Abstract
Coronavirus disease (COVID-19) was first reported in December 2019, Hubei Province, China. As on 9th December 2021, severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) has affected 266018810 people worldwide with 5265092 deaths. The outbreak of COVID-19 pandemic has caused severe public health crisis across the world. Nucleic acids have been emerging as potential drugs to treat a variety of diseases. Lipid nanoparticles (LNPs) have great potential to deliver nucleic acids including mRNAs. The two mRNA-based vaccines namely the BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna) have been given emergency use authorization (EUA) by the US-FDA to prevent SARS-CoV-2 caused COVID-19 and the vaccines were developed using LNPs. This article focuses on the potential application of LNPs in the development and delivery of mRNA vaccines for COVID-19.
Keywords: Lipid nanoparticles, COVID-19, Nanotechnology, mRNA, Vaccines, BNT162b2, mRNA-1273
Graphical abstract
1. COVID-19
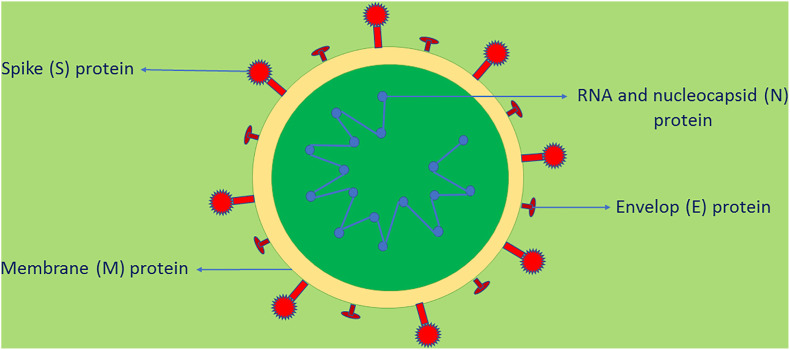
COVID-19 was first reported in December 2019, Hubei Province, China [1]. A group of patients with fever, cough, breathing difficulties and other symptoms were hospitalized and computed tomography scanning of patients showed varied opacities in the lungs when compared with images of healthy lungs [2]. Later it was found that the disease-causing pathogen has a similar genetic sequence to betacoronavirus B lineage and the virus was named as SARS-CoV-2. SARS-CoV-2 is a positive-sense single stranded RNA virus. It contains an RNA genome, four structural proteins namely spike (S), membrane (M), nucleocapsid (N) and envelope (E) proteins, and sixteen non-structural proteins (Fig. 1 ). The S protein (crown like) consist of two subunits namely S1 and S2 subunits. The S1 subunit contains receptor binding domain which binds with host cells’ ACE2 receptors and subsequently the S2 subunit catalyses the interaction (fusion) between the envelope of the virus and the cell membrane of the host, thus, gains entry into the host cells. Inside the cells, the RNA undergoes the process such as translation, replication and transcription into vital viral components. Finally, the virus is generated, packed and subsequently the viral particles are released [[3], [4], [5], [6]]. Since the S protein plays a crucial role for infection, it can be considered as a main target to prevent and treat COVID-19 [7].
Fig. 1.
Structure of SARS-CoV-2.
SARS-CoV-2 is a positive-sense single stranded RNA virus. T contains an RNA genome, four structural proteins namely spike (S), membrane (M), nucleocapsid (N) and envelope (E) proteins, and sixteen non-structural proteins. The S protein (crown like) consist of two subunits namely S1 and S2 subunits. The S1 subunit contains receptor binding domain which binds with host cells' ACE2 receptors and subsequently the S2 subunit catalyses the interaction (fusion) between the envelope of the virus and the cell membrane of the host, thus, gains entry into the host cells. Inside the cells, the RNA undergoes the process such as translation, replication and transcription into vital viral components. Finally, the virus is generated, packed and subsequently the viral particles are released [[3], [4], [5], [6]].
As on 9th October 2021, SARS-CoV-2 has affected 266018810 people worldwide with 5265092 deaths [8]. The COVID-19 pandemic has caused severe public health crisis and economic and social disturbance worldwide. Significant measures, which includes using personal protection equipment, wearing masks, maintaining social distancing and Emergency Use Authorization (EUA) of drugs, have been taken to avoid disease spreading and treatment. But these measures may not be useful in long term. Therefore, many vaccines have been developed and their safety and efficacy have been evaluated in clinical trials and several vaccines got EUA and many are in clinical trials. Nucleic acids have been emerging as potential drugs to treat a variety of diseases. Lipid nanoparticles (LNPs) have been studied extensively to deliver a wide range of drugs including vaccines. This article focuses on the potential application of LNPs in the development and delivery of mRNA vaccines for COVID-19.
2. Vaccines
Vaccines protect people from various diseases and have transformed public health and saved many lives every year. Widespread use of vaccine has completely eradicated the smallpox virus infection and reduced the incidence of measles, polio as well as other juvenile diseases considerably [9]. As per WHO, the current immunization programmes save 2 to 3 million lives every year and hence significantly reduce the mortality of children less than 5 years old worldwide [10]. A vaccine contains an antigen which is derived either from the pathogen or prepared synthetically and the antigens induce immune responses after administration and provide protection against the particular pathogen. The effectiveness of a vaccine is determined in clinical trials which relate the vaccine antigens’ immune responses to clinical end points such as the ability to prevent infection, reduce disease severity or decrease the rate of hospitalization [11]. Development of vaccine is a complex process with a time period of 10–15 years normally. Clinical trial studies of vaccines are costly and requires large sampling size with different ethnicities and age groups. Further, monitoring for a longer time is required to ensure the efficacy and safety. Fast development and large-scale manufacturing of vaccines are highly essential to control outbreaks like COVID-19. Therefore, it is important to develop more potent and versatile vaccine platforms [12]. Nucleic acid-based vaccines have been introduced as an alternative to traditional vaccine approaches.
