Abstract
Background:
Using data from a randomized trial, we evaluated the cost of HCV care facilitation that supports moving along the continuum of care for HIV/HCV co-infected individuals with substance use disorder.
Methods:
Participants were HIV patients residing in the community, initially recruited from eight US hospital sites. They received HCV care facilitation (n=51) or treatment as usual (n=62) for up to six months. We used micro-costing methods to evaluate costs from the healthcare sector and patient perspectives in 2017 USD. We conducted sensitivity analyses varying care facilitator caseloads and examined offsetting savings using participant self-reported healthcare utilization.
Results:
The average site start-up cost was $6,320 (site range: $4,320–$7,000), primarily consisting of training. The mean weekly cost per participant was $20 (site range: $4–$30) for care facilitation visits and contacts, $360 (site range: $130- $700) for supervision and client outreach, and $70 (site range: $20–$180) for overhead. In sensitivity analyses applying a weekly caseload of 10 participants per care facilitator (versus 1–6 observed in the trial), the total mean weekly care facilitation cost from the healthcare sector perspective decreased to $110. Weekly participant time and travel costs averaged $7. There were no significant differences in other healthcare service costs between participants in the intervention and control arms.
Conclusion:
Weekly HCV care facilitation costs were approximately $450 per participant, but approximately $110 at a real-world setting maximum caseload of 10 participants per week. No healthcare cost offsets were identified during the trial period, although future savings might result from successful HCV treatment.
Keywords: Hepatitis C, HIV, Substance Use Disorder, Cost, Care Facilitation
1. INTRODUCTION
Approximately 2.3 million people globally were HCV-HIV co-infected in 2016 and more than half reported substance use.(World Health Organization, 2017) In the United States 39.8% of people who inject drugs are HCV positive, and many are co-infected with HIV which worsens the prognosis of untreated HCV-liver disease.(Grebely et al., 2019; Matthews and Dore, 2008) The World Health Organization has set a goal of HCV elimination by 2030, and the United States has set a goal to decrease the number of acute HCV infections by 90% and the number of HCV-related deaths by 65% by 2030.(United States Department of Health & Human Services, 2021; Waheed et al., 2018) The advent of direct-acting antivirals (DAAs) may provide the opportunity to meet the goal of HCV elimination because they offer a cure for HCV with minimal side effects. DAAs have been proven to be safe and effective among people with co-morbid HIV and substance use disorder (SUD),(Grebely et al., 2017) and can cure HCV with well-tolerated oral regimens lasting up to 12 weeks among people who adhere to treatment. Although the prevalence of HCV in these populations make them high priority populations for treatment,(American Association for the Study of Liver Diseases & Infectious Diseases Society of America, 2019) a recent report indicates that commercially insured individuals with substance use and HIV are less likely to receive HCV treatment.(Harris et al., 2021) Social and structural barriers along the continuum of HCV care persist, however, making HCV elimination among these populations challenging. Barriers include stigma based on substance use and HIV/ HCV coinfection, difficulties accessing treatment (including lack of transportation), long waits for care, complex healthcare systems that are difficult to navigate, burdensome documentation requirements for prescribing DAAs, insurance restrictions on treatment such as sobriety or fibrosis severity, and lack of health insurance coverage.(Campbell et al., 2017; Clement et al., 2018; Goodyear et al., 2020; Gowda et al., 2018; Kapadia et al., 2018; Lin et al., 2017; Muncan et al., 2021; Pundhir et al., 2016; Sims et al., 2017; Spradling et al., 2020)
Lessons on how to overcome these barriers can be derived from successful interventions to improve the HIV care continuum. Active linkage to care strategies such as employing case managers or patient navigators have been shown to successfully link people living with HIV to care.(Craw et al., 2008; Gardner et al., 2005) The National Institute on Drug Abuse (NIDA) National Drug Abuse Treatment Clinical Trials Network (CTN) trial CTN-0049 adapted the Antiretroviral Treatment Access Study (ARTAS) patient navigation model to link hospitalized people who used substances and were living with HIV to outpatient care.(Metsch et al., 2016) Although this intervention did not result in a sustained improvement in HIV viral load suppression six months after the intervention ended, the short-term nature of HCV treatment holds promise for time-limited patient navigation interventions among people who use substances.(Masson et al., 2013) The CTN-0064 trial evaluated a care facilitator intervention to improve progress along the HCV continuum of care among HIV/HCV co-infected people who use substances and had previously been enrolled in the CTN-0049 trial.(Craw et al., 2008; Gardner et al., 2005; Masson et al., 2013) Continuum of care steps included: 1) receiving HCV viral load results, 2) HIV primary care engagement, 3) initiating ART, 4) having a liver fibrosis evaluation, 5) receiving an offer of HCV medications, 6) initiating HCV medications, 7) completing HCV treatment, and 8) achieving sustained virologic response (SVR) at 12 weeks. Participants randomized to the HCV care facilitation intervention completed significantly more steps compared with those receiving treatment as usual (average 2.44 steps and average 1.68 steps, respectively).(Metsch et al., 2021) A higher proportion of individuals in the HCV care facilitation arm received their HCV results (94.2% v 54.1% in the control arm), had their HCV status evaluated (53.7% v 32.1% in the control arm), and were prescribed treatment (26.8% v 13.2% in the control arm).
Understanding the cost of time-limited care facilitation interventions is necessary for healthcare organizations to adopt these interventions and seek reimbursement; therefore, we conducted a cost analysis alongside the CTN-0064 trial. Our objectives were to estimate 1) the cost of providing the care facilitation intervention delivered in this trial, 2) likely costs of the intervention in non-trial settings, and 3) cost-offsets in other healthcare service areas.
2. METHODS
2.1. Analytic Overview
We employed a micro-costing approach from the healthcare sector perspective and the patient perspective (one element of societal costs) to determine the cost of providing HCV care facilitation, consistent with the recommendations of the Second Panel on Cost-effectiveness in Health and Medicine.(Neumann et al., 2017) We also used participant self-reported data to compare healthcare service costs between the intervention and control arms in order to identify any possible cost savings. All costs are reported in 2017 US dollars. Data were analyzed using Microsoft Excel 2013 (Microsoft Core; Redmond, Washington) and Stata 15.1 (Stata Corp; College Station, Texas).
