Abstract
Background
Helicobacter pylori (H. pylori) infection affects ≈4.4 billion people worldwide. Several studies suggest that this pathogen impacts the digestive system, causing diverse and severe conditions, and results in extragastrointestinal disorders like vascular diseases. Our study aims to examine the association between H. pylori infection and carotid intima‐media thickness.
Methods and Results
Electronic databases (MEDLINE, Embase, CENTRAL, Web of Science, and Scopus) were searched for studies, comparing the thickness of the carotid intima‐media in H. pylori–infected and noninfected individuals listed until October 20, 2020. Statistical analyses were performed using the random effects meta‐analysis of model of weighted mean differences with the corresponding 95% CI using the DerSimonian and Laird method. The protocol was registered in advance in PROSPERO (International Prospective Register of Systematic Reviews; CRD42021224485). Thirteen studies were found meeting inclusion criteria for our systematic review and meta‐analysis, presenting data on the thickness of the carotid intima‐media considering the presence of H. pylori infection. Altogether, 2298 individuals’ data were included (1360 H. pylori positive, 938 negative). The overall carotid intima‐media thickness was significantly larger among infected patients compared with uninfected participants (weighted mean difference: 0.07 mm; 95% CI, 0.02–0.12; P=0.004; I2=91.1%; P<0.001). In case of the right common carotid artery, the intima‐media thickening was found to be significant as well (weighted mean difference, 0.08 mm; 95% CI, 0.02–0.13, P=0.007; I2=85.1%; P<0.001), while it showed no significance in the left common carotid artery (weighted mean difference, 0.12 mm; 95% CI, −0.05 to 0.28, P=0.176; I2=97.4%; P<0.001).
Conclusions
H. pylori infection is associated with increased carotid intima‐media thickness. Therefore, the infection may indirectly contribute to the development of major vascular events.
Keywords: atherosclerosis, carotid intima‐media thickness, Helicobacter pylori, infection, meta‐analysis
Subject Categories: Atherosclerosis, Vascular Disease, Stenosis
Nonstandard Abbreviations and Acronyms
- CIM
carotid intima‐media
- TC
total cholesterol
- WMD
weighted mean difference
Clinical Perspective
What Is New?
Data of 13 observational studies on carotid intima‐media thickness, comprising 2298 individuals (1360 Helicobacter pylori–positive and 938 negative cases), were included in our systematic review and meta‐analysis.
Weighted mean differences were calculated to determine significant differences in terms of carotid intima‐media thickness between the H. pylori–positive and –negative groups.
The analyses revealed statistically significant association of H. pylori infection and overall carotid intima‐media thickness as well as the right but not left common carotid arteries.
What Are the Clinical Implications?
H. pylori testing might be considered in individuals with a thicker carotid intima‐media, especially in those with other risk factors of cerebrovascular or cardiovascular diseases.
The screening and eradication of H. pylori infection in the general population should not be fully discarded as a potential intervention to contribute to the risk reduction of future cerebrovascular and cardiovascular events.
The inequality of the cost‐benefit ratio is a severe limitation of this approach.
Over half of the world’s population is affected by Helicobacter pylori infection; in 2015, the estimated number of infected individuals was 4.4 billion. 1 The prevalence of H. pylori is around 80% in middle‐aged adults in developing countries. 2 While 48.6% of the examined adults were H. pylori positive, only 32.6% of children (<18 years) were infected worldwide, according to a recent meta‐analysis based on 183 studies mainly from Asia, Europe, Latin America, and the Caribbean. 3
The relationship between atherosclerosis and H. pylori infection has been extensively studied. Karadag et al 4 examined carotid intima‐media thickness (CIMT), epicardial adipose tissue thickness, and biologic markers of inflammation (high‐sensitivity C‐reactive protein) in patients who were H. pylori positive and found a relationship between the infection and CIMT. 5
The published literature on the link between H. pylori infection and stroke is controversial. In 1996, a nested case‐control study by Whincup et al 6 found an association between H. pylori infection and increased risk of stroke; however, the relationship was not significant after adjustment for major risk factors. Later, Doheim et al 7 claimed in their meta‐analysis that anti–H. pylori IgG positivity increases the risk of stroke. In another meta‐analysis, Wang et al 8 concluded that patients with chronic H. pylori infection are more likely to have a stroke by a noncardioembolic cause. Despite these, another meta‐analysis claims no strong relationship between H. pylori infection and stroke. 9 Wasay et al 10 identified H. pylori gastritis as a nonindependent risk factor for a higher occurrence of stroke in their cohort study. A recent systematic review and meta‐analysis concluded that in infected individuals the possibility of acute coronary syndrome increased. 11 Schöttker et al 12 found no association between the infection and major vascular events, cardiovascular mortality, and all‐cause mortality in a population‐based cohort study.
Concerning the relationship between the proliferation of the carotid arterial wall layers and the propagation of cerebrovascular and cardiovascular diseases, O’Leary et al 13 support in a cohort that CIMT is directly associated with an increased risk of major vascular events in individuals aged >65 years and no history of cardiovascular disease. Centurión 14 emphasized the importance of the noninvasive measurement of atherosclerotic burdens and also a relation of CIMT to the severity of systemic atherosclerosis.
According to Wang et al, 15 patients diagnosed with type 2 diabetes showed endothelial vascular impairment and thickening of CIM layers compared with healthy individuals. Another cross‐sectional study associated time length in a range of patients with type 2 diabetes with increased CIMT. 16
The findings mentioned above suggest a link between H. pylori infection and thicker carotid artery wall layers. However, clear evidence of the association is missing, and the literature is full of contradictory findings.
Our study aimed to evaluate the association of H. pylori infection and CIMT in a meta‐analysis.
Methods
The authors declare that all supporting data are available within the article and its online supplemental files.
A systematic review and meta‐analysis of the studies was reported by the guidance of Preferred Reporting Items for Systematic Reviews and Meta‐Analyses Statement (Table S1). 17
Protocol
The protocol was registered in advance in PROSPERO (International Prospective Register of Systematic Reviews) under the number CRD42021224485. Deviating from the originally planned protocol, no analyses on cytotoxin‐associated gene A positivity and age distribution were performed. No hazard ratios were reported. According to the Cochrane Handbook, pairs were formed; if not, 2 comparable groups were published in a study. 18 Subgroup analysis was performed on the basis of geographic distribution. For dichotomous outcomes, odds ratios (ORs) with 95% CIs were calculated.
Systematic Search
The literature search of 5 electronic databases (MEDLINE, Embase, CENTRAL, Web of Science, and Scopus) from inception until October 20, 2020, was conducted. All fields/texts were searched except for Scopus ("article title, abstract, keywords"), and no filters were applied. The clinical question was based on the PECO (Population, Exposure, Comparison, Outcome) framework: In this study, P means the individuals whose CIMT was measured, E is the individuals who are H. pylori positive, C marks the comparison between participants who are H. pylori positive and H. pylori negative, and the primary O is the intima‐media thickness in mm given separately for overall (the mean of the right and left common carotid arteries) and right and left carotid. The secondary outcomes, as part of our substantial analyses, were laboratory parameters (triglyceride, total cholesterol [TC], high‐density lipoprotein [HDL], low‐density lipoprotein [LDL]), the number of patients with diabetes, age, and hypertension. The following search key was used to find the relevant studies: (Helicobacter OR pylori) AND (caroti* or [Cerebrovascular Disorders] OR [Cardiovascular Diseases]). The references of the eligible articles were also reviewed to identify additional eligible studies.
