Abstract
Although the individual and societal consequences of antibiotic resistance spiral upwards, coordinated action has not kept pace on a global scale. The COVID-19 pandemic has highlighted the need for resilient health systems and has resulted in an unprecedented rate of collaboration in scientific, medical, social, and political dimensions. The pandemic has also created a renewed awareness of the importance of infectious diseases and is a substantial entry point for reigniting the momentum towards containing the silent pandemic of antibiotic resistance. In this Viewpoint, we discuss the limitations in the current narrative on antibiotic resistance and how it could be improved, including concerted efforts to close essential data gaps. We discuss the need for capacity building and coordination at the national and global levels to strengthen the understanding of the importance of sustainable access to effective antibiotics for all health systems that could generate tangible links to current processes for global health and development.
Introduction
In 2001, WHO published a global strategy to contain antimicrobial resistance.1 A year later, a workshop identified several crucial barriers to the implementation of the strategy (eg, underdeveloped health infrastructures and scarcity of valid data);2 an action plan for continuing implementation of the strategy was therefore recommended. These challenges, many of which remain valid today, were discussed at the inaugural meeting of ReAct—an international network dedicated to the problem of antibiotic resistance—in 2005.3 A global action plan on antimicrobial resistance,4 which spurred other political initiatives, was not adopted until 2015. Antibiotics are used more than any other class of antimicrobials, and in the political declaration on antimicrobial resistance in 2016,5 the UN General Assembly identified resistance to antibiotics as the greatest and most urgent global threat. This declaration was followed by the recommendations from the ad hoc UN Interagency Coordination Group (IACG) on Antimicrobial Resistance in 2019,6 and the formation of the One Health Global Leaders Group on antimicrobial resistance in 2020.7 The issue of antimicrobial resistance has featured in several health-related international agendas, such as the Global Health Security Agenda, International Health Regulations, UN Sustainable Development Goals, health systems strengthening, primary health care, and universal health coverage.8, 9, 10, 11 However, the nature of antimicrobial resistance spanning diseases and sectors is a barrier to making a strong and sustained case, ultimately, to drive local impact. Substantial shortcomings remain in making investments, international collaboration, and collective action against antibiotic resistance proportionate to its global consequences.12, 13
The current COVID-19 pandemic has created a renewed awareness of the importance of infectious diseases, the speed at which health gains can be lost, and the need for resilient health systems. The pandemic offers opportunities to increase recognition that sustainable access to effective antibiotics is a fundamental component for both basic and specialised medicine, which is increasingly threatened by antibiotic resistance. However, when advancing action on antibiotic resistance, all stakeholders should reflect on the shortcomings of current messaging and advocacy, and move towards adapting and refocusing the message. Here, we have identified areas in which improvements could help reset the agenda on antibiotic resistance through a health systems perspective. A similar systems view is needed in the animal and environmental sectors with synergies sought between the three sectors in a One Health perspective.
Key messages.
-
•
Sustainable access to effective antibiotics is a crucial function of all health systems that are increasingly being threatened by antibiotic resistance.
-
•
The current narrative on antibiotic resistance is too technical, and its grouping with resistance to other pathogens within the broader concept of antimicrobial resistance has made this threat less visible.
-
•
Paucity of nationally relevant data on antibiotic use, resistance, and the health and economic burden is hindering a strong policy response. Concerted global efforts are needed to rapidly close these crucial evidence gaps.
-
•
Lessons from the COVID-19 pandemic could facilitate the understanding of the silent pandemic of antibiotic resistance and how it can be tackled from a health systems perspective.
-
•
High-income countries need to provide leadership by reducing inappropriate antibiotic use in all sectors and by coordinating funding streams to allocate adequate resources to strengthen capacity and governance for implementation of national action plans in less-resourced countries.
-
•
Preserving antibiotic effectiveness as a global resource requires engagement from the whole of society. Powerful and contextualised awareness campaigns for behavioural change are needed.
-
•
To secure a continuous supply of new antibiotics, the broken innovation system needs to be reformed and replaced with a needs-driven end-to-end approach, from solving scientific challenges to ensuring sustainable and affordable access to those in need.