The Columbia University Institutional Review Board, and the Institutional Review Board at each participating hospital reviewed and approved the CTN-0064 study protocol. The Weill Cornell Medical College Institutional Review Board reviewed and approved this cost study.
2.2. Overview of the Intervention Study
Individuals previously enrolled in CTN-0049 were recruited for CTN-0064 by outreach workers using locator information from CTN-0049. The median time between randomization in CTN-0049 and CTN-0064 was 3.3 years, (Metsch et al., 2021) so outreach workers relied on targeted street outreach, and database reviews when locator information was outdated. All participants in the study reported a history of injection drug use, and at baseline 87.6% self-reported substance use in the past 12 months or had a positive urine drug screen. Over half of the study population was male (58.4%) with a mean age of 51 (SD= 8.2), and the majority of the study population was non-Hispanic black (72.6%). The study population was primarily insured by Medicaid, Medicare, or other public insurance (82.3%), or uninsured (9.7%). At baseline only 6.2% of participants reported an annual income over $20,000, and approximately 16% reported experiencing unstable housing in the past six months.
CTN-0049 participants who were recruited for CTN-0064 who tested antibody positive for HCV at the initial visit were randomized between February 2016 and January 2017 to either treatment as usual where they were encouraged to maintain or re-enter HIV care and received referrals to social services (n=62), or care facilitation with motivational interviewing to help them progress along the HCV continuum of care (n=51), with 12-month follow up assessments completed at all sites by January 2018. Individuals who were HCV antibody positive at the initial visit but HCV RNA negative upon receipt of results could only complete step one in the HCV/HIV care continuum (i.e., receipt of HCV RNA test results). Participants receiving the intervention were expected to meet with the care facilitator twice each month, either in-person or over the phone during the six-month intervention period, to monitor progress along the HCV continuum of care, and discuss other social service needs as necessary. The care facilitator followed up with participants, providers, and participants’ collateral contacts (i.e., relatives, friends, partners etc.) over the phone between scheduled visits. For participants who were not in HIV care when they were randomized in CTN-0064 (n=21 in the control arm; n=15 in the intervention arm), re-engagement in HIV care after randomization was also considered progress along the HCV care continuum; some treating physicians would not consider initiating HCV treatment until participants had stable HIV viral loads. HIV care occurred at outpatient and community HIV clinics.
The care facilitation intervention took place in eight hospitals in cities across the US. Study sites were located in Atlanta, Georgia; Baltimore, Maryland; Boston, Massachusetts; Chicago, Illinois; Dallas, Texas; Miami, Florida; New York, New York; and Philadelphia, Pennsylvania.(Metsch et al., 2021) Start-up activities included in-person and webinar staff training. The central study team conducted an in-person two-day training for care facilitators and outreach workers on the needs of the HCV/HIV co-infected study population, HCV care facilitation, and motivational interviewing. On-site trainings were provided as needed for study staff who were unable to attend the two-day training, or for newly hired site staff. The in-person trainings were reinforced with pre-recorded webinar trainings. Throughout the intervention, care facilitators received local supervision and held case conferences with care facilitators at the other sites on weekly intervention and retention calls.
2.3. Site-Level Data Collection
Start-up costs, including training time and transportation, were determined from study training records. The cost of webinar development was not included, as central study staff indicated they would draw on existing resources from different government organizations if the study were repeated in a real-world setting.
Information on the number of care facilitator visits, whether the visit was in-person or by phone, and the duration of the visit were captured in care facilitators’ logs. After each care facilitator visit with a participant, care facilitators recorded their travel time and distance to the care facilitation visit; participant mode of transportation, distance, and travel time; and the length of the visit for both the care facilitator and participant. We calculated the number of care facilitation visits during the intervention and the duration of each visit using the care facilitator logs. A subset of care facilitation visits were audio recorded for study fidelity. The study team documented the duration of these recordings, and we compared this data to the duration recorded in care facilitators’ logs for the same visits. The duration of each recorded visit was consistent between the care facilitator logs and the audio recordings. Care facilitators also recorded non-visit contacts in care facilitator logs, which included phone calls with participants, providers, case managers, and participants’ collateral contacts between visits. We calculated the number of non-visit contacts and the duration of each contact using care facilitator logs.
We conducted in-person site visits at all eight participating hospitals between September 2016 and January 2017 that included semi-structured interviews with outreach workers, care facilitators, and study coordinators, with follow-up by email and/or phone. Data were collected on staff time for specific activities related to outreach and retention of participants, including scheduling initial and follow-up appointments, and clinical supervision including case conferencing (Supplemental Table 1).
2.4. Participant-Level Data Collection
Participants self-reported information on healthcare services utilization using the time-anchoring method at baseline, at the completion of the six-month interventions, and six months after the interventions.(National Institute on Drug Abuse, 2013) The time-anchoring method asks participants to recall the number of events within a given time frame, which acts as a reference point or anchor to yield more accurate results and minimize variation in length of recall. At each time point, participants reported emergency department visits, overnight inpatient stays, outpatient visits, and substance use-related healthcare visits received during the previous six months.
2.5. Unit Costs
Table 1 summarizes the unit costs used in the analysis. We assigned the relevant national wage and fringe benefit rates from the Bureau of Labor Statistics to study staff trainer and trainee time, and to time estimates reported during interviews and in care facilitator logs. We used the federal minimum wage to value participant time because approximately 90% of participants were unemployed or disabled, and unable to work.(United States Department of Labor, 2019) To estimate the cost of attending and delivering the training, we calculated the average cost of a flight from each site to the training site over a 3-month window to account for monthly variability in flight prices. We applied standard mileage reimbursement rates to the distance traveled by the care facilitators and participants, whether they drove or received a ride from a friend or family member.(Internal Revenue Service, 2018) For participants who reported taking public transportation, we applied the local public transportation fare to each reported one-way travel to or from a care facilitator visit. When participants reported using a taxi or rideshare service, we used local ride sharing pricing structures to estimate the cost of travel time and distance. We estimated the cost for emergency department visits, inpatient hospital stays, outpatient visits, psychologist visits, substance use disorder treatment visits, and case manager visits using published sources.(Agency for Healthcare Research and Quality, 2018; Centers for Medicare & Medicaid Services, 2017; Dunlap et al., 2018; King et al., 2016; Substance Abuse and Mental Health Services Administration)
Table 1.
