This genetic association study assesses the association of sickle cell trait with prepandemic health conditions in Million Veteran Program participants and assesses the severity and sequelae of COVID-19.
Key Points
Question
Is the presence of sickle cell trait (SCT) associated with worse outcomes of COVID-19?
Findings
In this genetic association study of 2729 persons with SCT and 129 848 who were SCT negative, individuals with SCT had a number of preexisting kidney conditions that were associated with unfavorable outcomes following COVID-19. The presence of SCT was associated with increased risk of mortality and acute kidney failure following COVID-19.
Meaning
Results strongly support the inclusion of SCT as an adverse prognostic factor for COVID-19.
Abstract
Importance
Sickle cell trait (SCT), defined as the presence of 1 hemoglobin beta sickle allele (rs334-T) and 1 normal beta allele, is prevalent in millions of people in the US, particularly in individuals of African and Hispanic ancestry. However, the association of SCT with COVID-19 is unclear.
Objective
To assess the association of SCT with the prepandemic health conditions in participants of the Million Veteran Program (MVP) and to assess the severity and sequelae of COVID-19.
Design, Setting, and Participants
COVID-19 clinical data include 2729 persons with SCT, of whom 353 had COVID-19, and 129 848 SCT-negative individuals, of whom 13 488 had COVID-19. Associations between SCT and COVID-19 outcomes were examined using firth regression. Analyses were performed by ancestry and adjusted for sex, age, age squared, and ancestral principal components to account for population stratification. Data for the study were collected between March 2020 and February 2021.
Exposures
The hemoglobin beta S (HbS) allele (rs334-T).
Main Outcomes and Measures
This study evaluated 4 COVID-19 outcomes derived from the World Health Organization severity scale and phenotypes derived from International Classification of Diseases codes in the electronic health records.
Results
Of the 132 577 MVP participants with COVID-19 data, mean (SD) age at the index date was 64.8 (13.1) years. Sickle cell trait was present in 7.8% of individuals of African ancestry and associated with a history of chronic kidney disease, diabetic kidney disease, hypertensive kidney disease, pulmonary embolism, and cerebrovascular disease. Among the 4 clinical outcomes of COVID-19, SCT was associated with an increased COVID-19 mortality in individuals of African ancestry (n = 3749; odds ratio, 1.77; 95% CI, 1.13 to 2.77; P = .01). In the 60 days following COVID-19, SCT was associated with an increased incidence of acute kidney failure. A counterfactual mediation framework estimated that on average, 20.7% (95% CI, −3.8% to 56.0%) of the total effect of SCT on COVID-19 fatalities was due to acute kidney failure.
Conclusions and Relevance
In this genetic association study, SCT was associated with preexisting kidney comorbidities, increased COVID-19 mortality, and kidney morbidity.
Introduction
The COVID-19 pandemic has caused more than 405 million confirmed cases and 5.7 million deaths worldwide (as of February 10, 2022).1 Certain demographic and preexisting medical conditions are associated with worse COVID-19 outcomes, including chronic kidney disease, chronic obstructive pulmonary disease, and sickle cell disease (SCD).2,3,4,5 Sickle cell disease has 2 copies of hemoglobin beta sickle alleles (rs334-T); sickle cell trait (SCT) has 1 rs334-T and 1 wild-type allele.
The US incidence estimate for SCT was 73.1 cases per 1000 Black newborns, 6.9 cases per 1000 Hispanic newborns, and 3.0 cases per 1000 White newborns.6 Although largely considered a benign condition, SCT has been associated with increased risk for adverse outcomes7 ranging from rare complications of exertion-related injuries8,9 and renal medullary carcinoma10 to more common medical conditions such as chronic kidney disease11,12 and venous thromboembolism.13,14,15
The Centers for Disease Control and Prevention (CDC) has advised that patients with SCD be regarded as highly susceptible to COVID-19.16 However, this cautionary advice does not extend to individuals with SCT. Sickle cell trait affects more than 3 million people in the US and 300 million people globally, but because it is not routinely assessed, there is a paucity of data on the association between SCT and COVID-19 outcomes. We addressed this issue in the Million Veteran Program (MVP) within the Department of Veterans Affairs (VA). The VA encompasses a comprehensive electronic health record (EHR) system with clinical data for pre–COVID-19 and post–COVID-19 conditions and genotyping results for SCT in more than 658 582 veterans. The objective was to examine the association of SCT with preexisting conditions, COVID-19 outcome severity, and post–COVID-19 conditions.
Methods
Data Sources
The MVP, a large multiethnic genetic biobank of US veterans,17 served as the primary cohort analyzed for this COVID-19 study. Directly genotyped (rs334-T) or imputed (rs33930165-T) markers in the hemoglobin beta gene were extracted and used for association testing (see eMethods in Supplement 1). All rs334-T–positive individuals had only 1 copy of the sickle allele and 1 copy of the nonsickle allele and therefore carried the SCT. There were no rs334-T homozygous, ie, sickle cell disease (SCD), individuals in our study population as individuals with SCD were not expected to enter the military (eFigure 1 in Supplement 1). The MVP received ethical and study protocol approval from the VA Central Institutional Review Board in accordance with the principles outlined in the Declaration of Helsinki. All individuals in the study provided written informed consent as part of the MVP.
COVID-19 Data Source and Severity Definition
COVID-19 severity and kidney complication assessment was obtained from the EHR data collected at a VA medical center through March 2021. All participants in this study were tested for COVID-19 at the VA using polymerase chain reaction–based methods.18 The index date was defined as a COVID-19 diagnosis date, ie, specimen date; and for a hospitalized patient, the admission date up to 15 days prior to the COVID-19 specimen date. The MVP Data Core and COVID-19 study team adapted the World Health Organization COVID-19 Disease Progression Scale to define COVID-19 outcomes.19,20,21 Detailed definitions of COVID-19 outcomes are provided in eMethods in Supplement 1.
