ABSTRACT
The growth of sulfate-reducing bacteria (SRB) and associated hydrogen sulfide production can be problematic in a range of industries such that inhibition strategies are needed. A range of SRB can reduce metal ions, a strategy that has been utilized for bioremediation, metal recovery, and synthesis of precious metal catalysts. In some instances, the metal remains bound to the cell surface, and the impact of this coating on bacterial cell division and metabolism has not previously been reported. In this study, Desulfovibrio desulfuricans cells (1g dry weight) enabled the reduction of up to 1500 mmol (157.5 g) palladium (Pd) ions, resulting in cells being coated in approximately 1 μm of metal. Thickly coated cells were no longer able to metabolize or divide, ultimately leading to the death of the population. Increasing Pd coating led to prolonged inhibition of sulfate reduction, which ceased completely after cells had been coated with 1200 mmol Pd g−1 dry cells. Less Pd nanoparticle coating permitted cells to carry out sulfate reduction and divide, allowing the population to recover over time as surface-associated Pd diminished. Overcoming inhibition in this way was more rapid using lactate as the electron donor, compared to formate. When using formate as an electron donor, preferential Pd(II) reduction took place in the presence of 100 mM sulfate. The inhibition of important metabolic pathways using a biologically enabled casing in metal highlights a new mechanism for the development of microbial control strategies.
IMPORTANCE Microbial reduction of sulfate to hydrogen sulfide is highly undesirable in several industrial settings. Some sulfate-reducing bacteria are also able to transform metal ions in their environment into metal phases that remain attached to their outer cell surface. This study demonstrates the remarkable extent to which Desulfovibrio desulfuricans can be coated with locally generated metal nanoparticles, with individual cells carrying more than 100 times their mass of palladium metal. Moreover, it reveals the effect of metal coating on metabolism and replication for a wide range of metal loadings, with bacteria unable to reduce sulfate to sulfide beyond a specific threshold. These findings present a foundation for a novel means of modulating the activity of sulfate-reducing bacteria.
KEYWORDS: Desulfovibrio desulfuricans, hydrogen sulfide, nanoparticles, palladium reduction, sulfate reduction
INTRODUCTION
Sulfate-reducing bacteria (SRB) are ubiquitous in anoxic natural and engineered environments where sulfate is present. SRB use sulfate as the terminal electron acceptor for growth, converting it to hydrogen sulfide (H2S) coupled to oxidation of a wide range of electron donors, including H2, lactate, formate, ethanol, methanol, and acetate (1). Sulfate reduction can be highly undesirable in several settings and industries. For example, during anaerobic digestion of agro-industrial wastewaters to produce methane biogas. Due to SRB having a thermodynamic advantage over methanogens for low concentrations of electron donors, the production of methane is inhibited as electron flow is diverted toward sulfate reduction (2, 3). In oil and gas reservoirs H2S formation can lead to souring (high acid gas content) of wells and must be separated from production streams using costly methods (4). In addition, H2S can cause corrosion of metal pipelines and safety problems for personnel who are involved in oil platform activities (5–8). Curtailing microbial growth, including by SRB, in oil and gas systems typically involves physical protection of the metal infrastructure using paints and coatings, pigging to remove biofilms on the insides of pipes, and treatment with chemicals like biocides and corrosion inhibitors (9) or metabolic inhibitors such as nitrate and other oxyanions (10). However, these approaches have limitations, for example, mechanical cleaning pigs getting stuck in pipelines (11), pipe coating defects leading to coating disbonding (12), and nitrate exacerbating corrosion (13). The development of novel methods for controlling biological sulfate reduction remains a high priority.
In addition to sulfate, SRB can also reduce a variety of other electron acceptors for growth, including different metals (14). For example, Desulfovibrio spp. can reduce a range of metal ions, including chromium (15), rhenium (16), molybdenum (17, 18), iron (19), technetium (20), gold (21), selenium (18), and uranium (18, 22, 23). Considerable attention has been given to the ability of multiple Desulfovibrio spp. to catalyze the reduction of Pd(II) to Pd(0) displayed as metallic nanoparticles on the outer cell surface, as observed in D. vulgaris (24), D. desulfuricans (25–29), and D. fructosivorans (30). Studies on the mechanism of deposition of Pd(0) have shown that this is a two-step process: an initial biosorption of Pd(II) followed by reduction when an electron donor is provided (27, 31). The reduction reaction occurs in the periplasm using either formate dehydrogenases (FDH) or hydrogenases to oxidize formate or hydrogen, respectively, in addition to cytochromes as redox partners (26). The use of formate may also give rise to hydrogen, generated endogenously and broken down by hydrogenases to yield electrons for metal reduction. This reaction is catalyzed by the formate hydrogenlyase (FHL) complex, which is well documented in facultatively anaerobic bacteria (25). Evidence of this process is observed in D. fructosivorans, where Pd nanoparticles formed in the periplasm of cells when using formate as an electron donor but failed to form in mutants lacking the major periplasmic hydrogenase (30). The Desulfovibrio cells must be viable for the reduction reaction to take place, with an absence of Pd(II) reduction observed in studies when using heat-killed cultures (26–28). Pd(0) nanoparticles that predominantly form in the surface periplasmic layer extend to the outer membrane to form heavy extracellular deposits that in turn further catalyze the reduction of any Pd(II) present (25, 30). Importantly, cell membrane integrity is maintained during the process of Pd(0) deposition (32). The first deposited Pd(0) particles can weaken the C–H bond of formate due to the interaction of a delocalized electron pair with the surface of Pd(0) (33), releasing CO2 and H2. Subsequent oxidation of H2 on the surface of Pd(0) particles yields electrons for the ongoing reduction of remaining Pd(II) in solution to Pd(0). Under these conditions, the Pd(0) catalyzed decomposition of formate is typically much faster than the microbial oxidation of formate. Therefore, the role of enzymes in this process primarily relates to the initial Pd(0) deposition, with further reduction and metal growth occurring autocatalytically via the Pd(0) nanoclusters at the expense of H2 or formate. In this scenario the bacterial cell has three functions: first, providing an enzyme catalyst for the initial stages of Pd(0) deposition; second, hosting Pd(0) particles that serve as nucleation sites for subsequent nanoparticle growth; and third, providing a scaffold for nanoparticles of Pd(0) able to further catalyze the reduction of remaining Pd(II) (26). In addition to this extracellular deposition of metal, intracellular synthesis of Pd nanoparticles in the cytoplasm has also been reported for D. desulfuricans (32) and D. fructosivorans (30).
The extensive use of precious metals in areas of catalysis and electronics and their increasingly high value has led to a growing interest in their recovery from waste sources. Palladium is sought-after owing to its ability to catalyze a wide range of chemical synthesis reactions for a range of industrial applications (34). The main research focus of bacterial reduction of soluble Pd(II) to insoluble metal Pd has therefore been as a nontoxic method to recover Pd from spent industrial leachates (25, 35) and also to synthesize nanoscale bioinorganic catalysts of considerable commercial potential (24, 26). Prior studies have not investigated the impact of the metal coating on bacterial cell division, mobility, or metabolism, which all remain poorly understood. This study investigates the loading of Pd metal on the cell surface of D. desulfuricans and how the resulting metal coating impacts sulfate reduction and cell division, paving the way for the potential development of a novel method for controlling biological sulfate reduction.
