Abstract
Regulatory agencies rely upon rodent in vivo acute oral toxicity data to determine hazard categorization, require appropriate precautionary labeling, and perform quantitative risk assessments. As the field of toxicology moves toward animal-free new approach methodologies (NAMs), there is a pressing need to develop a reliable, robust reference data set to characterize the reproducibility and inherent variability in the in vivo acute oral toxicity test method, which would serve to contextualize results and set expectations regarding NAM performance. Such a data set is also needed for training and evaluating computational models. To meet these needs, rat acute oral LD50 data from multiple databases were compiled, curated, and analyzed to characterize variability and reproducibility of results across a set of up to 2441 chemicals with multiple independent study records. Conditional probability analyses reveal that replicate studies only result in the same hazard categorization on average at 60% likelihood. Although we did not have sufficient study metadata to evaluate the impact of specific protocol components (eg, strain, age, or sex of rat, feed used, treatment vehicle, etc.), studies were assumed to follow standard test guidelines. We investigated, but could not attribute, various chemical properties as the sources of variability (ie, chemical structure, physiochemical properties, functional use). Thus, we conclude that inherent biological or protocol variability likely underlies the variance in the results. Based on the observed variability, we were able to quantify a margin of uncertainty of ±0.24 log10 (mg/kg) associated with discrete in vivo rat acute oral LD50 values.
Keywords: acute toxicity, in vivo variability, LD50, establishing confidence
Acute systemic toxicity studies are used by regulatory agencies to determine hazard categorization, assign appropriate labeling to alert consumers to potential toxicity hazards, and in risk assessment applications (Strickland et al., 2018). The in vivo regulatory test guidelines are used to determine a dose level expected to result in 50% lethality for tested animals following a single oral administration of test substance (oral LD50). There are regulatory needs for experimentally derived discrete point estimates of oral LD50 values (eg, when used to determine acceptable exposure limits), but in other instances a range of LD50 values may be acceptable (eg, to define personal protective equipment requirements; Morris-Schaffer and McCoy 2021; Strickland et al., 2018). To address these regulatory needs, multiple protocols have been internationally harmonized by the Organization for Economic Co-operation and Development (OECD) health effects test guidelines to identify potential acute oral toxicants (OECD, 2002a,b, 2008).
As the field of toxicology moves toward the development of new approach methodologies (NAMs) that do not require the use of laboratory animals, there is an inherent need for fully characterized reference test data against which NAMs can be compared. For acute oral toxicity, rodent LD50 values serve as the primary reference comparator, and therefore it is important to compile a resource of in vivo reference data and to characterize the variability of these values based on independently conducted studies for the same test substances. Simply put, a solid understanding of the reproducibility and inherent variability of the rat in vivo acute oral toxicity assays will provide a foundation to contextualize results and set expectations regarding NAM performance.
Several studies have previously evaluated the within- and between-laboratory reproducibility of the in vivo test method to demonstrate the impact of modifying protocol components such as rat strain, vehicle, or age of rat from a relatively small (n < 30) number of chemicals (Griffith, 1964; Hunter et al., 1979; Weil and Wright, 1967; Weil et al., 1966). Other studies compiled larger (n = 62–88) lists of reference chemicals with replicate LD50 values to establish a reported range as a measure of variability (Hoffmann et al., 2010; ICCVAM, 2006). However, with the expansive list of chemicals in global commerce that include substances registered by multiple manufacturers, far greater numbers of chemicals have been evaluated for acute oral toxicity numerous times. These data have become increasingly available through publicly accessible web-based resources, thereby providing an opportunity to collate large databases of toxicology study results.
Herein, we present the largest assembly to date of manually curated rat acute oral toxicity LD50 data comprising chemicals with more than one experimentally derived LD50 value retrieved from multiple international data sources. We have accounted for data redundancy, evaluated LD50 distribution/variance, applied cheminformatics analyses, and defined a margin of uncertainty that can be applied when considering in vivo acute oral toxicity data or predictions thereof. Given the long history of regulatory decisions based upon LD50, establishing this margin of uncertainty will more effectively build scientific confidence in results generated by NAMs as compared to in vivo results.
MATERIALS AND METHODS
Data Sources and Inventory Compilation
Data sources for rat acute oral toxicity were selected based on data accessibility or availability and included the following:
ChemProp (European Chemicals Agency [ECHA], https://echa.europa.eu/information-on-chemicals; last accessed November 2018)
Hazardous Substances Data Bank (National Library of Medicine, https://www.nlm.nih.gov/databases/download/hsdb.html; last accessed November 2018)
ChemIDplus (National Library of Medicine, https://chem.nlm.nih.gov/chemidplus/chemidlite.jsp; last accessed November 2018)
AcutoxBase (European Union Joint Research Centre, Kinsner-Ovaskainen et al., 2009)
eChemPortal (OECD, https://www.echemportal.org/echemportal/; last accessed November 2018)
Data compilation was restricted to rat LD50 values, retaining only LD50 values that were amenable to conversion into mg/kg units. Where unit conversion was required, molecular weights were retrieved from the U.S. Environmental Protection Agency (EPA) CompTox Chemicals Dashboard (Williams et al., 2017; https://comptox.epa.gov/dashboard; last accessed November 2018) using Chemical Abstracts Service Registry Numbers (CASRN) as input. In addition to species and LD50 unit filtering, data were also manually curated (described below). Compiled LD50 values were in the form of point estimates, limit tests, and acute toxic class ranges (OECD, 2002b).
The data set was compiled using CASRN as the primary chemical identifier. When more than one CASRN mapped to the same chemical structure (including deprecated CASRNs), these data entries were not collapsed/corrected, but rather kept separate to match the identifier used in the database of origin and to reflect unique experimental records; thus, unique chemical counts reflect unique CASRN, not necessarily unique chemical structures.
The initial compilation yielded a total of 15 688 unique chemicals with at least one rat LD50 value. The inclusion of multiple instances for the same LD50 value for any given chemical was limited to avoid overrepresenting studies that may have been reported in multiple data sources. A subset of chemicals for which at least 2 unique discrete point estimate LD50 values were available was selected and manually curated to create the foundation of the inventory used for the current study. Subsequently, data generously provided directly by ECHA were added to yield a final data set of 1885 chemicals and 5826 quantitative LD50 values (provided in Supplementary File 1). Additional acute oral systemic toxicity data that were not reported as discrete point estimate LD50 values, but rather as limit tests or acute toxic class ranges, were also retrieved and added to the discrete LD50 values to generate an expanded inventory for further hazard category-based analyses. When these values were added to the discrete LD50 data set, the expanded categorical data set contained 7574 entries representing 2441 chemicals (also provided in Supplementary File 1). This data set retained the requirement that chemicals have at least 2 unique entries.
Additional physicochemical data for various analyses conducted in this project were retrieved from a variety of sources. Chemical structures and properties were retrieved from the EPA Comptox Chemicals Dashboard (Williams et al., 2017) and generated by the Open Structure-activity/property Relationship App (OPERA; Mansouri et al., 2018). We used simplified molecular-input line-entry system (SMILES) strings for structure-related analyses. These were retrieved from the EPA CompTox Chemicals Dashboard with all queries conducted using CASRNs as input. ToxPrint (ChemoTyper v1.0; Yang et al., 2015) chemotypes fingerprints were used for cheminformatics assessments. Finally, product use information for each chemical was obtained from the EPA Consumer Product Categories (CPCat) database (Dionisio et al., 2018) to characterize the diversity of the data set and evaluate any trends associated with variability.
Curation of the Rat Acute Oral Systemic Toxicity Inventory
Curation of the compiled data set involved identifying unique LD50 values per CASRN in order to omit values that may have been the same data point present in multiple source databases to avoid overrepresentation. For example, a chemical might have 3 LD50 values of “>2000 mg/kg” based on limit test results. Because the majority of databases we used did not include primary source references for the original studies, it was impossible to determine whether this data point originated from 3 independent studies or arose from one study that was captured in multiple source databases. As such, only one value of >2000 mg/kg was kept in the final data set. Where available, qualifiers associated with limit tests were noted and tracked. For example, “>” or “<”, reflecting “greater than” or “less than” designations for limit test outcomes, respectively, were noted.
We removed each of the following: values derived from complex mixtures reported as individual substances; values from studies having an “unreported” exposure route (the required exposure route was oral); and values exceeding 10 000 mg/kg because they were considered to be unrealistically high. Data reported as ranges or sourced from acute toxic class or limit tests were separated into a unique inventory. These values were integrated with the main data set only for categorical analyses, where EPA or United Nations Globally Harmonized System of Classification and Labelling of Chemicals (GHS) hazard categories could be assigned despite the lack of discrete point estimate LD50 values.
