Key Points
Question
Is shortening the time to treatment for sepsis associated with increasing antimicrobial use, days of therapy, and broadness of coverage among the larger population of patients with potential infection?
Findings
In this cohort study of 1 559 523 patients hospitalized at 152 hospitals in 2 health care delivery systems, the median time to first antimicrobial administration for sepsis decreased by 37 minutes from 2013 to 2018. During this same period, antimicrobial use, days of therapy, and broadness of antibacterial coverage declined among patients with potential infection.
Meaning
These findings suggest that shortening the time to antibiotics administration for sepsis is feasible without leading to indiscriminate antimicrobial use, which can inform guidelines designed to accelerate early treatment for sepsis without having spillover effects onto other patients at risk for sepsis.
This cohort study assesses whether temporal changes in antimicrobial timing for sepsis are associated with increasing antimicrobial use, days of therapy, or broadness of antimicrobial coverage among all hospitalized patients at risk for sepsis.
Abstract
Importance
Some experts have cautioned that national and health system emphasis on rapid administration of antimicrobials for sepsis may increase overall antimicrobial use even among patients without sepsis.
Objective
To assess whether temporal changes in antimicrobial timing for sepsis are associated with increasing antimicrobial use, days of therapy, or broadness of antimicrobial coverage among all hospitalized patients at risk for sepsis.
Design, Setting, and Participants
This is an observational cohort study of hospitalized patients at 152 hospitals in 2 health care systems during 2013 to 2018, admitted via the emergency department with 2 or more systemic inflammatory response syndrome (SIRS) criteria. Data analysis was performed from June 10, 2021, to March 22, 2022.
Exposures
Hospital-level temporal trends in time to first antimicrobial administration.
Outcomes
Antimicrobial outcomes included antimicrobial use, days of therapy, and broadness of antibacterial coverage. Clinical outcomes included in-hospital mortality, 30-day mortality, length of hospitalization, and new multidrug-resistant (MDR) organism culture positivity.
Results
Among 1 559 523 patients admitted to the hospital via the emergency department with 2 or more SIRS criteria (1 269 998 male patients [81.4%]; median [IQR] age, 67 [59-77] years), 273 255 (17.5%) met objective criteria for sepsis. In multivariable models adjusted for patient characteristics, the adjusted median (IQR) time to first antimicrobial administration to patients with sepsis decreased by 37 minutes, from 4.7 (4.1-5.3) hours in 2013 to 3.9 (3.6-4.4) hours in 2018, although the slope of decrease varied across hospitals. During the same period, antimicrobial use within 48 hours, days of antimicrobial therapy, and receipt of broad-spectrum coverage decreased among the broader cohort of patients with SIRS. In-hospital mortality, 30-day mortality, length of hospitalization, new MDR culture positivity, and new MDR blood culture positivity decreased over the study period among both patients with sepsis and those with SIRS. When examining hospital-specific trends, decreases in antimicrobial use, days of therapy, and broadness of antibacterial coverage for patients with SIRS did not differ by hospital antimicrobial timing trend for sepsis. Overall, there was no evidence that accelerating antimicrobial timing for sepsis was associated with increasing antimicrobial use or impaired antimicrobial stewardship.
Conclusions and Relevance
In this multihospital cohort study, the time to first antimicrobial for sepsis decreased over time, but this trend was not associated with increasing antimicrobial use, days of therapy, or broadness of antimicrobial coverage among the broader population at-risk for sepsis, which suggests that shortening the time to antibiotics for sepsis is feasible without leading to indiscriminate antimicrobial use.
Introduction
Sepsis is a global health priority, contributing to approximately 50 million hospitalizations and 10 million deaths annually.1 Sepsis practice guidelines recommend prompt treatment for sepsis,2,3,4,5 because delays in antimicrobial therapy are associated with increased mortality.6,7,8 However, it is difficult to diagnose sepsis with certainty in real time,9 and as many as 1 in 3 patients who initiate treatment for bacterial sepsis are subsequently determined to have a noninfectious or nonbacterial cause of illness.10,11,12
Despite the difficulty of diagnosing sepsis in real time, a growing number of health system and government programs (eg, SEP-1 performance measure) incentivize rapid sepsis treatment,13 and there are growing concerns that our intense focus on early treatment may be increasing antimicrobial use.14,15,16 Thus, some experts argue that shortening time to antimicrobial administration for sepsis may increase antimicrobial use across the board and spur antimicrobial resistance.16,17 Conversely, other experts argue that efforts to accelerate sepsis treatment have resulted in antimicrobials being administered to the same patients, just several hours sooner, and thus saving lives without changing overall antimicrobial use.18
Given the global burden of sepsis,1,19 the urgency placed on rapid sepsis treatment,4,5 and the mounting threat of antimicrobial resistance,20,21 we need empirical data on whether (and to what extent) shortening the time to first antimicrobial for sepsis is associated with increasing antimicrobial use, days of therapy, or broadness of coverage among the broader population of patients at risk for sepsis. To address this gap, we measured temporal trends in antimicrobial prescribing. We further assessed whether hospital-level decreases in time to first antimicrobial administration for sepsis were associated with changes in antimicrobial use, days of therapy, and broadness of coverage in a cohort of patients admitted to hospitals in 2 large health care delivery systems.
Methods
Study Design and Cohort
This is an observational cohort study of adult patients hospitalized from 2013 to 2018 at the US Veterans Affairs (VA) and Kaiser Permanente Northern California (KPNC) health care systems. The study was reviewed by the University of Michigan, VA Ann Arbor, and KPNC institutional review boards and was deemed exempt from the need for consent under 45 CFR §46, category 4 (secondary use of identifiable data). This study follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. To focus on patients at risk for sepsis, we limited our cohort to patients admitted through the emergency department with 2 or more systemic inflammatory response syndrome (SIRS) criteria.22 Patient and hospitalization characteristics, including demographics, race, comorbidities, laboratory values, and hospital treatments, were extracted from the VA and KPNC electronic health records, as in prior work.23,24,25 Demographic and racial data are presented to convey the representativeness of the study cohort.
We classified patients as having sepsis on the basis of objective evidence of suspected infection and acute organ dysfunction, similar to the US Centers for Disease Control and Prevention’s Adult Sepsis Event definition.26,27 Specifically, patients with sepsis were identified by antimicrobial therapy administered within 12 hours of emergency department presentation, antimicrobial therapy continued for 4 or more consecutive days (or died while receiving consecutive days of therapy), and objective evidence of acute organ dysfunction within 48 hours of arrival,27 as described further in the eAppendix in the Supplement.
