Abstract
Background:
African Americans in the general population have been shown to be less likely than White ethnic groups to participate in advance care planning (ACP); however, ACP in the dialysis population has not been well explored.
Aim:
We examined the prevalence of African American patients on haemodialysis’ ACP discussions, and whether ACP impacts end-of-life (EOL) care preferences.
Design:
In-person interviewer-administered surveys of African American patients receiving in-centre haemodialysis
Setting/Participants:
101 participants at 3 large dialysis organisation units in Chicago
Outcomes:
Self-reported ACP and preferences for life-extending treatments at EOL
Results:
Most patients (69%) report no ACP discussions with their healthcare providers (HCP). Nearly all patients (92%) without prior ACP reported their HCP approached them about ACP. While the majority of patients indicated preference for aggressive life-extending care, prior conversations about EOL care wishes either with family members or a healthcare provider significantly decreased patients’ likelihood of choosing aggressive life-extending care across three scenarios (all p<0.05). Significantly more patients reported that common EOL scenarios related to increased dependence/disability were “not worth living through” compared to those associated with increased burden on family, decreased cognitive function, and severe pain/discomfort.
Conclusions:
African Americans with ESRD need more frequent, culturally-sensitive ACP discussions. Despite a preference for aggressive life-sustaining treatments, individuals with prior ACP discussions were significantly less likely to support aggressive EOL care. EOL care discussions that focus on the impact of life-extending care on patients’ independence could be more concordant with the values and priorities of the African American patients.
Keywords: Advance-Care Planning, African Americans, End-Stage Renal Disease, Haemodialysis, Patient-Reported Outcome Measures
Introduction
Haemodialysis is associated with an increased morbidity (Miskulin et al., 2009) and mortality (Chronic Kidney Disease Prognosis Consortium, 2010) that are reflected in extended hospital stays (Tam-Tham et al., 2020), lower quality of life (McClellan et al., 2010), and worsening functional dependence (Kurella Tamura et al., 2009). The five-year adjusted survival rate of patients with end-stage renal disease (ESRD) is only 42% (The United States Renal Data Service, 2018), thus advance-care planning (ACP) is an important intervention to help patients on haemodialysis understand their current health status and prognosis as well as assert more autonomy over their medical care. Despite its importance and benefits, however, ACP is underutilised among patients receiving haemodialysis, particularly racial and ethnic minorities (Davison, 2006).
Literature Review
In the United Kingdom (UK), Black, Asian, and Minority Ethnic (BAME) groups have a greater incidence of ESRD than White ethnic groups. However they are less likely to have a “Do Not Attempt Resuscitation” (DNAR) order in place, or documentation in regarding to discussions for discontinuing haemodialysis or receiving palliative care (McAdoo et al., 2012; Koffman and Higginson, 2016). Similarly, in the United States (US), African Americans, who comprise more than 30% of the US ESRD population, have been consistently shown to be less likely than their White counterparts to participate in ACP (Muni et al., 2011; Wicher and Meeker, 2012; Eneanya et al., 2016). African Americans are also less likely to stop haemodialysis after starting (Foley et al., 2018) and more likely to revoke hospice care to pursue life-sustaining treatments like mechanical ventilation (Johnson et al., 2008; Wicher and Meeker, 2012). In addition, the families of African American decedents are less likely than those of White decedents to report that EOL treatment wishes were met (Welch, Teno and Mor, 2005).
While preference for aggressive life-sustaining treatments could reflect missed or ineffective ACP, African Americans may be less open to ACP due to conflicts between spiritual beliefs and perceptions of palliative care, fear that they are not being offered treatments that other patients might receive, and family members’ resistance to accepting alternatives to life-sustaining treatments like hospice care (Crawley et al., 2000; Torke et al., 2005; Wicher and Meeker, 2012; Rhodes et al., 2015, 2017; Yancu et al., 2015). Latinx and Asian American populations may have low ACP rates because current ACP discussions may conflict with collectivistic values and the agency of family members to make final choices regarding EOL care or even override patients’ EOL care decisions (Gutheil and Heyman, 2006; Bito et al., 2007; Kwak and Salmon, 2007; Van Dorn, Swanson and Swartz, 2009). In the UK, racial and religious discrimination, difficulty discussing death, and language barriers have been found to be barriers to ACP for South Asian Sikh and Muslim patients (Worth et al., 2009). A separate study reported that BAME families in the UK may not fully disclose diagnoses to patients, limiting physicians’ options for discussing EOL care (Karim, Bailey and Tunna, 2016). A barrier to ACP that is shared among racial and ethnic minorities in the US and UK, however, is a lack of knowledge about what ACP is and how to complete relevant documents (Owens and Randhawa, 2004; Elkan et al., 2007; Karim, Bailey and Tunna, 2016; Vries et al., 2019). Due to the existence of these barriers to ACP and significant disparities in EOL care costs (Hanchate et al., 2009), several studies have stressed the importance of developing culturally sensitive interventions that can make ACP more acceptable to African American patients (Crawley et al., 2000; Torke et al., 2005; Rhodes et al., 2015; Yancu et al., 2015; Song et al., 2016). Some of these interventions include an EOL conversation game at community venues (Van Scoy et al., 2020) and a peer mentoring program through which information about ACP is offered through one-on-one relationships with trained patients on dialysis rather than via written documents (Perry et al., 2005). However, little is known about what factors shape the EOL health priorities of African American patients receiving haemodialysis and the preference for aggressive EOL care.
This study aimed to examine the ACP received by African American adults on haemodialysis and explore how prior ACP discussions were associated with views on common advanced illness scenarios and aggressive life-sustaining treatments. By understanding prior ACP care experience and treatment preferences, we can develop interventions aimed at improving rates and quality of ACP among African American patients with ESRD.
Materials and Methods
Study Setting and Participants
From June to September 2019, we approached adults receiving haemodialysis in three large dialysis organisation (LDO) units to participate in research examining their prior ACP discussions and views on end-of-life (EOL) care. We recruited participants who met the study criteria: receiving chronic in-centre haemodialysis, self-identifying as Black or African American, English-speaking, older than 18 years of age, and without significant cognitive impairment as determined by unit staff. Based on convenience sample, patients who met criteria were directly approached by the researcher (DA) who described the study and obtained written consent for participation. Each of the LDO units serve about 100 patients, and we aimed to approach half of the patients in order to eventually have a sample that was one-third of the population size. Consented patients were given surveys to complete. The surveys took about 15–30 minutes, and patients completed them in their dialysis chairs alone or with the help of the researcher who at patient request would read questions and record answers. The study took place over multiple days and across multiple sessions (morning, afternoon, and evening) at each unit to avoid bias. The study was approved by the Institutional Review Boards at the University of Chicago (IRB19–0450) and DaVita (IRB 026–2019).
Data Sources and Management
The instrument included questions about demographic and clinical characteristics, the FACIT-Sp (Version 4), which is a questionnaire that measures participants’ self-reported well-being, questions taken from a publicly available ACP workbook (Robert Pearlman et al., 2010) and a survey on EOL care planning from the National Kidney Foundation (NKF) (Weiner, 2010).
