ABSTRACT
Bidirectional nutrient flow between partners is integral to the cnidarian-dinoflagellate endosymbiosis. However, our current knowledge of the transporter proteins that regulate nutrient and metabolite trafficking is nascent. Four transmembrane transporters that likely play an important role in interpartner nitrogen and carbon exchange were investigated with immunocytochemistry in the model sea anemone Exaiptasia diaphana (“Aiptasia”; strain NZ1): ammonium transporter 1 (AMT1), V-type proton ATPase (VHA), facilitated glucose transporter member 8 (GLUT8), and aquaporin-3 (AQP3). Anemones lacking symbionts were compared with those in symbiosis with either their typical, homologous dinoflagellate symbiont, Breviolum minutum, or the heterologous species, Durusdinium trenchii and Symbiodinium microadriaticum. AMT1 and VHA were only detected in symbiotic Aiptasia, irrespective of symbiont type. However, GLUT8 and AQP3 were detected in both symbiotic and aposymbiotic states. All transporters were localized to both the epidermis and gastrodermis, though localization patterns in host tissues were heavily influenced by symbiont identity, with S. microadriaticum-colonized anemones showing the most distinct patterns. These patterns suggested disruption of fixed carbon and inorganic nitrogen fluxes when in symbiosis with heterologous versus homologous symbionts. This study enhances our understanding of nutrient transport and host-symbiont integration, while providing a platform for further investigation of nutrient transporters and the host-symbiont interface in the cnidarian-dinoflagellate symbiosis.
IMPORTANCE Coral reefs are in serious decline, in particular due to the thermally induced dysfunction of the cnidarian-dinoflagellate symbiosis that underlies their success. Yet our ability to react to this crisis is hindered by limited knowledge of how this symbiosis functions. Indeed, we still have much to learn about the cellular integration that determines whether a particular host-symbiont combination can persist, and hence whether corals might be able to adapt by acquiring new, more thermally resistant symbionts. Here, we employed immunocytochemistry to localize and quantify key nutrient transporters in tissues of the sea anemone Aiptasia, a globally adopted model system for this symbiosis, and compared the expression of these transporters when the host is colonized by native versus nonnative symbionts. We showed a clear link between transporter expression and symbiont identity, elucidating the cellular events that dictate symbiosis success, and we provide a methodological platform for further examination of cellular integration in this ecologically important symbiosis.
KEYWORDS: Carbon, Exaiptasia diaphana, Immunolocalization, Nitrogen, Symbiodiniaceae, Transporter Proteins
INTRODUCTION
Corals reefs are one of the most prominent, productive, and diverse ecosystems in the world’s oceans. The ecological persistence of coral reefs is based upon an endosymbiotic relationship between the cnidarian host and its dinoflagellate algal partners (family: Symbiodiniaceae) (1–4). Bidirectional nutrient flow has a crucial role in this endosymbiosis, with the cnidarian host being responsible for supplying inorganic carbon, nitrogen, phosphorus, and other nutrients to the algae, while the endosymbionts provide the host with photosynthetic products (5). This exchange facilitates the survival and proliferation of coral reefs in nutrient-poor tropical seas.
Glycerol was originally considered the main form in which photosynthetically fixed carbon is translocated from symbiont to host (6, 7), though more recent studies have suggested that glucose plays the greater role (8–10). Other constituents of the translocated photosynthate include fatty acids, such as palmitic, stearic, and oleic acids, lipids such as wax esters, triacylglycerol, and sterols (11–13). Nitrogen exchange is also crucial to the symbiosis, with ammonium being especially significant, as this is the primary form in which cnidarians excrete nitrogenous waste and is easily assimilated by the algal symbionts (14–16). Symbionts may then metabolize or store assimilated nitrogenous compounds and return a portion to the host as amino acids, creating an efficient recycling pathway that benefits the symbiosis (17–20). Regulation of this nutritional exchange is important for controlling host-symbiont biomass and hence maintaining the symbiosis in a stable state (21, 22).
All mobile compounds exchanged between partners must pass through multiple plasma membranes. Small uncharged molecules (e.g., CO2, O2, or NH3) permeate the membranes, while others are transported, either passively by channel proteins or actively by transporters or pumps (e.g., ATPases) (23). Moreover, each endosymbiont is located in a host-derived late-arrested phagosome, which, together with shed algal plasma membrane and thecal vesicles, forms the “symbiosome membrane complex” that acts as the interface between the partner organisms (24–28). Therefore, exchanged nutrients and metabolites must also pass through the symbiosome membrane, the algal plasma membrane, and in many instances, the chloroplast envelope. An abundance of certain intrinsic transporter proteins, such as those for ammonium and sugar, has previously been reported to be upregulated in symbiosis, suggesting their significance in a functional, integrated symbiosis (29–32). However, our current knowledge about transporter proteins that regulate nutrient and metabolite trafficking across the symbiosome is incomplete (33–36). For instance, we know little about the extent to which these same transporters are present elsewhere in the host’s tissues or the degree to which they differ across different cnidarian-dinoflagellate pairings.
The influence of symbiont identity on symbiosis function is an important consideration, given the taxonomic and physiological diversity within the family Symbiodiniaceae (1) and the findings that some corals can respond to changing temperatures by “shuffling” or “switching” their dinoflagellate endosymbionts in favor of more thermally resistant types (37–40; but see reference 41). Durusdinium trenchii is especially interesting in this regard, given its reputation as an opportunistic species that may colonize corals postbleaching but which may provide the host with less nutritional advantage than the native symbiont and even induce cellular stress (40, 42–45). We have similarly observed reduced benefits when Symbiodinium microadriaticum is inoculated into a nonnative host (46).
In the current study, immunocytochemistry was used to localize and quantify levels of specific transporter proteins in tissues of the sea anemone Exaiptasia diaphana (= E. pallida; commonly known as Aiptasia) when in symbiosis with its homologous (i.e., native) symbiont (Breviolum minutum) and two heterologous (i.e., nonnative) Symbiodiniaceae species (D. trenchii and S. microadriaticum). Four different transporters were investigated using immunohistochemical techniques: ammonium transporter 1 (AMT1; KXJ28516); V-type proton ATPase (VHA; KXJ14631); facilitated glucose transporter member 8 (GLUT8; KXJ23301); and aquaporin-3 (AQP3; KXJ09068). Sugar transporters GLUT8 and AQP3 have been previously predicted, via sequence homology, to be localized to the symbiosome membrane (36), while VHA and AMT1 are upregulated at the protein level in the symbiotic state (32, 45). Moreover, VHA has been immunolocalized to the symbiosome surface in the corals Acropora yongei and Stylophora pistillata (34). Here, we localized candidate transporters with respect to tissue layer (i.e., epi- and gastrodermis) and used a semiquantitative approach to establish the influence of symbiotic state and symbiont identity on transporter presence and localization. We hypothesized that our selected transporters would be downregulated in the presence of heterologous versus homologous symbiont species, reflecting impaired host-symbiont functional integration.
RESULTS
Colonization success.
No symbionts were detected by confocal examination of aposymbiotic anemones before colonization or in control aposymbiotic anemones at the end of the experiment (see Fig. S4 in the supplemental material). Sanger internal transcribed spacer 2 (ITS2) sequencing confirmed the genetic identities of symbionts in the colonized hosts. At the end of the experiment, symbiont density was approximately 3-fold greater in anemones hosting B. minutum than in those colonized by D. trenchii or S. microadriaticum (one-way analysis of variance [ANOVA], P = 2.2 × 10−7) (Fig. 1), consistent with previous studies (45, 46).
FIG 1.

Colonization densities of different symbiont species (B. minutum, D. trenchii, S. microadriaticum) in Aiptasia, 8 weeks postinoculation. Different letters above bars indicate significant differences between treatments (P < 0.05). Values are means ± standard errors (n = 24).
Localization of transporters in the native symbiosis.
