Abstract
Background
Resistance to cell death, a protective mechanism for removing damaged cells, is a “Hallmark of Cancer” that is essential for cancer progression. Increasing attention to cancer lipid metabolism has revealed a number of pathways that induce cancer cell death.
Scope of review
We summarize emerging concepts regarding lipid metabolic reprogramming in cancer that is mainly involved in lipid uptake and trafficking, de novo synthesis and esterification, fatty acid synthesis and oxidation, lipogenesis, and lipolysis. During carcinogenesis and progression, continuous metabolic adaptations are co-opted by cancer cells, to maximize their fitness to the ever-changing environmental. Lipid metabolism and the epigenetic modifying enzymes interact in a bidirectional manner which involves regulating cancer cell death. Moreover, lipids in the tumor microenvironment play unique roles beyond metabolic requirements that promote cancer progression. Finally, we posit potential therapeutic strategies targeting lipid metabolism to improve treatment efficacy and survival of cancer patient.
Major conclusions
The profound comprehension of past findings, current trends, and future research directions on resistance to cancer cell death will facilitate the development of novel therapeutic strategies targeting the lipid metabolism.
Keywords: Lipid metabolism, Cancer, Cell death, Therapeutic strategy
Highlights
-
•
Lipid metabolic reprogramming is crucial for various aspects of cancer cell death.
-
•
Cancer cell growth depends on lipid metabolic plasticity and sustained metabolic adaptations.
-
•
Lipid metabolism and the epigenome interact in a bidirectional manner, which regulate cancer cell death.
-
•
The therapeutic strategies targeting lipid metabolism can improve treatment efficacy and survival of cancer patients.
Abbreviations
- PCD
Programmed cell death
- TME
Tumor microenvironment
- LDL
Low-density lipoprotein
- LDLR
Low-density lipoprotein receptor
- LRP1
LDLR related protein 1
- ApoE
Apolipoprotein E
- ERK
Extracellular regulated protein kinase
- LXR
Liver X receptor
- IDOL
Inducible degrader of LDLR
- SR-B1
Scavenger receptor, class B type 1
- HDL
High-density lipoprotein
- ccRCC
Clear cell renal cell carcinoma
- CD36
Cluster of differentiation 36
- OS
Overall survival
- FATP4
Fatty acid transport protein 4
- oxLDL
Oxidized LDL
- CEBPβ
CCAAT/enhancer-binding protein β
- YAP
Yes-associated protein
- ABCA
ATP-binding cassette transporter A
- TRAIL
Tumor necrosis factor-related apoptosis-inducing ligand
- EGFR
Epidermal growth factor receptor
- Bcl-xL
B-cell lymphoma-extra large
- PARP-1
poly ADP ribose polymerase-1
- CAV-1
Caveolin-1
- ROCK1
Rho-associated coiled-coil kinase 1
- HMG-CoA
3-hydroxy-3-methylglutaryl-CoA
- HMGCR
3-hydroxy-3-methylglutaryl-CoA reductase
- RFS
relapse-free survival
- GPX4
Glutathione peroxidase 4
- CoQ10
Coenzyme Q10
- Alox12
Arachidonate lipoxygenase12
- AMPK
Adenosine 5‘-monophosphate (AMP) -activated protein kinase
- ACC1
Acetyl-CoA carboxylase1
- SREBPs
Sterol regulatory element binding proteins
- TAG
Triacylglycerol
- ROS
reactive oxygen species
- SCAP
SREBP-cleavage activating protein
- FASN
Fatty acid synthase
- ER
endoplasmic reticulum
- SOAT1/ACAT1
Sterol O-acyltransferase
- NADPH
nicotinamide adenine dinucleotide phosphate
- MUFA
Monounsaturated fatty acid
- SCD1
Stearoyl-CoA Desaturase-1
- PUFAs
Polyunsaturated fatty acids
- DDIT3
DNA-damage-inducible transcript 3
- FASN
Fatty acid synthase
- Mn
Manganese
- Cyt-c
Cytochrome c
- ECHS1
Enoyl CoA hydratase 1
- FAO
Fatty acid oxidation
- CPT1
Carnitine palmitoyltransferase 1
- mTORC1
Mammalian target of rapamycin
- ACSL4
Acyl CoA synthetase long chain family member 4
- HIF-1
Hypoxia inducible factor-1
- EGLN1/3
Egl-9 family hypoxia-inducible factor 1/3
- LSH
Lymphocyte-specific helicase
- HIF-1α
Hypoxia inducible factor-1α
- FADS2
fatty-acid desaturase 2
- HILPDA
Hypoxia inducible lipid droplet-associated protein
- PPARγ
Peroxisome proliferator-activated receptor γ
- PKD3
Protein kinase D3
- HER2
Human epidermal growth factor receptor 2
- HBXIP
Hepatitis B X-interacting protein
- LDs
Lipid droplets
- VLDL
Very low density lipoprotein
- hGX sPLA2
Human group X secreted phospholipase A2
- LPCAT2
Lysophosphatidylcholine acyltransferase 2
- ATG14
Adipose triglyceride lipase 14
- FABP7
Fatty acid-binding protein 7
- PLD1
Phospholipase D1
- DNMTs
DNA methyltransferases
- CpG islands
CpG-rich promoter regions
- Sp1
Specificity protein 1
- WDR76
WD repeat domain 76
- KMT2D
(K)-specific methyltransferase 2D
- ACACA
Acetyl CoA carboxylase
- EHMT2
Euchromatic histone-lysine N-methyltransferase 2
- SREBF2
Sterol regulatory element-binding protein 2
- SIRT6
Sirtuin 6
- NNMT
Nicotinamide N-methyltransferase
- FoxO1
Forkhead box O1A
- NNMT
Nicotinamide N-methyltransferase
- CHK2
Choline kinase 2
- PLIN2
Perilipinin-2
- RPS6
Ribosomal protein S6
- CAFs
Cancer-associated fibroblasts
- CAAs
Cancer-associated adipocytes
- TAM
Tumor-associated macrophages
- Treg cells
Regulatory T cells
- TCR
T cell receptor
- TILs
Tumor-infiltrating T lymphocytes
- LPA
Lysophosphatidic acid
- GGPP
Geranylgeranyl pyrophosphate
- 15-LOX
15-lipoxygenase
- LOX-1
Lectin-like oxidized low-density lipoprotein receptor-1
- DAPK2
Death associated protein kinase 2
- BNIP3
Bcl-2/adenovirus E1B 19kD interacting protein 3
- TOFA
5-(tetradecyloxy)-2-furoic acid
- ACL
ATP-citrate lyase
- HMGR
3-hydroxy-3-methyl glutaryl coenzyme A reductase
1. Introduction
One of the hallmarks of cancer cells is their ability to escape cell death [1]. A better understanding of cancer pathogenesis and therapies will come from elaborating cell death mechanisms. Programmed cell death (PCD) is a natural barrier in cancer progression, that starts with ancient apoptosis mechanisms and extends to additional PCD types, such as autophagy, pyroptosis, necroptosis, ferroptosis, cuproptosis, etc. Lipid metabolism is changed in cancer cells that are to a malignant phenotype transformation [2]. A complicated signature of lipid metabolic reprogramming in cancer cell death has emerged when functional techniques targeting metabolic pathways or enzymes are combined. Together with functional approaches in which specific pathways or enzymes are targeted, a complicated picture of lipid metabolic rewiring in cancer cell death has emerged. Lipid metabolism and the epigenetic modifying enzymes interact in a bidirectional manner which involves regulating cancer cell death. The rewiring of lipid metabolism is not limited to the cancer cells, but also affects other elements in the tumor microenvironment (TME), including physical factors, the healthy tissues or cells around the tumor, and so on. Acquired resistance to several cancer therapies includes increased resistance to cell death. Therefore, escaping cell death is not only a hallmark of cancer but also a constant requirement throughout the oncogenic process, posing a significant hurdle to cancer therapy. We summarize the ongoing research of small-molecule agents and natural compounds targeting lipid metabolism to spotlight the identified therapeutic opportunities for further development. The purpose of targeting lipid metabolism in cancer cells is to improve current treatment procedures and serve as adjuvant therapies.
2. Lipid metabolism reprogramming as an emerging hallmark of cancer cell death
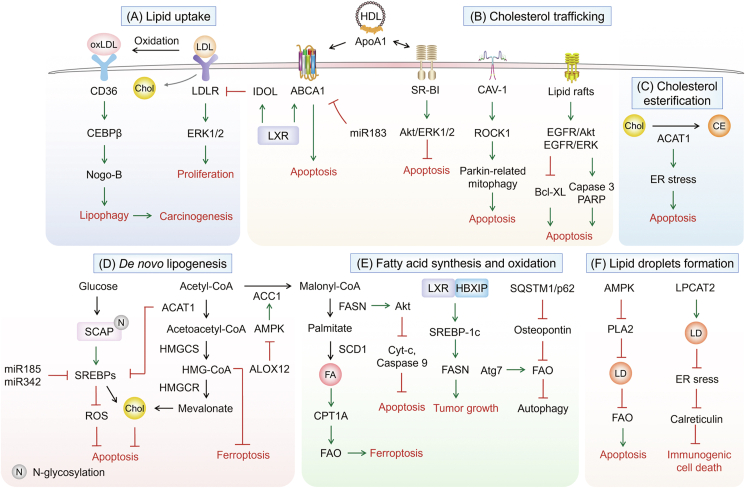
One of the distinguishing characteristics of cancer is metabolic reprogramming. Metabolic enzymes, upstream regulating molecules, together with downstream metabolic products are altered during various metabolic processes [3]. Rewiring of metabolic programming contributes to cancer cells shed from a primary tumor, overcomes the nutrient and energy deficiencies, and ultimately survives and forms metastases [4]. Lipid metabolism reprogramming is important for numerous aspects of cancer cell death, including lipid uptake and trafficking, de novo lipogenesis, cholesterol metabolism, fatty acid synthesis and oxidation, as well as phospholipid and neutral lipid metabolism (Figure 1).
Figure 1.
The role of lipid metabolism reprogramming in cancer cell death. (A) Increased exogenous lipid uptake by CD36 and LDLR promote carcinogenesis. (B) A network regulates the four systems and the entire transportation process. The elevated expression of ABCA1, HDL/ApoA-1, SR-B1 and CAV-1 accelerate cholesterol efflux, which lead to cancer cell apoptosis. (C) The increment of cholesterol esterification regulated by ACAT1 induces apoptosis. (D) Glucose increases N-glycosylation of SCAP, activates SREBPs, ultimately inhibits apoptosis of cancer cells. The biosynthesis pathway converts acetyl-CoA into cholesterol through HMGCs and HMGCR, which are the key speed-limiting enzymes. (E) The upregulation of enzymes promotes fatty acid synthesis and oxidation, then induces ferroptosis. (F) PLA2 and LPCAT2 induce LDs formation, stimulates cancer cell survival, and prevents apoptosis.
2.1. Lipid uptake and transport: greasing the wheels of the cancer machine
By screening clinical specimens and publicly available human datasets, highly expressed low-density lipoprotein (LDL) receptor (LDLR) in acute myeloid leukemia and breast cancer is associated with reduced recurrence-free survival, indicating LDLR is an independent adverse predictive marker [5,6]. Additionally, high mRNA levels of LDLR and LDLR related protein 1 (LRP1) are related to lower survival in pancreatic adenocarcinoma and worsened survival in bladder urothelial carcinoma patients, respectively [7]. In LDLR−/− and Apolipoprotein E (ApoE)−/− mice, silencing the LDLR induces the cleavage of caspase-3, results in the apoptosis. The silencing of LDLR profoundly reduces cholesterol uptake and alters its distribution, inhibits the extracellular regulated protein kinase 1/2 (ERK1/2) pathway, and suppresses cell growth in pancreatic cancer cells [8]. Activation of liver X receptor (LXR) reduces cholesterol synthesis and absorption, leads to LDLR degradation via upregulating inducible degrader of LDLR (IDOL), and thus ameliorates exogenous uptake [9]. LXR deficiency tends to accumulate cholesterol in prostate cancer mice fed with a high cholesterol diet. Regulating cholesterol homeostasis appears a valuable strategy to target human cancers. Blocking cholesterol uptake is one way to modulate the balance of cholesterol in cancer cells.
The scavenger receptor class B type 1 (SR-B1) functions as a high-density lipoprotein (HDL) receptor, which can selectively uptake cholesterol derived from HDL into tissues and cells [10]. Highly expressed SR-B1 is linked to apoptosis and poor prognosis in breast and prostate cancer [11,12]. However, the opposite has been observed in B-Cell lymphoma, osteosarcoma, lung, and ovarian malignancies, where low SR-B1 in particular is correlated with poor survival [13,14]. Knockdown of SR-B1 reduces tumor growth, and simultaneously decreases the activation of Akt and ERK1/2, thus promoting the apoptosis of breast cancer [15].
Cancer cells are forced to ingest fatty acids from the extracellular environment due to their increasing need for growth. Meanwhile, clear cell renal cell carcinoma (ccRCC) patients with elevated cluster of differentiation 36 (CD36) expression have lower progression-free survival and overall survival (OS), with hazard ratios [16]. Increased fatty acid transport protein 4 (FATP4) expression has been linked to short recurrence-free survival [17]. Fatty acid uptake is activated when CD36 and FATP4 are co-expressed. The uptake of oxidized LDL (oxLDL) mediated by CD36 triggers CCAAT/enhancer-binding protein β (CEBPβ) expression, then promotes Nogo-B interaction with Atg5 to induce lipid peroxidation, leads to lysophosphatidic acid-enhanced Yes-associated protein (YAP) oncogenic activity in non-alcoholic fatty liver disease-associated hepatocellular carcinoma [18].
Reduced expression of ATP-binding cassette transporter A (ABCA) transporters is association with poor prognosis in serous ovarian cancer and colorectal cancer, suggesting that lipid trafficking may be a key mechanism in epithelial ovarian cancer. High levels of ABCA1, ABCA6, ABCA8, and ABCA9 and low expression of ABCA5 result in shorter OS in patients with serous ovarian cancer [19]. Activation of LXR promotes cholesterol efflux in colon cancer cells by upregulating the expression of membrane transporters, such as ABCA1, ABCG5, ABCG8, and ABCG1. MiR-183 impedes apoptosis in colon cancer by targeting the 3'UTR of ABCA1 and degrading ABCA1 [20]. The increased expression of ApoA1, a protein involved in cholesterol efflux and the constitution of HDL, upregulates the expression of ABCA1 in the extracellular compartment and promotes ABCA1-dependent cholesterol efflux [21].
Lipid rafts are membrane microdomains contained in cholesterol and sphingolipids. These particular areas are implicated in carcinogenesis and metastasis and are connected with many proteins from both pro-oncogenic and apoptotic pathways [22]. Death receptors are one of the key regulatory proteins anchored to lipid rafts. Once activated, death receptors cause apoptotic signal pathways. Cancer cells can escape apoptosis by changing the cholesterol content of lipid rafts and destroying the folding and function of death receptors. Cholesterol deprivation in lipid rafts greatly reduces the cell death program by regulating Fas and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) [23]. Reduced membrane cholesterol levels promote lipid raft rearrangement with notably inhibition of epidermal growth factor receptor (EGFR) - mediated Akt and ERK pathways, downregulation of B-cell lymphoma-extra large (Bcl-xL), and upregulation of caspase-3 and Poly ADP ribose polymerase-1 (PARP) cleavages, thereby inducing apoptosis in prostate cancer cells [24].
Caveolins are membrane-associated scaffolding proteins, contributing to caveolae formation. Caveolins are a family of proteins that binds to lipids in plasma membrane, particularly cholesterol and sphingomyelin. The caveolin-1 (CAV-1) is positively correlated with better OS and later age of onset in breast cancer patients [25]. CAV-1 inhibits Parkin-related mitophagy through activation of the Rho-associated coiled-coil kinase 1 (ROCK1) pathway, promotes lung cancer cell death. CAV-1 also accelerates docetaxel-induced apoptosis in breast cancer cells by arresting the cell cycle in the G2/M phase. CAV-1 activates the Rho-associated coiled-coil kinase 1 (ROCK1) pathway to ameliorate Parkin-related mitophagy, thereby promoting lung cancer cell death [26]. In addition, CAV-1 promotes docetaxel-induced breast cancer cells apoptosis by arresting cell cycle in the G2/M phase [27]. CAV-1 sensitizes cisplatin-induced lung carcinoma cell death by accelerating superoxide anion formation [28]. Overall, increasing evidence suggest that lipid uptake and trafficking are common phenotypes across various cancer types involved in cancer cell death (Figure 1A, B).
