Abstract
Temperature is an important factor controlling CH4 production in anoxic rice soils. Soil slurries, prepared from Italian rice field soil, were incubated anaerobically in the dark at six temperatures of between 10 to 37°C or in a temperature gradient block covering the same temperature range at intervals of 1°C. Methane production reached quasi-steady state after 60 to 90 days. Steady-state CH4 production rates increased with temperature, with an apparent activation energy of 61 kJ mol−1. Steady-state partial pressures of the methanogenic precursor H2 also increased with increasing temperature from <0.5 to 3.5 Pa, so that the Gibbs free energy change of H2 plus CO2-dependent methanogenesis was kept at −20 to −25 kJ mol of CH4−1 over the whole temperature range. Steady-state concentrations of the methanogenic precursor acetate, on the other hand, increased with decreasing temperature from <5 to 50 μM. Simultaneously, the relative contribution of H2 as methanogenic precursor decreased, as determined by the conversion of radioactive bicarbonate to 14CH4, so that the carbon and electron flow to CH4 was increasingly dominated by acetate, indicating that psychrotolerant homoacetogenesis was important. The relative composition of the archaeal community was determined by terminal restriction fragment length polymorphism (T-RFLP) analysis of the 16S rRNA genes (16S rDNA). T-RFLP analysis differentiated the archaeal Methanobacteriaceae, Methanomicrobiaceae, Methanosaetaceae, Methanosarcinaceae, and Rice clusters I, III, IV, V, and VI, which were all present in the rice field soil incubated at different temperatures. The 16S rRNA genes of Rice cluster I and Methanosaetaceae were the most frequent methanogenic groups. The relative abundance of Rice cluster I decreased with temperature. The substrates used by this microbial cluster, and thus its function in the microbial community, are unknown. The relative abundance of acetoclastic methanogens, on the other hand, was consistent with their physiology and the acetate concentrations observed at the different temperatures, i.e., the high-acetate-requiring Methanosarcinaceae decreased and the more modest Methanosaetaceae increased with increasing temperature. Our results demonstrate that temperature not only affected the activity but also changed the structure and the function (carbon and electron flow) of a complex methanogenic system.
Methane is one of the most important greenhouse gases (7, 20, 49). With a contribution of about 15 to 20% to the anthropogenic CH4 emissions, rice fields are one of the major sources for CH4 (8, 26, 44). In addition, rice fields may be considered as a rather simple model system for vegetated wetland ecosystems. Methane is the final product of anaerobic degradation of organic matter, which is accomplished by a complex microbial community involving hydrolytic, fermenting, homoacetogenic, syntrophic, and methanogenic microorganisms (54, 60, 75, 76).
Temperature, salinity, redox potential, pH, availability of organic substrates, and nutrient concentration have been identified as the main factors influencing methanogenic degradation processes (9, 43). From these factors temperature is recognized as one of the most important (31, 53, 58). Temperature not only has an effect on the methane production itself but also has an effect on the decomposition of organic materials from which the methanogenic substrates are produced (4, 16, 32, 58, 68).
The most important precursors of CH4 in anoxic rice field soil are acetate and H2/CO2, which theoretically contribute >67 and <33%, respectively, when polysaccharides are anaerobically degraded (11). Most studies indeed have found a slightly higher contribution of acetate, suggesting that homoacetogenesis is involved in the fermentation of the saccharides (12, 51, 73). It is also assumed that the fraction of hydrogenotrophic methanogenesis decreases at low temperature, as the pathway of carbon and electron flow changes, when the temperature of the rice field soil (4, 16, 18), as well as of the lake sediment (56, 57), is shifted. Several authors demonstrated the existence of psychrotolerant homoacetogens which compete with methanogens for H2 at low temperatures (17, 33, 34, 45). Up to 10% of the acetate in paddy soil slurries was found to be produced from CO2 (51, 63). Even higher percentages (<40%) were found when acetoclastic methanogenesis was inhibited by methyl fluoride (12). Increased formation of acetate at low temperatures would result in increased contribution of acetoclastic methanogenesis to CH4 production. On the other hand, it was found that homoacetogenesis from H2/CO2 should hardly be possible under in situ conditions for thermodynamic reasons (4, 52). Hence, the effect of temperature on the flow of carbon and electrons in methanogenic rice field soil is not completely clear.
There have been several attempts to get insight into the methanogenic archaeal community of rice field soil (30, 36, 50). Clone libraries of 16S rRNA genes retrieved from rice field soil recently showed a much higher diversity of the methanogenic community than expected from earlier cultivation studies and also revealed several novel phylogenetic lineages (5, 23, 24, 41). The following major phylogenetic lineages have been identified in the archaeal community in Italian rice field soil (5, 23, 24, 41): among the methanogens, the families of Methanobacteriaceae, Methanomicrobiaceae, Methanosaetaceae, and Methanosarcinaceae; in addition, the euryarchaeotal Rices cluster I and II, which are probably methanogenic (38); the euryarchaeotal Rice clusters III and V, which are probably nonmethanogenic; and the crenarchaeotal Rice clusters IV and VI.
Recently, terminal restriction fragment length polymorphism (T-RFLP) analysis was developed as a rapid PCR-based screening method (39) and was successfully applied to rice field soil (5, 41, 48). This method reveals not only information about the diversity (species richness) of an ecosystem but also information on the relative abundance of the different T-RFs (species evenness), which makes it a powerful tool for analysis of microbial communities (39, 47). Clone libraries of 16S rRNA genes created from rice field soil, the temperature of which was shifted to either 15 or 30°C, showed a completely different composition of the archaeal community (5). However, a quantification of the various archaeal T-RFs in methanogenic soil over a wide range of temperatures has not yet been done.
In general, studies of temperature effects in rice field soil were mostly restricted to a few temperatures and to relatively short-term temperature shifts (4, 69). These shifts may cause transient effects, which are different from those which are established when the system has come to steady state (66). Quasi-steady state conditions in rice field soil are reached quite some time after sulfate and ferric iron have been reduced. This happens faster at high temperatures than at low temperatures (65, 72) and is characterized by relatively low concentrations of H2 and acetate and by constant and almost equal rates of CH4 and CO2 production (73, 74).
The purpose of the present study was to investigate the effect of temperature on the flow of carbon and electrons in rice field soil which is producing CH4 under steady-state conditions. The temperatures in Italian rice fields range typically from 15 to 30°C (58). We chose a slightly larger range (10 to 37°C) for our experiments. The data were used to calculate the thermodynamic properties for the methanogenic and homoacetogenic processes. The relative fraction of methanogenesis from H2/CO2 was estimated by from the conversion of 14CO2 to 14CH4 (14). In addition, T-RFLP analysis was used to quantify the composition of the archaeal community at different temperatures.