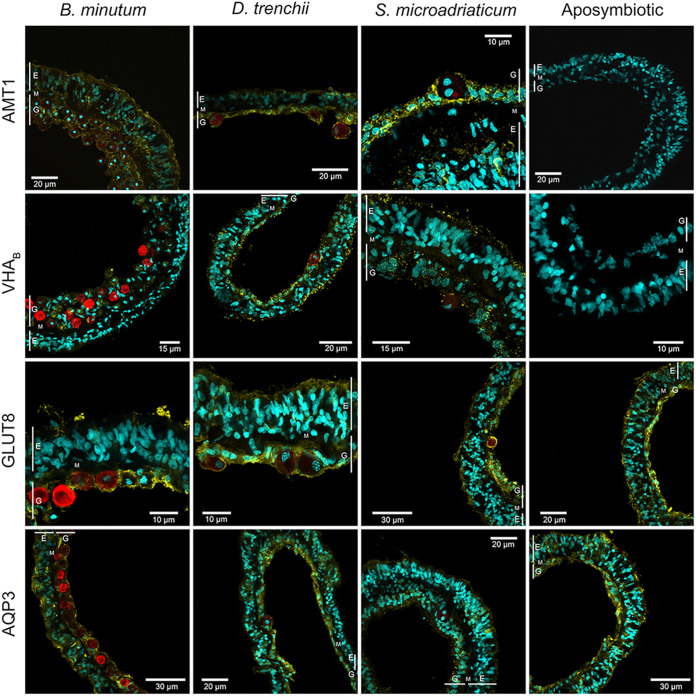
Symbiosis with the homologous B. minutum induced distinct changes in the abundances of the various nutrient transporters compared to the aposymbiotic state, as shown in immunoblots (see Fig. S2 in the supplemental material). These patterns were the same as those observed with immunolocalization (Fig. 2). Nevertheless, symbiont cell density did not correlate with the observed fluorescence intensity of any of the transporters in either tissue layer (see Fig. S5) (P > 0.05). AMT1 and VHA were exclusively detected in the symbiotic state, although predominantly in different tissues (Fig. 3). Specifically, AMT1 had 1.7-fold greater intensity in the epidermis than the gastrodermis (two-way mixed ANOVA, P = 1.03 × 10−6, Bonferroni post hoc test), while VHA intensity was 3.1-fold greater in the gastrodermis than in the epidermis (P = 4.85 × 10−7). In contrast, GLUT8 and AQP3 were present in both the aposymbiotic and symbiotic states, though their intensity patterns were dissimilar. Specifically, GLUT8 intensity was the same in both aposymbiotic and symbiotic anemones, with an even distribution in both host tissue layers, while AQP3 intensity was 4.7-fold higher in the aposymbiotic state (P = 3.27 × 10−27) and also 1.5-fold higher in the epidermis than gastrodermis of B. minutum-colonized anemones. Three-dimensional (3D) reconstructions of isolated symbiont-containing host cells indicated that VHA, AMT, and AQP transporters were present on the symbiosome membrane (single-cell samples for GLUT8 were not available); however, these transporters were not exclusively localized to the symbiosome. For improved clarity, 3D reconstructions are presented as videos and images available at GitHub (https://github.com/Amir-Mashini/Aiptasia-Transporters-Quantification-Project/tree/main/3D%20Reconstruction).
FIG 2.
Immunofluorescence localization of nutrient transporters in aposymbiotic and symbiotic Aiptasia hosting B. minutum, D. trenchii, and S. microadriaticum. Aposymbiotic anemones showed no visible presence of AMT1 or VHAB. Red, chlorophyll autofluorescence of symbionts; cyan, nuclear staining of host and symbionts using DAPI; yellow, anti-AMT1, anti-VHAB, anti-GLUT8, and anti-AQP3 localization using AlexaFluor555-conjugated secondary antibody. Host tissue layers are labeled as follows: E, epidermis; M, mesoglea; G, gastrodermis. Note: All images were obtained using a 100× oil immersion objective (Olympus). Images shown here are for localization only, rather than quantification purposes, and were taken with different microscope settings. All the patterns shown here were consistent across all samples. For comparative quantification, see Fig. 3.
FIG 3.
Fluorescence signal intensities of quantified transporters AMT1 (A), VHAB (B), GLUT8 (C), and AQP3 (D) in Aiptasia tentacles in different symbiotic states. Two-way mixed ANOVA was performed to compare intensity levels between symbiotic states and tissue layers. Asterisks indicate significant differences between tissue layers of each treatment (**, P < 0.01; ****, P < 0.0001). Whiskers show upper and lower quartiles. All images were obtained using a 100× oil immersion objective (Olympus).
Effects of symbiont identity on transporter localization.
The presence of heterologous symbionts influenced transporter localization (Fig. 3). Unlike in the native symbiosis, D. trenchii-colonized anemones had AMT1 distributed evenly across both tissue layers, although the fluorescence intensity was reduced by about half in the epidermis relative to that of B. minutum (P = 4.89 × 10−6). VHA in D. trenchii-colonized anemones exhibited a localization pattern similar to that in B. minutum-colonized anemones, with an intensity 2.1-fold greater in the gastrodermis than epidermis (P = 5.18 × 10−3), though this gastrodermal intensity was weaker than in the native symbiosis (P = 1.16 × 10−3); epidermal intensity was similar between the two symbioses. In contrast, levels of GLUT8 in D. trenchii-colonized anemones were similar across the gastrodermis and epidermis and similar in both intensity and pattern to those in anemones populated by B. minutum. AQP3 was likewise observed at similar levels in the epidermis and gastrodermis of D. trenchii-colonized anemones, though in both tissue layers this transporter had a higher intensity (2-fold) than in the native symbiosis (epidermis: P = 1.56 × 10−3; gastrodermis: P = 1.31 × 10−7).
S. microadriaticum-colonized hosts showed the most distinct protein localization patterns from those in the native symbiosis. For example, AMT1 levels were higher (1.5-fold) in the gastrodermis than the epidermis (P = 1.39 × 10−3). This difference resulted from a 36% decrease in AMT1 intensity in the epidermis and a 1.6-fold increase in the gastrodermis of S. microadriaticum-colonized anemones relative to the native symbiosis (P = 2 × 10−3 and P = 2.22 × 10−4, respectively). Unlike in B. minutum-colonized anemones, S. microadriaticum-colonized anemones exhibited an even distribution of VHA across both tissue layers, though the intensity was about 7 times less than in the presence of the homologous symbionts (epidermis: P = 1.16 × 10−3; gastrodermis: P = 2.22 × 10−8). Furthermore, in contrast to the distribution seen with B. minutum (and indeed D. trenchii), GLUT8 and AQP3 were relatively more abundant in the gastrodermis than epidermis of S. microadriaticum-colonized anemones (~1.5-fold; P = 4.49 × 10−3 and P = 3.5 × 10−3, respectively). Compared to the native symbiosis, GLUT8 intensity in both tissue layers of S. microadriaticum-colonized anemones was about 1.7-fold higher (epidermis: P = 6.22 × 10−16; gastrodermis: P = 9.65 × 10−21), while AQP3 intensity was significantly higher only in the gastrodermis (2.6-fold; P = 4.75 × 10−5).
DISCUSSION
This study is the first to report the cellular localization of AMT1, GLUT8, and AQP3 and their relative quantities in the tissue layers of a cnidarian-dinoflagellate model system. These metabolite transporters were selected because of their role in the translocation of nitrogen and fixed carbon, both of which are enormously important in a functional cnidarian-dinoflagellate symbiosis. Previously, only VHA and the sterol transporters NPC1 and NPC2 have been immunolocalized in symbiotic cnidarians (34, 35). Each transporter studied exhibited distinct patterns of abundance and localization in response to symbiotic state and/or symbiont identity, which are discussed below. We interpret these patterns in light of our antibody validation, which strongly suggests specific binding to our target transporters, even though some limited, nontargeted binding is always possible. Furthermore, while the homologous symbiont species reached a higher population density than the two heterologous species (which attained similar densities), this alone cannot explain the array of localization patterns observed across all three host-symbiont pairings.
Ammonium transport.
Nitrogen fluxes are crucial for corals in oligotrophic tropical waters (47–49). The dinoflagellate symbionts are the primary site of ammonium assimilation; however, it has been suggested that both partners can take up ammonium, including from the surrounding seawater (50–53). Accordingly, here AMT1 was localized to both host epidermis and gastrodermis, though it was only observed in the symbiotic state. Similarly, AMT1 has previously been found to be upregulated during symbiosis in Aiptasia, in both the transcriptome (30, 54) and proteome (32), and to be downregulated after heat shock and bleaching (55).