2.2. De novo synthesis and esterification of endogenous lipids are required for resistance to cancer cell death
Nearly 30 enzymatic processes convert acetyl CoA to cholesterol, such as the mevalonate pathway, squalene biosynthesis, etc. [29]. The primary rate-limiting enzymes in these enzymatic processes are 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase (HMGCR) and squalene epoxidase which decrease HMG-CoA to mevalonate and catalyze the oxidation of squalene to 2,3-epoxysqualene, respectively [30]. As a prognostic factor, the clinical significance of HMGCR in human malignancies is dependent on the environment and tumor type. In patients with ovarian, colorectal, or estrogen receptor-positive breast cancer, highly expressed HMGCR is associated with prolonged relapse-free survival and less aggressive phenotype [[31], [32], [33]]. However, high mRNA expression of HMGCR is correlated with poor prognosis and early recurrence in prostate cancer patients [34]. Silencing of HMGCR or farnesyl diphosphate synthase induces apoptosis in glioblastoma cells [35,36]. Inhibition of HMGCR depletes mevalonate-derived intermediates, and consequently inducing cell apoptosis [37]. Downregulation of HMGCR expression inhibits the mevalonate pathway and glutathione peroxidase 4 (GPX4), thereby triggering triple-negative breast cancer cell ferroptosis [38].
Increased squalene production protects against ferroptosis in cholesterol auxotroph cells by compensating for the loss of squalene epoxidase [39]. Inhibition of the squalene monooxygenase or squalene synthase activity, which is downstream of isopentenyl pyrophosphate, hampers ferroptosis of osteosarcoma, lung adenocarcinoma, and fibrosarcoma [40]. Coenzyme Q10 (CoQ10) supplementation substantially represses ferroptosis in cells. Suppression of isopentenyl pyrophosphate synthesis prevents Sec-tRNA maturation, which is essential for Sec incorporation into the selenoprotein GPX4 [41]. Depletion of arachidonate lipoxygenase12 (Alox12) activates AMPK, represses the activity of acetyl-CoA carboxylase1 (ACC1) and lipid synthesis, ultimately inhibits breast cancer growth [42].
Sterol regulatory element binding proteins (SREBPs) as transcriptional factors of the mevalonate pathway that modulate de novo lipogenesis, inducing lipogenic reprogramming of cancer cells (Figure 1D). MicroRNA-185 and 342 trigger apoptosis in prostate cancer via blocking the SREBP pathway and regulating the expression of genes involved in lipid biosynthesis [43]. Upregulated SREBP signaling enhances cancer cell growth by increasing phospholipid, triacylglycerol (TAG), and cholesterol syntheses. In contrast, SREBP depletion causes a loss of mono- and poly-unsaturated lipids, induces reactive oxygen species (ROS) accumulation and apoptosis in breast cancer. Increased glucose uptake facilitates N-glycosylation and stability of SREBP-cleavage activating protein (SCAP) in glioblastoma, accelerating SREBPs recruitment to the Golgi apparatus and subsequent proteolytic activation [44]. Importantly, SREBP ablation induces apoptosis in glioblastoma cells under lipoprotein-deplete conditions [45]. Induction of SREBP-1 driven by autophagy and FASN play crucial roles in leptin-stimulated metabolic reprogramming [46]. The regulation of lipid composition by SREBP is vital to maintain the balance between downstream protein and lipid biosynthesis and to prevent cancer cell death.
Phospholipids are necessary for membrane biogenesis. Compared to cholesterol, less is known about how its synthesis. Lipin-1 encoded by LPIN1, is substantially upregulated in basal-like triple-negative breast cancer and is linked to the poor outcomes of patients. Silencing LPIN1 induces apoptosis in basal-like breast cancer cells, whereas has no effect on human mammary gland epithelial cells and endoplasmic reticulum (ER)-positive breast cancer cells [47].
A previous study revealed that cholesterol esterification was a feature of glioblastoma [48]. Highly expressed sterol O-acyltransferase (SOAT1/ACAT1) is critical regulator of the cholesterol esterification and storage (Figure 1C). SOAT1 attenuates glioblastoma development and prolongs survival in xenograft models by inhibiting SREBP-1. The multiple mechanisms linking de novo synthesis of lipid and cholesteryl ester synthesis to oncogenic signaling networks clearly demonstrate their importance for cancer cell death.
2.3. Fatty acid synthesis and oxidation: a potential aspect of metabolic transformation in cancer cell death
Apart from being a source of ATP and nicotinamide adenine dinucleotide phosphate (NADPH), fatty acids are important structural components in cells. The monounsaturated fatty acid (MUFA) oleic acid (C18:1) is one of the most prevalent fatty acids in cells. During de novo fatty acid synthesis, stearoyl-CoA desaturase-1 (SCD1) form oleic and palmitoleic acid (C16:1) by catalyzing the formation of a double bond in stearic acid (C18:0) and palmitic acid (C16:0), respectively. In breast cancer cells cultivated under lipid-depleted circumstances, inhibition of fatty acid synthesis produces ER stress and apoptosis [49]. Exogenous oleic acid or other unsaturated fatty acids, such as the polyunsaturated fatty acids (PUFAs), arachidonic acid (C20:4), or linoleic (C18:2), is sufficient to prevent the activation of the DNA-damage-inducible transcript 3 (DDIT3/CHOP), ER stress and apoptosis via inhibition of SCD1 [50].
Fatty acid synthase (FASN) consumes NADPH, malonyl-CoA, and acetyl-CoA to produce fatty acids, contributing to maintain the metabolic state required for tumor growth [51]. Silencing of FASN induces the apoptosis of ovarian cancer by suppressing Akt phosphorylation [52]. The novel manganese (Mn) complex, labeled as PdpaMn, binds to TE domain of FASN, decreases the expression of FASN, releases mitochondrial cytochrome c (Cyt-c), activates caspase-9, and increases intrinsic apoptosis in breast cancer cells [53]. In patients with hepatocellular carcinoma, ACCα is a strong indicator of poor outcome. Inhibition of ACC drives caspase-mediated apoptosis of prostate cancer cells [54]. Inactivation of the AMPK-GATA3-Enoyl CoA hydratase 1 (ECHS1) pathway induces fatty acid synthesis, promotes the growth of ccRCC, and predicts poor survival in patients [55]. Lipid metabolic reprogramming is exploited to restore metabolic flexibility, maintains the balance between lipid saturation and unsaturation, and prevents cellular stress and death.
Depletion of palmitic acid induces apoptosis and is accompanied with the production of ROS and mitochondrial dysfunction. Fatty acid oxidation (FAO)-associated ROS production appears to be beneficial to cancer cells as it promotes proliferation and/or protects from apoptosis. Knockdown of carnitine palmitoyltransferase 1 (CPT1) induces apoptosis by generating metabolic stress, resulting in cancer cells more susceptible to radiation and anti-cancer drugs [56]. When cancer cells exposed to low glucose and hypoxic conditions, increased expression of CPT1C induced by AMPK and p53, protects cancer cells from death. Furthermore, selective inactivation of SQSTM1/p62 in adipocytes increases osteopontin levels and promotes FAO, leading to the enhanced aggressive ability of metastatic prostate cancer in vivo [57]. Symbiotic cooperation has been found in the metabolism of the tumors and the adipocytes, in which p62 deficiency blocks energy utilization pathways in adipose tissue by downregulating mammalian target of rapamycin (mTORC1) [58]. This effect provides more nutrients for FAO of cancer cell to support their invasive phenotype.
Cancer cells require higher levels of iron and lipids to facilitate growth than normal cells, making cancer cells more vulnerable to ferroptosis. Acyl CoA synthetase long chain family member 4 (ACSL4) is overexpressed in multiple cancer types, whereas significantly downregulated in ferroptosis-resistant cancer cells [59,60]. ACSL4 is rich in long polyunsaturated ω- 6 cell membrane of fatty acids, contributing to the generation of lipid peroxides. In ACSL4−/− Pfa1 cells, the ω-6 fatty acids are more effective than ω-3 fatty acids in triggering ferroptosis [61]. SCD1, a key enzyme in lipid metabolism that catalyzes the rate-limiting step in MUFA synthesis, protects ovarian cancer cells from ferroptosis [62]. The Egl-9 family hypoxia-inducible factor 1/3 (EGLN1/3), an iron-dependent enzyme, and c-Myc directly activate chromatin remodeling factor lymphoid-specific helicase (LSH) by inhibiting hypoxia inducible factor-1α (HIF-1α) in lung cancer. LSH suppresses ferroptosis by upregulating SCD1 and fatty acid desaturase 2 (FADS2) [63]. HIF-2 induces a ferroptosis susceptible cell state by activating the expression of hypoxia inducible lipid droplet-associated protein (HILPDA) [64]. In addition, lipophagy promotes RSL3-induced ferroptosis by decreasing lipid peroxidation and lipid storage in HepG2 cells [65]. Together, it can be concluded that fatty acid synthesis and oxidation is tightly linked to the regulation of ferroptosis (Figure 1E).
2.4. Lipogenesis and lipolysis are involved in cancer cell death
Lipid catabolism is identified as an important process in cancer cell death. In the absence of p53, loss of Atg7 affects FAO and makes it more susceptible to FAO suppression, suggesting that RAS-driven malignancies require autophagy to maintain mitochondrial function for lipid catabolism in the absence of p53 [66]. In the carcinogenesis process, impaired lipid catabolism causes defective survival in starvation and harms to tumor cell fitness. To maintain mitochondrial respiration and survive starvation, autophagy-deficient tumor-derived cell lines rely largely on exogenously provided glutamine [66]. In addition to maintaining mitochondrial lipid catabolism, autophagy-supplied substrates (especially glutamine) may support tumor metabolism and growth. Overexpression of peroxisome proliferator-activated receptor γ (PPARγ) downregulates the anti-apoptotic protein Bcl-2, resulting in cell cycle arrest, reinforces its anti-proliferative effect, and induces apoptosis and anoikis in hepatocellular carcinoma cells [67].
As light is shed into the role of adipogenesis transcription factors with oncogenic signal transduction network, the importance of adipogenesis for cancer cell death is appreciated more. In the presence of exogenous lipids or in the absence of serum derived lipids in TME, cancer cells activate lipogenesis in response to their high metabolic demand [68]. Silencing of protein kinase D3 (PKD3) not only reduces the level of matured-SREBP1, but also blocks the binding of SREBP1 to the promoter of the FASN gene, which in turn attenuates de novo lipogenesis to ameliorate the cell proliferation of prostate cancer [69]. An important feature of breast cancer cells that express the human epidermal growth factor receptor 2 (HER2) to maintain invasiveness is the persistent upregulation of de novo lipogenesis. Inhibition of HER2 abrogates its oncogenic activity and induces apoptosis [70]. Hepatitis B X-interacting protein (HBXIP)/LXRs/SREBP-1c pathway forms a positive-feedback loop, resulting in the enhanced lipogenesis and the sustained overexpression of FASN to potentiate breast cancer cell growth [71]. ACSL4 upregulates SREBP1 and its downstream lipogenic enzymes in hepatocellular carcinoma cells via c-Myc, thereby promoting de novo lipogenesis by accumulating intracellular triglycerides, cholesterols, and lipid droplets [72]. In cancer patients, lipolysis is one of the primary mechanisms that leads to adipose atrophy and cachexia. Melanoma cachexia factor, an inhibitor of adipose lipoprotein lipase secreted by the tumor, reduces extracellular lipolysis and depletes fat reserves in malignant melanoma [73]. Altogether, while lipogenesis and lipolysis are well understood in lipid metabolism, they still remain ill-defined in oncogenic lipid metabolism.
2.5. Cholesterol and lipid droplets (LDs) as metabolic determinants for cancer cell death
Clinical studies on the functions of cholesterol in carcinogenesis is contradictory, making it even more difficult to fully comprehend the relationship between cholesterol and human cancers [74]. Indeed, dietary cholesterol levels beyond a certain threshold are linked to an increased risk of cancer. However, the effects may be dependent on the specific circumstances. For instance, this impact, is only observed in postmenopausal breast cancer patients, not in premenopausal women [75]. High dietary cholesterol raises the risk of colorectal cancer compared to non-metastatic patients [76]. Hypercholesterolemia increases overall prostate cancer risk in the short term. The inverse risk association with advanced prostate cancer is observed with 20-years’ lag time. Higher serum HDL levels are correlated with worse survival in patients with surgery gastric cancer and prostate cancer [77,78]. Very low density lipoprotein (VLDL) reduces anchorage-dependent cell death and promotes lung metastasis in nude mice [79]. These findings highlight the complexity of evaluating cholesterol as a risk stratification or prognostic marker that takes consideration on the tissue origin, patient characteristics, dietary and lifestyle habits, and the length of time the patient was observed. Oxysterols, such as cholestane-3β-5α-6β-triol, 7-ketocholesterol, and 5α-cholestane-3β, 6β-diol, are 27-carbon oxidation products of cholesterol metabolism, that can inhibit cell-cycle progression and induce apoptosis in both human- and murine-derived cancer cell lines [80]. Oxysterols can be both friend and foe for cancer cells depending on the specific tumor environment. Collectively, understanding the fundamental variables that drive cholesterol pathways in cancer cells is crucial.
The formation and roles of LDs in regulating carcinogenesis and progression have been investigated extensively in the last decade. Elevated LDs in glioma patients are associated with poor survival [48]. The human group X secreted phospholipase A2 (hGX sPLA2) promotes LDs formation in invasive breast cancer cells, induces cell proliferation, and prevents cell death upon serum deprivation. Activation of AMPK inhibits LDs formation and cell growth induced by PLA2 [81]. Inhibition of PLA2 obviously reduces LDs, decreases FAO and oxidative phosphorylation, there by promoting cancer cell apoptosis [82]. Autophagy activates membrane glycerophospholipid metabolism and FAO for energy production. Induction of autophagy to ensure early and long-term metabolic adaptation contributes to increased growth of tumor cells in nutrient deprived environments. In addition, another possible source of amino acids and fatty acids is micropinocytosis, which involves the absorption of extracellular lysophospholipids and proteins.
Elevated LDs act an indirect function in preventing cell death during cancer therapy. An increment in the number of LDs has been found in drug-resistant cancer cells. Thus, LDs act as sequestering hydrophobic therapeutic agents, reduce drug effectiveness [83]. A positive correlation between increased content of LDs and resistance to an aminopeptidase inhibitor, accompanied with the activation of ERK/Akt/mTOR pathway are observed in drug-resistant cells derived from myeloid leukemia [84]. The protective role of LDs is described in colorectal cancer cells against chemotherapy-induced cell death. LDs biogenesis induced by lysophosphatidylcholine acyltransferase 2 (LPCAT2) in colorectal cancer cells impedes caspase cascade activation, impairs ER stress pathways, reduces calreticulin membrane exposure, and therefore inhibiting immunogenic cell death after treatment [85]. Increased expression of acylglycerol-3-phosphate acyltransferase 2 under hypoxia is directly involved in LDs accumulation and cell growth after etoposide treatment in different cancer cell lines [86]. Constitutive HIF signaling and numerous intracellular LDs are two characteristics of ccRCC. PLIN2, an LDs coat protein gene, is upregulated in primary ccRCC specimens and is linked to HIF2. HIF2-dependent PLIN2 expression enhances lipid storage to maintain ER homeostasis, which support ccRCC cells survival under nutrient and oxygen deprivation [87]. Interestingly, LDs-enriched cells in the same population contribute to sequester contribute to harmful chemicals like ROS or lipid peroxides, which can protect the overall cell population from cytotoxic stress [85]. These findings suggest that hindering LD biogenesis might improve cancer therapy efficiency (Figure 1F).
