Abstract
Background
The effectiveness of a nurse‐led in‐hospital monitoring protocol with mobile ECG (iECG) was investigated for detecting atrial fibrillation in patients post‐ischemic stroke or post‐transient ischemic attack. The study aimed to assess the cost‐effectiveness of using iECG during the initial hospital stay compared with standard 24‐hour Holter monitoring.
Methods and Results
A Markov microsimulation model was constructed to simulate the lifetime health outcomes and costs. The rate of atrial fibrillation detection in iECG and Holter monitoring during the in‐hospital phase and characteristics of modeled population (ie, age, sex, CHA2DS2‐VASc) were informed by patient‐level data. Costs related to recurrent stroke, stroke management, medications (new oral anticoagulants), and rehabilitation were included. The cost‐effectiveness analysis outcome was calculated as an incremental cost per quality‐adjusted life‐year gained. As results, monitoring patients with iECG post‐stroke during the index hospitalization was associated with marginally higher costs (A$31 196) and greater benefits (6.70 quality‐adjusted life‐years) compared with 24‐hour Holter surveillance (A$31 095 and 6.66 quality‐adjusted life‐years) over a 20‐year time horizon, with an incremental cost‐effectiveness ratio of $3013/ quality‐adjusted life‐years. Monitoring patients with iECG also contributed to lower recurrence of stroke and stroke‐related deaths (140 recurrent strokes and 20 deaths avoided per 10 000 patients). The probabilistic sensitivity analyses suggested iECG is highly likely to be a cost‐effective intervention (100% probability).
Conclusions
A nurse‐led iECG monitoring protocol during the acute hospital stay was found to improve the rate of atrial fibrillation detection and contributed to slightly increased costs and improved health outcomes. Using iECG to monitor patients post‐stroke during initial hospitalization is recommended to complement routine care.
Keywords: atrial fibrillation, cost‐effectiveness analysis, iECG monitoring, stroke
Subject Categories: Cerebrovascular Disease/Stroke, Atrial Fibrillation
Nonstandard Abbreviations and Acronyms
- ICER
incremental cost‐effectiveness ratio
- iECG
smartphone‐based handheld ECG device
- NOAC
new oral anticoagulant
Clinical Perspective
What Is New?
The potential health benefits in terms of number of recurrent strokes avoided, and long‐term costs associated with nurse‐led in‐hospital monitoring for atrial fibrillation post‐stroke were unknown.
What Are the Clinical Implications?
The findings from this study showed the potential in identifying atrial fibrillation post‐stroke could be translated into improved health outcomes accompanied with slightly increased cost, demonstrating values to complement routine practice.
Atrial fibrillation (AF) is associated with significantly increased risk of stroke. Among patients with established AF, patients with a history of stroke have the highest risk of future ischemic stroke. 1 Post‐stroke patients with a diagnosis of AF are reported to carry a 15% risk over the first year of experiencing a recurrent stroke. 2 , 3 In contrast, patients with AF with no history of stroke only had a 6% risk of suffering from such an event. 2 , 3 AF thus is an important risk factor for stroke secondary prevention. However, a considerable proportion of patients with stroke might have paroxysmal, asymptomatic AF undetected, which places them at substantially increased risk of experiencing another ischaemic event. This under‐diagnosis also precludes patients benefiting from new anticoagulants, which have proven to be significantly effective in reducing the risk of stroke recurrence by up to two thirds. 4 , 5 , 6 A systematic review and meta‐analysis found that the AF detection rate can be as high as 23.7% after 4 phases of sequential cardiac monitoring, while the diagnosis rate with in‐hospital Holter monitoring was only 4.5%. 7
The current stroke guidelines recommend that post‐stroke patients should be monitored by an ECG for at least 24 hours, 8 , 9 , 10 and longer term monitoring was associated with greater detection rate of AF post‐stroke or post‐transient ischemic attack (TIA). 11 , 12 Adherence to these guidelines is suboptimal: an analysis of stroke registry data indicated that only 30% of patients received 24‐hour Holter monitoring within 30 days of their stroke. 13
To improve the detection rate of undiagnosed AF, several alternative devices are available to overcome the limitations of traditional Holter monitoring. 14 A recent observational study prospectively examined the diagnostic performance of a smartphone‐based handheld ECG device (iECG) in patients hospitalized because of stroke or TIA. 15 It was found that the nurse‐led iECG surveillance after stroke before hospital discharge was more effective than routine 24‐hour Holter monitoring, by detecting AF earlier and changing subsequent management. 15
Given the demonstrated clinical effectiveness of iECG, the next important question is whether offering this new model of cardiac monitoring represents value‐for‐money. This study aimed to undertake a modeled economic evaluation based on recently published clinical data to assess the cost‐effectiveness of iECG in detecting AF for patients post‐stroke or post‐TIA during the acute in‐hospital phase.
Methods
Data Availability Statement
The data and methods that support the findings of this study are available from the corresponding author upon reasonable request.
Model Structure
A Markov microsimulation model, consisting of no further events, post‒non‐major stroke (defined by modified Rankin scale (score ≤2), post‐major stroke (modified Rankin scale score 3–5), and death (modified Rankin scale score=6), was constructed to simulate the long‐term outcomes after an index stroke/TIA (Figure 1). Following the study outcome, a proportion of patients being diagnosed with AF via iECG (ie, true positives) and routine Holter surveillance would initiate the new oral anticoagulant (NOAC) treatment. Over a lifetime horizon, patients post‐stroke/TIA may remain event‐free or experience a recurrent stroke (non‐major or major defined by modified Rankin scale score ≤2 or >2), or die from non‐stroke causes. Non‐major recurrent strokes were considered because of significant detection of clinically silent infarcts in patients with AF (15%). 16
Figure 1. Markov simulation model structure.