Based on expert judgment, manual curation removed any data identified as potentially erroneous. Such values were traced back to their source database to determine if they could be confirmed. For example, in some instances, 3 values for the same substance were reported that were both similar and very precise (eg, 22.2, 23.6, and 25). Upon further inspection, it was determined that these values were actually the mean and 95% confidence interval for that substance; in these cases only the mean value was retained in our data set and the other 2 values were excluded. In another example, 3 values were reported for the same substance that, upon further inspection, were determined to be separate values calculated based on either males, females, or a mean value calculated from both sexes; in these cases only one value was retained. In yet another example, multiple LD50 values for the same substance were flagged because they were the same as those associated with LD50 ranges used for hazard classification (eg, 300 mg/kg, 2000 mg/kg). The study metadata revealed that the results were actually a reported LD50 range (300–2000 mg/kg) generated from the acute toxic class protocol that was mistakenly recorded as 2 separate LD50 values, these data were kept only in the expanded data set.
Additional Systematic Evaluation for Possible Read-Across Data
The lack of metadata available for the retrieved data compiled herein created another source of uncertainty around the acute oral toxicity values. For example, some LD50 values were reported as experimental point estimate values, but manual curation identified the original toxicity assessment report to be based on a read-across analysis from an analog structure (see ECHA dossier on 3-methylpentane, https://www.echa.europa.eu/web/guest/registration-dossier/-/registered-dossier/24591/7/3/2; last accessed November 2018). Once this was discovered, we used cheminformatics tools to try to identify other instances where data points may have originated from read-across. For this, we tested 2 types of hypotheses for which the findings are described below. Results suggest that some of the data points in our compiled inventory may have originated from read-across studies. However, without clear metadata, as was the case for 3-methylpentane, it was not possible to prove with certainty that even the most similar chemicals are a result of a read-across.
Read-across analysis 1: Based on association of multiple CASRNs with the same set of LD50 values
We identified chemical clusters where multiple CASRNs were associated with the same set of LD50 values. In total, there are 47 clusters of chemicals sharing at least 2 LD50 values. The clusters were identified using the CASRNs matched to the same Distributed Structure-Searchable Toxicity Database identifiers (DTXSIDs; Grulke, 2019), names, original structures, and quantitative structure-activity relationship (QSAR)-ready structures (Supplemental File 2, “repeated_LD50.csv”; Mansouri, 2016). Upon evaluating these clusters of chemicals, we identified several cases of single chemicals with multiple CASRNs or different salts like boric acid and calcium borate, silver sulfate and silver chloride, sodium lactate and calcium lactate, magnesium vanadate (MgV2O6), and calcium vanadate (Ca(VO3)2), all of which would generally have resulted in the same QSAR-ready structure, and had the same set of LD50 values. We also found instances of chemicals that exist in different forms, like d-dilactide and 3,6-dimethyl-2,5-dioxo-1,4-dioxane, camphor and d-camphor, or citronellal and (3R)-3,7-dimethyloct-6-enal. Finally, there were cases where 2 different CASRNs represented different forms of a chemical that can coexist in the same solution, and the LD50 was assigned to both, such as p-xylene and m-xylene, or cinerin I and cinerin II.
On the other hand, we concluded that the equivalent LD50 values of ethane-1,2-diyl bis(sulfanylacetate) and 2-ethylhexyl thioglycolate were derived from independent studies, as the structures of these 2 substance looked too different for the values to have been generated by read-across. Similarly, although chloroxuron and dinitramine had very similar structures, their LD50 values (3000 and 3700 mg/kg, respectively) seemed more like limit test values, despite having been recorded as point estimates in the source databases. In conclusion, despite these chemical clusters appearing to have the exact same data, our review suggested that none were conclusively the result of read-across and could not be excluded from the compiled inventory for that reason.
Read-across analysis 2: Based on structural similarity
This analysis approach targeted chemical structures and compared them based on multiple chemicals sharing the same single LD50 (as opposed to groups of CASRNs sharing multiple LD50s, examined in the previous analysis). First, groups were formed by identifying CASRNs sharing a single LD50. Then, extended fingerprints generated using CDK were used to evaluate similarity using pairwise Tanimoto indices, see Supplemental File 3 (repeated_LD50_analogs.xlsx). For example, 6 CASRNs in the compiled inventory had an LD50 of 2 mg/kg. After generating the similarity matrix of the 6 chemicals, we pulled those with Tanimoto similarity scores between 0.7 and 0.95, representing the range of structures that are similar enough for read-across analysis but not actually the same structure. Out of the 6, only 2 were within the similarity range for read-across (0.9 in this example). We identified 41 such groups, some of which were likely candidates for read-across given the chemical similarity scores. However, there were no cases where we were able to definitively identify LD50 data points that were generated by read-across. Furthermore, out of many structures with the same LD50 values, only 2 groups of chemicals were in the appropriate similarity range, with the remaining structure too dissimilar to suspect read-across. Thus, if some substances had the same LD50 and were very different structurally, we cannot make the case that only the identical LD50 values from chemicals with similar structures resulted from read-across and should potentially be excluded. This possibility, however, is worth noting as an additional source of uncertainty in the data set that we have attempted to quantify.
Analysis Approaches
All analyses were conducted in R (version 3.6.0) unless stated otherwise, and all these analyses and figure generation code are provided in an R script in Supplementary File 4. A representative LD50 for each chemical was computed as the median of only replicate point estimate LD50 values (ie, not including limit tests or hazard categorical data). Similarly, variability was computed as the median absolute deviation (MAD) across log10 of the point estimate LD50 values only. The correlation between each of the experimental LD50 point estimate values and the chemical-specific median LD50 was assessed using Pearson’s correlation r2 and root mean-squared error (RMSE). Chemical-specific MADs were evaluated relative to chemical potency, number of replicate studies, and hazard categories. A global margin of error around the median LD50 was computed as ±2.5×MAD, as recommended for outlier detection (Leys et al., 2013). To assess the likelihood of hazard category concordance across repeat studies, conditional probabilities were calculated as described in Luechtefeld et al. (2016). The probability of a chemical being in category j (C2 = j) given that it has been previously categorized in category i (C1 = i) is calculated as:
where i and j are hazard categories, n(i) is the number of studies for chemicals classified in category i at least once, and n(i)j is the number of studies within n(i) where a chemical was classified in category j.
Chemical use categories, structural descriptors, toxicity-related fingerprints, and physicochemical properties were examined for trends associated with LD50 variability. MATLAB (version 9.4) was used for categorical analyses for concordance, ToxPrint, and physiochemical property analyses.
RESULTS
Rat Acute Oral Systemic Toxicity Inventory
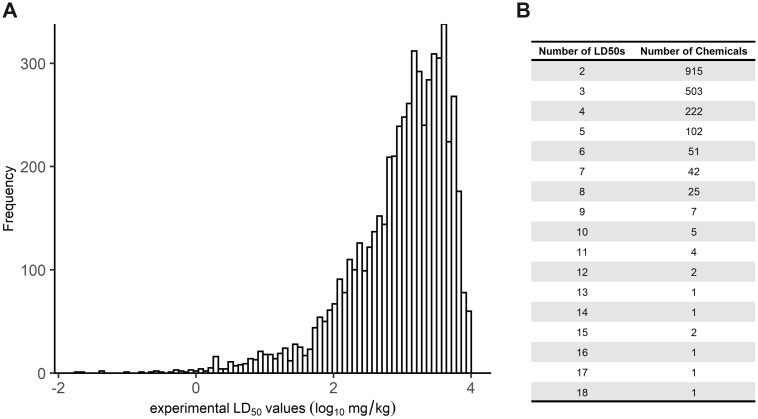
A data set of rat acute oral LD50 values, for chemicals with at least 2 independent experimentally derived discrete point estimate values, was compiled comprising 1885 chemicals and 5826 LD50 values. Figure 1A shows the distribution of these point estimate LD50 values, revealing that the 5826 LD50 values in the data set ranged from 0.02 to 10 000 mg/kg. Most values were above 1000 mg/kg (3349/5826 values, ie, 57.5%). Additionally, the number of unique LD50 values per chemical was assessed (Figure 1B) revealing that the majority of chemicals had 2 (915 chemicals) or 3 (503 chemicals) unique point estimate LD50 values. There were 18 chemicals with 10 or more point estimate LD50 values in the data set.
