Abstract
Methaemoglobinaemia is defined as elevated methaemoglobin in the blood which is characterised by conversion of some of the reduced ferrous iron elements [Fe2+] to the oxidised ferric [Fe3+] form which does not have capacity to bind and transport oxygen resulting in functional anaemia. Causes can be genetic mutations or acquired by medications such as dapsone, nitrates or benzocaine. Benzocaine is currently being used as a topical anaesthetic agent before certain procedures. We report a case of benzocaine spray–induced methaemoglobinaemia in a patient who underwent oesophagogastroduodenoscopy for evaluation of upper gastrointestinal bleeding.
Keywords: Endoscopy, Haematology (incl blood transfusion), Adult intensive care, Unwanted effects / adverse reactions
Background
Methaemoglobinaemia is a dangerous medical condition which can cause symptoms including, but not limited to, shortness of breath, headache, dizziness, nausea or vomiting. If severe, it can end with cyanosis, seizures or fatal cardiac arrhythmias.1 It is of high importance to recognise the clinical signs and symptoms of this condition in a timely manner in order to proceed with treatment which can be lifesaving and prevent deterioration. Since 1955, it has been reported that benzocaine as a preoperative anaesthetic agent can cause methaemoglobinaemia which can be fatal.2 We present a case of a patient who received preoperative benzocaine for oesophagogastroduodenoscopy (OGD) which resulted in asymptomatic methaemoglobinaemia that resolved with only one time injection of methylene blue (MB) with no consequences.
Case presentation
A man in his mid-50s with a history of Williams syndrome and class I obesity (weight: 77.8 kg, body mass index: 30 kg/m2) was admitted to the hospital for an elective cystoscopy, right ureteroscopy with laser lithotripsy and right ureteral stent exchange. During his hospital stay, anticoagulation with enoxaparin was resumed for treatment of his previous right lower limb thrombosis. During the hospital stay, it was incidentally found that he had black tarry stool with a haemoglobin drop from 11.6 to 7.5 g/dL. Gastroenterology was consulted and recommended stopping anticoagulation and pursuing an OGD which was performed under sedation by intravenous midazolam 3 mg and benzocaine spray 20% 2 puffs ‘brand name: HurriCaine 20%, dosage 2 puffs at once; maximum 4 puffs daily’. The procedure was performed successfully without initial complications, but while the patient was in the recovery room, he suddenly developed hypoxia with oxygen saturation of 92% that slowly dropped further to 85%. During hypoxia, his vital signs were blood pressure: 167/77 mm Hg, respiratory rate: 24 breath/min, heart rate: 111 beat/min and temperature: 36.6ºC. The patient was comfortable, alert, oriented and completely asymptomatic. On physical examination, he was not cyanotic, and he did not show any signs of respiratory distress, heart and lung auscultation revealed normal findings with no added sounds. His oxygen saturation did not respond to oxygen supply via non-rebreather mask.
Investigations
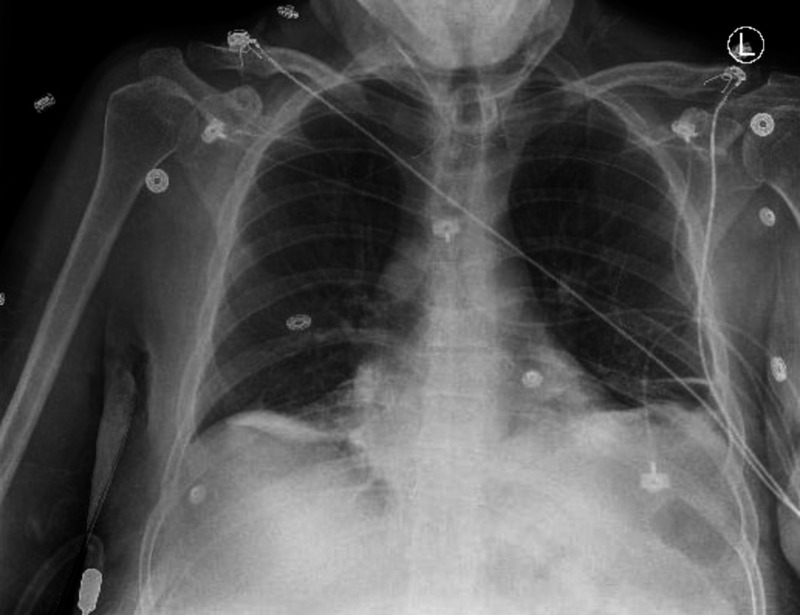
