Abstract
Background
Reduced cardiorespiratory fitness (CRF) is an independent risk factor for dependency, cognitive impairment and premature mortality. High-intensity interval training (HIIT) is a proven time-efficient stimulus for improving both CRF and other facets of cardiometabolic health also known to decline with advancing age. However, the efficacy of equipment-free, unsupervised HIIT to improve the physiological resilience of older adults is not known.
Methods
Thirty independent, community-dwelling older adults (71(SD: 5) years) were randomised to 4 weeks (12 sessions) equipment-free, supervised (in the laboratory (L-HIIT)) or unsupervised (at home (H-HIIT)) HIIT, or a no-intervention control (CON). HIIT involved 5, 1-minute intervals of a bodyweight exercise each interspersed with 90-seconds recovery. CRF, exercise tolerance, blood pressure (BP), body composition, muscle architecture, circulating lipids and glucose tolerance were assessed at baseline and after the intervention period.
Results
When compared to the control group, both HIIT protocols improved the primary outcome of CRF ((via anaerobic threshold) mean difference, L-HIIT: +2.27, H-HIIT: +2.29, both p < 0.01) in addition to exercise tolerance, systolic BP, total cholesterol, non-HDL cholesterol and m. vastus lateralis pennation angle, to the same extent. There was no improvement in these parameters in CON. There was no change in diastolic BP, glucose tolerance, whole-body composition or HDL cholesterol in any of the groups.
Conclusions
This is the first study to show that short-term, time-efficient, equipment-free, HIIT is able to elicit improvements in the CRF of older adults irrespective of supervision status. Unsupervised HIIT may offer a novel approach to improve the physiological resilience of older adults, combating age-associated physiological decline, the rise of inactivity and the additional challenges currently posed by the COVID-19 pandemic.
Trial registration
This study was registered at clinicaltrials.gov and coded: NCT03473990.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12877-022-03208-y.
Keywords: Exercise, Supervision, Cardiorespiratory fitness, HIIT, Cardiometabolic
Background
Cardiovascular disease is the most common cause of death worldwide, accounting for nearly one third of deaths each year [1]. Physical inactivity and advancing age are each associated with an increased risk of cardiovascular disease [2], with just a quarter of older adults in the UK meeting the minimum recommended activity levels needed to maintain health [3]. Considering the ongoing COVID-19 pandemic, physical activity levels amongst older adults are reported to have reduced further due to national lockdowns and shielding behaviours [4], lessening their physiological resilience to health-related challenges.
Cardiorespiratory fitness (CRF) is not only the “gold-standard” measure of physical fitness [5], but is also a recognised marker of cardiovascular health, and prognostic marker of both cardiovascular disease [6] and premature mortality [7, 8]. Known to decline with advancing age [9], reductions in CRF have been suggested to be due to declines in mitochondrial quality and reductions in maximal ATP production and aerobic respiration rate [10, 11]. Exemplifying the impact of physical activity on CRF, sedentary adults with lower CRF have a mortality risk that is 4.5 times higher than those that exercise regularly [12], with reduced CRF a stronger risk factor for all-cause mortality than hypertension, smoking and diabetes [13].
Beyond CRF, another important risk factor for premature mortality in older adults is sarcopenia; the age-associated loss of muscle mass and function [14, 15]. Exacerbated by physical inactivity [15], sarcopenia is a precursor to both falls and frailty syndromes, and is directly associated with lower CRF [16]. Another age-associated change in body composition which is also associated with physical inactivity is excess accumulation of body fat. Associated with cardiovascular disease and metabolic dysfunction (i.e., insulin resistance and dyslipidaemia), obesity in older age not only impacts morbidity and mortality, but also impacts quality of life and independence maintenance [17].
Exercise is a proven strategy to improve CRF, muscle mass and metabolic status in older adults [12, 18, 19], with aerobic [20] and resistance exercise training [21] each eliciting distinct favourable adaptations. However, high-intensity interval training (HIIT) appears to confer improvements commonly associated with both aerobic and resistance exercise training [22], whilst also addressing a commonly cited barrier to exercise uptake and adherence, “lack of time” [23]. Beyond “time”, older adults also frequently cite their age and “poor health” as barriers to exercise [24], despite a significant body of literature evidencing the benefits of all types of exercise training (aerobic: [25, 26], resistance: [21, 27], HIIT: [28, 29]) for even the oldest old [30]. Further, the Generation 100 randomised control trial has demonstrated that older adults can perform both aerobic exercise and HIIT without strict supervision [31, 32], suggesting that home-based, time-efficient HIIT without the need for specialist equipment may prove a promising strategy to improve the physiological resilience of older adults, especially at a time when access and desire to attend specialist exercise facilities (i.e., gyms and community classes) may be at an all-time low.
We have previously demonstrated that 4-weeks equipment-free, unsupervised HIIT can elicit comparable improvements in the CRF of middle-aged adults when compared to supervised, cycle ergometer-based HIIT [33], and that supervision status does not impact the efficacy of HIIT for improving CRF in young adults [34]. However, to date, no study has investigated the impact of short-term, equipment-free, unsupervised HIIT for improving multiple parameters of physiological resilience in older adults.
Methods
Subject characteristics
Thirty independent community-dwelling older (71(SD = 5) years) adults (14 male) who were disability free and able to perform activities of daily living without support or assistive devices, and who were not engaged in a formal exercise regime (defined as 2 or more sessions per week) were recruited to this study. Further exclusion criteria were taken from the American Thoracic Society/American College of Chest Physicians, and the Preoperative Exercise Testing and Training Society [35, 36] safety guidelines for exercise testing, which include, but are not limited to: numerous cardiac abnormalities, hemodynamic compromise, untreated resting hypertension, lower limb thrombus, pulmonary oedema, embolus or significant hypertension and orthopaedic or cognitive impairment leading to inability to perform the exercise or cooperate, respectively. After health screening and provision of informed consent, participants were randomised to each intervention group using random permuted blocks of: (i) laboratory (supervised) HIIT (L-HIIT), (ii) home (unsupervised) HIIT (H-HIIT) and (iii) a no-intervention control (CON). Ethical approval for the study was obtained from the University of Nottingham Faculty of Medicine and Health Sciences Research Ethics Committee (C16122016). The study was registered at clinicaltrials.gov (NCT03473990) and all aspects of the study were performed in accordance with the Declaration of Helsinki.
Assessment sessions
Assessment sessions were conducted 72 h before and 72 h after a 4-week intervention period. Participants were asked to fast from 2200 h the preceding night and to attend the research unit at 0900 h. Measures of height and weight were taken and resting BP was measured in accordance with established guidelines [37]. Before a two-hour oral glucose tolerance test (OGTT) was conducted [38], body composition was determined by dual-energy x-ray absorptiometry (DXA; Lunar Prodigy 2, GE Medical Systems, Buckinghamshire, UK). Baseline blood samples from the OGTT were subject to clinical chemistry analysis to determine cholesterol profiles. Plasma insulin concentrations from all OGTT samples were measured using an ultrasensitive enzyme linked immunosorbent assay (ELISA; Mercodia AB, Uppsala, Sweden), with blood glucose measured on a near-patient glucose analyser (YSI Life Sciences, Ohio, USA). During the OGTT, muscle architecture (fibre pennation angle (PA), muscle thickness (MT) and fascicle length (FL)) was assessed using B-mode ultrasound (MyLab™50; Esaote, Genoa, Italy) as previously described [39, 40].
