender (Proportion male) | ||||
|
Trauma severity |
Undefined Minor Moderate Severe |
Undefined Minor Moderate Severe |
Undefined Minor Moderate Severe |
Undefined Minor Moderate Severe |
|
Indicate methods of measurement for severity: e.g.: EEG,MRI, or CT scan findings, GCS range, etc. |
||||
| How were seizures identified? |
Not reported EEG Clinical findings |
Not reported EEG Clinical findings |
Not reported EEG Clinical findings |
Not reported EEG Clinical findings |
4. Treatment/Control/Comparison
| Control | Treatment 1 | Treatment 2 | |||
| Type of agent |
Placebo Usual care |
Traditional AED Newly licensed AED Other agent |
Traditional AED Newly licensed AED Other agent |
||
| Examples for types of agent: |
Traditional antiepileptic: antiepileptic Drug that has been on the market for many years (E.g. Carbamazepine, phenytoin, valproate) Newly licensed AED: antiepileptic Drug that has been licensed more recently (E.g. Levetiracetam, topiramate, lamotrigine, oxcarbazepine) Other agent: Any agent or drug that is not marketed as an AED (E.g.Magnesium sulphate |
||||
| Name of agent | |||||
|
Dose amount& administration method |
|
||||
| Therapeutic dose | |
||||
| Timing of doses | |
||||
|
Duration of treatment |
Short‐term Mid‐term Long‐term Additional comment: |
Short‐term Mid‐term Long‐term Additional comment: |
Short‐term Mid‐term Long‐term Additional comment: |
||
| Definitions for duration of treatment: |
Short‐term: Treatment less than or equal to 3 months post injury Mid‐term: Treatment less than or equal to 12 months post injury Long‐term: Treatment more than 12 months post injury |
||||
| First dose given before a first posttraumatic seizure? |
Not reported Yes No |
Not reported Yes No |
Not reported Yes No |
||
5. Primary outcomes
For the purpose of this review, seizure outcomes are classified according to the following definitions:
Early seizures are those that occur within one week of trauma.
Late seizures are those that occur later than one week post‐trauma.
Note that some studies only report seizure incidence in general and do not indicate when they occurred. Report this in the row labelled “Patients experiencing seizures (general)”.
| Control (n/N) | Treatment 1 (n/N) | Treatment 2 (n/N) | |
| Patients experiencing early seizures * | |||
| Patients experiencing late seizures | |
||
| Patients experiencing seizures (general) |
* If the study reports actuarial percentages, please note that here.
6. Secondary outcomes
| Control | Treatment 1 | Treatment 2 | |
| Mortality from any cause during follow‐up period | |||
| Mean number of early seizures (per patient) | |||
| Mean number of late seizures (per patient) | |||
| Mean number of seizures (general) (per patient) | |||
|
Time to first seizure (may report hazard ratio, CI) |
|||
|
Time to second seizure (hazard ratio, CI) |
|||
|
Other adverse effects (Eg. Skin rashes) (Define adverse effect and indicate incidence) (n/N) |
|
||
| Number of patients for which treatment was discontinued (include reasons) (n/N) | |
||
|
Other outcomes reported by study (e.g. Neurological findings, Status Epilepticus) |
|
Part II:Questions for assessing Risk of Bias (ROB)
A summary of the ROB domains, as described by the Cochrane Collaboration in chapter 8of the handbook, are included here for reference.
| Domain | Support for judgement |
| Selection bias. | |
| Random sequence generation. | Describe the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups. |
| Allocation concealment. | Describe the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment. |
| Performance bias. | |
| Blinding of participants and personnelAssessments should be made for each main outcome (or class of outcomes). | Describe all measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. Provide any information relating to whether the intended blinding was effective. |
| Detection bias. | |
| Blinding of outcome assessmentAssessments should be made for each main outcome (or class of outcomes). | Describe all measures used, if any, to blind outcome assessors from knowledge of which intervention a participant received. Provide any information relating to whether the intended blinding was effective. |
| Attrition bias. | |
| Incomplete outcome dataAssessments should be made for each main outcome (or class of outcomes). | Describe the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. State whether attrition and exclusions were reported, the numbers in each intervention group (compared with total randomized participants), reasons for attrition/exclusions where reported, and any re‐inclusions in analyses performed by the review authors. |
| Reporting bias. | |
| Selective reporting. | State how the possibility of selective outcome reporting was examined by the review authors, and what was found. |
| Other bias. | |
| Other sources of bias. | State any important concerns about bias not addressed in the other domains in the tool. If particular questions/entries were pre‐specified in the review’s protocol, responses should be provided for each question/entry. |
| Domain | Assessment | Support for judgement |
| Selection bias. | ||
| Random sequence generation. |
Low Unclear High |
|
| Allocation concealment. |
Low Unclear High |
|
For performance bias, detection bias and attrition bias, assessments should be made for each main outcome (or class of outcomes). Print off more copies of the following page, if more space is required.
Define outcome: _________________________________________
| Domain | Assessment | Support for judgement |
| Performance bias. | ||
| Blinding of participants and personnel |
Low Unclear High |
|
| Detection bias. | ||
| Blinding of outcome assessment |
Low Unclear High |
|
| Attrition bias. | ||
| Incomplete outcome data |
Low Unclear High |
|
| Domain | Assessment | Support for judgement |
| Reporting bias. | ||
| Selective reporting. |
Low Unclear High |
|
| Other bias. | ||
| Other sources of bias. |
Low Unclear High |
|
Part III. Additional details
Fill in any study shortcomings and other relevant details.
Appendix 8. Severity of trauma
| Mild | Moderate | Severe | Very severe |
| PTA < 1 hour | PTA 1‐24 hours | PTA 1‐7 days | PTA > 7 days |
| GCS 13‐15 | GCS 9‐12 | GCS 3‐8 | LOC > 48 hours |
| LOC < 15 minutes | LOC < 6 hours | LOC 6‐48 hours | |
| GCS: Glasgow Coma Score; LOC: loss of consciousness; PTA: post‐traumatic amnesia. | |||
Data and analyses
Comparison 1. Antiepileptic drug (AED) versus placebo or standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Early seizure | 5 | 987 | Risk Ratio (IV, Random, 95% CI) | 0.42 [0.23, 0.73] |
| 2 Late seizure | 6 | 1029 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.57, 1.46] |
| 3 All‐cause mortality | 5 | 1065 | Risk Ratio (IV, Random, 95% CI) | 1.08 [0.79, 1.46] |
| 4 Any serious event | 2 | 568 | Risk Ratio (IV, Random, 95% CI) | 1.63 [0.73, 3.66] |
| 5 Skin rash | 2 | 568 | Risk Ratio (IV, Random, 99% CI) | 1.65 [0.54, 5.04] |
| 6 Sensitivity analysis ‐ early seizure: age of population | 4 | 885 | Risk Ratio (IV, Random, 95% CI) | 0.36 [0.21, 0.60] |
| 7 Sensitivity analysis ‐ early seizure: study quality | 2 | 506 | Risk Ratio (IV, Random, 95% CI) | 0.48 [0.11, 2.18] |
| 8 Subgroup: late seizure: type of AED | 6 | 1029 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.57, 1.46] |
| 8.1 Late seizure ‐ phenytoin | 4 | 752 | Risk Ratio (IV, Random, 95% CI) | 0.83 [0.40, 1.70] |
| 8.2 Late seizure ‐ other AED | 2 | 277 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.46, 1.99] |
| 9 Subgroup ‐ late seizure: treatment duration | 6 | 1029 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.57, 1.46] |
| 9.1 Long treatment duration | 5 | 943 | Risk Ratio (IV, Random, 95% CI) | 1.08 [0.81, 1.46] |
| 9.2 Short treatment duration | 1 | 86 | Risk Ratio (IV, Random, 95% CI) | 0.14 [0.03, 0.55] |
| 10 Sensitivity analysis ‐ late seizure: age of population | 5 | 706 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.44, 1.48] |
| 11 Sensitivity analysis ‐ late seizure: comparison group | 5 | 903 | Risk Ratio (IV, Random, 95% CI) | 0.83 [0.48, 1.41] |
| 12 Sensitivity analysis ‐ late seizure: study quality | 1 | 323 | Risk Ratio (IV, Fixed, 95% CI) | 1.25 [0.79, 1.96] |
| 13 Subgroup Analysis ‐ all‐cause mortality: age of population | 5 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 13.1 All‐cause mortality ‐ children only | 2 | 143 | Risk Ratio (IV, Random, 95% CI) | 0.54 [0.25, 1.19] |
| 13.2 All‐cause mortality ‐ adults and children | 2 | 315 | Risk Ratio (IV, Random, 95% CI) | 1.43 [0.90, 2.27] |
| 13.3 All‐cause mortality ‐ adults only | 1 | 404 | Risk Ratio (IV, Random, 95% CI) | 1.13 [0.78, 1.62] |
| 14 Subgroup analysis ‐ all‐cause mortality: treatment duration | 5 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 14.1 All‐cause mortality ‐ short‐term treatment duration | 2 | 346 | Risk Ratio (IV, Random, 95% CI) | 0.69 [0.39, 1.24] |
| 14.2 All‐cause mortality ‐ long‐term treatment duration | 3 | 719 | Risk Ratio (IV, Random, 95% CI) | 1.24 [0.93, 1.65] |
| 15 Sensitivity analysis ‐ all‐cause mortality: type of AED | 4 | 914 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.65, 1.43] |
| 16 Sensitivity analysis ‐ all‐cause mortality: study quality | 2 | 506 | Risk Ratio (IV, Fixed, 95% CI) | 1.00 [0.72, 1.41] |
1.12. Analysis.
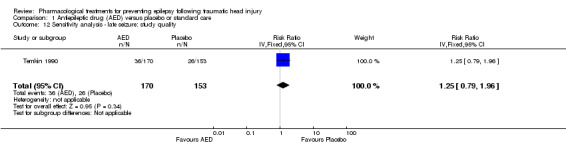
Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 12 Sensitivity analysis ‐ late seizure: study quality.
