Abstract
Background
One‐quarter of all strokes are subsequent events. It is not known whether higher levels of blood glucose are associated with an increased risk of subsequent stroke after high‐risk transient ischemic attack or minor ischemic stroke.
Methods and Results
We performed a secondary analysis of the POINT (Platelet Oriented Inhibition in New TIA and Minor Ischemic Stroke) trial to evaluate the relationship between serum glucose hyperglycemia (≥180 mg/dL) versus normoglycemia (<180 mg/dL) before enrollment in the trial and outcomes at 90 days. The primary end point was subsequent ischemic stroke modeled by a multivariable Cox model with adjustment for age, sex, race, ethnicity, study treatment assignment, index event, and key comorbidities. Of 4878 patients included in this study, 267 had a recurrent stroke. There was a higher hazard of subsequent stroke in patients with hyperglycemia compared with normoglycemia (adjusted hazard ratio [HR], 1.50 [95% CI, 1.05–2.14]). Treatment with dual antiplatelet therapy was not associated with a reduced hazard of subsequent stroke in patients with hyperglycemia (HR, 1.18 [95% CI, 0.69–2.03]), though the wide confidence interval does not exclude a treatment effect. When modeled as a continuous variable, there was evidence of a nonlinear association between serum glucose and the hazard of subsequent stroke (P<0.001).
Conclusions
Hyperglycemia on presentation is associated with an increased risk of subsequent ischemic stroke after high‐risk transient ischemic attack or minor stroke. A rapid, simple assay of serum glucose may be a useful biomarker to identify patients at particularly high risk of subsequent ischemic stroke.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT0099102.
Keywords: antithrombotic therapy, clinical trial, diabetes, hyperglycemia, ischemic stroke
Subject Categories: Cerebrovascular Disease/Stroke, Ischemic Stroke, Transient Ischemic Attack (TIA)
Nonstandard Abbreviations and Acronyms
- CHANCE
Clopidogel in High‐Risk Patients With Acute Nondisabling Events
- POINT
Platelet‐Oriented Inhibition in New TIA and Minor Ischemic Stroke
- SHINE
Stroke Hyperglycemia Insulin Network Effort
Clinical Perspective
What Is New?
This study demonstrates that higher serum glucose on admission is associated with an increased risk of subsequent stroke after a high‐risk transient ischemic attack or minor stroke.
What Are the Clinical Implications?
People with high serum glucose may be at higher risk of future stroke and may benefit from particularly cautious monitoring and follow‐up.
Every ischemic stroke represents a critical opportunity to prevent another, potentially more severe, stroke. The risk of subsequent stroke is as high as 17% in the 90 days following the index event, but this risk is front‐loaded within the first 7 days. 1 For this reason, there is a need to incorporate dynamic physiological metrics into risk stratification schemes, and not simply long‐term risk factors. Serum glucose is an intriguing potential predictor of recurrent stroke risk, because it is already assessed in the majority of patients with acute stroke using widely available, low‐cost assays.
Hyperglycemia, an elevation in serum glucose, is associated with an increase in lesion volume 2 , 3 and worse functional outcomes 3 , 4 , 5 , 6 , 7 , 8 after acute ischemic stroke. Several studies 9 , 10 have shown that a history of diabetes is associated with subsequent stroke after transient ischemic attack (TIA) or minor ischemic stroke. One prior study 11 suggested that stress hyperglycemia (serum glucose indexed against glycosylated albumin) was associated with subsequent stroke. However, it is not known whether serum glucose itself is associated with subsequent stroke risk.
The objective of this study was to determine whether serum glucose measured on presentation to the emergency department is associated with the risk of subsequent ischemic stroke within 90 days after a high‐risk TIA or minor ischemic stroke. We hypothesized that elevated admission serum glucose is associated with a higher risk of subsequent stroke.
Methods
This research is based on the National Institute of Neurological Disorders and Stroke’s archived clinical research data sets (POINT [Platelet‐Oriented Inhibition in New TIA and Minor Ischemic Stroke Trial], S. Claiborne Johnston [U01/NS062835]). The data supporting this study are available upon request from the National Institute of Neurological Disorders and Stroke Clinical Research Liaison (CRLiaison@ninds.nih.gov). The code supporting this analysis and the STrengthening the Reporting of OBservational studies in Epidemiology checklist for observational research 12 are included in Data S1. Because this study was performed using a deidentified, publicly available data set, it was deemed exempt from further review by the institutional review board of Duke University School of Medicine (number 00108046).
Study Design
We performed a secondary analysis using data from the POINT (Registration URL: https://www.clinicaltrials.gov; Unique identifier: NCT00991029). 13 The POINT compared clopidogrel/aspirin to aspirin alone with respect to the primary outcome of a composite of subsequent ischemic stroke, myocardial infarction, or vascular death within 90 days of randomization. It enrolled 4881 patients aged 18 years or older who presented with a high‐risk TIA (ABCD 2 score ≥4) or acute minor ischemic stroke (National Institutes of Health Stroke Scale score ≤3) between May 2010 and December 2017 at 269 hospitals. Patients were excluded if they received intravenous tissue‐type plasminogen activator, mechanical thrombectomy, had an indication for anticoagulation, or were planned for carotid endarterectomy.