After provision of a standardised meal and rest period, a cardiopulmonary exercise testing (CPET) was performed in accordance with the aforementioned guidelines [35, 36] on a cycle ergometer (Lode Corival, Lode BV, Groningen, Netherlands). Breath-by-breath data was collected using an inline gas analysis system (ZAN 680, nSpire Health, Colorado, USA). Each test began with a 3-minute period of observed rest on the cycle ergometer followed by a 2-minute warm-up of unloaded cycling. Participants were encouraged to exercise to volitional exhaustion, with termination criteria as per safety guidelines [35, 36]. VO2 peak was defined as the highest VO2 attained during the test with anaerobic threshold (AT) defined using a combination of the modified V-slope and ventilatory equivalents methods by two blinded experienced assessors [41].
Interventions
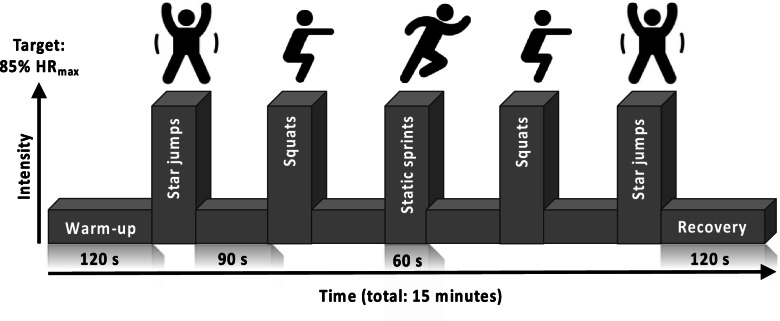
The HIIT protocol used was based on previous work demonstrating the efficacy of this temporal protocol to improve CRF when performed on a cycle ergometer [33, 42] and with bodyweight-based exercises [33, 34]. All sessions began with a warm-up period of two minutes walking on-the-spot before five, one-minute high-intensity efforts were performed, each interspersed by 90 s of active recovery. Following the final effort, a 2-minute recovery period concluded each session. Exercises were performed in a pyramid formation, designed to provide motivation to maintain intensity during fatigue (i.e., in efforts 4 and 5) (Fig. 1).
Fig. 1.
Schematic representation of the high intensity interval training (HIIT) protocol perform with and without supervision. Abbreviations: HRmax, age-predicted maximum heart rate (220-age); s, seconds
All participants assigned to a HIIT group attended the research unit for a HIIT introductory session before starting training. This session ensured that participants were comfortable with the temporal profile of the sessions, were coached for proper form during each exercise, and showed H-HIIT participants how to record engagement with each session. The aim for each effort during every session was to achieve a heart rate > 85% age-predicted maximum [43].
All participants were asked to maintain their habitual level of physical activity and dietary intake for the duration of the study. Participants in both HIIT groups completed 3 sessions each week for 4 weeks. L-HIIT participants completed this under one-to-one supervision at the research unit, with verbal encouragement throughout each session. H-HIIT participants completed their sessions at home with no supervision or feedback. Participants in the H-HIIT group were given a printed resource outlining the HIIT protocol and a fingertip pulse oximeter (iMDK, MediSave, UK) to assess HR at the end of each effort. Training adherence (number of sessions) and compliance (% of sessions where the target heart rate threshold was achieved) for the H-HIIT group was determined via instructed self-report using a training diary and the fingertip pulse oximeter, with attendance and HR recorded by the research team for the L-HIIT group.
Statistical analysis
Based on CRF data from a previous study [33], an a priori power calculation using the R pwr package, based on an improvement in CRF from 15.28 to 18.23ml/kg/min, provides an effect size of 1.12, which for n = 10 per group provides a power of greater than 80%. Descriptive statistics are reported as number (%) or mean (SD). Change between groups was determined using ANCOVA with baseline values as the continuous covariates [44]. Within group analysis for pre to post intervention change was performed using repeated measures t-tests. Effect estimates are presented as mean differences (MD) with 95% confidence intervals (CI). Due to lack of normality, equality of variance and heteroscedasticity for pennation angle, these data were log transformed. Analyses were conducted using Stata Version 16 (StataCorp LLC, TX, USA). The level of significance was set at p < 0.05.
Results
Participant characteristics
Thirty independent community-dwelling older adults were recruited to this study. Groups were matched for age, BMI, CRF, BP and cholesterol at baseline (Table 1).
Table 1.
Participant baseline characteristics
| L-HIIT (n = 10) | H-HIIT (n = 10) | CON (n = 10) | |
|---|---|---|---|
| Age (years) | 70 (5) | 71 (4) | 71 (7) |
| Male / Female | 5/5 | 3/7 | 6/4 |
| BMI | 26 (3) | 25 (3) | 26 (1) |
| AT | 13.40 (4) | 14.90 (6) | 14.27 (3) |
| VO2peak | 26.37 (8) | 25.47 (8) | 28.05 (4) |
| SBP | 127 (14) | 126 (10) | 128 (11) |
| DBP | 73 (7) | 68 (12) | 77 (7) |
| Total Cholesterol | 5.1 (1.5) | 5.7 (0.9) | 5.1 (0.7) |
| Co-morbidities | |||
| − BPH | 1 | - | - |
| − HTN | 2 | 2 | 2 |
| − Asthma | - | 1 | 1 |
| − Depression | - | 1 | - |
| − OA | - | 1 | 3 |
| − HCL | - | 1 | 1 |
| − Hypothyroidism | - | - | 1 |
| Medications | |||
| − Alpha-blocker | 1 | - | - |
| − ACE-inhibitor | 2 | 1 | 1 |
| − Statins | - | 1 | 1 |
| − Ca + channel blocker | - | 1 | 1 |
| − SSRI | - | 1 | - |
| − Thyroxine | - | - | 1 |
| − Beta-2 adrenergic receptor agonist | - | 1 | 1 |
Data depicts mean (SD) or for co-morbidities and medication class. Abbreviations: L-HIIT, laboratory (supervised) high intensity interval training; H-HIIT, home-based (unsupervised) HIIT; CON, no-intervention control group; BMI, body mass index (kg/m2); AT, anaerobic threshold (ml/kg/min); VO2 peak, peak oxygen uptake (ml/kg/min); SBP, systolic blood pressure (mmHg); DBP, diastolic BP; BPH, benign prostatic hyperplasia; HTN, hypertension; HCL, hypercholesterolaemia; OA, osteoarthritis; ACE, angiotensin converting enzyme; Ca+, calcium; SSRI, serotonin re-uptake inhibitor. There were no significant differences between groups in any numerical parameter
Study adherence
After randomisation, no participants were lost to follow-up. There were no adverse safety events reported in any group. Two participants (1 H-HIIT, 1 CON) did not have a post intervention DXA scan. Training adherence and compliance was 100% for both HIIT groups.
Cardiorespiratory fitness and exercise tolerance
There was a significant increase in AT in L-HIIT (MD + 2.27 (0.57 to 3.98) ml/kg/min; p = 0.007) and H-HIIT (MD + 2.29 (0.59 to 4) ml/kg/min; p = 0.006) when compared to CON, with no difference in change between the HIIT groups (MD -0.02; 95% CI -1.74 to 1.7; p = 1.0) (Fig. 2 A, Supplementary Information Table S1). Similarly, there was a significant increase in VO2peak in L-HIIT (MD + 3.05 (0.81 to 5.29) ml/kg/min; p = 0.005) and H-HIIT (MD + 3.45 (1.19 to 5.7) ml/kg/min; p = 0.002) when compared to CON, with no difference in change between the HIIT groups (MD -0.4 (-2.63 to 1.84) ml/kg/min; p = 0.96) (Fig. 2B, Table S1).
