Abstract
A highly inducible fungal promoter derived from the Penicillium chrysogenum endoxylanase (xylP) gene is described. Northern analysis and the use of a β-glucuronidase (uidA) reporter gene strategy showed that xylP expression is transcriptionally regulated. Xylan and xylose are efficient inducers, whereas glucose strongly represses the promoter activity. Comparison of the same expression construct as a single copy at the niaD locus in P. chrysogenum and at the argB locus in Aspergillus nidulans demonstrated that the xylP promoter is regulated similarly in these two species but that the level of expression is about 80 times higher in the Aspergillus species. The xylP promoter was found to be 65-fold more efficient than the isopenicillin-N-synthetase (pcbC) promoter in Penicillium and 23-fold more efficient than the nitrate reductase (niaD) promoter in Aspergillus under induced conditions. Furthermore, the xylP promoter was used for controllable antisense RNA synthesis of the nre-encoded putative major nitrogen regulator of P. chrysogenum. This approach led to inducible downregulation of the steady-state mRNA level of nre and consequently to transcriptional repression of the genes responsible for nitrate assimilation. In addition, transcription of nreB, which encodes a negative-acting nitrogen regulatory GATA factor of Penicillium, was found to be subject to regulation by NRE. Our data are the first direct evidence that nre indeed encodes an activator in the nitrogen regulatory circuit in Penicillium and indicate that cross regulation of the controlling factors occurs.
Utilization of filamentous fungi in industrial processes for production of pharmaceuticals is well established. A variety of fungal metabolites are commercially exploited for their antibiotic properties; e.g., the β-lactam antibiotics penicillin and cephalosporin are produced by Penicillium chrysogenum and Acremonium chrysogenum, respectively (3, 16). Furthermore, well-established large-scale fermentation technology and the capacity to secrete substantial amounts of proteins into the medium increased the industrial application of these organisms as hosts for homologous and heterologous protein production and secretion (14). For synthesis of proteins in large quantities efficient gene expression systems are required. Over the years a number of such expression systems have been developed, especially for Aspergillus and Trichoderma species, focusing on heterologous protein production. A second obvious application for gene expression systems is the improvement of strains of commercially employed producers of pharmaceuticals, such as P. chrysogenum. In this respect, promoters with various characteristics are needed for increasing the transcript levels of the penicillin biosynthetic genes or for directed metabolic engineering in order to improve specific cellular properties (39, 40). In contrast to Aspergillus, only a few homologous promoter systems have been analyzed in P. chrysogenum so far. Some examples are the largely constitutive promoters of the genes encoding phosphoglycerate kinase (pgkA) and NADP-dependent glutamate dehydrogenase (gdhA) (10, 24). Furthermore, the promoter of the isopenicillin-N-synthetase (pcbC) has been used for driving expression of the Tn5phleomycin resistance gene as a selection marker (11, 21, 29). The only highly regulatable homologous expression system described for P. chrysogenum utilizes the promoter of the acid phosphatase-encoding gene (phoA), but full induction requires phosphate starvation, which might induce production of proteases and therefore is not feasible for all purposes (15). Use of the Aspergillus nidulans glyceraldehyde-3-phosphate dehydrogenase gene (gpdA) promoter is an example of functioning of a constitutive heterologous promoter in Penicillium (29). Although several promoters have proven to be useful for expression of genes, there still is clearly a need for new, strongly inducible promoters in P. chrysogenum, especially promoters that can be induced independently of the promoters already available.
We have cloned and characterized a gene encoding a group F 1,4-β-endoxylanase (XYLP) which is expressed at a high level during growth on xylan, yielding up to 25% of the total proteins secreted into the culture medium, and is repressed by glucose (17, 18). Recently, we have employed the xylP promoter region successfully for overexpression of the nitrogen regulatory GATA factor NREB in Penicillium, but a detailed analysis of this promoter, which would permit general application, has not been performed so far (21).
In this paper we describe an extended promoter sequence analysis of xylP and a detailed characterization analysis of the regulation of xylP expression in Penicillium in which a β-glucuronidase (GUS) reporter strategy was used. Furthermore, the usefulness of this system was demonstrated by employing it for inducible synthesis of antisense RNA of nre, which encodes the putative major nitrogen regulator of P. chrysogenum (19). Additionally, we show the functionality of the xylP-based expression system in A. nidulans, indicating the suitability of this system for various other fungal systems.
MATERIALS AND METHODS
Strains, media, and transformation.
Vectors and plasmids were propagated in Escherichia coli DH5α (Life Technologies). All fungal strains used in this study were derived from A. nidulans WG355 (biA1 bgaO argB2) or from P. chrysogenum niaD mutant M20 (11, 51). Generally, P. chrysogenum was grown at 25°C in Vogel's minimal medium supplemented with different carbon and nitrogen sources (21). P. chrysogenum protoplasts were transformed as described by Cantoral et al. (5), and transformants were selected on minimal medium containing NaNO3 and glucose as the nitrogen and carbon sources, respectively. For A. nidulans the minimal medium described by Pontecorvo et al. (41) was used and incubation was performed at 37°C. Media were supplemented as required. Transformation of A. nidulans was carried out as described by Tilburn et al. (50). Screening of positive clones was performed by PCR and Southern blot analysis (46).
Recombinant DNA and RNA techniques.
For cloning procedures standard recombinant DNA techniques were used (46).
Fungal chromosomal DNA was isolated as described by Bainbridge et al. (1), with some modifications. The fungi were grown in 3 ml of complete medium as described by Kafer (27); Aspergillus cultures were grown for 24 h at 37°C, and Penicillium cultures were grown for 48 h at 25°C. Mycelia were collected by centrifugation, washed with 100 mM EDTA, and resuspended in 3 ml of lysis solution containing 2 mg of NOVOzym 234 (Sigma) per ml, 50 mM potassium phosphate buffer (pH 5.8), 700 mM KCl, and 100 mM EDTA. After incubation for 3 h at 30°C in a rotary shaker at 150 rpm, the resulting protoplasts were transferred into 1.5-ml Eppendorf tubes, pelleted by centrifugation for 10 min at 7,000 × g, and resuspended in 0.4 ml of extraction buffer containing 100 mM NaCl, 0.5% sodium dodecyl sulfate (SDS), 50 mM Tris-HCl (pH 7.5), and 100 mM EDTA. After incubation for 10 min at 65°C, the samples were extracted with phenol-chloroform-isoamyl alcohol (25:24:1) and then incubated with 20 μg of RNase (Boehringer Mannheim) for 10 min at 65°C, followed by incubation for 30 min at 20°C. The DNA was precipitated with 0.5 volume of isopropanol, pelleted by centrifugation, and dissolved in TE buffer.