Exposure
The independent variable in this analysis was hyperglycemia. This was defined as a random serum glucose on presentation ≥180 mg/dL (10 mmol/L). The threshold of 180 mg/dL was chosen a priori based on (1) the upper bound of the active control arm of the SHINE (Stroke Hyperglycemia Insulin Network Effort) trial 14 and (2) the upper bound of the serum glucose range recommended from the 2019 Guidelines for the Early Management of Acute Ischemic Stroke. 15 Serum glucose was assayed on presentation per trial protocol and documented before a determination was made on eligibility for the trial. It was recorded in millimoles per liter or milligrams per deciliter and stored pro forma by study investigators. We excluded patients in whom serum glucose level was unavailable.
End Points
The primary end point of this analysis was subsequent ischemic stroke. This was collected as a secondary outcome in POINT and defined as acute, focal infarction of the brain or retina as evidenced by (1) rapid onset of a new, focal neurological deficit with clinical or imaging evidence of infarction and not attributable to a nonischemic cause; or (2) rapid worsening of an existing focal neurological deficit that was judged by the investigator as attributed to new infarction. 13 Subsequent ischemic stroke was adjudicated by 2 neurologists based on study outcome visits complemented by neuroimaging and medical record review. Secondary end points for this study included major hemorrhage and a composite of subsequent ischemic stroke, myocardial infarction, and death. All definitions used in this article are per the POINT protocol. 13
Power Calculations
Because this study was performed on a data set of fixed size, sample size calculations were not performed in advance of data analysis. Instead, we calculated study power across a range of postulated hazard ratios and group proportions. With the known 267 ischemic stroke events in the data set and assuming 15% of subjects in the exposure (hyperglycemia) group, a Cox proportional hazards regression model would have 99% power to detect a hazard ratio (HR) of 2 between the groups, at an α of 0.05. We determined the study was likely to be adequately powered with study power in the extreme cases ranging from 61% (10% exposed; HR, 1.5) to 99.9% (25% exposed; HR, 2.5). Power calculations were performed using the powerSurvEpi package in R (version 4.03; R Foundation for Statistical Computing, Vienna, Austria).
Statistical Analysis
Our study sample was described using descriptive statistics with mean±SD or median±interquartile range as appropriate for continuous variables and frequencies/counts for categorical variables. Patients with or without hyperglycemia were compared on univariate analysis using the Student t test or Mann‐Whitney test for continuous variables and the χ2 or Fisher exact test for categorical variables, as appropriate. We compared the rate of subsequent ischemic stroke between patients with and without hyperglycemia on presentation using Kaplan‐Meier statistics. The log‐rank test was used to compare survival curves between groups.
We constructed a Cox proportional hazards regression model to calculate HRs for the primary end point between those with and without admission hyperglycemia. We adjusted for known predictors of subsequent stroke by including age, biological sex, hypertension, diabetes, coronary artery disease, congestive cardiac failure, tobacco exposure, valvular heart disease, carotid disease, treatment assignment (clopidogrel/aspirin versus placebo/aspirin based on the intent‐to‐treat analysis), and index event classification (high‐risk TIA or acute minor ischemic stroke). Additionally, we chose to include both race and ethnicity in multivariable modeling because each are known to predict subsequent stroke. 16 , 17 We tested the assumption of proportional hazards by inspection of Schoenfeld residuals plots. We fitted models containing the interaction terms hyperglycemia*clopidogrel and hyperglycemia*final adjudicated cause. No adjustment was performed in the clopidogrel interaction analysis because we expected equal distribution of covariates across groups. We reported the HRs with 95% CIs for clopidogrel within the stratifications of hyperglycemia or no hyperglycemia and for hyperglycemia within the subdivisions of diabetes or no diabetes and minor stroke or other adjudicated cause. These analyses were repeated for the secondary end points of major hemorrhage and the composite outcome of ischemic stroke, myocardial infarction, or vascular death.
Sensitivity/Subgroup Analyses
We performed several further analyses:
We used a continuous measurement of admission serum glucose as the independent variable within a fully adjusted proportional hazards regression model. To explore the potential for nonlinearity between glucose and subsequent stroke, glucose was modeled as a restricted cubic spline. We chose 5 knots within the restricted cubic spline function at the 5%, 27.5%, 50%, 72.5%, and 95% percentiles. We then performed proportional hazards regression modeling with adjustment for the same covariates as in our primary analysis. We tested for nonlinearity using a likelihood ratio test. The relative hazards of subsequent ischemic stroke were graphed.
We created a logistic regression model incorporating all covariates within our primary analysis, and we created a propensity score 18 to predict hyperglycemia versus normoglycemia. Using a caliper of 0.05, we matched hyperglycemic patients on a 1:1 ratio with a propensity‐score matched patient without hyperglycemia and replicated our primary analysis restricted to this subgroup.
We replicated the main analysis substituting “acute infarction on an imaging study that was attributed to the index event” for “final diagnosis of index event based on symptoms, signs, and imaging data.”
We performed subgroup analyses restricted to (1) patients with minor stroke as the index event and (2) patients with TIA as the index event.