Inclusion and Exclusion Criteria
Only peer‐reviewed observational studies (including cohort, cross‐sectional, and case‐control studies) were eligible for inclusion, which reported on at least 1 CIMT in mm of adult patients who were H. pylori positive and adult patients who were negative. No other cardiovascular markers were needed for inclusion. Pediatric studies (<18 years) and nonhuman studies were excluded. All methods of H. pylori infection determination were accepted.
Selection and Data Extraction
The selection process was conducted by 2 independent authors (O.S., E.O.). All results from the databases were transferred to a reference manager program (EndNote X9.3.3., Clarivate Analytics, Philadelphia, PA). After automatic and manual removal of duplicates, the records were screened on the basis of title, abstract, and full text following predetermined principles. Disagreements were resolved by a third investigator (L.S.). Cohen’s kappa coefficient was calculated to measure interrater reliability during the selection process. 19 The same independent investigators (O.S., E.O.) performed the data extraction onto a data collection sheet (Excel, Office 365, Microsoft, Redmond, WA). The following data were extracted from the eligible articles: first author, publication year, Digital Object Identifier, study design, the detection method of H. pylori, geographic location, age distribution, sex distribution, number of patients in each comparison group, number of patients with each event (eg, H. pylori positivity), laboratory parameters (triglyceride, TC, LDL, HDL), and number of patients with diabetes and hypertension in each group. The thickness of the carotid intima‐media (CIM) layers is given in mm for the right side, left side, and overall. Disagreements were resolved by a third investigator (L.S.).
Measurement of CIMT
Since the method of CIMT measurement shows wild differences, any approach was accepted. Generally, the measurement of CIMT shows moderate heterogeneity. There are slight differences in the definition of CIMT and the place of measurement by ultrasound. Characteristics of the CIMT measurements in the included articles are noted in Table S2.
Inclusion of Multiple Groups From One Study
As for studies with not just 2 comparable groups, we formed pairs according to the Cochrane Handbook. 18 In the case of 4 articles, 20 , 21 , 22 , 23 not only the presence or the absence of H. pylori was examined but also various comorbidities. These were alcoholic liver disease, 20 diabetes, 21 at least 2 risk factors for atherosclerosis (hypertension, hyperlipidemia, obesity, diabetes, smoking, female sex, personal history of atherosclerosis, and family history of premature atherosclerosis), 22 or early‐stage diabetic kidney disease. 23 We paired the H. pylori–positive group with the negative one, presenting the same comorbidity. In other cases, when the previously mentioned method could not be applied, we chose to compare the groups with the higher number of participants. 4 , 24 , 25
Management of Further Sources of Inaccuracy
In the study published by El Hadidy et al, 26 CIMT results were bigger with 1 decimal than in the other articles included in this meta‐analysis. Furthermore, no information was published on the definition of CIMT and the measurement area of CIM (Table S2). Influence diagnostics were performed in the case of right and left CIMT to determine this specific article’s effect on the outcome. Sensitivity analysis and statistical analyses were performed with and without their data to assess the effect of their questionable results on the pooled analysis.
Substantial and Subgroup Analyses
We performed substantial analyses to see how the characteristics of the included population affect the results. Laboratory parameters of triglyceride, TC, LDL, and HDL levels were compared between the H. pylori–positive and –negative groups. The pooled values were given in mmol/L. The numbers of patients with diabetes, age, and hypertension in the specific groups were also compared. We performed additional subgroup analyses based on the geographic distribution and detection method of the bacterium.
Statistical Analysis
For dichotomous outcomes (diabetes and hypertension) ORs with 95% CIs, and for continuous outcomes (CIMT, age, and laboratory parameters), weighted mean difference (WMD) with 95% CI were calculated on the basis of crude estimates. In one case, 27 the means and SDs were calculated from the median, minimum, and maximum values and the sample size according to Wan’s method. 28 We used the random effect model by DerSimonian and Laird 29 with the estimate of heterogeneity in all cases. An I2 test was performed to assess the heterogeneity. According to the Cochrane Handbook, 18 the interpretation of the I2 value is the following: If it is 0% to 40%, the heterogeneity is considered as “might not be important,” from 30% to 60% it is “moderate,” 50% to 90% means “substantial” heterogeneity, while from 75% to 100% the heterogeneity is “considerable.” Results were displayed graphically using forest plots. For our primary outcome (overall, right and left CIMT) we assessed publication bias by visually inspecting funnel plots to detect nonsymmetrical distribution of SEs around the study‐level effect estimates, and the Egger’s test, using a significance of P<0.05 to indicate significant asymmetry. These statistical analyses were performed in STATA version 16.0 (StataCorp, College Station, TX). Influence diagnostics are basic ways to detect and remove outliers in meta‐analyses. Studies without particularly high or low effect sizes can still exert a very high influence on our overall results and may lead to some concerns regarding the robustness of the pooled effect. 30 In our meta‐analysis, the following methods were applied: (1) Externally Standardized Residuals (rstudent), (2) DFFITS value, (3) Cook’s Distance, (4) Covariance Ratio, (5) Tau2, (6) Q values, (7) Hat value, (8) Study Weight. Influence diagnostics were performed with metafor package in R (R Foundation for Statistical Computing, Vienna, Austria). 31 As an extension to our subgroup analyses by meta‐regression, the effect of continuous and categorical characteristics and the effects of multiple factors were investigated simultaneously. The minimum number of the included studies was at least 10. 18 In our meta‐analysis, the groups of age difference, geographic location, and detection method met this criterion. Univariable meta‐regressions were performed in R with the package meta. 32
Risk‐of‐Bias Assessment
The risk of bias in the eligible studies was evaluated using the Quality in Prognosis Studies tool by 2 independent review authors (O.S., E.O.). 33 The assessment comprises 6 main domains, which were scored as low, moderate, or high risk of bias. If all domains were deemed as low risk, the overall assessment was a low risk of bias. If a study carried a domain with high risk or at least 3 domains with moderate risk, the overall assessment was a high risk of bias. All other cases were rated as moderate. Disagreements between the reviewers were resolved by consensus.
Results
Selection Process
The electronic literature search identified 4725 records. After the removal of duplicates, 2824 records were left; 106 full‐text articles were assessed for eligibility (Cohen’s kappa coefficient, 0.83), and after the selection process (Cohen’s kappa coefficient, 0.83), 13 studies were included both in qualitative and quantitative synthesis (Figure 1). 4 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 34 , 35 , 36 , 37 Table 1. presents the baseline characteristics of the enrolled studies, and Table S3 provides a further comparison of the H. pylori–positive and –negative groups.
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses flowchart of study selection and inclusion.
