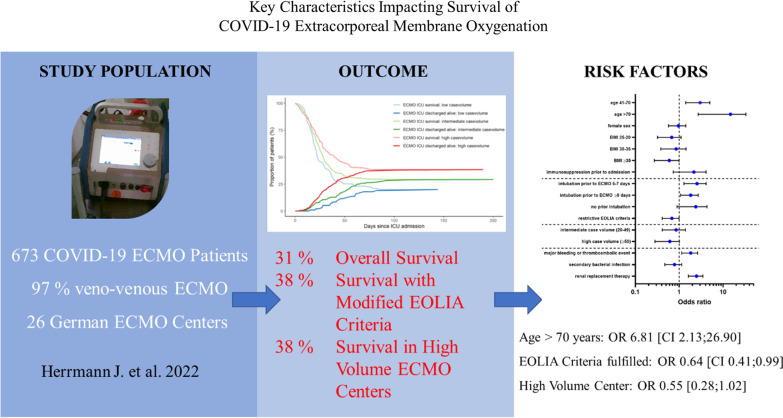
Abstract
Background
Severe COVID-19 induced acute respiratory distress syndrome (ARDS) often requires extracorporeal membrane oxygenation (ECMO). Recent German health insurance data revealed low ICU survival rates. Patient characteristics and experience of the ECMO center may determine intensive care unit (ICU) survival. The current study aimed to identify factors affecting ICU survival of COVID-19 ECMO patients.
Methods
673 COVID-19 ARDS ECMO patients treated in 26 centers between January 1st 2020 and March 22nd 2021 were included. Data on clinical characteristics, adjunct therapies, complications, and outcome were documented. Block wise logistic regression analysis was applied to identify variables associated with ICU-survival.
Results
Most patients were between 50 and 70 years of age. PaO2/FiO2 ratio prior to ECMO was 72 mmHg (IQR: 58–99). ICU survival was 31.4%. Survival was significantly lower during the 2nd wave of the COVID-19 pandemic. A subgroup of 284 (42%) patients fulfilling modified EOLIA criteria had a higher survival (38%) (p = 0.0014, OR 0.64 (CI 0.41–0.99)). Survival differed between low, intermediate, and high-volume centers with 20%, 30%, and 38%, respectively (p = 0.0024). Treatment in high volume centers resulted in an odds ratio of 0.55 (CI 0.28–1.02) compared to low volume centers. Additional factors associated with survival were younger age, shorter time between intubation and ECMO initiation, BMI > 35 (compared to < 25), absence of renal replacement therapy or major bleeding/thromboembolic events.
Conclusions
Structural and patient-related factors, including age, comorbidities and ECMO case volume, determined the survival of COVID-19 ECMO. These factors combined with a more liberal ECMO indication during the 2nd wave may explain the reasonably overall low survival rate. Careful selection of patients and treatment in high volume ECMO centers was associated with higher odds of ICU survival.
Trial registration
Registered in the German Clinical Trials Register (study ID: DRKS00022964, retrospectively registered, September 7th 2020, https://www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00022964.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-022-04053-6.
Keywords: COVID-19, Acute respiratory distress syndrome (ARDS), Intensive care, Extracorporeal life support, Case-volume relationship
Introduction
The COVID-19 pandemic is challenging intensive care providers due to severe and prolonged cases of COVID-19 induced acute respiratory distress syndrome (ARDS). Compromised gas exchange may deteriorate despite maximum medical care, whereas veno-venous extracorporeal membrane oxygenation (VV ECMO) offers the chance to uphold oxygenation, carbon dioxide removal and rest the lungs. Although ECMO provides a rescue strategy and bridge to recovery, its use is resource intensive and can be associated with serious complications. In Germany, ECMO utilization had already increased manifold prior to the COVID-19 pandemic [1].
Need and indications of ECMO support are not universally defined but vary between centers and resource availability. In a pandemic ECMO use likely follows a U-shaped curve. A high number of patients are treated as COVID-19 numbers rise, decrease when hospital strain exceeds their capabilities and may rise again as strain eases [2]. In contrast to other countries, the German health care system was not overloaded during the COVID-19 pandemic [3]. In this context, patients with a lower, but reasonable probability of survival received ECMO support and numerous low to high volume ECMO centers treated COVID-19 ARDS patients. Recent health insurance data including more than 4000 VV ECMO patients surprisingly revealed a hospital survival rate of only 34%, thus further detailed structural and patient related analyses are urgently needed [4].
The continuous provision of organizational structures for successful ECMO therapy is challenging and during the pandemic less experienced centers have been faced with an increased number of ECMO patients, necessitating careful planning and training [5]. In this regard, effectiveness of low volume centers had already been questioned in non-COVID ECMO. In previous studies, admission to hospitals treating more than 30 [6] or more than 50 [7] ECMO patients per year was associated with a lower mortality in veno-arterial ECMO (VA ECMO). However, volume-outcome relationships have been less frequently defined in VV ECMO. An analysis of the Extracorporeal Life Support Organization Registry found no significant association between center volumes and patient survival in non-COVID respiratory assist [6]. Nonetheless, a position paper of renowned experts recommended that respiratory ECMO programs should treat at least 20 patients per year, including 12 respiratory cases [8]. Moreover, a recent study found that centers with longer experience with COVID-19 ECMO had a lower mortality rate relative to centers that started COVID-19 ECMO at a later timepoint [9].
We performed a multicenter study aiming to delineate the characteristics of ECMO therapy for COVID-19 induced ARDS, as well as to identify structural and patient-related factors independently associated with early survival of intensive care unit (ICU) treatment.
Material and methods
Study design and patient population
This is a retrospective observational study. Consecutive patients with SARS-CoV-2 infection confirmed with real-time reverse transcriptase polymerase chain reaction (RT-PCR) testing suffering from ARDS treated with ECMO at 26 ECMO centers across Germany between January 1st 2020 and March 22nd 2021 were included (Additional file 1: Figure S1). Hospitals in Germany utilizing COVID-19 ECMO support were invited to enter patient data into the register. The register continuously collects observational, multi-center data to recognize structural- and patient-related risk factors, complications, treatment effects and the outcome of COVID-19 ECMO patients.
Indication of ECMO
Indications of ECMO support were at the discretion of the respective centers according to their in-house standards. Indications were classified as hypoxemia, hypercapnia, lung protective ventilation, right heart failure, left heart failure, cardiopulmonary resuscitation, or pulmonary embolism.
Data collection and variable definition
Data were documented by the treating physicians within a standardized electronic case report form (RedCap®, Vanderbilt University).
Bleeding events were assessed according to definitions by Schulman et al. [10] and Kaatz et al. [11]. Thromboembolic events were included if diagnosed by standardized ultrasound examinations or CT scans.
Outcomes and subgroups
The primary endpoint was survival at ICU discharge. Experience of the ECMO centers as a structural criterion was categorized according to the number of VV ECMO performed in 2019 as follows: low (< 20), intermediate (20–49) and high (≥ 50). In 6 centers this information was not available, thus, the center`s experience was estimated based in the number of COVID ECMOs in the observation period.
Statistical analysis
Descriptive statistics are expressed as median (IQR) for continuous variables and as frequencies for categorical variables (including a category for missing data). Differences between groups were tested using the Mann–Whitney U test (continuous variables), χ2 test (categorical variables) or Fisher’s exact test (categorical variables with observed frequencies < 5), as appropriate. In a subgroup analysis, modified EOLIA trial inclusion criteria were applied to evaluate the impact of liberal vs. restrictive patient selection [12]. These subgroup criteria were defined as use of ECMO, age ≤ 70 years, mechanical ventilation for less than 8 days prior to ECMO, body mass index ≤ 45 kg/m2, absence of malignancies and no history of myocardial infarction, congestive heart failure, chronic pulmonary disease, and moderate to severe liver or kidney disease. We used logistic regression analyses to determine variables associated with mortality during stay at ICU and estimated odds ratios (ORs) with corresponding 95% confidence intervals (CIs). We selected variables a priori based on clinical background knowledge and assigned them to blocks reflecting the clinical course over time. We adjusted the models block-wise in four additional blocks: 1. demographics, risk factors and comorbidities (age, sex, BMI and immunosuppression within 6 months prior to admission); 2. severity of disease (intubation prior to ECMO and EOLIA criteria); 3. ECMO case volume, and 4. complications (major bleeding or thromboembolic events, secondary bacterial infection and renal replacement therapy). The quality of the models was assessed using the Akaike information criterion (AIC). Due to the high number of missing values within distinct variables, missingness was considered as a separate category in the primary analysis. We applied the following sensitivity analyses for the model: EOLIA criteria fulfilled or not, and complete case analysis. Data analysis was performed with R version 4.1.0. Statistical significance was determined at an α level of 0.05 (two-tailed).