3. mRNA vaccines
In the year 1990, successful production of protein was observed in in vitro transcribed (IVT) mRNA injected mice [13]. Even though there are issues related to mRNA stability, advances in research especially in drug delivery approaches has made mRNA to become a promising approach in vaccine development as well as protein replacement therapy. mRNA can be expressed in non-dividing cells as it does not require to enter into the nucleus. mRNA is transiently active and does not integrate into the genome and is safer than viral vectors and DNA [12]. The mRNAs can be synthesized in a cell-free system and large-scale manufacturing are easy and fast with standardized and controlled conditions [14]. Synthetic mRNA has been studied for preventing and treating diseases. The structure of synthetic mRNA is same as that of natural mRNA molecule [15]. mRNAs are safer when compared with whole viral particles because of their non-infectious nature and are transient carriers of information [16]. mRNAs have self-adjuvanting characteristics as they have the ability to bind with toll-like receptor 7 (TLR7) and improve cellular immunity [17]. Further, various antigens, cell-signalling factors and modulators can be encoded [18].
Nucleic acid vaccines contain DNA or mRNA encoding disease specific antigens and after administration they use the host cells to produce immunogens. The immunogens, inside the host cells induces antibody production and T-cells activation. mRNA is a transient intermediator between genes and proteins. mRNAs have been shown the potential for cancer immunotherapies, protein replacement therapies, viral vaccines, genome editing and cell reprogramming [19]. The different types of mRNA vaccines are non-replicating mRNA, self-amplifying mRNA and circular mRNA. Non-replicating mRNA vaccines can use a simple structure and shorter length RNA molecule. A modified mRNA can significantly increase the biological activity. The modifications of biological macromolecules control the functional specificity. The Pfizer-BioNTech (BNT162b2) and Moderna (mRNA-1273) vaccines use pseudouridine modifications to ensure the stability of mRNA [20]. Both vaccines contain N1-methyl-pseudouridine-modified mRNA which encodes SARS-COVID-19 spike protein. The uridine in the IVT mRNA can be replaced with pseudouridine and this replacement can increase the RNA stability and decrease anti-RNA immune response [21]. In mRNA-LNPs, the mRNA is encapsulated in LNPs. After administration, mRNA-LNPs are delivered into the host cells’ cytosol and the mRNA is used as template for the synthesis of protein antigen [22]. As the protein antigen is generated in the cells after vaccination, the mRNA vaccines are able to induce both antibody production and T-cells induction. Further after administration, the antigen expression is transient which restricts its persistence in the host body [12,23]. Some important vaccine candidates for COVID-19 are given in Table 1 .
Table 1.
Some important COVID-19 vaccine candidates (www.clinicaltrials.gov) (Accessed November 1, 2021).
| Candidate | Type | Clinical trial identifier number | Developer | Delivery system and No. of doses | Clinical trial status | Actual enrolment | Estimated study completion date |
|---|---|---|---|---|---|---|---|
| BNT162b2 | mRNA | NCT04368728 | Pfizer-BioNtech | Lipid NPs | Phase III | 43998 (estimated) | May 2, 2023 |
| NCT04760132 | Two (separated by 3 weeks) | Phase IV | 10000 (estimated) | December 31, 2024 | |||
| mRNA-1273 | mRNA | NCT04470427 | Moderna | Lipid NPs | Phase III | 30420 | October 27, 2022 |
| NCT04760132 | Two (separated by 4 weeks) | Phase IV | 10000 (estimated) | December 31, 2024 | |||
| ChAdOx1 nCoV-19 | Non- replicating viral vector | NCT04400838 | AstraZeneca/Oxford university | Two (separated by 12 weeks) | Phase II/III | 12390 (estimated) | December 31, 2021 |
| Ad26.COV2.S | Non- replicating viral vector | NCT04505722 | Janssen Vaccines & Prevention B.V. | One | Phase III | 44325 | January 2, 2023 |
| Ad5-nCoV | Non- replicating viral vector | NCT04526990 | CanSino Biologicals Inc. | One | Phase III | 40000 (estimated) | January 30, 2022 |
| Gam-COVID-Vac | Non- replicating viral vector | NCT04530396 | Gamaleya Research Institute of Epidemiology and Microbiology | Two (separated by 3 weeks) | Phase III | 33758 | May 1, 2021 |
| Adsorbed COVID-19 (inactivated) vaccine | Inactivated | NCT04456595 | Sinovac Life Sciences Co., Ltd. | Two (separated by 2 weeks) | Phase III | 12688 | February 2022 |
| NCT04747821 | Phase IV | 27711 | February 2022 | ||||
| Inactivated SARS-CoV-2 vaccine (Vero cell) | Inactivated | NCT04560881 | Sinopharm | Two (separated by 3 weeks) | Phase III | 3000 | December 1, 2021 |
| BBV152 | Inactivated | NCT04641481 | Bharat Biotech International Limited | Two (separated by 4 weeks) | Phase III | 25800 | December 2022 |
| SARS-CoV-2rS/Matrix-M-1 Adjuvant | Protein subunit | NCT04611802 | Novavax | Two (separated by 3 weeks) | Phase III | 33000 (estimated) | June 30, 2023 |
4. Lipid nanoparticles
Dr. Alec D. Bangham, British haematologist, was the first person to describe about liposomes in the year 1961 at the Babraham Institute in Cambridge. G. Gregoriadis, in early 1970s suggested that liposomes can be used as a carrier for drug delivery. Liposomes are microscopic vesicles made of membrane and the membrane is composed of phospholipid bilayers which are similar to that of cell membranes. Liposomes can be used to deliver a wide range of substances which include hydrophilic or hydrophobic drugs, diagnostic substances, proteins, DNA and RNA. Liposomes protect the loaded drug from metabolic enzymes and the biological environment. The conventional liposomes are easily removed from the blood. Therefore, sterically stabilized liposomes (stealth liposomes) have been introduced to avoid easy removal from the blood and also to provide favourable pharmacokinetic properties after administration by coating the liposomes surface with a hydrophilic substance usually a derivative of PEG. Further, the lipid bilayer composition can be altered, hence enable them to form complex with genes and to transport the loaded drug to the cytosol using endosomal/lysosomal pathway [24,25]. Liposome-based formulations were introduced in the market in early 1990's. The term LNPs came into use in early 1990s. LNPs differ from liposomes that LNPs form micellar structures within their core and the core can be modified by varying the formulation parameters to obtain desirable characters [26]. Nanoparticles have been extensively studied for the delivery of a wide range of drugs and nucleic acids [[27], [28], [29], [30], [31], [32], [33], [34], [35], [36]]. Comparatively LNPs offer some advantages over other nanocarriers such as more stable and low cost and importantly large-scale industrial manufacturing by techniques such as microfluidics. LNPs showed comparatively less cytotoxicity and immunogenicity when compared with liposomes [37]. LNPs further exhibited high encapsulation efficiency of nucleic acids with increased transfection efficiency [38]. A study by Ndeupen et al. in mice showed that LNPs used nucleoside-modified mRNA vaccines produced inflammatory responses. LNPs produced inflammatory responses after intradermal and intramuscular injections which was characterized by neutrophil infiltration, activation of varied inflammatory pathways and production of different inflammatory cytokines and chemokines [39]. Complexation of nucleic acids with lipids having positive charge stabilizes the nucleic acids and decrease their degradation by nuclease and making them to be delivered into the cells. LNPs can be used to deliver a wide variety of drugs and nucleic acids such as DNA, mRNA and siRNA. The US FDA approved Patisiran (Onpattro®), contains a transthyretin-directed siRNA formulated in LNPs, to treat polyneuropathy caused by hereditary transthyretin-mediated amyloidosis [40].