MATERIALS AND METHODS
Soil samples.
The soil samples were collected in March 1998 (after plowing) from the yet-unflooded rice fields of the Italian Rice Research Institute in Vercelli, Italy. The soil was air dried and stored as dry lumps at room temperature. It was a sandy-loamy silt (27% sand, 58% silt, 15% clay). The dry lumps were broken and passed through a stainless steel sieve (2 mm, pore size). For preparation of slurries the soil samples were suspended at a weight ratio of 1:1 in distilled anoxic sterile water.
Incubation of soil slurries.
To obtain temperature effects at high resolution, soil slurries were incubated in a temperature gradient block, made from aluminum (length, 185 cm), that was heated at the one end and cooled at the other end (1, 27). The block contained four rows each with 31 holes into which test tubes with soil slurries were fitted. Test tubes (16 ml) were filled with 3 g (dry weight) of soil and 3 ml of anoxic water, closed with black butyl rubber stoppers, and flushed with N2. To obtain methanogenic conditions, the samples were preincubated for 2 days at 30°C. Thereafter, they were put into the temperature gradient block which was kept at 10 to 37°C in 1°C intervals (four replicates). The concentrations of CH4, CO2, and H2 in the gas headspace were determined weekly after the tubes were vigorously shaken for 30 s by hand to reach equilibration of the gas and liquid phase. At the end of incubation (after 89 to 127 days), liquid samples were taken for the analysis of fatty acids. Before sampling, the bottles were briefly mixed on a vortex mixer.
Other experiments were done analogously, using 60-ml serum bottles which were filled with 10 g (dry weight) of soil and 10 ml of anoxic water, closed with butyl rubber stoppers, and flushed with N2. The bottles were then preincubated at 10, 15, 20, 25, 30, and 37°C for 60 to 90 days (depending on temperature) until quasi-steady-state conditions were reached.
Radioactive experiments.
The serum bottles containing CH4-producing soil slurry were evacuated for several minutes and then flushed at least six times with N2 and evacuated to remove all CH4 and CO2. Finally, the bottles were filled with N2 to a pressure of 1.2 × 105 Pa. To estimate the fraction of CH4 produced from H2/CO2, ca. 50 kBq of NaH14CO3 (2 GBq mmol−1; Amersham) was added. The experiment was done twice with three and five replicates, respectively.
Analytical techniques.
CH4 and CO2 were analyzed by gas chromatography using a flame ionization detector (Shimadzu, Kyoto, Japan). CO2 was measured after conversion to CH4 with a methanizer (nickel catalyst at 350°C; Chrompack, Middleburg, The Netherlands). H2 was analyzed by gas chromatography using a HgO-to-Hg conversion detector (RGD2; Trace Analytical, Stanford, Calif.) (19). 14CH4 and 14CO2 were measured in a gas chromatograph equipped with a methanizer, a flame ionization detector, and a RAGA radioactivity gas proportional counter (Raytest, Straubenhardt, Germany) (18).
Liquid samples were transferred into 2-ml Eppendorf cups. The samples were centrifuged for 15 min (12,000 × g; 4°C), and the supernatant was stored frozen at −26°C until analysis. Before analysis the thawed samples were again centrifuged (15 min) and filtered through 0.2-μm (pore-size) membrane filters (Minisart SRP 15; Sartorius, Göttingen, Germany). Fatty acids were measured by high-pressure liquid chromatography (Sykam, Gilching, Germany) with a refraction index detector, having a detection limit of 3 to 5 μM (35). The pH of all soil slurry samples was measured prior to centrifugation and filtration using a glass electrode.
Calculations.
The apparent activation energy (Ea) of CH4 production was calculated by linear regression of the natural logarithm of the CH4 production rates (v) against the reciprocal temperature (T [in degrees Kelvin]) between 10 and 37°C using the logarithmic form of the Arrhenius equation: ln v = −(Ea/R)(1/T) + constant.
The fraction of CH4 produced from H2/CO2 (fH2/CO2) was determined from the specific radioactivities of CH4 (SRCH4) and CO2 (SRCO2) in the gas headspace of the bottles: fH2/CO2 = SRCH4/SRCO2 (14, 18).
Gibbs free-energy changes (ΔG) were calculated from the standard Gibbs free-energy changes (ΔG0); the concentrations of products and substrates at steady state were as described earlier (15). Values of ΔG0 of the reactions (Table 1) were calculated from the standard Gibbs free energies of formation (Gf0) of the reactants and products (62) and were corrected for the actual temperature (T) by the Van't Hoff equation (13). The standard reaction enthalpy changes (ΔH0; Table 1) were calculated from the enthalpies of formation (Hf0) of the reactants and products (37, 61). The standard reaction entropy changes (ΔS0; Table 1) were calculated from ΔG0, ΔH0, and the standard temperature (T = 298.14°K): ΔS0 = (ΔH0 − ΔG0)/T
TABLE 1.
Gibbs free energies, enthalpies, and entropies of reactions involved in methanogenesis, homoacetogenesis, and propionate degradation under standard conditionsa
| Reaction | kJ/reaction
|
||
|---|---|---|---|
| ΔG0 | ΔH0 | ΔS0 | |
| Methane production | |||
| 4 H2 (g) + CO2 (g) → CH4 (g) + 2 H2O (l) | −130.7 | −252.9 | −0.41 |
| CH3COO− (aq) + H+ (aq) → CH4 (g) + CO2 (g) | −75.7 | +17.7 | +0.314 |
| Homoacetogenesis | |||
| 4 H2 (g) + 2 CO2 (g) → CH3COO− (aq) + H+ (aq) + 2 H2O (l) | −55.1 | −270.6 | −0.723 |
| C6H12O6 (aq) → 3 CH3COO− (aq) + 3 H+ (aq) | −197.5 | −185.3 | +0.041 |
That is, 1 M, 105 Pa, 298.14°K.
DNA extraction from soil slurries.
The samples for molecular analysis were taken from soil slurries that had been incubated for 60 to 90 days at different temperatures. The extraction procedure was a modification of previously described protocols (5, 23, 46, 59). A slurry sample (1 ml) was mixed with 0.5 ml of sodium phosphate buffer (pH 8.0, 120 mM), 200 μl of sodium dodecyl sulfate (10%), and 1 g of glass beads (0.17 to 0.18 mm in diameter). After incubation (10 min, 65°C) and two 1-min cycles of bead beating (Mini-Bead-Beater; Biospec Products, Bartlesville, Okla.), the slurry was centrifuged (10 min, 13,000 × g). The DNA was extracted from the supernatant using chloroform-isoamyl alcohol (24:1 [vol/vol]), and the extracts were precipitated with a 0.1 volume of sodium acetate (3 M, pH 5.3) and 2 volumes of ethanol and then purified with cesium chloride as described elsewhere (59).