Exposure
The primary exposure was time to first systemic antimicrobial, calculated as the time from emergency department arrival to the time of first administration of an enteral or intravenous antimicrobial included in the Centers for Disease Control and Prevention’s Adult Sepsis Event definition.26 Time of administration was determined from physician order-entry and bar code medication administration data, as in prior work.8,24
We measured hospital-level temporal trends in time to first antimicrobial administration among patients with sepsis using multilevel linear regression models (sepsis hospitalizations nested within hospitals) with a random slope for calendar year, adjusted for patient characteristics (age, sex, comorbidities, individual SIRS criteria, and individual acute organ dysfunctions), as in prior work.24 We considered multiple alternative approaches to modeling temporal trends in antimicrobial timing, all of which yielded similar results.24
Outcomes
We examined several antimicrobial prescribing and clinical outcomes. Antimicrobial outcomes included antimicrobial use (cumulative proportion of patients receiving antimicrobial therapy within 12, 24, and 48 hours of emergency department arrival), antimicrobial duration (total days with antimicrobial therapy to day 30, inclusive of outpatient antimicrobial prescriptions dispensed within 1 calendar day of discharge to capture the full treatment course associated with hospitalization), and broadness of antibacterial coverage (using Spectrum Score,28,29 a validated score [range, 0-64, with higher scores representing broader coverage] that quantifies the broadness of any antibacterial regimen, as described in the eAppendix in the Supplement). We considered 2 thresholds for broad-spectrum coverage, a Spectrum Score of 40 or higher and a Spectrum Score of 45 or higher. A Spectrum Score of 40 or higher would include coverage with piperacillin-tazobactam (Spectrum Score, 42.25), vancomycin plus piperacillin-tazobactam (Spectrum Score, 44.5), or similar, whereas a Spectrum Score of 45 or higher would include coverage with vancomycin plus a carbapenem (Spectrum Score, 45.25), or similar.
Clinical outcomes included in-hospital mortality, 30-day mortality, length of hospitalization, and new culture positivity for a multidrug-resistant (MDR) pathogen. The MDR pathogens were a culture or swab positive for methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, carbapenem-resistant Enterobacteriaceae, extended-spectrum β-lactamase–producing Enterobacteriaceae, MDR Pseudomonas species (ie, a Pseudomonas species that is resistant to at least 1 antibacterial from at least 3 different antibacterial classes), or Acinetobacter species, which was collected during calendar days 2 to 90 after emergency department arrival and no positive culture or swab for that organism in the 180 calendar days before emergency department arrival. All outcomes were extracted from the electronic health record at VA and KNPC, as described further in the eAppendix in the Supplement.
Statistical Analysis
We completed a series of analyses to understand whether shortening antimicrobial timing for sepsis increases antimicrobial use. First, we measured temporal trends in antimicrobial prescribing outcomes among all hospitalized patients with SIRS in a series of linear and logistic regression models. We adjusted for patient characteristics (age, sex, comorbidities, individual SIRS criteria, and individual acute organ dysfunctions) and health care system to understand temporal trends independently of temporal changes in the patient case mix. This analysis allowed us to assess concurrent trends in antimicrobial prescribing practices. We also examined temporal trends in clinical outcomes, including mortality, length of hospitalization, and new MDR pathogens.
However, because analysis of concurrent temporal trends across a large-multihospital cohort may be subject to the Simpson paradox (wherein the cohort trend differs from individual hospital trends), we also assessed the association between temporal trends in antimicrobial prescribing at the hospital level. In this analysis, we leveraged the variation in trends across the hospitals to assess whether hospitals with greater decreases in antimicrobial timing for sepsis from 2013 to 2018 had increasing antimicrobial use (or less robust decreases) than hospitals with lesser decreases or flat antimicrobial timing for sepsis. Specifically, we calculated Spearman correlations and measured the associations between antimicrobial timing trend and other antimicrobial prescribing trends. The trends were estimated from regression models adjusted for patient characteristics, as described already; the association between trends was estimated using robust regression. Robust regression is an alternative to ordinary least-squares regression when data include outliers or influential data points.30,31 Rather than excluding outlier and influential hospitals or treating all hospitals equally, the robust regression weighted each hospital by the number of hospitalizations. In primary analyses, we assessed the association between antimicrobial timing trend for sepsis with antimicrobial prescribing trends for all patients with SIRS. In a sensitivity analysis, we examined the association with antimicrobial prescribing trends for patients with SIRS but without sepsis (to understand potential spillover effects of accelerating sepsis treatment onto antimicrobial prescribing for patients hospitalized without sepsis).
Data analysis was performed from June 10, 2021, to March 22, 2022. Data management and analysis were performed in SAS Enterprise Guide statistical software version 7.1 (SAS Institute) and Stata/MP statistical software version 16.1 (StataCorp). We considered P < .05 (2-sided) and r > 0.2 to be significant.32
Results
Study Cohort
During the study period, 1 559 523 hospitalized patients (representing 45 million patient days at-risk for antimicrobial therapy) were admitted to 152 hospitals via the emergency department with 2 or more SIRS criteria and were thus included in this study (eTable 1 in the Supplement). Of these patients, 273 255 (17.5%) met surveillance criteria for sepsis. Patient and hospitalization characteristics are presented in Table 1, stratified by health care system in eTables 2 and 3 in the Supplement. Patients hospitalized with sepsis had a median (IQR) age of 69 (61-79) years, 215 584 (78.9%) were male, 44 808 (16.4%) were Black, 191 358 (70.0%) were White, the median (IQR) length of hospitalization was 6 (4-10) days, and the 30-day mortality rate was 14.1% (38 552 patients). Patients hospitalized with SIRS had a median (IQR) age of 67 (59-77) years, 1 269 998 (81.4%) were male, 292 901 (18.8%) were Black, 1 086 826 (69.7%) were White, the median (IQR) length of hospitalization was 4 (3-7) days, and the 30-day mortality rate was 7.8% (121 690 patients).