Patient Characteristics and Other Measures
We used the FACIT-Sp (Version 4) to assess patients’ self-reported wellbeing (‘Functional Assessment of Chronic Illness Therapy-Spiritual Wellbeing (FACIT-Sp) Version 4’, no date). This questionnaire has a total of 39 items, each consisting of short statements such as “I have pain.” Patients answered the questions by indicating how much the statements have applied to them in the past week using a five-point scale ranging from “not at all” (0) to “very much” (4). The FACIT-Sp is divided into five sections with each corresponding to a domain of well-being: emotional (6 items), social (7 items), functional (7 items), physical (7 items), and spiritual (12 items). Based on scoring guidelines (‘Functional Assessment of Chronic Illness Therapy-Spiritual Wellbeing (FACIT-Sp) Version 4’, no date), scores for individual items were converted and summed to generate totals for each section, which are a maximum value of 24 for emotional well-being, 28 each for social, functional, and physical well-being, and 48 for spiritual well-being. Total scores ranged from 0 to 156, with higher scores indicating a more favourable perception of well-being. We used each section score as a continuous variable in logistic regression models. The FACIT-Sp has high reliability and validity for patients chronic illnesses, including those on haemodialysis (Cella et al., 1993; Peterman et al., 2002; Weisbord et al., 2003). In addition, we collected information on age, medical co-morbidities, income, and length of time on haemodialysis. We collapsed several of these variables into smaller groups for analysis. For example, for age, we grouped patients into categories of “18–65” and “>65” years old to separate older adults from the rest of the population. We collapsed time on haemodialysis into three categories: “<1 year,” “1–5 years,” and “>5 years” to separate patients who have been on haemodialysis for a short, medium, or long period of time and to have groups that are roughly split evenly.
End-of-Life and Life-Sustaining Care
We used an NKF survey to capture patients’ prior experiences with EOL care with healthcare providers and family, which topics were addressed and whether they would like additional opportunities to talk about EOL with healthcare providers (Weiner, 2010).
We used questions from an ACP workbook to identify patients’ preferences in twelve common EOL care scenarios as well as their views on life-sustaining treatments. This work was rigorously developed and tested in a predominantly White US Veteran population (Pearlman et al., 1993, 2005; Patrick et al., 1994) and later used for the purpose of investigating health outcome priorities of a predominantly White patient population with CKD (Ramer et al., 2018). For the EOL care scenarios, each item asked patients to choose whether they would find life in a certain scenario, if permanent, to be “difficult but acceptable,” “worth living, but just barely,” or “not worth living.” The first two responses were combined into one category because both indicate that patients found life to be worth living. Patients also were allowed to either respond “can’t answer now” or skip the question. Furthermore, we divided twelve scenarios into five thematic groups: decrease in cognitive function, severe discomfort or pain, burden on family, and increased dependence or disability; the twelfth scenario, “needing a breathing machine to keep me alive,” was its own theme because it encompasses all the domains and had the greatest proportion of people who reported life “not worth living.”
For patients’ views on life-sustaining treatments, each item asked patients to respond with “yes” or “no” to indicate whether they agreed with a particular statement. Patients were permitted to respond with “not sure” or skip questions if they had no answer or found any questions stressful. Our final analysis included items 1–3 that specifically measure patients’ preferences regarding life-sustaining care; answering “yes” for any of the three questions indicated a preference for aggressive life-sustaining treatments. Additionally, we combined the responses “no” and “not sure”.
Statistical Analysis
Baseline characteristics were summarised as means and standard deviations (SD) for continuous variables and counts with percentages for categorical variables. We tested the association between categorical variables with chi-square statistics and between continuous variables with two-tailed sample t-tests. We used the two-proportion Z-test to determine the differences in proportions of patients who responded “not worth living” to at least one of the scenarios in each of the five categories created from the twelve EOL scenarios. We determined the odds of patients preferring aggressive life-sustaining treatments by using bivariable and multivariable logistic regression models, with the following covariates: age, sex, income, education, wellbeing measured by the FACIT-Sp, number of hospitalisations in the last year, time on dialysis, prior EOL care experience with family and a healthcare provider, and desire to have new or additional EOL discussions with a healthcare provider. P values of 0.05 were considered statistically significant. All analyses were performed using STATA, version 15.0 (Stata Corp, College Station, TX).
Results
Respondent Characteristics
Out of the 145 patients we approached, 44 (30.3%) declined to participate. In Table 1, we report results for 101 participants. Overall, participants had a mean age of 57.8 years (SD 1.4), 52.4% identified as female, 62.4% had a high school degree, 54.5% had a yearly household income of less than $20,000, 85.1% have been on dialysis for more than 1 year, and 66.3% were insured by Medicaid and/or Medicaid. The most common comorbidities were hypertension (91.1%), diabetes (45.5%), congestive heart failure (35.6%), and arthritis (33.7%).
Table 1:
Demographic and Clinical Characteristics of Patients
| Overall | Never Had an EOL Care Discussion with Healthcare Team | Had at Least 1 EOL Care Discussion with Healthcare Team | P-Value | |
|---|---|---|---|---|
| Total | 101 | 69 | 32 | |
| Women | 53 (52.4) | 34 (49.3) | 19 (59.4) | 0.34 |
| Age, Years | 57.9 (1.4) | 58.7 (1.75) | 56.1 (2.35) | 0.38 |
| Highest Education Level | ||||
| Less Than High School | 9 (8.9) | 8 (11.6) | 1 (3.1) | 0.16 |
| High School or Some College | 63 (62.4) | 44 (63.8) | 19 (59.4) | 0.67 |
| College Graduate or Above | 29 (28.7) | 17 (24.6) | 12 (37.5) | 0.18 |
| Yearly Household Income | * | ** | *** | |
| <$20,000 | 49 (54.5) | 32 (51.6) | 17 (60.7) | 0.42 |
| $20,000–39,999 | 20 (22.2) | 14 (22.6) | 6 (21.4) | 0.90 |
| >$40,000 | 21 (23.3) | 16 (25.8) | 5 (17.9) | 0.41 |
| Time on Hemodialysis | ||||
| <1 Year | 15 (14.9) | 11 (15.9) | 4 (12.5) | 0.65 |
| 1–5 Years | 44 (43.6) | 31 (44.9) | 13 (40.6) | 0.68 |
| >5 Years | 42 (41.5) | 27 (39.2) | 15 (46.9) | 0.47 |
| Insurance Type | ||||
| Private | 16 (15.8) | 11 (15.9) | 5 (15.6) | 0.97 |
| Medicaid/Medical Assistance | 67 (66.3) | 46 (66.7) | 21 (65.6) | 0.92 |
| Medicare | 67 (66.3) | 47 (68.1) | 20 (62.5) | 0.58 |
| Comorbidities | ||||
| Hypertension | 92 (91.1) | 64 (92.8) | 28 (87.5) | 0.39 |
| Diabetes | 46 (45.5) | 33 (47.8) | 13 (40.6) | 0.50 |
| Congestive Heart Failure | 36 (35.6) | 26 (37.7) | 10 (31.3) | 0.53 |
| Arthritis | 34 (33.7) | 26 (37.7) | 8 (25.0) | 0.21 |
| Cancer | 13 (12.9) | 11 (15.9) | 2 (6.3) | 0.18 |
| Stroke | 12 (11.9) | 9 (13.0) | 3 (9.4) | 0.60 |
| Depression/Anxiety | 10 (9.9) | 6 (8.7) | 4 (12.5) | 0.55 |
| Coronary Artery Disease | 9 (8.9) | 7 (10.1) | 2 (6.3) | 0.52 |
| COPD | 7 (6.9) | 7 (10.1) | 0 (0.0) | 0.06 |
| Lupus | 7 (6.9) | 5 (7.2) | 2 (6.3) | 0.86 |
| Hepatitis | 4 (4.0) | 2 (2.9) | 2 (6.3) | 0.42 |
| Well-being | ||||
| FACIT-Sp Score (Out of 156) | 122.55 (22.07) | 125.04 (21.78) | 117.17 (22.08) | 0.04 |
| Emotional Well-being Score (Out of 24) | 20.11 (4.50) | 20.76 (4.31) | 18.72 (4.65) | 0.02 |
| Social Well-being Score (Out of 28) | 21.87 (5.30) | 22.31 (5.41) | 20.94 (5.03) | 0.11 |
| Functional Well-being Score (Out of 28) | 18.01 (6.76) | 18.77 (6.93) | 16.38 (6.15) | 0.04 |
| Physical Well-being Score (Out of 28) | 20.80 (5.49) | 21.01 (5.65) | 20.34 (5.18) | 0.29 |
| Spiritual Well-being Score (Out of 48) | 41.75 (7.87) | 42.20 (7.69) | 40.79 (8.31) | 0.20 |
Continuous variables expressed as mean (SD). Categorical variables expressed as N (%).