All hypothesis testing was 2‐sided, and the threshold for statistical significance was set at α=0.05. We did not perform imputation for missing data. Statistical analyses were performed using R (version 4.03).
Results
Patient Characteristics
Overall, 4878 patients were included in this analysis after 3 patients without recorded serum glucose values were excluded. The mean age of subjects in this analysis was 64.6±13.1 years, 45% were women, and 594 (12.2%) were hyperglycemic on presentation. Nine hundred sixty‐six (19.8%) patients were Black and 387 (7.9%) were Hispanic. Patients with hyperglycemia on presentation were more likely to be Hispanic (12.3% versus 7.3%, P<0.001) and to have hypertension (83.5% versus 67.1%, P<0.001), diabetes (85.7% versus 19.4%, P<0.001), congestive cardiac failure (4.5% versus 2.3%, P=0.002), coronary artery disease (13.1% versus 9.8%, P=0.01), or an index event consistent with minor ischemic stroke (56.7% versus 45.9%, P<0.001). Key demographic and clinical characteristics of the study population are presented in Table 1.
Table 1.
Demographics and Key Clinical Characteristics of Patients Included in This Study
| All, N=4878 | Hyperglycemia, n=594 | Normoglycemia, n=4284 | P value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, y, mean±SD | 64.6±13.1 | 62.6±11.5 | 64.8±13.3 | <0.001 |
| Women | 2194 (45%) | 248 (41.8%) | 1946 (45.4%) | 0.1 |
| Black* | 966 (19.8%) | 123 (20.7%) | 843 (19.7%) | 0.59 |
| Hispanic † | 387 (7.9%) | 73 (12.3%) | 314 (7.3%) | <0.001 |
| Comorbidities | ||||
| Hypertension ‡ | 3371 (69.1%) | 496 (83.5%) | 2875 (67.1%) | <0.001 |
| Diabetes § | 1340 (27.5%) | 509 (85.7%) | 831 (19.4%) | <0.001 |
| Congestive cardiac failure ‖ | 126 (2.6%) | 27 (4.5%) | 99 (2.3%) | 0.002 |
| Atrial fibrillation ¶ | 49 (1%) | 4 (0.7%) | 45 (1.1%) | 0.52 |
| Coronary artery disease # | 497 (10.2%) | 78 (13.1%) | 419 (9.8%) | 0.01 |
| Valvular disease** | 83 (1.7%) | 8 (1.3%) | 75 (1.8%) | 0.59 |
| Carotid disease †† | 208 (4.3%) | 31 (5.2%) | 177 (4.1%) | 0.26 |
| Active smoking ‡‡ | 1003 (20.6%) | 109 (18.4%) | 894 (20.9%) | 0.17 |
| Index stroke §§ | 2304 (47.2%) | 337 (56.7%) | 1967 (45.9%) | <0.001 |
| Assigned to clopidogrel | 2430 (49.8%) | 307 (51.7%) | 2123 (49.6%) | 0.35 |
| Subsequent stroke | 267 (5.5%) | 54 (9.1%) | 213 (5%) | <0.001 |
POINT indicates Platelet‐Oriented Inhibition in New TIA and Minor Ischemic Stroke.
Other racial groups represented in this sample included 3555 (72.9%) White patients, 144 (3%) Asian patients, 23 (0.5%) American Indian/Alaskan Native patients, 15 (0.3%) Native Hawaiian patients, 9 (0.2%) patients of >1 race, 26 (0.5%) patients labeled as “other,” and 140 (2.9%) patients who were “unknown/not reported.” Within the POINT study, the 140 “unknown” patients were not included in the denominator, hence the discrepancy between the percentages reported between that study and the present one.
There were 230 patients labeled as being of unknown ethnicity.
There were 21 patients labeled as unknown hypertension status.
There were 9 patients missing data on diabetes.
There were 7 patients labeled as unknown congestive cardiac failure status.
There were 4 patients labeled as unknown atrial fibrillation status.
There were 12 patients labeled as unknown coronary artery disease status.
There were 12 patients labeled as unknown valvular disease status.
There were 32 patients labeled as unknown carotid disease status.
There were 4 patients missing data on smoking status.
There were 3 patients who had missing data on index event (minor stroke vs transient ischemic attack).
Study End Points
During 90 days of follow‐up, 267 out of 4878 patients had a subsequent ischemic stroke. The cumulative incidence of subsequent ischemic stroke was 9.7% (95% CI, 7.2%–12.2%) in patients with hyperglycemia and 5.2% (95% CI, 4.5%–5.8%) in normoglycemic patients (P<0.001 by the log‐rank test) (Figure 1). The hazard of subsequent ischemic stroke was higher among patients with hyperglycemia than among normoglycemic patients (HR, 1.88 [95% CI, 1.39–2.53]; P<0.001) in an unadjusted proportional hazards regression model (Table 2). In a fully adjusted model (including age, biological sex, race, ethnicity, treatment assignment, index event classification, and vascular risk factors as covariates), a significant association remained between admission hyperglycemia and subsequent ischemic stroke (HR, 1.5 [95% CI, 1.05–2.14]; P=0.01). There was no significant association between hyperglycemia and major hemorrhage in a model adjusted for age, biological sex, race, ethnicity, treatment assignment, final adjudicated cause, and hypertension (HR, 0.47 [95% CI, 0.11–1.99]; P=0.31). There was a significant association between hyperglycemia and the composite of ischemic stroke, myocardial infarction, or vascular death (HR, 1.55 [95% CI, 1.10–2.20]; P=0.01).