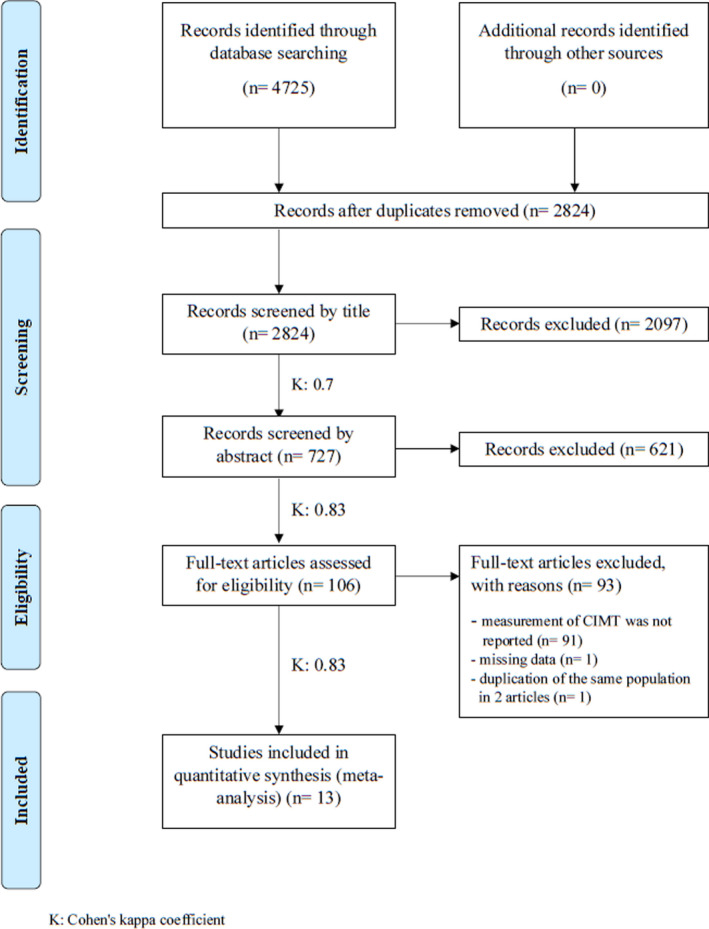
Table 1.
Basic Characteristics of the Included Studies
| HP detection method | Country | No. of patients |
Age (mean±SD) |
Sex (female %) | Comorbidities (considered) | ||
|---|---|---|---|---|---|---|---|
| Study | H. pylori positivity | H. pylori negativity | |||||
| Bao‐Ge et al 20 2017 I. | Urea breath test | China | 78 | 46.37±7.37* | 46.72±6.89* | 10.26 | Alcoholic liver disease |
| Bao‐Ge et al 20 2017 II. | Urea breath test | China | 82 | 46.74±6.69* | 46.66±6.75* | 10.98 | None |
| Başyığıt et al 34 2012 | Stool antigen, urea breath test | Turkey | 61 | 40.9±10.3 | 42.3±9.4 | 52.45 |
Hypertension, diabetes |
| Diomedi et al 24 2004 | Serum ELISA | Italy | 124 | 68.8±9.8 | 66.9±15.8 | 39.52 |
Hypertension, diabetes |
| El Hadidy et al 26 2009 | Serum ELISA | Egypt | 60 | NI | NI | 73.34 |
Hypertension, diabetes |
| Hamed et al 21 2008 I | Serum 2‐step immunometric assay | Egypt | 80 | 47.6±9.1 | 48.2±9.3 | 51.25 | Diabetes |
| Hamed et al 21 2008 II | Serum two‐step immunometric assay | Egypt | 60 | 46.2±9.7 | 50.2±6.5 | 40 | Diabetes |
| Judaki et al 35 2017 | Urea breath test, histology, culture | Iran | 80 | 45.64±8.32 | 46.52±5.52 | 48.75 | Hypertension |
| Karadag et al 4 2018 | Histology | Turkey | 45 | 50±8.2 | 52±7.9 | 53.34 | Hypertension |
| Köksal et al 22 2004 I | Serum ELISA | Turkey | 84 | 46.7±14.7 | 45.1±7.1 | 71.43 | Hypertension |
| Köksal et al 22 2004 II | Serum ELISA | Turkey | 50 | 45±11 | 45±10 | 68 | Hypertension |
| Mayr et al 25 2003 | Serum ELISA | Italy | 421 | 56.6 † | 55.7 † | 47.74 | None |
| Mete et al 27 2013 | Histology | Turkey | 134 | 49.8±8.7 | 50.2±9.33 | 58.21 | Hypertension |
| Shan et al 36 2018 | Serum ELISA | China | 395 | NI | NI | 57.47 | Hypertension |
| Xu et al 37 2016 | Urea breath test | China | 364 | 63.2±10.4 | 62.8±11.7 | 46.98 | None |
| Feng et al 23 2018 I | Urea breath test | China | 89 | 46.1±0.58* | 46.79±0.63* | 20.22 |
Hypertension, diabetes, early‐stage diabetic kidney disease |
| Feng et al 23 2018 II | Urea breath test | China | 91 | 46.64±0.54* | 46.61±0.53* | 21.98 |
Hypertension, diabetes, early‐stage diabetic kidney disease |
HP indicates, Helicobacter pylori; and NI, no information.
Mean±SE.
Mean without SD.
Characteristics of the Included Studies
Altogether, 2298 individuals were included: 1360 H. pylori–positive cases and 938 negative cases. All 13 included studies were single‐center, retrospective, observational studies. H. pylori positivity was determined by urea breath test in 377 cases, by serum ELISA in 786 cases, by histology in 127 cases, and using combined methods (stool antigen, urea breath test, histology, or cultivation) in 70 cases. In terms of the geographic distribution, 4‐4 studies were performed in China and Turkey, 2‐2 in Egypt and Italy, and 1 in Iran. In the way of CIMT measurement, used in included studies, no general practice was found (Table S2). Four of the included studies published data on plaques in the area of CIMT measurement, 21 , 24 , 27 , 34 but a standardized definition and clear inclusion/exclusion criteria are missing. Because of these, we were not able to perform any statistical analyses on plaques detected in the carotid arteries, but we would like to emphasize its importance in future studies.
H. pylori Infection and CIMT
A significant difference was found in the case of overall CIMT, which was thicker in H. pylori–positive patients based on the included 11 studies (1151 patients who were H. pylori–positive versus 692 patients who were H. pylori negative; WMD, 0.07 mm; 95% CI, 0.02–0.12; P=0.004; I2=91.1%; P<0.001; Figure 2). 4 , 20 , 21 , 22 , 23 , 24 , 25 , 27 , 34 , 35 , 37 In 4 studies, the right and the left CIMT were detailed separately. 26 , 27 , 34 , 36 Based on these, significantly increased CIMT was also detected in the right carotid artery; among H. pylori–infected individuals, it was 0.08 mm thicker than in the H. pylori–negative group (342 H. pylori–positive patients versus 308 patients who were H. pylori negative; WMD, 0.08 mm; 95% CI, 0.02–0.13; P=0.007; I2=85.1%; P<0.001; Figure 3), while no significant difference was found in the left carotid artery (342 patients who were H. pylori positive versus 308 patients who were H. pylori negative; WMD, 0.12 mm; 95% CI, −0.05–0.28; P=0.176; I2=97.4%; P<0.001; Figure 4).
Figure 2. Forest plot of studies comparing overall carotid intima‐media thickness between individuals who were Helicobacter pylori positive and negative.