N=90
N=62
N=28
Prior EOL Care Discussions
Of the 101 patients, 69 (68.3%) reported never having an EOL care discussion with a healthcare provider since starting haemodialysis either at their dialysis unit or doctor’s office, while 32 (31.7%) reported at least one EOL care discussion. Patients who had an EOL care discussion did not differ significantly from those who had not for most demographic and clinical characteristics (Table 1). However, patients with a prior EOL care discussion with a healthcare provider had significantly lower scores on the total FACIT-Sp (117.17 vs. 125.04), emotional well-being (18.72 vs. 20.76), and functional well-being (16.38 vs. 18.77) compared to their counterparts without an EOL care discussion, all p<0.05 (Table 1).
Of the 32 patients who reported at least one EOL care discussion with a healthcare provider (Table 2), the most commonly reported providers who engaged in these discussions were the dialysis unit social worker (62.5%) and primary care doctor (50%). The topics most frequently addressed were selecting a power of attorney (53.1%) and completing a living will (43.8%). Most patients (62.5%) reported only 1 or 2 discussions, and 25% reported more than 2 discussions. The timing of these discussions varied. While many patients (40.6%) reported having EOL care discussions when they started dialysis, large proportions had them during major health crises (25%) or more than a year after starting dialysis (25%). Most of these patients (74%) have spoken to their family about their EOL care wishes, and less than 40% expressed any desire to have additional EOL discussions.
Table 2:
Nature of EOL Discussions among People with EOL Care Planning Experience
| N=32 (%) | |
|---|---|
| Had These Discussions With: | |
| Dialysis Unit Social Worker | 20 (62.5) |
| Dialysis Unit Doctor | 6 (18.8) |
| Dialysis Unit Nurse | 5 (15.6) |
| Dialysis Unit Dietician | 2 (6.3) |
| Dialysis Unit Technician | 1 (3.1) |
| Primary Care Doctor | 16 (50) |
| Clinic Social Worker | 8 (25) |
| Clinic Nurse | 4 (12.5) |
| Clinic Pastor | 3 (9.4) |
| Home Nurse/Health Aide | 2 (6.3) |
| Spoke about These Topics: | |
| Caring for Loved Ones if I Am Unable | 11 (34.4) |
| Completing an Advance Directive | 11 (34.4) |
| Completing a Living Will | 14 (43.8) |
| CPR | 12 (37.5) |
| DNR | 11 (34.4) |
| Hospice Care | 9 (28.1) |
| Pain Control | 12 (37.5) |
| Selecting a Power of Attorney | 17 (53.1) |
| Selecting a Health Care Proxy | 12 (37.5) |
| Stopping Dialysis | 2 (6.3) |
| Had Informal Discussions | 9 (28.1) |
| Not Sure | 1 (3.1) |
| Had These Discussions When: | |
| I Started Dialysis | 13 (40.6) |
| During the First Year after I Started Dialysis | 4 (12.5) |
| More than a Year after I started Dialysis | 8 (25) |
| I Had a Major Health Crisis | 8 (25) |
| We Talk about EOL Care Regularly | 5 (15.6) |
| Had This Many Discussions: | |
| 1–2 | 20 (62.5) |
| >2 | 8 (25) |
| Not Sure | 3 (9.4) |
| Wants to Discuss EOL Care Additionally | 12* (38.7) |
| Has Spoken to Family about EOL Care Wishes | 23* (74.2) |
N=31
The majority (68.3%) of patients reported having no EOL care discussions with any healthcare provider since starting dialysis. Almost all of these patients (92.8%) reported that their healthcare team never approached them to discuss EOL care (Table 3). Just 17.4% of patients reported that they would not feel comfortable talking about EOL care if approached by a provider, and very few (5.8%) patients did not want to talk about EOL care. Despite this, only a small proportion of these patients (36.8%) expressed any interest in discussing EOL care with a member of their healthcare team. Almost half (45.6%) of patients who had not had an EOL care discussion with a health care provider reported having spoken to family about their EOL wishes.
Table 3:
Characteristics of Patients Without EOL Care Discussion Experience
| N=69 (%) | |
|---|---|
| Reason for Not Having Discussions: | |
| My Healthcare Team Never Talked to Me About EOL Care | 64 (92.8) |
| I Did Not Want to Talk about EOL Care When Asked | 4 (5.8) |
| Wants to Discuss EOL Care with a Member of Healthcare Team | 25* (36.8) |
| Would Not Feel Comfortable Talking about EOL Care if Asked | 12 (17.4) |
| Has Spoken to Family about EOL Care Wishes | 31* (45.6) |
N=68
Views on Common End-of-Life Scenarios
Patients’ responses to the twelve care scenarios, organised into five thematic categories, are presented in Table 4. Overall, more than 50% of respondents reported that all scenarios are “worth living through” except for two: “rely on a breathing machine to keep me alive” (48.5%) and “are a severe financial/emotional burden on family” (49.5%). Notably, a high proportion of patients (16–36%) reported uncertainty (“can’t answer now”) about their views. We found no significant association between previous EOL care experience with a healthcare provider and preferences in these care scenarios. Of respondents who indicated “not worth living” for at least one of the scenarios in the five thematic categories (Fig. 1), significantly more participants reported scenarios related to increased dependence/disability (31.7%) to be not worth living compared to those related to decrease in cognitive function (20.8%), severe discomfort/pain (19%), and burden to family (19.8%), all p<0.05.
