ABSTRACT
Burkholderia pseudomallei is a Gram-negative soil saprophyte with the potential to cause melioidosis, an opportunistic disease with a high mortality potential. Periodic case reports of melioidosis in or imported from Africa occur in the literature dating back decades. Furthermore, statistical models suggest Western sub-Saharan Africa as a high-risk zone for the presence of B. pseudomallei. A recent case report from the United Kingdom of a returning traveler from Ghana highlights the need for environmental studies in Ghana. We examined 100 soil samples from a rice farm in south-central Ghana. Soil was subjected to selective enrichment culture for B. pseudomallei using threonine-basal salt solution with colistin (TBSS-C50) and erythritol medium, as described in the literature. Bacterial cultures were identified with standard biochemical tests, a rapid antigen detection assay, and real-time PCR specific for B. pseudomallei. Of the 100 soil samples, 55% yielded cultures consistent with B. pseudomallei on Ashdown’s agar as well as by capsular polysaccharide antigen production. This is the first confirmatory report of culture-confirmed B. pseudomallei in the environment of Ghana. Our study emphasizes the need for further exploration of the burden of human melioidosis in Ghana. We recommend that local clinicians familiarize themselves with the diagnosis and clinical management of melioidosis, while laboratories develop capacity for the safe isolation and identification of B. pseudomallei.
IMPORTANCE We present the first confirmation of the presence of B. pseudomallei in the environment of Ghana. This study will bring attention to a disease with the potential to cause significant morbidity and mortality in Ghana, but which has gone completely unrecognized until this point. Furthermore, this work would encourage local clinicians to familiarize themselves with the diagnosis and clinical management of melioidosis and laboratories to develop capacity for the safe isolation and identification of B. pseudomallei.
KEYWORDS: Burkholderia pseudomallei, melioidosis, environment, soil, Ghana, West Africa, environmental microbiology
INTRODUCTION
Burkholderia pseudomallei, the causative agent of melioidosis, is an emerging opportunistic, Gram-negative, soil-dwelling bacterium causing diverse presentations, such as lung, skin, and liver infection, septic arthritis, and osteomyelitis. Bacteremia is seen in most patients, with multifocal infection being common (1). The mortality rate of recognized melioidosis in well-resourced areas ranges from 10% (Australia) to 40% (Thailand), although it can be much higher in low-resource settings and as high as 90% in untreated sepsis (1, 2). Recent in silico modeling predicts the global burden of melioidosis, once thought to be a few thousand cases per year, to infect 165,000 people, causing approximately 89,000 deaths (3). Consistent with this prediction, B. pseudomallei continues to emerge as a significant pathogen even 100 years after its discovery in Rangoon, Burma (Yangon, Myanmar), by the pathologist Alfred Whitmore in 1911 (4).
While our understanding of the presence of B. pseudomallei and burden of disease in Southeast Asia, the Americas, and northern Australia is improving, evidence in Africa is scant (5–8). Recently, a culture-confirmed case of melioidosis was reported from the United Kingdom in a Ghanaian patient who emigrated there for a period of 14 months (9). This, together with predictive environmental modeling, suggests that B. pseudomallei could be present in Ghana, necessitating further investigation.
A set of consensus guidelines have been developed to promote consistent conduct of environmental surveys for B. pseudomallei. These include the systematic sampling of a fixed-interval grid and the use of threonine-basal salt solution with colistin (TBSS-C50) medium for enrichment and the use of Ashdown’s agar for isolation (10, 11). While these methods are robust in the promotion of B. pseudomallei growth, they also allow the growth of near-neighbor species. The preponderance of near neighbors complicates soil culture for B. pseudomallei recovery (12). A recent study identified a significant increase in sensitivity when erythritol was used as the sole carbon source in the enrichment medium, precluding the growth of many near-neighbor species while promoting the growth of B. pseudomallei. Combining these two methods into a two-step enrichment improves the yield of B. pseudomallei isolates from environmental samples (13).