Ethics
The Ethics Committee of the Medical Faculty of the Julius-Maximilians-University of Wuerzburg approved the study protocol (131/20-me). Additional local ethics committee votes were obtained from each of the participating ECMO centers. According to German legislation, no informed consent for retrospective, anonymous data is required and informed consent was waived by the ethics committee. This study was registered in the German Clinical Trials Register (study ID: DRKS00022964, retrospectively registered, September 7th 2020, https://www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00022964).
Results
Patient population
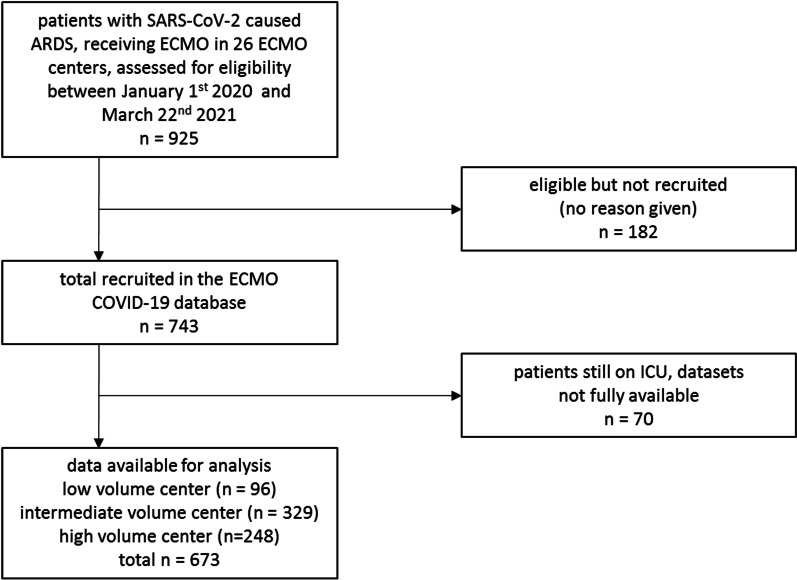
Of 925 patients treated between January 1st 2020 and March 22nd 2021, routine data from 743 patients were documented and 673 complete datasets were available at the first database closure on March 22, 2021 (Fig. 1). Intermediate volume centers treated 329 (49%), high volume centers 248 (37%), and low volume centers 96 (14%) patients.
Fig. 1.
Flowchart patient recruitment
Table 1(1) depicts demographics, risk factors and comorbidities. 535 (79%) were male, median BMI was 29 (IQR: 27–35). Most study patients were between 41 and 70 years (86%). The most frequent comorbidities were cardiovascular diseases (62%), diabetes mellitus (28%), and chronic pulmonary disease (15%). Immunosuppression was the only comorbidity significantly associated with mortality. 284 (42%) patients fulfilled modified EOLIA criteria, whereas 389 (58%) did not.
Table 1.
Clinical characteristics of study cohort
| n | Level | Overall—n (%) | Survivor—n (%) | Non-survivor—n (%) | p |
|---|---|---|---|---|---|
| 673 (100) | 211 (31.4) | 462 (68.6) | |||
| (1) Demographics, Risk factors, comorbidities | |||||
| Date of hospital admission | 04/2020–06/2020 | 186 (27.6) | 75 (35.5) | 111 (24.0) | 0.0019 |
| 07/2020–03/2021 | 487 (72.4) | 136 (64.5) | 351 (76.0) | ||
| Age [years] | 19–40 | 50 (7.4) | 29 (13.7) | 21 (4.6) | < 0.0001 |
| 41–70 | 578 (85.9) | 178 (84.4) | 400 (86.6) | ||
| > 70 | 42 (6.2) | 4 (1.9) | 38 (8.2) | ||
| Missing | 3 (0.4) | 0 (0.00) | 3 (0.7) | ||
| Sex | m | 535 (79.5) | 161 (76.3) | 374 (81,095) | 0.3661 |
| w | 131 (19.5) | 48 (22.8) | 83 (18.0) | ||
| Missing | 7 (1.0) | 2 (0.9) | 5 (1.1) | ||
| BMI [kg/m2] | < 25 | 81 (12.0) | 21 (10.0) | 60 (13.0) | 0.0414 |
| 25–30 | 263 (39.1) | 83 (39.3) | 180 (39.0) | ||
| 30–35 | 147 (21.8) | 43 (20.4) | 104 (22.5) | ||
| ≥ 35 | 157 (23.3) | 61 (28.9) | 96 (20.8) | ||
| Missing | 25 (3.7) | 3 (1.4) | 22 (4.8) | ||
| Cardiovascular disease | No | 226 (33.6) | 82 (38.9) | 144 (31.2) | 0.1323 |
| Yes | 416 (61.8) | 119 (56.4) | 297 (64.3) | ||
| Missing | 31 (4.6) | 10 (4.7) | 21 (4.6) | ||
| Chronic pulmonary disease | No | 564 (83.8) | 179 (84.8) | 385 (83.3) | 0.8043 |
| Yes | 103 (15.3) | 31 (14.7) | 72 (15.6) | ||
| Missing | 6 (0.9) | 1 (0.5) | 5 (1.1) | ||
| Diabetes mellitus | No | 483 (71.8) | 153 (72.5) | 330 (71.4) | 0.9401 |
| Yes | 186 (27.6) | 57 (27.0) | 129 (27.9) | ||
| Missing | 4 (0.6) | 1 (0.5) | 3 (0.7) | ||
| Moderate to severe kidney disease | No | 619 (92.0) | 192 (91.0) | 427 (92.4) | 0.6161 |
| Yes | 49 (7.3) | 18 (8.5) | 31 (6.7) | ||
| Missing | 5 (0.7) | 1 (0.5) | 4 (0.9) | ||
| Immunosuppression within 6 months prior to admission | No | 523 (77.7) | 158 (74.9) | 365 (79.0) | 0.0334 |
| Yes | 39 (5.8) | 8 (3.8) | 31 (6.7) | ||
| Unknown | 111 (16.5) | 45 (21.3) | 66 (14.3) | ||
| (2) Severity of disease, laboratory parameters (day 1) | |||||
| EOLIA criteria | Met | 284 (42.2) | 108 (51.2) | 176 (38.1) | 0.0014 |
| Not met | 389 (57.8) | 103 (48.8) | 286 (61.9) | ||
| Indication of ECMO | Hypoxemia | 419 (62.3) | 126 (59.7) | 293 (63.4) | 0.5229 |
| Hypercapnia | 141 (21.0) | 43 (20.4) | 98 (21.2) | ||
| Lung protective ventilation | 48 (7.1) | 16 (7.6) | 32 (6.9) | ||
| Right heart failure | 2 (0.3) | 1 (0.5) | 1 (0.2) | ||
| Left heart failure | 4 (0.6) | 1 (0.5) | 3 (0.6) | ||
| Cardiopulmonary resuscitation | 6 (0.9) | 0 (0.0) | 6 (1.3) | ||
| Pulmonary embolism | 3 (0.4) | 3 (1.4) | 0 (0.0) | ||
| Other | 9 (1.3) | 5 (2.4) | 4 (0.9) | ||
| Intubation prior to ECMO [days] | < 5 | 275 (40.9) | 102 (48.3) | 173 (37.4) | 0.0004 |
| 5–7 | 102 (15.2) | 20 (9.5) | 82 (17.7) | ||
| ≥ 8 | 179 (26.6) | 44 (20.9) | 135 (29.2) | ||
| No prior intubation | 75 (11.1) | 33 (15.6) | 42 (9.1) | ||
| Missing | 42 (6.2) | 12 (5.7) | 30 (6.5) | ||
| Creatinine [mg/dl] | ≤ 1.17 | 313 (46.5) | 99 (46.9) | 214 (46.3) | 0.8757 |
| > 1.17 | 304 (45.2) | 93 (44.1) | 211 (45.7) | ||
| Missing | 56 (8.3) | 19 (9.00) | 37 (8.0) | ||
| Mode of ventilation | Spontaneous | 18 (2.67) | 9 (4.27) | 9 (1.95) | 0.3042 |
| Assisted | 71 (10.55) | 19 (9.00) | 52 (11.26) | ||
| Controlled | 544 (80.83) | 170 (80.57) | 374 (80.95) | ||
| Missing | 40 (5.94) | 13 (6.16) | 27 (5.84) | ||
| Lung compliance [ml/cm H2O] | 25.5 [18.1, 34.2] | 26.0 [17.6, 35.7] | 25.4 [18.4, 33.9] | 0.7168 | |
| (3) ECMO and adjunct therapy | |||||
| Mode of ECMO | VV | 651 (96.7) | 205 (97.2) | 446 (96.5) | 0.5104 |
| VA | 12 (1.8) | 2 (0.9) | 10 (2.2) | ||
| VVA | 10 (1.5) | 4 (1.9) | 6 (1.3) | ||
| Cannula | Single lumen | 544 (80.8) | 153 (72.5) | 391 (84.6) | 0.0028 |
| Double lumen | 52 (7.7) | 22 (10.4) | 30 (6.5) | ||
| Unknown | 70 (10.4) | 33 (15.6) | 37 (8.0) | ||
| Missing | 7 (1.0) | 3 (1.4) | 4 (0.9) | ||
| Case volume ECMO center [n/year] | Low (< 20) | 96 (14.3) | 19 (9.0) | 77 (16.7) | 0.0024 |
| Intermediate (20–49) | 329 (48.9) | 97 (46.0) | 232 (50.2) | ||
| High (≥ 50) | 248 (36.9) | 95 (45.0) | 153 (33.1) | ||