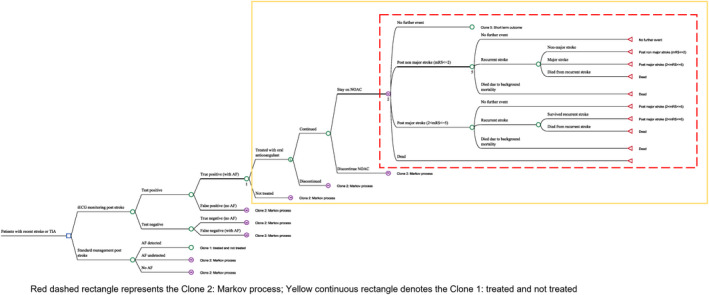
AF indicates atrial fibrillation; iECG, smartphone‐based handheld ECG device; mRS, modified Rankin Scale; NOAC, new oral anticoagulant; and TIA, transient ischemic attack.
Population
A hypothetical cohort of Australian patients who had survived an ischemic stroke/TIA as defined by the characteristic from the primary study in 2017 was simulated. Briefly, a total of 1079 patients (median age of 66 years with predominantly male participants) were recruited for the study from 8 hospitals across China and Australia. 15 The total number of ischemic strokes and deaths from stroke were sourced from publicly available data. 17 , 18 , 19 CHA2DS2‐VASc scores calculate the stroke risk for patients with AF. The post‐stroke CHA2DS2‐VASc score, age, and sex distribution of each simulated patient were defined by the patient‐level data from the primary observational study, 15 and the CHA2DS2‐VASc score was modified by increasing age over the timeframe of the simulation for patients with diagnosed AF. In the base case analysis, the simulated stroke survivors run through the Markov microsimulation model one‐by‐one and are followed for 20 years given the remaining life expectancy from corresponding general Australians. The AF detection rate by monitoring protocols was derived from patient‐level data from the original study. 15 Ethics approval for the primary study was obtained with patient consent waiver through the Melbourne Health Human Research Ethics Committee on March 25, 2015.
Transition Probability
The proportion of patients being diagnosed with AF using iECG or Holter monitoring was derived from the primary study (8.5% versus 2.8%). The annual probability of recurrent stroke for each hypothetical patient was derived directly from their CHA2DS2‐VASc score if they were comorbid with AF. 20 It was conservatively assumed that most patients would have a non‐major stroke in the event of a recurrent event, 21 , 22 even though AF‐related stroke would be expected to be more disabling. The compliance and adherence with NOAC treatment was also considered (15% discontinued in the first year and 2% thereafter). 23 All the transition probabilities are presented inTable 1 7 , 15 , 23 , 24 , 25 , 26 , 27 , 28 , 29 and Tables S1 to S2.
Table 1.
Probabilities and Utility Weights for the Markov Model Parameters
| Variable | Base case | Range | Reference |
|---|---|---|---|
| Sensitivity of iECG | 0.97 | 0.92–1.00 | Lowers et al 24 |
| Specificity of iECG | 0.92 | 0.89–0.93 | Lowers et al 24 |
| Prevalence of AF after a stroke/TIA | 0.0876 | ||
| Proportion of patients experienced gastro bleeding with anticoangulant treatment (per yearly cycle) | 0.004 | … | Connolly et al 2011 25 |
| Proportion of patients experienced intracranial bleeding with anticoangulant treatment (per yearly cycle) | 0.006 | … | Connolly et al 2011 25 |
| Probability of diagnosing AF using iECG | 0.085 | 0.05–0.10 | Yan et al 2020, 15 and Sposato et al 2015 7 |
| Probability of diagnosing AF not using iECG | 0.028 | … | Yan et al 2020 15 |
| Relative risk of background mortality for patients with AF and no AF | 1.66 | 1.59–1.73 | Miyasaka et al 2007 26 |
| Probability of treating with oral anticoagulant in the iECG group | 0.44 | … | Yan et al 2020 15 |
| Probability of treating with oral anticoagulant in the no iECG group | 0.625 | … | Yan et al 2020 15 |
| Probability of recurrent stroke without AF (per yearly cycle) | 0.021 | … | Mohan et al 2011 27 |
| Probability of having a non‐major stroke | 0.5 | … | Assumption* |
| Relative risk of all‐cause mortality for NOAC vs no NOAC | 0.79 | 0.62–1.02 | Connolly et al 2011 25 |
| Relative risk of stroke for NOAC vs no NOAC | 0.37 | 0.25–0.55 | Connolly et al 2011 25 |
| Discontinuation rate with NOAC | |||
| First year | 0.15 | … | Garkina et al 2016 23 |
| Second year onwards | 0.02 | … | Garkina et al 2016 23 |
| Baseline utility | 0.63 | 0.50–0.76 | Sturm et al 2002 28 |
| Utility post a major stroke | 0.35 | … | Sturm et al 2002 28 |
| Utility post a non‐major stroke | 0.55 | … | Sturm et al 2002 28 |
| Utility decrement from Holter monitoring | 0.0203 | … | Diekmann et al 2019 29 |
| Utility decrement from iECG monitoring | 0.0020 | … | Assumption |
AF indicates atrial fibrillation; iECG, smartphone‐based handheld ECG device; NOAC, new oral anticoagulant; and TIA, transient ischemic attack.
Assuming equal probability of having a major and non‐major stroke.
Costs
Costs relating to iECG monitoring, rehospitalization because of recurrent stroke, stroke management (ie, outpatient care with GP/specialist, medications, examinations, etc.), rehabilitation, NOAC, and adverse events because of NOAC (ie, gastrointestinal and intracranial bleeding) were included in the model (Table 2). It was assumed that formal diagnosis of AF in patients receiving iECG monitoring (ie, those who tested either true or false positive) would require a read over by a specialist referred by a GP for patients receiving iECG monitoring (equivalent to the cost of performing a 24‐hour Holter recording) and removal of the cost for specialist overread was tested in the sensitivity analysis. For the patients classified as false negatives, the same costs related to the formal AF diagnosis were also applied. For the intervention cost, in addition to the device cost of iECG, the opportunity cost of nurse’s time to deliver the iECG monitoring was also included (average 2.5 recordings per day for a duration of 4 days). 15 The distributions for key cost inputs are constructed to account for first‐order uncertainty (Table S3). 30
Table 2.