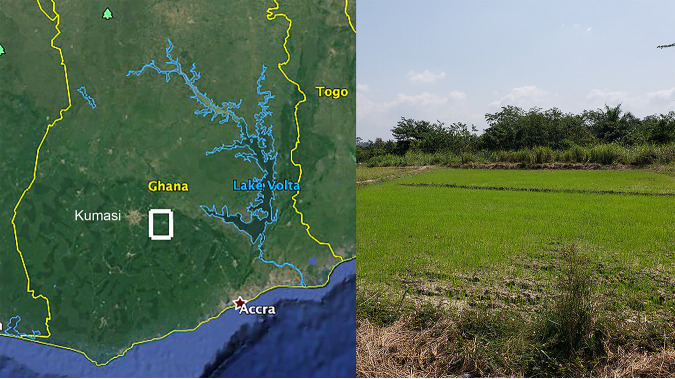
Here, we present preliminary findings of the first environmental sampling for the presence of B. pseudomallei in Ghana. The Konongo-Odumase area in the Anum Valley of the Ashanti Region, located in south-central Ghana, was selected for this initial exploratory study as the environment is suitable for rice farming and consistent with areas of known endemicity for B. pseudomallei (Fig. 1). This area has undergone rice farming development and land use changes in recent years. The region is a semideciduous forest zone comprised of many inland valley wetland bottomlands, with an annual rainfall of approximately 1,400 mm and with two peak rain seasons from April to August and September to November. Previous soil surveys of 60 field plots in this area found soils to be relatively deep, with textures that vary from sandy loam through silt loam and loam and consistent with those from other countries where melioidosis is endemic (14). In the areas we sampled, B. pseudomallei was widespread, although not evenly distributed, demonstrating that Ghana, and potentially all of West Africa, is an extensive area of endemicity for B. pseudomallei.
FIG 1.
Location and environment of sampling site.
RESULTS
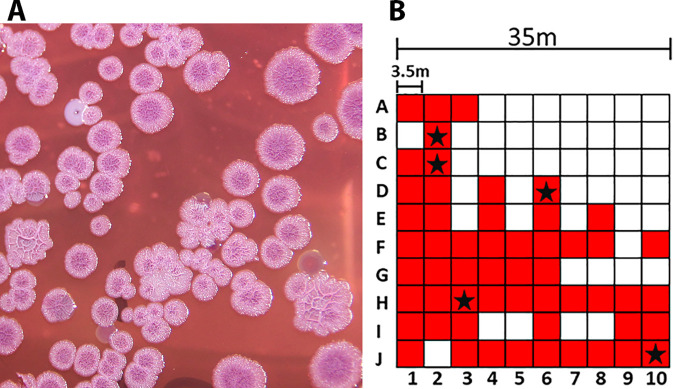
The 100 soil samples were 30 cm deep and yielded an average pH of 4.5 ± 0.49 and temperature of 29.75 ± 5.42°C, while ambient air temperature increased from 31°C to 32°C during the 66-min sampling time. Soil cultures yielded colonies resembling B. pseudomallei (Fig. 2A) from 55/100 sampling points; other colony morphologies, not resembling B. pseudomallei, were also present. The capsular polysaccharide (CPS) antigen was detected in the same 55 enrichment broth supernatants collected throughout the sampling area (Fig. 2B). Five isolates selected for confirmatory identification were verified by API profile with at least 92.6% confidence (Table 1, top), were oxidase positive (not shown), CPS positive (Fig. 3), and resistant to colistin (TBSS-C50) and gentamicin (Ashdown’s agar), but susceptible to amoxicillin-clavulanic acid, ceftazidime, doxycycline, imipenem, meropenem, and trimethoprim-sulfamethoxazole (Table 2, top). Other isolates selected for characterization were identified as Stenotrophomonas maltophilia and Achromobacter xylosoxidans (bottom portions of Tables 1 and 2). The overall pattern of positivity within the 1,225 m2 grid was not evenly distributed (Fig. 2B).
FIG 2.
Sampling results. (A) Presumptive B. pseudomallei colonies on Ashdown’s agar. Growth of enriched culture from sampling point C2 after 96 h of incubation at 35°C. (B) Fixed-interval sampling grid. The sampling points that produced colonies consistent with B. pseudomallei on Ashdown’s agar and were B. pseudomallei capsule positive are indicated by red shading. Those positively identified as B. pseudomallei are indicated with a star.
TABLE 1.