Total RNA was isolated from nitrogen-frozen and ground mycelia by using TRIzol reagent (Life Technologies) according to the supplier's instructions. Generally, 15 μg of total RNA was electrophoresed on 1.2% agarose–1.1 M formaldehyde gels and blotted onto Hybond N membranes (Amersham). Hybridization probes labeled with digoxigenin (Boehringer Mannheim) were generated by PCR amplification by using oligonucleotides described previously (21).
uidA reporter constructs and vector for nre antisense expression.
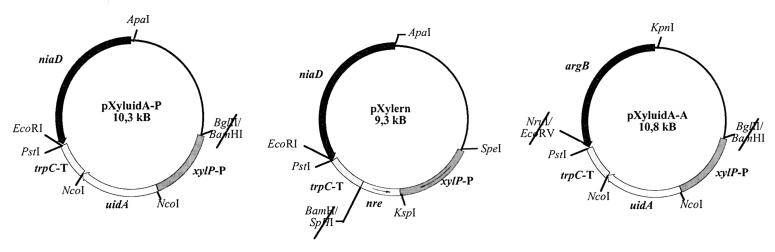
A vector containing the uidA reporter gene preceded by the xylP promoter and followed by the A. nidulans trpC terminator sequence was obtained as follows. The 1.7-kb upstream region of the xylanase promoter was amplified by PCR from a subcloned SalI fragment (18) by using synthetic oligonucleotides to create an NcoI site in the xylP start codon (5′-CCATGCCATGGTTGGTTCTTCGAGTCGA) and to generate a BglII site at bp −1680, destroying the SalI site at bp −1676 (5′-TTTGAAGATCTCGACGGAAGCGCGCAG). The PCR-amplified and BglII-NcoI-cleaved fragment was ligated into the 5.4-kb BglII-NcoI fragment of plasmid pNOM102 (44). In order to allow targeted integration in P. chrysogenum, the 4.2-kb BglII-PstI fragment of the resulting plasmid was inserted into the 6.1-kb BamHI-PstI fragment of vector pUN-pcbC, which contains a truncated niaD gene (11, 33). The resulting vector was called pXyluidA-P (Fig. 1).
FIG. 1.
Schematic representation of the vectors used for analysis of xylP promoter activity (pXyluidA-P, pXyluidA-A) and for expression of nre antisense RNA (pXylern). Construction of these vectors is described in Materials and Methods. The xylP promoter region is indicated by xylP-P, and the trpC termination region is indicated by trpC-T.
To analyze the xylP-uidA fusion in A. nidulans as a single-copy insertion at the argB locus, the truncated Penicillium niaD gene was replaced by a mutated argB allele. To do this, a 3.7-kb NruI-KpnI fragment from plasmid pTran3-1A (43) was cloned into EcoRV-KpnI-cleaved pBluescript KS (Stratagene). The resulting vector was digested with PstI and BamHI, ligated with the 4.2-kb BglII-PstI fragment carrying the xylP-uidA fusion described above, and termed pXyluidA-A (Fig. 1).
To express nre antisense RNA under control of the xylP promoter, the pXylern vector was constructed as follows (Fig. 1). The 1.7-kb PCR fragment of the xylP promoter region described above was inserted into plasmid pGEM-T (Promega), resulting in plasmid pXyl-Gem. A 0.8-kb SphI-KspI fragment (bp 468 to 1280) encoding NRE amino acids 138 to 407 was PCR amplified from an nre template (19), and the SphI site at bp 468 was inserted with the PCR primer. After SphI-KspI cleavage, this fragment was subcloned into the SphI and KspI sites of pXyl-Gem. To allow targeted integration in P. chrysogenum, the 2.5-kb SphI-SpeI fragment carrying the xylP-nre-antisense fusion was inserted into the 7-kb BamHI-SpeI pXyluidA-P fragment; the SphI and BamHI sites were previously filled in with Klenow polymerases.
The PCR fragments were verified by sequencing (46).
GUS activity assay and N-terminal amino acid sequence determination.
GUS activity was measured by using 4-methylumbelliferyl-β-d-glucuronide as the substrate as described by Jefferson (26). The amount of 4-methylumbelliferone produced was determined with a fluorometer (TKO 100; Hoefer Scientific Instruments) calibrated with a standard 4-methylumbelliferone solution.
For in-gel GUS detection 100 μg of soluble protein from a mycelial crude extract was subjected to native polyacrylamide gel electrophoresis (PAGE) in 10% Tris-glycine gels (NOVEX) by using an XCELL II MiniCell (NOVEX) operated at 120 V and 4°C for 2 h under nondenaturing conditions (32). After completion of electrophoresis, the gel was immediately rinsed for 10 min in 50 mM sodium phosphate buffer (pH 7.5) and then transferred into an assay solution containing 50 μg of the cyclohexylammonium salt of 5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid (Sigma) per ml in 50 mM sodium phosphate buffer (pH 7.5). The assay was performed at 37°C until the blue color of the GUS zone appeared. The reaction was stopped by rinsing the gel in water. The stained band was excised from the gel, reelectrophoresed under denaturing conditions, and stained with Coomassie blue (32). After Western blotting onto an Immobilon membrane (Pharmacia), the protein was N terminally sequenced as described by Lindner et al. (34).
Nucleotide sequence accession number.
The complete sequence of the xylP promoter has been deposited in the GenBank database under accession no. M98458.
RESULTS
Sequence analysis of the P. chrysogenum xylP promoter.