Host epidermal cells take up ammonium directly from the surrounding seawater (15). Given that photosynthesis drives host ammonium uptake (21, 56, 57), there is a clear link between AMT1 abundance and symbiont photophysiology. Consistent with this, B. minutum-colonized hosts exhibited greater fluorescence intensity of AMT1 in their epidermis than anemones colonized by either of the two heterologous symbionts, while AMT1 was below the level of detection in aposymbiotic anemones; indeed, AMT1 is absent in the proteome of aposymbiotic Aiptasia (32). Of note, D. trenchii and S. microadriaticum have previously been reported to be less nutritionally beneficial partners than B. minutum when experimentally introduced into Aiptasia, while D. trenchii is widely acknowledged as an opportunistic species (38, 40, 41, 45, 46, 58). Once assimilated, however, the host actively regulates nitrogen supply to the symbionts through the glutamine synthetase and glutamine 2-oxoglutarate amino-transferase pathways, thereby maintaining a low ammonium concentration in the gastrodermal cytoplasm (17, 18, 57). This might explain the opposite pattern of AMT1 expression seen in the gastrodermis versus epidermis, though why S. microadriaticum-colonized anemones exhibited a greater intensity of gastrodermal AMT1 than either the B. minutum- or D. trenchii-colonized anemones is unclear. One suggestion is that S. microadriaticum might be co-opting host metabolism, thereby enriching itself with nitrogen even though its population growth is still apparently restricted by the host. More detailed physiological examination of ammonium uptake and metabolism is needed, to more fully understand the transporter localization patterns observed here and what they can tell us about the consequences of hosting different symbiont species.
Proton pumps and acidification.
Proton transporters are central to pH homeostasis in corals (59). VHA, a member of the P-type ATPase superfamily, is found in all domains of life (60). VHA is primarily found in the vacuolar membrane as well as the Golgi complex, endosomes, and synaptic vesicles, where it acidifies these organelles by hydrolysis of ATP and transport of protons across the membrane (34, 61). Similar to our study, a proteomic survey reported significantly increased abundance (12-fold) of VHA in symbiotic versus aposymbiotic Aiptasia (32). In corals, VHA is abundant in the oral gastrodermis in the space immediately surrounding the endosymbionts, where it plays a role in the host’s carbon-concentrating mechanism by acidifying the symbiosome space and facilitating photosynthesis (34). Consistent with these previous observations, VHA in the current study was most abundant in the gastrodermis. In addition, VHA facilitates CO2 uptake by host epidermal cells, as exportation of protons decreases the pH of ambient seawater, increasing the dehydration of HCO3− to CO2 (62). The localization of VHA to the epidermis here was, therefore, unsurprising.
VHA levels were influenced by symbiont identity, being more abundant in both the gastrodermis and epidermis when anemones were in symbiosis with B. minutum or D. trenchii than with S. microadriaticum. Proton ATPases have been reported in other mutualistic associations, where they are actively excluded by symbionts through different mechanisms in parasitic symbioses to prevent digestion by the host via acidification of phagosomes (63–66). However, whether the low abundance of VHA in S. microadriaticum-colonized anemones is due to symbionts actively inhibiting host digestion or due to the host reducing the symbiosome CO2 concentration to limit less beneficial symbionts is unknown.
Carbon translocation.
In the current study, only hosts harboring heterologous symbionts had significantly higher levels of GLUT8 than aposymbiotic animals, with S. microadriaticum-colonized anemones possessing more GLUT8 in both the gastrodermis and epidermis than the other anemones. Translocation of fixed carbon to the host has been researched intensively (5, 67), and glucose has been proposed as the primary form of fixed carbon translocated, along with glycerol and other hexoses (7–10). SLC2 (GLUT) is a major facilitator superfamily of transmembrane transporters that mediates the transport of monosaccharides, polyols, and other small compounds across the membranes of eukaryotic cells (68, 69). GLUT8 is mostly localized to endosomal compartments; however, it can also be present at the cell surface (70–73). In cnidarians, GLUT8 has been hypothesized to have a primary role in glucose uptake by the host (36), and molecular evidence has indicated its upregulation in symbiosis (30, 32). Why a heterologous symbiont induces the greatest levels of GLUT8 is not clear, but it might be due to increased efforts by the host to acquire more fixed carbon when in symbiosis with less nutritionally beneficial symbionts (46). Indeed, the latter study showed that, when in symbiosis with Aiptasia, gross photosynthesis and the ratio of photosynthesis to respiration were similar in B. minutum- and D. trenchii-colonized anemones, but higher than in S. microadriaticum-colonized anemones (46).
Members of the aquaporin water channel family primarily render the lipid bilayer of cell membranes permeable to water, which is vital for cell osmoregulation and homeostasis (74). Aquaglyceroporins, a subset of the AQP family, are also permeable to glycerol and specific small, uncharged solutes (75, 76). AQP3 channels were previously predicted to be localized to the outer symbiosome surface (36). Aquaglyceroporins are involved in glycerol metabolism, and several reports have shown elevated levels during fasting and downregulation after feeding in other organisms (77–81). Similarly, in corals, inhibition of photosynthesis by 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) causes higher expression levels of AQP transcripts in host tissues, suggesting a potential cellular response to compensate for reduced availability of photosynthetic products (82). Consistent with this, we report elevated levels of AQP3 in the aposymbiotic state and enhanced AQP3 levels in the presence of the putatively less beneficial heterologous versus more beneficial homologous symbionts. This presumably reflects the reduced supply of photosynthetic carbon in the aposymbiotic state and the presence of less beneficial symbionts relative to the normal symbiotic state.
Conclusion.
Our findings demonstrate the effects of symbiotic state and symbiont identity on the quality and quantity of select metabolite transporters. Acquisition of novel symbionts has been proposed as a temporary strategy in response to different environmental stressors, such as increasing temperature (39, 83). However, D. trenchii and S. microadriaticum have been previously observed to be less beneficial when inoculated into Aiptasia (40, 43, 45, 46), while D. trenchii is a well-known opportunist on coral reefs, proliferating after coral bleaching events despite a relatively low level of nutritional benefit to the host (42, 58, 84). Consistent with this, our results suggest that anemones harboring heterologous symbionts show reduced metabolite transporter compatibility relative to anemones containing the native symbiont, at least with respect to the transporters targeted here. Further research investigating these and other transmembrane transporters, including their colocalization patterns and unequivocal confirmation of their presence on the symbiosome membrane, is needed in both model systems and reef corals to understand the mechanisms required for fully integrated metabolic exchange, symbiosis success, and their implications for coral responses to climate change.
MATERIALS AND METHODS
Experimental organisms.
Long-term cultures (>10 years) of three Symbiodiniaceae species were used: the homologous B. minutum (culture ID FLAp2; original host Aiptasia) and heterologous D. trenchii (culture ID Ap2; original host was an unknown sea anemone from Okinawa, Japan) and S. microadriaticum (culture ID CCMP2467; original host was the coral Stylophora pistillata). These cultures were grown in 0.22-μm-filtered f/2 culture medium at 25°C and 100 μmol photons m−2 s−1 irradiance (fluorescent OSRAM L 58W/840) on a 12 h:12 h light:dark cycle, and subcultured 8 weeks prior to experimental use. To confirm the identity of these cultures, DNA was extracted using the cetyltrimethylammonium bromide–phenol-chloroform method (85), and the ITS2 region of ribosomal DNA was amplified by PCR using ITSintfor2 (5′-GAATTGCAGAACTCCGTG-3′) and ITS2Rev2 (5′-CCTCCGCTTACTTATATGCTT-3′) as the forward and reverse primers, respectively (86, 87). The total volume of each PCR mixture was 25 μL, and each mixture contained 1 ng template DNA, 1 mM total deoxynucleoside triphosphate, 7.5 pmol of each primer, and 1.5 U of Taq DNA polymerase. A Qiagen thermocycler was used with the following cycling regimen: 3 min at 95°C for initial denaturation, 35 cycles of 15 s at 95°C, 15 s at 56°C, and 10 s at 72°C, and a final extension of 3 min at 72°C. Amplicons were directly sequenced (Sanger sequencing; Macrogen Inc., Seoul, South Korea), sequences were aligned with Geneious Prime v. 2019.2.3 (Biomatters Ltd., Auckland, New Zealand), and a custom BLAST search was performed against Symbiodiniaceae ITS2 sequences in Geosymbio (88).