5. mRNA-based lipid nanoparticle vaccines for COVID-19
Effective delivery of mRNA vaccines is a challenge. The positive charge and hydrophilic nature of nucleic acids hinder them to diffuse across the cell membranes. Degradation due to endogenous nucleases and phagocytic uptake further hinders their effective delivery. Hence, efficient delivery systems like nanocarriers are required for the effective delivery of nucleic acids at cellular level [23]. Several carriers have been studied for delivering mRNA which includes polymer derivatives, lipids and protein derivatives. The LNPs have been studied in a systematic way and successfully used for the delivery of siRNA and mRNA [[40], [41], [42]]. Many mRNA encapsulated LNPs are under clinical investigation for cancer, viral infections and genetic diseases [19,43]. LNPs composed of synthetic cationic lipids are widely used as a nonviral nucleic acid carriers. In endosomes, the pH is lower when compared with extracellular environment. Therefore, after the process of endocytosis, ionizable lipids become protonated and positively charged which may enhance destabilization of membrane and helps endosomal escape of LNPs and deliver the encapsulated material into the cytosol and there the mRNA is translated into antigenic proteins which stimulates the production of antibodies by the immune system [19,43,44]. The delivery of nucleic acids using LNPs involves adsorption of LNPs on the cell plasma membrane and subsequent uptake into the cell by endocytosis followed by nucleic acid release inside the cell. Adsorption of LNPs and their fusion with plasma membrane is promoted electrostatically due to the difference between the charges of cell membrane (negative charge) and the LNPs (positive charge). Once the LNPs entered into the cells, the release of nucleic acid from the cationic carrier is believed that the LNPs’ charge is neutralized by anionic lipids which are present in the cells. This stops electrostatic attraction between nucleic acids and lipids and also disrupt the structure of NPs resulting in the formation of nonlamellar structure [23,45,46].
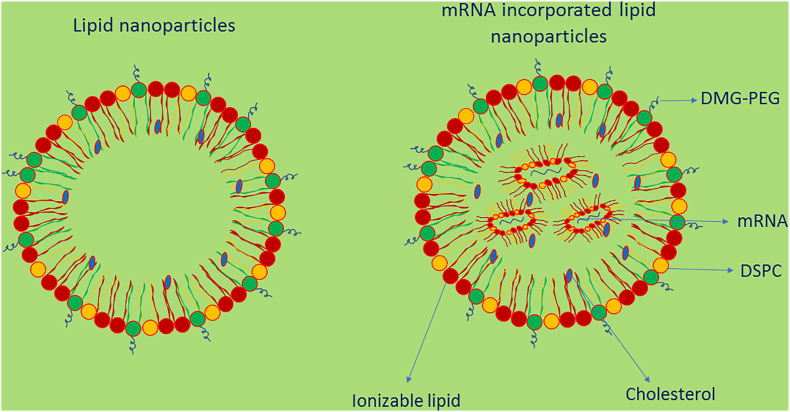
mRNA-LNPs are prepared by rapid mixing generally using microfluidic mixers [3] and one of the commonly used methods is ethanol dilution in which the ethanol solution is added to aqueous media which results in the formation of nanodroplets [47,48]. This method showed significant nucleic acid encapsulation efficiency and the encapsulated mRNA molecule is protected from degradation by nuclease enzymes [49]. LNPs contain structural lipids generally a phospholipid and cholesterol and a PEG-lipid (Fig. 2 ). The function of these lipids is to stabilize the particles, to control their size and provide blood compatibility, but it is important to modify their chemical properties and optimize their concentrations for effective mRNA delivery [[52], [53], [54]]. Structural modification of cholesterol derivatives increased the cellular uptake of LNPs and trafficking by 25-fold [55]. The PEG-lipid affects the particle size, prevent aggregation and destabilization and decrease adsorption of opsonins on LNPs’ surface hence prevents opsonization and RES removal and increase circulation half-life [[56], [57], [58]]. The PEG coating may help navigation through viscous media like lung mucous [59]. The chemical structure of PEG-lipid, the hydrophilic region and the hydrophobic region, influences the size, penetration across lipid membranes and immune reactions [59,60]. It is important that the structure and concentration of PEG-lipid to be optimized to maintain the stealth effect of LNPs [3]. Two mRNA-based vaccines namely the Pfizer-BioNTech (BNT162b2) and Moderna (mRNA-1273) have been given emergency use authorization (EUA) by the US-FDA to prevent SARS-CoV-2 causing COVID-19 disease [61,62].
Fig. 2.
Structure of lipid nanoparticles and mRNA-based lipid nanoparticles.