RESULTS AND DISCUSSION
Reduction of Pd(II) by D. desulfuricans.
Previous studies investigating the bioreduction of Pd(II) by D. desulfuricans have focused on Pd concentrations of approximately 12.5 mmol (1.31 g) Pd(II) g−1 dry cells (26–28). In this study, freshly cultured cells were exposed to 8, 16, 32, and 64 mmol Pd(II) g−1 wet cells (equivalent to 20, 40, 80, and 160 mmol Pd[II] g−1 dry cells) with formate as the electron donor, to determine whether higher concentrations of Pd ions could be reduced. For all Pd(II) concentrations, a visible change from a yellow-tinted Pd(II) initial solution to a colorless solution with black precipitates as the experiment progressed, denoted the reduction of soluble Pd(II) to insoluble metal Pd. Regardless of the level of palladium added, >99% Pd(II) was removed from the solution after 30 min incubation (Fig. S1). High-performance liquid chromatography (HPLC) analysis revealed that all the added formate was consumed during this reaction (data not shown). In contrast to this rapid reduction of Pd(II) by D. desulfuricans cells, when no cells were present, the reduction of metal ions with formate took approximately 8 h. All cells exposed to 20 mmol Pd(II) g−1 dry cells were coated with at least a thin layer of metal Pd nanoparticles, approximately 10 to 30 nm in diameter (Fig. 1B), smaller than the 50 nm surface-associated nanoparticles that have been previously reported (26). The presence of larger (100 to 300 nm) nanoparticles was also observed on cell surfaces (Fig. 1B) and at a greater density as the loading of Pd(II) increased (Fig. 1D to F). At every Pd(II) concentration tested, a small proportion of cells were heavily coated in a thick layer of metal (approximately 0.3 μm) (Fig. 1C). Even after vortexing samples before SEM analysis, few free Pd nanoparticles were observed, demonstrating that the metallic Pd produced was strongly bound to the cell surfaces. Energy-dispersive X-ray spectroscopy (EDX) confirmed that the nanoparticles observed on the cell surfaces consist of Pd (Fig. 2A), which was only minimally detected or absent in spectra corresponding to uncoated regions of the cell surface (Fig. 2B) and the background filter (Fig. 2C). A signal for gold was observed for all samples because this element was used to sputter coat the membrane filters before SEM analysis. Powder X-ray diffraction (PXRD) analysis revealed a diffractogram with peaks corresponding exactly to those of elemental Pd (Fig. 2D and F), as has been previously observed (25, 26).
FIG 1.
Uncoated D. desulfuricans cells (A) and cells coated in 20 (B, C), 40 (D), 80 (E), and 160 (F) mmol Pd g−1 dry cells.
FIG 2.
EDX and PXRD analysis of D. desulfuricans cells coated with 40 mmol Pd g−1 dry cells. SEM image and corresponding EDX spectrum of the metal coating (A), cell surface (B), and membrane filter (C). PXRD diffractograms of dried Pd-loaded biomass formed in the absence (D and E) and presence (F and G) of 100 mM sulfate.
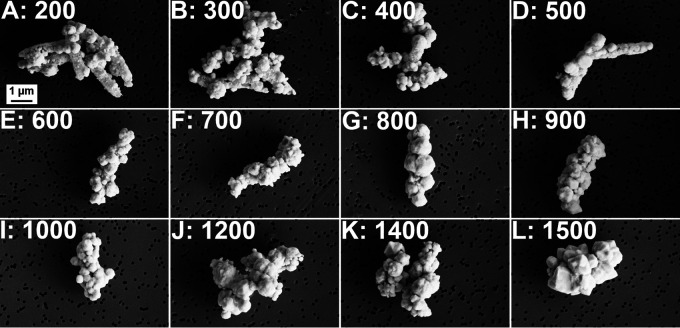
Complete metal reduction at palladium-biomass ratios 10 times greater than reported in other studies (26, 27) raises the question of upper bounds on these observed effects. Here, D. desulfuricans were exposed to Pd(II) concentrations from 200 mmol up to 1500 mmol g−1 dry cells (the solubility limit for this method) with formate as the electron donor. These high concentrations of Pd(II) led to substantial black precipitates forming in the medium and falling out of suspension. When these were vortexed and analyzed using SEM, images revealed individual cells heavily coated in Pd metal (Fig. 3A to L). At the highest palladium loading (1500 mmol g−1 dry cells), bacterial cells with a metal coating up to approximately 1 μm thick were observed (Fig. 3L). Regardless of the Pd(II) concentration tested, >99% of the supplied Pd(II) was reduced within 30 min (Fig. S1). When cells were exposed to greater than 500 mmol Pd(II) g−1 dry cells, SEM did not reveal any uncoated or lightly coated cells, as was observed with lower concentrations. In other studies, the concentration of Pd(II) was kept constant at 2 mM and the biomass of the bacteria was variable (26, 27) equating to concentrations of Pd(II) ranging from 9 to 40 mmol g−1 dry cells. This revealed that the minimum D. desulfuricans biomass needed for complete reduction of 2 mM Pd(II) ranged from 0.13 to 0.19 g/liter dry weight (the equivalent of 11 to 15 mmol Pd[II] g−1 dry cells). Only negligible amounts of palladium were reduced (<20%) at lower biomass levels (0.1 and 0.05 g/liter dry weight, equivalent to 20 and 40 mmol Pd(II) g−1 dry cells), respectively (26, 27). Here, Pd concentrations ranging from 20 to 1500 mmol g−1 dry cells – up to 100× higher than in the previous studies (26, 27) – were fully reduced. This complete reduction of higher Pd(II) concentrations could be a consequence of the longer incubation time in comparison to previous studies (30 min versus 5 min), enabling more time for the catalytic reduction of Pd(II) to take place. Greater Pd reduction may also result from using freshly cultured cells rather than resting cells (stored at 4°C for up to 48 h before use), resulting in higher efficiency for the initial reduction reaction. Resting cells were used in previous studies (25–28) to avoid possible precipitation of Pd(II) with sulfide produced from the reduction of sulfate in the growth medium. However, PXRD data in our study showed that the use of freshly cultured cells did not lead to any sulfide being present in the Pd nanoparticles formed by the D. desulfuricans (Fig. 2E). In addition, sulfur was not detected during EDX analysis (Fig. 2A).
FIG 3.
D. desulfuricans cells coated in 200 (A), 300 (B), 400 (C), 500 (D), 600 (E), 700 (F), 800 (G), 900 (H), 1000 (I), 1200 (J), 1400 (K), and 1500 (L) mmol Pd g−1 dry cells.