Table 1. Characteristics and Outcomes of Study Patients.
| Characteristics | Hospitalized patients, No. (%) | |
|---|---|---|
| SIRS (n = 1 559 523)a | Sepsis (n = 273 255) | |
| Health care system | ||
| Veterans Affairs | 1 100 393 (70.6) | 165 146 (60.4) |
| Kaiser Permanente Northern California | 459 130 (29.4) | 108 109 (39.6) |
| Demographics | ||
| Age, median (IQR), y | 67 (59-77) | 69 (61-79) |
| Sex | ||
| Male | 1 269 998 (81.4) | 215 584 (78.9) |
| Female | 289 525 (18.6) | 57 671 (21.1) |
| Self-reported race | ||
| Black or African American | 292 901 (18.8) | 44 808 (16.4) |
| White | 1 086 826 (69.7) | 191 358 (70.0) |
| Other or missingb | 179 796 (11.5) | 37 089 (13.6) |
| Comorbiditiesc | ||
| Congestive heart failure | 505 061 (32.4) | 90 827 (33.2) |
| Neurological disease | 263 388 (16.9) | 57 683 (21.1) |
| Chronic pulmonary disease | 700 590 (44.9) | 125 137 (45.8) |
| Liver disease | 275 630 (17.7) | 57 400 (21.0) |
| Any diabetes | 660 485 (42.4) | 131 329 (48.1) |
| Diabetes with complication | 447 677 (28.7) | 96 779 (35.4) |
| Any cancer | 300 572 (19.3) | 60 878 (22.3) |
| Metastatic cancer | 108 800 (7.0) | 24 466 (9.0) |
| Renal disease | 475 230 (30.5) | 104 143 (38.1) |
| Acute organ dysfunctiond | ||
| Lactate elevation | 268 897 (17.2) | 155 858 (57.0) |
| Kidney dysfunction | 335 804 (21.5) | 140 129 (51.3) |
| Vasopressor use | 52 971 (3.4) | 34 215 (12.5) |
| Hematological dysfunction | 80 408 (5.2) | 35 554 (13.0) |
| Hepatic dysfunction | 68 779 (4.4) | 29 004 (10.6) |
| Mechanical ventilation | 41 820 (2.7) | 25 020 (9.2) |
| Acute organ dysfunctions, No. | ||
| 0 | 960 381 (61.6) | 0 |
| 1 | 423 453 (27.2) | 177 039 (64.8) |
| 2 | 123 849 (7.9) | 62 263 (22.8) |
| ≥3 | 51 840 (3.3) | 33 953 (12.4) |
| Hospital outcomes | ||
| Length of hospitalization, median (IQR), d | 4 (3-7) | 6 (4-10) |
| In-hospital mortality | 54 562 (3.5) | 24 114 (8.8) |
| 30-d mortality | 121 690 (7.8) | 38 552 (14.1) |
Abbreviation: SIRS, systemic inflammatory response syndrome.
Patients with SIRS were admitted through the emergency department with 2 or more SIRS criteria. Patients with sepsis comprised the subset with 4 or more days of antimicrobial therapy and objective evidence of acute organ dysfunction.
Other includes Alaska Native, American Indian, Asian, Native Hawaiian, Pacific Islander, and unknown.
Comorbidities were defined using the Elixhauser Comorbidity Index and were identified from diagnostic codes during inpatient and outpatient health care encounters in the 540 days before emergency department presentation.
Acute organ dysfunction was determined from electronic health record data for the 24 hours before through 48 hours after presentation to the emergency department.
Temporal Trends in Antimicrobial Prescribing
Temporal trends in antimicrobial prescribing for patients with SIRS are presented in Table 2. Temporal trends for patients with sepsis are presented in Figure 1 and in eTable 4 in the Supplement.
Table 2. Antimicrobial Prescribing and Outcomes by Year Among Patients Hospitalized With SIRS, Adjusted for Patient Characteristicsa.
| Outcome | 2013-2018 (n = 1 559 523) | 2013 (n = 259 754) | 2014 (n = 257 041) | 2015 (n = 256 729) | 2016 (n = 257 500) | 2017 (n = 265 163) | 2018 (n = 263 336) | P value for trend |
|---|---|---|---|---|---|---|---|---|
| Receipt of antimicrobial therapy | ||||||||
| Time to first antimicrobial, hb | ||||||||
| Median (IQR) | 4.4 (3.8-4.9) | 4.7 (4.0-5.2) | 4.7 (4.0-5.1) | 4.5 (3.8-5.0) | 4.4 (3.7-4.9) | 4.2 (3.6-4.8) | 4.1 (3.5-4.7) | <.001 |
| Mean (95% CI) | 4.3 (4.3-4.3) | 4.5 (4.5-4.5) | 4.4 (4.4-4.4) | 4.4 (4.3-4.4) | 4.3 (4.3-4.3) | 4.2 (4.2-4.2) | 4.1 (4.1-4.1) | <.001 |
| Antimicrobial, % | ||||||||
| Within 12 h | 49.8 | 49.1 | 49.4 | 49.7 | 50.0 | 50.2 | 50.5 | <.001 |
| Within 24 h | 56.0 | 55.9 | 55.9 | 55.9 | 56.0 | 56.0 | 56.0 | .24 |
| Within 48 h | 59.9 | 60.0 | 60.0 | 59.9 | 59.8 | 59.8 | 59.7 | .002 |
| Days of therapy to day 30, mean (95% CI) | 5.5 (5.5-5.5) | 5.8 (5.8-5.8) | 5.7 (5.7-5.7) | 5.6 (5.6-5.6) | 5.5 (5.4-5.5) | 5.3 (5.3-5.4) | 5.2 (5.2-5.2) | <.001 |
| Broadness of antibacterial coverage | ||||||||
| Receipt of broad-spectrum coverage, %c | ||||||||
| Within 48 h | ||||||||
| Spectrum Score ≥40 | 37.2 | 38.8 | 38.2 | 37.5 | 36.9 | 36.2 | 35.6 | <.001 |
| Spectrum Score ≥45 | 26.4 | 27.3 | 27.0 | 26.6 | 26.3 | 25.9 | 25.6 | <.001 |
| Within 30 d | ||||||||
| Spectrum Score ≥40 | 44.3 | 46.6 | 45.7 | 44.8 | 43.9 | 43.0 | 42.1 | <.001 |
| Spectrum Score ≥45 | 34.7 | 36.3 | 35.6 | 35.0 | 34.3 | 33.7 | 33.1 | <.001 |
| Cumulative Spectrum Score, mean (95% CI) | ||||||||
| 48 h | 23.7 (23.7-23.8) | 24.2 (24.2-24.3) | 24.0 (24.0-24.1) | 23.8 (23.8-23.9) | 23.6 (23.6-23.7) | 23.4 (23.4-23.5) | 23.2 (23.2-23.3) | .70 |
| 30 d | 27.3 (27.3-27.3) | 28.1 (28.1-28.2) | 27.8 (27.8-27.8) | 27.5 (27.4-27.5) | 27.2 (27.1-27.2) | 26.8 (26.8-26.9) | 26.5 (26.4-26.6) | <.001 |
| Outcomes | ||||||||
| Mortality, % | ||||||||
| In-hospital | 3.5 | 4.3 | 3.9 | 3.6 | 3.4 | 3.1 | 2.9 | <.001 |
| 30-d | 7.8 | 9.0 | 8.5 | 8.0 | 7.6 | 7.2 | 6.8 | <.001 |
| Length of stay, d | ||||||||
| Among all patients, median (IQR) | 5.8 (4.8-7.1) | 5.9 (5.0-7.1) | 5.9 (5.0-7.2) | 5.8 (4.9-7.1) | 5.8 (4.8-7.1) | 5.7 (4.7-7.0) | 5.5 (4.5-6.9) | <.001 |
| Patients with live discharge, median (IQR) | 5.7 (4.8-7.0) | 5.8 (5.0-7.0) | 5.8 (4.9-7.1) | 5.7 (4.8-7.0) | 5.7 (4.7-7.0) | 5.6 (4.6-6.9) | 5.5 (4.5-6.8) | <.001 |
| New antimicrobial resistance, % | ||||||||
| New MDR cultured | 2.9 | 3.8 | 3.4 | 3.0 | 2.7 | 2.4 | 2.2 | <.001 |
| New MDR blood cultured | 0.4 | 0.5 | 0.5 | 0.4 | 0.4 | 0.3 | 0.3 | <.001 |
Abbreviations: MDR, multidrug resistant; SIRS, systemic inflammatory response syndrome.