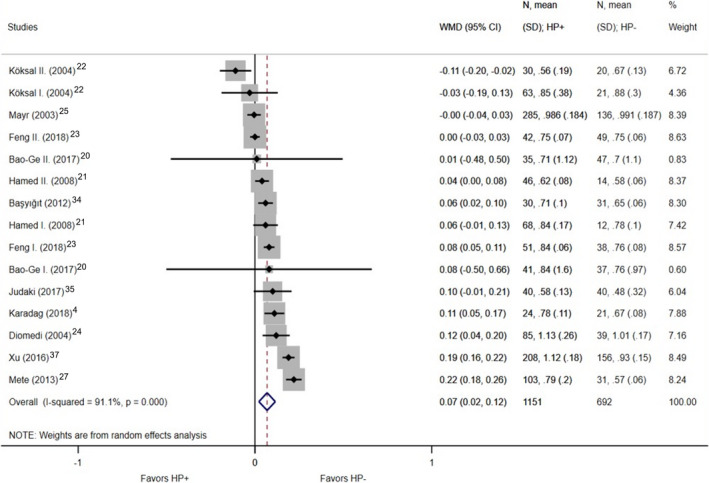
Black diamonds represent the weighted mean difference between the 2 groups we compared, and horizontal lines show the corresponding 95% CIs. Size of the gray squares reflects the weight of a particular study. The blue diamond is the overall or summary effect. The outer edges of the diamonds represent the CIs. HP indicates Helicobacter pylori; and WMD, weighted mean difference.
Figure 3. Forest plot of studies comparing right carotid intima‐media thickness between individuals who were Helicobacter pylori positive and negative.
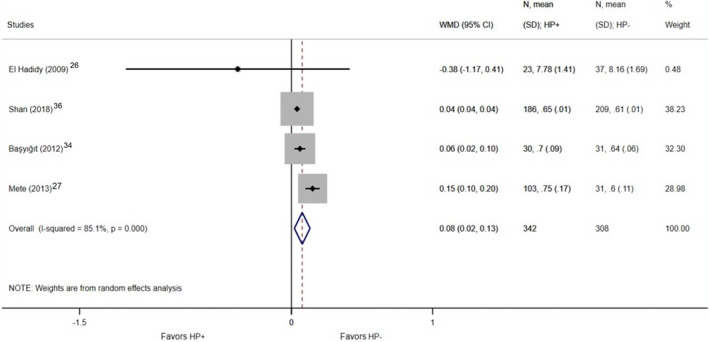
Black diamonds represent the weighted mean difference between the 2 groups we compared, and horizontal lines show the corresponding 95% CIs. Size of the gray squares reflects the weight of a particular study. The blue diamond is the overall or summary effect. The outer edges of the diamonds represent the CIs. HP indicates Helicobacter pylori; and WMD, weighted mean difference.
Figure 4. Forest plot of studies comparing left carotid intima‐media thickness between individuals who were Helicobacter pylori positive and negative.
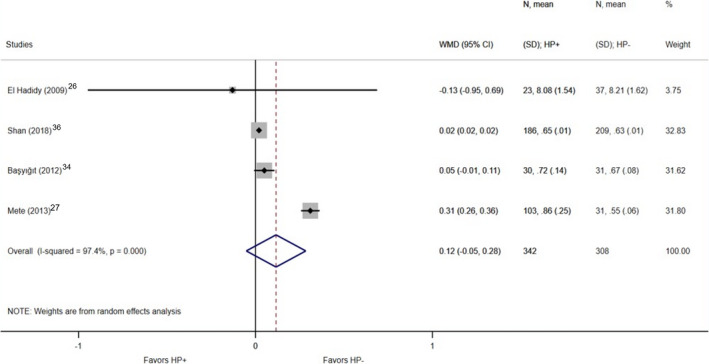
Black diamonds represent the weighted mean difference between the 2 groups we compared, and horizontal lines show the corresponding 95% CIs. Size of the gray squares reflects the weight of a particular study. The blue diamond is the overall or summary effect. The outer edges of the diamonds represent the CIs. HP indicates Helicobacter pylori; and WMD, weighted mean difference.
In case of the article by El Hadidy et al, 26 we faced several uncertainities: The values of CIMT were bigger with 1 decimal than in the other included articles regarding right and left CIMT; the definition of CIMT and the measurement area of the CIM is not reported, and it carries a high risk of bias. If such studies are detected, it is advisable to recalculate our meta‐analysis without them to see if this changes the interpretation of our results. 31 As for the influence diagnostics, based on all methods, in the case of the right carotid, the outliers, with higher values than the threshold value, are the articles by El Hadidy et al and Shan et al, 26 , 36 while in the case of left carotid, these are Mete et al and Shan et al. 27 , 36 Because of the small number of the included studies in the right and left CIMT analyses, only 1 article could be excluded at the same time. To keep it uniform and considering the above‐mentioned uncertainities, we decided to choose the same publication in both cases, which is the article by El Hadidy et al. 26 By sensitivity analysis and repeating the comparison without the data published by El Hadidy et al, 26 the significant difference was still detectable in the right carotid (319 patients who were H. pylori positive versus 271 patients who were H. pylori negative; right: WMD, 0.08 mm; 95% CI, 0.02–0.13; P=0.006; I2=89.5%; P<0.001; left: WMD, 0.13 mm; 95% CI, −0.05 to 0.30; P=0.153; I2=98.3%; P<0.001; Figure S1 through S4).
Meta‐Regression to Determine the Effect of Age, Geographic Location, and Detection Method
Meta‐regression could be performed if at least 10 articles were included in the specific groups. In the case of age, geographic location, and detection method, this condition is met among included individuals with published overall CIMT values. There were no significant regression of age (WMD, 0.0021; I2=91.12%; P=0.9033), geographic location (WMD, −0.0248; I2=93.84%; P=0.7326) and detection method (WMD, 0.0645; I2=91.11%; P=0.3183; Figure S5).
Diabetes, Age, Hypertension, and Laboratory Parameters of Included Individuals
There was no significant difference in the prevalence of diabetes in the 2 compared groups (OR, 1.15; 95% CI, 0.49–2.68; P=0.751; I2=0%; P=0.997; Figure S6A). 21 , 23 , 24 , 26 , 34 The mean age of individuals who were H. pylori positive did not differ from the mean age of the negative individuals (866 patients who were H. pylori positive versus 556 patients who were H. pylori negative; WMD, −0.35 years; 95% CI, −1.18 to 0.48; P=0.404; I2=0%; P=0.961; Figure S7). 4 , 20 , 21 , 22 , 23 , 24 , 27 , 34 , 35 , 37 These results correlates with the meta‐regression. There was no significant difference in the odds of patients diagnosed with hypertension in the 2 compared groups (OR, 0.91; 95% CI, 0.59–1.39; P=0.65; I2=0%; P=1; Figure S6B). 4 , 22 , 23 , 24 , 26 , 27 , 34 , 35 , 36 Laboratory parameters of TC, triglyceride, LDL, and HDL were compared from the listed studies. 20 , 21 , 22 , 23 , 26 , 27 , 34 , 37 The TC level was significantly higher in the positive group (634 patients who were H. pylori positive versus 428 patients who were H. pylori negative; WMD, 0.07 mmol/L; 95% CI, 0.01–0.13; P=0.017; I2=0%; P=0.826; Figure S8). No further significant differences were detected in case of laboratory parameters like triglyceride (664 patients who were H. pylori positive versus 459 patients who were H. pylori negative; WMD, 0.09 mmol/L; 95% CI, −0.01 to 0.19; P=0.088; I2=44.5%; P=0.063), LDL (664 patients who were H. pylori positive versus 459 patients who were H. pylori negative; WMD, 0.06 mmol/L; 95% CI, −0.00 to 0.13; P=0.058; I2=22.8%; P=0.233), or HDL (664 patients who were H. pylori positive versus 459 patients who were H. pylori negative; WMD, 0.09 mmol/L; 95% CI, −0.01 to 0.19; P=0.693; I2=88.5%; P<0.001; Figure S9 through 11).
