Abstract
Objectives
To clarify non-alcoholic fatty liver disease (NAFLD) prevalence, risk factors and clinical outcome in an exemplary Chinese population, a cohort of company employees was followed up for 11 years.
Design
Retrospective cohort study.
Setting
Between 2006 and 2016 in Ning bo, China.
Participants
13 032 company employees.
Results
Over 11 years, the prevalence of NAFLD increased from 17.2% to 32.4% (men 20.5%–37% vs women 9.8%–22.2%). Male peak prevalence was between 40 and 60 years of age, whereas highest prevalence in women was at an age of 60 years and older. Logistic and Cox regression revealed 16 risk factors, including body mass index (BMI), albumin, white blood cell, triglycerides (TG), high-density lipoprotein, glutamyl transpeptidase, alanine transaminase, creatinine, urea acid, glucose, systolic blood pressure, diastolic blood pressure, blood sedimentation, haemoglobin, platelet and apolipoprotein B2 (p<0.05 for all factors). The area under the curve of these variables for NAFLD is 0.88. However, cause-effect analyses showed that only BMI, gender and TG directly contributed to NAFLD development. Over an 11-year follow-up period, 12.6%, 37.7% and 14.2% of male patients with NAFLD and 11.6%, 44.7% and 22.6% of female patients with NAFLD developed diabetes, hypertension and hyperuricaemia, respectively. Except one male patient who developed cirrhosis, no patients with NAFLD progressed into severe liver disease.
Conclusion
Diabetes, hypertension and hyperuricaemia are the main clinical outcomes of NAFLD. Eleven years of NAFLD are not sufficient to cause severe liver disease. Age and obesity are direct risk factors for NAFLD. BMI, gender and TG are three parameters directly reflecting the occurrence of NAFLD.
Keywords: epidemiology, hypertension, diabetes & endocrinology, health & safety, health informatics
Strengths and limitations of this study.
This study dynamically follows up non-alcoholic fatty liver disease (NAFLD) prevalence in an eastern Chinese community for 11 years.
The study adopted first-order Markov models to evaluate the cause-effect link between NAFLD and risk factors.
The relatively low sensitivity of ultrasound for the detection of liver fat might underestimate the incidence of NAFLD in this cohort.
Given that the current study is a single-centre observation, multiple-centre studies are required to confirm the conclusions in the future.
The study population is a highly select, relatively homogenous group of well-educated professionals in privileged social positions and permanent employment. Thus, the conclusions might not be transferable to the general Chinese population.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is one of the most common causes of chronic liver disease globally.1 The global prevalence of NAFLD is currently around 25%.2 3 NAFLD is predicted to become the most frequent indication for liver transplantation by 2030 in Western countries.4 An analysis based on 18 million patients in 4 European cohorts showed that NAFLD and non-alcoholic steatohepatitis (NASH) increase the risk of end-stage liver diseases, for example, cirrhosis and hepatocellular carcinoma (HCC).5 Of note, NAFLD is not only a disease restricted to the liver, but also affects extra-hepatic organs. NAFLD is tightly associated with the occurrence of type 2 diabetes mellitus (T2DM), cardiovascular (CVD) and cardiac diseases, and chronic kidney disease.4
In China, the incidence of NAFLD has been increasing over the last two decades. A recent meta-analysis, based on 392 studies between 2008 and 2018, showed the national incidence of NAFLD in China to be at 29.2%.6 In Shanghai, the adult incidence of NAFLD has increased from 14.04% in 1995 to 43.65% in 2015.2 Being a vast country, Chinese living in different areas vary widely in lifestyle and economic status. Thus, the epidemiology, natural history and clinical outcomes of NAFLD in different areas of the country are worth further investigation.
It is well accepted that viral hepatitis is a major reason for progressive chronic liver diseases, for example, fibrosis, cirrhosis and ultimately, HCC. With regard to NAFLD, incidence and severity of associated chronic liver disease outcomes has not been monitored in large Chinese cohorts yet—especially over an extended time span. The current study therefore describes the prevalence of NAFLD in a large Eastern Chinese community over 11 years (2006–2016). We focused on three questions: (1) What is the annual incidence of NAFLD? (2) What are the risk factors for NAFLD? (3) What are the most frequent extrahepatic and intrahepatic clinical outcomes of NAFLD in this selected population?
Methods
Patient and public involvement
No patients were involved in this study.
Design and participants
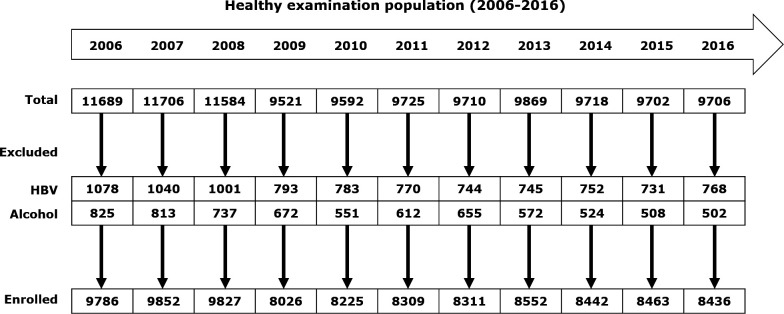
In this retrospective study, we analysed the ‘annual health examination database’ of the Zhenhai Lianhua Hospital from 2006 to 2016. This hospital is affiliated to Sinopec Zhenhai Refining & Chemical Company. Supported by the company, all employees were offered the opportunity to go to this hospital for an annual health examination. Over a period of 11 years, a total 13 032 employees received health examinations. From 2006 to 2016, 11689, 11706, 11584, 9521, 9592, 9725, 9710, 9869, 9718, 9702 and 9706 persons received health examinations, respectively (figure 1). To describe the longitudinal NAFLD occurrence in this cohort, we excluded subjects with the following conditions: (1) viral hepatitis B and C infection, which were identified by blood virus measurements (HBV–DNA and HCV–RNA) and (2) alcoholic liver disease, which was defined as previously described.7 8 NAFLD was defined as the presence of hepatic steatosis, determined by ultrasonography.