Specimens of Aiptasia (n ~ 400) were taken from a clonal laboratory stock (strain NZ1) and rendered aposymbiotic (i.e., symbiont-free) by incubating them in 0.19 mM menthol with 5 μM 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU), an inhibitor of photosynthetic electron transport, for 4 weeks in the dark at 25°C (89). The absence of symbionts was confirmed via a lack of chlorophyll autofluorescence, assessed by confocal microscopy (100× magnification; Olympus Provis AX70).
Inoculation of Aiptasia with symbiotic algae.
Aposymbiotic anemones were maintained in 0.22-μm-filtered seawater (FSW) in the dark at 25°C and starved for 1 week prior to one-time inoculation with algal cultures. One drop of symbiont cells at ~3 × 106 cells mL−1, mixed with a dilute suspension of Artemia sp. nauplii to induce phagocytosis, was pipetted onto each anemone’s oral disk (n ~ 100 anemones per symbiont species). Anemones were incubated in this symbiont suspension for 24 h before the FSW was changed. After this step, all anemones, including aposymbiotic anemones, were maintained for 8 weeks at 25°C under 100 μmol photons m−2 s−1 irradiance on a 12 h:12 h light:dark cycle. All anemones were fed and FSW was refreshed three times per week. At the end of the experiment, anemones were flash-frozen in liquid nitrogen or used for immediate downstream analysis. Symbiont genetic identity was confirmed again after the experiment, using the DNA extraction and Sanger sequencing method described above. Symbiont cell densities were measured by using a hemocytometer (Improved Neubauer), and one-way ANOVA was used to compare the population density of different species.
Polyclonal antibody design.
Prospective transporter proteins were selected from previous studies on Aiptasia (30, 32, 36), and their sequences and those of homologous proteins from other model organisms were searched against the available Aiptasia genome (31) and transcriptomes (30, 90), using BLAST at an E value threshold of <1 × 10−10. Highly similar sequences (>40% identical) were selected as candidates for antibody generation (Geneious Prime v2019.2.3; Biomatters Ltd.). A 40% similarity criterion was selected to ensure that similarity was higher than the standard threshold (>30%) (91).
Antigenic regions of each candidate transporter were predicted by EMBOSS (92), and epitopes were selected based on a set of criteria that prioritized (1) antigenicity, (2) whether the epitopes were external to the lipid bilayer, and (3) conservation level (see Fig. S1 in the supplemental material). In addition, the similarity of each selected epitope was compared to the protein sequences encoded on the genome of the animal used to generate the antibodies (Oryctolagus cuniculus, European rabbit) and on the Aiptasia and B. minutum genome and transcriptomes (30, 31, 93), to maintain high antigenicity and limit cross-reactivity.
Custom rabbit polyclonal antibodies against AMT1, GLUT8, and AQP3 were developed using synthetic peptide antigens for the candidate transporters (see Table S1 in the supplemental material) (Genscript USA, Inc.). The rabbits were immunized with 200 μg keyhole limpet hemocyanin (KLH)-conjugated peptide antigens, followed by three boosts of 200 μg antigen every 2 weeks (KLH conjugation is used to increase peptide antigenicity). Antibody titers were evaluated by enzyme-linked immunosorbent assay, 63 days after the initial injection. An affinity column with 5 mg peptide antigen was used for antibody purification (Genscript USA, Inc.), ensuring that only antibodies raised against the peptide were purified. Premade polyclonal rabbit anti-VHA subunit B was obtained from Genscript (catalog number A00960), because the selected regions of the protein used for antibody generation (KLH coupled) were highly similar to regions of the Aiptasia homolog (see Fig. S1 in the supplemental material).
Protein extraction.
Aposymbiotic and symbiotic anemones were flash frozen and those of the same type were pooled (n ~ 5 per pool) to yield a minimum of 1 μg/μL protein per sample. Total proteins of aposymbiotic anemones were extracted by homogenizing the frozen samples in protein extraction buffer (100 mM Tris, 100 mM NaCl, 10 mM EDTA, 5% SDS; pH 7.4) using a tissue homogenizer at 4°C and then denaturing the proteins at 80°C for 15 min in the presence of 5% β-mercaptoethanol. The homogenate was centrifuged at 20,238 × g for 10 min, and the supernatant was decanted and used for downstream analysis. Frozen symbiotic anemones were also mechanically homogenized in 500 μL FSW, and the resulting homogenate was centrifuged at 500 × g for 5 min to separate host (supernatant) and symbiont (pellet) fractions; total protein of the symbiotic host fraction was extracted as described above. Symbiont pellets were resuspended in 500 μL FSW and then passed through a 23-gauge needle. Symbiont suspensions were centrifuged again at 500 × g for 5 min to remove host contamination and finally resuspended in protein extraction buffer. Algal resuspensions were ultrasonicated for 1 min to break the thecal plates, before being submitted to the aforementioned extraction method. Protein concentrations were assayed with a Qubit 2.0 fluorometer using a protein assay kit (ThermoFisher Scientific).
Immunoblotting.
Expression of all candidate transporters was confirmed via immunoblotting with antibodies against AMT1, VHAB, GLUT8, and AQP3. A total of 30 μg extracted protein from each sample, including symbiotic host fraction, aposymbiotic host, and symbionts, was loaded and resolved by Tris-glycine SDS-PAGE with a molecular weight standard (PageRuler prestained protein ladder; ThermoFisher Scientific). The gel concentration was 10% polyacrylamide for AMT1, GLUT8, and VHAB and 15% polyacrylamide for AQP3. Resolved proteins were transferred to 0.45-μm polyvinylidene difluoride (PVDF) membranes overnight using Tris-glycine-methanol transfer buffer (94) in a wet blotting system (Mini-PROTEAN Tetra Cell; Bio-Rad). PVDF membranes were washed three times for 5 min with Tris-buffered saline containing 0.1% Tween 20 (TBST) and then blocked with fresh TBST buffer and 5% fat-free milk at room temperature for 1 h. Membranes were incubated with custom-made polyclonal primary antibodies overnight at 4°C with shaking (see Table S1 for specifics of each antibody incubation). Membranes were washed three times with TBST and then incubated with the secondary antibody (1:2,000 goat anti-rabbit IgG conjugated with AlexaFluor555; ab150078; Abcam) for 2 h in the dark at room temperature with shaking. The membranes were then washed three times with TBST, and the immunoreactive proteins were visualized with a Typhoon FLA 9500 laser scanner (GE Healthcare Life Sciences). As a control, the procedure was performed as described above but without inclusion of the primary antibodies. Immunizing peptide-blocking experiments were performed to check and validate specificity of all primary antibodies by incubating them with double the concentration of peptide antigen in TBST–5% milk powder buffer at room temperature for 2 h. The preimmune serum and preadsorbed primary antibodies were used for immunodetection; neither the preadsorbed serum nor secondary antibodies reacted on immunoblots. Moreover, antibodies in the preimmune sera reacted with nonspecific proteins of different molecular weights (see Fig. S2 in the supplemental material).
Immunolocalization.
Whole symbiotic anemones harboring different Symbiodiniaceae species and aposymbiotic anemones were fixed in freshly made 4% paraformaldehyde in FSW at 4°C overnight. The whole, fixed anemones were fully submerged in 10%, 20%, and 30% sucrose as a cryoprotectant at 4°C, embedded with OCT medium, and rapidly frozen in liquid nitrogen, and their tentacles were sectioned (12 μm) at −20°C (CM3050 cryostat; Leica Biosystems). The cryosections were mounted on positively charged slides and permeabilized with phosphate-buffered saline (PBS) buffer containing 0.1% Triton X-100 in a humidified bath, followed by three washes of PBS–0.1% Tween. Sections were immersed in blocking buffer (PBS–0.1% Tween containing 10% normal goat serum and 1% bovine serum albumin), and maintained for 2 h at room temperature. Sections were then incubated with different concentrations of primary antibodies in blocking buffer at 4°C overnight (see Table S1), followed by incubation with secondary antibodies (1:500) for 2 h at room temperature. Mounting medium containing 2 μg mL−1 DAPI, for DNA staining, was applied to sections before sealing with a coverslip. In the controls, the primary antibody was preadsorbed with the peptides used for monospecific antibody generation, as described above, except that the PBS–0.1% Tween buffer was used instead of TBST. Peptide antigen preadsorption and secondary antibody controls did not show any binding reaction with the cryosections (Fig. S3). Aposymbiotic and symbiotic negative controls were also included. These were treated as above, except that no antibody was added to the solutions. To assess differential patterns of each transporter among the different host tissue layers, cryosections were treated identically (i.e., primary antibody concentration and incubation time).
