Abstract
Background
Postoperative headache (POH) is frequent after cardiac surgery; however, few studies on risk factors for POH exist. The aims of the current study were to explore risk factors related to POH after elective cardiac surgery and to establish a predictive system.
Methods and Results
Adult patients undergoing elective open‐heart surgery under cardiopulmonary bypass from 2016 to 2020 in 4 cardiac centers were retrospectively included. Two thirds of the patients were randomly allocated to a training set and one third to a validation set. Predictors for POH were selected by univariate and multivariate analysis. POH developed in 3154 of the 13 440 included patients (23.5%) and the overall mortality rate was 2.3%. Eight independent risk factors for POH after elective cardiac surgery were identified, including female sex, younger age, smoking history, chronic headache history, hypertension, lower left ventricular ejection fraction, longer cardiopulmonary bypass time, and more intraoperative transfusion of red blood cells. A nomogram based on the multivariate model was constructed, with reasonable calibration and discrimination, and was well validated. Decision curve analysis revealed good clinical utility. Finally, 3 risk intervals were divided to better facilitate clinical application.
Conclusions
A nomogram model for POH after elective cardiac surgery was developed and validated using 8 predictors, which may have potential application value in clinical risk assessment, decision‐making, and individualized treatment associated with POH.
Keywords: cardiac surgery, headache, nomogram, prediction model, risk factor
Subject Categories: Cardiovascular Disease, Risk Factors, Cardiovascular Surgery, Quality and Outcomes
Nonstandard Abbreviations and Acronyms
- CPB
cardiopulmonary bypass
- ECS
elective cardiac surgery
- POH
postoperative headache
- RBC
red blood cell
Clinical Perspective
What Is New?
Postoperative headache is prevalent following elective cardiac surgery, portending poorer clinical outcomes.
Using data from 13 440 patients who underwent elective cardiac surgery in 4 tertiary care centers, we identified 6 preoperative and 2 intraoperative predictors for postoperative headache after elective cardiac surgery, including female sex, younger age, smoking history, chronic headache history, hypertension, lower left ventricular ejection fraction, longer cardiopulmonary bypass time, and more intraoperative transfusion of red blood cells.
This is the first report that describes the predictors for postoperative headache after elective cardiac surgery and the first construction and validation of a clinical prediction model worldwide.
What Are the Clinical Implications?
The nomogram model may be helpful for clinical decision‐making and risk modification through individualized risk estimation and identification of high‐risk patients.
Appropriate preventive measures and specific interventions targeting high‐risk patients identified by the nomogram may be more efficient and may yield more net clinical benefits.
Cardiovascular diseases remain the leading cause of morbidity, mortality, and disease burden in the world, with a steady increase in prevalence levels and health care costs. 1 , 2 Although much progress has been made in the development of pharmaceutical science and technology in recent years, surgical interventions remain an important and effective treatment option for severe cardiovascular diseases. 3 , 4 Elective procedures constitute the majority of cardiac operations performed every day all over the world; however, there is a significant proportion of patients who experience headache after elective cardiac surgery (ECS) according to clinical observations.
Postoperative headache (POH) is a common surgical complication, related to increased morbidity, decreased quality of life, prolonged hospitalization, and higher health care costs. 5 , 6 , 7 , 8 , 9 The prevalence rates of POH after surgery varied widely among different surgical types in the literature. 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 Presently, some studies aiming to explore risk factors for POH have been conducted in patients who underwent noncardiac surgery. 15 , 16 , 17 , 18 , 19 , 20 Several significant risk factors associated with POH have been reported, such as female sex and smoking history. 15 , 17 , 21 Nevertheless, there is little information regarding the development of POH in patients undergoing cardiac surgery, even if just used as a secondary outcome. 22 To the best of our knowledge, there are no previous studies aiming to explore risk factors for POH after ECS, not to mention the development and validation of a risk prediction model.
The purposes of this study were first to identify significant predictors for the development of POH in adults who underwent open ECS under cardiopulmonary bypass (CPB), and second to develop and validate an intuitive and practical nomogram model to provide help for early risk assessment and prevention.
METHODS
The data that support the findings of this study are available from the corresponding author on reasonable request.
Ethical Statement
This study was conducted in accordance with the ethical statement of the Declaration of Helsinki, and was approved by the ethics committee of Tongji Medical College of Huazhong University of Science and Technology (IORG number IORG0003571). Written informed consent was waived because of its retrospective, observational nature.
Study Population
We conducted a multicenter, retrospective, observational study that included consecutive adult patients (aged ≥18 years) who underwent open ECS under CPB in 4 tertiary care centers between 2016 and 2020. Exclusion criteria included: (1) emergent cardiac surgery; (2) intraoperative death or postoperative unconsciousness; and (3) organ transplantation, immune deficiency, or immunosuppression. The remaining patients were categorized into 2 groups based on whether they had at least 1 episode of headache during postoperative hospitalization.
Data Collection and Variables
Clinical data collection was completed using the electronic medical record management systems of the hospitals. Demographics, comorbidities, laboratory examination results, operative variables, and postoperative outcomes were collected and compared. Demographics included age, sex, body mass index, drinking, and smoking history. Comorbidities included hypertension, diabetes, chronic headache history, cerebrovascular disease, chronic obstructive pulmonary disease, peripheral vascular disease, gastrointestinal tract disease, renal insufficiency, pulmonary artery hypertension, atrial fibrillation, pericardial effusion, left ventricular ejection fraction (LVEF), and general and cardiac surgery history. Laboratory examination results included red blood cell (RBC) count, white blood cell count, hemoglobin, serum albumin, globulin, and creatinine levels. Operative variables included the volume of RBC transfusion, the lengths of CPB, and aortic cross clamp. Postoperative outcomes included mortality, readmission to intensive care unit (ICU), reintubation, tracheotomy, and the lengths of ICU and hospital stays.
Definitions of Important Variables
The diagnosis of POH in this study was based on a self‐reported or physician‐diagnosed headache identified in the electronic medical records, including disease course and nursing records. Smoking history was defined as previous daily or current smoking. Body mass index was calculated based on weight and height. Hypertension was defined as systolic blood pressure >140 mm Hg, diastolic blood pressure >90 mm Hg, or having a previous history of hypertension. Chronic headache history was defined as self‐reported or recorded migraine or any other kind of recurrent headache. Diabetes was defined as random glucose ≥11.1 mmol/L, fasting glucose ≥7.0 mmol/L, a prior history of diabetes, and/or receiving antidiabetic therapy. Chronic obstructive pulmonary disease was defined according to self‐report, previous diagnosis, or forced expiratory volume in the first second of expiration/forced vital capacity ≤0.7.
Statistical Analysis
We randomly divided cases into the training set and the validation set at a ratio of 2:1. The training set was used for model development, whereas the validation set was used for model validation. Continuous variables were presented as mean±SD when normally distributed and medians with interquartile ranges when non‐normally distributed. Categorical variables were expressed as frequencies with percentages. For univariate analysis, continuous variables were analyzed by Student t test when normally distributed and Mann‐Whitney U test when non‐normally distributed, and categorical data were analyzed by chi‐square test or Fisher exact test. Multiple imputation was used to handle missing data. Continuous variables were imputed using linear regression (or predictive mean matching if skewed), and categorical variables were imputed using logistic regression. Univariate logistic regression analysis was performed first to screen possible risk factors. Factors with P<0.1 were further analyzed to identify independent predictors by forward stepwise multivariate logistic regression. A nomogram based on the multivariate model was then established.