Venous blood gas (VBG) was done and showed pH of 7.39 (7.35–7.45), PCO2 of 37.9 mm Hg (36–45), PaO2 of 21.4 mm Hg (36–40), venous oxygen saturation of 43.8% (63%–75%) and methaemoglobin of 26.7% (normal <3%). Arterial blood gas (ABG) would be more informative in patients with hypoxia; however, our sample was accidentally venous but adequately diagnosed the patient’s condition. Chest X-ray (CXR) showed bibasilar subsegmental atelectasis that was worse in the right lung base (figure 1). The OGD showed hiatal hernia, distal oesophageal ring, diffuse gastritis, two non-bleeding gastric ulcers and marked duodenitis with multiple duodenal ulcers, which were managed conservatively with oral pantoprazole 40 mg daily. Of notice, blood gas analysis with co-oximetry must be obtained if methaemoglobinaemia is suspected; however, at our institution, methaemoglobin level is included in standard blood gas analysis. In case ABG obtained without methaemoglobin level, mismatches between SpO2 and either PaO2 (PaO2-saturation gap) or SaO2 are indicative of haemoglobinopathies, most often methaemoglobinaemia.3
Figure 1.
Chest X-ray after the incident showing bibasilar subsegmental atelectasis worse in the right lung base.
Differential diagnosis
Differential diagnosis for post-procedural hypoxia is wide, including, but not limited to, respiratory causes (atelectasis, pneumothorax, aspiration, broncho/laryngospasm), cardiac causes (acute pulmonary oedema, acute heart failure, myocardial infarction), vascular causes (acute pulmonary embolism, poor perfusion/cold extremities), anaphylaxis. Detailed history, monitor vital signs and physical examination, along with proper laboratory and radiological workup, and elevated methaemoglobin level on VBG were able to diagnose methaemoglobinaemia and exclude the abovementioned differential diagnoses. Negative allergic history and lack of reporting aspiration or asphyxia events by medical staff during the procedure were again verified. Physical examination showed tachypnoea with no snoring, wheezing or jugular venous distension. Chest examination showed good bilateral air entry and no murmurs, gallops or signs of pneumothorax. Skin was warm with strong peripheral pulses. The patient did not show skin oedema, hives or swelling suggesting anaphylaxis. Recent echocardiogram on admission showed an ejection fraction of 60%. Bilateral subsegmental atelectasis on CXR would not explain the degree of hypoxia. This holistic approach was sufficient for a spot diagnosis of methaemoglobinaemia.
Treatment
Immediately after the VBG results, 75 mg intravenous MB was given over 15 min and oxygen saturation improved gradually from 85% to 95%. Patient’s oxygen supply switched from non-rebreather mask to nasal cannula and to room air 2 hours after MB treatment.
Outcome and follow-up
The patient was transferred to the intensive care unit (ICU) for continuous monitoring. He continued to be haemodynamically stable without any signs of respiratory distress. While being on room air, repeat ABG showed methaemoglobin level of 3.4% and the following day level of 1.8%. PaO2 was 188 mm Hg and 109 mm Hg on the two ABGs, respectively (80–100). He was transferred out from the ICU and discharged from the hospital in a stable condition.