Lipophagy appears to have a dual function in cancer, despite poorly studied. LDs degradation via lipophagy improves hepatocarcinoma cell survival during starvation [88]. Lipophagy facilitates LDs degradation and lipolysis in androgen-sensitive prostate cancer cells during androgen limited condition, resulting in cell death during hormone treatment [89]. In colorectal cancer, inhibiting lipolysis causes both LDs accumulation and cell death, driving the transition of cancer stem cells to a more aggressive mesenchymal phenotype [78]. Additionally, overexpression of autophagy regulatory protein autophagy-related protein 14 (ATG14) increases LDs lipophagy, leading to HeLa cells more susceptible to apoptosis [90]. In addition, increased levels of circulating free fatty acids, monoacylglycerides, and diacylglycerides have been observed in patients with cachectic cancer, which promote tumor growth by fueling the metabolism of cancer cells [91].
3. The interplay between lipid metabolic plasticity and cancer cell death: new challenges and opportunities
Recent findings have been emphasized the metabolic plasticity of cancer cells and provided new insights into how metabolic rewiring is a key event in cancer cell persistence, dedifferentiation, and growth. During the process of metastasis, cancer cells are able to adapt to complicated environments. Metastatic cancer cells rely on a variety of nutrients and metabolites to meet their metabolic demands, a phenomenon known as metabolic flexibility, which might be a hurdle to overcoming various stages of the metastatic cascade [92]. The constant requirement for metabolic changes necessitates both flexibility (the capacity to use various metabolic substrates) and plasticity (the ability to process metabolic substrates in various ways) [93]. When confronted with harsh environments, such as hypoxia and acute nutritional depletion, cancer cells often exhibit metabolic plasticity, allowing them to use available resources as bioenergetic substrates. This metabolic flexibility allows maintained ATP production under a variety of physiological and pathological situations, and is primarily influenced by substrate concentration, blood flow, hormone levels, and oxygen delivery [94]. The metabolic vulnerabilities of Hs578T cells overexpressing fatty acid-binding protein 7 (FABP7) cause S/G2-phase arrest and cell death. When FABP7-overexpressing triple-negative breast cancer cells are confronted with serum starvation, the activation of PPARα ameliorates metabolic flexibility, thereby resulting in cancer cell death [95].
Given the role of lipids at different levels in cellular physiology, alterations in lipid metabolism can disturb tissue and organelle plasticity [68,96]. FAO acts as a metabolic cue that triggers proliferation in a -HB/GPR109A dependent autocrine manner under nutrient favorable conditions, while provides an efficient, alternate ATP source only when nutrients are limited. FAO has been identified as a dominant metabolic circuit in glioblastoma, allowing these cells to adapt to their dynamic environment through metabolic plasticity [97]. Phospholipase D1 (PLD1) is involved in regulating metabolic plasticity in breast cancer cells. Large amounts of membrane phospholipids are hydrolyzed by PLD1-regulated autophagy for FAO in mitochondria. Inhibition of PLD1 attenuates ATP generation and promotes ROS accumulation in low glucose conditions, resulting in breast cancer cell death [98].
Adipocytes provide energy to cancer cells, boost mitochondrial metabolism, thus promote tumor growth. In metastatic ovarian cancer, host obesity raises overall and organ-specific tumor burdens, increases the expression of SREBP1, and elevates intracellular lipid content. Aside from increased fatty acid transport, SREBP1-driven de novo fatty acid synthesis promotes ovarian cancer growth and metastasis in the microenvironment of obese host [99]. These findings emphasize metabolic plasticity and bioenergetic adaptation of ovarian cancer cells in the adipocyte-rich microenvironment.
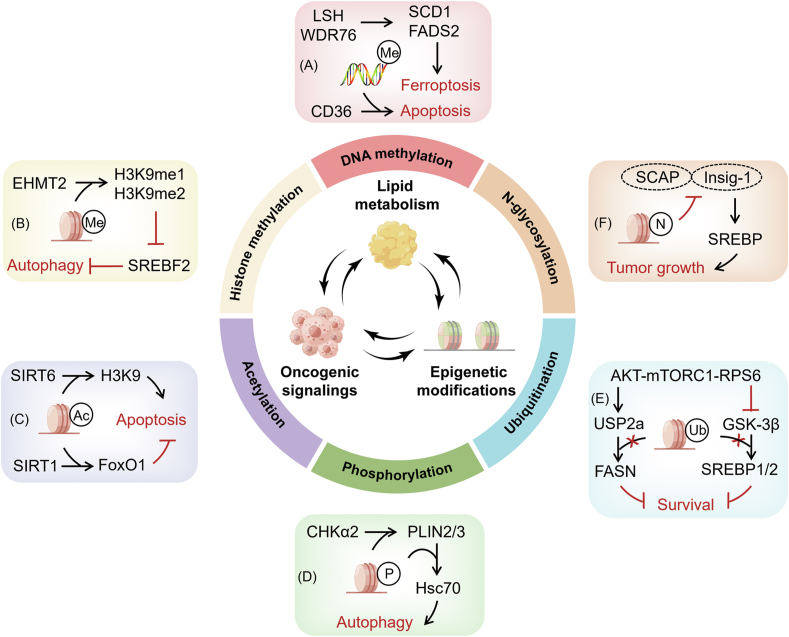
4. Epigenetic modification and lipid metabolism genes in cancer cell death: a bidirectional link
Recently, cellular metabolism has been identified as a context-dependent regulator in epigenetic alterations. Intermediate metabolites drive chromatin dynamics post-translational modifications that altered chromatin structures and functions [100]. Lipid metabolism and the epigenome have a bidirectional relationship and interact with the genetic and molecular factors that control cancer. In general, epigenetic regulation is achieved through two mechanisms: cytosine methylation in the DNA sequence and chemical changes of histones and other chromatin-associated proteins. Histone tail domains can be covalently modified via methylation, acetylation, phosphorylation, ubiquitination, and palmitoylation at certain amino acid locations to regulate the contacts with DNA [101].
The epigenetic regulators have been identified to be involved in cancer cell death by modulating metabolic genes and intermediators. Hypermethylation mediated by DNA methyltransferases (DNMTs) prevents the transcription of tumor-suppressor genes. High levels of FABP5 in prostate cancer cells can be attributed to hypomethylation of the CpG-rich promoter regions (CpG islands), and upregulation of the direct trans-acting factors specificity protein 1 (Sp1) and c-Myc [102]. Hypermethylation of CD36 correlates with its low expression in primary lung cancers, leads to lung cancer cell apoptosis [103]. Loss of PPARα in the intestine increases methylation of P21 mediated by DNMT1 and p27 methylation mediated by PRMT6 to trigger the initiation of colon carcer [104]. LSH suppresses ferroptosis and promotes lung carcinogenesis by altering the chromatin modification of metabolic genes [105]. WD repeat domain 76 (WDR76) is recruited by LSH to the promoters of metabolic genes, such as FADS2 and SCD1. The increased expression of FADS2 and SCD1 caused by DNA methylation and histone modification induces lipid ROS and iron accumulation, blocks ferroptosis, and promotes lung carcinogenesis [106]. Cancer-specific ABCA1 hypermethylation leads to increased intracellular cholesterol levels and hence promotes prostate cancer progression [107]. Histone lysine (K)-specific methyltransferase 2D (KMT2D) is repressed due to DNA methylation in human pancreatic cancers. Inactivation of KMT2D significantly changes the expression levels of genes involving in the uptake, biosynthesis, β-oxidation or degradation of lipids, such as HMGCR, FASN, and acetyl CoA carboxylase (ACACA), resulting in the loss of the H3K4me3 mark and promoting pancreatic carcinogenesis [108].
Recent studies suggest that metabolic dysfunction is caused by a direct relationship between metabolism and chromatin dynamics. The surface charge of histone proteins is affected by the enzymatic addition of methyl groups, ubiquitin, or phosphate to amino acids at the N terminus, contributing to the structural balance between euchromatin and heterochromatin [109]. Euchromatic histone-lysine N-methyltransferase 2 (EHMT2) as a histone methyltransferase can catalyzes di- and mono-methylation at histone H3 lysine 9 (H3K9me1 and H3K9me2, respectively), leading to gene silencing. Inhibition of EHMT2 using the specific blocker BIX attenuates the expression of H3K9me1 and H3K9me2 at the promoter of the sterol regulatory element-binding protein 2 (SREBF2) locus, upregulates the transcription of SREBF2 and its target genes, promotes cholesterol biosynthesis, and activates autophagy, resulting in non-small cell lung cancer cell death. Inhibition of 25HC-mediated cholesterol biosynthesis to attenuate autophagy restored Bix-induced cell death [110]. Hence, inhibiting EHMT2 may be a promising approach to trigger cell death by affecting the expression of SREBF2 and cholesterol synthesis.
Although ectopic expression of Sirtuin 6 (SIRT6) in liver cancer cells enhances apoptosis sensitivity, liver-specific deletion of SIRT6 profoundly increases promoter H3K9 acetylation level, leading to stronger glycolysis and triglyceride synthesis, reduced β-oxidation [111,112]. Nicotinamide N-methyltransferase (NNMT) expression is negatively associated with the acetylation state of forkhead box O1A (FoxO1), a well-studied SIRT1 target. Hepatocytes overexpressing NNMT improve SIRT1 stability while reducing FoxO1 acetylation [113]. Moreover, SIRT1 activates gluconeogenesis mediated by NNMT and attenuates cholesterol synthesis as well as lipogenesis [114]. In breast cancer patients receiving chemotherapy, high levels of NNMT are associated with poor survival and a poor therapeutic response. The overexpression of NNMT reduces the susceptibility of breast cancer cells to paclitaxel- and doxorubicin-induced apoptosis by enhancing the stability and activity of SIRT1. Inhibition of NNMT or downstream SIRT1 has also been shown to boost the effectiveness of breast cancer chemotherapy [115]. NNMT promotes resistance to 5-fluorouracil in colorectal cancer cells. NNMT increases ATP levels and decreases intracellular ROS production, leading to inactivation of the ASK1-MAPK pathway and reduction of 5-fluorouracil-induced mitochondrial apoptosis [116,117]. Interestingly, NNMT acts a negative autophagy regulator in hepatocellular carcinoma, inhibiting cancer cell death. Although NNMT is abundant in hepatocellular carcinoma tissues, it is considerably downregulated in surrounding normal [118]. Acetate primarily promotes the expression of lipogenic genes ACACA and FASN by raising H3K9, H3K27, and H3K56 acetylation levels at their promoter regions, resulting in increased de novo lipid synthesis [119]. Acetate is also an epigenetic metabolite that prevents cancer cells death under hypoxic conditions [119].
The binding of choline kinase 2 (CHK2) to LDs is promoted by glucose deprivation, which is mediated by KAT5-dependent CHK2 K247 acetylation and AMPK-dependent CHK2 S279 phosphorylation. Importantly, CHK2 modifies catalytic domain conformation functions and phosphorylates perilipinin-2 (PLIN2) at Y232 and PLIN3 at Y251 as a protein kinase. Phosphorylated PLIN2/3 dissociates from LDs and is degraded by Hsc70-mediated autophagy, thereby increasing FAO, LDs lipolysis, and brain tumor growth. Moreover, the acetylation of CHK2 K247, the phosphorylation of CHK2 S279, and the phosphorylation of PLIN2/3 are all positively related to a poor prognosis in glioblastoma patients [120].
Palmitate, a product of FASN, binds to G proteins, or RAS and RAS-homologous GTPases at C-terminal CAAX-boxes, affecting their intracellular distribution and activity [121]. The products of the mevalonate pathway are available for lipid-dependent post-translational modification of proteins, such as ubiquitination or N-glycosylation. The Akt-mTORC1-ribosomal protein S6 (RPS6) pathway inhibits the ubiquitination of FASN by upregulating the USP2a de-ubiquitinase and suppresses the ubiquitination of SREBP1, SREBP2 by repressing GSK-3β, then impedes proteasome degradation of SREBP1, SREBP2, FASN, and thus promotes de novo lipogenesis and hepatocellular carcinoma survival [122]. RAS and RHO GTPases have been shown to be prenylated, while insulin-like growth factor receptor has been found to be N-glycosylated [123]. Glycosylation stabilizes SCAP and decreases its interaction with insulin-induced gene 1 (Insig-1), allowing SCAP/SREBP to be transported to the Golgi and activated SREBP proteolytically. Blocking SCAP N-glycosylation inhibits glioblastoma growth driven by EGFRvIII [44]. In summary, cancer epigenetics, lipid metabolism, and oncogenic signaling are now widely considered to be intertwined through a multidirectional, crosstalk regulatory network (Figure 2).
Figure 2.
Crosstalk between lipid metabolism, oncogenic signaling, and epigenetic modifications and their links to metabolic disorders and cancer. Lipid metabolism and the epigenome interact in a bidirectional fashion with genetic and molecular drivers that regulate cancer. An in-depth understanding of the interplay between molecular drivers, metabolic reprogramming, and epigenetic modifications in cancer will clarify their relationships and contribute to the development of effective cancer therapies. (A) Hypermethylation of CD36 leads to apoptosis in lung cancer cells. Increased expression of FADS2 and SCD1 caused by DNA methylation and histone modifications blocks ferroptosis. (B) EHMT2 activates H3K9me1 and H3K9me2, upregulates the transcription of SREBF2, and then triggers autophagy. (C) Deletion of SIRT6 increases promoter H3K9 acetylation levels, resulting in enhanced apoptosis sensitivity. NNMT improves SIRT1 stability while reducing FoxO1 acetylation, lowering the susceptibility of apoptosis. (D) CHK2 phosphorylates PLIN2/3 dissociates from LDs and is degraded by Hsc70-mediated autophagy. (E) The Akt-mTORC1-RPS6 pathway inhibits FASN ubiquitination by upregulating the USP2a de-ubiquitinase and suppresses ubiquitination of SREBP1 and SREBP2 by repressing GSK-3β, thereby promoting hepatocellular carcinoma survival. (F) Glycosylation stabilizes SCAP and decreases its interaction with Insig-1, promoting glioblastoma growth.
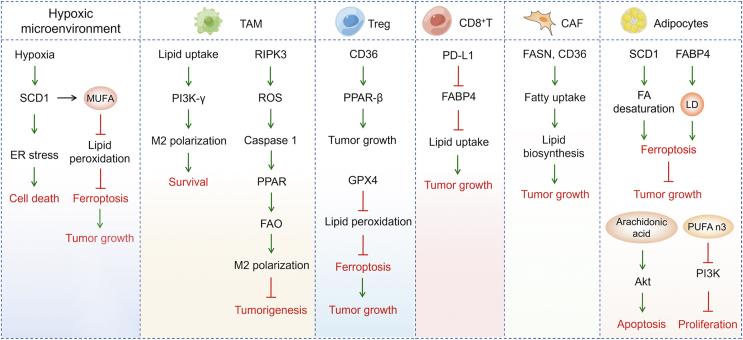
5. Lipid metabolism in the TME: emerging theranostic platforms for cancer cell death
During tumor evolution, metabolic pathways are reprogrammed and plasticized by the TME to meet the material and energy requirements for tumor growth. To understand TME, we pay attention to the effects of biomolecules presenting in the TME (including nutrients, cytokines, cell stroma) on lipid metabolism and cancer cell death (Figure 3).
Figure 3.
Exogenous lipids and lipid metabolism-related genes from the TME regulate cancer cell death. A complicated combination of cancer cells, immune cells, and stromal cells composes the TME. LD-acquired and stored unsaturated fatty acids assist cancer cells survival under hypoxia when de novo synthesis of unsaturated fatty acids is inhibited. Adipokines like FABP4 boost the expression of fatty acid transporters like CD36, making it easier for non-cancer cells to uptake fatty acids. Fatty acids promote tumor growth in immune cells attracted to the TME, such as TAM and T cells. Cancer cells enter the stromal compartment through the basement membrane, activating stromal cells such as CAFs and adipocytes and influencing lipid metabolism.
