Abstract
Background
Duchenne and Becker muscular dystrophy are progressive disorders associated with cardiac mortality. Guidelines recommend routine surveillance; we assess cardiac resource use and identify gaps in care delivery.
Methods and Results
Male patients, aged 1 to 18 years, with Duchenne and Becker muscular dystrophy between January 2013 and December 2017 were identified in the IBM MarketScan Research Database. The cohort was divided into <10 and 10 to 18 years of age. The primary outcome was rate of annual health care resource per person year. Resource use was assessed for place of service, cardiac testing, and medications. Adjusted incidence rate ratios (IRRs) were estimated using a Poisson regression model. Medication use was measured by proportion of days covered. There were 1386 patients with a median follow‐up time of 3.0 years (interquartile range, 1.9–4.7 years). Patients in the 10 to 18 years group had only 0.40 (95% CI, 0.35–0.45) cardiology visits per person year and 0.66 (95% CI, 0.62–0.70) echocardiography/magnetic resonance imaging per person year. Older patients had higher rates of inpatient admissions (IRR, 1.46; 95% CI, 1.03–2.09), outpatient cardiology visits (IRR, 2.0; 95% CI, 1.66–2.40), cardiac imaging (IRR, 1.59; 95% CI, 1.40–1.80), and Holter monitoring (IRR, 3.33; 95% CI, 2.35–4.73). A proportion of days covered >80% for angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers was observed in 13.6% (419/3083) of total person years among patients in the 10 to 18 years group.
Conclusions
Children 10 to 18 years of age have higher rates of cardiac resource use compared with those <10 years of age. However, rates in both age groups fall short of guidelines. Opportunities exist to identify barriers to resource use and optimize cardiac care for patients with Duchenne and Becker muscular dystrophy.
Keywords: Becker muscular dystrophy, Duchenne muscular dystrophy, heart failure, resource use
Subject Categories: Pediatrics, Cardiomyopathy, Heart Failure, Health Services
Nonstandard Abbreviations and Acronyms
- CCC
complex chronic condition
- DBMD
Duchenne and Becker muscular dystrophy
- DMD
Duchenne muscular dystrophy
- IRR
incidence rate ratio
- PDC
proportion of days covered
Clinical Perspective
What Is New?
Cardiac disease is one of the leading causes of morbidity and mortality among children with muscular dystrophy; however, cardiac surveillance is limited.
Older children with muscular dystrophy have higher rates of hospitalization and cardiac imaging compared with younger children.
Data of health care resource use may provide information on areas in need of improvement.
What Are the Clinical Implications?
An increase in cardiac surveillance for children with Duchenne or Becker muscular dystrophy is needed to meet clinical care guidelines.
Duchenne and Becker muscular dystrophy (DBMD) are the most common forms of childhood‐onset muscular dystrophy, with pooled prevalence estimates of 1.38 per 10 000 male individuals. 1 Although phenotypically distinct, both are attributable to X‐linked mutations in the dystrophin gene, which subsequently leads to progressive muscle fiber degeneration and weakness. Because of differences in diagnostic methods and variations in populations, there have been some small differences in published analyses on prevalence. Duchenne muscular dystrophy (DMD) has been estimated to have a prevalence of 1.2 per 10 000 male individuals in North America. 1 Affected male patients with DMD usually present with symptoms between 2 and 5 years of age. Loss of ambulation occurs around 10 years of age in those untreated with steroids, and in the setting of progressive respiratory insufficiency, noninvasive ventilatory support is typically initiated by the age of 18 years. 2 , 3 Becker muscular dystrophy (BMD) has a somewhat lower prevalence than DMD and is estimated to be 0.36 per 10 000 male individuals in North America. 1 It is characterized by variable amounts of functioning dystrophin and has a later age of onset and a milder clinical course, with ambulation usually preserved beyond age 16 years. 4
Cardiomyopathy represents a significant source of morbidity and mortality for patients with DBMD. Affected patients typically have a ventricular dysfunction, dilated phenotype, and electrocardiographic abnormalities, and may have fibrosis detected by cardiac magnetic resonance imaging (MRI). 5 , 6 , 7 More important, the findings of cardiomyopathy do not typically manifest until late childhood or even adolescence. 7 , 8 However, as the life expectancy among patients with DMD has improved with the introduction of noninvasive respiratory support and the growing use of glucocorticoids, the cardiac manifestations of the disease have become increasingly prominent, 2 , 9 , 10 , 11 with some studies estimating an increase in mortality attributable to a cardiac cause from 8% to 40% over the past 30 years. 12 It is in the context of evolving medical management and increase in life expectancy that there has rightly been a growing focus on standardization and optimization of cardiac management for patients with DBMD. 13 , 14
In the setting of skeletal muscle weakness and impaired ambulation, it can be difficult to identify symptoms of poor cardiac output in patients with muscular dystrophy. Therefore, surveillance is critical in diagnosing cardiomyopathy and initiating potentially disease‐modifying interventions. In 2017, the American Heart Association updated management guidelines, recommending cardiac evaluation in asymptomatic patients, including a physical examination, ECG, and noninvasive imaging every 2 years in patients <10 years of age, with an increase to an annual evaluation at 10 years of age and later. 15 The DMD care considerations, which focus specifically on the care of those with DMD, were originally published in 2010 and then updated in 2018. 16 , 17 The current care guidelines recommend annual evaluation for patients at even younger ages in the late ambulatory period. 16 With respect to recommendations on heart remodeling therapies, consideration of initiating an angiotensin‐converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB) before the age of 10 years among patients with DMD has been suggested, but there is no definitive guideline. Furthermore, it is unknown how many providers prescribe prophylactic ACEIs/ARBs. 15 , 18
There are limited data evaluating the use of cardiac care for patients with DBMD, yet doing so is especially important given the changing disease course and the recently revised guidelines. Therefore, our objectives were to describe the burden of cardiac disease and management in pediatric patients with DBMD and to assess the association between age and cardiac resource use.