Immunolabelled sections were visualized by confocal laser scanning microscopy (Fluoview FV3000, Olympus, Japan) at 100× magnification. A combination of 4′,6-diamidino-2-phenylindole (DAPI), AlexaFluor 555, and AlexaFluor 647 was used for fluorescence visualization of the DNA and nucleus (430 to 460 nm), the location of the proteins that reacted with the primary antibodies (570 to 589 nm), and chlorophyll autofluorescence (650 to 671nm). A total of 261 z-stacks (nominal thickness, >10 μm) were analyzed for semiquantitative comparisons of transporter abundance and localization in the host tissue layers. We analyzed two tentacles in each of 10 anemones for localization of each of the different transporters (Table S2). All z-stacks were acquired with the same settings (95–99).
In addition to the whole tissue sections, isolated host cells containing symbionts were examined. These cells were generated opportunistically during the inadvertent mechanical disruption of the sections on the slides caused by placement of the coverslips. Cells were selected based on the visibility of the host cell nucleus outside the enclosed symbiont cell(s) and ideally the presence of a dividing symbiont cell doublet where the symbiosome membrane was visible, stretched between each half of the doublet. Three-dimensional images were constructed from z-stacks (details as above) and converted into videos to enhance their clarity by using Olympus FV31S-SW software. These cellular reconstructions are presented only in those cases where immunolabelling of the symbiosomal region was sufficiently clear; given this condition, no single-cell videos of GLUT8 labeling are available.
Semiquantitative confocal analysis.
An analysis pipeline was developed to quantify the relative AlexaFluor555 intensity in each host tissue layer, as well as host tissue volume and the number of symbiont cells in each z-stack, using FIJI (ImageJ2) (100, 101). All z-stacks were split into different channels and 3D gaussian blur and background subtraction methods were used to smooth the images and remove noise from all channels prior to downstream analyses (102, 103). The AlexaFluor555 channel was manually segmented to acquire images from host gastrodermis tissue. Total volume and signal intensities were quantified in segmented and unsegmented AlexaFluor555 gastrodermis images using a 3D object counter plugin (104). Volumes and signal intensities of epidermal objects were calculated by subtracting the total of the gastrodermal values from the unsegmented AlexaFluor555 channel. Symbiont cells were designated regions of interest by creating a mask via thresholding intensities of the AlexaFluor647 channel, corresponding to chlorophyll autofluorescence. Overlapping symbiont cells were separated by applying a round of 3D watershed splitting (103). All symbiont objects were counted using the same plugin. Host tentacle volume for each z-stack was quantified as described above, using the brightfield channel.
AlexaFluor555 intensity and the number of symbiont cells were each normalized to host tissue volume, the latter for the estimation of symbiont density. Data normality was checked with the Shapiro-Wilk test, and a mixed two-way ANOVA was used to compare differential expression of transporters in the different host tissue layers in both aposymbiotic and symbiotic anemones. This was followed by a Bonferroni post hoc test to determine if significant differences occurred in the various pairwise comparisons. Correlations between homologous symbiont density and transporter signal intensity of epidermis and gastrodermis were investigated using the Spearman correlation coefficient. All data analyses were conducted in R 4.0.3 (105). The z-stack image analysis pipelines, FIJI scripts, data, and supplementary files are available at GitHub (https://github.com/Amir-Mashini/Aiptasia-Transporters-Quantification-Project).
ACKNOWLEDGMENTS
This research was supported by a grant from the Marsden Fund of the Royal Society Te Apārangi of New Zealand (grant number VUW1601), awarded to S.K.D., A.R.G., V.M.W., and C.A.O., and a Ph.D. scholarship from Victoria University of Wellington awarded to A.G.M.
Footnotes
Supplemental material is available online only.
Contributor Information
Simon K. Davy, Email: simon.davy@vuw.ac.nz.
Laura Villanueva, Royal Netherlands Institute for Sea Research.
REFERENCES
- 1.LaJeunesse TC, Parkinson JE, Gabrielson PW, Jeong HJ, Reimer JD, Voolstra CR, Santos SR. 2018. Systematic revision of Symbiodiniaceae highlights the antiquity and diversity of coral endosymbionts. Curr Biol 28:2570–2580.e6. 10.1016/j.cub.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Muscatine L, Porter JW. 1977. Reef corals: mutualistic symbioses adapted to nutrient-poor environments. Bioscience 27:454–460. 10.2307/1297526. [DOI] [Google Scholar]
- 3.Polovina JJ. 1984. Model of a coral reef ecosystem. Coral Reefs 3:1–11. 10.1007/BF00306135. [DOI] [Google Scholar]
- 4.Sheppard C, Davy SK, Pilling GM, Graham NAJ. 2018. Coral reefs, p 1–34. In The Biology of Coral Reefs, 2nd ed. Oxford University Press, Oxford, England. [Google Scholar]
- 5.Davy SK, Allemand D, Weis VM. 2012. Cell biology of cnidarian-dinoflagellate symbiosis. Microbiol Mol Biol Rev 76:229–261. 10.1128/MMBR.05014-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muscatine L. 1965. Symbiosis of Hydra and algae. III. Extracellular products of the algae. Comp Biochem Physiol 16:77–92. 10.1016/0010-406x(65)90165-9. [DOI] [PubMed] [Google Scholar]
- 7.Muscatine L. 1967. Glycerol excretion by symbiotic algae from corals and Tridacna and its control by the host. Science 156:516–519. 10.1126/science.156.3774.516. [DOI] [PubMed] [Google Scholar]
- 8.Burriesci MS, Raab TK, Pringle JR. 2012. Evidence that glucose is the major transferred metabolite in dinoflagellate–cnidarian symbiosis. J Exp Biol 215:3467–3477. 10.1242/jeb.070946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suescún-Bolívar LP, Traverse GMI, Thomé PE. 2016. Glycerol outflow in Symbiodinium under osmotic and nitrogen stress. Mar Biol 163:128. 10.1007/s00227-016-2899-6. [DOI] [Google Scholar]
- 10.Hillyer KE, Dias DA, Lutz A, Wilkinson SP, Roessner U, Davy SK. 2017. Metabolite profiling of symbiont and host during thermal stress and bleaching in the coral Acropora aspera. Coral Reefs 36:105–118. 10.1007/s00338-016-1508-y. [DOI] [Google Scholar]
- 11.Yamashiro H, Oku H, Higa H, Chinen I, Sakai K. 1999. Composition of lipids, fatty acids and sterols in Okinawan corals. Comp Biochem Physiol B 122:397–407. 10.1016/S0305-0491(99)00014-0. [DOI] [Google Scholar]
- 12.Dunn SR, Thomas MC, Nette GW, Dove SG. 2012. A lipidomic approach to understanding free fatty acid lipogenesis derived from dissolved inorganic carbon within cnidarian-dinoflagellate symbiosis. PLoS One 7:e46801. 10.1371/journal.pone.0046801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hillyer KE, Tumanov S, Villas-Bôas S, Davy SK. 2016. Metabolite profiling of symbiont and host during thermal stress and bleaching in a model cnidarian-dinoflagellate symbiosis. J Exp Biol 219:516–527. 10.1242/jeb.128660. [DOI] [PubMed] [Google Scholar]
- 14.Muller-Parker G, Hoegh-Guldberg O. 1994. Effect of ammonium enrichment on animal and algal biomass of the coral Pocillopora damicornis. Pacific Sci 48:273–283. [Google Scholar]
