Abstract
Early diagnosis and treatment of oral cancer are vital for patient survival. Since the oral cavity accommodates the second largest and most diverse microbiome community after the gut, the diagnostic and therapeutic approaches with low invasiveness and minimal damage to surrounding tissues are keys to preventing clinical intervention-related infections. Gold nanoparticles (AuNPs) are widely used in the research of cancer diagnosis and therapy due to their excellent properties such as surface-enhanced Raman spectroscopy, surface plasma resonance, controlled synthesis, the plasticity of surface morphology, biological safety, and stability. AuNPs had been used in oral cancer detection reagents, tumor-targeted therapy, photothermal therapy, photodynamic therapy, and other combination therapies for oral cancer. AuNPs-based noninvasive diagnosis and precise treatments further reduce the clinical intervention-related infections. This review is focused on the recent advances in research and application of AuNPs for early screening, diagnostic typing, drug delivery, photothermal therapy, radiotherapy sensitivity treatment, and combination therapy of oral cancer. Distinctive reports from the literature are summarized to highlight the latest advances in the development and application of AuNPs in oral cancer diagnosis and therapy. Finally, this review points out the challenges and prospects of possible applications of AuNPs in oral cancer diagnosis and therapy.
Keywords: Gold nanomaterials, Oral cancer, Diagnosis, Cancer therapy, Theranostics
Graphical abstract
1. Introduction
Oral cancer comprises the cancers of the lip, tongue, and mouth representing the 6th most common cancer worldwide [1,2], with nearly 355,000 new diagnoses and 177,000 death yearly [3]. The oral cancer incidence rate in young people and women has increased significantly in the past 25 years, but the mortality rate is reduced just slightly [4]. Histopathological analysis of surgical biopsy is still the gold standard for oral cancer diagnosis. However, the histopathological analysis is time-consuming, and the results mainly depend on the subjective judgment of pathologists [5]. Most importantly, surgery can only be performed in the confirmed case of tumor lesions in the mouth. In addition, precancerous lesions may occur in hidden anatomical sites such as the crypts at the base of the tongue, making it difficult for the precise diagnosis of the lesion sites based on the histological examination of surgical biopsy. Standard clinical imaging methods, such as computed tomography, magnetic resonance imaging (MRI), and ultrasound provide basic information about tumor location, size, and spreading. However, these methods are not good enough to distinguish the small size (<0.5 cm) tumors, as well as benign and malignant tumors [6]. Advanced optical coherence tomography (OCT) is not suitable for cancer imaging due to its less sensitivity to the recognition of tumor and normal tissue [7]. The enzymatic immunosorbent technique is time-consuming and the signal of the Raman spectrum to biological samples is weak [8]. Therefore, the development of highly sensitive and specific as well as minimum invasive diagnostic approaches for oral cancer is urgent.
Surgical removal or/and combined radio-chemotherapies are commonly adapted for oral cancer treatment [9]. However, these treatment methods cause various complications such as large-scale facial defects and scar formation, radioactive bone necrosis, trismus, dysphagia, erythema or ulcerative mucosa, facial nerve palsy, and other complications [[10], [11], [12]]. These complications are directly related to the patient's morbidity, aesthetic, and psychological problems [10,[12], [13], [14]]. In addition, the oral cavity accommodates the second largest and most diverse microbiome community after the gut [15,16]. Surgery in the oral cavity possesses a high risk of microbial infection that adversely affects surgical wound healing and cancer treatment [17]. Therefore, the development of less invasive, early-stage cancer-detecting, and cost-effective diagnostic and therapeutic approaches are highly demanded in oral oncology. Nanomaterial-based technologies hold promising potential for cancer diagnosis and therapy. Recent advances in nanotechnology have made great progress in the development of novel approaches for rapid diagnosis and precise treatment of various cancers, including oral cancer [[18], [19], [20], [21], [22]]. Gold nanoparticles (AuNPs) are commonly used to develop novel strategies for cancer diagnosis or/and treatment.
Gold is one of the most stable metals due to its stable metallic bonds. Gold had been used as a treatment for mental illness, plum diarrhea, and other diseases in medieval Europe [23]. In 1971, British researchers Faulk and Taylor developed a revolutionary method of antibody colloidal gold coupling for direct electron microscopy of salmonellae surface antigens that opened the door to biomedical applications of AuNPs [24]. In the last 50 years, remarkable advances had been achieved in the controllable fabrication of AuNPs with good biocompatibility and unique optical properties, such as enhanced computed tomography [[25], [26], [27], [28]], and photothermal conversion properties [[29], [30], [31]]. The major physical properties of AuNPs are localized surface plasmon resonance, radioactivity, and high X-ray absorption coefficient, which render the applications of surface plasma resonance (SPR), photothermal therapy (PTT), photodynamic therapy (PDT), computed tomography, MRI, etc. From the chemical aspect of view, AuNPs can form stable chemical bonds with S and N containing groups, allowing the attachment of organic ligands or polymers for a straightforward surface modification [21]. This endows AuNPs with good targeting and drug delivery capabilities, especially in tumor imaging, diagnosis, and treatment [10,[32], [33], [34]]. In 2005, El-Sayed et al. firstly introduced the anti-epidermal growth factor receptor (anti-EGFR) antibody to gold nanosurface for the diagnosis of oral cancer, providing excellent differentiation between the tumor cells and normal cells [35]. From 2005 to 2020, AuNPs' research has made a great leap in oral cancer diagnosis and therapy [35,36].
AuNPs play a unique role in cancer diagnosis and treatment in the oral cavity. Due to the irregular anatomical structure of the oral cavity, it is difficult to detect early lesions and surgical margins of oral cancer [37,38]. The localized surface plasmon resonance at the near-infrared wavelength of AuNPs improves the contrast of the oral cavity images [39]. Moreover, the combination of AuNPs and appropriate antibodies enhance Raman spectroscopy and detect the diseased tissue faster and more accurately [40,41]. AuNPs have been used in PTT that converts light energy into heat energy in the targeted area of skin and mucous membranes without damaging the surrounding healthy tissues, thereby destroying tumor cells [[42], [43], [44], [45]]. AuNPs-based minimum invasive theranostics avoid the risk of surgical wound-related infection in the oral cavity. In addition, AuNPs have the potential to deliver anti-cancer drugs to cancer sites enhancing radiotherapy efficacy [46,47]. From the diagnosis to the treatment of oral cancer, the use of AuNPs preserves the normal tissue to the greatest extent that helps to maintain the chewing function and facial aesthetic. Based on the size, shape, and physical properties, AuNPs are classified into gold nanospheres [48,49], gold nanorods (AuNRs) [50,51], gold nanoflowers (AuNFs) [52], gold nanocages (AuNCs) [53,54], gold nanotriangles (AuNTs) [55,56] et al. AuNPs also include composite nanomaterials composed of other materials, such as core-shell structured gold-silica nanomaterials [57,58], gold-coated iron oxide nanomaterials [128], etc. AuNPs with different morphology had been designed for potential application in oral cancer diagnosis and therapy (Fig. 1). Nano-scale size, easy synthesis/surface modification, and good biocompatibility are the key characteristics that make AuNPs popular in the field of cancer diagnosis and therapy. In recent years, AuNPs have been developed and applied in the diagnosis and treatment of various cancers such as oral, gastric, colorectal, and breast cancer [40,[59], [60], [61], [62]]. Modulation in physicochemical properties of AuNPs optimizes oral cancer screening, diagnosis, and treatment. Recently, researchers are focused on developing AuNPs-based strategies for diagnostic and therapeutic applications in oral cancer. Such strategies are expected to overcome the limitations of traditional oral cancer diagnostic and therapeutic approaches [63]. In this study, we summarized the latest basic and clinical advances in AuNPs-based oral cancer diagnosis and treatment. Finally, this review points out the challenges and prospects of possible applications of AuNPs in oral cancer diagnosis and therapy.
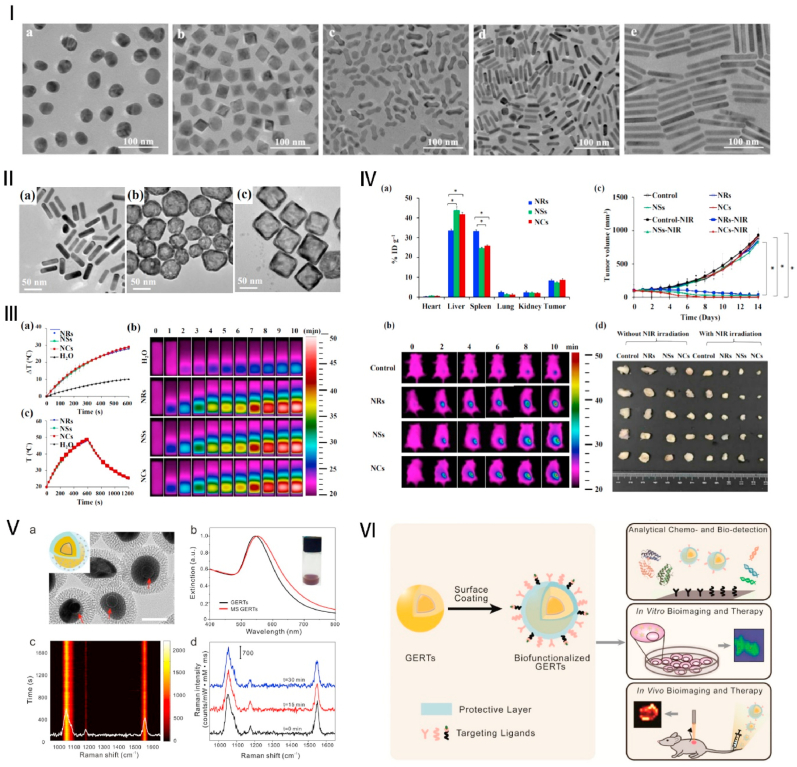
Fig. 1.
AuNPs with various morphologies. Ⅰ. TEM images of the AuNPs: gold nanospheres (a), gold nano-octahedras (b), gold arrow-headed (c), gold nanorods (d), and gold long nanorods (e). Source: Reprinted with permission from Ref. [64]. Copyright 2015, with permission from Elsevier. Ⅱ. TEM images of gold nanorods (NRs) (a), nanoshells (NSs) (b), and nanocages (NCs) (c); Ⅲ. Photothermal properties of PEGylated gold NRs, NSs, and NCs. (a) Temperature heating curves and (b) infrared thermal images for gold NRs, NSs, and NCs under 808 nm laser irradiation; (c) Heating and cooling curves of the above gold NRs, NSs, and NCs, which were irradiated by an 808 laser for 10 min, after that the laser was switched off. Ⅳ: In vivo phototherapeutic evaluation of AuNPs. (a) Biodistribution of gold NRs, NSs, and NCs in mouse organs; (b) Infrared thermal images of PBS, gold NRs, NSs, and NCs intravenously injected into a mouse tumor model under laser irradiation; (c) the tumor growth curves after being treated with various groups. (d) In vitro photos of the tumor after treatment. Source: Reprinted with permission from Ref. [65]. Copyright 2019, with permission from Elsevier. Ⅴ: Core-shell structure mesoporous silica-coated AuNPs (MS-AuNPs). (a) TEM of the nanoparticles (b), Extinction spectra of AuNPs and MS-AuNPs (c), Photostability measurement of time-resolved surface-enhanced Raman spectroscopy (SERS) spectra of solid MS-AuNPs (d), Three representative SERS spectra at selected irradiation times in panel (c). Source: Reprinted with permission from Ref. [57]. Copyright 2017, with permission from ACS. Ⅵ: Biofunctionalization and biomedical applications of AuNPs based gap-enhanced Raman tags. Source: Reprinted with permission from Ref. [58]. Copyright 2020, with permission from ivyspring.
2. Synthesis of AuNPs
AuNPs have been widely studied in biomedical applications, due to their unique physical and chemical properties. At the same time, researchers are increasingly interested in the synthesis of AuNPs. The synthesis of AuNPs mainly includes traditional chemical synthesis methods such as chemical reduction methods [66], electrochemical methods [67], ultrasonic chemistry methods [68], etc., and newly developed biosynthesis methods [69]. Generally, the preparation of AuNPs by the chemical reduction method includes two main parts: reductants, which are mainly used for REDOX reaction with gold-containing compounds such as tetrachloroauric acid, and stabilizers, which are designed to avoid the aggregation of the particles [70]. Herizchi R et al. reviewed the commonly used reductants and stabilizers, as well as the chemical synthesis methods of AuNPs represented by the Turkevich method, the Brust-Schiffrin method, the seeding growth method, and the ascorbic acid method in detail [70]. The chemical synthesis method can effectively control the morphology, size, structure, surface charge, uniformity, and dispersion. Therefore, the chemical synthesis method is more conducive to the later combination with other materials, drugs, antibodies, or proteins to enhance the function. However, when traditional synthesis methods are used, toxic by-products that harm the organism and the environment are produced, and the use of chemical solvents in the synthesis process makes subsequent extraction of nanoparticles difficult and presents significant obstacles to medical applications. For example, toluene is toxic, which is commonly used in the Brust-Schiffrin methods [71] and Cetyl trimethyl ammonium bromide (CTAB) is highly cytotoxic and is often used as a stabilizer in the seeding growth method [72]. In addition, some studies have found that small-sized AuNPs (5 nm) could induce significant pathological changes in the liver after being injected for 2 days [73]. The medium (20 nm) and large (50 nm) sized AuNPs preferentially target the spleen and cause significant pathological changes to the spleen architecture on day 2 that persists on day 8 as well [73]. Therefore, further systematic safety evaluation and further stabilization tests in biological fluids of synthesized AuNPs are required before they can be used in vivo or clinically.
Biosynthesis is a green and environment-friendly new synthetic method of gold nanoparticle synthesis. Lee KX et al. [74] and Kalimuthu Ket al. [75] reviewed the newest biosynthetic study of AuNPs. Biosynthetic AuNPs are generally safer to use in biomedical applications because they come from natural materials themselves [74]. Natural materials such as plants, fungi, microorganisms, enzymes, and biopolymers are currently used to synthesize various nanoparticles [74]. The advantage of biosynthesis is to reduce or eliminate the use and production of harmful chemicals or toxic by-products during conventional AuNPs chemical synthesis methods. In addition, the biosynthesis process does not need special temperature and pressure, and the reaction conditions are mild, compared with the traditional synthesis is more economic, and environmentally friendly. Although the biosynthesis of AuNPs has many significant advantages, there are still many problems hindering their clinical transformation. Most importantly, the mechanism of biosynthesis is not clear. Reducing substances such as organic compounds and functional groups in natural materials are complex and diverse, so more studies are needed to achieve more stable and controllable synthesis. In addition, systematic safety and biocompatibility studies are essential before clinical transformation. In a word, traditional chemical methods are more mature and controllable, but there are environmental, energy, and other problems. The newly developed biosynthesis is safer and environmentally friendly, but it is still not mature enough. We believe that with the rapid development of nanotechnology safer, environmentally friendly, economic, and cos-effective AuNPs can be obtained to better serve life science.
3. Application of AuNPs in oral cancer diagnosis
In early diagnosed oral cancer, surgical resection is highly efficient to cure cancer, with the 5-year survival rate exceeding 85% [76]. However, early clinical changes and lesions of oral cancer are mostly in hidden anatomical areas making it difficult for early diagnosis. At the same time, some precancerous lesions and diseases such as oral leukoplakia and erythema are difficult to distinguish from chronic inflammatory diseases such as lichen planus [77]. Clinicians’ subjective judgment can easily lead to misdiagnosis and delay in treatment. The majority of oral cancer are diagnosed only at the late stage resulting in poor treatment and prognosis [77]. The in vitro detection technology based on AuNPs is excellent in the early screening and diagnosis of diseases [78]. The detection and diagnosis techniques constructed with AuNPs find cancer cells and cancer tissues in a more timely and effective manner that improves the specificity and accuracy of oral cancer screening and diagnosis.
3.1. Early screening and prevention
The high mortality rate in oral cancer patients has been attributed to the difficulties in detecting the disease in an early treatable stage. Moreover, because early carcinomas are asymptomatic, most oral cancer cases are usually diagnosed in advanced stages [79]. Current diagnosis relies on clinical investigation and histopathological examinations, with low sensitivity and a high risk of undetected cancerous lesions in hidden areas [80]. Proteins, nucleic acids, and enzymes found in saliva could be potential biomarkers for cancer detection, as they have been shown to have distinct levels in cancer patients compared with healthy individuals. Therefore, saliva is developed as a clinical sample for the early diagnosis of oral cancer. Saliva plays an important role in the early diagnosis of oral diseases including oral cancer and periodontitis [[81], [82], [83]]. Saliva is more popular than other biological fluids because its' collection is easy and less stressful and does not require any sophisticated equipment or skilled personnel. In addition, direct contact between saliva and oral cancer lesions makes saliva a more specific and potentially sensitive biological sample for early screening and diagnosing. Khurshid Z et al. had concluded more than 100 salivary potential biomarkers (DNA, RNA, mRNA, protein markers) as early diagnostic markers for oral cancer, including cytokines (IL-8, IL-1β, TNF-α), defensin-1, P53, Cyfra 21–1, tissue polypeptide specific antigen, dual-specificity phosphatase, spermidine/spermineN1-acetyltransferase, profilin, cofilin-1, transferrin, osteopontin, and many more [3,[84], [85], [86]]. A recent review summarized 65 salivary-based biomarkers and their function in oral cancer [87]. In addition to the above summary, long non-coding RNAs can also be used as potential markers for oral cancer diagnosis [88]. The upregulated long non-coding RNAs TFAP2A-AS1, AC007271.3, ANRIL, BC200, BLACAT1, CASC9, CASC15, CCAT1, CCHE1, CEBPA-AS1, CRNDE, DANCR, DNM3OS, ELF3-AS1, FAL1, FALEC, FGD5-AS1, H19, HAS2-AS1, HCP5, HIFCAR/MIR31HG, HNF1A-AS1, HOTAIR, HOTTIP, HOXA11-AS, HOXC13-AS, HULC, JPX, LEF1-AS1, LINC00152, LINC00319, LINC00941, LINC00958, LINC00963, LncRNA-p23154, LUCAT1, MALAT1, MCM3AP-AS1, MIR4435-2HG-, MYOSLID, NEAT1, OIP5-AS1, PAPAS, PLAC2, PDIA3P, PVT1, RBM5-AS1, RC3H2, RP11-874J12.4, SNHG12, SNHG17, SNHG20, SNHG3, TIRY, TTN-AS1, TUG1, and UCA1, and downregulated long non-coding RNAs C5orf66-AS1, CASC2-, ENST00000470447.1, GAS5, LINC01315, MEG3, MORT, and SOX21-AS1, are related to the proliferation, migration, invasion, apoptosis, and cisplatin cytotoxicity of oral cancer [89,90]. In a word, with the rapid development of bioinformatics in recent years, more and more potential biomarkers of oral cancer will be discovered, such as new proteins, DNAs, RNAs, exosomes, etc.