Serum and oxygen limitations decrease the viability of renal cancer and glioblastoma cells, which are reversed by the addition of MUFA [49]. Furthermore, oleate rescues the hypoxic state resulting from SCD1 inhibition, suggesting that unsaturated lipids are essential for cancer cell survival under hypoxic conditions [49]. Human cervical, breast, and lung cancer cells have been demonstrated to compensate for hypoxic environments by scavenging unsaturated fatty acids [124]. Hypoxia can also up-regulate SCD1 levels in human glioblastoma cell lines, in addition to increasing the expression of proteins that regulate fatty acid uptake [125]. Inhibition of SCD1 causes a deficiency in unsaturated lipids, promotes ER stress and accelerates human glioblastoma cell death in a lipid-depleted microenvironment [45]. While fatty acid synthesis is sustained, loss of desaturation capacity due to reduced SCD1 activity disturbs the balance between saturated FA and MUFA, resulting in lipotoxicity, a mechanism by which excessive fatty acid accumulation in non-adipocytes causes cell dysfunction and death [126]. Metastatic melanoma cells in the oleate-rich lymphatic environment utilize oleate to protect themselves from ferroptosis in an ACLS3-dependent manner [127]. Hypoxia and nutrient starvation cause the pancreatic cancer cell ferroptosis resistance which is attributed to SCD1 upregulation. SCD1 induces MUFA TAMs synthesis and protected against lipid peroxidation [128]. The capability of the cells to burn lipid is directly related to their survival in response to common TME alterations, such as hypoxia-reoxygenation patterns in prostate cancer [129].
Cancers are not a simple population of cells to govern the cell cycle. More importantly, cancer cells interact with the surrounding healthy tissue and the TME. A co-evolution takes place between malignant cells, the immune system, cancer-associated fibroblasts (CAFs), tumor endothelial cells, adipocytes, cancer-associated adipocytes (CAAs), and the extracellular matrix throughout the neoplastic process (Figure 3). Tumor heterogeneity is influenced by the numerous types of immune cells infiltrating the initial tumor, the flexibility of the CAFs, and the tissue responsible for angiogenesis, in addition to intratumoral variety acquired through Darwinian evolution [130].
Abundant lipid contents are found in tumor-associated macrophages (TAM) derived from tumor-bearing mice and specimens of gastric cancer patients. Increased uptake of extracellular lipids from tumor cells causes lipid accumulation in TAM, which leads to the activation of PI3K, polarizing TAM to M2-like profile [131]. Deletion of RIPK3 in TAM significantly reduces ROS and inhibits caspase 1-mediated PPAR activation, facilitates FAO, and induces M2 polarization in the TME. Upregulating RIPK3 or blocking FAO reverses the immunosuppressive activity of TAM and dampens hepatocellular carcinoma tumorigenesis [132]. A-FABP expression in TAM promotes IL6/STAT3 signaling by activating the NF-κB/miR-29b pathway [133]. A-FABP, as a functional marker of protumor macrophages, provides a novel target for tumor immunotherapy.
CD36, as a central metabolic regulator, is selectively upregulated on intratumoral Treg cells. CD36 upregulates PPAR-β expression, maintains mitochondrial fitness, drives regulatory T cells (Treg cells) programming to adapt to the lactate rich TME. Genetic ablation of CD36 reduces intratumoral, Treg cells enhance tumor infiltrating lymphocyte antitumor activity without disrupting immune homeostasis. Furthermore, targeting CD36 elicits an antitumor response against programmed cell death protein 1 therapy [134]. Atg5-deficient dendritic cells exhibit an increased expression of CD36 and excessive lipid storage. When blocking CD36 with antibodies, CD4+ T-cell responses are significantly increased, along with improved phagocytosis and inhibition of tumor growth [135]. In addition, CD36 mediates fatty acids uptake by tumor infiltrating CD8+ T cells in the TME, promotes lipid peroxidation and ferroptosis, reduces the production of cytotoxic cytokine and impairs anti-cancer capacity [136]. When the T cell receptor (TCR)/CD28 are co-stimulated, the loss of GPX4 causes an excessive accumulation of lipid peroxides and ferroptosis in Treg cell. Moreover, Treg-specific ablation of GPX4 suppresses tumor growth and simultaneously enhancing antitumor immunity [137]. The survival of CD8+ tumor-infiltrating T lymphocytes (TILs) relies on FAO. Deprivation of fatty acid causes cell death of CD8+ TILs. In a tumor cell-T-cell coculture system, the ability of gastric adenocarcinoma cells to uptake lipids outcompeted CD8+ TILs cells, leading to cell death of CD8+ TILs. Alternatively, inhibiting XBP1 and reducing cholesterol content in both CD8+ T cells and the TME dramatically enhance the anticancer effectiveness of T cell-based immunotherapy [138]. Targeting PD-L1 reduces the expression of FABP4/5 in gastric adenocarcinoma cells. In contrast, blocking PD-L1 enhances FABP4/5 expression in CD8+ TILs, stimulates lipid uptake by CD8+ TILs, and improves the survival of CD8+ TILs.
Since cancer cells tend to be in low oxygen and nutrient poor environments, cancer cells are dominated by anabolic metabolism, whereas CAFs are dominated by catabolic metabolism. Previous study has shown that the anabolism of cancer cells is coupled with the catabolism of CAFs, which can provide crucial metabolic substances for highly proliferative cancer cell [139]. Cancer cells mutually promote lipidomic reprogramming with their CAFs, establishing a symbiotic relationship. Colorectal cancer cells uptake the phospholipids and fatty acids released by CAFs to promote their migration, which are partially reversed by knockdown of FASN in CAFs with SSO or utilizing CD36 monoclonal antibody in vivo.
The loss of CD36 inhibits fatty acid uptake from the TME, as well as cancer-mediated lipid biosynthesis from fatty acid precursors and the formation of carcinogenic lipid signaling pathways and attenuates prostate cancer growth. Exogenous fatty acids contribute to the formation of complex lipids in prostate cancer and are as important as glucose for energy supply [140]. During the activation phase, CAFs derived from pancreatic stellate cells in pancreatic ductal adenocarcinoma alter lipid metabolism with remodeling of intracellular liposomes. Meanwhile, neutral lipids are disappeared and intracellular lysophospholipid levels are dramatically raised in pancreatic stellate cells. Lysophosphatidylcholines are abundantly secreted into the TME and a subset is directly taken up by pancreatic ductal adenocarcinoma cells for membrane lipid formation; Another part of lysophosphatidylcholines is hydrolyzed by autotaxin secreted by pancreatic ductal adenocarcinoma cells to generate lysophosphatidic acid (LPA), which activates protein kinase B, also known as Akt pathway of pancreatic ductal adenocarcinoma cells via LPA receptor [141]. The autotaxin/LPA/LPA receptors signaling pathway affects tumor growth, proliferation, and metabolism and is a potential therapeutic target for various type of cancers, including liver, non-small cell lung, ovarian, pancreatic, and thyroid cancers [142].
Adipocytes in the TME serve as an energy source and metabolic regulator to promote colon cancer cell proliferation. By upregulating mitochondrial FAO, the uptake of fatty acid allows cancer cells to survive under nutritional deprivation conditions. Inhibition of autophagy attenuates the capacity of cancer cells to utilize fatty acids and prevents the growth-promoting effect of adipocytes [143]. SCD1 causes fatty acid desaturation and FABP4 derived from tumor endothelial cells as well as adipocytes in the TME enhances LDs in cancer cells, which synergistically protect from oxidative stress-induced ferroptosis. Lipid mobilization and desaturation trigger tumor intrinsic antioxidant and ferroptosis resistance, prompting tumor survival in the TME [144]. Arachidonic acid secreted by adipocytes directly activates Akt signaling pathway and inhibits cisplatin-induced apoptosis in ovarian cancer [145]. PUFA n-3 released from the adipocytes impairs the proliferation of chronic myeloid leukemia by inhibiting the PI3K pathway [146].
CAAs release adipokines and adipocytokines, which are directly involved in tumor development, after sense signals from malignant cells. Alternatively, adipocyte-derived substances regulate tumor development, metastasis, and cachexia by influencing nontumor cells in the TME [147]. A recent study found that ovarian cancer metastases to omental adipose tissue, where the cancer cells trigger fatty acid release from the adipocytes [91].
Cancer and host cells, particularly immune cells, have similar metabolic requirements, implying that targeting lipid metabolism should be based on the gene type, tumor type, and TME compositions of malignancies. While inhibiting lipid metabolism, strategies to retain antitumor immune cell activity should be prioritized. A full knowledge of the complex heterogeneous interactions in the TME, as well as the redundant mechanisms dependency between tumor and host cell lipid metabolism, will contribute to realize lipid metabolism targeted therapy.
6. Therapeutic strategies for alteration of lipid metabolism to induce cancer cell death
6.1. Small molecule agents trigger cancer cell death by targeting lipid metabolism
Small-molecule compounds have been used to cancer treatment for several years, which can modulate cell death pathways by targeting lipid metabolism (Table 1). Owing to the importance and diversity of cholesterol metabolism in cancer progression, hindering cholesterol metabolism by inhibiting the mevalonate pathway, has proven to be a viable anti-cancer approach. HMGCR inhibitors, such as statins, have been the most commonly utilized cholesterol metabolism-targeting drugs in clinical studies for cancer patients. Statins induce apoptosis in glioma cells upon geranylgeranyl pyrophosphate (GGPP) biosynthesis is inhibited, and consequently decrease the level of phosphorylated ERK1/2 and Akt [148]. Statins induce the programmed death of lung cancer cells, indicating a susceptibility for statin-specific apoptosis in vivo [149].
Table 1.
Targeting lipid metabolism with small-molecule agents to modulate cancer cell death.
| Target | Reagents | Drug development stage | Effects | Mechanisms | Cancer types | References |
|---|---|---|---|---|---|---|
| HMGCR | Statins | Pre-clinical and clinical | Cholesterol synthesis↓, Apoptosis↑ | Mevalonate pathway↓ | Glioma, lung cancer | [44,148,149,157,178] |
| LXR | GW3965 | Pre-clinical | Cholesterol efflux↑, cell death↑ | LDLR degradation, ABCA1↑ | Glioblastoma, pancreatic cancer and ccRCC | [8,150,151] |
| T0901317 | Pre-clinical | Apoptosis↑ | AKT↓, caspase-3↑ | Melanoma, prostate cancer | [8,[150], [151], [152], [153]] | |
| LXR623 | Pre-clinical | Cholesterol↓, apoptosis↑ | LDLR↓, ABCA1↑ | ccRCC | [[151], [152], [153]] | |
| SR9243 | Pre-clinical | Apoptosis↑ | SREBP-1c, FASN, SCD1↓ | ccRCC | [151] | |
| SOAT1 | Avasimibe | Pre-clinical | Fatty acid production and LDL uptake↓, apoptosis↑ | SREBP1↓ | Prostate cancer, glioblastoma, pancreatic cancer, liver cancer | [44,151,157,178] |
| FASN | TVB-3166 | Pre-clinical | Lipid biosynthesis↓, apoptosis↑ | PI3K-AKT-mTOR, β-catenin, c-Myc↓ | Non-small cell lung cancer | [51] |
| ACC | ND-654 | Pre-clinical | Lipogenesis↓, apoptosis↑ | ACC phosphorylation↓ | Liver cancer | [160] |
| – | Valproic acid | Pre-clinical | Lipogenesis↓, apoptosis↑ | CEBPα/SREBP-1 pathway↓ | Prostate cancer | [161] |
| – | CPI-613 | Pre-clinical | Lipid metabolism↓, autophagy, apoptosis ↑ | AMPK-ACC signaling↑ | Pancreatic cancer | [162] |
| – | VD3+ T0901317 | Cholesterol accumulation↓, apoptosis↑ | ABCA1-CHOP-BCL-2↑ | Breast cancer | [154] | |
| CPT-1 | CTPi + PMCTi | Pre-clinical | Fatty acid production↓, mitochondrial-dependent apoptosis↑ | / | Liver cancer | [163] |
↓: Downregulation; ↑: Upregulation; -: Not determined.
Targeting LDLR with the LXR agonist GW3965 improves cholesterol efflux and cell death in ccRCC, glioblastoma, and pancreatic cancer by increasing IDOL-mediated LDLR degradation and upregulating ABCA1 expression [8,150,151]. The synthetic LXR agonist T0901317 induces apoptosis in melanoma cells through activating the caspase-3 pathway [152]. T0901317 downregulates the Akt survival pathway to induce prostate cancer cells apoptosis in a lipid raft-dependent manner [153]. The combination of VD3 and T0901317 is more effective in inhibiting cholesterol storage and inducing apoptosis via triggering the ABCA1-CHOP-BCL-2 cascade in breast cancer cells [154]. LXR623 is a LXR agonist that downregulates the protein expression of the LDLR and upregulates ABCA1 levels, leading to lower intracellular cholesterol and apoptosis in ccRCC [151]. The LXR inverse agonist SR9243 downregulates fatty acid synthesis proteins, including FASN, SREBP-1c, and SCD1, resulting in decreased intracellular fatty acid concentration, thereby inducing apoptosis in ccRCC cells [151].
A case–control study found that statin usage was related with a reduced incidence of colorectal cancer and prostate cancer [155,156]. Avasimibe, an inhibitor of SOAT1, reduces SREBP1-mediated fatty acids production and LDL uptake in prostate cancer, pancreatic cancer, and glioblastoma via increasing intracellular cholesterol-induced ER stress, therefore suppressing tumor growth [48,157]. Besides, Avasimibe also enhances TCR aggregation and immunological synapse formation in CD8+ T cells by elevating the cholesterol content of the plasma membrane, thus enhancing the killing ability of CD8+ T cells on melanoma [158]. Avasimibe may be a safe and effective way to disrupt cholesterol metabolic homeostasis in cancer therapy and should be investigated further in clinical trials.
FASN inhibitor induces apoptosis and restored the sensitivity of ovarian cancer cells to platinum [159]. TVB-3166, an oral, selective, and effective FASN inhibitor, disrupts lipid raft structure, inhibits lipid biosynthesis, and represses oncogenic effector c-Myc expression, PI3K-Akt-mTOR, and β-catenin signal transduction in non-small cell lung cancer [51]. ND-654, a novel potent, liver-specific ACC inhibitor, mimics the effects of ACC phosphorylation and inhibits hepatic de novo lipogenesis and the progression of hepatocellular carcinoma by mimicking the effects of ACC phosphorylation. Using it alone or in combination with multi kinase inhibitor sorafenib can improve the survival of tumor-bearing mice [160]. Valproic acid markedly attenuates lipogenesis and induces apoptosis in prostate cancer cells via blocking the C/EBPα/SREBP-1 pathway [161]. CPI-613 (Devimistat), a new lipoate analog, inhibits mitochondrial metabolism, induces autophagy and inhibits lipid metabolism by activating AMPK-ACC signaling, and thus triggers ROS-related apoptosis of pancreatic cancer cells [162].
The DNA methylation inhibitor decitabine and the histone deacetylase inhibitor chidamide co-treatment decreases CD36 methylation, increases its mRNA expression levels, and promotes apoptosis of lung cancer cells [103]. The combination of citrate transporter (CTP) inhibitor and plasma membrane citrate transporter (PMCT) inhibitor impairs CPT-1 activity, reduces intracellular citrate levels and fatty acid contents, increases ROS production, and consequently induces HepG2 cell apoptosis through a mitochondrial-dependent pathway [163]. The aim of these combination therapy strategies is to identify interacting regulatory networks (such as proliferative signaling and lipid metabolic gene regulation), as well as activating stress responses in cancer cells, which can be recognized by the immune system to eradicate cancer cells.
6.2. Natural products target lipid metabolism to improve cancer therapy
Chemotherapeutic drugs have undeniably improved cancer treatment outcomes, yet significant side effects and the development of drug resistance may be obstacles to cancer treatment. Despite the development of various alternative therapies, current clinical outcomes are insufficiently robust, and new effective approaches must be continually sought. The utilization of phytochemical substances, which have potential pharmacological effects, is one of the emerging strategies. Natural substances have been shown to have anti-cancer properties, particularly in the regulation of lipid metabolism and the activation of cell death (Table 2). Therefore, active ingredients derived from natural product are the alternative approaches for therapeutic strategy against cancer.