Amplification of archaeal 16S rRNA genes.
The 16S rDNA fraction of the DNA samples was amplified by PCR with the archaeal group-specific primers described by Grosskopf et al. (23), which amplify from positions 109 to 934 (Escherichia coli 16S rRNA numbering (3) as described previously (5). The thermal profile used for amplification included 32 cycles of denaturation (45 s), primer annealing at 52°C (45 s), and elongation at 72°C (1 min).
T-RFLP analysis.
The principle of the T-RFLP analysis has been described by Liu et al. (39). The backward primer was labeled 5′ terminally with FAM (5-carboxyfluorescein). The SSU rDNA amplicons were purified by use of the QIAquick PCR purification kit (Qiagen, Hilden, Germany) according to the instructions of the manufacturer. Aliquots of the purified 16S rDNA were digested by TaqI (Promega, Mannheim, Germany) for 3 h at 65°C. Each 0.5-ml tube contained 2 to 6 μl of the 16S rDNA amplicons, 1 μl of the incubation buffer, and 1 μl of restriction enzyme (10 U) made up to a total volume of 10 μl with deionized water. Aliquots (2.5 μl) of the digested 16S rDNA were mixed with 2.0 μl of formamide and 0.5 μl of an internal lane standard consisting of 17 different 6-carboxy-X-rhodamine (ROX)-labeled fragments ranging in length from 29 to 928 nucleotides (GeneScan-1000 ROX; PE Applied Biosystems). The samples were denatured at 94°C for 3 min and then immediately stored on ice until being loaded onto the gel. The electrophoresis and analysis of the resulting bands were performed as described previously (5).
RESULTS
Development of steady-state conditions.
Incubation of slurries from rice field soil resulted in the production of CH4 after a lag phase of about 2 days. The production of CO2 started directly after preparation of the samples. In the same time initial peaks of H2 and acetate were observed. Hydrogen started to decrease after the first 24 h of incubation and reached a stable partial pressure within 2 weeks. For acetate this decrease took about 25 days (37°C) or even more than 50 days (10°C) until the steady-state concentration was reached. Other fatty acids, such as propionate, lactate, isobutyrate, butyrate, isovalerate, valerate, and caproate, were only observed in the beginning of the incubation and disappeared at all temperatures within the first month. After this time the CO2 accumulation decreased and reached a stable rate. Methane was first produced with high rates which dropped after a while to lower but stable production rates. This phase was reached after between 60 and 90 days at 37 and 10°C, respectively. This phase is referred to below as the steady state.
Steady-state conditions at different temperatures.
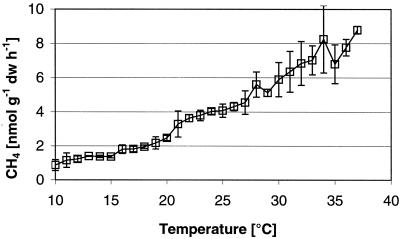
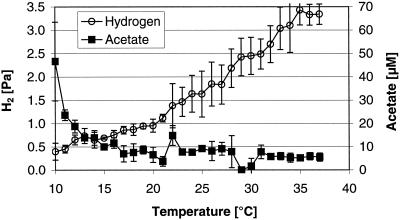
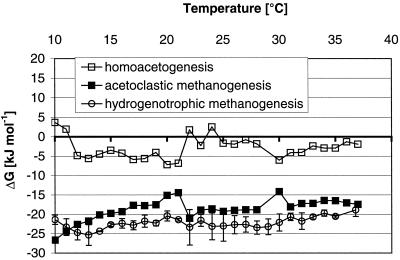
The steady-state CH4 production rates per gram of dry soil increased with increasing temperature from about 1 nmol g−1 h−1 (at 10°C) to 9 nmol g−1 h−1 (at 37°C) (Fig. 1). This almost linear increase resulted in an apparent activation energy of 61 ± 1 kJ mol−1, equivalent to Q10 values of 2.8 (10 to 20°C) and 1.8 (27 to 37°C). The steady-state pH values in the slurries slightly decreased with temperature and ranged from pH 7.4 to pH 6.9. Steady-state partial pressures of H2 linearly increased with temperature from <0.5 Pa (10°C) to ca. 3.5 Pa (≥35°C) (Fig. 2). In contrast, acetate steady-state concentrations were constant at about 5 μM at between 17 and 37°C but increased as the temperature decreased below 17°C, reaching about 50 μM at 10°C (Fig. 2). This increase resulted in a more negative ΔG for acetoclastic methanogenesis at temperatures that were lower than 17°C (−27 kJ mol of CH4−1 at 10°C; Fig. 3) than at 17 to 37°C, where the ΔG was constant at −15 to −20 kJ mol of CH4−1. In contrast to that, the ΔG of hydrogenotrophic methane production was constant at a level of −20 to −25 kJ mol of CH4−1 over the whole temperature range (10 to 37°C). The energy available for homoacetogenesis was always very low (ΔG > −7.2 kJ mol of acetate−1).
FIG. 1.
Methane production rates in anaerobically incubated rice field soil under steady-state conditions. Shown are the means ± the standard deviations (SD) (n = 4).
FIG. 2.
Hydrogen partial pressure in the gas phase and acetate concentration in the pore water of anaerobically incubated rice field soil under steady state conditions. Shown are the means ± the SD (n = 4).
FIG. 3.
Gibbs free energies of methane production and homoacetogenic H2 consumption calculated for the actual conditions of the anaerobically incubated rice field soil under steady state conditions. The typical SD (n = 4) is only shown for hydrogenotrophic methanogenesis as an example.
Radioactive experiments.
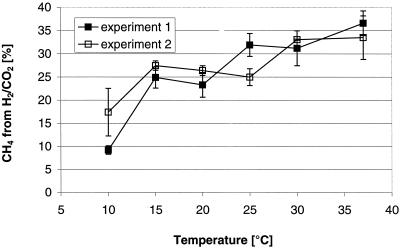
Two experiments with radiolabeled bicarbonate showed the theoretically expected contribution of hydrogenotrophic methanogenesis of about one-third to the total methanogenesis at 30 and 37°C (Fig. 4). But with decreasing temperatures this contribution decreased and resulted in a contribution of only 10 to 15% at 10°C. This means that as much as 85 to 90% of the CH4 was then produced via acetate.
FIG. 4.
Fraction of CH4 produced from H2/CO2 in rice field soil as determined in two independent experiments. Shown are the means ± the SD (n = 3 or 5, respectively).