A LNP contain an ionizable lipid, a stabilizing agent for stability, a phospholipid to stabilize the bilayer and PEG. The ionizable lipid allows self-assembly, increase the encapsulation of mRNA and helps to escape from endosomes. The PEG minimizes LNPs' nonspecific binding to proteins, increases circulation time in blood and helps to avoid RES uptake [50,51].
BNT162b2 is a lipid NP-formulated nucleotide-modified RNA vaccine which encodes a membrane anchored, perfusion stabilized full length SARS-CoV-2 spike protein [63]. The phase I/II trials showed that the vaccine was well tolerated in young (18–55 years of age) and older (65–85 years of age) adults [64]. The participant groups received two doses at a dose level of 10, 20 and 30 μg with a 21-day interval between the two doses. The highest neutralization titres were observed on samples collected on 28th day (7th day after the second dose) or 35th day (14th day after the second dose) [64]. The vaccine induced robust CD8+ and T helper type 1 CD4+ cell responses [65]. BNT162b2, at a dose level of 30 μg, was advanced to phase II/III clinical trials (NCT04368728) [60] and the ongoing trials showed promising results [66]. BNT162b2 showed partial protection quickly 12 days after the first dose and exhibited 95% efficacy after 7 days of second dose [66]. The phase III study results suggested that BNT162b2 vaccine is safe and effective against COVID-19 and the vaccine has been given EUA by many countries including the USA and UK [67,68].
mRNA-1273 is a lipid NP-formulated mRNA vaccine which encodes the perfusion stabilized full length SARS-CoV-2 spike protein [69]. The phase I clinical trials showed that the vaccine was well tolerated in young (18–55 years of age) and older (56–70 years and 71 years of age or older) adults (NCT04283461) [70]. The participant groups received two doses at a dose level of 25 and 100 μg with a 28-day interval between the two doses. The participants group who received 100 μg showed higher binding and neutralizing antibody titres when compared with participants group who received 25 μg [70]. The vaccine produced robust CD4+ type 1 T helper cell responses [3]. The interim results of ongoing phase III clinical trials at a dose level of 100 μg with a 28-day interval between the two doses showed that the vaccine is 94.1% effective [3] and the Moderna (mRNA-1273) vaccine has been given EUA by the US-FDA to prevent SARS-CoV-2 caused COVID-19 disease [71].
Both BNT162b2 and mRNA-1273 are lipid NP-formulated mRNA vaccines and contain mRNA encoding the SARS-CoV-2 spike protein and once delivered into the cytoplasm, the mRNA is translated into the spike protein, which results in producing immune response [37]. The composition of LNPs in both vaccines are similar and are ionizable cationic lipid, cholesterol, PEGylated lipid and distearoylphosphatidylcholine (DSPC) [63,71,72]. The ionizable lipid exhibits neutral charge at physiological pH and hence reduce the toxicity and helps to release the drug. Further, the interaction between the neutral lipids and the blood cell membranes is less, hence improve the LNPs’ biocompatibility [44]. At low pH, the ionizable lipid becomes positively charged as they are protonated which enables RNA complexation. The PEGylated lipid reduces opsonization process hence avoid uptake by phagocytes and increase their circulation time in blood. Cholesterol and distearoylphosphatidylcholine (DSPC) help to incorporate the drug into the LNPs [37,63,71,72]. The molar ratios of the positively charged lipid:PEGylated lipid:cholesterol:DSPC are 46.3:1.6:42.7:9.4 and 50:1.5:38.5:10 for the BNT162b2 and mRNA-1273 vaccines respectively [37,73]. Before administration, BNT162b2 requires thawing and subsequent dilution with saline solution, but mRNA-1273, after thawing, could be administered directly as it is without further dilution with saline solution. The storage conditions of BNT162b2 and mRNA-1273 are −60 to −80 °C and −15 to −25 °C respectively [3]. Table 2 shows the key features of Pfizer-BioNTech (BNT162b2) and Moderna (mRNA-1273) vaccines.
Table 2.
| Key features | Pfizer-BioNTech vaccine (BNT162b2) | Moderna vaccine (mRNA-1273) |
|---|---|---|
| mRNA | modRNA encoding the viral spike glycoprotein of SARS-CoV-2 | Synthetic mRNA encoding the spike glycoprotein of SARS-Co-V-2 |
| Carrier platform | Lipid nanoparticles | Lipid nanoparticles |
| Lipids | ALC-0315 | SM-102 PEG2000-DMG 1,2-distearoyl-sn-glycero-3-phosphocholine, Cholesterol |
| ALC-0159 | ||
| DSPC | ||
| Cholesterol | ||
| EUA approval by FDA | 11th December 2020 | 18th December 2020 |
| Dose | 0.3 ml containing 30 μg vaccine | 0.5 ml containing 100 μg vaccine |
| Number of injections | Two injections and second dose to be administered after 21-to-28-day of first dose | Two injections and second dose to be administered after 28-day of first dose |
| Efficacy | 95% against the SARS-CoV infection | 94.1% against the SARS-CoV infection |
| Stability/storage | −60 to −80 °C (6 months) | −15 to −25 °C (6 months) |
| 2–8 °C (5 days) | 2–8 °C (30 days) | |
| Directions | Supplied in the form of frozen suspension. | Supplied in the form of frozen suspension |
| Must be thawed followed by dilution with 1.8 ml of preservative free sterile saline solution (0.9% w/v). | Vaccine must be thawed before administration. | |
| The vaccine requires to be stored, after dilution, at 2–25 °C and administered within 6 h. | The vaccine requires to be stored, after thawing, at 2–25 °C and administered within 6 h. |
ALC-0315: ((4-hydroxybutyl)azanediyl)bis(hexane6,1-diyl)bis(2-hexyldecanoate).
ALC-0159: 2[(polyethylene glycol)-2000]-N,N-ditetradecylacetamide.
DSPC: 1,2-Distearoyl-sn-glycero-3-phosphocholine.
SM-102: Heptadecan-9-yl 8-((2-hydroxyethyl) (6-oxo-6-(undecyloxy)hexyl)amino) octane.
