Abstract
Background
Although advanced parental age has been definitively linked to pediatric acute lymphoblastic leukemia, studies of parental age and pediatric solid tumors have not reached firm conclusions. This analysis aimed to elucidate the relationship between parental age and pediatric solid tumors through meta-analysis of existing studies based in population registries.
Methods
We searched Medline (PubMed) and Embase for registry-based studies of parental age and solid tumors through March 2022. We performed random-effects meta-analysis to estimate pooled effects and 95% confidence intervals (CIs). All statistical tests were 2-sided.
Results
A total of 15 studies covering 10 childhood solid tumor types (30 323 cases and 3 499 934 controls) were included in this analysis. A 5-year increase in maternal age was associated with an increased risk of combined central nervous system tumors (odds ratio [OR] = 1.07, 95% CI = 1.04 to 1.10), ependymoma (OR = 1.19, 95% CI = 1.09 to 1.31), astrocytoma (OR = 1.10, 95% CI = 1.05 to 1.15), rhabdomyosarcoma (OR = 1.14, 95% CI = 1.03 to 1.25), and germ cell tumors (OR = 1.06, 95% CI = 1.00 to 1.12). A 5-year increase in paternal age was associated with an increased risk of non-Hodgkin lymphoma (OR = 1.06, 95% CI = 1.00 to 1.12).
Conclusions
This meta-analysis of registry-based analyses of parental age and childhood cancer supports the association between older maternal age and certain childhood solid cancers. There is also some evidence that paternal age may be associated with certain cancers such as non-Hodgkin lymphoma. However, as maternal and paternal age are highly correlated, disentangling potential independent causal effects of either factor will require large studies with extensive data on potential confounders.
In the last several decades, there has been a trend toward delayed childbearing across the globe (1). This has been attributed to a number of factors including higher educational attainment, women working outside the home, improved contraception methods, and access to assisted reproductive technologies (1). Within the United States, this rise in age is seen in both men and women and across races and ethnicities and geographic regions (2). Delaying childbearing can benefit families individually through greater socioeconomic attainment, however, the association between older parental age and adverse perinatal outcomes such as Down syndrome, preterm birth, and perinatal and neonatal death, among others, is well established in the literature (1,3).
Previous studies have shown a clear link between older parental age and acute lymphoblastic leukemia (ALL), the most common childhood cancer. The Childhood Cancer and Leukemia International Consortium (CLIC) recently published on this association in a pooled analysis, stratifying by original study type (questionnaire-based case-control studies vs registry-based linkage studies) (4). They found a positive association between both older maternal (odds ratio [OR] = 1.05, 95% confidence interval [CI] = 1.01 to 1.08) and paternal age (OR = 1.04, 95% CI = 1.01 to 1.07) per 5-year increase but only in the registry-based studies. This discrepancy likely reflects selection bias in participation in questionnaire-based case-control studies. CLIC has also investigated this association in acute myeloid leukemia (AML), again stratifying by original study design (5). They found a strong association (OR = 6.87, 95% CI = 2.12 to 22.25) between advanced maternal age and AML in children aged younger than 1 year, again only in the registry-based studies. There were no associations found for paternal age or in AML risk among older children (1-14 years).
The association between parental age and other childhood cancers has been studied without any firm conclusions (6-9). This likely results from limitations in study sizes among these rarer solid childhood tumors and, again, the likelihood of selection bias in questionnaire-based case-control studies. We planned to overcome these limitations by conducting a thorough literature review and meta-analysis of the current literature on the association between parental age and solid tumors in children focusing only on population-based studies to minimize bias.
Methods
Search Strategy
We searched for English-language publications in Medline (PubMed) and Embase through March 2022. We used the MeSH terms “neoplasms,” “maternal age,” “paternal age,” and “registries,” along with text words “child*,” “pediatric,” “paediatric,” “neoplas*,” “malignan*,” “cancer*,” “tumor*,” “sarcoma,” “lymphoma,” “maternal,” “paternal,” “parent*,” “age*,” “characteristic*,” “register*,” “registry,” and “registries.”
Finally, we also included the text words “leukemia” and “leukaemia” to capture any studies that only focused descriptions of their findings on these more common types of cancer but also presented other cancer types within their articles.
Study Selection
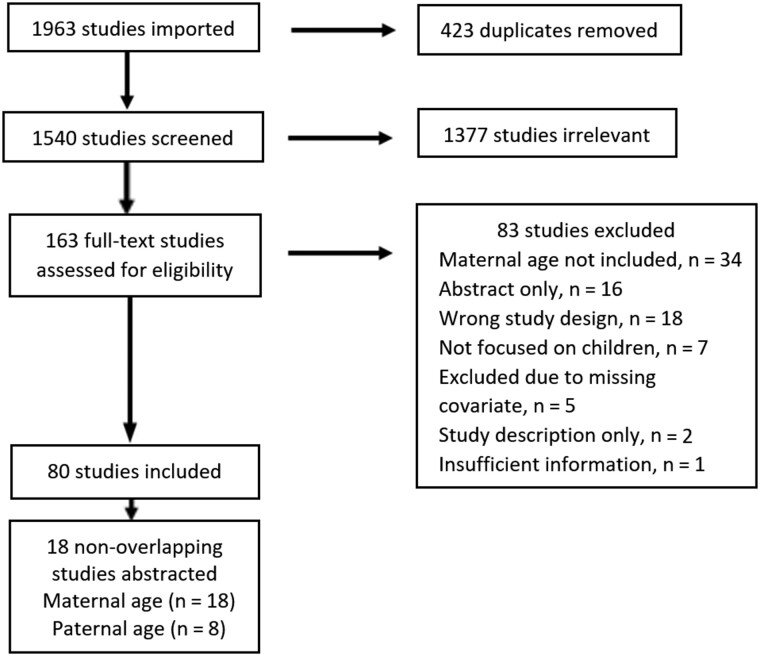
For inclusion, we required that articles used data derived from registry-based or birth certificate data and be published in peer-reviewed journals. Only articles that examined childhood (ages 0-19 years) cancer, with effect estimates for maternal or paternal age, or including information from which a crude estimate could be determined, were included. One author (KM) screened all titles, abstracts, and full-text publications based on these criteria and abstracted relevant effect estimates. Figure 1 outlines our search and selection process.
Figure 1.
Search results and study selection flowchart.
Many of the articles used similar datasets or individual datasets that had been pooled. We allowed for small overlap (1 year or less) between studies in similar or overlapping populations to minimize double-counting participants. As one author (LS) was an original investigator of the 5-state pooled dataset described in Johnson et al. (8), odds ratios were re-estimated excluding California data so as to allow for inclusion of the larger Wang et al. (10) study without double-counting some participants.