Figure 1.
Characterizing the LD50 data set. The curated rat acute oral systemic toxicity LD50 data set comprised chemicals with at least 2 point estimate LD50 values, resulting in an inventory of 5826 LD50 values representing 1885 chemicals. A, Histogram of the distribution of LD50 values in the data set. B, Summary of replicate LD50 values in the data set per chemical. The LD50 values ranged from 0.02 to 10 000 mg/kg, with most values between 1000 and 10 000 mg/kg as shown in the histogram. Most chemicals (1742/1885 chemicals) had 5 or fewer LD50 values.
Characterizing Acute Oral Toxicity Study Reproducibility and Variability
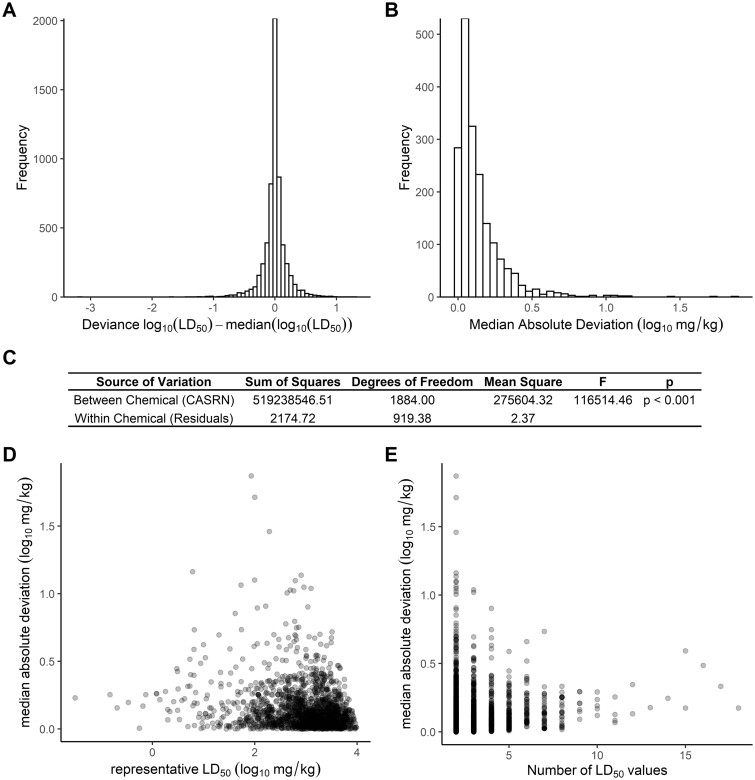
To assess reproducibility of discrete LD50 point estimates from replicate studies for a given chemical, a single representative LD50 value was calculated for each chemical to serve as a reference value. Given increased confidence due to manual curation, representative LD50 values were defined per chemical as the median of all point estimate LD50 values for a single chemical. In vivo study reproducibility was then assessed as a measure of how each experimentally derived LD50 value (at least 2 independent experiments per chemical) compared with the representative value (used as “truth”). Most of the 5826 experimental LD50 point estimate values were within one order of magnitude from the chemical-specific median LD50 value (representative value; Figure 2A). This plot visualizing the deviation from the median shows a peak at 0 where uneven replicate LD50 values resulted in the median being exactly one of the experimental values. The high Pearson’s correlation (r2 of 0.927) and low RMSE (0.197) confirm the appropriateness of the median LD50 representative value for the experimental data set.
Figure 2.
Evaluating variability of LD50 replicates. For each of the 1885 chemicals with at least 2 discrete point estimate LD50 values, a median value was used as the chemical-specific representative LD50. Deviation was derived by subtracting the chemical median from the individual LD50 values (A). MAD values computed per chemical are plotted as a histogram to visualize frequency of values (B). Welch’s ANOVA table (C) summarizes statistical analyses that confirm most variability is attributable to interchemical (between chemicals) rather than intrachemical (within chemical) variability. Variability among replicate point estimate LD50 values was assessed by calculating MAD across replicate LD50 values per chemical in log10 mg/kg units. Plots show MAD values as a function of chemical potency (D) or the number of replicate LD50 values available per chemical (E).
To quantify the variability, MAD was calculated for each chemical using discrete point estimate experimental LD50 values. The distribution of MAD (Figure 2B) reveals that most MAD values are below 0.5, though there are a few chemicals with high MAD values, the top10 most variable chemicals based on MAD are listed in Table 1. Iridium tetrachloride (CASRN 10025-97-5) had the highest MAD of 1.87 (log10 (mg/kg)), based on 2 independent LD50 point estimate values that were 3 orders of magnitude apart: 4.67 mg/kg and 1560 mg/kg. The median MAD across all 1885 chemicals was 0.0895 log10 (mg/kg).
Table 1.
Top 10 Most Variable Chemicals Based on Median Absolute Deviation
| CASRN | Chemical Name | Median Absolute Deviation (log10 (mg/kg)) | Number of LD50 Values | LD50 Values (mg/kg) |
|---|---|---|---|---|
| 10025-97-5 | Iridium tetrachloride | 1.871 | 2 | 4.67, 1560 |
| 97-18-7 | Bithionol | 1.713 | 2 | 7, 1430 |
| 62-56-6 | Thiourea | 1.459 | 2 | 20, 1860 |
| 7487-94-7 | Mercuric chloride | 1.163 | 2 | 1, 37 |
| 104-12-1 | 4-Chlorophenyl isocyanate | 1.137 | 2 | 138, 4710 |
| 7719-12-2 | Phosphorous trichloride | 1.101 | 2 | 18, 550 |
| 72-54-8 | p,p'-DDD | 1.096 | 2 | 113, 3400 |
| 83-26-1 | Pindone | 1.063 | 2 | 10.3, 280 |
| 2374-14-3 | 2,4,6-Trimethyl-2,4,6-tris(3,3,3-trifluoropropyl)cyclotrisiloxane | 1.047 | 2 | 180, 4659 |
| 117-18-0 | 2,3,5,6-Tetrachloronitrobenzene | 1.039 | 3 | 250, 1256, 7500 |
The variability of LD50 values was significantly different between chemicals (Brown-Forsythe p < .001). To confirm that interchemical variability was the primary source of variance rather than intrachemical replicate variability, a Welch’s ANOVA was performed as this approach does not assume equal variances and adjusts for differing group sizes in the derivation of the residual degrees of freedom (Welch, 1951). The Welch’s ANOVA confirmed that in the linear model between log10 LD50 and chemical, most variability is attributable to inter-chemical variability (Figure 2C).
To assess whether any trends existed between variability and either the number of studies for a chemical or the chemical’s potency (eg, LD50), we evaluated the chemical-specific MADs relative to both of these factors (Figure 2D and 2E). Most chemicals had a relatively low MAD (represented by the y-axis spread of Figures 2D and 2E). We found that the MADs had low correlation with chemical potency (linear trend r2 = 0.046) and that MAD did not increase or decrease as the number of study replicates increased (Jonckheere-Terpstra test p > .05). It should, however, be noted that the sample distributions were rather biased, with relatively few chemicals having a high number of repeated studies. Thus, we suggest that variability, represented as high MADs over multiple studies, may be associated either with potency or with the number of replicate studies available. In general, it is difficult to conclude that the variability observed was simply due the number of times the rat acute oral toxicity assay was conducted.
Impact of Variability on Hazard Categorization
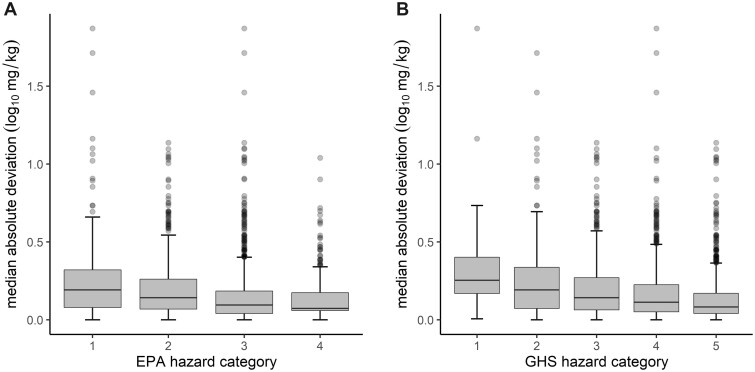
Acute oral toxicity studies are frequently used to derive hazard categorizations for safety labeling. Accordingly, we mapped the chemical-specific representative point estimate LD50 values to hazard categories using both the EPA (EPA, 2018) and GHS (UN, 2021) hazard classification systems. The association between hazard categories (ie, potency) and quantitative variability for the 1885 chemicals was evaluated by generating boxplots of the chemical-specific MADs for all chemicals per hazard category (Figure 3). A generally decreasing median was observed in MAD distributions across hazard categories, which were significant as tested by a one-sided Jonckheere-Terpstra trend test (p value .0002 for both the EPA and GHS systems; Figure 3A and 3B).