The impact of acidic pH on the reduction of Pd(II) by D. desulfuricans using formate as an electron donor has been previously investigated, with optimal palladium reduction taking place at pH 7 and decreasing under more acidic conditions down to pH 3 (26, 27). However, no study has previously examined the impact of more alkaline pH values on this reaction. In this study, the alkaline nature of the palladium source led to the pH increasing from 7 to 7.5 in solutions containing 800 mmol to 1000 mmol Pd(II) g−1 dry cells and went as high as pH 8 in solutions containing 1200 to 1500 mmol Pd(II) g−1 dry cells. However, even at these elevated alkaline pH values, greater than 99% of the Pd(II) was reduced, indicating that the D. desulfuricans cells were unaffected by this pH shift. To survive and grow under alkaline or acidic conditions, SRB have developed several strategies to combat environmental challenges (36). Previous studies investigating the impact of pH on Desulfovibrio spp. growth and sulfate reduction have shown inhibition only occurs at pH extremes, i.e., lower than pH 5 and higher than pH 9 (37). Specifically, D. desulfuricans has been shown to grow well at a pH range of 6.8 to 8.2 (38), and tolerate pH up to 9.2 (39) or 9.9 (40).
Previous research has shown that the presence of 100 mM nitrate inhibited Pd(II) reduction by D. desulfuricans grown on formate by 50% (26). The mechanism for this inhibition was not explained, but as D. desulfuricans is one of only several SRB that can use nitrate reduction for growth (41–43), this electron acceptor may have been preferentially reduced. Due to this potential inhibition of Pd(II) reduction with a competing oxidant, unlike experiments described above that omitted sulfate during Pd(II) reduction, here sulfate was included to test whether cells could reduce 150 mmol Pd(II) g−1 dry cells in the presence of sulfate concentrations ranging from 20 to 100 mM. This is an important consideration for any in situ coating of SRB with metals, where high concentrations of sulfate could be present. D. desulfuricans could still reduce >99% Pd(II) in the presence of 100 mM sulfate (Fig. S2), showing that this electron acceptor does not inhibit the initial metal bioreduction reaction nor the subsequent catalytic reduction reaction. PXRD analysis of Pd nanoparticles formed in the presence of 100 mM sulfate showed minor amounts of sulfide in their composition (presence of Na2S2 peak), illustrating that a very small concentration of sulfate was reduced in parallel with the Pd(II) reduction (Fig. 2G). The inhibition of Pd(II) reduction by nitrate observed by Yong et al., 2002a (26), could therefore have been a consequence of the shorter incubation time (5 min versus 30 min) allowing less time for the reduction of Pd(II) to take place in the presence of a competing electron acceptor. Alternatively, because nitrate reduction is more energetically favorable than sulfate reduction, the presence of nitrate may be a more powerful inhibitor of Pd(II) reduction.
Impact of Pd metal coating on biological sulfate reduction.
As biological sulfate reduction can be problematic in a range of industries, the formation of Pd metal nanoparticles on D. desulfuricans cells was investigated as an inhibition strategy. After complete Pd(II) reduction, coated cells were exposed to a fresh medium containing 20 mM sulfate, and formate as an electron donor, with no additional Pd(II) present. Inhibition of sulfate reduction was proportional to coating with palladium in amounts ranging from 8 to 800 mmol g−1 dry cells (Fig. 4A). Sulfate consumption, in stoichiometric agreement with sulfide production (Fig. S3A), commenced in uncoated cell cultures within the first 24 h, while cells coated in 8 and 80 mmol Pd g−1 dry cells experienced an extended lag of 2 and 3 days, respectively (Fig. 4A; Fig. S3A). Whereas uncoated cells reduced all available sulfate within 4 days, cells coated in 8 and 80 mmol Pd g−1 dry cells were much slower, requiring 8 and 11 days, respectively. While a maximum sulfate reduction rate of 8.32 mM day−1 was achieved by the uncoated control cells between days 2 and 3, maximum rates for cells coated in 8 and 80 mmol Pd g−1 dry cells were 6.13 and 4.3 mM day−1, respectively. Even when D. desulfuricans cells were heavily coated in Pd (800 mmol), they were still able to reduce approximately 25% of the available sulfate over 12 days (conversion of 5 mM sulfate into sulfide; Fig. S3A). In this treatment, 8 days were required to reduce 25% of the sulfate after an initial 4-day lag phase, and rates of sulfate reduction were much lower, ranging from 0.53 up to a maximum of 1.55 mM day−1 (an average of 0.74 mM day−1). In contrast, rates of sulfate reduction in the uncoated control and cultures coated in 8 and 80 mmol Pd g−1 dry cells all increased approximately 7-fold during growth, i.e., from 1.25 to 8.32 mM day−1, from 0.94 to 6.13 mM day−1 and from 0.6 to 4.3 mM day−1, respectively. For cells coated in Pd, the dilution of metal coating on cell surfaces as the cells divided would also have contributed to higher rates of sulfate reduction. In the control and cultures coated with 8 and 80 mmol Pd g−1 dry cells, the optical density value at 600 nm (OD600) reached a peak of approximately 0.3, decreasing once all the sulfates had been reduced. Slightly higher starting measurements of OD600 were observed for the cultures coated in 8 and 80 mmol Pd g−1 cells, reflecting the presence of Pd nanoparticles in these samples. In contrast, no significant increase in OD600 was observed in cultures coated with 800 mmol Pd g−1 dry cells. The large clumps of Pd coated cells in these tubes could not be taken up through the syringe needle, resulting in these samples having the lowest starting OD600 value (Fig. 4B). A small increase in OD600 (from 0.015 to 0.05) was observed between days 0 and 5, caused by the large clumps of Pd coated cells breaking up in the shaking incubator. The lack of turbidity observed in cultures coated with 800 mmol Pd g−1 cells, suggests a lack of cell division by D. desulfuricans under these conditions. Consistent with OD600 data, SEM carried out on day 12 revealed that D. desulfuricans coated with 8 and 80 mmol Pd g−1 dry cells were still able to divide, giving rise to a large population of uncoated cells (Fig. 4F and H). SEM did not reveal any uncoated cells in the D. desulfuricans culture treated with 800 mmol Pd g−1 dry cells (Fig. 4J), consistent with heavily metal-coated cells being unable to divide. The small amount of sulfate reduced to sulfide in this situation suggests that some formate and sulfate were still able to physically pass through the metal coating and enter the cell to be metabolized.
FIG 4.
Sulfate reduction (A) and cell growth (OD600) (B) of uncoated D. desulfuricans cells (green circles), and cells coated in 8 (yellow triangles), 80 (gray squares), and 800 mmol Pd g−1 dry cells (orange diamonds) when using 80 mM formate as an electron donor. Error bars are SD (n = 3). SEM images (day 0 and day 12) of uncoated D. desulfuricans cells (C and D), and cells coated in 8 (E and F), 80 (G and H), and 800 mmol Pd g−1 dry cells (I and J).
Effect of electron donor type on sulfate reduction inhibition.