Discussion
Carboxyhaemoglobin and methaemoglobin are normally found in small fractions in human’s blood, while most haemoglobin exists in the reduced state that is capable of carrying oxygen. The physiologic level of methaemoglobin is <=1% of total haemoglobin concentration, if more than 1% of haemoglobin is oxidised to methaemoglobin, oxygen-carrying capacity is affected.4 5
Although most patients remain asymptomatic until the methaemoglobin fraction is greater than 20% when patients start to complain of dyspnoea, anxiety, lightheadedness, dizziness, headache and nausea,6 7 it was reported that when methaemoglobin concentration exceeds 15%, cyanosis with a gray-brown hue that is unresponsive to oxygen may occur.1 When methaemoglobin levels are between 30% and 50%, tachypnoea, confusion and loss of consciousness can occur. When levels exceed 50%, patients can have more profound dyspnoea, hypoxia, bradycardia, seizures, metabolic acidosis, cardiac arrhythmias and coma. Levels above 70% are often rapidly fatal.8 A study was performed to evaluate methaemoglobinaemia incidence related to benzocaine use as a topical analgesic for transoesophageal echocardiography (TOE). The authors reported 19 methaemoglobinaemia cases during 90 months among 28 478 TOEs, with a mean±SD methaemoglobin level of 32%±15%. All patients were found to have cyanosis and hypoxia after the procedure.9 Our patient with a methaemoglobin fraction of 26.7% was in the typical range where patients have symptoms, but he remained completely asymptomatic which is unusual.
A similar case report published for a 51-year-old female patient who developed benzocaine-induced methaemoglobinaemia of 32.4% after taking benzocaine spray and lozenges for nasogastric tube discomfort relief. The patient had typical hypoxaemia to 79% on room air and tachycardia with a heart rate up to 124 beat/min. She had only symptoms of generalised fatigue despite significantly elevated methaemoglobin concentration.10
Benzocaine has been implicated to cause acquired methaemoglobinaemia when used as ointments, rectal suppositories or perineal ointments in infants,11 12 and when used as a lubricant in endotracheal, bronchoscopic and oesophageal procedures.13 14 Benzocaine can cause methaemoglobinaemia even if used in the recommended dose range as given in our patient. A study that included 242 patients who received local anaesthetics (LA) recommended abandoning benzocaine use because it can be impossible to predict susceptibility of developing methaemoglobinaemia after its exposure due to the unclear therapeutic window of the medication in susceptible individuals who might be at unexpected risk and the availability of safer options (lidocaine and more cautiously prilocaine).15
Topical anaesthetics are classified based on biochemical structure into two main categories: ester anaesthetics (EA) and amide anaesthetics (AA). Benzocaine spray belongs to the EA group and has been shown to induce methaemoglobinaemia in statistically significant more cases when compared with lidocaine spray (belongs to AA).16 Benzocaine metabolism leads to N-hydroxy metabolite which contains the alanine group that has been implicated in oxidising ferrous ions and increasing methaemoglobin production even when benzocaine is used at appropriate dosing.17 However, AA lacks this metabolism profile and is considered safer, so several institutions mandate the use of lidocaine spray as LA for procedures.18
Physiologic spontaneous formation of methaemoglobin in blood is controlled by the presence of counteracted regulatory enzymes; as nicotinamide adenine dinucleotide reductase hydrogen (NADH) methaemoglobin reductase (cytochrome-b5 reductase) and nicotinamide adenine dinucleotide phosphate reductase hydrogen (NADPH) methaemoglobin reductase.19 MB as a treatment for methaemoglobinaemia has a role of accepting an electron from NADPH by the enzyme NADPH methaemoglobin reductase, then transferring the electron to the excessive oxidised ferric iron, facilitating its conversion to its physiologic reduced state (ferrous iron).20
Intravenous MB is the treatment of choice in symptomatic patients or when methaemoglobin level is >20% even in asymptomatic patients.21 The recommended dosage of MB infusion is 1 mg/kg over 5–30 min.22 It is worth mentioning that MB can paradoxically cause methaemoglobinaemia if given in large doses (>7 mg/kg) or infused quickly (<5 min) by directly oxidising haemoglobin to methaemoglobin.23 24 Unfortunately, no clear guidelines on follow-up after MB infusion exist. It is recommended to measure methaemoglobin level 1 hour after MB infusion to assess response. Repeated dose of intravenous MB 1 mg/kg can be repeated if a patient continues to be symptomatic or methaemoglobin levels remain >20%. Rebound methaemoglobinaemia can occur up to 18 hours later, as benzocaine is a lipophilic agent and distributed in fat tissue. Frequent measurements of methaemoglobin level are recommended in the first 18–24 hours,25 which was the pursued practice in our case.