Fig. 2.
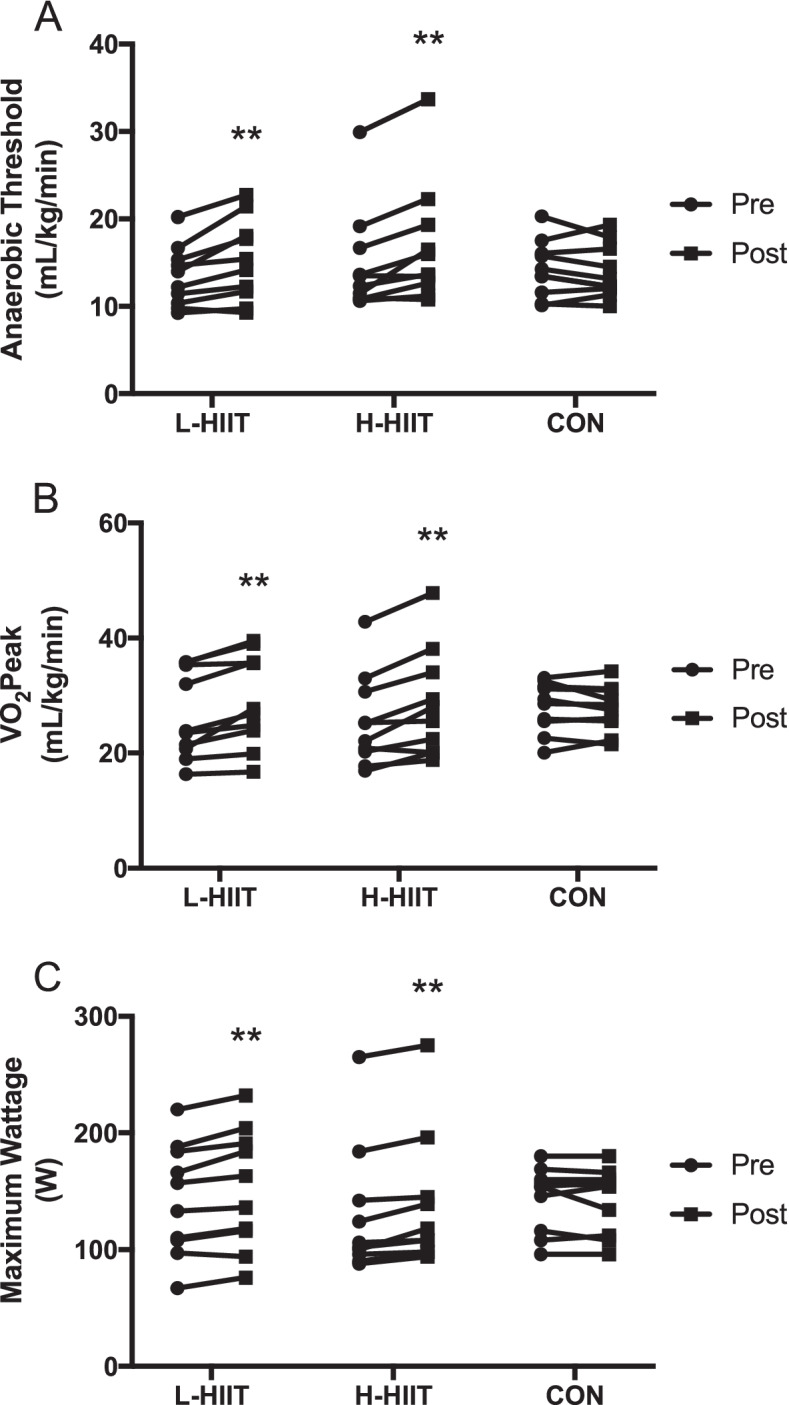
Anaerobic threshold (A), VO2peak (B) and maximum wattage (C) in older individuals before and after laboratory (L-HIIT; n = 10) or home-based (H-HIIT; n = 10) high intensity interval training (HIIT) or a no intervention control (CON; n = 10) period. **= p < 0.01 versus pre-intervention
Exercise tolerance, determined as maximum wattage (Wmax), increased in both L-HIIT (MD + 10.64 (2.9 to 18.38) W; p = 0.005) and H-HIIT (MD + 10.57 (2.98 to 18.16) W; p = 0.005) when compared to CON, with no difference in change between the HIIT groups (MD + 0.07 (-7.68 to 7.82) W; p = 1) (Fig. 2 C, Table S1).
Cardiometabolic status
There was a significant reduction in resting systolic BP in L-HIIT (MD -6 (-12 to -1) mmHg; p = 0.02) and H-HIIT (MD -6 (-11 to 0) mmHg; p = 0.05) when compared to CON, with no difference in change between the HIIT groups (MD -1 (-6 to 5) mmHg; p = 0.97) (Fig. 3 A, Table S1). There were no significant changes in resting diastolic BP in any group (Table S1).
Fig. 3.
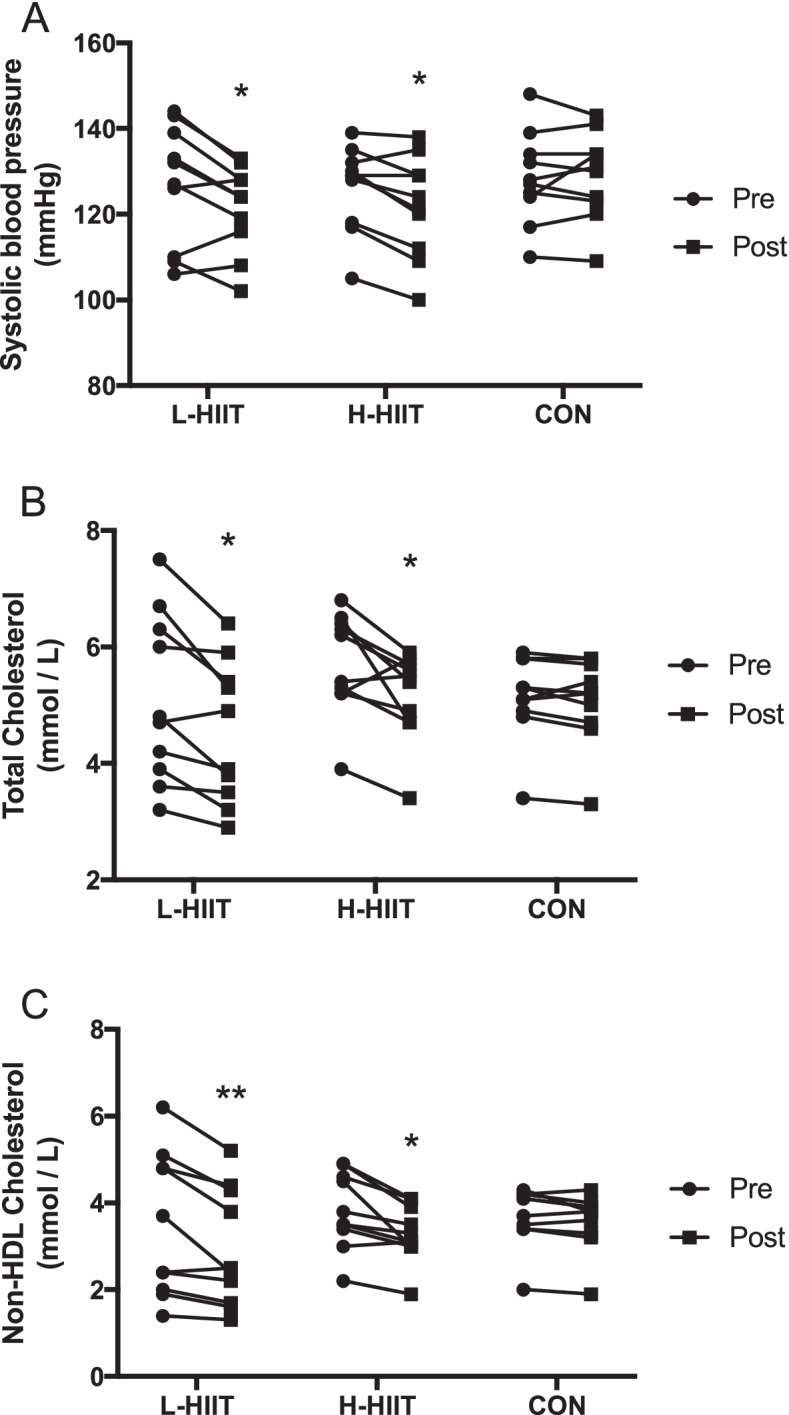
Systolic blood pressure (A), total cholesterol (B) and non-HDL cholesterol (C) in older individuals before and after laboratory (L; n = 10) or home-based (H; n = 10) high intensity interval training (HIIT) or a no intervention control (CON; n = 10) period. *=p < 0.05, **= p < 0.01 versus pre-intervention
There was a significant reduction in both total (L-HIIT (MD -0.51 (-1.01 to -0.01) mmol/L; p = 0.04) and H-HIIT (MD -0.46 (-0.87 to -0.05) mmol/L; p = 0.05)) (Fig. 3B) and non-HDL (L-HIIT (MD -0.46 (-0.81 to -0.11) mmol/L; p = 0.008) and H-HIIT (MD -0.38 (-0.73 to -0.03) mmol/L; p = 0.03) (Fig. 3 C) cholesterol in L-HIIT and H-HIIT when compared to CON, with no difference in change between the HIIT groups (Table S1). HDL cholesterol did not change in any group (Table S1). Neither glucose nor insulin area-under-the-curve (AUC) during the OGTT, or the homeostatic model assessment of insulin resistance (HOMA-IR) changed in any group (Table S1).