Table 4:
Patients’ Self-Reported Preferences for Common EOL Scenarios
| Theme | Common End-of-Life Scenario | Total (N=101) | Has Never Had EOL Discussion with Healthcare Team (N=69) | Has Had EOL Discussions with Healthcare Team (N=32) |
P Value |
|---|---|---|---|---|---|
| Decrease in Cognitive Function | Can No Longer Recognize Family/Friends
|
51 (50.5) 14 (13.9) 36 (35.6) |
33 (47.8) 11 (15.9) 25 (36.3) |
18 (56.2) 3 (9.4) 11 (34.4) |
0.60 |
|
| |||||
Can No Longer Think Clearly
|
56 (55.4) 20 (19.8) 25 (24.8) |
39 (56.5) 14 (20.3) 16 (23.2) |
17 (53.1) 6 (18.8) 9 (28.1) |
0.87 | |
|
| |||||
| Increase in Dependence or Disability | Can No Longer Get Outside—Spend All Day at Home
|
75 (74.3) 10 (9.9) 16 (15.8) |
50 (72.5) 8 (11.6) 11 (15.9) |
25 (78.1) 2 (6.3) 5 (15.6) |
0.70 |
|
| |||||
Need Someone to Help Take Care of Me All the Time
|
70 (69.3) 13 (12.9) 18 (17.8) |
45 (65.2) 10 (14.5) 14 (20.3) |
25 (78.1) 3 (9.4) 4 (12.5) |
0.42 | |
|
| |||||
Can No Longer Control Bladder/Bowels
|
65 (64.3) 15 (14.9) 21 (20.8) |
44 (63.8) 11 (15.9) 14 (20.3) |
21 (65.6) 4 (12.5) 7 (21.9) |
0.90 | |
|
| |||||
Can No Longer Walk but Get around in a Wheelchair
|
79 (78.2) 3 (3.0) 19 (18.8) |
53 (76.8) 2 (2.9) 14 (20.3) |
26 (81.3) 1 (3.1) 5 (15.6) |
0.86 | |
|
| |||||
Live in a Nursing Home Permanently
|
52 (51.5) 29 (28.7) 20 (19.8) |
36 (52.2) 22 (31.9) 11 (15.9) |
16 (50.0) 7 (21.9) 9 (28.1) |
0.30 | |
|
| |||||
| Severe Discomfort or Pain | Have Severe Discomfort Most of the Time (Nausea, Diarrhea)
|
70 (69.3) 12 (11.9) 19 (18.8) |
48 (69.6) 8 (11.6) 13 (18.8) |
22 (68.7) 4 (12.5) 6 (18.8) |
0.99 |
|
| |||||
Are in Severe Pain Most of the Time
|
61 (60.4) 19 (18.8) 21 (20.8) |
43 (62.3) 12 (17.4) 14 (20.3) |
18 (56.2) 7 (21.9) 7 (21.9) |
0.82 | |
|
| |||||
| Burden on Family | Can No Longer Contribute to Family’s Wellbeing
|
* 57 (57.0) 15 (15.0) 28 (28.0) |
** 41 (60.3) 11 (16.2) 16 (23.5) |
16 (50.0) 4 (12.5) 12 (37.5) |
0.35 |
|
| |||||
Are a Severe Financial/Emotional Burden on Family
|
* 50 (50.0) 17 (17.0) 33 (33.0) |
** 35 (51.5) 11 (16.2) 22 (32.3) |
15 (46.8) 6 (18.8) 11 (34.4) |
0.90 | |
|
| |||||
Rely on Breathing Machine to Keep Me Alive
|
49 (48.5) 27 (26.7) 25 (24.8) |
33 (47.8) 20 (29.0) 16 (23.2) |
16 (50.0) 7 (21.9) 9 (28.1) |
0.72 | |
N=100
N=68
Figure 1:
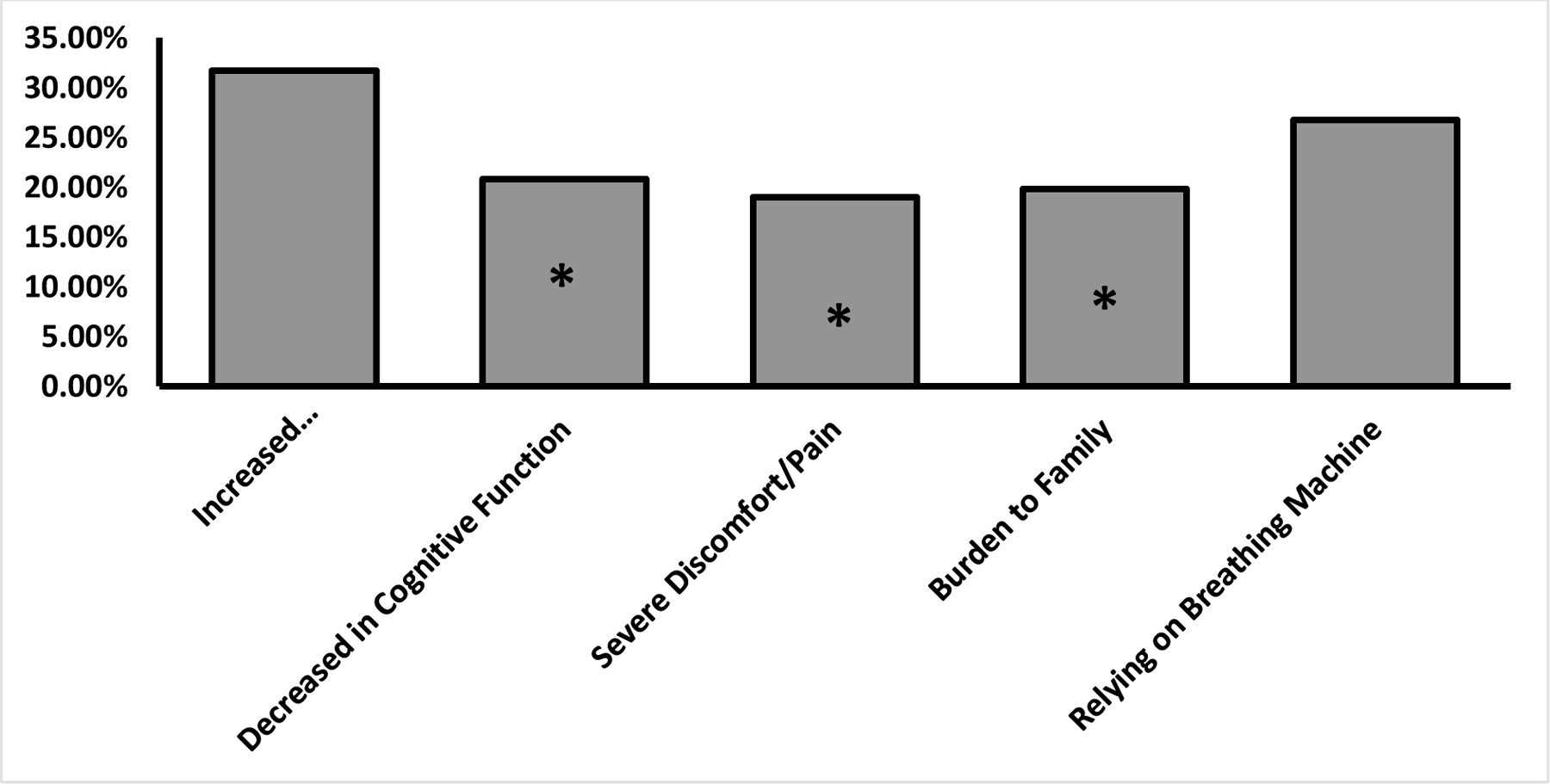
Proportion of Patients Responding “Not Worth Living” to at Least 1 Scenario in Each Theme. *P<0.05
Views on Aggressive Life-Sustaining Care
We evaluated respondents’ views on three questions about life-sustaining care (Table 5): 1) “I believe that it is always wrong to withhold treatments that could keep me alive,” 2) “I believe that it is always wrong to withdraw treatments that could keep me alive after they’ve been started,” and 3) I believe it is wrong to withhold nutrition and fluids given through tubes, even if I am terminally ill or in a permanent coma.” The percentage of patients who answered “yes” to the three questions were 78%, 71%, and 63%, respectively. We used bivariable and multivariable logistic regression models to further study factors associated with preference for life-sustaining care. In bivariable analysis, female sex (OR 0.37, 95% CI [0.15, 0.92]) and having more than 3 hospitalisations in the past year (OR 0.25, 95% CI [0.07, 0.84]) each decreased the likelihood of agreeing that it is always wrong to withdraw treatment. Age greater than 65 years (OR 0.34, 95% CI [0.15, 0.79]) decreased odds of reporting that withholding nutrition and fluids is wrong.