Comorbidity Analysis
Association was examined between SCT status and a broad spectrum of common comorbidities and median laboratory values obtained from the EHR focusing on the period prior to the onset of the pandemic. Median laboratory values were calculated from longitudinal EHR data for each individual (eg, the median of all creatinine values for an individual in the EHR). Conceptually, this analysis is similar to examination of comorbidity indices in epidemiology studies to determine if specific comorbid conditions, represented by frequently used laboratory measures or disease codes, are enriched among cases. In the recent genetic literature, this type of analysis is called a phenome-wide association study (PheWAS)22 and laboratory-wide association study (LabWAS), which enables us to determine if SCT affects more than 1 organ system or laboratory measure, a phenomenon called pleiotropy. We derived 1866 preexisting conditions and 64 laboratory measurements from the EHR prior to onset of COVID-19 (from the time of enrollment at VA through September 2019).
Phenotyping of Preindex and Postindex Kidney Conditions for COVID-19
To further evaluate the associations between SCT, kidney disorders, and COVID-19, we curated a list of kidney conditions. These kidney sequelae were extracted by using 1 of the following: natural language processing, International Classification of Diseases codes, Current Procedural Terminology codes, or laboratory data. The list extracted includes many kidney conditions: acute kidney failure (AKF), prior end-stage renal disease, chronic kidney disease, chronic kidney failure, nephrosis, and stable and normal kidney function. Using the date of COVID-19 diagnosis as the partition, conditions within a 2-year window prior to COVID-19 diagnosis were preindex conditions, and those within 60 days after the COVID-19 diagnosis date were postindex conditions (eTable 1 in Supplement 2).
Statistical Analysis
The PheWAS assessed whether hemoglobin beta alleles had shared genetic architecture with preexisting conditions and COVID-19, and LabWAS analyzed median laboratory measures using clinical data from the EHR. We derived 1866 preexisting conditions and 64 laboratory measurements from the EHR prior to onset of COVID-19 (from the time of enrollment at VA through September 2019). We applied logistic and linear regression for models to preexisting comorbidities and laboratory measures, respectively. Firth logistic regression23,24 implemented with the R package “brglm2” (version 0.7.1)25 was used to examine the association between hemoglobin beta alleles and COVID-19 outcomes. Because genetic ancestral groups show considerable heterogeneity within and across groups, the preexisting comorbidity association analyses were conducted separately in each ancestral group.26 All the models were adjusted for sex, age, age squared, and the first 20 ancestry-specific principal components derived from the genetic data to account for confounding due to population stratification. Adjustments for demographic and population structure are standard corrections for bias and confounding27; additional details can be found in eMethods in Supplement 1. Lastly, summary statistics from each ancestry were meta-analyzed using random-effects meta-analysis as implemented in the R package “metafor” (version 2.4-0).28 P values presented are 2-tailed, and the level of significance was .05.
We used counterfactual mediation modeling to investigate whether postindex AKF caused mortality in participants of African ancestry with SCT. Please see the eMethods in Supplement 1 for specific details on the mediator model.