Body composition and muscle architecture
There was a significant decrease in total fat mass in L-HIIT and H-HIIT (Fig. 4 A, Table S1), although only the reduction in L-HIIT was different to CON (Table S1). Percentage body fat decreased in both L-HIIT and H-HIIT, but these changes were not significantly different to CON (Table S1). Similarly, whole-body lean mass increased in both L-HIIT and H-HIIT, but these changes were not significantly different to CON (Fig. 4B, Table S1).
Fig. 4.
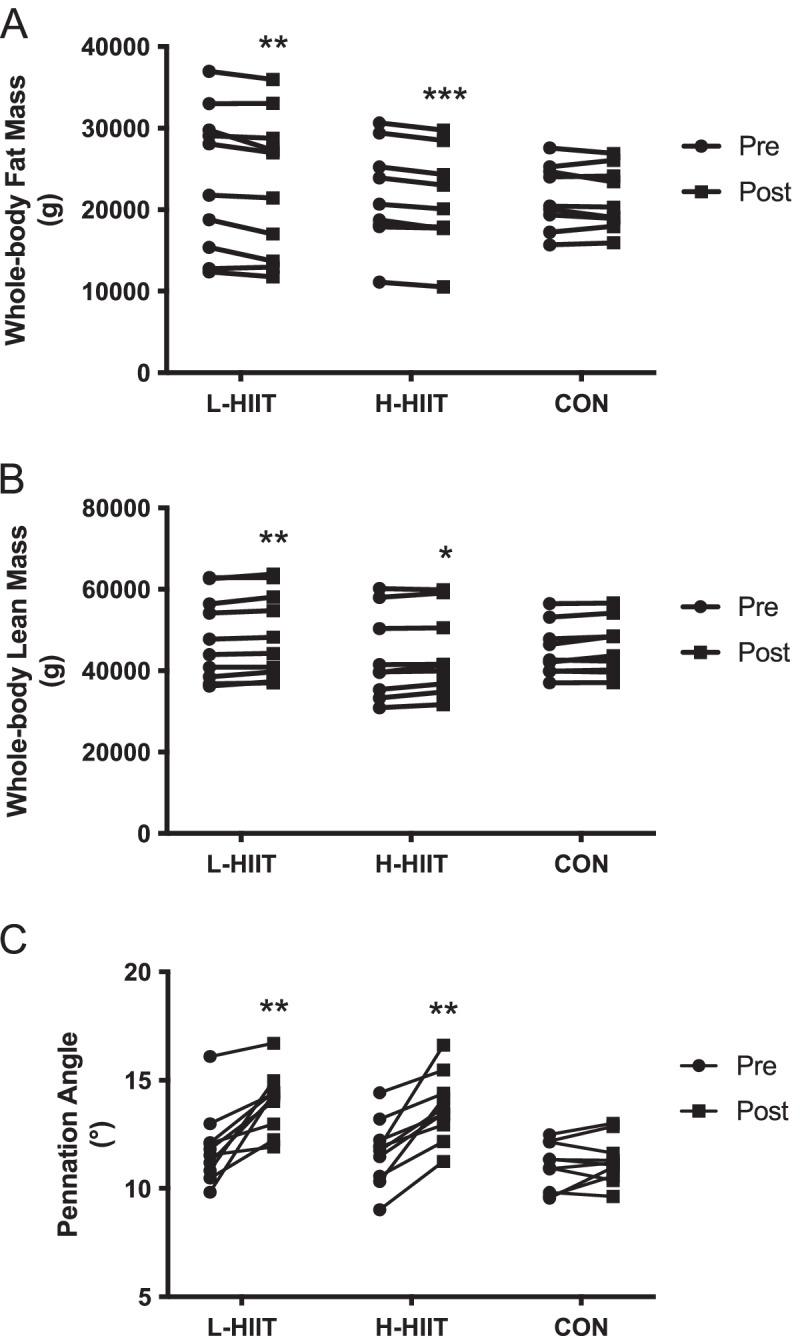
Whole-body fat (A) and lean (B) mass and m. vastus lateralis pennation angle (C) in older individuals before and after laboratory (L; n = 10) or home-based (H; n = 10) high intensity interval training (HIIT) or a no intervention control (CON; n = 10) period. **= p < 0.01, ***=p < 0.01 versus pre-intervention
There was a significant increase in m. vastus lateralis PA in L-HIIT (MD + 1.95 (0.72 to 3.17) º; p = 0.001) and H-HIIT (MD + 1.8 (0.59 to 3.01) º; p = 0.002) when compared to CON, with no difference in change between the HIIT groups (MD + 0.14 (-1.05 to 1.34) º; p = 0.99) (Fig. 4 C, Table S1). Neither m. vastus lateralis MT nor FL changed in any group (Table S1).
Discussion
Whilst the positive effects of HIIT on CRF in older adults are well-established [20, 42, 45], to our knowledge, this is the first study to show that just four-weeks, time-efficient, equipment-free HIIT is able to elicit improvements in the CRF, body composition and cardiovascular health of older adults. Further, these benefits are also achievable via unsupervised, home-based exercise. The rapidity of these improvements demonstrate the potential for this exercise regime to be used in time-constrained clinical settings commonly associated with advancing age (i.e., prior to cancer surgery [42, 45]), as well as to improve the general health and physiological resilience of an ageing population [4].