Cost Inputs
| Cost Input | Unit Cost (2017 USD) | Reference(s) |
|---|---|---|
| Intervention Costs | ||
| Personnel time, $ per hour | ||
| Outreach worker | 17.05 | (U.S. Bureau of Labor Statistics, ;, 2017) |
| Care facilitator | 20.36 | (U.S. Bureau of Labor Statistics, ;, 2017) |
| Project manager | 33.91 | (U.S. Bureau of Labor Statistics, ;, 2017) |
| Motivational interviewing trainer | 53.69 | (U.S. Bureau of Labor Statistics, ;, 2017) |
| Fringe and overhead rates | ||
| Fringe benefits | 0.464 | (U.S. Bureau of Labor Statistics, 2017) |
| Overhead | 0.0673–0.33 | Unpublished data from CTN-0049 Project HOPE (NCT01612169)(U.S. National Library of Medicine, 2012) |
| Patient costs | ||
| Patient time, $ per hour | 7.25 | (United States Department of Labor, 2019) |
| Transportation | ||
| Driving, $ per mile | 0.55 | (Internal Revenue Service, 2018) |
| Public transportation | 1.80–2.75 | One-way (site specific) |
| Healthcare Costs | ||
| Service utilization costs* | ||
| Emergency department visits | 989.47 | (Agency for Healthcare Research and Quality, 2018) |
| Inpatient hospital stay (per night) | 4254.74 | (Agency for Healthcare Research and Quality, 2018) |
| Outpatient visit (community clinic or private doctor) | 1127.79 | (Agency for Healthcare Research and Quality, 2018) |
| Psychologist visit counseling session | 85.42 | (Centers for Medicare & Medicaid Services, 2017) |
| Psychologist visit medical session | 44.12 | (Centers for Medicare & Medicaid Services, 2017) |
| Residential drug treatment facility (per day) | 125.82 | (Substance Abuse and Mental Health Services Administration) |
| Outpatient substance use treatment | 44.17 | (Substance Abuse and Mental Health Services Administration) |
| Individual visit with substance use provider | 153.27 | (Substance Abuse and Mental Health Services Administration) |
| Group session with substance use provider | 17.47 | (King et al., 2016) |
| Support group or group counseling | 8.51 | (Dunlap et al., 2018) |
| Case manager visit | 97.88 | (Agency for Healthcare Research and Quality, 2018) |
| Medication cost, $ per dose | ||
| Methadone per day (1 ML of 10 mg/ ML dosage) | 3.42 | (Veterans Affairs Office of Acquisition and Logistics (OAL), 2016) |
| ** Buprenorphine HCL 8mg/ Naloxone HCL 2mg sublingual film: average dose 16 mg | 6.00 | (Veterans Affairs Office of Acquisition and Logistics (OAL), 2016) |
| *** Oral Naltrexone (50mg/ tab) | 0.98 | (Veterans Affairs Office of Acquisition and Logistics (OAL), 2016) |
Service Utilization costs from the Medical Expenditure Panel Survey (MEPS) are fee-for-service payment
Collected as “Buprenorphine (Suboxone),” used the price of generic buprenorphine.
Oral naltrexone was reported for treatment of opioid use disorder. The cost of oral naltrexone is calculated as $29.45 per 30 tablets. The recommended dose for opioid treatment is 50 mg of oral naltrexone per day. Apply $29.45 cost per monthly pick up.
2.6. Overhead Rates
We calculated overhead rates for each of the sites using data collected during the CTN-0049 trial at the same sites. The categories of overhead resources used in the CTN-0049 patient navigation intervention were similar to those used in the CTN-0064 care facilitation intervention; we therefore assumed that the overhead rate, defined as the ratio of the overhead costs to intervention labor costs, would be similar for both studies. Overhead resources included recurrent goods and services (e.g., office supplies and cellphone service plans); equipment (e.g., laptops, desktops and cellphones); and facilities.
2.7. Analysis
We calculated the cost of care facilitator visits and non-visit contacts by applying the appropriate wage and fringe rates to the times reported in the care facilitator logs. We calculated the cost of outreach and supervision by applying the appropriate wage and fringe rates to time estimates provided in site interviews. Overhead rates were multiplied by the sum of care facilitator visit and non-visit contact costs, outreach costs, and supervision costs to calculate overhead costs. Overhead rates were not applied to start-up training costs as most of these activities occurred offsite.
We report the mean start-up and intervention costs by site and summary statistics across sites. We also report participant costs for all participants across sites. In sensitivity analyses we adjusted intervention costs based on care facilitator caseload. The number of participants per site in the base case analysis reflects the study sample and study design. In a sensitivity analysis, we varied the number of participants per site to the peak weekly caseload per site and estimated the cost per participant if the site maintained their peak weekly caseload for the entire study duration. During interviews, staff estimated that the maximum weekly caseload in a real-world setting (i.e., not limited to study participants) would be approximately ten participants per care facilitator. Therefore, we also estimated the cost per participant if the caseload reached ten participants per week throughout the study at all sites, which represents the maximum number of participants that care facilitators reported they could reasonably manage weekly on an ongoing basis in a real-world setting. This assumes that even with these consistently higher caseloads, the duration and frequency of recurring meetings would remain the same and the cost of outreach activities could continue to be shared across participants.