The semantic barrier
The plethora of terms describing microbial drug resistance has created a language barrier, which continues to be discussed.14, 15, 16 We believe that the grouping of bacterial resistance with resistance in other microbes (ie, viruses, fungi, and parasites) under the umbrella of antimicrobial resistance has diffused the focus on antibiotic resistance. Although resistance is a problem with all antimicrobial drugs, antibiotics are used more than any other class of antimicrobials and need a more prominent position in the narrative. Although ReAct, the US Centers for Disease Control and Prevention, and the European Centre for Disease Prevention and Control are focusing on antibiotic resistance, WHO uses both antibiotic resistance and antimicrobial resistance, often interchangeably. Moreover, in 2020, World Antibiotic Awareness Week was renamed World Antimicrobial Awareness Week. The term antimicrobial resistance could cause confusion as we believe that most people who use it are referring to antibiotic resistance, especially given its use in the One Health context. To avoid this equivocation, clarity is needed when antimicrobial resistance is discussed.
Evidence building to strengthen the narrative
Resetting the agenda for antibiotic resistance needs to build on relevant data, informing both policy makers and the public about the serious consequences of a lack of effective antibiotics. Scarce data regarding the magnitude of the problem has been considered one of the biggest barriers for national champions to convince policy makers.17 A review on antimicrobial resistance estimated 700 000 annual deaths globally and 10 million deaths by 2050 from antimicrobial-resistant infections.18 These infections included malaria and HIV, as well as tuberculosis and a few other bacteria, which has obscured the distinction between antimicrobial resistance and antibiotic resistance, and has created opportunities for criticisms regarding usefulness and reliability.19, 20 Nevertheless, the figures from the antimicrobial resistance review have been uncritically used as an indicator of the burden of antibiotic resistance.
The first large study on the mortality and economic burden of antibiotic resistance was done in the EU by the European Centre for Disease Prevention and Control in collaboration with ReAct21 and estimated that there were 25 000 attributable deaths from antibiotic resistance in 2007. This study was later updated, giving an estimate of 33 110 attributable deaths.22 Although not fully comparable, national-level data have also been published for the USA (35 900 deaths in 2019)23 and Thailand (19 122 deaths in 2010),24 yielding an annual total of 88 132 deaths from these regions, constituting approximately 10% of the world population.
Improved methodological approaches are needed to comprehensively estimate global deaths caused by antibiotic-resistant infections.20 Access to nationally relevant surveillance data on resistance is also crucial. The Global Antimicrobial Surveillance System25 is an important initiative. However, the slow expansion of this system, especially in low-income and middle-income countries (LMICs), is concerning, with reports indicating that data from the surveillance system are not used nationally.26 Basic data generation on the resistance situation is needed, particularly in LMICs. A systematic review showed that a suitable report of bacterial resistance was absent from more than 40% of the countries in the African region.27 Furthermore, concerted efforts are required to develop tools for monitoring antibiotic use. Point prevalence studies are useful as initial steps to fill these gaps.28
Changing patients' demand for antibiotics is an important factor to reduce inappropriate antibiotic use.15, 29 Reliable and understandable information on the detrimental and persisting effects of antibiotics on the commensal bacteria in our microbiomes could help to implement such behavioural change and reduce antibiotic use.12 Globally, there is a continuous shift towards the increase of resistant strains, with faecal carriage of extended-spectrum β-lactamase-producing bacteria reaching 60–70% in some WHO regions by 2011.30 Although the carriage of resistant bacteria in the normal flora is not directly harmful, many infections are emanating from these flora. Such infections include wound, urinary tract, and invasive infections in people with cancer and other immunocompromised patients, making their treatment increasingly difficult as resistance in these bacteria becomes more common.