- 15.Pernice M, Meibom A, Van Den Heuvel A, Kopp C, Domart-Coulon I, Hoegh-Guldberg O, Dove S. 2012. A single-cell view of ammonium assimilation in coral-dinoflagellate symbiosis microbe-microbe and microbe-host interactions. ISME J 6:1314–1324. 10.1038/ismej.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ezzat L, Fine M, Maguer JF, Grover R, Ferrier-Pagès C. 2017. Carbon and nitrogen acquisition in shallow and deep holobionts of the scleractinian coral S. pistillata. Front Mar Sci. 10.3389/fmars.2017.00102. [DOI] [Google Scholar]
- 17.Wang JT, Douglas AE. 1998. Nitrogen recycling or nitrogen conservation in an alga-invertebrate symbiosis? J Exp Biol 201:2445–2453. 10.1242/jeb.201.16.2445. [DOI] [PubMed] [Google Scholar]
- 18.Wang JT, Douglas AE. 1999. Essential amino acid synthesis and nitrogen recycling in an alga-invertebrate symbiosis. Mar Biol 135:219–222. 10.1007/s002270050619. [DOI] [Google Scholar]
- 19.Reynaud S, Martinez P, Houlbrèque F, Billy I, Allemand D, Ferrier-Pagès C. 2009. Effect of light and feeding on the nitrogen isotopic composition of a zooxanthellate coral: role of nitrogen recycling. Mar Ecol Prog Ser 392:103–110. 10.3354/meps08195. [DOI] [Google Scholar]
- 20.Kopp C, Pernice M, Domart-Coulon I, Djediat C, Spangenberg JE, Alexander DTL, Hignette M, Meziane T, Meibom A. 2013. Highly dynamic cellular-level response of symbiotic coral to a sudden increase in environmental nitrogen. mBio 4:e00052-13. 10.1128/mBio.00052-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falkowski PG, Dubinsky Z, Muscatine L, McCloskey L. 1993. Population control in symbiotic corals. Bioscience 43:606–611. 10.2307/1312147. [DOI] [Google Scholar]
- 22.Rädecker N, Pogoreutz C, Gegner HM, Cárdenas A, Roth F, Bougoure J, Guagliardo P, Wild C, Pernice M, Raina JB, Meibom A, Voolstra CR. 2021. Heat stress destabilizes symbiotic nutrient cycling in corals. Proc Natl Acad Sci USA 118:e2022653118. 10.1073/pnas.2022653118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lodish H, Berk A, Kaiser CA, Amon A, Ploegh H, Bretscher A, Krieger M, Martin KC. 2016. Molecular Cell Biology, 8th ed. Macmillan Learning, New York, N.Y. [Google Scholar]
- 24.Hohman TC, McNeil PL, Muscatine L. 1982. Phagosome-lysosome fusion inhibited by algal symbionts of Hydra viridis. J Cell Biol 94:56–63. 10.1083/jcb.94.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fitt WK, Trench RK. 1983. Endocytosis of the symbiotic dinoflagellate Symbiodinium microadriaticum Freudenthal by endodermal cells of the scyphistomae of Cassiopeia xamachana and resistance of the algae to host digestion. J Cell Sci 64:195–212. 10.1242/jcs.64.1.195. [DOI] [PubMed] [Google Scholar]
- 26.Neckelmann N, Muscatine L. 1983. Regulatory mechanisms maintaining the Hydra-Chlorella symbiosis. Proc R Soc London Ser B Biol Sci 219:193–210. [Google Scholar]
- 27.Wakefield TS, Farmer MA, Kempf SC. 2000. Revised description of the fine structure of in situ “Zooxanthellae” genus Symbiodinium. Biol Bull 199:76–84. 10.2307/1542709. [DOI] [PubMed] [Google Scholar]
- 28.Wakefiel TS, Kempf SC. 2001. Development of host- and symbiont-specific monoclonal antibodies and confirmation of the origin of the symbiosome membrane in a cnidarian-dinoflagellate symbiosis. Biol Bull 200:127–143. 10.2307/1543306. [DOI] [PubMed] [Google Scholar]
- 29.Peng SE, Wang YB, Wang LH, Chen WNU, Lu CY, Fang LS, Chen CS. 2010. Proteomic analysis of symbiosome membranes in Cnidaria - dinoflagellate endosymbiosis. Proteomics 10:1002–1016. 10.1002/pmic.200900595. [DOI] [PubMed] [Google Scholar]
- 30.Lehnert EM, Mouchka ME, Burriesci MS, Gallo ND, Schwarz JA, Pringle JR. 2014. Extensive differences in gene expression between symbiotic and aposymbiotic cnidarians. G3 (Bethesda) 4:277–295. 10.1534/g3.113.009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baumgarten S, Simakov O, Esherick LY, Liew YJ, Lehnert EM, Michell CT, Li Y, Hambleton EA, Guse A, Oates ME, Gough J, Weis VM, Aranda M, Pringle JR, Voolstra CR, Knowlton N. 2015. The genome of Aiptasia, a sea anemone model for coral symbiosis. Proc Natl Acad Sci USA 112:11893–11898. 10.1073/pnas.1513318112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oakley CA, Ameismeier MF, Peng L, Weis VM, Grossman AR, Davy SK. 2016. Symbiosis induces widespread changes in the proteome of the model cnidarian Aiptasia. Cell Microbiol 18:1009–1023. 10.1111/cmi.12564. [DOI] [PubMed] [Google Scholar]
- 33.Barott KL, Perez SO, Linsmayer LB, Tresguerres M. 2015. Differential localization of ion transporters suggests distinct cellular mechanisms for calcification and photosynthesis between two coral species. Am J Physiol Regul Integr Comp Physiol 309:R235–R246. 10.1152/ajpregu.00052.2015. [DOI] [PubMed] [Google Scholar]
- 34.Barott KL, Venn AA, Perez SO, Tambutteeé S, Tresguerres M, Somero GN. 2015. Coral host cells acidify symbiotic algal microenvironment to promote photosynthesis. Proc Natl Acad Sci USA 112:607–612. 10.1073/pnas.1413483112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dani V, Priouzeau F, Mertz M, Mondin M, Pagnotta S, Lacas-Gervais S, Davy SK, Sabourault C. 2017. Expression patterns of sterol transporters NPC1 and NPC2 in the cnidarian–dinoflagellate symbiosis. Cell Microbiol 19. 10.1111/cmi.12753. [DOI] [PubMed] [Google Scholar]
- 36.Sproles AE, Kirk NL, Kitchen SA, Oakley CA, Grossman AR, Weis VM, Davy SK. 2018. Phylogenetic characterization of transporter proteins in the cnidarian-dinoflagellate symbiosis. Mol Phylogenet Evol 120:307–320. 10.1016/j.ympev.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 37.Buddemeier RW, Fautin DG. 1993. Coral bleaching as an adaptive mechanism. Bioscience 43:320–326. 10.2307/1312064. [DOI] [Google Scholar]
- 38.Berkelmans R, Van Oppen MJH. 2006. The role of zooxanthellae in the thermal tolerance of corals: a “nugget of hope” for coral reefs in an era of climate change. Proc Biol Sci 273:2305–2312. 10.1098/rspb.2006.3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cunning R, Silverstein RN, Baker AC. 2015. Investigating the causes and consequences of symbiont shuffling in a multi-partner reef coral symbiosis under environmental change. Proc Biol Sci 282:20141725. 10.1098/rspb.2014.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matthews JL, Crowder CM, Oakley CA, Lutz A, Roessner U, Meyer E, Grossman AR, Weis VM, Davy SK. 2017. Optimal nutrient exchange and immune responses operate in partner specificity in the cnidarian-dinoflagellate symbiosis. Proc Natl Acad Sci USA 114:13194–13199. 10.1073/pnas.1710733114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gabay Y, Parkinson JE, Wilkinson SP, Weis VM, Davy SK. 2019. Inter-partner specificity limits the acquisition of thermotolerant symbionts in a model cnidarian-dinoflagellate symbiosis. ISME J 13:2489–2499. 10.1038/s41396-019-0429-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pettay DT, Wham DC, Smith RT, Iglesias-Prieto R, LaJeunesse TC. 2015. Microbial invasion of the Caribbean by an Indo-Pacific coral zooxanthella. Proc Natl Acad Sci USA 112:7513–7518. 10.1073/pnas.1502283112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matthews JL, Oakley CA, Lutz A, Hillyer KE, Roessner U, Grossman AR, Weis VM, Davy SK. 2018. Partner switching and metabolic flux in a model cnidarian–dinoflagellate symbiosis. Proc R Soc Lond B Biol Sci 285:20182336. 10.1098/rspb.2018.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silverstein RN, Cunning R, Baker AC. 2017. Tenacious D: Symbiodinium in clade D remain in reef corals at both high and low temperature extremes despite impairment. J Exp Biol 220:1192–1196. 10.1242/jeb.148239. [DOI] [PubMed] [Google Scholar]