Internal validation of the nomogram was assessed using bootstrap with 1000 replicates. External validation was performed in the validation set. Calibration was assessed by both visual inspection and Hosmer‐Lemeshow goodness‐of‐fit test. Discrimination was assessed by the area under the receiver operating characteristic curve. Clinical usefulness was assessed by decision curve analysis. The area under the curves of the training and validation sets were compared using the Delong method. 23 Propensity score matching was applied to balance important patient characteristics between groups using a greedy‐nearest‐neighbor matching algorithm without replacement, with an algorithm of 1:1 matching (caliper width of 0.02 on the propensity score scale).
Statistical analyses were performed using R software (version 4.0.4, www.R‐project.org/) and SPSS (version 26.0, IBM). Two‐tailed P values <0.05 were considered statistically significant.
RESULTS
Demographic Characteristics
Among the 15 207 adults who underwent cardiac surgery, 1189 patients experienced emergency procedures; 548 patients experienced immunosuppression, immune deficiency, or organ transplantation; and 30 patients died intraoperatively or lapsed into unconsciousness postoperatively. The remaining 13 440 patients met the inclusion criteria and were evaluated further (Figure 1). The mean age of the included patients was 51.61±13.15 years and 53.9% were men. The overall morbidity rate of POH after ECS was 23.5%.
Figure 1. Flow chart of the study.
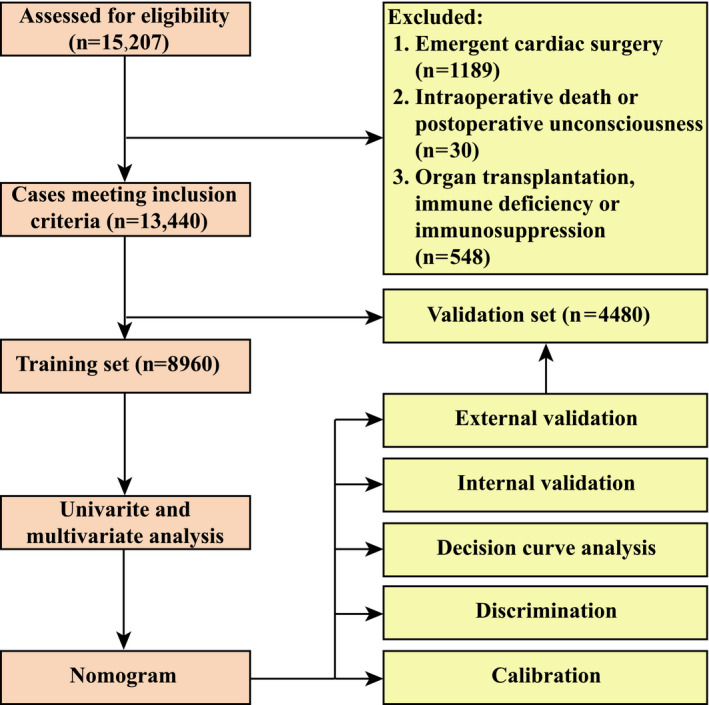
The population involved in this study had multiple comorbidities and different patient details. Smoking history was found in 27.0%, hypertension in 24.9%, drinking history in 20.1%, atrial fibrillation in 19.4%, chronic obstructive pulmonary disease in 11.5%, chronic headache history in 10.8%, gastrointestinal tract disease in 8.2%, diabetes in 7.9%, and cardiac surgery history in 6.9% of patients. Isolated valve surgery was performed for 62.5% of the patients, mixed valve and coronary surgery for 11.0%, isolated coronary artery bypass grafting for 9.3%, and other types for 17.2%. The average lengths of CPB and aortic cross clamp were 108 minutes (interquartile range, 86–139 minutes) and 67 minutes (interquartile range, 47–88 minutes), respectively. RBC was transfused in 72.0% of the patients, with an average volume of 1.0 units (interquartile range, 0–3.0 units). There was no significant difference with respect to baseline characteristics and surgical factors between the training and validation sets (Table 1).
Table 1.
Comparison of Characteristics Between the Training and Validation Sets
| Characteristics |
Training set n=8960 (%) |
Validation set n=4480 (%) |
P value |
|---|---|---|---|
| Demographics | |||
| Men | 4822 (53.8) | 2420 (54.0) | 0.826 |
| Age, y | 51.51±13.13 | 51.79±13.19 | 0.241 |
| Body mass index, kg/m2 | 23.11±3.29 | 23.14±3.31 | 0.560 |
| Smoking history | 2395 (26.7) | 1239 (27.7) | 0.254 |
| Drinking history | 1812 (20.2) | 896 (20.0) | 0.761 |
| Underlying conditions | |||
| Hypertension | 2243 (25.0) | 1107 (24.7) | 0.683 |
| Diabetes | 682 (7.6) | 377 (8.4) | 0.103 |
| Chronic headache history | 966 (10.8) | 487 (10.9) | 0.875 |
| Chronic obstructive pulmonary disease | 1047 (11.7) | 495 (11.0) | 0.275 |
| Cerebrovascular disease | 1576 (17.6) | 774 (17.3) | 0.653 |
| Peripheral vascular disease | 1993 (22.2) | 965 (21.5) | 0.354 |
| Renal insufficiency | 652 (7.3) | 325 (7.3) | 0.963 |
| Gastrointestinal tract disease | 753 (8.4) | 349 (7.8) | 0.221 |
| Atrial fibrillation | 1718 (19.2) | 890 (19.9) | 0.339 |
| Cardiac surgery history | 626 (7.0) | 303 (6.8) | 0.631 |
| General surgery history | 2577 (28.8) | 1344 (30.0) | 0.136 |
| Pulmonary artery hypertension | 2610 (29.1) | 1308 (29.2) | 0.936 |
| Pericardial effusion | 1146 (12.8) | 603 (13.5) | 0.277 |
| NYHA class III or IV | 1509 (16.8) | 779 (17.4) | 0.426 |
| LVEF, % | 62 (57–67) | 62 (57–67) | 0.682 |
| Laboratory values | |||
| White blood cell count, ×109/L | 5.68 (4.73–6.79) | 5.64 (4.70–6.84) | 0.577 |
| RBC count, ×1012/L | 4.29 (3.93–4.66) | 4.30 (3.94–4.66) | 0.440 |
| Hemoglobin, g/L | 118 (106–129) | 130 (118–141) | 0.259 |
| Red cell distribution width, % | 14.1 (11.6–15.7) | 14.1 (11.6–15.7) | 0.182 |
| Serum creatinine, μmol/L | 60.5 (53.1–71.1) | 60.5 (53.2–71.4) | 0.522 |
| Serum albumin, g/L | 40.6 (38.3–42.9) | 40.7 (38.3–42.9) | 0.918 |
| Serum globulin, g/L | 24.2 (21.6–27.1) | 24.3 (21.5–27.1) | 0.551 |
| Operative variables | |||
| Surgical types | 0.649 | ||
| Isolated valve surgery | 5598 (62.4) | 2800 (62.5) | |
| Isolated coronary artery bypass grafting | 850 (9.5) | 400 (8.9) | |
| Mixed valve and coronary surgery | 992 (11.1) | 493 (11.0) | |
| Other types | 1520 (17.0) | 787 (17.6) | |
| Cardiopulmonary bypass time, min | 100 (77–130) | 99 (76–129) | 0.210 |
| Aortic cross clamp time, min | 67 (48–89) | 66 (47–87) | 0.190 |
| Transfusion of RBCs, units | 1 (0–3) | 1 (0–3) | 0.126 |
LVEF indicates left ventricular ejection fraction; NYHA, New York Heart Association; and RBC, red blood cell.