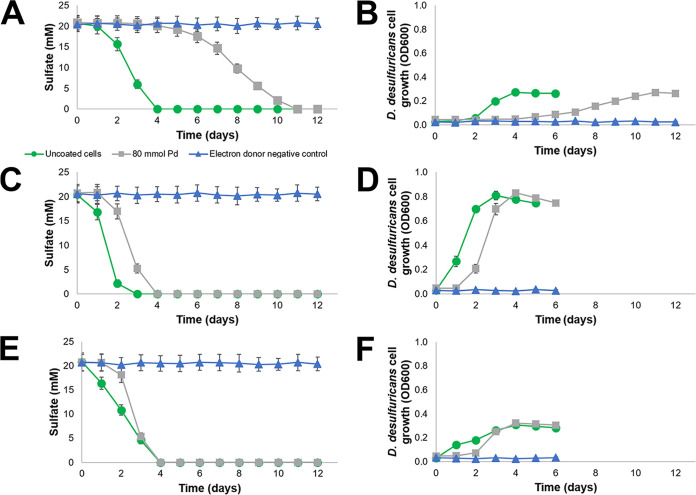
The ability of D. desulfuricans to reduce sulfate despite a significant coating of Pd suggests that inhibition depends on preventing the transmission of metabolites through this outer layer of metal. Accordingly, the molecular weights of different electron donors could influence their ability to pass through the metal layer. Inhibition of sulfate reduction by D. desulfuricans cells coated with 80 mmol Pd g−1 dry cells was compared to uncoated cells supplied with formate, lactate, or hydrogen as the electron donor (Fig. 5). For these experiments, formate was used as the electron donor for Pd(II) reduction and the establishment of coated cells, and then fresh media containing sulfate and either formate (80 mM), lactate (40 mM), or hydrogen (0.6 mM added every 24 h) was provided to Pd coated cells to test sulfate reduction inhibition.
FIG 5.
Sulfate reduction and cell growth by D. desulfuricans with formate (A and B) lactate (C and D), or hydrogen (E and F) as the electron donor. Uncoated cells (green circles), cells coated in 80 mmol Pd g−1 dry cells (gray squares), and electron donor negative-control (blue triangles). Error bars are SD (n = 3).
Using formate as the electron donor resulted in the greatest inhibition, with metal-coated cells requiring 11 days for complete sulfate reduction, compared to only 4 days for the uncoated control cells (i.e., 2.8 times longer) (Fig. 5A and Fig. S3B). Whereas sulfate reduction commenced in uncoated cell cultures within the first 24 h, Pd coated cells experienced a 3-day lag. A maximum sulfate reduction rate of 9.67 mM day−1 was achieved by the uncoated control cells between days 2 and 3, whereas the maximum reduction rate for cells coated in 80 mmol Pd g−1 dry cells was 4.89 mM day−1 between days 7 and 8. For both experimental conditions, the OD600 value reached a peak of approximately 0.3, decreasing once all the sulfate had been reduced.
Providing Pd coated cells with lactate gave rise to a less pronounced impact of the metal coating on sulfate reduction. Pd coated cells catalyzed complete reduction of sulfate 1.3 times slower than uncoated cells (Fig. 5C and Fig. S3C). Sulfate reduction with lactate commenced in uncoated cell cultures within the first 24 h, and within the first 48 h for metal coated cells. Once sulfate reduction had commenced, both the uncoated and metal-coated cells took 3 days to reduce all the sulfate and displayed comparable maximum sulfate reduction rates (14.68 and 11.72 mM day−1, respectively). Lactate resulted in much stronger cell growth over the experimental period, with far greater turbidity than was observed in the formate-amended cultures (Fig. 5).
Supplying precoated Desulfovibrio cells with hydrogen (i.e., introducing 0.6 mM H2 gas every 24h) introduced a 1-day lag to the onset of sulfate reduction compared to uncoated cells (Fig. 5E and F). Both coated and uncoated cells completed sulfate reduction after 4 days of incubation (Fig. 4E and Fig. S3D), indicating that the concentration of H2 being added daily, and not the metal coating, controlled the extent of sulfate reduction. This is illustrated by the steady rate of sulfate reduction in the uncoated control (ranging from 4.43 to 6.16 mM day−1), which is close to the stoichiometric maximum of 7.44 mM for this amount of H2. Pd coated cells did not reduce any sulfate in the first 24 h and less than 3 mM over the following 24 h, leading to a buildup of H2, followed by a rapid reduction of 12.78 mM sulfate in the 24h period between days 2 and 3 that saw the metal-coated cells catch up with the uncoated control culture. Due to the role of hydrogenases in Pd(II) reduction by Desulfovibrio spp. (30, 44), the likely deposition of Pd metal localized to these enzymatic sites would be expected to inhibit sulfate reduction when using H2 as an electron donor. However, due to the rate-limiting supply of H2 in the control culture, no such inhibition was observed. Hydrogen resulted in similar cell growth dynamics as formate, with Pd-coated cells reaching similar levels of turbidity but in a shorter period.
Different mechanisms could explain the prolonged inhibition of sulfate reduction in the presence of formate compared with lactate. Oxidation of these compounds generates reductants through the periplasmic formate dehydrogenases (FDH) (45) and lactate dehydrogenase (LDH) enzyme (46, 47), respectively. If Pd deposition is localized to these enzymatic oxidation sites, or there is a semipermeable metal layer around the cell, oxidation of the electron donor will be impeded, ultimately preventing respiration via sulfate reduction. In this study, because D. desulfuricans cells in the presence of lactate did not catalyze metal ion reduction, formate was always provided for Pd(II) reduction and the generation of Pd coated cells. The utilization of FDH, or hydrogenases via the FHL complex, for palladium reduction, would have likely led to metal deposition near these periplasmic oxidation sites. When metal-coated cells were transferred to a new medium and formate was supplied again – this time as the electron donor for sulfate reduction – the likely presence of increased Pd metal close to FDH or hydrogenase oxidation sites may have led to greater inhibition of sulfate reduction, compared to when providing lactate (Fig. 5), which utilizes LDH.
Alternatively, lactate oxidation has been reported to enable higher energy generation and more robust growth of sulfate-reducing Desulfovibrio spp. compared to metabolism through formate oxidation (47), as was observed in growth curves in the present study (Fig. 5B and D). Increased cell growth with lactate as electron donor would likely have led to a more rapid emergence of uncoated bacterial populations compared to parallel cultures presented with formate, and thus growth by less coated or eventually uncoated cells in these cultures.
Pd coating of cells for complete inhibition of sulfate reduction.