Comparison 2. Neuroprotective agent versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Early seizure | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 2 Late seizure | 1 | 498 | Risk Ratio (IV, Random, 95% CI) | 1.07 [0.53, 2.17] |
| 3 All‐cause mortality | 1 | 466 | Risk Ratio (IV, Random, 95% CI) | 1.2 [0.80, 1.81] |
Comparison 3. Antiepileptic drug (AED) versus other AED.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Early seizure | 2 | 431 | Risk Ratio (IV, Random, 95% CI) | 0.66 [0.20, 2.12] |
| 2 Late seizure | 2 | 378 | Risk Ratio (IV, Random, 95% CI) | 0.77 [0.46, 1.30] |
| 3 All‐cause mortality | 2 | 431 | Risk Ratio (IV, Random, 95% CI) | 0.53 [0.30, 0.94] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Glotzner 1983.
| Methods | RCT; single center Enrolled: 1978‐1979 Duration of treatment: long‐term; 18‐24 months Follow‐up: 18‐24 months Setting: Neurosurgery Department of University of Wurzburg Type of agent: traditional AED |
|
| Participants | 151 participants > 15 years of age, 88.5% male, were admitted due to moderate and severe TBI. Severity determined by GCS and presence of retrospective amnesia Carbamazepine: 75 participants Placebo: 76 participants Pre‐existing epilepsy was excluded |
|
| Interventions |
Carbamazepine: participants were treated according to serum levels 300‐600 µg. First dose given immediately after accident (no dosage given), other details not specified Placebo: details not provided First dose given before FPS NOTE: 61% of all 139 participants received additional phenobarbital for brain edema (administered in first week). Mean cumulative dosage: 2780 µg in placebo group vs. 1500 µg in intervention group. 59% of all 139 participants received diazepam: 248 µg in placebo group vs. 150 µg in carbamazepine group: acute phase only |
|
| Outcomes |
Seizure identification: EEG and clinical findings |
|
| Notes | Imbalance at baseline: carbamazepine group had more permanent vegetative states 26% vs. 13% placebo Unable to confirm the data with respect to late seizures; therefore, not included in analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence predicable ‐ based on birthdays. carbamazepine = even birthdays, placebo = odd birthdays |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: 6 from carbamazepine group, 5 from placebo group (poor description of reasons) |
| Selective reporting (reporting bias) | High risk | Very detailed description of severity of injury (over 50 baseline descriptive tables) but no adverse events reported |
| Other bias | High risk | Majority of participants received phenobarbital or diazepam, or both (both active AEDs) in the first week for edema treatment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Is not stated if participants or physicians were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study indicated as "blinded"; blinding of assessment not specifically reported |
Manaka 1992.
| Methods | RCT; multicenter; parallel group study Enrolled: 1983‐1985 Duration of treatment: long‐term; 2 years Follow‐up: 5 years Setting: Japan Type of agent: traditional AED |
|
| Participants | 244 participants ages 7‐88 years admitted due to TBI Analyzed: Group I with severe TBI (mean age 38 ± 19.9 years):
Group II with mild TBI: 65 participants; mean age 29.3 ± 19.6 years): treatment not described Proportion male: not reported Did not specify if pre‐existing epilepsy was excluded |
|
| Interventions |
Group IA: received phenobarbital, 10‐25 µg/mL, started 4 weeks after TBI. Full dose for 2 years, tapered in third year Group IB: some participants received nothing, some participants received anticonvulsants Group II: intervention not specified First dose given before an FPS: not reported |
|
| Outcomes |
Seizure identification: not specified |
|
| Notes | Baseline characteristics not reported Drug not administered until approximately 2 months post‐injury; some early seizures occurred Control group appeared to include participants who were taking other anticonvulsant drugs |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No further details provided; therefore, no confirmation method was carried out appropriately |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment process not described: used envelope method; no further details provided. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up at 5 years was 25%. 52/244 excluded due to drop‐out or "against" protocol. No mention of which groups these participants were in, their characteristics, if they were randomized or if they received treatment prior to drop‐out. Intention‐to‐treat not performed No Table 1 to clearly describe participant characteristics No clear description of drug protocol or control protocol for Group IB and Group II |
| Selective reporting (reporting bias) | High risk | Did not report on adverse events or mortality. Baseline characteristics were not reported; therefore, cannot assess balance of baseline characteristics |
| Other bias | High risk | Co‐intervention in control group. Group IB had some participants who received anticonvulsant medication and other participants who did not receive anticonvulsant medication. Not sure what proportion received medication or what drugs were |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No mention of blinding participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding outcome assessors |
McQueen 1983.
| Methods | RCT; double‐blind, multicenter (2 sites), parallel‐group study Enrolled: Newcastle, UK: November 1977 to October 1979; Edinburgh, UK: 1 January 1977 to October 1979 Duration of treatment: mid‐term; 12 months Follow‐up: 2 years Setting: Neurological Surgery Departments Type of agent: traditional AED |
|
| Participants | 164 participants ages 5‐65 years admitted due to TBI Phenytoin: 84 participants; 35% were 5‐15 year olds; 79% male Placebo: 80 participants; 18% were 5‐15 year olds; 80% male 85% of participants had injuries associated with high risk of post‐traumatic epilepsy Pre‐existing epilepsy was excluded |
|
| Interventions |
Phenytoin: child (5‐15 years 5 mg/kg; adults 300 mg) Placebo: Therapeutic dose: during follow‐up, adjusted to achieve plasma concentration 40‐80 µmol/L Dose administration: capsule of phenytoin 50 or 100 mg and matching placebo capsules Timing of dose: single or divided daily dose; not precisely reported but participants received a full dose every 24 hours First dose given before post‐traumatic seizure; early seizure was an exclusion criteria |
|
| Outcomes |
Seizures diagnosed based on clinical findings |
|
| Notes | Potentially significant difference in participant characteristics at baseline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment was administered by the hospital pharmacist using a prepared list of random treatment allocation ‐ but report did not indicate how the list was made. |
| Allocation concealment (selection bias) | Low risk | Treatment was administered by the hospital pharmacist using a prepared list of random treatment allocation ‐ but report did indicate how the list was made. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up over 2 years was 1%. Authors explained causes of participants lost to follow‐up. These participants were counted in the treatment group to which they were originally assigned |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. Prespecified outcomes reported |
| Other bias | High risk | Potentially significant difference at baseline. In phenytoin group, more 5‐15 year olds than in placebo group. In phenytoin group, more participants admitted in 8‐10 days post injury. In placebo group, more participants admitted > 30 days post‐injury Low compliance in treatment group. 80% were dispensed capsules for up to 6 months, 68% for up to 9 months, 49% for up to 12 months When tested, the level of phenytoin in the plasma of the phenytoin group often below the therapeutic level with only 48% of participants achieving plasma concentrations of > 40 µmol/L on at least 1 occasion, 36% had plasma concentrations of 20‐39 µmol/L, 12% in range 10‐19 µmol/L |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The trial was conducted 'double‐blind' with prescribed treatment known only to the hospital pharmacy and the trial co‐ordinators, who had no responsibility for participant care or follow‐up |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors involved in the prescribed treatment were not involved in follow‐up |
Pechadre 1991.
| Methods | RCT Enrolment: January 1982 to March 1985 Duration of treatment: short‐term and mid‐term; 3 months and 1 year Follow‐up: 2 years Setting: France Type of agent: traditional AED |
|
| Participants | 86 participants aged 5‐60 years, 80% males admitted due to severe TBI Phenytoin: 34 participants); mean age 26 years; 74% male Placebo: 52 participants; mean age 30.3 years; 85% male Pre‐existing epilepsy was excluded Severity determined by EEG and repeat CT scans |
|
| Interventions |
Phenytoin: 10 mg/kg by slow intravenous pump 40 mg/minute Placebo: Therapeutic dose: determined by serum results at 48 hours and 7 days ‐ adjusted therapeutic does using formula by Young 1979. Dose administration: capsule of phenytoin 50 or 100 mg and matching placebo capsules Timing of doses: 4 divided doses on first day; on second day, oral phenytoin (gastric tube in some participants), mean dose 8 mg/kg in 2 divided doses. Treated within 24 hours of accident and upon arrival in ICU Not reported if first dose was given before post‐traumatic seizure |
|
| Outcomes |
Seizures diagnosed based on clinical findings and EEG |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomized by day of arrival even or odd day (predictable sequence) |
| Allocation concealment (selection bias) | Unclear risk | Did not describe allocation concealment. Predictable sequence of randomization |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 5 participants excluded from analysis due to death in first 5 days ‐ group allocation not indicated. Loss to follow‐up other than mortality was not discussed |
| Selective reporting (reporting bias) | High risk | Indicated that some participants received phenytoin for > 3 months but did not describe outcomes by length of treatment. Adverse events and mortality not reported for included participants |
| Other bias | Unclear risk | No clear description of the control group |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Did not mention blinding strategies ‐ but given nature of randomization, it would be easy to determine which participants were in control/treatment groups |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Given the allocation by date of enrolment ‐ it is unlikely that treatment was blinded as the assessors could easily determine which participants were in which group by date of admission |
Szaflarski 2010.