Nomogram Development
In the training set, univariate analysis was first used to filtrate possible indicators for POH after ECS and the results are presented in Table 2. Colinearity between variables was checked before further analysis. Variables with P<0.1 in the univariate analysis were further analyzed using multivariate logistic regression. By a stepwise forward regression procedure, 8 significant predictors were identified in the final multivariate model, including female sex, younger age, smoking history, chronic headache history, hypertension, lower LVEF, longer CPB time, and more intraoperative transfusion of RBC (Table 3). A nomogram based on these risk factors was then established to predict the risk of POH after ECS (Figure 2). Regression coefficients of these factors were combined into a score ranging from 0 to 100 depending on their relative importance.
Table 2.
Univariate Analysis of Possible Risk Factors for POH After Elective Cardiac Surgery in the Training Set
| Characteristics |
Without POH n=2161 (%) |
With POH n=6799 (%) |
P value |
|---|---|---|---|
| Demographics | |||
| Men | 3719 (54.7) | 1103 (51.0) | 0.003 |
| Age, y | 51.81±13.13 | 50.58±13.08 | <0.001 |
| Body mass index, kg/m2 | 23.08±3.28 | 23.21±3.32 | 0.113 |
| Smoking history | 1660 (24.4) | 735 (34.0) | <0.001 |
| Drinking history | 1323 (19.5) | 489 (22.6) | 0.001 |
| Underlying conditions | |||
| Hypertension | 1501 (22.1) | 742 (34.3) | <0.001 |
| Diabetes | 483 (7.1) | 199 (9.2) | 0.001 |
| Chronic headache history | 600 (8.8) | 366 (16.9) | <0.001 |
| Chronic obstructive pulmonary disease | 708 (10.4) | 339 (15.7) | <0.001 |
| Cerebrovascular disease | 1206 (17.7) | 370 (17.1) | 0.512 |
| Peripheral vascular disease | 1513 (22.3) | 480 (22.2) | 0.968 |
| Renal insufficiency | 418 (6.1) | 234 (10.8) | <0.001 |
| Gastrointestinal tract disease | 592 (8.7) | 161 (7.5) | 0.067 |
| Atrial fibrillation | 1191 (17.5) | 527 (24.4) | <0.001 |
| Cardiac surgery history | 398 (5.9) | 228 (10.6) | <0.001 |
| General surgery history | 1928 (28.4) | 649 (30.0) | 0.134 |
| Pulmonary artery hypertension | 1946 (28.6) | 664 (30.7) | 0.061 |
| Pericardial effusion | 799 (11.8) | 347 (16.1) | <0.001 |
| NYHA class III or IV | 908 (13.4) | 601 (27.8) | <0.001 |
| LVEF, % | 63 (59–67) | 59 (54–63) | <0.001 |
| Laboratory values | |||
| White blood cell count, ×109/L | 5.64 (4.70–6.75) | 5.77 (4.78–6.85) | 0.009 |
| RBC count, ×1012/L | 4.31 (3.95–4.67) | 4.24 (3.88–4.62) | <0.001 |
| Hemoglobin, g/L | 130 (119–142) | 127 (116–139) | <0.001 |
| Red cell distribution width, % | 14.1 (11.6–15.7) | 14.3 (11.7–15.8) | 0.023 |
| Serum creatinine, μmol/L | 70.9 (60.7–83.3) | 71.3 (59.8–85.6) | 0.205 |
| Serum albumin, g/L | 40.7 (38.5–42.9) | 40.4 (38.0–42.7) | <0.001 |
| Serum globulin, g/L | 24.2 (21.6–27.0) | 24.4 (21.8–27.4) | 0.004 |
| Operative variables | |||
| Surgical types | <0.001 | ||
| Isolated valve surgery | 4358 (64.1) | 1240 (57.4) | |
| Isolated coronary artery bypass grafting | 612 (9.0) | 238 (11.0) | |
| Mixed valve and coronary surgery | 594 (8.7) | 398 (18.4) | |
| Other types | 1235 (18.2) | 285 (13.2) | |
| Cardiopulmonary bypass time, min | 94 (73–119) | 127 (104–156) | <0.001 |
| Aortic cross clamp time, min | 62 (44–82) | 85 (66–108) | <0.001 |
| Transfusion of RBCs, units | 1.0 (0–2.5) | 2.5 (1.0–4.5) | <0.001 |
LVEF indicates left ventricular ejection fraction; POH, postoperative headache; and RBC, red blood cell.
Table 3.
Multivariate Analysis of Independent Risk Factors for POH After Elective Cardiac Surgery
| Characteristics | Coefficient | SE | OR (95% CI) | P value |
|---|---|---|---|---|
| Female sex | 1.071 | 0.076 | 2.920 (2.518–3.386) | <0.001 |
| Age, y | −0.056 | 0.003 | 0.945 (0.941–0.950) | <0.001 |
| Smoking history | 1.004 | 0.080 | 2.729 (2.332–3.195) | <0.001 |
| Chronic headache history | 1.282 | 0.092 | 3.605 (3.011–4.317) | <0.001 |
| Hypertension | 1.019 | 0.071 | 2.771 (2.410–3.186) | <0.001 |
| LVEF, % | −0.089 | 0.004 | 0.915 (0.908–0.922) | <0.001 |
| Cardiopulmonary bypass time, min | 0.018 | 0.001 | 1.019 (1.017–1.020) | <0.001 |
| Intraoperative transfusion of RBCs, units | 0.322 | 0.015 | 1.379 (1.340–1.420) | <0.001 |
LVEF indicates left ventricular ejection fraction; OR, odds ratio; POH, postoperative headache; and RBC, red blood cell.
Figure 2. Nomogram for the prediction of postoperative headache in patients undergoing elective cardiac surgery.
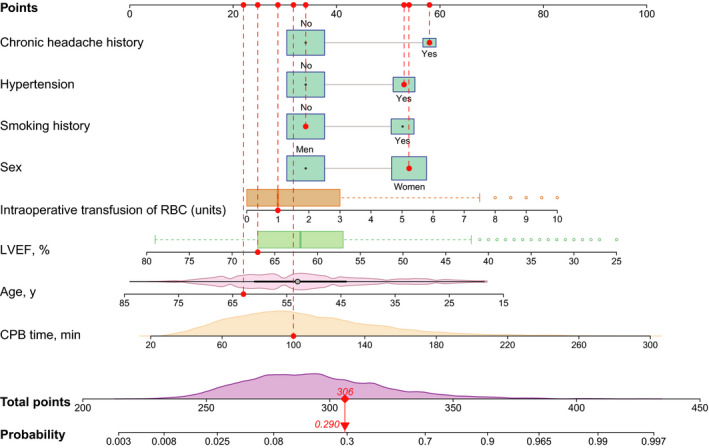
A specific case is presented to show the use of the nomogram. This was a 63‐year‐old woman who did not smoke but had a chronic headache history, hypertension, and a left ventricular ejection fraction (LVEF) of 67%. The duration of cardiopulmonary bypass (CPB) was 100 minutes and the transfused red blood cell (RBC) count was 1 unit. The corresponding points of the predictors are represented by red dots. The total points for this patient was 306, corresponding to a probability of 0.290 for developing postoperative headache.