Figure 1.
Flow chart depicting the enrolment of a population with non-alcoholic fatty liver disease for follow-up in Ningbo Zhenhai Lianhua Hospital, China. HBV, hepatitis B virus.
Measures
Online supplemental table 1 shows all parameters measured in the annual health examinations. Ultrasonography was performed by the same three experienced doctors (LC, FL and JY) with an Ultrasonograph B, GE, Voluson 730 pro. Blood biochemistry and serum HBV levels were measured by an Olympus AU640 autoanalyzer (Olympus, Kobe, Japan) and an ImmunoAssay Analyzer VitrosECI (Johnson & Johnson, USA), respectively. All methods were carried out in accordance with relevant guidelines and regulations.
bmjopen-2021-054891supp001.pdf (109.2KB, pdf)
Statistical analysis
For population characteristics, variables were described as means and SD or proportions as appropriate. Student’s t-test or non-parametric test was used to analyse differences between two groups as mentioned. χ2 test was used to verify the differences of nominative variables between two groups. Multivariate analysis of risk factors for NAFLD was performed using logistic regression analysis. Combined receiver operating characteristic (ROC) curve and area under curve (AUC) analyses were used to evaluate the diagnostic performance of biomarkers based on the logistic regression model. Multivariate Cox regression model was performed to calculate HRs of variables to identify independent prognostic variables. First-order Markov models were used to analyse the cause-effect link between NAFLD and risk factors. L1 penalised logistic regression was applied to select predictive predictors. R package ‘glmnet’ contains functions to select predictors using L1 penalised logistic regression. Statistical analyses were performed using SPSS V.22.0 and R V.3.5.3. P values that were less than 0.05 were considered statistically significant. Figures were generated by R package such as ‘forestplot’, ‘ROCR’, ‘bnlearn’ or ‘survival’.
Results
Prevalence of NAFLD from 2006 to 2016
We retrospectively analysed 9786, 9852, 9827, 8026, 8225, 8309, 8311, 8552, 8442, 8463 and 8436 persons who received health examinations from 2006 to 2016, respectively. Online supplemental table 2 shows the 11-year annual NAFLD incidence in this population. In 2006, NAFLD was diagnosed in 17.2% of persons, and gradually increased over the examination period to 19% (2007), 22% (2008), 22.4% (2009), 22.7% (2010), 23.4% (2011), 24.5% (2012), 25.4% (2013), 27.9% (2014), 30.8% (2015) and 32.4% (2016), respectively (online supplemental table 2). Both men and women demonstrated continuously increasing NAFLD prevalence (online supplemental table 2). Compared with female Chinese, male Chinese demonstrated significantly higher NAFLD prevalence, for example, in 2006, the prevalence of NAFLD in men and women was 20.5% and 9.8%, respectively. Eleven years later, the prevalence had increased to 37% in men and 22.2% in women (online supplemental table 2). Noteworthy, the prevalence of NAFLD in men and women was correlating with age. The peak prevalence of NAFLD in men emerged in those aged between 40 and 60 years. In 2006, the prevalence of NAFLD in men aged between 40–50 and 50–60 years was 24.2% and 24.6%, respectively. In 2016, prevalence reached 42.8% and 46.6% (online supplemental table 2) for men. Distinct from men, the peak NAFLD prevalence in women emerged at an age above 60 years. In 2006, the prevalence of NAFLD in women older than 60 and 70 years was 25.6% and 22.9%, respectively. Eleven years later, these values had increased to 53.4% and 30.9% (online supplemental table 2).
Among the observed population, 5606 persons received annual health examinations for 11 years, and thus prevalence of NAFLD was analysed in these individuals. As shown in table 1, the prevalence of NAFLD increased from 17% in 2006 to 35.2% in 2016. The highest prevalence rates for NAFLD in 3795 men emerged in those older than 40 years. In 2006, the NAFLD prevalence in men aged between 40 and 50, 50 and 60 and 60 and 70 years was 24.2%, 25.4% and 19.8%, respectively. In 2016, these values reached 43.3%, 46.8% and 42.3% (table 1). Different from men, the peak NAFLD prevalence in 1811 women emerged at an age of more than 60 years. In 2006, the prevalence of NAFLD in women older than 60 and 70 years was 22.1% and 28%, respectively. Eleven years later, these values had increased to 31.8% and 28.6%(table 1).
Table 1.