| Duration of ECMO support [h] | 312.5 [144.0, 528.0] | 336.0 [178.2, 560.8] | 300.0 [120.0, 502.5] | 0.0046 | |
| Prone positioning | No | 240 (35.7) | 64 (30.3) | 176 (38.1) | 0.1314 |
| Yes | 333 (49.5) | 111 (52.6) | 222 (48.1) | ||
| Missing | 100 (14.9) | 36 (17.1) | 64 (13.9) | ||
| Therapeutic Anticoagulation | No | 53 (7.9) | 22 (10.4) | 31 (6.7) | 0.0968 |
| Yes | 620 (92.1) | 189 (89.6) | 431 (93.3) | ||
| (4) Complications during ECMO | |||||
| Major bleeding or thromboembolic event | No | 231 (34.3) | 97 (46.0) | 134 (29.0) | < 0.0001 |
| Yes | 442 (65.7) | 114 (54.0) | 328 (71.0) | ||
| Secondary bacterial infection (respiratory tract or bloodstream) | No | 239 (35.5) | 81 (38.4) | 158 (34.2) | 0.2921 |
| Yes | 434 (64.5) | 130 (61.6) | 304 (65.8) | ||
| Renal replacement therapy | No | 282 (41.9) | 123 (58.3) | 159 (34.4) | < 0.0001 |
| Yes | 391 (58.1) | 88 (41.7) | 303 (65.6) | ||
Clinical characteristics of study cohort. Clinical characteristics in total population (n = 673) and survivor (n = 211) vs. non-survivor (n = 462). Parameters were slit up into blocks: (1) Demographics, Risk factors, Comorbidities; (2) Severity of Disease, Laboratory Parameters (day 1); (3) ECMO and adjunct therapy; (4) Complications during ECMO. Descriptive statistics are expressed as frequencies for categorical variables (including a category for missing data). Lung compliance is expressed as median (IQR) and data are missing for 34%, since the parameter was recorded in controlled ventilated patients only. Differences between groups were tested using the Mann–Whitney U test (continuous variables), χ2 test (categorical variables) or Fisher’s exact test (categorical variables with observed frequencies < 5), as appropriate
In low volume centers, less patients fulfilled modified EOLIA criteria and had higher SOFA scores at the time of ECMO initiation. During therapy, high volume centers reported significantly less bleeding/thromboembolic events, less secondary bacterial infections, and a lower need for renal replacement therapy (Table 2).
Table 2.
Case volume-dependent characteristics in study cohort
| Low (< 20) n/(%) | Intermediate (20–49) n/(%) | High (≥ 50) n/(%) | p | |
|---|---|---|---|---|
| Total | 96 (14.3) | 329 (48.9) | 248 (36.9) | |
| Survivor | 19 (19.8) | 97 (29.5) | 95 (38.3) | 0.0024 |
| EOLIA criteria fulfilled | 27 (28.1) | 146 (44.4) | 111 (44.8) | 0.0106 |
| SOFA (day 1) | ||||
| 0–9 | 32 (33.3) | 31 (9.4) | 64 (25.8) | 0.0005 |
| 9–12 | 20 (20.8) | 91 (27.7) | 21 (8.5) | |
| 12–15 | 15 (15.6) | 71 (21.5) | 4 (1.6) | |
| 15–21 | 10 (10.4) | 75 (22.8) | 1 (0.4) | |
| Missing | 19 (19.8) | 61 (18.5) | 158 (64.7) | |
| Intubation prior to ECMO [days] | ||||
| < 5 | 25 (26.0) | 171 (52.0) | 79 (32.9) | 0.0005 |
| 5–7 | 21 (21.9) | 51 (15.5) | 30 (12.1) | |
| ≥ 8 | 46 (47.9) | 87 (26.4) | 46 (18.5) | |
| No prior | 1 (1.0) | 9 (2.7) | 65 (26.2) | |
| Missing | 3 (3.1) | 11 (3.3) | 28 (11.3) | |
| Major bleeding or thromboembolic events | 70 (72.9) | 254 (77.2) | 112 (45.2) | < 0.0001 |
| Secondary bacterial infection (respiratory tract or bloodstream) | 69 (71.9) | 253 (76.9) | 112 (45.2) | < 0.0001 |
| Renal replacement therapy | 64 (66.7) | 208 (63.2) | 119 (48.0) | 0.0002 |
Survival, disease severity according to SOFA score, time between intubation and ECMO cannulation, incidence of complications (Major bleeding or thromboembolic events, secondary bacterial infection, renal replacement therapy) dependent on annual case volume. Results for SOFA score are given within ranges (0–9, 9–12, 12–15, 15–21). Descriptive statistics are expressed as frequencies for categorical variables (including a category for missing data). Differences between groups were tested using the Mann–Whitney U test (continuous variables), χ2 test (categorical variables) or Fisher’s exact test (categorical variables with observed frequencies < 5), as appropriate
Pre-ECMO characteristics
Additional file 1: Table S1 shows parameters of gas exchange prior to ECMO initiation. Median PaO2/FiO2 ratio prior to ECMO initiation was 72 mmHg (IQR: 58–99), indicating severe ARDS according to the BERLIN definition [13]. There was no statistically significant difference in PaO2 (69 mmHg vs. 67 mmHg), SpO2 (92% vs. 91%) and pH (7.28 vs. 7.27) between survivors vs. non-survivors prior to ECMO (data not shown). On average, SARS-CoV-2 was diagnosed 2 days (IQR: 0–6) prior to ICU admission. Duration between hospital to ICU admission was 1 day (IQR: 0–3) (data not shown).
ECMO support
Table 1 and Additional file 1: Table S1 show severity of disease, parameters of mechanical ventilation, ECMO support and adjunct therapies. ECMO support started with a median of 5 days (IQR 1–9) after endotracheal intubation (data not shown). Mobile teams transported 493 patients (73%) from peripheral hospitals to designated ARDS/ECMO centers. Time between intubation and commencement of ECMO support was longer in low compared to high volume centers. Duration between intubation and start of ECMO support was significantly different between survivors (2 days, IQR 1–8) and non-survivors (5 days, IQR 2–9). Most patients were cannulated due to hypoxemia (62%) or hypercapnia (21%). In almost 97% of the cases, VV ECMO was the mode of choice. The most frequent cannulation side was “cervical and femoral” (60%) (data not shown).
During ECMO support prone positioning was used in 49%, neuromuscular blockers in 42%, therapeutic anticoagulation in 92% and glucocorticoids in 67% of the patients. 58% received renal replacement therapy.
