Abstract
Background:
Antipsychotics are the treatment of choice in the therapy of schizophrenia. These drugs can be associated with changes in heart rate, but this question has never been examined systematically.
Objective:
We aimed to analyse changes in heart rate during treatment with antipsychotics using the frequency of tachycardia and bradycardia events.
Design:
For this systematic review and meta-analysis, we included all randomized controlled trials for the acute treatment of schizophrenia comparing antipsychotics head-to-head or with placebo.
Data Sources and Methods:
We searched Embase, MEDLINE, PsycINFO, PubMed, BIOSIS, Cochrane Central Register of Controlled Trials (CENTRAL), WHO International Clinical Trials Registry Platform and ClinicalTrials.gov (last search June 2021). Two authors independently selected studies and extracted data. We conducted pairwise meta-analyses using a random-effects model. Outcomes were tachycardia and bradycardia events.
Results:
We found 469 trials meeting the inclusion criteria. Seventy-seven studies with 16,907 participants provided data on tachycardia or bradycardia events. We found no significant differences between antipsychotics and placebo or between antipsychotics for bradycardia events based on sparse data. Antipsychotics had a higher risk for tachycardia events compared with placebo [N = 37, n = 7827, risk ratio (RR) = 1.83, 95% confidence interval (CI) = 1.40–2.41], with large differences between the individual substances (iloperidone RR = 14.05, chlorpromazine RR = 4.84, loxapine RR = 4.52, risperidone RR = 3.38, quetiapine RR = 2.64, paliperidone RR = 1.65). Some head-to-head comparisons were also significantly different: olanzapine versus haloperidol RR = 2.87, chlorpromazine versus thiothixene RR = 2.92, quetiapine versus lurasidone RR = 3.22, risperidone versus aripiprazole RR = 4.37, iloperidone versus ziprasidone RR = 4.65).
Conclusion:
Many studies do not report data for cardiac outcomes, but the available evidence indicates that treatment with antipsychotics raises the risk for tachycardia. Therefore, especially patients with cardiac risk factors should be monitored closely during antipsychotic treatment.
Registration:
PROSPERO: CRD42014014919
Keywords: schizophrenia, antipsychotics, meta-analysis, tachycardia, bradycardia
Introduction
Schizophrenia is a debilitating disease with a huge burden for patients and their relatives. 1 Antipsychotics are the treatment of choice, but can cause severe side effects. 2 Some antipsychotics (e.g. clozapine and olanzapine) are associated with higher cardiometabolic risks compared with other (e.g. aripiprazole). 3 There is evidence that people with schizophrenia have a higher risk of cardiovascular disease–related deaths compared with the general population, which maybe increased by the use of antipsychotics. 4 Furthermore, patients with schizophrenia often have many risk factors for cardiovascular events such as tobacco addiction or metabolic syndrome. 5 Often, they do not seek medical treatment at all 6 and there is evidence that they obtain fewer cardiac interventions than the general population. 7 A large cohort study found that second-generation antipsychotic agents raise the risk of sudden cardiac death. 8 These drugs influence repolarisation and heart rate, which can result in fatal arrhythmias. 9 In general, higher heart rates are associated with a higher mortality due to cardiovascular diseases even in the general population. 10 In the past, several retrospective trials have examined the risk for cardiac events during antipsychotic treatment and the prolongation of the QTc interval. But the evidence of randomized clinical trials in terms of heart rate has never been summarized systematically. We therefore aim to systematically summarize the available evidence of this cardiac side effect in randomized controlled trials of acute treatment of schizophrenia.
Methods
This analysis follows the PRISMA-Guideline 11 and is part of a larger already published project 2 (PRISMA checklist Supplemental Appendix 1). The according protocol was registered a priori at PROSPERO under the registration number: CRD42014014919 (Supplemental Appendix 2).