We multiplied self-reported non-study healthcare service utilization by unit costs to calculate the costs for separate healthcare service categories for each participant during the six-month intervention period, and during the following six months. Following established guidelines,(Neumann et al., 2017) the costs of non-study healthcare utilization were estimated separately as potential cost offsets from the healthcare sector perspective and were not included in the weekly estimated costs per participant. Then we used a two-part linear multivariable regression model to predict mean non-study healthcare costs for the care facilitation and treatment as usual groups, controlling for baseline characteristics (age, sex, race/ethnicity, insurance status, educational level, and marital status), and baseline service utilization, for the two time periods (completion of the six-month trial and six months after trial completion). Standard errors were estimated via nonparametric bootstrap of the multivariable regression, and a two-tailed t-test was used to estimate whether there were significant differences by study arm.(Glick et al., 2014)
3. RESULTS
3.1. Start-Up Costs
The average start-up cost per site was $6,320 (range: $4,320–$7,000) (Table 2). The average cost of the centralized training varied by site depending on the number of staff attending (average: $3,380, range: $570–$5,320). The cost of onsite training at each study site varied by the number of newly hired staff members attending, and the cost of the trainer’s travel (average: $2,410, range: $0–$3,230). The cost of the webinar training ($520) did not vary by site because the same number of staff members were required to attend the webinars at all sites.
Table 2.
Site level start-up costs, 2017 US $
| All Sites | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cost component | Site A | Site B | Site C | Site D | Site E | Site F | Site G | Site H | Mean | Median |
| Onsite training | 2,510 | 2,370 | - | 3,230 | 2,580 | 3,070 | 2,690 | 2,860 | 2,410 | 2,640 |
| Central training | 3,670 | 3,730 | 5,320 | 570 | 3,760 | 2,790 | 3,620 | 3,620 | 3,380 | 3,650 |
| Webinar training | 520 | 520 | 520 | 520 | 520 | 520 | 520 | 520 | 520 | 520 |
| Total start-up cost | 6,710 | 6,620 | 5,840 | 4,320 | 6,870 | 6,380 | 6,830 | 7,000 | 6,32 0 | 6,660 |
3.2. Care Facilitation Intervention Costs (Healthcare Sector Perspective)
The average weekly cost per participant for all visits and contacts was $20 (site range: $4–$30) (Table 3a). Participants had an average of 9.6 visits with the care facilitator in-person or over the phone (site range: 2.7–17.00) out of the expected 12 visits over the course of an average of 23.6 weeks (range: 21.0–25.7). On average, each visit with the care facilitator cost $40 (range: $20–$90) (Table 3a) and lasted approximately 54 minutes (Supplemental Table 1). This included the cost of the care facilitator’s time with the participant (average: 33 minutes, range: 8–100 minutes), and round-trip travel time and transportation to the visit (average: 21 minutes, range: 2–84 minutes) (Supplemental Table 1). At six out of eight sites, care facilitators also recorded telephone contacts with participants, providers, case managers, social workers, and participants’ contacts (site average of those who reported telephone contacts: 10.5 contacts site range: 0.2–32.5). On average, each of these contacts resulted in an additional cost of $7 per intervention participant at sites that reported telephone contacts (range: $1–$15).
Table 3.
Care facilitator intervention costs, 2017 US $
| All Sites | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| a. Contact cost per participant | Site A | Site B | Site C | Site D | Site E | Site F | Site G | Site H | Mean | Median |
| Average number of visits | 17. | 7. | 13. | 12. | 8.2 | 6.2 | 2.6 | 9.0 | 9.6 | 8.63 |
| per participant* | 00 | 85 | 92 | 00 | 5 | 5 | 7 | 0 | 2 | |
| Cost per visit, $ | 30 | 30 | 40 | 30 | 30 | 90 | 20 | 40 | 40 | 30 |
| Average number of other contacts per participant** | 18.00 | 4.29 | 32.54 | 0.20 | 3.33 | 0.00 | 0.00 | 4.33 | 7.84 | 3.81 |
| Cost per contact, $ | 10 | 1 | 1 | 15 | 10 | 0 | 0 | 6 | 5 | 4 |
| Total contact cost for intervention period, $ | 630 | 350 | 670 | 420 | 310 | 670 | 80 | 410 | 440 | 420 |
| Weekly contact cost per participant, $ | 20 | 10 | 30 | 20 | 10 | 30 | 4 | 20 | 20 | 20 |
| All Sites | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| b. Contact cost per participant | Site A | Site B | Site C | Site D | Site E | Site F | Site G | Site H | Mean | Median |
| Weekly scheduling and outreach per intervention participant, $* | 130 | 300 | 60 | 100 | 320 | 90 | 180 | 60 | 150 | 120 |
| Weekly supervision per intervention participant, $** | 200 | 240 | 70 | 50 | 380 | 160 | 440 | 120 | 210 | 180 |
| Weekly outreach and supervision cost per intervention participant, $ | 330 | 530 | 130 | 160 | 710 | 250 | 620 | 180 | 360 | 290 |
| All Sites | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| c. Total Intervention cost per participant (healthcare sector perspective) | Site A | Site B | Site C | Site D | Site E | Site F | Site G | Site H | Mean | Median |
| Total weekly cost per participant (without overhead), $ | 350 | 550 | 150 | 170 | 720 | 280 | 620 | 200 | 380 | 320 |
| Overhead Rate (%) | 17.61 | 33.10 | 15.95 | 9.30 | 6.73 | 7.66 | 29.57 | 30.98 | 18.86 | 16.78 |
| Weekly cost per participant (with overhead), $ | 420 | 730 | 180 | 190 | 770 | 300 | 800 | 260 | 450 | 370 |
face-to-face or by telephone
other contacts include telephone contacts with participants, providers, case managers, social workers, and participants’ friends and families between visits. The average number of other contacts among sites that reported other contacts was 10.5 visits with range 0.2– 32.5, and average cost of $7 with a site range $1–$15.
Initial scheduling, reminder calls, and community outreach were done by the outreach worker.
Supervision includes weekly site team meetings, local supervision, case conferencing, a care facilitator call, and retention call. These tasks represent costs and activities that would occur outside a research setting.
Weekly supervision and outreach costs per participant reflect the limited number of participants that were enrolled at each site. On average, sites incurred $360 (range: $130–$700) in supervision and outreach costs per participant weekly (Table 3b). The average cost of supervision was $210 per intervention participant, per week (range: $50–$440). This cost varied by the number of staff attending supervision and case conference meetings and the frequency and duration of these meetings. The average cost of outreach was $150 per participant weekly (range: $60–$320). The cost varied by the amount of time spent locating this hard-to-reach population using street outreach and reviewing hospital and other databases.