Early detection of oral cancer is vital for effective treatment. In recent years, a series of studies on early diagnosis of oral cancer have been developed based on SERS, OCT, and DR enhanced by AuNPs. We summarized the AuNPs designed for early screening of OSCC and the outcomes in Table 1. SERS was developed based on Raman imaging technology. During light scattering, most photons undergo an elastic process called Rayleigh scattering, while about one in ten million photons undergo an inelastic process called Raman scattering, in which they exchange energy with the scattering material [91]. Raman scattering shows sharp and clear fingerprint peaks of specific chemical groups and can be easily separated from the background signal of the aqueous solution, making it an ideal analytical method for biological applications [92]. However, the weak Raman scattering strength of many biological samples hinders the further application of this technique. The research showed that the Raman scattering can be enhanced by using specific AuNPs as SERS active substrates [93]. SERS can be used as an optical imaging technology by monitoring specific molecules. The key advantage of SERS imaging lies in its high spatial resolution, which can reach <0.5 μm in the visible range, allowing high-resolution sample mapping. Another advantage is that multiple analyses can be performed for several analytes in the same sample [94]. Parallel access to large amounts of valuable data saves clinicians time and costs. We summarized the study on the early diagnosis of oral tumors based on SERS enhanced by AuNPs. Chakraborty D et al. had reported a non-invasive AuNPs-based enzyme-linked immunosorbent assay (ELISA) to detect osteopontin in oral cancer [39]. This modified version of ELISA has a wide linear detection range (0.31–20 ng/mL), good repeatability, and specificity for the tested interference in saliva [39]. AuNRs (detection limit: 0.02 ng/mL) or AuNSs (detection limit: 0.03 ng/mL) based ELISA kits shows higher accuracy of oral cancer screening compared to traditional ELISA kits (detection limit: 0.14 ng/mL) [39]. In addition, Liu Q et al. proposed a highly sensitive, noninvasive, and rapid cancer-screening platform encompassing exfoliated cytology and surface-enhanced Raman scattering (SERS) technology (Fig. 2) [95]. The filter paper adsorbed SERS matrix consisting of plasma AuNRs has been designed for cancer screening. Raman spectroscopy reflects the molecular changes in cancer, showing a high specificity of the biochemical components of cells and tissues. Due to changes in specific biomolecules in cancer cells, different and repeatable SERS spectra can be obtained from normal cells and cancer cells. A diagnostic algorithm based on the ratio of spectral values has high sensitivity and specificity for distinguishing exfoliated cells in normal and cancer tissues [95].
Table 1.
AuNPs developed for early screening and prevention of oral cancer.
| Nanomaterial | Size (nm) | Principle | Sample/target | Effect | Diagnosis ability in cancer vs control group | Differences in abnormal vs normal group | Ref. |
|---|---|---|---|---|---|---|---|
| AuNSs AuNRs |
13.6–17.2 1.3 × 5.6 |
SPR | Saliva/OPN | Limit of detection ↑ | 0.02 vs 0.14 0.03 vs 0.14 ng mL−1 |
/ | [39] |
| AuNRs | 13.72 × 49.73 | SERS | Exfoliative cells/CAL-27 | Specificity↑ Sensitivity↑ |
100% vs 60% 100% vs 70% |
/ | [95] |
| AuNPs | 15 | SERS | Saliva/spectrum | Potential to detect peaks | / | New peaks at 1097 and 1627 cm-1 in the cancer group | [80] |
| AuNPs | 20 | SERS | Saliva/(S100P) mRNA | S100P mRNA detection limited | 10 nM (S100P) mRNA | Three times higher in cancer group | [96] |
| AuNCs | 15 | OCT | Hamster/cheek pouch tumor | Visualization of tumor sites | / | OCT and DvOCT signal inversion | [97] |
| AuNPs | 70 | OCT | Hamster/cheek pouch tumor | Pinpointed pathological structures of early-stage oral cancer | / | Increase optical scattering intensity 33% | [98] |
| AuNRs | 15 × 50 | DR | OSCC | Sensitivity and specificity significant increase | Sensitivity 100%, specificity 89% | / | [99] |
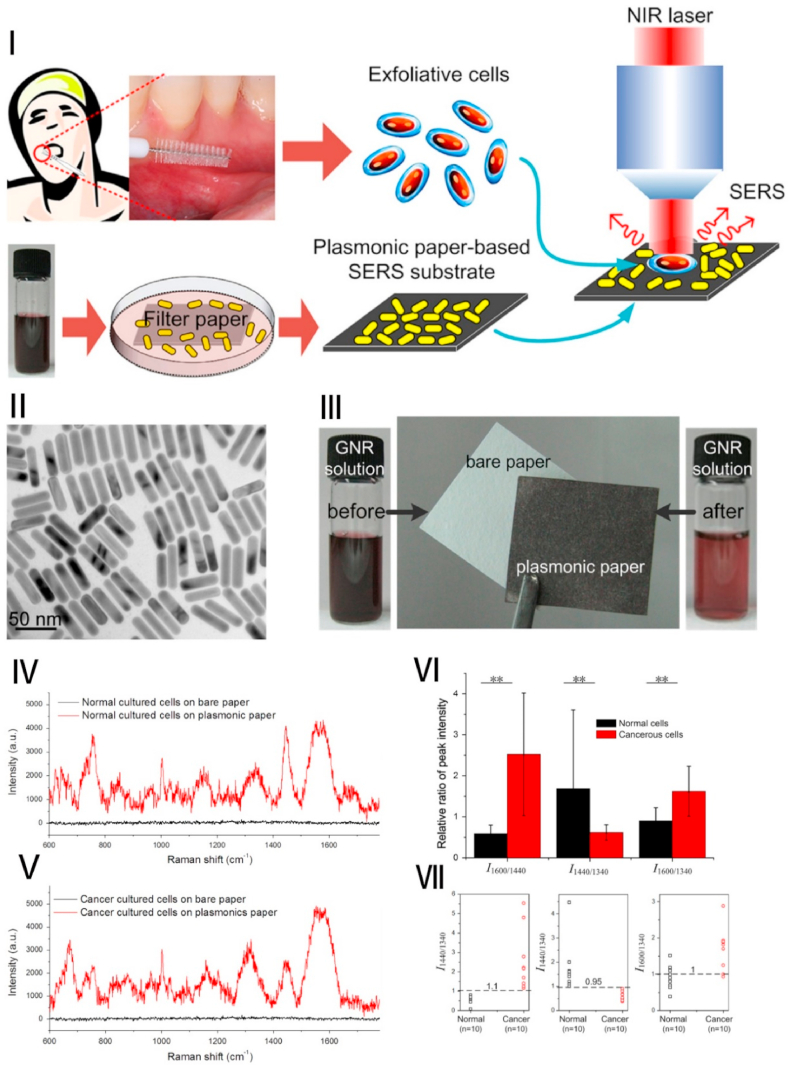
Fig. 2.
Early, sensitive, noninvasive, and rapid cancer screening based on AuNRs: (Ⅰ) The schematic diagram showed the preparation of SERS substrate and the SERS detection of exfoliated cells obtained from oral cancer patients; (Ⅱ) TEM images of AuNRs; (Ⅲ) the plasmonic paper preparation and the color changes of AuNR solution before and after exposure; (Ⅳ, Ⅴ) The Raman spectra detected from the two different cells (normal cells, oral keratinocyte, and cancerous cultured cells) on the plasmonic paper and bare paper; (Ⅵ) Comparison of SERS signal intensity ratio of oral exfoliated cells; (Ⅶ、Ⅷ) Scatter plot of Raman signal intensity ratio tested from the different treated group. Source: Reprinted from Ref. [95] Copyright 2014, with permission from Elsevier.
Fălămaș A et al. developed SERS spectra in saliva from healthy volunteers and oral cancer patients [80]. Principal components analysis (PCA) based on multiple SERS strips is mainly assigned to amino acids and proteins that distinguish saliva between healthy controls and oral cancer patients [80]. PCA was used to identify saliva samples, and SERS spectra of the healthy group and oral cancer group could be distinguished. This proves that SERS technology based on AuNPs can be successfully used for the detection of oral cancer without labels [80]. Priezzhev AV et al. had developed a saliva-based sensitive and portable SERS system for early diagnosis of oral cancer [100]. Saliva samples (healthy group and patient group) were added to the AuNPs fabricated membrane and then excited with a laser at 633 nm. SERS spectra were obtained in 2 min [100]. New peaks were observed in the patient samples at 1097 cm-1 and 1627 cm-1, while these peaks were not found in the normal group [100]. This study demonstrates the potential for rapid, sensitive, and portable diagnosis of oral cancer based on AuNPs. In addition to the above-mentioned methods, Han S et al. reported an assay based on SERS of AuNPs for the sensitive detection of S100P mRNA in saliva [96]. S100 calcium-binding protein P (S100P) mRNA (S100PmRNA) is one of the potential biomarkers for oral cancer [96]. The results showed that the amount of S100P mRNA detected in oral cancer patients is 3-fold higher compared to the healthy group. This nanoparticle-based SERS detection of S100P mRNA has the potential to achieve non-invasive early detection of oral cancer enabling point-of-care immediate medical treatment [96].
OCT, a technique for producing two-dimensional cross-sectional images using the interference of light, was first described in 1991 and is often referred to as an optical analog of ultrasound, which detects reflected light from tissue rather than sound [101]. OCT imaging technology provides cross-sectional and 3D image resolutions of a few microns and penetrations to depths of one to 2 mm [102]. Contrast agents can improve the quality of OCT in situ images to a level comparable to biopsy, reducing the need for tissue removal, sectioning, and staining [103]. The addition of contrast agents to the OCT system helps to enhance the final image contrast, which helps to better distinguish normal and tumor tissue. AuNPs have the characteristics of good biocompatibility, easy to synthesize, systematic or local administration, etc., which are the most widely used contrast agents at present [104]. A non-invasive high-resolution imaging modality of in vivo OCT imaging identifies the site of early oral dysplasia through the acid decomposition of gold nanoclusters [97]. Based on OCT, Kim CS et al. used acid-degradable gold nanoclusters to pinpoint slightly acidic tumor tissue [104]. They developed a novel platform to formulate AuNPs into water-soluble microneedles [104]. Afterward, AuNPs were effectively and precisely controlled and conveniently delivered to the hamster's oral tissue in vivo [104]. The results showed that OCT detection with AuNPs improved resolution by 150%. However, due to absorption and scattering limitations, the penetration depth is only a few millimeters [105], so further investigation and improvements are needed for better clinical use. In recent years, a new optical technique, diffuse reflectance spectroscopy (DR), has been developed for biological imaging. DR can identify tissue characteristics by measuring their intrinsic light absorption and scattering properties at different wavelengths [106]. The optical fingerprint of tissue is obtained by irradiating the selected spectrum, and the fingerprint represents specific quantitative biochemical morphological information [106]. DR has the advantages of completeness, simplicity, and great diagnostic potential. In addition, the DR spectra of tissues also contain abundant information on biochemical and ultrastructural changes [107]. The observed characteristics of the DR spectrum depend on metabolic rate, blood vessels, intravascular oxygenation, and tissue morphology [106]. As a result, DR can provide detailed information about the underlying biological composition of tissues, and thus has the potential to distinguish tumor tissue from normal tissue. AuNPs can enhance the scattering and absorption of tissue signals due to their unique optical properties. In addition, AuNPs can improve the specificity of DR signals and distinguish healthy and abnormal parts in tissues by targeting abnormal parts [108]. The DR method is based on the accumulation of AuNPs in tumors, which leads to significant changes in the optical properties of tumors. To achieve this goal, AuNPs are coupled to the anti-epidermal growth factor receptor (anti-EGFR), which is a cell surface receptor belonging to the ErbB family of tyrosine kinases that play an important role in regulating cell proliferation [109]. Based on DR measurements of AuNRs bio-conjugated to anti-EGFR specifically attached to OSCC cells [99]. The DR measurements revealed a significant increase in light absorption in rats with OSCC compare with rats without cancer (p < 0.02, sensitivity 100%, specificity 89%) [99]. The aforementioned studies indicate that the detection system based on AuNPs could be effective in the early detection of oral cancer. In summary, AuNPs-based detection techniques have great potential in the early diagnosis of oral cancer, but the accuracy and applicability of these nanomedicine methods in clinical applications need to be studied in vivo. Early and accurate diagnosis and detection of OSCC and highly dysplastic lesions still remains a key challenge.
3.2. Diagnosis of advanced stage OSCC
Besides distinguishing normal tissues from cancerous tissues, AuNPs also detect the size and degree of tumors that facilitate clinicians to evaluate the scope of surgery and the progress of the disease [40,110]. The correct judgment of the boundary between tumor and normal tissue as well as the degree of malignancy of the tumor guides the operation and postoperative treatment. The AuNPs designed for detection of tumor edge location and staging of oral cancer, their mode of action, and the outcomes are summarized in Table 2.
Table 2.
AuNPs designed for the diagnosis and staging of oral cancer.
| Type | Size (nm) | Principle | Sample | Purpose | Result | Ref |
|---|---|---|---|---|---|---|
| AuNRs | Major axis 55 Minor axis 16 |
SERS | Sliced tissues | Discriminate benign from malignant oral lesions | The significantly lower intensity in the benign group | [40] |
| AuNPs | 35 | SERS | Cell lines HOC 313 clone 8 and HSC 3 | Distinguish between cancerous and noncancerous cells | [35] | |
| AuNPs | 55 | SERS | Serum | Develop new diagnoses method to detect OSCC | Sensitivity 80.7%, specificity 84.1%, and | [111] |
| AuNPs | 55 | SERS | Serum | Detect the tumor stages and histologic classification of OSCC | accuracy 85.9% | [112] |
| AuNRs | Major axis 25 Minor axis 11 |
AirSEM & DR | OSCC tissue sections | Determine tumor margins | Accuracy margins of 1 mm | [110] |
| AuNPs | 60 | NIR absorption imaging | Oral cancer cell lysate | Specific and quantitative analysis | Rapid and quantitative detection of oral cancer cells | [113] |
As discussed above, AuNPs can effectively enhance the signal of SERS, but AuNPs bind non-specifically to tumor cells, so they cannot effectively enhance the signal of tumor cells. AuNPs can be targeted to tumor cells by coupling specific tumor cells' surface markers such as aptamer, peptide, antibody, and protein [114], and the changes in scattering imaging and absorption spectrum caused by targeting tumor cells make it possible to diagnose tumors based on AuNPs. In terms of oral cancer, the antibody-conjugated AuNPs have a promising potential to apply in molecular biosensor technology to diagnose and visualize the live oral epithelial cancer cells in vivo and in vitro [35]. El-Sayed IH et al. reported that AuNPs conjugated anti-EGFR antibodies bind to the surface of oral malignant tumor cells with 600% higher affinity, specificity, and uniformity compared to non-cancer cells [35]. Wang JH et al. developed a diagnostic platform based on rose-bengal-conjugated AuNRs (RB-AuNRs) [113]. Rose Bengal (RB), the 4,5,6,7-tetrachloro- 20,40,50,70-tetraiododerivative of fluorescein, is a specific agent of cancer cells [113]. RB has been shown to have specificity to oral cancer through inhibition of DNA polymerases of oral cancer cells [113]. The plasmon resonance peak shift of the AuNR surface and its changes in near-infrared light absorption allow highly sensitive diagnosis and rapid screening of oral cancer [113].
Based on the above diagnostic properties, we investigated the use of AuNPs-based oral cancer diagnostic techniques for oral cancer edge location and for differentiating benign and malignant tumors. Accurate edge positioning can more accurately guide the surgical resection range. Fixler D et al. prepared an EGFR conjugated AuNRs and added on to different oral tissue lesion sections [40]. This technique distinguishes precancerous lesions and malignant diseases according to the reflectance in lesions, i.e., the deeper the disease progression the higher the reflectivity. This staining differentiates the reflectivity of benign lesions and mild dysplasia from moderate and severe dysplasia and aggressive lesions [40]. This non-ionizing optical detection method provides a highly sensitive and cost-effective tool for early cancer diagnosis and accurate detection of tumor margins and residual tumor tissue Ankri R et al. used EGFR-modified AuNRs in combination with a new high-resolution air scanning electron microscope to locate the edge of OSCC tumors and visualize the tumor tissue (Fig. 3) [110]. This technique facilitates the tumor margin mapping in squamous cell carcinoma.
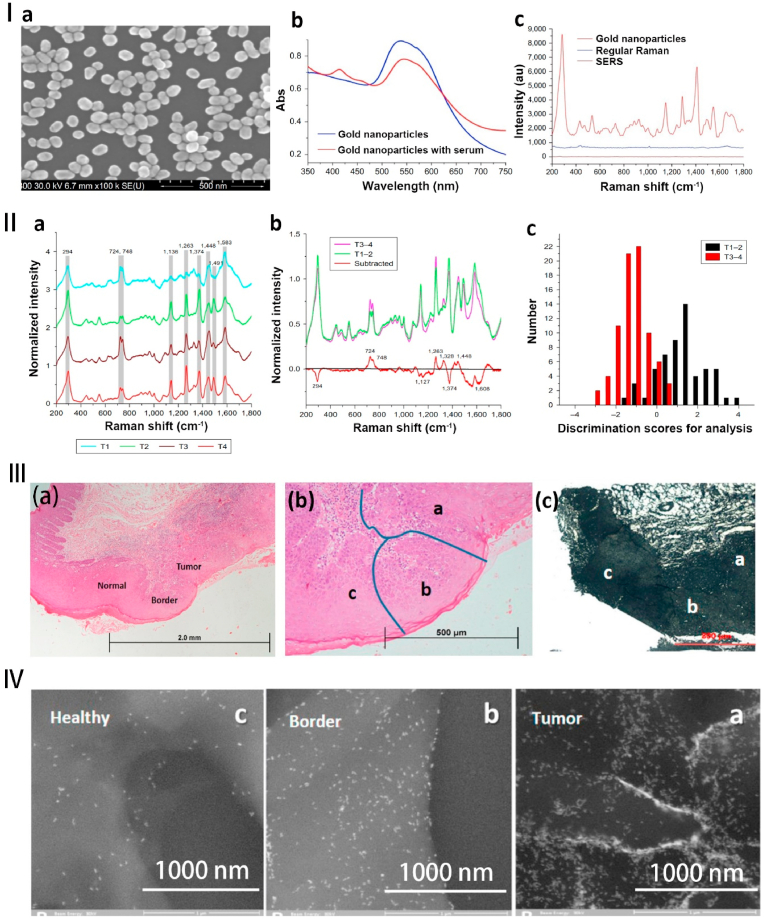
Fig. 3.