The nomogram‐predicted probability of POH after ECS ranged widely. By summing all the points, the personalized risk of POH after ECS can be easily and directly evaluated. Young women with a history of smoking, hypertension, chronic headache, lower LVEF, longer CPB duration, and more intraoperative RBC transfusion may have higher scores and therefore are at a higher risk of POH. A specific example is illustrated in Figure 2.
Nomogram Validation and Assessment
The results of internal and external validations indicated that the nomogram had good predicative performance. The calibration was good by both visual inspection and goodness‐of‐fit test, with a Hosmer‐Lemeshow chi‐square statistic of 8.772 (P=0.362, Figure 3A) in the training set and 8.121 (P=0.422, Figure 3B) in the validation set. The discrimination was assessed by plotting receiver operating characteristic curves, with an area under the curve of 0.833 (95% CI, 0.823–0.842) in the training set and 0.838 (95% CI, 0.824–0.852) in the validation set (Figure 3C). The 2 receiver operating characteristic curves showed no significant difference (P=0.516). The clinical usefulness was evaluated by plotting decision and clinical impact curves. The decision curves suggested that compared with strategies that either no or all patients received intervention, more significant clinical net benefits could be achieved by the nomogram (Figure 3D). The clinical impact curves also indicated reasonable predictive power and showed good clinical utility (Figure 3E and 3F).
Figure 3. Assessment of the nomogram model for postoperative headache (POH) after elective cardiac surgery (ECS).
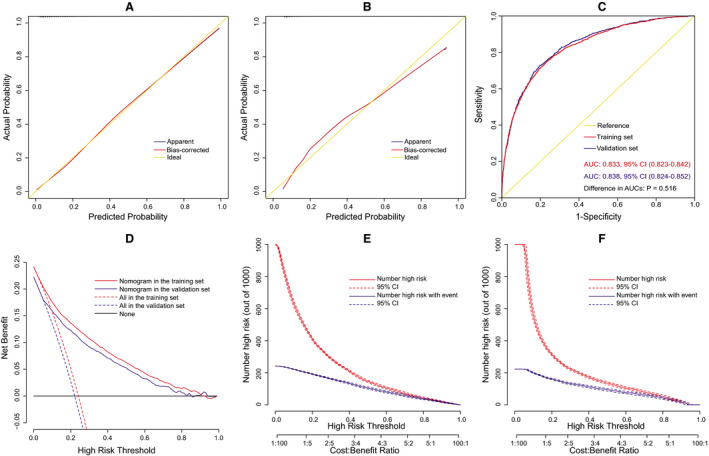
Calibration plots in the training set (A) and the validation set (B), receiver operating characteristic (ROC) curves in the 2 sets (C), decision curves in the 2 sets (D), and clinical impact curves in the training set (E) and the validation set (F). AUC indicates area under the curve.
Risk Stratification
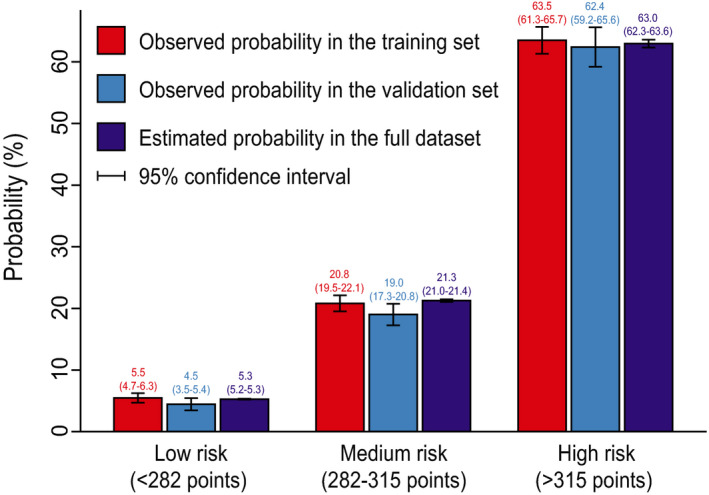
Based on the nomogram and clinical practice, we performed risk stratification (Table 4). Three risk intervals were defined as low‐, medium‐, and high‐risk groups, corresponding to predicted probabilities of <0.1, 0.1 to 0.4, and >0.4. The scores on the nomogram were <282 points, 282 to 315 points, and >315 points, respectively. In this study, 37.1% of the patients were divided into the low‐risk group, 42.3% into the medium‐risk group, and 20.6% into the high‐risk group. Comparison of the 3 risk groups between the predicted probabilities by the nomogram and the observed probabilities in the training and validation sets are plotted in Figure 4, demonstrating favorable consistency.
Table 4.
Risk Intervals of POH Based on the Nomogram
| Risk intervals |
Low risk (<282 points) |
Medium risk (282–315 points) |
High risk (>315 points) |
|---|---|---|---|
| Estimated probability, % | <10 | 10–40 | >40 |
| Observed probability, % (95% CI) | 5.1 (4.5–5.8) | 20.2 (19.2–21.2) | 63.1 (61.3–64.9) |
| No. of patients (%) | 4983 (37.1) | 5687 (42.3) | 2770 (20.6) |
POH indicates postoperative headache.
Figure 4. Bar chart showing the consistency between predicted and observed probabilities.
Outcomes
The results of univariate analysis showed that patients with POH had higher probabilities of pneumonia, reintubation, tracheotomy, readmission to ICU, and mortality, and longer lengths of ICU and hospital stay (Table 5). To further evaluate the relationship between POH and outcomes, propensity score matching was performed, yielding 2390 matched pairs of patients. In this study population, the differences of pneumonia, reintubation, and lengths of ICU and hospital stay remained significant between patients with and without POH. However, the statistical differences between the 2 groups were eliminated with regard to tracheotomy, readmission to ICU, and mortality, despite the fact that absolute number and rates were slightly higher in patients with POH. Details of comparison are presented in Table 6.
Table 5.
Univariate Analysis of Outcomes in Patients With and Without POH After Elective Cardiac Surgery
| Variables |
All patients N=13 440 (%) |
Without POH n=10 286 (%) |
With POH n=3154 (%) |
P value |
|---|---|---|---|---|
| Pneumonia | 954 (7.1) | 401 (3.9) | 553 (17.5) | <0.001 |
| Reintubation | 465 (3.5) | 220 (2.1) | 245 (7.8) | <0.001 |
| Tracheotomy | 186 (1.4) | 83 (0.8) | 103 (3.3) | <0.001 |
| Readmission to ICU | 440 (3.3) | 253 (2.5) | 187 (5.9) | <0.001 |
| ICU stay, d | 3 (2–4) | 3 (2–4) | 4 (2–6) | <0.001 |
| Hospital stay, d | 14 (11–18) | 13 (10–17) | 16 (12–21) | <0.001 |
| Mortality | 306 (2.3) | 138 (1.3) | 168 (5.3) | <0.001 |
ICU indicates intensive care unit; and POH, postoperative headache.
Table 6.