- 45.Sproles AE, Oakley CA, Matthews JL, Peng L, Owen JG, Grossman AR, Weis VM, Davy SK. 2019. Proteomics quantifies protein expression changes in a model cnidarian colonised by a thermally tolerant but suboptimal symbiont. ISME J 10.1038/s41396-019-0437-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gabay Y, Weis VM, Davy SK. 2018. Symbiont identity influences patterns of symbiosis establishment, host growth, and asexual reproduction in a model cnidarian-dinoflagellate symbiosis. Biol Bull 234:1–10. 10.1086/696365. [DOI] [PubMed] [Google Scholar]
- 47.Miller DJ, Yellowlees D. 1989. Inorganic nitrogen uptake by symbiotic marine cnidarians: a critical review. Proc R Soc London B 237:109–125. [Google Scholar]
- 48.Grover R, Maguer J-FF, Allemand D, Ferrier-Pagès C. 2008. Uptake of dissolved free amino acids by the scleractinian coral Stylophora pistillata. J Exp Biol 211:860–865. 10.1242/jeb.012807. [DOI] [PubMed] [Google Scholar]
- 49.Rädecker N, Raina JB, Pernice M, Perna G, Guagliardo P, Kilburn MR, Aranda M, Voolstra CR. 2017. Aiptasia as a model to study metabolic diversity and specificity in cnidarian-dinoflagellate symbioses. bioRxiv 10.1101/223933. [DOI]
- 50.Dudler N, Miller DJ. 1988. Characterization of two glutamate dehydrogenases from the symbiotic microalga Symbiodinium microadriaticum isolated from the coral Acropora formosa. Mar Biol 97:427–430. 10.1007/BF00397773. [DOI] [Google Scholar]
- 51.Grover R, Maguer JF, Reynaud-Vaganay S, Ferrier-Pagès C. 2002. Uptake of ammonium by the scleractinian coral Stylophora pistillata: effect of feeding, light, and ammonium concentrations. Limnol Oceanogr 47:782–790. 10.4319/lo.2002.47.3.0782. [DOI] [Google Scholar]
- 52.Lipschultz F, Cook CB. 2002. Uptake and assimilation of 15N-ammonium by the symbiotic sea anemones Bartholomea annulata and Aiptasia pallida: conservation versus recycling of nitrogen. Mar Biol 140:489–502. [Google Scholar]
- 53.Bednarz VN, Grover R, Ferrier-Pagès C. 2020. Elevated ammonium delays the impairment of the coral-dinoflagellate symbiosis during labile carbon pollution. Aquat Toxicol 218:105360. 10.1016/j.aquatox.2019.105360. [DOI] [PubMed] [Google Scholar]
- 54.Maor-Landaw K, van Oppen MJH, McFadden GI. 2020. Symbiotic lifestyle triggers drastic changes in the gene expression of the algal endosymbiont Breviolum minutum (Symbiodiniaceae). Ecol Evol 10:451–466. 10.1002/ece3.5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cleves PA, Krediet CJ, Lehnert EM, Onishi M, Pringle JR. 2020. Insights into coral bleaching under heat stress from analysis of gene expression in a sea anemone model system. Proc Natl Acad Sci USA 117:28906–28917. 10.1073/pnas.2015737117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoegh-Guldberg O. 1994. Population dynamics of symbiotic zooxanthellae in the coral Pocillopora damicornis exposed to elevated ammonium [(NH4)2 SO4] concentrations. Pacific Sci 48:263–27. [Google Scholar]
- 57.Xiang T, Lehnert E, Jinkerson RE, Clowez S, Kim RG, DeNofrio JC, Pringle JR, Grossman AR. 2020. Symbiont population control by host-symbiont metabolic interaction in Symbiodiniaceae-cnidarian associations. Nat Commun 11:108. 10.1038/s41467-019-13963-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stat M, Gates RD. 2010. Clade D Symbiodinium in scleractinian corals: a “nugget” of hope, a selfish opportunist, an ominous sign, or all of the above? J Mar Biol 2011:9. [Google Scholar]
- 59.Capasso L, Ganot P, Planas-Bielsa V, Tambutté S, Zoccola D. 2021. Intracellular pH regulation: characterization and functional investigation of H+ transporters in Stylophora pistillata. BMC Mol Cell Biol 22:18. 10.1186/s12860-021-00353-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chan H, Babayan V, Blyumin E, Gandhi C, Hak K, Harake D, Kumar K, Lee P, Li TT, Liu HY, Lo TCT, Meyer CJ, Stanford S, Zamora KS, Saier MH. 2010. The P-Type ATPase superfamily. J Mol Microbiol Biotechnol 19:5–104. 10.1159/000319588. [DOI] [PubMed] [Google Scholar]
- 61.Graham LA, Flannery AR, Stevens TH. 2003. Structure and assembly of the yeast V-ATPase. J Bioenerg Biomembr 35:301–312. 10.1023/a:1025772730586. [DOI] [PubMed] [Google Scholar]
- 62.Furla P, Allemand D, Orsenigo MN. 2000. Involvement of H+-ATPase and carbonic anhydrase in inorganic carbon uptake for endosymbiont photosynthesis. Am J Physiol Regul Integr Comp Physiol 278:R870–R881. 10.1152/ajpregu.2000.278.4.R870. [DOI] [PubMed] [Google Scholar]
- 63.Smith SE, Gianinazzi-Pearson V. 1988. Physiological interactions between symbionts in vesicular-arbuscular mycorrhizal plants. Annu Rev Plant Physiol Plant Mol Biol 39:221–244. 10.1146/annurev.pp.39.060188.001253. [DOI] [Google Scholar]
- 64.Sturgill-Koszycki S, Schlesinger PH, Chakraborty P, Haddix PL, Collins HL, Fok AK, Allen RD, Gluck SL, Heuser J, Russell DG. 1994. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science 263:678–681. 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- 65.Vinet AF, Fukuda M, Turco SJ, Descoteaux A. 2009. The Leishmania donovani lipophosphoglycan excludes the vesicular proton-ATPase from phagosomes by impairing the recruitment of Synaptotagmin V. PLoS Pathog 5:e1000628. 10.1371/journal.ppat.1000628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wong D, Bach H, Sun J, Hmama Z, Av-Gay Y. 2011. Mycobacterium tuberculosis protein tyrosine phosphatase (PtpA) excludes host vacuolar-H+-ATPase to inhibit phagosome acidification. Proc Natl Acad Sci USA 108:19371–19376. 10.1073/pnas.1109201108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Muscatine L, Hand C. 1958. Direct evidence for the transfer of materials from symbiotic algae to the tissues of a coelenterate. Proc Natl Acad Sci USA 44:1259–1263. 10.1073/pnas.44.12.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Augustin R. 2010. The protein family of glucose transport facilitators: it’s not only about glucose after all. IUBMB Life 62:315–333. 10.1002/iub.315. [DOI] [PubMed] [Google Scholar]
- 69.Mueckler M, Thorens B. 2013. The SLC2 (GLUT) family of membrane transporters. Mol Aspects Med 34:121–138. 10.1016/j.mam.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ibberson M, Riederer BM, Uldry M, Guhl B, Roth J, Thorens B. 2002. Immunolocalization of GLUTX1 in the testis and to specific brain areas and vasopressin-containing neurons. Endocrinology 143:276–284. 10.1210/endo.143.1.8587. [DOI] [PubMed] [Google Scholar]
- 71.Ibberson M, Uldry M, Thorens B. 2000. GLUTX1, a novel mammalian glucose transporter expressed in the central nervous system and insulin-sensitive tissues. J Biol Chem 275:4607–4612. 10.1074/jbc.275.7.4607. [DOI] [PubMed] [Google Scholar]
- 72.Flessner LB, Moley KH. 2009. Similar [DE]XXXL[LI] motifs differentially target GLUT8 and GLUT12 in Chinese hamster ovary cells. Traffic 10:324–333. 10.1111/j.1600-0854.2008.00866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.DeBosch BJ, Chen Z, Saben JL, Finck BN, Moley KH. 2014. Glucose transporter 8 (GLUT8) mediates fructose-induced de novo lipogenesis and macrosteatosis. J Biol Chem 289:10989–10998. 10.1074/jbc.M113.527002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Campbell EM, Ball A, Hoppler S, Bowman AS. 2008. Invertebrate aquaporins: a review. J Comp Physiol B 178:935–955. 10.1007/s00360-008-0288-2. [DOI] [PubMed] [Google Scholar]