Data Extraction and Quality Assessment
Two authors (KM, AD) extracted descriptive information from the methods of each publication including International Classification of Childhood Cancer, Third Edition (ICCC-3) grouping, ICCC-3 subgrouping if applicable, year of publication, population characteristics, age range for cancer diagnoses, and time frame for cancer diagnoses. The maximally adjusted effect estimate, as well as adjustment factors, were extracted from each study. Data was presented in multiple ways in the manuscripts included. When available, estimates associated with 5-year increases in maternal or paternal age were extracted. If age categories were presented, estimates from all categories were recorded. Any discrepancies in data extraction were reviewed by a third author (EM).
Synthesis of Data and Analysis
In studies that only included categorical estimates, these age categories were collapsed using methods described in Greenland and Longnecker (11) and Berlin, Longnecker, and Greenland (12). Briefly, all participants within each age category were given the midpoint age within that group. In the oldest age category, participants were assigned an age of 1.2 times that of the lower endpoint age and, in the youngest age category, the mean value between the upper endpoint and a value that was determined to be a possible lower bound—in this case age 12 years. We then determined the odds ratio for a 5-year increase in parental age.
For each childhood cancer grouping, the effect of a 5-year increase in age was estimated using random-effects meta-analysis (13). Where possible, the analyses were conducted by ICCC-3 subgroup, but as certain studies only classified cases by broader diagnostic groups, some analyses were conducted by both levels. For example, central nervous system (CNS) tumors were analyzed in the broader CNS group as well as in individual subgroups such as ependymoma and astrocytoma. In these random-effects meta-analysis models, the weights given to each study are inversely related to the total variance within that study. We additionally estimated the effect of a 5-year increase in parental age on all cancers included together to assess the overall effect of delayed childbearing on childhood cancer. For each cancer category, random-effects models were used to estimate summary odds ratios and 95% confidence intervals. Statistical significance of a summary effect was assessed using these confidence intervals to determine if the effect was statistically significantly different from the null. We used the I2 statistic, a measure of heterogeneity ranging from 0% to 100% and corresponding P value to assess between-study heterogeneity (Pheterogeneity). Publication bias was assessed by funnel plot asymmetry and Egger test for small study effects. All tests were 2-sided with P values of less than .05 indicating statistical significance.
Several studies included in this analysis only provided crude estimates of the association between parental age and cancer risk. To assess the effect that these studies had on the meta-analysis, the analysis was reconducted excluding these crude estimates.
Additionally, as birth records are often missing paternal age (14), a sensitivity analysis was conducted to estimate the effect of missing paternal age on results. This sensitivity analysis was conducted using the 5-state pooling data from the Johnson et al. (8) study, one of the larger studies in this analysis, by assigning fathers with missing ages to the youngest and oldest age categories to determine the bounds within which the true effect estimate might lie.
Data analysis was conducted using Stata (version 16.1).
Results
Our initial search yielded 1540 nonduplicate records from Medline and Embase (Figure 1). We conducted a review of titles and abstracts, screening for manuscripts that either directly examined the association between parental age and childhood cancer and for manuscripts that may contain sufficient data in tables allowing for calculation of crude odd ratios. A total of 163 records were deemed eligible for a full-text review. Of these studies, 83 were excluded during full-text review, and 80 studies were identified for further review. From these studies, we analyzed those with nonoverlapping dates, regions, and tumor types of inclusion. If multiple studies were conducted in the same dataset, we chose the study that presented the most fully adjusted model or that had the most recent date of publication. Estimates of associations were abstracted from 18 publications covering 10 childhood solid tumor types and 33 847 cases and 3 675 858 controls (Table 1). Each study’s quality was assessed using the Newcastle-Ottawa Scale (NOS) (15). The NOS rates study quality based on selection of cases and controls, the comparability of cases and controls, and exposure ascertainment and has a maximum score of 9. We considered a study high quality if it had a score of 7 or higher. Using this criterion, all included studies with adjusted effect estimates were considered high quality. The average NOS of all studies included was 7.2.
Table 1.
Characteristics of studies on parental age and solid childhood cancers
| Study | Study population | Parental age variable | Odds ratio available | Adjustment variables | Cancer type(s) | No. of controls |
|---|---|---|---|---|---|---|
| Contreras ZA et al. (16) |
|
Maternal, paternal | Adjusted | Parental place of birth, parity, other parent’s age | Lymphoma (n = 578), CNS (n = 1548), neuroblastoma (n = 346), retinoblastoma (n = 163), renal tumor (n = 293), hepatic tumor (n = 73), bone tumor (n = 266), soft tissue sarcoma (n = 342), germ cell tumor (n = 166), other/nonspecific (n = 342) | 585 594 |
| Johnson KJ et al. (8) |
|
Maternal, paternal | Adjusted | Maternal race, sex, birthweight, gestational age, birth order, birth year category, plurality, state, other parent’s age | Lymphoma (n = 1248), CNS (n = 2863), neuroblastoma (n = 993), retinoblastoma (n = 399), renal tumor (n = 776), hepatic tumor (n = 181), bone tumor (n = 492), soft tissue sarcoma (n = 810), germ cell tumor (n = 395) | 49 236 |
| Wang R et al. (10) |
|
Maternal, paternal | Adjusted | Other parent’s age, birth weight, length of gestation, birth order, maternal country of birth, maternal smoking during pregnancy | Lymphoma (n = 2760), CNS (n = 4582), neuroblastoma (n = 1233), retinoblastoma (n = 590), renal tumor (n = 1006), hepatic tumor (n = 327), bone tumor (n = 1020), soft tissue sarcoma (n = 1488), germ cell tumor (n = 1450), other/nonspecific (n = 1604) | 87 593 |
| Petridou et al. (17) |
|
Maternal | Adjusted | Sex, maternal education, gestational age, birth order | Lymphoma (n = 684) | 2 334 346 |
| Wong and Dockerty (18) |
|
Maternal, paternal | Adjusted | Parity, social class, marital status, other parent’s age, urban or nonurban status | Lymphoma (n = 236) | 585 |
| Cantwell MM et al. (19) |
|
Maternal, paternal | Crude | — | CNS (n = 155) | 420 436 |
| Danysh HE et al. (20) |
|
Maternal | Crude | — | CNS (n = 315) | 1575 |
| de Paula Silva N et al. (21) |
|
Maternal | Crude | — | CNS (n = 119), other/nonspecific (n = 221) | 1580 |
| Bluhm E et al. (22) |
|
Maternal | Conditional | Sex, birth year and month | Neuroblastoma (n = 245) | 1225 |
| Kumar SV et al. (23) |
|
Maternal | Crude | — | Neuroblastoma (n = 252), retinoblastoma (n = 121)a, renal tumor (n = 143), hepatic tumor (n = 55)a | 2855 |
| Schuz J et al. (24) | Denmark, Norway, Sweden (diagnosed 1985-2006), Finland (diagnosed 1987-2006) | Maternal, paternal | Conditional | Birth month and year, sex, and country | Renal tumor (n = 690) | 3298 |
| de Fine Licht S et al. (26) |
|
Maternal | Conditional | Sex, age, country | Hepatic tumor (n = 155) | 775 |
| Troisi R et al. (27) | Norway (birth years 1970-2009), Sweden (birth years 1974-2009), Denmark (birth years 1980-2010); younger than 43 years | Maternal | Conditional | Birth year and sex | Bone tumor (n = 510) | 9140 |
| Ghali MH et al. (28) |
|
Maternal, paternal | Crude | — | Soft tissue sarcoma (n = 103) | 205 |
| Stephansson O et al. (29) |
|
Maternal | Adjusted | Birth weight, gestational age, and parity | Germ cell tumor (n = 152) | 1491 |
| Fahmideh et al. (25) |
|
Maternal | Adjusted | Birth year, sex, maternal race and ethnicity, maternal education, tumor malignancy | CNS (n = 217) | 2170 |
| Lombardi et al. (30) |
|
Maternal | Crude | — | CNS (n = 157) | 123 154 |
| Deziel et al. (31) |
|
|
Adjusted | Sex, race and ethnicity, gestational age, other parent’s age, maternal education, maternal birthplace, birth order, mode of delivery, history of miscarriage, history of pregnancy complications, previous c-section | Other or nonspecific (n = 1012) | 50 600 |
| Total | NA | NA | NA | NA | Lymphoma (n = 5506), CNS (n = 9927), neuroblastoma (n = 3069), retinoblastoma (n = 1273), renal tumor (n = 2908), hepatic tumor (n = 791), bone tumor (n = 2288), soft tissue sarcoma (n = 2743), germ cell tumor (n = 2163), other or nonspecific (n = 3179) | 3 675 858 |
Odds ratios could not be estimated for these cancers as certain cell counts were omitted from the publication because of confidentiality concerns. However, these cases were included in the estimated effect of all cancers pooled. CNS = central nervous system; NA = not applicable.