Unit Costs of Markov Model Parameters
| Cost item | Unit cost | Reference |
|---|---|---|
| Gastrointestinal bleeding | $4777 | AR‐DRG G61A, G61B 31 |
| Intracranial bleeding |
$23 648 ($19 060–28 235) |
AR‐DRG B70A 31 |
| Hospitalization for a major stroke |
$17 724 ($14 212–21 235) |
Cost weight 8.0 round 20 (2015–2016) 31 |
| Dying immediately from acute stroke |
$11 541 ($9302–13 779) |
Cost weight 8.0 round 20 (2015–2016) 31 |
| Hospitalization for a non‐major stroke |
$6666 ($5372–7959) |
Cost weight 8.0 round 20 (2015–2016) 31 |
| Monitoring with iECG | $22 | Orchard et al 32 |
| Nurse’s time to administer iECG monitoring | $5.6 | Calculated as 10 min (10 recordings ×1 min/recording) times with the average hourly wage (A$33.59) for a nurse |
| Monitoring with 24‐h Holter | $170.15 | MBS 11709 |
| Specialist consultation | $86.85 | MBS 104 |
| GP consultation | $38.75 | MBS 23 |
| Management post a non‐major stroke | $1559 | Arona et al 2018 33 |
| Management post a major stroke |
$11 368 ($9162–13 573) |
Arona et al 2018 33 |
| Novel oral anticoagulant medication per year | $1273 | PBS 10414D |
| Rehabilitation for a major stroke |
$67 158 ($60 340–73 976) |
Costing data from Royal Melbourne Hospital, Australia |
| Rehabilitation for a non‐major stroke | $7170 | Gao et al 2019 34 |
Utility Weights
Utility weights corresponding to each modeled health state (no further event, post‒non‐major stroke, and post‐major stroke) were derived from the published literature. A disutility (utility decrement attributable to an event) was applied if a patient experienced a recurrent stroke and/or adverse event attributable to NOAC. Moreover, a utility decrement was applicable to all patients as per the monitoring strategy to account for the quality‐of‐life impact from either wearing a Holter or being monitored frequently by iECG. The utility weights and disutility are shown in Table 1.
Further details on methods are presented in Data S1.
Cost‐Effectiveness Analysis
The primary outcome measure for the cost‐effectiveness analysis was the quality‐adjusted life‐year (QALY) gained. Utility weights were assigned to the corresponding life‐years lived. Current clinical practice (ie, Holter monitoring over the acute hospital stay) to identify AF in patients post‐stroke was adopted as the comparator. The incremental cost‐effectiveness ratio (ICER) representing the ratio between incremental cost (ie, total costs related to iECG and Holter monitoring) and incremental QALYs (ie, total QALYs related to these 2 management groups) was calculated. Both costs and QALYs were discounted at a rate of 3%. 37 All the costs were expressed in Australian dollars valued for the 2018 reference year. An often cited willingness‐to‐pay per QALY threshold (A$50 000/QALY) was adopted to determine the cost‐effectiveness of iECG surveillance compared with the usual care over a 20‐year time horizon. 38
Sensitivity Analyses
Both deterministic and probabilistic sensitivity analyses were performed to examine the model parameter uncertainty around the cost‐effectiveness of iECG. For the deterministic sensitivity analyses, individual key model parameters were varied within a plausible range (informed by the published literature) one at a time to explore their impact on the ICER. A lifetime time horizon (ie, simulated until all patients were dead) was examined in the sensitivity analysis. The results from deterministic sensitivity analyses are presented in a Tornado diagram.
Probabilistic sensitivity analyses were undertaken which incorporated the distributions of main model parameters (ie, probabilities, utility weights, and costs) on the assumption that they were all independent of each other (eg the variation in transition probability was not correlated with changes in utility). Monte Carlo simulations randomly sampled 2000 parameters from a given distribution and then parameterised the Markov microsimulation model for each hypothetical patient (Table 1 and Table S4).
Estimation at the National Level
The 5‐year budget impact of implementing this nurse‐led AF monitoring protocol during the acute phase was further explored to examine its national impact. The number of ischemic stroke patients who survived an acute incident event and had no prior history of AF for the next 5 years from 2017 onwards were simulated. Costs were discounted at 3% per annum. 37
Results
The results of the observational study were reported in detail elsewhere. 15 Briefly, following screening in the stroke ward, the study recruited 1079 patients who underwent iECG monitoring, and 294 patients who had both iECG and 24‐hour Holter outcomes concurrently with a median CHA2DS2‐VASc score of 4 (interquartile range, 3–5). During routine observations of vital signs (typically every 2 to 4 hours), trained nursing staff performed iECG recordings on patients up to the time of hospital discharge. AF was detected in 8.5% by iECG versus 2.8% by 24‐hour Holter recording (P<0.001). Median time from stroke onset to AF detect was 3 days (interquartile range, 2–6) for iECG and 7 days (interquartile range, 6–10) for Holter (P=0.02). Among patients detected with AF, the anticoagulant treatment during the hospital stay was initiated for 44% versus 63% in the iECG and Holter monitoring groups, respectively (P>0.05).
In 2017, there were 56 000 stroke events in Australia with ≈80% being ischemic strokes; 80% survived this acute incident event. It was assumed that one third of patients with stroke had a prior history of AF. Therefore, in the baseline cohort, there were 25 088 stroke survivors modeled in 2017.