Participants and interventions
We included randomized controlled trials (RCTs) in adults with acute symptoms of schizophrenia or related disorders (such as schizophreniform or schizoaffective disorders). We excluded studies in patients with treatment resistance, first episode, predominant negative or depressive symptoms, concomitant medical illnesses and relapse-prevention studies. We included trials with a minimum duration of 3 weeks. Interventions were all second-generation antipsychotics approved in Europe or the United States and placebo. Based on a survey among 50 international schizophrenia experts, 12 we also included the following older antipsychotics: benperidol, chlorpromazine, clopenthixol, flupenthixol, fluphenazine, haloperidol, levomepromazine, loxapine, molindone, penfluridol, perazine, perphenazine, pimozide, sulpiride, thioridazine, thiothixene, trifluoperazine and zuclopenthixol. Intramuscular formulations were excluded as they are mainly used for relapse prevention (long-acting) or in emergency situations (short-term). We included target to maximum doses according to the ‘International Consensus Study of Antipsychotics’ for flexible dose studies. 13 We included all flexible dose studies because investigators can titrate the dose for the individual patient. As the examined outcomes are based on the objective measurement of heart rates, we included double-blind, single-blind and open studies, but excluded open studies in a sensitivity analysis. Studies from mainland China were excluded due to quality concerns. 14 We excluded studies with high risk of bias for randomization and allocation.
Search
We searched the following databases without language restrictions: Medline, Cochrane Central register of Controlled Trials (CENTRAL), Embase, Biosis, PsycINFO, Pubmed, Clinicaltrials.gov and WHO International Trial Registry. The references of included studies were screened for additional studies. The search strategy was based on the update of a previously conducted analysis and the full search strategy is presented in the supplement (last search June 2021, Supplemental Appendix 3).
Outcomes
We extracted bradycardias and tachycardias reported as adverse events as stated by the original authors, but also reported with the adverse event terms ‘low heart frequency’ and ‘low pulse’ or ‘high heart frequency’ and ‘high pulse rate’.
Data extraction and analysis
At least two reviewers (MH, TA, JST, Natalie Peters, Lio Baeckers, Angelika Kapfhammer, Dongfang Wang and Shimeng Dong) screened the search results independently, retrieved full-text articles, and checked inclusion criteria. In case of doubt, a third reviewer (SL) was involved. Two reviewers independently extracted data and entered them in electronic forms in Microsoft Access 2010 (MH, TA, JST, Natalie Peters and Lio Baeckers). An algorithm checked for conflicting data entries.
We calculated pairwise random-effects meta-analyses combining the event rates in intervention and control groups. 15 Effect sizes were risk ratios and number-needed-to-harm (NNTH). All effect sizes were presented along with their 95% confidence intervals (CIs). We assessed between study heterogeneity with the I2-statistic and the chi-square test for homogeneity, with I2 > 50% and chi-square p < 0.05 indicating significant heterogeneity. 16 We used contour-enhanced funnel plots 17 and the trim and fill method 18 to explore small trial bias, if at least 10 studies were available. All statistical analysis were conducted with the statistical programme R version 3.6.1 19 using the package meta. 20 The p values lower than 0.05 were considered statistically significant.
Sensitivity analysis
We excluded open and single-blind studies in a sensitivity analysis. We also applied a fixed-effects model and checked for probable changes when a random-effects model was applied. We decided post hoc to exclude long-term studies (duration >13 weeks) in a sensitivity analysis to check for higher event rates in short-term studies.
Risk of bias
Risk of bias was assessed independently by two reviewers (Maximilian Huhn, Thomas Arndt, Natalie Peter, Lio Baeckers and Johannes Schneider) using the criteria stated by the Cochrane Collaboration 21 (Supplemental Appendix 6).