Maternal Age
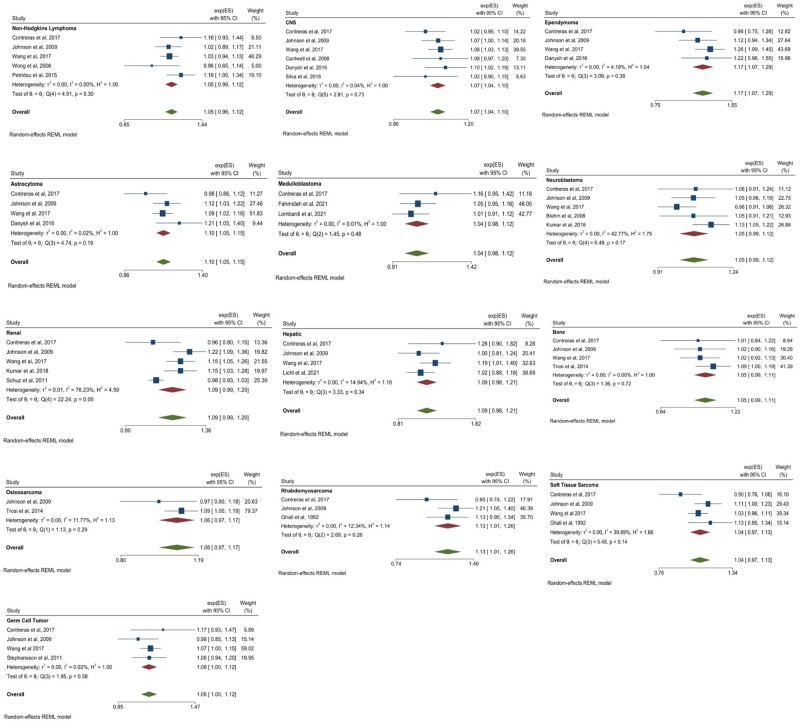
There were several statistically significant associations between maternal age at birth and the odds of certain cancer types. A 5-year increase in age was statistically significantly associated with an increased odds of CNS tumors (OR = 1.07, 95% CI = 1.04 to 1.10; I2 = 0.04%, Pheterogeneity = .73), ependymoma (OR = 1.17, 95% CI = 1.07 to 1.29; I2 = 4.19%, Pheterogeneity = .38), astrocytoma (OR = 1.10, 95% CI = 1.05 to 1.15; I2 = 0.02%, Pheterogeneity = .19), rhabdomyosarcoma (OR = 1.13, 95% CI = 1.01 to 1.26; I2 = 12.34%, Pheterogeneity = .26), and germ cell tumors (OR = 1.06, 95% CI = 1.00 to 1.12 [lower bound rounded down to 1.00]; I2 = 0.02%, Pheterogeneity = .58). No other statistically significant associations were observed; however, several point estimates and confidence intervals were suggestive of an association. Results were considered suggestive of an association if 95% confidence interval lower bounds were between 0.97 and 1.00 for a point estimate above 1.00 and upper bounds between 1.00 and 1.03 for a point estimate below the null. Such suggestive positive associations between a 5-year increase in maternal age and cancer odds were observed for non-Hodgkin lymphoma (OR = 1.05, 95% CI = 0.99 to 1.12; I2 = 0.00%, Pheterogeneity = .30), medulloblastoma (OR = 1.04, 95% CI = 0.98 to 1.12; I2 = 0.01%, Pheterogeneity = .46), neuroblastoma (OR = 1.05, 95% CI = 0.99 to 1.12; I2 = 42.77%, Pheterogeneity = .17), combined renal tumors (OR = 1.09, 95% CI = 0.99 to 1.20; I2 = 78.23%, Pheterogeneity < .001), combined hepatic tumors (OR = 1.09, 95% CI = 0.98 to 1.21; I2 = 14.94%, Pheterogeneity = .34), combined bone tumors (OR = 1.05, 95% CI = 0.99 to 1.11; I2 = 0.00%, Pheterogeneity = .72), osteosarcoma (OR = 1.06, 95% CI = 0.97 to 1.17; I2 = 11.77%, Pheterogeneity = .29), and soft tissue sarcomas (OR = 1.04, 95% CI = 0.97 to 1.13; I2 = 39.89%, Pheterogeneity = .14). These statistically significant and suggestive meta-analysis results are displayed in Figure 2. Full results may be found in Supplementary Figure 1 (available online). The overall effect of a 5-year increase in maternal age on the odds of combined childhood lymphoma and solid tumors was also statistically significant (OR = 1.05, 95% CI = 1.03 to 1.07).
Figure 2.
Maternal statistically significant and suggestive meta-analysis results for a 5-year increase in maternal age at birth. Error bars represent 95% confidence intervals. Between-study heterogeneity is presented in terms of the τ2, I2, and H2 statistic. Test of θi = θj refers to the Cochran Q test of between-study homogeneity. Random effects modeling using restricted maximum likelihood methods was used to produce summary estimates. All tests were 2-sided. CI = confidence interval; ES = estimate (β); REML = restricted maximum likelihood.