Prevalence of NAFLD in 5606 persons with 11-year follow-up (2006–2016)
| 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | |
| Total (n) | 5606 | 5606 | 5606 | 5606 | 5606 | 5606 | 5606 | 5606 | 5606 | 5606 | 5606 |
| NAFLD (n) | 951 | 1068 | 1247 | 1322 | 1378 | 1466 | 1524 | 1574 | 1700 | 1883 | 1976 |
| (%) | 17 | 19.1 | 22.2 | 23.6 | 24.6 | 26.2 | 27.2 | 28.1 | 30.3 | 33.6 | 35.2 |
| Male (n) | 3795 | 3795 | 3795 | 3795 | 3795 | 3795 | 3795 | 3795 | 3795 | 3795 | 3795 |
| NAFLD (n) | 776 | 871 | 1014 | 1075 | 1117 | 1206 | 1244 | 1283 | 1387 | 1517 | 1569 |
| (%) | 20.4 | 23 | 26.7 | 28.3 | 29.4 | 31.8 | 32.8 | 33.8 | 36.5 | 40 | 41.3 |
| ≤30 years (n) | 668 | 466 | 366 | 297 | 246 | 204 | 142 | 67 | 14 | 4 | 0 |
| NAFLD (n) | 97 | 71 | 57 | 46 | 35 | 35 | 30 | 17 | 3 | 1 | 0 |
| (%) | 14.5 | 15.2 | 15.6 | 15.5 | 14.2 | 17.2 | 21.1 | 25.4 | 21.4 | 25 | 0 |
| >30, ≤40 years (n) | 1212 | 1315 | 1342 | 1258 | 1180 | 1091 | 1034 | 981 | 896 | 778 | 668 |
| NAFLD (n) | 231 | 286 | 349 | 349 | 338 | 330 | 330 | 331 | 331 | 301 | 261 |
| (%) | 19.1 | 21.7 | 26 | 27.7 | 28.6 | 30.2 | 31.9 | 33.7 | 36.9 | 38.7 | 39.1 |
| >40, ≤50 years (n) | 873 | 863 | 842 | 928 | 988 | 1022 | 1097 | 1128 | 1170 | 1201 | 1212 |
| NAFLD (n) | 211 | 215 | 239 | 278 | 314 | 353 | 388 | 411 | 454 | 524 | 525 |
| (%) | 24.2 | 24.9 | 28.4 | 30 | 31.8 | 34.5 | 35.4 | 36.4 | 38.8 | 43.6 | 43.3 |
| >50, ≤60 years (n) | 562 | 623 | 696 | 741 | 776 | 826 | 796 | 798 | 820 | 851 | 873 |
| NAFLD (n) | 143 | 184 | 231 | 262 | 276 | 314 | 292 | 286 | 320 | 363 | 409 |
| (%) | 25.4 | 29.5 | 33.2 | 35.4 | 35.6 | 38 | 36.7 | 35.8 | 39 | 42.7 | 46.8 |
| >60, ≤70 years (n) | 368 | 382 | 373 | 348 | 346 | 356 | 398 | 465 | 496 | 521 | 562 |
| NAFLD (n) | 73 | 85 | 97 | 95 | 96 | 108 | 125 | 156 | 178 | 210 | 238 |
| (%) | 19.8 | 22.3 | 26 | 27.3 | 27.7 | 30.3 | 31.4 | 33.5 | 35.9 | 40.3 | 42.3 |
| >70 years | 112 | 146 | 176 | 223 | 259 | 296 | 328 | 356 | 399 | 440 | 480 |
| NAFLD (n) | 21 | 30 | 41 | 45 | 58 | 66 | 79 | 82 | 101 | 118 | 136 |
| (%) | 18.8 | 20.5 | 23.3 | 20.2 | 22.4 | 22.3 | 24.1 | 23 | 25.3 | 26.8 | 28.3 |
| Female (n) | 1811 | 1811 | 1811 | 1811 | 1811 | 1811 | 1811 | 1811 | 1811 | 1811 | 1811 |
| NAFLD (n) | 175 | 197 | 233 | 247 | 261 | 260 | 280 | 291 | 313 | 366 | 407 |
| (%) | 9,7 | 10,9 | 12,9 | 13,6 | 14,4 | 14,4 | 15,5 | 16,1 | 17,3 | 20,2 | 22,5 |
| ≤30 years (n) | 85 | 44 | 22 | 13 | 9 | 9 | 5 | 2 | 0 | 0 | 0 |
| NAFLD (n) | 3 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| (%) | 3.5 | 2.3 | 4.5 | 7.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| >30, ≤40 years (n) | 582 | 578 | 541 | 480 | 403 | 334 | 277 | 213 | 173 | 124 | 85 |
| NAFLD (n) | 17 | 21 | 25 | 22 | 24 | 18 | 18 | 13 | 15 | 14 | 12 |
| (%) | 2.9 | 3.6 | 4.6 | 4.6 | 6 | 5.4 | 6.5 | 6.1 | 8.7 | 11.3 | 14.1 |
| >40, ≤50 years (n) | 485 | 469 | 482 | 496 | 541 | 580 | 612 | 638 | 617 | 608 | 582 |
| NAFLD (n) | 31 | 31 | 35 | 41 | 47 | 54 | 61 | 66 | 65 | 81 | 86 |
| (%) | 6.4 | 6.6 | 7.3 | 8.3 | 8.7 | 9.3 | 10 | 10.3 | 10.5 | 13.3 | 14.8 |
| >50, ≤60 years (n) | 365 | 400 | 422 | 450 | 461 | 469 | 456 | 452 | 467 | 476 | 485 |
| NAFLD (n) | 56 | 67 | 85 | 86 | 90 | 88 | 88 | 83 | 88 | 98 | 109 |
| (%) | 15.3 | 16.8 | 20.1 | 19.1 | 19.5 | 18.8 | 19.3 | 18.4 | 18.8 | 20.6 | 22.5 |
| >60, ≤70 years (n) | 244 | 260 | 262 | 267 | 266 | 266 | 280 | 301 | 314 | 337 | 365 |
| NAFLD (n) | 54 | 62 | 61 | 66 | 69 | 66 | 70 | 81 | 82 | 104 | 116 |
| (%) | 22.1 | 23.8 | 23.3 | 24.7 | 25.9 | 24.8 | 25 | 26.9 | 26.1 | 30.9 | 31.8 |
| >70 years (n) | 50 | 60 | 82 | 105 | 131 | 153 | 181 | 205 | 240 | 266 | 294 |
| NAFLD (n) | 14 | 15 | 26 | 31 | 31 | 34 | 43 | 48 | 63 | 69 | 84 |
| (%) | 28 | 25 | 31.7 | 29.5 | 23.7 | 22.2 | 23.8 | 23.4 | 26.3 | 25.9 | 28.6 |
NAFLD, non-alcoholic fatty liver disease.