Table 5:
Associations between Views on Aggressive EOL Care and Co-Variates
| Scenario 1: Is It Always Wrong to Withhold Treatments That Could Keep Me Alive? | Scenario 2: Is It Always Wrong to Withdraw Treatments That Could Keep Me Alive After They’ve Been Started? | Scenario 3: Is It Wrong to Withhold Nutrition and Fluids Given through Tubes, Even If I Am Terminally Ill or in a Permanent Coma? | ||||
|---|---|---|---|---|---|---|
| Bivariable | Multivariable | Bivariable | Multivariable | Bivariable | Multivariable | |
| Co-Variates | OR [95% CI] | OR [95% CI] | OR [95% CI] | OR [95% CI] | OR [95% CI] | OR [95% CI] |
| Age | ||||||
| 18–65 Years | Reference | Reference | Reference | Reference | Reference | Reference |
| >65 Years | 0.41 [0.16, 1.06] | 0.53 [0.13, 2.25] | 0.67 [0.28, 1.61] | 0.59 [0.14, 2.49] | 0.34 [0.15, 0.79]* | 0.26 [0.07, 0.93]* |
| Gender | ||||||
| Male | Reference | Reference | Reference | Reference | Reference | Reference |
| Female | 0.45 [0.17, 1.18] | 0.22 [0.05, 0.94]* | 0.37 [0.15, 0.92]* | 0.19 [0.05, 0.75]* | 0.74 [0.33, 1.67] | 0.46 [0.14, 1.57] |
| Education | ||||||
| Less Than High School | Reference | Reference | Reference | Reference | Reference | Reference |
| High School/Some College | 0.70 [0.13, 3.69] | 0.15 [0.01, 2.55] | 0.76 [0.14, 4.01] | 0.07 [0.01, 1.74] | 0.74 [0.17, 3.24] | 0.24 [0.02, 2.99] |
| College Graduate or Above | 1.79 [0.27, 11.86] | 2.03 [0.13, 31.08] | 0.54 [0.09, 3.12] | 0.38 [0.02, 6.89] | 1.11 [0.23, 5.47] | 1.68 [0.14, 20.71] |
| Yearly Household Income | ||||||
| <$20,000 | Reference | Reference | Reference | Reference | Reference | Reference |
| $20,000–39,999 | 2.27 [0.57, 8.96] | 3.21 [0.47, 21.86] | 1.20 [0.37, 3.93] | 1.38 [0.27, 7.09] | 0.82 [0.27, 2.47] | 1.09 [0.27, 4.38] |
| >$40,000 | 1.28 [0.39, 4.17] | 0.85 [0.14, 5.28] | 0.80 [0.27, 2.40] | 0.68 [0.11, 4.06] | 0.40 [0.14, 1.15] | 0.12 [0.02, 0.71]* |
| Number of Hospitalizations in the Last Year (Not ED Visits) | ||||||
| None | Reference | Reference | Reference | Reference | Reference | Reference |
| 1 | 0.66 [0.19, 2.29] | 0.52 [0.10, 2.79] | 0.99 [0.28, 3.51] | 1.49 [0.25, 8.77] | 0.79 [0.27, 2.28] | 0.62 [0.14, 2.75] |
| 2–3 | 1.05 [0.27, 4.09] | 0.28 [0.03, 2.32] | 0.59 [0.18, 1.94] | 0.05 [0.01, 0.50]* | 2.07 [0.62, 6.86] | 1.80 [0.28, 11.62] |
| >3 | 0.33 [0.09, 1.19] | 0.19 [0.03, 1.31] | 0.25 [0.07, 0.84]* | 0.10 [0.02, 0.73]* | 0.68 [0.21, 2.19] | 0.80 [0.16, 4.07] |
| Time on Hemodialysis | ||||||
| <1 Year | Reference | Reference | Reference | Reference | Reference | Reference |
| 1–5 Years | 1.20 [0.31, 4.61] | 2.16 [0.27, 17.30] | 1.19 [0.34, 4.21] | 2.55 [0.29, 22.34] | 2.14 [0.63, 7.29] | 2.10 [0.35, 12.52] |
| >5 Years | 1.47 [0.37, 5.79] | 3.85 [0.41, 35.99] | 1.78 [0.48, 6.55] | 7.27 [0.69, 76.88] | 1.63 [0.48, 5.50] | 2.23 [0.36, 13.68] |
| FACIT Sp Subcategories | ||||||
| Emotional Wellbeing | 1.05 [0.95, 1.15] | 1.04 [0.82, 1.32] | 1.04 [0.95, 1.14] | 1.22 [0.96, 1.55] | 1.01 [0.92, 1.10] | 1.10 [0.89, 1.36] |
| Social Wellbeing | 0.98 [0.90, 1.07] | 0.95 [0.81, 1.12] | 1.01 [0.94, 1.10] | 0.98 [0.85, 1.13] | 1.01 [0.93, 1.09] | 1.07 [0.93, 1.23] |
| Functional Wellbeing | 1.01 [0.95, 1.09] | 0.93 [0.81, 1.07] | 1.00 [0.94, 1.06] | 0.93 [0.82, 1.06] | 1.00 [0.94, 1.06] | 0.98 [0.87, 1.11] |
| Physical Wellbeing | 1.01 [0.93, 1.10] | 0.98 [0.84, 1.14] | 0.97 [0.90, 1.06] | 0.89 [0.76, 1.04] | 0.96 [0.89, 1.04] | 0.91 [0.78, 1.05] |
| Spiritual Wellbeing | 1.04 [0.98, 1.10] | 1.09 [0.95, 1.24] | 1.03 [0.98, 1.08] | 1.02 [0.89, 1.16] | 1.04 [0.98, 1.09] | 1.00 [0.89, 1.12] |
| Had at Least One EOL Care Discussion with Healthcare Team? | ||||||
| No | Reference | Reference | Reference | Reference | Reference | Reference |
| Yes | 0.68 [0.26, 1.78] | 0.42 [0.10, 1.74] | 0.42 [0.17, 1.03] | 0.11 [0.02, 0.55]* | 0.74 [0.31, 1.76] | 0.54 [0.16, 1.84] |
| Would Like to Have EOL Discussions (New/Additional) with Healthcare Team? | ||||||
| No | Reference | Reference | Reference | Reference | Reference | Reference |
| Yes | 0.73 [0.28, 1.89] | 0.18 [0.03, 0.99]* | 0.44 [0.18, 1.06] | 0.08 [0.01, 0.49]* | 0.83 [0.36, 1.91] | 0.50 [0.13, 1.96] |
| Have Had EOL Discussions with Family? | ||||||
| No | Reference | Reference | Reference | Reference | Reference | Reference |
| Yes | 0.50 [0.19, 1.31] | 0.20 [0.05, 0.86]* | 0.77 [0.32, 1.85] | 0.55 [0.14, 2.16] | 0.59 [0.26, 1.35] | 0.24 [0.07, 0.81]* |
P<0.05
In multivariable analysis, identifying as female was associated with a decreased likelihood of responding “yes” to Scenarios 1 “wrong to withdraw” and 2 “wrong to withhold” (OR 0.22, 95% CI [0.05, 0.94] and OR 0.19, 95% CI [0.05, 0.75]), respectively. Previous EOL care experience with a healthcare provider (OR 0.11, 95% CI [0.02, 0.55]) and having had 2 to 3 or more than 3 hospitalisations in the past year (OR 0.05, 95% CI [0.01, 0.50] and OR 0.10, 95% CI [0.02, 0.73]) were associated with decreased likelihood of saying “yes” to Scenario 2. Additionally, age greater than 65 years (OR 0.26, 95% CI [0.07, 0.93]) and an income level of >$40,000 (OR 0.12, 95% CI [0.02, 0.71]) were associated with a lower likelihood of responding “yes” to Scenario 3, “wrong to withhold nutrition/fluid”. Patients who reported a desire to initiate or have additional EOL care discussions with a healthcare provider were significantly less likely to have responded “yes” to Scenarios 1 and 2 (OR 0.18, 95% CI [0.03, 0.99] and OR 0.08, 95% CI [0.01, 0.49]). Lastly, prior experience discussing EOL care wishes with family was associated with a decreased likelihood of answering “yes” to Scenarios 1 and 3 (OR 0.20, 95% CI [0.05, 0.86] and OR 0.24, 95% CI [0.07, 0.81]).