Paternal Age
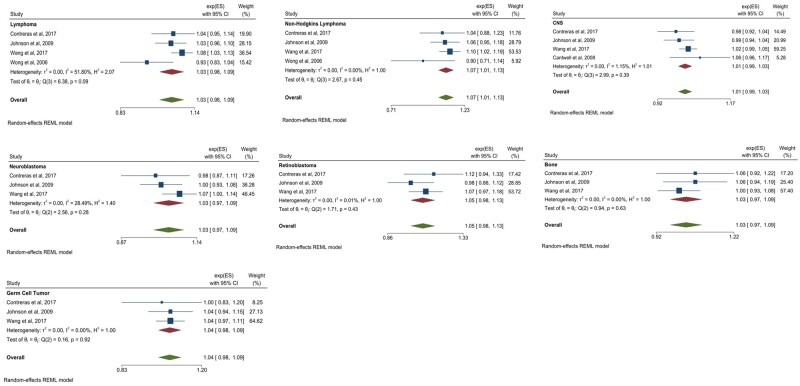
A 5-year increase in paternal age at birth was associated with a statistically significant increase in the odds of non-Hodgkin lymphoma (OR = 1.07, 95% CI = 1.01 to 1.13; I2 = 0.00%, Pheterogeneity = .45). Using the same criteria for suggestive results as outlined in the maternal results, suggestive positive associations were observed between a 5-year increase in paternal age and combined lymphoma (OR = 1.03, 95% CI = 0.98 to 1.09; I2 = 51.80%, Pheterogeneity = .09), combined CNS tumors (OR = 1.01, 95% CI = 0.99 to 1.03; I2 = 1.15%, Pheterogeneity = .39), neuroblastoma (OR = 1.03, 95% CI = 0.97 to 1.09; I2 = 28.49%, Pheterogeneity = .28), retinoblastoma (OR = 1.05, 95% CI = 0.98 to 1.13; I2 = 0.01%, Pheterogeneity = .43), combined bone tumors (OR = 1.03, 95% CI = 0.97 to 1.09; I2 = 0.00%, Pheterogeneity = .63), and germ cell tumors (OR = 1.04, 95% CI = 0.98 to 1.09; I2 = 0.00%, Pheterogeneity = .92). These statistically significant and suggestive paternal meta-analysis results are displayed in Figure 3. Full results may be found in Supplementary Figure 2 (available online). The overall effect of a 5-year increase in paternal age on the odds of combined childhood lymphoma and solid tumors was also statistically significant (OR = 1.02, 95% CI = 1.01 to 1.04).
Figure 3.
Paternal statistically significant and suggestive meta-analysis results for a 5-year increase in paternal age at birth. Error bars represent 95% confidence intervals. Between-study heterogeneity is presented in terms of the τ2, I2, and H2 statistic. Test of θi = θj refers to the Cochran Q test of between-study homogeneity. Random effects modeling using restricted maximum likelihood methods was used to produce summary estimates. All tests were 2-sided. CI = confidence interval; ES = estimate (β); REML = restricted maximum likelihood.
Publication Bias
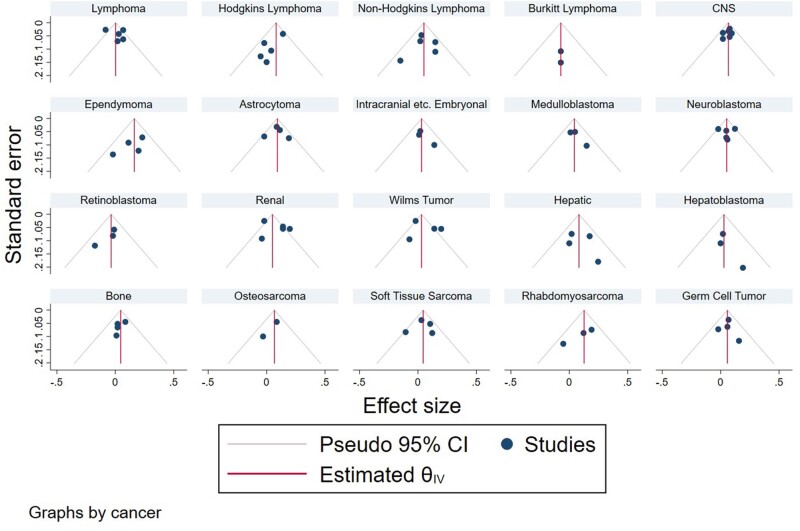
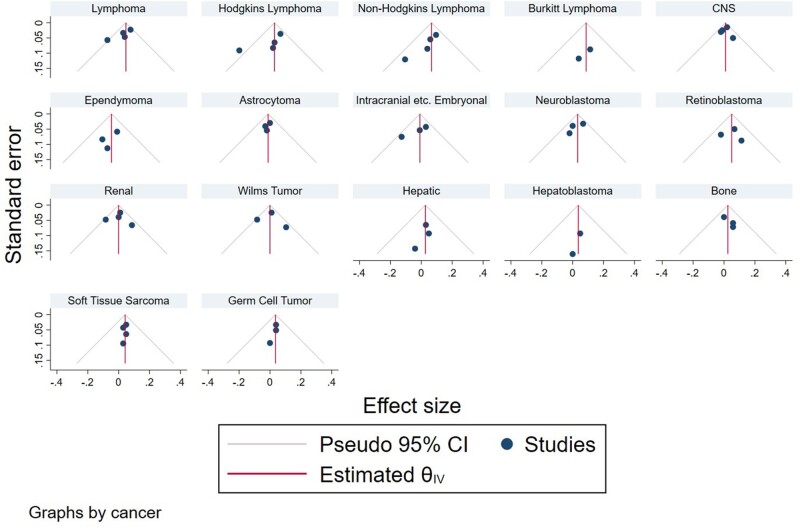
Publication bias was assessed through visual inspection of funnel plots as well as Egger test for small study effects. In the case that there was not publication bias, funnel plots are roughly symmetrical, and 95% of studies fall between the pseudo 95% confidence lines. In this analysis, there is evidence of potential publication bias in the studies of maternal age and any lymphoma, Hodgkin lymphoma, and renal tumors based on visual evaluation of funnel plots by cancer type (Figure 4). However, none of this asymmetry was statistically significant at the 0.05 level as determined by Egger test. There was no evidence of strong publication bias for the other cancer types investigated by maternal age.
Figure 4.
Funnel plots for maternal age publications by cancer type. θ is the log summary odds ratio. CI = confidence interval; CNS = central nervous system; IV = inverse variance.
There is some evidence of publication bias in paternal studies of lymphomas based on visual inspection of funnel plots by cancer type. This asymmetry was statistically significant as determined by Egger test, indicating the possibility of publication bias in studies of paternal age and lymphoma. There was not strong evidence of publication bias for the other cancers investigated with paternal age (Figure 5).
Figure 5.