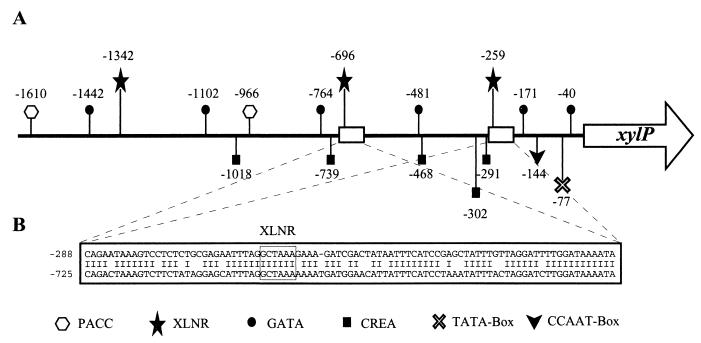
Initially, only 510 bp of the 5′ noncoding region of xylP was analyzed (18). In order to characterize the complete 1,676-bp promoter fragment employed for overexpression of nreB (21), the region between an SalI site and the xylP translation start codon was sequenced in its entirety and was analyzed to determine the presence of putative transcription factor binding sites. The xylP promoter region displays characteristic features of fungal promoters, including a putative 5′-TATAA box (2), a putative HAP complex-binding 5′-CCAAT box (4), three 5′-GGCTAAA consensus binding sites for the transcriptional activator XLNR necessary for expression of the xylanolytic system of Aspergillus niger (52), five 5′-SYGGRG sites that possibly mediate carbon catabolite repression by a CREA-homologue (9), two 5′-GCCARG consensus target sequences for the wide-domain pH-regulatory PACC transcription factor (49), and six 5′-HGATAR motifs (Fig. 2A). The last sequences are possible targets for GATA factors which have been shown to be involved in regulatory circuits as different as nitrogen metabolism, iron metabolism, sexual development, blue light signal transduction, and circadian rhythmicity (47). Several GATA factor-encoding genes have already been identified in P. chrysogenum (19, 21, 22).
FIG. 2.
Analysis of the xylP promoter region. (A) Schematic representation of putative binding site positions (in base pairs) for fungal transcription factors. (B) Duplicated region containing a putative binding site for an XLNR homologue.
Remarkably, a 91-bp sequence starting at position −288 seems to be duplicated at position −725 with a level of identity of 80% (Fig. 2B). The presence of a perfectly conserved XLNR consensus sequence in this region reveals a possible means of evolution to enhance regulation of promoters by duplication of transcription factor binding sites.
Induction characteristics of the xylP promoter in P. chrysogenum and A. nidulans.
In order to avoid interference of different xylanase activities in enzymatic assays, xylP expression was studied by using the GUS-encoding uidA gene of E. coli as a reporter. GUS was chosen because P. chrysogenum has a very low endogenous background level of this enzyme activity. The uidA coding region, trailed by the A. nidulans trpC transcription termination region, was fused with the xylP promoter exactly at the start codon (Fig. 1). The presence of a truncated niaD gene on plasmid pXyluidA-P allowed targeted integration of a single copy of this reporter construct at the nitrate reductase-encoding niaD locus of the P. chrysogenum niaD mutant M20 (11).
To evaluate the functionality of the Penicillium xylP promoter in A. nidulans, exactly the same reporter construct was targeted as a single copy to the argB locus of A. nidulans argB2 mutant WG355 by using plasmid pXyluidA-A, which carries a defective argB copy (42) instead of the truncated niaD copy in pXyluidA-P (Fig. 1). P. chrysogenum and A. nidulans were transformed with the reporter constructs as described in Materials and Methods, and transformants carrying a single copy of the vector at the appropriate site were identified by Southern blot analysis. The resulting P. chrysogenum strain (strain QXU-P1) and A. nidulans WXU-A1 were chosen for further analysis.
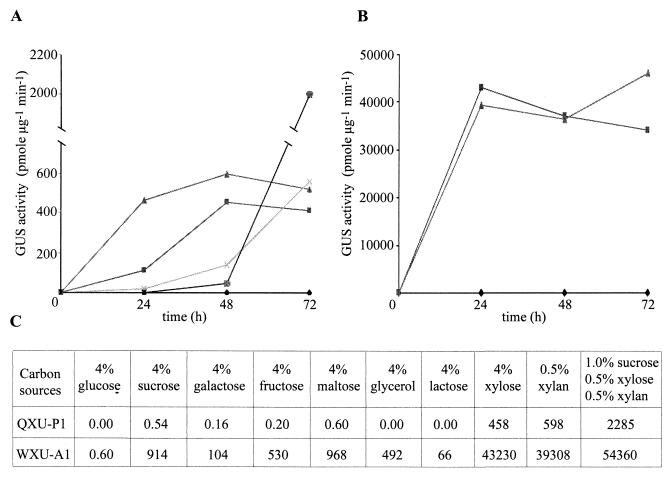
It has already been shown that xylP is not expressed during growth on glucose but is highly induced by xylan as a sole carbon source (18). To investigate induction of the xylP promoter in P. chrysogenum in more detail, QXU-P1 was grown in shake flasks on various carbon sources and GUS activity was measured after different time periods (Fig. 3A). After 24 h of growth on xylan expression of uidA was about five times higher than expression on xylose, whereas after 48 and 72 h of growth the levels of expression differed by only 25%. The significantly lower inducibility of the xylP promoter by xylose than by xylan at 24 h (Fig. 3A) was also observed in a Northern analysis of uidA expression (Fig. 4). Interestingly, the endogenous xylP gene showed even lower induction by xylose at this time point. On glucose no GUS activity was detected (Fig. 3A). On glucose-xylose and glucose-xylan the levels of uidA expression increased most significantly after 72 h of growth, when the glucose had been consumed. Remarkably, in glucose-xylan cultures GUS activity at 72 h was threefold greater than GUS activity on xylan or xylose alone.
FIG. 3.
Effects of carbon sources on reporter gene expression. P. chrysogenum QXU-P1 and A. nidulans WXU-A1 were grown in 500 ml of minimal medium containing 4% glucose as the sole carbon source. Subsequently, mycelia were harvested by filtration and washed with a 0.9% NaCl solution. A 3-g mycelial pad was transferred into 200 ml of minimal medium containing one of the carbon sources studied. The effect of glucose, xylose, and xylan on uidA expression was determined at various times for QXU-P1 (A) and WXU-A1 (B). Symbols: ⧫, 4% glucose; ■, 4% xylose; ▴, 0.5% xylan; ×, 4% glucose and 4% xylose; ●, 4% glucose and 0.5% xylan. In addition, the effects of various carbohydrates on GUS expression were measured 48 h after mycelia were transferred into media (C). The nitrogen source was 30 mM ammonium. GUS activities are given in picomoles of 4-methylumbelliferone per minute per microgram of total soluble cellular protein, and the values represent the means based on three independent experiments; the standard deviations did not exceed 10%.
FIG. 4.
Northern analysis of xylP and uidA expression in P. chrysogenum QXU-P1. Portions (15 μg) of total RNA, isolated 24 h after transfer of mycelia into 4% glucose (lane 1), 4% xylose (lane 2), or 0.5% xylan (lane 3), were subjected to Northern analysis. The blot was probed with digoxigenin-labeled fragments of uidA, xylP, and, as a control for loading and RNA quality, actP (GenBank accession no. U61733), the γ-actin-encoding gene of P. chrysogenum.