Despite MB being the first-line treatment for methaemoglobinaemia, its availability can be limited. Alternative options including hyperbaric oxygen (HBO) which works by promoting reduction of methaemoglobin to oxyhaemoglobin to allow for immediate reoxygenation.26 Examples include two methaemoglobinaemia cases successfully treated with HBO.27 28
Patient’s perspective.
“I was scared when the doctors told me about my condition after the procedure, I felt that the situation was more serious when they told me that I need to go to the ICU for continuous monitoring. What made it better is that I was totally fine and felt no pain, shortness of breath or any other complaints. Doctors reassured me that my condition had stabilized, and I was glad that everything went well.”
Learning points.
Benzocaine is a topical anaesthetic used before invasive procedures and is historically known to induce methaemoglobinaemia in certain patients with risk factors.
Methaemoglobinaemia is a life-threatening condition that if recognised early, is easily treated and has generally benign outcomes.
If methaemoglobin concentration is high, symptoms occur. Our patient had high methaemoglobin concentration and despite that, he remained asymptomatic.
In general, avoiding benzocaine is recommended due to its unclear therapeutic window and availability of safer alternatives.
Footnotes
Contributors: AJ did majority of editing, assigned roles to coauthors, obtained the consent from the patient’s sister and addressed the article after reviewers' comments. AAS provided efforts in the discussion part, proofreading the case presentation as well because he took care of the patient during admission and wrote cover letter. NM helped in editing and review, and made sure that references are accurate and another doctor who took care of the patient. SA worked on learning points and reviewed the manuscript to make sure that vocabulary and grammar are accurate again.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained from next of kin.
References
- 1.Ludlow JT, Wilkerson RG, Nappe TM. Methemoglobinemia. In: StatPearls. Treasure Island (FL): StatPearls Publishing, 2021. [PubMed] [Google Scholar]
- 2.Kaufman MR, Aouad RK. Benzocaine-Induced methemoglobinemia. J Emerg Med 2017;53:912–3. 10.1016/j.jemermed.2017.09.004 [DOI] [PubMed] [Google Scholar]
- 3.Koduri PR, Kedar PS, Warang P. Erythrocytosis, methemoglobinemia, and the saturation gap. Ann Hematol 2015;94:509–10. 10.1007/s00277-014-2179-9 [DOI] [PubMed] [Google Scholar]
- 4.Mansouri A, Lurie AA. Concise review: methemoglobinemia. Am J Hematol 1993;42:7–12. 2. 10.1002/ajh.2830420104 [DOI] [PubMed] [Google Scholar]
- 5.Hegedus F, Herb K. Benzocaine-induced methemoglobinemia. Anesth Prog 2005;52:136–9. 10.2344/0003-3006(2005)52[136:BM]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Umbreit J. Methemoglobin--it's not just blue: a concise review. Am J Hematol 2007;82:134–44. 10.1002/ajh.20738 [DOI] [PubMed] [Google Scholar]
- 7.Jaffé ER. Methemoglobin pathophysiology. Prog Clin Biol Res 1981;51:133–51. [PubMed] [Google Scholar]
- 8.Wilkerson RG. Getting the blues at a rock concert: a case of severe methaemoglobinaemia. Emerg Med Australas 2010;22:466–9. 10.1111/j.1742-6723.2010.01336.x [DOI] [PubMed] [Google Scholar]
- 9.Kane GC, Hoehn SM, Behrenbeck TR, et al. Benzocaine-induced methemoglobinemia based on the Mayo clinic experience from 28 478 transesophageal echocardiograms: incidence, outcomes, and predisposing factors. Arch Intern Med 2007;167:1977–82. 10.1001/archinte.167.18.1977 [DOI] [PubMed] [Google Scholar]
- 10.Sewell CR, Rivey MP. A case report of Benzocaine-Induced methemoglobinemia. J Pharm Pract 2018;31:507–9. 10.1177/0897190017723211 [DOI] [PubMed] [Google Scholar]