Funnel plots for paternal age publications by cancer type. θ is the log summary odds ratio. CI = confidence interval; CNS = central nervous system; IV = inverse variance .
Sensitivity Analysis Excluding Crude Estimates
Comparisons between the estimated associations observed when the crude estimates were included vs the estimated associations observed with the exclusion of these studies may be found in Table 2. No large differences in odds ratios were observed.
Table 2.
Odds ratio estimates including and excluding studies with crude estimates onlya
| Cancer | Maternal OR including crude estimates (95% CI) | Maternal OR excluding crude estimates (95% CI) | Paternal OR including crude estimates (95% CI) | Paternal OR excluding crude estimates (95% CI) |
|---|---|---|---|---|
| CNS tumor | 1.07 (1.04 to 1.10) | 1.07 (1.03 to 1.10) | 1.01 (0.99 to 1.03) | 1.01 (0.98 to 1.03) |
| Ependymoma | 1.17 (1.07 to 1.29) | 1.15 (1.01 to 1.31) | NA | NA |
| Astrocytoma | 1.10 (1.05 to 1.15) | 1.08 (1.03 to 1.14) | NA | NA |
| Medulloblastoma | 1.04 (0.98 to 1.12) | 1.07 (0.98 to 1.17) | NA | NA |
| Neuroblastoma | 1.05 (0.99 to 1.12) | 1.02 (0.97 to 1.07) | NA | NA |
| Renal tumor | 1.09 (0.99 to 1.20) | 1.08 (0.96 to 1.21) | NA | NA |
| Wilms tumor | 1.07 (0.95 to 1.21) | 1.04 (0.89 to 1.22) | NA | NA |
| Soft tissue sarcoma | 1.04 (0.97 to 1.13) | 1.03 (0.93 to 1.13) | 1.04 (1.00 to 1.09) | 1.04 (1.00, 1.09) |
| Rhabdomyosarcoma | 1.13 (1.01 to 1.26) | 1.10 (0.87 to 1.38) | NA | NA |
CI = confidence interval; CNS = central nervous system; NA = not applicable; OR = odds ratio.
Sensitivity Analysis of Missing Paternal Age
The sensitivity analysis of the Johnson et al. (8) study estimated the effect of missing paternal age by assigning missing fathers to the youngest and oldest age categories. This analysis did not detect any meaningful differences (>10% change) in estimated effect sizes because of missing paternal age with the exception of the effect maternal age on ependymoma risk in which the point estimate changed by 11.2%. However, this sensitivity analysis investigated the most extreme cases—that all missing fathers belonged to the youngest and oldest categories—which is highly unlikely. Additionally, none of the new estimates were statistically significantly different from the original estimates.
Discussion
Overall, through this meta-analysis of population-based studies of parental age and childhood solid tumors, we observed statistically significant associations between higher maternal age and CNS tumors, ependymoma, astrocytoma, rhabdomyosarcoma, and germ cell tumors as well as between higher paternal age and non-Hodgkin lymphoma. Increasing maternal age has been shown to be a risk factor for a number of adverse perinatal outcomes; it has also been shown to be associated with ALL (4) and AML (5). This analysis provides evidence that increasing maternal age may be associated with an increased odds of many solid tumors as well, particularly CNS tumors. Though several point estimates highlighted in this analysis were relatively small, each point estimate is reported in reference to a 5-year increase in parental age; thus, the differences across the full reproductive age span would be much greater. For example, a 5-year increase in maternal age was associated with a 7% increase in odds of combined CNS tumors in this analysis (OR = 1.07), but a 20-year difference in maternal age (for example, comparing a mother aged 20 years to a mother aged 40 years) is associated with a 31% increase (OR = 1.31).
A priori, we expected to observe an association between paternal age and childhood cancer risk. The rate of germline mutations increases with paternal age, which has been hypothesized to be due to increased number of cell division during spermatogenesis (32). On average, it has been found that with each year in paternal age, the number of mutations in the child increases approximately linearly by 2.9 mutations (33), and on an average, the father transmits 3.44 times more de novo mutations than the mother (34). As germline pathogenic or likely pathogenic variants are associated with childhood cancer risk (35-37), it seemed likely that paternal age influences cancer risk in offspring through this increase in de novo mutations. Previous large, pooled analyses such as the previously mentioned CLIC analyses of ALL (4) (16 720 cases and 42 632 controls) and AML (5) (3182 cases and 8377 controls) have reported that higher paternal age is associated with an increased risk of ALL, though there was no association seen with AML. Our analysis demonstrated a statistically significant association between paternal age and non-Hodgkin lymphoma but no other cancers. As the mechanism through which paternal age may influence childhood cancer risk is both plausible and well understood, the abundance of null findings in this analysis was contrary to expectation. However, at present, there are a limited number of registry-based studies of paternal age available for analysis, and certain registries do not contain paternal age data, thus it is possible that there may be an association that is undetectable with current literature. It is also difficult to disentangle the independent associations of maternal and paternal age on childhood cancer risk, given their strong correlation (38). However, several of the included studies, and notably the largest studies included in this meta-analysis (8,10,16), did adjust for the other parent’s age in all effect estimates. Furthermore, exclusion of studies that only reported crude estimates did not substantially change observed effect estimates.
Somewhat against our expectations, maternal age showed more and greater associations with childhood cancer than paternal age. The observed association between increasing maternal age and odds of childhood lymphoma and solid tumors could be due to multiple potential biological mechanisms. The rate of germline de novo mutations increases with both maternal and paternal age (39), although the association with maternal age is much less strong (34), and certain cancer predisposition syndromes may occur as a result of de novo germline mutations (40). Chromosomal abnormalities and nondisjunction such as Down syndrome are also associated with older maternal age (41). Down syndrome is associated with an increased risk of leukemia (42), and chromosomal abnormalities are associated with an increased risk of any cancer (43); thus, this represents a pathway through which advanced maternal age may affect childhood cancer risk. Advanced maternal age is also associated with an increase in nonchromosomal birth defects (44), which are linked with an increased risk of cancer, including strong associations with CNS tumors and germ cell tumors (43). Additionally, mitochondrial DNA heteroplasmy, or the occurrence of more than 1 mitochondrial DNA haplotype in a cell or tissue, is associated with maternal age (45,46) and has been linked to an increased risk of conditions such as cancer (47). Lastly, advanced maternal age influences patterns of DNA methylation in offspring, and some of the CpG sites found to be influenced by maternal age are also potentially associated with cancer, such as KLHL35 (48). As proof of principle, transgenerational inheritance of aberrant epigenetic patterns from mother to son has been reported in a case of epimutation, which silenced the MLH1 DNA mismatch repair gene, resulting in Lynch syndrome in the affected son (49).