The ability of cells to achieve some respiration despite an 800 mmol Pd g−1 dry cells coating raises the question of whether complete inhibition of sulfate reduction through metal coating could be achieved. To test this, D. desulfuricans cells were coated in 800, 1000, 1200, and 1400 mmol Pd g−1 dry cells. Coated cells were exposed to a fresh medium containing 20 mM sulfate, and formate as an electron donor, with no additional Pd(II) present. Cellular coatings of ≥1200 Pd completely inhibited sulfate reduction (Fig. 6A and Fig. S3E). Cells coated in 800 and 1000 mmol Pd g−1 dry cells both reduced a small amount of sulfate (~4 mM) over the first 13 days of the experiment. It is likely that encapsulated cells remained viable and were able to reduce small amounts of sulfate as low concentrations of formate reached the cell interior for oxidation via sulfate respiration. A range of bacteria, including Desulfovibrio vulgaris, have been shown to regulate their cell size by growing the same amount between divisions, providing a remarkably strong cell-size control that prevents large size deviations from occurring within a few generations (48, 49). If Desulfovibrio spp. must increase by a specific size increment before division can take place, then cells encased in a thick layer of Pd metal coating might be prevented from ever meeting such a requirement. With essentially no space to divide, metal-covered cells may be dormant or dead, entombed in a Pd metal sarcophagus. No increase in turbidity was observed in any of the 800 to 1400 mmol Pd g−1 dry cells coated cultures throughout the experimental period, consistent with SEM images taken on day 34 showing no evidence of dividing cells (Fig. 6B to I).
FIG 6.
(A) Sulfate reduction by D. desulfuricans cells coated in 800 (green circles), 1000 (yellow triangles), 1200 (gray squares), and 1400 mmol Pd g−1 dry cells (orange diamonds). Error bars are SD (n = 3). SEM images (day 0 and day 34) of D. desulfuricans cells coated in 800 (B and C), 1000 (D and E), 1200 (F and G), and 1400 mmol Pd g−1 dry cells (H and I).
To further understand the mechanism of sulfate reduction inhibition in D. desulfuricans cells heavily coated in Pd metal, 0.5 mL freshly cultured uncoated D. desulfuricans cells (OD600 = 0.6) were added on day 34 to the tubes where cells had been coated in 1200 and 1400 mmol Pd g−1 dry cells, to give a final wet cell concentration of 0.0125 g/liter. Following 9 days of incubation of this mixed culture, all sulfate was reduced to sulfide (Fig. S4). Due to their high surface-area-to-volume ratio, zero-valent metal nanoparticles may have a strong adsorption capability for both organic and inorganic compounds (50). However, sulfate reduction by this mixed culture demonstrated that the inhibition of similar metabolism in Pd coated cells cultured alone is due to metal encapsulation, not the removal of important trace elements or salts needed for sulfate reduction and cell growth through adsorption onto Pd(0). Because formate was still present in the medium and was the only electron donor present, the addition of freshly cultured cells leading to sulfate reduction similarly demonstrates that formate is also not broken down abiotically by metallic Pd on coated cell surfaces (Fig. S4). Despite the ability of Pd(0) to catalyze the decomposition of formate (33), this reaction does not appear to take place in the absence of Pd(II). The poor sulfate-reducing capabilities of D. desulfuricans cells coated in Pd metal concentrations greater than 800 mmol g−1 dry cells (Fig. 4 and 6) are contradictory to this organism’s ability to rapidly reduce concentrations of Pd(II) up to 1500 mmol g−1 dry cells (Fig. 3 and Fig. S1). Despite a thick coating of metal rapidly forming on bacterial cell surfaces, their ability to continue reducing Pd(II) further supports the existence of two synergistic mechanisms for palladium reduction (26). First, the bacterial cell provides enzymes (FDH and hydrogenases) for the initial stages of metal ion reduction. Second, the accumulated Pd(0) then provides a catalytic surface for the release of H2 from formate and its subsequent oxidation, facilitating the chemical reduction of yet more Pd(II). The latter does not depend on microbial growth substrates passing through the metallic cellular exterior and cell envelope for normal microbial metabolism by periplasmic enzymes. The chemical reaction rate is fast and independent of loading at the time scales resolved in these experiments (~30 min).
Prognosis.
This work shows the impact of a Pd metal coating on the sulfate-reducing capabilities of D. desulfuricans. Metals occur naturally in the environment through the breakdown of minerals, and industries such as metallurgical plants, petroleum refining, and coal mines can lead to additional environmental inputs of metals such as lead, cadmium, arsenic, zinc, and chromium. The formation of metal nanoparticles can be beneficial to microbial populations as a means of removal of toxic metal ions from their environment. However, if reduced metal species accumulate on cell surfaces and this effect becomes too great, this survival mechanism can change from favorable to deadly. In this study, we have shown that D. desulfuricans cells can enable the reduction of extremely high concentrations of metals – up to 1500 mmol Pd(II) g−1 dry cells. This demonstrates an increased potential for recovery of Pd metal from wastewaters using bacteria. At such high Pd(II) concentrations, cells become deeply encapsulated in metal (up to 1 μm thick), leading to the inhibition of cellular respiration and cell division, and ultimately death of the population. Despite the high cost associated with using Pd ions for inhibition of sulfate reduction, downstream recovery of the Pd metal on cell surfaces could favorably shift the economics of this potential biotechnology. To find less expensive alternatives, further work will need to take place to determine the amount and subsequent impact of coating with other metals and metal combinations on the sulfate-reducing capability of D. desulfuricans and the metabolic capabilities of other bacteria. Ultimately, the discovery of the inhibition of important metabolic pathways through a biological coating of metal nanoparticles could lead to the development of new methods for the control of problematic microorganisms, such as SRB or pathogenic bacteria.
MATERIALS AND METHODS
Cell preparation.
D. desulfuricans (ATCC 29577) was cultured anaerobically (N2 headspace pressurized to 1.5 bar) at 30°C for 72 h in Postgate Medium C (PGMC) (51) containing (g/liter): sodium lactate, 6.0; Na2SO4, 4.5; CaCl2 2H2O, 0.06; sodium citrate, 0.3; NH4Cl, 1.0; KH2PO4, 0.5; MgSO4 7H2O, 2.0; yeast extract, 1.0, FeSO4 7H2O, 0.004, ascorbic acid, 0.1; sodium thioglycolate, 0.1, pH adjusted to 7.5 with 1 M NaOH. The active culture was transferred to fresh PGMC and incubated for a further 48 h (OD600 = 0.6, beginning of stationary-phase). Cells were harvested by centrifugation and washed twice in degassed (N2) filter-sterilized 20 mM MOPS (morpholinepropanesulfonic acid)-NaOH buffer (pH 7). The cell pellet was then weighed and resuspended in MOPS-NaOH buffer to a biomass density of 0.25 g/liter. To calculate the equivalent dry weight, preweighed bacterial pellets were dried in an oven at 70°C for 72 h before reweighing, giving a dry weight to wet weight ratio of 1:2.5.
Palladium(II) reduction by D. desulfuricans.
To determine the maximum concentration of Pd(II) reduction by D. desulfuricans, aliquots (2.5 mL) of the prepared cell culture (0.1 g/liter dry weight) were transferred to sterile anaerobic Hungate tubes (N2 headspace pressurized to 1.5 bar) supplemented with sodium formate and palladium (as tetraamminepalladium[II] chloride salt) to a ratio of 12.5:1 (as previously defined (26)). This resulted in complete Pd(II) reduction and complete utilization of formate. Pd concentrations ranged from 200 to 1500 mmol g−1 dry cells. Cultures were incubated in triplicate in the dark on a shaking incubator (120 rpm) at 30°C for 30 min.