| Methods | RCT; double‐blind, single‐center, parallel group study Enrolled: not reported Duration of treatment: short‐term; 7 days Duration of follow‐up: outcomes assessed at 3 and 6 months Setting: USA; neuroscience ICU Type of agent: traditional AED and newly licensed AEDs |
|
| Participants | 52 participants with severe TBI or subarachnoid hemorrhage ages 17‐80 years. Randomization up to 24 hours post admission, at a 2 : 1 ratio levetiracetam : phenytoin Levetiracetam: 34 participants; 30 with TBI; ages 17‐75 years, median 44 years; 77% male Phenytoin: 18 participants; 16 with TBI; ages 18‐80 years, median 25 years; 72% male Inclusion: TBI or subarachnoid hemorrhage, GCS (3‐8 inclusive) or GCS of ≤ 5 and abnormal CT scan showing intracranial pathology, hemodynamically stable, at least 1 reactive pupil, ages ≥ 17 years and informed consent Exclusion: spinal cord injury, previous brain injury, known hypersensitivity to anticonvulsant, hemodynamically unstable, anoxic events Report did not indicate exclusion of pre‐existing seizures prior to study inclusion but author confirmed exclusion by email |
|
| Interventions |
Levetiracetam: loading dose 20 mg rounded to nearest 250 mg over 60 minutes. Maintenance dose of 1000 mg, IV every 12 hours over 15 minutes. Therapeutic dose: up to 1500 mg (3000 mg/day). Duration of treatment: 1‐7 days Phenytoin: loading dose of 20 mg/kg IV, maximum 2000 mg over 60 minutes and then phenytoin maintenance of 5 mg/kg/day rounded to nearest 100 mg, dose every 12 hours. Therapeutic dose: 10‐20 µg/dL. Duration of treatment range: 1‐7 days Not reported if drug was given before first seizure |
|
| Outcomes |
Seizures identified based on clinical findings. Continuous EEG monitoring for first 72 hours |
|
| Notes | Baseline characteristics appeared balanced between study groups People with TBI or subarachnoid hemorrhage recruited and it was not possible to obtain data exclusively for the people with TBI, which represented 89% of the participants |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details on method of randomization. Randomized at a 2 : 1 ratio of levetiracetam : phenytoin |
| Allocation concealment (selection bias) | Low risk | Participants randomized and treatment group assigned by the pharmacy |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Participants were analyzed as survivors and as per their treatment group assignment. No indication of any loss to follow‐up other than death |
| Selective reporting (reporting bias) | Low risk | All expected and pre‐specified outcomes were reported |
| Other bias | Unclear risk | No report of drug levels to assess efficacy |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Physicians "partially" blinded. Were not "told" group assignment, but PHT levels could be reviewed. Physicians were also unblinded if a seizure occurred to optimize treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | EEG monitoring occurred for 72 hours. Electrophysiologist was blinded to the group assignment and diagnosis The clinical research co‐ordinator remained blinded to participant medication and conducted all assessments |
Temkin 1990.
| Methods | RCT; double‐blind, single center, parallel group study Enrolled: November 1983 to December 1987 Duration of treatment: mid‐term; 12 months Follow‐up: 2 years Setting: USA, Level 1 trauma center Type of Agent: traditional AED |
|
| Participants | 404 participants with severe TBI, mean age 34 ± 18 years Phenytoin: 208 participants; mean age 34 ± 18 years; 78% male Placebo: 196 participants; mean age 34 ± 17 years; 75% male Eligibility ‐ meet at least 1 of following criteria: cortical contusion visible on CT scan; a subdural, epidural or intracerebral hematoma; a depressed skull fracture; penetrating head wound; seizure within 24 hours of injury or a GCS ≤ 10 on admission. If any criteria met ‐ estimated 20% chance of seizure Excluded participants with previous documented unprovoked seizures |
|
| Interventions |
Phenytoin (Dilantin): initial dose 20 mg/kg IV within 24 hours of injury Therapeutic dose: total 40‐80 µmol/L, 10‐20 mg/L Dose administration: daily dose varied based on individual serum level; range 200‐1200 mg to maintain serum levels Placebo: given daily First dose not given before an FPS |
|
| Outcomes |
Seizure identification based on clinical findings. Clinicians who were blinded to treatment diagnosed seizures primarily on basis of clinical manifestations especially involuntary movements; alterations in consciousness; or abnormal motor, sensory or psychosensory phenomena. Participants and caregivers were trained to recognize subtle manifestations of seizures |
|
| Notes | Baseline characteristics were comparable between groups Additional data regarding the group without prior seizure history was received from Dr. Temkin |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization process not reported |
| Allocation concealment (selection bias) | Unclear risk | Concealment of allocation process not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomized participants were analyzed in the groups they were allocated to. Withdrawal from treatment well reported. 24% of participants lost to follow‐up; 23% in phenytoin group and 26% in placebo group over 24 months |
| Selective reporting (reporting bias) | Low risk | Expected outcomes of interest appear to be reported |
| Other bias | Low risk | Study groups similar baseline characteristics with respect to demographic characteristics, cause of injury, and severity of injury. 70% of participants had therapeutic levels of phenytoin |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Dose modified by unblinded study staff. Similar "mock" adjustments made to placebo group. Treatment code was not broken unless phenytoin appeared to be responsible for reaction and the participant's condition warranted such action |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Clinicians who were blinded to treatment diagnosed seizures primarily on basis of clinical manifestations |
Temkin 1999.
| Methods | RCT; double‐blind, single‐center, parallel‐group study Enrolled: November February 1991 to December 1995 Duration of treatment: varied 2 groups short‐term; 1 week and 1 month treatments; 1 group mid‐term ‐ 6 month treatment Follow‐up: 2 years Setting: USA, level 1 trauma center Type of agent: traditional AED |
|
| Participants | 379 participants with TBI randomized to:
Qualifying injury had 1 of the following characteristics: immediate posttraumatic seizures. Depressed skull fracture, penetrating brain injury, or CT evidence of cortical contusion or subdural, epidural, intracerebral hematoma Excluded people with previous documented unprovoked seizures |
|
| Interventions |
Phenytoin (1 week): loading dose IV 20 mg/kg ‐ administered within 24 hours. Maintenance dose 5 mg/kg/day in two divided doses. Therapeutic dose: 40‐80 µmol/L (10‐20 µg/mL) Valproate (1 and 6 months): loading dose IV 20 mg/kg. Maintenance dose 15 mg/kg/day in 4 divided doses. Therapeutic dose ‐ 277‐693 µmol/L (40‐100 µg/mL) First dose not given before an FPS |
|
| Outcomes |
Seizure identification: based on clinical findings. Early seizures were witnessed by medical personnel. Late seizures recognized by participants and caregivers who reported them to study neurologist. A blinded study neurologist reviewed all suspected seizures; if in doubt, the event was not counted as a seizure |
|
| Notes | For early seizures the valproate group was considered as 1 group regardless of length of time to be treated All participants were included in the analysis of late seizures regardless of whether they had experienced early seizures Baseline characteristics were comparable between groups |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated blocked randomization list, generated by statistician and kept in locked part of pharmacy |
| Allocation concealment (selection bias) | Low risk | Allocation by pharmacist |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 87% followed for full 2 years. But not all participants followed through with assigned treatment. Of randomized cases: 75% of 1 month and 70% of 6 month valproate group followed up for 2 years. 79% of phenytoin group followed for 2 years Because most participants were unconscious or had cognitive impairments during the first week, early seizures without a prominent motor component were likely to be overlooked 113 participants initially randomized were subsequently found to be ineligible after randomization for issues such as prior history of epilepsy. These participants were only observed for 28 days for incidence of adverse effects and for mortality |
| Selective reporting (reporting bias) | Unclear risk | Although most expected and pre‐specified outcomes appeared to be reported, denominators of counts not reported clearly |
| Other bias | Low risk | Valproate concentrations were at or above the target valproate range in 97% of participants in first week; 90% in first month; 85% in fifth month Phenytoin concentrations were at or above the target phenytoin range in 91% of participants in the first week Compliance: 16% stopped taking blinded medication before 1 month because of participant preference or mild adverse effects. 21% stopped before 6 months compliance reported for each group |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical‐appearing IV solutions and call‐backs to check placebo "drug levels" to maintain blind conditions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Neurologist blinded to the assignment made the final determination on seizure diagnosis for the study |
Temkin 2007.
| Methods | RCT; double‐blind, single‐center, parallel group study Enrolled: August 1998 to October 2004 Duration of treatment: short‐term; 5 days Follow‐up: 6 months Setting: USA, level 1 trauma center Type of agent: "other" |
|
| Participants | 499 participants older than 14 years were admitted due to moderate or severe TBI Treatment 1 (high dose magnesium sulfate; MgSO4):
Treatment 2 (low‐dose magnesium sulfate; MgSO4):
Moderate to severe was defined as: the need for intracranial surgery within 8 hours of injury; a post‐resuscitation GCS score of 3‐12; or intubated, a GCS motor score of 1‐5 without pharmacologic paralysis Pre‐injury seizures were not excluded from the study, but participants with pre‐injury seizures were excluded from seizure outcome analysis |
|
| Interventions |
Treatment 1 (high dose): magnesium sulfate (MgSO4) high dose 1.2‐2.5 mmol/L. Initial IV load of 0.425 mmol/kg over 15 minutes followed by continuous infusion (0.10 mmol/kg/hour) to maintain target range for 5 days. Therapeutic dose 1.25‐2.5 mmol/L Treatment 2 (low dose): magnesium sulfate (MgSO4) low dose 1.0‐1.85 mmol/L. Initial IV load of 0.30 mmol/kg over 15 minutes followed by continuous infusion (0.05 mmol/kg/hour) to maintain target range for 5 days. Therapeutic dose 1.0‐1.85 mmol/L In both treatments, agent was administered within 8 hours of injury. Infusion rate adjusted daily by pharmacist Placebo: saline, magnesium sulfate given below normal levels Not reported if drug was given before first seizure 96% of participants received phenytoin for the first week as part of clinical care |
|
| Outcomes |
Seizure identification not explicitly reported, but, at 1 and 3 months, health status measures were assessed by telephone and as a part of a formal in‐person comprehensive examination at 6 months that included neuropsychologic testing (panel). A family member who knew the person prior to injury also participated in assessment at 6‐month test |
|
| Notes | Participants who died before day 8 were excluded from the late seizure analysis Author contacted: contacted for participant details within outcome categories to determine if history of seizure was excluded. Response summary: participants with history of seizure were deleted from early and late seizure outcome. Author provided counts for primary and secondary outcomes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was stratified by sex and age. Randomization was blocked (2‐4 people) to ensure balance. Computer‐generated list kept in a restricted area of the pharmacy |
| Allocation concealment (selection bias) | Low risk | Pharmacist randomly assigned participant sequentially when order received |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Primary analysis excluded people with fixed dilated pupils and people who were randomized but died before receiving the drug (7 in magnesium sulfate group, 8 in placebo group). In secondary analysis, all participants were analyzed in the groups they were assigned (ITT analysis) 93% were followed for 6 months. 72% had a full neurologic assessment at 6 months Loss to follow‐up similar in both the Mg (18) and placebo (19) groups and for similar reasons |
| Selective reporting (reporting bias) | Unclear risk | Did not report mortality and seizures in a conventional way for these common study outcomes |
| Other bias | Unclear risk | Co‐intervention: phenytoin administered to 96% of participants in the first week Most characteristics were "quite" well balanced at baseline, but the lower magnesium dose had more participants with hematomas and with worse abbreviated‐injury‐scale‐head scores. Noted significant differences in P values between group in age, severity and gender Drug treatment as specified was given in 95% of cases 25 participants stopped taking study drug before the 5 days Pre‐injury seizures were not excluded, but participants with pre‐injury seizures were excluded from seizure outcome analysis Total mean magnesium concentrations were 2.15 mmol/L (SD 0.35) in high‐dose group, 1.45 mmol/L (SD 0.2) in low‐dose group and 0.9 mmol/L (SD 0.1) in placebo group |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, doctors and nurses treating participants were all blinded to assignment. Clinicians were not allowed to order any tests of magnesium concentration during the infusion or for 2 days post drug treatment. Masking was broken in 8% of cases ‐ usually when clinician ordered routine laboratory tests. Research nurse became aware of 4% of cases. Participants remained constantly unaware of assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research nurses and professionals involved in assessment of outcome were all masked to treatment assignment. There was no formal assessment of the success of the masking. Outcome examiners remained consistently unaware of assignment |
Young 1983.