Results
SCT in MVP Participants of African and Hispanic Ancestry
Of the 132 577 MVP participants with COVID-19 data, mean (SD) age at the index date was 64.8 (13.1) years. Demographic and clinical characteristics of the study participants are shown in eTables 2 and 3 in Supplement 2. The prevalence of the sickle allele (rs334-T) comprised 7.8% of study participants of African ancestry and 1% of study participants of Hispanic ancestry. Given that the prevalence of SCT differed by ancestry, we conducted ancestry-specific analyses. However, main findings were focused on individuals of African ancestry. The study design with the number of individuals selected for different analyses is presented in Figure 1.
Figure 1. Flowchart With the Number of Individuals Selected for the Association Study.
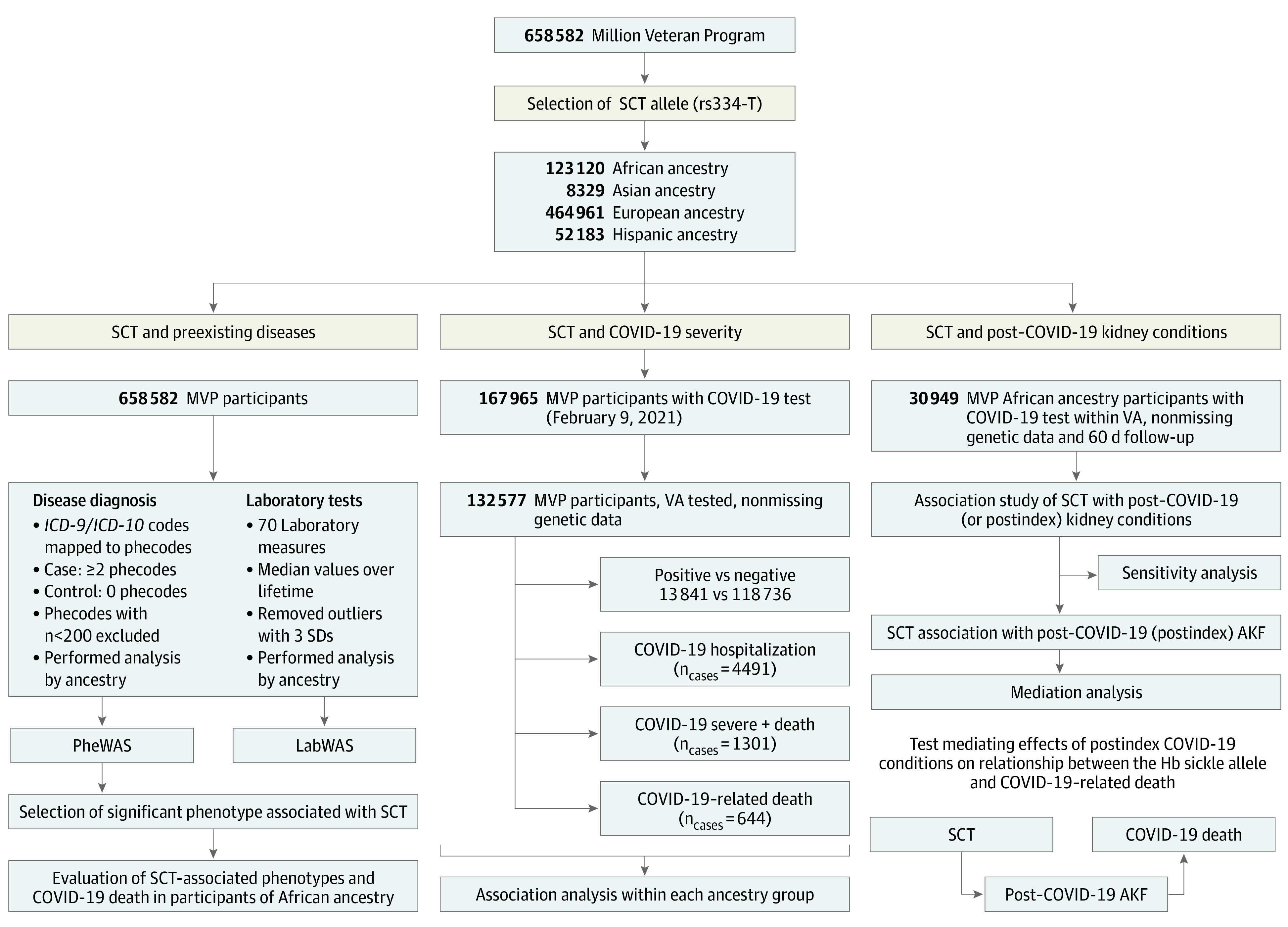
Left, phenome-wide association study (PheWAS) and laboratory-wide association study (LabWAS) analysis; middle, outcome severity; right, association study with clinical outcomes that occurred within 60 days of COVID-19 testing/diagnosis. Individuals tested within the Department of Veterans Affairs (VA) who had nonmissing genotyping and ethnic information were included. Phecodes that showed association with sickle cell trait (SCT) were tested for their association with COVID-19–related death in individuals of African ancestry (left lower); SCT-related conditions post–COVID-19 were tested for their mediation of SCT-related death in COVID-19 (right lower). AKF indicates acute kidney failure; Hb, hemoglobin; ICD-9/ICD-10, International Classification of Diseases, Ninth Revision/Tenth Revision; MVP, Million Veteran Program.
SCT Comorbidities and Risk Factors Associated With COVID-19 Severity
To determine preexisting conditions in individuals with SCT that may be associated with poor COVID-19 outcome, we performed PheWAS to test for associations between rs334-T and preexisting conditions preceding the COVID-19 pandemic among 658 358 MVP participants. We identified 31 phecodes, with significant association (adjusted P < 1.48 × 10−5) in individuals of African ancestry (Figure 2; eTable 4 in Supplement 2). The most significant association observed was sickle cell anemia/trait-related condition (phecode: 282.5; odds ratio [OR], 93.17; 95% CI, 78.60-110.44; P = 1 × 10−300). We identified several associations with conditions reported as risk factors associated with COVID-19 severity and mortality, such as chronic kidney disease (OR, 1.45; 95% CI, 1.36-1.55; P = 1.8 × 10−28), type 2 diabetes with kidney complications (OR, 1.33; 95% CI, 1.23-1.43; P = 3.7 × 10−13), pulmonary embolism (OR, 1.43; 95% CI, 1.27-1.60; P = 1.73 × 10−9), pulmonary heart disease (OR, 1.30; 95% CI, 1.19-1.42; P = 5.3 × 10−9), and hypertensive kidney disease (OR, 1.19; 95% CI, 1.12-1.26; P = 2.77 × 10−9). The full summary statistics for the association study are presented in eTable 4 in Supplement 2.
Figure 2. Association Study of rs334-T With Prepandemic Comorbidities in the Million Veteran Program (MVP).
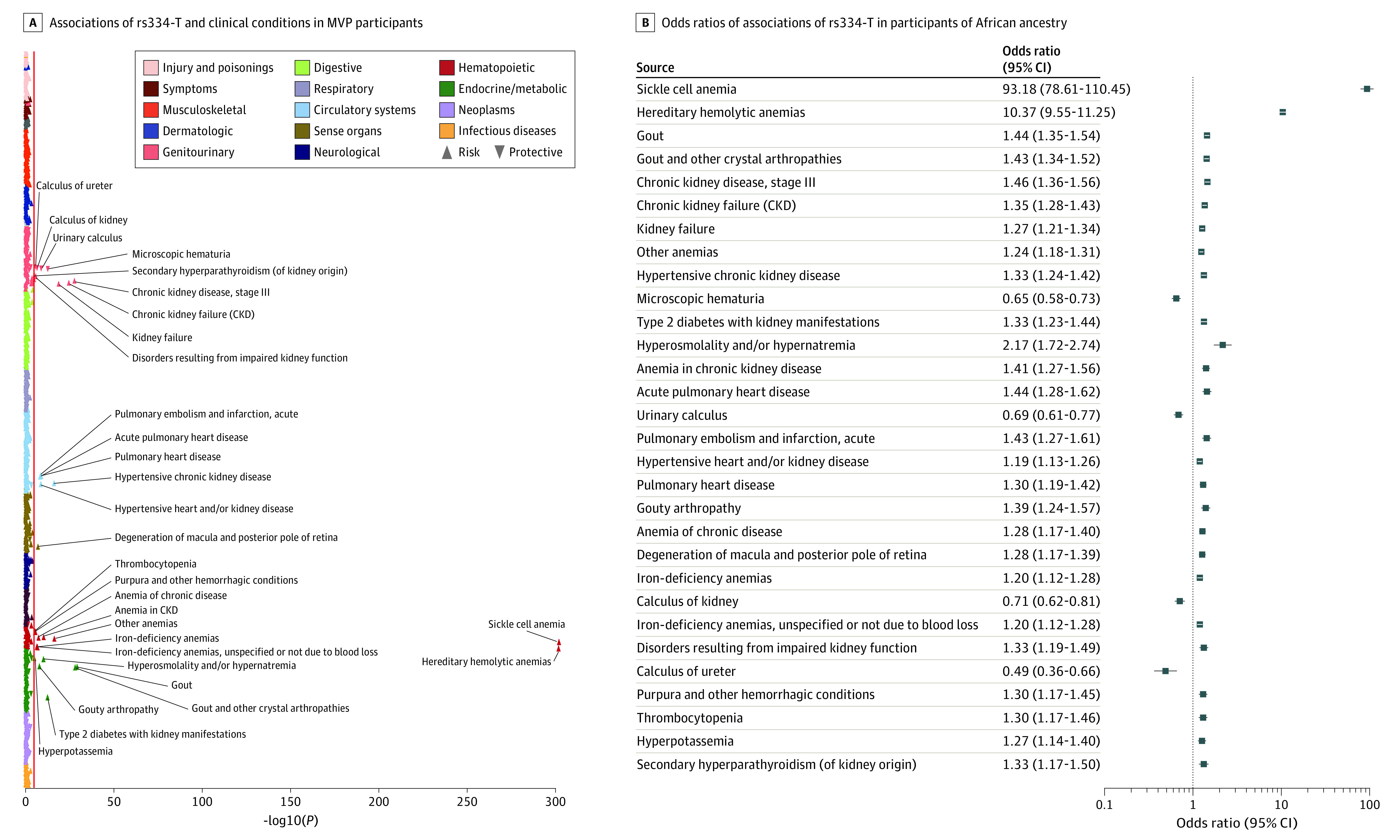