Subgroup Analyses Based on Geographic Location
We performed subgroup analyses on overall CIMT for geographic localization. Three studies published data on 704 subjects from China; 377 were H. pylori positive and 327 negatives. 20 , 23 , 37 Four studies were from Turkey with 374 participants, 4 , 22 , 27 , 34 250 were infected and 124 were not infected. There were no significant differences between the CIMT of infected and noninfected subjects in neither Chinese (WMD, 0.09 mm; 95% CI, −0.01 to 0.19; P=0.094; I2=94.5%; P<0.001) nor Turkish studies (WMD, 0.06 mm; 95% CI, −0.04 to 0.16; P=0.259; I2=92.9%; P<0.001), if the individuals were compared with noninfected controls from the same country (Figure S12 and S13). These results correlate with the meta‐regression.
Even in the geographically uniform subgroup analyses, there was a considerable degree of remaining heterogeneity within subgroups.
Subgroup Analyses Based on H. pylori Detection Method
We performed subgroup analyses among individuals involved in the analysis of overall CIMT based on the detection method of the pathogen. In 3 studies, urea breath test identified 377 positive and 327 negative individuals. 20 , 23 , 37 Serology was used in 4 studies, identifying 577 positive and 242 negative individuals. 21 , 22 , 24 , 25 In the case of the urea breath test (WMD, 0.09 mm; 95% CI, −0.01 to 0.19; P=0.094; I2=94.5%; P<0.001) and serum ELISA (WMD, 0.02 mm; 95% CI, −0.03 to 0.07, P=0.456, I2=73.8%, P=0.002), there was no significant differences in the CIMTs (Figure S14 and S15). These results correlate with the meta‐regression.
Risk‐of‐Bias Assessment
The Quality in Prognosis Studies tool was applied for our primary outcomes, the overall, right, and left CIMT. The 6 main domains were scored as low, moderate, or high risk of bias. The overall risk of bias for overall CIMT was low in 10 studies, 4 , 20 , 21 , 22 , 23 , 24 , 27 , 34 , 35 , 37 while it was moderate in 1. 25 , 38 As for studies publishing data on right and left CIMT, in both cases, 3 studies carried low risk, 27 , 34 , 36 and 1 study carried a high risk of bias. 26 Details are shown in Figure 5 and Figure S16.
Figure 5. Result of risk‐of‐bias assessment for primary outcomes.
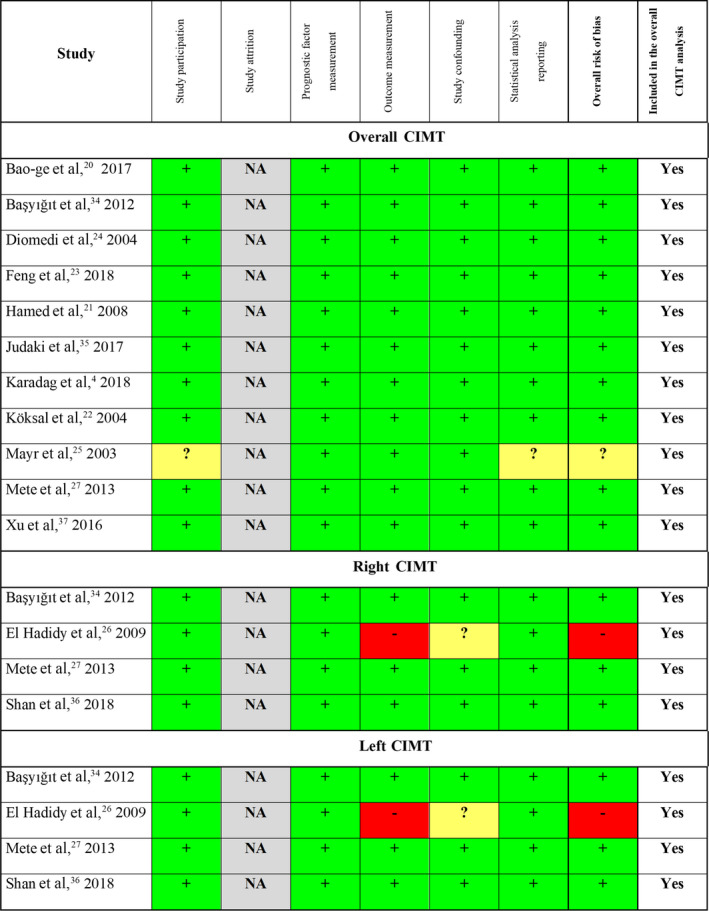
If all domains were deemed as low risk, the overall assessment was a low risk of bias (green, +). When a study carried a domain with high risk or at least 3 domains with moderate risk, the overall risk was defined as high (red, ‐). All other cases were rated as moderate (yellow, ?). CIMT indicates carotid intima‐media thickness; and NA, not applicable.
Publication Bias
The visual assessment of the funnel plot for overall CIMT showed relatively big study samples and small SE, suggesting that publication bias is unlikely. The Egger test revealed no small‐study effect (P=0.971). As for right and left CIMT, the sample numbers are low (Figure S17).
Discussion
Our meta‐analysis supported that H. pylori infection is associated with the overall CIMT. The thickening was more detectable in the right carotid artery. The substantial analyses found higher TC levels among infected patients. Still, there were no significant differences between the H. pylori–positive and –negative groups regarding the other investigated laboratory parameters (triglyceride, LDL, and HDL) influencing atherosclerosis.