Cost‐Effectiveness Analysis
Monitoring stroke survivors with iECG was associated with both higher cost and benefits (QALY): the total cost and QALYs of patients managed by iECG and 24‐hour Holter were A$31 196 and 6.70 versus A$31 095 and 6.66, respectively. Increased costs related to the early initiation of NOAC (A$563 versus $222) and its associated adverse events (A$70 versus $28) could be partly offset by the cost savings resulting from lower costs of hospitalization (A$2792 versus A$2888), management (A$22 238 versus A$22 240), and rehabilitation (A$5506 versus A$5717). Not surprisingly, iECG‐monitored patients also experienced fewer recurrent strokes over the lifetime horizon compared with those managed by 24‐hour Holter (3440 versus 3580 per 10 000 stroke patients). Therefore, monitoring AF in patients post‐stroke with iECG during the acute phase has an ICER of A$3013/QALY in comparison with traditional 24‐hour Holter recording (Table 3).
Table 3.
Base Case Results From the Cost‐Effectiveness Analysis
| iECG | Usual care | Difference | ES* | ICER | |
|---|---|---|---|---|---|
| Total cost | $31 196 | $31 095 | $101 | 0.002 | |
| Management | $22 238 | $22 240 | −$3 | … | |
| Rehabilitation | $5506 | $5717 | −$211 | … | |
| Hospitalization | $2792 | $2888 | −$96 | … | |
| NOAC | $563 | $222 | $342 | … | |
| Adverse events | $70 | $28 | $43 | … | |
| iECG device | $27 | $0 | $27 | … | |
| No. of recurrent stroke † | 0.344 | 0.358 | −0.014 | 0.019 | $7374 |
| No. of stroke‐related death † | 0.064 | 0.066 | −0.002 | 0.008 | $56 275 |
| QALY | 6.697 | 6.663 | 0.034 | 0.012 | $3013 |
| LY | 11.51 | 11.47 | 0.037 | 0.008 | $2733 |
ES indicates effect size (calculated as standardized mean difference); ICER, incremental cost‐effectiveness ratio; iECG, smartphone‐based handheld ECG device; LY, life‐year; NOAC, new oral anticoagulant; and QALY, quality‐adjusted life‐year.
The average number of events across all simulated cohort since not all patients would experience an event over the modeled time horizon.
Effect size <0.1 is considered trivial.
Subgroup Cost‐Effectiveness Analysis
In the subgroup of patients aged ≥65 years, the same rate of detection was 10.3% in the iECG and 4.0% in the 24‐Holter recording groups (P<0.001). Similarly, iECG again led to higher costs and benefits. Results of the subgroup analysis are summarized in Table S5 and Data S2.
Sensitivity Analysis
The 1‐way deterministic sensitivity analyses identified that the base case result was most sensitive to the increased relative risk for background mortality, the acquisition cost of NOAC, proportion of patients being treated with NOAC after AF detection, and cost of management for a major stroke (Figure 2).
Figure 2. Tornado diagram for the 1‐way deterministic sensitivity analyses.
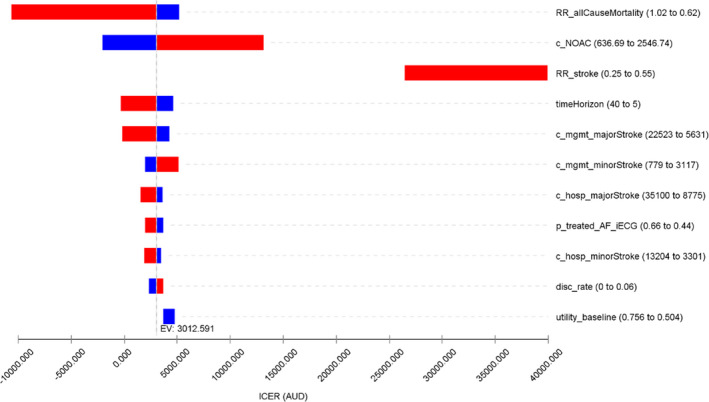
Incremental net monetary benefit was calculated according to the willingness‐to‐pay/quality‐adjusted life‐year threshold of $50 000. The expected value at base case suggests that smartphone‐based handheld ECG device (iECG) is associated with an incremental cost‐effectiveness ratio of $3013/quality‐adjusted life‐year in the base case scenario. RR_stroke and utility_baseline do not align with the base case line as they both impact the results of iECG and standard care arms. c_NOAC indicates cost of new oral anticoagulant medications; c_hosp_majorStroke, cost of hospitalization for a major stroke; c_hosp_minorStroke: cost of hospitalization for a minor stroke; c_mgmt_majorStroke, annual management cost post a major stroke; c_mgmt_minorStroke, annual cost of management post a minor stroke; disc_rate, discount rate; EV, expected value; ICER, incremental cost‐effectiveness ratio; p_treated_AF_iECG, probability of initiating oral anticoagulant treatment after AF detection by iECG; RR_allCauseMortality, relative risk of all cause mortality for oral anticoagulant treated vs non‐oral anticoagulant treated patients; RR_stroke, relative risk of stroke for oral anticoagulant treated vs non‐oral anticoagulant treated patients; timeHorizon, long‐term modeled time horizon; and utility_baseline, utility weight for being post an ischemic stroke at baseline.
The probabilistic sensitivity analysis showed that iECG monitoring has a 100% probability of being a cost‐effective strategy to monitor AF in patients post‐stroke using the $50 000/QALY willingness‐to‐pay threshold, with 9.5% of results showing it was a dominant strategy (ie, less costly and more effective) (Figure 3).
Figure 3. Incremental cost‐effectiveness plane from the probabilistic sensitivity analysis.
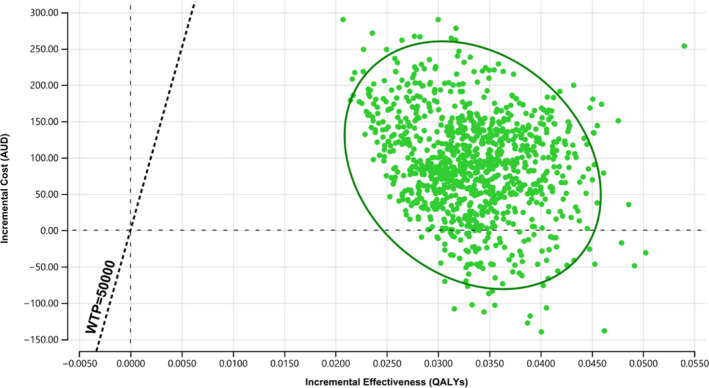
One hundred percent of results suggesting smartphone‐based handheld ECG device being the cost‐effective monitoring strategy with 9.5% indicating less costly and more effective using the $50 000/quality‐adjusted life‐year willingness‐to‐pay threshold. AUD indicates Australian dollar; QALY, quality‐adjusted life‐year; and WTP, willingness‐to‐pay.