Diagnosis of oral cancer based on AuNPs. Ⅰ. (a) The SEM images of the AuNPs. (b) The UV–vis absorption of the AuNPs and the AuNPs with serum. (c) Raman spectra of blank, serum, and AuNPs. Ⅱ. (a) The normalized mean SERS spectra of the different tumor size groups (T1, T2, T3, and T4). (b) The normalized average SERS spectra of subtracted, T1–2 and T3–4. (c) The histogram of discrimination scores of T1–2 and T3–4. Source: Reprinted with permission from Ref. [112]. Republished with permission of 2018 International Journal of Nanomedicine. Ⅲ. SERS of blood serum based on AuNPs for tumor stages detection and histologic grades classification of OSCC. (a) Hematoxylin-eosin (H&E) staining for OSCC can identify tumors, boundaries, and normal sites ( × 40). (b) higher magnification ( × 100). (c) the airSEM image of the same tumor area (a) tumor, (b) border, and (c) normal sites. Ⅳ. The higher magnification airSEM images of the different sites (a–c) in the same area. From the tumor to the healthy site, the concentration of nanoparticles gradually decreases. Source: Reprinted with permission from Ref. [110]. Reproduced with permission. Copyright 2016, ACS.
The evaluation of tumor size, lymph node stage, and pathological status assists in preoperative judgment, prediction, and postoperative recovery of OSCC. Adding AuNPs to OSCC or normal human serum showed 80.7% sensitivity, 84.1% specificity, and 81.1% overall accuracy rate of OSSC diagnosis (Fig. 3). This technique successfully diagnoses and classifies OSCC, mucoepidermoid carcinoma (MEC), and normal groups [111]. The increase in the content of nucleic acids and proteins in OSCC serum correlates with the increase in tumor volume with the higher accuracy rate in the classification. Xue L et al. used the characteristics of enhanced SERS of AuNPs and combined the principal component analysis and linear discriminant analysis (PCA-LDA) in patients with OSSC and MEC [112]. PCA-LDA classifies and diagnoses Raman spectra of AuNPs added OSCC serum. As the result of the PCA-LDA classification, the total accuracy of ∼80% shows that the SERS had a giant potential to detect and diagnose the different T stages of OSCC. The accuracy was higher in the classification of T1–2 and T3–4 stages [112].
AuNPs-based strategies hold promising potential for the development of simple, highly specific, sensitive, and less invasive tools for diagnosis or/and treatment of OSCC. Such approaches not only avoid the complex surgery and use of expensive equipment in cancer diagnosis and treatment but also detect malignant lesions at the molecular level, which is more objective and efficient compared to traditional histological examination. of tumor stages T1-2 and T3-4 [112]. Therefore, the use of AuNPs nanoparticles in OSSC analysis not only diagnoses OSSC but also determines the tumor edge location, size, and grade.
4. Application in oral cancer treatment
At present, the main methods of cancer treatment are surgery, radiotherapy, and chemotherapy. Each of these methods has certain shortcomings such as damage to nerves, blood vessels, and bone in the oral maxillofacial region, and other series of quality-of-life issues [[115], [116], [117]]. Therefore, more precise, low-invasive, and highly effective approaches for oral cancer treatment are in urgent need. AuNPs mainly accomplish the above treatment goals through drug delivery, photo-, or radio-sensitization, and multi-functional treatment platforms. The AuNPs fabricated for oral cancer treatment, their effect in alone or in combination therapy, and in vitro or in vivo outcomes are listed in Table 3.
Table 3.
AuNPs fabricated for oral cancer treatment.
| Type | Treatment strategies | Combination with | Cell line/model | Result | Ref. |
|---|---|---|---|---|---|
| AuNPs | Anti-angiogenic and anti-metastatic | Anti-angiogenic drug quinacrine | H-357 cells/xenograft mice model | Compared with quinacrine and/or AuNPs, mice exposed to AuNPs-based drugs simultaneously gained weight and significantly reduced tumor volume. | [118] |
| AuNPs | Reverse multidrug resistance | Folate and bilirubin | KB-ChR-8-5 cells/tumor xenograft | Targeted therapy and reduce drug side effects The tumor volume of the saline group, bilirubin group, and AuNPs-based drug-treated group was 2.124, 1.405, and 0.954 g, respectively. |
[119] |
| AuNPs | PTT and chemotherapy | Conjugated to DOX through pH-sensitive and resistant linkers | OSCC (HSC-3) | The apoptotic indices of combination group, single DOX, and single AuNPs is 33.3, 12.6, and 19.3 respectively. | [120] |
| AuNPs | Induce cell apoptosis | Mouse anti-human programmed death-ligand 1 (PD-L1) | OSCC | Induce apoptosis of SCC-25 oral squamous cell carcinoma cells. PDL1 conjugated AuNPs decreased cell viability in about 50% of nontreated control cells. |
[121] |
| AuNPs | Cause apoptosis and death of the CSCs | Quinacrine | Cancer stem cells (CSCs) | Caused apoptosis through modulating replication fork. The positive apoptosis-related protein Bax increased (6.1 times) and the negative apoptosis-related protein Bcl-XL decreased (by 5 times). | [122] |
| AuNPs | Chemotherapy and radiotherapy | Cetuximab and cisplatin-conjugated | OSCC | Combination therapy improves radiotherapy sensitivity. The sensitization enhancement ratio was calculated as 1.2 and 1.9, for AuNPs and AuNP + Cetuximab, respectively. | [123] |
| AuNPs | Selectively induce necrosis and apoptosis | / | Gingival fibroblasts and dysplastic keratinocytes | AuNPs induced selective toxic effects against dysplastic cells and induced antioxidant and anti-inflammatory effects. | [124] |
| AuNRs | PTT | Trehalose | human OSCC cell line/tumor mouse model | Precise treatment, at 6 weeks, tumor volume in the PBS group was 6-fold larger than that in the treatment group. | [125] |
| AuNRs | PTT | Folate | human oral epidermoid cancer cells (KB cells) | Target and precise treatment, in the laser combined with AuNPs treatment group, cell death reached 56%. | [126] |
| AuNPs | PTT and radiotherapy | Folate | KB cells | Combination of PTT and radiotherapy led to the highest early apoptotic ratio of 27.76%. | [42] |
| AuNPs | PTT and radiotherapy | Gold-coated iron oxide nanoparticles | KB cells | Multimodal cancer therapy improved the apoptosis of cancer cells, in the combinatorial treatment group, the cell viability substantially decreased to 19%. | [127] |
| AuNFs | PTT and chemotherapy | Coated with two layers of silica | CAL27 | A combination of PTT and chemotherapy has better potential in the treatment of oral tumors. | [128] |
| AuNRs | PTT and PDT | Conjugated with Rose Bengal | CAL27/DMBA-induced hamster tumor model | Combined PDT-PTT therapy resulted in 95.5% tumor suppression at day 10, compared with 46.5% and 65.5% for PDT and PTT alone. | [129] |
| AuNRs | PTT and gene therapy | Coated with siRNA oligos targeting BAG3 | CAL-27 | PTT effect was enhanced by inhibition of heat shock response-related proteins. In the combination group, tumor volume decreased to 39.5% on day 18 compared with 79.5% in the gene therapy group alone. | [130] |
| AuNPs | PTT and chemotherapy | Conjugated with podoplanin (PDPN) antibody- and doxorubicin (DOX)- | CAL27 | Show the synergetic effect of PTT and chemotherapy. The combination group had the highest inhibitory efficiency of 82.6% compared with 51% in the drug group alone. | [131] |
| AuNPs | Photothermal-chemical-radiotherapy | Alginate hydrogel co-loaded with cisplatin and AuNPs | KB cells | Develop a multi-functional platform combining photothermal-chemical-radiotherapy. The gene expression in trimodal therapy group up-regulation of Bax pro-apoptotic factor (by 4.5-fold) and the down-regulation of Bcl-2 anti-apoptotic factor (by 0.3-fold). | [132] |
| Ultrafine AuNPs |
Modulating microenvironment | / | Cancer-associated fibroblasts (CAFs) | Blocking dominant cells in the microenvironment to eradicate tumors | [133] |
| AuNPs | PTT and PDT | Black phosphorous nanosheets and cisplatin | OSCC | Effectively inhibit the metastasis and growth of OSCC, Combination therapy can improve the overall survival rate of golden hamsters. | [134] |
| AuNPs | Gum Arabic-encapsulated | OTSCC | As a promising carrier for chemotherapies to diminish intratumoral hypoxia-stimulated resistance | [135] |
4.1. Construction of anticancer nano-drugs
With the development of nano-technology, nano-drugs show great potential in anti-tumor application [136]. AuNPs are one of the most widely studied medical nanomaterials because of their excellent physicochemical properties, making AuNPs suitable for biochemical applications [137]. Firstly, nanoparticles with various morphologies and structures can be synthesized, such as nanospheres, nanorods, nanowires, nanoarrows, etc. Each of which has its unique properties, behaviors, and applications. Secondly, AuNPs can be composed of pure gold, composites (grafted with polyethylene glycol, cysteine, etc.), or doped with other metals (e.g., Ag, Se, Mo, Mn, Pt, Fe3O4, ect.) to obtain new hybrid materials, which can be further covered, functionalized, or combined with drugs or other molecules for cell targeting and drug delivery [138]. In addition, AuNPs have high direct anticancer activity, such as photothermal and photodynamic, etc [137]. Therefore, AuNPs have great potential to construct anticancer nano-drugs via loading chemical drugs, antibody drugs, gene drugs as well as other anti-cancer drugs [139]. AuNPs can load chemotherapy drugs and immunotherapy drugs by binding to amines and sulfhydryl groups [140]. Drugs conjugation with AuNPs can improve the antitumor effect of drugs by enhanced permeability and retention and active targeting effect, enriching drugs into tumor cells [[118], [119], [120]]. Satapathy SR et al. developed gold-conjugated biologically active quinacrine hybrid nanoparticles (QAuNP) [118]. This hybrid nanoparticle gives targeted controlled release of drugs, significantly inhibits OSCC-tumor stem cells proliferation and blood vessel formation, induces apoptosis, and reduces tumor size in a xenograft mouse model. Similarly, Rathinaraj P et al. developed a folate-gold-bilirubin (FGB) nanoconjugate for multidrug-resistant oral cancer cells [119]. The combination of AuNPs with folic acid ligands and the potential anti-cancer drug bilirubin targets the overexpressed folate receptors in cancer cells and causes the death of multidrug-resistant oral cancer cells in vitro [119]. Besides, FGB shows higher toxicity to tumor xenograft models than bilirubin alone, suggesting its promising potential to treat multi-drug resistant oral cancer [119]. Recently, AuNPs be used to treat oral cancer co-conjugated with cisplatin and cetuximab [123], which is an effective target for epidermal growth factor receptor (EGFR) [23]. This research results show a co-delivery of cisplatin and CTX has a high anti-cancer effect through increasing cytotoxicity and overcoming resistance to radiotherapy [123]. Essawy MM et al. studied AuNPs coupling DOX-related anti-oral tumor effect, and the results showed that compared with pH-sensitive AuNPs, DOX pH resistant AuNPs treated animals had significantly improved tumor shrinkage rate and survival rate [120]. In addition, gold nano-loaded PDL-1 showed the ability to promote OSCC cell apoptosis [121]. Gum Arabic, a complex mixture of glycoproteins encompassing Arabic acid and FDA approval for human consumption, was used to encapsulate AuNPs, which reduced CAL-27 cells viability with IC50 of 392.3 and 247.3 μg/mL after 24 h and 48 h, respectively [135]. They also demonstrated that Gum Arabic encapsulates AuNPs to cause CAL-27 cells apoptosis via inducing high hypoxia [135]. Tumor metastasis to local or remote recurrence caused by tumor stem cells is a major factor in tumor treatment failure. AuNPs with quinacrine can induce oral cancer stem cell apoptosis by inhibiting DNA replication [122]. In addition to delivering anti-cancer drugs, AuNPs themselves have also shown anti-tumor effects [124]. Baldea I et al. reported AuNPs phytosynthesized with cornus mas extract can selectively induce the death of dysplastic oral cells without damaging normal cells [124]. AuNPs induce cell necrosis and apoptosis by activating p53/BAX/BCL2 and inhibiting PI3K/AKT pathway [124]. Moreover, Xia C et al. reported the size of AuNPs have influence for the anti-tumor effect and their research prove that the ultrafine AuNPs (3 nm in diameter) could effectively inhibit the growth of OSCC tumors in vivo [133]. Overall, AuNPs have broad research and application prospects to construct nanodrugs in oral tumors treatement.
4.2. PTT and immunotherapy
PTT is a non-invasive therapeutic tool to eradicate tumors by absorbing light energy to convert it into heat energy. PTT provides an alternative route for cancer treatment by spatiotemporal control of the photothermal effect without being influenced by cell type [[141], [142], [143]]. The PTT increases the temperature in targeted tumor cells by > 20 °C [144]. During PTT, temperature exceeding 42 °C kills malignant cells without damaging nearby healthy tissues [145]. PTT improves the specific therapeutic effect within the light-exposed area without generating systemic toxicity. Due to their high efficiency of photothermal conversion properties, AuNPs are the commonly used nanomaterials for PTT of various cancers [146,147]. Vines JB et al. had nicely summarized the application of AuNPs in photothermal cancer therapy [148]. Liao YT et al. used cysteine-and-peptide-modified alginate AuNRs for PTT of oral cancer and tested its potential in vitro and mouse xenograft model [125]. AuNRs-based PTT decreased tumor volume in mouse xenograft by 5-fold suggesting its accuracy and efficacy. Mehdizadeh A et al. coupled AuNRs with folic acid to develop nanostructures (F-GNR) for targeted PTT therapy [126]. Combined plasmonic PTT of 20 μM F-GNR with seven pulsed lasers and a 6 h incubation period shows 56% lethality of human oral epidermoid cancer cells (KB cells) [126]. These reports suggest that the AuNPs-mediated photothermal conversion could be an effective component of PTT-based oral cancer therapy. However, the oral cavity is an open and dynamic functional organ, playing the functions of language, swallowing, taste, and so on. Therefore, the treatment of oral cancer needs to adopt precise treatment strategies, to better protect the normal oral function of patients. PTT is a minimally invasive way of treating cancer that uses light absorbers to produce photothermal damage to tumors [149], which can protect the normal tissue and organ function of patients as much as possible compared with traditional surgery, chemotherapy, and radiotherapy. Therefore, the development of more accurate oral photoheat treatment technology and photoheat treatment adjuvant based on AuNPs has important clinical significance for the treatment of oral cancer patients. This also puts forward higher requirements for oral cancer PTT based on AuNRs. Oral cancer treatment based on AuNPs needs to have targeting function, which can accurately target oral cancer cells and combine with normal cells as little as possible.
Tumor immunotherapy is an effective and vital anti-tumor strategy, which improves prognosis and patient survival. Immunotherapy is a new anti-cancer strategy that recognizes and destroys cancer cells by activating the immune system, thereby preventing the proliferation of cancer cells. However, the controlled regulation of the complex immune system is the key challenge in the implementation of cancer immunotherapy. Immune therapies also exert serious side effects such as autoimmunity and non-specific inflammation [150]. Moreover, conventional immunostimulants cannot target solid tumor tissues [151]. AuNPs have immunoregulatory and drug delivery potential [152]. AuNPs can be transported by the enhanced permeability and retention agents and specifically accumulate in tumor tissues and cells, which is very beneficial for the targeted delivery of immune adjuvants [153]. In addition, compared with other nanomaterials, AuNPs have more significant advantages. First, AuNPs have good plasticity which leads to changes in the distribution, metabolism, and immunogenicity of AuNPs [154]. Secondly, AuNPs can be well surface modified and can be bonded with various functional molecules, such as PEG, folic acid, and so on, through gold-sulfur bonds and other chemical structures. By modifying PEG, RGD, and immune adjuvant simultaneously, AuNPs can improve the performance in many aspects, and comprehensively enhance the effectiveness of immunoreagent targeted delivery and immune system activation [155]. Most importantly, AuNPs have a good photothermal effect, and heat-related signals can stimulate inflammation of tumor tissues and secrete immune factors, to better coordinate with immunotherapy drugs to achieve efficient and low-toxicity anti-tumor immunotherapy [156]. In recent years, several studies have focused on using AuNPs to enhance the efficacy of cancer immunotherapy. He J et al. had summarized the recent research progress of AuNPs-based immunotherapy [152]. AuNPs can be used as good carriers to deliver tumor-related antigens, genes, and antibodies to enhance the effect of immunotherapy by modulating the immune response of the body to tumor cells [152]. Choi BBR et al. developed AuNPs conjugated with PD-L1 as a promising tool for cancer treatment via inducing T cells to enter a resting state by binding to T cell surface antibody PD1 [121]. These PD-L1 conjugated AuNPs induce apoptosis in SCC-25 OSCC cells. However, the author didn't report the immune regulation effect for T cells or OSCCs in this study. AuNPs improve the anti-tumor effect via regulating the tumor immune microenvironment, which can affect tumor progression via regulating dendritic cells (DCs) and macrophages [152,156]. Even though there is a lack of research about the use AuNPs in oral tumor immunotherapy, shreds of literature had shown the immune regulating potential of AuNPs oral cavity. AuNPs prevent 5-fluorouracil (5FU)-induced oral mucositis in hamsters and improve the parameters of inflammation and oxidative stress through the downregulation of inflammation gene expression including TNF-α, IL-1β, COX-2, NF-κB, TGF-β, and SMAD 2/3 [157]. Ni C et al. reported that 45 nm AuNPs can inhibit periodontal inflammation through regulation of macrophage phenotype and expression of inflammatory factors [158]. In the microenvironment of oral tumors, tumor-associated CAFs are the dominant population of stromal cells, whose function is closely related to tumor cell metastasis [[159], [160], [161]]. Ultrafine (3-nm) AuNPs block the communication between CAFs and OSCC tumor cells through inhibit the migration of CAFs and reduce the activety of CAFs [133]. In vivo experiments have confirmed that ultrafine nanoparticles have a good anti-oral tumor effect [133]. Therefore, AuNPs have great research and application prospects in oral cancer treatment via modulating the immune system or microenvironment.
However, compared with other cancer, it has been found in clinical treatment that oral wound infection commonly caused by bacteria during treatment may lead to systemic complications, thus affecting the treatment and recovery of the disease, and even promoting the development of the disease and increased mortality [162]. Therefore, antibacterial therapy is an important challenge to be overcome in the photothermal treatment of oral cancer. An antibacterial platform based on AuNPs is also widely reported. Therefore, in the follow-up study of immunotherapy of oral cancer based on AuNPs, more attention should be paid to the effect of antibacterial properties, to better serve the clinical practice [163]. In addition, there are conflicting results regarding the pharmacokinetics (biological distribution, clearance, and toxicity) of AuNPs [164]. This paradoxical result may be due to differences in the shape, size, and surface chemistry of AuNPs, as well as related research conditions [164]. These conflicting data suggest that clinical use of AuNPs remains a challenge, requiring a clearer understanding of the toxicity, fate, and long-term exposure in vivo of AuNPs.