Severe complications were frequent during ECMO therapy. Major bleeding or thromboembolic events occurred in 66% of the patients. Moreover, secondary bacterial respiratory tract or blood stream infections occurred in 64%. Major bleeding or thromboembolic events and renal replacement therapy were significantly associated with poor survival (Table 1). During therapy, high volume centers reported significantly less bleeding/thromboembolic events, less secondary bacterial infections, and a lower need for renal replacement therapy (Table 2).
Outcome
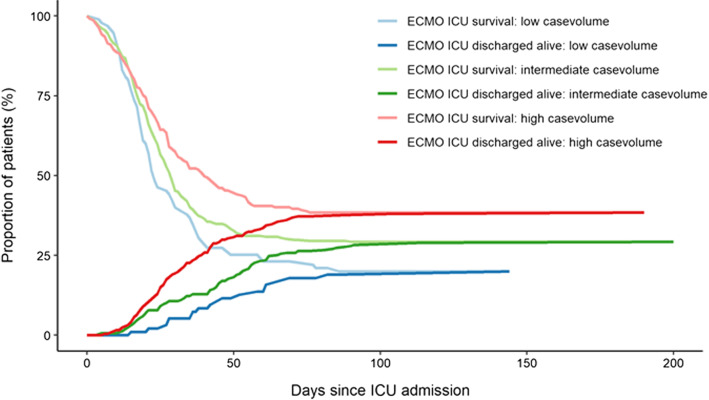
Overall survival to ICU discharge was 31.4%. During the 1st wave of the COVID-19 pandemic (04/2020-06/2020) 40.3% survived to ICU discharge, while survival was significantly lower during the 2nd wave of the COVID-19 pandemic (07/2020-03/2021, 27.9%, p = 0.0019; Table 1(1), Additional file 1: Figure S2). ICU survival was higher, when ECMO therapy started within 5 days after endotracheal intubation (Table 1). Survival was significantly different between patients fulfilling modified EOLIA inclusion criteria (38.0%) and patients not fulfilling these criteria (26.5%) (p = 0.0014) (Table 1). Characteristics of the respective patient cohorts are found in Additional file 1: Table S2 and S3. Moreover, survival differed according to age group, ranging from 58.0% (19–40 years) to 9.5% (71–80 years) (Table 1). Survival also significantly differed according to case volume and was 20% for low volume centers, 30% for intermediate volume centers and 38% for high volume centers (p = 0.0024), respectively (Fig. 2).
Fig. 2.
Volume-outcome relationship of COVID-19 ECMO. Case volume. Case volume vs. survival in low (n = 96, survival 20%), intermediate (n = 329, survival 30%) and high volume (n = 248, survival 38%) ECMO centers. Annual case volumes prior to the pandemic were defined as low (< 20/year), intermediate (20–49/year) and high (> 50/year). Lower lines (ECMO ICU discharged alive) depict the percentage of patents discharged alive from the ECMO providing ICU. ICU discharge destinations were mainly other ICUs (40%), rehabilitation facilities (33%), or general wards (23%) (data not shown)
Factors affecting survival
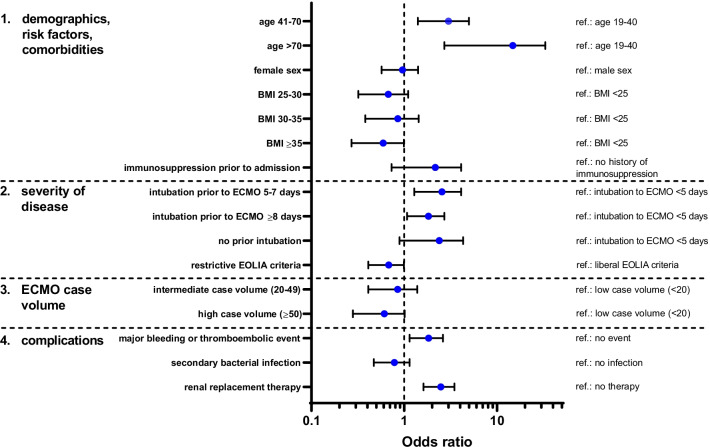
Results of the univariate logistic regression and block wise logistic regression, containing aspects of demographics, comorbidities, disease severity, ECMO therapy and complications are depicted in Table 3 and Fig. 3. Independent risk factors for non-survival included higher age with an OR of 2.48 (CI 1.32–4.17)) in patients aged 41–70 years and 6.81 (CI 2.13–26.90) in patients aged 71–80 compared to 19–40 years. Time periods of 5–7 days between intubation and ECMO initiation resulted in higher mortality (OR 2.39; CI 1.35–4.37) compared to < 5 days, whereas ≥ 8 days was not an independent risk factor (OR 1.30; CI 0.76–2.22). Patients fulfilling modified EOLIA criteria had an improved chance of survival with an OR of 0.64 (CI 0.41–0.99). Higher case volume of the ARDS/ECMO center in the preceding year also led to improved chances of survival with an OR of 0.55 (CI 0.28–1.02) for high compared to low volume centers. Interestingly, BMI ≥ 35 compared to BMI < 25 was also associated with higher chances of survival (OR 0.51; CI 0.26–0.97). Furthermore, major bleeding or thromboembolic events resulted in an OR of 1.70 (CI 1.12–2.57) for non-survival. Renal replacement therapy (OR 2.35; CI 1.60–3.46) was also an independent risk factor of mortality.
Table 3.
Blockwise logistic regression
| Blocks adjusted | Block 1 | Block 1–2 | Block 1–3 | Block 1–4 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Units | OR | CI 95 | OR | CI 95 | OR | CI 95 | OR | CI 95 |
| 1. Demographics, risk factors, comorbidities | |||||||||
| Age [years] | 19–40 | Ref | Ref | Ref | Ref | ||||
| 41–70 | 3.11 | [1.71;5.78] | 2.75 | [1.49;5.15] | 2.71 | [1.46;5.10] | 2.48 | [1.32;4.71] | |
| > 70 | 12.31 | [4.07;46.66] | 8.70 | [2.78;33.78] | 8.28 | [2.63;32.25] | 6.81 | [2.13;26.90] | |
| Missing | 2,329,070.78 | [0.00;NA] | 2,976,862.44 | [0.00;NA] | 3,313,285.99 | [0.00;NA] | 1,813,873.83 | [0.00;NA] | |
| Sex | m | Ref | Ref | Ref | Ref | ||||
| w | 0.87 | [0.57;1.34] | 0.79 | [0.51;1.24] | 0.78 | [0.50;1.23] | 0.87 | [0.55;1.38] | |
| Missing | 0.89 | [0.18;6.34] | 1.06 | [0.22;7.66] | 0.97 | [0.20;7.03] | 1.12 | [0.21;8.39] | |
| BMI [kg/m2] | < 25 | Ref | Ref | Ref | Ref | ||||
| 25–30 | 0.63 | [0.35;1.13] | 0.65 | [0.35;1.17] | 0.65 | [0.35;1.18] | 0.62 | [0.33;1.13] | |
| 30–35 | 0.70 | [0.37;1.32] | 0.71 | [0.37;1.35] | 0.73 | [0.38;1.40] | 0.74 | [0.38;1.43] | |
| ≥ 35 | 0.54 | [0.29;0.99] | 0.57 | [0.30;1.07] | 0.57 | [0.30;1.06] | 0.51 | [0.26;0.97] | |
| Missing | 2.49 | [0.74;11.48] | 3.16 | [0.91;14.85] | 3.13 | [0.89;14.84] | 3.25 | [0.90;15.77] | |
| Immunosuppression within 6 months prior to admission | No | Ref | Ref | Ref | Ref | ||||
| Yes | 1.82 | [0.84;4.44] | 1.69 | [0.76;4.16] | 1.75 | [0.78;4.34] | 1.44 | [0.63;3.63] | |
| Unknown | 0.61 | [0.39;0.95] | 0.73 | [0.42;1.31] | 0.76 | [0.42;1.36] | 0.77 | [0.41;1.44] | |
| 2. Severity of disease | |||||||||
| Intubation prior to ECMO [days] | < 5 | Ref | Ref | Ref | |||||
| 5–7 | 2.67 | [1.54;4.81] | 2.65 | [1.52;4.81] | 2.39 | [1.35;4.37] | |||
| ≥ 8 | 1.31 | [0.79;2.18] | 1.26 | [0.75;2.10] | 1.30 | [0.76;2.22] | |||
| No prior intubation | 0.88 | [0.45;1.73] | 1.12 | [0.55;2.33] | 1.90 | [0.87;4.25] | |||
| Missing | 1.57 | [0.76;3.43] | 1.86 | [0.88;4.13] | 1.93 | [0.89;4.42] | |||
| EOLIA criteria | Liberal | Ref | Ref | Ref | |||||
| Restrictive | 0.62 | [0.40;0.95] | 0.62 | [0.40;0.95] | 0.64 | [0.41;0.99] | |||
| 3. ECMO Case volume | Low | Ref | Ref | ||||||
| Medium | 0.81 | [0.44;1.44] | 0.79 | [0.42;1.43] | |||||
| High | 0.53 | [0.28;0.97] | 0.55 | [0.28;1.02] | |||||
| 4. Complications | |||||||||
| Major bleeding or thromboembolic event | No | Ref | |||||||
| Yes | 1.70 | [1.12;2.57] | |||||||
| Secondary bacterial infection (respiratory tract or bloodstream) | No | Ref | |||||||
| Yes | 0.75 | [0.48;1.16] | |||||||
| Renal replacement therapy | No | Ref | |||||||
| Yes | 2.35 | [1.60;3.46] | |||||||
| AIC | 815.24 | 804.94 | 803.01 | 780.89 | |||||
Variables associated with mortality during ICU stay and odds ratios (ORs) with corresponding 95% confidence intervals (CIs) in blockwise logistic regression. Variables were selected a priori based on clinical background and assigned to blocks reflecting the clinical course over time. References for each variable are indicated (ref). Models were adjusted in four blocks: 1. Demographics, Risk factors and Comorbidities; 2. Severity of disease; 3. ECMO case volume, and 4. Complications. Step-wise integration of blocks into the model is depicted in the table from the left (block 1 only) to the right (block 1–4). The quality of the models was assessed using the Akaike information criterion (AIC). Statistical significance was determined at an α level of 0.05 (two-tailed)
Fig. 3.