Outcomes in this table were estimated from linear or logistic regression models adjusted for age, sex, 30 Elixhauser comorbidities, individual SIRS criteria, and individual acute organ dysfunctions. Data for the 273 255 patients with sepsis are presented in eTable 4 in the Supplement.
Time to first antimicrobial use is reported for the 777 083 patients who received antimicrobial therapy within 12 hours.
A Spectrum Score of 40 or higher would include coverage with piperacillin-tazobactam (Spectrum Score, 42.25), vancomycin plus piperacillin-tazobactam (Spectrum Score, 44.5), or similar, whereas a Spectrum Score of 45 or higher would include coverage with vancomycin plus a carbapenem (Spectrum Score 45.25) or similar.
MDR is defined as a culture or swab positive for methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, carbapenem-resistant Enterobacteriaceae, extended-spectrum β-lactamase–producing Enterobacteriaceae, MDR Pseudomonas species (ie, a Pseudomonas species that is resistant to at least 1 antibacterial from at least 3 different antibacterial classes), or Acinetobacter, which was collected during calendar days 2 to 90 after emergency department arrival and no positive culture or swab for that organism in the 180 calendar days before emergency department arrival.
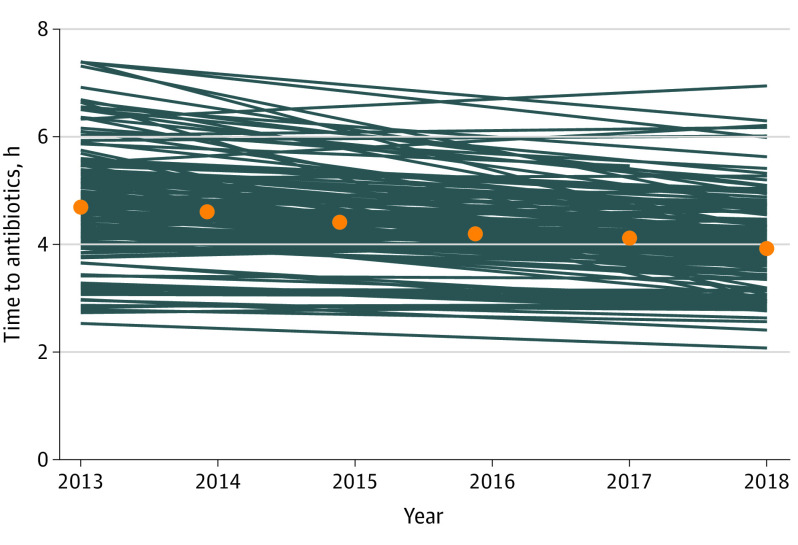
Figure 1. Temporal Trends in Time to First Antimicrobial Administration Among Patients With Sepsis, by Hospital.
Orange dots indicate median time to first antimicrobial by year; blue lines denote temporal trend in median time to first antimicrobial per hospital. Median time to first antimicrobial, after adjustment for patient characteristics, decreased by 7.3 minutes per year (from a median [IQR] of 4.7 [4.1-5.3] hours in 2013 to 3.9 [3.6-4.4] hours in 2018).
Among patients with sepsis, the median (IQR) time to first antimicrobial administration decreased by 37 minutes, from 4.7 (4.1-5.3) hours in 2013 to 3.9 (3.6-4.4) hours in 2018, whereas the mean days of antimicrobial therapy decreased from 11.4 days (95% CI, 11.4-11.5 days) to 10.5 days (95% CI, 10.5-10.6 days). Mean broadness of antimicrobial coverage and receipt of broad-spectrum coverage both decreased over time.
Among all patients with SIRS, receipt of an antimicrobial within 12 hours of emergency department arrival increased from 49.1% in 2013 to 50.5% in 2018. However, cumulative receipt of an antimicrobial within 24 hours was stable, and cumulative receipt of an antimicrobial within 48 hours decreased from 60.0% in 2013 to 59.7% in 2018. Mean days of antimicrobial therapy decreased from 5.82 days (95% CI, 5.80-5.83 days) to 5.22 days (95% CI, 5.20-5.24 days). Mean broadness of antimicrobial coverage and receipt of broad-spectrum coverage also decreased over time.
Temporal Trends in Clinical Outcomes
Among patients with SIRS, in-hospital mortality decreased from 4.3% in 2013 to 2.9% in 2018, whereas 30-day mortality decreased from 9.0% to 6.8%. The median (IQR) length of stay decreased from 5.9 (5.0-7.1) days to 5.5 (4.5-6.9) days. New MDR culture positivity within 90 days decreased from 3.8% to 2.2%, whereas new MDR blood culture decreased from 0.5% to 0.3%.
Among patients with sepsis, 30-day mortality decreased from 16.3% in 2013 to 12.3% in 2018. As with patients with SIRS, length of hospitalization, new MDR culture positivity, and new MDR blood culture positivity also decreased among patients with sepsis over the study period for each (eTable 4 in the Supplement).