Another aspect to consider is that resistant bacteria are spreading globally through contacts between humans, animals, and the environment, via travel and trade.31, 32, 33 Although antibiotic resistance is not a disease per se, the pandemic dimensions are obvious and it could be described as an already ongoing silent pandemic driven by the continuous selection of resistant bacteria in the microbiome.12, 34 We believe that the current situation with COVID-19 is the appropriate time to start describing antibiotic resistance as a pandemic.17, 35, 36
A health systems approach to antibiotic resistance
The responses sparked by the COVID-19 pandemic span from an unprecedented collaboration for rapid innovation of diagnostics and vaccines to increasing understanding of the need for preventive measures and equitable access to essential medicines and basic supplies. The same perspective needs to be applied to address the much less visible threat to all health systems caused by antibiotic resistance. As this problem will continue while health care is dependent on antibiotics, a long-term coordinated global and national response is needed in which gaps in crucial functions are minimised, avoiding a fragmented approach. This response must be an essential part of health systems strengthening, in which the implementation of national action plans (NAPs) is a key function. Countries were urged to develop such plans during the 2015 World Health Assembly at which the global action plan was adopted. NAPs were to be aligned with the objectives of the global action plan and put in place by 2017. The work on antibiotic resistance should have a strong and dedicated focus within a system-wide approach, and synergies should be sought with other national programmes, if appropriate.
Health system pillars to manage antibiotic resistance
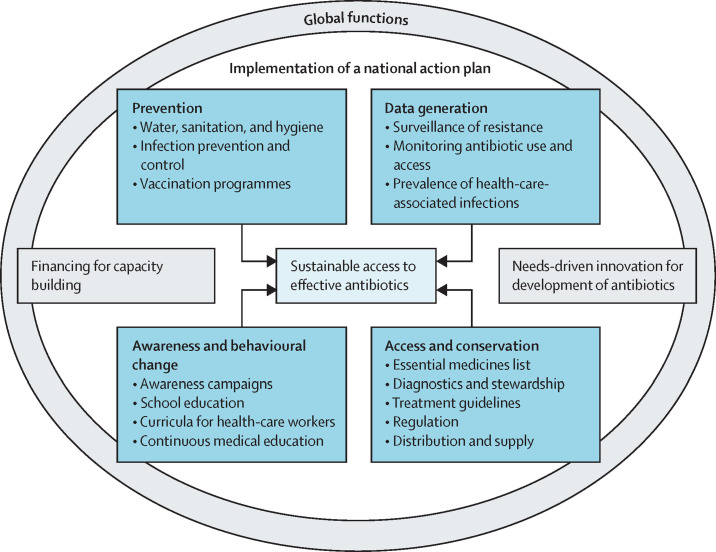
We recognise four main pillars needed for implementing NAPs: prevention, data generation, access and conservation, and awareness and behavioural change (figure ). Although activities addressing these areas are ongoing in many countries, they are often time-bound projects that are not integrated into national policies or systems. Such a fragmented approach is ineffective. A health systems approach for sustainable access to antibiotics implies not just coordinated specific actions, but concerted efforts towards human resources, universal health care, and optimal health-care infrastructure. A few examples of actions needed within these pillars in which lessons could be learned from the COVID-19 pandemic are explained further.
Figure.
Four key pillars in the implementation of a national action plan on antimicrobial resistance, supported by global functions
The COVID-19 pandemic generated unprecedented awareness regarding infection prevention including water, sanitation, and hygiene. A third of health-care facilities in low-resource settings have little access to running water and soap for handwashing.37 Improvement in this area, triggered by the current pandemic, minimises the risk for spread of resistant bacteria in these settings. There is also an urgent need to make existing bacterial vaccines available, and develop new ones for the prevention and control of antibiotic resistance.38, 39
COVID-19 proves that urgency enables science to be rapidly translated into action on the ground. The quick development of diagnostic tests allowed for the tracing of individual cases and an unprecedented generation and dissemination of global data. Many countries do not have basic data on antibiotic resistance. Although investments are needed to fill this gap, very few donors have stepped up support. Investments will fall short if the data generated are not used effectively to inform not only global trends, but also national policies—eg, comparing antibiotic use data, updating treatment guidelines and essential medicines lists, optimising procurement, educating health-care workers, and raising community awareness. An inexpensive rapid point-of-care diagnostic test would be a major game changer for reducing antibiotic misuse in viral conditions, including COVID-19 and influenza.
The COVID-19 pandemic has shown the world the impact of not having effective treatment or vaccines for infectious diseases, and the need for sustainable and equitable procurement and distribution channels once such technologies become available. Although access to effective antibiotics is an essential component of all health systems, the situation is complex, because expanding access needs to avoid excess use, but this is hampered by minimal laboratory diagnostic capacity, leading to inappropriate use and risk of treatment failure. Reliable supply of essential antibiotics will enable the use of the WHO Access, Watch, Reserve classification,40 thus optimising health-care spending and reducing risks for resistance development.