The expression profile of the xylP-driven uidA gene in A. nidulans is similar to that in P. chrysogenum: strong induction by xylose or xylan and repression by glucose. One major difference is the level of expression. In A. nidulans the xylP promoter is about 80 times more efficient than it is in P. chrysogenum under the same conditions (Fig. 3).
To test additional carbon sources for their xylP-inducing capacity, QXU-P1 and WXU-A1 were grown on different carbohydrates for 48 h (Fig. 3C). With both Penicillium and Aspergillus strains strongest expression of uidA was found during growth on a combination of different carbon sources (sucrose, xylose, and xylan). The highest levels of induction other than the levels obtained with xylose and xylan were observed in cultures containing sucrose and maltose, in which the level of GUS activity reached about 0.1% of the xylan induction level in P. chrysogenum and up to 2% of the xylan induction level in A. nidulans.
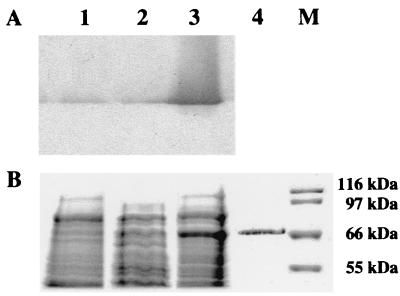
In order to analyze uidA expression in A. nidulans at the protein level, 100-μg portions of soluble protein from mycelial extracts were subjected to SDS-PAGE. In Coomassie blue-stained gels containing crude protein extracts, a 67-kDa protein appeared in a sucrose-xylose-xylan-induced culture of strain WXU-A1; this protein was not present in induced mycelia of control strain WG355 and repressed strain WXU-A1 (Fig. 5). To prove that this protein was E. coli GUS, the same extracts were subjected to native PAGE and GUS was detected by an in-gel activity assay as described in Materials and Methods. Subsequently, the stained band was excised from the native gel and reelectrophoresed by performing SDS-PAGE (Fig. 5, lane 4). The migration pattern, as well as N-terminal sequence determination after Western blotting, which yielded the sequence Val-Arg-Pro-Val-Glu, confirmed the identity as E. coli GUS. The amount of GUS was estimated to be 0.2 μg, suggesting that complete induction of this expression system results in production of approximately 0.2% of the total soluble cellular protein.
FIG. 5.
PAGE analysis of GUS expression. A. nidulans WXU-A1 (lane 3), containing the xylP promoter-controlled uidA expression cassette, and control strain WG355 (lane 1) were grown in minimal medium containing 1% sucrose, 0.5% xylose, and 0.5% xylan as the carbon sources for 48 h. Additionally, WXU-A1 was grown with glucose as the carbon source for 48 h (lane 2). Portions (100 μg) of soluble cellular proteins from mycelial extracts were subjected to in-gel GUS activity staining subsequent to native PAGE (A). The same crude extracts plus the excised zymogram stained GUS band of WXU-A1 (lane 4) were also subjected to SDS-PAGE and stained with Coomassie blue (B). Lane M contained a molecular mass marker.
Comparison of xylP promoter efficiency with the efficiencies of other promoters of P. chrysogenum and A. nidulans.
To relate the efficiency of the xylP promoter to the efficiencies of other promoters used in P. chrysogenum, the pcbC promoter-controlled uidA gene was inserted at the niaD locus in P. chrysogenum M20 by using the pUN-pcbC vector described by Feng et al. (11). The pcbC gene encodes the isopenicillin-N-synthetase, which is necessary for the second step in penicillin biosynthesis. GUS activity in transformant QPU-P6 was measured after 48 h of growth on xylan and ammonium as the carbon and nitrogen sources, respectively. Comparison of the uidA expression levels demonstrated that the xylan-induced xylP promoter is about 65 times more efficient than the pcbC promoter under these conditions.
To compare the efficiency of the xylP promoter with that of an A. nidulans promoter, construct pTRAN3-1A (43) was targeted to the argB locus in A. nidulans WG355, yielding strain WNU-A3. pTRAN3-1A carries the uidA reporter gene under control of the A. nidulans niaD promoter and normally drives expression of nitrate reductase in a strictly nitrate-induced and nitrogen metabolite-repressed manner (8, 43). Expression analysis indicated that the Penicillium xylP promoter yields about 23 times more GUS activity than the niaD promoter under fully induced conditions (with nitrate as the sole nitrogen source).
Use of the xylP promoter for nre antisense RNA expression.
As an application of an expression system in P. chrysogenum, the xylP promoter was used for controllable nre antisense RNA synthesis. The nre gene encodes an AREA-NIT2 homologue and therefore the putative major nitrogen regulatory factor of P. chrysogenum (8, 19, 37). Its function was indicated by heterologous complementation of a Neurospora crassa nit-2 mutant, but direct proof of this function in P. chrysogenum is still not available (19). Several attempts to disrupt nre in Penicillium by targeted integration failed (Haas, unpublished data), probably due to a very low degree of homologous recombination in this fungus (6). In an alternative approach the vector pXylern was constructed (Fig. 1). In this plasmid the central part of nre encoding amino acids 138 to 408 was cloned in antisense orientation downstream of the xylP promoter. Using the same strategy that was used for the other vectors described in this study, we integrated this construct as a single copy at the niaD locus of P. chrysogenum M20.