| Methods | RCT; double‐blind, single‐center; parallel‐group study Enrolled: December 1976 to November 1979 Duration of treatment: short‐term 7 days and long‐term 18 months Follow‐up: 1 week to 18 months Setting: USA, neurologic services Type of agent: traditional AED Results of trial reported in 3 reports. Report A: Young 1983. Journal of Neurosurgery 1983;58(2):231‐5; all participants; early seizure only. 1‐week follow‐up. Report B: Journal of Neurosurgery 1983;58(2):236‐41; all participants; late seizures only, 18‐month follow‐up. Report C: Child's Brain 1983;10(3):1985‐92, subanalysis of Report B ‐ all participants aged < 17 years |
|
| Participants |
Report A: 244 participants of all ages with severe TBI Phenytoin: 136 participants; mean age 24.4 ± 1.29 years; 6 (4.4%) ages 0‐4 years ; 26 (19.1%) ages 5‐15 years; 80.9% male GCS: 14 (10.3%) had GCS 3‐4; 56 (41.2%) had GCS of 5‐7; 56 (48.5%) had GCS ≥ 8. 7 had pre‐randomized seizures (mean age 12 years) Placebo: 108 participants; mean age 25.8 ± 1.47 years; 5 (4.6%)ages 0‐4 years; 17 (15.7%)ages 5‐15 years; 84.3% male GCS: 17 (15.7%) had GCS 3‐4; 46 (42.6%) had GCS 5‐7; 45 (41.7%) had GCS ≥ 8. 3 had pre‐randomized seizures (mean age 12 years) Report B: 214 participants of all ages, mean age of 25.2 years, with severe TBI. 4.7% aged < 5 years, 17.3% ages 5‐16 years, 78.0% > 16 years Phenytoin: 119 participants; mean age 24.4 ± 1.29 years; 6 (4.4%) ages 0‐4 years; 26 (19.1%) ages 5‐15 years; 80.9% male GCS: 9 (8.6%) had GCS 3‐4; 40 (38.1%) had GCS 5‐7; 56 (53.3%) had GCS ≥ 8 Phenobarbital: 20 participants; received phenytoin initially; mean age 21.6 ± 3.01, 75% male. GCS: 0 (0%) had GCS 3‐4; 11 (55.5%) had GCS 5‐7; 9 (45.0%) had GCS of ≥ 8 Placebo: 95 participants; mean age 26.3 ± 2.03 years; 82.4% male GCS: 8 (10.8%) had GCS 3‐4; 33 (44.6%) had GCS 5‐7; 33 (44.6%) had GCS ≥ 8 Report C: 46 participants all age of 17 years with severe TBI. Randomized: 27 to treatment, 19 to placebo. 4 died and 1 early seizure excluded from analysis Follow‐up: Phenytoin: 20 participants; mean age 9.3 ± 0.81 years; 72% male. GCS: 1 (5.0%) had GCS 3‐4; 5 (25.0%) had GCS 5‐7; 14 (70.0%) had GCS ≥ 8. (5 switched to phenobarbital) Phenobarbital: 5 participants; received phenytoin initially. Mean age 9.0 ± 1.92 years, % male unknown GCS: 0 (0%) had GCS 3‐4; 4 (80.0%) had GCS 5‐7; 1 (20.0%) had GCS ≥ 8 Placebo: 16 participants; mean age 9.2 ± 1.15 years; 93.8% male GCS: 2 (12.5%) had GCS 3‐4; 4 (25.0%) had GCS 5‐7; 10 (62.5%) had GCS ≥ 8 Included people with penetrating head wound or blunt head injury providing > 10% chance of developing seizures. Participants had: intracranial hematomas; frontal, temporal, or parietal depressed skull fracture; and other blunt head injuries causing unconsciousness for at least 6 hours or major focal neurologic deficits. Some seizures occurred prior to first dose |
|
| Interventions | Phenytoin: initial dose 11 mg/kg at 25 mg/minute plus 13 mg/kg intramuscularly If levels were adequate 8.8 mg/kg administered daily or adjusted as needed. Therapeutic dose: plasma concentrations 10‐20 µg/ml. Timing of dose: administered with 24 hours of admission Placebo: identical IV of phenytoin diluent (10% ethanol, propylene glycol 40% and water 50%) or placebo capsule |
|
| Outcomes |
|
|
| Notes | Method of identification of early seizure was not reported. Identification of late seizure by interview, exam, written and telephone follow‐ups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported; "randomized" but did not say how |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | A number of participants were lost to follow‐up or excluded from the analysis. Report A: 1 participant who had a drug reaction was excluded from the results. Report B: 179/214 followed for 18 months. 4 participants had early seizures in placebo group and were eliminated as they were administered phenytoin (3 people) or phenobarbital (1 person). 11 participants lost to follow‐up: 3 in drug group, 8 in placebo. 20 participants died in first week: 11 in drug group and 9 in placebo group. Report C: participants were analyzed in the group they were assigned regardless of outcome |
| Selective reporting (reporting bias) | High risk | Results indicated that deaths occurred beyond those reported in first 7 days. Median time to death was reported with ranges from 8 to 450 days but number of deaths between week 2 and 18 months not reported. Types of adverse reactions not reported despite 20 participants switched to phenobarbital. Did not include lost to follow‐up in time to event analysis |
| Other bias | Unclear risk |
|
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | State: "only the clinical pharmacist or the clinical nurse on the team was aware of which participant was receiving active drug or the placebo and made dosing adjustments" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | State: "In all cases the physician evaluators were blinded as to the drug received"; however, unclear if blinding was broken when participants were switched from phenytoin to phenobarbital |
Young 2004.
| Methods | RCT; double‐blind, multicenter, parallel‐group study Enrolled: December 1992 to November 1997 Duration of treatment: short‐term; 5 days Follow‐up: 30 days; median time 34.5 days (interquartile range 30‐50 days) Setting: USA, urban pediatric trauma centers Type of agent: traditional AED |
|
| Participants | 103 participants aged < 10 years, range of 3.3‐9.4 years, median 6.1 years with moderate and severe TBI. 68% male Phenytoin: 47 participants*; age range 3.7‐9.6 years, median 6.4 years; 67% male Placebo: 56 participants; age range 2.6‐8.8 years, median 5.9 years; 68% male Severity of TBI determined by: acute blunt head injury, with marked alteration in level of consciousness as defined by GCS (≤ 10 in children aged ≥ 4 years; ≥ 9 in children aged < 4 years, and pulse rate > 60 beats/minute |
|
| Interventions |
Phenytoin: initial IV dose 18 mg/kg over 20 minutes, maintenance 2 mg/kg every 8 hours for 48 hours (5 doses). Drug administered within 60 minute of arrival in emergency room Placebo: dilutent alone. First dose was administered prior to first traumatic seizure |
|
| Outcomes |
Seizures were identified with EEG and clinical finding |
|
| Notes | Excluded participants who had post‐trauma seizures before randomization * 1 participant in phenytoin group was removed from the study at the request of the family |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Enrolment was intended to be consecutive. Participants stratified into 6 groups according to age and initial GCS. Within each of the 6 stratified groups and study site, participants were randomly allocated to phenytoin or placebo by using randomized permuted blocks to ensure baseline similarity of treatment groups |
| Allocation concealment (selection bias) | Low risk | A code kept locked in an office off site was available only to the principal investigator linking each vial to the contents of the vial (phenytoin or placebo). Sealed envelopes with the identity of the study medication were kept with the vials and in the participant's medical record. The envelopes were to be opened at the end of the 48‐hour observation period, if a participant experienced a seizure or if the attending neurosurgeon wished to withdraw the participant from the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 68% completed entire observation period; 6 seizures, 9 deaths, 1 surgery, 12 discharged home, 5 protocol violations or neurosurgeons request Of 82/102 remaining participants, 62 (76%) returned for 30‐day follow‐up. Telephone follow‐up obtained from 4 others. Total follow‐up including deaths = 86/102 (84%). Randomized participants were analyzed in the groups they were allocated to. 10 lost to follow‐up from phenytoin group and 6 lost to follow‐up from placebo group |
| Selective reporting (reporting bias) | Low risk | Expected outcomes analyzed and reported |
| Other bias | Unclear risk | Median serum phenytoin levels: 16.2 mg/L (range 3.3‐61) Serum levels in the participants who had post‐traumatic seizures was 2.3, 34, 13 mg/L Ideal study therapeutic dose of phenytoin not stated Emergency room administration of benzodiazepines and barbiturates: differences approached significance between the groups (see report, Table 3) Administration of paralytic agents in the pediatric ICU and potential seizures were not monitored by EEG. Unlikely to introduce bias due to blinding, but number of seizures reported may underestimate the true early seizure rate 18% of participants had been receiving anticonvulsant medications at some point since hospital discharge and prior to 30 day follow‐up |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study medication and identical‐appearing placebo were prepared by the pharmacy. A code kept locked in an office off site was available only to the principal investigator linking each vial to the contents of the vial (phenytoin or placebo). Group assignment concealed until end of 48‐hour observation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Group assignment concealed until end of 48‐hour observation (low risk) Unclear for secondary outcomes as the groups would have been unblinded for 30‐day assessment (unclear risk) |
AED: antiepileptic drug; CT: computed tomography; EEG: electroencephalography; FPS: first post‐traumatic seizure; GCS: Glasgow Coma Scale; ICU: intensive care unit; ITT: intention to treat; IV: intravenous; RCT: randomized controlled trial; SD: standard deviation; TBI: traumatic brain injury.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anderson 2000 | Not RCT or quasi‐randomized trial |