Results
Descriptives of the sample
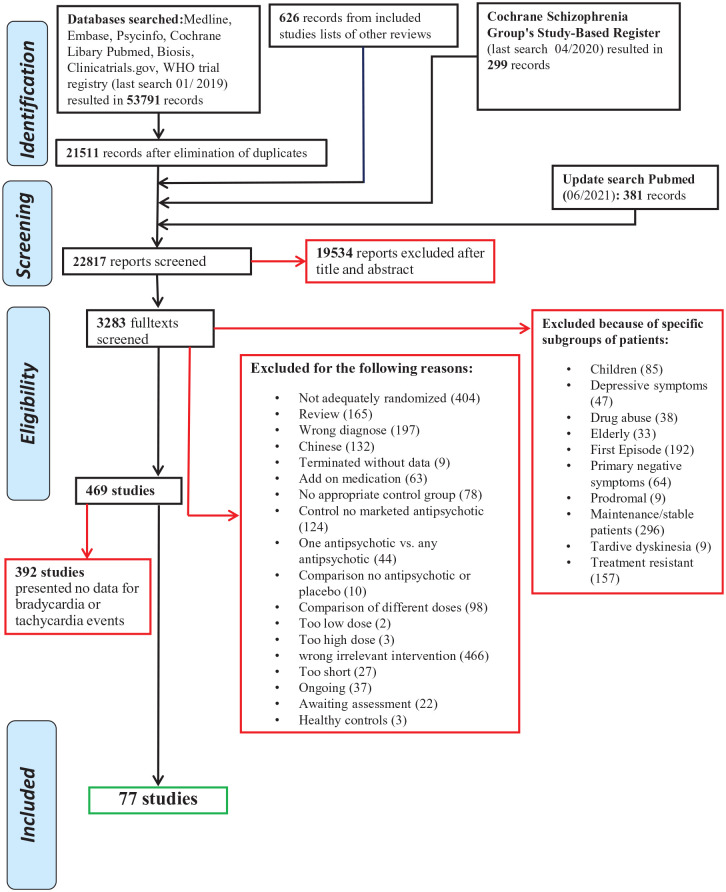
The searches resulted in 55,097 hits. After elimination of duplications, titles and abstracts were screened for matching the inclusion criteria and 3283 full texts retrieved. After full-text screening, 77 studies with 16,907 participants provided data on bradycardia or tachycardia events. The detailed PRISMA flowchart can be found in Figure 1. A detailed list of the included studies can be found in Supplemental Appendix 4. The studies were published between 1968 and 2019. Seventy-three studies were double-blind, two single-blind and two open. Mean trial duration was 8 (SD = 5.94) weeks. Thirty-nine studies were placebo controlled. The sample had the following characteristics: 10,372 participants were male and 6535 female. Mean age was 37.34 (SD = 4.12) years and the duration of illness in years was 12.80 (SD = 5.94).
Figure 1.
PRISMA flow diagram.
Source: Moher et al.
Bradycardia
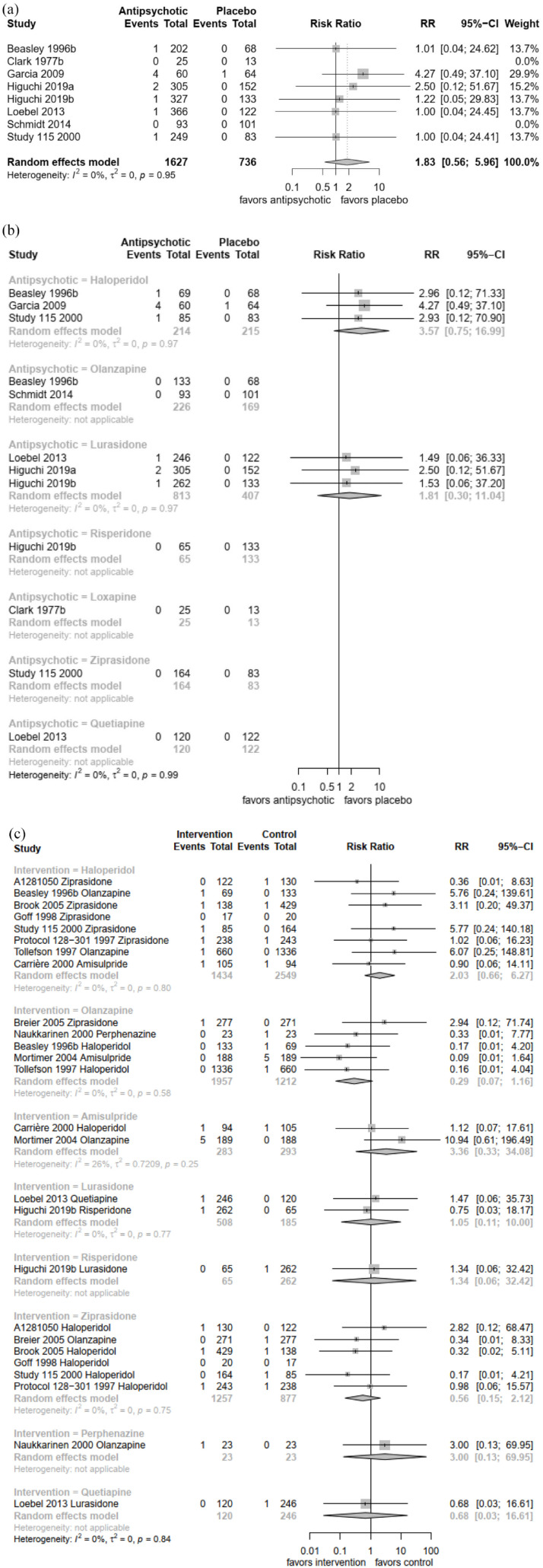
Seventeen studies with 6866 participants reported data for bradycardia events. Bradycardias were overall quite rare and reported for 0.4% of patients. Eight studies with 2363 participants reported bradycardia events for the comparison of antipsychotics with placebo. The overall risk ratio of antipsychotics compared with placebo was not significant [N = 8, n = 2363, risk ratio (RR) = 1.83, 95% confidence interval (CI) = 0.56–5.96, p = 0.32] [Figure 2(a)]. There was no significant difference between haloperidol and placebo (N = 3, n = 429, RR = 3.57, 95% CI = 0.75–16.99, p = 0.11) and lurasidone and placebo (N = 3, n = 1220, RR = 1.81, 95% CI = 0.30–11.0, p = 0.52) [Figure 2(b)]. The comparisons of loxapine, olanzapine, quetiapine, risperidone and ziprasidone could not be calculated because there were no events in both arms.
Figure 2.
(a) Antipsychotic drugs versus placebo (overall) – bradycardia events. (b) Antipsychotic drugs versus placebo (individual antipsychotics) – bradycardia events. (c) Antipsychotic drugs head-to-head comparison – bradycardia events.