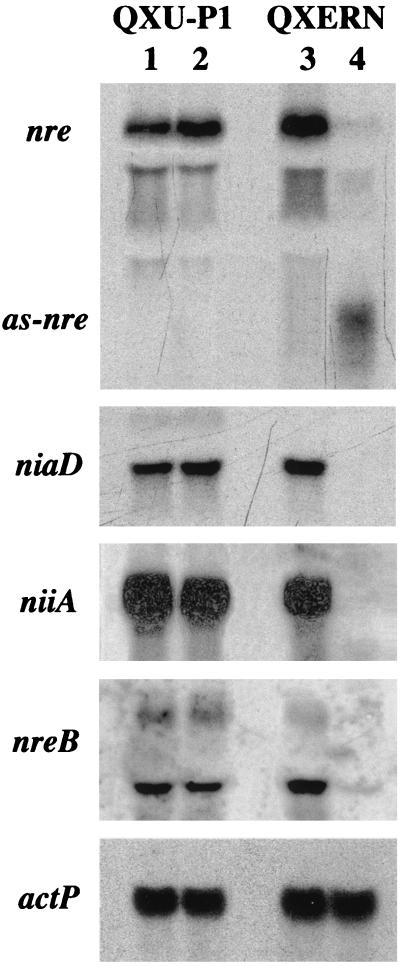
The GATA factors AREA and NIT2 are necessary for utilization of nitrogen sources other than glutamine or ammonia in A. nidulans and N. crassa, respectively. Consequently, downregulation of NRE should result in reduced growth on such compounds (8, 37). Unexpectedly, five transformants tested to determine their growth rates on the secondary nitrogen sources nitrate, proline, and hypoxanthine exhibited no differences compared to Penicillium control strain QXU-P1 in liquid media or on solid media. Furthermore, nre antisense RNA expression did not lead to a difference in resistance to chlorate (8, 37), which is toxic to nitrate reductase-carrying strains (data not shown). One of the nre antisense RNA-expressing transformants, QXERN1, was further analyzed for expression of niaD and niiA (Fig. 6). These two genes encode the nitrate assimilatory enzymes nitrate reductase and nitrite reductase, respectively, and are known to be subject to nitrogen metabolite repression in this fungus (20). Northern analysis revealed that induction of nre antisense RNA synthesis by growth on sucrose-xylose-xylan for 48 h led to a dramatic decrease in the nre steady-state mRNA level and subsequently to transcriptional repression of niaD and niiA in QXERN1 (Fig. 6). In addition, transcription of nreB was found to be downregulated by nre antisense expression, nreB encodes a second GATA factor that has been suggested to be negatively involved in the nitrogen regulatory circuit of Penicillium (21). The specificity of the antisense RNA-mediated repression of nitrogen-regulated genes is indicated by two lines of evidence: (i) the level of expression of niaD, niiA, and nreB in QERN1 during growth on glucose, which represses nre antisense synthesis, did not differ from the level of expression in control strain QXU-P1; and (ii) the actin-encoding actP gene was found to be constitutively expressed under all growth conditions.
FIG. 6.
Northern analysis of nre antisense RNA expression effects. P. chrysogenum QXERN, containing the nre antisense RNA expression cassette, and control strain QXU-P1 were grown for 48 h in minimal medium containing 30 mM nitrate as the sole nitrogen source and 4% glucose (lanes 1 and 3) or 1% sucrose, 0.5% xylose, and 0.5% xylan (lanes 2 and 4) as carbon sources. Subsequently, 15 μg of isolated total RNA was subjected to Northern analysis. The blot was probed with digoxigenin-labeled fragments of nre, niaD, niiA, and actP. as-nre, nre antisense RNA.
Two further experiments proved that expression of the nitrate assimilation gene cluster is not complete, which explains the growth that occurs on nitrate and probably the other secondary nitrogen sources; overexposure of the niaD blot shown in Fig. 6 revealed the presence of a low level of the niaD transcript, and Northern analysis performed 24 h after nre antisense induction revealed only about 50% reduced niaD expression, indicating that the nre antisense effect increases with time (data not shown).
The data described above are the first direct proof that NRE functions as an activator in nitrogen metabolism by P. chrysogenum. NRE-dependent expression of nreB indicates that cross regulation of the GATA factors is involved in this regulatory circuit.
DISCUSSION
Xylanases play an important role in the natural degradation of hemicellulosic material, as well as in biotechnological processes like paper pulp bleaching or bioconversion of biomass into fuels and chemicals (31). The P. chrysogenum xylP gene was one of the first group F xylanase-encoding genes cloned in filamentous fungi. Homologues have been identified in various filamentous fungi, including Aspergillus kawachii, Aspergillus ryzae, A. nidulans, Penicillium simplicissimum, Thermoascus aurantiacus, Fusarium oxysporum, Magnaporthe grisea, Trichoderma reesei, and Claviceps purpurea (12, 18, 25, 28, 35, 45, 48, 53, 54).
In this study we demonstrated the suitability of the strongly regulatable xylP promoter for use as an expression system in P. chrysogenum and A. nidulans. The expression system was found to be regulated similarly in the two fungi: strong inducibility by xylose or xylan and tight repression by glucose. The presence of five SYGGRG motifs in the promoter indicates that xylP expression is subject to CREA–CRE1-mediated carbon catabolite repression, as demonstrated for xylanases from A. nidulans and T. reesei (9, 38, 56). High-level induction by xylose, which is a more defined and cheaper compound than xylan, does not apply to all xylanases but has been demonstrated for EXLA and XYN1 from Aspergillus awamori and T. reesei, respectively (13, 56). The presence of XLNR consensus binding sites in the promoter suggests that there is an induction process similar to that described for A. niger (52). Furthermore, the CCAAT box indicates that HAP complex-mediated regulation occurs, as described for other xylanases (4, 56). In A. nidulans two xylanases show an opposite pattern of expression with respect to ambient pH which is PACC mediated (36). Despite two PACC consensus sites in the regulatory region, it is unlikely that xylP is pH regulated because uidA expression was found to be similar during growth on ammonia (leading to an acidic ambient pH) or nitrate (leading to an alkaline ambient pH) as the nitrogen source and xylose as the carbon source (data not shown).
The efficiency of the xylP promoter was compared with the efficiencies of the pcbC promoter in P. chrysogenum and the niaD promoter in A. nidulans by using the same reporter gene and the same chromosomal integration locus. The xylP promoter yielded about 65-fold more GUS activity than the Penicillium pcbC promoter, which normally controls expression of the isopenicillin-N-synthetase. Kolar et al. (30) have demonstrated that the Penicillium pcbC promoter is rather weak compared to the A. nidulans gpdA promoter during growth in minimal medium, but this study was performed with A. nidulans. In A. nidulans the xylP promoter is about 23 times stronger than the niaD promoter under induced conditions. Although making a direct comparison of promoter strengths is difficult since the resulting mRNAs possess different 5′ and 3′ untranslated regions, which might result in different mRNA stabilities or different translational efficiencies, it is obvious that the xylP promoter is highly inducible and very efficient.
During the last decade various antisense technologies have become important as instruments for the development of agricultural products, as therapeutic tools in human medicine, and for investigation of gene function (23). In fungi the antisense RNA approach is rather rarely exploited, probably due to the relatively efficient gene disruption techniques available. Nevertheless, there are two obvious applications. (i) Using antisense technology, expression of a given gene usually cannot be completely turned off but can only be reduced. However, this obvious disadvantage might be an advantage for certain applications; if the gene studied is essential, an antisense approach still allows functional characterization of the gene product, especially if the antisense expression is controllable (55). (ii) If the organism studied has weak homologous recombination, which seems to be the case in P. chrysogenum (6), antisense technology is a faster and more efficient approach to investigate a gene's function.