Table 2.
Representative natural compounds regulating lipid metabolism in cancer cell death.
| Compounds | Sources | Drug development stage | Effects | Mechanisms | Cancer types | References |
|---|---|---|---|---|---|---|
| Celastrol | Tripterygium wilfordii Hook. F. | Pre-clinical | Lipid accumulation↓, lipophagy↑ | LXRα/ABCA1↑, CAV-1/LOX-1↓ | Clear cell renal cell carcinoma | [164,165] |
| Platycodin D | Platycodon grandiflorus | Pre-clinical | LDL uptake↑, autophagy↓ | LDLR↑ | Glioblastoma | [166] |
| Berberine | Ranunculaceae | Pre-clinical | Lipid synthesis↓, apoptosis↑ | FASN↓ | Breast cancer | [167] |
| Betulinic acid | Betulaceae | Pre-clinical | De novo FA synthesis↓, apoptosis↑ | SCD-1↓ | Cervical cancer | [168,169] |
| GL22 | Ganoderma mushrooms | Pre-clinical | Disrupted FA transport, cell death↑ | FABPs↓ | Liver cancer | [175] |
| Peiminine | Fritillaria thunbergii | Pre-clinical | PUFAs accumulation, apoptosis and autophagy↑ | PI3K/Akt/mTOR↑ | Colorectal cancer | [170,171,177] |
| Resveratrol | Polygonum cuspidatum, Vitis | Phase I-III | Lipogenesis ↓, apoptosis↑ | Fas/CAV-1, caspase-8 DAPK2, BNIP3↑ | Breast cancer, non-small lung adenocarcinoma | [173,174] |
| Citral | Cymbopogon citratus (D.C.) citral | Pre-clinical | Lipogenesis↓, apoptosis↑ | FASN, ACC, HMGR, SREBP1, Bcl-2↓, BAX↑ | Prostate cancer | [176] |
| Curcumin | Curcuma longa L. | Phase I-II | LD formation↓ | cPLA2α↓ | Glioblastoma | [170,171,177] |
↓: Downregulation; ↑: Upregulation.
Celastrol is a triterpene extracted from Tripterygium wilfordii Hook. f., a traditional Chinese medicine with anti-cancer properties. Celastrol triggers lipophagy by activating LXRα signal, enhances ABCA1-induced cholesterol efflux, impedes EMT, and inhibits ccRCC cell proliferation and tumor growth [164]. Celastrol also suppresses tumor growth through reduction of lipid storage and caveolae abundance, inhibition of the binding of lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) and CAV-1 in ccRCC cells [165].
Platycodin D, a triterpenoid saponin derived from Platycodon grandiflorus, inhibits autophagy in glioblastoma multiforme cells. Platycodin D prevents the degradation and fusion of lysosomal between autophagosomes and lysosomes by triggering free cholesterol sequestration in lysosomes. Moreover, Platycodin D enhances the uptake of exogenous LDL by upregulating LDLR, resulting in increased cholesterol storage in lysosomes and glioblastoma multiforme cell death [166].
Berberine, a lipid-lowering botanic ingredient with a wide range of therapeutic potential for metabolic disorders, is a viable option against cancer. Berberine inhibits lipid metabolism in breast cancer cells via suppressing the expression of AMPK and FASN. The mitochondrial membrane potential hyperpolarization was alleviated by the presence of compound C or TOFA, indicating that berberine induced apoptosis even under AMPK inactivation and lipid synthesis inhibition. Moreover, the altered mitochondrial function in the presence of compound C and 5-(tetradecyloxy)-2-furoic acid (TOFA; an inhibitor of acetyl-CoA carboxylase) and the significant upregulation of ATP-citrate lyase (ACL) in the absence or presence of compound C and TOFA suggest the interference of berberine on the metabolism reprogramming of cancer [167].
Betulinic acid is a natural compound that preferentially kills cancer cells by opening the mitochondrial permeability transition pore. Betulinic acid inhibits the activity of SCD1, reduces cardiolipin synthesis, prevents the binding of cardiolipin to Cyt-c and represses its release from the inner mitochondrial membrane, activates the intrinsic apoptotic pathway, thereby blocking cell growth of cervical cancer, lung cancer, breast cancer [168,169].
Peiminine induces autophagy and apoptosis of colorectal cancer cells by suppressing PI3K/Akt/mTOR and AMPK pathways and dephosphorylating mTOR [170]. One of the most significant changes in colorectal cancer cells treated with peiminine is the accumulation of several essential PUFAs, such as oleate (18:1n9) and linoleate (18:2n6), indicating that colorectal cancer cells need to produce lipid mediators, as measured by an established metabolomic profiling platform combining ultra-performance liquid chromatography/tandem mass spectrometry with gas chromatography/mass spectrometry [171].
Resveratrol is a natural polyphenolic compound with anti-cancer properties. Resveratrol inhibits lipogenesis and induces apoptosis in cancer stem-like cells by decreasing FAS expression [172]. The formation of Fas/CAV-1 complexes is enabled by resveratrol-induced autophagic degradation of p62, which subsequently stimulates caspase-8-mediated Beclin-1 cleavage, resulting in the translocation of the Beclin-1 C-terminal fragment to the mitochondrial apoptosis of non-small lung adenocarcinoma [173]. Resveratrol induces breast cancer cell death in cancer stem-like cells of ductal carcinoma in situ by upregulating pro-apoptotic genes death associated protein kinase 2 (DAPK2) and Bcl-2/adenovirus E1B 19kD interacting protein 3 (BNIP3), as well as downregulating lipogenic genes. Furthermore, resveratrol can block the lipogenic gene expression in cancer stem-like cells and obviously suppresses their ability to generate ductal carcinoma in situ in animal models, providing powerful evidence for the application of resveratrol for chemoprevention against ductal carcinoma in situ [174].
A novel natural triterpene GL22 isolated from Ganoderma leucocontextum decreases the expression of FABPs, which may explain why hepatic carcinoma cells lose cardiolipin, undergo mitochondrial dysfunction, and cell death [175]. The citral isomers (cis-citral and trans-citral) derived from the Cymbopogon citratus (D.C.) citral suppresses lipogenesis by activating AMPK phosphorylation and downregulating the levels of FASN, ACC, 3-hydroxy-3-methyl glutaryl coenzyme A reductase (HMGR), and SREBP1, as well as promote apoptosis of prostate cancer cells by downregulating Bcl-2 expression and upregulating BAX [176].
Curcumin is a typical dietary supplement with a wide range of pharmacological effects. However, the solubility and stability issues of curcumin remain the major obstacles to commercialization. Notably, decreasing cPLA2 dramatically improves the therapeutic effect of the anti-cancer drug curcumin in glioblastoma cells [177]. Therefore, interfering with drug sequestration into intracellular LDs to promote cell death after antineoplastic drug treatment might be meaningful for multidrug resistance.
Taken together, these encouraging discoveries might help fill in the gaps between cell death subroutines and natural products, allowing for the development of novel cancer therapeutic strategies.
7. Conclusions
To understand how lipid metabolic reprogramming supports cancer cell death, and to determine which reprogramming modalities are most important for therapeutic liabilities. The finding of lipid metabolic plasticity will be facilitated by advanced animal models that elucidate key processes in human cancer development. These animal studies require the assessment of cancer metabolism in situ tumors by advanced technologies, such as metabolic isotope tracers and metabolomics to obtain subtype selective metabolic properties of tumors that compensate for the deficiencies of lipid metabolism in human tumors. Although animal models are useful for mechanistic exploration and hypothesis testing, cooperative and multidisciplinary research is required for translational medicine.
The link between lipid metabolism and immune cell interactions remains a hot topic of research in the context of cancer and the TME. Since secondary tumors are the leading cause of cancer-related fatalities, therapy options to prevent this from happening are crucial. Whether the effects of lipids on metastatic cancer cells are directly against cancer cells, as with CD36-targeting drugs, or whether broader therapies that alter the TME are an option, further studies are needed for therapeutic exploitation. Given the growing number of small-molecule inhibitors that are getting better at tissue-targeting specificity, there are still numerous potentials for lipid-targeted therapeutic approaches that can be translated alone or in combination with conventional or immuno-cancer treatments.
Natural product-derived ingredients, in addition to being used as a monotherapy, may also be used as chemosensitizers, which are critical for establishing novel anti-cancer approaches. Due to our current limited understanding of the efficacy of natural product-derived compounds, further preclinical and clinical studies are urgently needed to realize the potential of natural product-derived compounds in cancer. We are on the verge of exploiting hitherto overlooked features and prospective targets in cancer-fighting strategies from oncologists. Targets and drug combinations that regulate multiple cancer hallmarks beyond cancer metabolism, accurate patient stratification based on genotype and biomarker analyses to guide early proof-of-concept clinical trials, and clinical trials incorporating target engagement assays as well as assessment of adaptive therapeutic responses and escape mechanisms can all be part of a more productive approach. In the best-case scenario, research along these lines may pave the way for detecting predictors of lipid metabolism activity in human cancers, leading to strategic clinical trial designs and the most effective therapies.
Funding
This work was supported by the National Natural Sciences Foundation of China (81973668, 81774130), Key Project of the Educational Department of Hunan Province (20A375), Scientific Research Project of Changsha Science and Technology Bureau (kq2004060), Key Guidance Project of Hunan Provincial Health Commission (202213055529), Science-Pharmacy Joint Fund of the Natural Science Foundation of Hunan Province, Hunan University of Chinese Medicine Innovation Foundation for Postgraduate (2021CX08) and the First-Class Discipline of Pharmaceutical Science of Hunan.
Acknowledgement
L Qin, A Dai and W Wei designed the article, C Zhang drafted the manuscript, N Zhu, H Li and Y Gong performed the literature search, J Gu and Y Shi drew the figures, D Liao revised the manuscript.
Contributor Information
Wei Wang, Email: wangwei402@hotmail.com.
Aiguo Dai, Email: daiaiguo2003@163.com.
Li Qin, Email: lqin@hnucm.edu.cn.
Conflict of interest
None declared.
References
- 1.Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discovery. 2022;12(1):31–46. doi: 10.1158/2159-8290.CD-21-1059. [DOI] [PubMed] [Google Scholar]
- 2.Faubert B., Solmonson A., DeBerardinis R.J. Metabolic reprogramming and cancer progression. Science. 2020;368(6487) doi: 10.1126/science.aaw5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng C., Geng F., Cheng X., Guo D. Lipid metabolism reprogramming and its potential targets in cancer. Cancer Communications. 2018;38(1):27. doi: 10.1186/s40880-018-0301-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luo X., Cheng C., Tan Z., Li N., Tang M., Yang L., et al. Emerging roles of lipid metabolism in cancer metastasis. Molecular Cancer. 2017;16(1):76. doi: 10.1186/s12943-017-0646-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gallagher E.J., Zelenko Z., Neel B.A., Antoniou I.M., Rajan L., Kase N., et al. Elevated tumor LDLR expression accelerates LDL cholesterol-mediated breast cancer growth in mouse models of hyperlipidemia. Oncogene. 2017;36(46):6462–6471. doi: 10.1038/onc.2017.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Floeth M., Elges S., Gerss J., Schwöppe C., Kessler T., Herold T., et al. Low-density lipoprotein receptor (LDLR) is an independent adverse prognostic factor in acute myeloid leukaemia. British Journal of Haematology. 2021;192(3):494–503. doi: 10.1111/bjh.16853. [DOI] [PubMed] [Google Scholar]
- 7.Gonias S.L., Karimi-Mostowfi N., Murray S.S., Mantuano E., Gilder A.S. Expression of LDL receptor-related proteins (LRPs) in common solid malignancies correlates with patient survival. PLoS One. 2017;12(10) doi: 10.1371/journal.pone.0186649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guillaumond F., Bidaut G., Ouaissi M., Servais S., Gouirand V., Olivares O., et al. Cholesterol uptake disruption, in association with chemotherapy, is a promising combined metabolic therapy for pancreatic adenocarcinoma. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(8):2473–2478. doi: 10.1073/pnas.1421601112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bovenga F., Sabbà C., Moschetta A. Uncoupling nuclear receptor LXR and cholesterol metabolism in cancer. Cell Metabolism. 2015;21(4):517–526. doi: 10.1016/j.cmet.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Shen W.J., Azhar S., Kraemer F.B. SR-B1: a unique multifunctional receptor for cholesterol influx and efflux. Annual Review of Physiology. 2018;80:95–116. doi: 10.1146/annurev-physiol-021317-121550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schörghofer D., Kinslechner K., Preitschopf A., Schütz B., Röhrl C., Hengstschläger M., et al. The HDL receptor SR-BI is associated with human prostate cancer progression and plays a possible role in establishing androgen independence. Reproductive Biology and Endocrinology. 2015;13:88. doi: 10.1186/s12958-015-0087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan B., Wu C., Wang X., Wang D., Liu H., Guo L., et al. High scavenger receptor class B type I expression is related to tumor aggressiveness and poor prognosis in breast cancer. Tumour Biology. 2016;37(3):3581–3588. doi: 10.1007/s13277-015-4141-4. [DOI] [PubMed] [Google Scholar]
- 13.Kuijjer M.L., Rydbeck H., Kresse S.H., Buddingh E.P., Lid A.B., Roelofs H., et al. Identification of osteosarcoma driver genes by integrative analysis of copy number and gene expression data. Genes Chromosomes & Cancer. 2012;51(7):696–706. doi: 10.1002/gcc.21956. [DOI] [PubMed] [Google Scholar]
- 14.Chang X.L., Liu L., Wang N., Chen Z.J., Zhang C. The function of high-density lipoprotein and low-density lipoprotein in the maintenance of mouse ovarian steroid balance. Biology of Reproduction. 2017;97(6):862–872. doi: 10.1093/biolre/iox134. [DOI] [PubMed] [Google Scholar]
- 15.Danilo C., Gutierrez-Pajares J.L., Mainieri M.A., Mercier I., Lisanti M.P., Frank P.G. Scavenger receptor class B type I regulates cellular cholesterol metabolism and cell signaling associated with breast cancer development. Breast Cancer Research. 2013;15(5):R87. doi: 10.1186/bcr3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu W.H., Qu Y.Y., Wang J., Wang H.K., Wan F.N., Zhao J.Y., et al. Elevated CD36 expression correlates with increased visceral adipose tissue and predicts poor prognosis in ccRCC patients. Journal of Cancer. 2019;10(19):4522–4531. doi: 10.7150/jca.30989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim Y.S., Jung J., Jeong H., Lee J.H., Oh H.E., Lee E.S., et al. High membranous expression of fatty acid transport protein 4 is associated with tumorigenesis and tumor progression in clear cell renal cell carcinoma. Disease Markers. 2019;2019 doi: 10.1155/2019/5702026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tian Y., Yang B., Qiu W., Hao Y., Zhang Z., Yang B., et al. ER-residential Nogo-B accelerates NAFLD-associated HCC mediated by metabolic reprogramming of oxLDL lipophagy. Nature Communications. 2019;10(1):3391. doi: 10.1038/s41467-019-11274-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hedditch E.L., Gao B., Russell A.J., Lu Y., Emmanuel C., Beesley J., et al. ABCA transporter gene expression and poor outcome in epithelial ovarian cancer. Journal of the National Cancer Institute. 2014;106(7):dju149. doi: 10.1093/jnci/dju149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bi D.P., Yin C.H., Zhang X.Y., Yang N.N., Xu J.Y. MiR-183 functions as an oncogene by targeting ABCA1 in colon cancer. Oncology Reports. 2016;35(5):2873–2879. doi: 10.3892/or.2016.4631. [DOI] [PubMed] [Google Scholar]