The average total intervention cost per participant, per week was $450 (range $150–$720) (Table 3c), including $70 for overhead, and the average intervention cost per participant over the six-month intervention was $10,720 (range: $4,530–$18,650).
3.3. Sensitivity Analysis
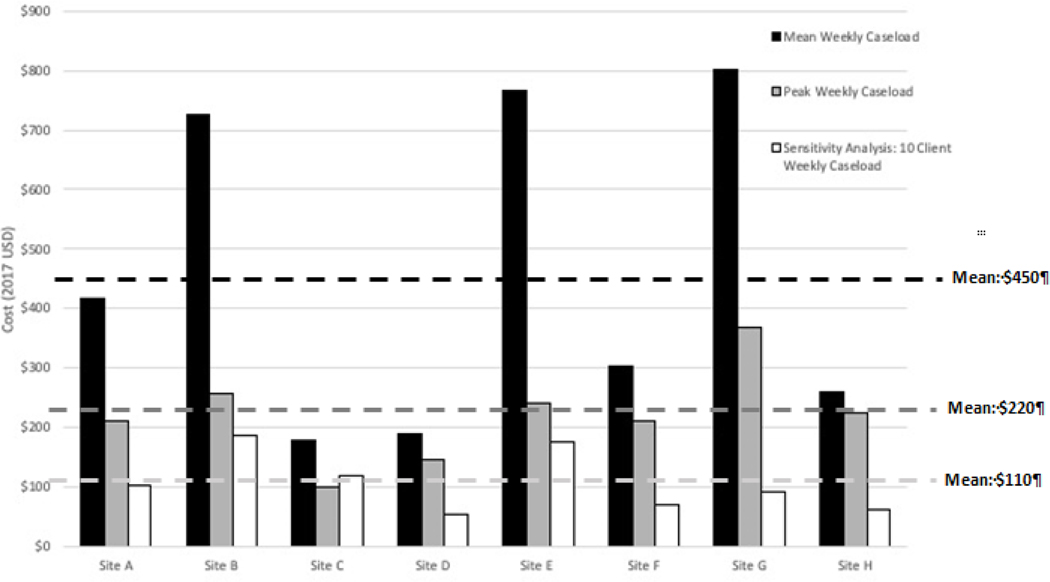
We varied the care facilitator caseload to examine its effect on the total cost per participant (Figure 1). Care facilitators had an average caseload of two participants per week (site range: 1–6). At the peak of intervention implementation at each site, the average weekly caseload for each site ranged from 2 to 12 participants across sites. When we applied the peak caseload of each site to its weekly caseload for 24-weeks, the weekly cost per participant decreased from $450 to $220. When we increased the average caseload to ten participants across all sites for the 24-week intervention to represent the real-world sensitivity analysis, the weekly cost per participant further decreased to $110.
Figure 1. Weekly cost per participant decreased with increasing caseloads.
The mean weekly cost per participant at an average weekly caseload was $450, the mean weekly cost per participant at a peak weekly caseload was $220, and the mean weekly cost per participant at realistic caseload of 10 clients per week was $110. All costs are in 2017 USD. Note: The average weekly caseload was 2 participants per week (site range: 1–6). The average peak weekly caseload was 5 participants per week (site range: 2–12), which represents the peak number of participants at a site in a given week. The sensitivity capacity caseload was 10 participants per week at every site, estimated from site interviews and discussions with the study team.
3.4. Participant Costs
Each care facilitator visit, including visit time and travel, cost the participant an average of $17 (range $2–$60) (Table 4). The average cost to the participant for non-visit reminders and contacts with care facilitators was less than $1 per contact. The average weekly cost to the participant was $8 and the total average cost to the participant over the intervention period was $177 (range: $5–$470).
Table 4.
Care facilitation participant costs, 2017 US $
| Participant Level Costs and Visits n=49 * | ||||
|---|---|---|---|---|
| Mean (SD) | Median | Min | Max | |
| Average number of visits perparticipant | 10.67 (5.92) | 11 | 1 | 20 |
| Cost of participant time pervisit, $ | 8 (4) | 8 | 2 | 20 |
| Cost of participant travel time pervisit, $ | 5 (4) | 4 | 0 | 23 |
| Cost of participant travel distance pervisit, $ | 4 (7) | 3 | 0 | 46 |
| Total cost pervisit, $ | 17 (9) | 15 | 2 | 60 |
| Average number of non-visit contacts perparticipant | 11.35 (21.17) | 3 | 0 | 103 |
| Cost per non visit contact perparticipant, $ | 1.00 (1) | <1 | 0 | 4 |
| Total cost of visits over intervention period, $ | 177 (120) | 162 | 5 | 465 |
| Total cost of non-visit contacts over the intervention period, $ | 7 (15) | 2 | 0 | 81 |
| Weekly cost of care facilitator visits per participant, $ | 4 (2) | 4 | <1 | 10 |
| Weekly cost of participant travel time per participant, $ | 4 (4) | 3 | 0 | 20 |
| Weekly cost of non-visit contacts per participant, $ | <1 (<1) | 0 | 0 | 3 |
| Total Weekly Cost per participant for all contacts, $ | 8 (4) | 7 | 0 | 20 |
2 participants were omitted because they reported no visits with the care facilitator
3.5. Cost Offsets
The estimated cost of non-study healthcare utilization was approximately $6,820 per participant in the care facilitation arm, versus $4,980 per participant in the treatment as usual arm during the intervention period, and $5,120 versus $6,160, respectively, during the six-month follow up period (Supplemental Table 2). These costs did not differ significantly in either period or over the entire 12-month period.
4. DISCUSSION
HCV care facilitation provided to people who used substances and were co-infected with HIV was costly in this trial, at approximately $450 per participant, per week over six months. The cost per participant varied substantially by site. While the number of visits and time associated with each visit contributed to variation in contact costs by site, between-site cost differences were largely driven by differences in weekly supervision and client outreach activities. Sites varied in the how they conducted and staffed these activities, as well as the average caseloads that the activities supported. The costs of recurring supervision and outreach were distributed evenly across the caseload when estimating the average weekly cost per participant. When we increased the caseload in sensitivity analysis, these supervision and outreach costs were distributed among more participants so the average weekly cost per participant decreased. If sites maintained their peak caseload the average cost per participant of HCV care facilitation fell by about half, and at an estimated real-world maximum caseload of ten participants per week this cost fell by almost three quarters.