For the subgroup of patients aged ≥65 years, a similar pattern was seen: the base case ICER was most sensitive to the variation in the relative risk for background mortality, relative risk of NOAC in preventing stroke, and the acquisition cost of NOAC (Figure S1). The probabilistic sensitivity analysis indicated that this nurse‐led monitoring protocol had a 100% probability of being cost‐effective using the $50 000/QALY willingness‐to‐pay threshold (Figure S2).
Estimation at the National Level
If rolled out to a national population, over 5 years, the total costs associated with iECG device (and time associated with iECG monitoring) and NOAC treatment (including related adverse events) were A$3.19 million and A$45.5 million. Meanwhile, the cost offset from avoided hospitalizations, rehabilitation, and long‐term management was $36.6 million, which would result in a net cost to the health system of A$12.0 million over 5 years (Table S6).
Discussion
A Markov microsimulation model was constructed to maximize the use of data from a key observational study and to reflect the heterogeneity of the patients with stroke. The results showed that monitoring patients post‐stroke with iECG led to both higher health care costs and health benefits compared with 24‐hour Holter recording, making iECG a highly cost‐effective management strategy for secondary prevention of stroke using the $50 000/QALY willingness‐to‐pay threshold.
Cost‐effectiveness analyses examining the economic credentials of other cardiac monitoring interventions in detecting AF post‐stroke have been performed. A within‐trial economic evaluation reported that the prolonged surveillance with 24‐hour Holter recording for 10 days was associated with comparable costs and QALYs 39 ; Kamel et al evaluated the long‐term cost‐effectiveness of 7‐day outpatient cardiac surveillance for a cohort of patients (mean age of 70 years) with stroke history and reported an ICER of $13 000/QALY. The meta‐analyzed AF detection rate for the surveillance strategy was 5.9% which is lower than the rate used in the current study. 40 Yong et al assessed the ambulatory cardiac surveillance after cryptogenic stroke for 7, 14, and 30 days and reported that, whilst 30 days monitoring was cost‐effective, shorter term (7 or 14 or 7 days) monitoring was cost saving. 41 Another study evaluated the AF screening in the primary care setting for members of the general population (regardless of comorbidities) aged >65 years with a handheld, single‐lead ECG device; they reported the screening program would save €764 and improve QALYs by 0.27 per participant. 42 For prolonged monitoring with an insertable device, generally it was considered cost‐effective but not cost‐saving because of the high acquisition and insertion cost of the device: an economic analysis based on the The Cryptogenic Stroke and Underlying AF trial reported it was cost‐effective (ICER £13 926 and QALY gain of 0.15) in patients with cryptogenic stroke. 43 Another 3 modeled economic analyses also yielded similar conclusions in patients with cryptogenic stroke or high risk of stroke. 44 , 45 , 46 An Australian‐based study that examined an opportunistic screening of AF in community pharmacies reported an ICER of A$5988 per QALY gained compared with the practice without such screening. 32 Although the results from these studies are not directly comparable, all indicated that cardiac monitoring for the purpose of AF detection in various settings is highly likely to be cost‐effective or even cost‐saving.
The results from this study have important implications for future clinical practice. It is a requirement for any new medical technology to demonstrate effectiveness, safety, and cost‐effectiveness before receiving a public subsidy (ie, subsidized by the Commonwealth Department of Health in Australia). The evidence generated could inform policy making around post‐stroke management during the index hospital stay. In the key observational study, cardiac monitoring was delivered by nurses during their routine work (ie, vital sign observation monitoring) and imposed no extra workload. Managing patients with iECG offers great potential to detect more patients with AF in a timely manner and to result in significant cost‐savings in terms of hospitalization, rehabilitation, and management, and improved health outcomes for patients. It is possible that our nurse‐lead in‐hospital iECG monitoring protocol could become cost‐saving with the current cost of NOAC having a moderate discount of 30% in the next 5 years. Moreover, in the current modeled analysis, the iECG device was costed for each individual patient. Since the surveillance was provided by stroke unit nurses, the number of devices required could be markedly reduced, meaning the estimated cost of devices is likely to be overestimated.
Some limitations warrant mentioning. First, the subsequent cost associated with false positives (patients without AF but incorrectly diagnosed by iECG) was not fully captured (only the cost of a following Holter to confirm the diagnosis was included). Even though the built‐in algorithm allows the expedited diagnosis of AF, final diagnosis of AF would be established by a specialist. The false positives would have been ruled out. Second, the analysis did not take a societal perspective as recommended for gold standard evaluations. However, monitoring with iECG is likely to reduce the risk of recurrent stroke and to avoid potential disability because of stroke, thus the inclusion of the costs of productivity losses and informal care would further strengthen the cost‐effectiveness profile of iECG in this setting. Thirdly, the cohort study on which the diagnostics yields of iECG were based upon may have inherent imbalance that confounds the results. However, the baseline characteristics in terms of age, sex, and comorbidities were well‐balanced in the primary study. Lastly, the short‐term in hospital monitoring might fail to identify some patients with AF, however there is insufficient clinical data to allow for that in the modeling.
Conclusions
iECG monitoring during the acute hospital stay by a nurse was found to significantly improve the rate of AF detection and was cost‐effective, contributing to marginally increased costs and improved health outcomes. Using iECG to monitor patients post‐stroke is recommended to complement the standard routine care.
Sources of Funding
None.
Disclosures
None.
Supporting information
For Sources of Funding and Disclosures, see page 8.