Analysis of the Relationship Between POH and Outcomes After Propensity Score Matching
| Variables |
Without POH n=2390 (%) |
With POH n=2390 (%) |
P value |
|---|---|---|---|
| Pneumonia | 171 (7.2) | 339 (14.2) | <0.001 |
| Reintubation | 104 (4.4) | 135 (5.6) | 0.040 |
| Tracheotomy | 44 (1.8) | 51 (2.1) | 0.468 |
| Readmission to ICU | 96 (4.0) | 102 (4.3) | 0.663 |
| ICU stay, d | 3 (2–4) | 3 (2–5) | <0.001 |
| Hospital stay, d | 14 (11–19) | 15 (11–20) | <0.001 |
| Mortality | 78 (3.3) | 87 (3.6) | 0.476 |
ICU indicates intensive care unit; and POH, postoperative headache.
DISCUSSION
POH has been thought to be associated with increased risk of morbidity and prolonged hospitalizations, 5 , 7 , 8 which was further affirmed by the results of the present study. The morbidity rate of POH after ECS was 23.5% and the overall mortality rate was 2.3%. The rates of tracheotomy, readmission to ICU, and mortality were significantly higher in patients with POH by univariate analysis, but the difference disappeared after propensity score matching. Nevertheless, the rates of pneumonia and reintubation remained significantly higher and the lengths of ICU and hospital stay remained significantly longer in patients with POH. The increased risk of these poor outcomes in patients with POH after ECS highlighted the need to identify significant risk factors and high‐risk populations.
Globally, much research has been conducted to explore risk factors for POH in patients who underwent noncardiac surgery, 8 , 15 , 17 , 18 , 20 whereas available reports involving cardiac surgery are limited in this area. As far as we know, this is the first report that describes the predictors for POH after ECS and the construction of a clinical prediction model worldwide. In this study, we used data from 13 440 patients who underwent ECS in 4 tertiary care centers to develop and validate a prediction model for POH. By multivariate logistic regression analysis, 6 preoperative and 2 intraoperative predictors were considered to be closely related to the occurrence of POH. A nomogram was then established on the basis of these predictors, which demonstrated excellent discrimination, calibration, and clinical utility. Finally, we defined 3 risk groups to better facilitate clinical application according to the nomogram and clinical practice.
The reported predictors for POH differed greatly in different types of surgery; however, female sex and younger age have been widely recognized as independent risk factors in the literature, 15 , 17 , 19 , 24 , 25 , 26 which was in agreement with our results. Kim and colleagues 27 conducted a large retrospective study in patients undergoing spinal and neuraxial anesthesia procedures, finding that there were significant differences for sex (P=0.034) and age (P=0.016) between patients with or without headache after surgery. Female sex and younger age were identified as independent risk factors for POH by multivariate logistic regression analysis. Similar results were also observed in a prospective study conducted by Liang and colleagues 19 in patients undergoing endoscopic endonasal procedures with lumbar drainage. They found that patients in the headache group were significantly younger than those in the control group (33.0 versus 53.5 years, P=0.014), and there was a near‐significant trend toward a higher incidence of headache in women (85.7% versus 47.9%, P=0.062). A review conducted by Bezov and colleagues 28 indicated that the risk of POH was highest in 20‐ to 30‐year‐olds, who were 3 to 5 times more likely to develop POH than those older than 60 years. They also reported that women had almost twice the risk of developing a POH in comparison with men.
The age‐ and sex‐related difference in POH are multifactorial, and some possible mechanisms have been proposed, including pain perception, psychosocial factors, and hormones influence. 28 In terms of pain perception, younger patients and women seem to be more sensitive than older patients and men. 29 Furthermore, younger patients and women are more likely to report pain, which may be because such emotional expression was more socially acceptable in these patients and therefore they were more likely to report them. 26 In addition, fluctuating hormones in the menstrual cycle and hormone‐related differences in cerebral vessel reactivity have long been recognized to be responsible for the development of various headaches. 30 , 31 , 32
Chronic headache history was another independent risk factor for the development of POH after ECS in our analysis, consistent with previous reports. 20 , 25 , 33 , 34 Riley and colleagues 35 reviewed patients who underwent thoracoabdominal aortic aneurysm repair, finding that patients with preoperative headache history were more likely to develop a POH than those without (27.9% versus 8.3%, P≤0.001). Ryzenman and colleagues 25 conducted a detailed questionnaire in patients undergoing acoustic neuroma surgery and reported that preoperative headache could be an independent predictive factor for POH, and the frequency of POH was significantly higher in those with preoperative headache (odds ratio [OR], 1.4; P<0.01). In addition, the pain levels of POH were higher and the quality of life poorer in patients with headache history. 36 Therefore, taking prophylactic medications early may be a proper strategy to reduce the significantly increased probability of POH in patients with headache history. 37
In addition to the above nonmodifiable risk factors, several preoperative modifiable independent risk factors were also identified in this study, including smoking, lower LVEF, and hypertension. This was in agreement with the results of previous studies. 37 Matsota and colleagues 15 conducted a prospective study in patients with elective surgery and reported that smoking was an independent predictor for developing POH, with 1.74‐fold increased odds (P=0.006). The exact mechanism remains to be explored; nevertheless, smoking cessation may be appropriate in both high‐risk patients and the general population for the maintenance of general health and primary prevention. Lower LVEF often implies poorer cardiac function, which may result in a decrease of blood pressure and insufficient blood and oxygen supply. This is similar to the results from Matsota et al’s study, in which they identified intraoperative hypotension as an independent risk factor for POH, with an OR value of 2.12 (P=0.008). 15 Therefore, although not yet verified, we speculate that preoperative improvement in cardiac function may contribute to decreased POH. Hypertension has been reported to be strongly associated with migraine headache, 38 and the severity and frequency of headache may be relieved by some antihypertensive medications, such as β‐blockers, calcium channel blockers, angiotensin‐converting enzyme inhibitors, and angiotensin receptor blockers. 37 , 38 , 39 , 40 , 41 Recently, Siewert and colleagues conducted a genetic correlation analysis and identified a close genetic correlation between migraine headache and blood pressure, which revealed that neurovascular processes associated with increased blood pressure may be the basis of a headache. 42
Two intraoperative variables, CPB time and transfusion of RBC, were also identified as independent risk factors for POH in this study. It is not surprising that CPB time is closely related to the occurrence of POH, as longer CPB always implies longer surgery time, larger intake doses of anaesthesia drugs, and prolonged forced position. Hora and colleagues 16 reported that surgery duration lasting >4 hours was significantly related to the occurrence of POH (OR, 3.7; P=0.019). Matsota and colleagues found that the administration of sevoflurane was significantly associated with a 3.66‐fold increased risk of POH in patients with previous headache history and a 6.90‐fold increased risk of POH in patients without headache history. 15 Furthermore, the process of CPB itself can lead to brain damage and POH in several ways, such as hemodilution, cerebral edema, hypoxia, and embolism. 43 , 44 , 45 Caldas and colleagues 44 conducted a systematic review in patients undergoing cardiac surgery with CPB and reviewed the literature on cerebral hemodynamic abnormalities, demonstrating that the impairment of cerebral autoregulation during CPB may play an important role in the development of neurological complications. Thus, better brain protection during CPB may be an effective strategy to reduce postoperative complications and POH. 45
Transfusion of RBC has been reported to be associated with adverse outcomes in the literature despite it being common and lifesaving during cardiac surgery. 46 , 47 The underlying pathophysiological mechanisms may be multifactorial. A randomized clinical trial conducted by Sharon and colleagues 48 found that headache developed in a plasma treatment group while it did not in the placebo group. Experimental hypoxia has been confirmed to be associated with migraine headache attacks, 49 and we speculate that the decreased oxygen‐carrying capacity of the transfused RBC may be a responsible reason for POH, which may result in insufficient oxygen supply to the brain. Moreover, the red cell distribution width is always increased after RBC transfusion, which may impair microcirculation attributable to the decrease of RBC deformability. 49 Previous studies have shown that red cell distribution width was a predictor of perioperative stroke and multiple organ dysfunction syndrome after cardiac surgery, which may also associate with the development of POH. 50 , 51 In addition, massive blood transfusion implies massive blood loss. The reduction in the total amount of hemoglobin and RBCs and the dilution of the blood can also lead to insufficient oxygen supply to the brain. Clinical practice guidelines for blood transfusion management have been published and updated and a restrictive transfusion strategy has been recommended to reduce the risk of adverse events. 52 , 53
Several other predictors for POH have been reported in other diseases, such as body mass index. 15 , 28 However, these variables were not identified as independent risk factors for POH after ECS in our analysis. In contrast, the identification of intraoperative RBC transfusion volume as an independent risk factor for POH has never been reported, even in surgery performed for other diseases. The huge discrepancies in independent predictors for POH between ECS and surgery for other diseases further underscored the need of this study.