The comparison of haloperidol with amisulpride/olanzapine/ziprasidone showed no significant difference for the pooled comparison (N = 8, n = 3983, RR = 2.03, 95% CI = 0.66–6.27, p = 0.22) nor for the comparison with olanzapine (N = 2, n = 2198, RR = 5.92, 95% CI = 0.62–56.56, p = 0.12) or ziprasidone (N = 5, n = 1586, RR = 1.62, 95% CI = 0.37–7.11, p = 0.52) or amisulpride (N = 1, n = 199, RR = 0.90, 95% CI = 0.06–14.11, p = 0.94) alone [Figure 2(c)]. We found also no significant differences for lurasidone, perphenazine, quetiapine and risperidone compared with other drugs.
Tachycardia
Seventy-three studies with 15,732 participants reported data for tachycardia events. Tachycardias were reported for 4.8% of patients. Treatment with antipsychotics had a significant higher risk for tachycardias than placebo (N = 37, n = 7827, RR = 1.83, 95% CI = 1.40–2.41, p < 0.001, NNTH = 43) [Figure 3(a)], but there were large differences between the individual substances. The following drugs had a higher relative risk for tachycardia compared with placebo. The ranking is from highest to lowest risk RR: iloperidone 14.05 (95% CI = 1.93–102.26, p < 0.01), chlorpromazine 4.84 (95% CI = 1.82–18.29, p = 0.02), loxapine 4.52 (95% CI = 1.06–19.28, p = 0.04), risperidone 3.27 (95% CI = 1.11–10.32, p = 0.03), quetiapine 2.64 (95% CI = 1.13–6.16, p = 0.02), paliperidone 1.65 (95% CI = 1.08–2.51, p = 0.02) [Figure 3(b)]. We found no significant differences for the following antipsychotics: aripiprazole, fluphenazine, haloperidol, loxapine, lurasidone, olanzapine, perphenazine, thioridazine, trifluoperazine, ziprasidone and zotepine [Figure 3(b)]. The following antipsychotics differed significantly from each other: aripiprazole was better than risperidone: RR 0.23 (95% CI = 0.1–0.541, p < 0.01), haloperidol better than olanzapine: RR 0.35 (95% CI = 0.13–0.95, p = 0.04), lurasidone better than quetiapine: RR 0.31 (95% CI = 0.12–0.78, p = 0.01), thiothixene better than chlorpromazine: RR 0.34 (95% CI = 0.14–0.85, p = 0.02) and ziprasidone better than iloperidone: RR 0.21 (95% CI = 0.07–0.7, p = 0.01) [Figure 3(c)].