In addition to being able to elicit improvements in a number of physiological parameters key to optimal health across the life-course, the HIIT regime employed in this study also addresses the issue of “lack of time”, a commonly cited barrier to exercise for older adults [46, 47]. The improvements reported herein were achieved with a total time commitment of < 50 min per week; one third of the current guidelines for moderate continuous exercise [48]. That the benefits achieved were largely indistinguishable between the two HIIT groups, contests the need for supervision to optimise the benefits of exercise [49], a notion supported by previous work reporting on middle-aged adults [50]. Although there appears to be no data reporting the impact of HIIT supervision in older adults, in young [34] and middle-aged [33] adults, and in adults with type 1 diabetes [51] and heightened cardiovascular disease risk [52], supervision was not shown to enhance adaptation. It should however be noted that in the latter two studies telehealth monitoring and feedback was employed, compared to the self-report of adherence and absence of feedback for the home-based group in this study. Conversely, improvements in functional balance were reported as superior with supervision during strength and coordination training [53, 54], suggesting that the influence of supervision is likely impacted by the type of exercise training and/or the assessed outcomes.
Another key benefit to the HIIT regime employed in this study is that no facilities or equipment are required, removing two further barriers (access to facilities and reticence to exercise in public [23, 55, 56]) to exercise for older adults. In addition, our HIIT protocol only included three exercises, addressing concerns about complexity [49], each of which are easily adaptable for those with, for example, joint restrictions which commonly occur in older age [57]. It is our belief, that in a pragmatic roll-out of equipment-free, unsupervised HIIT to improve the health of older adults, any exercise which can be performed safely to raise heart to > 85% of age-predicted maximum could be substituted into the temporal regime reported herein.
That we observed significant improvements in AT, VO2peak and exercise tolerance adds to the growing body of literature reporting that HIIT is safe and effective for improving CRF in older adults [42, 58–60]. In addition, HIIT is able to elicit these gains in a shorter time-frame than that commonly reported for aerobic exercise training [61]. For example, the improvements we report herein, using an equipment free protocol (irrespective of supervision) are not dissimilar to those previously seen in older adults with HIIT of the same temporal profile but on a specialised cycle ergometer; findings which have been replicated in comorbid octogenarians [30]. Not only are improvements in CRF associated with reduced cardiovascular disease risk and all-cause mortality in older adults [7], but the magnitude of improvement observed in this study is above the reported minimum clinically important difference in AT (1.5 ml/kg/min) to reduce post-operative risk [62]. This finding exemplifies the broad potential of this equipment-free HIIT regime to improve the physiological resilience of older adults in different situations.
Aligned to the reduction in CVD risk associated with improved CRF [7], reductions in both SBP and cholesterol (total and non-HDL) are also associated with an reduced risk of all-cause mortality in older adults [63]. Our finding of reduced resting SBP, irrespective of supervision, is in agreement with previous HIIT studies of older adults, albeit with most of these based having a longer training period [18], greater time-commitment [64] and supervision [45]. In addition, the magnitude of our reductions in resting SBP are superior to those reported with 3-months, time-intense aerobic exercise training in older adults [65], and similar to that elicited by 6-weeks HIIT on a cycle ergometer [20]. Our reduction in cholesterol aligns with previous suggestions that higher-intensity exercise is required to reduce non-HDL cholesterol effectively [66], further supporting the notion that HIIT may be an alternative option to traditional exercise training modalities to reduce cardiovascular disease risk in older adults.
To our surprise, despite a wealth of evidence suggesting that insulin sensitivity can be improved in older adults via HIIT [58, 67, 68], and that HIIT can combat the severity of type 2 diabetes [69, 70], we were not able to show improvement in any marker of insulin sensitivity. This may be due to a longer time period of training being required and/or due to improvements in insulin sensitivity being more pronounced in subjects with existing insulin resistance [71].
Importantly, given the largely inevitable and progressive development of sarcopenia and the relationship between low muscle mass and all-cause mortality, falls, frailty [72] and dependency [73] in older adults, the HIIT regime employed in this study was able to elicit favourable changes in body composition. These changes include increased whole-body lean mass, increased muscle pennation angle, reduced fat mass and reduced body mass index. Muscle pennation angle has been shown to be associated with sarcopenia progression [74] and is an accepted marker of force and contractile capabilities [75] which corresponds to both muscle cross-sectional area and muscle strength [76]. Increased fat mass and obesity are both related to the development of cardiometabolic disorders [77], and as such improvements in these parameters further exemplifies the potential of this intervention for maintaining whole-body health across the life course. It must however be acknowledged that for the majority of these parameters the changes seen in the HIIT groups were not statistically different to that observed in the control group- perhaps owing to a lack of statistical power to determine between group differences. Furthermore, the magnitude of reduction in fat mass seen in this study is not consistent with numerous studies (including those in older adults) [61, 67], where an average 6% reduction was seen with ten weeks of HIIT [78]. That our individuals were only slightly overweight on average, and/or that our HIIT regime was considerably shorter may explain this discrepancy, whilst also highlighting the potential for this regime to have even more impact in older individuals with less favourable body composition profiles [67].
As with all studies, we recognise some limitations to this work. Whilst participants were asked to maintain their current level of physical activity and habitual dietary intake for the duration of the study, this was not measured, and as such alterations in these aspects may have influenced outcomes of this study. In addition, our participants were older community-dwelling volunteers with only well-controlled comorbidities. The ability of multimorbid, frail, dependent and/or very elderly individuals to undertake unsupervised equipment-free HIIT has been poorly studied to date and given the ageing population should be considered in future work. Finally, while our home-based intervention was delivered without supervision or engagement, participants did attend for an in-person instruction session (and so that study-related resources could be provided). As such, future studies considering the ‘roll-out’ of home-based HIIT may wish to explore the impact of remote delivery of the instruction session to facilitate a wholly home-based intervention.
Conclusions
In conclusion, four-weeks, time-efficient, equipment-free HIIT is able to elicit improvements in the CRF, body composition and cardiometabolic health of older adults irrespective of supervision. This application of HIIT at home may provide a novel approach to increase the physiological resilience of older adults by combating the rise of inactivity and associated poor health in a growing section of society; both of which are magnified by current challenges associated with the COVID-19 pandemic.
Supplementary Information
Acknowledgements
Not applicable.
Abbreviations
- CRF
cardiorespiratory fitness
- HIIT
high-intensity interval training
- L-HIIT
Lab HIIT, H-HIIT: Home HIIT
- CON
control (group)
- BMI
body mass index
- AT
anaerobic threshold
- VO2peak
peak volume of oxygen
- SBP
systolic blood pressure
- OGTT
oral glucose tolerance test
- DXA
dual-energy x-ray absorptiometry
- ELISA
enzyme linked immunosorbent assay
- PA
pennation angle
- MT
muscle thickness
- FL
fascicle length
- CPET
cardiopulmonary exercise test
- HRmax
maximum heart rate
- MD
mean difference
- CI
confidence interval
- Wmax
maximum wattage
- BP
bloos pressure
- HDL
high-density lipoprotein
- AUC
area under-the-curve
- HOMA-IR
homeostatic model assessment of insulin resistance
Authors’ contributions
TSS, JNL and BEP were responsible for study conception; TSS, TI and AG were responsible for data acquisition; TSS, BD, JJB and BEP were responsible for data interpretation; TSS, PJA and BEP were responsible for manuscript writing. All authors read and approved the final manuscript.