Risk factors for mortality. Adjusted odds ratios (OR) and 95% confidence intervals (CI) of risk factors for mortality according to the final model of block wise logistic regression (Table 3, Block 1–4). References for each variable are indicated (ref)
Discussion
In our study analyzing 673 patients treated in 26 German ECMO centers without resource constraints, 31.4% survived COVID-19 ECMO to ICU discharge. Patients younger than 40 years of age, without the need of renal replacement therapy, treated in a high volume ECMO center were most likely to survive COVID-19 ECMO. Moreover, patients without significant comorbidities, fulfilling modified EOLIA criteria had a significantly higher chance of survival. This emphasizes the importance of patient selection, identifying those that benefit the most.
VV ECMO use has rapidly increased during the COVID-19 pandemic with first studies indicating high chances of survival [14, 15]. Data from the Extracorporeal Life Support Organization (ELSO) Registry, Greater Paris, the US and Chile showed 90-day-survival rates ranging between 46 and 65% [14–18]. Accordingly, a recent meta-analysis reported a survival rate of 62.9% until hospital discharge [19]. In the current retrospective analysis, survival was lower but comparable with preliminary analyses of German health care insurance data, reporting in-hospital mortalities of 73% and 66% [4, 20]. However, survival depends on multiple factors including local resource allocation, patient inclusion criteria, timing of ECMO initiation, as well as experience of the centers. These factors varied between health care systems. Most importantly, none of the participating German ECMO centers experienced resource constraints or had to triage during the pandemic.
In our cohort, a high proportion of comorbid patients aged above 60 years were treated with ECMO. Although age per se is not an adequate cutoff parameter for any therapeutic intervention, our data indicate a very poor outcome in the elderly. Risk of non-survival progressively increased with age. Only 9.5% of COVID-19 patients older than 70 years survived ECMO therapy. Multiple studies have confirmed that increasing age is associated with a higher risk of death which has been explained by an increasing number of comorbidities [5, 14, 15, 17, 20]. However, only immunosuppression within 6 months prior to admission was associated with decreased survival. Therefore, increasing age can be considered as an independent risk factor and COVID-19 patients older than 70 years are significantly less likely to survive ECMO treatment.
To further delineate the impact of patient selection, we applied modified inclusion criteria of the EOLIA trial to our dataset by excluding patients older than 70 years and significant comorbidities. These patients accounted for a total of 42% of the study cohort, whereas survival significantly increased to 38.0%. Nevertheless, survival was still lower compared to a similar cohort from Greater Paris [15]. Hence, additional factors must explain this discrepancy. A larger proportion of patients not fulfilling the modified EOLIA criteria were treated at low volume centers (72%) compared to high volume centers (55%), suggesting that high volume centers used stricter ECMO inclusion criteria. High volume centers selected patients with lower scores of organ dysfunction/failure, started ECMO support earlier and had lower complication rates. Most importantly, chance of survival was doubled in high compared to low volume centers. It has previously been demonstrated that ECMO treatment in specialized high-volume ECMO centers benefits survival in non-COVID-19 ARDS [22, 23]. Similar, non ECMO critically ill patients also benefit from treatment in high volume centers [24]. Furthermore, early adopting hospitals starting COVID-19 ECMO prior to May 2020 have been shown to achieve better outcomes compared to new centers performing [9]. Most patients were treated during the 2nd wave of the pandemic when first virus variants were observed [21]. In situations requiring specific care and technical complex therapies such as ECMO, the outcome-volume relationship is pronounced, and organizational challenges may exaggerate during the COVID-19 pandemic. Hence, our data support the conclusion that patients should be treated in high-volume centers whenever possible.
In addition, commencement of ECMO therapy < 5 days after intubation is desirable. Mortality increased if ECMO started later than this time point. These results are in line with data from Greater Paris [15]. However, considering the results of the multivariate analysis, the effect of early ECMO initiation is interdependent with other contextual factors. In similar fashion, our results regarding a beneficial effect of class II obesity are limited. BMI > 35 was only compared to BMI < 25 and not against healthy weight and underweight patients. Nevertheless, our result is in line with current evidence suggesting improved 90-day survival in obese COVID-19 ECMO patients [25] or corroborating that obesity is not a risk factor for a worse outcome [26].
Considering complications of ECMO therapy, major bleeding or thromboembolic events were frequent and contributed to a poor outcome. ECMO requires systemic anticoagulation and bleeding dominates coagulation abnormalities [27]. Thromboembolic events did not significantly alter survival, although they have been associated with a higher risk of death in COVID-19 [28].
The use of renal replacement therapy was also associated with higher odds of non-survival. Contribution of ECMO to acute kidney injury is unknown [29], however, independent of ECMO, kidney failure is a marker of disease severity and associated with increased COVID-19 in-hospital mortality [30, 31]. Early detection of renal dysfunction in COVID-19 ARDS is crucial and the presence of renal replacement can be included as a risk factor of non-survival when evaluating the chances of bridging to recovery.
Strengths of our study include a large patient sample on a nationwide level recruited from low to high volume ECMO centers without resource constraints. Highly granular patient data collected during the entire course of ECMO support allowed a comprehensive analysis of risk factors. Sensitivity analysis allowed the evaluation of data quality and block wise regression permitted the identification of independent risk factors of non-survival (Additional file 1: Table S4). Limitations include the impossibility of external validation of the submitted patient data or structural criteria of the participating centers. Approximately 200 German hospitals performed COVID-19 ECMO [32], of which 26 entered data in the COVID-19 ECMO register, mainly due to the lack of staffing. Our dataset did not include changes of ECMO or cannulation modes during treatment. Moreover, our observation period was limited to intensive care in the ECMO providing ICU and did not include onset of COVID-19 related symptoms, time-to-event data, long-term follow-up, or cause of death.
Conclusion
Careful selection of patients and high standards of care are necessary to maximize and justify ECMO support in COVID-19 ARDS. Survival of COVID-19 ECMO was underwhelming in elderly patients, in patients not fulfilling modified EOLIA inclusion criteria as well as those treated in low volume ECMO centers. These factors combined with a more liberal ECMO indication during the 2nd wave may explain the reasonably overall low survival rate. The observed volume-outcome relationship further suggests that ECMO allocation should prefer intermediate to high volume centers and patients should be transported to these centers whenever possible.