| Anderson 2003 | Secondary publication of Temkin 1999, no further relevant information reported |
| Asikainen 1999 | Not treatment of interest |
| Carter 2009 | Not RCT or quasi‐randomized |
| Chiaretti 2000 | Not RCT or quasi‐randomized |
| Coplin 2002 | Not population of interest |
| De Santis 1992a | Not RCT or quasi‐randomized |
| De Santis 1992b | Not RCT or quasi‐randomized |
| De Santis 1998 | Not RCT or quasi‐randomized |
| Dikmen 1991 | Secondary publication of Temkin 1990, no further relevant information reported |
| Dikmen 1995 | Not treatment of interest |
| Dikmen 2000 | Secondary publication of Temkin 1999, no further relevant information reported |
| Dizdarevic 2012 | Not treatment of interest; not population of interest |
| Dolati 2012 | Not RCT or quasi‐randomized |
| Englander 2003 | Not RCT or quasi‐randomized |
| Formisano 2007 | Not RCT or quasi‐randomized |
| Glotzner 1998 | Outcome Information unavailable (no response from author) |
| Haltiner 1997 | Not RCT or quasi‐randomized: single‐ arm trial |
| Holland 1995 | Contacted author: not population of interest (all postoperati on ) |
| Inaba 2013 | Not RCT or quasi‐randomized |
| Jallon 1984 | Not RCT or quasi‐randomized |
| Japan Follow‐up... 1991 | Not RCT or quasi‐randomized |
| Johnson 2009 | Not RCT or quasi‐randomized |
| Jones 2008 | Not RCT or quasi‐randomized |
| Kieslich 2001 | Not RCT or quasi‐randomized |
| Kirmani 2013 | Not RCT or quasi‐randomized |
| Klein 2008 | Not RCT or quasi‐randomized |
| Klein 2012a | Not RCT or quasi‐randomized |
| Klein 2012b | Not RCT or quasi‐randomized |
| Kobayashi 1997 | Not RCT or quasi‐randomized |
| Lopes 2009 | Not treatment of interest |
| Maas 2006 | Outcomes of interest not recorded, author unable to provide |
| Meo 2009 | Not RCT or quasi‐randomized |
| Murri 1980 | Not RCT or quasi‐randomized |
| Murri 1992 | Not treatment of interest. Compare d different doses of same drug |
| Nakamura 1995 | Not RCT or quasi‐randomized |
| Nakamura 1999 | Not population of interest |
| NCT00566046 | Study terminated due to lack of enrolment; n o outcome data available |
| NCT00598923 | Status of trial unknown, further information unavailable (no response from author) |
| NCT01110187 | Study terminated due to lack of enrolment; n o outcome data available |
| North 1980 | Not population of interest. Postoperative participants |
| North 1983 | Not population of interest. Postoperative participants |
| Ohman 2001 | Not population of interest. Included some participants with pre‐existing seizures (excluded following confirmation by author) |
| Ohno 1993 | Not RCT or quasi‐randomized |
| Pearl 2009 | Not RCT or quasi‐randomized |
| Pearl 2013 | Not RCT or quasi‐randomized |
| Penry 1979 | Outcome data unavailable from author |
| Popek 1969 | Not RCT or quasi‐randomized |
| Popek 1972 | Not RCT or quasi‐randomized |
| Richard 1998 | Not RCT or quasi‐randomized |
| Schutze 1999 | Not RCT or quasi‐randomized |
| Servit 1981 | Not RCT or quasi‐randomized |
| Smith 1994 | Not RCT or quasi‐randomized. No control participants |
| Steinbaugh 2012 | Secondary publication of Szaflarski 2010, no further relevant information reported |
| Szaflarski 2007 | Not RCT or quasi‐randomized. No control participants |
| Temkin 2003 | Not RCT or quasi‐randomized |
| Thapa 2010 | Not RCT or quasi‐randomized |
| Tomovic 1997 | Author did not respond with outcome data |
| Van den Berghe 2005 | Not population of interest. Participa nt population not traumatic brain injury |
| Virant‐Young 2009 | Not RCT or quasi‐randomized |
| Watson 2004 | Not RCT or quasi‐randomized |
| Wohns 1979 | Not RCT or quasi‐randomized |
| Young 1979 | Not RCT or quasi‐randomized. No control group |
RCT: randomized controlled trial .
Characteristics of ongoing studies [ordered by study ID]
NCT01048138.
| Trial name or title | Use of Biperiden for the Prevention of Post‐Traumatic Epilepsy |
| Methods | Placebo‐controlled, randomized, double‐blind study |
| Participants | 132 |
| Interventions | Biperiden lactate and placebo |
| Outcomes | Onset of post‐traumatic epilepsy, quality of life; cognitive level |
| Starting date | 2013 |
| Contact information | Luiz Eugenio Mello, Federal University of São Paulo, lemello@unifesp.br |
| Notes | ClinicalTrials.gov/show/NCT01048138 |
NCT01673828.
| Trial name or title | Allopregnanolone for the Treatment of Traumatic Brain Injury |
| Methods | Double‐blind, placebo‐controlled, randomized, dose‐finding, 2‐stage adaptive, clinical trial comparing allopregnanolone to placebo when administered intravenously for 5 days beginning within 8 hours after injury |
| Participants | 136 |
| Interventions | Allopregnanolone and placebo |
| Outcomes | Extended Glasgow Outcome Scale (GOS‐E) Score; mortality; depression; late post‐traumatic epilepsy; Neurobehavioral Rating Scale Revised (NRS‐R); Test of Adult Reading; Tests of Executive Function; Tests of Learning, Delayed Recall, and Recognition; Test of Working Memory; Tests of Psychomotor and Processing Speed; depression; quality of life; anxiety |
| Starting date | 2013 |
| Contact information | University of California, Davis Medical Center. Nancy Rudisill nancy.rudisill@ucdmc.ucdavis.edu / Steffany Lim steffany.lim@ucdmc.ucdavis.edu |
| Notes | ClinicalTrials.gov/show/NCT01673828 |
NCT02027987.
| Trial name or title | Traumatic neuroprotection and epilepsy prevention of Valproate acid |
| Methods | 160 participants who were in a vegetative or minimally conscious state 4 to 16 weeks after TBI and who were receiving inpatient rehabilitation. Participants were randomly assigned to receive VPA or placebo for 4 weeks and were followed for 2 weeks after the treatment was discontinued. The rate of functional recovery on the Disability Rating Scale (DRS; range, 0 to 29, with higher scores indicating greater disability) was compared over the 4 weeks of treatment (primary outcome) and during the 2‐week washout period with the use of mixed‐effects regression models. |
| Participants | 160 |
| Interventions | Valproate acid and placebo |
| Outcomes | DRS scores; time of break out and state of epilepsy; brain magnetic resonance imaging scan; the blood concentration of valproate acid |
| Starting date | 2013 |
| Contact information | Hu S Jie, Xijing Hospital hushijie@fmmu.edu.cnhushijie1979@126.com |
| Notes | ClinicalTrials.gov/show/NCT02027987 |
Differences between protocol and review
We found too few studies; therefore, we produced no funnel plots or orbit tables. Other sources of bias were also considered in addition to the bias domains listed in protocol. We performed no subgroup analysis by trauma severity as only one study reported including participants of moderate to severe trauma, all the other studies included severe only. The subgroup analyses for age were broken down by proportion of participants aged over 17 years and under 17 years, such that studies that exclusively included adults or exclusively children were separated out. Data were not provided in a manner that we were able to separate by mean age under six years, age between six and 17 years and age over 17 years as planned in protocol. We performed no subgroup analysis for the early seizure outcome due to insufficient data. However, we added a sensitivity analysis for age of population for early seizure. Subgroup analysis was only performed by type of AED and treatment duration for the late seizure outcome due to insufficient data for other analyses. We added a sensitivity analysis for age of population, and type of control group (placebo versus usual care) comparison for late seizure. Insufficient data were available to perform a subgroup analysis by type of AED for all‐cause mortality; however, we performed a sensitivity analysis. Time‐to‐event data were not provided in the papers; therefore, we did not use hazard ratios to summarize time‐to‐event outcomes. Time from first seizure to second seizure was not recorded or analyzed as these data were not provided in the papers. No trial reported comparing a pharmacologic agent other than an AED with a placebo on adverse events; therefore, we completed no analysis. We performed no imputation for missing data, as this was not relevant based on data collected. We performed no sensitivity analysis for analysis based on imputation.
Contributions of authors
KT initiated the topic.
KT, BP and LA all contributed to drafting of the protocol.
Tracey Daley along with the Cochrane Epilepsy Group Trials Search Co‐ordinator devised the search strategy and carried out the literature searches.
KT, BP and LA reviewed and approved protocol.
KT, HA and BP all contributed to the data extraction, and drafted, reviewed and approved the text.
Sources of support
Internal sources
Research Office, Department of Medicine, Dalhousie University, Canada.
Research Methods Unit, CDHA, Canada.
External sources
-
Nova Scotia Health Research Foundation's (NSHRF) Systematic Review grant, Canada.
Funding was provided for research assistant.
Declarations of interest
Leslie Anne Campbell and Bernhard Pohlmann‐Eden were supported by a Nova Scotia Health Research Foundation Knowledge Programs Grant.
Bernhard Pohlmann‐Eden is a member of the Advisory Board of UCB Pharma. He has also acted as a consultant for UCB Pharma Canada. Berhard has received speaker's honoraria from UCB Pharma and Desitin Pharam Germany on several occasions.