For Pd(II) reduction in the presence of sulfate, sodium sulfate was added to the MOPS-NaOH buffer to give sulfate concentrations of 25, 50, and 100 mM.
Cells were harvested by centrifugation (4816 × g for 20 min) and the supernatant was removed for residual Pd analysis using an Agilent 8900 inductively coupled plasma triple quadrupole mass spectrometer (ICPQQQ). Samples were prepared by dilution in 2% HNO3 made up of trace metals grade concentrated HNO3 and 18.2 MΩ cm−1 MilliQ laboratory water. Elements were calibrated between 10 mg/liter and 0.001 mg/liter. Data quality assurance and control included calibration checks, sample duplicates, reagent controls, and sample blanks. The supernatant was also analyzed for residual formate using a Thermo Scientific Dionex UltiMate 3000 HPLC System. Separation was achieved over an Aminex HPX-87H organic acid column (Bio-Rad, USA) under isocratic conditions (0.05 mM H2SO4) at 60°C with a run time of 20 min. Samples were filtered (0.2 μm) before analysis.
For SEM analysis, the centrifuged cell pellet was fixed overnight in 0.1 M sodium cacodylate with 2.5% glutaraldehyde. Fixed samples (0.1 mL) were vacuum filtered onto 0.1 μm isopore polycarbonate membrane filters (EMD Millipore) previously sputtered with ~5 nm of gold. Samples were washed by increasing concentrations of ethanol in DI water (0%, 25%, 50%, 75%, 90%, 100% in 10 mL for 10 min each). Membrane filters were finally transferred into hexamethyldisilazane for 1 h, then air-dried overnight. Samples were mounted and imaged using a Sigma VP scanning electron microscope (Zeiss) equipped with an INCA x-act EDX system (Oxford Instruments). All imaging was performed under high vacuum conditions using an accelerating voltage of 10 kV to capture secondary electron (SE) images. When X-ray analysis was desired, accelerating voltage was increased to 10 kV to allow for good excitation of relevant X-ray lines.
For powder X-ray diffraction (PXRD) analysis of Pd loaded biomass, a centrifuged cell pellet was washed with MilliQ water and left to air dry. PXRD patterns were collected using a Bruker D8 Advance ECO instrument equipped with a Cu Kα source (λ = 1.54178 Å, kV = 40, mA = 25) and an LYNXEYE XE detector. The samples were suspended in acetone and drop cast on a low background Si holder. The measurement was collected from 10 to 80° 2θ at a scan rate of 2.3 s per 0.05° step. GSAS-II was used to refine these diffraction patterns with reported structures as a starting point.
Sulfate reduction inhibition experiments.
D. desulfuricans was cultured in PGMC as described above. Aliquots of cells (1 mL of 0.25 g/liter wet cells in MOPS-NaOH buffer) were transferred to sterile anaerobic Hungate tubes (N2 headspace pressurized to 1.5 bar) and sodium formate and tetraamminepalladium(II) chloride to a ratio of 12.5:1 added, to give the appropriate Pd concentrations. For Pd-free controls, only formate was added. Cultures were incubated in triplicate in the dark on a shaking incubator (120 rpm) at 30°C for 30 min. After Pd(II) reduction, minimal media (52), with 20 mM sulfate and 1 g/liter yeast extract, was added to the Hungate tubes to give a final volume of 20 mL and a wet cell concentration of 0.0125 g/liter, with sodium formate (4:1 formate to sulfate), sodium lactate (2:1 lactate to sulfate) or hydrogen (0.6 mM) added as electron donors. Due to cells heavily coated with Pd metal falling out of suspension, Hungate tubes were incubated (30°C) in the dark, horizontally on a shaking incubator at 75 rpm for the duration of the experiment. Every 24 h samples were taken to measure OD600 and concentrations of sulfate and sulfide. Hydrogen (10 mL) was also added to the relevant tubes each day. For SEM analysis 400 μL of the sample was taken on day 0 and the final day of the incubation. Samples were centrifuged (9,391 × g) for 10 min and fixed overnight in 0.1 M sodium cacodylate with 2.5% glutaraldehyde. Samples were then prepared for SEM as described above.
To determine the OD600 value, 200 μL of culture was added to an ultramicro cuvette and analyzed using a spectrophotometer at 600 nm wavelength.
To determine sulfide concentrations, 50 μL of the sample was added to 950 μL of a 5 mM CuSO4 5H2O 50 mM HCl solution, inverted, and analyzed using a spectrophotometer at 480 nm wavelength (53). Values were based on standards (0 to 20 mM) set up using Na2S in N2 degassed water.
To determine sulfate concentrations, 100 μL of the sample was added to 900 μL DI water, filtered (0.2 μm), and frozen at minus 20°C. Defrosted samples were vortexed and analyzed using a Dionex ICS-5000 reagent-free ion chromatography system (Thermo Scientific, CA, USA) equipped with an anion-exchange column (Dionex IonPac AS22; 4 × 250 mm; Thermo Scientific), an EGC-500 K2CO3 eluent generator cartridge and a conductivity detector. An isocratic separation method was used with a constant flow rate of 1.3 mL/min while maintaining column temperature at 30°C. Standards (0 to 2 mM) were made using (NH4)2SO4 to match the sulfate source in the minimal media.
ACKNOWLEDGMENTS
This research was supported by funding from the Canada Excellence Research Chairs Program (S.L.B.), the Canada Foundation for Innovation (C.R.J.H.), and a Campus Alberta Innovates Program Chair (C.R.J.H.).
We thank Brian Baillie of the Department of Chemical and Petroleum Engineering, University of Calgary, for ICPQQQ analysis, and Benjamin Gelfand, Department of Chemistry, University of Calgary, for PXRD analysis.
We declare no conflict of interest.
Footnotes
Supplemental material is available online only.
Contributor Information
Steven L. Bryant, Email: Steven.Bryant@ucalgary.ca.