Figure 3.
MAD of LD50 values per chemical in each hazard category. The MAD (log10 mg/kg units) across all LD50 values, per chemical, was computed for each of the 1885 chemicals having at least 2 independently reported LD50 values. The boxplots reflect the distribution of MADs for each chemical classified into each respective hazard category for EPA categorization (A) and GHS categorization (B) schema, respectively, where lower hazard category numbers correspond to lower/more potent LD50 values. These distributions demonstrate that MAD, as a measure of variability, may be correlated with hazard category (ie, more potently toxic chemicals have higher variability; Jonckheere-Terpstra trend test p value = .0002 for both classification systems).
When limit tests and acute toxic class range values were added to the discrete LD50 data set, the expanded categorical data set contained 7574 entries representing 2441 chemicals. Note that the categorical data set encompassed the entirety of the discrete point estimate LD50 values (5826 entries representing 1885 chemicals). Because not all data points in this categorical data set were discrete values, a median or MAD per chemical could not be calculated for evaluating reproducibility or quantifying variability by hazard category. Instead, chemicals were grouped based the number of hazard categories they could be assigned to using the available replicate study data.
Concordance across hazard category determinations was computed and evaluated as compared with the number of experimental replicates and number of entries per hazard category (Supplementary Figure 1), revealing that increased study replication does not correlate with category concordance. In fact, disparate hazard categorization (ie, chemicals mapping to more than one hazard category) was seen for nearly 40% of chemicals with either classification schema. For example, of the 2441 chemicals in the expanded categorical data set, 809 were mapped to more than one EPA hazard category and 969 were mapped to more than one GHS category. The chemicals with the most variable LD50 values were associated with as many as 3 EPA hazard categories (19 chemicals) or with 3 (33 chemicals) and even 4 (2 chemicals) GHS hazard categories. The majority of data points in the expanded categorical acute oral toxicity data set were associated with EPA hazard Category III or GHS Categories 4 and 5 (Table 2), reflecting the general bias of the data set toward less potent chemicals for hazard category evaluation.
Table 2.
Hazard Categorization of Expanded Inventory
| EPA Category | GHS Category | Number of LD50 Entriesa | Number of Chemicalsa |
|---|---|---|---|
| I (LD50 ≤ 50 mg/kg) | 1 (LD50 ≤ 5 mg/kg) | 104 | 53 |
| I (LD50 ≤ 50 mg/kg) | 2 (5 < LD50 ≤ 50 mg/kg) | 342 | 183 |
| II (50 < LD50 ≤ 500 mg/kg) | 3 (50 < LD50 ≤ 300 mg/kg) | 1166 | 556 |
| II (50 < LD50 ≤ 500 mg/kg) | 4 (300 < LD50 ≤ 2000 mg/kg) | 528 | 354 |
| III (500 < LD50 ≤ 5000 mg/kg) | 4 (300 < LD50 ≤ 2000 mg/kg) | 2567 | 1309 |
| III (500 < LD50 ≤ 5000 mg/kg) | 5 (LD50 > 2000 mg/kg) | 2079 | 1032 |
| IV (LD50 > 5000 mg/kg) | 5 (LD50 > 2000 mg/kg) | 788 | 458 |
Note that although the sum of LD50 entries equals the total number of chemicals in the inventory (7574 entries), the total number of chemicals does not equal the sum of unique chemicals in the inventory (2441 chemicals) because many chemicals have entries (eg, LD50 values) in more than one row depending on whether replicate study results mapped to different categories.
The impact of variability in hazard category assignment across independently conducted replicate acute oral toxicity studies was summarized by conditional probabilities analysis. Briefly, conditional probabilities are a measure of the probability that, upon repeat testing, a chemical will be categorized into a given hazard category once a prior study has already categorized the chemical. This approach has similarly been applied to ocular and dermal toxicity studies to evaluate the reproducibility of these in vivo methods in identifying hazard categories (Luechtefeld et al., 2016; Rooney et al., 2021). Using the expanded categorical data set, the conditional probabilities were computed across the experimental data and summarized for EPA hazard categorization (Table 3) and GHS categorization (Table 4). For the EPA hazard system, an assignment to Category III was most likely to be replicated by subsequent studies, with a probability of 79.8%, whereas replication of a Category IV outcome was only 54.6%. Similarly striking results were observed for the GHS schema where an assignment to category 5 was most likely to be reproduced with the highest probability being 75%. These results suggest that reproducibility of the rat acute oral toxicity study for hazard categorization is closer to 50%–60% as studies are replicated and not nearly as consistent as might be expected.
Table 3.
Conditional Probabilities for EPA Hazard Category Reproducibility
| Conditional Probability of Subsequent Study Categorization |
|||||
|---|---|---|---|---|---|
| I | II | III | IV | ||
| First Study Hazard Category | I | 57.9% | 34.5% | 6.2% | 1.3% |
| II | 5.7% | 66.5% | 27.5% | 0.4% | |
| III | 0.5% | 11% | 79.8% | 8.7% | |
| IV | 0.1% | 0.6 | 44.7% | 54.6% | |
Bold values represent conditional probability of subsequent studies identifying the same hazard category as the first study.
Table 4.
Conditional Probabilities for GHS Hazard Category Reproducibility
| Conditional Probability of Subsequent Study Categorization |
||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| First Study Hazard Category | 1 | 53.3% | 34.9% | 1.5% | 5.1% | 5.1% |
| 2 | 7.7% | 48.9% | 33.2% | 8.9% | 1.3% | |
| 3 | 0.2% | 7.1% | 61.9% | 28.9% | 1.9% | |
| 4 | 0.1% | 1% | 11.0% | 66.1% | 21.8% | |
| 5 | 0% | 0.2% | 1% | 23.8% | 75% | |
Bold values represent conditional probability of subsequent studies identifying the same hazard category as the first study.
Evaluating Possible Chemical Sources of Variability
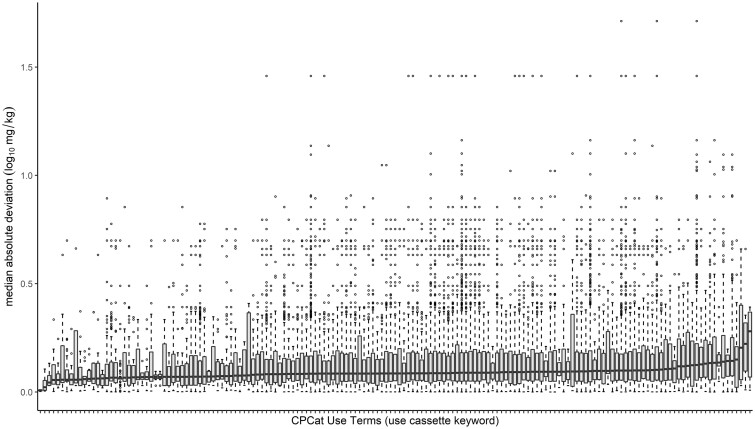
An investigation to assess whether chemical use was correlated with variability was also conducted. Chemical use categories were retrieved from the CPCat database (Dionisio et al., 2018). Of the 1885 chemicals with discrete point estimate LD50 values, 1619 had chemical use information. There were 181 unique use terms represented across the 1619 chemicals with retrieved use information. Chemicals were often associated with more than one use term; in fact, the number of use terms per chemical ranged from one to 128. Similarly, we also assessed how many chemicals were associated with each use term present in the data set, revealing a range of 1 to 1093 chemicals being associated with any term. There were 5 use terms associated with over 500 chemicals: manufacturing (1093 chemicals), industrial manufacturing (1048 chemicals), pesticide (901 chemicals), consumer use (713 chemicals), and personal care (518 chemicals). This analysis was repeated with the expanded categorical data set, yielding the same results (data not shown). To evaluate whether variability was associated with use terms, we focused on 161 use terms with at least 3 associated chemicals (Figure 4). An examination of this data subset revealed that the range of LD50 variability was comparable for all use terms represented in the data set. The lowest median MAD category (left-most boxplot in Figure 4) was UV stabilizer, which mapped to 3 chemicals having a combined median MAD of 0.0081 log10 (mg/kg). Conversely, the highest median MAD was 0.280 log10 (mg/kg) for the use term friction agent. There was no use term enriched with chemicals with more variable MADs, suggesting that use category is not correlated with variability in acute oral toxicity studies (Brown-Forsythe p = .27).