Funding
This work was funded by the Medical Research Council (MRC) and Versus Arthritis via the MRC-Versus Arthritis Centre for Musculoskeletal Ageing Research (MR/P021220/1). The funding body had no role in the study design, collection, analysis, data interpretation or manuscript writing.
Availability of data and materials
The datasets from this study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Ethical approval for this study was obtained from the University of Nottingham Faculty of Medicine and Health Sciences Research Ethics Committee (C16122016), with all participants providing written, informed consent. All aspects of the study were performed in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
No author has a competing interest to declare.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ryan A, Heath S, Cook P. Managing dyslipidaemia for the primary prevention of cardiovascular disease. BMJ. 2018;360:k946. doi: 10.1136/bmj.k946. [DOI] [PubMed] [Google Scholar]
- 2.LaMonte MJ, Eisenman PA, Adams TD, Shultz BB, Ainsworth BE, Yanowitz FG. Cardiorespiratory Fitness and Coronary Heart Disease Risk Factors. Circulation. 2000;102:1623–1628. doi: 10.1161/01.cir.102.14.1623. [DOI] [PubMed] [Google Scholar]
- 3.McPhee JS, French DP, Jackson D, Nazroo J, Pendleton N, Degens H. Physical activity in older age: perspectives for healthy ageing and frailty. Biogerontol. 2016;17:567–580. doi: 10.1007/s10522-016-9641-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paul Greenhaff P, Charlotte Bolton P, Faulkner J, Carolyn Greig P, Stephen Harridge PD, Lambrick D, et al. A National COVID-19 Resilience Programme: Improving the health and wellbeing of older people during the pandemic Expert Panel. https://static.physoc.org/app/uploads/2021/02/16145936/A-National-COVID-19-Resilience-Programme-report-web-version.pdf
- 5.Gonzales TI, Westgate K, Strain T, Hollidge S, Jeon J, Christensen DL, et al. Cardiorespiratory fitness assessment using risk-stratified exercise testing and dose–response relationships with disease outcomes. Sci Rep. 2021;11:15315. doi: 10.1038/s41598-021-94768-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ross R, Blair SN, Arena R, Church TS, Després J-P, Franklin BA, et al. Importance of Assessing Cardiorespiratory Fitness in Clinical Practice: A Case for Fitness as a Clinical Vital Sign: A Scientific Statement From the American Heart Association. Circulation. 2016;134. 10.1161/CIR.0000000000000461 [DOI] [PubMed]
- 7.Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, et al. Cardiorespiratory Fitness as a Quantitative Predictor of All-Cause Mortality and Cardiovascular Events in Healthy Men and Women. JAMA. 2009;301:2024. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 8.Al-Mallah MH, Keteyian SJ, Brawner CA, Whelton S, Blaha MJ. Rationale and design of the Henry Ford Exercise Testing Project (the FIT project) Clin Cardiol. 2014;37:456–61. doi: 10.1002/clc.22302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hakola L, Komulainen P, Hassinen M, Savonen K, Litmanen H, Lakka TA, et al. Cardiorespiratory fitness in aging men and women: the DR’s EXTRA study. Scand J Med Sci Sports. 2011;21:679–687. doi: 10.1111/j.1600-0838.2010.01127.x. [DOI] [PubMed] [Google Scholar]
- 10.Seo DY, Lee SR, Kim N, Ko KS, Rhee BD, Han J. Age-related changes in skeletal muscle mitochondria: the role of exercise. Integr Med Res. 2016;5:182–186. doi: 10.1016/j.imr.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nair KS. Aging muscle. Am J Clin Nutr. 2005;81:953–63. doi: 10.1093/ajcn/81.5.953. [DOI] [PubMed] [Google Scholar]
- 12.Myers J. Exercise and Cardiovascular Health. Circulation. 2003;107. 10.1161/01.CIR.0000048890.59383.8D [DOI] [PubMed]
- 13.Booth FW, Roberts CK, Laye MJ. Lack of Exercise Is a Major Cause of Chronic Diseases. Yet to Come Compr Physiol. 2012;7:1143–1211. doi: 10.1002/cphy.c110025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee DH, Keum N, Hu FB, Orav EJ, Rimm EB, Willett WC, et al. Predicted lean body mass, fat mass, and all cause and cause specific mortality in men: prospective US cohort study. BMJ. 2018;362:k2575. doi: 10.1136/bmj.k2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boo S-H, Joo MC, Lee JM, Kim SC, Yu YM, Kim M-S. Association between skeletal muscle mass and cardiorespiratory fitness in community-dwelling elderly men. Aging Clin Exp Res. 2019;31:49–57. doi: 10.1007/s40520-018-0987-9. [DOI] [PubMed] [Google Scholar]
- 17.Batsis JA, Zagaria AB. Addressing Obesity in Aging Patients. Med Clin North Am. 2018;102:65–85. doi: 10.1016/j.mcna.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruseghini P, Calabria E, Tam E, Milanese C, Oliboni E, Pezzato A, et al. Effects of eight weeks of aerobic interval training and of isoinertial resistance training on risk factors of cardiometabolic diseases and exercise capacity in healthy elderly subjects. Oncotarget. 2015;6:16998. doi: 10.18632/oncotarget.4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marzetti E, Calvani R, Tosato M, Cesari M, Di Bari M, Cherubini A, et al. Physical activity and exercise as countermeasures to physical frailty and sarcopenia. Aging Clin Exp Res. 2017;29:35–42. doi: 10.1007/s40520-016-0705-4. [DOI] [PubMed] [Google Scholar]
- 20.Herrod PJJ, Blackwell JEM, Boereboom CL, Atherton PJ, Williams JP, Lund JN, et al. The time course of physiological adaptations to high-intensity interval training in older adults. Aging Med. 2020;3:245–251. doi: 10.1002/agm2.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snijders T, Leenders M, de Groot LCPGM, van Loon LJC, Verdijk LB. Muscle mass and strength gains following 6 months of resistance type exercise training are only partly preserved within one year with autonomous exercise continuation in older adults. Exp Gerontol. 2019;121:71–78. doi: 10.1016/j.exger.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 22.MacInnis MJ, Gibala MJ. Physiological adaptations to interval training and the role of exercise intensity. J Physiol. 2017;595:2915. doi: 10.1113/JP273196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korkiakangas EE, Alahuhta MA, Laitinen JH. Barriers to regular exercise among adults at high risk or diagnosed with type 2 diabetes: a systematic review. Health Promot Int. 2009;24:416–427. doi: 10.1093/heapro/dap031. [DOI] [PubMed] [Google Scholar]
- 24.Cohen-Mansfield J, Marx MS, Guralnik JM. Motivators and Barriers to Exercise in an Older Community-Dwelling Population. J Aging Phys Act. 2003;11:242–253. [Google Scholar]