Methods
The present study contains deidentified data and thus was deemed to be exempt from review by The Children’s Hospital of Philadelphia Institutional Review Board, according to the Common Rule. The study materials linked to this research are available from the corresponding author on reasonable request and following approval by the IBM MarketScan Research Databases.
Data Sources
The IBM MarketScan Research Databases are a family of research data sets that capture deidentified patient‐ and insurance claim–level health data (medical, drug, and dental), productivity (workplace absence, short‐ and long‐term disability, and workers’ compensation), laboratory results, health risk assessments, hospital discharges, and electronic medical records. The database has collected information on >245 million unique patients since 1995, and data are contributed by large employers, health plans, and government and public organizations. 19 It includes claims from both inpatient and outpatient encounters for all patient enrollment periods, thus representing a longitudinal characterization of resource use. This analysis used the following individual databases:
IBM MarketScan Commercial Claims and Encounters database, which includes data from active employees, early retirees, COBRA continuees, and dependents insured by employer‐sponsored health plans;
IBM MarketScan Multi‐State Medicaid Database, which contains pooled health care data of >44 million Medicaid enrollees from multiple states.
The MarketScan Research Databases have been effectively used in a variety of other studies to characterize patient populations with chronic disease, such as Fontan physiology, HIV, and cirrhosis, and to describe trends in resource use. 20 , 21 , 22 , 23
Study Sample Selection
Male patients ≤18 years of age with at least one hereditary progressive muscular dystrophy diagnosis (International Classification of Diseases, Ninth Revision [ICD‐9], code 359.1; International Classification of Diseases, Tenth Revision [ICD‐10], code G71.01) were identified between January 1, 2013, and December 31, 2017. As illustrated in Figure 1, the date of the first hereditary progressive muscular dystrophy diagnosis was designated as the index date. All patients were required to have continuous enrollment for at least 12 months after the index date. To limit the number of patients with other hereditary progressive muscular dystrophies who tend to have earlier and more severe presentations, patients aged <1 year on the index date were excluded. Patients were also excluded if they had any of the following diagnoses indicated via ICD‐9/ICD‐10 codes in any patient claims: mitochondrial disorder, fatty oxidation disorders, lipid storage disorders, metabolic disorder, spinal muscular atrophy, congenital myotonia, myotonic muscular dystrophy, and myasthenia gravis. Similar methods have been used in other retrospective studies on DMBD. 24 , 25 , 26
Figure 1. Patient selection.
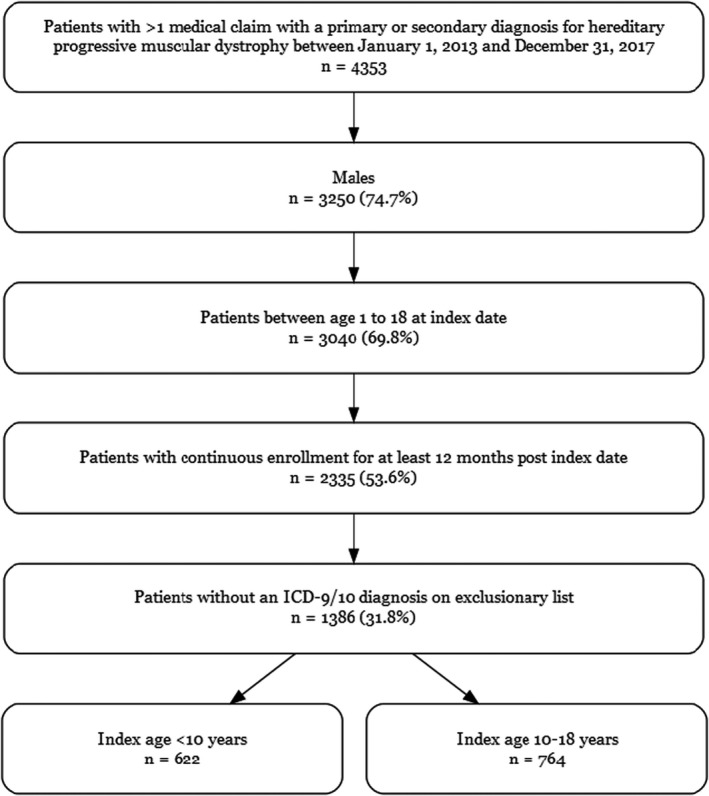
ICD‐9 indicates International Classification of Diseases, Ninth Revision; and ICD‐10, International Classification of Diseases, Tenth Revision.
Patients were grouped into 2 cohorts based on their index age: <10 years and 10 to 18 years. These cohorts were selected as 10 years is traditionally considered the beginning of the early nonambulatory stage, and current guidelines use this age as a cutoff with respect to recommendations for frequency of cardiac evaluation. 15 , 16
Characteristics
Patient‐level characteristics included duration of follow‐up, age at time of claim, insurance plan type, geographic region (Commercial Claims and Encounters database only), race (Multi‐State Medicaid Database only), comorbidities, such as heart failure and renal failure (defined by presence of ICD‐9/ICD‐10 diagnosis code), complex chronic conditions (CCCs), and death. CCCs were used to identify comorbid conditions and have been defined as “medical conditions that can be reasonably expected to last at least 12 months and that involve either several different organ systems or one organ system severely affected enough to require specialty pediatric care and some period of hospitalization in a tertiary care.” 27 CCCs, heart failure, respiratory failure, and in‐hospital death were captured in any patient claim and attributed to the entire patient study period.