4.3. AuNPs based combined therapy
A single treatment method is often not effective to treat cancer. A combination of multiple approaches exerts synergistic effects against the tumor and gives better results. In recent years, AuNPs-based combination treatment for cancer had been developed [42,127]. Recent reports from the literature had found that AuNPs-based combination therapies, including radiotherapy, chemotherapy, drug delivery, gene therapy, PTT, or PDT are more effective in oral cancer treatment [42,[127], [128], [129],131,132]. Here, we have reviewed the recently developed AuNPs-based combination therapy for oral cancer treatment.
4.3.1. Combination of PTT and radiotherapy
Nanoradiation sensitization has been a hot topic of cancer therapy in the last decade. Nanoradiation sensitization increases the intracellular radiation deposition through high atomic number (HIGH Z) nanomaterials that improve the treatment efficacy and reduce side effects [165,166]. However, during radiotherapy, tumor recurrence, cancer cell resistance to radiation, and limitations on damage to healthy tissue have led to the need to find new therapies to improve treatment effectiveness [167]. PTT combined with radiotherapy can significantly inhibit tumor growth. At present, studies have proved that combined hyperthermia and radiotherapy have a good synergistic effect [167]. AuNPs are promising radiation sensitizers due to their ultra-small size and strong ability to adsorb, scatter, and re-emit radiation [[168], [169], [170]]. AuNPs increase the radiation sensitivity of tumors and reduce the radiation dose [168]. Folic acid-coupled AuNPs (F-AuNPs) in combination with PTT and radiotherapy had shown cytotoxic and apoptotic effects in oral epidermal cancer cells [42]. A combination of F-AuNPs (20 μM) and X-ray enhances the mortality rate of KB cells by 7-fold compared to normal radiation alone [42]. Gold-coated iron oxide nanoparticles (Au@Fe2O3 NPs) in combination with 6 mv X-ray, and near infrared laser (NIR) treatment of KB cells in human oral epidermal cancer robustly induce cancer cell apoptosis and drop cancer cell survival to 19% [127]. These results indicate higher effectiveness of gold-plated iron oxide nanoparticle-based thermal radiation therapy for oral cancer compared to routine photothermal or radiotherapy.
4.3.2. Combination of PTT and chemotherapy
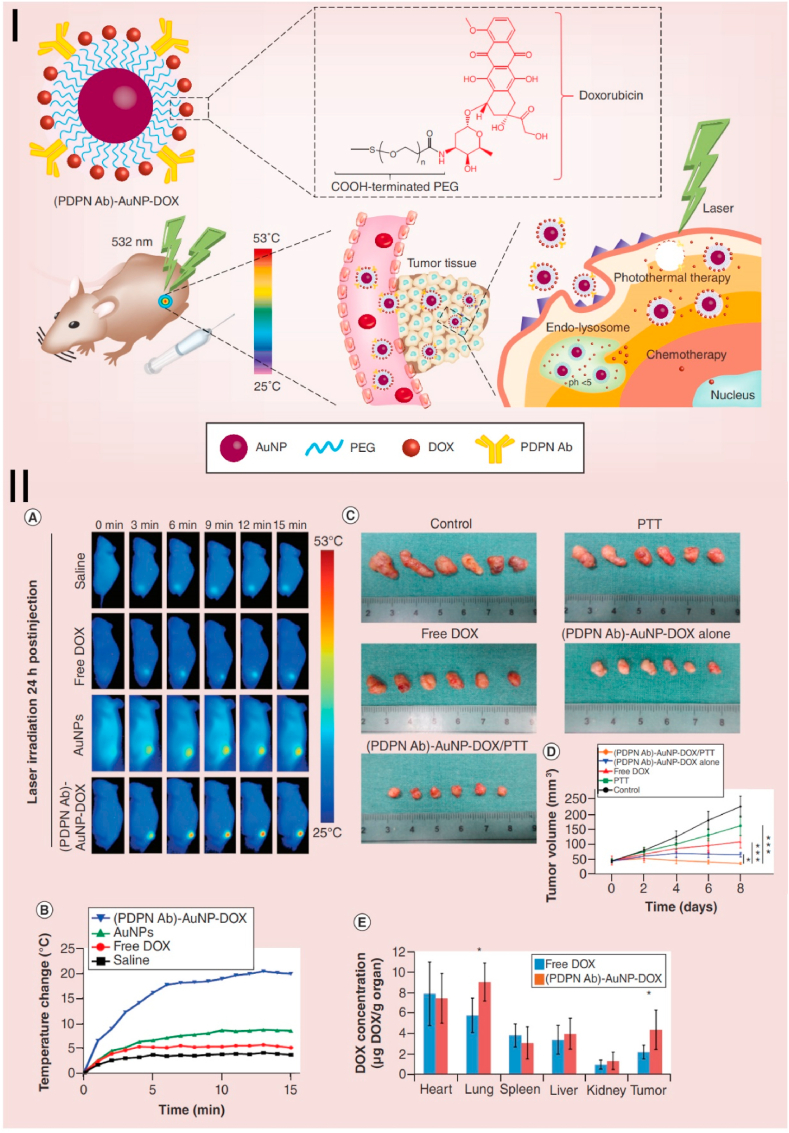
Chemotherapy is one of the three traditional effective treatments for cancer. The use of high-dose chemotherapy drugs is prone to drug resistance and accompanied by greater toxic and side effects [171,172]. These toxic side effects result in the limited efficacy of single chemotherapy. It is noteworthy that the combination of PTT and chemotherapy with nanoplatform has many advantages, such as increasing drug targeting, accumulating chemotherapy drugs at tumor sites, inhibiting DNA repair, controlling drug release, and killing effect on cancer cells [173]. Therefore, combined photothermal therapy and chemotherapy are considered to be effective means to improve the treatment effect and reduce the toxicity and adverse side effects [174,175]. AuNPs are widely studied in combination with PTT and chemotherapy because of their drug delivery and photothermal properties [[176], [177], [178], [179]]. Song W et al. have developed a novel nanocarrier with multi-layered silica combined with AuNFs that showed good photothermal and doxorubicin delivery effects and has a good inhibitory effect on human tongue squamous carcinoma cell line Cal27 [128]. The drug-loading efficiency was 9.78%, and the entrapment efficiency was 62.6% [128]. The amount and rate of drug release directly correlate with the low pH value and intensity of NIR irradiation (808 nm). With NIR laser irradiation, the DOX release rate was much faster in the pH 5.5 solution than in the pH 7.4 solution. 60% of the drug was released after 120 h in the pH 5.5 PBS solution [128]. Liu Z et al. have fabricated a PDPN antibody and doxorubicin conjugated multifunctional AuNPs (PDPN Ab-AuNP-DOX) nanometer system for the combined chemo PTT of oral cancer (Fig. 4) [131]. This system shows low toxicity and high drug loading, cell absorption efficiency, and anti-tumor efficacy in both in vitro and in vivo models. The result of the combination of PDPN Ab-AuNP-DOX with laser irradiation shows an excellent anti-tumor effect [131]. Therefore, gold nanocarrier has the potential for targeted release of various anti-cancer drugs in the tumor milieu and could be a promising tool for photothermal chemotherapy targeting malignant tumors including oral cancer.
Fig. 4.
Combined chemotherapy and PTT based on AuNRs in the human OSCC. Ⅰ. The illustration of the synthesis and chemo-photothermal cancer therapy of PDPN antibody-AuNPs-doxorubicin. Ⅱ. In vivo antitumor effect and biological distribution of PDPN antibody-AuNPs -doxorubicin in a xenograft tumor model. (A) Thermal images of tumors after treatment with saline, free DOX, AuNPs, and (PDPN Ab)-AuNP-DOX, followed by laser irradiation. (B) the temperature curve of mouse tumor after being treated with different groups. (C) The excised solid tumors image and (D) tumor volume changes of different treatment groups. (E) DOX levels were detected in different organs of mice after DOX and (PDPN Ab)-AuNP-DOX were injected into the tail vein at an equivalent DOX dose. Source: Reprinted from Ref. [131] Copyright 2020, with permission from Futuremedicine).
4.3.3. Combination of PTT and PDT
PDT refers to a method of treating diseases using photosensitizer drugs activated by specific wavelengths of visible light [144,180,181]. When the photosensitizer enters the body, it selectively accumulates in the tumor tissue. After the photosensitizer is excited by selective light of the appropriate wavelength, a series of photochemical reactions occur in the tumor tissue to produce some intermediate active substances, mainly reactive oxygen species (ROS) leading to cell death through apoptosis [182]. PDT alone has some limitations, such as the radiation effects of photosensitizers can also have toxic side effects on normal cells [183]. Therefore, the combination of PTT and PDT to improve the therapeutic effect and reduce adverse reactions has become a research hotspot. Bengal Red (RB) is a classical photosensitizer used in PDT [184]. Wang B et al. have developed an RB-conjugated Au-NRs (RB-GNRs)-based photodynamic-photothermal combined treatment system for oral cancer [129]. Under the irradiation of 532 nm laser, the RB-AuNRs complex produces ROS and exerts an anti-cancer effect [129]. At the same time, the AuNRs exert excellent light-to-heat conversion under 810 nm laser irradiation [129]. RB-AuNRs complex effectively damages oral tumor cells in vitro and animal models [129]. After irradiation with 532 nm light for 90 s, the RB-GNRs induce about 13%–73% of Cal-27 cells being killed in a dose-dependent manner at concentrations between 0.5 and 2 nM. After irradiation with 810 nm NIR light for 5 min, RB-GNRs display the highest cytotoxicity (89.1%, p < 0.0001) in Cal-27 cells. After the combined PDT-PTT treatment in hamster cheek pouches model, a 95.5% tumor inhibition rate is achieved on the 10th day. The combined PDT and PTT treatment employing RB-GNRs is thus the most effective modality for oral cancer therapy [129]. Black phosphorus nanosheets (BPNSs) are considered to be a highly efficient photosensitizer due to their unique electronic structure and layer-dependent band [185] and has been used as PDT agent to generate singlet oxygen [186]. Zeng JJ et al. develop a novel composite material of BPNSs and AuNPs for PTT and PDT treatment of OSCC [134]. Their results indicate combination composite of AuNPs−BPNSs loaded with cisplatin could more effectively inhibit the metastasis and growth of OSCC [134]. Combination AuNPs has been demonstrated to enhance the synergistic anti-tumor effects of PTT and PDT [134]. Therefore, AuNPs-based PTD and PTT combination could be effective, minimally invasive, and non-destructive oral cancer therapy strategies.
4.3.4. Combination of PTT and gene therapy
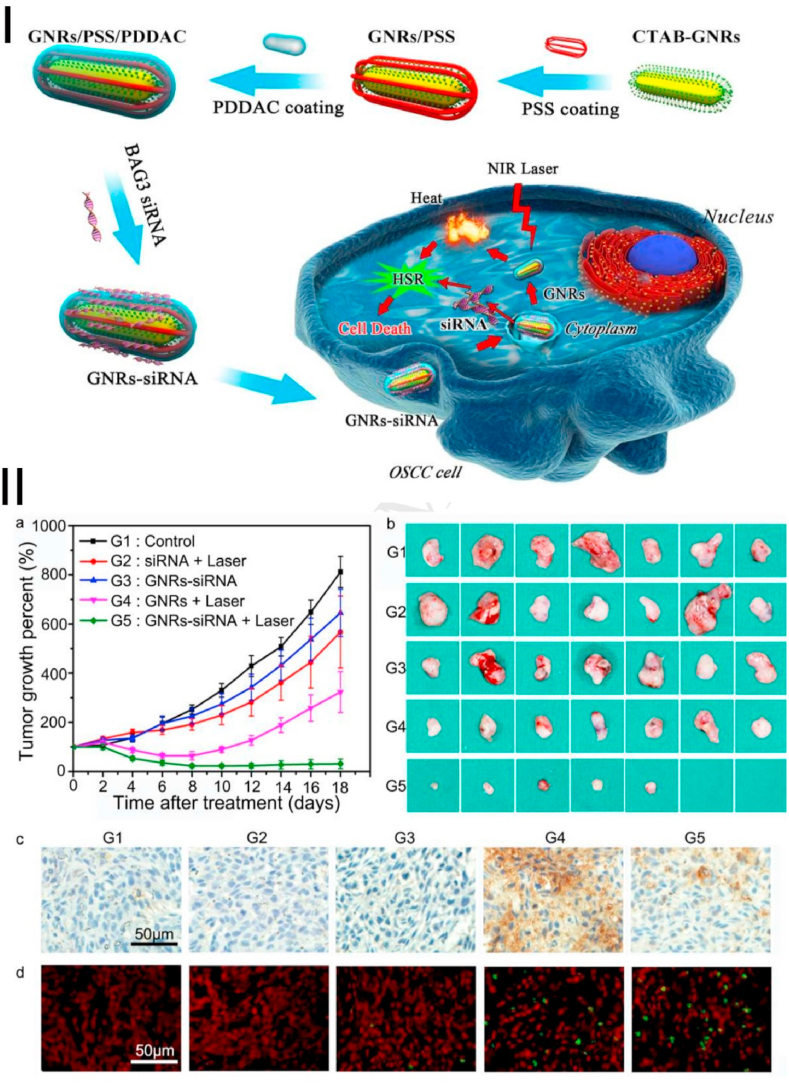
Gene therapy is an emerging tumor therapy strategy that has taken root by introducing exogenous genes into cells to treat genetic or non-genetic diseases. Especially in cancer therapy, more than 100 treatments have entered the stage of clinical research [187,188]. Genetic drugs (RNA and DNA) are extremely easy to degrade in the body, so a series of nano-delivery platforms have been developed to protect nucleic acid molecules in the cell to function [189]. Some of the properties of nanoplatforms themselves, such as photothermal properties, could further enhance the effect of gene therapy. PTT/gene therapy based on AuNPs has been extensively studied and proved to have good antitumor effects [190]. Therefore, the combination of gene therapy and PTT has been widely reported in recent years [[190], [191], [192]]. AuNPs are widely used in combination with PTT and gene therapy research because of their excellent gene delivery performance [45,191,193]. However, the heat shock response in the cellular defense mechanism is activated in the PTT mediated by nanomaterials, which can easily lead to treatment resistance of cancer cells and reduce the treatment effect. Wang B et al. have developed a gene photothermal cooperative therapy platform, which uses AuNRs to deliver BAG3 (Bcl-2 associated athanogene domain 3), a gene that effectively blocks heat shock response, to improve the PTT efficiency (Fig. 5) [130]. This platform improves the effect of PTT by silencing heat shock genes which decreases the “heat resistance” effect of OSCC. Real-time PCR and Western blots showed a significant reduction of BAG3 in CAL-27 cells [130]. This result made CAL-27 cells more sensitive to PTT, which leads to more cell apoptosis under NIR (810 nm) light irradiation [130]. However, the biosafety of AuNRs-siRNA was not evaluated in this study.
Fig. 5.
Combined gene therapy and photothermal therapy PTT based on AuNRs in the human OSCC. Ⅰ. Schematic design of the preparation of Au NRs-siRNA and application in oral cancer PTT therapy. Ⅱ. Au NRs-siRNA inhibited tumor growth in the xenograft model after irradiation by laser. (a) Average percentage growth of tumors in different groups. (b) The excised solid tumors image from the different groups after treatment 18 days. The expression of BAG3 (c) and apoptotic cells (d) is determined by immunochemistry and TUNEL assay after 24 h treated with different groups. Source: Reprinted from Ref. [130] Copyright 2016, with permission from Elsevier).
4.3.5. Multimodal combination therapy
Multimodal combination therapy has become a new hotspot in oncology clinical research because of its potential synergistic effect [194,195]. Multimodal combination therapy can achieve a better response in a shorter period than one or two treatments alone, thus reducing the incidence of drug resistance [196]. In our previous study, we reported AuNRs were synthesized in the cavity of hollow silica nanoparticles to construct rattle-structured rough nanocapsules, which were used for complementary gene/chemo/photo therapy and play a high-efficiency anti-tumor effect [45]. Multimodal combination therapy has also been extensively studied in oral tumors [[197], [198], [199]]. AuNPs have become a promising platform for the diagnosis and treatment of nanomedicine with multiple therapeutic and diagnostic functions [[200], [201], [202], [203]]. Alamzadeh Z et al. proposed an alginate hydrogel, cisplatin, and AuNPs multifunctional nanoplatforms for thermo-chemo-radiotherapy [132]. This system uses a combination of PTT (532 nm laser), chemotherapy, and radiotherapy (6 mv x Radiation) for oral cancer therapy. This combo therapy reduced human mouth epidermal carcinoma(KB)cell viability to 17%, increases the intracellular reactive oxygen species level by 4.4-fold, and upregulates Bax (pro-apoptotic) expression by 4.5-fold. Results showed the synergistic effect of alginate hydrogel, cisplatin, and AuNPs to produce powerful anti-cancer effects [132]. However, the in vivo anti-OSCC therapeutic efficacy of this multimodel combination therapy should be further evaluated.
4.4. Imaging-guided cancer therapy
In recent years, accurate and effective imaging-guided tumor therapies have attracted extensive attention in the field of nanomedicine [[204], [205], [206], [207], [208]]. AuNPs integrate imaging and therapeutic functions simultaneously, making it possible to construct image-guided therapies [[209], [210], [211]]. AuNPs have unique optical properties, which can enhance the scattering and absorption of tissue signals. By targeting tumor cells, the signal contrast between tumor cells and normal cells can be improved so that tumor cells can be observed under a microscope. According to this imaging principle, Huang XH et al. have coupled AuNRs with monoclonal anti-EGFR (AuNRs-EGFR) [212]. Under microscope observation, the AuNRs-EGFR complex distinguishes normal cells from malignant oral cancer cells. At the same time, the laser-generated heat effectively kills cancer cells. This theranostic platform combined with imaging and treatment ability simultaneously achieves effective oral cancer cell diagnosis and selective PTT [212]. In addition, the combination of other imaging technologies, such as MRI and computed tomography, can better realize image-guided tumor treatment [213]. MRI is an effective way to provide image guidance for particle-mediated thermal ablation because the fine soft-tissue contrast of MRI makes it possible to identify lesions and key structures for planning and targeting therapy [213]. Superparamagnetic Iron Oxide (SPIO) nanoparticles are a good MRI sensitizer and can provide real-time temperature-sensitive feedback during therapy [214]. In oral cancer research, Melancon MP et al. reported that the multifunctional superparamagnetic iron oxide coated with gold nanoshells can simultaneously visualize and treat oral cancer by selective ablation [215]. This theranostic approach not only increases the efficacy of thermal ablation but also reduces thermal damage to surrounding normal tissues that improve the safety and effectiveness of oral cancer treatment.