Figure 3.
(a) Antipsychotic drugs versus placebo (overall) – tachycardia events. Results are presented separately for short- and long-term studies. (b) Antipsychotic drugs versus placebo (individual antipsychotics)– tachycardia events. (c) Antipsychotic drugs head-to-head comparison – tachycardia events.
Risk of bias
The percentage of studies with low/unclear/high risk of bias for the individual items was randomization (57.1%, 42.9%, 0%), allocation (42.93%, 57.1%, 0%), blinding of patients and personnel (57.1%, 36.4%, 6.5%), rater blinding (59.7%, 32.54%, 7.8%), missing outcomes (67.5%, 20.8%, 11.7%), selective reporting (72.7%, 10.4%, 16.9%) and other bias (89.6%,5.2%, 5.2%), whereas selective reporting was based on overall symptom scales like positive and negative syndrome scale (Supplemental Appendix 5).
Assessment of heterogeneity
We found no significant heterogeneity for any comparison concerning the outcome bradycardia. Concerning tachycardia, only the comparison of risperidone with placebo (τ2 = 1.2088, p = 0.04; I2 = 52%) and quetiapine with other antipsychotics (τ2 = 0.7570, p = 0.01; I2 = 77%) revealed significant heterogeneity. Many comparisons consisted only of one study, so heterogeneity assessment was not applicable.
Sensitivity analysis
Using a fixed-effects model instead of a random-effects model in a sensitivity analysis changed the results only in case of tachycardia. Chlorpromazine compared with any antipsychotic using a fixed-effects model was statistically significant and RR raised from 1.36 (95% CI = 0.96–1.94, p = 0.025) to 1.46 (95% CI = 1.03–2.08, p = 0.003). Results for all other comparisons did not change materially. We did not conduct the a priori planned sensitivity analysis exclusion of open studies as there were only two open studies in the complete data set. Exclusion of long-term studies (duration > 13 weeks) did not change the results for bradycardia or tachycardia (Supplemental Appendix 7).
Publication bias
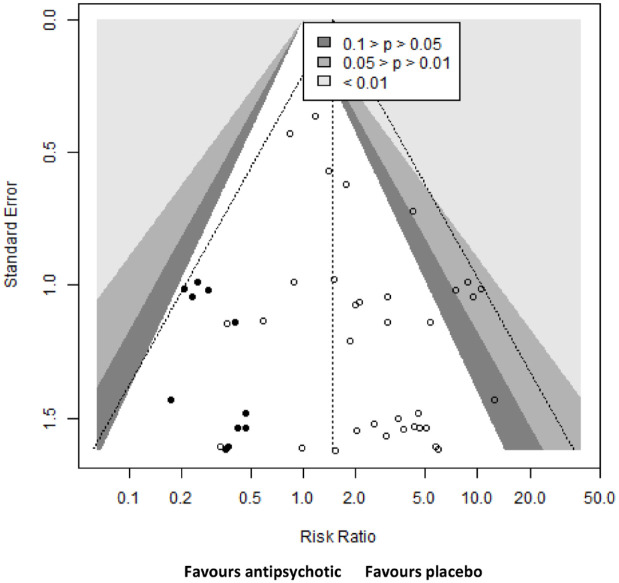
We did not assess small study bias for bradycardia events as no comparison had 10 or more studies. We did a contour-enhanced funnel plots for tachycardia events, which revealed a possibility that small trials with a lower relative risk for tachycardia events in the antipsychotic group could be missing (Figure 4).
Figure 4.
Contour-enhanced funnel plot for all antipsychotics compared with placebo.