Body mass index (BMI) and NAFLD incidence
Given the tight link between obesity and NAFLD, we paid special attention to the population with high BMI. We focused on the 5606 persons with complete follow-up and analysed the prevalence of NAFLD in those with BMI>25. In total, out of the 5606 persons, 2445 presented with a BMI of >25. The prevalence of NAFLD in this overweight subpopulation was far higher than in the general population. In 2006, 45.2% of individuals (n=1104; man vs woman: 47.3% vs 37.1%) with BMI>25 were suffering from NAFLD (online supplemental table 3). In 2016, values reached 67.1% (n=1414; man vs woman: 69% vs 59.2%, (online supplemental table 3). Impressively, the NAFLD prevalence in both genders was very high at any age, even in those below the age of 30 years. In 2006, among 213 overweight men, younger than 30 years, 52.6% were also diagnosed for NAFLD (online supplemental table 3). This number increased to 63% in 2016 (online supplemental table 3). In 2006, there were 15 overweight women aged less than 30 years. Among them, three presented as NAFLD (20%). In 2016, 7 out of 16 overweight women aged less than 30 years were identified. The NALFD prevalence had increased to 43.8% (online supplemental table 3). In those older than 40 years, NAFLD prevalence increased from 36.6%–45.4% in 2006 to 53%–65.6% in 2016 (online supplemental table 3).
Risk factors relevant to NAFLD occurrence
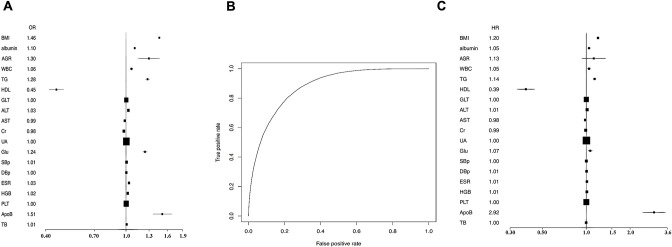
Next, we analysed risk factors relevant to NAFLD occurrence. Logistic regression analysis was performed on 26 parameters, including gender, age, BMI, albumin to globulin ratio, white blood cell count (WBC), low-density lipoprotein (LDL), triglycerides (TG), high-density lipoprotein (HDL), glutamyl transpeptidase (GLT), alanine transaminase (ALT), aspartate transaminase (AST), creatinine (Cr), alkaline phosphatase, blood urea nitrogen (BUN), uric acid (UA), blood glucose (Glu), systolic blood pressure (SBp), diastolic blood pressure (DBp), blood sedimentation (ESR), haemoglobin (HGB), platelets (PLT), apolipoprotein A1 (ApoA1), apolipoprotein B2 (ApoB), total bilirubin and total protein (TP). We performed variable selection by penalised logistic regression using R package glmnet. Cross validation selected 16 variables as potential predictors. These were BMI, albumin, WBC, TG, HDL, GLT, ALT, Cr, UA, Glu, SBp, DBp, ESR, HGB, PLT and ApoB (online supplemental table 4). The corresponding forest plot is shown in figure 2A. Among these variables, ApoB and BMI displayed the most robust positive correlation with NAFLD occurrence, while HDL had a strong negative correlation with NAFLD incidence (online supplemental table 4). The AUC of these variables for NAFLD is 0.88 (see ROC curve in figure 2B). We further performed a time-dependent Cox regression to calculate the HRs of these parameters for NAFLD occurrence. Cox regression confirmed that the 16 parameters were significantly relevant to NAFLD incidence (online supplemental table 5 and figure 2C). Furthermore, ApoB and HDL were the most robust positive and negative risk factors for NAFLD (figure 2C).
Figure 2.