Jeremy D. Semrau, University of Michigan-Ann Arbor
REFERENCES
- 1.Liamleam W, Annachhatre AP. 2007. Electron donors for biological sulfate reduction. Biotechnol Adv 25:452–463. 10.1016/j.biotechadv.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Harada H, Uemura S, Momonoi K. 1994. Interaction between sulfate-reducing bacteria and methane-producing bacteria in UASB reactors fed with low strength wastes containing different levels of sulfate. Wat Res 28:355–367. 10.1016/0043-1354(94)90273-9. [DOI] [Google Scholar]
- 3.Lovley DR, Dwyer DF, Klug MJ. 1982. Kinetic analysis of competition between sulfate reducers and methanogens for hydrogen in sediments. Appl Environ Microbiol 43:1373–1379. 10.1128/aem.43.6.1373-1379.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basafa M, Hawboldt K. 2019. Reservoir souring: sulfur chemistry in offshore oil and gas reservoir fluids. J Petrol Explor Prod Technol 9:1105–1118. 10.1007/s13202-018-0528-2. [DOI] [Google Scholar]
- 5.Muyzer G, Stams AJM. 2008. The ecology and biotechnology of sulphate-reducing bacteria. Nat Rev Microbiol 6:441–454. 10.1038/nrmicro1892. [DOI] [PubMed] [Google Scholar]
- 6.Enning D, Garrelfs J. 2014. Corrosion of iron by sulfate-reducing bacteria: new views of an old problem. Appl Environ Microbiol 80:1226–1236. 10.1128/AEM.02848-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mattorano DA, Merinar T. 1999. Respiratory protection on offshore drilling rigs. Appl Occup Environ Hyg 14:141–148. 10.1080/104732299303052. [DOI] [PubMed] [Google Scholar]
- 8.Lahme S, Hubert C. 2017. Corrosion risks associated with (bio) chemical processes in sour systems due to nitrate injection or oxygen ingress, p 87–109. In Skovhus TL, Enning D, Lee JS (ed), Microbiologically Influenced Corrosion in the Upstream Oil and Gas Industry, 1st ed, CRC Press, New York, NY. [Google Scholar]
- 9.Sharma M, Liu H, Chen S, Cheng F, Voordouw G, Gieg L. 2018. Effect of selected biocides on microbiologically influenced corrosion caused by Desulfovibrio ferrophilus IS5. Sci Rep 8:16620. 10.1038/s41598-018-34789-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlson HK, Hubert CRJ. 2019. Mechanisms and monitoring of oil reservoir souring control by nitrate or perchlorate injection. In Handbook of Hydrocarbon and Lipid Microbiology. T.J. McGenity (ed) Springer Nature, Switzerland. [Google Scholar]
- 11.Quarini J, Shire S. 2007. A review of fluid-driven pipeline pigs and their applications. Proceedings of the Institution of Mechanical Engineers, Part E: J Process Mechanical Engineering 221:1–10. 10.1243/0954408JPME108. [DOI] [Google Scholar]
- 12.Li SY, Kim YG, Jeon KS, Kho YT. 2000. Microbiologically influenced corrosion of underground pipelines under the disbonded coatings. Metals and Materials 6:281–286. 10.1007/BF03028224. [DOI] [Google Scholar]
- 13.Lahme S, Enning DE, Callbeck CM, Menendez-Vega D, Curtis TP, Head IM, Hubert CRJ. 2019. Metabolites of an oil field sulfide-oxidizing nitrate-reducing Sulfurimonas sp. cause severe corrosion. Applied & Environ Microbiol 85:e01891-18. 10.1128/AEM.01891-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lovley DR. 1993. Dissimilatory metal reduction. Annu Rev Microbiol 47:263–290. 10.1146/annurev.mi.47.100193.001403. [DOI] [PubMed] [Google Scholar]
- 15.Chardin B, Dolla A, Chaspoul F, Fardeau ML, Gallice P, Bruschi M. 2002. Bioremediation of chromate: thermodynamic analysis of the effects of Cr(VI) on sulfate reducing bacteria. Appl Microbiol Biotechnol 60:352–360. 10.1007/s00253-002-1091-8. [DOI] [PubMed] [Google Scholar]
- 16.Xu H, Barton LL, Zhang P, Wang Y. 2000. TEM investigation of U6þ and Re7þ reduction by Desulfovibrio desulfuricans, a sulfate-reducing bacterium. Mater Res Soc Symp Proc 608:299–304. [Google Scholar]
- 17.Tucker MD, Barton LL, Thomson BM. 1997. Reduction and immobilization of molybdenum by Desulfovibrio desulfuricans. J Environ Qual 26:1146–1152. 10.2134/jeq1997.00472425002600040029x. [DOI] [Google Scholar]
- 18.Tucker MD, Barton LL, Thomson BM. 1998. Reduction of Cr, Mo, Se, and U by Desulfovibrio desulfuricans immobilized in polyacrylamide gels. J Ind Microbiol Biotechnol 20:13–19. 10.1038/sj.jim.2900472. [DOI] [PubMed] [Google Scholar]
- 19.Park HS, Lin S, Voordouw G. 2008. Ferric iron reduction by Desulfovibrio vulgaris Hildenborough wild type and energy metabolism mutants. Antonie Van Leeuwenhoek 93:79–85. 10.1007/s10482-007-9181-3. [DOI] [PubMed] [Google Scholar]
- 20.Lloyd JR, Ridley J, Khizniak T, Lyalikova NN, Macaskie LE. 1999. Reduction of technetium by Desulfovibrio desulfuricans: biocatalyst characterization and use in a flowthrough bioreactor. Appl Environ Microbiol 65:2691–2696. 10.1128/AEM.65.6.2691-2696.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deplanche K, Macaskie LE. 2008. Biorecovery of Gold by Escherichia coli and Desulfovibrio desulfuricans. Biotechnol Bioeng 99:1055–1064. 10.1002/bit.21688. [DOI] [PubMed] [Google Scholar]
- 22.Payne RB, Gentry DM, Rapp-Giles BJ, Casalot L, Wall JD. 2002. Uranium reduction by Desulfovibrio desulfuricans strain G20 and a cytochrome c3 mutant. Appl Environ Microbiol 68:3129–3132. 10.1128/AEM.68.6.3129-3132.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lovley DR, Phillips EJ. 1992. Reduction of uranium by Desulfovibrio desulfuricans. Appl Environ Microbiol 58:850–856. 10.1128/aem.58.3.850-856.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baxter-Plant VS, Mikheenko IP, Macaskie LE. 2003. Sulphate-reducing bacteria, palladium and the reductive dehalogenation of chlorinated aromatic compounds. Biodegradation 14:83–90. 10.1023/a:1024084611555. [DOI] [PubMed] [Google Scholar]
- 25.Lloyd JR, Yong P, Macaskie LE. 1998. Enzymatic recovery of elemental palladium by using sulfate-reducing bacteria. Appl Environ Microbiol 64:4607–4609. 10.1128/AEM.64.11.4607-4609.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yong P, Rowson NA, Farr JPG, Harris IR, Macaskie LE. 2002a. Bioreduction and biocrystallization of palladium by Desulfovibrio desulfuricans NCIMB 8307. Biotechnol Bioeng 80:369–379. 10.1002/bit.10369. [DOI] [PubMed] [Google Scholar]
- 27.Yong P, Rowson NA, Farr JPG, Harris IR, Macaskie LE. 2002b. Bioaccumulation of palladium by Desulfovibrio desulfuricans. J Chem Technol Biotechnol 77:593–601. 10.1002/jctb.606. [DOI] [PubMed] [Google Scholar]