Figure 1. Kaplan‐Meier curves depicting cumulative risk of subsequent ischemic stroke in patients with and without hyperglycemia.
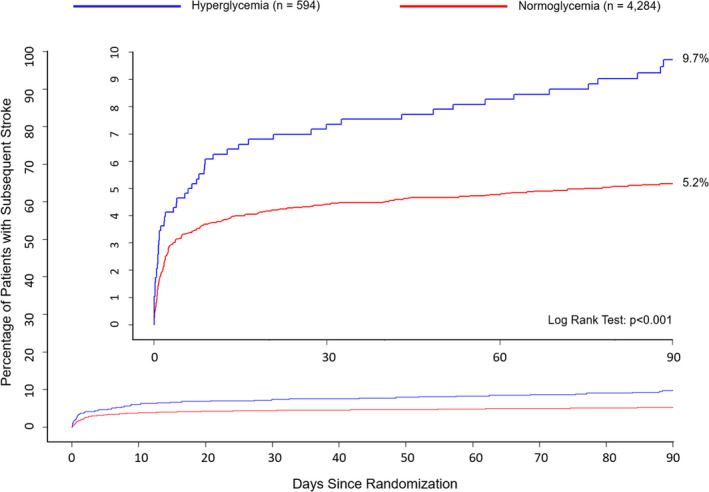
Table 2.
Association Between Hyperglycemia and Subsequent Ischemic Stroke
| Model | HR* (95% CI) |
|---|---|
| 1. Unadjusted | 1.87 (1.39–2.53) |
| 2. Model 1+age, biological sex, race, and ethnicity | 1.93 (1.43–2.61) |
| 3. Model 2+treatment assignment † and index event ‡ | 1.77 (1.31–2.39) |
| 4. Model 3+vascular risk factors § (excluding diabetes) | 1.71 (1.26–2.32) |
| 5. Model 4+vascular risk factors (including diabetes) | 1.5 (1.05–2.14) |
HR indicates hazard ratio.
HR is for the comparison of hyperglycemia vs normoglycemia.
Treatment assignment includes aspirin/clopidogrel compared with aspirin/placebo (on intention‐to‐treat basis).
Index event denotes minor ischemic stroke compared with high‐risk transient ischemic attack/other diagnosis.
Vascular risk factors include hypertension, congestive cardiac failure, atrial fibrillation, coronary artery disease, valvular disease, carotid disease, and active smoking.
Interaction Analyses
In patients with hyperglycemia, treatment with dual antiplatelet therapy was not associated with a reduced hazard of subsequent ischemic stroke in an unadjusted Cox model (HR, 1.18 [95% CI, 0.69–2.03]). In patients with normoglycemia, treatment with dual antiplatelet therapy was associated with a lower hazard of subsequent ischemic stroke (HR, 0.63 [95% CI, 0.48–0.83]). The P value for interaction was 0.04. We observed similar results for the composite end point of stroke, myocardial infarction, and vascular death (Table 3). The low number of major hemorrhages observed in this sample did not permit an interaction analysis. There was no significant interaction between hyperglycemia and final adjudicated cause (minor stroke versus TIA) on these end points (Table S1).
Table 3.
Association Between Treatment Assignment (Clopidgrel Versus Placebo) and Key Study End Points in Patients With and Without Hyperglycemia
| Outcome | Aspirin/clopidogel, n=2430 | Aspirin/placebo, n=2448 | HR (95% CI)* | P value | P value for interaction |
|---|---|---|---|---|---|
| Ischemic stroke | |||||
| <180 mg/dL | 82/2123 | 131/2161 | 0.63 (0.48–0.83) | <0.001 | 0.04 |
| ≥180 mg/dL | 30/307 | 24/287 | 1.18 (0.84–02.03) | 0.50 | |
| Major hemorrhage | |||||
| <180 mg/dL | 21/2123 | 10/2161 | 2.14 (1.01–4.54) | <0.05 | … |
| ≥180 mg/dL | 2/307 | 0/287 | … | … | |
| Primary end point † | |||||
| <180 mg/dL | 89/2123 | 134/2161 | 0.67 (0.51–0.87) | 0.003 | 0.06 |
| ≥180 mg/dL | 32/207 | 26/287 | 1.17 (0.70–1.96) | 0.55 | |
HRs are for the association between clopidogrel and the end point within the <180 mg/dL and ≥180 mg/dL strata. The interaction term is derived from a model including all patients in the study sample, which includes the term clopidogrel*hyperglycemia. HR indicates hazard ratio.
Unadjusted HR.
Subsequent ischemic stroke, myocardial infarction, ischemic vascular death.