A, Plot showing associations of rs334-T and clinical conditions derived from the electronic health records data prior to COVID-19 in MVP participants of African ancestry. The clinical conditions are shown on the y-axis and organized by broader disease categories. The P value (−log10) of each association is shown on the x-axis. The direction of each triangle represents the direction of effect of the associations, with the upward triangle representing increased risk and the downward represents reduced risk. The red line indicates the significance threshold based on the Bonferroni correction (P < 1 × 10−5). B, The plot shows the odds ratio and 95% CI of the Bonferroni significant associations of rs334-T in participants of African ancestry. CKD indicates chronic kidney disease.
Association studies with laboratory parameters identified previously known associations with several hematologic traits such as mean corpuscular hemoglobin, mean corpuscular volume, hemoglobin, hematocrit, and red blood cell distribution width11,29 (eFigure 2 in Supplement 1; eTable 5 in Supplement 2). Among the kidney function laboratory measures, increased creatinine levels and decreased estimated glomerular filtration rate were associated with SCT in participants of African and Hispanic ancestry, consistent with the presence of kidney problems reported by PheWAS (Table 1 and eTable 5 in Supplement 2). The full summary statistics for association with laboratory measurement are presented in eTable 5 in Supplement 2.
Table 1. Association of Sickle Cell Trait With Kidney and Hematologic Laboratory Measurements Through Laboratory-Wide Association Study Analysis in African Ancestry Individuals.
| Laboratory measurement | Sample size | Model 1a | Model 2b | ||
|---|---|---|---|---|---|
| β | P value | β | P value | ||
| Hematologic trait measures | |||||
| Mean corpuscular volume | 112 329 | −2.97 | 1.00 × 10−300 | −2.99 | 1.00 × 10−300 |
| Mean corpuscular hemoglobin concentration | 112 411 | 0.31 | 1.12 × 10−265 | 0.32 | 8.28 × 10−264 |
| Mean corpuscular hemoglobin | 112 343 | −0.73 | 2.84 × 10−189 | −0.73 | 4.94 × 10−180 |
| Hematocrit | 112 313 | −0.93 | 7.37 × 10−121 | −0.84 | 2.65 × 10−101 |
| Red blood cell distribution width | 110 682 | 0.12 | 3.96 × 10−26 | 0.11 | 7.5 × 10−20 |
| Red blood cell | 112 818 | 0.04 | 3.41 × 10−20 | 0.06 | 9.16 × 10−30 |
| Platelet | 112 297 | −7.91 | 5.02 × 10−42 | −7.75 | 1.12 × 10−38 |
| Hemoglobin | 112 417 | −0.18 | 1.45 × 10−39 | −0.15 | 1.51 × 10−27 |
| Kidney function measures | |||||
| Creatinine | 111 609 | 0.03 | 1.53 × 10−48 | 0.02 | 1.12 × 10−24 |
| Estimated glomerular filtration rate | 109 365 | −3.02 | 1.00 × 10−38 | −1.71 | 3.24 × 10−17 |
Model 1: Models adjusted by sex, age, age squared, and first 20 principal components.
Model 2: Models adjust by sex, age, age squared, first 20 principal components, and chronic kidney disease.
Next, we examined the association of the 31 preexisting conditions identified from the aforementioned comorbidity association studies with COVID-19 outcomes among MVP participants of African ancestry (March 2020 to February 2021). We observed 13 of these preexisting conditions were associated with COVID-19–related death (eTable 6 in Supplement 2). The most significant associations were with kidney disorders such as chronic kidney failure (OR, 1.95; 95% CI, 1.44-2.62; P = 9.3 × 10−7), chronic kidney disease (OR, 1.94; 95% CI, 1.44-2.62; P = 1.2 × 10−5), and kidney dialysis (OR, 2.07; 95% CI, 1.24-3.45; P = .005). Other clinical conditions associated with COVID-19–related death included type 2 diabetes with kidney manifestation (OR, 2.19; 95% CI, 1.60-2.98; P = 7.42 × 10−7), hypertensive heart and/or kidney disease (OR, 1.86; 95% CI, 1.40-2.46; P = 1.64 × 10−5), and hyperkalemia (OR, 2.62; 95% CI, 1.56-3.90; P = 2.07 × 10−6). Therefore, our subsequent analyses focused on kidney conditions among patients with COVID-19.