Supplementary Information
Additional file 1. Supplemental data including participating centers, complete case analysis, delineation of the first and second wave of the pandemic, details on clinical course, as well as patient characteristics according to the use of modified EOLIA criteria.
Acknowledgements
We very much thank the collaborators who contributed to this study: Viktoria Keck (Department of Anaesthesiology, Intensive Care, Emergency and Pain Medicine, University Hospital Wuerzburg, Germany), Kerstin Müller (Department of Anaesthesiology, Intensive Care, Emergency and Pain Medicine, University Hospital Wuerzburg, Germany), Melanie Kerinn (University Medical Center Hamburg-Eppendorf, Department of Intensive Care, Hamburg Germany), Lisa Krebs (University Medical Center Hamburg-Eppendorf, Department of Intensive Care, Hamburg Germany), Andrea Conrad (University Medical Center Hamburg-Eppendorf, Department of Intensive Care, Hamburg Germany), Grit Ringeis (University Medical Center Hamburg-Eppendorf, Department of Intensive Care, Hamburg Germany), Michael Weyand (Department of Cardiac Surgery, University Hospital Erlangen, Friedrich-Alexander University, Erlangen-Nuernberg (FAU), Erlangen), Christian Heim (Department of Cardiac Surgery, University Hospital Erlangen, Friedrich-Alexander University, Erlangen-Nuernberg (FAU), Erlangen), Rene Tandler (Department of Cardiac Surgery, University Hospital Erlangen, Friedrich-Alexander University, Erlangen-Nuernberg (FAU), Erlangen), Stefanie Schmidt (Department of Anaesthesiology, University Hospital Erlangen, Friedrich-Alexander University, Erlangen-Nuernberg (FAU), Erlangen), Lena Garczarek (Department of Anesthesiology and Intensive Care Medicine, University Hospital Essen, University Duisburg-Essen, Essen, Germany), Anke Hoffmann (Department of Anesthesiology, Klinikum Dortmund, Dortmund, Germany), Daniela Nickoleit-Bitzenberger (Medical ICU, Pulmonology and Infectiology, Klinikum Dortmund, Klinikum University Witten/Herdecke, Germany), Bernhard Schaaf Medical ICU, Pulmonology and Infectiology, Klinikum Dortmund, Klinikum University Witten/Herdecke, Germany), Christoph Benk (Department of Cardiovascular Surgery (University Heart Center Freiburg - Bad Krozingen), Medical Center - University of Freiburg, Faculty of Medicine, University of Freiburg, Germany), Alexander Supady (Department of Cardiology and Angiology I (Heart Center Freiburg - Bad Krozingen), Medical Center - University of Freiburg, Faculty of Medicine, University of Freiburg, Germany; Department of Medicine III (Interdisciplinary Medical Intensive Care), Medical Center - University of Freiburg, Faculty of Medicine, University of Freiburg, Germany), Xavier Bemtgen (Department of Cardiology and Angiology I (Heart Center Freiburg - Bad Krozingen), Medical Center - University of Freiburg, Faculty of Medicine, University of Freiburg, Germany; Department of Medicine III (Interdisciplinary Medical Intensive Care), Medical Center - University of Freiburg, Faculty of Medicine, University of Freiburg, Germany), Asieb Sekandarzad (Department of Cardiology and Angiology I (Heart Center Freiburg - Bad Krozingen), Medical Center - University of Freiburg, Faculty of Medicine, University of Freiburg, Germany; Department of Medicine III (Interdisciplinary Medical Intensive Care), Medical Center - University of Freiburg, Faculty of Medicine, University of Freiburg, Germany), Annabelle Fluegler (Department of Cardiology and Angiology I (Heart Center Freiburg - Bad Krozingen), Medical Center - University of Freiburg, Faculty of Medicine, University of Freiburg, Germany; Department of Medicine III (Interdisciplinary Medical Intensive Care), Medical Center - University of Freiburg, Faculty of Medicine, University of Freiburg, Germany), Johannes Scheumann (Department of Cardiovascular Surgery (University Heart Center Freiburg - Bad Krozingen), Medical Center - University of Freiburg, Faculty of Medicine, University of Freiburg, Germany), Michael Berchthold-Herz (Department of Cardiovascular Surgery (University Heart Center Freiburg - Bad Krozingen), Medical Center - University of Freiburg, Faculty of Medicine, University of Freiburg, Germany), Andrea Kernchen (Department of Anesthesiology, University Medical Center Göttingen, Germany, Robert-Koch-Str. 40, D-37085 Göttingen), Lars- Olav Harnisch (Department of Anesthesiology, University Medical Center Göttingen, Germany, Robert-Koch-Str. 40, D-37085 Göttingen), Antje Seidel (Department of Anesthesiology and Intensive Care Medicine, University of Leipzig Medical Center, Leipzig, Germany), Christian Schlegel (Department of Anesthesiology and Intensive Care Medicine, University of Leipzig Medical Center, Leipzig, Germany), Sebastian Rasch (Klinik und Poliklinik für Innere Medizin II, Klinikum rechts der Isar der Technischen Universität München, Munich, Germany), Carsten Hullermann (Department of Cardiology I – Coronary and Peripheral Vascular Disease, Heart Failure, University Hospital Muenster, Muenster, Germany), Jan Padberg (Department of Cardiology I – Coronary and Peripheral Vascular Disease, Heart Failure, University Hospital Muenster, Muenster, Germany), Ulrich Göbel (Department of Anaesthesiology and Critical Care Medicine, St. Franziskus-Hospital Muenster, Germany).
German ECMO COVID Study Group
1Martha E. Hübsch, 1Quirin Notz, 1Daniel Röder, 1Peter Kranke, 2Michaela L. Merten, 4Dominik Jarczak, 4Kevin Roedl, 5Jens-Christian Schewe, 5Stefan F. Ehrentraut, 5Stefan Kreyer, 6Ixchel Castellanos, 7Thorsten Brenner, 7Frank Herbstreit, 7Florian Espeter, 7Jan Wiefhoff, 9Björn Ellger, 10Florian J. Raimann, 10Michael Sonntagbauer, 11,12Tobias Wengenmayer, 11,12Dawid Staudacher, 14Ruslan Natanov, 16Caroline Rolfes, 16Christian Reyher, 16Iuliu-Emilian Torje, 17Patricia Glaser, 20Vanessa Rembold, 21Rainer Haseneder, 23Jan Sackarnd, 23Johannes Lepper 25Andre Becker, 25Guy Danziger, 25Carlos Metz, 26Valbona Mirakaj, 26Stefanie Calov, 28Anna Grau, 29Kirsten Haas, 28Katrin Ungethüm, 30Karl Bihlmaier.