Study Outcomes
The primary outcome measure in the study was the rate of annual health care resource use during the follow‐up period. Resource use was assessed for place of service (inpatient, outpatient cardiology, and emergency department visits), cardiac tests (echocardiogram/MRI, ECG, and Holter monitoring), and medications (ACEIs/ARBs and steroids). Medication use was measured by proportion of days covered (PDC), which was determined by summing the number of days patients were covered on a particular drug during each follow‐up year divided by 365 days and multiplied by 100. To capture different patterns of use, patients were stratified into 5 PDC categories: 0%, >0% to <20%, 20% to <50%, 50% to <80%, and ≥80%.
Statistical Analysis
Patient demographic and clinical characteristics at time of the index date were summarized using descriptive statistics. Frequencies and percentages were reported for categorical variables (insurance plan type, geographic region, race, comorbidities, CCCs, and death), and differences between the 2 age‐group cohorts were assessed with χ2 or Fisher exact tests, as appropriate; median and interquartile range were reported for continuous variables (follow‐up duration), and differences between the 2 cohorts were determined using Wilcoxon rank‐sum tests. Resource use was assessed between the 2 age groups; the age of the patient at the time of the claim was used to assign the event to the specific age group. Thus, if a patient aged 9 years old turned 10 years of age during the study period, claims observed following this date were assigned to the 10 to 18 years group.
Crude incidence rates for the primary outcome were calculated by dividing the total number of events by the total number of person‐years contributed by people at risk during the time period of interest. Because patients within age group cohorts can have repeated events, repeated measures Poisson regression models, adjusted for follow‐up time, were used to estimate incidence rates and incidence rate ratios (IRRs) of resource use (place of service and cardiac tests). P<0.05 was considered to indicate a significant difference. SAS version 9.4 (SAS Institute Inc, Cary, NC) was used for data management and all statistical analyses.
Results
Patient Characteristics
Patient attrition is displayed in Figure 1. Of the 4353 patients who had at least 1 medical claim with a primary or secondary diagnosis for hereditary progressive muscular dystrophy, 2335 (53.6%) satisfied the inclusion criteria: male sex, age 1 to 18 years at the index date, and continuous enrollment for at least 12 months after the index date. After excluding patients who also carried diagnoses of other neuromuscular diseases, 1386 patients (31.8%) remained in the study.
Patients of White race (53.2%) were the most commonly represented race (using the Medicaid database only as race information was not available in the Commercial database) (Table 1). The most commonly represented region was the South (36.1%, using the Commercial database only as region was not widely available in the Medicaid database). Of the 1386 patients, 622 (44.8%) were aged <10 years at the index date. The median follow‐up duration was 3.0 (interquartile range, 1.9–4.7) years, with patients <10 years having a slightly longer duration of follow‐up (3.9 [interquartile range, 2.0–5.3] years versus 2.5 [interquartile range, 1.7–4.0] years; P<0.001). There was no significant difference between the 2 groups with regard to type of insurance, region, or race.
Table 1.
Patient Demographic and Clinical Characteristics
| Characteristics |
Overall (n=1386) |
Index age <10 y (n=622) |
Index age 10–18 y (n=764) |
P value |
|---|---|---|---|---|
| Race or ethnicity, n (%)* | 0.448 | |||
| White | 379 (53.2) | 163 (50.5) | 216 (55.5) | |
| Black | 82 (11.5) | 39 (12.1) | 43 (11.1) | |
| Hispanic | 50 (7.0) | 28 (8.7) | 22 (5.7) | |
| Other † | 7 (1.0) | 4 (1.2) | 3 (0.8) | |
| Unknown | 194 (27.3) | 89 (27.5) | 105 (26.9) | |
| Region, n (%)* | 0.209 | |||
| Northeast | 116 (17.2) | 49 (16.4) | 67 (8.8) | |
| North central | 166 (24.6) | 62 (20.7) | 104 (13.6) | |
| South | 243 (36.1) | 115 (38.5) | 128 (16.8) | |
| West | 133 (19.7) | 66 (22.1) | 67 (8.8) | |
| Unknown | 16 (2.4) | 7(2.3) | 9 (2.4) | |
| Insurance, n (%) | 0.707 | |||
| Commercial | 674 (48.6) | 299 (48.1) | 375 (49.1) | |
| Medicaid | 712 (51.4) | 323 (51.9) | 389 (50.9) | |
| Follow‐up duration, y, median (Q1–Q3) | 3.0 (1.9–4.7) | 3.9 (2.0–5.3) | 2.5 (1.7–4.0) | <0.001 |
| Heart failure, n (%) | 75 (5.4) | 18 (2.9) | 57 (7.5) | <0.001 |
| Dilated or other cardiomyopathy, n (%) | 211 (15.2) | 40 (6.4) | 171 (22.4) | <0.001 |
| Hypertrophic cardiomyopathy, n (%) | 10 (0.7) | 2 (0.3) | 8 (1.1) | 0.200 |
| Respiratory failure, n (%) | 144 (10.4) | 34 (5.5) | 110 (14.4) | <0.001 |
| CCCs, n (%) | ||||
| ≥1 CCC | 431 (31.1) | 180 (28.9) | 251 (32.9) | 0.117 |
| CCC categories, n (%) | ||||
| Renal | 43 (3.1) | 24 (3.9) | 19 (2.5) | 0.143 |
| Gastrointestinal | 110 (7.9) | 43 (6.9) | 67 (8.8) | 0.203 |
| Hematologic/immunologic | 35 (2.5) | 22 (3.5) | 13 (1.7) | 0.030 |
| Metabolic | 215 (15.5) | 99 (15.9) | 116 (15.2) | 0.708 |
| Malignancy | 52 (3.8) | 23 (3.7) | 29 (3.8) | 0.924 |
| Premature/neonatal | 18 (1.3) | 14 (2.3) | 4 (0.5) | 0.005 |
| Transplant | 5 (0.4) | 2 (0.3) | 3 (0.4) | 0.999 |
| Technology dependence | 169 (12.2) | 50 (8.0) | 119 (15.6) | <0.001 |
| Death, n (%) | 14 (1.0) | 0 (0.0) | 14 (1.8) | <0.001 |
CCC indicates complex chronic condition; Q1, quartile 1; and Q3, quartile 3.