T-RFLP analysis.
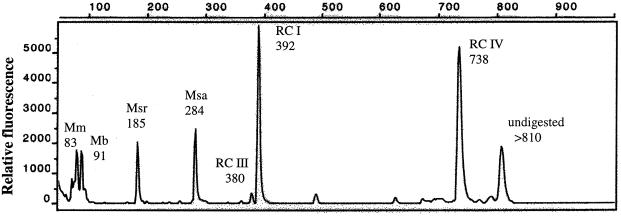
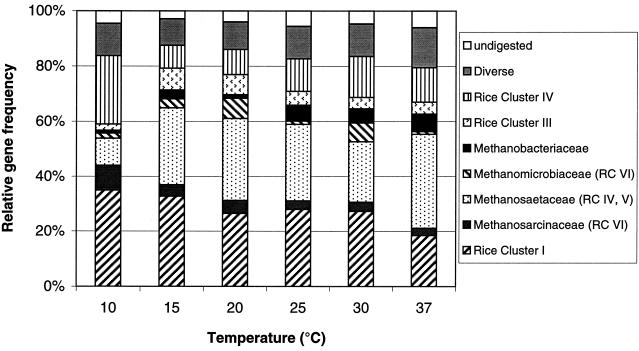
A typical T-RFLP pattern of the archaeal 16S rRNA genes amplified from total community DNA extracted from soil slurry at 10°C is shown in Fig. 5. The marked fragments (T-RFs) correspond to phylogenetic lineages that were characterized by cloning and sequencing (for details, see references 5 and 41). Some of the T-RFs can come from more than one phylogenetic lineage. The relative frequency of the individual T-RFs among the total archaeal community was determined from the relative peak areas using all peaks of ≥1% of the total peak area. Ramakrishnan et al. (48) showed that the relative proportion of T-RFs was independent of the number of PCR cycles between 22 and 44 cycles. The results obtained at the different temperatures using 32 PCR cycles are summarized in Fig. 6. The T-RFs characteristic for the euryarchaeotal Rice cluster I and the Methanosaetaceae came out to be the most dominant ones in rice field soil. The abundance of Rice cluster I, as well as of Methanosarcinaceae, decreased with increasing temperature, while the Methanosaetaceae became more dominant at higher temperature. The quantification of the Methanosarcinaceae and Methanomicrobiaceae can be biased, since their T-RFs are also characteristic for some members of Rice cluster VI. Similarly, the T-RF of Methanosaetaceae may also contain Rice clusters IV and V (5, 41). Other T-RFs were characteristic for Methanobacteriaceae, the euryarchaeotal Rice cluster III, and the crenarchaeotal Rice cluster IV. Fragments which could not be assigned to any known lineage were collected as “diverse.” Fragments with a length of 810 bp or longer were considered to be undigested DNA.
FIG. 5.
T-RFLP pattern of 16S rDNA extracted from anoxic rice field soil at 10°C. The numbers indicate the length of the fragment in base pairs. Mm, Methanomicrobiaceae; Mb, Methanobacteriaceae; Msr, Methanosarcinaceae; Msa, Methanosaetaceae; RC I, Rice cluster I; RC III, Rice cluster III; RC IV, Rice cluster IV.
FIG. 6.
Archaeal population in anaerobically incubated rice field soil under steady-state conditions. The figure shows the relative abundances of T-RFs used as a measure of the composition of the archaeal microbial community. The frequencies were calculated from three replicates using the areas of all detected fragments that were >1% of the total area. The mean SD of the relative abundances of the T-RFs was 3.2 ± 0.4%. Note that the T-RFs characteristic for Methanomicrobiacea, Methanosaetacea, and Methanosarcinacea may also contain T-RFs of those rice clusters indicated in parentheses.
DISCUSSION
Our experiments focused on the quasi-steady-state conditions in a methanogenic rice field soil as affected by different constant temperatures. These conditions are artificial, since rice fields normally undergo diel temperature changes. However, they serve as a model for the principle effect of temperature on the function of a methanogenic microbial community. Our experiments demonstrate that temperature not only affected the rate of CH4 production in anoxic rice soil but also influenced the pathway of carbon and electron flow during methanogenic degradation of organic matter and the composition of the methanogenic microbial community. With increasing temperature, the carbon flow via acetate decreased relative to that via H2/CO2. The steady-state concentrations of H2 increased, thus resulting in thermodynamic homeostasis of H2-dependent methanogenesis. Acetate concentrations, on the other hand, decreased, thus resulting in decreasing energy available for acetoclastic methanogenesis. Simultaneously, the acetoclastic methanogenic microbial community was increasingly dominated by Methanosaetaceae relative to Methanosarcinaceae. Our results for the first time provide a rather comprehensive picture of how temperature affects the microbial structure and the function of a methanogenic environment.
Influence of temperature on CH4 production rates.
In soil slurry that was preincubated at 30°C, CH4 production started after a lag phase of 2 days, during which ferric iron and sulfate were reduced and organic matter was predominantly degraded to CO2. This initial reduction phase was followed by relatively high CH4 production rates which progressively slowed down until a quasi-steady state was reached. The sequential reduction process and the different phases of CH4 production have been characterized in detail for various rice field soils (71, 74). A three-phase conceptual model, with reduction phase (I), methanogenic phase (II), and steady-state phase (III) was proposed by Yao et al. (74) and put into a process model by vanHulzen et al. (64). The model indicates that the CH4 production rate is first limited by the methanogens themselves (phase II) and later on by the carbon mineralization rate (phase III). The effect of temperature on the lag phase until the quasi-steady state is reached has been characterized (64, 72), and our results agree. Quasi-steady-state CH4 production was reached after 60 to 90 days, depending on the incubation temperature. Production rates of CH4 during the quasi-steady state increased with temperature, with an apparent activation energy of 61 kJ mol−1 or a Q10 of 1.8 to 2.8, which is consistent with earlier results obtained with rice soil microcosms and carbon-limited soil slurries (58, 64, 72).
Steady-state concentrations of hydrogen.
The H2 partial pressures showed a clear increase with temperature. Similar results have been obtained with freshwater sediments (66), marine sediments (25), and also with pure cultures of methanogens (13). As a consequence of the increase of H2 with temperature, the Gibbs free-energy available for H2-dependent methanogenesis stayed constant at a level of −20 to −25 kJ mol of CH4−1 for the whole temperature range. This free energy should be sufficient for the generation of one-third ATP (approximately −23 kJ mol of CH4−1) which is expected to be the minimum energy required for growth (55). Similar values were observed in various rice field soils from the major rice-growing areas (70), in rice soil supplemented with straw (22), and also in various other methanogenic environments (reviewed in reference 11). All these data are consistent with the concept that H2-dependent methanogenesis is thermodynamically controlled. Hence, the Gibbs free energies that are calculated from the H2 partial pressures (10, 13, 25), rather than the H2 partial pressures themselves (40), are characteristic for environments in which the anaerobic degradation is dominated by methanogenesis versus sulfate reduction, iron reduction, etc.