Bacterial identification of B. pseudomallei and other organisms identified in the same environmenta
| Organism | Sampling point | API 20 NE profile | Confidence % | Reaction to: |
||
|---|---|---|---|---|---|---|
| CPS | Ox | PCR | ||||
| B. pseudomallei | B2 | 1 1 5 6 5 7 6 | 99.9 | POS | POS | POS |
| C2 | 1 1 5 6 5 7 6 | 99.9 | POS | POS | POS | |
| D6 | 1 1 5 6 5 7 4 | 92.6 | POS | POS | POS | |
| H3 | 1 1 5 6 5 7 6 | 99.9 | POS | POS | POS | |
| J10 | 1 1 5 6 5 7 4 | 92.6 | POS | POS | POS | |
| Other organisms | ||||||
| S. maltophilia | D10 | 0 4 7 2 3 4 5 | 99.7 | NEG | POS | ND |
| A. xylosxidans | F3 | 1 0 4 0 4 7 7 | 94.5 | NEG | POS | ND |
Shown are results for Burkholderia pseudomallei colonies and Stenotrophomonas maltophilia and Achromobacter xylosoxidans. The API profiles are provided, along with the identification and confidence in that identification provided by APIWEB. The reactions to B. pseudomallei capsule (CPS), oxidase test (Ox), and PCR specific for the B. pseudomallei type III secretion system are also presented (19). POS, positive; NEG, negative; ND, not done.
FIG 3.
Active Melioidosis Detect lateral flow immunoassay (AMD LFI) results from the five colonies positively identified as B. pseudomallei. Suspected colonies were suspended in sterile saline to a McFarland standard of 0.5, and 25 mL of the suspension was added to the AMD LFI followed by chase buffer. The control line is indicated by “C” and the test line by “T.” Samples with a line at both positions are considered positive for B. pseudomallei capsular polysaccharide.
TABLE 2.
Antibiotic susceptibility profiles for B. pseudomallei and other organisms identified in the same environmenta
| Organism | Sampling point | Zone of inhibition (mm) for antimicrobial agent |
|||||
|---|---|---|---|---|---|---|---|
| AMC (20/10 μg) | CAZ (30 μg) | DO (30 μg) | IPM (10 μg) | MEM (10 μg) | SXT (1.25/23.75 μg) | ||
| B. pseudomallei | B2 | 28 | 32 | 30 | 41 | 28 | 30 |
| C2 | 28 | 30 | 28 | 42 | 28 | 30 | |
| D6 | 28 | 31 | 28 | 41 | 30 | 30 | |
| H3 | 28 | 32 | 30 | 40 | 30 | 30 | |
| J10 | 28 | 32 | 30 | 41 | 28 | 30 | |
| Other organisms | |||||||
| S. maltophilia | D10 | 0 | 0 | 0 | 20 | 0 | 35 |
| A. xylosoxidans | F3 | 22 | 25 | 22 | 22 | 35 | 38 |
Shown are results for Burkholderia pseudomallei colonies and Stenotrophomonas maltophilia and Achromobacter xylosoxidans. Each disk and its content (in parentheses) are provided, as are the zones of inhibition for amoxicillin-clavulanic acid (AMC), ceftazidime (CAZ), doxycycline (DO), imipenem (IPM), meropenem (MEM), and trimethoprim-sulfamethoxazole (SXT).
DISCUSSION
This is the first definitive description of the presence of B. pseudomallei in Ghana, supporting the predictive environmental modeling for the region and the recent case report in the United Kingdom of a returning traveler with a rural environmental exposure history in Ghana (8, 9). The presence of B. pseudomallei was positively confirmed through culture, the expression of B. pseudomallei capsular polysaccharide, biochemical testing, and PCR in five points throughout our sampling site. These five isolates also displayed antibiotic sensitivity profiles that were consistent with B. pseudomallei. In total, 55% of the 100 points sampled produced colonies that were consistent with B. pseudomallei (Fig. 2A) and were positive for B. pseudomallei capsule. While near neighbors such as Burkholderia thailandensis produce similar colony morphologies on Ashdown’s agar, and some strains have been shown to produce B. pseudomallei capsular polysaccharide, near-neighbor species lack the ability to utilize erythritol as the sole carbon source and would have been selected against during the enrichment process (13, 15). Nonetheless, the number of sampling points with confirmed B. pseudomallei remains uncertain, and additional characterization of each sampling point would be required to definitively characterize the full distribution of B. pseudomallei across this sampling site.