Simulation modeling has indicated that interventions to improve progression along the HCV care continuum are likely to be cost-effective.(Linas et al., 2014; Linas et al., 2016; Schackman et al., 2018) Nevertheless, the cost of HCV care facilitation in this study, of approximately $2,600 per participant for a six-month intervention (using our real-world estimate of $110 per week per participant) was considerably higher than for a care coordination intervention that was implemented in HCV mono-infected and HIV/HCV co-infected patients who were primarily already engaged in clinical care;(Behrends et al., 2019) the cost per participant of that intervention over 5.6 months, excluding overhead, ranged from $522 to $656 at two different healthcare systems.(Behrends et al., 2019) The cost of that intervention included care facilitation, case conferencing and supervision, but did not require community outreach for participant enrollment and retention. At many facilities, reimbursement using Medicare’s chronic care management fee schedule would have been insufficient to cover even this cost.(Fluegge et al., 2019)
In this study the cost associated with locating and scheduling participants for care facilitator visits was high because of the study inclusion criteria, transient nature of this patient population, and the elapsed time from the conclusion of CTN-0049 until the start of CTN-0064. For every $1 spent on care facilitation visits, at least $1 was spent to stay in touch with participants. This reflects the hard-to-reach vulnerable HIV/HCV co-infected population included in the study, who were originally recruited for the previous study in hospital settings with detectable HIV viral loads. While there are limits to the savings that can be obtained from streamlining outreach efforts, there are opportunities to reduce costs with higher caseloads because outreach activities such as visiting locations and searching databases can yield contact with multiple participants.
Supervision costs in real-world settings might also be lower than those in this study, which included weekly team meetings, local supervision meetings, local case conferences, and cross-site care facilitator case conferences; however, we cannot be certain to what extent this level of intensity in supervision affected participant outcomes. Although start-up training costs were not included in the estimated intervention cost per participant, start-up training for staff is another opportunity to reduce care facilitation program implementation costs in a real-world setting because several of the training topics would likely be available by webinar or training for new staff could be delivered onsite by existing local staff.
While we followed established micro-costing methods to conduct these analyses, the small number of participants at many of the study sites resulted in wide variability of results between sites. We did not find any significant healthcare-sector cost offsets, but future savings might result from successful HCV treatment. The infrequent reports of criminal activity or incarceration also limited our ability to identify societal cost offsets over the intervention and follow-up period. We did not conduct a budget impact analysis to estimate potential revenue from the care facilitator intervention that could offset program costs. However, there are potential resources that vary by state and site, such as Ryan White funding and 340b program revenues, that could offset some programmatic costs and should be investigated in future research. Finally, we also did not examine cost-effectiveness, which would require simulation modeling to estimate the impact of the trial outcomes on subsequent life expectancy and quality of life.
5. CONCLUSION
HCV care facilitation programs to move patients along the care continuum are an important strategy to meet the US viral hepatitis C elimination objectives. The weekly cost of HCV care facilitation was estimated to be $450 per HIV and HCV co-infected individual recruited from a previous trial. The weekly cost of HCV care facilitation decreased to $110 per participant in real-world settings for HIV and HCV co-infected people who use drugs, and are difficult to reach and retain. Although there are considerable outreach and supervision costs associated with care facilitation for this population, the cost per participant decreases with higher caseloads in real-world settings compared to costs incurred in an intervention trial.
Supplementary Material
Highlights.
Care facilitation weekly cost was $450 per HIV/HCV co-infected trial participant
The weekly cost was lower ($110 per person) assuming a more realistic caseload
Outreach and supervision represented the largest share of care facilitation costs
No healthcare cost offsets were identified during the trial period
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the contribution of James Kahn, MDW, MPH for sharing costing resources and results from the CTN-0049 trial. We would like to acknowledge the important contributions of the CTN-0064 staff, clinical providers, care facilitators, outreach workers and site coordinators who provided patient data for this study.
Role of Funding Sources
Funding/Support: This work was supported by grants from the National Institute on Drug abuse and the National Drug Abuse Treatment Clinical Trials Network (CTN): UG1DA013720, UG1DA015815, UG1DA013035, HHSN271201400028C/N01DA142237, UG1DA013034, UG1DA015831, UG1DA020024, and assistance from the Miami (P30A1073961), and Emory (P30AI050409), Centers for AIDS Research; Center for Health Economics of Treatment Interventions for Substance Use Disorder, HCV, and HIV (P30DA040500); Center for Drug Use and HIV/HCV Research (P30DA011041); and Training Programs in Substance Abuse Epidemiology (T32DA031099) and Comparative and Cost-Effectiveness Research (T32NR014205).
Role of the Funder/Sponsor: The funding organizations and sponsoring agencies had no further role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Support: Supported by the National Drug Abuse Treatment Clinical Trials Network (CTN): UG1DA013720, UG1DA015815, UG1DA013035, HHSN271201400028C/N01DA142237, UG1DA013034, UG1DA015831, UG1DA020024, and assistance from the Miami (P30A1073961), and Emory (P30AI050409), Centers for AIDS Research; Center for Health Economics of Treatment Interventions for Substance Use Disorder, HCV, and HIV (P30DA040500); Center for Drug Use and HIV/HCV Research (P30DA011041); and Training Programs in Substance Abuse Epidemiology (T32DA031099) and Comparative and Cost-Effectiveness Research (T32NR014205).
Footnotes
Conflict of Interest
All authors declare that they have no conflict of interest.
Presented at the 8th International Conference on Hepatitis Care in Substance Users, Montreal, Canada September 11-September 13, 2019 and American Psychopathological Association Annual Conference, New York, NY, March 5- March 7, 2020.
Clinical Trial Number: NCT01612169 (CTN-0049), NCT1612169 (CTN-0064)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agency for Healthcare Research and Quality, 2018. Medical Expenditure Panel Survey. https://meps.ahrq.gov/mepsweb/. (Accessed February 13 2019). [PubMed]
- American Association for the Study of Liver Diseases & Infectious Diseases Society of America, 2019. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. https://www.hcvguidelines.org/. (Accessed March 14 2018).