1Department of Anaesthesiology, Intensive Care, Emergency and Pain Medicine, University Hospital Wuerzburg, Oberduerrbacherstr. 6, 97080 Wuerzburg, Germany; 2Department of Pneumology and Critical Care Medicine, Cologne-Merheim Hospital, ARDS and ECMO center, Kliniken der Stadt Köln, Witten/Herdecke University Hospital, Cologne, Germany; 3Department of Anesthesiology and Operative Intensive Care Medicine (CCM, CVK), Charité - Universitätsmedizin Berlin, Berlin, Germany; 4University Medical Center Hamburg-Eppendorf, Department of Intensive Care, Hamburg Germany; 5Department of Anesthesiology and Intensive Care Medicine, University Hospital Bonn, Bonn, Germany; 6Department of Anaesthesiology, University Hospital Erlangen, Friedrich-Alexander University, Erlangen-Nuernberg (FAU), Erlangen, Germany; 7Department of Anesthesiology and Intensive Care Medicine, University Hospital Essen, University Duisburg-Essen, Essen, Germany; 8Department of Anesthesiologie and Intensive Care Medicine, Klinikum Dortmund, Klinikum University Witten/Herdecke, Germany; 9Department of Anesthesiology, Intensive Care Medicine and Pain Therapy, Clinic Centre Westfalen, Dortmund Germany; 10Department of Anaesthesiology, Intensive Care Medicine and Pain Therapy, University Hospital Frankfurt, Goethe University Frankfurt, Frankfurt, Germany; 11Department of Cardiology and Angiology I (Heart Center Freiburg - Bad Krozingen), Medical Center - University of Freiburg, Faculty of Medicine, University of Freiburg, Germany; 12Interdisciplinary Medical Intensive Care (IMIT), Medical Center - University of Freiburg, Faculty of Medicine, University of Freiburg, Germany; 13Department of Anesthesiology, University Medical Center Göttingen, Germany, Robert-Koch-Str. 40, D-37085 Göttingen; 14Department of Cardiothoracic, Transplanatation and Vascular Surgery, Hannover Medical School, Hannover, Germany; 15Department of Internal Medicine, Division I (Hematology/Oncology), University Hospital of Cologne, Cologne, Germany; 16Department of Anesthesiology and Critical Care Medicine, ARDS/ECMO-center, Campus Kassel of the University of Southampton, Kassel, Germany; 17Departement of Anaesthesia, perioperative Medicine and Interdisciplinary Intensive Care Medicine, ECLS/ECMO-Center, Asklepios Klinik Langen, Germany ; 18Department of Anesthesiology and Intensive Care Medicine, University of Leipzig Medical Center, Leipzig, Germany; 19 ARDS and ECMO-Centre Munich-Nymphenburg, Germany; 20Klinik für Pneumologie, Internistische Intensiv- und Beatmungsmedizin, München Klinik Harlaching, Munich, Germany ; 21Department of Anaesthesiology and Intensive Care Medicine, Technical University of Munich, School of Medicine, Munich, Germany; 22University of Munich, School of Medicine, University hospital rechts der Isar, Department of Internal Medicine II, Ismaninger Str. 22, 81675 Munich, Germany; 23Department of Cardiology I – Coronary and Peripheral Vascular Disease, Heart Failure, University Hospital Muenster, Muenster, Germany; 24Department of Intensive Care Medicine, Imland Klinik Rendsburg, Rendsburg, Germany; 25Department of Internal Medicine V- Pneumology, Allergology and Critical Care Medicine, Saarland University, Homburg, Germany; 26Department of Anesthesiology and Intensive Care Medicine, University Hospital Tübingen, Eberhard Karls University Tübingen, Tübingen, Germany; 27Department of Medical Intensive Care and Emergency Medicine, St. Franziskus-Hospital Muenster, Germany; 28Institute of Clinical Epidemiology and Biometry, University of Würzburg, Germany; 29Clinical Trial Center Würzburg, Universitätsklinikum Würzburg, Germany; 30Department of Internal Medicine 4 - Nephrology and Hypertension, University Hospital Erlangen, Friedrich-Alexander University, Erlangen-Nuernberg (FAU), Erlangen, Germany
Author contributions
JH, CL, PK, PM and PH conceived the study. JH, MEH, QN, DR, AG, CP, J-CS, SFE, SK, IC, AW, KB, KS, TB, FH, FE, JW, RKE, DO, BE, GL, FJR, MS, VZ, TW, DS, OM, SK, DJ, KR, CK, RN, MK, RM, CR, CR, I-ET, MG, PG, FF, FB, MF, VR, MH, RH, TL, JS, JL, FR, NH, PML, AB, GD, CM, PR, VM, SC, SB, CK and MLM were involved in data generation. JH, CL, PM, PH, AG, KH, KU, MK, CK and SW-C were involved in analysis of the data. JH, CL, MK, PM and PH wrote the manuscript. All authors contributed, read and approved the final version of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This publication was supported by the Open Access Publication Fund of the University of Wuerzburg.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Declarations
Ethical approval and consent to participate
The Ethics Committee of the Medical Faculty of the Julius-Maximilians-University of Wuerzburg approved the study protocol (131/20-me). Additional local ethics committee votes were obtained from each of the participating ECMO centers. According to German legislation, no informed consent for retrospective, anonymous data is required and informed consent was waived by the ethics committee.
Consent for publication
Not applicable.
Competing interests
Christian Karagiannidis reports support for the present manuscript by Xenios (advisory board member), Getinge (personal fees); personal fees by Bayer, Xenios, Pfizer, Getinge; President of the German Society of internal Intensive Care Medicine; Lead of the German ICU Registry. Stefan Kluge reports consulting fees by Baxter, Fresenius Medical Care; payment by Baxter, Fresenius Medical Care. Karsten Schmidt reports research grants from Stiftung Universitätsmedizin Essen (Microcirculation/Sepsis/Covid-19), Heidelberger Stiftung Chirurgie (Microcirculation/Sepsis); payment for lectures (Buthylcholinesterase and Inflammation) by Dr. Franz Köhler Chemie GmbH. Onnen Moerer reports research grants from CSL Behring: Unrestricted Grant from CS Behring (research related to extracorporeal membrane oxygenation); Member of the national CEOsys network Germany (Covid ecosystem), which is funded by the Federal Ministry of Education and Research (BMBF). [FKZ 01KX2021]; Participation as speaker in workshops on haemodynamic monitoring at the European Medical School in Oldenburg, Germany; Expert testimonies for legal proceedings related to Critical Care or Anesthesiology cases on malpractice accusation by district courts and conciliation committees. Matthias Kochanek reports payment for lectures for Astellas, MSD, Gilead, Pfizer; stock or stock options by Biontech, Moderna; member of DGIIN, DGIM, DIVI. Tobias Lahmer reports payment for lectures and presentations by Gilead, MSD, Pfizer, ADVITOS GmbH. Peter Heuschmann reports research grants from German Ministry of Research and Education, European Union, Charité – Universitätsmedizin Berlin, Berlin Chamber of Physicians, German Parkinson Society, University Hospital Würzburg, Robert Koch Institute, German Heart Foundation, Federal Joint Committee (G-BA) within the Innovationfond, German Research Foundation, Bavarian State (ministry for science and the arts), German Cancer Aid, Charité – Universitätsmedizin Berlin (within Mondafis; supported by an unrestricted research grant to the Charité from Bayer), University Göttingen (within FIND-AF randomized; supported by an unrestricted research grant to the University Göttingen from Boehringer-Ingelheim), University Hospital Heidelberg (within RASUNOA-prime; supported by an unrestricted research grant to the University Hospital Heidelberg from Bayer, BMS, Boehringer-Ingelheim, Daiichi Sankyo), outside the submitted work. Peter Kranke reports research grants for CEOsys projects in the Network University Medicine (NUM) (institutional grants); chairman of the ESAIC guideline committee. Thorsten Brenner reports research grants from Deutsche Forschungsgemeinschaft (DFG), Dietmar Hopp Stiftung, Innovationsfonds/Gemeinsamer Bundesauschuss (G-BA), Stiftung Universitäts-medizin Essen; payments by CSL Behring GmbH, Schöchl medical education GmbH, Boehringer Ingelheim Pharma GmbH, Biotest AG, Baxter Deutschland GmbH, Astellas Pharma GmbH, B. Braun Melsungen AG, MSD Sharp & Dohme GmbH, Lücke Kongresse GmbH, Akademie für Infektionsmedizin e.V.; 2 patents on biomarkers in sepsis/septic shock with BRAHMS GmbH. Frank Herbstreit reports speaker honoria by Biotest; paid expert witness at several courts; International Anesthesia Research Society: Support for Attendance of Annual Meeting; Senior Editor: Anesthesia & Analgesia. All other authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Patrick Meybohm, Email: Meybohm_P@ukw.de.