Kara Thompson is a Biostatistician for the Department of Medicine, therefore, additional funding received from Nova Scotia Health Research Foundation was to provide a research assistant to help with work on project.
Hannah Abel provided research assistance in the review.
New
References
References to studies included in this review
Glotzner 1983 {published data only}
- Glotzner FL, Haubitz I, Miltner F, Kapp G, Pflughaupt K‐W. Seizure prevention using carbamazepine following severe brain injuries [Anfallsprophylaxe mit Carbamazepin nach schweren Schadelhirnverletzungen]. Neurochirurgia 1983;26(3):66‐79. [DOI] [PubMed] [Google Scholar]
Manaka 1992 {published data only}
- Manaka S. Cooperative prospective study on posttraumatic epilepsy: risk factors and the effect of prophylactic anticonvulsant. Japanese Journal of Psychiatry and Neurology 1992;46(2):311‐5. [DOI] [PubMed] [Google Scholar]
McQueen 1983 {published data only}
- Harris P, Kalbag RM, McQueen JK, Blackwood DHR, Johnson AL. The incidence and the possible pharmacological prophylaxis of post‐traumatic epilepsy [abstract]. Acta Neurochirurgica 1984;70(1‐2):132. [Google Scholar]
- McQueen JK, Blackwood DHR, Harris P, Kalbag RM, Johnson AL. Low risk of late post‐traumatic seizures following severe head injury: implications for clinical trials of prophylaxis. Journal of Neurology, Neurosurgery, and Psychiatry 1983;46(10):899‐904. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pechadre 1991 {published data only}
- Lauxerois M, Pechadre JC, Colnet G, Commun C, Bonnard M. Hydantoins in the prevention of early posttraumatic epileptic crises [Prevention des crises d'epilepsie posttraumatiques precoces par les hydantoines]. Epilepsies 1990;2(1):5‐10. [Google Scholar]
- Pechadre JC, Lauxerois M, Colnet G, Commun C, Bonnard M, Gilbert J, et al. Prevention of late post‐traumatic epilepsy by phenytoin in severe brain injuries, 2 years' follow‐up [Prevention de l'epilepsie post‐traumatique tardive par phenytoine dans les traumatismes craniens graves, suivi durant 2 ans]. Presse Medicale 1991;20(18):841‐5. [PubMed] [Google Scholar]
Szaflarski 2010 {published data only}
- NCT00618436. Assess the safety and efficacy of a seizure medication, levetiracetam (LEV; Keppra), in neuroscience intensive care unit patients. www.clinicaltrials.gov/ct2/show/NCT00618436 (accessed 11 February 2014).
- Steinbaugh LA, Lindsell CJ, Shutter LA, Szaflarski JP. Initial EEG predicts outcomes in a trial of levetiracetam vs. fosphenytoin for seizure prevention. Epilepsy & Behavior 2012;23(3):280‐4. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Sangha KS, Lindsell CJ, Shutter LA. Prospective, randomized, single‐blinded comparative trial of intravenous levetiracetam versus phenytoin for seizure prophylaxis. Neurocritical Care 2010;12(2):165‐72. [DOI] [PubMed] [Google Scholar]
Temkin 1990 {published data only}
- Haltiner AM, Newell DW, Temkin NR, Dikmen SS, Winn HR. Side effects and mortality associated with use of phenytoin for early posttraumatic seizure prophylaxis. Journal of Neurosurgery 1999;91(4):588‐92. [DOI] [PubMed] [Google Scholar]
- Temkin NR, Dikmen SS, Keihm J, Chabal S, Winn HR. Does phenytoin prevent posttraumatic seizures ‐ 1‐year follow‐up results of a randomized double‐blind‐study in 407 patients [abstract]. Journal of Neurosurgery 1989;70(2):314A‐315A. [Google Scholar]
- Temkin NR, Dikmen SS, Wilensky AJ, Keihm J, Chabal S, Winn HR. A randomized double‐blind study of phenytoin for the prevention of post‐traumatic seizures. New England Journal of Medicine 1990;323(8):497‐502. [DOI] [PubMed] [Google Scholar]
Temkin 1999 {published data only}
- NCT00004817. Phase III double blind trial of valproate sodium for prophylaxis of post traumatic seizures. www.clinicaltrials.gov/ct2/show/record/NCT00004817 (accessed 11 February 2014).
- Temkin NR, Dikmen SS, Anderson GD, Wilensky AJ, Holmes, Cohen W, et al. Valproate therapy for prevention of posttraumatic seizures: a randomized trial. Journal of Neurosurgery 1999;91(4):593‐600. [DOI] [PubMed] [Google Scholar]
Temkin 2007 {published data only}
- Temkin NR, Anderson GC, Winn HR, Ellenbogen RG, Britz GW, Schuster J, et al. Magnesium sulfate for neuroprotection after traumatic brain injury: a randomized controlled trial. Lancet Neurology 2007;6(1):29‐38. [DOI] [PubMed] [Google Scholar]
Young 1983 {published data only}
- Young B, Rapp RP, Norton JA, Haack D, Tibbs PA, Bean JB. Failure of prophylactically administered phenytoin to prevent early posttraumatic seizures. Journal of Neurosurgery 1983;58(2):231‐5. [DOI] [PubMed] [Google Scholar]
- Young B, Rapp RP, Norton JA, Haack D, Tibbs PA, Bean JR. Failure of prophylatically administered phenytoin to prevent late posttraumatic seizures. Journal of Neurosurgery 1983;58(2):236‐41. [DOI] [PubMed] [Google Scholar]
- Young B, Rapp RP, Norton JA, Haack D, Walsh JW. Failure of prophylatically administered to prevent post‐traumatic seizures in children. Child's Brain 1983;10(3):1985‐92. [DOI] [PubMed] [Google Scholar]
Young 2004 {published data only}
- Young KD, Okada PJ, Sokolove PE, Palchak MJ, Panacek EA, Baren JM, et al. A randomized, double‐blinded, placebo‐controlled trial of phenytoin for the prevention of early posttraumatic seizures in children with moderate to severe blunt head injury. Annals of Emergency Medicine 2004;43(4):435‐46. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anderson 2000 {published data only}
- Anderson GD, Lin Y, Temkin NR, Fischer JH, Winn RH. Incidence of intravenous site reactions in neurotrauma patients receiving valproate or phenytoin. Annals of Pharmacotherapy 2000;34(6):697‐702. [DOI] [PubMed] [Google Scholar]
Anderson 2003 {published data only}
- Anderson GD, Temkin NR, Chandler WL, Winn HR. Effect of valproate on hemostatic function in patients with traumatic brain injury. Epilepsy Research 2003;57(2‐3):111‐19. [DOI] [PubMed] [Google Scholar]
Asikainen 1999 {published data only}
- Asikainen I, Kaste M, Sarna Seppo. Early and late posttraumatic seizures in traumatic brain injury rehabilitation patients: brain injury factors causing late seizures and influence of seizures on long‐term outcome. Epilepsia 1999;40(5):584‐9. [DOI] [PubMed] [Google Scholar]
Carter 2009 {published data only}
- Carter D, Askari R, Frawley B, Rogers S. Evaluation of the use of phenytoin and levetiracetam for seizure prophylaxis in patients with traumatic brain injury. Critical Care Medicine 2009;37(12 Suppl):A77, Abstract no: 185. [Google Scholar]
Chiaretti 2000 {published data only}
- Chiaretti A, Benedictis R, Polidori G, Piastra M, Iannelli A, Rocco C. Early post‐traumatic seizures in children with head injury. Child's Nervous System 2000;16(12):862‐6. [DOI] [PubMed] [Google Scholar]
Coplin 2002 {published data only}
- Coplin WM, Rhoney DH, Rebuck JA, Clements EA, Cochran MS, O'Neil BJ. Randomized evaluation of adverse events and length‐of‐stay with routine emergency department use of phenytoin or fosphenytoin. Neurological Research 2002;24(8):842‐8. [DOI] [PubMed] [Google Scholar]
De Santis 1992a {published data only}
- Santis A, Sganzeria E, Spagnoll D, Bello L, Tiberio F. Risk factors for late posttraumatic epilepsy. Acta Neurochirurgica. Supplementum 1992;55:64‐7. [DOI] [PubMed] [Google Scholar]
De Santis 1992b {published data only}
- Santis A, Bello L, Spagnoli D, Sganzeria E. Antiepileptic prophylaxis in head injured patients. Bollettino Lega Italiana contro l'Epilessia 1992;no. 79‐80:305‐6. [Google Scholar]
De Santis 1998 {published data only}
- Santis A, Lanterma AL, Ceccarelli G, Kouhpouros N, Villani RM. Role of PB in preventing late PTE [abstract]. Epilepsia 1998;39(Suppl s2):48. [Google Scholar]
Dikmen 1991 {published data only}
- Dikmen SS, Temkin NR, Miller B, Machamer J, Winn HR. Neurobehavioral effects of phenytoin prophylaxis of posttraumatic seizures. JAMA 1991;265(10):1271‐7. [PubMed] [Google Scholar]
- Dikmen SS, Temkin NR, Winn HR. Neurobehavioral effects of phenytoin prophylaxis in posttraumatic seizures: a double‐blind comparison [abstract]. Epilepsia 1989;30(5):667. [Google Scholar]