Sensitivity Analyses
Incorporating Serum Glucose as a Continuous Variable
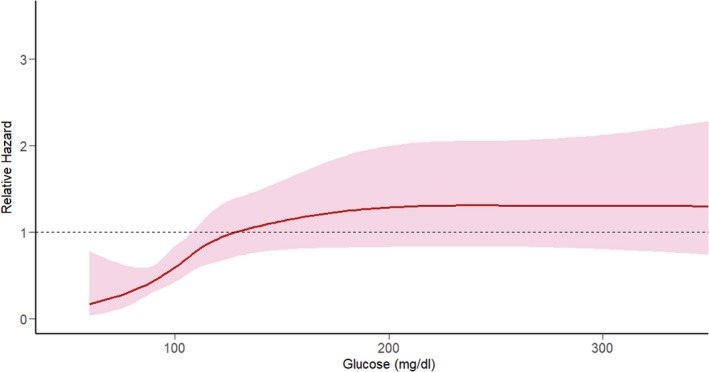
There was evidence of a nonlinear relationship between serum glucose and subsequent stroke risk (P<0.001). Figure 2 assesses glucose as a restricted cubic spline rather than as a categorical variable. The restricted cubic spline for the risk of subsequent stroke was positively sloped with a gradual inflection in the 100 to 150 mg/dL range with a plateau at approximately 200 mg/dL.
Figure 2. Relative hazard of subsequent ischemic stroke modeled on serum glucose as a restricted cubic spline and adjusted for all covariates used in the primary analysis.
Propensity Score–Matched Cohort
We compared the hazard of subsequent stroke between 554 (out of 594) patients with hyperglycemia and 554 propensity score–matched controls with normoglycemia. The association with subsequent stroke was not evident in this analysis (HR, 1.42 [95% CI, 0.92–2.12]). There was satisfactory matching of propensity scores across groups (Figure S1).
Alternative Definition of Index Event
Incorporating “acute infarction on an imaging study that was attributed to the index event” instead of final adjudicated cause, the association with subsequent stroke persisted (adjusted HR, 1.47 [95% CI, 1.03–2.11]; P=0.03).
Subgroup Analyses
In the 2327 patients whose index event was a TIA, there was a higher hazard of subsequent stroke in an unadjusted model (HR, 2.35 [95% CI, 1.28–4.31]) but a nonsignificant association in a fully adjusted model (HR, 1.67 [95% CI, 0.83–3.35]). In the 2304 patients whose index event was an acute minor ischemic stroke, there was a higher hazard of subsequent stroke in an unadjusted model (HR, 1.5 [95% CI, 1.05–2.15]) but not in a fully adjusted model (HR, 1.48 [95% CI, 0.95–2.29]).
Discussion
We found that patients with hyperglycemia had a higher risk of subsequent ischemic stroke than patients with normoglycemia within the POINT clinical trial. The association between hyperglycemia and subsequent ischemic stroke persisted even after adjustment for demographics and clinical covariates that are known to predict subsequent stroke. The benefits of clopidogrel/aspirin were not apparent in the small subgroup of patients with hyperglycemia, with an interaction observed between clopidogrel and serum glucose on subsequent stroke.
There are several possible explanations for this association. First, hyperglycemia on presentation may be a marker of undiagnosed or poorly controlled diabetes, signifying a population known to be at high risk of subsequent stroke. Second, hyperglycemia may act as a surrogate for overall illness 19 , 20 and thus a marker of an inflammatory prothrombotic state. Third, it increases the likelihood of developing infection, itself a risk factor for stroke 21 ; thus, hyperglycemia may act as an intermediate step in the development of subsequent stroke. Fourth, there may be a causal relationship between hyperglycemia and stroke. There are several mechanisms described linking transient short‐term hyperglycemia with thrombus formation. 22 , 23 In subjects with and without diabetes, there is a linear correlation between fasting serum glucose and coagulation factor VII. 22 In healthy individuals, elevated thrombin–antithrombin complex and tissue factor are observed after only 3 hours of induced hyperglycemia and further accentuated by an induced inflammatory response. 24 Transient hyperglycemia is typically followed by transient hyperinsulinemia in healthy subjects, and this combined elevation of serum glucose and insulin have been shown to have an additive effect on enhancing circulating tissue factor and other components of the coagulation system. 25 Acute hyperglycemia also has deleterious effects on the vascular endothelium, and increased extracellular glucose increases the propensity to platelet activation and endothelial dysfunction. 26 , 27 , 28 The mechanisms linking hyperglycemia to thrombus formation independent of platelet function may explain the apparent lack of effect of dual antiplatelet therapy in the subgroup of patients with hyperglycemia, although given the low number, this may also represent a type I error.
One previous study 11 examined glycemic control as a predictor of subsequent stroke in the CHANCE (Clopidogel in High‐Risk Patients With Acute Nondisabling Events) trial. 29 Using the glucose/glycosylated albumin ratio they found that patients in the highest quartile had an HR of 1.46 (95% CI, 1.06–2.01) of subsequent stroke compared with patients in the lowest quartile. This study was performed in an exclusively Chinese population and relied on 2 separate assays (serum glucose and glycosylated albumin), calculation of a ratio, then classification into quartiles. The current study overcomes the limitations inherent in this prior study by (1) focusing on 1 simple, rapid measurement of serum glucose, and (2) testing our hypothesis in a population more diverse and representative with respect to race, ethnicity, and national origin.