Association of SCT With Severity of COVID-19
We examined the SCT association with 4 outcomes of COVID-19: susceptibility, hospitalization, severe conditions where individuals required ventilator support or intensive care, and death due to COVID-19. We determined that SCT was associated with increased risk of death from COVID-19 in African ancestry individuals (OR, 1.77; 95% CI, 1.13-2.77; P = .01) (Table 2). Our main focus was the African ancestry individuals from the MVP where SCT has more prevalence. We also studied the Hispanic ancestral group in the MVP, but the reduced allele frequencies and low sample size led to weaker, though consistent, observations. These results are presented in eTable 7 in Supplement 2. The meta-analysis of the estimates across 2 ancestral groups provided more statistical power and a stronger association between SCT and COVID-19–related deaths (OR, 1.77; 95% CI, 1.13-2.77; P = .005) (eTable 7 in Supplement 2).
Table 2. Association of Sickle Cell Trait (rs334) and COVID-19 Outcomes in 31 287 African Ancestry Individuals.
| COVID-19 outcome | Wild type, No. | Sickle cell trait, No. | Odds ratio (95% CI) | P value |
|---|---|---|---|---|
| Susceptibility | ||||
| Negative | 25 326 | 2212 | 1.07 (0.95-1.21) | .30 |
| Positive | 3426 | 323 | ||
| Hospitalization | ||||
| Not hospitalized | 2160 | 193 | 1.17 (0.91-1.50) | .20 |
| Hospitalized | 1266 | 130 | ||
| Severe + death | ||||
| Not severe | 3053 | 278 | 1.33 (0.95-1.88) | .10 |
| Severe | 373 | 45 | ||
| Related death | ||||
| Survivors | 3271 | 298 | 1.77 (1.13-2.77) | .01 |
| Deaths | 155 | 25 | ||
In contrast, rs33930165-T, which is an HbC allele with a prevalence of 1.7% among African ancestry individuals, was not associated with any COVID-19 outcomes (eTable 8 in Supplement 2). Association analyses showed that the HbC allele was not associated with the myriad of clinical/kidney conditions associated with SCT (eFigures 3 and 4 in Supplement 1; eTables 9 and 10 in Supplement 2).
Association of SCT With Kidney Outcomes Within 60 Days of COVID-19
Given the association of SCT with prepandemic kidney comorbidities and the association of these kidney conditions with COVID-19 death in African ancestry individuals, we investigated the incidence of AKF and declining kidney function within 60 days of COVID-19 diagnosis and their interaction with SCT. Among 31 287 African ancestry individuals tested for COVID-19, 66.8% had stable and normal kidney function, 31% had declining kidney function, and 27.4% had kidney impairments (includes AKF, prior end-stage kidney failure, chronic kidney disease, chronic kidney failure or nephrosis) within 2 years prior to COVID-19 diagnosis. We observed a statistically significant increase in postindex AKF in individuals with SCT with COVID-19 compared with individuals without SCT (OR, 1.40; 95% CI, 1.09-1.90; P = .02) (Table 3). The interaction model suggests a significant interaction effect of COVID-19 with SCT on AKF (P = .02; Table 3). In separate models, after adjusting for preexisting kidney impairment based on International Classification of Diseases codes in stepwise regression analysis or declining kidney function based on primarily laboratory values, the ORs for AKF remained largely unchanged with a nominally significant P value in all the models (Table 3).
Table 3. Development of Acute Kidney Failure and Declining Kidney Function Within 60 Days of COVID-19a.
| Model | Adjustment (preindex) | SCT | COVID-19+/− with SCT, P value for interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome | COVID-19 | ||||||||
| Negative | Positive | ||||||||
| No. | OR (95% CI) | P value | No. | OR (95% CI) | P value | ||||
| I | +AKI = 1, 2, 3 or prior ESRD (kidney function) | AKF | 24 691 | 0.92 (0.78-1.10) | .37 | 3448 | 1.41 (1.04-1.90) | .03 | .02 |
| IIA | No | AKF | 24 691 | 0.95 (0.81-1.13) | .58 | 3448 | 1.40 (1.06-1.86) | .02 | .02 |
| IIB | +AKF, +CKF, +prior ESRD | AKF | 24 691 | 0.92 (0.77-1.09) | .31 | 3448 | 1.46 (1.09-1.95) | .01 | .007 |
| IIC | +AKF, +CKF, +prior ESRD +CKD, +nephrosis | AKF | 24 691 | 0.89 (0.75-1.05) | .16 | 3448 | 1.33 (0.99-1.79) | .06 | .02 |
| III | No | Worsening kidney function (from AKI = 0 to AKI = 1, 2, 3) | 13 403 | 1.13 (0.90-1.42) | .31 | 2027 | 1.77 (1.17-2.68) | .007 | .06 |
Abbreviations: AKF, acute kidney failure; AKI, acute kidney injury; CKD, chronic kidney disease; CKF, chronic kidney failure; ESRD, end-stage renal disease; OR, odds ratio; SCT, sickle cell trait.
A stepwise regression analysis and the interaction of COVID-19 with SCT.
We then examined a subset of patients with stable and normal kidney function prior to COVID-19 and determined whether SCT was associated with increased risk of declining kidney function with COVID-19. We observed a statistically significant increase in the odds for declining kidney function after COVID-19 in individuals with SCT compared with individuals without SCT with COVID-19 (OR, 1.77; 95% CI, 1.16-2.67; P = .007). The same association was not observed in COVID-19–negative patients (OR, 1.13; 95% CI, 0.90-1.42; P = .31). However, this differential effect of SCT on postindex kidney function decline in COVID-19–positive vs COVID-19–negative patients was not statistically significant (P for interaction = .06; model III in Table 3).