Dikmen 1995 {published data only}
- Dikmen SS, Machamer JE, Winn HR, Temkin NR. Neuropsychological outcome at 1‐year post head‐injury. Neuropsychology 1995;9(1):80‐90. [Google Scholar]
Dikmen 2000 {published data only}
- Dikmen SS, Machamer JE, Winn HR, Anderson DG, Temkin NR. Neuropsychological effects of valproate in traumatic brain injury: a randomized trial. Neurology 2000;54(4):895‐902. [DOI] [PubMed] [Google Scholar]
Dizdarevic 2012 {published data only}
- Dizdarevic K, Hamdan A, Omerhodzic I, Kominlija‐Smajic E. Modified Lund concept versus cerebral perfusion pressure‐targeted therapy: a randomised controlled study in patients with secondary brain ischaemia. Clinical Neurology and Neurosurgery 2012;114(2):142‐8. [DOI] [PubMed] [Google Scholar]
Dolati 2012 {published data only}
- Dolati P, Akhtar SM, Ziabary SM. Determination of the efficacy of phenytoin on prevention of late post‐traumatic seizure. Journal of Neurology 2012;259(1):S15. [Google Scholar]
Englander 2003 {published data only}
- Englander J, Bushnik T, Duong TT, Cifu DX, Zafonte R, Wright J, et al. Analyzing risk factors for late posttraumatic seizures: a prospective, multicenter investigation. Archives of Physical Medicine & Rehabilitation 2003;84(3):365‐73. [DOI] [PubMed] [Google Scholar]
Formisano 2007 {published data only}
- Formisano R, Barba C, Buzzi MG, Newcomb‐Fernadez J, Menniti‐Ippolito F, Zafonte R, et al. The impact of prophylactic treatment on post‐traumatic epilepsy after severe traumatic brain injury. Brain Injury 2007;21(5):499‐504. [DOI] [PubMed] [Google Scholar]
Glotzner 1998 {published data only}
- Glotzner FL. Epilepsy prophylaxis with carbamazepine in severe brain injury [abstract]. Epilepsia 1998;39(Suppl 2):48. [Google Scholar]
Haltiner 1997 {published data only}
- Haltiner AM, Temkin NR, Dikmen SS. Risk of seizure recurrence after the first late posttraumatic seizure. Archives of Physical Medicine & Rehabilitation 1997;78(8):835‐40. [DOI] [PubMed] [Google Scholar]
- Haltiner AM, Temkin NR, Winn HR, Dikmen SS. The impact of posttraumatic seizures on 1‐year neuropsychological and psychosocial outcome of head injury. Journal of the International Neuropsychological Society 1996;2(6):494‐504. [DOI] [PubMed] [Google Scholar]
Holland 1995 {published data only}
- Holland JP, Stapleton SR, Moore AJ, Marsh HT, Uttley D, Bell BA. A randomised double blind study of sodium valproate for the prevention of seizures in neurosurgical patients [abstract]. Journal of Neurology, Neurosurgery, and Psychiatry 1995;58(1):116. [Google Scholar]
Inaba 2013 {published data only}
- Inaba K, Menaker J, Branco BC, Gooch J, Okoye OT, Herrold J, et al. A prospective multicenter comparison of levetiracetam versus phenytoin for early posttraumatic seizure prophylaxis. Journal of Trauma and Acute Care Surgery 2013;74(3):766‐71. [DOI] [PubMed] [Google Scholar]
Jallon 1984 {published data only}
- Jallon P, Ducolomnier A, Boucetta M, Desgeorges M. Epidemiological approach of post‐traumatic epilepsy [L'epilepsie post‐traumatique approche epidemiologique]. Revue Internationale des Services de Sante des Armees de Terre, de Mer et de l'Air 1994;56(6):505‐12. [Google Scholar]
Japan Follow‐up... 1991 {published data only}
- Japan Follow‐up Group for Posttraumatic Epilepsy. [The factors influencing posttraumatic epilepsy: a multicentric cooperative study]. No shinkei geka [Neurological Surgery] 1991;19(12):1151‐9. [PUBMED: 1766540] [PubMed] [Google Scholar]
Johnson 2009 {published data only}
- Johnson D, Rottman K, Santos M. Retrospective comparison of the cost and effectiveness of levetiracetam versus phenytoin for seizure prophylaxis. Critical Care Medicine 2009;37(12 Suppl):A475, Abstract no: 968. [Google Scholar]
Jones 2008 {published data only}
- Jones KE, Puccio AM, Harshman KJ, Falcione B, Benedict N, Jankowitz BT, et al. Levetiracetam versus phenytoin for seizure prophylaxis in severe traumatic brain injury. Neurosurgery Focus 2008;25(4):1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kieslich 2001 {published data only}
- Kieslich M, Born A, Jacobi G. Anticonvulsive prophylaxis of posttraumatic epilepsy in childhood [Antikonvulsive Prophylaxe der posttraumatischen Epilepsie im Kindersalter]. Aktuelle Neurologie 2001;28(7):313‐8. [Google Scholar]
Kirmani 2013 {published data only}
- Kirmani BF, Mungall D, Ling G. Role of intravenous levetiracetam in seizure prophylaxis of severe traumatic brain injury patients. Fontiers in Neurology 2013;4:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klein 2008 {published data only}
- Klein P, Herr D, Pearl PL, Sandoval F, Trzcinski S, Nogay C, et al. Safety, tolerability and pharmacokinetics of levetiracetam in patients with acute traumatic brain injury with high risk [abstract]. Epilepsia 2008;49(Suppl s7):81, Abstract no: 1.187. [Google Scholar]
Klein 2012a {published data only}
- Klein P, Herr D, Pearl PL, Natale J, Levine Z, Nogay C, et al. Results of phase 2 safety and feasibility study of treatment with levetiracetam for prevention of posttraumatic epilepsy. Archives of Neurology 2012;69(10):1290‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klein 2012b {published data only}
- Klein P, Herr D, Pearl PL, Natale J, Levine Z, Nogay C, et al. Results of phase II pharmacokinetic study of levetiracetam for prevention of post‐traumatic epilepsy. Epilepsy & Behavior 2012;24:457‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kobayashi 1997 {published data only}
- Kobayashi M. Cooperative multicentre study on postraumatic epilepsy. Brain and Nerve 1997;49(8):723‐7. [PubMed] [Google Scholar]
Lopes 2009 {published data only}
- Lopes J, Santos A, Martins da Silva A. Post traumatic epilepsy: a follow‐up of 20 years. Epilepsia 2009;50(Suppl 4):160, Abstract no: E411. [Google Scholar]
Maas 2006 {published data only}
- Maas AIR, Murry G, Henney H 3rd, Kassem N, Legrand V, Mangelus M, et al. Efficacy and safety of dexanabinol in severe traumatic brain injury: results of a phase III randomised, placebo‐controlled, clinical trial. Lancet Neurology 2006;5(1):38‐45. [DOI] [PubMed] [Google Scholar]
Meo 2009 {published data only}
- Meo R, Bilo L, Leva MF. Levetiracetam in patients with post‐traumatic epilepsy: observations in a case series. Epilepsia 2009;50(Suppl. 11):106, Abstract no: 1.215. [Google Scholar]
Murri 1980 {published data only}
- Murri L, Parenti G, Bonuccelli U, Lenzi B, Tacca M. Phenobarbital prophylaxis of post traumatic epilepsy. Italian Journal of Neurological Sciences 1980;1(4):225‐30. [PubMed] [Google Scholar]
Murri 1992 {published data only}
- Murri L, Arrigo A, Bonuccelli U, Rossi G, Parenti G. Phenobarbital in the prophylaxis of late posttramatic seizures. Italian Journal of Neurological Sciences 1992;13(9):755‐60. [DOI] [PubMed] [Google Scholar]
Nakamura 1995 {published data only}
- Nakamura N. Cooperative multicentre study on postraumatic epilepsy. Brain and Nerve 1995;47(12):1170‐6. [PubMed] [Google Scholar]
Nakamura 1999 {published data only}
- Nakamura N, Ishijima B, Mayanagi Y, Manaka S. A randomized controlled trial of zonisamide in postoperative epilepsy: a report of the cooperative group study. Japanese Journal of Neurosurgery 1999;8(10):647‐56. [Google Scholar]
NCT00566046 {published data only}
- NCT00566046. Prospective, randomized, double‐blind study assessing the effects of levetiracetam compared to placebo in the prevention of early epileptic seizures and late epilepsy in patients with severe traumatic brain injury. www.clinicaltrials.gov/ct/show/NCT00566046 (accessed 11 February 2014).
NCT00598923 {published data only}
- NCT00598923. Preventing epilepsy after traumatic brain injury with topiramate (PEPTO). www.clinicaltrials.gov/ct/show/NCT00598923 (accessed 11 February 2014).
NCT01110187 {published data only}
- NCT01110187. A pilot study of NSICU assessment of seizure prophylaxis with lacosamide. clinicaltrials.gov/show/NCT01110187 (accessed 11 February 2014).