Risk of tachycardia events of all antipsychotics compared with placebo. Circles represent effect sizes of individual studies measured as risk ratio. Missing studies were estimated using the trim-and-fill method. Original effect sizes are represented by empty circles and estimated ones by filled circles.
Discussion
The analysis presents bradycardia and tachycardia events from 77 studies with 16,907 participants using antipsychotics for the treatment of acute schizophrenia. Based on few data, we found significant differences in bradycardia events neither between antipsychotics and placebo nor between individual antipsychotics. Overall bradycardia is primarily dangerous for people at risk, for example, after a myocardial infarction or with already existing arrhythmia (e.g. branch blocks). 22 In contrast, bradycardia can be even physiological in young or exercised people. 23 Nevertheless, bradycardias can cause dizziness and are responsible for 3–10% of syncopes. 24
The risk for tachycardia events was 1.81 times higher for antipsychotics compared with placebo with a number needed to harm of 43. There are some substances that have a significantly higher risk for tachycardia than placebo. Some of the reported tachycardias may be reflex tachycardias caused by an adequate autonomic reflex to orthostatic hypotension. Undermining this hypothesis is the fact that antipsychotics with a higher risk for orthostatic hypotension like iloperidone and chlorpromazine have a higher risk for tachycardias. 25
The antipsychotic substance most often associated with tachycardia is clozapine. 26 This may be related to the vagolytic effects of clozapine. Unfortunately, we did not find an RCT that compared clozapine with placebo, only one comparison with olanzapine 27 and one with chlorpromazine. 28 Neither found significant differences in tachycardia risks. Haloperidol that can be associated with prolongation of the QTC interval, especially when given intravenously, 29 had a significantly lower risk of tachycardia compared with olanzapine based on three studies with 2454 participants (RR = 0.35, p = 0.04).
We conducted the analysis using state-of-the-art methods and following the PRISMA guidelines. Nevertheless, there are some limitations. The primary outcome of most included studies was efficacy of antipsychotics. So bradycardia and tachycardia events were only reported as adverse events. It is unclear whether changes in heart rate did not occur in most of the studies or whether the studies did not report these events. No study presented a bradycardia or tachycardia definition in beats per minute, so we had to rely on the original author’s definition of these events. Of the 469 studies examining acute treatment with antipsychotics, only 77 studies with 15,732 participants reported data for tachycardia events and even less for bradycardia events (17 studies with 6866 participants). Bradycardias and tachycardias could be missed as the heart rate of patients is not monitored 24 h. This could lead to an underestimation of the ‘real’ event rate. We did not analyse mean heart rate, which would be interesting and allow to test for factors that could mitigate changes in heart rate like weight gain. Unfortunately, authors often did not state whether they counted all bradycardias and tachycardias irrespectively if the underlying rhythm was sinus rhythm or not. So tachycardia in the presence of atrial fibrillation could be counted even it is most likely not caused by the intake of antipsychotic drugs. We also did not analyse other arrhythmias, except QTc prolongation which is published elsewhere, 2 because they are even rarer than bradycardias. To pool the results, we needed a homogeneous sample. Therefore, we had to exclude studies focused on elderly patients, although bradycardias and tachycardias are more dangerous for this population, 30 but only few studies were excluded on this basis. 31 So the generalizability of our results is limited, especially as patients with concomitant somatic medial illness are typically excluded from randomized controlled trials of antipsychotics in schizophrenia. The risk-of-bias assessment was focused on overall efficacy measures, but only the risk-of-bias items ‘missing outcomes’ and ‘selective reporting’ are not outcome-specific.
Conclusion
There is evidence that treatment with antipsychotics increases the risk for tachycardia. Therefore, especially patients with risk factors should undergo electrocardiography and be monitored closely during antipsychotic treatment. 32 In case of pre-existing cardiovascular disease, an antipsychotic agent with low risk profile should be used.