Discussion
In our study of urban African American patients receiving haemodialysis, we found low rates of ACP discussions with healthcare providers. Prior work has found similarly low rates among both African American and White patients with ESRD (Davison, 2006). Furthermore, dialysis unit professionals have reported ACP as a significant unmet need among patients with ESRD (Culp et al., 2016; O’Hare et al., 2016; Sharma et al., 2017). Thus, it was not surprising that more than 90% of patients with no ACP experience in our study stated that they were never approached by a healthcare provider.
Interestingly, only 36.7% of “never-approached” patients expressed any desire to discuss ACP their healthcare team. This was unexpected because most patients receiving haemodialysis, including African Americans, look forward to discussing their preferences for care and assume that their nephrologists have a timely plan on introducing ACP (Yancu et al., 2015; Ladin et al., 2018). Yet, this may be explained by findings that many patients with advanced kidney disease are optimistic about their prognosis and, consequently, are less likely to participate in ACP and more likely to engage in intensive treatments (O’Hare et al., 2019). Indeed, we found that patients without ACP experience with providers had significantly higher scores on several measures of self-reported wellbeing, including the total FACIT-Sp as well as the emotional and functional wellbeing domains, when compared to patients with ACP experience. This difference could indicate that patients in our study without EOL care experience may feel that they are healthy and thus have no need for ACP, despite the poor prognosis that a diagnosis of ESRD portends.
Among the patients who have discussed ACP with healthcare providers, only 15.6% reported speaking regularly about ACP with their team and only 38.7% wanted additional discussions. When ACP is done appropriately, conversations occur with family members, take place on multiple occasions over time, and lead to greater concordance between patients’ values and their medical care (Ramer et al., 2018; Oskoui et al., 2020). However, there is evidence that very few EOL discussions that take place among CKD patients exhibit features of quality ACP, including talking about personal values and implications of treatment options on daily life (Culp et al., 2016; Oskoui et al., 2020). Of note, only two patients in our study reported speaking about the topic of stopping haemodialysis in their discussions with providers. In addition, among the patients who have discussed EOL care with healthcare providers, less than 75% had spoken to their family about their wishes, and only 15.6% reported speaking about EOL care with their team regularly. As a result, it is possible that many patients in our study do not want to discuss EOL care additionally due to the inadequate ACP. Alternatively, patients just may not want to talk about ACP issues, especially when discussions may be taking place in an environment where there is very little patient privacy during haemodialysis treatments.
The majority of patients in our study reported that they believe withholding life-extending treatments is always wrong, consistent with prior work demonstrating that African Americans tend to prefer aggressive life-extending care (Johnson et al., 2008; Wicher and Meeker, 2012; Foley et al., 2018). However, we found that patients with prior ACP discussions either with family or healthcare providers were less likely to answer that refusing life-extending care is always wrong. For African American patients receiving haemodialysis, speaking about their core values and EOL care preferences with family and healthcare providers may provide them with the knowledge and clarity to reconsider whether pursuing aggressive life-sustaining care is truly concordant with their wishes.
When presented with a dozen common advanced illness scenarios, the majority of patients found nearly all of them to be worth living through. However, for scenarios involving increased disability or dependence, a significantly higher proportion of patients responded “not worth living” compared to those involving decreased cognitive function, severe pain or discomfort, and negative impact of illness on family members. Thus, our results support several studies in predominantly White participants on haemodialysis that identified maintaining independence as an important health priority (Ramer et al., 2018; Oskoui et al., 2020). However, other categories were also important. For example, a similarly low proportion of patients reported that being a “severe financial/emotional burden on family” (50%) and “rely on a breathing machine to keep me alive” (48.5%) were worth living through. Framing future ACP discussions in a way that can focus more on the deleterious impact of life-extending care on patients’ independence and family wellbeing could be more concordant with the values and priorities of the African American patients in this study. This is consistent with research findings recommending that ACP for dialysis patients should be restructured to be more centred on topics relevant to daily living, such as impact on ability to work (Urquhart-Secord et al., 2016; Ladin et al., 2018).
Our study had several strengths. This is one of the few studies that delved broadly into EOL care views and experiences of African American patients receiving haemodialysis. Our survey instrument included a wide range of questions that captured prior ACP experiences and views on EOL scenarios and life-sustaining treatments. Second, the patient participation rate was high, which limits selection bias. Third, in-person survey administration allowed participants to ask for clarifications and have the survey read to them, which helped reduce response bias.
We also report several limitations to this research. First, the study population was comprised of African American patients from three clinics from a university-affiliated, national dialysis chain on the South Side of Chicago. As a result, this reduces generalisability. Second, we did not verify existence of ACP documents, such as advance directives and power of attorney forms, in patients’ medical records. While the practise at these units is for all new patients to receive ACP information, health literacy has been found to be a stronger predictor of ACP knowledge than prior ACP experience (Nouri et al., 2019). As a result, patients may have misunderstood what ACP discussions entailed or forgotten about prior discussions, especially if they occurred long ago, when answering the surveys. However, the researcher (DA) spent much time explaining what ACP is, describing commonly discussed topics, and answering questions about what counted as ACP. Third, patients’ responses to questions about views on EOL scenarios and life-sustaining treatments may not reflect how they would respond if they did encounter such situations. Finally, although the ACP scenarios used were from a rigorously developed ACP workbook, the psychometric properties were not publicly available, nor have they been validated in an African American population.
Implications for Practise
A significant finding of our study is that nearly 40% of patients who had never discussed EOL care topics with their providers were willing to participate in ACP. Given that such a significant number of patients are missing out on the benefits of ACP, providers should be more proactive about engaging patients rather than waiting for acute changes in medical condition or for patients to express interest. Moreover, to improve low rates of ACP, EOL care discussions can be mandated or incentivised to overcome clinical inertia and competing clinical demands. A possible solution is to make ACP discussions and documentation a reported quality metric in the Centers for Medicare and Medicaid Services’ evaluations of dialysis clinics.
However, one issue is that dialysis care providers are often uncertain about who has the responsibility and authority to conduct ACP (O’Hare et al., 2016). A potential workflow would be for front-line clinical staff such as dialysis technicians to first gauge interest in ACP because they have the most direct and prolonged contact with patients and frequently have close relationships with them (Patient Care Technicians at the Dialysis Center, no date). If interested, patients can be referred to their nephrologists, social workers, or advance care providers to participate in ACP formally. For ACP to work successfully, providers may need additional training with the understanding that there are barriers to ACP among African American patients, such as medical mistrust, conflicts with spirituality, and lack of knowledge (Collins et al., 2018; Berzoff et al., 2020). A few studies have recommended including faith-based community leaders, avoiding medical jargon, and allowing for time to build and maintain trustworthy relationships with providers to help African American patients suffering from chronic illnesses have more meaningful ACP (Rhodes et al., 2015, 2017). Our study indicates that focused discussions about how aggressive life-extending treatments can negatively impact patients’ independence and familial wellbeing can be important additions in ACP with African American patients with ESRD.