There are some important limitations to the current analysis. Though one of the strengths of meta-analyses is the ability to pool multiple studies to increase the sample size, we are still limited by the rarity of some of the cancer types we included, and the relatively limited number of registry-based studies of certain cancer types. If the effect of parental age on cancer incidence is small, current pooling efforts still may not be enough to detect statistically significant effect estimates. We are also limited to what has been published in the current literature. If studies found null associations, these may not be reported in the literature. We tried to minimize the potential for publication bias by using a more liberal inclusion criteria for full text review, however, the funnel plots assessing publication bias appear to show publication bias may be present for certain cancers and parental age. Additionally, there was some evidence of within-study heterogeneity in some of the cancers investigated. This heterogeneity may be partially explained by the inclusion of differing confounders in the individual studies. Additionally, as included studies were conducted in a variety of regions including North and South America, Europe, and Oceania, it is likely that lifestyle and genetic factors that may contribute to cancer risk in children as well as the detection of such cancers may vary. For example, smoking prevalence and age at initiation of smoking varies regionally across the globe (53), and parental smoking is a potential risk factor for multiple childhood cancers (50-52,54-56). Included studies also vary by years of diagnosis, which may additionally add heterogeneity in risk factor prevalence and cancer detection. Many existing registry-based studies were missing paternal age data more often than maternal age—for example, Johnson et al. (8) reported that though maternal age was available for almost all subjects, paternal age was missing in 10% of cases and 11% of controls. We evaluated the influence that this missingness could have on effect estimates in a sensitivity analysis of the 5-state pooling data from the Johnson et al. (8) study, one of the larger studies in this analysis. We assigned fathers with missing ages to both the youngest and oldest age categories to determine the bounds within which the true effect estimate might lie. We found that in this case, missing ages for fathers were unlikely to have a meaningful impact on effect estimates. It is also possible that there are unmeasured confounders affecting these results. Socioeconomic status is associated with parental age (57) and childhood cancer risk (58,59), and only 2 of the included studies controlled for a socioeconomic status proxy: maternal education (17,18). There are also other potential confounders that are associated with both offspring cancer risk and parental age, such as parental smoking (51,60) and maternal prenatal vitamin use (61,62), which are generally not available in registry-based studies. In the case of smoking, older mothers are less likely to smoke than younger mothers (63), and very young paternal age is associated with substance use (64), whereas parental smoking is associated with an increased risk of cancer in children (51,65). In the case of vitamin use, older mothers are more likely to take supplements than younger mothers (61), although maternal vitamin use may decrease the risk of certain childhood cancers (62). In both of the mentioned situations, uncontrolled confounding could lessen observed associations. However, it is important to note that these trends in smoking and vitamin use may not hold constant across all birth years and regions investigated. Another factor limiting this analysis is that some registry-based studies did not have access to paternal age because of the respective registries used, for example, the Swedish and Danish birth registries used in a number of studies included in this analysis do not collect data on paternal age, and thus, these studies could neither estimate the effect of paternal age on the odds of cancer nor control for paternal age in their analyses of maternal age. Maternal and paternal age are strongly correlated variables (38), and a number of studies included in our analysis did not control for paternal age in their analyses of maternal age and cancer risk. Thus, it is possible that some of the observed effect of maternal age on cancer risk is actually due to paternal age. However, removing studies with only crude estimates did not have undue effects on estimates. Of the additional studies that did not control for paternal age in their analysis of maternal age, only one, Stephan sson et al. (29), was a study of a cancer where a statistically significant result was observed—germ cell tumors.
We also must emphasize several strengths to this analysis. We chose to include only population-based registry studies, leading to more valid findings as recall bias is not a concern and selection bias should be minimal. Additionally, we had access to the 5-state pooling data, allowing for re-analysis of the data without the California data, which allowed for the inclusion of the Wang et al. (10) paper and the data from the Johnson et al. (8) paper, thus increasing sample size.
In conclusion, this meta-analysis of registry-based analyses of parental age and childhood cancer supports the association between older maternal age and certain childhood solid cancers—namely, CNS tumors, ependymoma, astrocytoma, rhabdomyosarcoma, and germ cell tumors—in addition to the previously investigated association between maternal age and leukemia. There was also some evidence of an association between older paternal age and non-Hodgkin lymphoma. The observed associations are also supported by numerous potential biological mechanisms such as germline mutations, DNA methylation, and chromosomal and nonchromosomal birth defects. However, the number of registry-based studies of certain cancers and parental age is relatively limited at this time; thus, further research into the association between parental age and certain cancers is warranted in the future. Additionally, mechanistic studies may also be used to investigate the relationship between parental age and cancer risk, for example, an analysis of tumor profiles and parental age could determine whether or not mutation profiles differ with age.
Funding
This work was supported by the National Cancer Institute [T32 CA099936].
Notes
Role of the funder: The funder had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Disclosures: The authors declare no potential conflicts of interest.
Author contributions: Conceptualization: LS; Data Curation: AD, JS; Formal Analysis: AD, JS, KM; Supervision: EM, LS; Writing—original draft: AD, KM; Writing—review and editing: AD, KM, HK, EM, LS
Data Availability
The data underlying this meta-analysis are available in the article itself and the referenced articles.
Supplementary Material
Contributor Information
Allison Domingues, Division of Epidemiology & Clinical Research, Department of Pediatrics, University of Minnesota, Minneapolis, MN, USA.
Kristin J Moore, Program in Health Disparities Research, Department of Family Medicine & Community Health, University of Minnesota Medical School, University of Minnesota, Minneapolis, MN, USA.
Jeannette Sample, Division of Epidemiology & Clinical Research, Department of Pediatrics, University of Minnesota, Minneapolis, MN, USA.
Harmeet Kharoud, Department of Epidemiology, School of Public Health, University of Minnesota, Minneapolis, MN, USA.
Erin L Marcotte, Division of Epidemiology & Clinical Research, Department of Pediatrics, University of Minnesota, Minneapolis, MN, USA; Masonic Cancer Center, University of Minnesota, Minneapolis, MN, USA.
Logan G Spector, Division of Epidemiology & Clinical Research, Department of Pediatrics, University of Minnesota, Minneapolis, MN, USA; Masonic Cancer Center, University of Minnesota, Minneapolis, MN, USA.