Figure 4.
Evaluation of LD50 median absolute deviation (MAD) per chemical use category. The 1619 chemicals with at least 2 discrete point estimate LD50 values could be mapped to chemical use categories in CPCat. Chemicals were associated with anywhere from 1 to 128 use terms among 181 unique use categories present in the data set. The 161 use category terms having at least 3 chemicals mapped are all represented across this figure (ie, each respective boxplot along the x-axis). The boxplots represent the distribution of LD50 MADs (in log10 mg/kg units) for the chemicals associated with each use term, respectively. Although the MAD across the chemicals mapped to each use term increases from left to right across the plot, the range of MADs (boxplots) generally overlap, confirming that there is no use term with significantly lower/higher variable chemicals.
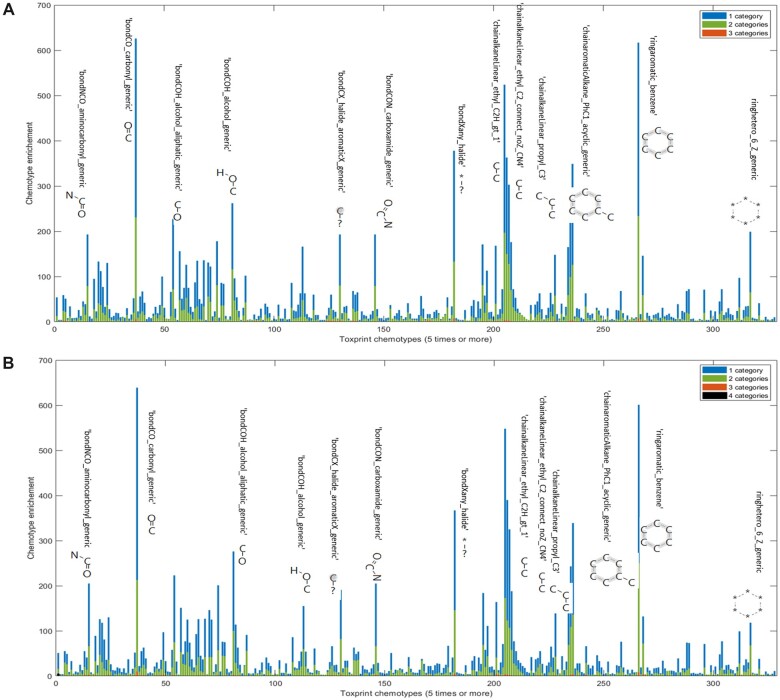
To investigate whether chemical structure was associated with an enrichment of greater variability in acute oral LD50 data, the publicly available set of 729 ToxPrint chemotypes (Yang et al., 2015) were utilized to define chemicals in terms of structural features. This set of chemical fingerprints was selected because it was developed specifically with toxicology and annotation of motifs associated with mechanisms of toxicity in mind. The ToxPrint chemotypes encompass 3 sets of substructures, all of which were included for our analysis: generic structural fragments, Ashby-Tennant genotoxic carcinogen rules, and cancer threshold of toxicological concern (TTC) categories. All 2441 chemicals in our expanded categorical data set were included in this analysis. The data set chemicals were represented by 408 of the 729 total ToxPrint chemotypes, suggesting a somewhat limited chemical space compared with all possible annotations available. Of the 408 ToxPrint chemotypes represented, 224 ToxPrint chemotypes had at least 5 chemicals mapped and were included for variability assessment (Figure 5). This analysis revealed that enrichment of any ToxPrint chemotype was proportional to the number of chemicals per variability class (classes were defined based on the number of hazard categories that the chemical was mapped to, as defined in the figure legend) and that there were no significant differentiators (ie, ToxPrint substructure/fragment) associated with higher or lower LD50 variability, as defined by the number of categories to which a chemical was classified across experimental replicates.
Figure 5.
Evaluation of LD50 variability per ToxPrint chemotype. The expanded categorical inventory of 2441 chemicals was mapped to ToxPrint chemotypes. Chemicals were associated with 408 ToxPrint chemotypes represented, of which 224 ToxPrint chemotypes had at least 5 chemicals mapped and are represented in this figure. Evaluations for both EPA hazard category (A) and GHS hazard category (B) schema yielded comparable results in that enrichment of ToxPrint chemotypes were proportional to the number of chemicals per variability class, rather than the class itself (1 category being low variability class, 2 being moderate, and 3–4 categories being high variability class).
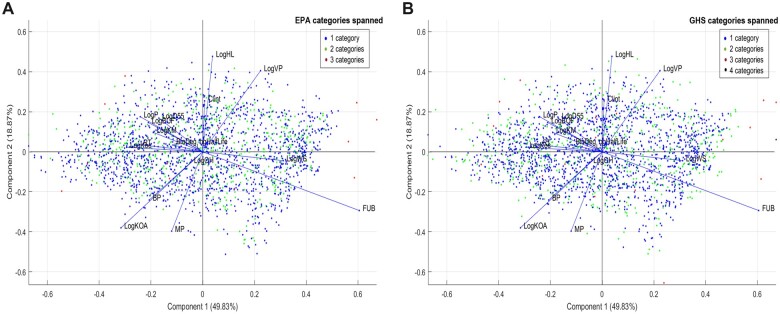
To further explore factors that may have affected variability, physicochemical properties for the 2441 chemicals in the expanded categorical data set were retrieved from OPERA and evaluated using principal component analysis (PCA) for any association with variability, as measured by the number of hazard categories that each chemical was classified to based on replicate LD50 study data. There were 17 physiochemical properties included in the analysis, including melting point, boiling point, and solubility properties. The PCA analysis yielded comparable results whether considering EPA or GHS categorization (Figure 6), with no clear clustering or trend for any physiochemical property, confirming that physiochemical properties were not correlated with variability in this data set of acute oral LD50 values.
Figure 6.
Evaluation of LD50 variability and physiochemical properties. The physiochemical properties for the expanded categorical inventory of 2441 chemicals, grouped into the same variability class groupings as Figure 7 (1 category being low variability class, 2 being moderate, and 3–4 categories being high variability) were retrieved from OPERA. PCA was conducted for both EPA hazard category (A) and GHS hazard category (B) schema yielded comparable results. Overall, there was no discernable physiochemical property related to variability.
Defining a Margin of Uncertainty for Interpreting LD50 Data
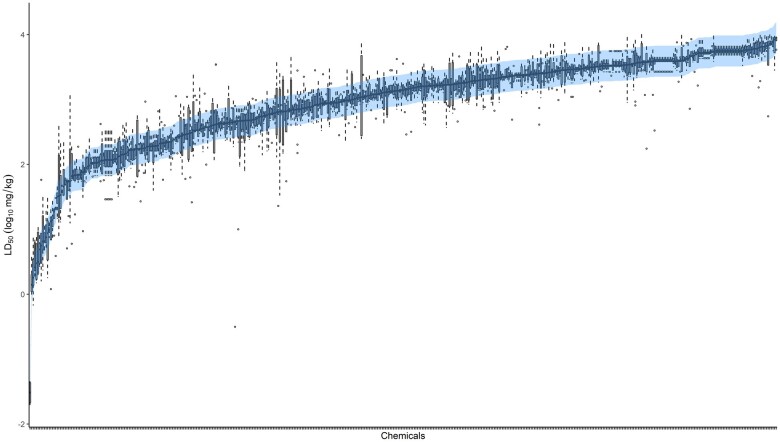
Given the variability documented herein for chemicals that have been evaluated in several independently conducted rat acute oral systemic toxicity studies and the impact on hazard categorization, it is apparent that some degree of uncertainty should be associated with experimentally derived LD50 values. In an effort to determine a margin of uncertainty based on experimental LD50 values, we leveraged our inventory of discrete point estimate rat acute oral LD50 values. MADs for the 1885 chemicals in the data set with 2 or more point estimate LD50 values were bootstrapped 5000 times, and the result provided a representative MAD (0.0953 ± 0.002 log10 (mg/kg)) that takes into account the range of MADs in the data set. A margin of ±2.5 × MAD was computed as ±0.24 log10 (mg/kg), providing a moderately conservative margin of uncertainty for acute oral LD50 values (Leys et al., 2013).