PEG2000-DMG: 1,2-dimyristoyl-rac-glycero-3-methoxypolyethylene glycol 2000.
modRNA-nucleoside modified messenger RNA.
EUA- Emergency Use Authorization.
6. Future perspectives
The introduction of mRNA vaccines has revolutionized the development of vaccine because of their rapid development, high effectiveness and low-cost manufacturing [12]. But it is difficult to transport mRNA vaccines across the plasma membranes. The nucleases easily degrade mRNA. Further, the long-term storage of mRNA vaccines is a challenge as the half-life decreases with increase in temperature. LNPs are one of the successful carriers to deliver mRNA vaccines. It is beyond doubt that technological advances in LNPs and mRNA drastically reduced the time required for the development of mRNA-LNPs vaccines for COVID-19. The recent successful use of LNPs in the development with unparalleled speed of COVID-19 mRNA vaccines with effectiveness in disease prevention by BNT162b2 (Pfizer-BioNtech) and mRNA-1273 (Moderna) show the potential application of LNPs to deliver mRNA-based vaccines. Optimization of IVT mRNA is expected to reduce intrinsic immunogenicity and enhance the stability as well as translational efficiency [23]. The storage conditions of these vaccines (−60 to −80 °C for Pfizer-BioNTech and −15 to −25 °C for Moderna vaccine) may restrict their use in remote places with inadequate storage facilities [23]. Therefore, developing mRNA-LNPs vaccines which don't need ultra-cold/frozen storage conditions would minimize transportation related issues [19]. It has been reported that advanced cancer can be treated using artificial intelligence enabled nanomedicines [76]. The application of artificial intelligence can be used in the development of mRNA-LNPs. Therefore, it is believed that a combination of artificial intelligence and LNPs would be very effective for the rapid development and delivery of superior mRNA-LNPs vaccines to control outbreaks like SARS-CoV-2.
Author statement
Barnabas Wilson: Conceptualization, designing, writing and editing. Kannoth Mukundan Geetha: Conceptualization, designing, writing and editing.
Declaration of competing interest
None.
References
- 1.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ai T., Yang Z., Hou H., Zhan C., Chen C., Lv W., Tao Q., Sun Z., Xia L. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296:E32–E40. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim J., Eygeris Y., Gupta M., Sahay G. Self- assembled mRNA vaccines. Adv. Drug Deliv. Rev. 2021;170:83–112. doi: 10.1016/j.addr.2020.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang S., Hillyer C., Du L. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol. 2020;41:355–359. doi: 10.1016/j.it.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poland G.A., Ovsyannikova I.G., Crooke S.N., Kennedy R.B. SARS-CoV-2 vaccine development: current status. Mayo Clin. Proc. 2020;95:2172–2188. doi: 10.1016/j.mayocp.2020.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tregoning J.S., Brown E.S., Cheeseman H.M., Flight K.E., Higham S.L., Lemm N.-M., Pierce B.F., Stirling D.C., Wang Z., Pollock K.M. Vaccines for COVID-19. Clin. Exp. Immunol. 2020;202:162–192. doi: 10.1111/cei.13517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samrat S.K., Tharappel A.M., Li Z., Li H. Prospect of SARS-CoV-2 spike protein: potential role in vaccine and therapeutic development. Virus Res. 2020;288 doi: 10.1016/j.virusres.2020.198141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases
- 9.Younger D.S., Younger A.P.J., Guttmacher S. Childhood vaccination: implications for global and domestic public health. Neurol. Clin. 2016;34:1035–1047. doi: 10.1016/j.ncl.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization . WHO; 2020. Child Mortality and Causes of Death.https://www.who.int/gho/child_health/mortality/mortality_under_five_text/en/ [Google Scholar]
- 11.Pollard A.J., Bijker E.M. A guide to vaccinology: from basic principles to new developments. Nat. Rev. Immunol. 2021;21:129. doi: 10.1038/s41577-020-00479-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines - a new era in vaccinology. Nat. Rev. Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolff J.A., Malone R.W., Williams P., Chong W., Acsadi G., Jani A., Felgner P.L. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 14.Granados-Riveron J.T., Aquino-Jarquin G. Engineering of the current nucleoside-modified mRNA-LNP vaccines against SARS-CoV-2. Biomed. Pharmacother. 2021;142 doi: 10.1016/j.biopha.2021.111953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Niessen A.G.O., Poleganov M.A., Rechner C., Plaschke A., Kranz L.M., Fesser S., Diken M., Löwer M., Vallazza B., Beissert T., Bukur V., Kuhn A.N., Türeci Ö., Sahin U. Improving mRNA-based therapeutic gene delivery by expression-Augmenting 3' UTRs Identified by Cellular Library Screening. Mol. Ther. 2019;27:824–836. doi: 10.1016/j.ymthe.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nanomedicine and the COVID-19 vaccines. Nat. Nanotechnol. 2020;15:963. doi: 10.1038/s41565-020-00820-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fotin-Mleczek M., Duchardt K.M., Lorenz C., Pfeiffer R., Ojkic-Zrna S., Probst J., Kallen K.J. Messenger RNA-based vaccines with dual activity induce balanced TLR-7 dependent adaptive immune responses and provide antitumor activity. J. Immunother. 2011;34:1–15. doi: 10.1097/CJI.0b013e3181f7dbe8. [DOI] [PubMed] [Google Scholar]