4.5. Clinical trials and applications
Very few FDA-approved clinical trials of AuNPs are being carried out for cancer theranostic applications. CYT-6091 is the first AuNPs-based cancer therapy that reached early-phase clinical trials in 2005 [216]. This citrate-coated 27 nm AuNPs bound with thiolated PEG and TNF-a (Aurimmune; CytImmune Sciences, Rockville, MD) showed tumor targeting and tumor toxicity dual effects. Controllable fever was the only side effect observed in the Phase I trial enrolling 29 patients with solid cancers unresponsive to conventional therapies. A clinical trial of CD24-Gold Nanocomposite expression using Real-time quantitative polymerase chain reaction is going to be conducted in Egypt for diagnostic and prognostic accuracy of AuNPs in salivary gland tumors (NCT04907422) [217]. We did not find FDA-approved clinical trials of AuNPs for oral cancer theranostic applications in the literature.
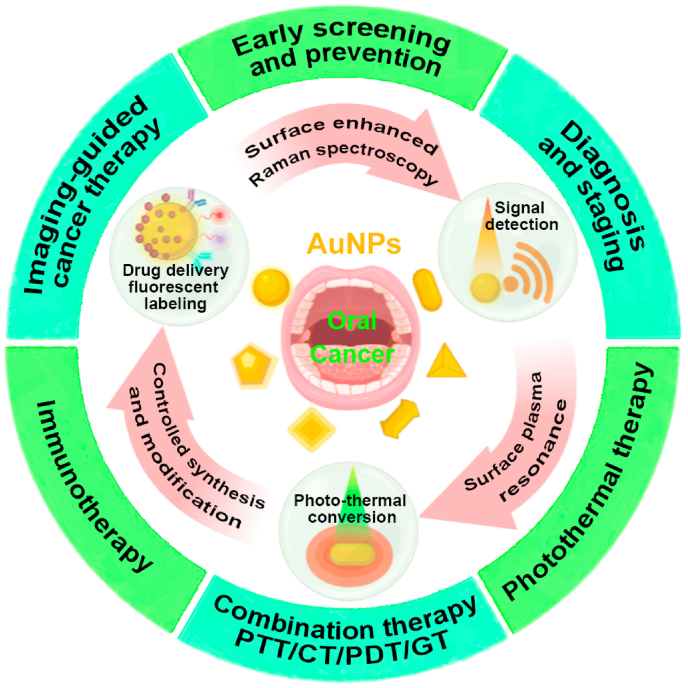
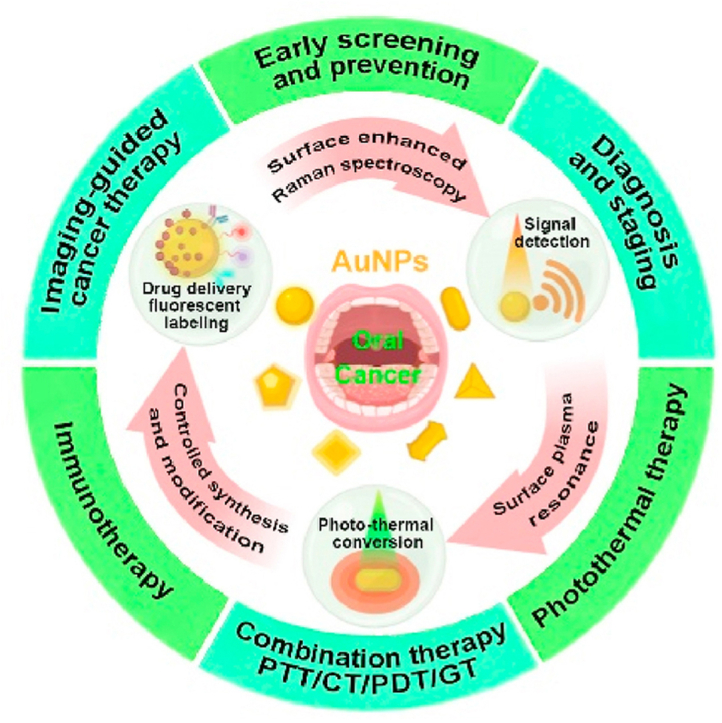
5. Summary, challenges, and prospects
In the last decade, a variety of AuNPs had been fabricated for oral cancer diagnosis and therapy applications and their efficacy had been widely tested in vitro and in animal studies. Fig. 6 summarizes the possible potentials of AuNPs for diagnostic and therapeutic applications in oral cancer. AuNPs showed the promising potential for the application in oral cancer early diagnosis and detection of tumor staging. Similarly, literature had well-reported AuNPs applications in drug delivery, PTT, and combination therapies such as PTT + radiotherapy, PTT + chemotherapy, PTT + gene therapy, and PTT + PDT for oral cancer. AuNPs also showed efficacy in multimodal combination therapy and image-guided cancer therapy of oral cancer. However, AuNPs-based immunotherapies had been developed and their efficacy had been tested in other cancers but not in oral cancer. AuNPs-based oral cancer theranostics had not entered the clinical trials and clinical applications yet. The advances in AuNPs development technologies and promising results of AuNPs for oral cancer diagnosis and therapy from in vitro and animal studies indicate the possible clinical application potential of AuNPs in oral cancer theranostics in near future.
Fig. 6.
Scheme of AuNPs for diagnostic and therapeutic applications in oral cancer. CT: chemotherapy, GT: gene therapy.
Although AuNPs show great potential in the diagnosis and treatment of tumors, biosafety remains an important challenge that needs to be addressed. Although many studies have found that AuNPs have good biocompatibility and in vivo safety, some studies have found that AuNPs are toxic in vivo. These contradictory results may be due to the different synthesis methods of AuNPs, or the different morphology, size, and structure of AuNPs. Therefore, the biosafety problems caused by various properties of AuNPs need to be addressed before possible clinical trials.
AuNPs have tremendous potential for application in oral cancer such as early screening, diagnostic typing, drug delivery, PTT, radiotherapy, combined therapy, and theranostics. The research on the application of AuNPs in oral cancer diagnosis and therapy is in a very early stage. Further research works are mandatory to improve biological safety and minimize the short and long-term systemic adverse effects of AuNPs to facilitate the preclinical and clinical trials in oral cancer diagnosis and treatment. Based on the recent advances in AuNPs-related research in cancer theranostics, the possibility of preclinical and clinical application in oral cancer diagnosis and therapy is very considerable in near future.
Author statement
Qing Zhang: Conceptualization, Investigation, Writing Original Draft and Review & Editing, Funding acquisition. Dan Hou: Writing Original Draft and Review & Editing. Xueying Wen: Writing Original Draft, Visualization. Mengyu Xin: Investigation. Ziling Li: Investigation. Lihong Wu: Conceptualization, Funding acquisition. Janak L. Pathak: Conceptualization, Writing Review & Editing, Supervision, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was funded by the National Natural Science Foundation of China (31801152 and 82150410451), International Science and Technology Innovation Cooperation Program of the State Key Research and Development Program (2021YFE0108000), Health Science and Technology Project of Guangzhou City (Grant No. 20202A011026), High-level University Construction Funding of Guangzhou Medical University (02-412-B205002-1003017 and 06-410-2106035), and University Student Laboratory Open Project of Guangzhou Medical University (2020–27).
Contributor Information
Lihong Wu, Email: wcanhong@163.com.
Janak L. Pathak, Email: j.pathak@gzhmu.edu.cn.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Miranda-Filho A., Bray F. Global patterns and trends in cancers of the lip, tongue and mouth. Oral Oncol. 2020;102 doi: 10.1016/j.oraloncology.2019.104551. [DOI] [PubMed] [Google Scholar]
- 3.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 4.Varela-Centelles P., Estany-Gestal A., Bugarin-Gonzalez R., Seoane-Romero J.M. Oral cancer awareness in Spain: a pilot study. Oral Dis. 2018;24(1–2):124–127. doi: 10.1111/odi.12756. [DOI] [PubMed] [Google Scholar]
- 5.Chen X.J., Zhang X.Q., Liu Q., Zhang J., Zhou G. Nanotechnology: a promising method for oral cancer detection and diagnosis. J. Nanobiotechnol. 2018;16 doi: 10.1186/s12951-018-0378-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Popovtzer R., Agrawal A., Kotov N.A., Popovtzer A., Balter J., Carey T.E., Kopelman R. Targeted gold nanoparticles enable molecular CT imaging of cancer. Nano Lett. 2008;8(12):4593–4596. doi: 10.1021/nl8029114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kah J.C.Y., Kho K.W., Lee C.G.L., Sheppard C.J.R., Shen Z.X., Soo K.C., Olivo M.C. Early diagnosis of oral cancer based on the surface plasmon resonance of gold nanoparticles. Int. J. Nanomed. 2007;2(4):785–798. [PMC free article] [PubMed] [Google Scholar]
- 8.Reddy P.S., Ramaswamy P., Sunanda C. Role of gold nanoparticles in early detection of oral cancer. J. Indian Acad. Oral Med. Radiol. 2010;22(1):30. [Google Scholar]
- 9.Wong T., Wiesenfeld D. Oral cancer. Aust. Dent. J. 2018;63(Suppl 1):S91–S99. doi: 10.1111/adj.12594. [DOI] [PubMed] [Google Scholar]
- 10.Gaetti-Jardim E., Jr., Jardim E.C.G., Schweitzer C.M., da Silva J.C.L., Oliveira M.M., Masocatto D.C., Dos Santos C.M. Supragingival and subgingival microbiota from patients with poor oral hygiene submitted to radiotherapy for head and neck cancer treatment. Arch. Oral Biol. 2018;90:45–52. doi: 10.1016/j.archoralbio.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Elad S., Zadik Y. Chronic oral mucositis after radiotherapy to the head and neck: a new insight, Support. Care Cancer. 2016;24(11):4825–4830. doi: 10.1007/s00520-016-3337-5. [DOI] [PubMed] [Google Scholar]
- 12.Sugiyama S., Iwai T., Oguri S., Koizumi T., Mitsudo K., Tohnai I. Facial nerve paralysis after super-selective intra-arterial chemotherapy for oral cancer. Int. J. Oral Maxillofac. Surg. 2017;46(6):682–686. doi: 10.1016/j.ijom.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal P., Shiva Kumar H.R., Rai K.K. Trismus in oral cancer patients undergoing surgery and radiotherapy. J Oral Biol Craniofac Res. 2016;6(Suppl 1):S9–S13. doi: 10.1016/j.jobcr.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Owosho A.A., Tsai C.J., Lee R.S., Freymiller H., Kadempour A., Varthis S., Sax A.Z., Rosen E.B., Yom S.K., Randazzo J., Drill E., Riedel E., Patel S., Lee N.Y., Huryn J.M., Estilo C.L. The prevalence and risk factors associated with osteoradionecrosis of the jaw in oral and oropharyngeal cancer patients treated with intensity-modulated radiation therapy (IMRT): the Memorial Sloan Kettering Cancer Center experience. Oral Oncol. 2017;64:44–51. doi: 10.1016/j.oraloncology.2016.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belusic-Gobic M., Zubovic A., Predrijevac A., Harmicar D., Cerovic R., Udovic Gobic S., Zubovic L. Microbiology of wound infection after oral cancer surgery. J. Cranio-Maxillo-Fac. Surg. 2020;48(7):700–705. doi: 10.1016/j.jcms.2020.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Kitamoto S., Nagao-Kitamoto H., Hein R., Schmidt T.M., Kamada N. The bacterial connection between the oral cavity and the gut diseases. J. Dent. Res. 2020;99(9):1021–1029. doi: 10.1177/0022034520924633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caselli E., Fabbri C., D'Accolti M., Soffritti I., Bassi C., Mazzacane S., Franchi M. Defining the oral microbiome by whole-genome sequencing and resistome analysis: the complexity of the healthy picture. BMC Microbiol. 2020;20(1):120. doi: 10.1186/s12866-020-01801-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beik J., Khademi S., Attaran N., Sarkar S., Shakeri-Zadeh A., Ghaznavi H., Ghadiri H. A nanotechnology-based strategy to increase the efficiency of cancer diagnosis and therapy: folate-conjugated gold nanoparticles. Curr. Med. Chem. 2017;24(39):4399–4416. doi: 10.2174/0929867324666170810154917. [DOI] [PubMed] [Google Scholar]
- 19.Chen X.Y., Zhang Q., Li J.L., Yang M., Zhao N.N., Xu F.J. Rattle-structured rough nanocapsules with in-situ-formed reil gold nanorod cores for complementary gene/chemo/photothermal therapy. ACS Nano. 2018;12(6):5646–5656. doi: 10.1021/acsnano.8b01440. [DOI] [PubMed] [Google Scholar]
- 20.Song W.T., Anselmo A.C., Huang L. Nanotechnology intervention of the microbiome for cancer therapy. Nat. Nanotechnol. 2019;14(12):1093–1103. doi: 10.1038/s41565-019-0589-5. [DOI] [PubMed] [Google Scholar]
- 21.Zhao N.N., Yan L.M., Zhao X.Y., Chen X.Y., Li A.H., Zheng D., Zhou X., Dai X.G., Xu F.J. Versatile types of organic/inorganic nanohybrids: from strategic design to biomedical applications. Chem. Rev. 2019;119(3):1666–1762. doi: 10.1021/acs.chemrev.8b00401. [DOI] [PubMed] [Google Scholar]
- 22.Goldberg M.S. Improving cancer immunotherapy through nanotechnology. Nat. Rev. Cancer. 2019;19(10):587–602. doi: 10.1038/s41568-019-0186-9. [DOI] [PubMed] [Google Scholar]
- 23.Pricker S.P. Medical uses of gold compounds: past, present and future. Gold Bull. 1996;29(2):53–60. [Google Scholar]
- 24.Faulk W.P., Taylor G.M. Communication to the editors: an immunocolloid method for the electron microscope. J Immunochemistry. 1971;8(11):1081–1083. doi: 10.1016/0019-2791(71)90496-4. [DOI] [PubMed] [Google Scholar]
- 25.Dong Y.C., Hajfathalian M., Maidment P.S.N., Hsu J.C., Naha P.C., Si-Mohamed S., Breuilly M., Kim J., Chhour P., Douek P., Litt H.I., Cormode D.P. Effect of gold nanoparticle size on their properties as contrast agents for computed tomography. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-50332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimm M.A., Shevtsov M., Werner C., Sievert W., Zhiyuan W., Schoppe O., Menze B.H., Rummeny E.J., Proksa R., Bystrova O., Martynova M., Multhoff G., Stangl S. Gold nanoparticle mediated multi-modal CT imaging of Hsp70 membrane-positive tumors. Cancers. 2020;12(5) doi: 10.3390/cancers12051331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashton J.R., Castle K.D., Qi Y., Kirsch D.G., West J.L., Badea C.T. Dual-energy CT imaging of tumor liposome delivery after gold nanoparticle-augmented radiation therapy. Theranostics. 2018;8(7):1782–1797. doi: 10.7150/thno.22621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y.J., Li S.B., Duan S., Lu B.A., Yang J., Panneerselvam R., Li C.Y., Fang P.P., Zhou Z.Y., Phillips D.L., Li J.F., Tian Z.Q. Probing the electronic structure of heterogeneous metal interfaces by transition metal shelled gold nanoparticle-enhanced Raman spectroscopy. J. Phys. Chem. C. 2016;120(37):20684–20691. [Google Scholar]
- 29.Song J.B., Wang F., Yang X.Y., Ning B., Harp M.G., Culp S.H., Hu S., Huang P., Nie L.M., Chen J.Y., Chen X.Y. Gold nanoparticle coated carbon nanotube ring with enhanced Raman scattering and photothermal conversion property for theranostic applications. J. Am. Chem. Soc. 2016;138(22):7005–7015. doi: 10.1021/jacs.5b13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y.L., Kangas J., Wang Y.R., Khosla K., Pasek-Allen J., Saunders A., Oldenburg S., Bischof J. Photothermal conversion of gold nanoparticles for uniform pulsed laser warming of vitrified biomaterials. Nanoscale. 2020;12(23):12346–12356. doi: 10.1039/d0nr01614d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tódor I.S., Marişca O.T., Rugină D., Diaconeasa Z., Leopold L.F., Coman C., Antonescu E., Szabó L., Iancu S.D., Bálint Z. Photothermal property assessment of gold nanoparticle assemblies obtained by hydroxylamine reduction. Colloid Polym. Sci. 2020;298(10):1369–1377. [Google Scholar]
- 32.Lopes T.S., Alves G.G., Pereira M.R., Granjeiro J.M., Leite P.E.C. Advances and potential application of gold nanoparticles in nanomedicine. J. Cell. Biochem. 2019;120(10):16370–16378. doi: 10.1002/jcb.29044. [DOI] [PubMed] [Google Scholar]
- 33.Hu X., Zhang Y., Ding T., Liu J., Zhao H. Multifunctional gold nanoparticles: a novel nanomaterial for various medical applications and biological activities. Frontiers in Bioengineering Biotechnology advances. 2020;8:990. doi: 10.3389/fbioe.2020.00990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ge X., Fu Q., Su L., Li Z., Zhang W., Chen T., Yang H., Song J. Light-activated gold nanorod vesicles with NIR-II fluorescence and photoacoustic imaging performances for cancer theranostics. Theranostics. 2020;10(11):4809. doi: 10.7150/thno.44376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El-Sayed I.H., Huang X., El-Sayed M.A. Surface plasmon resonance scattering and absorption of anti-EGFR antibody conjugated gold nanoparticles in cancer diagnostics: applications in oral cancer. Nano Lett. 2005;5(5):829–834. doi: 10.1021/nl050074e. [DOI] [PubMed] [Google Scholar]
- 36.Yang W., Liang H., Ma S., Wang D., Huang J, Gold nanoparticle based photothermal therapy: development and application for effective cancer treatment. Sustainable Materials and Technologies. 2019;22 [Google Scholar]
- 37.Slaughter D.P., Southwick H.W., Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6(5):963–968. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 38.Morikawa T., Bessho H., Nomura T., Kozakai A., Kosugi A., Shibahara T. Setting of the surgical margin using optical instrument for treatment of early tongue squamous cell carcinoma. J Oral Maxillofac Surg Med Pathol. 2019;31(1):8–12. [Google Scholar]
- 39.Chakraborty D., Viveka T.S., Arvind K., Shyamsundar V., Kanchan M., Alex S.A., Chandrasekaran N., Vijayalakshmi R., Mukherjee A. A facile gold nanoparticle-based ELISA system for detection of osteopontin in saliva: towards oral cancer diagnostics. Clin. Chim. Acta. 2018;477:166–172. doi: 10.1016/j.cca.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 40.Hirshberg A., Allon I., Novikov I., Ankri R., Ashkenazy A., Fixler D. Gold nanorods reflectance discriminate benign from malignant oral lesions. Nanomedicine. 2017;13(4):1333–1339. doi: 10.1016/j.nano.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 41.Yan E., Cao M., Wang Y., Hao X., Pei S., Gao J., Wang Y., Zhang Z., Zhang D. Gold nanorods contained polyvinyl alcohol/chitosan nanofiber matrix for cell imaging and drug delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2016;58:1090–1097. doi: 10.1016/j.msec.2015.09.080. [DOI] [PubMed] [Google Scholar]
- 42.Neshastehriz A., Tabei M., Maleki S., Eynali S., Shakeri-Zadeh A. Photothermal therapy using folate conjugated gold nanoparticles enhances the effects of 6MV X-ray on mouth epidermal carcinoma cells. J. Photochem. Photobiol., B. 2017;172:52–60. doi: 10.1016/j.jphotobiol.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 43.Norouzi H., Khoshgard K., Akbarzadeh F. In vitro outlook of gold nanoparticles in photo-thermal therapy: a literature review. Laser Med. Sci. 2018;33(4):917–926. doi: 10.1007/s10103-018-2467-z. [DOI] [PubMed] [Google Scholar]