References
- 1. Wolf PA, Abbott RD, Kannel WB. Atrial‐fibrillation as an independent risk factor for stroke ‐ the Framingham‐Study. Stroke. 1991;22:983–988. doi: 10.1161/01.Str.22.8.983 [DOI] [PubMed] [Google Scholar]
- 2. Cardiogenic brain embolism . The second report of the cerebral embolism task force. Arch Neurol. 1989;46:727–743. [PubMed] [Google Scholar]
- 3. Stroke Risk in Atrial Fibrillation Working G . Independent predictors of stroke in patients with atrial fibrillation: a systematic review. Neurology. 2007;69:546–554. doi: 10.1212/01.wnl.0000267275.68538.8d [DOI] [PubMed] [Google Scholar]
- 4. EAFT (European Atrial Fibrillation Trial) Study Group . Secondary prevention in non‐rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. Lancet. 1993;342:1255–1262. doi: 10.1016/0140-6736(93)92358-Z [DOI] [PubMed] [Google Scholar]
- 5. Ntaios G, Papavasileiou V, Diener HC, Makaritsis K, Michel P. Nonvitamin‐K‐antagonist oral anticoagulants versus warfarin in patients with atrial fibrillation and previous stroke or transient ischemic attack: an updated systematic review and meta‐analysis of randomized controlled trials. Int J Stroke. 2017;12:589–596. doi: 10.1177/1747493017700663 [DOI] [PubMed] [Google Scholar]
- 6. Ntaios G, Papavasileiou V, Diener HC, Makaritsis K, Michel P. Nonvitamin‐K‐antagonist oral anticoagulants in patients with atrial fibrillation and previous stroke or transient ischemic attack: a systematic review and meta‐analysis of randomized controlled trials. Stroke. 2012;43:3298–3304. doi: 10.1161/STROKEAHA.112.673558 [DOI] [PubMed] [Google Scholar]
- 7. Sposato LA, Cipriano LE, Saposnik G, Ruiz Vargas E, Riccio PM, Hachinski V. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: a systematic review and meta‐analysis. Lancet Neurol. 2015;14:377–387. doi: 10.1016/S1474-4422(15)70027-X [DOI] [PubMed] [Google Scholar]
- 8. Jauch EC, Saver JL, Adams HP, Bruno A, Connors JJ, Demaerschalk BM, Khatri P, McMullan PW, Qureshi AI, Rosenfield K, et al. Guidelines for the early management of patients with acute ischemic stroke: executive summary a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44. doi: 10.1161/STR.0b013e318284056a [DOI] [PubMed] [Google Scholar]
- 9. National Stroke Foundation Australia. Clinical Guidelines for Stroke Management 2017 updates . Available from: https://informme.org.au/en/Guidelines/Clinical‐Guidelines‐for‐Stroke‐Management‐2017. Accessed on 14 Dec 2021.
- 10. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–e110. doi: 10.1161/STR.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 11. Gladstone DJ, Spring M, Dorian P, Panzov V, Thorpe KE, Hall J, Vaid H, O'Donnell M, Laupacis A, Côté R, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370:2467–2477. doi: 10.1056/NEJMoa1311376 [DOI] [PubMed] [Google Scholar]
- 12. Sanna T, Diener H‐C, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA, Rymer MM, Thijs V, Rogers T, Beckers F, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478–2486. doi: 10.1056/NEJMoa1313600 [DOI] [PubMed] [Google Scholar]
- 13. Edwards JD, Kapral MK, Fang J, Saposnik G, Gladstone DJ, Investigators of the Registry of the Canadian Stroke N . Underutilization of ambulatory ECG monitoring after stroke and transient ischemic attack: missed opportunities for atrial fibrillation detection. Stroke. 2016;47:1982–1989. doi: 10.1161/STROKEAHA.115.012195 [DOI] [PubMed] [Google Scholar]
- 14. Schnabel RB, Haeusler KG, Healey JS, Freedman B, Boriani G, Brachmann J, Brandes A, Bustamante A, Casadei B, Crijns HJGM, et al. Searching for atrial fibrillation poststroke: a white paper of the AF‐SCREEN international collaboration. Circulation. 2019;140:1834–1850. doi: 10.1161/CIRCULATIONAHA.119.040267 [DOI] [PubMed] [Google Scholar]
- 15. Yan B, Tu H, Lam C, Swift C, Ho MS, Mok VCT, Sui YI, Sharpe D, Ghia D, Jannes J, et al. Nurse led smartphone electrographic monitoring for atrial fibrillation after ischemic stroke: SPOT‐AF. J Stroke. 2020;22:387–395. 10.5853/jos.2020.00689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Conen D, Rodondi N, Müller A, Beer JH, Ammann P, Moschovitis G, Auricchio A, Hayoz D, Kobza R, Shah D, et al. Relationships of overt and silent brain lesions with cognitive function in patients with atrial fibrillation. J Am Coll Cardiol. 2019;73:989–999. doi: 10.1016/j.jacc.2018.12.039 [DOI] [PubMed] [Google Scholar]
- 17. Bronnum‐Hansen H, Davidsen M, Thorvaldsen P, Danish MSG. Long‐term survival and causes of death after stroke. Stroke. 2001;32:2131–2136. doi: 10.1161/hs0901.094253 [DOI] [PubMed] [Google Scholar]
- 18. National Stroke Foundation Australia . No postcode untouched Stroke in Australia. Available from https://strokefoundation.org.au/What‐we‐do/Research/No‐postcode‐untouched. 2017. Accessed on 14 Dec 2021
- 19. Australian Institute of Health and Welfare . Stroke and its management in Australia: an update. Cardiovascular disease series no. 37. Cat. no. CVD 61. Canberra: AIHW. 2013. Accessed on 14 Dec 2021. [Google Scholar]