- Behrends CN, Eggman AA, Gutkind S, Bresnahan MP, Fluegge K, Laraque F, Litwin AH, Meissner P, Shukla SJ, Perumalswami PV, Weiss J, Wyatt BE, Schackman BR, 2019. A Cost Reimbursement Model for Hepatitis C Treatment Care Coordination. J Public Health Manag Pract 25(3), 253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell CA, Canary L, Smith N, Teshale E, Ryerson AB, Ward JW, 2017. State HCV incidence and policies related to HCV preventive and treatment services for persons who inject drugs--United States, 2015–2016. Morbidity and Mortality Weekly Report (MMWR) 66(18), 465–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Medicare & Medicaid Services, 2017. Physician Fee Schedule Search. https://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx. (Accessed April 1, 2019.
- Clement ME, Collins LF, Wilder JM, Mugavero MJ, Barker T, Naggie S, 2018. Hepatitis C virus elimination in the human immunodeficiency virus-coinfected population: Leveraging the existing human immunodeficiency virus infrastructure. Infect Dis Clin North Am 32(2), 407–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craw JA, Gardner LI, Marks G, Rapp RC, Bosshart J, Duffus WA, Rossman A, Coughlin SL, Gruber D, Safford LA, Overton J, Schmitt K, 2008. Brief strengths-based case management promotes entry into HIV medical care: results of the antiretroviral treatment access study-II. J Acquir Immune Defic Syndr 47(5), 597–606. [DOI] [PubMed] [Google Scholar]
- Dunlap LJ, Zarkin GA, Orme S, Meinhofer A, Kelly SM, O’Grady KE, Gryczynski J, Mitchell SG, Schwartz RP, 2018. Re-engineering methadone—Cost-effectiveness analysis of a patient-centered approach to methadone treatment. Journal of Substance Abuse Treatment 94, 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluegge K, Bresnahan MP, Laraque F, Litwin AH, Perumalswami PV, Shukla SJ, Weiss JJ, Winters A, 2019. Evaluating reimbursement of integrated support services using chronic care management (CCM) codes for treatment of hepatitis C among Medicare beneficiaries. J Healthc Risk Manag. [DOI] [PubMed] [Google Scholar]
- Gardner LI, Metsch LR, Anderson-Mahoney P, Loughlin AM, del Rio C, Strathdee S, Sansom SL, Siegal HA, Greenberg AE, Holmberg SD, 2005. Efficacy of a brief case management intervention to link recently diagnosed HIV-infected persons to care. Aids 19(4), 423–431. [DOI] [PubMed] [Google Scholar]
- Glick HA, Doshi JA, Sonnad SS, 2014. Economic Evaluation in Clinical Trials, Second Edition ed. Oxford University Press, Great Britain. [Google Scholar]
- Goodyear T, Lianping T, Carrieri P, Small W, Knight R, 2020. “Everybody living with a chronic disease is entitled to be cured”: challenges and opportunities in scaling up access to direct-acting antiviral hepatitis C virus treatment among people who inject drugs. Int J Drug Policy 81, 102766. [DOI] [PubMed] [Google Scholar]
- Gowda C, Lott S, Grigorian M, Carbonari DM, Saine ME, Trooskin S, Roy JA, Kostman JR, Urick P, Lo Re III, V., 2018. Absolute insurer denial of direct-acting antiviral therapy for hepatitis C: A national specialty pharmacy cohort study. Open forum infectious diseases 5(6), ofy076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebely J, Hajarizadeh B, Dore GJ, 2017. Direct-acting antiviral agents for HCV infection affecting people who inject drugs. Nat Rev Gastroenterol Hepatol 14(11), 641–651. [DOI] [PubMed] [Google Scholar]
- Grebely J, Larney S, Peacock A, Colledge S, Leung J, Hickman M, Vickerman P, Blach S, Cunningham EB, Dumchev K, Lynskey M, Stone J, RTrickey A, Razavi H, Mattick RP, Farrell M, Dore GJ, Degenhardt L, 2019. Global, regional, and country-level estimates of hepatitis C infection among people who have recently injected drugs. Addiction 114(1), 150–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AM, Khan MA, Osinubi A, Nelson NP, Thompson WW, 2021. Hepatitis C treatment among commercially or Medicaid-insured individuals, 2014–2018. Am J Prev Med 61(5), 716–723. [DOI] [PubMed] [Google Scholar]
- Internal Revenue Service, 2018. Standard Mileage Rates for 2018 Up from Rates for 2017. [Google Scholar]
- Kapadia SN, Jeng PJ, Schackman BR, Bao Y, 2018. State medicaid hepatitis C treatment eligibility criteria and use of direct-acting antivirals. Clin Infect Dis 66(10), 1618–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JB, Sainski-Nguyen AM, Bellows BK, 2016. Office-Based Buprenorphine Versus Clinic-Based Methadone: A Cost-Effectiveness Analysis. J Pain Palliat Care Pharmacother 30(1), 55–65. [DOI] [PubMed] [Google Scholar]
- Lin M, Kramer J, White D, Cao Y, Tavakoli-Tabasi S, Madu S, Smith D, Asch SM, El-Serag HB, Kanwal F, 2017. Barriers to hepatitis C treatment in the era of direct-acting anti-viral agents. Aliment Pharmacol Ther 46(10), 992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linas BP, Barter DM, Leff JA, Assoumou SA, Salomon JA, Weinstein MC, Kim AY, Schackman BR, 2014. The hepatitis C cascade of care: identifying priorities to improve clinical outcomes. PLoS One 9(5), e97317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linas BP, Morgan JR, Pho MT, Leff JA, Schackman BR, Horsburgh CR, Assoumou SA, Salomon JA, Weinstein MC, Freedberg KA, Kim AY, 2016. Cost Effectiveness and Cost Containment in the Era of Interferon-Free Therapies to Treat Hepatitis C Virus Genotype 1. Open forum infectious diseases 4(1), ofw266-ofw266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson CL, Delucchi KL, McKnight C, Hettema J, Khalili M, Min A, Jordan AE, Pepper N, Hall J, Hengl NS, Young C, Shopshire MS, Manuel JK, Coffin L, Hammer H, Shapiro B, Seewald RM, Bodenheimer HC, Sorensen JL, Des Jarlais DC, Perlman DC, 2013. A randomized trial of a hepatitis care coordination model in methadone maintenance treatment. American Journal of Public Health 103(10), e81–e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews GV, Dore GJ, 2008. HIV and heptatitis C coinfection. J Gastroenterol Hepatol 23(7), 1000–1008. [DOI] [PubMed] [Google Scholar]