Association of Hospital-Level Temporal Trends
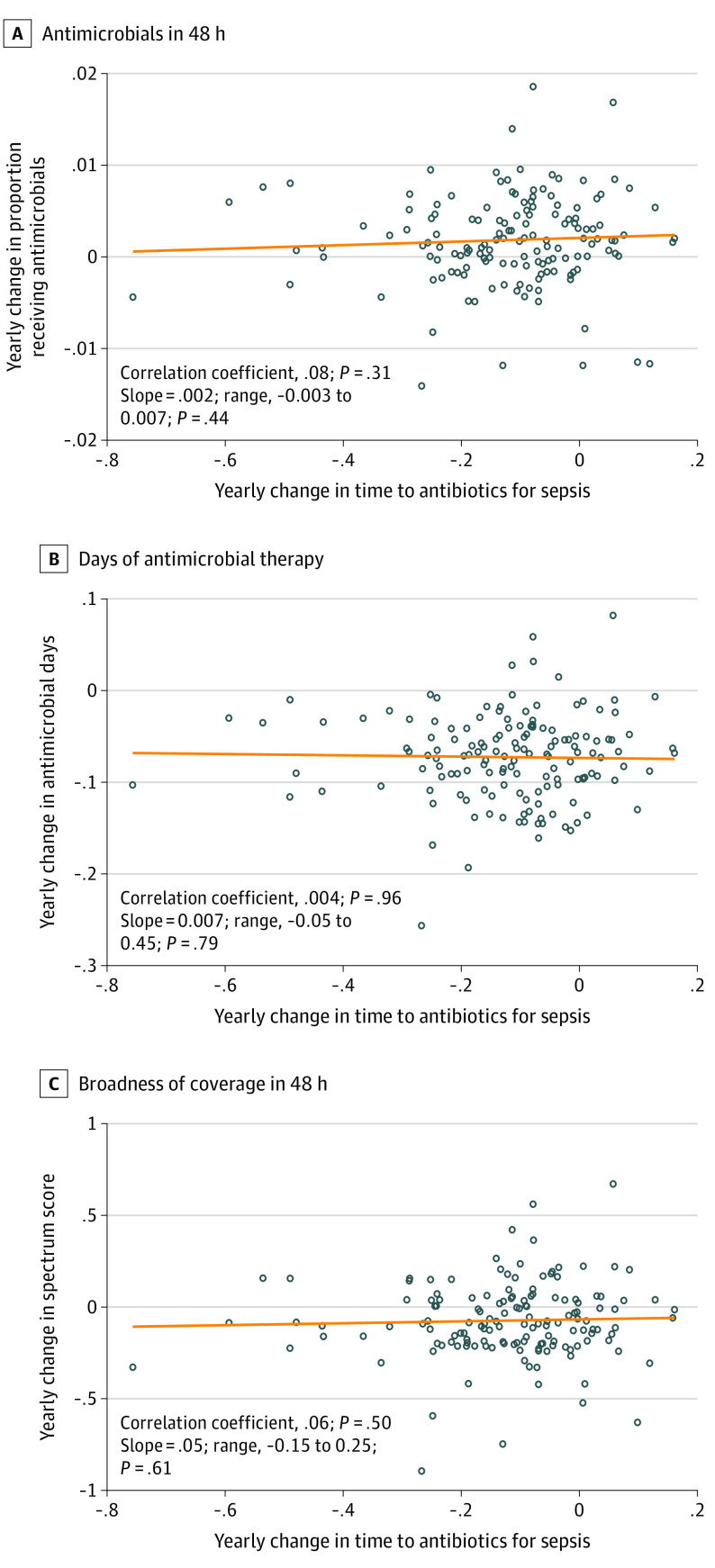
Although median time to first antimicrobial administration for sepsis decreased by 37 minutes from 2013 to 2018, temporal trends varied across the 152 hospitals (Figure 1). We leveraged this variation in hospital trends to assess whether hospitals with steeper decreases in antimicrobial timing had increasing antimicrobial use, days of therapy, or broadness of antibacterial coverage compared with hospitals with stable or less steep decreases in antimicrobial timing for sepsis. The associations between these temporal trends are presented in Table 3 and Figure 2.
Table 3. Association and Correlation Between Hospital-Level Trends in Antimicrobial Timing for Patients With Sepsis and Hospital-Level Trends in Antimicrobial Prescribinga.
| Outcome | Spearman correlation, r | P value | Change in outcome per 1-h decrease in median time to first antimicrobial, % (95% CI)b | P value |
|---|---|---|---|---|
| Antimicrobial receipt | ||||
| Within 12 h | 0.072 | .39 | 0.1 (−0.4 to 0.6) | .63 |
| Within 24 h | 0.075 | .37 | 0.2 (−0.3 to 0.7) | .47 |
| Within 48 h | 0.083 | .32 | 0.2 (−0.3 to 0.7) | .44 |
| Days of antimicrobial therapy | 0.004 | .96 | −0.7 (−5.9 to 4.5)c | .79 |
| Receipt of broad-spectrum coveraged | ||||
| Within 48 h | ||||
| Spectrum Score ≥40 | 0.028 | .73 | 0.008 (−0.3 to 0.3) | .95 |
| Spectrum Score ≥45 | 0.064 | .44 | 0.06 (−0.1 to 0.3) | .58 |
| Within 30 d | ||||
| Spectrum Score ≥40 | 0.066 | .43 | 0.1 (−0.3 to 0.5) | .62 |
| Spectrum Score ≥45 | 0.080 | .34 | 0.1 (−0.2 to 0.5) | .53 |
| Broadness of antimicrobial coverage | ||||
| Within 48 h | 0.056 | .50 | 0.05 (−0.15 to 0.25)c | .61 |
| Within 30 d | 0.029 | .73 | 0.03 (−0.20 to 0.25)c | .82 |
This table presents the correlation and association of hospital’s temporal trend in antimicrobial timing in patients with sepsis (ie, hospital trends shown in Figure 1) and antimicrobial prescribing trends among all hospitalized patients with systemic inflammatory response syndrome (SIRS). Conceptually, this analysis answers the question, as a hospital speeds up antimicrobial delivery for patients with sepsis, what is the association with broader antimicrobial prescribing trends among all patients with SIRS? A sensitivity analysis examining the association with trends in patients with SIRS but without sepsis is presented in eTable 5 in the Supplement. Data on antimicrobial receipt within 48 hours, days of antimicrobial therapy, and broadness of coverage within 48 hours are also presented visually in Figure 2.
Associations are shown for patients with sepsis and were determined from robust regression.
Data represent an absolute change of a continuous outcome, not a percentage change.