Counterfactual Mediation Analysis
Our results show that SCT was significantly associated with death in COVID-19–positive patients, and individuals with SCT had a higher risk of AKF due to COVID-19. Therefore, we used mediation analysis to examine how much of the effect of SCT on COVID-19–related death was mediated through AKF due to COVID-19. On average, 22% (95% bootstrap CI, −3% to 83%) of the total effect of SCT on COVID-19–related death was mediated through AKF within 60 days of COVID-19.
Discussion
Sickle cell disease is a multisystem disorder.30 Multisystem anomalies were not known to be common in the heterozygous HbS state, nor had they been comprehensively investigated in SCT. We showed that individuals with SCT were predisposed to multisystem alterations, particularly kidney disease. Multiple correlated chronic kidney conditions derived from the EHR were associated with SCT, corroborated by a decrease in median laboratory values for estimated glomerular filtration rate and elevation in the baseline creatinine level. Veterans of African ancestry (n = 123 120) with preexisting kidney codes or displaying signs of the multisystem disorder showed significant association with COVID-19 death.
In addition, SCT was significantly associated with a diagnosis of AKF within 60 days of COVID-19. The increased risk of AKF persisted despite adjustment for preexisting kidney conditions or declining kidney function based on laboratory values. These observations indicated that pre–COVID-19 kidney impairment only explained a small fraction of increased risk for post–COVID-19 AKF, suggesting that the mechanisms for AKF might be different for individuals with SCT and COVID-19. The association of SCT with kidney dysfunction both before and after COVID-19 indicates an active role of sickle hemoglobin in the pathogenesis of kidney function abnormalities. Mediation analysis found that an AKF diagnosis within 60 days of COVID-19 accounted for more than 20% of COVID-19 deaths in individuals of African ancestry with SCT. In summary, there was an increased risk of death from COVID-19 among SCT carriers.
Chronic kidney disease at 3% to 13% prevalence was among the most common comorbidities in the hospitalized patients with COVID-19, which also included hypertension (48%-57%), diabetes (17%-34%), cardiovascular disease (21%-28%), chronic pulmonary disease (4%-10%), and malignant neoplasm (6%-8%).31 Many of these conditions have been shown to be associated with severe COVID-19 outcomes in prior studies. The polymerization of hemoglobin beta sickle protein in SCD contributes to vaso-occlusion.32 In individuals with SCT, sickling due to low oxygenation tension in the kidneys may cause kidney dysfunction,33,34 which can be exacerbated by COVID-19, in addition to other potential mechanisms.35 Of note, gout has a known association with SCD,36 but its linkage to SCT identified through association studies has not been previously reported.
Earlier studies on the association of SCT and COVID-19 outcome have been limited by sample size.37,38,39 A large EHR-based case-control study of mostly women (80%) and younger adults had not found worse outcomes for individuals with SCT and COVID-19.40 Consistent with our findings, a recent report found increased risk of hospitalization and death from COVID-19 for individuals with SCD and SCT.4 Studies show that COVID-19 disproportionately affects certain populations, including the medically underserved and racial and ethnic minority groups, and places them at higher risk.41 Our findings suggest that SCT can further contribute to worse outcomes in individuals of African ancestry, and there is a need for new treatment strategies to improve clinical outcomes of COVID-19 in individuals with SCT.
The presence of an HbC allele was not associated with worse COVID-19 outcomes. The lack of associations of HbC with multiple medical/kidney comorbidities may explain the difference in COVID-19 outcomes.
Limitations
The MVP participants were predominantly male but represented one of the largest African ancestry cohorts available. No patient with SCD was present in the MVP, as this condition would generally preclude enlistment in the armed forces. The PheWAS association study was designed as a broad screen to test for potentially clinically relevant associations between genes and clinical conditions, with limited power to detect associations among uncommon conditions, particularly when stratified by genetic ancestry. Despite our best statistical efforts and adjustment, residual confounding and misclassification may still exist. Our work can be strengthened by replication studies. The molecular subtypes of COVID-19, vaccination, and treatment approaches evolved organically during the study period.
Conclusions
In this genetic association study, SCT was associated with increased COVID-19 mortality and a number of preexisting chronic medical conditions in African ancestry individuals. Our findings support the inclusion of SCT as an adverse prognostic factor for COVID-19 and development of SCT-tailored interventions. Our work has broad implications for the detection and clinical management of SCT.
eMethods.
eFigure 1. A Hybridization Intensity Distribution Plot of Human β Hemoglobin A (HbA, the wild type allele) and Hemoglobin S (HbS, the alternative sickle allele rs334) in a Representative Batch (probeset AX-42810399, MVP 1.0 Axiom Array; out of 161 batches) of 4353 MVP Participants
eFigure 2. LabWAS of rs334-T in the MVP
eFigure 3. Phenome-Wide Association Studies of the Hb C allele (rs33930165-T) in MVP Participants From AFR Ancestry
eFigure 4. Laboratory-Wide Association Studies of the Hb C allele (rs33930165-T) in MVP Participants From AFR Ancestry
eAppendix. VA Million Veteran Program COVID-19 Science Initiative Membership & Acknowledgements
eTable 1. The ICD-10 Codes Used in the Definition of the Post-Index Conditions of AMI, AKF, CKD, CKF and Nephrosis
eTable 2. Demographics and Clinical Characteristics of Study Participants in the Million Veteran Programs
eTable 3. The demographics and associated clinical conditions were documented within two years prior to the index dates for MVP subjects in this study.
eTable 4. Phenome-Wide Association Studies of the Hb S allele (rs334-T) in 4 Major Ancestry Groups in the MVP.
eTable 5. Laboratory-Wide Association Studies of the Hb S allele(rs334-T) in 4 Major Ancestry Groups in the MVP.