- Metsch LR, Feaster DJ, Gooden L, Matheson T, Stitzer M, Das M, Jain MK, Rodriguez AE, Armstrong WS, Lucas GM, Nijhawan AE, Drainoni ML, Herrera P, Vergara-Rodriguez P, Jacobson JM, Mugavero MJ, Sullivan M, Daar ES, McMahon DK, Ferris DC, Lindblad R, VanVeldhuisen P, Oden N, Castellon PC, Tross S, Haynes LF, Douaihy A, Sorensen JL, Metzger DS, Mandler RN, Colfax GN, del Rio C, 2016. Effect of Patient Navigation With or Without Financial Incentives on Viral Suppression Among Hospitalized Patients With HIV Infection and Substance Use: A Randomized Clinical Trial. Jama 316(2), 156–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metsch LR, Feaster DJ, Gooden LK, Masson C, Perlman DC, Jain MK, Matheson T, Nelson CM, Jacobs P, Tross S, Haynes LF, Lucas GM, Colasanti JA, Rodriguez A, Drainoni M-L, Osorio G, Nijhawan AE, Jacobson JM, Sullivan M, Metzger D, Vergara-Rodriguez P, Lubelcheck R, Duan R, Batycki JN, Matthews AG, Munoz F, Jelstrom E, Mandler R, del Rio C, 2021. Care Facilitation Advances Movement Along the Hepatitis C Care Continuum for Persons With Human Immunodeficiency Virus, Hepatitis C, and Substance Use: A Randomized Clinical Trial (CTN-0064), Open Forum Infectious Diseases. Oxford University Press US, p. ofab334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muncan B, Jordan AE, Perlman DC, Frank D, Ompad DC, Walters SM, 2021. Acceptability and effectiveness of hepatitis C care at syringe service programs for people who inject drugs in New York city. Substance Use & Misuse 56(5), 728–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Drug Abuse, 2013. Seek, Test, Treat and Retain for vulnerable populations: Data harmonization measure. Service Utilization. https://www.drugabuse.gov/sites/default/files/sttrfiles/Service_UtilizationV.pdf. (Accessed 1 March 2018. [Google Scholar]
- Neumann PJ, Ganiats TG, Russell LB, Sanders GD, Siegel JE, 2017. Cost-Effectiveness in Health and Medicine, Second Edition ed. Oxford University Press, New York, NY. [Google Scholar]
- Pundhir P, North CS, Faturnde O, Jain MK, 2016. Health beliefs and comorbidities associated with appointment-keeping behavior among HCV and HIV/HCV patients. J Community Health 41(1), 30–37. [DOI] [PubMed] [Google Scholar]
- Schackman BR, Gutkind S, Morgan JR, Leff JA, Behrends CN, Delucchi KL, McKnight C, Perlman DC, Masson CL, Linas BP, 2018. Cost-effectiveness of hepatitis C screening and treatment linkage intervention in US methadone maintenance treatment programs. Drug and alcohol dependence 185, 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims OT, Guo Y, Shoreibah MG, Venkata K, Fitzmorris P, Kommineni V, Romano J, Massoud OI, 2017. Short article: Alcohol and substance use, race, and insurance status predict nontreatment for hepatitis C virus in the era of direct acting antivirals: a retrospective study in a large urban tertiary center. Eur J Gastroenterol Hepatol 29(11), 1219–1222. [DOI] [PubMed] [Google Scholar]
- Spradling PR, Zhong Y, Moorman AC, Rupp LB, Lu M, Gordon SC, Teshale E, Schmidt MA, Daida YG, Boscarino JA, Chronic Hepatitis Cohort Study (CHeCS) Investigators, 2020. Psychosocial obstacles to hepatitis C treatment initiation among patients in care: A hitch in the cascade of cure. Hepatol Commun 5(3), 400–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, The Alcohol and Drug Services Study (ADSS) Cost Study: Costs of Substance Abuse Treatment in the Specialty Sector, Analytic Series A-20. Substance Abuse and Mental Health Services Administration, Rockville, MD. [Google Scholar]
- U.S. Bureau of Labor Statistics, 2017. Employer Costs for Employee Compensation Historical Listing. https://www.bls.gov/ncs/ect/sp/ececqrtn.pdf. (Accessed April 1 2019).
- U.S. Bureau of Labor Statistics, 2017. United States Department of Labor Bureau of Labor Statistics. http://www.bls.gov/. (Accessed March 1 2018).
- U.S. National Library of Medicine, 2012. Project HOPE: Hospital Visit as Opportunity for Prevention and Engagement for HIV-Infected Drug Users (NCT01612169). https://clinicaltrials.gov/ct2/show/NCT01612169?term=Project+HOPE%3A+Hospital+Visit+as+Opportunity+for+Prevention+and+Engagement+for+HIV-Infected+Drug+Users&rank=1. (Accessed April 9 2021).
- United States Department of Health & Human Services, 2021. Viral Hepatitis National Strategic Plan https://www.hhs.gov/sites/default/files/Viral-Hepatitis-National-Strategic-Plan-2021-2025.pdf. (Accessed April 9 2021).
- United States Department of Labor, 2019. Minimum Wage. https://www.dol.gov/general/topic/wages/minimumwage. (Accessed August 2 2019).
- Veterans Affairs Office of Acquisition and Logistics (OAL), 2016. The Federal Supply Schedule. Online. [Google Scholar]
- Waheed Y, Siddiq M, Jamil Z, Najmi MH, 2018. Hepatitis elimination by 2030: Progress and challenges. World J Gastroenterol 24(44), 4959–4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, 2017. Global Hepatitis Report 2017. Geneva, Switzerland. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.