- 44.Sun L., Wang X., Gong F., Yin K., Zhu W., Yang N., Bai S., Liao F., Shao M., Cheng L. Silicon nanowires decorated with platinum nanoparticles were applied for photothermal-enhanced sonodynamic therapy. Theranostics. 2021;11(19):9234. doi: 10.7150/thno.58755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen X., Zhang Q., Li J., Yang M., Zhao N., Xu F.-J. Rattle-structured rough nanocapsules with in-situ-formed gold nanorod cores for complementary gene/chemo/photothermal therapy. ACS Nano. 2018;12(6):5646–5656. doi: 10.1021/acsnano.8b01440. [DOI] [PubMed] [Google Scholar]
- 46.Zhu X., Shi J., Ma H., Chen R., Li J., Cao S. Hierarchical hydroxyapatite/polyelectrolyte microcapsules capped with AuNRs for remotely triggered drug delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;99:1236–1245. doi: 10.1016/j.msec.2019.02.078. [DOI] [PubMed] [Google Scholar]
- 47.Kim J.E., Choi J.H., Colas M., Kim D.H., Lee H. Gold-based hybrid nanomaterials for biosensing and molecular diagnostic applications. Biosens. Bioelectron. 2016;80:543–559. doi: 10.1016/j.bios.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 48.Yadid M., Feiner R., Dvir T. Gold nanoparticle-integrated scaffolds for tissue engineering and regenerative medicine. Nano Lett. 2019;19(4):2198–2206. doi: 10.1021/acs.nanolett.9b00472. [DOI] [PubMed] [Google Scholar]
- 49.Gonzalez-Rubio G., de Oliveira T.M., Altantzis T., La Porta A., Guerrero-Martinez A., Bals S., Scarabelli L., Liz-Marzan L.M. Disentangling the effect of seed size and crystal habit on gold nanoparticle seeded growth. Chem. Commun. 2017;53(82):11360–11363. doi: 10.1039/c7cc06854a. [DOI] [PubMed] [Google Scholar]
- 50.Chen Y.S., Zhao Y., Yoon S.J., Gambhir S.S., Emelianov S. Miniature gold nanorods for photoacoustic molecular imaging in the second near-infrared optical window. Nat. Nanotechnol. 2019;14(5):465–472. doi: 10.1038/s41565-019-0392-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fan Z., Bosman M., Huang Z., Chen Y., Ling C., Wu L., Akimov Y.A., Laskowski R., Chen B., Ercius P. Heterophase fcc-2H-fcc gold nanorods. Nat. Commun. 2020;11(1):1–8. doi: 10.1038/s41467-020-17068-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu F., Liu Y., Wu Y., Song D., Qian J., Zhu B. Chlorin e6 and polydopamine modified gold nanoflowers for combined photothermal and photodynamic therapy. J. Mater. Chem. B. 2020;8(10):2128–2138. doi: 10.1039/c9tb02646k. [DOI] [PubMed] [Google Scholar]
- 53.Feng Y., Cheng Y., Chang Y., Jian H., Zheng R., Wu X., Xu K., Wang L., Ma X., Li X., Zhang H. Time-staggered delivery of erlotinib and doxorubicin by gold nanocages with two smart polymers for reprogrammable release and synergistic with photothermal therapy. Biomaterials. 2019;217 doi: 10.1016/j.biomaterials.2019.119327. [DOI] [PubMed] [Google Scholar]
- 54.Xu X., Chong Y., Liu X., Fu H., Yu C., Huang J., Zhang Z. Multifunctional nanotheranostic gold nanocages for photoacoustic imaging guided radio/photodynamic/photothermal synergistic therapy. Acta Biomater. 2019;84:328–338. doi: 10.1016/j.actbio.2018.11.043. [DOI] [PubMed] [Google Scholar]
- 55.Park S., Lee W.J., Park S., Choi D., Kim S., Park N. Reversibly pH-responsive gold nanoparticles and their applications for photothermal cancer therapy. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-56754-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang X., El-Sayed M.A. Gold nanoparticles: optical properties and implementations in cancer diagnosis and photothermal therapy. J. Adv. Res. 2010;1(1):13–28. [Google Scholar]
- 57.Zhang Y., Qiu Y., Lin L., Gu H., Xiao Z., Ye J. Ultraphotostable mesoporous silica-coated gap-enhanced Raman tags (GERTs) for high-speed bioimaging. ACS Appl. Mater. Interfaces. 2017;9(4):3995–4005. doi: 10.1021/acsami.6b15170. [DOI] [PubMed] [Google Scholar]
- 58.Khlebtsov N.G., Lin L., Khlebtsov B.N., Ye J. Gap-enhanced Raman tags: fabrication, optical properties, and theranostic applications. Theranostics. 2020;10(5):2067. doi: 10.7150/thno.39968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao X., Pan J., Li W., Yang W., Qin L., Pan Y. Gold nanoparticles enhance cisplatin delivery and potentiate chemotherapy by decompressing colorectal cancer vessels. Int. J. Nanomed. 2018;13:6207–6221. doi: 10.2147/IJN.S176928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu W., Qian J., Hou G., Wang Y., Wang J., Sun T., Ji L., Suo A., Yao Y. PEGylated hydrazided gold nanorods for pH-triggered chemo/photodynamic/photothermal triple therapy of breast cancer. Acta Biomater. 2018;82:171–183. doi: 10.1016/j.actbio.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 61.Singh P., Pandit S., Mokkapati V., Garg A., Ravikumar V. I. Mijakovic, gold nanoparticles in diagnostics and therapeutics for human cancer. Int. J. Mol. Sci. 2018;19(7) doi: 10.3390/ijms19071979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kubota T., Kuroda S., Kanaya N., Morihiro T., Aoyama K., Kakiuchi Y., Kikuchi S., Nishizaki M., Kagawa S., Tazawa H., Fujiwara T. HER2-targeted gold nanoparticles potentially overcome resistance to trastuzumab in gastric cancer. Nanomedicine. 2018;14(6):1919–1929. doi: 10.1016/j.nano.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 63.Tian Y., Qiang S., Wang L. Gold nanomaterials for imaging-guided near-infrared in vivo cancer therapy. Front. Bioeng. Biotechnol. 2019;7:398. doi: 10.3389/fbioe.2019.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yan P., Wang R., Zhao N., Zhao H., Chen D.-F., Xu F.-J. Polycation-functionalized gold nanoparticles with different morphologies for superior gene transfection. Nanoscale. 2015;7(12):5281–5291. doi: 10.1039/c5nr00481k. [DOI] [PubMed] [Google Scholar]
- 65.Feng Y., Chang Y., Sun X., Cheng Y., Zheng R., Wu X., Wang L., Ma X., Li X., Zhang H. Differential photothermal and photodynamic performance behaviors of gold nanorods, nanoshells and nanocages under identical energy conditions. Biomater. Sci. 2019;7(4):1448–1462. doi: 10.1039/c8bm01122b. [DOI] [PubMed] [Google Scholar]
- 66.De Souza C.D., Nogueira B.R., Rostelato M.E.C. Review of the methodologies used in the synthesis gold nanoparticles by chemical reduction. J. Alloys Compd. 2019;798:714–740. [Google Scholar]
- 67.Ma H., Yin B., Wang S., Jiao Y., Pan W., Huang S., Chen S., Meng F. Synthesis of silver and gold nanoparticles by a novel electrochemical method. ChemPhysChem. 2004;5(1):68–75. doi: 10.1002/cphc.200300900. [DOI] [PubMed] [Google Scholar]
- 68.Fuentes-García J., Santoyo-Salzar J., Rangel-Cortes E., Goya G.F., Cardozo-Mata V., Pescador-Rojas J.A. Effect of ultrasonic irradiation power on sonochemical synthesis of gold nanoparticles. Ultrason. Sonochem. 2021;70 doi: 10.1016/j.ultsonch.2020.105274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ahmed S., Ikram S. Biosynthesis of gold nanoparticles: a green approach. J. Photochem. Photobiol. B Biol. 2016;161:141–153. doi: 10.1016/j.jphotobiol.2016.04.034. [DOI] [PubMed] [Google Scholar]
- 70.Herizchi R., Abbasi E., Milani M., Akbarzadeh A. Current methods for synthesis of gold nanoparticles. Artif. Cell Nanomed. Biotechnol. 2016;44(2):596–602. doi: 10.3109/21691401.2014.971807. [DOI] [PubMed] [Google Scholar]
- 71.Booth S.G., Uehara A., Chang S.-Y., La Fontaine C., Fujii T., Okamoto Y., Imai T., Schroeder S.L., Dryfe R. The significance of bromide in the Brust–Schiffrin synthesis of thiol protected gold nanoparticles. Chem. Sci. 2017;8(12):7954–7962. doi: 10.1039/c7sc03266h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wei M.-Z., Deng T.-S., Zhang Q., Cheng Z., Li S. Seed-mediated synthesis of gold nanorods at low concentrations of CTAB. ACS Omega. 2021;6(13):9188–9195. doi: 10.1021/acsomega.1c00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ibrahim K.E., Al-Mutary M.G., Bakhiet A.O., Khan H.A. Histopathology of the liver, kidney, and spleen of mice exposed to gold nanoparticles. Molecules. 2018;23(8):1848. doi: 10.3390/molecules23081848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee K.X., Shameli K., Yew Y.P., Teow S.-Y., Jahangirian H., Rafiee-Moghaddam R., Webster T.J. Recent developments in the facile bio-synthesis of gold nanoparticles (AuNPs) and their biomedical applications. Int. J. Nanomed. 2020;15:275. doi: 10.2147/IJN.S233789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kalimuthu K., Cha B.S., Kim S., Park K.S. Eco-friendly synthesis and biomedical applications of gold nanoparticles: a review. Microchem. J. 2020;152 [Google Scholar]
- 76.Cicciu M., Cervino G., Fiorillo L., D'Amico C., Oteri G., Troiano G., Zhurakivska K., Lo Muzio L., Herford A.S., Crimi S., Bianchi A., Di Stasio D., Rullo R., Laino G., Laino L. Early diagnosis on oral and potentially oral malignant lesions: a systematic review on the VELscope((R)) fluorescence method. Dent. J. 2019;7(3) doi: 10.3390/dj7030093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gigliotti J., Madathil S., Makhoul N. Delays in oral cavity cancer. Int. J. Oral Maxillofac. Surg. 2019;48(9):1131–1137. doi: 10.1016/j.ijom.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 78.Zhou W., Gao X., Liu D., Chen X. Gold nanoparticles for in vitro diagnostics. Chem. Rev. 2015;115(19):10575–10636. doi: 10.1021/acs.chemrev.5b00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Williams J. Oral cancer and precancer: clinical features. Br. Dent. J. 1990;168(1):13–17. doi: 10.1038/sj.bdj.4807061. [DOI] [PubMed] [Google Scholar]
- 80.Fălămaș A., Rotaru H., Hedeșiu M. Surface-enhanced Raman spectroscopy (SERS) investigations of saliva for oral cancer diagnosis. Laser Med. Sci. 2020;35(6) doi: 10.1007/s10103-020-02988-2. [DOI] [PubMed] [Google Scholar]
- 81.P. Panta, D.T. Wong, Saliva-based point-Of-Care in Oral Cancer Detection: Current Trend and Future Opportunities, Oral Cancer Detection, Springer2019, pp. 297-314.
- 82.He W., You M., Wan W., Xu F., Li F., Li A. Point-of-care periodontitis testing: biomarkers, current technologies, and perspectives. Trends Biotechnol. 2018;36(11):1127–1144. doi: 10.1016/j.tibtech.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 83.He W., You M., Li Z., Cao L., Xu F., Li F., Li A. Upconversion nanoparticles-based lateral flow immunoassay for point-of-care diagnosis of periodontitis. Sensor. Actuator. B Chem. 2021;334 [Google Scholar]
- 84.Subramani V.N., Narasimhan M., Thiyagarajan M., Munuswamy B.D., Jayamani L. Expression of osteopontin in oral squamous cell carcinoma and its surgical margins-an immunohistochemical study. J. Clin. Diagn. Res. 2015;9(11):ZC66. doi: 10.7860/JCDR/2015/12777.6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Khurshid Z., Zafar M.S., Khan R.S., Najeeb S., Slowey P.D., Rehman I.U. Role of salivary biomarkers in oral cancer detection. Adv. Clin. Chem. 2018;86:23–70. doi: 10.1016/bs.acc.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 86.Mukama O., Wu W., Wu J., Lu X., Liu Y., Liu Y., Liu J., Zeng L. A highly sensitive and specific lateral flow aptasensor for the detection of human osteopontin. Talanta. 2020;210 doi: 10.1016/j.talanta.2019.120624. [DOI] [PubMed] [Google Scholar]
- 87.Kumar N., Gowri V., Khan R., Ranjan P., Sadique M. Efficiency of nanomaterials for electrochemical diagnostics based point-of-care detection of non-Invasive oral cancer biomarkers. Adv. Mater. Lett. 2021;12(8):1–20. [Google Scholar]
- 88.Li Y., Cao X., Li H. Identification and validation of novel long non-coding RNA biomarkers for early diagnosis of oral squamous cell carcinoma. Front. Bioeng. Biotechnol. 2020;8:256. doi: 10.3389/fbioe.2020.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jie G., Peng S., Cui Z., He C., Feng X. Long non-coding RNA TFAP2A-AS1 plays an important role in oral squamous cell carcinoma: research includes bioinformatics analysis and experiments. BMC Oral Health. 2022;22(1):1–11. doi: 10.1186/s12903-022-02203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lei C.-S., Kung H.-J., Shih J.-W. Long non-coding rnas as functional codes for oral cancer: translational potential, progress and promises. Int. J. Mol. Sci. 2021;22(9):4903. doi: 10.3390/ijms22094903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Du Z., Qi Y., He J., Zhong D., Zhou M. Recent advances in applications of nanoparticles in SERS in vivo imaging. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2021;13(2) doi: 10.1002/wnan.1672. [DOI] [PubMed] [Google Scholar]
- 92.Lyon L.A., Keating C.D., Fox A.P., Baker B.E., He L., Nicewarner S.R., Mulvaney S.P., Natan M.J. Raman spectroscopy. Anal. Chem. 1998;70(12):341–362. doi: 10.1021/a1980021p. [DOI] [PubMed] [Google Scholar]
- 93.Joseph V., Matschulat A., Polte J., Rolf S., Emmerling F., Kneipp J. SERS enhancement of gold nanospheres of defined size. J. Raman Spectrosc. 2011;42(9):1736–1742. [Google Scholar]
- 94.Blanco-Formoso M., Alvarez-Puebla R.A. Cancer diagnosis through SERS and other related techniques. Int. J. Mol. Sci. 2020;21(6):2253. doi: 10.3390/ijms21062253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu Q., Wang J., Wang B., Li Z., Huang H., Li C., Yu X., Chu P.K. Paper-based plasmonic platform for sensitive, noninvasive, and rapid cancer screening. Biosens. Bioelectron. 2014;54:128–134. doi: 10.1016/j.bios.2013.10.067. [DOI] [PubMed] [Google Scholar]
- 96.Han S., Locke A.K., Oaks L.A., Cheng Y.L., Cote G.L. Nanoparticle-based assay for detection of S100P mRNA using surface-enhanced Raman spectroscopy. J. Biomed. Opt. 2019;24(5):1–9. doi: 10.1117/1.JBO.24.5.055001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim C.S., Ingato D., Wilder-Smith P., Chen Z., Kwon Y.J. Stimuli-disassembling gold nanoclusters for diagnosis of early stage oral cancer by optical coherence tomography. Nano Converg. 2018;5(1):3. doi: 10.1186/s40580-018-0134-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim C.S., Ahn Y.C., Wilder-Smith P., Oh S., Chen Z., Kwon Y.J. Efficient and facile delivery of gold nanoparticles in vivo using dissolvable microneedles for contrast-enhanced optical coherence tomography. Biomed. Opt Express. 2010;1(1):106–113. doi: 10.1364/BOE.1.000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sudri S., Duadi H., Altman F., Allon I., Ashkenazy A., Chakraborty R., Novikov I., Fixler D., Hirshberg A. Diffusion reflection method for early detection of oral squamous cell carcinoma specifically targeted by circulating gold-nanorods bio-conjugated to anti-epidermal growth factor receptor. Int. J. Nanomed. 2021;16:2237. doi: 10.2147/IJN.S300125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kho K.W., Malini O., Shen Z.X., Soo K.C. International Society for Optics and Photonics; 2005. Surface Enhanced Raman Spectroscopic (SERS) Study of Saliva in the Early Detection of Oral Cancer, Optical Diagnostics and Sensing V; pp. 84–91. [Google Scholar]
- 101.di Ruffano L.F., Dinnes J., Deeks J.J., Chuchu N., Bayliss S.E., Davenport C., Takwoingi Y., Godfrey K., O'Sullivan C., Matin R.N. Optical coherence tomography for diagnosing skin cancer in adults. Cochrane Database Syst. Rev. 2018;12 doi: 10.1002/14651858.CD013189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Braz A.K.S., de Araujo R.E., Ohulchanskyy T.Y., Shukla S., Bergey E.J., Gomes A.S., Prasad P.N. In situ gold nanoparticles formation: contrast agent for dental optical coherence tomography. J. Biomed. Opt. 2012;17(6) doi: 10.1117/1.JBO.17.6.066003. [DOI] [PubMed] [Google Scholar]
- 103.T.S. Troutman, Plasmon Resonant Nanostructures of Gold for Biomedical Applications, The University of Arizona2008.