In our analysis, the overall CIMT of the individuals who were H. pylori positive individuals was 0.07 mm bigger than in the negative group. According to the measurements on individuals with no cardiovascular risk factors by Jarauta et al, 39 the normal CIMT was found to be 0.59 to 0.95 mm in men, and 0.52 to 0.93 mm in women. Willeit et al 40 claim that the average of CIMT is 0.65 to 0.9 mm in adults, and the thickness is increasing by 0 to 0.04 mm/y. Intimal thickening and later atherosclerosis in areas of low and oscillatory shear stress are attributable to prolonged endothelial exposure. 41 A 3‐year study by Pessin et al 42 draw attention to the importance of the detection of silent carotid artery diseases, as 50% of their patients suffered moderate to severe carotid stroke without any warning clinical symptoms such as transient ischemic attack. A clinical trial scanning both carotid and femoral arteries of asymptomatic individuals found, that a future cardiovascular event can be predicted using arterial morphology classification and ultrasound arterial score. 43 Another study claims that patients with a thicker CIM should be examined for coronary artery lesions. 44 In 2008, the American Society of Echocardiography consensus statement concluded that the measurement of carotids could specify the cardiovascular disease risk assessment only with limitations in general clinical practice. 45 In addition, a recent guideline, published in 2016 by the European Society of Cardiology, did not recommend the screening of carotid arteries to estimate cardiovascular risk. 46
A significant difference was found when comparing the thickness of the right carotid between the H. pylori–positive and –negative groups. Both by inclusion and exclusion of the unclear data provided by El Hadidy, 26 CIM was 0.08 mm thicker among individuals who were H. pylori infected. We found no difference of CIMT between positive and negative participants in the subgroup analysis of the studies with detailed data on the left CIMT. Luo et al 47 examined the right and left carotid arteries separately. He found that the thickness of the right carotid artery is more likely to be associated with altered hemodynamic parameters, whereas the change in the left carotid thickening rather correlates with changes in biochemical indices. The anatomic difference can be the other explanation. 48 High shear stress in the left carotid artery results in greater hemodynamic stress in the left cerebral hemisphere. 49 According to a cohort study, the plaques in the right carotid artery were found to be more stable because of pronounced calcification, while the left carotid plaques were more vulnerable, often with intraplaque hemorrhage. 50 However, the Rotterdam Study suggests that the difference in recognizability can be responsible for the higher number of reported left‐sided clinical strokes and transient ischemic attacks. 51 As for cardiac relevance, both mean right and left CIMTs were significantly higher among individuals with coronary artery disease than in the control group. Furthermore, the thickness of the common carotid artery on both sides suggested a positive correlation to more severe coronary artery disease. 52
Presumably, several factors and mechanisms influence the thickening of the carotid arteries. According to the review of the major risk factors on CIMT, age, sex, hypertension, systolic blood pressure, smoking, body mass index, diabetes, dyslipidemia, impaired TC and LDL‐ and HDL‐cholesterol levels, and fasting glucose level seem to have the most important influence. Furthermore, biological markers were also found to be associated with CIMT. 53 Besides these, Libby et al 54 also noted alteration in the endothelium, triglyceride, and inflammation. Vijayvergiya et al, 55 going into the details of possible mechanisms in H. pylori–related atherosclerosis, identified the elevated level of cytokines in chronic H. pylori infection. Furthermore, their decrease after eradication was also observed. This suggests that H. pylori causing chronic inflammation might play a role in atherosclerotic plaque formation and endothelial dysfunction. Similarly, several studies found that H. pylori positivity was associated with dyslipidemia with higher TC, triglyceride, LDL, and apolipoprotein‐B, and lower HDL and apolipoprotein‐A levels, and reverse changes were observed after eradication. At the same time, other research could not show the effect on laboratory parameters.
Our meta‐analysis has some limitations, including small sample size and different diagnostic methods. All the included studies were retrospective, which have their limitations, and according to our risk‐of‐bias assessment, the presence of moderate and high‐risk domains was detected. Statistical heterogeneity could be explained by the clinical heterogeneity caused by slight differences in CIM definition and measurement, geographic distribution, and different detection methods. We performed subgroup analyses to reduce the heterogeneity and examine the causative role of the latter 2 factors on our primary outcomes. However, the heterogeneity remained high, which may limit the generalizability of the meta‐analysis. Our sample size may also limit the testing of the effects of multiple covariates. Lack of standardized imaging approaches and the inclusion of common carotid artery areas with plaques may result in bias as well. The inclusion of multiple groups from one study and pair formation may also limit our results. No publication bias was found.
As for basic research, further studies might give additional evidence on molecular changes induced by chronic inflammation such as H. pylori infection leading to plaque formation. Studies with high sample numbers in measurements of separate right and left CIMTs are warranted. By performing future clinical studies among patients who are H. pylori infected, we would have a more accurate view of this arising role.
In clinical practice, H. pylori testing might be considered in individuals with thicker CIM, especially in those with other risk factors of cerebrovascular or cardiovascular diseases. A recent meta‐analysis based on 100.667 participants’ data estimated that by reducing the progression of CIM thickening by 0.01, 0.02, 0.03 or 0.04 mm/y, the relative risk of cardiovascular disease would be 0.84 (0.75–0.93), 0.76 (0.67–0.85), 0.69 (0.59–0.79), or 0.63 (0.52–0.74), respectively. 40 On the other hand, the screening and eradication of H. pylori infection in the general population should not be fully discarded as a potential intervention to contribute to the risk reduction of future cerebrovascular and cardiovascular events. However, the inequality of the cost‐benefit ratio is a severe limitation of this approach, as the benefits are unlikely to outweigh the costs.
In conclusion, infection with H. pylori is associated with the thickening of the CIM, as it was found to be more prominent in individuals who were H. pylori positive. By understanding the molecular changes and performing large‐sample‐size randomized clinical trials, the pathomechanism could also be further clarified. The early screening and eradication of the bacteria in individuals with a thicker CIMT should be considered.
Sources of Funding
This work will be funded by the Economic Development and Innovation Operational Programme Grant (GINOP‐2.3.2‐15‐2016‐00048 ‐ STAY ALIVE and GINOP‐2.3.4‐15‐2020‐00010 Competence Center for Health Data Analysis, Data Utilisation and Smart Device and Technology Development at the University of Pécs), the New National Excellence Program of the Ministry for Innovation and Technology from the source of the National Research, Development and Innovation Fund (ÚNKP‐20‐3), and Tandem Funding of University of Pécs (granted to Dr Szabó, KA‐2020‐32).
Disclosures
None.
Supporting information
Tables S1–S3
Figures S1–S17
Acknowledgments
All authors provided critical input and approved the final form of the manuscript. Dr Simon: conceptualization, methodology, validation, investigation, and writing (original draft); Görbe: formal analysis and writing (review and editing); Dr Hegyi: writing (review and editing); Dr Szakó: conceptualization, methodology, validation, investigation, and supervision; Dr Oštarijaš: conceptualization, methodology, validation, and investigation; Dr Dembrovszky: conceptualization, methodology, validation, and supervision; Dr Kiss: conceptualization, methodology, validation, and writing (review and editing); Dr Czopf: writing (review and editing) and supervision; Dr Erőss: conceptualization, methodology, validation, and writing (review and editing); Dr Szabó: conceptualization, methodology, validation, writing (review and editing), and supervision.
Supplemental Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.022919
For Sources of Funding and Disclosures, see page 12.