Steady-state concentrations of acetate.
In contrast to H2, the acetate concentration did not increase with temperature but instead decreased by up to about 20°C and then stayed constant at a very low level. Consequently, the energy available for acetoclastic methanogenesis decreased with temperature. Acetate apparently does not have the same regulating function for methanogenesis as does H2. This conclusion is consistent with observations by Westermann (66), who found a constant concentration of acetate and propionate at between 2 and 37°C. It is also consistent with the study of various rice field soils in which only the Gibbs free energies of H2-dependent but not of acetate-dependent methanogenesis regulated total CH4 production (70).
Contribution of H2/CO2 and acetate to total CH4 production.
Our results show that the relative contribution of H2/CO2-dependent methanogenesis increased with temperature. Consequently, acetate-dependent methanogenesis decreased. Our results are in contrast to observations in a tidal freshwater estuarine sediment in which the production of CH4 from CO2 was constant at about 25% at between 8 and 32°C (2). However, our results confirm earlier studies on anoxic rice soil which, however, were restricted to only two different temperatures (4, 16). There are three possible explanations for the observed decrease of H2/CO2 to CH4 production with decreasing temperature.
(i) The contribution of homoacetogenesis to the total flux of carbon and electrons increases with decreasing temperature, thus causing a relative increase of acetate as methanogenic precursor.
(ii) The composition of the methanogenic microbial community changes with temperature and so does the relative activity of different physiological groups of microorganisms.
(iii) The fluidity of the microbial cytoplasmic membrane changes with temperature so that the turnover of acetate is affected differently than the turnover of H2.
Membrane fluidity.
Concerning the last point, it is well known that microorganisms adapt to decreasing temperature by changing the lipid composition to alleviate the increasing viscosity of the cytoplasmic membrane. Nevertheless, temperature decrease often results in decreased efficiency of transport proteins and decreased specific affinity of substrate utilization (42). Since acetate has to be transported over the membrane, while H2 is freely diffusible, one might expect that decreasing temperature inhibits the utilization of acetate more than that of H2.
This very effect has been found in cultures of Methanosarcina barkeri, in which the specific affinity for H2 was better compensated for at decreasing temperatures than that for acetate (67). In conclusion, decreasing temperatures may in particular affect acetate utilization negatively and thus explain why steady-state acetate concentrations in methanogenic rice soil increased. However, it does not explain why acetate became a more important methanogenic precursor than H2. It also should be noted that the ability for temperature compensation is different for different microbial species, and thus a complex microbial community, containing both psychrophiles and mesophiles, can probably better adapt to temperature changes than can a single species.
The archaeal community.
The community of methanogenic archaea was indeed rather complex and changed in composition with changing temperature. The results from the T-RFLP analysis showed the presence of all the archaeal groups that had previously been detected in Italian rice field soil (5, 23, 24, 41). The most obvious effect was the increase of the relative abundance of Methanosaetaceae with increasing temperature, in contrast to a decrease of Methanosarcinaceae, which were most common at the lowest temperature. Although the T-RFLP pattern may to some extent be biased since the T-RFs characteristic for Methanosaetaceae and Methanosarcinaceae may partially be caused by T-RFs of Rice clusters IV, V, and VI (5, 41), the pattern is nevertheless intriguing, since it is consistent with the observation that acetate concentrations have been much lower at high versus low temperatures and the fact that Methanosaetaceae have a lower threshold for acetate than do Methanosarcinaceae (28, 29). Therefore, Methanosarcinaceae may be unable to grow at the low acetate concentrations observed at high temperatures and be replaced by the better-adapted Methanosaetaceae. However, it cannot be completely ruled out that the observed pattern of T-RFs was biased by archaeal lineages other than Methanosaetaceae and Methanosarcinaceae.
At a first glance, our results seem to be in contradiction to earlier observations by Chin et al. (5), who found that Methanosarcinaceae dominated in rice soil at 30°C while Methanosaetaceae dominated at 15°C. These authors also reported the dominance of the Methanosaetaceae over Methanosarcinaceae at 15°C (and vice versa at 30°C) in anaerobic cellulose-degrading enrichment cultures inoculated with rice field soil (6). In all of these previous experiments, however, acetate concentrations were always >100 μM and thus not discriminative for the lower thresholds of the Methanosaetaceae. Under these conditions, the temperature itself or an unknown factor related to temperature change was obviously selective for Methanosaetaceae and Methanosarcinaceae at low and high temperatures, respectively. The capacity for compensation of the changing membrane fluidity may play an important role (see above). Hence, it appears that temperature affects the microorganisms both directly (proximally) and indirectly (distally). We assume that the proximal temperature effect was responsible for the selection of methanogens in the relatively short-term experiments by Chin and Conrad (4) and Chin et al. (5), while distal effects (via acetate concentration) were responsible for the selection under the quasi-steady-state conditions in our experiments.
Temperature also affected the relative abundance of the euryarchaeotal Rice cluster I, which decreased with increasing temperature. Rice cluster I probably contains methanogenic archaea because of the phylogenetic position of this cluster (24) and of its presence in methanogenic enrichment cultures (38). However, the physiological properties of this group, and thus the function in the methanogenic system, are unknown.
Although temperature did exhibit a clear effect on the composition of the methanogenic archaeal community, these structural differences do not explain the observed effects on the carbon and electron flow, in particular why acetate dominated over H2/CO2 as the methanogenic precursor especially at low temperatures.
Homoacetogenesis.
It is possible that H2/CO2-dependent acetate production was less temperature-sensitive than H2/CO2-dependent methanogenesis. This would explain the relative decrease of H2 as a methanogenic precursor with decreasing temperature. It was shown, however, that homoacetogens require a Gibbs free energy of at least −5 to −6 kJ mol of H2−1 (13) which corresponds to −20 to −24 kJ mol of acetate−1. The most negative ΔG values for homoacetogenesis observed in our study were ca. −7 kJ mol of acetate−1. Similar values, in the same unfavorable range, have been observed before in anoxic rice soil (4, 22, 52). Therefore, we have to assume that homoacetogenesis from H2/CO2 plays only a minor role in anoxic rice field soil. This conclusion is consistent with radiotracer studies which found only <10% of the acetate being produced from 14CO2 (51, 63). However, since homoacetogens are highly versatile organisms (21), we assume that psychrotolerant homoacetogens are involved in the production of acetate from saccharides or other organic substrates (4). The standard Gibbs free-energy change of acetate production by homoacetogenic sugar fermentation is only slightly affected by a temperature change, as shown by the small standard entropy change (ΔS0 = 0.04 kJ mol−1 K−1; Table 1) because of ΔG0T = ΔG0 − ΔS0 (T − 298.14). The consumption of acetate by acetoclastic methanogenesis, on the other hand, is becoming less exergonic at decreasing temperature (ΔS0 = 0.31 kJ mol−1 K−1; Table 1) and thus has to be compensated for by an increasing acetate concentration, being in agreement with our observations.