- 104.Kim C.S., Wilder-Smith P., Ahn Y.C., Liaw L.H., Chen Z., Kwon Y.J. Enhanced detection of early-stage oral cancer in vivo by optical coherence tomography using multimodal delivery of gold nanoparticles. J. Biomed. Opt. 2009;14(3) doi: 10.1117/1.3130323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Das A., Raposo G.C.C., Lopes D.S., da Silva E.J., Carneiro V.S.M., Mota C.C.B.d.O., Amaral M.M., Zezell D.M., Barbosa-Silva R., Gomes A.S.L. Exploiting nanomaterials for optical coherence tomography and photoacoustic imaging in nanodentistry. Nanomaterials. 2022;12(3):506. doi: 10.3390/nano12030506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Evers D.J., Nachabe R., Vranken Peeters M.-J., van der Hage J.A., Oldenburg H.S., Rutgers E.J., Lucassen G.W., Hendriks B.H., Wesseling J., Ruers T.J. Diffuse reflectance spectroscopy: towards clinical application in breast cancer. Breast Cancer Res. Treat. 2013;137(1):155–165. doi: 10.1007/s10549-012-2350-8. [DOI] [PubMed] [Google Scholar]
- 107.Einstein G., Udayakumar K., Aruna P.R., Koteeswaran D., Ganesan S. Diffuse reflectance spectroscopy for monitoring physiological and morphological changes in oral cancer. Optik. 2016;127(3):1479–1485. [Google Scholar]
- 108.Olshinka A., Ad-El D., Didkovski E., Weiss S., Ankri R., Goldenberg-Cohen N., Fixler D. Diffusion reflection measurements of antibodies conjugated to gold nanoparticles as a method to identify cutaneous squamous cell carcinoma borders. Materials. 2020;13(2):447. doi: 10.3390/ma13020447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sahu A., Verma S., Varma M., Yadav M.K. Impact of ErbB receptors and anticancer drugs against breast cancer: a review. Curr. Pharmaceut. Biotechnol. 2022;23(6):787–802. doi: 10.2174/1389201022666210719161453. [DOI] [PubMed] [Google Scholar]
- 110.Ankri R., Ashkenazy A., Milstein Y., Brami Y., Olshinka A., Goldenberg-Cohen N., Popovtzer A., Fixler D., Hirshberg A. Gold nanorods based air scanning electron microscopy and diffusion reflection imaging for mapping tumor margins in squamous cell carcinoma. ACS Nano. 2016;10(2):2349–2356. doi: 10.1021/acsnano.5b07114. [DOI] [PubMed] [Google Scholar]
- 111.Tan Y., Yan B., Xue L., Li Y., Luo X., Ji P. Surface-enhanced Raman spectroscopy of blood serum based on gold nanoparticles for the diagnosis of the oral squamous cell carcinoma. Lipids Health Dis. 2017;16(1):73. doi: 10.1186/s12944-017-0465-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xue L., Yan B., Li Y., Tan Y., Luo X., Wang M. Surface-enhanced Raman spectroscopy of blood serum based on gold nanoparticles for tumor stages detection and histologic grades classification of oral squamous cell carcinoma. Int. J. Nanomed. 2018;13:4977–4986. doi: 10.2147/IJN.S167996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang J.H., Wang B., Liu Q., Li Q., Huang H., Song L., Sun T.Y., Wang H., Yu X.F., Li C., Chu P.K. Bimodal optical diagnostics of oral cancer based on Rose Bengal conjugated gold nanorod platform. Biomaterials. 2013;34(17):4274–4283. doi: 10.1016/j.biomaterials.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 114.Sperling R.A., Parak W.J. Surface modification, functionalization and bioconjugation of colloidal inorganic nanoparticles. Phil. Trans. Math. Phys. Eng. Sci. 2010;368(1915):1333–1383. doi: 10.1098/rsta.2009.0273. [DOI] [PubMed] [Google Scholar]
- 115.Nguyen N.P., Sallah S., Karlsson U., Antoine J.E. Combined chemotherapy and radiation therapy for head and neck malignancies: quality of life issues. Cancer. 2002;94(4):1131–1141. doi: 10.1002/cncr.10257. [DOI] [PubMed] [Google Scholar]
- 116.Moore K.A., Ford P.J., Farah C.S. Support needs and quality of life in oral cancer: a systematic review. Int. J. Dent. Hyg. 2014;12(1):36–47. doi: 10.1111/idh.12051. [DOI] [PubMed] [Google Scholar]
- 117.Nori P., Kline-Quiroz C., Stubblefield M.D. Cancer rehabilitation: acute and chronic issues, nerve injury, radiation sequelae, surgical and chemo-related, Part 2, med. Clin. North Am. 2020;104(2):251–262. doi: 10.1016/j.mcna.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 118.Satapathy S.R., Nayak A., Siddharth S., Das S., Nayak D., Kundu C.N. Metallic gold and bioactive quinacrine hybrid nanoparticles inhibit oral cancer stem cell and angiogenesis by deregulating inflammatory cytokines in p53 dependent manner. Nanomedicine. 2018;14(3):883–896. doi: 10.1016/j.nano.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 119.Rathinaraj P., Muthusamy G., Prasad N.R., Gunaseelan S., Kim B., Zhu S. Folate–gold–bilirubin nanoconjugate induces apoptotic death in multidrug-resistant oral carcinoma cells. Eur. J. Drug Metab. Pharmacokinet. 2020;45(2):285–296. doi: 10.1007/s13318-019-00600-9. [DOI] [PubMed] [Google Scholar]
- 120.Essawy M.M., El-Sheikh S.M., Raslan H.S., Ramadan H.S., Kang B., Talaat I.M., Afifi M.M. Function of gold nanoparticles in oral cancer beyond drug delivery: implications in cell apoptosis. Oral Dis. 2021;27(2):251–265. doi: 10.1111/odi.13551. [DOI] [PubMed] [Google Scholar]
- 121.Choi B.B.R., Choi J.H., kyu Kim U., Hwang D.S., Kim G.C. Gold nanoparticles conjugated with programmed death-ligand 1 antibodies induce apoptosis of SCC-25 oral squamous cell carcinoma cells via programmed death-ligand 1/signal transducer and transcription 3 pathway. Arch. Oral Biol. 2021;125 doi: 10.1016/j.archoralbio.2021.105085. [DOI] [PubMed] [Google Scholar]
- 122.Hembram K.C., Dash S.R., Das B., Sethy C., Chatterjee S., Bindhani B.K., Kundu C.N. Quinacrine based gold hybrid nanoparticles caused apoptosis through modulating replication fork in oral cancer stem cells. Mol. Pharm. 2020;17(7):2463–2472. doi: 10.1021/acs.molpharmaceut.0c00197. [DOI] [PubMed] [Google Scholar]
- 123.Sürer Ş.İ., Elçitepe T.B., Akçay D., Daşkın E., Kocal G.Ç., Alıcıkuş Z.A., Eskiizmir G., Yapıcı K., Başbınar Y. A promising, novel radiosensitizer nanodrug complex for oral cavity cancer: cetuximab and cisplatin-conjugated gold nanoparticles. Balkan Med. J. 2021;38(5) doi: 10.5152/balkanmedj.2021.21013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Baldea I., Florea A., Olteanu D., Clichici S., David L., Moldovan B., Cenariu M., Achim M., Suharoschi R., Danescu S. Effects of silver and gold nanoparticles phytosynthesized with Cornus mas extract on oral dysplastic human cells. Nanomedicine. 2020;15(1):55–75. doi: 10.2217/nnm-2019-0290. [DOI] [PubMed] [Google Scholar]
- 125.Liao Y.T., Liu C.H., Chin Y., Chen S.Y., Liu S.H., Hsu Y.C., Wu K.C.W. Correction: biocompatible and multifunctional gold nanorods for effective photothermal therapy of oral squamous cell carcinoma. J. Mater. Chem. B. 2020;8(35) 8084-8084. [Google Scholar]
- 126.Mehdizadeh A., Pandesh S., Shakeri-Zadeh A., Kamrava S.K., Habib-Agahi M., Farhadi M., Pishghadam M., Ahmadi A., Arami S., Fedutik Y. The effects of folate-conjugated gold nanorods in combination with plasmonic photothermal therapy on mouth epidermal carcinoma cells. Laser Med. Sci. 2014;29(3):939–948. doi: 10.1007/s10103-013-1414-2. [DOI] [PubMed] [Google Scholar]
- 127.Hosseini V., Mirrahimi M., Shakeri-Zadeh A., Koosha F., Ghalandari B., Maleki S., Komeili A., Kamrava S.K. Multimodal cancer cell therapy using Au@Fe2O3 core-shell nanoparticles in combination with photo-thermo-radiotherapy. Photodiagnosis Photodyn. Ther. 2018;24:129–135. doi: 10.1016/j.pdpdt.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 128.Song W., Gong J., Wang Y., Zhang Y., Zhang H., Zhang W., Zhang H., Liu X., Zhang T., Yin W. Gold nanoflowers with mesoporous silica as “nanocarriers” for drug release and photothermal therapy in the treatment of oral cancer using near-infrared (NIR) laser light. J. Nanopart. Res. 2016;18(4):101. [Google Scholar]
- 129.Wang B., Wang J.H., Liu Q., Huang H., Chen M., Li K., Li C., Yu X.F., Chu P.K. Rose-bengal-conjugated gold nanorods for in vivo photodynamic and photothermal oral cancer therapies. Biomaterials. 2014;35(6):1954–1966. doi: 10.1016/j.biomaterials.2013.11.066. [DOI] [PubMed] [Google Scholar]
- 130.Wang B.K., Yu X.F., Wang J.H., Li Z.B., Li P.H., Wang H., Song L., Chu P.K., Li C. Gold-nanorods-siRNA nanoplex for improved photothermal therapy by gene silencing. Biomaterials. 2016;78:27–39. doi: 10.1016/j.biomaterials.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 131.Liu Z., Shi J., Zhu B., Xu Q. Development of a multifunctional gold nanoplatform for combined chemo-photothermal therapy against oral cancer. Nanomedicine. 2020;15:661–676. doi: 10.2217/nnm-2019-0415. 07. [DOI] [PubMed] [Google Scholar]
- 132.Alamzadeh Z., Beik J., Mirrahimi M., Shakeri-Zadeh A., Ebrahimi F., Komeili A., Ghalandari B., Ghaznavi H., Kamrava S.K., Moustakis C. Gold nanoparticles promote a multimodal synergistic cancer therapy strategy by co-delivery of thermo-chemo-radio therapy. Eur J Pharm Sci. 2020;145 doi: 10.1016/j.ejps.2020.105235. [DOI] [PubMed] [Google Scholar]
- 133.Xia C., Pan J., Wang J., Pu Y., Zhang Q., Hu S., Hu Q., Wang Y. Functional blockade of cancer-associated fibroblasts with ultrafine gold nanomaterials causes an unprecedented bystander antitumoral effect. Nanoscale. 2020;12(38):19833–19843. doi: 10.1039/d0nr04682e. [DOI] [PubMed] [Google Scholar]
- 134.Zeng J.J., Tang Z.G., Jiao Z., Yu J.G. Black phosphorous nanosheets–gold nanoparticles–cisplatin for photothermal/photodynamic treatment of oral squamous cell carcinoma. T NONFERR METAL SOC. 2021;31(9):2812–2822. [Google Scholar]
- 135.Gamal-Eldeen A.M., Baghdadi H.M., Afifi N.S., Ismail E.M., Alsanie W.F., Althobaiti F., Raafat B.M. Gum Arabic-encapsulated gold nanoparticles modulate hypoxamiRs expression in tongue squamous cell carcinoma. Mol Cell Toxicol. 2021;17(2):111–121. [Google Scholar]
- 136.Gmeiner W.H., Ghosh S. Nanotechnology for cancer treatment. Nanotechnol. Rev. 2014;3(2):111–122. doi: 10.1515/ntrev-2013-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hu X., Zhang Y., Ding T., Liu J., Zhao H. Multifunctional gold nanoparticles: a novel nanomaterial for various medical applications and biological activities. Front. Bioeng. Biotechnol. 2020:990. doi: 10.3389/fbioe.2020.00990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Medici S., Peana M., Coradduzza D., Zoroddu M.A. Elsevier; 2021. Gold Nanoparticles and Cancer: Detection, Diagnosis and Therapy, Semin. Cancer Biol. pp. 27–37. [DOI] [PubMed] [Google Scholar]
- 139.Wang W., Wang J., Ding Y. Gold nanoparticle-conjugated nanomedicine: design, construction, and structure–efficacy relationship studies. J. Mater. Chem. B. 2020;8(22):4813–4830. doi: 10.1039/c9tb02924a. [DOI] [PubMed] [Google Scholar]
- 140.Awotunde O., Okyem S., Chikoti R., Driskell J.D. Role of free thiol on protein adsorption to gold nanoparticles. Langmuir. 2020;36(31):9241–9249. doi: 10.1021/acs.langmuir.0c01550. [DOI] [PubMed] [Google Scholar]
- 141.Ahmad R., Fu J., He N., Li S. Advanced gold nanomaterials for photothermal therapy of cancer. J. Nanosci. Nanotechnol. 2016;16(1):67–80. doi: 10.1166/jnn.2016.10770. [DOI] [PubMed] [Google Scholar]
- 142.Wei W., Zhang X., Zhang S., Wei G., Su Z. Biomedical and bioactive engineered nanomaterials for targeted tumor photothermal therapy: a review. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;104 doi: 10.1016/j.msec.2019.109891. [DOI] [PubMed] [Google Scholar]
- 143.Liu S., Pan X., Liu H. Two-dimensional nanomaterials for photothermal therapy. Angew Chem. Int. Ed. Engl. 2020;59(15):5890–5900. doi: 10.1002/anie.201911477. [DOI] [PubMed] [Google Scholar]
- 144.Govindaraju S., Yun K. Synthesis of gold nanomaterials and their cancer-related biomedical applications: an update. 3 Biotech. 2018;8(2):1–13. doi: 10.1007/s13205-018-1137-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Qi Z., Shi J., Zhang Z., Cao Y., Li J., Cao S. PEGylated graphene oxide-capped gold nanorods/silica nanoparticles as multifunctional drug delivery platform with enhanced near-infrared responsiveness. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;104 doi: 10.1016/j.msec.2019.109889. [DOI] [PubMed] [Google Scholar]
- 146.Huang X.H., Jain P.K., El-Sayed I.H., El-Sayed M.A. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Laser Med. Sci. 2008;23(3):217–228. doi: 10.1007/s10103-007-0470-x. [DOI] [PubMed] [Google Scholar]
- 147.Riley R.S., Day E.S. Gold nanoparticle-mediated photothermal therapy: applications and opportunities for multimodal cancer treatment. Wires Nanomed Nanobi. 2017;9(4) doi: 10.1002/wnan.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Vines J.B., Yoon J.H., Ryu N.E., Lim D.J., Park H. Gold nanoparticles for photothermal cancer therapy. Front. Chem. 2019;7:167. doi: 10.3389/fchem.2019.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bai G., Yuan P., Cai B., Qiu X., Jin R., Liu S., Li Y., Chen X. Stimuli-Responsive scaffold for breast cancer treatment combining accurate photothermal therapy and adipose tissue regeneration. Adv. Funct. Mater. 2019;29(36) [Google Scholar]
- 150.Riley R.S., June C.H., Langer R., Mitchell M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019;18(3):175–196. doi: 10.1038/s41573-018-0006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Connor D.M., Broome A.-M. Gold nanoparticles for the delivery of cancer therapeutics. Adv. Cancer Res. 2018;139:163–184. doi: 10.1016/bs.acr.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 152.He J., Liu S., Zhang Y., Chu X., Lin Z., Zhao Z., Qiu S., Guo Y., Ding H., Pan Y. The application of and strategy for gold nanoparticles in cancer immunotherapy. Front. Pharmacol. 2021;12:1430. doi: 10.3389/fphar.2021.687399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Luo D., Wang X., Burda C., Basilion J.P. Recent development of gold nanoparticles as contrast agents for cancer diagnosis. Cancers. 2021;13(8):1825. doi: 10.3390/cancers13081825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.He J.-s., Liu S.-j., Zhang Y.-r., Chu X.-d., Lin Z.-b., Zhao Z., Qiu S.-h., Guo Y.-g., Ding H., Pan Y.-l. The application of and strategy for gold nanoparticles in cancer immunotherapy. Front. Pharmacol. 2021;12:1430. doi: 10.3389/fphar.2021.687399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lopez-Campos F., Candini D., Carrasco E., Francés M.A.B. Nanoparticles applied to cancer immunoregulation. Rep. Practical Oncol. Radiother. 2019;24(1):47–55. doi: 10.1016/j.rpor.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liu Y., Crawford B.M., Vo-Dinh T. Gold nanoparticles-mediated photothermal therapy and immunotherapy. Immunotherapy. 2018;10(13):1175–1188. doi: 10.2217/imt-2018-0029. [DOI] [PubMed] [Google Scholar]
- 157.Vilar C.J.F., Ribeiro S.B., de Araújo A.A., Guerra G.C.B., de Araújo Júnior R.F., Brito G.A.d.C., Leitão R.F.C., Pontes D.d.L., Gasparotto L.H.D.S., Oliveira M.M.B. Effect of gold nanoparticle on 5-fluorouracil-induced experimental oral mucositis in hamsters. Pharmaceutics. 2020;12(4):304. doi: 10.3390/pharmaceutics12040304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ni C., Zhou J., Kong N., Bian T., Zhang Y., Huang X., Xiao Y., Yang W., Yan F. Gold nanoparticles modulate the crosstalk between macrophages and periodontal ligament cells for periodontitis treatment. Biomaterials. 2019;206:115–132. doi: 10.1016/j.biomaterials.2019.03.039. [DOI] [PubMed] [Google Scholar]
- 159.Quail D.F., Joyce J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013;19(11):1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Scherz-Shouval R., Santagata S., Mendillo M.L., Sholl L.M., Ben-Aharon I., Beck A.H., Dias-Santagata D., Koeva M., Stemmer S.M., Whitesell L. The reprogramming of tumor stroma by HSF1 is a potent enabler of malignancy. Cell. 2014;158(3):564–578. doi: 10.1016/j.cell.2014.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Li X., Fan Q., Li J., Song J., Gu Y. MiR-124 down-regulation is critical for cancer associated fibroblasts-enhanced tumor growth of oral carcinoma. Exp. Cell Res. 2017;351(1):100–108. doi: 10.1016/j.yexcr.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 162.Fan Z., Liu B., Wang J., Zhang S., Lin Q., Gong P., Ma L., Yang S. A novel wound dressing based on Ag/graphene polymer hydrogel: effectively kill bacteria and accelerate wound healing. Adv. Funct. Mater. 2014;24(25):3933–3943. [Google Scholar]