References
- 1. Bergh C, Pinborg A, Wennerholm U-B.. Parental age and child outcomes. Fertil Steril. 2019;111(6):1036–1046. doi: 10.1016/j.fertnstert.2019.04.026. [DOI] [PubMed] [Google Scholar]
- 2. Khandwala YS, Zhang CA, Lu Y, Eisenberg ML.. The age of fathers in the USA is rising: an analysis of 168 867 480 births from 1972 to 2015. Hum Reprod. 2017;32(10):2110-2116. doi: 10.1093/HUMREP/DEX267 [DOI] [PubMed] [Google Scholar]
- 3. Jacobsson B, Ladfors L, Milsom I.. Advanced maternal age and adverse perinatal outcome. Obstet Gynecol. 2004;104(4):727-733. [DOI] [PubMed] [Google Scholar]
- 4. Petridou ET, Georgakis MK, Erdmann F, et al. Advanced parental age as risk factor for childhood acute lymphoblastic leukemia: results from studies of the Childhood Leukemia International Consortium. Eur J Epidemiol. 2018;33(10):965-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Panagopoulou P, Skalkidou A, Marcotte E, et al. Parental age and the risk of childhood acute myeloid leukemia: results from the Childhood Leukemia International Consortium. Cancer Epidemiol. 2019;59:158-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dockerty JD, Draper G, Vincent T, Rowan SD, Bunch KJ.. Case-control study of parental age, parity and socioeconomic level in relation to childhood cancers. Int J Epidemiol. 2001;30(6):1428-1437. doi: 10.1093/IJE/30.6.1428 [DOI] [PubMed] [Google Scholar]
- 7. Yip BH, Pawitan Y, Czene K.. Parental age and risk of childhood cancers: a population-based cohort study from Sweden. Int J Epidemiol. 2006;35(6):1495-1503. doi: 10.1093/IJE/DYL177 [DOI] [PubMed] [Google Scholar]
- 8. Johnson KJ, Carozza SE, Chow EJ, et al. Parental age and risk of childhood cancer: a pooled analysis. Epidemiology. 2009;20(4):475-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Urhoj SK, Raaschou-Nielsen O, Hansen AV, Mortensen LH, Andersen PK, Andersen AMN.. Advanced paternal age and childhood cancer in offspring: a nationwide register-based cohort study. Int J Cancer. 2017;140(11):2461-2472. [DOI] [PubMed] [Google Scholar]
- 10. Wang R, Metayer C, Morimoto L, et al. Parental age and risk of pediatric cancer in the offspring: a population-based record-linkage study in California. Am J Epidemiol. 2017;186(7):843-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Greenland S, Longnecker MP.. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135(11):1301-1309. [DOI] [PubMed] [Google Scholar]
- 12. Berlin J, Longnecker MP, Greenland S.. Meta-analysis of epidemiologic dose-response data. Epidemiology. 1993;4(3):218-228. [DOI] [PubMed] [Google Scholar]
- 13. STATA META-ANALYSIS REFERENCE MANUAL RELEASE 17, 17th ed. StataCorp LLC; 2021.
- 14. Landry DJ, Forrest JD.. How old are U.S. fathers? Fam Plann Perspect. 1995;27(4):159-161, 165. [PubMed] [Google Scholar]
- 15. Ottawa Hospital Research Institute. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed April 4, 2022.
- 16. Contreras ZA, Hansen J, Ritz B, Olsen J, Yu F, Heck JE.. Parental age and childhood cancer risk: a Danish population-based registry study. Cancer Epidemiol. 2017;49:202-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Petridou ET, Sergentanis TN, Skalkidou A, et al. Maternal and birth anthropometric characteristics in relation to the risk of childhood lymphomas: a Swedish nationwide cohort study. Eur J Cancer Prev. 2015;24(6):535-541. [DOI] [PubMed] [Google Scholar]
- 18. Wong DI, Dockerty JD.. Birth characteristics and the risk of childhood leukaemias and lymphomas in New Zealand: a case-control study. BMC Blood Disord. 2006;6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cantwell MM, Forman MR, Middleton RJ, Murray LJ.. Association of early life factors and brain tumour risk in a cohort study. Br J Cancer. 2008;99(5):796-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Danysh HE, Zhang K, Mitchell LE, Scheurer ME, Lupo PJ.. Maternal residential proximity to major roadways at delivery and childhood central nervous system tumors. Environ Res. 2016;146:315-322. [DOI] [PubMed] [Google Scholar]
- 21. de Paula Silva N, de Souza Reis R, Garcia Cunha R, et al. Maternal and birth characteristics and childhood embryonal solid tumors: a population-based report from Brazil. PLoS One. 2016;11(10):e0164398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bluhm E, McNeil DE, Cnattingius S, Gridley G, Ghormli LE, Fraumeni JF.. Prenatal and perinatal risk factors for neuroblastoma. Int J Cancer. 2008;123(12):2885-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kumar SV, Lupo PJ, Pompeii LA, Danysh HE.. Maternal residential proximity to major roadways and pediatric embryonal tumors in offspring. Int J Environ Res Public Health. 2018;15(3):505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schüz J, Schmidt LS, Kogner P, et al. Birth characteristics and Wilms tumors in children in the Nordic countries: a register-based case-control study. Int J Cancer. 2011;128(9):2166-2173. [DOI] [PubMed] [Google Scholar]
- 25. Adel Fahmideh M, Peckham-Gregory EC, Schraw JM, et al. Maternal and perinatal factors are associated with risk of pediatric central nervous system tumors and poorer survival after diagnosis. Sci Rep. 2021;11(1):10410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. de Fine Licht S, Schmidt LS, Rod NH, et al. Hepatoblastoma in the Nordic countries. Int J Cancer. 2012;131(4):E555-E561. [DOI] [PubMed] [Google Scholar]
- 27. Troisi R, Stephansson O, Jacobsen J, et al. Perinatal characteristics and bone cancer risk in offspring—a Scandinavian population-based study. Acta Oncol (Madr). 2014;53(6):830-838. [DOI] [PubMed] [Google Scholar]
- 28. Ghali MH, Yoo K‐Y, Flannery JT, Dubrow R.. Association between childhood rhabdomyosarcoma and maternal history of stillbirths. Int J Cancer. 1992;50(3):365-368. [DOI] [PubMed] [Google Scholar]
- 29. Stephansson O, Wahnström C, Pettersson A, et al. Perinatal risk factors for childhood testicular germ-cell cancer: a Nordic population-based study. Cancer Epidemiol. 2011;35(6):e100-e104. [DOI] [PubMed] [Google Scholar]
- 30. Lombardi C, Thompson S, Ritz B, Cockburn M, Heck JE.. Residential proximity to pesticide application as a risk factor for childhood central nervous system tumors. Environ Res. 2021;197:111078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deziel NC, Zhang Y, Wang R, et al. Birth characteristics and risk of pediatric thyroid cancer: a population-based record-linkage study in California. Thyroid. 2021;31(4):596–606. doi:10.1089/thy.2020.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kong A, Frigge ML, Masson G, et al. Rate of de novo mutations and the importance of father’s age to disease risk. Nature 2012;488(7412):471-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rahbari R, Wuster A, Lindsay SJ, et al. ; for the UK10K Consortium. Timing, rates and spectra of human germline mutation. Nat Genet. 2016;48(2):126-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wong WSW, Solomon BD, Bodian DL, et al. New observations on maternal age effect on germline de novo mutations. Nat Commun. 2016;7(1):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saletta F, Pozza LD, Byrne JA.. Genetic causes of cancer predisposition in children and adolescents. Transl Pediatr. 2015;4(2):67-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Spector LG, Pankratz N, Marcotte EL.. Genetic and nongenetic risk factors for childhood cancer. Pediatr Clin North Am. 2015;62(1):11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Roth AJ, Stallard J.. Childhood cancer survivors found to have inherited mutations increasing risk of the disease. Memorial Sloan Kettering Cancer Center; 2017. https://www.mskcc.org/news/aacr17-childhood-cancer-survivors-found-have-inherited-mutations-increasing-risk-disease. Accessed August 25, 2021.