North 1980 {published data only}
- North BJ, Hanieh A, Challen RG, Penhall RK, Hann CS, Frewin DB. Postoperative epilepsy: a double‐blind trial of phenytoin after craniotomy. Lancet 1980;1(8165):384‐6. [DOI] [PubMed] [Google Scholar]
North 1983 {published data only}
- North BJ, Penhall RK, Hanieh A, Frewin DB, Taylor WB. Phenytoin and postoperative epilepsy: a double‐blind study. Journal of Neurosurgery 1983;58(5):672‐7. [DOI] [PubMed] [Google Scholar]
Ohman 2001 {published data only}
- Ohman J, Braakman R, Legout V, Traumatic Brain Injury Study Group. Repinotan (BAY x 3702): a 5HT1A agonist in traumatically brain injured patients. Journal of Neurotrauma 2001;18(12):1313‐21. [DOI] [PubMed] [Google Scholar]
Ohno 1993 {published data only}
- Ohno K, Maehara T, Ichimura K, Suzuki R, Hirakawa K. Low incidence of seizures in patients with chronic subdural haematoma. Journal of Neurology, Neurosurgery, and Psychiatry 1993;56(11):1231‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pearl 2009 {published data only}
- Pearl PL, Tsuchida T, McCarter R, Pettiford JM, Sandoval F, Trzcinski S, et al. Results of phase II NIH study of levetiracetam for the prevention of pediatric post‐traumatic epilepsy (PTE). Annals of Neurology 2009;66(Suppl S1):S122, Abstract no: 81. [Google Scholar]
Pearl 2013 {published data only}
- Pearl PL, McCarter R, McGavin CL, Yu Y, Sandoval F, Trzcinsky S, et al. Results of phase II levetiracetam trial following head injury in children at risk for posttraumatic epilepsy. Epilepsia 2013;59(9):135‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Penry 1979 {published data only}
- Penry JK, White BG, Bracket CE. A controlled prospective study of the pharmacologic prophylaxis of posttraumatic epilepsy. Neurology 1979;29(4):600‐1. [Google Scholar]
Popek 1969 {published data only}
- Popek K, Musil F. Clinical attempt to prevent post‐traumatic epilepsy following severe brain injuries in adults [Klindky pokus o prevenci posttraumaticke epidelpsie po tezkych zranenick mozku u dospelych]. Casopis Lekaru Ceskych 1969;108(5):133‐47. [PUBMED: 4976099] [PubMed] [Google Scholar]
Popek 1972 {published data only}
- Popek K. Preventative treatment of post‐traumatic epilepsy following severe brain injury. Closing report summarizing results of the research done in the framework of the state research plan for the years 1965‐1969; edited by Z. Servit [Prevence posttraumaticke eplilepsi po tezkych zranenich mozku]. Ceskoslovenska Neurologie 1972;35(4):169‐74. [PUBMED: 5044144] [PubMed] [Google Scholar]
Richard 1998 {published data only}
- Richard I, Francois C, Louis F, Greve M, Perrouin‐Verbe B, Mathe JF. Post‐traumatic epilepsy: retrospective analysis of 90 severe traumatic brain injuries [Epilepsie post‐traumatique: analyse retrospective d'une serie de 90 traumatismes craniens graves]. Annales de Readaptation et de Medecine Physique 1998;41(7):409‐15. [Google Scholar]
Schutze 1999 {published data only}
- Schutze M, Dauch AW, Guttinger M, Hampel‐Christiansen M, Firsching R. Risk factors for posttraumatic fits and epilepsies [Riskofaktoren fur posttraumatische Anfalle und Epilepsie]. Zentralblatt fur Neurochirurgie 1999;60(4):163‐7. [PubMed] [Google Scholar]
Servit 1981 {published data only}
- Servit Z, Musil F. Prophylactive treatment of post traumatic epilepsy: results of a long‐term follow‐up in Czechoslovakia. Epilepsia 1981;22(3):315‐20. [DOI] [PubMed] [Google Scholar]
Smith 1994 {published data only}
- Smith KR Jr, Goulding PM, Wilderman D, Goldfader PR, Holterman‐Hommes P, Wei F. Neurobehavioral effects of phenytoin and carbamazepine in patients recovering from brain trauma: a comparative study. Archives of Neurology 1994;51(7):653‐60. [DOI] [PubMed] [Google Scholar]
Steinbaugh 2012 {published data only}
- Steinbaugh LA, Lindsell CJ, Shutter LA, Szaflarski JP. Initial EEG predicts outcomes in a trial of levetiracetam vs. fosphenytoin for seizure prevention. Epilepsy & Behavior 2012;23:280‐4. [DOI] [PubMed] [Google Scholar]
Szaflarski 2007 {published data only}
- Szaflarski JP, Meckler JM, Szaflarski M, Shutter LA, Privitera MD, Yates SL. Levetiracetam use in critically ill patients. Neurocritical Care 2007;7(2):140‐7. [PUBMED: 17607530] [DOI] [PubMed] [Google Scholar]
Temkin 2003 {published data only}
- Temkin NR. Risk factors for posttraumatic seizures in adults. Epilepsia 2003;44(Suppl s10):18‐20. [DOI] [PubMed] [Google Scholar]
Thapa 2010 {published data only}
- Thapa A, Chandra SP, Sinha S, Sreenivas V, Sharma BS, Tripathi M. Post‐traumatic seizures ‐ a prospective study from a tertiary level trauma center in a developing country. Seizure 2010;19(4):211‐6. [DOI] [PubMed] [Google Scholar]
Tomovic 1997 {published data only}
- Tomovic M, Ilic T, Mihajlovic M, Minic LJ, Jovicic A. Prophylaxis or therapy for patients with open cranio‐cerebral trauma in order to prevent post‐traumatic epilepsy. Journal of the Neurological Sciences 1997;150(Suppl 1):S214, Abstract no: 4‐06‐07. [Google Scholar]
Van den Berghe 2005 {published data only}
- Berghe G, Schoonheydt K, Becx P, Bruyninckx F, Wouters PJ. Insulin therapy protects the central and peripheral nervous system of intensive care patients. Neurology 2005;64(8):1348‐53. [PUBMED: 15851721] [DOI] [PubMed] [Google Scholar]
Virant‐Young 2009 {published data only}
- Virant‐Young D, Alexander R, Umstead G, Savoy‐Moore R, Himes D, Nikolavsky M, et al. Retrospective analysis of phenytoin and levetiracetam for seizure prophylaxis after traumatic brain injury. Critical Care Medicine 2009;37(12 suppl):A443, Abstract no: 904. [Google Scholar]
Watson 2004 {published data only}
- Watson NF, Barber JK, Doherty MJ, Miller JW, Temkin NR. Does glucocorticoid administration prevent late seizures after head injury?. Epilepsia 2004;45(6):690‐4. [DOI] [PubMed] [Google Scholar]
Wohns 1979 {published data only}
- Wohns RNW, Wyler AR. Prophylactic phenytoin in severe head injuries. Journal of Neurosurgery 1979;51(4):507‐9. [PUBMED: 113510] [DOI] [PubMed] [Google Scholar]
Young 1979 {published data only}
- Bertch KE, Norton JA, Young AB, Rapp RP, Tibbs PA. A comparative study of laboratory parameters in head‐injured patients receiving either phenytoin or placebo for 24 months. Drug Intelligence & Clinical Pharmacy 1985;19(7‐8):561‐6. [DOI] [PubMed] [Google Scholar]
- Rapp RP, Norton JA, Young B, Tibbs PA. Cutaneous reactions in head‐injured patients receiving phenytoin for seizure prophylaxis. Neurosurgery 1983;13(3):272‐5. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT01048138 {published data only}
- Use of Biperiden for the Prevention of Post‐Traumatic Epilepsy. Ongoing study 2013.
NCT01673828 {published data only}
- Allopregnanolone for the Treatment of Traumatic Brain Injury. Ongoing study 2013.
NCT02027987 {published data only}
- Traumatic neuroprotection and epilepsy prevention of Valproate acid. Ongoing study 2013.
Additional references
Annegers 1998
- Annegers JF, Hauser WA, Coan SP, Rocca WA. A population‐based study of seizures after traumatic brain injuries. New England Journal of Medicine 1998;338(1):20‐4. [DOI] [PubMed] [Google Scholar]
Beghi 2003
- Beghi E. Overview of studies to prevent post traumatic epilepsy. Epilepsia 2003;44(Suppl 10):21‐6. [DOI] [PubMed] [Google Scholar]
Bertch 1985
- Bertch KE, Norton JA, Young AB, Rapp RP, Tibbs PA. A comparative study of laboratory parameters in head‐injured patients receiving either phenytoin or placebo for 24 months. Drug Intelligence & Clinical Pharmacy 1985;19(7‐8):561‐6. [DOI] [PubMed] [Google Scholar]
Brandt 2006
- Brandt C, Heile A, Potschka H, Stoehr T, Loscher W. Effects of the novel antiepileptic drug lacosamide on the development of amygdala kindling in rats. Epilepsia 2006;47(11):1803‐9. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jennett 1981
- Jennett B, Teasdale G. Management of head injuries. Philadelphia: F.A. Davis, 1981. [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI: ] [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Löscher 2002
- Löscher W. Animal models of epilepsy for the development of antiepileptogenic and disease‐modifying drugs. A comparison of the pharmacology of kindling and post‐status epilepticus models of temporal lobe epilepsy. Epilepsy Research 2002;50(1‐2):105‐23. [PUBMED: 12151122] [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Schünemann 2011
- Schünemann HG, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and summary of findings tables. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Slemmer 2008
- Slemmer JE, Shacka JJ, Sweeney MI, Weber JT. Antioxidants and free radical scavengers for the treatment of stroke, traumatic brain injury and aging. Current Medicinal Chemistry 2008;15(4):404‐14. [DOI] [PubMed] [Google Scholar]
Teasell 2007
- Teasell R, Bayona N, Marshall S, Cullen N, Bayley M, Chundamala J, et al. A systematic review of rehabilitation of moderate to severe acquired brain injuries. Brain Injury 2007;21(2):107‐12. [DOI] [PubMed] [Google Scholar]
Temkin 2001
- Temkin N. Antiepileptogenesis and seizure prevention trials with antiepileptic drugs: meta‐analysis of controlled trials. Epilepsia 2001;42(4):515‐24. [DOI] [PubMed] [Google Scholar]
Temkin 2009
- Temkin N. Preventing and treating posttraumatic seizures: the human experience. Epilepsia 2009;50(Suppl 2):10‐13. [DOI] [PubMed] [Google Scholar]
Whitehead 1991
- Whitehead A, Whitehead J. A general parametric approach to the meta‐analysis of randomised clinical trials. Statistics in Medicine 1991;10(11):1665‐77. [DOI] [PubMed] [Google Scholar]
Williamson 2002
- Williamson PR, Tudur Smith C, Hutton JL, Marson AG. Aggregate data meta‐analysis with time to event outcomes. Statistics in Medicine 2002;21(22):3337‐51. [DOI] [PubMed] [Google Scholar]
Willmore 2009
- Willmore LJ, Ueda Y. Posttraumatic epilepsy: hemorrhage, free radicals and the molecular regulation of glutamate. Neurochemical Research 2009;34(4):688‐97. [DOI] [PubMed] [Google Scholar]
Wyllie 2010
- Wyllie E. Wyllie's Treatment of Epilepsy. 5th Edition. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins, 2010. [Google Scholar]
Young 1979
- Young B, Rapp R, Brooks WH, Madauss W, Norton JA. Posttraumatic epilepsy prophylaxis. Epilepsia 1979;20(6):671‐81. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Schierhout 2001
- Schierhout G, Roberts IG. Anti‐epileptic drugs for preventing seizures following acute traumatic brain injury. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD000173] [DOI] [PubMed] [Google Scholar]
Thompson 2012
- Thompson K, Pohlmann‐Eden B, Campbell LA. Pharmacological treatments for preventing epilepsy following traumatic head injury. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD009900] [DOI] [PMC free article] [PubMed] [Google Scholar]