- 18.Reichmuth A.M., Oberli M.A., Jaklenec A., Langer R., Blankschtein D. mRNA vaccine delivery using lipid nanoparticles. Ther. Deliv. 2016;7:319–334. doi: 10.4155/tde-2016-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hou X., Zaks T., Langer R., Dong Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021 doi: 10.1038/s41578-021-00358-0. (Epub Ahead of Print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang E., Liu X., Li M., Zhang Z., Song L., Zhu B., Wu X., Liu J., Zhao D., Li Y. Advances in COVID-19 mRNA vaccine development. Signal Transduct. Targeted Ther. 2022;7:94. doi: 10.1038/s41392-022-00950-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karikó K., Muramatsu H., Welsh F.A., Ludwig J., Kato H., Akira S., Weissman D. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 2008;16:1833–1840. doi: 10.1038/mt.2008.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kowalski P.S., Rudra A., Miao L., Anderson D.G. Delivering the messenger: advances in technologies for therapeutic mRNA delivery. Mol. Ther. 2019;27:710–728. doi: 10.1016/j.ymthe.2019.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y., Tenchov R., Smoot J., Liu C., Watkins S., Zhou Q. A comprehensive review of the global efforts on COVID-19 vaccine development. ACS Cent. Sci. 2021;7:512–533. doi: 10.1021/acscentsci.1c00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bangham A.D. Liposomes: the Babraham connection. Chem. Phys. Lipids. 1993;64:275–285. doi: 10.1016/0009-3084(93)90071-a. [DOI] [PubMed] [Google Scholar]
- 25.Daraee H., Etemadi A., Kouhi M., Alimirzalu S., Akbarzadeh A. Application of liposomes in medicine and drug delivery. Artif. Cells Nanomed. Biotechnol. 2016;44:381–391. doi: 10.3109/21691401.2014.953633. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell M.J., Billingsley M.M., Haley R.M., Wechsler M.E., Peppas N.A., Langer R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021;20:101–124. doi: 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson B., Samanta M.K., Santhi K., Sampathkumar K.P., Paramakrishnan N., Suresh B. Targeted delivery of tacrine into the brain with polysorbate 80-coated poly(n-butylcyanoacrylate) nanoparticles. Eur. J. Pharm. Biopharm. 2008;70:75–84. doi: 10.1016/j.ejpb.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 28.Wilson B., Samanta M.K., Santhi K., Sampathkumar K.P., Paramakrishnan N., Suresh B. Poly(n-butylcyanoacrylate) nanoparticles coated with polysorbate 80 for the targeted delivery of rivastigmine into the brain to treat Alzheimer's disease. Brain Res. 2008;1200:159–168. doi: 10.1016/j.brainres.2008.01.039. [DOI] [PubMed] [Google Scholar]
- 29.Wilson B., Samanta M.K., Santhi K., Sampathkumar K.P., Ramasamy M., Suresh B. Chitosan nanoparticles as a novel delivery system for anti-Alzheimer’s drug tacrine. Nanomed. Nanotechnol. Biol. Med. 2010;6:144–152. doi: 10.1016/j.nano.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Wilson B., Ambika T.V., Dharmeshkumar P., Jenita J.J.L., Priyadarshini S.R.B. Nanoparticles based on albumin: preparation, characterization and the use for 5-flurouracil delivery. Int. J. Biol. Macromol. 2012;51:874–878. doi: 10.1016/j.ijbiomac.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 31.Wilson B., Lavanya Y., Priyadarshini S.R.B., Ramasamy M., Jenita J.J.L. Albumin nanoparticles for the delivery of gabapentin: preparation, characterization and pharmacodynamic studies. Int. J. Pharm. 2014;473:73–79. doi: 10.1016/j.ijpharm.2014.05.056. [DOI] [PubMed] [Google Scholar]
- 32.Wilson B., Paladugu L., Priyadarshini S.R.B., Jenita J.J.L. Development of albumin-based nanoparticles for the delivery of abacavir. Int. J. Biol. Macromol. 2015;81:763–767. doi: 10.1016/j.ijbiomac.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 33.Wilson B., Selvam J., Mukundan G.K., Premakumari K.B., Jenita J.L. Albumin nanoparticles coated with polysorbate 80 for the targeted delivery of antiepileptic drug levetiracetam into the brain. Drug Deliv. Transl. Res. 2020;10:1853–1861. doi: 10.1007/s13346-020-00831-3. [DOI] [PubMed] [Google Scholar]
- 34.Wilson B., Geetha K.M. Neurotherapeutic applications of nanomedicine for treating Alzheimer's disease. J. Contr. Release. 2020;325:25–37. doi: 10.1016/j.jconrel.2020.05.044. [DOI] [PubMed] [Google Scholar]
- 35.Wilson B., Alobaid B.N.M., Geetha K.M., Jenita J.L. Chitosan nanoparticles to enhance nasal absorption and brain targeting of sitagliptin to treat Alzheimer's disease. J. Drug Deliv. Sci. Technol. 2021;61 [Google Scholar]
- 36.Shankar J., Geetha K.M., Wilson B. Potential applications of nanomedicine for treating Parkinson's disease. J. Drug Deliv. Sci. Technol. 2021;66 [Google Scholar]
- 37.Tenchov R., Bird R., Curtze A.E., Zhou Q. Lipid nanoparticles-from liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano. 2021 doi: 10.1021/acsnano.1c04996. (Epub Ahead of Print) doi: [DOI] [PubMed] [Google Scholar]
- 38.Billingsley M.M., Singh N., Ravikumar P., Zhang R., June C.H., Mitchell M.J. Ionizable lipid nanoparticle-mediated mRNA delivery for human CAR T cell engineering. Nano Lett. 2020;20:1578–1589. doi: 10.1021/acs.nanolett.9b04246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ndeupen S., Qin Z., Jacobsen S., Bouteau A., Estanbouli H., Igyarto B.Z. The mRNA-LNP platform's lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience. 2021;24 doi: 10.1016/j.isci.2021.103479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akinc A., Maier M.A., Manoharan M., Fitzgerald K., Jayaraman M., Barros S., Ansell S., Du X.Y., Hope M.J., Madden T.D., Mui B.L., Semple S.C., Tam Y.K., Ciufolini M., Witzigmann D., Kulkarni J.A., van der Meel R., Cullis P.R. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat. Nanotechnol. 2019;14:1084–1087. doi: 10.1038/s41565-019-0591-y. [DOI] [PubMed] [Google Scholar]