To demonstrate the range (±0.24 log10 (mg/kg)) against the experimental data, we highlight the uncertainty region surrounding the median LD50 in Figure 7. For chemicals with relatively consistent LD50 values across independent studies, the highlighted region encompasses all experimental LD50 values (represented by the boxplot). However, for those chemicals with a larger range of LD50 values (and higher variability), the highlighted region helps narrow down a high confidence range for each chemical’s LD50 estimate. For instance, converting the values back to linear range, which is consistent with experimental practice and application to classification schema, a highly toxic chemical with an LD50 of 5 mg/kg would have a confidence range of 2.9 to 8.7 mg/kg and for a low toxicity chemical with an LD50 of 5000 mg/kg it would be 2877–8689 mg/kg.
Figure 7.
Defining an acute oral toxicity LD50 margin of uncertainty. For illustration purposes, only chemicals with at least 4 LD50 values (467 chemicals) are shown in this plot. Each chemical has a boxplot wherein the box limits represent the 25th and 75th percentiles of the data, dashed lines represent the bounds for outlier detection defined by the 1.5 interquartile range rule, and circles indicate outliers. The MADs across point estimates, per chemical, were used as input for bootstrapping (sampling 5000 times), from which the median was used to compute a margin of uncertainty. This interval equates to ±0.24 (in log10 mg/kg units) and is shown centered around the median of LD50 values per chemical (shaded area). The defined range generally encompasses the distribution of experimental LD50 values and serves as a reasonable range for evaluating acceptable LD50 estimates per chemical.
DISCUSSION
The current study presents the most diverse and comprehensive compilation of curated rat acute oral LD50 data based on the consolidation of multiple resources, as well as providing broad coverage in terms of chemical structure and usage. The internationally sourced LD50 data contained instances of redundancy, which were limited to unique values per chemical through manual curation efforts. The compiled data allowed for comprehensive characterization of the variability and performance for the rat acute oral toxicity study. The data set is available for download via the National Toxicology Program’s Integrated Chemical Environment (https://ice.ntp.niehs.nih.gov/; last accessed January 2022).
The original data compilation, prior to the manual curation efforts undertaken here, served as the basis for several predictive modeling efforts, including the Collaborative Acute Toxicity Modeling Suite (CATMoS) collaboration (Mansouri et al., 2021), in which an appropriate representative LD50 value was calculated for each compound and data were used to develop a consensus model for predicting rat acute oral LD50 (Bercu et al., 2021; Borba et al., 2020; Helman et al., 2019; Kim et al., 2020; Lunghini et al., 2019). However, for the purpose of evaluating variability among replicate study results, additional data curation was conducted to increase confidence and fidelity in subsequent analyses. Our curation processes revealed several data cleaning challenges that should be considered in future data set compilation efforts. First, many values obtained from online resources overrepresented the actual number of studies conducted. For example, there were several instances where 3 LD50 values were extracted from the online resource, but upon closer inspection, those values represented one LD50 and the upper and lower bounds of its associated 95% confidence interval from a single study. Similarly, we noted multiple examples where 3 LD50 values were extracted, but they were all obtained from the same study and represented an LD50 for males, females, and both sexes combined. Second, some “extreme” LD50 values were chemical specific, and sometimes associated with chemicals that have multiple isomers that mapped to the same CASRN, which could introduce variability as different individual studies may have been testing different isomers. Furthermore, “extreme” LD50 values were also identified as being the result of unit transcription errors, for example, where mg/kg was recorded as g/kg. We also observed instances where a reported range of LD50 values was extracted as a single value, thereby creating a nonsensical “extreme” LD50 value, (eg, 500–2000 mg/kg recorded as 5 002 000 mg/kg). Collectively, these examples point to the critical need to carefully curate and review information from computationally derived data sets. However, it is important to note that there were many manually curated chemicals for which the high variability across multiple LD50 values could not be attributed to errors in the extraction process, and thus all seemingly reliable values were reported as experimental values herein.
Using the curated data set, analyses of data-rich chemicals (those having multiple unique LD50 values) revealed that independent experimental studies can yield LD50 values orders of magnitude apart. The variability among LD50 estimates for a single compound bears significant implications in regulatory applications due to disparities in hazard classification and labeling. We interrogated every source of variability that could be attributed to the chemicals themselves. We compared a wide range of parameters across the entire chemical space to determine if there were any distinct between “high variability” chemicals (ie, highest MADs or the most hazard categories classified per chemical) and “low variability” chemicals (ie, lowest MADs or only one hazard category classified per chemical). Chemical parameters such as functional use or chemical and physicochemical structural properties (ToxPrint and OPERA) could not account for the variability observed.
Accordingly, it is likely that variability among LD50 estimates for a single compound in our data set reflects biological variability inherent in animal models and/or the test method itself. It is important to note that although there are different iterations of the acute oral systemic toxicity test method with minor protocol differences (eg, up-and-down procedure, limit tests), they share key commonalities (species, dosing route, etc.), are all considered acceptable by regulatory authorities, and the results are used interchangeably for hazard and risk assessment purposes. Because this work is intended to support regulatory application, it was deemed appropriate to pool all obtained LD50 values despite the lack of experimental metadata. In the absence of complete study metadata, we were not able to consider known sources of biologic variability such as rat strain (Kacew and Festing, 1996; Walden and Schiller, 1985), sex (Fonsart et al., 2008), age (Gaines and Linder, 1986), or vehicle. Such sources of variability have the potential to influence acute LD50 estimates across an order of magnitude and may explain some of the variability observed in our analyses. For example, the acute LD50 of 2,3,7,8-tetrachlorodibenzodioxin (TCDD) is 9.8–17.7 µg/kg in Long Evans rats, but is greater than 7200 µg/kg in Wistar Hannover rats (Pohjanvirta et al., 1993). Similarly, sex differences can result in LD50 estimates that differ by several-fold between males and females. For example, female Sprague Dawley rats exhibit an oral LD50 for colchicine that is 2-fold lower than males (Wiesenfeld et al., 2007), whereas the acute LD50 of 3,4-methylenedioxymethamphetamine is 2.4-fold lower in male Sprague Dawley rats compared with females (Fonsart et al., 2008). In addition to strain and sex, rat age may also influence sensitivity toward a test chemical. The acute oral toxicity of 57 pesticides was compared across male and female adult or weanling Sherman rats, and LD50 estimates were significantly lower for adult rats compared with weanlings for 18 pesticides (up to 1.87-fold different; Gaines and Linder, 1986).
There are other factors not typically standardized across studies nor included in study metadata that may alter biological responses and potentially contribute to LD50 variability. Rodents are sensitive to their environment and previous work has shown that numerous factors can influence their biology, such as time of day/circadian cycle (Ede, 1973), and physical stressors such as restraint (Pearl et al., 1966) or noise level (Lauer et al., 2009), among others. For example, simply moving a rack of cages from the colony room into a new location, such as a procedure room, can induce a 12%–15% increase in thymus weight (Drozdowicz et al., 1990), a 36% decrease in lymphocyte count (Drozdowicz et al., 1990), or a 30%–40% increase in serum glucose in mice (Tabata et al., 1998). Animal handling can also influence physiology; picking rats up by their tails rather than by their body can predispose them to convulsions (Everds et al., 2013).
Rodent biology is sensitive to environmental factors but the potential for these stressors to impact acute oral LD50 estimates is not well characterized. There is evidence that stressors can directly impact key aspects of toxicology, such as alteration of stability or absorption of chemicals by changing gastrointestinal secretion or motility; disruption of vasoconstriction or vasodilation in different tissues, which affects distribution of chemicals; and impairment of metabolism of chemicals (Vogel, 1987, 1993). Standardized test methodology cannot feasibly encompass every single factor that may contribute to animal stress or otherwise serve as a potential source of variability. Given the numerous potential sources of variability in rodent studies, it is reasonable to conclude that even well-standardized test methods will not be able to control or account for every potential source of variability.