Conclusion.
We found that temperature affected the carbon and electron flow in methanogenic rice soil under quasi-steady-state conditions and also affected the composition of the archaeal community. Acetate became the increasingly more important methanogenic precursor when the temperature decreased. This resulted in higher steady-state concentrations of acetate, which in turn allowed proliferation of the fast growing acetoclastic Methanosarcinaceae, while slow-growing but more modest Methanosaetaceae were selected by the low acetate concentrations at higher temperatures. It is unclear, however, why acetate became relatively more important at low temperatures. Possibly, psychrotolerant homoacetogens prevailed over fermenting bacteria, thus reducing the product spectrum to just acetate. Such a prevalence is thermodynamically reasonable (see above) but does not offer a mechanistic explanation. Without a mechanistic explanation we are unable to extrapolate from the situation encountered in anoxic rice soil to other methanogenic environments. In particular, we will need to study how temperature affects the community of fermenting, syntrophic, and homoacetogenic bacteria.
ACKNOWLEDGMENT
We thank K. J. Chin, H. Lüdemann, and T. Lukow for their help and for technical instructions during the T-RFLP analysis.
The Fonds der Chemischen Industrie provided financial support. This study was part of the Sonderforschungsbereich 395 of the Deutsche Forschungsgemeinschaft “Interaction, Adaptation, and Catalytic Capacity of Terrestrial Microorganisms.”
REFERENCES
- 1.Arnosti C, Jørgensen B B, Sagemann J, Thamdrup B. Temperature dependence of microbial degradation of organic matter in marine sediments. Mar Ecol Prog Ser. 1998;165:59–70. [Google Scholar]
- 2.Avery G B, Jr, Martens C S. Controls on the stable isotopic composition of biogenic methane produced in a tidal freshwater estaurine sediment. Geochim Cosmochim Acta. 1999;63:1075–1082. [Google Scholar]
- 3.Brosius J, Palmer M L, Kennedy P J, Noller H R. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci USA. 1978;75:4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chin K J, Conrad R. Intermediary metabolism in methanogenic paddy soil and the influence of temperature. FEMS Microbiol Ecol. 1995;18:85–102. [Google Scholar]
- 5.Chin K J, Lukow T, Conrad R. Effect of temperature on structure and function of the methanogenic archaeal community in an anoxic rice field soil. Appl Environ Microbiol. 1999;65:2341–2349. doi: 10.1128/aem.65.6.2341-2349.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chin K J, Lukow T, Stubner S, Conrad R. Structure and function of the methanogenic archaeal community in stable cellulose-degrading enrichment cultures at two different temperatures (15 and 30°C) FEMS Microbiol Ecol. 1999;30:313–326. doi: 10.1111/j.1574-6941.1999.tb00659.x. [DOI] [PubMed] [Google Scholar]
- 7.Cicerone R J, Oremland R S. Biogeochemical aspects of atmospheric methane. Blob Biogeochem Cycles. 1988;2:299–327. [Google Scholar]
- 8.Cole C V, Cerri C, Minami K, Mosier A, Rosenberg N, Sauerbeck D, Dumanski D, Duxbury J, Freney J, Gupta R, Heinemeyer O, Kolchugina T, Lee J, Paustian K, Powlson D, Sampson N, Tiessen H, Van Noordwijk M, Zhao Q. Agricultural options for mitigation of greenhouse gas emissions. In: Watson R T, Zinyowera M C, Moss R H, editors. Climate change 1995: impacts, adaptations, and mitigation of climate change: scientific-technical analyses. Cambridge, England: Intergovernmental Panel on Climate Change/Cambridge University Press; 1996. pp. 745–771. [Google Scholar]
- 9.Conrad R. Mechanisms controlling methane emission from wetland rice fields, 317–335. In: Oremland R S, editor. The biogeochemistry of global change: radiative trace gases. New York, N.Y: Chapman & Hall; 1993. [Google Scholar]
- 10.Conrad R. Anaerobic hydrogen metabolism in aquatic sediments. Mitt Internat Verein Limnol. 1996;25:15–24. [Google Scholar]
- 11.Conrad R. Contribution of hydrogen to methane production and control of hydrogen concentrations in methanogenic soils and sediments. FEMS Microbiol Ecol. 1999;28:193–202. [Google Scholar]
- 12.Conrad R, Klose M. How specific is the inhibition by methyl fluoride of acetoclastic methanogenesis in anoxic rice field soil? FEMS Microbiol Ecol. 1999;30:47–56. [Google Scholar]
- 13.Conrad R, Wetter B. Influence of temperature on energetics of hydrogen metabolism in homoacetogenic, methanogenic, and other anaerobic bacteria. Arch Microbiol. 1990;155:94–98. [Google Scholar]
- 14.Conrad R, Schütz H. Methods of studying methanogenic activities in aquatic environments. In: Austin B, editor. Methods in aquatic bacteriology. Chichester, England: Wiley; 1988. pp. 301–343. [Google Scholar]
- 15.Conrad R, Schink B, Phelps T J. Thermodynamics of H2-consuming and H2-producing metabolic reactions in diverse methanogenic environments under in situ conditions. FEMS Microbiol Ecol. 1986;38:353–360. [Google Scholar]
- 16.Conrad R, Schütz H, Babbel M. Temperature limitation of hydrogen turnover and methanogenesis in anoxic paddy soil. FEMS Microbiol Ecol. 1987;45:281–289. [Google Scholar]
- 17.Conrad R, Bak F, Seitz H J, Thebrath B, Mayer H-P, Schütz H. Hydrogen turnover by psychrotrophic homoacetogenic and mesophilic bacteria in anoxic paddy soil and lake sediment. FEMS Microbiol Ecol. 1989;62:285–294. [Google Scholar]
- 18.Conrad R, Mayer H-P, Wüst M. Temporal change of gas metabolism by hydrogen-syntrophic methanogenic bacterial associations in anoxic paddy soil. FEMS Microbiol Ecol. 1989;62:265–274. [Google Scholar]
- 19.Cord-Ruwisch R, Seitz H-J, Conrad R. The capacity of hydrogenotrophic anaerobic bacteria to compete for traces of hydrogen depends on the redox potential of the terminal electron acceptor. Arch Microbiol. 1988;149:350–357. [Google Scholar]