Awareness campaigns have been instrumental in conveying important facts about COVID-19 to change the behaviour of the general public. Although awareness campaigns on antibiotic resistance have been launched both globally and nationally, their effectiveness remains to be evaluated.41, 42 As alluded to earlier, the narrative for antibiotic resistance needs drastic improvement to enable a strong civil society movement and political commitment. However, there is very little evidence for how this can be achieved, and for identifying the type of messaging that would be the most effective in the long term. Social and behavioural scientists need to be involved in developing and evaluating contextualised awareness campaigns.
National governance mechanisms
Securing the need for sustainable access to effective antibiotics must be part of a national governance mechanism at the highest decision making level. Approximately 80 countries have an NAP uploaded to the WHO library, and many countries have made substantial steps for their implementation. High-income countries should lead by example; however, unnecessary antibiotic use remains high in many of these countries.43 A major problem, especially in LMICs, is the scarcity of incentives to fund and support implementation.26, 44 A discussion document from the IACG45 identified five main barriers: political will, finance, coordination, monitoring, and data and technical capacity. A 2020 article44 discussed continuing challenges of NAP implementation in Africa and indicated that minimal allocations for NAPs in national budgets and the absence of country and region-specific studies are major barriers. There is a wealth of supporting tools from WHO46 and other sources including the ReAct Toolbox.47 Efficient use of such tools requires sufficient human resources. However, many governments do not have full-time personnel to coordinate NAP activities; instead, they have focal points fulfilling other duties concurrently.
Global action needed
Responsibility for securing equitable access to effective antibiotics in health-care systems falls on national governments, and NAP implementation should ultimately be financed from sources within a country as part of existing health budget lines. However, preserving lifesaving antibiotics must also be a global responsibility. The need for global governance is evident given the slow progress despite country commitments.
All countries need to come together in collective action and take greater responsibility for their domestic management of antibiotic use. High-income countries must set an example and mobilise resources to support countries with weaker economies. WHO needs to strengthen its leadership by applying a health systems perspective on antibiotic resistance, with adequate resources to coordinate activities across programmes. An important priority for the Global Leaders Group on antimicrobial resistance7 should be to move implementation of the IACG recommendations forward and bring together donors globally to strengthen and coordinate funding streams to manage antibiotic resistance. In this way, the antibiotic resistance agenda can be reset, rekindling the agenda into a sustained movement.
There are two major areas for global collective action to transform failing systems towards sustainability: funding for capacity building and developing a sustainable global model for research and development of novel antibiotics and alternatives (figure). Antibiotic resistance has not been visible in the global funding landscape, and the absence of a go-to place for countries to receive support is a critical barrier.48 Instead, countries have been seeking funding for smaller projects from different sources, complicating coordinated planning and implementation. Calls have been made for dedicated funds to address antibiotic resistance. The IACG recommendations urged existing financing mechanisms, such as the Global Fund to Fight AIDS, Tuberculosis and Malaria, to give the issue greater priority in their resource allocations, including assessing the need to expand their scope and mandate.6 Antibiotic resistance should be a powerful component in existing funding streams for the Sustainable Development Goals, universal health coverage, and water, sanitation, and hygiene. A newly established Multi-Partner Trust Fund49 on antimicrobial resistance will help fill some gaps in funding for capacity building, but currently, only three countries have pledged support. Therefore, tangible funding for NAP implementation needs rapid expansion.