- 163.Khandelwal P., Singh D.K., Poddar P. Advances in the experimental and theoretical understandings of antibiotic conjugated gold nanoparticles for antibacterial applications. ChemistrySelect. 2019;4(22):6719–6738. [Google Scholar]
- 164.Adewale O.B., Davids H., Cairncross L., Roux S. Toxicological behavior of gold nanoparticles on various models: influence of physicochemical properties and other factors. Int. J. Toxicol. 2019;38(5):357–384. doi: 10.1177/1091581819863130. [DOI] [PubMed] [Google Scholar]
- 165.Xie J., Gong L., Zhu S., Yong Y., Gu Z., Zhao Y. Emerging strategies of nanomaterial-mediated tumor radiosensitization. Adv. Mater. 2019;31(3) doi: 10.1002/adma.201802244. [DOI] [PubMed] [Google Scholar]
- 166.Zhang Y., Huang F., Ren C., Liu J., Yang L., Chen S., Chang J., Yang C., Wang W., Zhang C., Liu Q., Liang X.J., Liu J. Enhanced radiosensitization by gold nanoparticles with acid-triggered aggregation in cancer radiotherapy. Adv. Sci. 2019;6(8) doi: 10.1002/advs.201801806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hosseini V., Mirrahimi M., Shakeri-Zadeh A., Koosha F., Ghalandari B., Maleki S., Komeili A., Kamrava S.K. Multimodal cancer cell therapy using Au@ Fe2O3 core–shell nanoparticles in combination with photo-thermo-radiotherapy. Photodiagnosis Photodyn. Ther. 2018;24:129–135. doi: 10.1016/j.pdpdt.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 168.Jia T.T., Yang G., Mo S.J., Wang Z.Y., Li B.J., Ma W., Guo Y.X., Chen X., Zhao X., Liu J.Q., Zang S.Q. Atomically precise gold-levonorgestrel nanocluster as a radiosensitizer for enhanced cancer therapy. ACS Nano. 2019;13(7):8320–8328. doi: 10.1021/acsnano.9b03767. [DOI] [PubMed] [Google Scholar]
- 169.Kefayat A., Ghahremani F., Motaghi H., Mehrgardi M.A. Investigation of different targeting decorations effect on the radiosensitizing efficacy of albumin-stabilized gold nanoparticles for breast cancer radiation therapy. Eur. J. Pharm. Sci. 2019;130:225–233. doi: 10.1016/j.ejps.2019.01.037. [DOI] [PubMed] [Google Scholar]
- 170.Samani R.K., Tavakoli M.B., Maghsoudinia F., Motaghi H., Hejazi S.H., Mehrgardi M.A. Trastuzumab and folic acid functionalized gold nanoclusters as a dual-targeted radiosensitizer for megavoltage radiation therapy of human breast cancer. Eur. J. Pharm. Sci. 2020;153 doi: 10.1016/j.ejps.2020.105487. [DOI] [PubMed] [Google Scholar]
- 171.Deng X., Liang S., Cai X., Huang S., Cheng Z., Shi Y., Pang M., Ma P., Lin J. Yolk-shell structured Au Nanostar@Metal-organic framework for synergistic chemo-photothermal therapy in the second near-infrared window. Nano Lett. 2019;19(10):6772–6780. doi: 10.1021/acs.nanolett.9b01716. [DOI] [PubMed] [Google Scholar]
- 172.Patel T.H., Norman L., Chang S., Abedi S., Liu C., Chwa M., Atilano S.R., Thaker K., Lu S., Jazwinski S.M., Miceli M.V., Udar N., Sofa D., Kenney M.C. European mtDNA variants are associated with differential responses to cisplatin, an anticancer drug: implications for drug resistance and side effects. Front. Oncol. 2019;9 doi: 10.3389/fonc.2019.00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Phung D.C., Nguyen H.T., Phuong Tran T.T., Jin S.G., Yong C.S., Truong D.H., Tran T.H., Kim J.O. Combined hyperthermia and chemotherapy as a synergistic anticancer treatment. Journal of Pharmaceutical Investigation. 2019;49(5):519–526. [Google Scholar]
- 174.Majidinia M., Mirza-Aghazadeh-Attari M., Rahimi M., Mihanfar A., Karimian A., Safa A., Yousefi B. Overcoming multidrug resistance in cancer: recent progress in nanotechnology and new horizons. IUBMB Life. 2020;72(5):855–871. doi: 10.1002/iub.2215. [DOI] [PubMed] [Google Scholar]
- 175.Qing G.C., Zhao X.X., Gong N.Q., Chen J., Li X.L., Gan Y.L., Wang Y.C., Zhang Z., Zhang Y.X., Guo W.S., Luo Y., Liang X.J. Thermo-responsive triple-function nanotransporter for efficient chemo-photothermal therapy of multidrug-resistant bacterial infection. Nat. Commun. 2019;10 doi: 10.1038/s41467-019-12313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Salem D.S., Sliem M.A., El-Sesy M., Shouman S.A., Badr Y. Improved chemo-photothermal therapy of hepatocellular carcinoma using chitosan-coated gold nanoparticles. J. Photochem. Photobiol., B. 2018;182:92–99. doi: 10.1016/j.jphotobiol.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 177.Liu Y., Zhang X., Luo L., Li L., Zhu R.Y., Li A., He Y., Cao W., Niu K., Liu H., Yang J., Gao D. Gold-nanobranched-shell based drug vehicles with ultrahigh photothermal efficiency for chemo-photothermal therapy. Nanomedicine. 2019;18:303–314. doi: 10.1016/j.nano.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 178.Mirrahimi M., Abed Z., Beik J., Shiri I., Dezfuli A.S., Mahabadi V.P., Kamrava S.K., Ghaznavi H., Shakeri-Zadeh A. A thermo-responsive alginate nanogel platform co-loaded with gold nanoparticles and cisplatin for combined cancer chemo-photothermal therapy. Pharmacol. Res. 2019;143:178–185. doi: 10.1016/j.phrs.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 179.Wang Z., Chang Z.M., Shao D., Zhang F., Chen F., Li L., Ge M.F., Hu R., Zheng X., Wang Y., Dong W.F. Janus gold triangle-mesoporous silica nanoplatforms for hypoxia-activated radio-chemo-photothermal therapy of liver cancer. ACS Appl. Mater. Interfaces. 2019;11(38):34755–34765. doi: 10.1021/acsami.9b12879. [DOI] [PubMed] [Google Scholar]
- 180.Dos Santos A.F., De Almeida D.R.Q., Terra L.F., Baptista M.S., Labriola L. Photodynamic therapy in cancer treatment-an update review. J Cancer Metastasis Treat. 2019;5:10–20517. [Google Scholar]
- 181.Yanovsky R.L., Bartenstein D.W., Rogers G.S., Isakoff S.J., Chen S.T. Photodynamic therapy for solid tumors: a review of the literature. Photodermatol. Photoimmunol. Photomed. 2019;35(5):295–303. doi: 10.1111/phpp.12489. [DOI] [PubMed] [Google Scholar]
- 182.Kim H.S., Lee D.Y. Near-infrared-Responsive cancer photothermal and photodynamic therapy using gold nanoparticles. Polymers. 2018;10(9) doi: 10.3390/polym10090961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Oh J., Yoon H., Park J.-H. Nanoparticle platforms for combined photothermal and photodynamic therapy. Biomedical Engineering Letters. 2013;3(2):67–73. [Google Scholar]
- 184.Paulino T.P., Ribeiro K.F., Thedei G., Jr., Tedesco A.C., Ciancaglini P. Use of hand held photopolymerizer to photoinactivate Streptococcus mutans. Arch. Oral Biol. 2005;50(3):353–359. doi: 10.1016/j.archoralbio.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 185.Guo T., Wu Y., Lin Y., Xu X., Lian H., Huang G., Liu J.Z., Wu X., Yang H.H. Black phosphorus quantum dots with renal clearance property for efficient photodynamic therapy. Small. 2018;14(4) doi: 10.1002/smll.201702815. [DOI] [PubMed] [Google Scholar]
- 186.Chen W., Ouyang J., Liu H., Chen M., Zeng K., Sheng J., Liu Z., Han Y., Wang L., Li J. Black phosphorus nanosheet-based drug delivery system for synergistic photodynamic/photothermal/chemotherapy of cancer. Adv. Mater. 2017;29(5) doi: 10.1002/adma.201603864. [DOI] [PubMed] [Google Scholar]
- 187.Hidai C., Kitano H. Nonviral gene therapy for cancer: a review. Diseases. 2018;6(3) doi: 10.3390/diseases6030057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Dachs G.U., Dougherty G.J., Stratford I.J., Chaplin D.J. Targeting gene therapy to cancer: a review. Oncol. Res. 1997;9(6–7):313–325. [PubMed] [Google Scholar]
- 189.Zhang Q., Shen C., Zhao N., Xu F.J. Redox-responsive and drug-embedded silica nanoparticles with unique self-destruction features for efficient gene/drug codelivery. Adv. Funct. Mater. 2017;27(10) [Google Scholar]
- 190.Kim J., Kim J., Jeong C., Kim W.J. Synergistic nanomedicine by combined gene and photothermal therapy. Adv. Drug Deliv. Rev. 2016;98:99–112. doi: 10.1016/j.addr.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 191.Huang S.N., Duan S.F., Wang J., Bao S.J., Qiu X.J., Li C.M., Liu Y., Yan L.J., Zhang Z.Z., Hu Y.R. Folic-acid-mediated functionalized gold nanocages for targeted delivery of anti-miR-181b in combination of gene therapy and photothermal therapy against hepatocellular carcinoma. Adv. Funct. Mater. 2016;26(15):2532–2544. [Google Scholar]
- 192.Kong L.D., Xing L.X., Zhou B.Q., Du L.F., Shi X.Y. Dendrimer-modified MoS2 nanoflakes as a platform for combinational gene silencing and photothermal therapy of tumors. Acs Appl Mater Inter. 2017;9(19):15995–16005. doi: 10.1021/acsami.7b03371. [DOI] [PubMed] [Google Scholar]
- 193.Zhang J., Zhao T., Han F., Hu Y., Li Y. Photothermal and gene therapy combined with immunotherapy to gastric cancer by the gold nanoshell-based system. J. Nanobiotechnol. 2019;17(1):1–11. doi: 10.1186/s12951-019-0515-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hanada S., Tsuruta T., Haraguchi K., Okamoto M., Sugiyama H., Koido S. Long-term survival of pancreatic cancer patients treated with multimodal therapy combined with WT1-targeted dendritic cell vaccines. Hum. Vaccines Immunother. 2019;15(2):397–406. doi: 10.1080/21645515.2018.1524238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Prasongsook N., Kumar A., Chintakuntlawar A.V., Foote R.L., Kasperbauer J., Molina J., Garces Y., Ma D., Wittich M.A.N., Rubin J., Richardson R., Morris J., Hay I., Fatourechi V., McIver B., Ryder M., Thompson G., Grant C., Richards M., Sebo T.J., Rivera M., Suman V., Jenkins S.M., Smallridge R.C., Bible K.C. Survival in response to multimodal therapy in anaplastic thyroid cancer. J. Clin. Endocrinol. Metab. 2017;102(12):4506–4514. doi: 10.1210/jc.2017-01180. [DOI] [PubMed] [Google Scholar]
- 196.Su Y., Zhang X., Lei L., Liu B., Wu S., Shen J. Tumor microenvironment-activatable cyclic cascade reaction to reinforce multimodal combination therapy by destroying the extracellular matrix. ACS Appl. Mater. Interfaces. 2021;13(11):12960–12971. doi: 10.1021/acsami.1c02011. [DOI] [PubMed] [Google Scholar]
- 197.Zhang H., Dziegielewski P.T., Biron V.L., Szudek J., Al-Qahatani K.H., O'Connell D.A., Harris J.R., Seikaly H. Survival outcomes of patients with advanced oral cavity squamous cell carcinoma treated with multimodal therapy: a multi-institutional analysis. J. Otolaryngol. Head Neck Surg. 2013;42:30. doi: 10.1186/1916-0216-42-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Pantvaidya G., Sivasanker M., Ranganathan P., Pai P., D'Cruz A. Prospective cross-sectional study assessing prevalence and factors affecting trismus after multimodal treatment for oral cancers. Head Neck. 2019;41(2):286–290. doi: 10.1002/hed.25464. [DOI] [PubMed] [Google Scholar]
- 199.Dhawan A., Duggal P., Bhullar R.S., Kaur T., Sandhu A., Kaur K. Efficacy of multimodal therapy in the survival outcomes of advanced-stage (stage III-stage IV) oral carcinoma patients: an institutional experience in asian Indian population. J. Maxillofac. Oral Surg. 2018;17(1):89–94. doi: 10.1007/s12663-017-1011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mieszawska A.J., Mulder W.J.M., Fayad Z.A., Cormode D.P. Multifunctional gold nanoparticles for diagnosis and therapy of disease. Mol. Pharm. 2013;10(3):831–847. doi: 10.1021/mp3005885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Beik J., Shiran M.B., Abed Z., Shiri I., Ghadimi-Daresajini A., Farkhondeh F., Ghaznavi H., Shakeri-Zadeh A.J.M.p. Gold nanoparticle-induced sonosensitization enhances the antitumor activity of ultrasound in colon tumor-bearing mice. Med. Phys. 2018;45(9):4306–4314. doi: 10.1002/mp.13100. [DOI] [PubMed] [Google Scholar]
- 202.Fan W.P., Yung B., Huang P., Chen X.Y. Nanotechnology for multimodal synergistic cancer therapy. Chem. Rev. 2017;117(22):13566–13638. doi: 10.1021/acs.chemrev.7b00258. [DOI] [PubMed] [Google Scholar]
- 203.Yang X., Yang M.X., Pang B., Vara M., Xia Y.N. Gold nanomaterials at work in biomedicine. Chem. Rev. 2015;115(19):10410–10488. doi: 10.1021/acs.chemrev.5b00193. [DOI] [PubMed] [Google Scholar]
- 204.Sun W., Zhang X., Jia H.R., Zhu Y.X., Guo Y., Gao G., Li Y.H., Wu F.G. Water-dispersible candle soot–derived carbon nano-onion clusters for imaging-guided photothermal cancer therapy. Small. 2019;15(11) doi: 10.1002/smll.201804575. [DOI] [PubMed] [Google Scholar]
- 205.Chen W., Wang X., Zhao B., Zhang R., Xie Z., He Y., Chen A., Xie X., Yao K., Zhong M. CuS–MnS 2 nano-flowers for magnetic resonance imaging guided photothermal/photodynamic therapy of ovarian cancer through necroptosis. Nanoscale. 2019;11(27):12983–12989. doi: 10.1039/c9nr03114f. [DOI] [PubMed] [Google Scholar]
- 206.Wang P., Wang X., Luo Q., Li Y., Lin X., Fan L., Zhang Y., Liu J., Liu X. Fabrication of red blood cell-based multimodal theranostic probes for second near-infrared window fluorescence imaging-guided tumor surgery and photodynamic therapy. Theranostics. 2019;9(2):369. doi: 10.7150/thno.29817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Liu Y., Wu S., Liu Y., Zhang H., Zhang M., Tang Z., Wang Y., Gong T., Yao Z., Fang X. Cathodic protected Mn2+ by NaxWO3 nanorods for stable magnetic resonance imaging-guided tumor photothermal therapy. Biomaterials. 2020;234 doi: 10.1016/j.biomaterials.2020.119762. [DOI] [PubMed] [Google Scholar]
- 208.Zhang C.C., Sun W.J., Wang Y., Xu F., Qu J., Xia J.D., Shen M.W., Shi X.Y. Gd-/CuS-Loaded functional nanogels for MR/PA imaging-guided tumor-targeted photothermal therapy. Acs Appl Mater Inter. 2020;12(8):9107–9117. doi: 10.1021/acsami.9b23413. [DOI] [PubMed] [Google Scholar]
- 209.Yang K.K., Liu Y.J., Wang Y., Ren Q.L., Guo H.Y., Matson J.B., Chen X.Y., Nie Z.H. Enzyme-induced in vivo assembly of gold nanoparticles for imaging-guided synergistic chemo-photothermal therapy of tumor. Biomaterials. 2019:223. doi: 10.1016/j.biomaterials.2019.119460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wang Z.H., Sun X., Huang T., Song J.B., Wang Y.D. A sandwich nanostructure of gold nanoparticle coated reduced graphene oxide for photoacoustic imaging-guided photothermal therapy in the second NIR window. Front. Bioeng. Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.00655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Yin H.Q., Cao P.P., Wang X.Y., Li Y.H., Yin X.B. Computed tomography imaging-guided tandem catalysis-enhanced photodynamic therapy with gold nanoparticle functional covalent organic polymers. ACS Appl. Bio Mater. 2020;3(4):2534–2542. doi: 10.1021/acsabm.0c00244. [DOI] [PubMed] [Google Scholar]
- 212.Huang X.H., El-Sayed I.H., Qian W., El-Sayed M.A. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc. 2006;128(6):2115–2120. doi: 10.1021/ja057254a. [DOI] [PubMed] [Google Scholar]
- 213.Liu R., Guo H., Ouyang Z., Fan Y., Cao X., Xia J., Shi X., Guo R. Multifunctional core–shell tecto dendrimers incorporated with gold nanoparticles for targeted dual mode CT/MR imaging of tumors. ACS Appl. Bio Mater. 2021;4(2):1803–1812. doi: 10.1021/acsabm.0c01525. [DOI] [PubMed] [Google Scholar]
- 214.Yang L., Fu S., Cai Z., Liu L., Xia C., Gong Q., Song B., Ai H. Integration of PEG-conjugated gadolinium complex and superparamagnetic iron oxide nanoparticles as T 1–T 2 dual-mode magnetic resonance imaging probes. Regenerative Biomaterials. 2021;8(6) doi: 10.1093/rb/rbab064. rbab064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Melancon M.P., Lu W., Zhong M., Zhou M., Liang G., Elliott A.M., Hazle J.D., Myers J.N., Li C., Stafford R.J. Targeted multifunctional gold-based nanoshells for magnetic resonance-guided laser ablation of head and neck cancer. Biomaterials. 2011;32(30):7600–7608. doi: 10.1016/j.biomaterials.2011.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Libutti S., Paciotti G., Myer L., Haynes R., Gannon W., Walker M., Seidel G., Byrnes A., Yuldasheva N., Tamarkin L. Results of a completed phase I clinical trial of CYT-6091: a pegylated colloidal gold-TNF nanomedicine. J. Clin. Oncol. 2009;27(15_suppl) 3586-3586. [Google Scholar]
- 217.Diagnostic and Prognostic Accuracy of Gold Nanoparticles in Salivary Gland Tumours.