To the Editor:
Hepatocellular carcinoma (HCC)1 is a major cause of cancer mortality worldwide (1). There remains a need for noninvasive tests to identify patients in the community at risk of developing HCC, especially that resulting from underlying nonalcoholic fatty liver disease (NAFLD) (2). The Enhanced Liver Fibrosis (ELF) test consists of 3 direct markers of liver extracellular matrix: hyaluronic acid (HA), tissue inhibitor of matrix metalloproteinases-1 (TIMP-1), and amino-terminal propeptide of type III procollagen (PIIINP). The ELF score has been validated in diagnosis of advanced fibrosis and predicting complications in patients with established chronic liver disease (3, 4). In this prospective study, we evaluated the use of the ELF score as a predictor of HCC in a population-based cohort of Chinese in Singapore.
The Singapore Chinese Health Study recruited 63257 Chinese men and women, of age between 45 and 74 years, in Singapore between 1993 and 1998 (5) and blood samples were collected using plain tubes from 32543 subjects between 1999 and 2005. Serum from each blood sample was prepared by centrifugation and stored at −80 °C for an average of 14 years before chemical analysis. After a mean of 11.5 years of follow-up, among the 394 incident cases of HCC identified in this cohort through linkage with the Singapore Cancer Registry, 60 cases were randomly sampled from those who had donated blood samples. Each of these cases was then matched to a control for year at recruitment (±1 years), year of birth (±2 years), sex, dialect group (Cantonese, Hokkien), and date of biospecimen collection (± 6 months).
The 3 ELF analytes in the stored serum were assayed on the same day, using 2-site sandwich assays on the Siemens ADVIA Centaur XP system, in the Core Laboratory in National University Hospital Singapore. ELF scores were calculated as follows:
ELF score = 2.278 + 0.851 ln(HA concentration) + 0.751 ln (PIIINP concentration) + 0.394 ln(TIMP-1 concentration).
We used the ROC analysis and Youden index to derive the optimal cutoff value for the ELF score in its diagnostic ability to discriminate between cases and controls. Multivariable logistic regression models were used to compute the odds ratio (OR) for the association between a higher ELF score and HCC risk.
Among the 60 cases, the mean (SD) time interval from blood draw to the time of cancer diagnosis was 3.1 (2.3) years, the mean age at diagnosis was 68.3 (7.4) years, and, among cases, 44 (73.8%) were men. Positivity for Hepatitis B serology was present in 34 cases (56.7%) and 8 (13.3%) controls, whereas only 1 in each group was positive for Hepatitis C serology.
The mean and median (interquartile range) values for HA concentrations were 229.7 ng/mL and 228.1 ng/mL (110.3–504.2) in cases and 75.1 ng/mL and 70.7 ng/mL (38.0–115.2) in controls, respectively. The corresponding values for TIMP-1 concentrations were 325.0 ng/mL and 303.9 ng/mL (260.0–358.8) in cases and 240.8 ng/mL and 244.6 ng/mL (223.2–267.4) in controls and those for PIIINP concentrations were 13.1 ng/mL and 11.3 ng/mL (8.1–16.4) in cases and 6.8 ng/mL and 6.9 ng/mL (5.3–8.7) in controls. All P values for differences between cases and controls were <0.001. An ELF cutoff score of 9.89 had the highest predictive value for incident HCC with a sensitivity of 85.3% and specificity of 79.5%, and the area under the ROC curve (AUC) for a model that included the matching factors (age, sex, dialect group) and ELF score was 0.89 (0.83–0.94; P < 0.001; model 3). In a clinical model that included age, sex, dialect group, body mass index (BMI), alcohol consumption, and history of diabetes, AUC was 0.68 (95% CI, 0.58–0.78; model 1) and adding information on viral hepatitis serology improved AUC to 0.82 (0.75–0.89; model 2; P < 0.001). Finally, inclusion of ELF score further improved the AUC to 0.93 (0.89–0.97; Model 4; P < 0.001; Fig. 1). In all subjects, after adjusting for age, sex, BMI, smoking status, alcohol, coffee drinking, and diabetes, an ELF score of ≥9.89 increased the risk by about 24-fold (OR, 24.54; 95% CI, 4.62–130.26]. This risk estimate was much higher in those negative for viral hepatitis serology (OR, 54.37; 95% CI, 6.98–423.49) than in those who were positive (OR, 4.22; 95% CI, 0.66–26.86).