- 25.Seals DR, Nagy EE, Moreau KL. Aerobic exercise training and vascular function with ageing in healthy men and women. J Physiol. 2019;597:4901–4914. doi: 10.1113/JP277764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye Y, Lin H, Wan M, Qiu P, Xia R, He J, et al. The Effects of Aerobic Exercise on Oxidative Stress in Older Adults: A Systematic Review and Meta-Analysis. Front Physiol. 2021;12:701151. doi: 10.3389/fphys.2021.701151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen F-T, Etnier JL, Chan K-H, Chiu P-K, Hung T-M, Chang Y-K. Effects of Exercise Training Interventions on Executive Function in Older Adults: A Systematic Review and Meta-Analysis. Sports Med. 2020;50:1451–1467. doi: 10.1007/s40279-020-01292-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wyckelsma VL, Levinger I, McKenna MJ, Formosa LE, Ryan MT, Petersen AC, et al. Preservation of skeletal muscle mitochondrial content in older adults: relationship between mitochondria, fibre type and high-intensity exercise training. J Physiol. 2017;595:3345–3359. doi: 10.1113/JP273950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marriott CFS, Petrella AFM, Marriott ECS, Boa Sorte Silva NC, Petrella RJ. High-Intensity Interval Training in Older Adults: a Scoping Review. Sport Med - Open. 2021;7:49. doi: 10.1186/s40798-021-00344-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blackwell JEM, Gharahdaghi N, Brook MS, Watanabe S, Boereboom CL, Doleman B, et al. The physiological impact of high-intensity interval training in octogenarians with comorbidities. J Cachexia Sarcopenia Muscle. 2021;12:866–879. doi: 10.1002/jcsm.12724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reitlo LS, Sandbakk SB, Viken H, Aspvik NP, Ingebrigtsen JE, Tan X, et al. Exercise patterns in older adults instructed to follow moderate- or high-intensity exercise protocol - the generation 100 study. BMC Geriatr. 2018;18:208. doi: 10.1186/s12877-018-0900-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Letnes JM, Berglund I, Johnson KE, Dalen H, Nes BM, Lydersen S, et al. Effect of 5 years of exercise training on the cardiovascular risk profile of older adults: the Generation 100 randomized trial. Eur Heart J. Published Online First: 8 November 2021. 10.1093/eurheartj/ehab721 [DOI] [PMC free article] [PubMed]
- 33.Blackwell J, Atherton PJ, Smith K, Doleman B, Williams JP, Lund JN, et al. The efficacy of unsupervised home-based exercise regimens in comparison to supervised laboratory-based exercise training upon cardio-respiratory health facets. Physiol Rep. 2017;5. 10.14814/phy2.13390 [DOI] [PMC free article] [PubMed]
- 34.Sian TS, Inns T, Gates A, Doleman B, Gharahdaghi N, Atherton PJ, et al. Short-Term, Equipment-Free High Intensity Interval Training Elicits Significant Improvements in Cardiorespiratory Fitness Irrespective of Supervision in Early Adulthood. Front Sport Act Living. 2021;3:207. doi: 10.3389/fspor.2021.697518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weisman IM, Marciniuk D, Martinez FJ, Sciurba F, Sue D, Myers Bruce, Johnson J, et al. ATS/ACCP Statement on Cardiopulmonary Exercise Testing. Am J Respir Crit Care Med. 2003;167:211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 36.Levett DZH, Jack S, Swart M, Carlisle J, Wilson J, Snowden C, et al. Perioperative cardiopulmonary exercise testing (CPET): consensus clinical guidelines on indications, organization, conduct, and physiological interpretation. Br J Anaesth. 2018;120:484–500. doi: 10.1016/j.bja.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 37.British and Irish Hypertension Society. Blood pressure measurement; using automated blood pressure monitors. 2017. https://bihsoc.org/wp-content/uploads/2017/11/BP-Measurement-Poster-Automated-2017.pdf
- 38.World Health Organisation (WHO), International Diabetets Federation (IDF). Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: Report of a WHO/IDF Consultation. 2006.
- 39.Franchi MV, Longo S, Mallinson J, Quinlan JI, Taylor T, Greenhaff PL, et al. Muscle thickness correlates to muscle cross-sectional area in the assessment of strength training-induced hypertrophy. Scand J Med Sci Sports. 2018;28:846–853. doi: 10.1111/sms.12961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rueden CT, Schindelin J, Hiner MC, DeZonia BE, Walter AE, Arena ET, et al. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics. 2017;18:529. doi: 10.1186/s12859-017-1934-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaczmarek S, Habedank D, Obst A, Dörr M, Völzke H, Gläser S, et al. Interobserver variability of ventilatory anaerobic threshold in asymptomatic volunteers. Multidiscip Respir Med. 2019;14:20. doi: 10.1186/s40248-019-0183-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boereboom C, Phillips BE, Williams JP, Lund JN. A 31-day time to surgery compliant exercise training programme improves aerobic health in the elderly. Tech Coloproctol. 2016;20:375–382. doi: 10.1007/s10151-016-1455-1. [DOI] [PubMed] [Google Scholar]
- 43.Fox SM, Naughton JP, Haskell WL. Physical activity and the prevention of coronary heart disease. Ann Clin Res. 1971;3:404–32. [PubMed] [Google Scholar]
- 44.de Boer MR, Waterlander WE, Kuijper LDJ, Steenhuis IHM, Twisk JWR. Testing for baseline differences in randomized controlled trials: an unhealthy research behavior that is hard to eradicate. Int J Behav Nutr Phys Act. 2015;12:4. doi: 10.1186/s12966-015-0162-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blackwell JEM, Doleman B, Boereboom CL, Morton A, Williams S, Atherton P, et al. High-intensity interval training produces a significant improvement in fitness in less than 31 days before surgery for urological cancer: a randomised control trial. Prostate Cancer Prostatic Dis. 2020;23(4):696-704. [DOI] [PMC free article] [PubMed]
- 46.Trost SG, Owen N, Bauman AE, Sallis JF, Brown W. Correlates of adults’ participation in physical activity: review and update. Med Sci Sports Exerc. 2002;34:1996–2001. doi: 10.1097/00005768-200212000-00020. [DOI] [PubMed] [Google Scholar]
- 47.Costello E, Kafchinski M, Vrazel J, Sullivan P. Motivators, Barriers, and Beliefs Regarding Physical Activity in an Older Adult Population. J Geriatr Phys Ther. 2011;34:138–147. doi: 10.1519/JPT.0b013e31820e0e71. [DOI] [PubMed] [Google Scholar]
- 48.World Health Organisation (WHO). Physical Activity. 2018.