- 21.Aguirre-Portolés C., Feliu J., Reglero G., Ramírez de Molina A. ABCA1 overexpression worsens colorectal cancer prognosis by facilitating tumour growth and caveolin-1-dependent invasiveness, and these effects can be ameliorated using the BET inhibitor apabetalone. Molecular Oncology. 2018;12(10):1735–1752. doi: 10.1002/1878-0261.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Y.F., Jan Y.H., Liu Y.P., Yang C.J., Su C.Y., Chang Y.C., et al. Squalene synthase induces tumor necrosis factor receptor 1 enrichment in lipid rafts to promote lung cancer metastasis. American Journal of Respiratory and Critical Care Medicine. 2014;190(6):675–687. doi: 10.1164/rccm.201404-0714OC. [DOI] [PubMed] [Google Scholar]
- 23.Song J.H., Tse M.C., Bellail A., Phuphanich S., Khuri F., Kneteman N.M., et al. Lipid rafts and nonrafts mediate tumor necrosis factor related apoptosis-inducing ligand induced apoptotic and nonapoptotic signals in non small cell lung carcinoma cells. Cancer Research. 2007;67(14):6946–6955. doi: 10.1158/0008-5472.CAN-06-3896. [DOI] [PubMed] [Google Scholar]
- 24.Oh H.Y., Lee E.J., Yoon S., Chung B.H., Cho K.S., Hong S.J. Cholesterol level of lipid raft microdomains regulates apoptotic cell death in prostate cancer cells through EGFR-mediated Akt and ERK signal transduction. The Prostate. 2007;67(10):1061–1069. doi: 10.1002/pros.20593. [DOI] [PubMed] [Google Scholar]
- 25.Wang S., Wang N., Zheng Y., Yang B., Liu P., Zhang F., et al. Caveolin-1 inhibits breast cancer stem cells via c-Myc-mediated metabolic reprogramming. Cell Death & Disease. 2020;11(6):450. doi: 10.1038/s41419-020-2667-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y., Fu Y., Hu X., Chen S., Miao J., Wang Y., et al. Caveolin-1 knockdown increases the therapeutic sensitivity of lung cancer to cisplatin-induced apoptosis by repressing Parkin-related mitophagy and activating the ROCK1 pathway. Journal of Cellular Physiology. 2020;235(2):1197–1208. doi: 10.1002/jcp.29033. [DOI] [PubMed] [Google Scholar]
- 27.Kang J., Park J.H., Lee H.J., Jo U., Park J.K., Seo J.H., et al. Caveolin-1 modulates docetaxel-induced cell death in breast cancer cell subtypes through different mechanisms. Cancer Research Treatment. 2016;48(2):715–726. doi: 10.4143/crt.2015.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pongjit K., Chanvorachote P. Caveolin-1 sensitizes cisplatin-induced lung cancer cell apoptosis via superoxide anion-dependent mechanism. Molecular and Cellular Biochemistry. 2011;358(1–2):365–373. doi: 10.1007/s11010-011-0988-x. [DOI] [PubMed] [Google Scholar]
- 29.Mullen P.J., Yu R., Longo J., Archer M.C., Penn L.Z. The interplay between cell signalling and the mevalonate pathway in cancer. Nature Reviews Cancer. 2016;16(11):718–731. doi: 10.1038/nrc.2016.76. [DOI] [PubMed] [Google Scholar]
- 30.Xu H., Zhou S., Tang Q., Xia H., Bi F. Cholesterol metabolism: new functions and therapeutic approaches in cancer. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer. 2020;1874(1) doi: 10.1016/j.bbcan.2020.188394. [DOI] [PubMed] [Google Scholar]
- 31.Bengtsson E., Nerjovaj P., Wangefjord S., Nodin B., Eberhard J., Uhlén M., et al. HMG-CoA reductase expression in primary colorectal cancer correlates with favourable clinicopathological characteristics and an improved clinical outcome. Diagnostic Pathology. 2014;9:78. doi: 10.1186/1746-1596-9-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brennan D.J., Brändstedt J., Rexhepaj E., Foley M., Pontén F., Uhlén M., et al. Tumour-specific HMG-CoAR is an independent predictor of recurrence free survival in epithelial ovarian cancer. BMC Cancer. 2010;10:125. doi: 10.1186/1471-2407-10-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gustbée E., Tryggvadottir H., Markkula A., Simonsson M., Nodin B., Jirström K., et al. Tumor-specific expression of HMG-CoA reductase in a population-based cohort of breast cancer patients. BMC Clinical Pathology. 2015;15:8. doi: 10.1186/s12907-015-0008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Longo J., Mullen P.J., Yu R., van Leeuwen J.E., Masoomian M., Woon D.T.S., et al. An actionable sterol-regulated feedback loop modulates statin sensitivity in prostate cancer. Molecular Metabolism. 2019;25:119–130. doi: 10.1016/j.molmet.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X., Huang Z., Wu Q., Prager B.C., Mack S.C., Yang K., et al. MYC-regulated mevalonate metabolism maintains brain tumor-initiating cells. Cancer Research. 2017;77(18):4947–4960. doi: 10.1158/0008-5472.CAN-17-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abate M., Laezza C., Pisanti S., Torelli G., Seneca V., Catapano G., et al. Deregulated expression and activity of farnesyl diphosphate synthase (FDPS) in glioblastoma. Scientific Reports. 2017;7(1) doi: 10.1038/s41598-017-14495-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mo H., Jeter R., Bachmann A., Yount S.T., Shen C.L., Yeganehjoo H. The potential of isoprenoids in adjuvant cancer therapy to reduce adverse effects of statins. Frontiers in Pharmacology. 2018;9:1515. doi: 10.3389/fphar.2018.01515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yao X., Xie R., Cao Y., Tang J., Men Y., Peng H., et al. Simvastatin induced ferroptosis for triple-negative breast cancer therapy. Journal of Nanobiotechnology. 2021;19(1):311. doi: 10.1186/s12951-021-01058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garcia-Bermudez J., Baudrier L., Bayraktar E.C., Shen Y., La K., Guarecuco R., et al. Squalene accumulation in cholesterol auxotrophic lymphomas prevents oxidative cell death. Nature. 2019;567(7746):118–122. doi: 10.1038/s41586-019-0945-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shimada K., Skouta R., Kaplan A., Yang W.S., Hayano M., Dixon S.J., et al. Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nature Chemical Biology. 2016;12(7):497–503. doi: 10.1038/nchembio.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang W.S., Stockwell B.R. Ferroptosis: death by lipid peroxidation. Trends in Cell Biology. 2016;26(3):165–176. doi: 10.1016/j.tcb.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang Z., Xia L., Zhou X., Wei C., Mo Q. ALOX12 inhibition sensitizes breast cancer to chemotherapy via AMPK activation and inhibition of lipid synthesis. Biochemical and Biophysical Research Communications. 2019;514(1):24–30. doi: 10.1016/j.bbrc.2019.04.101. [DOI] [PubMed] [Google Scholar]
- 43.Wen Y.A., Xiong X., Zaytseva Y.Y., Napier D.L., Vallee E., Li A.T., et al. Downregulation of SREBP inhibits tumor growth and initiation by altering cellular metabolism in colon cancer. Cell Death & Disease. 2018;9(3):265. doi: 10.1038/s41419-018-0330-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng C., Ru P., Geng F., Liu J., Yoo J.Y., Wu X., et al. Glucose-mediated N-glycosylation of SCAP is essential for SREBP-1 activation and tumor growth. Cancer Cell. 2015;28(5):569–581. doi: 10.1016/j.ccell.2015.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Griffiths B., Lewis C.A., Bensaad K., Ros S., Zhang Q., Ferber E.C., et al. Sterol regulatory element binding protein-dependent regulation of lipid synthesis supports cell survival and tumor growth. Cancer & Metabolism. 2013;1(1):3. doi: 10.1186/2049-3002-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pham D.V., Tilija Pun N., Park P.H. Autophagy activation and SREBP-1 induction contribute to fatty acid metabolic reprogramming by leptin in breast cancer cells. Molecular Oncology. 2021;15(2):657–678. doi: 10.1002/1878-0261.12860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He J., Zhang F., Tay L.W.R., Boroda S., Nian W., Levental K.R., et al. Lipin-1 regulation of phospholipid synthesis maintains endoplasmic reticulum homeostasis and is critical for triple-negative breast cancer cell survival. The FASEB Journal. 2017;31(7):2893–2904. doi: 10.1096/fj.201601353R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Geng F., Cheng X., Wu X., Yoo J.Y., Cheng C., Guo J.Y., et al. Inhibition of SOAT1 suppresses glioblastoma growth via blocking SREBP-1-mediated lipogenesis. Clinical Cancer Research. 2016;22(21):5337–5348. doi: 10.1158/1078-0432.CCR-15-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Young R.M., Ackerman D., Quinn Z.L., Mancuso A., Gruber M., Liu L., et al. Dysregulated mTORC1 renders cells critically dependent on desaturated lipids for survival under tumor-like stress. Genes & Development. 2013;27(10):1115–1131. doi: 10.1101/gad.198630.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Minville-Walz M., Pierre A.S., Pichon L., Bellenger S., Fèvre C., Bellenger J., et al. Inhibition of stearoyl-CoA desaturase 1 expression induces CHOP-dependent cell death in human cancer cells. PLoS One. 2010;5(12) doi: 10.1371/journal.pone.0014363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ventura R., Mordec K., Waszczuk J., Wang Z., Lai J., Fridlib M., et al. Inhibition of de novo Palmitate Synthesis by Fatty Acid Synthase Induces Apoptosis in Tumor Cells by Remodeling Cell Membranes, Inhibiting Signaling Pathways, and Reprogramming Gene Expression. EBioMedicine. 2015;2(8):808–824. doi: 10.1016/j.ebiom.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tomek K., Wagner R., Varga F., Singer C.F., Karlic H., Grunt T.W. Blockade of fatty acid synthase induces ubiquitination and degradation of phosphoinositide-3-kinase signaling proteins in ovarian cancer. Molecular Cancer Research. 2011;9(12):1767–1779. doi: 10.1158/1541-7786.MCR-10-0467. [DOI] [PubMed] [Google Scholar]
- 53.Wang Q., Du X., Zhou B., Li J., Lu W., Chen Q., et al. Mitochondrial dysfunction is responsible for fatty acid synthase inhibition-induced apoptosis in breast cancer cells by PdpaMn. Biomedicine & Pharmacotherapy. 2017;96:396–403. doi: 10.1016/j.biopha.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 54.Brusselmans K., De Schrijver E., Verhoeven G., Swinnen J.V. RNA interference-mediated silencing of the acetyl-CoA-carboxylase-alpha gene induces growth inhibition and apoptosis of prostate cancer cells. Cancer Research. 2005;65(15):6719–6725. doi: 10.1158/0008-5472.CAN-05-0571. [DOI] [PubMed] [Google Scholar]
- 55.Qu Y.Y., Zhao R., Zhang H.L., Zhou Q., Xu F.J., Zhang X., et al. Inactivation of the AMPK-GATA3-ECHS1 pathway induces fatty acid synthesis that promotes clear cell renal cell carcinoma growth. Cancer Research. 2020;80(2):319–333. doi: 10.1158/0008-5472.CAN-19-1023. [DOI] [PubMed] [Google Scholar]
- 56.Schlaepfer I.R., Rider L., Rodrigues L.U., Gijón M.A., Pac C.T., Romero L., et al. Lipid catabolism via CPT1 as a therapeutic target for prostate cancer. Molecular Cancer Therapeutics. 2014;13(10):2361–2371. doi: 10.1158/1535-7163.MCT-14-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang J., Duran A., Reina-Campos M., Valencia T., Castilla E.A., Müller T.D., et al. Adipocyte p62/SQSTM1 suppresses tumorigenesis through opposite regulations of metabolism in adipose tissue and tumor. Cancer Cell. 2018;33(4):770–784. doi: 10.1016/j.ccell.2018.03.001. e776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Amaravadi R.K., Kimmelman A.C., Debnath J. Targeting autophagy in cancer: recent advances and future directions. Cancer Discovery. 2019;9(9):1167–1181. doi: 10.1158/2159-8290.CD-19-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xia H., Lee K.W., Chen J., Kong S.N., Sekar K., Deivasigamani A., et al. Simultaneous silencing of ACSL4 and induction of GADD45B in hepatocellular carcinoma cells amplifies the synergistic therapeutic effect of aspirin and sorafenib. Cell Death & Disease. 2017;3 doi: 10.1038/cddiscovery.2017.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sánchez-Martínez R., Cruz-Gil S., García-Álvarez M.S., Reglero G., Ramírez de Molina A. Complementary ACSL isoforms contribute to a non-Warburg advantageous energetic status characterizing invasive colon cancer cells. Scientific Reports. 2017;7(1) doi: 10.1038/s41598-017-11612-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Doll S., Proneth B., Tyurina Y.Y., Panzilius E., Kobayashi S., Ingold I., et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nature Chemical Biology. 2017;13(1):91–98. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tesfay L., Paul B.T., Konstorum A., Deng Z., Cox A.O., Lee J., et al. Stearoyl-CoA desaturase 1 protects ovarian cancer cells from ferroptotic cell death. Cancer Research. 2019;79(20):5355–5366. doi: 10.1158/0008-5472.CAN-19-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Olenchock B.A., Moslehi J., Baik A.H., Davidson S.M., Williams J., Gibson W.J., et al. EGLN1 inhibition and rerouting of α-ketoglutarate suffice for remote ischemic protection. Cell. 2016;164(5):884–895. doi: 10.1016/j.cell.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zou Y., Palte M.J., Deik A.A., Li H., Eaton J.K., Wang W., et al. A GPX4-dependent cancer cell state underlies the clear-cell morphology and confers sensitivity to ferroptosis. Nature Communications. 2019;10(1):1617. doi: 10.1038/s41467-019-09277-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bai Y., Meng L., Han L., Jia Y., Zhao Y., Gao H., et al. Lipid storage and lipophagy regulates ferroptosis. Biochemical and Biophysical Research Communications. 2019;508(4):997–1003. doi: 10.1016/j.bbrc.2018.12.039. [DOI] [PubMed] [Google Scholar]
- 66.Guo J.Y., Karsli-Uzunbas G., Mathew R., Aisner S.C., Kamphorst J.J., Strohecker A.M., et al. Autophagy suppresses progression of K-ras-induced lung tumors to oncocytomas and maintains lipid homeostasis. Genes & Development. 2013;27(13):1447–1461. doi: 10.1101/gad.219642.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peyrou M., Ramadori P., Bourgoin L., Foti M. PPARs in liver diseases and cancer: epigenetic regulation by MicroRNAs. PPAR Research. 2012;2012 doi: 10.1155/2012/757803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Röhrig F., Schulze A. The multifaceted roles of fatty acid synthesis in cancer. Nature Reviews Cancer. 2016;16(11):732–749. doi: 10.1038/nrc.2016.89. [DOI] [PubMed] [Google Scholar]
- 69.Li L., Hua L., Fan H., He Y., Xu W., Zhang L., et al. Interplay of PKD3 with SREBP1 promotes cell growth via upregulating lipogenesis in prostate cancer cells. Journal of Cancer. 2019;10(25):6395–6404. doi: 10.7150/jca.31254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ravacci G.R., Brentani M.M., Tortelli T.C., Torrinhas R.S., Santos J.R., Logullo A.F., et al. Docosahexaenoic acid modulates a HER2-associated lipogenic phenotype, induces apoptosis, and increases trastuzumab action in HER2-overexpressing breast carcinoma cells. BioMed Research International. 2015;2015 doi: 10.1155/2015/838652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao Y., Li H., Zhang Y., Li L., Fang R., Li Y., et al. Oncoprotein HBXIP modulates abnormal lipid metabolism and growth of breast cancer cells by activating the LXRs/SREBP-1c/FAS signaling cascade. Cancer Research. 2016;76(16):4696–4707. doi: 10.1158/0008-5472.CAN-15-1734. [DOI] [PubMed] [Google Scholar]