The efficiency of the antisense approach depends on the strength of antisense expression. As an interesting application requiring high-level expression, the xylP promoter was therefore employed for controllable nre antisense RNA synthesis. The nre gene encodes a homologue of the Aspergillus GATA factor AREA and hence the putative positive-acting nitrogen regulator of Penicillium, but final data to prove this have not been obtained since no nre mutant is available (8, 19). nre antisense expression led to an inducible decrease in nre mRNA and consequently to repression of nitrate assimilatory gene cluster transcription. Remarkably, no decrease in the growth rate was seen with this strain when it was grown on nitrate or other secondary nitrogen sources under nre antisense-inducing conditions. A likely explanation for this is that this antisense approach does not lead to a complete block of gene expression—as seen for niaD and most antisense applications—and that the remaining expression is still sufficient to maintain about the same growth rate. The data also indicate that in P. chrysogenum the level of transcription of the nitrate catabolic genes is not the limiting factor for growth under the conditions tested. In this respect it is noteworthy that expression of the essential protein phosphatase 2A can be significantly decreased by an RNA antisense strategy in N. crassa; nevertheless, hyphal growth and conidiation can still be maintained (55).
In addition, transcription of nreB, which encodes a second nitrogen regulatory GATA factor of Penicillium, was found to be downregulated by nre antisense expression. Recently, we have shown that NREB acts negatively in the nitrogen regulatory circuit and that its expression is subject to nitrogen regulation (21). NRE-dependent nreB expression indicates that cross regulation of the two GATA factors is involved in nitrogen regulation in Penicillium. This resembles the situation in Saccharomyces cerevisiae. In this yeast expression of the four GATA factors controlling nitrogen catabolic gene expression is interdependent; two factors act negatively, and two factors act in a positive manner (7).
The obvious advantage of an antisense RNA strategy is the possibility of being able to study a gene's function under conditions that are not possible when a classical gene disruption approach is used; e.g., by analogy to Aspergillus and Neurospora (8, 37), a nre loss-of-function mutant cannot grow on nitrate as a sole nitrogen source, and therefore, the response of target genes cannot be investigated under these growth conditions. The antisense strategy provided the first direct proof that NRE is an activator in the nitrogen regulatory circuit of Penicillium.
In conclusion, this study shows that the xylP-based expression system is a valuable tool in P. chrysogenum and probably in a variety of other filamentous fungi since its functionality could also be demonstrated in A. nidulans. Furthermore, RNA antisense expression proved to be a powerful tool for conducting gene regulation studies in Penicillium.
ACKNOWLEDGMENTS
This project was funded by the Austrian Science Foundation (grant FWF-P11164-MOB to H.H.).
We are grateful to Peter Punt and Bo Feng for providing plasmids pTRAN3-1A, pNOM102, and pUN-pcbC. We thank Herbert Lindner for N-terminal amino acid sequence determinations.
REFERENCES
- 1.Bainbridge B W, Spreadbury C L, Scalise F G, Cohen J. Improved methods for the preparation of high molecular weight DNA from large and small scale cultures of filamentous fungi. FEMS Microbiol Lett. 1990;66:113–118. doi: 10.1016/0378-1097(90)90267-t. [DOI] [PubMed] [Google Scholar]
- 2.Ballance D J. Sequences important for gene expression in filamentous fungi. Yeast. 1986;2:229–236. doi: 10.1002/yea.320020404. [DOI] [PubMed] [Google Scholar]
- 3.Brakhage A A. Molecular regulation of beta-lactam biosynthesis in filamentous fungi. Microbiol Mol Biol Rev. 1998;62:547–585. doi: 10.1128/mmbr.62.3.547-585.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brakhage A A, Andrianopoulos A, Kato M, Steidl S, Davis M A, Tsukagoshi N, Hynes M J. HAP-Like CCAAT-binding complexes in filamentous fungi: implications for biotechnology. Fungal Genet Biol. 1999;27:243–252. doi: 10.1006/fgbi.1999.1136. [DOI] [PubMed] [Google Scholar]
- 5.Cantoral I M, Diez B, Barredo J L, Alvarez E, Martin J F. High frequency transformation of Penicillium chrysogenum. J Biotechnol. 1987;5:494–497. [Google Scholar]
- 6.Casqueiro J, Gutierrez S, Banuelos O, Hijarrubia M J, Martin J F. Gene targeting in Penicillium chrysogenum: disruption of the lys2 gene leads to penicillin overproduction. J Bacteriol. 1999;181:1181–1188. doi: 10.1128/jb.181.4.1181-1188.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coffman J A, Rai R, Loprete D M, Cunningham T, Svetlov V, Cooper T G. Cross regulation of four GATA factors that control nitrogen catabolic gene expression in Saccharomyces cerevisiae. J Bacteriol. 1997;179:3416–3429. doi: 10.1128/jb.179.11.3416-3429.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crawford N M, Arst H N., Jr The molecular genetics of nitrate assimilation in fungi and plants. Annu Rev Genet. 1993;27:115–146. doi: 10.1146/annurev.ge.27.120193.000555. [DOI] [PubMed] [Google Scholar]
- 9.Cubero B, Scazzocchio C. Two different, adjacent and divergent zinc finger binding sites are necessary for CREA-mediated carbon catabolite repression in the proline gene cluster of Aspergillus nidulans. EMBO J. 1994;13:407–415. doi: 10.1002/j.1460-2075.1994.tb06275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diez B, Mellado E, Rodriguez M, Bernasconi E, Barredo J L. The NADP-dependent glutamate dehydrogenase gene from Penicillium chrysogenum and the construction of expression vectors for filamentous fungi. Appl Microbiol Biotechnol. 1999;52:196–207. doi: 10.1007/s002530051509. [DOI] [PubMed] [Google Scholar]