- 41.Anderson E.J., Rouphael N.G., Widge A.T., Jackson L.A., Roberts P.C., Makhene M., Chappell J.D., Denison M.R., Stevens L.J., Pruijssers A.J., McDermott A.B., Flach B., Lin B.C., Doria-Rose N.A., O'Dell S., Schmidt S.D., Corbett K.S., Swanson P.A., 2nd, Padilla M., Neuzil K.M., Bennett H., Leav B., Makowski M., Albert J., Cross K., Edara V.V., Floyd K., Suthar M.S., Martinez D.R., Baric R., Buchanan W., Luke C.J., Phadke V.K., Rostad C.A., Ledgerwood J.E., Graham B.S., Beigel J.H. mRNA-1273 Study Group, Safety and immunogenicity of SARS- CoV-2 mRNA-1273 vaccine in older adults. N. Engl. J. Med. 2020;383:2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Marc G.P., Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck R.W., Jr., Hammitt L.L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D.B., Mather S., Dormitzer P.R., Şahin U., Jansen K.U., Gruber W.C. C4591001 clinical trial group, safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gebre M.S., Brito L.A., Tostanoski L.H., Edwards D.K., Carfi A., Barouch D.H. Novel approaches for vaccine development. Cell. 2021;184:1589–1603. doi: 10.1016/j.cell.2021.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meng C., Chen Z., Li G., Welte T., Shen H. Nanoplatforms for mRNA therapeutics. Adv. Ther. 2021;4 [Google Scholar]
- 45.Koynova R., Wang L., MacDonald R.C. An intracellular lamellar - nonlamellar phase transition rationalizes the superior performance of some cationic lipid transfection agents. Proc. Natl. Acad. Sci. U.S.A. 2006;103:14373–14378. doi: 10.1073/pnas.0603085103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koynova R., Tenchov B. In: Bielke W., Erbacher C., editors. vol. 296. Springer-Verlag; Berlin, Heidelberg: 2010. Cationic Lipids: molecular structure/transfection activity relationships and interactions with biomembranes; pp. 51–93. (Nucleic Acid Transfection). [DOI] [PubMed] [Google Scholar]
- 47.Leung A.K., Tam Y.Y.C., Chen S., Hafez I.M., Cullis P.R. Microfluidic mixing: a general method for encapsulating macromolecules in lipid nanoparticle systems. J. Phys. Chem. B. 2015;119:8698–8706. doi: 10.1021/acs.jpcb.5b02891. [DOI] [PubMed] [Google Scholar]
- 48.Jahn A., Stavis S.M., Hong J.S., Vreeland W.N., Devoe D.L., Gaitan M. Microfluidic mixing and the formation of nanoscale lipid vesicles. ACS Nano. 2010;4:2077–2087. doi: 10.1021/nn901676x. [DOI] [PubMed] [Google Scholar]
- 49.Blakney A.K., McKay P.F., Yus B.I., Aldon Y., Shattock R.J. Inside out: optimization of lipid nanoparticle formulations for exterior complexation and in vivo delivery of saRNA. Gene Ther. 2019;26:363–372. doi: 10.1038/s41434-019-0095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guevara M.L., Persano F., Persano S. Advances in lipid nanoparticles for mRNA-based cancer immunotherapy. Front. Chem. 2020;8 doi: 10.3389/fchem.2020.589959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khurana A., Allawadhi P., Khurana I., Allwadhi S., Weiskirchen R., Banothu A.K., Chhabra D., Joshi K., Bharani K.K. Role of nanotechnology behind the success of mRNA vaccines for COVID-19. Nano Today. 2021;38 doi: 10.1016/j.nantod.2021.101142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng X., Lee R.J. The role of helper lipids in lipid nanoparticles (LNPs) designed for oligonucleotide delivery. Adv. Drug Deliv. Rev. 2016;99:129–137. doi: 10.1016/j.addr.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 53.Li B., Luo X., Deng B., Wang J., McComb D.W., Shi Y., Gaensler K.M.L., Tan X., Dunn A.L., Kerlin B.A., Dong Y. An orthogonal array optimization of lipid-like nanoparticles for mRNA delivery in vivo. Nano Lett. 2015;15:8099–8107. doi: 10.1021/acs.nanolett.5b03528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kauffman K.J., Dorkin J.R., Yang J.H., Heartlein M.W., DeRosa F., Mir F.F., Fenton O.S., Anderson D.G. Optimization of lipid nanoparticle formulations for mRNA delivery in vivo with fractional factorial and definitive screening designs. Nano Lett. 2015;15:7300–7306. doi: 10.1021/acs.nanolett.5b02497. [DOI] [PubMed] [Google Scholar]
- 55.Patel S., Ashwanikumar N., Robinson E., Xia Y., Mihai C., Griffith J.P., Hou S., Esposito A.A., Ketova T., Welsher K., Joyal J.L., Almarsson Ö., Sahay G. Naturally occurring cholesterol analogues in lipid nanoparticles induce polymorphic shape and enhance intracellular delivery of mRNA. Nat. Commun. 2020;11:983. doi: 10.1038/s41467-020-14527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heyes J., Hall K., Tailor V., Lenz R., MacLachlan I. Synthesis and characterization of novel poly(ethylene glycol)-lipid conjugates suitable for use in drug delivery. J. Contr. Release. 2006;112:280–290. doi: 10.1016/j.jconrel.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 57.Leung A.K.K., Tam Y.Y.C., Cullis P.R. Lipid nanoparticles for short interfering RNA delivery. Adv. Genet. 2014;88:71–110. doi: 10.1016/B978-0-12-800148-6.00004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jokerst J.V., Lobovkina T., Zare R.N., Gambhir S.S. Nanoparticle PEGylation for imaging and therapy. Nanomed. Lond. 2011;6:715–728. doi: 10.2217/nnm.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hashizaki K., Taguchi H., Itoh C., Sakai H., Abe M., Saito Y., Ogawa N. Effects of poly(ethylene glycol) (PEG) concentration on the permeability of PEG-grafted liposomes. Chem. Pharm. Bull. (Tokyo) 2005;53:27–31. doi: 10.1248/cpb.53.27. [DOI] [PubMed] [Google Scholar]
- 60.Abu Lila A.S., Kiwada H., Ishida T. The accelerated blood clearance (ABC) phenomenon: clinical challenge and approaches to manage. J. Contr. Release. 2013;172:38–47. doi: 10.1016/j.jconrel.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 61.https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine
- 62.https://www.modernatx.com/covid19vaccine-eua
- 63.Emergency Use Authorization (Eua) of the Pfizer-Biontech Covid-19 Vaccine to Prevent Coronavirus Disease 2019 (COVID-19) in individuals 16 Years of Age and Older. https://www.fda.gov/media/144414/download (Accessed 22 December 2020).