- 20.Crutzen P J. The role of methane in atmospheric chemistry and climate. In: von Engelhardt W, Leonhardt-Marek S, Breves G, Giesecke D, editors. Ruminant physiology: digestion, metabolism, growth and reproduction. Stuttgart, Germany: Enke; 1995. pp. 291–315. [Google Scholar]
- 21.Drake H L. Acetogenesis. London, England: Chapman & Hall; 1994. [Google Scholar]
- 22.Glissmann K, Conrad R. Fermentation pattern of methanogenic degradation of rice straw in anoxic paddy soil. FEMS Microbiol Ecol. 2000;31:117–126. doi: 10.1111/j.1574-6941.2000.tb00677.x. [DOI] [PubMed] [Google Scholar]
- 23.Grosskopf R, Janssen P H, Liesack W. Diversity and structure of the methanogenic community in anoxic paddy soil microcosms as examined by cultivation and direct 16S rRNA gene sequence retrieval. Appl Environ Microbiol. 1998;64:960–969. doi: 10.1128/aem.64.3.960-969.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grosskopf R, Stubner S, Liesack W. Novel euryarchaeotal lineages detected on rice roots and in the anoxic bulk soil of flooded rice microcosms. Appl Environ Microbiol. 1998;64:4983–4989. doi: 10.1128/aem.64.12.4983-4989.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoehler T M, Alperin M J, Albert D B, Martens C S. Thermodynamic control on hydrogen concentrations in anoxic sediments. Geochim Cosmochim Acta. 1998;62:1745–1756. [Google Scholar]
- 26.Intergovernmental Panel on Climate Change. Climate change 1992. Supplementary Report to the IPCC Scientific Assessment. Cambridge, United Kingdom: Intergovernmental Panel on Climate Change; 1992. [Google Scholar]
- 27.Isaksen M F, Bak F, Jørgensen B B. Thermophilic sulfate-reducing bacteria in cold marine sediment. FEMS Microbiol Ecol. 1994;14:1–8. [Google Scholar]
- 28.Jetten M S M, Stams A J M, Zehnder A J B. Acetate threshold values and acetate activating enzymes in methanogenic bacteria. FEMS Microbiol Ecol. 1990;73:339–344. [Google Scholar]
- 29.Jetten M S M, Stams A J M, Zehnder A J B. Methanogeneis from acetate: a comparison of the acetate metabolism in Methanothrix soehngenii and Methanosarcina spp. FEMS Microbiol Rev. 1992;88:181–198. [Google Scholar]
- 30.Joulian C, Ollivier B, Patel B K C, Roger P A. Phenotypic and phylogenetic characterization of dominant culturable methanogens isolated from ricefield soils. FEMS Microbiol Ecol. 1998;25:135–145. [Google Scholar]
- 31.Khalil M A K, Rasmussen R A, Shearer M J, Dalluge R W, Ren L, Duan C-H. Factors affecting methane emissions from rice fields. J Geophys Res. 1998;103:25219–25231. [Google Scholar]
- 32.Kimura M, Minoda T, Murase J. Water-soluble organic materials in paddy soil ecosystem. Soil Sci Plant Nutr. 1993;39:713–724. [Google Scholar]
- 33.Kotsyurbenko O R, Nozhevnikova A N, Zavarzin G A. Methanogenic degradation of organic matter by anaerobic bacteria at low temperature. Chemosphere. 1993;27:1745–1761. [Google Scholar]
- 34.Kotsyurbenko O R, Nozhevnikova A N, Soloviova T I, Zavarzin G A. Methanogenesis at low temperatures by microflora of tundra wetland soil. Antonie Leeuwenhoek. 1996;69:75–86. doi: 10.1007/BF00641614. [DOI] [PubMed] [Google Scholar]
- 35.Krummböck M, Conrad R. Metabolism of position la: belled glucose in anoxic-methanogenic paddy soil and lake sediment. FEMS Microbiol Ecol. 1991;85:247–256. [Google Scholar]
- 36.Kudo Y, Nakajima T, Miyaki T, Oyaizu H. Methanogen flora of paddy soils in Japan. FEMS Microbiol Ecol. 1997;22:39–48. [Google Scholar]
- 37.Lange N A. Handbook of chemistry. New York, N.Y: McGraw-Hill; 1979. [Google Scholar]
- 38.Lehmann-Richter S, Großkopf R, Liesack W, Frenzel P, Conrad R. Methanogenic archaea and CO2-dependent methanogenesis on washed rice roots. Environ Microbiol. 1999;1:159–166. doi: 10.1046/j.1462-2920.1999.00019.x. [DOI] [PubMed] [Google Scholar]
- 39.Liu W-T, Marsh T L, Cheng H, Forney L J. Characterization of microbial diversity by determining terminal restriction fragment length polymorphisms of genes encoding 16S rRNA. Appl Environ Microbiol. 1997;63:4516–4522. doi: 10.1128/aem.63.11.4516-4522.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lovley D R, Goodwin S. Hydrogen concentrations as an indicator of the predominant terminal electron-accepting reactions in aquatic sediments. Geochim Cosmochim Acta. 1988;52:2993–3003. [Google Scholar]
- 41.Lueders T, Friedrich M. Archaeal population dynamics during sequential reduction processes in rice field soil. Appl Environ Microbiol. 2000;66:2732–2742. doi: 10.1128/aem.66.7.2732-2742.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nedwell D B. Effect of low temperature on microbial growth: lowered affinity for substrates limits growth at low temperature. FEMS Microbiol Ecol. 1999;30:101–111. doi: 10.1111/j.1574-6941.1999.tb00639.x. [DOI] [PubMed] [Google Scholar]
- 43.Neue H-U, Roger P A. Rice agriculture: factors controlling emissions. In: Khalil M A K, editor. Atmospheric methane: sources, sinks and role in global change. Berlin, Germany: Springer-Verlag; 1993. pp. 254–298. [Google Scholar]
- 44.Neue H-U, Sass R L. Trace gas emissions from rice fields. In: Prinn R G, editor. Global atmospheric-biospheric chemistry. New York, N.Y: Plenum Press; 1994. pp. 119–147. [Google Scholar]
- 45.Nozhevnikova A N, Kotsyurbenko O R, Simankova M V. Acetogenesis at low temperature. In: Drake H L, editor. Acetogenesis. London, England: Chapman & Hall; 1994. pp. 416–431. [Google Scholar]
- 46.Ogram A, Sayler G S, Barkay T. The extraction and purification of microbial DNA from sediments. J Microbiol Methods. 1987;7:57–66. [Google Scholar]
- 47.Osborn A M, Moore E R B, Timmis K N. An evaluation of terminal-restriction fragment length polymorphism (T-RFLP) analysis for the study of microbial community structure and dynamics. Environ Microbiol. 2000;2:39–50. doi: 10.1046/j.1462-2920.2000.00081.x. [DOI] [PubMed] [Google Scholar]