- 11.Feng B, Friedlin E, Marzluf G. A reporter gene analysis of penicillin biosynthesis gene expression in Penicillium chrysogenum and its regulation by nitrogen and glucose catabolite repression. Appl Environ Microbiol. 1994;60:4432–4439. doi: 10.1128/aem.60.12.4432-4439.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giesbert S, Lepping H B, Tenberge K B, Tudzynski P. The xylanolytic system of Claviceps purpurea: cytological evidence for secretion of xylanases in infected rye tissue and molecular characterization of two xylanase genes. Phytopathology. 1998;88:1020–1030. doi: 10.1094/PHYTO.1998.88.10.1020. [DOI] [PubMed] [Google Scholar]
- 13.Gouka R J, Hessing J G, Punt P J, Stam H, Musters W, Van den Hondel C A. An expression system based on the promoter region of the Aspergillus awamori 1,4-beta-endoxylanase A gene. Appl Microbiol Biotechnol. 1996;46:28–35. doi: 10.1007/s002530050779. [DOI] [PubMed] [Google Scholar]
- 14.Gouka R J, Punt P J, Van den Hondel C A. Efficient production of secreted proteins by Aspergillus: progress, limitations and prospects. Appl Microbiol Biotechnol. 1997;47:1–11. doi: 10.1007/s002530050880. [DOI] [PubMed] [Google Scholar]
- 15.Graessle S, Haas H, Friedlin E, Kurnsteiner H, Stöffler G, Redl B. Regulated system for heterologous gene expression in Penicillium chrysogenum. Appl Environ Microbiol. 1997;63:753–756. doi: 10.1128/aem.63.2.753-756.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gutierrez S, Fierro F, Casqueiro J, Martin J F. Gene organization and plasticity of the beta-lactam genes in different filamentous fungi. Antonie Leeuwenhoek. 1999;75:81–94. doi: 10.1023/a:1001861025070. [DOI] [PubMed] [Google Scholar]
- 17.Haas H, Herfurth E, Stöffler G, Redl B. Purification, characterization and partial amino acid sequences of a xylanase produced by Peniillium chrysogenum. Biochim Biophys Acta. 1992;1117:279–286. doi: 10.1016/0304-4165(92)90025-p. [DOI] [PubMed] [Google Scholar]
- 18.Haas H, Friedlin E, Stöffler G, Redl B. Cloning and structural organization of a xylanase-encoding gene from Penicillium chrysogenum. Gene. 1993;126:237–242. doi: 10.1016/0378-1119(93)90372-a. [DOI] [PubMed] [Google Scholar]
- 19.Haas H, Bauer B, Redl B, Stöffler G, Marzluf G A. Molecular cloning and analysis of nre, the major nitrogen regulatory gene of Penicillium chrysogenum. Curr Genet. 1995;27:150–158. doi: 10.1007/BF00313429. [DOI] [PubMed] [Google Scholar]
- 20.Haas H, Marx F, Graessle S, Stöffler G. Sequence analysis and expression of the Penicillium chrysogenum nitrate reductase encoding gene (niaD) Biochim Biophys Acta. 1996;1309:81–84. doi: 10.1016/s0167-4781(96)00150-9. [DOI] [PubMed] [Google Scholar]
- 21.Haas H, Angermayr K, Zadra I, Stöffler G. Overexpression of nreB, a new GATA factor-encoding gene of Penicillium chrysogenum, leads to repression of the nitrate assimilatory gene cluster. J Biol Chem. 1997;272:22576–22582. doi: 10.1074/jbc.272.36.22576. [DOI] [PubMed] [Google Scholar]
- 22.Haas H, Angermayr K, Stöffler G. Molecular analysis of a Penicillium chrysogenum GATA factor encoding gene (sreP) exhibiting significant homology to the Ustilago maydis urbs1 gene. Gene. 1997;184:33–37. doi: 10.1016/s0378-1119(96)00570-7. [DOI] [PubMed] [Google Scholar]
- 23.Hawkins J W, Nellen W. Diversification of antisense research and development: review of the Ringberg meeting. April 1994. Mechanisms of antisense-mediated gene silencing. Antisense Res Dev. 1994;4:127–129. doi: 10.1089/ard.1994.4.127. [DOI] [PubMed] [Google Scholar]
- 24.Hoskins I C, Roberts C F. Expression of the 3-phosphoglycerate kinase gene (pgkA) of Penicillium chrysogenum. Mol Gen Genet. 1994;243:270–276. doi: 10.1007/BF00301062. [DOI] [PubMed] [Google Scholar]
- 25.Ito K, Ikemasu T, Ishikawa T. Cloning and sequencing of the xynA gene encoding xylanase A of Aspergillus kawachii. Biosci Biotechnol Biochem. 1992;56:906–912. doi: 10.1271/bbb.56.906. [DOI] [PubMed] [Google Scholar]
- 26.Jefferson R A. Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep. 1987;5:387–405. [Google Scholar]
- 27.Kafer E. Meiotic and mitotic recombination in Aspergillus and its chromosomal aberrations. Adv Genet. 1977;19:33–131. doi: 10.1016/s0065-2660(08)60245-x. [DOI] [PubMed] [Google Scholar]
- 28.Kitamoto N, Yoshino S, Ito M, Kimura T, Ohmiya K, Tsukagoshi N. Repression of the expression of genes encoding xylanolytic enzymes in Aspergillus oryzae by introduction of multiple copies of the xynF1 promoter. Appl Microbiol Biotechnol. 1999;50:558–563. doi: 10.1007/s002530051334. [DOI] [PubMed] [Google Scholar]
- 29.Kolar M, Punt P J, van den Hondel C A, Schwab H. Transformation of Penicillium chrysogenum using dominant selection markers and expression of an Escherichia coli lacZ fusion gene. Gene. 1988;62:127–134. doi: 10.1016/0378-1119(88)90586-0. [DOI] [PubMed] [Google Scholar]
- 30.Kolar M, Holzmann K, Weber G, Leitner E, Schwab H. Molecular characterization and functional analysis in Aspergillus nidulans of the 5′-region of the Penicillium chrysogenum isopenicillin N synthetase gene. J Biotechnol. 1991;17:67–80. doi: 10.1016/0168-1656(91)90027-s. [DOI] [PubMed] [Google Scholar]
- 31.Kulkarni N, Shendye A, Rao M. Molecular and biotechnological aspects of xylanases. FEMS Microbiol Rev. 1999;23:411–456. doi: 10.1111/j.1574-6976.1999.tb00407.x. [DOI] [PubMed] [Google Scholar]
