Enrollment of populations that bear the real‐world burden of ischemic strokes is critical to development of generalizable and clinically relevant randomized clinical trials (RCTs). Despite well‐recognized historical patterns of underrepresentation for women, older adults, and certain racial/ethnic minorities, 1 knowledge gaps remain regarding representation in modern trials. Interestingly, preliminary evidence suggests more equitable enrollment among RCTs with acute rather than chronic interventions. 2 , 3 We aimed to determine representativeness by age, sex, race, and ethnicity of participants in acute ischemic stroke RCTs performed in the United States or Canada.
We conducted a systematic review and meta‐analysis in accordance with Preferred Reporting Items for Systematic Reviews and Meta‐Analyses and prospectively registered with PROSPERO: International Prospective Register of Systematic Reviews (CRD42021247730). All screening, data extraction, and quality assessment steps were undertaken independently by 2 reviewers (E.K.A. and M.H.A.), with areas of disagreement resolved by consensus.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Our search was conducted in ClinicalTrials.gov among trials that (1) registered with the study type listed as “interventional,” the condition listed as “stroke” (or one of the terms affiliated with “stroke”), the location listing indicating trial conduct in the United States and/or Canada, the age category for enrollees listed as “adult” and/or “older adult,” and the trial phase listed as “phase 2,” “phase 3,” and/or phase “not applicable”; (2) reported a start date on or after January 1, 2010; and (3) reported a primary completion or termination date on or before December 31, 2020. ClincalTrials.gov entries and associated full‐text, English language publications were screened for ischemic stroke RCTs, with acute interventions (≤72 hours following stroke onset), ≥5 sites, ≥80% of sites located in the United States or Canada, and ≥100 participants. Publications were excluded if exclusively reporting secondary, interim, or post hoc analyses.
Data were extracted from eligible trials using a standardized extraction form, with all data collected directly from the source article or its supplementary materials. The outcome of interest was representation among trial participants. Specifically, for representation by sex, race, and ethnicity, the proportion of participants in each of the extracted groups was assessed. Participants whose sex, race, or ethnicity was listed as “unknown” were excluded from the denominator of the calculated proportions for sex, race, or ethnicity, respectively. In accordance with recommendations, 4 “sex” was distinguished from “gender identity.” Documentation of gender identity was extracted for descriptive purposes. For age, representation was evaluated using the reported mean (+/−1.35 SDs) or median (+/−1 interquartile range).
As this review focuses on the representativeness of stroke trial participants, assessment of methodological quality concentrated on the completeness of reporting for the age, sex, racial, and ethnic profiles of participants within the publication or its supplementary materials.
In order to efficiently characterize and convey participant representation in modern acute ischemic stroke trials, a combination of qualitative and quantitative data synthesis was employed. Data were quantitatively synthesized via meta‐analysis using a generalized linear mixed model (random intercept logistic regression model), which is a recommended approach for meta‐analysis of single proportions. CIs for individual studies were derived based on the Clopper‐Pearson method. Quantitative data synthesis and forest plot construction was performed using the meta R package. Statistical heterogeneity was expressed in terms of the I² statistic.
Of 548 screened ClincalTrials.gov entries, 12 RCTs met eligibility criteria (total: 7955 participants). Age and sex were reported in all assessed trials. However, race and ethnicity were documented in just 42% (5 publications), and only 33% (4 publications) provided a level of granularity beyond White/non‐White for race. Gender identity was not described in any included publications. As shown in Figure, relatively proportionate representation was observed by age (12 studies; heterogeneity in reporting precluded meta‐analysis) and sex (12 studies; 48% female, CI, 45%–51%; I2=70%). Overall, non‐White participants comprised 23% of reporting trials’ populations (5 studies; CI, 17%–29%; I2=93%). Black and Asian participants accounted for 19% (CI, 12%–28%) and 2% (CI, 1%–4%), respectively (4 studies; I2=96% and 45%). Hispanic participants encompassed 11% of reporting trials’ populations (5 studies; CI, 8%–15%; I2=97%).
Figure 1. Representation in the 12 eligible US or Canadian acute ischemic stroke trials published between 2010 and 2020.