- 72.Chen J., Ding C., Chen Y., Hu W., Yu C., Peng C., et al. ACSL4 reprograms fatty acid metabolism in hepatocellular carcinoma via c-Myc/SREBP1 pathway. Cancer Letters. 2021;502:154–165. doi: 10.1016/j.canlet.2020.12.019. [DOI] [PubMed] [Google Scholar]
- 73.Zaidi N., Lupien L., Kuemmerle N.B., Kinlaw W.B., Swinnen J.V., Smans K. Lipogenesis and lipolysis: the pathways exploited by the cancer cells to acquire fatty acids. Progress in Lipid Research. 2013;52(4):585–589. doi: 10.1016/j.plipres.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kuzu O.F., Noory M.A., Robertson G.P. The role of cholesterol in cancer. Cancer Research. 2016;76(8):2063–2070. doi: 10.1158/0008-5472.CAN-15-2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim J.H., Lee J., Jung S.Y., Kim J. Dietary factors and female breast cancer risk: a prospective cohort study. Nutrients. 2017;9(12):1331. doi: 10.3390/nu9121331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang J., McDowell A., Kim E.K., Seo H., Lee W.H., Moon C.M., et al. Development of a colorectal cancer diagnostic model and dietary risk assessment through gut microbiome analysis. Experimental and Molecular Medicine. 2019;51(10):1–15. doi: 10.1038/s12276-019-0313-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Murtola T.J., Kasurinen T.V.J., Talala K., Taari K., Tammela T.L.J., Auvinen A. Serum cholesterol and prostate cancer risk in the Finnish randomized study of screening for prostate cancer. Prostate Cancer and Prostatic Diseases. 2019;22(1):66–76. doi: 10.1038/s41391-018-0087-0. [DOI] [PubMed] [Google Scholar]
- 78.Tamura T., Inagawa S., Hisakura K., Enomoto T., Ohkohchi N. Evaluation of serum high-density lipoprotein cholesterol levels as a prognostic factor in gastric cancer patients. Journal of Gastroenterology and Hepatology. 2012;27(10):1635–1640. doi: 10.1111/j.1440-1746.2012.07189.x. [DOI] [PubMed] [Google Scholar]
- 79.Lu C.W., Lo Y.H., Chen C.H., Lin C.Y., Tsai C.H., Chen P.J., et al. VLDL and LDL, but not HDL, promote breast cancer cell proliferation, metastasis and angiogenesis. Cancer Letters. 2017;388:130–138. doi: 10.1016/j.canlet.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 80.Levy D., Correa de Melo T., Ohira B.Y., Fidelis M.L., Ruiz J.L.M., Rodrigues A., et al. Oxysterols selectively promote short-term apoptosis in tumor cell lines. Biochemical and Biophysical Research Communications. 2018;505(4):1043–1049. doi: 10.1016/j.bbrc.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 81.Pucer A., Brglez V., Payré C., Pungerčar J., Lambeau G., Petan T. Group X secreted phospholipase A(2) induces lipid droplet formation and prolongs breast cancer cell survival. Molecular Cancer. 2013;12(1):111. doi: 10.1186/1476-4598-12-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lue H.W., Podolak J., Kolahi K., Cheng L., Rao S., Garg D., et al. Metabolic reprogramming ensures cancer cell survival despite oncogenic signaling blockade. Genes & Development. 2017;31(20):2067–2084. doi: 10.1101/gad.305292.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schlaepfer I.R., Hitz C.A., Gijón M.A., Bergman B.C., Eckel R.H., Jacobsen B.M. Progestin modulates the lipid profile and sensitivity of breast cancer cells to docetaxel. Molecular and Cellular Endocrinology. 2012;363(1–2):111–121. doi: 10.1016/j.mce.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Verbrugge S.E., Al M., Assaraf Y.G., Kammerer S., Chandrupatla D.M., Honeywell R., et al. Multifactorial resistance to aminopeptidase inhibitor prodrug CHR2863 in myeloid leukemia cells: down-regulation of carboxylesterase 1, drug sequestration in lipid droplets and pro-survival activation ERK/Akt/mTOR. Oncotarget. 2016;7(5):5240–5257. doi: 10.18632/oncotarget.6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cotte A.K., Aires V., Fredon M., Limagne E., Derangère V., Thibaudin M., et al. Lysophosphatidylcholine acyltransferase 2-mediated lipid droplet production supports colorectal cancer chemoresistance. Nature Communications. 2018;9(1):322. doi: 10.1038/s41467-017-02732-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Triantafyllou E.A., Georgatsou E., Mylonis I., Simos G., Paraskeva E. Expression of AGPAT2, an enzyme involved in the glycerophospholipid/triacylglycerol biosynthesis pathway, is directly regulated by HIF-1 and promotes survival and etoposide resistance of cancer cells under hypoxia. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2018;1863(9):1142–1152. doi: 10.1016/j.bbalip.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 87.Qiu B., Ackerman D., Sanchez D.J., Li B., Ochocki J.D., Grazioli A., et al. HIF2α-Dependent lipid storage promotes endoplasmic reticulum homeostasis in clear-cell renal cell carcinoma. Cancer Discovery. 2015;5(6):652–667. doi: 10.1158/2159-8290.CD-14-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lu G.D., Ang Y.H., Zhou J., Tamilarasi J., Yan B., Lim Y.C., et al. CCAAT/enhancer binding protein α predicts poorer prognosis and prevents energy starvation-induced cell death in hepatocellular carcinoma. Hepatology. 2015;61(3):965–978. doi: 10.1002/hep.27593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kaini R.R., Sillerud L.O., Zhaorigetu S., Hu C.A. Autophagy regulates lipolysis and cell survival through lipid droplet degradation in androgen-sensitive prostate cancer cells. The Prostate. 2012;72(13):1412–1422. doi: 10.1002/pros.22489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mukhopadhyay S., Schlaepfer I.R., Bergman B.C., Panda P.K., Praharaj P.P., Naik P.P., et al. ATG14 facilitated lipophagy in cancer cells induce ER stress mediated mitoptosis through a ROS dependent pathway. Free Radical Biology and Medicine. 2017;104:199–213. doi: 10.1016/j.freeradbiomed.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 91.Nieman K.M., Kenny H.A., Penicka C.V., Ladanyi A., Buell-Gutbrod R., Zillhardt M.R., et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nature Medicine. 2011;17(11):1498–1503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bergers G., Fendt S.M. The metabolism of cancer cells during metastasis. Nature Reviews Cancer. 2021;21(3):162–180. doi: 10.1038/s41568-020-00320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fendt S.M., Frezza C., Erez A. Targeting metabolic plasticity and flexibility dynamics for cancer therapy. Cancer Discovery. 2020;10(12):1797–1807. doi: 10.1158/2159-8290.CD-20-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Obre E., Rossignol R. Emerging concepts in bioenergetics and cancer research: metabolic flexibility, coupling, symbiosis, switch, oxidative tumors, metabolic remodeling, signaling and bioenergetic therapy. The International Journal of Biochemistry & Cell Biology. 2015;59:167–181. doi: 10.1016/j.biocel.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 95.Kwong S.C., Jamil A.H.A., Rhodes A., Taib N.A., Chung I. Metabolic role of fatty acid binding protein 7 in mediating triple-negative breast cancer cell death via PPAR-α signaling. The Journal of Lipid Research. 2019;60(11):1807–1817. doi: 10.1194/jlr.M092379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhao W., Prijic S., Urban B.C., Tisza M.J., Zuo Y., Li L., et al. Candidate antimetastasis drugs suppress the metastatic capacity of breast cancer cells by reducing membrane fluidity. Cancer Research. 2016;76(7):2037–2049. doi: 10.1158/0008-5472.CAN-15-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kant S., Kesarwani P., Prabhu A., Graham S.F., Buelow K.L., Nakano I., et al. Enhanced fatty acid oxidation provides glioblastoma cells metabolic plasticity to accommodate to its dynamic nutrient microenvironment. Cell Death & Disease. 2020;11(4):253. doi: 10.1038/s41419-020-2449-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cai M., He J., Xiong J., Tay L.W., Wang Z., Rog C., et al. Phospholipase D1-regulated autophagy supplies free fatty acids to counter nutrient stress in cancer cells. Cell Death & Disease. 2016;7(11):e2448. doi: 10.1038/cddis.2016.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang J., Stack M.S. Lipid regulatory proteins as potential therapeutic targets for ovarian cancer in obese women. Cancers. 2020;12(11):3469. doi: 10.3390/cancers12113469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sun L., Zhang H., Gao P. Metabolic reprogramming and epigenetic modifications on the path to cancer. Protein Cell. 2021 doi: 10.1007/s13238-021-00846-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.De Fabiani E., Mitro N., Gilardi F., Galmozzi A., Caruso D., Crestani M. When food meets man: the contribution of epigenetics to health. Nutrients. 2010;2(5):551–571. doi: 10.3390/nu2050551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kawaguchi K., Kinameri A., Suzuki S., Senga S., Ke Y., Fujii H. The cancer-promoting gene fatty acid-binding protein 5 (FABP5) is epigenetically regulated during human prostate carcinogenesis. Biochemical Journal. 2016;473(4):449–461. doi: 10.1042/BJ20150926. [DOI] [PubMed] [Google Scholar]
- 103.Sun Q., Zhang W., Wang L., Guo F., Song D., Zhang Q., et al. Hypermethylated CD36 gene affected the progression of lung cancer. Gene. 2018;678:395–406. doi: 10.1016/j.gene.2018.06.101. [DOI] [PubMed] [Google Scholar]
- 104.Luo Y., Xie C., Brocker C.N., Fan J., Wu X., Feng L., et al. Intestinal PPARα protects against colon carcinogenesis via regulation of methyltransferases DNMT1 and PRMT6. Gastroenterology. 2019;157(3):744–759. doi: 10.1053/j.gastro.2019.05.057. e744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jiang Y., Mao C., Yang R., Yan B., Shi Y., Liu X., et al. EGLN1/c-Myc induced lymphoid-specific helicase inhibits ferroptosis through lipid metabolic gene expression changes. Theranostics. 2017;7(13):3293–3305. doi: 10.7150/thno.19988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu Y., Zhang S., Gong X., Tam S., Xiao D., Liu S., et al. The epigenetic regulators and metabolic changes in ferroptosis-associated cancer progression. Molecular Cancer. 2020;19(1):39. doi: 10.1186/s12943-020-01157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee B.H., Taylor M.G., Robinet P., Smith J.D., Schweitzer J., Sehayek E., et al. Dysregulation of cholesterol homeostasis in human prostate cancer through loss of ABCA1. Cancer Research. 2013;73(3):1211–1218. doi: 10.1158/0008-5472.CAN-12-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Koutsioumpa M., Hatziapostolou M., Polytarchou C., Tolosa E.J., Almada L.L., Mahurkar-Joshi S., et al. Lysine methyltransferase 2D regulates pancreatic carcinogenesis through metabolic reprogramming. Gut. 2019;68(7):1271–1286. doi: 10.1136/gutjnl-2017-315690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang K.C., Chang H.Y. Molecular mechanisms of long noncoding RNAs. Molecular Cell. 2011;43(6):904–914. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim H., Choi S.Y., Lim J., Lindroth A.M., Park Y.J. EHMT2 inhibition induces cell death in human non-small cell lung cancer by altering the cholesterol biosynthesis pathway. International Journal of Molecular Sciences. 2020;21(3):1002. doi: 10.3390/ijms21031002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kim H.S., Xiao C., Wang R.H., Lahusen T., Xu X., Vassilopoulos A., et al. Hepatic-specific disruption of SIRT6 in mice results in fatty liver formation due to enhanced glycolysis and triglyceride synthesis. Cell Metabolism. 2010;12(3):224–236. doi: 10.1016/j.cmet.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Marquardt J.U., Fischer K., Baus K., Kashyap A., Ma S., Krupp M., et al. Sirtuin-6-dependent genetic and epigenetic alterations are associated with poor clinical outcome in hepatocellular carcinoma patients. Hepatology. 2013;58(3):1054–1064. doi: 10.1002/hep.26413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hong S., Moreno-Navarrete J.M., Wei X., Kikukawa Y., Tzameli I., Prasad D., et al. Nicotinamide N-methyltransferase regulates hepatic nutrient metabolism through Sirt1 protein stabilization. Nature Medicine. 2015;21(8):887–894. doi: 10.1038/nm.3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ye X., Li M., Hou T., Gao T., Zhu W.G., Yang Y. Sirtuins in glucose and lipid metabolism. Oncotarget. 2017;8(1):1845–1859. doi: 10.18632/oncotarget.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang Y., Zeng J., Wu W., Xie S., Yu H., Li G., et al. Nicotinamide N-methyltransferase enhances chemoresistance in breast cancer through SIRT1 protein stabilization. Breast Cancer Research. 2019;21(1):64. doi: 10.1186/s13058-019-1150-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hsu Y.C., Chen H.Y., Yuan S., Yu S.L., Lin C.H., Wu G., et al. Genome-wide analysis of three-way interplay among gene expression, cancer cell invasion and anti-cancer compound sensitivity. BMC Medicine. 2013;11:106. doi: 10.1186/1741-7015-11-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang J., Wang Y., Li G., Yu H., Xie X. Down-regulation of nicotinamide N-methyltransferase induces apoptosis in human breast cancer cells via the mitochondria-mediated pathway. PLoS One. 2014;9(2) doi: 10.1371/journal.pone.0089202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shin J.H., Park C.W., Yoon G., Hong S.M., Choi K.Y. NNMT depletion contributes to liver cancer cell survival by enhancing autophagy under nutrient starvation. Oncogenesis. 2018;7(8):58. doi: 10.1038/s41389-018-0064-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gao X., Lin S.H., Ren F., Li J.T., Chen J.J., Yao C.B., et al. Acetate functions as an epigenetic metabolite to promote lipid synthesis under hypoxia. Nature Communications. 2016;7 doi: 10.1038/ncomms11960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu R., Lee J.H., Li J., Yu R., Tan L., Xia Y., et al. Choline kinase alpha 2 acts as a protein kinase to promote lipolysis of lipid droplets. Molecular Cell. 2021;81(13):2722–2735. doi: 10.1016/j.molcel.2021.05.005. e2729. [DOI] [PubMed] [Google Scholar]
- 121.Eisenberg S., Laude A.J., Beckett A.J., Mageean C.J., Aran V., Hernandez-Valladares M., et al. The role of palmitoylation in regulating Ras localization and function. Biochemical Society Transactions. 2013;41(1):79–83. doi: 10.1042/BST20120268. [DOI] [PubMed] [Google Scholar]
- 122.Calvisi D.F., Wang C., Ho C., Ladu S., Lee S.A., Mattu S., et al. Increased lipogenesis, induced by AKT-mTORC1-RPS6 signaling, promotes development of human hepatocellular carcinoma. Gastroenterology. 2011;140(3):1071–1083. doi: 10.1053/j.gastro.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Siddals K.W., Marshman E., Westwood M., Gibson J.M. Abrogation of insulin-like growth factor-I (IGF-I) and insulin action by mevalonic acid depletion: synergy between protein prenylation and receptor glycosylation pathways. Journal of Biological Chemistry. 2004;279(37):38353–38359. doi: 10.1074/jbc.M404838200. [DOI] [PubMed] [Google Scholar]
- 124.Kamphorst J.J., Cross J.R., Fan J., de Stanchina E., Mathew R., White E.P., et al. Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(22):8882–8887. doi: 10.1073/pnas.1307237110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lewis C.A., Brault C., Peck B., Bensaad K., Griffiths B., Mitter R., et al. SREBP maintains lipid biosynthesis and viability of cancer cells under lipid- and oxygen-deprived conditions and defines a gene signature associated with poor survival in glioblastoma multiforme. Oncogene. 2015;34(40):5128–5140. doi: 10.1038/onc.2014.439. [DOI] [PubMed] [Google Scholar]