for the German ECMO COVID Study Group:
Martha E. Hübsch, Quirin Notz, Daniel Röder, Peter Kranke, Michaela L. Merten, Dominik Jarczak, Kevin Roedl, Jens-Christian Schewe, Stefan F. Ehrentraut, Stefan Kreyer, Ixchel Castellanos, Thorsten Brenner, Frank Herbstreit, Florian Espeter, Jan Wiefhoff, Björn Ellger, Florian J. Raimann, Michael Sonntagbauer, Tobias Wengenmayer, Dawid Staudacher, Ruslan Natanov, Caroline Rolfes, Christian Reyher, Iuliu-Emilian Torje, Patricia Glaser, Vanessa Rembold, Rainer Haseneder, Jan Sackarnd, Johannes Lepper, Andre Becker, Guy Danziger, Carlos Metz, Valbona Mirakaj, Stefanie Calov, Anna Grau, Kirsten Haas, Katrin Ungethüm, and Karl Bihlmaier
References
- 1.Karagiannidis C, Brodie D, Strassmann S, Stoelben E, Philipp A, Bein T, et al. Extracorporeal membrane oxygenation: evolving epidemiology and mortality. Intensive Care Med. 2016;42(5):889–896. doi: 10.1007/s00134-016-4273-z. [DOI] [PubMed] [Google Scholar]
- 2.Abrams D, Lorusso R, Vincent JL, Brodie D. ECMO during the COVID-19 pandemic: when is it unjustified? Crit Care (Lond, Engl) 2020;24(1):507. doi: 10.1186/s13054-020-03230-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karagiannidis C, Mostert C, Hentschker C, Voshaar T, Malzahn J, Schillinger G, et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med. 2020;8(9):853–862. doi: 10.1016/S2213-2600(20)30316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedrichson B, Kloka JA, Neef V, Mutlak H, Old O, Zacharowski K, et al. Extracorporeal membrane oxygenation in coronavirus disease 2019: a nationwide cohort analysis of 4279 runs from Germany. Eur J Anaesthesiol. 2022;39(5):445–451. doi: 10.1097/EJA.0000000000001670. [DOI] [PubMed] [Google Scholar]
- 5.Ramanathan K, Antognini D, Combes A, Paden M, Zakhary B, Ogino M, et al. Planning and provision of ECMO services for severe ARDS during the COVID-19 pandemic and other outbreaks of emerging infectious diseases. Lancet Respir Med. 2020;8(5):518–526. doi: 10.1016/S2213-2600(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbaro RP, Odetola FO, Kidwell KM, Paden ML, Bartlett RH, Davis MM, et al. Association of hospital-level volume of extracorporeal membrane oxygenation cases and mortality. Analysis of the extracorporeal life support organization registry. Am J Respir Crit Care Med. 2015;191(8):894–901. doi: 10.1164/rccm.201409-1634OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becher PM, Goßling A, Schrage B, Twerenbold R, Fluschnik N, Seiffert M, et al. Procedural volume and outcomes in patients undergoing VA-ECMO support. Crit Care (Lond, Engl) 2020;24(1):291. doi: 10.1186/s13054-020-03016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Combes A, Brodie D, Bartlett R, Brochard L, Brower R, Conrad S, et al. Position paper for the organization of extracorporeal membrane oxygenation programs for acute respiratory failure in adult patients. Am J Respir Crit Care Med. 2014;190(5):488–496. doi: 10.1164/rccm.201404-0630CP. [DOI] [PubMed] [Google Scholar]
- 9.Barbaro RP, MacLaren G, Boonstra PS, Combes A, Agerstrand C, Annich G, et al. Extracorporeal membrane oxygenation for COVID-19: evolving outcomes from the international Extracorporeal Life Support Organization Registry. Lancet (London, England) 2021;398(10307):1230–1238. doi: 10.1016/S0140-6736(21)01960-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost: JTH. 2005;3(4):692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 11.Kaatz S, Ahmad D, Spyropoulos AC, Schulman S. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost: JTH. 2015;13(11):2119–2126. doi: 10.1111/jth.13140. [DOI] [PubMed] [Google Scholar]
- 12.Combes A, Hajage D, Capellier G, Demoule A, Lavoué S, Guervilly C, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378(21):1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 13.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 14.Barbaro RP, MacLaren G, Boonstra PS, Iwashyna TJ, Slutsky AS, Fan E, et al. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet (London, England) 2020;396(10257):1071–1078. doi: 10.1016/S0140-6736(20)32008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lebreton G, Schmidt M, Ponnaiah M, Folliguet T, Para M, Guihaire J, et al. Extracorporeal membrane oxygenation network organisation and clinical outcomes during the COVID-19 pandemic in Greater Paris, France: a multicentre cohort study. Lancet Respir Med. 2021;9(8):851–862. doi: 10.1016/S2213-2600(21)00096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaefi S, Brenner SK, Gupta S, O'Gara BP, Krajewski ML, Charytan DM, et al. Extracorporeal membrane oxygenation in patients with severe respiratory failure from COVID-19. Intensive Care Med. 2021;47(2):208–221. doi: 10.1007/s00134-020-06331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saeed O, Tatooles AJ, Farooq M, Schwartz G, Pham DT, Mustafa AK, et al. Characteristics and outcomes of patients with COVID-19 supported by extracorporeal membrane oxygenation: a retrospective multicenter study. J Thorac Cardiovasc Surg. 2021;163(6):2107–2116. doi: 10.1016/j.jtcvs.2021.04.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diaz RA, Graf J, Zambrano JM, Ruiz C, Espinoza JA, Bravo SI, et al. Extracorporeal membrane oxygenation for COVID-19-associated severe acute respiratory distress syndrome in Chile: a nationwide incidence and cohort study. Am J Respir Crit Care Med. 2021;204(1):34–43. doi: 10.1164/rccm.202011-4166OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramanathan K, Shekar K, Ling RR, Barbaro RP, Wong SN, Tan CS, et al. Extracorporeal membrane oxygenation for COVID-19: a systematic review and meta-analysis. Crit Care (Lond, Engl) 2021;25(1):211. doi: 10.1186/s13054-021-03634-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karagiannidis C, Strassmann S, Merten M, Bein T, Windisch W, Meybohm P, et al. High in-hospital mortality in COVID patients receiving ECMO in Germany—a critical analysis. Am J Respir Crit Care Med. 2021;204(8):991–994. doi: 10.1164/rccm.202105-1145LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Challen R, Brooks-Pollock E, Read JM, Dyson L, Tsaneva-Atanasova K, Danon L. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: matched cohort study. BMJ (Clin Res Ed) 2021;372:n579. doi: 10.1136/bmj.n579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet (London, England) 2009;374(9698):1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 23.Noah MA, Peek GJ, Finney SJ, Griffiths MJ, Harrison DA, Grieve R, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1) JAMA. 2011;306(15):1659–1668. doi: 10.1001/jama.2011.1471. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen YL, Wallace DJ, Yordanov Y, Trinquart L, Blomkvist J, Angus DC, et al. The volume-outcome relationship in critical care: a systematic review and meta-analysis. Chest. 2015;148(1):79–92. doi: 10.1378/chest.14-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daviet F, Guilloux P, Hraiech S, Tonon D, Velly L, Bourenne J, et al. Impact of obesity on survival in COVID-19 ARDS patients receiving ECMO: results from an ambispective observational cohort. Ann Intensive Care. 2021;11(1):157. doi: 10.1186/s13613-021-00943-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galvagno SM, Jr, Pelekhaty S, Cornachione CR, Deatrick KB, Mazzeffi MA, Scalea TM, et al. Does weight matter? Outcomes in adult patients on venovenous extracorporeal membrane oxygenation when stratified by obesity class. Anesth Analg. 2020;131(3):754–761. doi: 10.1213/ANE.0000000000004454. [DOI] [PubMed] [Google Scholar]
- 27.Aubron C, DePuydt J, Belon F, Bailey M, Schmidt M, Sheldrake J, et al. Predictive factors of bleeding events in adults undergoing extracorporeal membrane oxygenation. Ann Intensive Care. 2016;6(1):97. doi: 10.1186/s13613-016-0196-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malas MB, Naazie IN, Elsayed N, Mathlouthi A, Marmor R, Clary B. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: a systematic review and meta-analysis. EClinicalMedicine. 2020;29:100639. doi: 10.1016/j.eclinm.2020.100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kilburn DJ, Shekar K, Fraser JF. The complex relationship of extracorporeal membrane oxygenation and acute kidney injury: causation or association? Biomed Res Int. 2016;2016:1094296. doi: 10.1155/2016/1094296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ronco C, Reis T, Husain-Syed F. Management of acute kidney injury in patients with COVID-19. Lancet Respir Med. 2020;8(7):738–742. doi: 10.1016/S2213-2600(20)30229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friedrichson B, Mutlak H, Zacharowski K, Piekarski F. Insight into ECMO, mortality and ARDS: a nationwide analysis of 45,647 ECMO runs. Crit Care (Lond, Engl) 2021;25(1):38. doi: 10.1186/s13054-021-03463-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplemental data including participating centers, complete case analysis, delineation of the first and second wave of the pandemic, details on clinical course, as well as patient characteristics according to the use of modified EOLIA criteria.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.