A Spectrum Score of 40 or higher would include coverage with piperacillin-tazobactam (Spectrum Score, 42.25), vancomycin plus piperacillin-tazobactam (Spectrum Score, 44.5), or similar, whereas a Spectrum Score of 45 or higher would include coverage with vancomycin plus a carbapenem (Spectrum Score, 45.25), or similar. For all antimicrobial prescribing trends assessed, there was no correlation and no association with trend in antimicrobial timing for sepsis. From 2013 to 2018, antimicrobial use declined. This table shows that trend of reduced antimicrobial use did not differ among hospitals with greater vs lesser declines in antimicrobial timing for sepsis. In short, speeding up time to antimicrobials with sepsis was not associated with increased antimicrobial use, and also not associated with impeding antimicrobial stewardship.
Figure 2. Association Between Hospital-Level Temporal Trends in Antimicrobial Timing for Sepsis and Hospital-Level Trends in Antimicrobial Use, Days of Therapy, and Broadness of Antibacterial Coverage.
In each scatterplot, the exposure (annual change in time to first antimicrobial among hospitalized patients with sepsis) is on the x-axis, and the outcome (annual change in antimicrobial use, days of therapy, and broadness of coverage) is on the y-axis. Each dot represents an individual hospital. The orange line shows the fitted association between temporal trends, estimated from a robust regression in which hospitals were weighted according to number of hospitalized patients. The line at 0 shows no change. The Spearman correlation between temporal trends and the slope from the robust regression are shown in each panel. Broadness of antibacterial coverage was measured using Spectrum Score (range, 0-64, in which higher scores represent broader coverage). There was no association between hospital temporal trends in antimicrobial timing among patients with sepsis and trends in antimicrobial use (A), days of antimicrobial therapy (B), or broadness of antibacterial coverage (C).
For all antimicrobial prescribing trends examined, there was no correlation and no association with the temporal trend in antimicrobial timing for sepsis. Temporal trends in antimicrobial use, days of therapy, and broadness of antibacterial coverage did not differ according to magnitude of decrease in antimicrobial timing for sepsis. Findings were consistent in the sensitivity analysis examining prescribing trends among patients with SIRS but without sepsis (eTable 5 in the Supplement).
Discussion
In this cohort study of more than 1.5 million patients hospitalized with SIRS and 45 million patient-days at risk for antimicrobial therapy, decreases in antimicrobial timing for sepsis from 2013 to 2018 were not associated with an increasing antimicrobial use among patients with potential infection. Rather, from 2013 to 2018, both antimicrobial timing for sepsis and the overall antimicrobial exposure decreased in our cohort. Specifically, the proportion of patients with potential infection receiving antimicrobial therapy within 48 hours, mean days of antimicrobial therapy (inclusive of antimicrobials prescribed at discharge), and the broadness of antibacterial coverage all decreased. Importantly, decreases in these measures were observed consistently across hospitals, even among hospitals with steeper decreases in antimicrobial timing for sepsis. Over the same period, mortality and MDR culture positivity also decreased. Taken together, our findings provide no evidence to indicate that accelerating antimicrobial timing for sepsis was associated with increases in indiscriminate antimicrobial use or impeded efforts to promote antimicrobial stewardship in VA and KPNC hospitals.
These findings are important because many initiatives over the past decade have focused on decreasing time to antimicrobials for sepsis, recognizing that faster antimicrobial timing is associated with improved survival.7,8,33 However, there has been growing concern that such initiatives may also increase overall antimicrobial exposure and thereby exacerbate antimicrobial adverse effects and resistance.15,16,17,34 Recent estimates suggest that MDR infections contribute to more than 600 000 hospitalizations21 and 70 000 deaths20 annually in the US, so this concern should not be taken lightly. However, before this study, there were limited data on the association of shortening antimicrobial timing for sepsis with broader antimicrobial exposure. In an interrupted time series of hospitalized patients at 111 hospitals, Pakyz et al35 found that unadjusted days of broad-spectrum antimicrobial therapy per 100 000 patient-days increased immediately after SEP-1 rollout among patients hospitalized for severe sepsis and septic shock, but days of therapy were stable among all-cause hospitalizations. In a study of 26 hospitals, Anderson et al36 found that, although unadjusted days of antimicrobial therapy per 100 000 patient-days were greater after SEP-1 implementation (vs before), antimicrobial use was increasing before SEP-1 implementation and actually decreased slightly during the year after SEP-1 implementation.
Our study expands on this prior work and has 4 key differences: (1) we focused on antimicrobial prescribing trends among a broad population of hospitalized patients with SIRS; (2) we used granular electronic health data for risk adjustment to ensure that temporal trends were not confounded by changes in case-mix or illness severity; (3) we correlated hospital-level trends to ensure that the cohort-wide trends were not being confounded as in the Simpson paradox; and (4) we evaluated several measures of antimicrobial administration and downstream sequelae that extended well beyond the index hospitalization. As such, our study not only describes temporal trends in antimicrobial prescribing, but directly correlates broader antimicrobial prescribing trends to antimicrobial timing trends for sepsis.
Our findings are pertinent to current debates about the impact of incentivizing rapid antimicrobial therapy for sepsis. On the basis of mounting concerns that antimicrobial timing targets may increase antimicrobial use, the American College of Emergency Physicians and other professional societies requested in September 2021 that the National Quality Forum de-endorse the Severe Sepsis and Septic Shock Early Management Bundle that serves as the backbone for the Centers for Medicare & Medicaid Services SEP-1 performance measure.37 Our study suggests that the concern for increasing antimicrobial use was reasonable, as use of antimicrobials within 12 hours increased over time. Importantly, the modest increases in early antimicrobial use were no longer evident even by 24 to 48 hours. Indeed, antimicrobial use within 48 hours, days of antimicrobial therapy, and broadness of antibacterial coverage all decreased, whereas overall outcomes also improved.
Strengths and Limitations
There are several strengths to our study. First, we examined all patients with SIRS at 152 hospitals in 2 heterogeneous health care systems over a 6-year period. We leveraged objective electronic health record data to classify SIRS positivity and sepsis, such that our measurements of temporal trends in antimicrobial prescribing were examined among a stable population of hospitalized patients and not biased by temporal trends in recognition, diagnosis, or labeling of patients.38 Furthermore, we were able to adjust for granular patient characteristics to ensure that the changes over time were not merely a reflection of changing case mix or illness severity, but rather true changes in antimicrobial prescribing practices. We also measured the provision of antimicrobials after hospital discharge, which is increasingly recognized as a source of antimicrobial overuse.39,40 Finally, we used a validated metric, the Spectrum Score,28,29 for assessing broadness of antibacterial coverage.