Supplemental Material
Supplemental material, sj-docx-1-tpp-10.1177_20451253221097261 for Effects of antipsychotics on heart rate in treatment of schizophrenia: a systematic review and meta-analysis by Maximilian Huhn, Thomas Arndt, Johannes Schneider-Thoma and Stefan Leucht in Therapeutic Advances in Psychopharmacology
Acknowledgments
We thank Natalie Peter, Lio Baeckers, Angelika Kapfhammer, Dongfang Wang and Shimeng Dong for their help with data extraction. This analysis was part of the doctoral thesis of Thomas Arndt.
Footnotes
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contribution(s): Maximilian Huhn: Conceptualization; Data curation; Formal analysis; Methodology; Validation; Writing – original draft; Writing – review & editing.
Thomas Arndt: Data curation; Formal analysis; Investigation; Writing – review & editing.
Johannes Schneider-Thoma: Data curation; Formal analysis; Writing – original draft.
Stefan Leucht: Conceptualization; Funding acquisition; Project administration; Supervision; Validation; Writing – original draft.
ORCID iD: Maximilian Huhn  https://orcid.org/0000-0003-4011-1189
https://orcid.org/0000-0003-4011-1189
Funding: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This study was funded by the German Ministry of Education and Research (grant number FKZ01KG1406).
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: MH has received honoraria as a consultant for Recordati. In the past 3 years, SL has received honoraria as a consultant/advisor and/or for lectures from Angelini, Boehringer Ingelheim, Gedeon Richter, Janssen, Johnson & Johnson, Lundbeck, LTS Lohmann, MSD, Otsuka, Recordati, SanofiAventis, Sandoz, Sunovion, TEVA, Eisai, Rovi, Medichem and Mitsubishi. All other authors declare no competing interests.
Availability of data and materials: Not applicable.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Maximilian Huhn, Department of Psychiatry and Psychotherapy, Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Ismaningerstr. 22, 81675 München, Germany; Department of Psychiatry, Psychosomatic Medicine and Psychotherapy, Social Foundation Bamberg, Teaching Hospital of the University of Erlangen, Erlangen, Germany.
Thomas Arndt, Department of Psychiatry and Psychotherapy, Klinikum rechts der Isar, School of Medicine, Technical University of Munich, München, Germany.
Johannes Schneider-Thoma, Department of Psychiatry and Psychotherapy, Klinikum rechts der Isar, School of Medicine, Technical University of Munich, München, Germany.
Stefan Leucht, Department of Psychiatry and Psychotherapy, Klinikum rechts der Isar, School of Medicine, Technical University of Munich, München, Germany.
References
- 1. Kassebaum NJ, Arora M, Barber RM, et al. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016; 388: 1603–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet 2019; 394: 939–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hert M, Detraux J, van Winkel R, et al. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol 2011; 8: 114–126. [DOI] [PubMed] [Google Scholar]
- 4. Correll CU, Solmi M, Veronese N, et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry 2017; 16: 163–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goff DC, Sullivan LM, McEvoy JP, et al. A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophr Res 2005; 80: 45–53. [DOI] [PubMed] [Google Scholar]
- 6. Ali RA, Jalal Z, Paudyal V. Barriers to monitoring and management of cardiovascular and metabolic health of patients prescribed antipsychotic drugs: a systematic review. BMC Psychiatry 2020; 20: 581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lawrence DM, Holman CDJ, Jablensky AV, et al. Death rate from ischaemic heart disease in Western Australian psychiatric patients 1980-1998. Br J Psychiatry 2003; 182: 31–36. [DOI] [PubMed] [Google Scholar]
- 8. Ray WA, Chung CP, Murray KT, et al. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med 2009; 360: 225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fayek M, Kingsbury SJ, Zada J, et al. Cardiac effects of antipsychotic medications. Psychiatr Serv 2001; 52: 607–609. [DOI] [PubMed] [Google Scholar]