When combined with the recent publication of a case report of melioidosis in Ghana, our findings support that Ghana is a previously unknown area of endemicity for B. pseudomallei. Even where B. pseudomallei is prevalent, melioidosis is a disease that is not readily detected by routine laboratory testing. Agricultural intensification and expansion in areas where environmental risks remain largely unknown may result in the undetected emergence of diseases in patients that present to health and laboratory services unprepared for differential screening and specific diagnostic testing. A recent study presenting details on 200 Gram-negative clinical isolates from a nearby hospital did not identify any B. pseudomallei isolates, although two isolates of Burkholderia cepacia were found (16). Investments should be made in building the capacity of medical laboratory personnel to improve their ability to identify and diagnose B. pseudomallei, and clinicians should familiarize themselves with the clinical management of melioidosis.
We are presently engaged in a more thorough characterization of numerous sampling sites in this region to include complete genome sequencing and phylogenetic analyses of isolates. Additionally, our study team is engaged in an observational study of sepsis at the nearby Komfo Anokye Teaching Hospital (KATH). Improved laboratory capability and retrospective analyses of patient samples and data will contribute to our understanding of the burden of melioidosis in south-central Ghana.
MATERIALS AND METHODS
Study site.
We identified a rice farming area in the Anum River Valley of the Ashanti Region of Ghana for soil sampling at approximately 6°37′17.2″N and 1°17′15.5″W. The farms consist of approximately 50- by 50-m plots, with individual plots in different stages of the rice growing cycle, including newly planted rice, freshly harvested rice, and previously harvested rice. The rice growing area is bisected by a tributary of the Anum River, and plots (or groups of plots) are irrigated to different degrees and range from dried hardened soil to waterlogged.
Institutional ethics and community outreach.
A study protocol was drafted and registered with the Komfo Anokye Teaching Hospital (KATH) Research and Development Unit as well as the KATH Institutional Review Board (IRB). The village chief was engaged and provided permission to approach community landowners. All landowners provided written informed consent to sample their property.
Environmental sampling.
Samples were collected according to the consensus guidelines for environmental sampling of B. pseudomallei (10). Briefly, each 35-m2 site was divided into 10-by-10 grids of 3.5 m2 each. At the center of each square of the grid, an auger was inserted to a depth of 30 cm, and approximately 10 g of soil was collected at each point. At each corner of the sampling site, soil temperature and pH were recorded at the sampling depth of 30 cm, in addition to ambient temperature at the start and completion of sampling. The sampling was conducted in the month of January, between the two wet seasons in the region.
Soil enrichment and screening.
Soil samples were enriched for B. pseudomallei according to the consensus guidelines, with one additional step as previously described by Trinh et al. (10, 13). Briefly, 10 g of soil was used to inoculate 10 mL of TBSS-C50. The tubes were vortexed to mix thoroughly and incubated at 40°C for 48 h. Following incubation, the tubes were again mixed by vortexing, and the solids were allowed to settle for 1 h at room temperature. One milliliter of the resulting supernatant was then transferred to 9 mL of erythritol enrichment medium and incubated at 40°C for an additional 96 h (13). A 1.2-mL sample of the resulting cultures was then combined with 0.3 mL of 80% sterile glycerol and frozen at −80°C for storage.
Identification and characterization of B. pseudomallei.
Frozen cultures were thawed in biosafety level 3 (BSL3) containment and screened with an antigen capture immunoassay for the B. pseudomallei capsule using the 4C4 capture antibody as previously described (17, 18). The thawed cultures were also used to inoculate Ashdown’s agar plates, which were incubated at 35°C for 96 h. Colonies presenting a morphology consistent with B. pseudomallei (i.e., flat, wrinkled, and purple colonies), as well as other morphologies, were streaked for isolation onto tryptic soy agar (TSA) plates and incubated at 37°C for 24 h. Individual colonies with identical morphology were selected for identification and suspended in 0.85% NaCl to a McFarland standard of ~0.5. The resulting suspension was used to inoculate API 20 NE strips (bioMérieux), and Mueller-Hinton (MH) agar plates for disk diffusion antimicrobial susceptibility testing. An additional 25 μL of the suspension from select colonies was added to the Active Melioidosis Detect lateral flow immunoassay (AMD LFI) (InBios) and read according to the manufacturer’s instructions (18). Antibiotic sensitivity disks (Oxoid) were added to the MH plates, which along with the API 20 NE strips, were incubated at 37°C. Following 24 h of incubation, each API 20 NE strip was read according to the manufacturer’s instructions, and the antibiotic zones of inhibition were measured. API 20 NE strips were reevaluated following an additional 24 h of incubation. API profiles were entered into APIWEB (https://apiweb.biomerieux.com) for final identification. Isolates with API profiles identified as B. pseudomallei were confirmed by PCR specific for the B. pseudomallei type III secretion system as described elsewhere (19).