eTable 6. Associations of Pre-COVID-19 Comorbidities and COVID-19 Related Death in the African Ancestry Participants
eTable 7. Association of Sickle Cell Trait (rs334) and 4 COVID-19 Outcomes by Ancestry and Combined Results Through Meta-analysis
eTable 8. Hb C allele Association Results With COVID-19 Outcome Severity
eTable 9. Phenome-Wide Association Studies of the Hb C allele (rs33930165) in MVP Participants From African Ancestry
eTable 10. Laboratory-Wide Association Studies of the Hb C allele (rs33930165) in MVP Participants From African Ancestry
Nonauthor Collaborators
References
- 1.Johns Hopkins Coronavirus Resource Center. Accessed February 11, 2022. https://coronavirus.jhu.edu/
- 2.Minniti CP, Zaidi AU, Nouraie M, et al. Clinical predictors of poor outcomes in patients with sickle cell disease and COVID-19 infection. Blood Adv. 2021;5(1):207-215. doi: 10.1182/bloodadvances.2020003456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panepinto JA, Brandow A, Mucalo L, et al. Coronavirus disease among persons with sickle cell disease, United States, March 20-May 21, 2020. Emerg Infect Dis. 2020;26(10):2473-2476. doi: 10.3201/eid2610.202792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clift AK, Saatci D, Coupland CAC, Dambha-Miller H, Hippisley-Cox J; International Investigator Group for Ethnicity and COVID-19 . Sickle cell disorders and severe COVID-19 outcomes: a cohort study. Ann Intern Med. 2021;174(10):1483-1487. doi: 10.7326/M21-1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gandhi RT, Lynch JB, Del Rio C. Mild or moderate COVID-19. N Engl J Med. 2020;383(18):1757-1766. doi: 10.1056/NEJMcp2009249 [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention . Incidence of sickle cell trait in the US. Published December 15, 2020. Accessed February 11, 2022. https://www.cdc.gov/ncbddd/sicklecell/features/keyfinding-trait.html
- 7.Pecker LH, Lanzkron S. Sickle cell disease. Ann Intern Med. 2021;174(1):ITC1-ITC16. doi: 10.7326/AITC202101190 [DOI] [PubMed] [Google Scholar]
- 8.Kark JA, Posey DM, Schumacher HR, Ruehle CJ. Sickle-cell trait as a risk factor for sudden death in physical training. N Engl J Med. 1987;317(13):781-787. doi: 10.1056/NEJM198709243171301 [DOI] [PubMed] [Google Scholar]
- 9.Nelson DA, Deuster PA, Kurina LM. Sickle cell trait and rhabdomyolysis among US Army soldiers. N Engl J Med. 2016;375(17):1696. doi: 10.1056/NEJMoa1516257 [DOI] [PubMed] [Google Scholar]
- 10.Elliott A, Bruner E. Renal medullary carcinoma. Arch Pathol Lab Med. 2019;143(12):1556-1561. doi: 10.5858/arpa.2017-0492-RS [DOI] [PubMed] [Google Scholar]
- 11.Raffield LM, Ulirsch JC, Naik RP, et al. ; NHLBI Trans-Omics for Precision Medicine (TOPMed) Consortium, Hematology & Hemostasis, Diabetes, and Structural Variation TOPMed Working Groups . Common α-globin variants modify hematologic and other clinical phenotypes in sickle cell trait and disease. PLoS Genet. 2018;14(3):e1007293. doi: 10.1371/journal.pgen.1007293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naik RP, Derebail VK, Grams ME, et al. Association of sickle cell trait with chronic kidney disease and albuminuria in African Americans. JAMA. 2014;312(20):2115-2125. doi: 10.1001/jama.2014.15063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Little I, Vinogradova Y, Orton E, Kai J, Qureshi N. Venous thromboembolism in adults screened for sickle cell trait: a population-based cohort study with nested case-control analysis. BMJ Open. 2017;7(3):e012665. doi: 10.1136/bmjopen-2016-012665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pecker LH, Naik RP. The current state of sickle cell trait: implications for reproductive and genetic counseling. Hematology Am Soc Hematol Educ Program. 2018;2018(1):474-481. doi: 10.1182/asheducation-2018.1.474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Folsom AR, Tang W, Roetker NS, et al. Prospective study of sickle cell trait and venous thromboembolism incidence. J Thromb Haemost. 2015;13(1):2-9. doi: 10.1111/jth.12787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention . Underlying medical conditions associated with higher risk for severe COVID-19: information for healthcare providers. Published December 22, 2021. Accessed February 11, 2022. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html
- 17.Gaziano JM, Concato J, Brophy M, et al. Million Veteran Program: a mega-biobank to study genetic influences on health and disease. J Clin Epidemiol. 2016;70:214-223. doi: 10.1016/j.jclinepi.2015.09.016 [DOI] [PubMed] [Google Scholar]
- 18.Ioannou GN, Locke E, Green P, et al. Risk factors for hospitalization, mechanical ventilation, or death among 10 131 US veterans with SARS-CoV-2 infection. JAMA Netw Open. 2020;3(9):e2022310. doi: 10.1001/jamanetworkopen.2020.22310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection . A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20(8):e192-e197. doi: 10.1016/S1473-3099(20)30483-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song RJ, Ho YL, Schubert P, et al. ; VA Million Veteran Program COVID-19 Science Initiative . Phenome-wide association of 1809 phenotypes and COVID-19 disease progression in the Veterans Health Administration Million Veteran Program. PLoS One. 2021;16(5):e0251651. doi: 10.1371/journal.pone.0251651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patterson OV, Jones M, Yao Y, et al. Extraction of vital signs from clinical notes. Stud Health Technol Inform. 2015;216:1035. [PubMed] [Google Scholar]
- 22.Denny JC, Ritchie MD, Basford MA, et al. PheWAS: demonstrating the feasibility of a phenome-wide scan to discover gene-disease associations. Bioinformatics. 2010;26(9):1205-1210. doi: 10.1093/bioinformatics/btq126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Firth D. Bias reduction of maximum likelihood estimates. Biometrika. 1993;80(1):27-38. doi: 10.1093/biomet/80.1.27 [DOI] [Google Scholar]