Our study should also be interpreted in the context of several limitations. First, although we found no evidence in our 152-hospital cohort that shortening the time to antibiotics for sepsis was associated with greater antimicrobial exposure, we cannot exclude that some hospitals could experience such spillover effects. With any performance improvement or public reporting program, it is important to consider and mitigate the possibility of unintended consequences. Rather than abandoning antimicrobial timing targets in sepsis, overall antimicrobial use should be monitored simultaneously.9 Indeed, both VA and KPNC have antimicrobial stewardship programs,41 which likely helped to facilitate the simultaneous decreases in antimicrobial timing for sepsis and broader antimicrobial use. Second, the changes in antimicrobial prescribing over time were small, and it is unclear what constitutes an important change. Regardless, our findings are reassuring that accelerating antimicrobial timing for sepsis was not associated with increasing antimicrobial exposure. In addition, we chose to evaluate a cohort defined by SIRS criteria because these criteria are commonly used to assess for infection,42 have higher sensitivity for infection than other criteria,43 and identify a stable population over time. However, they do not capture the full extent of antimicrobial use.
Conclusions
In this study of more than 1.5 million patients hospitalized with potential infection at 152 US hospitals, decreasing time to antibiotics administration for sepsis was not associated with increasing antimicrobial use. These findings indicate that it is possible to shorten time to treatment for sepsis without increasing indiscriminate antimicrobial use. These findings can inform guidelines designed to accelerate early treatment for sepsis without spillover effects onto other patients at risk for sepsis.
eAppendix. Supplemental Methods
eTable 1. Study Flow
eTable 2. VA Patients Hospitalized With Potential Infection and Sepsis
eTable 3. KPNC Patients Hospitalized With Potential Infection and Sepsis
eTable 4. Antimicrobial Prescribing and Outcomes Among Patients With Sepsis by Year, Adjusted for Patient Characteristics
eTable 5. Relationship Between Hospital-Level Trends in Antimicrobial Timing for Patients With Sepsis and Hospital-Level Trends in Antimicrobial Prescribing for Patients With SIRS but Without Sepsis
eReferences
References
- 1.Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395(10219):200-211. doi: 10.1016/S0140-6736(19)32989-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47(11):1181-1247. doi: 10.1007/s00134-021-06506-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021;49(11):e1063-e1143. doi: 10.1097/CCM.0000000000005337 [DOI] [PubMed] [Google Scholar]
- 4.Levy MM, Evans LE, Rhodes A. The surviving sepsis campaign bundle: 2018 update. Crit Care Med. 2018;46(6):997-1000. doi: 10.1097/CCM.0000000000003119 [DOI] [PubMed] [Google Scholar]
- 5.Levy MM, Evans LE, Rhodes A. The surviving sepsis campaign bundle: 2018 update. Intensive Care Med. 2018;44(6):925-928. doi: 10.1007/s00134-018-5085-0 [DOI] [PubMed] [Google Scholar]
- 6.Seymour CW, Gesten F, Prescott HC, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017;376(23):2235-2244. doi: 10.1056/NEJMoa1703058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peltan ID, Brown SM, Bledsoe JR, et al. ED door-to-antibiotic time and long-term mortality in sepsis. Chest. 2019;155(5):938-946. doi: 10.1016/j.chest.2019.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu VX, Fielding-Singh V, Greene JD, et al. The timing of early antibiotics and hospital mortality in sepsis. Am J Respir Crit Care Med. 2017;196(7):856-863. doi: 10.1164/rccm.201609-1848OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prescott HC, Iwashyna TJ. Improving sepsis treatment by embracing diagnostic uncertainty. Ann Am Thorac Soc. 2019;16(4):426-429. doi: 10.1513/AnnalsATS.201809-646PS [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor SP, Rozario N, Kowalkowski MA, et al. Trends in false-positive code sepsis activations in the emergency department. Ann Am Thorac Soc. 2020;17(4):520-522. doi: 10.1513/AnnalsATS.201910-757RL [DOI] [PubMed] [Google Scholar]
- 11.Klein Klouwenberg PM, Cremer OL, van Vught LA, et al. Likelihood of infection in patients with presumed sepsis at the time of intensive care unit admission: a cohort study. Crit Care. 2015;19:319. doi: 10.1186/s13054-015-1035-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shappell CN, Klompas M, Ochoa A, Rhee C; CDC Prevention Epicenters Program . Likelihood of bacterial infection in patients treated with broad-spectrum IV antibiotics in the emergency department. Crit Care Med. 2021;49(11):e1144-e1150. doi: 10.1097/CCM.0000000000005090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention . State policy approaches to sepsis prevention and early recognition. 2018. Accessed May 24, 2022. https://www.cdc.gov/hai/pdfs/sepsis/vs-sepsis-policy-final.pdf
- 14.Weingart S. We are complicit: a glimpse into the current state of severe sepsis/septic shock quality measures. June 11, 2015. Accessed May 24, 2022. https://emcrit.org/emcrit/current-state-of-severe-sepsis-quality-measures/
- 15.Klompas M, Rhee C. The CMS sepsis mandate: right disease, wrong measure. Ann Intern Med. 2016;165(7):517-518. doi: 10.7326/M16-0588 [DOI] [PubMed] [Google Scholar]
- 16.Rhee C, Gohil S, Klompas M. Regulatory mandates for sepsis care: reasons for caution. N Engl J Med. 2014;370(18):1673-1676. doi: 10.1056/NEJMp1400276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klompas M, Calandra T, Singer M. Antibiotics for sepsis-finding the equilibrium. JAMA. 2018;320(14):1433-1434. doi: 10.1001/jama.2018.12179 [DOI] [PubMed] [Google Scholar]
- 18.Townsend SR, Rivers EP, Duseja R. Centers for Medicare and Medicaid Services measure stewards’ assessment of the Infectious Diseases Society of America’s position paper on SEP-1. Clin Infect Dis. 2021;72(4):553-555. doi: 10.1093/cid/ciaa458 [DOI] [PubMed] [Google Scholar]
- 19.Reinhart K, Daniels R, Kissoon N, Machado FR, Schachter RD, Finfer S. Recognizing sepsis as a global health priority: a WHO resolution. N Engl J Med. 2017;377(5):414-417. doi: 10.1056/NEJMp1707170 [DOI] [PubMed] [Google Scholar]