Region was reported in the Commercial database only; race or ethnicity was reported in the Medicaid database only.
The “Other” race/ethnicity category includes those not identified in the above mentioned groups.
Overall, cardiopulmonary disease was common, with 1 in 6 patients having a diagnosis of cardiomyopathy, 1 in 20 patients having heart failure, and 1 in 10 patients having respiratory failure (Table 1). When comparing age groups, there was a higher percentage of patients aged 10 to 18 years with dilated or other cardiomyopathy (171 [22.4%] versus 40 [6.4%]; P<0.001), heart failure (57 [7.5%] versus 18 [2.9%]; P<0.001), and respiratory failure (110 [14.4%] versus 34 [5.5%]; P<0.001). With respect to complex chronic conditions, 431 (31.1%) of all patients had ≥1 CCCs, with the most commonly reported CCC being metabolic (215 [15.5%]). Metabolic CCCs encompass a variety of diagnoses, including hypocalcemia, dyslipidemia, and disordered carbohydrate metabolism, which can be seen in patients with DBMD. Glucocorticoid therapy and loss of ambulation/progressive musculoskeletal weakness have predisposed this patient population to poor nutritional status and compromised bone health. 28 , 29 The frequency of CCCs among both groups was comparable. In the <10 years age group, there were higher rates of hematologic/immunologic conditions and prematurity, whereas those 10 to 18 years had higher rates of technology dependence (119 [15.6%] versus 50 [8.0%]; P<0.001). During the study period, there were 14 deaths (1.8% of 764 patients) in the cohort, all of which occurred in the 10 to 18 years age group.
Resource Use
The 1386 patients contributed 4829 total person‐years. The <10 years and 10 to 18 years cohorts contributed 1746 and 3083 person‐years, respectively. Visit and testing rates based on these person‐years are shown in Table 2.
Table 2.
Visit and Testing Rates
| Variable | IR (95% CI)* | IRR (95% CI)* | P value | |
|---|---|---|---|---|
| <10 y | 10–18 y | 10–18 y vs <10 y | ||
| Visits | ||||
| Emergency department | 0.47 (0.39–0.56) | 0.34 (0.30–0.38) | 0.71 (0.58–0.87) | 0.0010 |
| Admissions | 0.09 (0.06–0.12) | 0.13 (0.11–0.15) | 1.46 (1.03–2.09) | 0.035 |
| Cardiology | 0.20 (0.17–0.23) | 0.40 (0.35–0.45) | 2.00 (1.66–2.40) | <0.001 |
| Tests | ||||
| Echocardiogram/MRI | 0.42 (0.37–0.47) | 0.66 (0.62–0.70) | 1.59 (1.40–1.80) | <0.001 |
| ECG | 0.37 (0.34–0.41) | 0.63 (0.58–0.67) | 1.68 (1.50–1.89) | <0.001 |
| Holter monitoring | 0.04 (0.03–0.06) | 0.14 (0.12–0.16) | 3.33 (2.35–4.73) | <0.001 |
The unit of IR is per person per year. IR indicates incidence rate; IRR, IR ratio; and MRI, magnetic resonance imaging.
Estimates adjusted for follow‐up time.
Of the 1386 patients, 638 (46.0%) had at least 1 emergency visit during their follow‐up period. Children who were 10 to 18 years of age had a lower rate of annual emergency department visits (IRR, 0.71; 95% CI, 0.58–0.87) compared with those who were younger. A total of 289 (20.9%) patients had at least 1 hospital admission, and 550 patients (39.7%) had at least 1 outpatient cardiology visit during follow‐up. Children who were 10 years or older had ≈1.5 times the rate of inpatient admissions (IRR, 1.46; 95% CI, 1.03–2.09) and twice the rate of outpatient cardiology visits (IRR, 2.00; 95% CI, 1.66–2.40) compared with children <10 years of age (Table 2).
With respect to cardiac testing, 68.6% (951/1386) of patients had at least one echocardiogram or MRI scan; the overall rate of echocardiograms/MRIs was 0.57 per person year (Figure 2). The rate of annual cardiac imaging among 10‐ to 18‐year‐old children was just 0.66 tests per person year (Table 2); those in the 10‐ to 18‐year‐old group had a higher rate of cardiac imaging (IRR, 1.59; 95% CI, 1.40–1.80) than those <10 years of age. A total of 64.4% (892/1386) of patients had at least one ECG, and the overall rate of ECGs was 0.54 per person year. Older children had 1.68 times the rate of ECGs (IRR, 1.68; 95% CI, 1.50–1.89) compared with those <10 years of age. A total of 18.2% (252/1386) of patients had a Holter monitor during follow‐up, with a rate of 0.10 monitors per person year. Patients in the 10‐ to 18‐year‐old group had triple the rate of Holter monitor testing (IRR, 3.33; 95% CI, 2.35–4.73) compared with patients <10 years of age.
Figure 2. Resource use.