- 75.Carbrey JM, Gorelick-Feldman DA, Kozono D, Praetorius J, Nielsen S, Agre P. 2003. Aquaglyceroporin AqP9: solute permeation and metabolic control of expression in liver. Proc Natl Acad Sci USA 100:2945–2950. 10.1073/pnas.0437994100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ishibashi K, Kondo S, Hara S, Morishita Y. 2011. The evolutionary aspects of aquaporin family. Am J Physiol Regul Integr Comp Physiol 300:R566–R576. 10.1152/ajpregu.90464.2008. [DOI] [PubMed] [Google Scholar]
- 77.Kuriyama H, Shimomura I, Kishida K, Kondo H, Furuyama N, Nishizawa H, Maeda N, Matsuda M, Nagaretani H, Kihara S, Nakamura T, Tochino Y, Funahashi T, Matsuzawa Y. 2002. Coordinated regulation of fat-specific and liver-specific glycerol channels, aquaporin adipose and aquaporin 9. Diabetes 51:2915–2921. 10.2337/diabetes.51.10.2915. [DOI] [PubMed] [Google Scholar]
- 78.Maeda N, Funahashi T, Hibuse T, Nagasawa A, Kishida K, Kuriyama H, Nakamura T, Kihara S, Shimomura I, Matsuzawa Y. 2004. Adaptation to fasting by glycerol transport through aquaporin 7 in adipose tissue. Proc Natl Acad Sci USA 101:17801–17806. 10.1073/pnas.0406230101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hibuse T, Maeda N, Nagasawa A, Funahashi T. 2006. Aquaporins and glycerol metabolism. Biochim Biophys Acta 1758:1004–1011. 10.1016/j.bbamem.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 80.Gambardella C, Gallus L, Amaroli A, Terova G, Masini MA, Ferrando S. 2012. Fasting and re-feeding impact on leptin and aquaglyceroporin 9 in the liver of European sea bass (Dicentrarchus labrax). Aquaculture 354–355:1–6. 10.1016/j.aquaculture.2012.04.043. [DOI] [Google Scholar]
- 81.Liao S, Gan L, Lv L, Mei Z. 2021. The regulatory roles of aquaporins in the digestive system. Genes Dis 8:250–258. 10.1016/j.gendis.2019.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yuyama I, Ito Y, Watanabe T, Hidaka M, Suzuki Y, Nishida M. 2012. Differential gene expression in juvenile polyps of the coral Acropora tenuis exposed to thermal and chemical stresses. J Exp Mar Bio Ecol 430–431:17–24. 10.1016/j.jembe.2012.06.020. [DOI] [Google Scholar]
- 83.Reich HG, Robertson DL, Goodbody-Gringley G. 2017. Do the shuffle: changes in Symbiodinium consortia throughout juvenile coral development. PLoS One 12:e0171768. 10.1371/journal.pone.0171768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kemp DW, Hernandez-Pech X, Iglesias-Prieto R, Fitt WK, Schmidt GW. 2014. Community dynamics and physiology of Symbiodinium spp. before, during, and after a coral bleaching event. Limnol Oceanogr 59:788–797. 10.4319/lo.2014.59.3.0788. [DOI] [Google Scholar]
- 85.Baker AC. 1999. The symbiosis ecology of reef-building corals. PhD dissertation. University of Miami, Coral Gables, FL. [Google Scholar]
- 86.Lajeunesse TC, Trench RK. 2000. Biogeography of two species of Symbiodinium (Freudenthal) inhabiting the intertidal sea anemone Anthopleura elegantissima (Brandt). Biol Bull 199:126–134. 10.2307/1542872. [DOI] [PubMed] [Google Scholar]
- 87.Stat M, Pochon X, Cowie ROM, Gates RD. 2009. Specificity in communities of Symbiodinium in corals from Johnston Atoll. Mar Ecol Prog Ser 386:83–96. 10.3354/meps08080. [DOI] [Google Scholar]
- 88.Franklin EC, Stat M, Pochon X, Putnam HM, Gates RD. 2012. GeoSymbio: a hybrid, cloud-based web application of global geospatial bioinformatics and ecoinformatics for Symbiodinium–host symbioses. Mol Ecol Resour 12:369–373. 10.1111/j.1755-0998.2011.03081.x. [DOI] [PubMed] [Google Scholar]
- 89.Matthews JL, Sproles AE, Oakley CA, Grossman AR, Weis VM, Davy SK. 2016. Menthol-induced bleaching rapidly and effectively provides experimental aposymbiotic sea anemones (Aiptasia sp.) for symbiosis investigations. J Exp Biol 219:306–310. 10.1242/jeb.128934. [DOI] [PubMed] [Google Scholar]
- 90.Lehnert EM, Burriesci MS, Pringle JR. 2012. Developing the anemone Aiptasia as a tractable model for cnidarian-dinoflagellate symbiosis: the transcriptome of aposymbiotic A. pallida. BMC Genomics 13:271–210. 10.1186/1471-2164-13-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pearson WR. 2013. An introduction to sequence similarity (“homology”) searching. Curr Protoc Bioinforma 42:3.1.1–3.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rice PM, Bleasby AJ, Ison JC, Mullan L, Bottu G. 2011. EMBOSS User’s Guide: Practical Bioinformatics. Cambridge University Press, Cambridge, United Kingdom. [Google Scholar]
- 93.Parkinson JE, Baumgarten S, Michell CT, Baums IB, LaJeunesse TC, Voolstra CR. 2016. Gene expression variation resolves species and individual strains among coral-associated dinoflagellates within the genus Symbiodinium. Genome Biol Evol 8:665–680. 10.1093/gbe/evw019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Towbin H, Gordon J. 1984. Immunoblotting and dot immunobinding. Current status and outlook. J Immunol Methods 72:313–340. 10.1016/0022-1759(84)90001-2. [DOI] [PubMed] [Google Scholar]
- 95.Arena ET, Rueden CT, Hiner MC, Wang S, Yuan M, Eliceiri KW. 2017. Quantitating the cell: turning images into numbers with ImageJ. Wiley Interdiscip Rev Dev Biol 6:e260. 10.1002/wdev.260. [DOI] [PubMed] [Google Scholar]
- 96.Kelly JG, Hawken MJ. 2017. Quantification of neuronal density across cortical depth using automated 3D analysis of confocal image stacks. Brain Struct Funct 222:3333–3353. 10.1007/s00429-017-1382-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pike JA, Styles IB, Rappoport JZ, Heath JK. 2017. Quantifying receptor trafficking and colocalization with confocal microscopy. Methods 115:42–54. 10.1016/j.ymeth.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 98.Miura K, Sladoje N. 2019. Bioimage Data Analysis Workflows. Springer, Cham, Switzerland. [Google Scholar]
- 99.Jonkman J, Brown CM, Wright GD, Anderson KI, North AJ. 2020. Tutorial: guidance for quantitative confocal microscopy. Nat Protoc 15:1585–1611. 10.1038/s41596-020-0313-9. [DOI] [PubMed] [Google Scholar]
- 100.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. 2012. Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rueden CT, Schindelin J, Hiner MC, DeZonia BE, Walter AE, Arena ET, Eliceiri KW. 2017. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics 18:529. 10.1186/s12859-017-1934-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kelley JB, Paschal BM. 2019. Fluorescence-based quantification of nucleocytoplasmic transport. Methods 157:106–114. 10.1016/j.ymeth.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tivey TR, Parkinson JE, Weis VM. 2020. Host and symbiont cell cycle coordination is mediated by symbiotic state, nutrition, and partner identity in a model cnidarian-dinoflagellate symbiosis. mBio 11:e02626-19. 10.1128/mBio.02626-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bolte S, Cordelières FP. 2006. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc 224:213–232. 10.1111/j.1365-2818.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- 105.R Core Team. 2020. R: a Language and Environment for Statistical Computing. 4.0.3. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 to S5 and Tables S1 and S2. Download aem.00412-22-s0001.pdf, PDF file, 1.1 MB (1.1MB, pdf)