- 10. Greenland P, Daviglus ML, Dyer AR, et al. Resting heart rate is a risk factor for cardiovascular and noncardiovascular mortality: the Chicago Heart Association Detection Project in Industry. Am J Epidemiol 1999; 149: 853–862. [DOI] [PubMed] [Google Scholar]
- 11. Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015; 162: 777–784. [DOI] [PubMed] [Google Scholar]
- 12. Leucht S, Huhn M, Rothe P, et al. Which are the most important first-generation antipsychotic drugs? Survey of international schizophrenia experts. npj Schizophr 2016; 2: 25. [Google Scholar]
- 13. Gardner DM, Murphy AL, O’Donnell H, et al. International consensus study of antipsychotic dosing. Am J Psychiatry 2010; 167: 686–693. [DOI] [PubMed] [Google Scholar]
- 14. Woodhead M. 80% of China’s clinical trial data are fraudulent, investigation finds. BMJ 2016; 355: 5396. [DOI] [PubMed] [Google Scholar]
- 15. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 16. Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Peters JL, Sutton AJ, Jones DR, et al. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol 2008; 61: 991–996. [DOI] [PubMed] [Google Scholar]
- 18. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000; 56: 455–463. [DOI] [PubMed] [Google Scholar]
- 19. R Core Team. R: a language and environment for statistical computing. Manual, Vienna: R Core Team, 2019, https://www.R-project.org/ [Google Scholar]
- 20. Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health 2019; 22: 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions, version 5.1.0, 2011, https://handbook-5-1.cochrane.org/
- 22. Da Costa D, Brady WJ, Edhouse J. Bradycardias and atrioventricular conduction block. BMJ 2002; 324: 535–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zehender M, Meinertz T, Keul J, et al. ECG variants and cardiac arrhythmias in athletes: clinical relevance and prognostic importance. Am Heart J 1990; 119: 1378–1391. [DOI] [PubMed] [Google Scholar]
- 24. Wyss E, Candinas R. Bradyarrhythmiebedingte Synkopen. Ther Umsch Rev Ther 1997; 54: 144–150. [PubMed] [Google Scholar]
- 25. Gugger JJ. Antipsychotic pharmacotherapy and orthostatic hypotension: identification and management. CNS Drugs 2011; 25: 659–671. [DOI] [PubMed] [Google Scholar]
- 26. Safferman A, Lieberman JA, Kane JM, et al. Update on the clinical efficacy and side effects of clozapine. Schizophr Bull 1991; 17: 247–261. [DOI] [PubMed] [Google Scholar]
- 27. Kluge M, Schuld A, Himmerich H, et al. Clozapine and olanzapine are associated with food craving and binge eating: results from a randomized double-blind study. J Clin Psychopharmacol 2007; 27: 662–666. [DOI] [PubMed] [Google Scholar]
- 28. Leon CA, Estrada H. Efectos Terapeuticos De La Clozapina (1) Sobre Los Sintomas De Psicosis. (Evaluacion Clinica Utilizando El Metodo Doble Ciego) [Therapeutic effects of clozapine on psychotic symptoms. (Clinical evaluation using the double blind method)]. Rev Colomb Psiquiatr 1974; 3: 309–320. (in Spanish) [Google Scholar]
- 29. Beach SR, Celano CM, Sugrue AM, et al. QT prolongation, torsades de pointes, and psychotropic medications: a 5-year update. Psychosomatics 2018; 59: 105–122. [DOI] [PubMed] [Google Scholar]
- 30. Manolio TA, Furberg CD, Rautaharju PM, et al. Cardiac arrhythmias on 24-h ambulatory electrocardiography in older women and men: the Cardiovascular Health Study. J Am Coll Cardiol 1994; 23: 916–925. [DOI] [PubMed] [Google Scholar]
- 31. Krause M, Huhn M, Schneider-Thoma J, et al. Antipsychotic drugs for elderly patients with schizophrenia: a systematic review and meta-analysis. Eur Neuropsychopharmacol 2018; 28: 1360–1370. [DOI] [PubMed] [Google Scholar]
- 32. De Hert M, Vancampfort D, Correll CU, et al. Guidelines for screening and monitoring of cardiometabolic risk in schizophrenia: systematic evaluation. Br J Psychiatry 2011; 199: 99–105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tpp-10.1177_20451253221097261 for Effects of antipsychotics on heart rate in treatment of schizophrenia: a systematic review and meta-analysis by Maximilian Huhn, Thomas Arndt, Johannes Schneider-Thoma and Stefan Leucht in Therapeutic Advances in Psychopharmacology