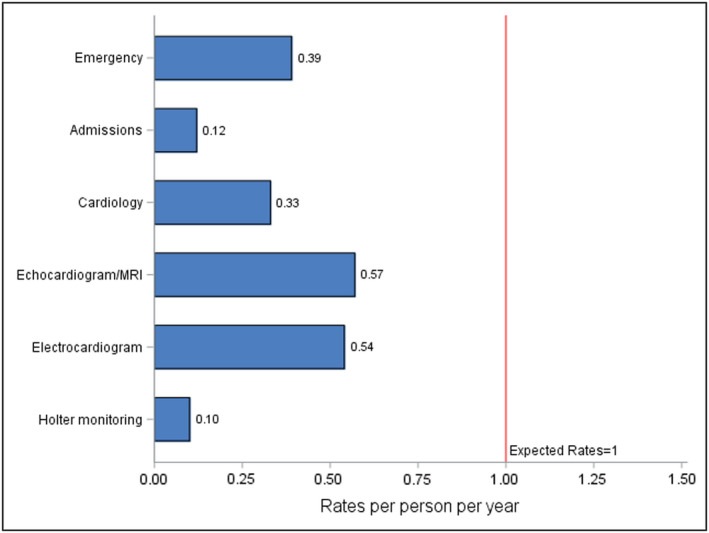
MRI indicates magnetic resonance imaging.
Medication
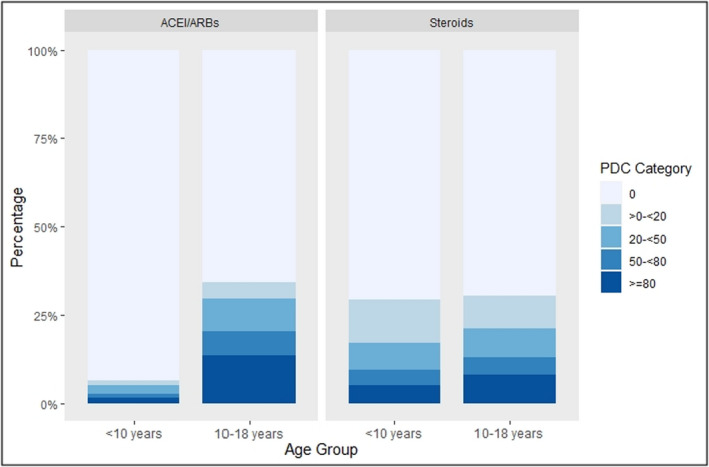
With regard to medications, use of an ACEI or ARB was rare. Only 9.3% (448) of all person years had an ACEI or ARB prescription with PDC ≥80% during follow‐up (Figure 3). Among those <10 years of age, 1.6% (28/1746) of person years had a PDC ≥80% for ACEI/ARB use, whereas among those in the 10‐ to 18‐year group, only 13.6% (419/3083) of total person years had a PDC ≥80%. Steroid use was also rare, with 7.1% of the total person years (345) having a PDC ≥80%. For those <10 years of age, PDC ≥80% was seen in 5.2% (91/1746) of all person years; and for those 10 to 18 years of age, PDC ≥80% was seen in 8.2% (253/3083) of total person years.
Figure 3. Proportion of days covered (PDC) for angiotensin‐converting enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs) and steroids by age group.
Discussion
This is the largest observational study to date assessing pediatric cardiac resource use among patients with DBMD. Prior work exploring resource use in this population has relied on surveys and, thus, may have provided a biased estimate. 30 , 31 , 32 The present study addresses this limitation by using a national claims database. Challenges on identification and selection of participants attributable to lack of specific ICD‐9 codes for DBMD were addressed using methods previously described to include a cohort of patients who represent the population of interest. 24 , 25 Through these methods, the present study provides a more accurate assessment of cardiac resource use in the United States among patients with DBMD and was able to highlight 3 notable findings: older patients have a higher rate of cardiac resource use; the rate of cardiac testing is greater than the rate of outpatient cardiac evaluation; and the rate of resource use and use of cardiac reverse remodeling medications suggests a need to improve access to care to meet current guideline recommendations.
Consistent with previous findings, cardiac and respiratory comorbidities increased with age in our cohort, and there were greater inpatient admissions among older children. 8 , 16 , 24 The higher rate of inpatient admissions among older patients may be attributable to progression of disease. Interestingly, the rate of emergency department visits was lower among those 10 to 18 years of age and may be attributable to challenges surrounding transportation. As patients age and have greater dependence on technology, it may be increasingly difficult for caregivers to take patients to seek medical care, thus leading to lower rates of emergency department visits. However, further study would be needed to better understand this pattern of use.
The most striking observations in the present study lie in the low frequency of cardiac evaluation and testing seen in our cohort. Although it is not surprising that children aged 10 to 18 years had higher rates of outpatient cardiology visits than those aged <10 years, it is notable that the adjusted rate among the older age groups was just 0.40 visits per person year. The rate of cardiac imaging among older children was also just 0.66 echocardiographic studies or MRIs per person year. Applying current practice care guidelines, the expected rate for both cardiac evaluation and testing would be ≈1 visit/test per patient year starting at 10 years of age. 15 Similar patterns were observed for both ECG and Holter monitoring, wherein children in the older cohort had higher rates compared with younger children, but the absolute rate was surprisingly low.
As the most recent guidelines were published toward the end of the observation period in 2017 and 2018, the present study is not a measure of adherence to the most updated clinical guidelines but rather a reflection of the significant change that is needed to meet such recommendations. In a patient population where the report of symptomatic heart failure is limited by musculoskeletal weakness, regular cardiac surveillance is crucial in identifying those at higher risk of cardiac disease, as well as those who have already developed cardiac dysfunction. Nearly 30 years ago, Nigro et al found that among a cohort of 328 patients with DMD, nearly 62% of patients had cardiac involvement by the age of 10 years, with nearly universal cardiac involvement by the age of 18 years. 8 This and other work have underscored the significant burden of cardiac pathology among these patients relatively early in the course of disease. 13 , 33 , 34 Although the introduction of glucocorticoids has extended life expectancy for patients with DMD, it has also ushered in an era where complications related to disease progression are a growing clinical concern. 9 Cardiac pathology, one of the leading causes of death for these patients, is one such area in need of attention.