ACKNOWLEDGMENTS
We thank the Chief and Elders of Nobewam for their approval and community access and the rice farmers of Nobewam for access to their land. We greatly appreciate the Komfo Anokye Teaching Hospital staff for their participation in and logistical support of this study.
Field work was conducted under the Joint West Africa Research Group, funded through the Defense Health Program with programmatic oversight from the Military Infectious Diseases Research Program.
Identification and characterization of suspected isolates were funded through the Armed Forces Health Surveillance Division (AFHSD), Global Emerging Infections Surveillance (GEIS) Branch, ProMIS ID P0008_22_NM.
We declare no conflict of interest.
K.L.S. is a federal employee of the United States government. This work was prepared as part of his official duties. The views expressed in this work reflect the results of research conducted by the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
Contributor Information
Kevin L. Schully, Email: kevin.l.schully.civ@mail.mil.
Pablo Ivan Nikel, Novo Nordisk Foundation Center for Biosustainability.
REFERENCES
- 1.Currie BJ. 2015. Melioidosis: evolving concepts in epidemiology, pathogenesis, and treatment. Semin Respir Crit Care Med 36:111–125. 10.1055/s-0034-1398389. [DOI] [PubMed] [Google Scholar]
- 2.Limmathurotsakul D, Wongratanacheewin S, Teerawattanasook N, Wongsuvan G, Chaisuksant S, Chetchotisakd P, Chaowagul W, Day NP, Peacock SJ. 2010. Increasing incidence of human melioidosis in northeast Thailand. Am J Trop Med Hyg 82:1113–1117. 10.4269/ajtmh.2010.10-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Limmathurotsakul D, Golding N, Dance DA, Messina JP, Pigott DM, Moyes CL, Rolim DB, Bertherat E, Day NP, Peacock SJ, Hay SI. 2016. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat Microbiol 1:15008. 10.1038/nmicrobiol.2015.8. [DOI] [PubMed] [Google Scholar]
- 4.Whitmore Kc A. 1912. An account of the discovery of a hitherto underscribed infective disease occurring among the population of Rangoon. Indian Med Gaz 47:262–267. [PMC free article] [PubMed] [Google Scholar]
- 5.Steinmetz I, Wagner GE, Kanyala E, Sawadogo M, Soumeya H, Teferi M, Andargie E, Yeshitela B, Yaba Atse-Achi L, Sanogo M, Bonfoh B, Rakotozandrindrainy R, Pongombo Shongo C, Shongoya Pongombo M, Kasamba Ilunga E, Lichtenegger S, Assig K, May J, Bertherat E, Owusu M, Owusu-Dabo E, Adu-Sarkodie Y. 2018. Melioidosis in Africa: time to uncover the true disease load. Trop Med Infect Dis 3:62. 10.3390/tropicalmed3020062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarovich DS, Garin B, De Smet B, Kaestli M, Mayo M, Vandamme P, Jacobs J, Lompo P, Tahita MC, Tinto H, Djaomalaza I, Currie BJ, Price EP. 2016. Phylogenomic analysis reveals an Asian origin for African Burkholderia pseudomallei and further supports melioidosis endemicity in Africa. mSphere 1:e00089-15. 10.1128/mSphere.00089-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wall RA, Mabey DC, Corrah PT, Peters L. 1985. A case of melioidosis in West Africa. J Infect Dis 152:424–425. 10.1093/infdis/152.2.424a. [DOI] [PubMed] [Google Scholar]