The nomogram may play an immense role in individualized risk evaluation, risk stratification, identification of high‐risk patients, and risk modification. Appropriate treatment aiming at high‐risk populations identified by the nomogram may bring more net benefits. In addition to the preoperative precautions mentioned above, such as smoking cessation, improvements in cardiac function, and blood pressure control with appropriate antihypertensive drugs, prevention efforts during operations are also important. Bezov and colleagues 28 concluded that operator experience was a modifiable risk factor for POH. Indeed, this is the case, as indirectly evidenced by our results. Besides the difficulty of the procedure itself, although not comprehensive, we acknowledge that CPB time and blood loss can largely reflect the surgery proficiency and experience of the operator. There is no doubt that a well‐trained operator requires shorter surgical time and less intraoperative transfusions than those who are unskilled and inexperienced in the same situation. Hence, improving the operating experience and proficiency of the operators may be a feasible and effective measure to reduce the incidence of POH and other adverse events. In addition, current clinical evidence reveals that minimally invasive CPB has advantages over traditional methods in the reduction of blood dilution and better preservation of hematocrit, which may effectively reduce blood transfusion requirements. 54
For the past few years, patients after cardiac surgery did not routinely use analgesics in our hospitals. Short‐acting analgesics were used to solve pain problems only when patients truly needed them. Some patients may complain of wound pain or headache; however, language‐comforting and short‐acting analgesics were sufficient to address these problems in most cases and did not affect observations of other indicators. We cannot deny that the use of short‐acting drugs may lead to underestimation of the true incidence of POH, but it may be difficult to solve this problem at this stage.
Previous studies have reported that POH was associated with adverse outcomes after surgery such as readmission, poor quality of life, and additional economic burden, and they believed that it was essential to implement continuous assessment and targeted intervention. 6 , 9 , 21 In this study, we found that POH was significantly associated with higher rates of pneumonia and reintubation and prolonged ICU and hospital stay. There are no previous studies reporting the specific pathogenesis and association; however, we speculate that the fact that patients with POH are more reluctant to cough or perform some airway cleaning work may be responsible for the elevated probability of pneumonia, which may significantly increase the risks of reintubation and prolonged ICU and hospital stay.
Several limitations of this study should be mentioned. First, this was a retrospective study and the diagnosis of POH was based on databases and medical records. Although we have reviewed the entire course of the diseases, we still cannot guarantee that all patients with headache were recorded, which may lead to an underestimation of true incidence. Second, some possible predictors that may be associated with POH development were not available in this study, such as anxiety disorders and operator experience. The bias may have occurred as a result of individual technical expertise as the operations were not completed by a single surgeon. Even so, the nomogram performed well in discrimination, calibration, and clinical utility. Third, all recorded end point events were limited to the hospitalization, and there was no long‐term follow‐up after hospital discharge, which may result in underestimation of true morbidity. Fourth, the primary end point of this study was POH, but we failed to evaluate the severity and type of POH because of retrospective limitations. Prospective study with long‐term follow‐up and more detailed assessment of headache type and severity may be more meaningful in subsequent work.
CONCLUSIONS
POH was prevalent in patients undergoing ECS. As far as we know, this work represents the first attempt of the identification of independent predictors and the development and validation of a clinical risk prediction model for POH after ECS. Six preoperative and 2 intraoperative factors were identified as independent predictors by multivariate analysis. A nomogram on the basis of these predictors was constructed, which had excellent performance in terms of discrimination, calibration, and clinical usefulness. Three risk groups were divided to better facilitate clinical application. The results of this work may be helpful for clinical risk assessment, decision‐making, and individualized treatment.
Sources of Funding
This work was supported by the National Natural Science Foundation of China (grant number 81800413 and 81801586).
Disclosures
None.
Acknowledgments
The authors would like to thank John S for the language help.
Author contributions: Conception and design: Xinling Du, Xiaofan Huang, Anchen Zhang, and Dashuai Wang; administrative support: Xinling Du, Hongfei Wang, and Sheng Le; provision of study materials or patients: Xinling Du, Xiaofan Huang, Anchen Zhang, and Hongfei Wang; collection and assembly of data: Dashuai Wang, Anchen Zhang, Jia Wu, Fei Xie, and Ximei Li; data analysis and interpretation: Dashuai Wang, Sheng Le, and Xiaofan Huang; article writing: all authors; final approval of article: all authors.
For Sources of Funding and Disclosures, see page 11.
Contributor Information
Xinling Du, Email: xinlingdu@hust.edu.cn.
Xiaofan Huang, Email: dr_xfhuang@hust.edu.cn.