- 32.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 33.Liang S H, Wu T S, Lee R, Chu F S, Linz J E. Analysis of mechanisms regulating expression of the ver-1 gene, involved in aflatoxin biosynthesis. Appl Environ Microbiol. 1997;63:1058–1065. doi: 10.1128/aem.63.3.1058-1065.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindner H, Sarg B, Hoertnagl B, Helliger W. The microheterogeneity of the mammalian H1(0) histone. Evidence for an age-dependent deamidation. J Biol Chem. 1998;273:13324–13330. doi: 10.1074/jbc.273.21.13324. [DOI] [PubMed] [Google Scholar]
- 35.MacCabe A P, Fernandez-Espinar M T, de Graaff L H, Visser J, Ramon D. Identification, isolation and sequence of the Aspergillus nidulans xlnC gene encoding the 34-kDa xylanase. Gene. 1996;175:29–33. doi: 10.1016/0378-1119(96)00116-3. [DOI] [PubMed] [Google Scholar]
- 36.MacCabe A P, Orejas M, Perez-Gonzalez J A, Ramon D. Opposite patterns of expression of two Aspergillus nidulans xylanase genes with respect to ambient pH. J Bacteriol. 1998;180:1331–1333. doi: 10.1128/jb.180.5.1331-1333.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marzluf G A. Genetic regulation of nitrogen metabolism in the fungi. Microbiol Mol Biol Rev. 1997;61:17–32. doi: 10.1128/mmbr.61.1.17-32.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orejas M, MacCabe A P, Perez Gonzalez J A, Kumar S, Ramon D. Carbon catabolite repression of the Aspergillus nidulans xlnA gene. Mol Microbiol. 1999;31:177–184. doi: 10.1046/j.1365-2958.1999.01157.x. [DOI] [PubMed] [Google Scholar]
- 39.Ostergaard S, Olsson L, Nielsen J. Metabolic engineering of Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 2000;64:34–50. doi: 10.1128/mmbr.64.1.34-50.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Penalva M A, Rowlands R T, Turner G. The optimization of penicillin biosynthesis in fungi. Trends Biotechnol. 1998;16:483–489. doi: 10.1016/s0167-7799(98)01229-3. [DOI] [PubMed] [Google Scholar]
- 41.Pontecorvo G, Roper J A, Hemmons L M, MacDonald K D, Bufton A W J. The genetics of Aspergillus nidulans. Adv Genet. 1953;5:141–238. doi: 10.1016/s0065-2660(08)60408-3. [DOI] [PubMed] [Google Scholar]
- 42.Punt P J, Dingemanse M A, Kuyvenhoven A, Soede R D, Pouwels P H, van den Hondel C A. Functional elements in the promoter region of the Aspergillus nidulans gpdA gene encoding glyceraldehyde-3-phosphate dehydrogenase. Gene. 1990;93:101–109. doi: 10.1016/0378-1119(90)90142-e. [DOI] [PubMed] [Google Scholar]
- 43.Punt P J, Greaves P A, Kuyvenhoven A, van Deutekom J C T, Kinghorn J R, Pouwels P H, Van den Hondel C A. A twin-reporter vector for simultaneous analysis of expression signals of divergently transcribed, contiguous genes in filamentous fungi. Gene. 1991;104:119–122. doi: 10.1016/0378-1119(91)90476-r. [DOI] [PubMed] [Google Scholar]
- 44.Roberts I N, Oliver R P, Punt P J, Van den Hondel C A. Expression of the Escherichia coli β-glucuronidase gene in industrial and phytopathogenic filamentous fungi. Curr Genet. 1989;15:177–180. doi: 10.1007/BF00435503. [DOI] [PubMed] [Google Scholar]
- 45.Ruiz-Roldan M C, Di Pietro A, Huertas-Gonzalez M D, Roncero M I. Two xylanase genes of the vascular wilt pathogen Fusarium oxysporum are differentially expressed during infection of tomato plants. Mol Gen Genet. 1999;261:530–536. doi: 10.1007/s004380050997. [DOI] [PubMed] [Google Scholar]
- 46.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 47.Scazzocchio C. The fungal GATA factors. Curr Opin Microbiol. 2000;3:126–131. doi: 10.1016/s1369-5274(00)00063-1. [DOI] [PubMed] [Google Scholar]
- 48.Schmidt A, Schlacher A, Steiner W, Schwab H, Kratky C. Structure of the xylanase from Penicillium simplicissimum. Protein Sci. 1998;7:2081–2088. doi: 10.1002/pro.5560071004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suarez T, Penalva M A. Characterization of a Penicillium chrysogenum gene encoding a PacC transcription factor and its binding sites in the divergent pcbAB-pcbC promoter of the penicillin biosynthetic cluster. Mol Microbiol. 1996;20:529–540. doi: 10.1046/j.1365-2958.1996.5421065.x. [DOI] [PubMed] [Google Scholar]
- 50.Tilburn J, Sarkar S, Widdick D A, Espeso E A, Orejas M, Mungroo J, Penalva M A, Arst H N., Jr The Aspergillus PACC zinc finger transcription factor mediates regulation of both acid- and alkaline-expressed genes by ambient pH. EMBO J. 1995;14:779–790. doi: 10.1002/j.1460-2075.1995.tb07056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Gorcom R F, Punt P J, Pouwels P H, Van den Hondel A system for the analysis of expression signals in Aspergillus. Gene. 1986;48:211–217. doi: 10.1016/0378-1119(86)90079-x. [DOI] [PubMed] [Google Scholar]
- 52.Van Peij N N, Visser J, de Graaff L H. Isolation and analysis of xlnR, encoding a transcriptional activator co-ordinating xylanolytic expression in Aspergillus niger. Mol Microbiol. 1998;27:131–142. doi: 10.1046/j.1365-2958.1998.00666.x. [DOI] [PubMed] [Google Scholar]
- 53.Viswamitra M A, Bhanumoorthy P, Ramakumar S, Manjula M V, Vithayathil P J, Murthy S K, Naren A P. Crystallization and preliminary X-ray diffraction analysis of crystals of Thermoascus aurantiacus xylanase. J Mol Biol. 1993;232:987–988. doi: 10.1006/jmbi.1993.1444. [DOI] [PubMed] [Google Scholar]
- 54.Wu S C, Kaufman S, Darvill A G, Albersheim P. Purification, cloning and characterization of two xylanases from Magnaporthe grisea, the rice blast fungus. Mol Plant-Microbe Interact. 1995;8:506–514. doi: 10.1094/mpmi-8-0506. [DOI] [PubMed] [Google Scholar]
- 55.Yatzkan E, Szoor B, Feher Z, Dombradi V, Yarden O. Protein phosphatase 2A is involved in hyphal growth of Neurospora crassa. Mol Gen Genet. 1998;259:523–531. doi: 10.1007/s004380050844. [DOI] [PubMed] [Google Scholar]
- 56.Zeilinger S, Mach R L, Schindler M, Herzog P, Kubicek C P. Different inducibility of expression of the two xylanase genes xyn1 and xyn2 in Trichoderma reesei. J Biol Chem. 1996;271:25624–25629. doi: 10.1074/jbc.271.41.25624. [DOI] [PubMed] [Google Scholar]