- 20. Hohnloser SH, Duray GZ, Baber U, Halperin JL. Prevention of stroke in patients with atrial fibrillation: current strategies and future directions. Eur Heart J Suppl. 2008;10:H4–H10. doi: 10.1093/eurheartj/sun029 [DOI] [Google Scholar]
- 21. Saposnik G, Fang J, O'Donnell M, Hachinski V, Kapral MK, Hill MD. Investigators of the Registry of the Canadian Stroke Network for the Stroke Outcome Research Canada Working G . Escalating levels of access to in‐hospital care and stroke mortality. Stroke. 2008;39:2522–2530. doi: 10.1161/STROKEAHA.107.507145 [DOI] [PubMed] [Google Scholar]
- 22. Krueger H, Lindsay P, Cote R, Kapral MK, Kaczorowski J, Hill MD. Cost avoidance associated with optimal stroke care in Canada. Stroke. 2012;43:2198–2206. doi: 10.1161/STROKEAHA.111.646091 [DOI] [PubMed] [Google Scholar]
- 23. Garkina SV, Vavilova TV, Lebedev DS, Mikhaylov EN. Compliance and adherence to oral anticoagulation therapy in elderly patients with atrial fibrillation in the era of direct oral anticoagulants. J Geriatr Cardiol. 2016;13:807–810. doi: 10.11909/j.issn.1671-5411.2016.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lowres N, Neubeck L, Salkeld G, Krass I, McLachlan AJ, Redfern J, Bennett AA, Briffa T, Bauman A, Martinez C, et al. Feasibility and cost‐effectiveness of stroke prevention through community screening for atrial fibrillation using iPhone ECG in pharmacies. The SEARCH‐AF Study. Thromb Haemost. 2014;111:1167–1176. doi: 10.1160/th14-03-0231 [DOI] [PubMed] [Google Scholar]
- 25. Connolly SJ, Eikelboom J, Joyner C, Diener H‐C, Hart R, Golitsyn S, Flaker G, Avezum A, Hohnloser SH, Diaz R, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–817. doi: 10.1056/NEJMoa1007432 [DOI] [PubMed] [Google Scholar]
- 26. Miyasaka Y, Barnes ME, Bailey KR, Cha SS, Gersh BJ, Seward JB, Tsang TS. Mortality trends in patients diagnosed with first atrial fibrillation: a 21‐year community‐based study. J Am Coll Cardiol. 2007;49:986–992. doi: 10.1016/j.jacc.2006.10.062 [DOI] [PubMed] [Google Scholar]
- 27. Mohan KM, Wolfe CD, Rudd AG, Heuschmann PU, Kolominsky‐Rabas PL, Grieve AP. Risk and cumulative risk of stroke recurrence: a systematic review and meta‐analysis. Stroke. 2011;42:1489–1494. doi: 10.1161/STROKEAHA.110.602615 [DOI] [PubMed] [Google Scholar]
- 28. Sturm JW, Osborne RH, Dewey HM, Donnan GA, Macdonell RA, Thrift AG. Brief comprehensive quality of life assessment after stroke: the assessment of quality of life instrument in the North East Melbourne Stroke Incidence Study (NEMESIS). Stroke. 2002;33:2888–2894. doi: 10.1161/01.str.0000040407.44712.c7 [DOI] [PubMed] [Google Scholar]
- 29. Diekmann S, Hörster L, Evers S, Hiligsmann M, Gelbrich G, Gröschel K, Wachter R, Hamann GF, Kermer P, Liman J, et al. Economic evaluation of prolonged and enhanced ECG Holter monitoring in acute ischemic stroke patients. Curr Med Res Opin. 2019;35:1859–1866. doi: 10.1080/03007995.2019.1646000 [DOI] [PubMed] [Google Scholar]
- 30. Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD. Model parameter estimation and uncertainty analysis: a report of the ISPOR‐SMDM modeling good research practices task force working group‐6. Med Decis Making. 2012;32:722–732. doi: 10.1177/0272989x12458348 [DOI] [PubMed] [Google Scholar]
- 31. Independent Hospital Pricing Authority Australia . National Hospital Cost Data Collection, Public Hospitals Cost Report, Round 20 (Financial year 2015‐16). Cost weight 8.0. March 2018. Avalable from https://www.ihpa.gov.au/publications/national‐hospital‐cost‐data‐collection‐public‐hospitals‐cost‐report‐round‐20‐0. Accessed on 15 Dec 2021
- 32. Orchard J, Li J, Freedman B, Webster R, Salkeld G, Hespe C, Gallagher R, Patel A, Kamel B, Neubeck L, et al. Atrial fibrillation screen, management, and guideline‐recommended therapy in the rural primary care setting: a cross‐sectional study and cost‐effectiveness analysis of eHealth tools to support all stages of screening. J Am Heart Assoc. 2020;9:e017080. doi: 10.1161/JAHA.120.017080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Arora N, Makino K, Tilden D, Lobotesis K, Mitchell P, Gillespie J. Cost‐effectiveness of mechanical thrombectomy for acute ischemic stroke: an Australian payer perspective. J Med Econ. 2018;21:799–809. doi: 10.1080/13696998.2018.1474746 [DOI] [PubMed] [Google Scholar]
- 34. Gao L, Sheppard L, Wu O, Churilov L, Mohebbi M, Collier J, Bernhardt J, Ellery F, Dewey H, Moodie M. Economic evaluation of a phase III international randomised controlled trial of very early mobilisation after stroke (AVERT). BMJ Open. 2019;9:e026230. doi: 10.1136/bmjopen-2018-026230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Medicare Benefits Schedule Australia . Items could be searched from: http://www.mbsonline.gov.au/internet/mbsonline/publishing.nsf/Content/Home. Accessed on 14 Dec 2021
- 36. Pharmaceutical Benefits Scheme Australia . Items could be searched from https://www.pbs.gov.au/pbs/home. Accessed on 14 Dec 2021.