Fig. 1. ROC curves comparing different models in predicting risk of HCC in 120 participants (60 case-control pairs).
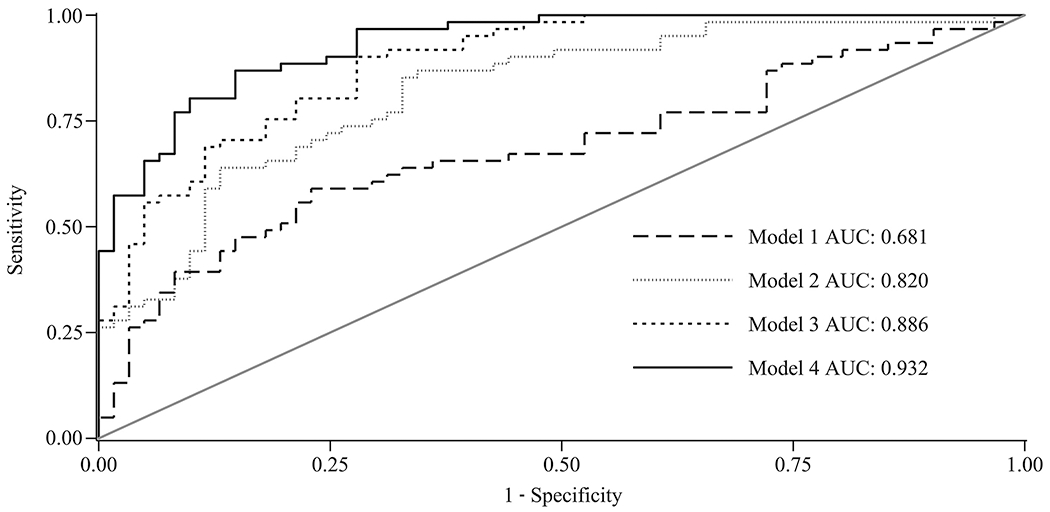
Model 1 is a clinical model that incorporated matching factors (age, sex, and dialect group) plus BMI, alcohol, and diabetes. Model 2 included variables in model 1 plus viral hepatitis serology status (positivity for either HBV or HCV). Model 3 included ELF score (continuous) and matching factors. Model 4 included variables in model 2 plus ELF score (continuous).
Although limited by lack of comparators of liver fibrosis such as fibroscan, platelet counts, or other blood fibrosis markers, this study has demonstrated the potential utility of the ELF score as a predictor of HCC in the general population with high diagnostic sensitivity, specificity, and accuracy. It was more robust in predicting nonviral related HCC and, thus, has implications for identifying at-risk patients with NAFLD in the community for targeted surveillance.
Research Funding
J.-M. Yuan, NIH Grant# R01CA144034, NIH Grant # UM1CA182876.
Y. Wang, statistical analysis; J.-M. Yuan, financial support, administrative support, provision of study material or patients.
Footnotes
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest:
Employment or Leadership: None declared.
Consultant or Advisory Role: None declared.
Stock Ownership: None declared.
Honoraria: None declared.
Expert Testimony: None declared.
Patents: None declared.
Nonstandard abbreviations: HCC, hepatocellular carcinoma; NAFLD, nonalcoholic fatty liver disease; ELF, Enhanced Liver Fibrosis; HA, hyaluronic acid; TIMP-1, tissue inhibitor of matrix metalloproteinases-1; PIIINP, amino-terminal propeptide of type III procollagen; OR, odds ratio; AUC, area under the ROC curve; BMI, body mass index.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser R, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- 2.Ekstedt M, Hagstrom H, Nasr P, Fredrikson M, Stal P, Kechagias S, Hultcrantz R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015;61:1547–54. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg WM, Voelker M, Thiel R, Becka M, Burt A, Schuppan D, et al. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology 2004;127:1704–13. [DOI] [PubMed] [Google Scholar]
- 4.Parkes J, Roderick P, Harris S, Day C, Mutimer D, Collier J, et al. Enhanced liver fibrosis test can predict clinical outcomes in patients with chronic liver disease. Gut 2010;59:1245–51. [DOI] [PubMed] [Google Scholar]
- 5.Hankin JH, Stram DO, Arakawa K, Park S, Low SH, Lee HP, Yu MC. Singapore Chinese Health Study: development, validation, and calibration of the quantitative food frequency questionnaire. Nutr Cancer 2001;39:187–95. [DOI] [PubMed] [Google Scholar]