- 20.Burnham JP, Olsen MA, Kollef MH. Re-estimating annual deaths due to multidrug-resistant organism infections. Infect Control Hosp Epidemiol. 2019;40(1):112-113. doi: 10.1017/ice.2018.304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jernigan JA, Hatfield KM, Wolford H, et al. Multidrug-resistant bacterial infections in U.S. hospitalized patients, 2012-2017. N Engl J Med. 2020;382(14):1309-1319. doi: 10.1056/NEJMoa1914433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bone RC, Sibbald WJ, Sprung CL. The ACCP-SCCM consensus conference on sepsis and organ failure. Chest. 1992;101(6):1481-1483. doi: 10.1378/chest.101.6.1481 [DOI] [PubMed] [Google Scholar]
- 23.Wang XQ, Vincent BM, Wiitala WL, et al. Veterans Affairs patient database (VAPD 2014-2017): building nationwide granular data for clinical discovery. BMC Med Res Methodol. 2019;19(1):94. doi: 10.1186/s12874-019-0740-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wayne MT, Seelye S, Molling D, et al. Temporal trends and hospital variation in time-to-antibiotics among veterans hospitalized with sepsis. JAMA Netw Open. 2021;4(9):e2123950. doi: 10.1001/jamanetworkopen.2021.23950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vincent BM, Wiitala WL, Burns JA, Iwashyna TJ, Prescott HC. Using Veterans Affairs Corporate Data Warehouse to identify 30-day hospital readmissions. Health Serv Outcomes Res Methodol. 2018;18:143-154. doi: 10.1007/s10742-018-0178-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention . Hospital toolkit for adult sepsis surveillance. March 2018. Accessed January 30, 2019. https://www.cdc.gov/sepsis/pdfs/Sepsis-Surveillance-Toolkit-Mar-2018_508.pdf
- 27.Wayne MT, Molling D, Wang XQ, et al. Measurement of sepsis in a national cohort using three different methods to define baseline organ function. Ann Am Thorac Soc. 2021;18(4):648-655. doi: 10.1513/AnnalsATS.202009-1130OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madaras-Kelly K, Jones M, Remington R, Caplinger C, Huttner B, Samore M. Description and validation of a spectrum score method to measure antimicrobial de-escalation in healthcare associated pneumonia from electronic medical records data. BMC Infect Dis. 2015;15:197. doi: 10.1186/s12879-015-0933-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madaras-Kelly K, Jones M, Remington R, Hill N, Huttner B, Samore M. Development of an antibiotic spectrum score based on Veterans Affairs culture and susceptibility data for the purpose of measuring antibiotic de-escalation: a modified Delphi approach. Infect Control Hosp Epidemiol. 2014;35(9):1103-1113. doi: 10.1086/677633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li G. Robust regression. In: Hoaglin DC, Mosteller F, Tukey JW, eds. Exploring Data Tables, Trends, and Shapes. Wiley; 1985. [Google Scholar]
- 31.John Fox . Applied Regression Analysis, Linear Models, and Related Models. Sage publications, Inc; 1997. [Google Scholar]
- 32.Schober P, Boer C, Schwarte LA. Correlation coefficients: appropriate use and interpretation. Anesth Analg. 2018;126(5):1763-1768. doi: 10.1213/ANE.0000000000002864 [DOI] [PubMed] [Google Scholar]
- 33.Levy MM, Gesten FC, Phillips GS, et al. Mortality changes associated with mandated public reporting for sepsis: the results of the New York State Initiative. Am J Respir Crit Care Med. 2018;198(11):1406-1412. doi: 10.1164/rccm.201712-2545OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singer M. Antibiotics for sepsis: does each hour really count, or is it incestuous amplification? Am J Respir Crit Care Med. 2017;196(7):800-802. doi: 10.1164/rccm.201703-0621ED [DOI] [PubMed] [Google Scholar]
- 35.Pakyz AL, Orndahl CM, Johns A, et al. Impact of the Centers for Medicare and Medicaid Services sepsis core measure on antibiotic use. Clin Infect Dis. 2021;72(4):556-565. doi: 10.1093/cid/ciaa456 [DOI] [PubMed] [Google Scholar]
- 36.Anderson DJ, Moehring RW, Parish A, et al. The Impact of CMS SEP-1 core measure implementation on antibacterial utilization: a retrospective multicenter longitudinal cohort study with interrupted time-series analysis. Clin Infect Dis. Published online November 5, 2021. doi: 10.1093/cid/ciab937 [DOI] [PubMed] [Google Scholar]
- 37.American College of Emergency Physicians . Comments on severe sepsis and septic shock: early management bundle (SEP-1). September 9, 2021. Accessed November 28, 2021. https://www.acep.org/by-medical-focus/sepsis/
- 38.Lindenauer PK, Lagu T, Shieh MS, Pekow PS, Rothberg MB. Association of diagnostic coding with trends in hospitalizations and mortality of patients with pneumonia, 2003-2009. JAMA. 2012;307(13):1405-1413. doi: 10.1001/jama.2012.384 [DOI] [PubMed] [Google Scholar]
- 39.Vaughn VM, Gandhi TN, Chopra V, et al. Antibiotic overuse after hospital discharge: a multi-hospital cohort study. Clin Infect Dis. 2021;73(11):e4499-e4506. doi: 10.1093/cid/ciaa1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaughn VM, Hersh AL, Spivak ES. Antibiotic overuse and stewardship at hospital discharge: the Reducing Overuse of Antibiotics at Discharge (ROAD) Home Framework. Clin Infect Dis. 2022;74(9):1696-1702. doi: 10.1093/cid/ciab842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelly AA, Jones MM, Echevarria KL, et al. A report of the efforts of the Veterans Health Administration national antimicrobial stewardship initiative. Infect Control Hosp Epidemiol. 2017;38(5):513-520. doi: 10.1017/ice.2016.328 [DOI] [PubMed] [Google Scholar]
- 42.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810. doi: 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gando S, Shiraishi A, Abe T, et al. ; Japanese Association for Acute Medicine (JAAM) Sepsis Prognostication in Intensive Care Unit and Emergency Room (SPICE) (JAAM SPICE) Study Group . The SIRS criteria have better performance for predicting infection than qSOFA scores in the emergency department. Sci Rep. 2020;10(1):8095. doi: 10.1038/s41598-020-64314-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Supplemental Methods
eTable 1. Study Flow
eTable 2. VA Patients Hospitalized With Potential Infection and Sepsis
eTable 3. KPNC Patients Hospitalized With Potential Infection and Sepsis
eTable 4. Antimicrobial Prescribing and Outcomes Among Patients With Sepsis by Year, Adjusted for Patient Characteristics
eTable 5. Relationship Between Hospital-Level Trends in Antimicrobial Timing for Patients With Sepsis and Hospital-Level Trends in Antimicrobial Prescribing for Patients With SIRS but Without Sepsis
eReferences