References
- 1. Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, Malfertheiner P, Graham DY, Wong VWS, Wu JCY, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta‐analysis. Gastroenterology. 2017;153:420–429. doi: 10.1053/j.gastro.2017.04.022 [DOI] [PubMed] [Google Scholar]
- 2. Wang F, Meng W, Wang B, Qiao L. Helicobacter pylori‐induced gastric inflammation and gastric cancer. Cancer Lett. 2014;345:196–202. doi: 10.1016/j.canlet.2013.08.016 [DOI] [PubMed] [Google Scholar]
- 3. Zamani M, Ebrahimtabar F, Zamani V, Miller WH, Alizadeh‐Navaei R, Shokri‐Shirvani J, Derakhshan MH. Systematic review with meta‐analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol Ther. 2018;47:868–876. [DOI] [PubMed] [Google Scholar]
- 4. Karadag Z, Sehitoglu T, Cure MC, Rakici H, Ayvaz MA, Bedir R, Kizilkaya B, Şahin OZ, Cure E. Helicobacter pylori can be related to carotid intima‐media thickness, epicardial adipose tissue thickness and serum neutrophil gelatinase‐associated lipocalin (NGAL) levels. Bratisl Med J. 2018;119:302–307. doi: 10.4149/BLL_2018_057 [DOI] [PubMed] [Google Scholar]
- 5. Qu B, Qu T. Causes of changes in carotid intima‐media thickness: a literature review. Cardiovasc Ultrasound. 2015;13:46. doi: 10.1186/s12947-015-0041-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Whincup PH, Mendall MA, Perry IJ, Strachan DP, Walker M. Prospective relations between Helicobacter pylori infection, coronary heart disease, and stroke in middle aged men. Heart. 1996;75:568–572. doi: 10.1136/hrt.75.6.568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Doheim MF, Altaweel AA, Elgendy MG, Elshanbary AA, Dibas M, Ali A, Dahy TM, Sharaf AK, Hassan AE. Association between Helicobacter pylori infection and stroke: a meta‐analysis of 273,135 patients. J Neurol. 2020. doi: 10.1007/s00415-020-09933-x [DOI] [PubMed] [Google Scholar]
- 8. Wang ZW, Li Y, Huang LY, Guan QK, Xu DW, Zhou WK, Zhang XZ. Helicobacter pylori infection contributes to high risk of ischemic stroke: evidence from a meta‐analysis. J Neurol. 2012;259:2527–2537. doi: 10.1007/s00415-012-6558-7 [DOI] [PubMed] [Google Scholar]
- 9. Yu M, Zhang Y, Yang Z, Ding J, Xie C, Lu N. Association between Helicobacter pylori infection and stroke: a meta‐analysis of prospective observational studies. J Stroke Cerebrovasc Dis. 2014;23:2233–2239. doi: 10.1016/j.jstrokecerebrovasdis.2014.04.020 [DOI] [PubMed] [Google Scholar]
- 10. Wasay M, Jafri W, Khealani B, Azam I, Hussaini A. Helicobacter pylori gastritis and risk of ischaemic stroke. J Pak Med Assoc. 2008;58:368–370. [PubMed] [Google Scholar]
- 11. Fang Y, Fan C, Xie H. Effect of Helicobacter pylori infection on the risk of acute coronary syndrome: a systematic review and meta‐analysis. Medicine. 2019;98:e18348. doi: 10.1097/MD.0000000000018348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schöttker B, Adamu MA, Weck MN, Müller H, Brenner H. Helicobacter pylori infection, chronic atrophic gastritis and major cardiovascular events: a population‐based cohort study. Atherosclerosis. 2012;220:569–574. doi: 10.1016/j.atherosclerosis.2011.11.029 [DOI] [PubMed] [Google Scholar]
- 13. O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK Jr. Carotid‐artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular health study collaborative research group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103 [DOI] [PubMed] [Google Scholar]
- 14. Centurión OA. Carotid intima‐media thickness as a cardiovascular risk factor and imaging pathway of atherosclerosis. Crit Pathw Cardiol. 2016;15:152–160. doi: 10.1097/HPC.0000000000000087 [DOI] [PubMed] [Google Scholar]
- 15. Wang M, Sui J, Wang S, Wang X. Correlations of carotid intima‐media thickness with endothelial function and atherosclerosis degree in patients with type 2 diabetes mellitus. Clin Hemorheol Microcirc. 2019;72:431–439. doi: 10.3233/CH-180486 [DOI] [PubMed] [Google Scholar]
- 16. Lu J, Ma X, Shen Y, Wu Q, Wang R, Zhang L, Mo Y, Lu W, Zhu W, Bao Y, et al. Time in range is associated with carotid intima‐media thickness in type 2 diabetes. Diabetes Technol Ther. 2020;22:72–78. doi: 10.1089/dia.2019.0251 [DOI] [PubMed] [Google Scholar]
- 17. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org
- 19. McHugh ML. Interrater reliability: the kappa statistic. Biochem Med. 2012;22:276–282. doi: 10.11613/BM.2012.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bao‐Ge Q, Hui W, Yi‐Guo J, Ji‐Liang S, Zhong‐Dong W, Ya‐Fei W, Xing‐Hai H, Yuan‐Xun L, Jin‐Dun P, Guang‐Ying R. The correlation and risk factors between carotid intima‐media thickening and alcoholic liver disease coupled with helicobacter pylori infection. Sci Rep. 2017;7:43059. doi: 10.1038/srep43059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hamed SA, Amine NF, Galal GM, Helal SR, Tag El‐Din LM, Shawky OA, Ahmed EA, Abdel Rahman MS. Vascular risks and complications in diabetes mellitus: the role of Helicobacter pylori infection. J Stroke Cerebrovasc Dis. 2008;17:86–94. doi: 10.1016/j.jstrokecerebrovasdis.2007.10.006 [DOI] [PubMed] [Google Scholar]
- 22. Köksal A, Ekmekçi Y, Karadeniz Y, Köklü S, Apan T, Yilmaz M, Sezikli M, Unal B, Demirel T, Yildiz A. Helicobacter pylori seropositivity and atherosclerosis risk factors. Dig Dis. 2004;22:386–389. [DOI] [PubMed] [Google Scholar]
- 23. Feng L, Deng C, Li Y. Assessment of the relationship between carotid intima‐media thickening and early‐stage diabetic kidney disease coupled with Helicobacter pylori infection. Dis Markers. 2018;2018:3793768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Diomedi M, Pietroiusti A, Silvestrini M, Rizzato B, Cupini LM, Ferrante F, Magrini A, Bergamaschi A, Galante A, Bernardi G. Caga‐positive Helicobacter pylori strains may influence the natural history of atherosclerotic stroke. Neurology. 2004;63:800–804. doi: 10.1212/01.WNL.0000138025.82419.80 [DOI] [PubMed] [Google Scholar]
- 25. Mayr M, Kiechl S, Mendall MA, Willeit J, Wick G, Xu Q. Increased risk of atherosclerosis is confined to CagA‐positive Helicobacter pylori strains: prospective results from the Bruneck Study. Stroke. 2003;34:610–615. [DOI] [PubMed] [Google Scholar]
- 26. El Hadidy EHM, Abdul‐Aziz MY, Mokhtar A‐R, Abo El Ata MM, El Gwad SSA. Helicobacter pylori infection and vascular complications in patients with type 2 diabetes mellitus. J Taibah Univ Med Sci. 2009;4:62–72. doi: 10.1016/S1658-3612(09)70082-4 [DOI] [Google Scholar]
- 27. Mete R, Oran M, Alpsoy S, Gunes H, Tulubas F, Turan C, Topcu B, Aydin M, Yildirim O. Carotid intima‐media thickness and serum paraoxonase‐1 activity in patients with Helicobacter pylori. Eur Rev Med Pharmacol Sci. 2013;17:2884–2889. [PubMed] [Google Scholar]