Currently, direct serum glucose measurements are not incorporated in stroke risk classification schemes. However, the presence/absence of diabetes is included in scores used to predict subsequent stroke after TIA 9 , 10 and risk of stroke in atrial fibrillation. 30 Subsequent stroke risk may be estimated based on imaging characteristics or cause classification. 31 The ABCD2 9 and California 10 scores aim to predict the risk of stroke after an index TIA at 7 and 90 days, respectively, by combining data on vascular risk factors and characteristics of the presenting stroke. However, there is a heightened risk of stroke within a short period of time after the index event, 1 which suggests that more short‐term, dynamic factors are likely at play. Serum glucose may be a useful measure for identifying patients at high‐risk of early recurrence. By contrast, assay of glycosylated hemoglobin is reflective only of glycemic control over a period of approximately 2.5 months. 32 Additionally, measurement of serum glucose can be performed rapidly, is inexpensive, and does not require calculation. For this reason, its use is proposed in 2 scoring systems for predicting hemorrhage after intravenous tissue plasminogen activator (IV tissue‐type plasminogen activator) use (the TAG 33 and SEDAN scores 34 ).
The SHINE trial 14 randomized 1151 patients with hyperglycemia on presentation to either intensive therapy via continuous intravenous insulin infusion (target glucose 80–130 mg/dL) or standard therapy (target glucose 80–179 mg/dL) via an insulin sliding scale administered subcutaneously. There was no difference in the primary outcome (proportion of patients with a favorable score on the modified Rankin Scale at 90 days) between the 2 groups and more episodes of hypoglycemia in the intensive versus standard therapy groups (11.2% versus 3.2%). Subsequent ischemic stroke was not ascertained as a secondary outcome in this trial, but there were an equivalent number of ischemic strokes (16) reported across each arm as a serious adverse event. Although not specifically designed to test the hypothesis that control of serum glucose reduced the risk of subsequent stroke, the results suggest that elevated serum glucose may be a marker of overall sickness/illness severity and not a target for therapy itself.
There are limitations inherent in this study. First, because this is a secondary analysis of data already collected from a well‐phenotyped clinical trial population with high‐risk TIA or acute minor ischemic stroke, our results should be used for hypothesis generation only. Second, the subgroup of patients with hyperglycemia was small (12.2% of the study sample), which limits our power to observe true effects. In particular, our finding that dual antiplatelet therapy was not associated with a reduced risk of subsequent stroke should be interpreted with caution and should not be evoked as a reason to deviate from guideline‐based care in this population (the most recent American Heart Association stroke secondary prevention guidelines advocate for the use of dual antiplatelet therapy for 21 to 90 days for patients with noncardioembolic minor ischemic stroke or high‐risk TIA 35 ). Third, the POINT excluded patients who received IV tissue‐type plasminogen activator, underwent mechanical thrombectomy, had an indication for anticoagulation, or were planned for a revascularization procedure, and so our results may not apply to these groups of patients. Fourth, hypo‐ or hyperglycemia can cause acute, focal neurological deficits. Although abnormalities in serum glucose were not listed as exclusion criteria for POINT, the trial protocol did require that the study investigator believed the primary reason for the presenting neurological deficit to be focal brain ischemia. Thus, it is possible that some patients with hypo‐ or hyperglycemia and concurrent stroke were erroneously excluded from the trial. Finally, glucose levels were not taken with reference to the time of a patient’s last meal and thus are classified as random levels.
Conclusions
There was a higher rate of subsequent ischemic stroke and no clear benefit to dual antiplatelet therapy in patients with hyperglycemia on admission. This study may provide further support for developing innovative secondary prevention strategies in this high‐risk patient population.
Sources of Funding
None.
Disclosures
S.Y. previously received funding (paid to the Department of Neurology at the Warren Alpert Medical School of Brown University) from Medtronic for outcomes adjudication within a clinical study. A.D.H. is funded by the National Institute of Neurological Disorders and Stroke (K23NS105924). Y.X. is funded by the National Institute on Aging (R01AG062770, R01AG066672), has research funding from Daiichi Sankyo and Janssen, and has received honoraria from Boehringer Ingelheim and Portola. S.C.J. reports receiving research support from Sanofi and AstraZeneca. W.F. is funded by the National Institute of Neurological Disorders and Stroke (U01/NS102353 and P2C HD086844‐060) and MicroTransponder Inc. The remaining authors have no disclosures to report.
Supporting information
Data S1
Table S1
Figure S1
Supplemental Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.023223
For Sources of Funding and Disclosures, see page 8.