Penalised logistic regression and Cox regression analysis were performed for risk factors and HRs of non-alcoholic fatty liver disease (NAFLD). The following parameters were available from 5606 participants: gender, age, BMI, albumin, albumin to globulin ratio (AGR), white blood cell (WBC), low-density lipoprotein (LDL), triglycerides (TG), high-density lipoprotein (HDL), glutamyl transpeptidase (GLT), alanine transaminase (ALT), aspartate transaminase (AST), creatinine (Cr), alkaline phosphatase (ALP), blood urea nitrogen, uric acid (UA), blood glucose (Glu), systolic blood pressure (SBp), diastolic blood pressure (DBp), blood sedimentation (ESR), haemoglobin (HGB), platelets (PLT), apolipoprotein A1 (ApoA1), apolipoprotein B2 (ApoB), total bilirubin (TB) and total protein (TP). Cross validation selected 16 variables to be potential predictors. The corresponding forest plot is shown in (A). The AUC of these above 16 variables for NAFLD is 0.88 (B). Cox regression confirmed that the 16 variables were relevant for NAFLD incidence, including BMI, albumin, WBC, TG, HDL, GLT, ALT, Cr, UA, Glu, SBp, DBp, ESR, HGB, PLT and ApoB. The corresponding forest plot is shown (C).
Cause-effect link between risk factors and NAFLD occurrence
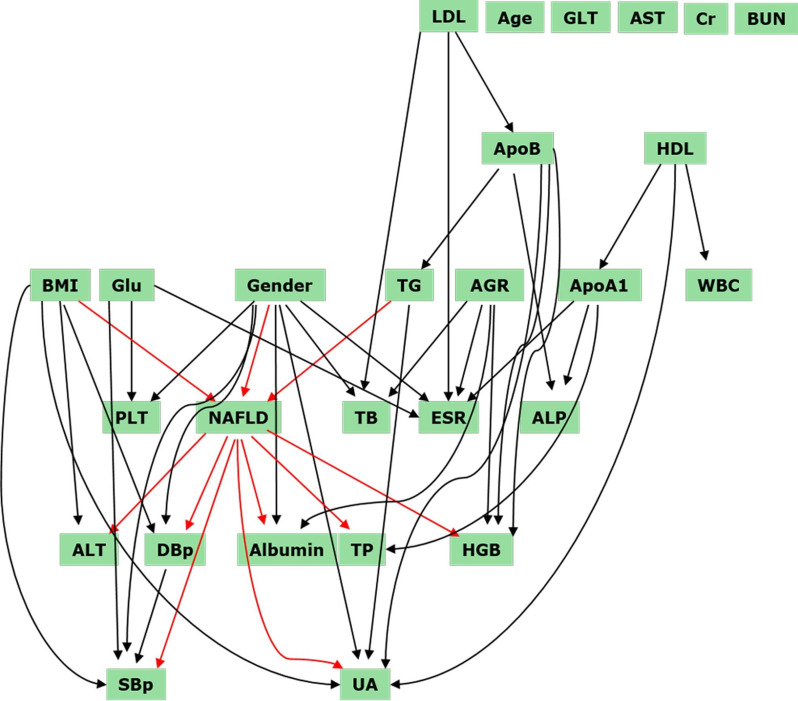
Although the afore-mentioned parameters were regarded as ‘risk factors’ according to statistical models, it did not necessarily mean that all of them contributed to NAFLD occurrence. Based on 11 years of longitudinal data, it was possible to construct a dynamic Bayesian network to identify the risk factors most relevant to NAFLD occurrence. As shown in figure 3, these parameters constituted a complicated, but clear intercross paradigm. Only three parameters, BMI, gender and TG, directly pointed to NAFLD. In addition, ApoB impacted the incidence of NAFLD through contributing to TG. Furthermore, LDL can indirectly contribute to NAFLD through influencing ApoB. Very impressively, the dynamic Bayesian network pointed out that NAFLD directly leads to alterations of seven parameters: ALT, DBp, SBp, TP, albumin, HGB and UA. Intriguingly, age, GLT, AST, Cr and BUN did not interact with any other parameter in our model, indicating that these factors correlate by incidence, but there is no causal interaction.
Figure 3.
Dynamic Bayesian network analyses were performed to show the cause-effect link between non-alcoholic fatty liver disease (NAFLD) and its potential risk factors. Three variables, body mass index (BMI), gender and triglycerides (TG) directly pointed to NAFLD. Apolipoprotein B2 (ApoB) impacted on the incidence of NAFLD through TG abundance. Low-density lipoprotein (LDL) indirectly contributed to NAFLD through ApoB. NAFLD directly led to alterations of seven clinical parameters: alanine transaminase (ALT), diastolic blood pressure (DBp), systolic blood pressure (SBp), total protein (TP), albumin, haemoglobin (HGB) and uric acid (UA). ALP, alkaline phosphatase; ApoA1, apolipoprotein A1; BUN, blood urea nitrogen; Cr, creatinine; ESR, blood sedimentation; GLT, glutamyl transpeptidase; Glu, blood glucose; HDL, high-density lipoprotein; PLT, platelets; TB, total bilirubin; WBC, white blood cell; AGR, albumin to globulin ratio.
Outcome of NAFLD
Subsequently, we examined clinical outcomes of NAFLD over the 11 years. Table 2 summarises the incidence of intrahepatic and extrahepatic diseases of 696 NAFLD patients during the follow-up period. Among the NAFLD population, only one male patient with NAFLD developed liver cirrhosis within the 11 years. However, this time span witnessed significantly increased extrahepatic diseases, including diabetes, hypertension and hyperuricaemia. In the NAFLD population, there were 64 (12.6%) men and 22 (11.6%) women, 191 (37.7%) men and 85 (44.7%) women, 72 (14.2%) men and 43 (22.6%) women who developed into type 2 diabetes, hypertension and hyperuricaemia, respectively (table 2).
Table 2.