The rate of cardiac imaging is greater than the rate of outpatient cardiac visits for both age groups. This observation suggests that noncardiac providers, such as primary care clinicians, acute care clinicians, or neurologists, may be delivering cardiac care to this patient population. It is also possible that patients are receiving cardiac care in settings other than an outpatient cardiology visit, such as during a hospitalization or in conjunction with another specialty visit. There is no evidence to suggest that receipt of cardiac care in these circumstances fails to provide appropriate surveillance or management. However, it does underscore a resource use pattern for a complex cohort of patients who have multisystem involvement and require multidisciplinary input as their disease progresses. It highlights how approaches on improvement of delivery and access to care may need to consider models that facilitate multidisciplinary participation. Such multidisciplinary clinics that involve cardiology have been shown to lower cardiac‐related hospitalizations and duration of hospitalizations among adult patients with muscular dystrophy and may be of similar value for children. 35
It is also noteworthy that in the present study, use of ACEIs/ARBs was low among both age groups, with older patients in the 10 to 18 years group representing a larger portion of patients using these interventions. The use of cardiac reverse remodeling agents in this population is an area of growing interest as patients are now living into the third and fourth decade of life. There is evidence to suggest that early implementation of ACEIs/ARBs and aldosterone antagonists may delay the decline of myocardial function, increase survival, and reduce heart failure hospitalization. 18 , 36 , 37 In a randomized, double‐blinded, placebo‐controlled study of patients with DMD, among patients with earlier use of ACEI, there were fewer cases of decreased ventricular function and reduced mortality. 18 , 38 In light of such findings, the 2018 DMD Care Considerations and American Heart Association guidelines suggest starting an ACEI or ARB around the age of 10 years for patients with DMD. 15 , 16 The findings in this study demonstrate a need not only to enhance cardiac evaluation, but also a need to understand practice variation and to improve standardization of care with respect to initiation of cardiac reverse remodeling agents.
Although MarketScan has many strengths, the present study does have several limitations, many of which are inherent to retrospective studies and the use of administrative claims‐level data sets. Although we used methods to define our cohort similar to those found in studies using claims data for patients with DBMD, it is possible that we included patients with other pathology and this may have affected our results. Furthermore, we recognize that the present analyses are pooled estimates for DMD and BMD. ICD‐9/ICD‐10 diagnostic codes unfortunately do not differentiate between these 2 distinct entities. As the DMD‐specific guidelines cannot be applied to patients with BMD, conclusions from this study should be interpreted within the context of the mixed study cohort. However, as DMD has been demonstrated to be more prevalent than BMD, the present population is likely to be weighted with more patients with DMD. Although there is now a “Duchenne or Becker muscular dystrophy” ICD‐10 code, the next revision may consider differentiating these entities further for research purposes. There may also be clinician and institutional differences with respect to coding practices for diagnosis, testing, and medications; and we may have thus not captured all events, such as cardiac imaging obtained for a funded clinical research study. Furthermore, data on important patient characteristics are limited as race and region are collected only in MarketScan Medicaid and Commercial claims, respectively.
The present study demonstrates a gap between cardiac resource use among patients with DBMD and the recently published recommendations on frequency of evaluation, including arrhythmia monitoring, noninvasive imaging, and initiation of ACEI/ARB for patients with DMD. Although resource use in some areas was greater among older patients, our findings suggest that significant efforts must be implemented to meet current guidelines. It is unclear from the present study whether the observed gap is the result of practice variation before modification of the guidelines or if barriers on access to care are hindering the DBMD community from receiving the recommended care. However, as cardiac disease continues to be a significant source of morbidity and mortality, identifying and addressing limitations and barriers will be essential for improving cardiac care for patients with DBMD.
Sources of Funding
This study was funded by National Institutes of Health T32 HL1007915.
Disclosures
Dr. Brandsema collaborated in the Consultant for Audentes, AveXis/Novartis, Biogen, Cytokinetics, Genentech, Marathon, Momenta/Janssen, NS Pharma, Pfizer, PTC Therapeutics, Sarepta, Scholar Rock, WaVe Speaker for veXis/Novartis and Biogen, Medical advisory council member for Cure SMA and site investigator for clinical trials with Alexion, Astellas, AveXis/Novartis, Biogen, Catabasis, CSL Behring, Cytokinetics, Fibrogen, Genentech, Ionis, Lilly, Pfizer, PTC Therapeutics, Sarepta, Summit, WaVe. The remaining authors have no disclosures to report.
Acknowledgments
We would like to acknowledge IBM MarketScan Research Database as the data source.
Presented in part as a poster at the Muscular Dystrophy Association Clinical and Scientific Conference, which was held virtually as a series of events throughout the summer of 2020 due to the pandemic.
For Sources of Funding and Disclosures, see page 9.