References
- 1. Coull AJ, Lovett JK, Rothwell PM; Study OV . Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services. BMJ. 2004;328:326. doi: 10.1136/bmj.37991.635266.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yaghi S, Dehkharghani S, Raz E, Jayaraman M, Tanweer O, Grory BM, Henninger N, Lansberg MG, Albers GW, Havenon A. The effect of hyperglycemia on infarct growth after reperfusion: an analysis of the defuse 3 trial. J Stroke Cerebrovasc Dis. 2021;30:105380. 10.1016/j.jstrokecerebrovasdis.2020.105380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Masrur S, Cox M, Bhatt DL, Smith EE, Ellrodt G, Fonarow GC, Schwamm L. Association of acute and chronic hyperglycemia with acute ischemic stroke outcomes post‐thrombolysis: findings from get with the guidelines‐stroke. J Am Heart Assoc. 2015;4:e002193. doi: 10.1161/JAHA.115.002193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001;32:2426–2432. doi: 10.1161/hs1001.096194 [DOI] [PubMed] [Google Scholar]
- 5. Bruno A, Biller J, Adams HP, Clarke WR, Woolson RF, Williams LS, Hansen MD. Acute blood glucose level and outcome from ischemic stroke. Trial of org 10172 in acute stroke treatment (TOAST) investigators. Neurology. 1999;52:280–284. doi: 10.1212/WNL.52.2.280 [DOI] [PubMed] [Google Scholar]
- 6. Weir CJ, Murray GD, Dyker AG, Lees KR. Is hyperglycaemia an independent predictor of poor outcome after acute stroke? Results of a long‐term follow up study. BMJ. 1997;314:1303–1306. doi: 10.1136/bmj.314.7090.1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rinkel LA, Nguyen TTM, Guglielmi V, Groot AE, Posthuma L, Roos YBWEM, Majoie CBLM, Lycklama à Nijeholt GJ, Emmer BJ, van der Worp HB, et al. High admission glucose is associated with poor outcome after endovascular treatment for ischemic stroke. Stroke. 2020;51:3215–3223. doi: 10.1161/STROKEAHA.120.029944 [DOI] [PubMed] [Google Scholar]
- 8. Chamorro Á, Brown S, Amaro S, Hill MD, Muir KW, Dippel DWJ, van Zwam W, Butcher K, Ford GA, den Hertog HM, et al. Glucose modifies the effect of endovascular thrombectomy in patients with acute stroke. Stroke. 2019;50:690–696. doi: 10.1161/STROKEAHA.118.023769 [DOI] [PubMed] [Google Scholar]
- 9. Rothwell PM, Giles MF, Flossmann E, Lovelock CE, Redgrave JN, Warlow CP, Mehta Z. A simple score (ABCD) to identify individuals at high early risk of stroke after transient ischaemic attack. Lancet. 2005;366:29–36. doi: 10.1016/S0140-6736(05)66702-5 [DOI] [PubMed] [Google Scholar]
- 10. Johnston SC, Gress DR, Browner WS, Sidney S. Short‐term prognosis after emergency department diagnosis of tia. JAMA. 2000;284:2901–2906. doi: 10.1001/jama.284.22.2901 [DOI] [PubMed] [Google Scholar]
- 11. Pan Y, Cai X, Jing J, Meng X, Li H, Wang Y, Zhao X, Liu L, Wang D, Johnston SC, et al. Stress hyperglycemia and prognosis of minor ischemic stroke and transient ischemic attack: the Chance study (clopidogrel in high‐risk patients with acute nondisabling cerebrovascular events). Stroke. 2017;48:3006–3011. doi: 10.1161/STROKEAHA.117.019081 [DOI] [PubMed] [Google Scholar]
- 12. Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M, STROBE Initiative . Strengthening the reporting of observational studies in epidemiology (strobe): explanation and elaboration. PLoS Med. 2007;4:e297. doi: 10.1371/journal.pmed.0040297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johnston SC, Easton JD, Farrant M, Barsan W, Conwit RA, Elm JJ, Kim AS, Lindblad AS, Palesch YY; Clinical Research Collaboration NuETTN, and the POINT Investigators . Clopidogrel and aspirin in acute ischemic stroke and high‐risk tia. N Engl J Med. 2018;379:215–225. doi: 10.1056/NEJMoa1800410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Johnston KC, Bruno A, Pauls Q, Hall CE, Barrett KM, Barsan W, Fansler A, Van de Bruinhorst K, Janis S, Durkalski‐Mauldin VL. Intensive vs standard treatment of hyperglycemia and functional outcome in patients with acute ischemic stroke: the shine randomized clinical trial. JAMA. 2019;322:326–335. doi: 10.1001/jama.2019.9346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 16. Kamel H, Zhang C, Kleindorfer DO, Levitan EB, Howard VJ, Howard G, Soliman EZ, Johnston SC. Association of black race with early recurrence after minor ischemic stroke or transient ischemic attack: secondary analysis of the point randomized clinical trial. JAMA Neurol. 2020;77:601–605. doi: 10.1001/jamaneurol.2020.0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morgenstern LB, Smith MA, Sánchez BN, Brown DL, Zahuranec DB, Garcia N, Kerber KA, Skolarus LE, Meurer WJ, Burke JF, et al. Persistent ischemic stroke disparities despite declining incidence in Mexican Americans. Ann Neurol. 2013;74:778–785. doi: 10.1002/ana.23972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rosenbaum PR, Rubin DR. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. doi: 10.1093/biomet/70.1.41 [DOI] [Google Scholar]