The first global analysis on the status of the antibiotic pipeline50 was a major contribution to the pivotal conference in 2009 (organised during the Swedish presidency of the EU) on new incentives for antibiotic development. The need for public sector involvement was clearly identified. However, more than a decade later, the antibiotic pipeline remains unresponsive to global needs.51 In the past 5 years, normative guidance, notably the Priority Pathogens List by WHO,52 has been developed. These guidelines aim to ensure that antibiotic research and development is needs-driven. Although public and philanthropic investments supporting the development of antibacterial compounds have increased, coordinated public sector engagement with vision and intent is required to solve multiple shortcomings throughout the whole system. Major scientific challenges continue in early stages of drug discovery, and financing for preclinical and clinical development are neither sufficient nor targeted enough. Production, procurement, and access to new and old antibiotics all require novel approaches. Finally, increased focus must be given to the formidable challenge of introducing new antibiotics into health systems without propagating the historical mistakes of overuse and misuse of antibiotics while ensuring equitable access.53, 54 Governments adopted the landmark UN Political Declaration on antimicrobial resistance in 2016,5 highlighting the importance of the principle of delinkage—ie, separating the cost of research and development from both the end product price and sales volume. This approach has yet to be implemented in a globally coordinated manner. There is an urgent need for governments to jointly define and take an end-to-end approach towards reforming the system to ensure affordable sustainable access to those in need.55
Declaration of interests
All authors report grants from ReAct, during the conduct of the study. OC is a former member of the Strategic and Technical advisory group on antimicrobial resistance for WHO and a former member of the ad hoc UN Interagency Coordination Group on Antimicrobial Resistance. ADS has received financial support for research and policy work related to antibiotic resistance from the Open Society Foundation, the Greenwall Foundation, and WHO; and has served as co-convener of the ad hoc UN Interagency Coordination Group on Antimicrobial Resistance and participated in multiple WHO expert consultations on antimicrobial resistance.
Acknowledgments
Acknowledgments
ReAct is funded primarily by the Swedish International Development Cooperation Agency. We have not received any specific grants or payments for writing this article.
Contributors
This Viewpoint builds on continuous dialogue within ReAct on the need for a strengthened global collective action to secure sustainable access to effective antibiotics. The authors are members of ReAct's Global Leaders Group. OC, SJC, and MM produced the first draft of the manuscript. All authors reviewed and edited drafts of the paper and approved the final manuscript.
References
- 1.WHO . World Health Organization; Geneva: 2001. WHO global strategy for containment of antimicrobial resistance. [Google Scholar]
- 2.WHO . World Health Organization; Geneva: 2002. Implementation workshop on the WHO global strategy for containment of antimicrobial resistance. [Google Scholar]
- 3.Cars O, Nordberg P. Antibiotic resistance–the faceless threat. Int J Risk Saf Med. 2005;17:103–110. [Google Scholar]
- 4.WHO . World Health Organization; Geneva: 2015. Global action plan on antimicrobial resistance. [DOI] [PubMed] [Google Scholar]
- 5.UN General Assembly Political declaration of the high-level meeting of the General Assembly on antimicrobial resistance. Oct 19, 2016. https://undocs.org/A/RES/71/3
- 6.UN Interagency Coordination Group on Antimicrobial Resistance . World Health Organization; Geneva: 2019. No time to wait: securing the future from drug-resistant infections. [Google Scholar]
- 7.WHO Global Leaders Group Terms of Reference. Aug 10, 2020. https://www.who.int/news-room/articles-detail/global-leaders-group-terms-of-reference
- 8.Wernli D, Haustein T, Conly J, Carmeli Y, Kickbusch I, Harbarth S. A call for action: the application of The International Health Regulations to the global threat of antimicrobial resistance. PLoS Med. 2011;8 doi: 10.1371/journal.pmed.1001022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Heijden M, Sandgren A, Pränting M, et al. When the drugs don't work: antibiotic resistance as a global development problem. Feb 28, 2019. https://www.reactgroup.org/wp-content/uploads/2019/02/When-the-Drugs-Don't-Work-Antibiotic-Resistance-as-a-Global-Development-Problem-Feb-2019.pdf