- 8.Cuadros J, Gil H, Miguel JD, Marabe G, Gomez-Herruz TA, Lobo B, Marcos R, Anda P. 2011. Case report: melioidosis imported from West Africa to Europe. Am J Trop Med Hyg 85:282–284. 10.4269/ajtmh.2011.11-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mabayoje DA, Kenna DTD, Dance DAB, NicFhogartaigh C. 2022. Melioidosis manifesting as chronic femoral osteomyelitis in patient from Ghana. Emerg Infect Dis 28:201–204. 10.3201/eid2801.211800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Limmathurotsakul D, Dance DA, Wuthiekanun V, Kaestli M, Mayo M, Warner J, Wagner DM, Tuanyok A, Wertheim H, Yoke Cheng T, Mukhopadhyay C, Puthucheary S, Day NP, Steinmetz I, Currie BJ, Peacock SJ. 2013. Systematic review and consensus guidelines for environmental sampling of Burkholderia pseudomallei. PLoS Negl Trop Dis 7:e2105. 10.1371/journal.pntd.0002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashdown LR. 1979. An improved screening technique for isolation of Pseudomonas pseudomallei from clinical specimens. Pathology 11:293–297. 10.3109/00313027909061954. [DOI] [PubMed] [Google Scholar]
- 12.Birnie E, van 't Hof S, Bijnsdorp A, Mansaray Y, Huizenga E, van der Ende A, Hugenholtz F, Grobusch MP, Wiersinga WJ. 2019. Identification of Burkholderia thailandensis with novel genotypes in the soil of central Sierra Leone. PLoS Negl Trop Dis 13:e0007402. 10.1371/journal.pntd.0007402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trinh TT, Assig K, Tran QTL, Goehler A, Bui LNH, Wiede C, Folli B, Lichtenegger S, Nguyen TT, Wagner GE, Kohler C, Steinmetz I. 2019. Erythritol as a single carbon source improves cultural isolation of Burkholderia pseudomallei from rice paddy soils. PLoS Negl Trop Dis 13:e0007821. 10.1371/journal.pntd.0007821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gumma M, Thenkabail P, Fujii H, Namara R. 2009. Spatial models for selecting the most suitable areas of rice cultivation in the Inland Valley Wetlands of Ghana using remote sensing and geographic information systems. J Appl Remote Sens 3:033537. 10.1117/1.3182847. [DOI] [Google Scholar]
- 15.Glass MB, Gee JE, Steigerwalt AG, Cavuoti D, Barton T, Hardy RD, Godoy D, Spratt BG, Clark TA, Wilkins PP. 2006. Pneumonia and septicemia caused by Burkholderia thailandensis in the United States. J Clin Microbiol 44:4601–4604. 10.1128/JCM.01585-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agyepong N, Govinden U, Owusu-Ofori A, Essack SY. 2018. Multidrug-resistant Gram-negative bacterial infections in a teaching hospital in Ghana. Antimicrob Resist Infect Control 7:37. 10.1186/s13756-018-0324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nuti DE, Crump RB, Dwi Handayani F, Chantratita N, Peacock SJ, Bowen R, Felgner PL, Davies DH, Wu T, Lyons CR, Brett PJ, Burtnick MN, Kozel TR, AuCoin DP. 2011. Identification of circulating bacterial antigens by in vivo microbial antigen discovery. mBio 2:e00136-11. 10.1128/mBio.00136-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Houghton RL, Reed DE, Hubbard MA, Dillon MJ, Chen H, Currie BJ, Mayo M, Sarovich DS, Theobald V, Limmathurotsakul D, Wongsuvan G, Chantratita N, Peacock SJ, Hoffmaster AR, Duval B, Brett PJ, Burtnick MN, Aucoin DP. 2014. Development of a prototype lateral flow immunoassay (LFI) for the rapid diagnosis of melioidosis. PLoS Negl Trop Dis 8:e2727. 10.1371/journal.pntd.0002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Novak RT, Glass MB, Gee JE, Gal D, Mayo MJ, Currie BJ, Wilkins PP. 2006. Development and evaluation of a real-time PCR assay targeting the type III secretion system of Burkholderia pseudomallei. J Clin Microbiol 44:85–90. 10.1128/JCM.44.1.85-90.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]