Clinical outcome of patients with NAFLD
| 2006–2016 | |||||
| Cirrhosis | HCC | Diabetes, n (%) | Hypertension, n (%) | Hyperuricaemia, n (%) | |
| Male (n=506) | 1 | 0 | 64 (12.6) | 191 (37.7) | 72 (14.2) |
| Female (n=190) | 0 | 0 | 22 (11.6) | 85 (44.7) | 43 (22.6) |
| 2007–2016 (outcome of new NAFLD) | ||||||||
| Cirrhosis | HCC | Diabetes | Hypertension | Hyperuricaemia | ||||
| n (%) | P value | n (%) | P value | n (%) | P value | |||
| Male | ||||||||
| NAFLD n=138 | 0 | 0 | 14 (10.1) | 0.028 | 47(34.1) | <0.001 | 34 (24.6) | <0.001 |
| Non-NAFLD n=2786 | 0 | 0 | 157 (5.6) | 259 (9.3) | 284 (10.2) | |||
| Female | ||||||||
| NAFLD n=47 | 0 | 0 | 5 (10.6) | 0.014 | 21 (44.7) | <0.001 | 8 (17) | <0.001 |
| Non-NAFLD n=1761 | 0 | 0 | 54 (3.1) | 324 (18.4) | 84 (4.8) | |||
HCC, hepatocellular carcinoma; NAFLD, non-alcoholic fatty liver disease.
Given that 2006 is the starting point of data collection, patients diagnosed for NAFLD in this year had by no means just manifested their disease, but rather patients had possibly developed NAFLD several years prior to inclusion. To clarify the exact clinical outcomes of NAFLD over one decade, we focused on the following two cohorts of individuals with annual health examinations for 11 years: (1) patients who were diagnosed as non-NAFLD in 2006, but were NAFLD in 2007 (new NAFLD cohort) and (2) who were non-NAFLD in both 2006 and 2007 (non-NAFLD cohort). As shown in figure 2, 185 new NAFLD cases (138 men and 47 women) and 4547 non-NAFLD (2786 men and 1761 women) persons were found in 2007. Between 2007 and 2016, neither NAFLD nor non-NAFLD individuals developed liver cirrhosis or cancer. However, the one-decade follow-up reveals different prevalences of diabetes, hypertension and hyperuricaemia: in patients with NAFLD, there were 14 (10.1%) men and 5 (10.6%) women, 47 (34.1%) men and 21 (44.7%) women, 34 (24.6%) men and 8 (17%) women who developed type 2 diabetes, hypertension and hyperuricaemia, respectively(table 2). In non-NAFLD individuals, 157 (5.6%) men and 54 (3.4%) women, 259 (9.3%) men and 324 (18.4%), 284 (10.2%) men and 84 women (4.8%) developed type 2 diabetes, hypertension and hyperuricaemia, respectively (table 2). For all three diseases, statistically significant differences were determined between the two cohorts of population (all p<0.05, table 2). These results suggest that diabetes, hypertension and hyperuricaemia are the main clinical outcomes of NAFLD.
Discussion
This 11-year follow-up retrospective study reports the following: (1) NAFLD prevalence has substantially increased in the examined Eastern Chinese population. (2) The prevalence of NAFLD differs by gender and age. Middle-aged men and elderly women are the two populations at highest risk for NAFLD. (3) Gender, BMI and TG are the parameters directly associated with NAFLD occurrence. Regardless of gender and age, persons with high BMI (≥25) have a high risk for NAFLD development. (4) NAFLD directly leads to alterations of seven clinical parameters: ALT, DBp, SBp, TP, albumin, HGB and UA. (5) Within 11 years, a significant part of the NAFLD population develops three clinically relevant diseases: T2DM, hypertension and hyperuricaemia. (6) Within 11 years, NAFLD does not cause severe liver disease, such as cirrhosis or HCC, in patients.
The most impressive observation of the current study is that among 918 affected persons, no patient progressed towards HCC and only 1 male patient with NAFLD developed liver cirrhosis within the 11 years. Furthermore, among 185 new NAFLD cases diagnosed in 2007, none developed liver cirrhosis or liver cancer. Liver cirrhosis and HCC are commonly regarded as the most severe and costly clinical outcomes of NAFLD.9 In the USA and Europe, it is estimated that 10%–15% of patients with NAFLD develop advanced fibrosis.10 In China, a study with biopsy-proven NAFLD revealed 1.97%–2.97% cirrhosis prevalence.11 In addition, NAFLD is regarded as the third most common cause of cancer-related death worldwide.12 In a study based on 4949 US patients with HCC, 701 patients had NAFLD.13 It was estimated that the cumulative incidence of HCC among patients with NAFLD and cirrhosis ranges from 2.4% to 12.8% over a median follow-up period of 3.2–7.2 years14 (Global Health Observatory data). Mortality and global health estimates were obtained from: http://www.who.int/gho/mortality_burden_disease/en/, last accessed on 1 July 2020. Given that the above conclusions were based on cross-sectional investigations and statistical models, it has been unknown to date over which period a patient with NAFLD develops liver cirrhosis or HCC (personal risk assessment). Our 11-year follow-up provides therefore a valuable and comprehensive dataset. In this study, most patients were diagnosed with NAFLD when they received a routine health examination. Before the examination, these people did not have any symptoms or signs of NAFLD. Therefore, they belong to patients with NAFLD at a very early stage (although for 2006, the duration of pre-existing NAFLD cannot be determined). Except for a single person, no serious liver problems were observed within this time period. These data suggest that for the vast majority of patients with early stage NAFLD, 11 years are not sufficient to develop liver cirrhosis or cancer. Nasr et al followed up 129 patients with NAFLD with varying fibrosis stages on two occasions (mean time 13.7 and 9.3 years). Liver biopsy analyses showed that 9.3% of patients developed end-stage liver disease and 34% advanced fibrosis.15 The patients with NAFLD observed by Nasr et al actually belonged to the NASH category, because they suffered from fibrosis and elevated ALT and/or AST levels. As our study was based on examinations of healthy individuals, liver biopsy is not justifiable. Very likely, the current cohort included a portion of NASH patients. They also did not show significant progression towards cirrhosis or HCC.