- 10.Bloom G, Merrett GB, Wilkinson A, Lin V, Paulin S. Antimicrobial resistance and universal health coverage. BMJ Glob Health. 2017;2 doi: 10.1136/bmjgh-2017-000518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomson G, Vlad I. The need to look at antibiotic resistance from a health systems perspective. Ups J Med Sci. 2014;119:117–124. doi: 10.3109/03009734.2014.902879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cars O, Högberg LD, Murray M, et al. Meeting the challenge of antibiotic resistance. BMJ. 2008;337 doi: 10.1136/bmj.a1438. [DOI] [PubMed] [Google Scholar]
- 13.Laxminarayan R, Van Boeckel T, Frost I, et al. The Lancet Infectious Diseases Commission on antimicrobial resistance: 6 years later. Lancet Infect Dis. 2020;20:e51–e60. doi: 10.1016/S1473-3099(20)30003-7. [DOI] [PubMed] [Google Scholar]
- 14.Krockow EM. Nomen est omen: why we need to rename ‘antimicrobial resistance’. JAC Antimicrob Resist. 2020;2 doi: 10.1093/jacamr/dlaa067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wellcome . The Wellcome Trust; London: 2019. Reframing resistance: how to communicate about antimicrobial resistance effectively. [Google Scholar]
- 16.Mendelson M, Balasegaram M, Jinks T, Pulcini C, Sharland M. Antibiotic resistance has a language problem. Nature. 2017;545:23–25. doi: 10.1038/545023a. [DOI] [PubMed] [Google Scholar]
- 17.Wellcome . The Wellcome Trust; London: 2020. The global response to AMR: momentum, success, and critical gaps. [Google Scholar]
- 18.Review on Antimicrobial Resistance . The Wellcome Trust; London: 2016. Tackling drug-resistant infections globally: final report and recommendations—review on antimicrobial resistance. [Google Scholar]
- 19.de Kraker MEA, Stewardson AJ, Harbarth S. Will 10 million people die a year due to antimicrobial resistance by 2050? PLoS Med. 2016;13 doi: 10.1371/journal.pmed.1002184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Limmathurotsakul D, Dunachie S, Fukuda K, et al. Improving the estimation of the global burden of antimicrobial resistant infections. Lancet Infect Dis. 2019;19:e392–e398. doi: 10.1016/S1473-3099(19)30276-2. [DOI] [PubMed] [Google Scholar]
- 21.European Centre for Disease Prevention and Control. European Medicines Agency . European Centre for Disease Prevention and Control; Stockholm: 2009. The bacterial challenge: time to react—a call to narrow the gap between multidrug-resistant bacteria in the EU and the development of new antibacterial agents. [Google Scholar]
- 22.Cassini A, Högberg LD, Plachouras D, et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis. 2019;19:56–66. doi: 10.1016/S1473-3099(18)30605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.US Department of Health and Human Services . US Centers for Disease Control and Prevention; Atlanta, GA: 2019. Antibiotic resistance threats in the United States. [Google Scholar]
- 24.Lim C, Takahashi E, Hongsuwan M, et al. Epidemiology and burden of multidrug-resistant bacterial infection in a developing country. eLife. 2016;5 doi: 10.7554/eLife.18082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WHO Global Antimicrobial Resistance Surveillance System (GLASS) https://www.who.int/glass/en/
- 26.ReAct . ReAct; Zambia: 2019. ReAct Africa and South Centre Conference 2019: achieving universal health coverage while addressing antimicrobial resistance. [Google Scholar]
- 27.Tadesse BT, Ashley EA, Ongarello S, et al. Antimicrobial resistance in Africa: a systematic review. BMC Infect Dis. 2017;17:616. doi: 10.1186/s12879-017-2713-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mölstad S, Löfmark S, Carlin K, et al. Lessons learnt during 20 years of the Swedish strategic programme against antibiotic resistance. Bull World Health Organ. 2017;95:764–773. doi: 10.2471/BLT.16.184374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pearson M, Chandler C. Knowing antmicrobial resistance in practice: a multi-country qualitative study with human and animal healthcare professionals. Glob Health Action. 2019;12 doi: 10.1080/16549716.2019.1599560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woerther P-L, Burdet C, Chachaty E, Andremont A. Trends in human fecal carriage of extended-spectrum β-lactamases in the community: toward the globalization of CTX-M. Clin Microbiol Rev. 2013;26:744–758. doi: 10.1128/CMR.00023-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ludden C, Lötsch F, Alm E, et al. Cross-border spread of blaNDM-1- and blaOXA-48-positive Klebsiella pneumoniae: a European collaborative analysis of whole genome sequencing and epidemiological data, 2014 to 2019. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.20.2000627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Voor in 't Holt AF, Mourik K, Beishuizen B, et al. Acquisition of multidrug-resistant Enterobacterales during international travel: a systematic review of clinical and microbiological characteristics and meta-analyses of risk factors. Antimicrob Resist Infect Control. 2020;9:71. doi: 10.1186/s13756-020-00733-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris SR, Feil EJ, Holden MTG, et al. Evolution of MRSA during hospital transmission and intercontinental spread. Science. 2010;327:469–474. doi: 10.1126/science.1182395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jørgensen PS, Wernli D, Folke C, Carroll SP. Changing antibiotic resistance: sustainability transformation to a pro-microbial planet. Curr Opin Environ Sustain. 2017;25:66–76. [Google Scholar]
- 35.Cantón R, Coque TM. The CTX-M beta-lactamase pandemic. Curr Opin Microbiol. 2006;9:466–475. doi: 10.1016/j.mib.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 36.Chaudhry D, Tomar P. Antimicrobial resistance: the next BIG pandemic. Int J Community Med Public Health. 2017;4 [Google Scholar]
- 37.WHO . World Health Organization; Geneva: 2019. Water, sanitation and hygiene in health care facilities: status in low-and middle-income countries and way forward. [Google Scholar]
- 38.Vekemans J, Hasso-Agopsowicz M, Kang G, et al. Leveraging vaccines to reduce antibiotic use and prevent antimicrobial resistance: a World Health Organization action framework. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab062. published online Jan 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ginsburg AS, Klugman KP. Vaccination to reduce antimicrobial resistance. Lancet Glob Health. 2017;5:e1176–e1177. doi: 10.1016/S2214-109X(17)30364-9. [DOI] [PubMed] [Google Scholar]
- 40.WHO Adopt AWaRe: handle antibiotics with care. https://adoptaware.org/
- 41.WHO . World Health Organization; Geneva: 2016. Evaluation of antibiotic awareness campaigns. [Google Scholar]
- 42.Clift C. Chatham House, The Royal Institute of International Affairs; London: 2019. Review of progress on antimicrobial resistance: background and analysis. [Google Scholar]
- 43.WHO Library of national action plans. https://www.who.int/antimicrobial-resistance/national-action-plans/library/en/
- 44.Mpundu M. Moving from paper to action—the status of National AMR Action Plans in African countries. https://revive.gardp.org/moving-from-paper-to-action-the-status-of-national-amr-action-plans-in-african-countries/
- 45.UN Interagency Coordination Group on Antimicrobial Resistance Antimicrobial resistance: national action plans. June, 2018. https://www.who.int/antimicrobial-resistance/interagency-coordination-group/IACG_AMR_National_Action_Plans_110618.pdf?ua=1
- 46.WHO Supporting countries with national action plan implementation. https://www.who.int/activities/supporting-countries-with-national-action-plan-implementation
- 47.ReAct Toolbox for action on antibiotic resistance. https://www.reactgroup.org/toolbox/
- 48.ReAct. Dag Hammarskjöld Foundation . Dag Hammarskjöld Foundation; Uppsala: 2018. Antimicrobial resistance and sustainable development: a planetary threat but a financing orphan. [Google Scholar]
- 49.Multi-Partner Trust Fund Trust fund factsheet—antimicrobial resistance multi-partner trust fund. http://mptf.undp.org/factsheet/fund/AMR00
- 50.Freire-Moran L, Aronsson B, Manz C, et al. Critical shortage of new antibiotics in development against multidrug-resistant bacteria—time to react is now. Drug Resist Updat. 2011;14:118–124. doi: 10.1016/j.drup.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 51.WHO . World Health Organization; Geneva: 2019. 2019 antibacterial agents in clinical development: an analysis of the antibacterial clinical development pipeline. [Google Scholar]
- 52.WHO . World Health Organization; Geneva: 2017. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. [Google Scholar]
- 53.Zorzet A. Overcoming scientific and structural bottlenecks in antibacterial discovery and development. Ups J Med Sci. 2014;119:170–175. doi: 10.3109/03009734.2014.897277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.So AD, Shah TA. New business models for antibiotic innovation. Ups J Med Sci. 2014;119:176–180. doi: 10.3109/03009734.2014.898717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.ReAct Europe Ensuring sustainable access to effective antibiotics for everyone–everywhere: how to address the global crisis in antibiotic research and development. https://www.reactgroup.org/antibiotics-sustainable-access-everyone-everywhere.pdf