- 24.Kosmidis I, Firth D. Jeffreys-prior penalty, finiteness and shrinkage in binomial-response generalized linear models. Biometrika. 2021;108(1):71-82. doi: 10.1093/biomet/asaa052 [DOI] [Google Scholar]
- 25.Kosmidis I. brglm2: Bias reduction in generalized linear models. Accessed May 17, 2022. https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.374.1508&rep=rep1&type=pdf
- 26.Fang H, Hui Q, Lynch J, et al. ; VA Million Veteran Program . Harmonizing genetic ancestry and self-identified race/ethnicity in genome-wide association studies. Am J Hum Genet. 2019;105(4):763-772. doi: 10.1016/j.ajhg.2019.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wellcome Trust Case Control Consortium . Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447(7145):661-678. doi: 10.1038/nature05911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1-48. doi: 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 29.Hodonsky CJ, Jain D, Schick UM, et al. Genome-wide association study of red blood cell traits in Hispanics/Latinos: the Hispanic Community Health Study/Study of Latinos. PLoS Genet. 2017;13(4):e1006760. doi: 10.1371/journal.pgen.1006760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sundd P, Gladwin MT, Novelli EM. Pathophysiology of sickle cell disease. Annu Rev Pathol. 2019;14:263-292. doi: 10.1146/annurev-pathmechdis-012418-012838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782-793. doi: 10.1001/jama.2020.12839 [DOI] [PubMed] [Google Scholar]
- 32.Kalpatthi R, Novelli EM. Measuring success: utility of biomarkers in sickle cell disease clinical trials and care. Hematology Am Soc Hematol Educ Program. 2018;2018(1):482-492. doi: 10.1182/asheducation-2018.1.482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henry ER, Cellmer T, Dunkelberger EB, et al. Allosteric control of hemoglobin S fiber formation by oxygen and its relation to the pathophysiology of sickle cell disease. Proc Natl Acad Sci U S A. 2020;117(26):15018-15027. doi: 10.1073/pnas.1922004117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu ZZ, Bullen A, Li Y, Singh P. Renal oxygenation in the pathophysiology of chronic kidney disease. Front Physiol. 2017;8:385. doi: 10.3389/fphys.2017.00385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conran N, De Paula EV. Thromboinflammatory mechanisms in sickle cell disease—challenging the hemostatic balance. Haematologica. 2020;105(10):2380-2390. doi: 10.3324/haematol.2019.239343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaspar CDW, Beach I, Newlin J, Sisler I, Feig D, Smith W. Hyperuricemia is associated with a lower glomerular filtration rate in pediatric sickle cell disease patients. Pediatr Nephrol. 2020;35(5):883-889. doi: 10.1007/s00467-019-04432-2 [DOI] [PubMed] [Google Scholar]
- 37.Balanchivadze N, Kudirka AA, Askar S, et al. Impact of COVID-19 infection on 24 patients with sickle cell disease: one center urban experience, Detroit, MI, USA. Hemoglobin. 2020;44(4):284-289. doi: 10.1080/03630269.2020.1797775 [DOI] [PubMed] [Google Scholar]
- 38.Kehinde TA, Osundiji MA. Sickle cell trait and the potential risk of severe coronavirus disease 2019—a mini-review. Eur J Haematol. 2020;105(5):519-523. doi: 10.1111/ejh.13478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tafti D, Kluckman M, Dearborn MC, Hunninghake J, Clayton S. COVID-19 in patients with hematologic-oncologic risk factors: complications in three patients. Cureus. 2020;12(12):e12064. doi: 10.7759/cureus.12064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh A, Brandow AM, Panepinto JA. COVID-19 in individuals with sickle cell disease/trait compared with other Black individuals. Blood Adv. 2021;5(7):1915-1921. doi: 10.1182/bloodadvances.2020003741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among Black patients and White patients with COVID-19. N Engl J Med. 2020;382(26):2534-2543. doi: 10.1056/NEJMsa2011686 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eFigure 1. A Hybridization Intensity Distribution Plot of Human β Hemoglobin A (HbA, the wild type allele) and Hemoglobin S (HbS, the alternative sickle allele rs334) in a Representative Batch (probeset AX-42810399, MVP 1.0 Axiom Array; out of 161 batches) of 4353 MVP Participants
eFigure 2. LabWAS of rs334-T in the MVP
eFigure 3. Phenome-Wide Association Studies of the Hb C allele (rs33930165-T) in MVP Participants From AFR Ancestry
eFigure 4. Laboratory-Wide Association Studies of the Hb C allele (rs33930165-T) in MVP Participants From AFR Ancestry
eAppendix. VA Million Veteran Program COVID-19 Science Initiative Membership & Acknowledgements
eTable 1. The ICD-10 Codes Used in the Definition of the Post-Index Conditions of AMI, AKF, CKD, CKF and Nephrosis
eTable 2. Demographics and Clinical Characteristics of Study Participants in the Million Veteran Programs
eTable 3. The demographics and associated clinical conditions were documented within two years prior to the index dates for MVP subjects in this study.
eTable 4. Phenome-Wide Association Studies of the Hb S allele (rs334-T) in 4 Major Ancestry Groups in the MVP.
eTable 5. Laboratory-Wide Association Studies of the Hb S allele(rs334-T) in 4 Major Ancestry Groups in the MVP.
eTable 6. Associations of Pre-COVID-19 Comorbidities and COVID-19 Related Death in the African Ancestry Participants
eTable 7. Association of Sickle Cell Trait (rs334) and 4 COVID-19 Outcomes by Ancestry and Combined Results Through Meta-analysis
eTable 8. Hb C allele Association Results With COVID-19 Outcome Severity
eTable 9. Phenome-Wide Association Studies of the Hb C allele (rs33930165) in MVP Participants From African Ancestry
eTable 10. Laboratory-Wide Association Studies of the Hb C allele (rs33930165) in MVP Participants From African Ancestry
Nonauthor Collaborators