- 19. Van den Berghe G. Dynamic neuroendocrine responses to critical illness. Front Neuroendocrinol. 2002;23:370–391. doi: 10.1016/S0091-3022(02)00006-7 [DOI] [PubMed] [Google Scholar]
- 20. Inzucchi SE. Clinical practice. Management of hyperglycemia in the hospital setting. N Engl J Med. 2006;355:1903–1911. doi: 10.1056/NEJMcp060094 [DOI] [PubMed] [Google Scholar]
- 21. Elkind MSV, Boehme AK, Smith CJ, Meisel A, Buckwalter MS. Infection as a stroke risk factor and determinant of outcome after stroke. Stroke. 2020;51:3156–3168. doi: 10.1161/STROKEAHA.120.030429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ceriello A, Giugliano D, Quatraro A, Dello Russo P, Torella R. Blood glucose may condition factor vii levels in diabetic and normal subjects. Diabetologia. 1988;31:889–891. doi: 10.1007/BF00265372 [DOI] [PubMed] [Google Scholar]
- 23. Rao AK, Chouhan V, Chen X, Sun L, Boden G. Activation of the tissue factor pathway of blood coagulation during prolonged hyperglycemia in young healthy men. Diabetes. 1999;48:1156–1161. doi: 10.2337/diabetes.48.5.1156 [DOI] [PubMed] [Google Scholar]
- 24. Stegenga ME, van der Crabben SN, Blümer RM, Levi M, Meijers JC, Serlie MJ, Tanck MW, Sauerwein HP, van der Poll T. Hyperglycemia enhances coagulation and reduces neutrophil degranulation, whereas hyperinsulinemia inhibits fibrinolysis during human endotoxemia. Blood. 2008;112:82–89. doi: 10.1182/blood-2007-11-121723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vaidyula VR, Rao AK, Mozzoli M, Homko C, Cheung P, Boden G. Effects of hyperglycemia and hyperinsulinemia on circulating tissue factor procoagulant activity and platelet cd40 ligand. Diabetes. 2006;55:202–208. doi: 10.2337/diabetes.55.01.06.db05-1026 [DOI] [PubMed] [Google Scholar]
- 26. Nieuwdorp M, van Haeften TW, Gouverneur MC, Mooij HL, van Lieshout MH, Levi M, Meijers JC, Holleman F, Hoekstra JB, Vink H, et al. Loss of endothelial glycocalyx during acute hyperglycemia coincides with endothelial dysfunction and coagulation activation in vivo. Diabetes. 2006;55:480–486. doi: 10.2337/diabetes.55.02.06.db05-1103 [DOI] [PubMed] [Google Scholar]
- 27. D'Onofrio N, Sardu C, Paolisso P, Minicucci F, Gragnano F, Ferraraccio F, Panarese I, Scisciola L, Mauro C, Rizzo MR, et al. Microrna‐33 and sirt1 influence the coronary thrombus burden in hyperglycemic stemi patients. J Cell Physiol. 2020;235:1438–1452. doi: 10.1002/jcp.29064 [DOI] [PubMed] [Google Scholar]
- 28. Worthley MI, Holmes AS, Willoughby SR, Kucia AM, Heresztyn T, Stewart S, Chirkov YY, Zeitz CJ, Horowitz JD. The deleterious effects of hyperglycemia on platelet function in diabetic patients with acute coronary syndromes mediation by superoxide production, resolution with intensive insulin administration. J Am Coll Cardiol. 2007;49:304–310. doi: 10.1016/j.jacc.2006.08.053 [DOI] [PubMed] [Google Scholar]
- 29. Wang Y, Wang Y, Zhao X, Liu L, Wang D, Wang C, Wang C, Li H, Meng X, Cui L, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11–19. doi: 10.1056/NEJMoa1215340 [DOI] [PubMed] [Google Scholar]
- 30. Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the national registry of atrial fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864 [DOI] [PubMed] [Google Scholar]
- 31. Amarenco P, Lavallée PC, Labreuche J, Albers GW, Bornstein NM, Canhão P, Caplan LR, Donnan GA, Ferro JM, Hennerici MG, et al. One‐year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. 2016;374:1533–1542. doi: 10.1056/NEJMoa1412981 [DOI] [PubMed] [Google Scholar]
- 32. Nathan DM, Singer DE, Hurxthal K, Goodson JD. The clinical information value of the glycosylated hemoglobin assay. N Engl J Med. 1984;310:341–346. doi: 10.1056/NEJM198402093100602 [DOI] [PubMed] [Google Scholar]
- 33. Montalvo M, Mistry E, Chang AD, Yakhkind A, Dakay K, Azher I, Kaushal A, Mistry A, Chitale R, Cutting S, et al. Predicting symptomatic intracranial haemorrhage after mechanical thrombectomy: the TAG score. J Neurol Neurosurg Psychiatry. 2019;90:1370–1374. doi: 10.1136/jnnp-2019-321184 [DOI] [PubMed] [Google Scholar]
- 34. Strbian D, Engelter S, Michel P, Meretoja A, Sekoranja L, Ahlhelm FJ, Mustanoja S, Kuzmanovic I, Sairanen T, Forss N, et al. Symptomatic intracranial hemorrhage after stroke thrombolysis: the SEDAN score. Ann Neurol. 2012;71:634–641. doi: 10.1002/ana.23546 [DOI] [PubMed] [Google Scholar]
- 35. Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi‐Hill D, Kamel H, Kernan WN, Kittner SJ, Leira EC, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021;52:e364–e467. doi: 10.1161/STR.0000000000000375 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Table S1
Figure S1