- 64.Walsh E.E., Frenck R.W., Falsey A.R., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Mulligan M.J., Bailey R., Swanson K.A., Li P., Koury K., Kalina W., Cooper D., Fontes-Garfias C., Shi P.-Y., Türeci Ö., Tompkins K.R., Lyke K.E., Raabe V., Dormitzer P.R., Jansen K.U., Şahin U., Gruber W.C. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N. Engl. J. Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sahin U., Vogler I., Derhovanessian E., Kranz L.M., Vormehr M., Quandt J., Bidmon N., Ulges A., Baum A., Pascal K., Maurus D., Brachtendorf S., Lörks V., Sikorski J., Koch P., Hilker R., Becker D., Eller A.K., Grützner J., Tonigold M., Boesler C., Rosenbaum C., Heesen L., Kühnle M.C., Poran A., Dong J.Z., Luxemburger U., Brück A.K., Langer D., Bexon M., Bolte S., Palanche T., Schultz T.A., Baumann S., Mahiny A.J., Boros G., Reinholz J., Szabó G.T., Karikó K., Shi P.Y., Fontes-Garfias C., Perez J.L., Cutler M., Cooper D., Kyratsous C.A., Dormitzer P.R., Jansen K.U., Türeci Ö. 2020. BNT162b2 Induces SARS-CoV-2 Neutralising Antibodies and T Cells in Humans.https://www.medrxiv.org/content/10.1101/2020.12.09.20245175v1 Preprint. [Google Scholar]
- 66.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Marc G.P., Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck R.W., Jr., Hammitt L.L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D.B., Mather S., Dormitzer P.R., Şahin U., Jansen K.U., Gruber W.C. For the C4591001 clinical trial group, safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ledford H. US authorization of first COVID vaccine marks new phase in safety monitoring. Nature. 2020;588:377–378. doi: 10.1038/d41586-020-03542-4. [DOI] [PubMed] [Google Scholar]
- 68.Ledford H., Cyranoski D., Van Noorden R. The UK has approved a COVID vaccine —here’s what scientists now want to know. Nature. 2020;588:205–206. doi: 10.1038/d41586-020-03441-8. [DOI] [PubMed] [Google Scholar]
- 69.Corbett K.S., Edwards D.K., Leist S.R., Abiona O.M., Boyoglu-Barnum S., Gillespie R.A., Himansu S., Schäfer A., Ziwawo C.T., DiPiazza A.T., Dinnon K.H., Elbashir S.M., Shaw C.A., Woods A., Fritch E.J., Martinez D.R., Bock K.W., Minai M., Nagata B.M., Hutchinson G.B., Wu K., Henry C., Bahi K., Garcia-Dominguez D., Ma L., Renzi I., Kong W.-P., Schmidt S.D., Wang L., Zhang Y., Phung E., Chang L.A., Loomis R.J., Altaras N.E., Narayanan E., Metkar M., Presnyak V., Liu C., Louder M.K., Shi W., Leung K., Yang E.S., West A., Gully K.L., Stevens L.J., Wang N., Wrapp D., Doria-Rose N.A., Stewart-Jones G., Bennett H., Alvarado G.S., Nason M.C., Ruckwardt T.J., McLellan J.S., Denison M.R., Chappell J.D., Moore I.N., Morabito K.M., Mascola J.R., Baric R.S., Carfi A., Graham B.S. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature. 2020;586:567–571. doi: 10.1038/s41586-020-2622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anderson E.J., Rouphael N.G., Widge A.T., Jackson L.A., Roberts P.C., Makhene M., Chappell J.D., Denison M.R., Stevens L.J., Pruijssers A.J., McDermott A.B., Flach B., Lin B.C., Doria-Rose N.A., O'Dell S., Schmidt S.D., Corbett K.S., Swanson P.A., Padilla M., Neuzil K.M., Bennett H., Leav B., Makowski M., Albert J., Cross K., Edara V.V., Floyd K., Suthar M.S., Martinez D.R., Baric R., Buchanan W., Luke C.J., Phadke V.K., Rostad C.A., Ledgerwood J.E., Graham B.S., Beigel J.H. Safety and immunogenicity of SARS-CoV-2mRNA-1273 vaccine in older adults. N. Engl. J. Med. 2020;383:2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Emergency Use Authorization (Eua) of the Moderna Covid-19 Vaccine to Prevent Coronavirus Disease 2019 (Covid-19) in individuals 18 Years of Age and Older. https://www.fda.gov/media/144638/download (Accessed 22 December 2020).
- 72.K. Miller, What's in the Pfizer and Moderna Covid-19 Vaccines? https://www.prevention.com/health/a35002158/pfizer-vs-modernacovid-19-vaccine-ingredients/(Accessed 22 December 2020).
- 73.DeFrancesco L. Whither COVID-19 vaccines? Nat. Biotechnol. 2020;38:1132–1145. doi: 10.1038/s41587-020-0697-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pfizer-Biontech COVID-19 Vaccine - bnt162b2 injection, suspension. https://dailymed.nlm.nih.gov/dailymed/drugInfo. cfm?setid=908ecbe7-2f1b-42dd-94bf-f917ec3c5af8 (Accessed December 22, 2020).
- 75.Vaccines and Related Biological Products Advisory Committee Meeting. Moderna COVID-19 vaccine. FDA Briefing Document. https://www.fda.gov/media/144434/download (Accessed December 22, 2020).
- 76.Wilson B., Geetha K.M. Artificial intelligence and related technologies enabled nanomedicine for advanced cancer treatment. Nanomedicine (London) 2020;15:433–435. doi: 10.2217/nnm-2019-0366. [DOI] [PubMed] [Google Scholar]