Conclusion
In sum, our findings show that African American patients receiving haemodialysis have low rates of EOL care discussions with healthcare providers, and many believe that withholding life-sustaining treatments at the EOL is wrong. However, ACP experience with family members and healthcare providers may be associated with a decreased preference for life-extending care, and ACP that is focused on maintaining independence and reducing disability may enrich EOL care conversations among African Americans with ESRD. Future research and clinical innovation should focus on engaging racial and ethnic minority patients on haemodialysis in frequent, high-quality ACP.
ACKNOWLEDGEMENTS:
The authors would like to thank the patients who generously participated in this study. We would also like to thank the staff of the Woodlawn, Kenwood, and Stony Island DaVita Dialysis Clinics for their cooperation during data collection. Finally, we would like to thank Christina Zhou and Akilah King, MSW, for their assistance with data acquisition.
Support: This work was funded by the National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK K23 DK103111). Mr. Ahn was supported by the National Kidney Foundation of Illinois Medical Student Award and the University of Chicago Pritzker School of Medicine Summer Research Program. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, and approval of the manuscript.
Footnotes
Financial Disclosure: The authors declare that they have no relevant financial interests or other conflicts of interest.
Prior Presentation: This work was presented at the National Kidney Foundation of Illinois Citywide Grand Rounds in Chicago, IL in September 2019.
References
- Berzoff J et al. (2020) ‘Advance Care Planning Training for Renal Social Workers’, Journal of Social Work in End-of-Life & Palliative Care, 16(1), pp. 5–18. doi: 10.1080/15524256.2020.1721396. [DOI] [PubMed] [Google Scholar]
- Bito S et al. (2007) ‘Acculturation and End-of-Life Decision Making: Comparison of Japanese and Japanese-American Focus Groups’, Bioethics, 21(5), pp. 251–262. doi: 10.1111/j.1467-8519.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- Cella DF et al. (1993) ‘The Functional Assessment of Cancer Therapy scale: development and validation of the general measure.’, Journal of Clinical Oncology, 11(3), pp. 570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- Chronic Kidney Disease Prognosis Consortium (2010) ‘Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis’, The Lancet, 375(9731), pp. 2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins JW et al. (2018) ‘Cultural Aspects of End-of-Life Care Planning for African Americans: An Integrative Review of Literature’, Journal of Transcultural Nursing, 29(6), pp. 578–590. doi: 10.1177/1043659617753042. [DOI] [PubMed] [Google Scholar]
- Crawley L et al. (2000) ‘Palliative and End-of-Life Care in the African American Community’, JAMA, 284(19), pp. 2518–2521. doi: 10.1001/jama.284.19.2518. [DOI] [PubMed] [Google Scholar]
- Culp S et al. (2016) ‘Unmet Supportive Care Needs in U.S. Dialysis Centers and Lack of Knowledge of Available Resources to Address Them’, Journal of Pain and Symptom Management, 51(4), pp. 756–761.e2. doi: 10.1016/j.jpainsymman.2015.11.017. [DOI] [PubMed] [Google Scholar]
- Davison SN (2006) ‘Facilitating Advance Care Planning for Patients with End-Stage Renal Disease: The Patient Perspective: Table 1.’, Clinical Journal of the American Society of Nephrology, 1(5), pp. 1023–1028. doi: 10.2215/CJN.01050306. [DOI] [PubMed] [Google Scholar]
- Elkan R et al. (2007) ‘The reported views and experiences of cancer service users from minority ethnic groups: a critical review of the literature’, European Journal of Cancer Care, 16(2), pp. 109–121. doi: 10.1111/j.1365-2354.2006.00726.x. [DOI] [PubMed] [Google Scholar]
- Eneanya ND et al. (2016) ‘Racial Disparities in End-of-Life Communication and Preferences among Chronic Kidney Disease Patients’, American Journal of Nephrology, 44(1), pp. 46–53. doi: 10.1159/000447097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley RN et al. (2018) ‘Race, Ethnicity, and End-of-Life Care in Dialysis Patients in the United States’, Journal of the American Society of Nephrology, 29(9), pp. 2387–2399. doi: 10.1681/ASN.2017121297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ‘Functional Assessment of Chronic Illness Therapy-Spiritual Wellbeing (FACIT-Sp) Version 4’ (no date). FACIT.org. Available at: https://www.facit.org/FACITOrg/Questionnaires. [Google Scholar]
- Gutheil IA and Heyman JC (2006) ‘“They Don’t Want to Hear Us”: Hispanic Elders and Adult Children Speak about End-of-Life Planning’, Journal of Social Work in End-of-Life & Palliative Care, 2(1), pp. 55–70. doi: 10.1300/J457v02n01_05. [DOI] [PubMed] [Google Scholar]
- Hanchate A et al. (2009) ‘Racial and Ethnic Differences in End-of-Life Costs: Why Do Minorities Cost More Than Whites?’, Archives of Internal Medicine, 169(5), p. 493. doi: 10.1001/archinternmed.2008.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KS et al. (2008) ‘Racial Differences in Hospice Revocation to Pursue Aggressive Care’, Archives of Internal Medicine, 168(2), pp. 218–224. doi: 10.1001/archinternmed.2007.36. [DOI] [PubMed] [Google Scholar]
- Karim K, Bailey M and Tunna K (2016) ‘Nonwhite ethnicity and the provision of specialist palliative care services: factors affecting doctors’ referral patterns’, Palliative Medicine doi: 10.1191/026921600701536390. [DOI] [PubMed] [Google Scholar]
- Koffman J and Higginson IJ (2016) ‘Accounts of carers’ satisfaction with health care at the end of life: A comparison of first generation black Caribbeans and white patients with advanced disease’, Palliative Medicine doi: 10.1191/026921601678320322. [DOI] [PubMed] [Google Scholar]
- Kurella Tamura M et al. (2009) ‘Functional Status of Elderly Adults before and after Initiation of Dialysis’, New England Journal of Medicine, 361(16), pp. 1539–1547. doi: 10.1056/NEJMoa0904655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak J and Salmon JR (2007) ‘Attitudes and Preferences of Korean-American Older Adults and Caregivers on End-of-Life Care’, Journal of the American Geriatrics Society, 55(11), pp. 1867–1872. doi: 10.1111/j.1532-5415.2007.01394.x. [DOI] [PubMed] [Google Scholar]
- Ladin K et al. (2018) ‘“End-of-Life Care? I’m not Going to Worry About That Yet.” Health Literacy Gaps and End-of-Life Planning Among Elderly Dialysis Patients’, The Gerontologist Edited by Bowers BJ, 58(2), pp. 290–299. doi: 10.1093/geront/gnw267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdoo SP et al. (2012) ‘Measuring the quality of end of life management in patients with advanced kidney disease: results from the pan-Thames renal audit group’, Nephrology Dialysis Transplantation, 27(4), pp. 1548–1554. doi: 10.1093/ndt/gfr514. [DOI] [PubMed] [Google Scholar]
- McClellan WM et al. (2010) ‘Physical and Psychological Burden of Chronic Kidney Disease among Older Adults’, American Journal of Nephrology, 31(4), pp. 309–317. doi: 10.1159/000285113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskulin D et al. (2009) ‘Key Comorbid Conditions that Are Predictive of Survival among Hemodialysis Patients’, Clinical Journal of the American Society of Nephrology, 4(11), pp. 1818–1826. doi: 10.2215/CJN.00640109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muni S et al. (2011) ‘The Influence of Race/Ethnicity and Socioeconomic Status on End-of-Life Care in the ICU’, Chest, 139(5), pp. 1025–1033. doi: 10.1378/chest.10-3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouri SS et al. (2019) ‘Health Literacy Matters More Than Experience for Advance Care Planning Knowledge Among Older Adults’, Journal of the American Geriatrics Society, 67(10), pp. 2151–2156. doi: 10.1111/jgs.16129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hare AM et al. (2016) ‘Provider Perspectives on Advance Care Planning for Patients with Kidney Disease: Whose Job Is It Anyway?’, Clinical Journal of the American Society of Nephrology, 11(5), pp. 855–866. doi: 10.2215/CJN.11351015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hare AM et al. (2019) ‘Assessment of Self-reported Prognostic Expectations of People Undergoing Dialysis: United States Renal Data System Study of Treatment Preferences (USTATE)’, JAMA Internal Medicine, 179(10), pp. 1325–1333. doi: 10.1001/jamainternmed.2019.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskoui T et al. (2020) ‘Advance Care Planning Among Older Adults With Advanced Non–Dialysis-Dependent CKD and Their Care Partners: Perceptions Versus Reality?’, Kidney Medicine, 2(2), pp. 116–124. doi: 10.1016/j.xkme.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens A and Randhawa G (2004) ‘“It’s different from my culture; they’re very different”: providing community-based, “culturally competent” palliative care for South Asian people in the UK’, Health & Social Care in the Community, 12(5), pp. 414–421. doi: 10.1111/j.1365-2524.2004.00511.x. [DOI] [PubMed] [Google Scholar]
- Patient Care Technicians at the Dialysis Center (no date). Available at: https://www.davita.com/treatment-services/dialysis/in-center-hemodialysis/patient-care-technicians-at-the-dialysis-center (Accessed: 22 May 2020).