The curated data set compiled herein was used to establish a margin of uncertainty that could help in the assessment of NAMs. By bootstrapping MADs 5000 times, a representative MAD was obtained in order to compute a margin of uncertainty range of ±0.24 log10 (mg/kg). This result is within the same order of magnitude established in previous efforts such as Hoffmann et al. (2010), who noted that most of the 88 chemicals with rat data they evaluated had standard deviations less than 0.5 log10 (mg/kg) and calculated a median transformed standard deviation for all chemicals of 0.17 log10 (mg/kg). Recent work examining systemic effect levels from subacute, subchronic, chronic, multi-generation reproductive, and developmental toxicity studies found that unexplained variance ranged from 0.20 to 0.39 log10 (mg/kg), even after accounting for study protocol descriptors (Ly Pham et al., 2020).
With the application of rat acute oral toxicity data for hazard category determination, it is important to understand reproducibility, how uncertain a hazard category may be, and the likelihood of repeated studies resulting in the same outcome. By applying the conditional probability analyses, it is apparent that specific potency ranges are more likely to be reproduced than others. This analysis was conducted using the same conditional probabilities approach as has been applied to ocular and dermal toxicity data to help identify which hazard categories’ determination may be no better than a coin toss (Luechtefeld et al., 2016; Rooney et al., 2021). Analyses conducted with the current data set suggest that acute oral toxicity in rats is also largely only 50%–60% reproducible for most hazard categories in both the EPA and GHS categorization schema based on percentages computed using the conditional probabilities approach on the expanded data sets comprising multiple studies per chemical to yield hazard category outcomes. This striking result is an important takeaway from this study to help characterize reproducibility of the existing in vivo study. Understanding the limitations and variance inherent in the method provides important context for subsequently using these in vivo data for training and evaluating alternative predictive methods.
Our analyses provide a global margin of uncertainty of ±0.24 log10 (mg/kg) characterizing the variability in the rat acute oral systemic toxicity study based on curated reference animal data. Applying this margin of uncertainty onto predictions made with alternative methods that predict rat acute oral LD50 values can serve as an approach to help identify acceptable LD50 ranges to target. For example, in the Collaborative Acute Toxicity Modeling Suite (CATMoS; Mansouri et al., 2021), international predictive modeling efforts were undertaken to create consensus predictions for rat acute oral toxicity LD50s and hazard categories. To provide context to the predicted values in that modeling exercise, a 95% confidence interval allowed for the identification of outlier predictions and generation of consensus hazard category predictions. Such applications of this data set, or the margin of uncertainty generated herein, could substantially improve confidence in predicted values.
Understanding uncertainty and characterizing reference data helps provide much needed context to assess “gold standard” in vivo regulatory test methods. In fact, an ad hoc committee of the National Academies of Sciences, Engineering, and Medicine was recently charged with providing a review of the variability and relevance of existing laboratory mammalian toxicity tests for human health risk assessment to inform approaches for validation and establishing scientific confidence in new approaches. Herein we have compiled an extensive list of rat acute oral LD50 data from a large number of chemicals; we believe our extensive curation efforts allow these analyses to best represent the true variability of the acute oral lethality test. Experimental replication, chemical use, and chemical structure were not found to be sources of variability for disparate LD50 values arising from independently conducted acute oral toxicity tests. Finally, the margin of uncertainty derived herein can be leveraged to provide context for alternative approaches based on the understanding that the precision of NAMs that predict the LD50 is inherently limited by the precision of animal data.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the ICCVAM Acute Toxicity Workgroup for their valuable feedback and support, Catherine Sprankle, ILS, and technical reviewers Dr Richard Judson and Dr Pei-Li Yao for editorial review of the manuscript, and ECHA for generously contributing data to the project.
FUNDING
This project was funded in part with federal funds from the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH) under Contract No. HHSN273201500010C to Integrated Laboratory Systems, LLC in support of the National Toxicology Program Interagency Center for the Evaluation of Alternative Toxicological Methods.
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Agnes L Karmaus, Integrated Laboratory Systems, LLC, Morrisville, North Carolina 27560, USA.
Kamel Mansouri, National Toxicology Program Interagency Center for the Evaluation of Alternative Toxicological Methods, National Institute of Environmental Health Sciences, Research Triangle Park, North Carolina 27709, USA.
Kimberly T To, Integrated Laboratory Systems, LLC, Morrisville, North Carolina 27560, USA.
Bevin Blake, National Toxicology Program Interagency Center for the Evaluation of Alternative Toxicological Methods, National Institute of Environmental Health Sciences, Research Triangle Park, North Carolina 27709, USA.
Jeremy Fitzpatrick, Center for Computational Toxicology and Exposure, Office of Research and Development, U.S. Environmental Protection Agency, Research Triangle Park, North Carolina 27711, USA.
Judy Strickland, Integrated Laboratory Systems, LLC, Morrisville, North Carolina 27560, USA.
Grace Patlewicz, Center for Computational Toxicology and Exposure, Office of Research and Development, U.S. Environmental Protection Agency, Research Triangle Park, North Carolina 27711, USA.
David Allen, Integrated Laboratory Systems, LLC, Morrisville, North Carolina 27560, USA.
Warren Casey, National Toxicology Program Interagency Center for the Evaluation of Alternative Toxicological Methods, National Institute of Environmental Health Sciences, Research Triangle Park, North Carolina 27709, USA.
Nicole Kleinstreuer, National Toxicology Program Interagency Center for the Evaluation of Alternative Toxicological Methods, National Institute of Environmental Health Sciences, Research Triangle Park, North Carolina 27709, USA.
Disclaimer: This article may be the work product of an employee or group of employees of NIEHS, NIH, or other organizations. However, the statements, opinions or conclusions contained therein do not necessarily represent the statements, opinions, or conclusions of NIEHS, NIH, U.S. government, or other organizations. Integrated Laboratory Systems, LLC staff do not represent NIEHS, the National Toxicology Program, or the official positions of any federal agency.
REFERENCES
- Bercu J., Masuda-Herrera M. J., Trejo-Martin A., Hasselgren C., Lord J., Graham J., Schmitz M., Milchak L., Owens C., Hari Lal S., et al. (2021). A cross-industry collaboration to assess if acute oral toxicity (Q)SAR models are fit-for-purpose for GHS classification and labelling. Regul. Toxicol. Pharm. 120, 104843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borba J. V. V. B., Alves V., Braga R., Korn D., Overdahl K., Silva A. C., Hall S., Overdahl E., Strickland J., Allen D., et al. (2020). STopTox: An in-silico alternative to animal testing for acute Systemic and TOPical TOXicity. ChemRxiv. Cambridge Open Engage, Cambridge. 10.26434/chemrxiv.13283930.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionisio K. L., Phillips D., Price P. S., Grulke C. M., Williams A., Biryol D., Hong T., Isaacs K. K. (2018). The Chemical and Products Database, a resource for exposure-relevant data on chemicals in consumer products. Sci. Data 5, 180125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drozdowicz C., Bowman T., Webb M., Lang C. M. (1990). Effect of in-house transport on murine plasma corticosterone concentration and blood lymphocyte populations. Am. J. Vet. Res. 51, 1841–1846. [PubMed] [Google Scholar]
- Ede M. C. (1973). Circadian rhythms of drug effectiveness and toxicity. Clin. Pharmacol. Ther. 14, 925–935. [DOI] [PubMed] [Google Scholar]
- EPA. (2018). Label review manual. EPA Office of Pesticide Programs, Washington, DC. https://www.epa.gov/sites/production/files/2018-04/documents/lrm-complete-mar-2018.pdf.