In contrast to hepatic complications, patients with NAFLD showed a significant risk for the development of extrahepatic diseases, including diabetes, hypertension and hyperuricaemia. In 696 patients with NAFLD, 11 years witnessed the development of 86 cases (12.4%) type 2 diabetes, 276 (40%) cases of hypertension and 115 (16.5%) patients with hyperuricaemia, respectively. Interestingly, in 222 NASH patients, the prevalence of these three diseases was 12 (5.4%), 46 (20.7%) and 33 (14.9%) only. In general, men had a higher probability to develop these diseases than women. These results are consistent with previous reports from the USA and Europe.16–18 Whether NAFLD is associated with the risk of severe heart or brain diseases such as acute myocardial infarction (AMI) and stroke is worth further investigation. A recent matched cohort study analysed databases from four European countries, which included 17.7 million patients with NAFLD or NASH.19 These patients had a mean follow-up of 2.1–5.5 years. The study showed that the diagnosis of NAFLD appears not to be associated with AMI or stroke risk after adjustment for established CVD risk factors. Nevertheless, the authors mentioned that CVD risk assessment in adults with a diagnosis of NAFLD is important.19 Follow-up for 5 years might be not sufficient to reach a conclusion for this issue.
An important issue is the cause-and-effect relationship between NAFLD and its clinical outcomes such as diabetes, hypertension and hyperuricaemia. A dynamic Bayesian network in the current study provides direct evidence on this issue: NAFLD directly results in alterations of several parameters, including DBp, SBp and UA, suggesting that NAFLD directly contributes to the occurrence of hypertension and hyperuricaemia. The underlying mechanisms require further investigation.
The current dynamic Bayesian network analysis does not confirm a direct cause-and-effect relationship between NAFLD and type 2 diabetes mellitus. There are plenty of studies showing the close relationship between type 2 diabetes and NALFD.20 Pathophysiologically, insulin resistance is a key event in both NAFLD and diabetes progression.20 However, genome-wide association studies have not yet identified the exact impact of insulin resistance on the variants associated with NAFLD severity.20 21 Clarification of the cause-and-effect relationship between NAFLD and diabetes requires further long-term follow-up studies.
To date, there are a large number of studies investigating risk factors for NAFLD.22 These studies tried to identify single, or multiple combined biomarkers to predict NAFLD occurrence. Given that most studies were based on cross-sectional designs, or with only short follow-up periods, it is difficult to clarify the causality between the proposed predictors and NAFLD morbidity. Our 11-year dataset provides a chance to shed led on this issue. Here, the dynamic causal relationships between variables, including risk parameters and clinical outcomes, were identified by a first-order Markov model, which was displayed by a dynamic Bayes network. The dynamic Bayes model discriminates causal relationship through time sequence. When a variable change is closely related to a previous variance alteration, a causal relationship between the two variables is assumed. Based on logistic and Cox regression and dynamic Bayesian network analyses, we confirmed three direct risk factors for NAFLD occurrence: gender, BMI and TG. These findings are supported by the following data: (1) Men have higher NAFLD prevalence than women in this population (37% vs 22.2% in 2016); (2) In overweight people with a BMI>25, NAFLD prevalence reached 69% in men and 59.2% in women. Given that TG are a major energy source, but are leading to obesity, it is not surprising that this parameter directly reflects the risk for NAFLD development. These findings provide robust evidence supporting the use of BMI to monitor or predict NAFLD.
Conclusion
This 11-year follow-up study documents the rapid increase in NAFLD prevalence in an Eastern Chinese population. In contrast to previous reports, we does not observe that one decade of NAFLD is sufficient to lead to severe hepatic clinical outcomes. It is worthy to note that our population represents a biased selection because they are on the well-off, well-educated side of the Chinese people, while previous studies were often based on hospital populations, who suffered from negative selection bias and thus came up with higher estimates. In addition, given there are differences in NAFLD profiles between Eastern and Western populations, it would be interesting to know the natural development of NAFLD in a Western population. A key point for clarifying the true history of NAFLD is to follow a population starting from the early phases of the disease. Consistent with previous studies, NAFLD is tightly associated with multiple extrahepatic diseases relevant to the metabolic syndrome. In the future, follow-up of the current cohort for another one and two decades will provide further valuable data to clarify the extended natural history of NAFLD. Last but not least, a large portion of the men and women in this study were educated above the average and have a position in the company that provided them with better food choices as well as regular sport. On the other hand, the relatively low sensitivity of ultrasound for the detection of liver fat might underestimate the incidence of NAFLD in this cohort.
Supplementary Material
Acknowledgments
We are grateful to Drs Changxi Chen, Jingyi Yuan and Yongjun Chen for support and discussion.
Footnotes
XT, YS and JD contributed equally.