- Patrick DL et al. (1994) ‘Measuring Preferences for Health States Worse than Death’, Medical Decision Making, 14(1). doi: 10.1177/0272989X9401400102. [DOI] [PubMed] [Google Scholar]
- Pearlman RA et al. (1993) ‘Insights Pertaining to Patient Assessments of States Worse Than Death’, Journal of Clinical Ethics, 4(1), pp. 33–41. [PubMed] [Google Scholar]
- Pearlman RA et al. (2005) ‘Improvements in Advance Care Planning in the Veterans Affairs System: Results of a Multifaceted Intervention’, Archives of Internal Medicine, 165(6), p. 667. doi: 10.1001/archinte.165.6.667. [DOI] [PubMed] [Google Scholar]
- Perry E et al. (2005) ‘Peer Mentoring: A Culturally Sensitive Approach to End-of-Life Planning for Long-Term Dialysis Patients’, American Journal of Kidney Diseases, 46(1), pp. 111–119. doi: 10.1053/j.ajkd.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Peterman AH et al. (2002) ‘Measuring spiritual well-being in people with cancer: The functional assessment of chronic illness therapy—spiritual well-being scale (FACIT-Sp)’, Annals of Behavioral Medicine, 24(1), pp. 49–58. doi: 10.1207/S15324796ABM2401_06. [DOI] [PubMed] [Google Scholar]
- Ramer SJ et al. (2018) ‘Health Outcome Priorities of Older Adults with Advanced CKD and Concordance with Their Nephrology Providers’ Perceptions’, Journal of the American Society of Nephrology, 29(12), pp. 2870–2878. doi: 10.1681/ASN.2018060657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes RL et al. (2015) ‘Barriers to End-of-Life Care for African Americans From the Providers’ Perspective: Opportunity for Intervention Development’, American Journal of Hospice and Palliative Medicine®, 32(2), pp. 137–143. doi: 10.1177/1049909113507127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes RL et al. (2017) ‘The Desires of Their Hearts: The Multidisciplinary Perspectives of African Americans on End-of-Life Care in the African American Community’, American Journal of Hospice and Palliative Medicine®, 34(6), pp. 510–517. doi: 10.1177/1049909116631776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlman Robert et al. (2010) Your Life, Your Choices: Planning for Future Medical Decisions [Google Scholar]
- Sharma RK et al. (2017) ‘Association of Racial Differences With End-of-Life Care Quality in the United States’, JAMA Internal Medicine, 177(12), pp. 1858–1860. doi: 10.1001/jamainternmed.2017.4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M-K et al. (2016) ‘Racial Differences in Outcomes of an Advance Care Planning Intervention for Dialysis Patients and Their Surrogates’, Journal of Palliative Medicine, 19(2), pp. 134–142. doi: 10.1089/jpm.2015.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam-Tham H et al. (2020) ‘Association of Initiation of Dialysis With Hospital Length of Stay and Intensity of Care in Older Adults With Kidney Failure’, JAMA Network Open, 3(2). doi: 10.1001/jamanetworkopen.2020.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The United States Renal Data Service (2018) 2018 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. [Google Scholar]
- Torke AM et al. (2005) ‘Medical Care at the End of Life: Views of African American Patients in an Urban Hospital’, Journal of Palliative Medicine, 8(3), pp. 593–602. doi: 10.1089/jpm.2005.8.593. [DOI] [PubMed] [Google Scholar]
- Urquhart-Secord R et al. (2016) ‘Patient and Caregiver Priorities for Outcomes in Hemodialysis: An International Nominal Group Technique Study’, American Journal of Kidney Diseases, 68(3), pp. 444–454. doi: 10.1053/j.ajkd.2016.02.037. [DOI] [PubMed] [Google Scholar]
- Van Dorn RA, Swanson JW and Swartz MS (2009) ‘Preferences for Psychiatric Advance Directives Among Latinos: Views on Advance Care Planning for Mental Health’, Psychiatric Services, 60(10), pp. 1383–1385. doi: 10.1176/ps.2009.60.10.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Scoy LJ et al. (2020) ‘Association of Participation in an End-of-Life Conversation Game With Advance Care Planning Behavior and Perspectives Among African American Individuals’, JAMA Network Open, 3(5), pp. e204315–e204315. doi: 10.1001/jamanetworkopen.2020.4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vries K. de et al. (2019) ‘Advance care planning for older people: The influence of ethnicity, religiosity, spirituality and health literacy’:, Nursing Ethics doi: 10.1177/0969733019833130. [DOI] [PubMed] [Google Scholar]
- Weiner S (2010) ‘End-of-Life Care Discussions: A Survey of Dialysis Patients and Professionals’, Journal of Pain and Symptom Management, 39(2), pp. 395–396. doi: 10.1016/j.jpainsymman.2009.11.129. [DOI] [Google Scholar]
- Weisbord SD et al. (2003) ‘Symptom burden, quality of life, advance care planning and the potential value of palliative care in severely ill haemodialysis patients’, Nephrology Dialysis Transplantation, 18(7), pp. 1345–1352. doi: 10.1093/ndt/gfg105. [DOI] [PubMed] [Google Scholar]
- Welch LC, Teno JM and Mor V (2005) ‘End-of-Life Care in Black and White: Race Matters for Medical Care of Dying Patients and their Families’, Journal of the American Geriatrics Society, 53(7), pp. 1145–1153. doi: 10.1111/j.1532-5415.2005.53357.x. [DOI] [PubMed] [Google Scholar]
- Wicher CP and Meeker MA (2012) ‘What Influences African American End-of-life Preferences?’, Journal of Health Care for the Poor and Underserved, 23(1), pp. 28–58. doi: 10.1353/hpu.2012.0027. [DOI] [PubMed] [Google Scholar]
- Worth A et al. (2009) ‘Vulnerability and access to care for South Asian Sikh and Muslim patients with life limiting illness in Scotland: prospective longitudinal qualitative study’, BMJ, 338. doi: 10.1136/bmj.b183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancu CN et al. (2015) ‘Accepting Transitions: African Americans Discuss End of Life’, American Journal of Hospice and Palliative Medicine®, 32(4), pp. 380–387. doi: 10.1177/1049909114528567. [DOI] [PubMed] [Google Scholar]