- 38. Carslake D, Tynelius P, Van Den Berg G, Davey Smith G, Rasmussen F.. Associations of parental age with health and social factors in adult offspring. Methodological pitfalls and possibilities. Sci Rep. 2017;7(1):1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Girard SL, Bourassa CV, Perreault L-PL, et al. Paternal age explains a major portion of de novo germline mutation rate variability in healthy individuals. PLoS One. 2016;11(10):e0164212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gonzalez KD, Buzin CH, Noltner KA, et al. High frequency of de novo mutations in Li-Fraumeni syndrome. J Med Genet. 2009;46(10):689-693. [DOI] [PubMed] [Google Scholar]
- 41. Hassold T, Hunt P.. Maternal age and chromosomally abnormal pregnancies: what we know and what we wish we knew. Curr Opin Pediatr. 2009;21(6):1–11.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mateos MK, Barbaric D, Byatt S-A, Sutton R, Marshall GM.. Down syndrome and leukemia: insights into leukemogenesis and translational targets. Transl Pediatr. 2015;4(2):76-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lupo PJ, Schraw JM, Desrosiers TA, et al. Association between birth defects and cancer risk among children and adolescents in a population-based assessment of 10 million live births. JAMA Oncol. 2019;5(8):1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gill SK, Broussard C, Devine O, Green RF, Rasmussen SA, Reefhuis J; and the National Birth Defects Prevention Study. Association between maternal age and birth defects of unknown etiology—United States, 1997-2007. Birth Defects Res A Clin Mol Teratol. 2012;94(12):1010-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zaidi AA, Wilton PR, Su MSW, et al. Bottleneck and selection in the germline and maternal age influence transmission of mitochondrial DNA in human pedigrees. Proc Natl Acad Sci USA. 2019;116(50):25172-25178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rebolledo-Jaramillo B, Su MSW, Stoler N, et al. Maternal age effect and severe germ-line bottleneck in the inheritance of human mitochondrial DNA. Proc Natl Acad Sci USA. 2014;111(43):15474-15479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wallace DC, Chalkia D. Mitochondrial DNA genetics and the heteroplasmy conundrum in evolution and disease. Cold Spring Harb Perspect Biol. 2013;5(11):a021220. doi: 10.1101/cshperspect.a021220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Markunas CA, Wilcox AJ, Xu Z, et al. Maternal age at delivery is associated with an epigenetic signature in both newborns and adults. PLoS One. 2016;11(7):e0156361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hitchins MP, Lin VA, Buckle A, et al. Epigenetic inactivation of a cluster of genes flanking MLH1 in microsatellite-unstable colorectal cancer. Cancer Res. 2007;67(19):9107-9116. [DOI] [PubMed] [Google Scholar]
- 50. Tettamanti G, Ljung R, Mathiesen T, Schwartzbaum J, Feychting M. Maternal smoking during pregnancy and the risk of childhood brain tumors: Results from a Swedish cohort study. 2015;40:67–72. doi: 10.1016/j.canep.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 51. Heck JE, Contreras ZA, Park AS, Davidson TB, Cockburn M, Ritz B.. Smoking in pregnancy and risk of cancer among young children: a population-based study. Int J Cancer. 2016;139(3):613-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rios P, Bailey HD, Poulalhon C, et al. Parental smoking, maternal alcohol consumption during pregnancy and the risk of neuroblastoma in children. A pooled analysis of the ESCALE and ESTELLE French studies. Int J Cancer. 2019;145(11):2907-2916. [DOI] [PubMed] [Google Scholar]
- 53. Reitsma MB, Flor LS, Mullany EC, Gupta V, Hay SI, Gakidou E.. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and initiation among young people in 204 countries and territories, 1990-2019. Lancet Public Heal. 2021;6(7):e472-e481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rumrich IK, Viluksela M, Vähäkangas K, Gissler M, Surcel H-M, Hänninen O.. Maternal smoking and the risk of cancer in early life—a meta-analysis. PLoS One. 2016;11(11):e0165040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chu P, Wang H, Han S, et al. Maternal smoking during pregnancy and risk of childhood neuroblastoma: systematic review and meta-analysis. J Can Res Ther. 2016;12(2):999. [DOI] [PubMed] [Google Scholar]
- 56. Müller-Schulte E, Kurlemann G, Harder A.. Tobacco, alcohol and illicit drugs during pregnancy and risk of neuroblastoma: systematic review. Arch Dis Child Fetal Neonatal Ed. 2018;103(5):F467-F473. [DOI] [PubMed] [Google Scholar]
- 57. Van Roode T, Sharples K, Dickson N, Paul C.. Life-course relationship between socioeconomic circumstances and timing of first birth in a birth cohort. PLoS One. 2017;12(1):e0170170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kehm RD, Spector LG, Poynter JN, Vock DM, Osypuk TL.. Socioeconomic status and childhood cancer incidence: a population-based multilevel analysis. Am J Epidemiol. 2018;187(5):982-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Carozza SE, Puumala SE, Chow EJ, et al. Parental educational attainment as an indicator of socioeconomic status and risk of childhood cancers. Br J Cancer. 2010;103(1):136-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jha P, Ranson MK, Nguyen SN, Yach D.. Estimates of global and regional smoking prevalence in 1995, by age and sex. Am J Public Health. 2002;92(6):1002-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Branum AM, Bailey R, Singer BJ.. Dietary supplement use and folate status during pregnancy in the United States. J Nutr. 2013;143(4):486-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Preston-Martin S, Pogoda JM, Mueller BA, et al. Prenatal vitamin supplementation and pediatric brain tumors: huge international variation in use and possible reduction in risk. Childs Nerv Syst. 1998;14(10):551-557. [DOI] [PubMed] [Google Scholar]
- 63.Drake P, Driscoll AK, Mathews TJ. Cigarette smoking during pregnancy: United States, 2016. NCHS Data Brief. 2018;(305):1–8. [PubMed]
- 64.Thornberry TP, Wei EH, Stouthamer-Loeber M, Van Dyke J. Teenage Fatherhood and Delinquent Behavior. Office of Juvenile Justice and Delinquency Prevention [Internet]; 2021. https://ojjdp.ojp.gov/library/publications/teenage-fatherhood-and-delinquent-behavior.
- 65.Chunxia D, Meifang W, Jianhua Z, et al. Tobacco smoke exposure and the risk of childhood acute lymphoblastic leukemia and acute myeloid leukemia: A meta-analysis. Medicine (Baltimore). 2019;98(28):e16454. doi: 10.1097/MD.0000000000016454. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this meta-analysis are available in the article itself and the referenced articles.