References
- 1. Romitti PA, Zhu Y, Puzhankara S, James KA, Nabukera SK, Zamba GKD, Ciafaloni E, Cunniff C, Druschel CM, Mathews KD, et al. Prevalence of Duchenne and Becker muscular dystrophies in the United States. Pediatrics. 2015;135:513–521. doi: 10.1542/peds.2014-2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moxley RT, Pandya S, Ciafaloni E, Fox DJ, Campbell K. Change in natural history of Duchenne muscular dystrophy with long‐term corticosteroid treatment: implications for management. J Child Neurol. 2010;25:1116–1129. doi: 10.1177/0883073810371004 [DOI] [PubMed] [Google Scholar]
- 3. Koeks Z, Bladen CL, Salgado D, van Zwet E, Pogoryelova O, McMacken G, Monges S, Foncuberta ME, Kekou K, Kosma K, et al. Clinical outcomes in Duchenne muscular dystrophy: a study of 5345 patients from the TREAT‐NMD DMD Global Database the Creative Commons Attribution Non‐Commercial License (CC BY‐NC 4.0). J Neuromuscul Dis. 2017;4:293–306. doi: 10.3233/JND-170280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Flanigan KM. Duchenne and Becker muscular dystrophies. Neurol Clin. 2014;32:671–688. doi: 10.1016/j.ncl.2014.05.002 [DOI] [PubMed] [Google Scholar]
- 5. Puchalski MD, Williams RV, Askovich B, Sower CT, Hor KH, Su JT, Pack N, Dibella E, Gottliebson WM. Late gadolinium enhancement: precursor to cardiomyopathy in Duchenne muscular dystrophy? Int J Cardiovasc Imaging. 2009;25:57–63. doi: 10.1007/s10554-008-9352-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mavrogeni S. Cardiac involvement in Duchenne and Becker muscular dystrophy. World J Cardiol. 2015;7:410. doi: 10.4330/wjc.v7.i7.410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Connuck DM, Sleeper LA, Colan SD, Cox GF, Towbin JA, Lowe AM, Wilkinson JD, Orav EJ, Cuniberti L, Salbert BA, et al. Characteristics and outcomes of cardiomyopathy in children with Duchenne or Becker muscular dystrophy: a comparative study from the Pediatric Cardiomyopathy Registry. Am Heart J. 2008;155:998–1005. doi: 10.1016/j.ahj.2008.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nigro G, Comi LI, Politano L, Bain RJI. The incidence and evolution of cardiomyopathy in Duchenne muscular dystrophy. Int J Cardiol. 1990;26:271–277. doi: 10.1016/0167-5273(90)90082-G [DOI] [PubMed] [Google Scholar]
- 9. Landfeldt E, Thompson R, Sejersen T, McMillan HJ, Kirschner J, Lochmüller H. Life expectancy at birth in Duchenne muscular dystrophy: a systematic review and meta‐analysis. Eur J Epidemiol. 2020;35:643–653. doi: 10.1007/s10654-020-00613-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim S, Campbell KA, Fox DJ, Matthews DJ, Valdez R. Corticosteroid treatments in males with Duchenne muscular dystrophy: treatment duration and time to loss of ambulation. J Child Neurol. 2015;30:1275–1280. doi: 10.1177/0883073814558120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ryder S, Leadley RM, Armstrong N, Westwood M, de Kock S, Butt T, Jain M, Kleijnen J. The burden, epidemiology, costs and treatment for Duchenne muscular dystrophy: an evidence review. Orphanet J Rare Dis. 2017;12:79. doi: 10.1186/s13023-017-0631-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kieny P, Chollet S, Delalande P, Le Fort M, Magot A, Pereon Y, Perrouin VB. Evolution of life expectancy of patients with Duchenne muscular dystrophy at AFM Yolaine de Kepper centre between 1981 and 2011. Ann Phys Rehabil Med. 2013;56:443–454. doi: 10.1016/j.rehab.2013.06.002 [DOI] [PubMed] [Google Scholar]
- 13. Wittlieb‐Weber CA, Knecht KR, Villa CR, Cunningham C, Conway J, Bock MJ, Gambetta KE, Lal AK, Schumacher KR, Law SP, et al. Risk factors for cardiac and non‐cardiac causes of death in males with Duchenne muscular dystrophy. Pediatr Cardiol. 2020;1:3. doi: 10.1007/s00246-020-02309-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ogata H, Ishikawa Y, Ishikawa Y, Minami R. Beneficial effects of beta‐blockers and angiotensin‐converting enzyme inhibitors in Duchenne muscular dystrophy. J Cardiol. 2009;53:72–78. doi: 10.1016/j.jjcc.2008.08.013 [DOI] [PubMed] [Google Scholar]
- 15. Feingold B, Mahle WT, Auerbach S, Clemens P, Domenighetti AA, Jefferies JL, Judge DP, Lal AK, Markham LW, Parks WJ, et al. Management of cardiac involvement associated with neuromuscular diseases: a scientific statement from the American Heart Association. Circulation. 2017;136:e200–e231. doi: 10.1161/CIR.0000000000000526 [DOI] [PubMed] [Google Scholar]
- 16. Birnkrant DJ, Bushby K, Bann CM, Alman BA, Apkon SD, Blackwell A, Case LE, Cripe L, Hadjiyannakis S, Olson AK, et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol. 2018;17:30026–30033. doi: 10.1016/S1474-4422(18)30025-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, Kaul A, Kinnett K, McDonald C, Pandya S, et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: implementation of multidisciplinary care. Lancet Neurol. 2010;9:177–189. doi: 10.1016/S1474-4422(09)70272-8 [DOI] [PubMed] [Google Scholar]
- 18. Duboc D, Meune C, Lerebours G, Devaux JY, Vaksmann G, Bécane HM. Effect of perindopril on the onset and progression of left ventricular dysfunction in Duchenne muscular dystrophy. J Am Coll Cardiol. 2005;45:855–857. doi: 10.1016/j.jacc.2004.09.078 [DOI] [PubMed] [Google Scholar]
- 19. IBM Watson Health IBM MarketScan Research Databases for Life Sciences Researchers (White Paper). 2018:1–16. IBM. Available at: https://www.ibm.com/downloads/cas/0NKLE57Y. Accessed October 01, 2001. [Google Scholar]