- Everds N. E., Snyder P. W., Bailey K. L., Bolon B., Creasy D. M., Foley G. L., Rosol T. J., Sellers T. (2013). Interpreting stress responses during routine toxicity studies: A review of the biology, impact, and assessment. Toxicol. Pathol. 41, 560–614. [DOI] [PubMed] [Google Scholar]
- Fonsart J., Menet M.-C., Declèves X., Galons H., Crété D., Debray M., Scherrmann J.-M., Noble F. (2008). Sprague–Dawley rats display metabolism-mediated sex differences in the acute toxicity of 3,4-methylenedioxymethamphetamine (MDMA, ecstasy). Toxicol. Appl. Pharmacol. 230, 117–125. [DOI] [PubMed] [Google Scholar]
- Gaines T. B., Linder R. E. (1986). Acute toxicity of pesticides in adult and weanling rats. Fundam. Appl. Toxicol. 7, 299–308. [DOI] [PubMed] [Google Scholar]
- Griffith J. (1964). Interlaboratory variations in the determination of acute oral LD20. Toxicol. Appl. Pharmacol. 6, 726–730. [DOI] [PubMed] [Google Scholar]
- Grulke C. M., Williams A. J., Thillanadarajah I., Richard A. M. (2019). EPA’s DSSTox database: History of development of a curated chemistry resource supporting computational toxicology research. Comput. Toxicol. 12, 100096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helman G., Shah I., Patlewicz G. (2019). Transitioning the generalized read-across approach (GenRA) to quantitative predictions: A case study using acute oral toxicity data. Comput. Toxicol. 12, 100097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann S., Kinsner-Ovaskainen A., Prieto P., Mangelsdorf I., Bieler C., Cole T. (2010). Acute oral toxicity: Variability, reliability, relevance and interspecies comparison of rodent LD50 data from literature surveyed for the ACuteTox project. Regul. Toxicol. Pharmacol. 58, 395–407. [DOI] [PubMed] [Google Scholar]
- Hunter W. J., Lingk W., Recht P. J. (1979). Intercomparison study on the determination of single administration toxicity in rats. J. Assoc. Off. Anal. Chem. 62, 864–873. [PubMed] [Google Scholar]
- Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM). (2006). ICCVAM test method evaluation report. In vitro cytotoxicity test for estimating starting doses for acute oral systemic toxicity tests (NIH Publication No. 07-4519). National Institute of Environmental Health Sciences, Research Triangle Park, NC.
- Kacew S., Festing M. F. (1996). Role of rat strain in the differential sensitivity to pharmaceutical agents and naturally occurring substances. J. Toxicol. Environ. Health 47, 1–30. [DOI] [PubMed] [Google Scholar]
- Kim B. C., Joe D., Woo Y., Kim Y., Yoon G. (2020). Extension of pQSAR: Ensemble model generated by random forest and partial least squares regressions. IEEE Access 8, 180087–180099. [Google Scholar]
- Kinsner-Ovaskainen A., Rzepka R., Rudowski R., Coecke S., Cole T., Prieto P. (2009). Acutoxbase, an innovative database for in vitro acute toxicity studies. Toxicol. In Vitro 23, 476–485. [DOI] [PubMed] [Google Scholar]
- Lauer A. M., May B. J., Hao Z. J., Watson J. (2009). Analysis of environmental sound levels in modern rodent housing rooms. Lab. Anim. 38, 154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leys C., Ley C., Klein O., Bernard P., Licata L. (2013). Detecting outliers: Do not use standard deviation around the mean, use absolute deviation around the median. J. Exp. Soc. Psych. 49, 764–766. [Google Scholar]
- Luechtefeld T., Maertens A., Russo D. P., Rovida C., Zhu H., Hartung T. (2016). Analysis of public oral toxicity data from REACH registrations 2008-2014. ALTEX 33, 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunghini F., Marcou G., Azam P., Horvath D., Patoux R., Van Miert E., Varnek A. (2019). Consensus models to predict oral rat acute toxicity and validation on a dataset coming from the industrial context. SAR QSAR Environ. Res. 30, 879–897. [DOI] [PubMed] [Google Scholar]
- Ly Pham, L., Watford, S., Pradeep, P., Martin, M. T., Thomas, R., Judson, R., Setzer, R. W., and Paul Friedman, K. (2020). Variability in in vivo studies: Defining the upper limit of performance for predictions of systemic effect levels. Comput. Toxicol., 15, 1-100126. [DOI] [PMC free article] [PubMed]
- Mansouri K., Grulke C. M., Judson R. S., Williams A. J. (2018). OPERA models for predicting physicochemical properties and environmental fate endpoints. J. Cheminform. 10, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri K., Grulke C. M., Richard A. M., Judson R. S., Williams A. J. (2016). An automated curation procedure for addressing chemical errors and inconsistencies in public datasets used in QSAR modeling. SAR QSAR Environ. Res. 27, 911–937. [DOI] [PubMed] [Google Scholar]
- Mansouri K., Karmaus A., Fitzpatrick J., Patlewicz G., Pradeep P., Alberga D., Alepee N., Allen T. E. H., Allen D., Alves V., et al. (2021). CATMoS: Collaborative acute toxicity modeling suite. Environ. Health Perspect. 129, 47013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris-Schaffer K., McCoy M. J. (2021). A review of the LD50 and its current role in hazard communication. ACS Chem. Health Saf .28, 25–33. [Google Scholar]
- OECD. (2002a). Test no. 420: Acute oral toxicity-fixed dose procedure. In OECD Guidelines for the Testing of Chemicals, Section 4: Health Effects. Organisation for Economic Co-operation and Development, Paris.
- OECD. (2002b). Test no. 423: Acute oral toxicity-acute toxic class method. In OECD Guidelines for the Testing of Chemicals, Section 4: Health Effects. Organisation for Economic Co-operation and Development, Paris.
- OECD. (2008). Test no. 425: Acute oral toxicity: Up-and-down procedure. In OECD Guidelines for the Testing of Chemicals, Section 4: Health Effects. Organisation for Economic Co-operation and Development, Paris.
- Pearl W., Balazs T., Buyske D. A. (1966). The effect of stress on serum transaminase activity in the rat. Life Sci. 5, 67–74. [DOI] [PubMed] [Google Scholar]
- Pohjanvirta R., Unkila M., Tuomisto J. (1993). Comparative acute lethality of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), 1,2,3,7,8-pentachlorodibenzo-p-dioxin and 1,2,3,4,7,8-hexachlorodibenzo-p-dioxin in the most TCDD-susceptible and the most TCDD-resistant rat strain. Pharmacol. Toxicol. 73, 52–56. [DOI] [PubMed] [Google Scholar]
- Rooney J. P., Choksi N. Y., Ceger P., Daniel A. B., Truax J., Allen D. G., Kleinstreuer N. C. (2021). Analysis of variability in the rabbit skin irritation assay. Regul. Toxicol. Pharmacol. 122, 104920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland J., Clippinger A. J., Brown J., Allen D., Jacobs A., Matheson J., Lowit A., Reinke E. N., Johnson M. S., Quinn M. J., et al. (2018). Status of acute systemic toxicity testing requirements and data uses by us regulatory agencies. Reg. Toxicol. Pharmacol. 94, 183–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata H., Kitamura T., Nagamatsu N. (1998). Comparison of effects of restraint, cage transportation, anaesthesia and repeated bleeding on plasma glucose levels between mice and rats. Lab. Anim. 32, 143–148. [DOI] [PubMed] [Google Scholar]
- United Nations (UN). (2021). Globally Harmonised System of Classification and Labelling of Chemicals (GHS), 9th rev ed. United Nations, New York: . [Google Scholar]
- Vogel W. H. (1987). Stress—The neglected variable in experimental pharmacology and toxicology. Trends Pharmacol. Sci. 8, 35–38. [Google Scholar]
- Vogel W. H. (1993). The effect of stress on toxicological investigations. Hum. Exp. Toxicol. 12, 265–271. [DOI] [PubMed] [Google Scholar]
- Walden R., Schiller C. M. (1985). Comparative toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in four (sub)strains of adult male rats. Toxicol. Appl. Pharmacol. 77, 490–495. [DOI] [PubMed] [Google Scholar]
- Weil C., Wright G. J. T. (1967). Intra-and interlaboratory comparative evaluation of single oral test. Pharmacol. 11, 378–388. [Google Scholar]
- Weil C. S., Carpenter C. P., West J. S., Smyth H. F. Jr (1966). Reproducibility of single oral dose toxicity testing. Am. Ind. Hyg. Assoc. J. 27, 483–487. [DOI] [PubMed] [Google Scholar]
- Wiesenfeld P. L., Garthoff L. H., Sobotka T. J., Suagee J. K., Barton C. N. (2007). Acute oral toxicity of colchicine in rats: Effects of gender, vehicle matrix and pre-exposure to lipopolysaccharide. J. Appl. Toxicol. 27, 421–433. [DOI] [PubMed] [Google Scholar]
- Williams A. J., Grulke C. M., Edwards J., McEachran A. D., Mansouri K., Baker N. C., Patlewicz G., Shah I., Wambaugh J. F., Judson R. S., et al. (2017). The CompTox Chemistry Dashboard: A community data resource for environmental chemistry. J. Cheminform. 9, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Tarkhov A., Marusczyk J., Bienfait B., Gasteiger J., Kleinoeder T., Magdziarz T., Sacher O., Schwab C. H., Schwoebel J., et al. (2015). New publicly available chemical query language, CSRML, to support chemotype representations for application to data mining and modeling. J. Chem. Inf. Model 55, 510–528. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.