- 28. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 30. Harrer M, Cuijpers P, Furukawa TA, Ebert DD. Doing Meta‐Analysis with R: A Hands‐On Guide. Boca Raton, FL and London: Chapmann & Hall/CRC Press; 2021: ISBN 978‐0‐367‐61007‐4. [Google Scholar]
- 31. Viechtbauer W. Conducting meta‐analyses in R with the metafor package. J Stat Softw. 2010;1:2010. [Google Scholar]
- 32. Balduzzi S, Rücker G, Schwarzer G. How to perform a meta‐analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22:153–160. doi: 10.1136/ebmental-2019-300117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158:280–286. doi: 10.7326/0003-4819-158-4-201302190-00009 [DOI] [PubMed] [Google Scholar]
- 34. Başyığıt S, Akbaş H, Süleymanlar İ, Kemaloğlu D, Koç S, Süleymanlar G. The assessment of carotid intima‐media thickness, lipid profiles and oxidative stress markers in Helicobacter pylori‐positive subjects. Turk J Gastroenterol. 2012;23:646–651. doi: 10.4318/tjg.2012.0441 [DOI] [PubMed] [Google Scholar]
- 35. Judaki A, Norozi S, Ahmadi MRH, Ghavam SM, Asadollahi K, Rahmani A. Flow mediated dilation and carotid intima media thickness in patients with chronic gastritis associated with Helicobacter pylori infection. Arq Gastroenterol. 2017;54:300–304. doi: 10.1590/s0004-2803.201700000-39 [DOI] [PubMed] [Google Scholar]
- 36. Shan J, Bai X, Han L, Yuan Y, Yang J, Sun X. Association between atherosclerosis and gastric biomarkers concerning Helicobacter pylori infection in a Chinese healthy population. Exp Gerontol. 2018;112:97–102. doi: 10.1016/j.exger.2018.09.009 [DOI] [PubMed] [Google Scholar]
- 37. Xu Y, Wang Q, Liu Y, Cui R, Lu K, Zhao Y. Association between Helicobacter pylori infection and carotid atherosclerosis in patients with vascular dementia. J Neurol Sci. 2016;362:73–77. doi: 10.1016/j.jns.2016.01.025 [DOI] [PubMed] [Google Scholar]
- 38. Glurich I, Grossi S, Albini B, Ho A, Shah R, Zeid M, Baumann H, Genco RJ, De Nardin E. Systemic inflammation in cardiovascular and periodontal disease: comparative study. Clin Diagn Lab Immunol. 2002;9:425–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jarauta E, Mateo‐Gallego R, Bea A, Burillo E, Calmarza P, Civeira F. Carotid intima‐media thickness in subjects with no cardiovascular risk factors. Rev Esp Cardiol. 2010;63:97–102. [DOI] [PubMed] [Google Scholar]
- 40. Willeit P, Tschiderer L, Allara E, Reuber K, Seekircher L, Gao LU, Liao X, Lonn E, Gerstein HC, Yusuf S, et al. Carotid intima‐media thickness progression as surrogate marker for cardiovascular risk: meta‐analysis of 119 clinical trials involving 100 667 patients. Circulation. 2020;142:621–642. doi: 10.1161/CIRCULATIONAHA.120.046361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gokaldas R, Singh M, Lal S, Benenstein RJ, Sahni R. Carotid stenosis: from diagnosis to management, where do we stand? Curr Atheroscler Rep. 2015;17:480. doi: 10.1007/s11883-014-0480-7 [DOI] [PubMed] [Google Scholar]
- 42. Pessin MS, Hinton RC, Davis KR, Duncan GW, Roberson GH, Ackerman RH, Mohr JP. Mechanisms of acute carotid stroke. Ann Neurol. 1979;6:245–252. doi: 10.1002/ana.410060311 [DOI] [PubMed] [Google Scholar]
- 43. Belcaro G, Nicolaides AN, Laurora G, Cesarone MR, De Sanctis M, Incandela L, Barsotti A. Ultrasound morphology classification of the arterial wall and cardiovascular events in a 6‐year follow‐up study. Arterioscler Thromb Vasc Biol. 1996;16:851–856. doi: 10.1161/01.ATV.16.7.851 [DOI] [PubMed] [Google Scholar]
- 44. Kotsis VT, Pitiriga V, Stabouli SV, Papamichael CM, Toumanidis ST, Rokas SG, Zakopoulos NA. Carotid artery intima‐media thickness could predict the presence of coronary artery lesions. Am J Hypertens. 2005;18:601–606. doi: 10.1016/j.amjhyper.2004.11.019 [DOI] [PubMed] [Google Scholar]
- 45. Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, Najjar SS, Rembold CM, Post WS. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography carotid intima‐media thickness task force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21:93–111; quiz 189–190. doi: 10.1016/j.echo.2007.11.011 [DOI] [PubMed] [Google Scholar]
- 46. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney M‐T, Corrà U, Cosyns B, Deaton C, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the sixth joint task force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37:2315–2381. doi: 10.1093/eurheartj/ehw106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Luo X, Yang Y, Cao T, Li Z. Differences in left and right carotid intima‐media thickness and the associated risk factors. Clin Radiol. 2011;66:393–398. doi: 10.1016/j.crad.2010.12.002 [DOI] [PubMed] [Google Scholar]
- 48. Rodríguez Hernández SA, Kroon AA, van Boxtel MP, Mess WH, Lodder J, Jolles J, de Leeuw PW. Is there a side predilection for cerebrovascular disease? Hypertension. 2003;42:56–60. doi: 10.1161/01.HYP.0000077983.66161.6F [DOI] [PubMed] [Google Scholar]
- 49. Hedna VS, Bodhit AN, Ansari S, Falchook AD, Stead L, Heilman KM, Waters MF. Hemispheric differences in ischemic stroke: is left‐hemisphere stroke more common? J Clin Neurol. 2013;9:97–102. doi: 10.3988/jcn.2013.9.2.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Selwaness M, van den Bouwhuijsen Q, van Onkelen RS, Hofman A, Franco OH, van der Lugt A, Wentzel JJ, Vernooij M. Atherosclerotic plaque in the left carotid artery is more vulnerable than in the right. Stroke. 2014;45:3226–3230. doi: 10.1161/STROKEAHA.114.005202 [DOI] [PubMed] [Google Scholar]
- 51. Portegies ML, Selwaness M, Hofman A, Koudstaal PJ, Vernooij MW, Ikram MA. Left‐sided strokes are more often recognized than right‐sided strokes: the Rotterdam Study. Stroke. 2015;46:252–254. doi: 10.1161/STROKEAHA.114.007385 [DOI] [PubMed] [Google Scholar]
- 52. Azarkish K, Mahmoudi K, Mohammadifar M, Ghajarzadeh M. Mean right and left carotid intima‐media thickness measures in cases with/without coronary artery disease. Acta Med Iran. 2014;52:884–888. [PubMed] [Google Scholar]
- 53. Nezu T, Hosomi N, Aoki S, Matsumoto M. Carotid intima‐media thickness for atherosclerosis. J Atheroscler Thromb. 2016;23:18–31. doi: 10.5551/jat.31989 [DOI] [PubMed] [Google Scholar]
- 54. Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, Tokgözoğlu L, Lewis EF. Atherosclerosis. Nat Rev Dis Primers. 2019;5:56. doi: 10.1038/s41572-019-0106-z [DOI] [PubMed] [Google Scholar]
- 55. Vijayvergiya R, Vadivelu R. Role of Helicobacter pylori infection in pathogenesis of atherosclerosis. World J Cardiol. 2015;7:134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3
Figures S1–S17