- 20. Klimchak AC, Szabo SM, Qian C, Popoff E, Iannaccone S, Gooch KL. Characterizing demographics, comorbidities, and costs of care among populations with Duchenne muscular dystrophy with Medicaid and commercial coverage. J Manag Care Spec Pharm. 2021;27:1426–1437. doi: 10.18553/jmcp.2021.27.10.1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. O’Byrne ML, Kim S, Hornik CP, Yerokun BA, Matsouaka RA, Jacobs JP, Jacobs ML, Jonas RA. Effect of obesity and underweight status on perioperative outcomes of congenital heart operations in children, adolescents, and young adults. Circulation. 2017;136:704–718. doi: 10.1161/circulationaha.116.026778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Agarwal A, Thombley R, Broberg CS, Harris IS, Foster E, Mahadevan VS, John A, Vittinghoff E, Marcus GM, Dudley RA. Age‐ and lesion‐related comorbidity burden among US adults with congenital heart disease: a population‐based study. J Am Heart Assoc. 2019;8: e013450. doi: 10.1161/JAHA.119.013450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Konerman MA, Rogers M, Kenney B, Singal AG, Tapper E, Sharma P, Saini S, Nallamothu B, Waljee A. Opioid and benzodiazepine prescription among patients with cirrhosis compared to other forms of chronic disease. BMJ Open Gastroenterol. 2019;6: e000271. doi: 10.1136/BMJGAST-2018-000271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mayer OH, Karafilidis J, Higgins K, Griffin B. Descriptive characteristics of males with Duchenne muscular dystrophy in those covered by government (Medicaid) and non‐government (private) health plans. Eur Neurol Rev. 2018;14:88–93. doi: 10.17925/USN.2018.14.2.88 [DOI] [Google Scholar]
- 25. Soslow JH, Hall M, Burnette B, Hor K, Chisolm J, Spurney C, Godown J, Xu M, Slaughter JC, Markham LW, et al. Creation of a novel algorithm to identify patients with Becker and Duchenne muscular dystrophy within an administrative database and application of the algorithm to assess cardiovascular morbidity. Cardiol Young. 2019;29:290–296. doi: 10.1017/S1047951118002226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen X, Agiro A, Martin AS, Lucas AM, Haynes K. Chart validation of an algorithm for identifying hereditary progressive muscular dystrophy in healthcare claims. BMC Med Res Methodol. 2019;19:174. doi: 10.1186/s12874-019-0816-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD‐10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. doi: 10.1186/1471-2431-14-199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Feudtner C, Christakis DA, Connell FA. Pediatric deaths attributable to complex chronic conditions: a population‐based study of Washington State, 1980–1997. Pediatrics. 2000;106(suppl 1):205–209. doi: 10.1542/peds.106.S1.205 [DOI] [PubMed] [Google Scholar]
- 29. Birnkrant DJ, Bushby K, Bann CM, Apkon SD, Blackwell A, Brumbaugh D, Case LE, Clemens PR, Hadjiyannakis S, Pandya S, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol. 2018;17:251–267. doi: 10.1016/S1474-4422(18)30024-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Landfeldt E, Lindgren P, Bell CF, Schmitt C, Guglieri M, Straub V, Lochmüller H, Bushby K. Compliance to care guidelines for Duchenne muscular dystrophy. J Neuromuscul Dis. 2015;2:63–72. doi: 10.3233/JND-140053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Teoh LJ, Geelhoed EA, Bayley K, Leonard H, Laing NG. Health care utilization and costs for children and adults with Duchenne muscular dystrophy. Muscle Nerve. 2016;53:877–884. doi: 10.1002/mus.24965 [DOI] [PubMed] [Google Scholar]
- 32. Pandya SK, Campbell KA, Andrews JG, Meaney FJ, Ciafaloni E. Health services received by individuals with Duchenne/Becker muscular dystrophy. Muscle Nerve. 2016;53:191–197. doi: 10.1002/mus.24727 [DOI] [PubMed] [Google Scholar]
- 33. Silva MC, Magalhães TA, Meira ZMA, Rassi CHRE, Andrade ACDS, Gutierrez PS, Azevedo CF, Gurgel‐Giannetti J, Vainzof M, Zatz M, et al. Myocardial fibrosis progression in Duchenne and Becker muscular dystrophy: a randomized clinical trial. JAMA Cardiol. 2017;2:190. doi: 10.1001/jamacardio.2016.4801 [DOI] [PubMed] [Google Scholar]
- 34. Chiang DY, Allen HD, Kim JJ, Valdes SO, Wang Y, Pignatelli RH, Lotze TE, Miyake CY. Relation of cardiac dysfunction to rhythm abnormalities in patients with Duchenne or Becker muscular dystrophies. Am J Cardiol. 2016;117:1349–1354. doi: 10.1016/j.amjcard.2016.01.031 [DOI] [PubMed] [Google Scholar]
- 35. Nikhanj A, Yogasundaram H, Miskew Nichols B, Richman‐Eisenstat J, Phan C, Bakal JA, Siddiqi ZA, Oudit GY. Cardiac intervention improves heart disease and clinical outcomes in patients with muscular dystrophy in a multidisciplinary care setting. J Am Heart Assoc. 2020;9:e014004. doi: 10.1161/JAHA.119.014004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Raman SV, Hor KN, Mazur W, Halnon NJ, Kissel JT, He X, Tran T, Smart S, McCarthy B, Taylor MD, et al. Eplerenone for early cardiomyopathy in Duchenne muscular dystrophy: a randomised, double‐blind, placebo‐controlled trial. Lancet Neurol. 2015;14:153–161. doi: 10.1016/S1474-4422(14)70318-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Porcher R, Desguerre I, Amthor H, Chabrol B, Audic F, Rivier F, Isapof A, Tiffreau V, Campana‐Salort E, Leturcq F, et al. Association between prophylactic angiotensin‐converting enzyme inhibitors and overall survival in Duchenne muscular dystrophy—analysis of registry data. Eur Heart J. 2021;42:1976–1984. doi: 10.1093/eurheartj/ehab054 [DOI] [PubMed] [Google Scholar]
- 38. Duboc D, Meune C, Pierre B, Wahbi K, Eymard B, Toutain A, Berard C, Vaksmann G, Weber S, Bécane HM. Perindopril preventive treatment on mortality in Duchenne muscular dystrophy: 10 years’ follow‐up. Am Heart J. 2007;154:596–602. doi: 10.1016/j.ahj.2007.05.014 [DOI] [PubMed] [Google Scholar]