- 48.Ramakrishnan B, Lueders T, Conrad R, Friedrich M. Effect of soil aggregate size on methanogenesis and archaeal community structure in anoxic rice field soil. FEMS Microbiol Ecol. 2000;32:261–270. doi: 10.1111/j.1574-6941.2000.tb00719.x. [DOI] [PubMed] [Google Scholar]
- 49.Rasmussen R A, Khalil M A K. Atmospheric methane (CH4): trends and seasonal cycles. J Geophys Res. 1981;86:9826–9832. [Google Scholar]
- 50.Reichardt W, Mascarina G, Padre B, Doll J. Microbial communities of continuously cropped, irrigated rice fields. Appl Environ Microbiol. 1997;63:233–238. doi: 10.1128/aem.63.1.233-238.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rothfuss F, Conrad R. Vertical profiles of CH4 concentrations, dissolved substrates and processes involved in CH4 production in a flooded Italian rice field. Biogeochemistry. 1993;18:137–152. [Google Scholar]
- 52.Roy R, Klüber H D, Conrad R. Early initiation of methane production in anoxic rice soil despite the presence of oxidants. FEMS Microbiol Ecol. 1997;24:311–320. [Google Scholar]
- 53.Sass R L, Fisher F M, Turner F T, Jund M F. Methane emission from rice fields as influenced by solar radiation, temperature, and straw incorporation. Global Biogeochem Cycles. 1991;5:335–350. [Google Scholar]
- 54.Schink B. Syntrophism among prokaryotes. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K H, editors. The prokaryotes. New York, N.Y: Springer-Verlag; 1992. pp. 276–299. [Google Scholar]
- 55.Schink B. Energetics of syntrophic cooperation in methanogenic degradation. Microbiol Mol Biol Rev. 1997;61:262–280. doi: 10.1128/mmbr.61.2.262-280.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schulz S, Conrad R. Influence of temperature on pathways to methane production in the permanently cold profundal sediment of Lake Constance. FEMS Microbiol Ecol. 1996;20:1–14. [Google Scholar]
- 57.Schulz S, Matsuyama H, Conrad R. Temperature dependence of methane production from different precursors in a profundal sediment (Lake Constance) FEMS Microbiol Ecol. 1997;22:207–213. [Google Scholar]
- 58.Schütz H, Seiler W, Conrad R. Influence of soil temperature on methane emission from rice paddy fields. Biogeochemistry. 1990;11:77–95. [Google Scholar]
- 59.Smalla K, Cresswell N, Mendonca-Hagler L C, Wolters A, van Elsas J D. Rapid D ectraction protocol from soil for polymerase chain reaction-mediated amplification. J Appl Bacteriol. 1993;74:78–85. [Google Scholar]
- 60.Stams A J M. Metabolic interactions between anaerobic bacteria in methanogenic environments. Antonie Leeuwenhoek. 1994;66:271–294. doi: 10.1007/BF00871644. [DOI] [PubMed] [Google Scholar]
- 61.Stumm W, Morgan J J. Aquatic chemistry. 3rd ed. New York, N.Y: John Wiley & Sons, Inc.; 1996. [Google Scholar]
- 62.Thauer R K, Jungermann K, Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977;41:100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thebrath B, Mayer H-P, Conrad R. Bicarbonate-dependent production and methanogenic consumption of acetate in anoxic paddy soil. FEMS Microbiol Ecol. 1992;86:295–302. [Google Scholar]
- 64.VanHulzen J B, Segers R, van Bodegom P M, Leffelaar P A. Temperature effects on soil methane production: an explanation for observed variability. Soil Biol Biochem. 1999;31:1919–1929. [Google Scholar]
- 65.VanBodegom P M, Stams A J M. Effects of alternative electron acceptors and temperature on methanogenesis in rice paddy soils. Chemosphere. 1999;39:167–182. [Google Scholar]
- 66.Westermann P. The effect of incubation temperature on steady-state concentrations of hydrogen and volatile fatty acids during anaerobic degradation in slurries from wetland sediments. FEMS Microbiol Ecol. 1994;13:295–302. [Google Scholar]
- 67.Westermann P, Ahring B K, Mah R A. Temperature compensation in Methanosarcina barkeri by modulation of hydrogen and acetate affinity. Appl Environ Microbiol. 1989;55:1262–1266. doi: 10.1128/aem.55.5.1262-1266.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamane I, Sato K. Effect of temperature on the decomposition of organic substances in flooded soil. Soil Sci Plant Nutr. 1967;13:94–100. [Google Scholar]
- 69.Yang S-S, Chang H-L. Effect of environmental conditions on methane production and emission from paddy soil. Agric Ecosyst Environ. 1998;69:69–80. [Google Scholar]
- 70.Yao H, Conrad R. Thermodynamics of methane production in different rice paddy soils from China, the Philippines and Italy. Soil Biol Biochem. 1999;31:463–473. [Google Scholar]
- 71.Yao H, Conrad R. Effect of soil characteristics on sequential reduction and methane production in sixteen rice paddy soils from China, the Philippines, and Italy. Biogeochemistry. 1999;47:269–295. [Google Scholar]
- 72.Yao, H., and R. Conrad. Effect of temperature on reduction of iron and production of carbon dioxide and methane in anoxic wetland rice soils. Biol. Fertil. Soils, in press.
- 73.Yao, H., and R. Conrad. Electron balance during steady state production of CH4 and CO2 in anoxic rice soil. Eur. J. Soil Sci., in press.
- 74.Yao H, Conrad R, Wassmann R, Neue H U. Effect of soil characteristics on sequential reduction and methane production in sixteen rice paddy soils from China, the Philippines, and Italy. Biogeochemistry. 1999;47:269–295. [Google Scholar]
- 75.Zehnder A J B. Ecology of methane formation. In: Mitchell R, editor. Water pollution microbiology. Vol. 2. New York, N.Y: Wiley; 1978. pp. 349–376. [Google Scholar]
- 76.Zinder S H. Physiological ecology of methanogens. In: Ferry J G, editor. Methanogenesis: ecology, physiology, biochemistry & genetics. New York, N.Y: Chapman & Hall; 1993. pp. 128–206. [Google Scholar]