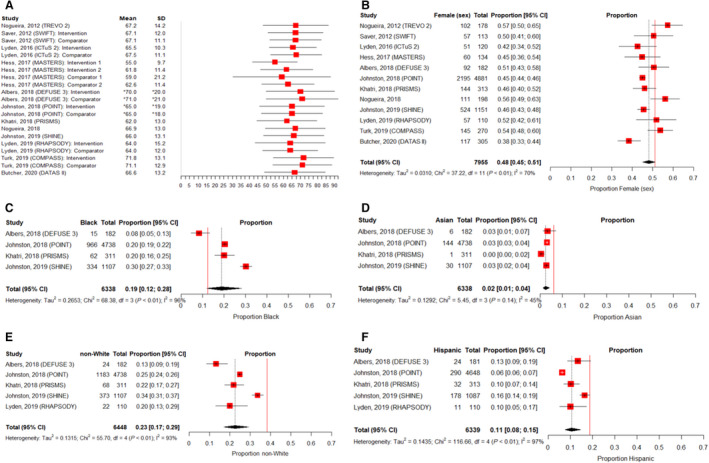
A, Measures of central tendency and distribution for age by trial/trial intervention group.† B, Pooled proportion of female (sex) participants (pooled proportion of acute ischemic stroke RCT participants=48%; proportion of the general US population=51%).‡ C, Pooled proportion of Black participants (pooled proportion of acute ischemic stroke RCT participants=19%; proportion of the general US population=12.4%).‡ D, Pooled proportion of Asian participants (pooled proportion of acute ischemic stroke RCT participants=2%; proportion of the general US population=6%).‡ E, Pooled proportion of non‐White participants (pooled proportion of acute ischemic stroke RCT participants=23%; proportion of the general US population=38.4%).‡ F, Pooled proportion of Hispanic participants (pooled proportion of acute ischemic stroke RCT participants=11%; proportion of the general US population=18.7%)‡. *In accordance with the format of age reporting in the RCT publications, age median and interquartile range were presented in place of mean and SD. †Error bars=mean +/− 1.35 SDs or median +/− 1 interquartile range. ‡To provide a visual comparator, the vertical, solid red line indicates the approximate proportion of each of the assessed groups within the general US population based on 2019 and 2020 census data. 6 , 7 RCT indicates randomized controlled trial.
Although age and sex were routinely documented, less than half of the assessed US or Canadian acute ischemic stroke RCTs provided details about race and ethnicity. It is likely that this information was collected but underreported, impeding interpretation of the generalizability of these trials. Notably, these findings parallel the underreporting of race and ethnicity observed in cardiovascular trials, 1 suggesting the need for comprehensive changes to RCT publication procedures and expectations. Core standards for reporting on race and ethnicity have been established by the National Institutes of Health, 5 and bolstered by recent guidance from organizations like the New England Journal of Medicine. 4 However, our findings underscore the need to drive uptake within the stroke research community. Echoing calls for increased transparency, 4 , 5 we recommend further emphasis on complete and granular reporting of racial and ethnic profiles in order to facilitate ongoing assessments of representation among modern stroke trials.
Sources of Funding
Drs Abbasi and Kasner are supported by NINDS U24NS107224.
Disclosures
Dr Kasner receives research support from WL Gore, Bristol‐Myers Squibb, and Medtronic and consulting fees from Janssen, Bristol‐Myers Squibb, Medtronic, and DiaMedica. The remaining authors have no disclosures to report.
For Sources of Funding and Disclosures, see page 3.
References
- 1. Sardar MR, Badri M, Prince CT, Seltzer J, Kowey PR. Underrepresentation of women, elderly patients, and racial minorities in the randomized trials used for cardiovascular guidelines. JAMA Intern Med. 2014;174:1868–1870. doi: 10.1001/jamainternmed.2014.4758 [DOI] [PubMed] [Google Scholar]
- 2. Kasner SE, Del Giudice A, Rosenberg S, Sheen M, Luciano JM, Cucchiara BL, Messé SR, Sansing LH, Baren JM. Who will participate in acute stroke trials? Neurology. 2009;72:1682–1688. doi: 10.1212/WNL.0b013e3181a55fbe [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carcel C, Harris K, Peters SAE, Sandset EC, Balicki G, Bushnell CD, Howard VJ, Reeves MJ, Anderson CS, Kelly PJ, et al. Representation of women in stroke clinical trials: a review of 281 trials involving more than 500,000 participants. Neurology. 2021;97:e1768–e1774. doi: 10.1212/WNL.0000000000012767 [DOI] [PubMed] [Google Scholar]
- 4. Editors . Striving for diversity in research studies. N Engl J Med. 2021;385:1429–1430. doi: 10.1056/NEJMe2114651 [DOI] [PubMed] [Google Scholar]
- 5. NIH Policy and Guidelines on The Inclusion of Women and Minorities as Subjects in Clinical Research | grants.nih.gov . NIH. 2017; Available from: https://grants.nih.gov/policy/inclusion/women‐and‐minorities/guidelines.htm
- 6. US Census Bureau . Age and Sex Composition in the United States. 2019. [Internet]. Census.gov. Available from: https://www.census.gov/data/tables/2019/demo/age‐and‐sex/2019‐age‐sex‐composition.html
- 7. US Census Bureau . Race and Ethnicity in the United States. 2010. [Internet]. Census.gov. Available from: https://www.census.gov/library/visualizations/interactive/race‐and‐ethnicity‐in‐the‐united‐state‐2010‐and‐2020‐census.html