- 49.Hardcastle SJ, Ray H, Beale L, Hagger MS. Why sprint interval training is inappropriate for a largely sedentary population. Front Psychol 2014;5. 10.3389/FPSYG.2014.01505 [DOI] [PMC free article] [PubMed]
- 50.Hunter JR, Gordon BA, Lythgo N, Bird SR, Benson AC. Exercise at an onsite facility with or without direct exercise supervision improves health-related physical fitness and exercise participation: An 8-week randomised controlled trial with 15-month follow-up. Heal Promot J Aust. 2018;29:84–92. doi: 10.1002/hpja.2. [DOI] [PubMed] [Google Scholar]
- 51.Scott SN, Shepherd SO, Strauss JA, Wagenmakers AJM, Cocks M. Home-based high‐intensity interval training reduces barriers to exercise in people with type 1 diabetes. Exp Physiol. 2020;105:571–578. doi: 10.1113/EP088097. [DOI] [PubMed] [Google Scholar]
- 52.Scott SN, Shepherd SO, Hopkins N, Dawson EA, Strauss JA, Wright DJ, et al. Home-hit improves muscle capillarisation and eNOS/NAD(P)Hoxidase protein ratio in obese individuals with elevated cardiovascular disease risk. J Physiol. 2019;597:4203–4225. doi: 10.1113/JP278062. [DOI] [PubMed] [Google Scholar]
- 53.Youssef EF, Shanb AA elhameed. Supervised Versus Home Exercise Training Programs on Functional Balance in Older Subjects. Malays J Med Sci. 2016;23:83–93. doi: 10.21315/mjms2016.23.6.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lacroix A, Hortobágyi T, Beurskens R, Granacher U. Effects of Supervised vs. Unsupervised Training Programs on Balance and Muscle Strength in Older Adults: A Systematic Review and Meta-Analysis. Sport Med. 2017;47:2341–2361. doi: 10.1007/s40279-017-0747-6. [DOI] [PubMed] [Google Scholar]
- 55.Morgan F, Battersby A, Weightman AL, Searchfield L, Turley R, Morgan H, et al. Adherence to exercise referral schemes by participants – what do providers and commissioners need to know? A systematic review of barriers and facilitators. BMC Public Health. 2016;16:227. doi: 10.1186/s12889-016-2882-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ashton LM, Hutchesson MJ, Rollo ME, Morgan PJ, Collins CE. Motivators and Barriers to Engaging in Healthy Eating and Physical Activity: A Cross-Sectional Survey in Young Adult Men. Am J Mens Health. 2017;11:330–343. doi: 10.1177/1557988316680936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu Y, Yang Y, Liu H, Wu W, Wu X, Wang T. A systematic review and meta-analysis of fall incidence and risk factors in elderly patients after total joint arthroplasty. Medicine (Baltimore) 2020;99:e23664. doi: 10.1097/MD.0000000000023664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hwang C-L, Yoo J-K, Kim H-K, Hwang M-H, Handberg EM, Petersen JW, et al. Novel all-extremity high-intensity interval training improves aerobic fitness, cardiac function and insulin resistance in healthy older adults. Exp Gerontol. 2016;82:112–9. doi: 10.1016/j.exger.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bruseghini P, Tam E, Calabria E, Milanese C, Capelli C, Galvani C. High Intensity Interval Training Does Not Have Compensatory Effects on Physical Activity Levels in Older Adults. Int J Environ Res Public Health 2020;17. 10.3390/IJERPH17031083 [DOI] [PMC free article] [PubMed]
- 60.Knowles A-M, Herbert P, Easton C, Sculthorpe N, Grace FM. Impact of low-volume, high-intensity interval training on maximal aerobic capacity, health-related quality of life and motivation to exercise in ageing men. Age (Dordr) 2015;37:25. doi: 10.1007/s11357-015-9763-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Silveira-Coswig V, Barbalho M, Raiol R, Boscolo F, Vecchio D, Ramirez-Campillo R, et al. Effects of high vs moderate-intensity intermittent training on functionality, resting heart rate and blood pressure of elderly women. J Transl Med. 2020;18:88. doi: 10.1186/s12967-020-02261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dunne DFJ, Jack S, Jones RP, Jones L, Lythgoe DT, Malik HZ, et al. Randomized clinical trial of prehabilitation before planned liver resection. Br J Surg. 2016;103:504–512. doi: 10.1002/bjs.10096. [DOI] [PubMed] [Google Scholar]
- 63.SPRINT Research Group TSR. Wright JT, Williamson JD, Whelton PK, Snyder JK, Sink KM, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373:2103–16. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grace F, Herbert P, Elliott AD, Richards J, Beaumont A, Sculthorpe NF. High intensity interval training (HIIT) improves resting blood pressure, metabolic (MET) capacity and heart rate reserve without compromising cardiac function in sedentary aging men. Exp Gerontol. 2018;109:75–81. doi: 10.1016/j.exger.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 65.Herrod PJJ, Doleman B, Blackwell JEM, O’Boyle F, Williams JP, Lund JN, et al. Exercise and other nonpharmacological strategies to reduce blood pressure in older adults: a systematic review and meta-analysis. J Am Soc Hypertens. 2018;12:248–267. doi: 10.1016/j.jash.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 66.Mann S, Beedie C, Jimenez A. Differential effects of aerobic exercise, resistance training and combined exercise modalities on cholesterol and the lipid profile: review, synthesis and recommendations. Sports Med. 2014;44:211–21. doi: 10.1007/s40279-013-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Søgaard D, Lund MT, Scheuer CM, Dehlbaek MS, Dideriksen SG, Abildskov CV, et al. High-intensity interval training improves insulin sensitivity in older individuals. Acta Physiol. 2018;222:e13009. doi: 10.1111/apha.13009. [DOI] [PubMed] [Google Scholar]
- 68.Robinson MM, Dasari S, Konopka AR, Johnson ML, Manjunatha S, Esponda RR, et al. Enhanced Protein Translation Underlies Improved Metabolic and Physical Adaptations to Different Exercise Training Modes in Young and Old Humans. Cell Metab. 2017;25:581. doi: 10.1016/j.cmet.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Little JP, Gillen JB, Percival ME, Safdar A, Tarnopolsky MA, Punthakee Z, et al. Low-volume high-intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. J Appl Physiol. 2011;111:1554–60. doi: 10.1152/japplphysiol.00921.2011. [DOI] [PubMed] [Google Scholar]
- 70.Francois ME, Little JP. Effectiveness and Safety of High-Intensity Interval Training in Patients With Type 2 Diabetes. Diabetes Spectr. 2015;28:39–44. doi: 10.2337/diaspect.28.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jelleyman C, Yates T, O’Donovan G, Gray LJ, King JA, Khunti K, et al. The effects of high-intensity interval training on glucose regulation and insulin resistance: a meta-analysis. Obes Rev. 2015;16:942–961. doi: 10.1111/obr.12317. [DOI] [PubMed] [Google Scholar]
- 72.Beard JR, Officer A, de Carvalho IA, Sadana R, Pot AM, Michel J-P, et al. The World report on ageing and health: a policy framework for healthy ageing. Lancet (London, England) 2016;387:2145–2154. doi: 10.1016/S0140-6736(15)00516-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet (London, England) 2013;381:752–62. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Giovannini S, Brau F, Forino R, Berti A, D’Ignazio F, Loreti C, et al. Sarcopenia: Diagnosis and Management, State of the Art and Contribution of Ultrasound. J Clin Med. 2021;10. 10.3390/jcm10235552 [DOI] [PMC free article] [PubMed]
- 75.Lee D, Li Z, Sohail QZ, Jackson K, Fiume E, Agur A. A three-dimensional approach to pennation angle estimation for human skeletal muscle. Comput Methods Biomech Biomed Engin. 2015;18:1474–1484. doi: 10.1080/10255842.2014.917294. [DOI] [PubMed] [Google Scholar]
- 76.Aagaard P, Andersen JL, Dyhre-Poulsen P, Leffers AM, Wagner A, Magnusson SP, et al. A mechanism for increased contractile strength of human pennate muscle in response to strength training: changes in muscle architecture. J Physiol. 2001;534:613–23. doi: 10.1111/j.1469-7793.2001.t01-1-00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gluvic Z, Zaric B, Resanovic I, Obradovic M, Mitrovic A, Radak D, et al. Link Between Metabolic Syndrome and Insulin Resistance. Curr Vasc Pharmacol. 2017;15. 10.2174/1570161114666161007164510 [DOI] [PubMed]
- 78.Wewege M, van den Berg R, Ward RE, Keech A. The effects of high-intensity interval training vs. moderate-intensity continuous training on body composition in overweight and obese adults: a systematic review and meta-analysis. Obes Rev. 2017;18:635–646. doi: 10.1111/obr.12532. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets from this study are available from the corresponding author on reasonable request.