Contributors: Conception and design: XT, YS, JD, H-LW, JJ and TH. Ultrasonography: LC and FL. Blood assays: YZ and HZ. Other examinations and data collection: XT, KH, TZ, JD, ZZ and TH. Statistical analyses: YS and JJ. Drafting the article: H-LW. Reviewing and editing the article critically: XT, YS, CM, RL, SD, H-LW, JJ and TH. Guarantor of the work: TH.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s)
Ethics approval
This study involves human participants and was approved by the Ethics Committee of Zhenhai Lianhua Hospital No. 2016(001). Informed consent was obtained from all subjects. Participants gave informed consent to participate in the study before taking part.
References
- 1.Younossi ZM, Tampi R, Priyadarshini M, et al. Burden of illness and economic model for patients with nonalcoholic steatohepatitis in the United States. Hepatology 2019;69:564–72. 10.1002/hep.30254 [DOI] [PubMed] [Google Scholar]
- 2.Younossi ZM, Loomba R, Anstee QM, et al. Diagnostic modalities for nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, and associated fibrosis. Hepatology 2018;68:349–60. 10.1002/hep.29721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan J-G, Kim S-U, Wong VW-S. New trends on obesity and NAFLD in Asia. J Hepatol 2017;67:862–73. 10.1016/j.jhep.2017.06.003 [DOI] [PubMed] [Google Scholar]
- 4.Byrne CD, Targher G. Nafld: a multisystem disease. J Hepatol 2015;62:S47–64. 10.1016/j.jhep.2014.12.012 [DOI] [PubMed] [Google Scholar]
- 5.Alexander M, Loomis AK, van der Lei J, et al. Risks and clinical predictors of cirrhosis and hepatocellular carcinoma diagnoses in adults with diagnosed NAFLD: real-world study of 18 million patients in four European cohorts. BMC Med 2019;17:95. 10.1186/s12916-019-1321-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou F, Zhou J, Wang W, et al. Unexpected rapid increase in the burden of NAFLD in China from 2008 to 2018: a systematic review and meta-analysis. Hepatology 2019;70:1119–33. 10.1002/hep.30702 [DOI] [PubMed] [Google Scholar]
- 7.European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO) . EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64:1388–402. 10.1016/j.jhep.2015.11.004 [DOI] [PubMed] [Google Scholar]
- 8.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American association for the study of liver diseases. Hepatology 2018;67:328–57. 10.1002/hep.29367 [DOI] [PubMed] [Google Scholar]
- 9.Younossi Z, Tacke F, Arrese M, et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 2019;69:2672–82. 10.1002/hep.30251 [DOI] [PubMed] [Google Scholar]
- 10.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73–84. 10.1002/hep.28431 [DOI] [PubMed] [Google Scholar]
- 11.Shen F, Zheng R-D, Shi J-P, et al. Impact of skin capsular distance on the performance of controlled attenuation parameter in patients with chronic liver disease. Liver Int 2015;35:2392–400. 10.1111/liv.12809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ascha MS, Hanouneh IA, Lopez R, et al. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010;51:1972–8. 10.1002/hep.23527 [DOI] [PubMed] [Google Scholar]
- 13.Younossi ZM, Otgonsuren M, Henry L, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology 2015;62:1723–30. 10.1002/hep.28123 [DOI] [PubMed] [Google Scholar]
- 14.White DL, Kanwal F, El-Serag HB. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol 2012;10:e1342:1342–59. 10.1016/j.cgh.2012.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nasr P, Ignatova S, Kechagias S, et al. Natural history of nonalcoholic fatty liver disease: a prospective follow-up study with serial biopsies. Hepatol Commun 2018;2:199–210. 10.1002/hep4.1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Estes C, Razavi H, Loomba R, et al. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018;67:123–33. 10.1002/hep.29466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williamson RM, Price JF, Glancy S, et al. Prevalence of and risk factors for hepatic steatosis and nonalcoholic fatty liver disease in people with type 2 diabetes: the Edinburgh type 2 diabetes study. Diabetes Care 2011;34:1139–44. 10.2337/dc10-2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Targher G, Bertolini L, Padovani R, et al. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care 2007;30:1212–8. 10.2337/dc06-2247 [DOI] [PubMed] [Google Scholar]
- 19.Alexander M, Loomis AK, van der Lei J, et al. Non-Alcoholic fatty liver disease and risk of incident acute myocardial infarction and stroke: findings from matched cohort study of 18 million European adults. BMJ 2019;367:l5367. 10.1136/bmj.l5367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tilg H, Moschen AR, Roden M. Nafld and diabetes mellitus. Nat Rev Gastroenterol Hepatol 2017;14:32–42. 10.1038/nrgastro.2016.147 [DOI] [PubMed] [Google Scholar]
- 21.Zhou Y, Llauradó G, Orešič M, et al. Circulating triacylglycerol signatures and insulin sensitivity in NAFLD associated with the E167K variant in TM6SF2. J Hepatol 2015;62:657–63. 10.1016/j.jhep.2014.10.010 [DOI] [PubMed] [Google Scholar]
- 22.Miyake T, Kumagi T, Furukawa S, et al. Non-Alcoholic fatty liver disease: factors associated with its presence and onset. J Gastroenterol Hepatol 2013;28 Suppl 4:71–8. 10.1111/jgh.12251 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-054891supp001.pdf (109.2KB, pdf)
Data Availability Statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information.