REFERENCES
- 1. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019. J Am Coll Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, Abbasi‐Kangevari M, Abbastabar H, Abd‐Allah F, Abdelalim A, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:e72–e227. doi: 10.1161/CIR.0000000000000923 [DOI] [PubMed] [Google Scholar]
- 4. Patel MR, Calhoon JH, Dehmer GJ, Grantham JA, Maddox TM, Maron DJ, Smith PK. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 appropriate use criteria for coronary revascularization in patients with stable ischemic heart disease. J Am Coll Cardiol. 2017;69:2212–2241. doi: 10.1016/j.jacc.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 5. Sabab A, Sandhu J, Bacchi S, Jukes A, Zacest A. Postoperative headache following treatment of vestibular schwannoma: a literature review. J Clin Neurosci. 2018;52:26–31. doi: 10.1016/j.jocn.2018.04.003 [DOI] [PubMed] [Google Scholar]
- 6. Jang MK, Oh EG, Lee H, Kim EH, Kim S. Postoperative symptoms and quality of life in pituitary macroadenomas patients. J Neurosci Nurs. 2020;52:30–36. doi: 10.1097/JNN.0000000000000483 [DOI] [PubMed] [Google Scholar]
- 7. Rocha‐Filho PAS. Post‐craniotomy headache: a clinical view with a focus on the persistent form. Headache. 2015;55:733–738. doi: 10.1111/head.12563 [DOI] [PubMed] [Google Scholar]
- 8. Jang MK, Park CG, Jang S, Kim EH. Prevalence and impact of postoperative headaches in nonfunctioning pituitary macroadenoma patients: a longitudinal cohort study. World Neurosurg. 2020;133:e633–e639. doi: 10.1016/j.wneu.2019.09.123 [DOI] [PubMed] [Google Scholar]
- 9. Pogoda L, Nijdam JS, Smeeing DPJ, Voormolen EHJ, Ziylan F, Thomeer HGXM. Postoperative headache after surgical treatment of cerebellopontine angle tumors: a systematic review. Eur Arch Otorhinolaryngol. 2021;278:3643–3651. doi: 10.1007/s00405-021-06627-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Su H, Li B, Wang J, Tian C, Cao X, Du Z, Liu X, Liu R, Yu S. Headache attributed to cranial venous sinus stenting: a case series and literature review. Cephalalgia. 2019;39:1277–1283. doi: 10.1177/0333102419847752 [DOI] [PubMed] [Google Scholar]
- 11. Guenther F, Swozil F, Heber S, Buchfelder M, Messlinger K, Fischer MJ. Pre‐ and postoperative headache in patients with meningioma. Cephalalgia. 2019;39:533–543. doi: 10.1177/0333102418793636 [DOI] [PubMed] [Google Scholar]
- 12. Kisser U, Adderson‐Kisser C, Förster M, Stelter K, Nicolo MS, Schrötzlmair F, Müller JM, Hempel JM. Prevalence of headache in cochlear implant patients. Otol Neurotol. 2016;37:1555–1559. doi: 10.1097/MAO.0000000000001244 [DOI] [PubMed] [Google Scholar]
- 13. Molnár L, Simon É, Nemes R, Fülesdi B, Molnár C. Postcraniotomy headache. J Anesth. 2014;28:102–111. doi: 10.1007/s00540-013-1671-z [DOI] [PubMed] [Google Scholar]
- 14. Rocha‐Filho P, Gherpelli J, de Siqueira J, Rabello GD. Post‐craniotomy headache: a proposed revision of IHS diagnostic criteria. Cephalalgia. 2010;30:560–566. doi: 10.1111/j.1468-2982.2009.02010.x [DOI] [PubMed] [Google Scholar]
- 15. Matsota PK, Christodoulopoulou TC, Batistaki CZ, Arvaniti CC, Voumvourakis KI, Kostopanagiotou GG. Factors associated with the presence of postoperative headache in elective surgery patients: a prospective single center cohort study. J Anesth. 2017;31:225–236. doi: 10.1007/s00540-016-2285-z [DOI] [PubMed] [Google Scholar]
- 16. de Oliveira Ribeiro MDC, Pereira CU, Sallum AM, Martins‐Filho PRS, DeSantana JM, Da Silva NM, Hora EC. Immediate post‐craniotomy headache. Cephalalgia. 2013;33:897–905. doi: 10.1177/0333102413479833 [DOI] [PubMed] [Google Scholar]
- 17. Bitargil M, El Kılıç H. Our experience regarding patients with headache, vomiting, and urinary retention following endothermal ablation of the greater saphenous vein under spinal anesthesia: gender type, age interval, and procedural risk factors are important. Vascular. 2020;28:591–596. doi: 10.1177/1708538120911302 [DOI] [PubMed] [Google Scholar]
- 18. Yilmaz G, Akca A, Kiyak H, Salihoglu Z. Elevation in optic nerve sheath diameter due to the pneumoperitoneum and Trendelenburg is associated to postoperative nausea, vomiting and headache in patients undergoing laparoscopic hysterectomy. Minerva Anestesiol. 2020;86:270–276. doi: 10.23736/S0375-9393.19.13920-X [DOI] [PubMed] [Google Scholar]
- 19. Liang B, Shetty SR, Omay SB, Almeida JP, Ni S, Chen Y, Ruiz‐Treviño AS, Anand VK, Schwartz TH. Predictors and incidence of orthostatic headache associated with lumbar drain placement following endoscopic endonasal skull base surgery. Acta Neurochir. 2017;159:1379–1385. doi: 10.1007/s00701-017-3247-4 [DOI] [PubMed] [Google Scholar]
- 20. Gill PS, Guest C, Rabey PG, Buggy DJ. Perioperative headache and day case surgery. Eur J Anaesth. 2003;20:401–403. doi: 10.1017/S0265021503000619 [DOI] [PubMed] [Google Scholar]
- 21. Platzbecker K, Zhang MB, Kurth T, Rudolph MI, Eikermann‐Haerter K, Burstein R, Eikermann M, Houle T. The association between migraine and hospital readmission due to pain after surgery: a hospital registry study. Cephalalgia. 2019;39:286–295. doi: 10.1177/0333102418786457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang EH, Sunderland S, Edwards NY, Chima NS, Yarnold CH, Schwarz SK, Coley MA. A Single prophylactic dose of ondansetron given at cessation of postoperative propofol sedation decreases postoperative nausea and vomiting in cardiac surgery patients: a randomized controlled trial. Anesth Analg. 2020;131:1164–1172. doi: 10.1213/ANE.0000000000004730 [DOI] [PubMed] [Google Scholar]
- 23. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. doi: 10.2307/2531595 [DOI] [PubMed] [Google Scholar]
- 24. Takenaka S, Makino T, Sakai Y, Kashii M, Iwasaki M, Yoshikawa H, Kaito T. Prognostic impact of intra‐ and postoperative management of dural tear on postoperative complications in primary degenerative lumbar diseases. Bone Joint J. 2019;101‐B:1115–1121. doi: 10.1302/0301-620X.101B9.BJJ-2019-0381.R1 [DOI] [PubMed] [Google Scholar]
- 25. Ryzenman JM, Pensak ML, Tew JM. Headache: a quality of life analysis in a cohort of 1,657 patients undergoing acoustic neuroma surgery, results from the Acoustic Neuroma Association. Laryngoscope. 2005;115:703–711. doi: 10.1097/01.mlg.0000161331.83224.c5 [DOI] [PubMed] [Google Scholar]
- 26. Myles PS, Hunt JO, Moloney JT. Postoperative 'minor' complications. Comparison between men and women. Anaesthesia. 2004;52:300–306. doi: 10.1111/j.1365-2044.1997.89-az0091.x [DOI] [PubMed] [Google Scholar]
- 27. Kim JE, Kim SH, Han RJ, Kang MH, Kim JH. Postdural puncture headache related to procedure: incidence and risk factors after neuraxial anesthesia and spinal procedures. Pain Med. 2021;22:1420–1425. doi: 10.1093/pm/pnaa437 [DOI] [PubMed] [Google Scholar]