- 126.Williams K.J., Argus J.P., Zhu Y., Wilks M.Q., Marbois B.N., York A.G., et al. An essential requirement for the SCAP/SREBP signaling axis to protect cancer cells from lipotoxicity. Cancer Research. 2013;73(9):2850–2862. doi: 10.1158/0008-5472.CAN-13-0382-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ubellacker J.M., Tasdogan A., Ramesh V., Shen B., Mitchell E.C., Martin-Sandoval M.S., et al. Lymph protects metastasizing melanoma cells from ferroptosis. Nature. 2020;585(7823):113–118. doi: 10.1038/s41586-020-2623-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gao J., Zhang Z., Liu Y., Zhang Z., Wang M., Gong A., et al. Stearoyl-CoA desaturase 1 potentiates hypoxic plus nutrient-deprived pancreatic cancer cell ferroptosis resistance. Oxidative Medicine and Cellular Longevity. 2021;2021 doi: 10.1155/2021/6629804. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 129.Deep G., Schlaepfer I.R. Aberrant lipid metabolism promotes prostate cancer: role in cell survival under hypoxia and extracellular vesicles biogenesis. International Journal of Molecular Sciences. 2016;17(7):1061. doi: 10.3390/ijms17071061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Junttila M.R., de Sauvage F.J. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 2013;501(7467):346–354. doi: 10.1038/nature12626. [DOI] [PubMed] [Google Scholar]
- 131.Luo Q., Zheng N., Jiang L., Wang T., Zhang P., Liu Y., et al. Lipid accumulation in macrophages confers protumorigenic polarization and immunity in gastric cancer. Cancer Science. 2020;111(11):4000–4011. doi: 10.1111/cas.14616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wu L., Zhang X., Zheng L., Zhao H., Yan G., Zhang Q., et al. RIPK3 orchestrates fatty acid metabolism in tumor-associated macrophages and hepatocarcinogenesis. Cancer Immunology Research. 2020;8(5):710–721. doi: 10.1158/2326-6066.CIR-19-0261. [DOI] [PubMed] [Google Scholar]
- 133.Hao J., Yan F., Zhang Y., Triplett A., Zhang Y., Schultz D.A., et al. Expression of adipocyte/macrophage fatty acid-binding protein in tumor-associated macrophages promotes breast cancer progression. Cancer Research. 2018;78(9):2343–2355. doi: 10.1158/0008-5472.CAN-17-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang H., Franco F., Tsui Y.C., Xie X., Trefny M.P., Zappasodi R., et al. CD36-mediated metabolic adaptation supports regulatory T cell survival and function in tumors. Nature Immunology. 2020;21(3):298–308. doi: 10.1038/s41590-019-0589-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Oh D.S., Lee H.K. Autophagy protein ATG5 regulates CD36 expression and anti-tumor MHC class II antigen presentation in dendritic cells. Autophagy. 2019;15(12):2091–2106. doi: 10.1080/15548627.2019.1596493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ma X., Xiao L., Liu L., Ye L., Su P., Bi E., et al. CD36-mediated ferroptosis dampens intratumoral CD8(+) T cell effector function and impairs their antitumor ability. Cell Metabolism. 2021;33(5):1001–1012. doi: 10.1016/j.cmet.2021.02.015. e1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xu C., Sun S., Johnson T., Qi R., Zhang S., Zhang J., et al. The glutathione peroxidase Gpx4 prevents lipid peroxidation and ferroptosis to sustain Treg cell activation and suppression of antitumor immunity. Cell Reports. 2021;35(11) doi: 10.1016/j.celrep.2021.109235. [DOI] [PubMed] [Google Scholar]
- 138.Ma X., Bi E., Lu Y., Su P., Huang C., Liu L., et al. Cholesterol induces CD8(+) T cell exhaustion in the tumor microenvironment. Cell Metabolism. 2019;30(1):143–156. doi: 10.1016/j.cmet.2019.04.002. e145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li Z., Sun C., Qin Z. Metabolic reprogramming of cancer-associated fibroblasts and its effect on cancer cell reprogramming. Theranostics. 2021;11(17):8322–8336. doi: 10.7150/thno.62378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gong J., Lin Y., Zhang H., Liu C., Cheng Z., Yang X., et al. Reprogramming of lipid metabolism in cancer-associated fibroblasts potentiates migration of colorectal cancer cells. Cell Death & Disease. 2020;11(4):267. doi: 10.1038/s41419-020-2434-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Auciello F.R., Bulusu V., Oon C., Tait-Mulder J., Berry M., Bhattacharyya S., et al. A stromal lysolipid-autotaxin signaling Axis promotes pancreatic tumor progression. Cancer Discovery. 2019;9(5):617–627. doi: 10.1158/2159-8290.CD-18-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kaffe E., Magkrioti C., Aidinis V. Deregulated lysophosphatidic acid metabolism and signaling in liver cancer. Cancers. 2019;11(11):1626. doi: 10.3390/cancers11111626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wen Y.A., Xing X., Harris J.W., Zaytseva Y.Y., Mitov M.I., Napier D.L., et al. Adipocytes activate mitochondrial fatty acid oxidation and autophagy to promote tumor growth in colon cancer. Cell Death & Disease. 2017;8(2) doi: 10.1038/cddis.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Luis G., Godfroid A., Nishiumi S., Cimino J., Blacher S., Maquoi E., et al. Tumor resistance to ferroptosis driven by Stearoyl-CoA Desaturase-1 (SCD1) in cancer cells and Fatty Acid Biding Protein-4 (FABP4) in tumor microenvironment promote tumor recurrence. Redox Biology. 2021;43 doi: 10.1016/j.redox.2021.102006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yang J., Zaman M.M., Vlasakov I., Roy R., Huang L., Martin C.R., et al. Adipocytes promote ovarian cancer chemoresistance. Scientific Reports. 2019;9(1) doi: 10.1038/s41598-019-49649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Beaulieu A., Poncin G., Belaid-Choucair Z., Humblet C., Bogdanovic G., Lognay G., et al. Leptin reverts pro-apoptotic and antiproliferative effects of α-linolenic acids in BCR-ABL positive leukemic cells: involvement of PI3K pathway. PLoS One. 2011;6(10) doi: 10.1371/journal.pone.0025651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cao Y. Adipocyte and lipid metabolism in cancer drug resistance. Journal of Clinical Investigation. 2019;129(8):3006–3017. doi: 10.1172/JCI127201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yanae M., Tsubaki M., Satou T., Itoh T., Imano M., Yamazoe Y., et al. Statin-induced apoptosis via the suppression of ERK1/2 and Akt activation by inhibition of the geranylgeranyl-pyrophosphate biosynthesis in glioblastoma. Journal of Experimental & Clinical Cancer Research. 2011;30(1):74. doi: 10.1186/1756-9966-30-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Galland S., Martin P., Fregni G., Letovanec I., Stamenkovic I. Attenuation of the pro-inflammatory signature of lung cancer-derived mesenchymal stromal cells by statins. Cancer Letters. 2020;484:50–64. doi: 10.1016/j.canlet.2020.05.005. [DOI] [PubMed] [Google Scholar]
- 150.Guo D., Reinitz F., Youssef M., Hong C., Nathanson D., Akhavan D., et al. An LXR agonist promotes glioblastoma cell death through inhibition of an EGFR/AKT/SREBP-1/LDLR-dependent pathway. Cancer Discovery. 2011;1(5):442–456. doi: 10.1158/2159-8290.CD-11-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wu G., Wang Q., Xu Y., Li J., Zhang H., Qi G., et al. Targeting the transcription factor receptor LXR to treat clear cell renal cell carcinoma: agonist or inverse agonist? Cell Death & Disease. 2019;10(6):416. doi: 10.1038/s41419-019-1654-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhang W., Jiang H., Zhang J., Zhang Y., Liu A., Zhao Y., et al. Liver X receptor activation induces apoptosis of melanoma cell through caspase pathway. Cancer Cell International. 2014;14(1):16. doi: 10.1186/1475-2867-14-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pommier A.J., Alves G., Viennois E., Bernard S., Communal Y., Sion B., et al. Liver X Receptor activation downregulates AKT survival signaling in lipid rafts and induces apoptosis of prostate cancer cells. Oncogene. 2010;29(18):2712–2723. doi: 10.1038/onc.2010.30. [DOI] [PubMed] [Google Scholar]
- 154.Munir M.T., Ponce C., Santos J.M., Sufian H.B., Al-Harrasi A., Gollahon L.S., et al. VD(3) and LXR agonist (T0901317) combination demonstrated greater potency in inhibiting cholesterol accumulation and inducing apoptosis via ABCA1-CHOP-BCL-2 cascade in MCF-7 breast cancer cells. Molecular Biology Reports. 2020;47(10):7771–7782. doi: 10.1007/s11033-020-05854-0. [DOI] [PubMed] [Google Scholar]
- 155.Voorneveld P.W., Reimers M.S., Bastiaannet E., Jacobs R.J., van Eijk R., Zanders M.M.J., et al. Statin use after diagnosis of colon cancer and patient survival. Gastroenterology. 2017;153(2):470–479. doi: 10.1053/j.gastro.2017.05.011. e474. [DOI] [PubMed] [Google Scholar]
- 156.Murtola T.J., Siltari A. Statins for prostate cancer: when and how much? Clinical Cancer Research. 2021;27(18):4947–4949. doi: 10.1158/1078-0432.CCR-21-1891. [DOI] [PubMed] [Google Scholar]
- 157.Li J., Gu D., Lee S.S., Song B., Bandyopadhyay S., Chen S., et al. Abrogating cholesterol esterification suppresses growth and metastasis of pancreatic cancer. Oncogene. 2016;35(50):6378–6388. doi: 10.1038/onc.2016.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yang W., Bai Y., Xiong Y., Zhang J., Chen S., Zheng X., et al. Potentiating the antitumour response of CD8(+) T cells by modulating cholesterol metabolism. Nature. 2016;531(7596):651–655. doi: 10.1038/nature17412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bauerschlag D.O., Maass N., Leonhardt P., Verburg F.A., Pecks U., Zeppernick F., et al. Fatty acid synthase overexpression: target for therapy and reversal of chemoresistance in ovarian cancer. Journal of Translational Medicine. 2015;13:146. doi: 10.1186/s12967-015-0511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lally J.S.V., Ghoshal S., DePeralta D.K., Moaven O., Wei L., Masia R., et al. Inhibition of acetyl-CoA carboxylase by phosphorylation or the inhibitor ND-654 suppresses lipogenesis and hepatocellular carcinoma. Cell Metabolism. 2019;29(1):174–182. doi: 10.1016/j.cmet.2018.08.020. e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pang B., Zhang J., Zhang X., Yuan J., Shi Y., Qiao L. Inhibition of lipogenesis and induction of apoptosis by valproic acid in prostate cancer cells via the C/EBPα/SREBP-1 pathway. Acta Biochimica et Biophysica Sinica. 2021;53(3):354–364. doi: 10.1093/abbs/gmab002. [DOI] [PubMed] [Google Scholar]
- 162.Gao L., Xu Z., Huang Z., Tang Y., Yang D., Huang J., et al. CPI-613 rewires lipid metabolism to enhance pancreatic cancer apoptosis via the AMPK-ACC signaling. Journal of Experimental & Clinical Cancer Research. 2020;39(1):73. doi: 10.1186/s13046-020-01579-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Poolsri W.A., Phokrai P., Suwankulanan S., Phakdeeto N., Phunsomboon P., Pekthong D., et al. Combination of mitochondrial and plasma membrane citrate transporter inhibitors inhibits de novo lipogenesis pathway and triggers apoptosis in hepatocellular carcinoma cells. BioMed Research International. 2018;2018 doi: 10.1155/2018/3683026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhang C.J., Zhu N., Long J., Wu H.T., Wang Y.X., Liu B.Y., et al. Celastrol induces lipophagy via the LXRα/ABCA1 pathway in clear cell renal cell carcinoma. Acta Pharmacologica Sinica. 2021;42(9):1472–1485. doi: 10.1038/s41401-020-00572-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang C.J., Zhu N., Wang Y.X., Liu L.P., Zhao T.J., Wu H.T., et al. Celastrol attenuates lipid accumulation and stemness of clear cell renal cell carcinoma via CAV-1/LOX-1 pathway. Frontiers in Pharmacology. 2021;12 doi: 10.3389/fphar.2021.658092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lee S.J., Choi Y.J., Kim H.I., Moon H.E., Paek S.H., Kim T.Y., et al. Platycodin D inhibits autophagy and increases glioblastoma cell death via LDLR upregulation. Molecular Oncology. 2021 doi: 10.1002/1878-0261.12966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tan W., Zhong Z., Wang S., Suo Z., Yang X., Hu X., et al. Berberine regulated lipid metabolism in the presence of C75, compound C, and TOFA in breast cancer cell line MCF-7. Evidence Based Complement Alternate Medicine. 2015;2015 doi: 10.1155/2015/396035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Potze L., Di Franco S., Grandela C., Pras-Raves M.L., Picavet D.I., van Veen H.A., et al. Betulinic acid induces a novel cell death pathway that depends on cardiolipin modification. Oncogene. 2016;35(4):427–437. doi: 10.1038/onc.2015.102. [DOI] [PubMed] [Google Scholar]
- 169.Schug Z.T., Frezza C., Galbraith L.C., Gottlieb E. The music of lipids: how lipid composition orchestrates cellular behaviour. Acta Oncologica. 2012;51(3):301–310. doi: 10.3109/0284186X.2011.643823. [DOI] [PubMed] [Google Scholar]
- 170.Lyu Q., Tou F., Su H., Wu X., Chen X., Zheng Z. The natural product peiminine represses colorectal carcinoma tumor growth by inducing autophagic cell death. Biochemical and Biophysical Research Communications. 2015;462(1):38–45. doi: 10.1016/j.bbrc.2015.04.102. [DOI] [PubMed] [Google Scholar]
- 171.Zheng Z., Xu L., Zhang S., Li W., Tou F., He Q., et al. Peiminine inhibits colorectal cancer cell proliferation by inducing apoptosis and autophagy and modulating key metabolic pathways. Oncotarget. 2017;8(29):47619–47631. doi: 10.18632/oncotarget.17411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pandey P.R., Okuda H., Watabe M., Pai S.K., Liu W., Kobayashi A., et al. Resveratrol suppresses growth of cancer stem-like cells by inhibiting fatty acid synthase. Breast Cancer Research and Treatment. 2011;130(2):387–398. doi: 10.1007/s10549-010-1300-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhang J., Ma K., Qi T., Wei X., Zhang Q., Li G., et al. P62 regulates resveratrol-mediated Fas/Cav-1 complex formation and transition from autophagy to apoptosis. Oncotarget. 2015;6(2):789–801. doi: 10.18632/oncotarget.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pandey P.R., Xing F., Sharma S., Watabe M., Pai S.K., Iiizumi-Gairani M., et al. Elevated lipogenesis in epithelial stem-like cell confers survival advantage in ductal carcinoma in situ of breast cancer. Oncogene. 2013;32(42):5111–5122. doi: 10.1038/onc.2012.519. [DOI] [PubMed] [Google Scholar]
- 175.Liu G., Wang K., Kuang S., Cao R., Bao L., Liu R., et al. The natural compound GL22, isolated from Ganoderma mushrooms, suppresses tumor growth by altering lipid metabolism and triggering cell death. Cell Death & Disease. 2018;9(6):689. doi: 10.1038/s41419-018-0731-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Balusamy S.R., Perumalsamy H., Veerappan K., Huq M.A., Rajeshkumar S., Lakshmi T., et al. Citral induced apoptosis through modulation of key genes involved in fatty acid biosynthesis in human prostate cancer cells: in silico and in vitro study. BioMed Research International. 2020;2020 doi: 10.1155/2020/6040727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhang I., Cui Y., Amiri A., Ding Y., Campbell R.E., Maysinger D. Pharmacological inhibition of lipid droplet formation enhances the effectiveness of curcumin in glioblastoma. European Journal of Pharmaceutics and Biopharmaceutics. 2016;100:66–76. doi: 10.1016/j.ejpb.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 178.Jiang Y., Sun A., Zhao Y., Ying W., Sun H., Yang X., et al. Proteomics identifies new therapeutic targets of early-stage hepatocellular carcinoma. Nature. 2019;567(7747):257–261. doi: 10.1038/s41586-019-0987-8. [DOI] [PubMed] [Google Scholar]