- 37. Gold MR. Cost‐effectiveness in health and medicine. USA: Oxford University Press; 1990. [Google Scholar]
- 38. Wang S, Gum D, Merlin T. Comparing the ICERs in medicine reimbursement submissions to NICE and PBAC‐does the presence of an explicit threshold affect the ICER proposed? Value Health. 2018;21:938–943. doi: 10.1016/j.jval.2018.01.017 [DOI] [PubMed] [Google Scholar]
- 39. Diekmann S, Hörster L, Evers S, Hiligsmann M, Gelbrich G, Gröschel K, Wachter R, Hamann GF, Kermer P, Liman J, et al. Economic evaluation of prolonged and enhanced ECG Holter monitoring in acute ischemic stroke patients. Curr Med Res Opin. 2019;1: doi: 10.1080/03007995.2019.1646000 [DOI] [PubMed] [Google Scholar]
- 40. Kamel H, Hegde M, Johnson DR, Gage BF, Johnston SC. Cost‐effectiveness of outpatient cardiac monitoring to detect atrial fibrillation after ischemic stroke. Stroke. 2010;41:1514–1520. doi: 10.1161/STROKEAHA.110.582437 [DOI] [PubMed] [Google Scholar]
- 41. Yong JH, Thavorn K, Hoch JS, Mamdani M, Thorpe KE, Dorian P, Sharma M, Laupacis A, Gladstone DJ, Committee ES. Potential cost‐effectiveness of ambulatory cardiac rhythm monitoring after cryptogenic stroke. Stroke. 2016;47:2380–2385. doi: 10.1161/STROKEAHA.115.011979 [DOI] [PubMed] [Google Scholar]
- 42. Jacobs MS, Kaasenbrood F, Postma MJ, van Hulst M, Tieleman RG. Cost‐effectiveness of screening for atrial fibrillation in primary care with a handheld, single‐lead electrocardiogram device in the Netherlands. Europace. 2018;20:12–18. doi: 10.1093/europace/euw285 [DOI] [PubMed] [Google Scholar]
- 43. Diamantopoulos A, Sawyer LM, Lip GY, Witte KK, Reynolds MR, Fauchier L, Thijs V, Brown B, Quiroz Angulo ME, Diener HC. Cost‐effectiveness of an insertable cardiac monitor to detect atrial fibrillation in patients with cryptogenic stroke. Int J Stroke. 2016;11:302–312. doi: 10.1177/1747493015620803 [DOI] [PubMed] [Google Scholar]
- 44. Rinciog CI, Sawyer LM, Diamantopoulos A, Elkind MSV, Reynolds M, Tsintzos SI, Ziegler PD, Quiroz ME, Wolff C, Witte KK. Cost‐effectiveness of an insertable cardiac monitor in a high‐risk population in the UK. Open Heart. 2019;6:e001037. doi: 10.1136/openhrt-2019-001037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thijs V, Guarnieri C, Makino K, Tilden D, Huynh M. Cost‐Effectiveness of long‐term continuous monitoring with an insertable cardiac monitor to detect atrial fibrillation in patients with cryptogenic stroke: an Australian payer perspective. J Neurol Neurosur Ps. 2018;89:E6. doi: 10.1136/jnnp-2018-ANZAN.12 [DOI] [Google Scholar]
- 46. Ziegler PD, Rogers JD, Ferreira SW, Nichols AJ, Richards M, Koehler JL, Sarkar S. Long‐term detection of atrial fibrillation with insertable cardiac monitors in a real‐world cryptogenic stroke population. Int J Cardiol. 2017;244:175–179. doi: 10.1016/j.ijcard.2017.06.039 [DOI] [PubMed] [Google Scholar]
- 47. Australian Bureau of Statistics . 3302055001DO001_20152017 Life Tables, States, Territories and Australia, 2015‐2017. 2018.
- 48. Miyasaka Y, Barnes ME, Bailey KR, Cha SS, Gersh BJ, Seward JB, Tsang TSM. Mortality trends in patients diagnosed with first atrial fibrillation ‐ A 21‐year community‐based study. J Am Coll Cardiol. 2007;49:986–992. doi: 10.1016/j.jacc.2006.10.062 [DOI] [PubMed] [Google Scholar]
- 49. Jabaudon D, Sztajzel J, Sievert K, Landis T, Sztajzel R, et al. Usefulness of ambulatory 7‐day ECG monitoring for the detection of atrial fibrillation and flutter after acute stroke and transient ischemic attack. Stroke. 2004;35:1647–1651. doi: 10.1161/01.STR.0000131269.69502.d9 [DOI] [PubMed] [Google Scholar]
- 50. Gladstone DJ, Spring M, Dorian P, Panzov V, Thorpe KE, Math M, Hall J, Vaid H, O'Donnell M, Laupacis A, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370:2467–2477. doi: 10.1056/NEJMoa1311376 [DOI] [PubMed] [Google Scholar]
- 51. Sanna T, Diener HC, Passman RS, Lazzaro VD, Bernstein RA, Morillo CA, Rymer MM, Thijs V, Rogers T, Beckers F, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014; 370:2478–2486. doi: 10.1056/NEJMoa1313600 [DOI] [PubMed] [Google Scholar]
- 52. Grond M, Jauss M, Hamann G, Stark E, Veltkamp R, Nabavi D, Horn M, Weimar C, Köhrmann M, Wachter R, et al. Improved detection of silent atrial fibrillation using 72‐hour Holter ECG in patients with ischemic stroke: a prospective multicenter cohort study. Stroke. 2013;44:3357–3364. doi: 10.1161/strokeaha.113.001884 [DOI] [PubMed] [Google Scholar]
- 53. Rizos T, Guntner J, Jenetzky E, Marquardt L, Reichardt C, Becker R, Reinhardt R, Hepp T, Kirchhof P, Aleynichenko E, et al. Continuous stroke unit electrocardiographic monitoring versus 24‐hour Holter electrocardiography for detection of paroxysmal atrial fibrillation after stroke. Stroke. 2012;43:2689–2694. doi: 10.1161/STROKEAHA.112.654954 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and methods that support the findings of this study are available from the corresponding author upon reasonable request.