- 28. Bezov D, Lipton RB, Ashina S. Post‐dural puncture headache: part I diagnosis, epidemiology, etiology, and pathophysiology. Headache. 2010;50:1144–1152. doi: 10.1111/j.1526-4610.2010.01699.x [DOI] [PubMed] [Google Scholar]
- 29. Novais A, Monteiro S, Roque S, Correia‐Neves M, Sousa N. How age, sex and genotype shape the stress response. Neurobiol Stress. 2017;6:44–56. doi: 10.1016/j.ynstr.2016.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hipolito Rodrigues MA, Maitrot‐Mantelet L, Plu‐Bureau G, Gompel A. Migraine, hormones and the menopausal transition. Climacteric. 2018;21:256–266. doi: 10.1080/13697137.2018.1439914 [DOI] [PubMed] [Google Scholar]
- 31. Gupta S, McCarson KE, Welch KM, Berman NE. Mechanisms of pain modulation by sex hormones in migraine. Headache. 2011;51:905–922. doi: 10.1111/j.1526-4610.2011.01908.x [DOI] [PubMed] [Google Scholar]
- 32. Anne ME. Oestrogen and attacks of migraine with and without aura. Lancet Neurol. 2004;3:354–361. doi: 10.1016/S1474-4422(04)00768-9 [DOI] [PubMed] [Google Scholar]
- 33. Valentinis L, Tuniz F, Valent F, Mucchiut M, Little D, Skrap M, Bergonzi P, Zanchin G. Headache attributed to intracranial tumours: a prospective cohort study. Cephalalgia. 2010;30:389–398. doi: 10.1111/j.1468-2982.2009.01970.x [DOI] [PubMed] [Google Scholar]
- 34. Wang D, Huang X, Wang H, Le S, Du X. Predictors and nomogram models for postoperative headache in patients undergoing heart valve surgery. J Thorac Dis. 2021;13:4236–4249. doi: 10.21037/jtd-21-644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Riley SP, Donnelly MJ, Khatib D, Warren C, Schroeder KM. Post‐dural puncture headaches following spinal drain placement during thoracoabdominal aortic aneurysm repair: incidence, associated risk factors, and treatment. J Anesth. 2015;29:544–550. doi: 10.1007/s00540-015-1990-3 [DOI] [PubMed] [Google Scholar]
- 36. Yabuki S, Takatsuki K, Otani K, Nikaido T, Watanabe K, Kato K, Kobayashi H, Handa J, Konno S, Antonaci F. Headache in patients with cervical spondylotic myelopathy. Pain Res Manag. 2020;2020:1–6. doi: 10.1155/2020/8856088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Evers S. Treatment of migraine with prophylactic drugs. Expert Opin Pharmacother. 2008;9:2565–2573. doi: 10.1517/14656566.9.15.2565 [DOI] [PubMed] [Google Scholar]
- 38. Rist PM, Winter AC, Buring JE, Sesso HD, Kurth T. Migraine and the risk of incident hypertension among women. Cephalalgia. 2018;38:1817–1824. doi: 10.1177/0333102418756865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ripa P, Ornello R, Pistoia F, Carolei A, Sacco S. The renin–angiotensin system: a possible contributor to migraine pathogenesis and prophylaxis. Expert Rev Neurother. 2014;14:1043–1055. doi: 10.1586/14737175.2014.946408 [DOI] [PubMed] [Google Scholar]
- 40. Diener HC, Küper M, Kurth T. Migraine‐associated risks and comorbidity. J Neurol. 2008;255:1290–1301. doi: 10.1007/s00415-008-0984-6 [DOI] [PubMed] [Google Scholar]
- 41. Charles A. The pathophysiology of migraine: implications for clinical management. Lancet Neurol. 2018;17:174–182. doi: 10.1016/S1474-4422(17)30435-0 [DOI] [PubMed] [Google Scholar]
- 42. Siewert KM, Klarin D, Damrauer SM, Chang KM, Tsao PS, Assimes TL, Davey Smith G, Voight BF, Gormley P, Anttila V, et al. Cross‐trait analyses with migraine reveal widespread pleiotropy and suggest a vascular component to migraine headache. Int J Epidemiol. 2020;49:1022–1031. doi: 10.1093/ije/dyaa050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhu S, Sai X, Lin J, Deng G, Zhao M, Nasser MI, Zhu P. Mechanisms of perioperative brain damage in children with congenital heart disease. Biomed Pharmacother. 2020;132:110957. doi: 10.1016/j.biopha.2020.110957 [DOI] [PubMed] [Google Scholar]
- 44. Caldas JR, Haunton VJ, Panerai RB, Hajjar LA, Robinson TG. Cerebral autoregulation in cardiopulmonary bypass surgery: a systematic review. Interact Cardiovasc Thorac Surg. 2018;26:494–503. doi: 10.1093/icvts/ivx357 [DOI] [PubMed] [Google Scholar]
- 45. Salameh A, Dhein S, Dähnert I, Klein N. Neuroprotective strategies during cardiac surgery with cardiopulmonary bypass. Int J Mol Sci. 2016;17:1945. doi: 10.3390/ijms17111945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sultan I, Bianco V, Aranda‐Michel E, Kilic A, Serna‐Gallegos D, Navid F, Wang Y, Gleason TG. The use of blood and blood products in aortic surgery is associated with adverse outcomes. J Thorac Cardiovasc Surg. 2021:S0022‐5223:00452‐9. doi: 10.1016/j.jtcvs.2021.02.096 [DOI] [PubMed] [Google Scholar]
- 47. Wang D, Huang X, Wang H, Le S, Yang H, Wang F, Du X. Risk factors for postoperative pneumonia after cardiac surgery: a prediction model. J Thorac Dis. 2021;13:2351–2362. doi: 10.21037/jtd-20-3586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sha SJ, Deutsch GK, Tian LU, Richardson K, Coburn M, Gaudioso JL, Marcal T, Solomon E, Boumis A, Bet A, et al. Safety, tolerability, and feasibility of young plasma infusion in the plasma for Alzheimer symptom amelioration study. JAMA Neurol. 2019;76:35. doi: 10.1001/jamaneurol.2018.3288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Arngrim N, Schytz HW, Britze J, Amin FM, Vestergaard MB, Hougaard A, Wolfram F, de Koning PJH, Olsen KS, Secher NH, et al. Migraine induced by hypoxia: an MRI spectroscopy and angiography study. Brain. 2016;139:723–737. doi: 10.1093/brain/awv359 [DOI] [PubMed] [Google Scholar]
- 50. Duchnowski P, Hryniewiecki T, Kuśmierczyk M, Szymanski P. Red cell distribution width as a predictor multiple organ dysfunction syndrome in patients undergoing heart valve surgery. Biol Open. 2018;7:bio036251. doi: 10.1242/bio.036251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Duchnowski P, Hryniewiecki T, Kuśmierczyk M, Szymański P. Red cell distribution width is a prognostic marker of perioperative stroke in patients undergoing cardiac valve surgery. Interact Cardiovasc Thorac Surg. 2017;25:925–929. doi: 10.1093/icvts/ivx216 [DOI] [PubMed] [Google Scholar]
- 52. Carson JL, Guyatt G, Heddle NM, Grossman BJ, Cohn CS, Fung MK, Gernsheimer T, Holcomb JB, Kaplan LJ, Katz LM, et al. Clinical practice guidelines from the AABB: red blood cell transfusion thresholds and storage. JAMA. 2016;316:2025–2035. doi: 10.1001/jama.2016.9185 [DOI] [PubMed] [Google Scholar]
- 53. Apfelbaum JL, Nuttall GA, Connis RT, Miller RD, Nickinovich DG, Nussmeier NA, Rosenberg AD. Practice guidelines for perioperative blood management: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Management*. Anesthesiology. 2015;122:241–275. doi: 10.1097/ALN.0000000000000463 [DOI] [PubMed] [Google Scholar]
- 54. Anastasiadis K, Argiriadou H, Deliopoulos A, Antonitsis P. Minimal invasive extracorporeal circulation (MiECC): the state‐of‐the‐art in perfusion. J Thorac Dis. 2019;11:S1507–S1514. doi: 10.21037/jtd.2019.01.66 [DOI] [PMC free article] [PubMed] [Google Scholar]