- 28.Yong P, Farr JPG, Harris IR, Macaskie LE. 2002c. Palladium recovery by immobilized cells of Desulfovibrio desulfuricans using hydrogen as the electron donor in a novel electrobioreactor. Biotechnol Lett 24:205–212. 10.1023/A:1014141610562. [DOI] [Google Scholar]
- 29.Mabbett AN, Sanyahumbi D, Yong P, Macaskie LE. 2006. Biorecovered precious metals from industrial wastes: single-step conversion of a mixed metal liquid waste to a bioinorganic catalyst with environmental application. Environ Sci Technol 40:1015–1021. 10.1021/es0509836. [DOI] [PubMed] [Google Scholar]
- 30.Mikheenko IP, Rousset M, Dementin S, Macaskie LE. 2008. Bioaccumulation of palladium by Desulfovibrio fructosivorans wild-type and hydrogenase-deficient strains. Appl Environ Microbiol 74:6144–6146. 10.1128/AEM.02538-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Vargas I, Macaskie LE, Guibal E. 2004. Biosorption of palladium and platinum by sulfate-reducing bacteria. J Chem Technol Biotechnol 79:49–56. 10.1002/jctb.928. [DOI] [Google Scholar]
- 32.Rhodin TN, Ertl G. 1979. The nature of the surface chemical bond. North-Holland, Oxford, p 104–108. [Google Scholar]
- 33.Omajali JB, Mikheenko IP, Merroun ML, Wood J, Macaskie LE. 2015. Characterization of intracellular palladium nanoparticles synthesized by Desulfovibrio desulfuricans and Bacillus benzeovorans. J Nanopart Res 17:264. 10.1007/s11051-015-3067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartings M. 2012. Reactions coupled to palladium. Nat Chem 4:764. 10.1038/nchem.1437. [DOI] [PubMed] [Google Scholar]
- 35.Yong P, Rowson NA, Farr JPG, Harris IR, Macaskie LE. 2003. A novel electrobiotechnology for the recovery of precious metals from spent automotive catalysts. Environ Technol 24:289–297. 10.1080/09593330309385561. [DOI] [PubMed] [Google Scholar]
- 36.Tran TTT, Kannoorpatti K, Padovan A, Thennadil S. 2021. Sulphate-reducing bacteria’s response to extreme pH environments and the effect of their activities on microbial corrosion. Appl Sci 11:2201. 10.3390/app11052201. [DOI] [Google Scholar]
- 37.Kushkevych I, Dordević D, Vítězová M. 2019. Analysis of pH dose-dependent growth of sulfate-reducing bacteria. Open Med 14:66–74. 10.1515/med-2019-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilmour CC, Elias DA, Kucken AM, Brown SD, Palumbo AV, Schadt CW, Wall JD. 2011. Sulfate-reducing bacterium Desulfovibrio desulfuricans ND132 as a model for understanding bacterial mercury methylation. Appl Environ Microbiol 77:3938–3951. 10.1128/AEM.02993-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Birnbaum SJ, Wireman JW. 1984. Bacterial sulfate reduction and pH: implications for early diagenesis. Chem Geol 43:143–149. 10.1016/0009-2541(84)90144-X. [DOI] [Google Scholar]
- 40.Fukunaga S, Yoshikawa H, Fujiki K, Asano H, Yoshikawa H. 1994. Experimental investigation on the active range of sulfate-reducing bacteria for geological disposal. MRS Online Proceedings Library 353:173–180. 10.1557/PROC-353-173. [DOI] [Google Scholar]
- 41.Marietou A, Richardson D, Cole J, Mohan S. 2005. Nitrate reduction by Desulfovibrio desulfuricans: a periplasmic nitrate reductase system that lacks NapB, but includes a unique tetraheme c-type cytochrome, NapM. FEMS Microbiol Lett 248:217–225. 10.1016/j.femsle.2005.05.042. [DOI] [PubMed] [Google Scholar]
- 42.Bursakov S, Liu MY, Payne WJ, LeGall J, Moura I, Moura JJ. 1995. Isolation and preliminary characterization of a soluble nitrate reductase from the sulfate reducing organism Desulfovibrio desulfuricans ATCC 27774. Anaerobe 1:55–60. 10.1016/S1075-9964(95)80444-7. [DOI] [PubMed] [Google Scholar]
- 43.Dalsgaard T, Bak F. 1994. Nitrate reduction in a sulfate-reducing bacterium, Desulfovibrio desulfuricans, isolated from rice paddy soil: sulfide inhibition, kinetics, and regulation. Appl Environ Microbiol 60:291–297. 10.1128/aem.60.1.291-297.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caffrey SM, Park HS, Voordouw JK, He Z, Zhou J, Voordouw G. 2007. Function of periplasmic hydrogenases in the sulfate-reducing bacterium Desulfovibrio vulgaris hildenborough. J Bacteriol 189:6159–6167. 10.1128/JB.00747-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stams AJM, Hansen TA. 1982. Oxygen-labile L(+) lactate dehydrogenase activity in Desulfovibrio desulfuricans. FEMS Microbiology Lett 13:389–394. 10.1016/0378-1097(82)90197-5. [DOI] [Google Scholar]
- 46.Vita N, Valette O, Brasseur G, Lignon S, Denis Y, Ansaldi M, Dolla A, Pieulle L. 2015. The primary pathway for lactate oxidation in Desulfovibrio vulgaris. Front Microbiol 6:606. 10.3389/fmicb.2015.00606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sebban C, Blanchard L, Bruschi M, Guerlesquin F. 1995. Purification and characterization of the formate dehydrogenase from Desulfovibrio vulgaris Hildenborough. FEMS Microbiol Lett 133:143–149. 10.1111/j.1574-6968.1995.tb07875.x. [DOI] [PubMed] [Google Scholar]
- 48.Campos M, Surovtsev IV, Kato S, Paintdakhi A, Beltran B, Ebmeier SE, Jacobs-Wagner CA. 2014. Constant size extension drives bacterial cell size homeostasis. Cell 159:1433–1446. 10.1016/j.cell.2014.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fievet A, Ducret A, Mignot T, Valette O, Robert L, Pardoux R, Dolla AR, Aubert C. 2015. Single-cell analysis of growth and cell division of the anaerobe Desulfovibrio vulgaris hildenborough. Front Microbiol 6:1378. 10.3389/fmicb.2015.01378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li L, Hu J, Shi X, Fan M, Luo J, Wei X. 2016. Nanoscale zero-valent metals: a review of synthesis, characterization, and applications to environmental remediation. Environ Sci Pollut Res 23:17880–17900. 10.1007/s11356-016-6626-0. [DOI] [PubMed] [Google Scholar]
- 51.Widdel F, Bak F. 1992. Gram-negative mesophilic sulfate-reducing bacteria. In: Balows A, Trüper HG, Dworkin M, Harder W, Schleifer KH (ed). The Prokaryotes. Springer, New York, NY. [Google Scholar]
- 52.Badziong W, Thauer RK, Zeikus JG. 1978. Isolation and characterization of Desulfovibrio growing on hydrogen plus sulfate as the sole energy source. Arch Microbiol 116:41–49. 10.1007/BF00408732. [DOI] [PubMed] [Google Scholar]
- 53.Cord-Ruwisch R. 1985. A quick method for the determination of dissolved and precipitated sulfides in cultures of sulfate-reducing bacteria. J Microbiological Methods 4:33–36. 10.1016/0167-7012(85)90005-3. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 to S4. Download aem.00580-22-s0001.pdf, PDF file, 0.9 MB (984.8KB, pdf)