We recently demonstrated dried blood spots (DBS) as an acceptable matrix for SARS-CoV-2 antibody testing using the Siemens sCOVG and COV2T assays [1]. DBS as a testing matrix may be useful for eliminating the need for phlebotomy, particularly for seroprevalence studies and underserved populations. Further, the use of DBS for testing may be applicable to other common viruses including HIV [2]. A recent study demonstrated that the Abbott Architect SARS-CoV-2 IgG assay, using different parameters for DBS than the FDA cleared assay, performed comparably to plasma [3]. However, instrument parameters are often not open for laboratories to modify. Therefore, we sought to validate the use of DBS on the Architect SARS-CoV-2 IgG assay using our previously published extraction method.
This study was approved by the Washington University Institutional Review Board. Remnant whole blood collected in lithium heparin syringes (n = 139) was obtained and 75 µl pipetted to each of the five available spots on the DBS cards. The card was dried at room temperature for 4 h before being frozen at −80˚C until extraction. The remaining whole blood was then centrifuged, the plasma layer separated, and then frozen at −80˚C until analysis. Prospectively collected paired plasma and DBS from a fingerstick (n = 29) were obtained as previously described (1). All DBS specimens were extracted from cards by transferring to cryovials via hole punch and eluted in 1% Triton-X in PBS. Samples were rocked gently at room temperature for 4 h prior to centrifugation and removal of the supernatant into a separate tube for analysis. All specimens were assessed using the Abbott Architect SARS-CoV-2 IgG assay which detects antibodies to viral nucleocapsid protein.
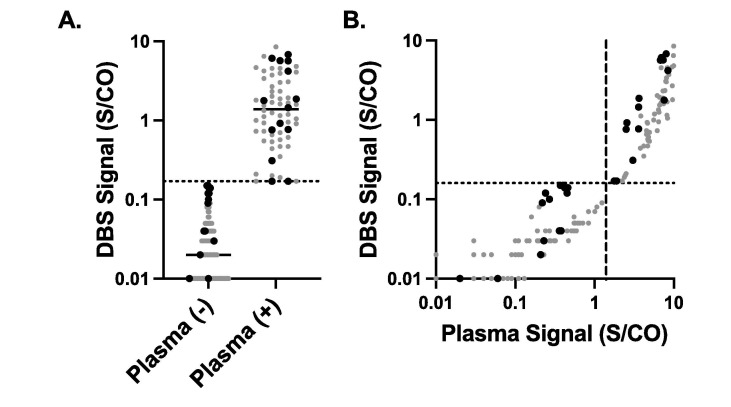
The area under the curve between the plasma and DBS result was 0.999 (0.998–1.0). At a cutoff of 0.16 S/CO for DBS specimens, the positive percent agreement with plasma specimens (using the manufacturer cutoff of ≥ 1.4 S/CO) was 98.5% (95% CI; 92.0–99.9) and negative percent agreement was 100.0% (96.6–100.0, Fig. 1A). The manufacturer defined cutoff of ≥ 1.4 S/CO for the plasma and DBS had a positive percent agreement of 49.3% (37.7–60.9) with the same negative percent agreement. The Pearson r of the S/CO between the plasma and the DBS specimens was 0.819 (p < 0.001, Fig. 1B). No difference was observed in recovery between remnant or prospectively collected specimens. Using a specimen with a mean recovery of 0.16 S/CO in diluent, the intraday precision was 2.6% (n = 20) and the total imprecision was 3.32% (n = 20).
Fig. 1.
Association between paired plasma and DBS SARS-CoV-2 IgG Paired results from 168 plasma and DBS cards. A. DBS results based on negative or positive plasma SARS-CoV-2 IgG. Gray circles are remnants and black triangles are prospectively collected plasma / DBS cards. B. Correlation between the plasma result and DBS result. Dashed line is manufacturer cutoff for plasma and dotted line is the proposed cutoff of 0.16 S/CO for DBS.
We demonstrate the accuracy of DBS testing relative to paired plasma samples; DBS specimens had near perfect agreement with paired plasma results when a cutoff of ≥ 0.16 was applied. Importantly the total imprecision at this cutoff concentration was also quite low, implying that the lowered cutoff is reproducible. These results demonstrate the feasibility of DBS for serological testing for SARS-CoV-2 IgG. Together with our previous studies, we demonstrate comparable results validation DBS for SARS-CoV-2 serology across multiple assays, instrument platforms, and antigenic targets.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Omosule C.L., Conklin J., Seck S., Howell R., Hock K.G., Ballman C., Freeman J., Du Toit L., Dubberke E., Farnsworth C.W. Qualitative and quantitative detection of SARS-CoV-2 antibodies from dried blood spots. Clin Biochem. 2022 doi: 10.1016/j.clinbiochem.2021.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Solomon S.S., Solomon S., Rodriguez I.I., McGarvey S.T., Ganesh A.K., Thyagarajan S.P., Mahajan A.P., Mayer K.H. Dried blood spots (DBS): a valuable tool for HIV surveillance in developing/tropical countries. Int J STD AIDS. 2002;13(1):25–28. doi: 10.1258/0956462021924578. [DOI] [PubMed] [Google Scholar]
- 3.Anderson M., Holzmayer V., Vallari A., Taylor R., Moy J., Cloherty G. Expanding access to SARS-CoV-2 IgG and IgM serologic testing using fingerstick whole blood, plasma, and rapid lateral flow assays. J Clin Virol. 2021;141:104855. doi: 10.1016/j.jcv.2021.104855. [DOI] [PMC free article] [PubMed] [Google Scholar]