- 11.Spielman FJ, Anderson JA, Terry WC. Benzocaine-induced methemoglobinemia during general anesthesia. J Oral Maxillofac Surg 1984;42:740–3. 10.1016/0278-2391(84)90424-5 [DOI] [PubMed] [Google Scholar]
- 12.Ferraro-Borgida MJ, Mulhern SA, DeMeo MO, et al. Methemoglobinemia from perineal application of an anesthetic cream. Ann Emerg Med 1996;27:785–8. 10.1016/S0196-0644(96)70203-2 [DOI] [PubMed] [Google Scholar]
- 13.Novaro GM, Aronow HD, Militello MA, et al. Benzocaine-induced methemoglobinemia: experience from a high-volume transesophageal echocardiography laboratory. J Am Soc Echocardiogr 2003;16:170–5. 10.1067/mje.2003.5 [DOI] [PubMed] [Google Scholar]
- 14.Clary B, Skaryak L, Tedder M, et al. Methemoglobinemia complicating topical anesthesia during bronchoscopic procedures. J Thorac Cardiovasc Surg 1997;114:293–5. 10.1016/S0022-5223(97)70163-6 [DOI] [PubMed] [Google Scholar]
- 15.Guay J. Methemoglobinemia related to local anesthetics: a summary of 242 episodes. Anesth Analg 2009;108:837–45. 10.1213/ane.0b013e318187c4b1 [DOI] [PubMed] [Google Scholar]
- 16.Vallurupalli S, Manchanda S. Risk of acquired methemoglobinemia with different topical anesthetics during endoscopic procedures. Local Reg Anesth 2011;4:25–8. 10.2147/LRA.S22711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guertler AT, Pearce WA. A prospective evaluation of benzocaine-associated methemoglobinemia in human beings. Ann Emerg Med 1994;24:626–30. 10.1016/S0196-0644(94)70272-1 [DOI] [PubMed] [Google Scholar]
- 18.Moos DD, Cuddeford JD. Methemoglobinemia and benzocaine. Gastroenterol Nurs 2007;30:342–5. 10.1097/01.SGA.0000296253.78457.f4 [DOI] [PubMed] [Google Scholar]
- 19.Ash-Bernal R, Wise R, Wright SM. Acquired methemoglobinemia: a retrospective series of 138 cases at 2 teaching hospitals. Medicine 2004;83:265–73. 10.1097/01.md.0000141096.00377.3f [DOI] [PubMed] [Google Scholar]
- 20.Sikka P, Bindra VK, Kapoor S, et al. Blue cures blue but be cautious. J Pharm Bioallied Sci 2011;3:543–5. 10.4103/0975-7406.90112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ludlow JT, Wilkerson RG, Nappe TM. Methemoglobinemia. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2022. https://www.ncbi.nlm.nih.gov/books/NBK537317/ [PubMed] [Google Scholar]
- 22.Bistas E, Sanghavi D. Methylene Blue. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2022. [PubMed] [Google Scholar]
- 23.McRobb CM, Holt DW. Methylene blue-induced methemoglobinemia during cardiopulmonary bypass? A case report and literature review. J Extra Corpor Technol 2008;40:206–14. [PMC free article] [PubMed] [Google Scholar]
- 24.AHFS Drug Information. McEvoy GK. American Society of Health-System pharmacists. Available: http://online.statref.com/document.aspx?.fxid?.1&docid?.1208 [Accessed 11 Jun 2007].
- 25.OpenAnesthesia . Methemoglobinemia: diagnosis. Available: https://www.openanesthesia.org/methemoglobinemia_diagnosis/
- 26.Lindenmann J, Matzi V, Kaufmann P, et al. Hyperbaric oxygenation in the treatment of life-threatening isobutyl nitrite-induced methemoglobinemia--a case report. Inhal Toxicol 2006;18:1047–9. 10.1080/08958370600904629 [DOI] [PubMed] [Google Scholar]
- 27.Cho Y, Park SW, Han S-K, et al. A case of methemoglobinemia successfully treated with hyperbaric oxygenation monotherapy. J Emerg Med 2017;53:685–7. 10.1016/j.jemermed.2017.04.036 [DOI] [PubMed] [Google Scholar]
- 28.Altintop I, Sanri E, Tatli M, et al. Methemoglobinemia treated with hyperbaric oxygen therapy: a case report. Turk J Emerg Med 2018;18:176–8. 10.1016/j.tjem.2018.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]