Abstract
Background
A systemic proinflammatory state plays a central role in the development of heart failure with preserved ejection fraction. Low‐level transcutaneous vagus nerve stimulation suppresses inflammation in humans. We conducted a sham‐controlled, double‐blind, randomized clinical trial to examine the effect of chronic low‐level transcutaneous vagus nerve stimulation on cardiac function, exercise capacity, and inflammation in patients with heart failure with preserved ejection fraction.
Methods and Results
Patients with heart failure with preserved ejection fraction and at least 2 additional comorbidities (obesity, diabetes, hypertension, or age ≥65 years) were randomized to either active (tragus) or sham (earlobe) low‐level transcutaneous vagus nerve stimulation (20 Hz, 1 mA below discomfort threshold), for 1 hour daily for 3 months. Echocardiography, 6‐minute walk test, quality of life, and serum cytokines were assessed at baseline and 3 months. Fifty‐two patients (mean age 70.4±9.2 years; 70% female) were included (active, n=26; sham, n=26). Baseline characteristics were balanced between the 2 arms. Adherence to the protocol of daily stimulation was >90% in both arms (P>0.05). While the early mitral inflow Doppler velocity to the early diastolic mitral annulus velocity ratio did not differ between groups, global longitudinal strain and tumor necrosis factor‐α levels at 3 months were significantly improved in the active compared with the sham arm (−18.6%±2.5% versus −16.0%±2.4%, P=0.002; 8.9±2.8 pg/mL versus 11.3±2.9 pg/mL, P=0.007, respectively). The reduction in tumor necrosis factor‐α levels correlated with global longitudinal strain improvement (r=−0.73, P=0.001). Quality of life was better in the active arm. No device‐related side effects were observed.
Conclusions
Neuromodulation with low‐level transcutaneous vagus nerve stimulation over 3 months resulted in a significant improvement in global longitudinal strain, inflammatory cytokines, and quality of life in patients with heart failure with preserved ejection fraction.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03327649.
Keywords: autonomic modulation, heart failure with preserved ejection fraction, inflammation
Subject Categories: Heart Failure, Autonomic Nervous System
Nonstandard Abbreviations and Acronyms
- E/A
ratio of early to late mitral inflow Doppler velocity
- E/e′
early mitral inflow Doppler velocity to the early diastolic mitral annulus velocity
- e′
early diastolic mitral annulus velocity
- GLS
global longitudinal strain
- HFpEF
heart failure with preserved ejection fraction
- IL
interleukin
- LLTS
low‐level transcutaneous vagus nerve stimulation
Clinical Perspective
What Is New?
We conducted a sham‐controlled, double‐blind, randomized clinical trial to examine the effect of chronic low‐level transcutaneous vagus nerve stimulation on cardiac function, exercise capacity, and inflammation in a subgroup of patients with heart failure with preserved ejection fraction with a predominantly inflammatory‐metabolic phenotype.
In this patient population, neuromodulation with low‐level transcutaneous vagus nerve stimulation over 3 months resulted in a significant improvement in global longitudinal strain, inflammatory cytokines, and quality of life.
What Are the Clinical Implications?
Our results support the emerging paradigm of noninvasive neuromodulation to treat selected patients with heart failure with preserved ejection fraction and provide the basis for further randomized trials.
The long‐term adherence and efficacy of this approach remain to be determined in future studies.
Heart failure with preserved ejection fraction (HFpEF), one of the most vexing cardiovascular conditions, is a heterogeneous disease with distinct phenotypes, with differential response to therapy. 1 , 2 Notably, a distinct phenotype of HFpEF, associated with diabetes, obesity, and inflammation has been described. 1 , 2 , 3 In fact, recent animal and human studies suggest that a systemic proinflammatory state, produced by comorbidities, including diabetes, obesity, and aging, plays a central role in the development of HFpEF. 4 , 5 , 6 , 7 Therefore, attenuating the proinflammatory state is an attractive therapeutic target for a subgroup of patients with HFpEF.
Vagus nerve stimulation exerts prominent anti‐inflammatory effects in multiple experimental models of systemic inflammation and sepsis, 8 , 9 , 10 , 11 as well as in humans with rheumatoid arthritis 12 and atrial fibrillation undergoing cardiac surgery. 13 Moreover, low‐level transcutaneous vagus nerve stimulation (LLTS), delivered at the tragus of the ear, where the auricular branch of the vagus nerve is located, 14 for just 1 hour, significantly suppressed atrial fibrillation and decreased systemic inflammatory cytokines. 15 , 16 Importantly, we have recently shown that LLTS ameliorated diastolic dysfunction in a well‐established animal model of HFpEF 17 and acutely improved left ventricular (LV) strain in humans. 18 However, the chronic effects of LLTS in patients with HFpEF remain unknown. In this randomized, sham‐controlled, double‐blind study, we examined the effect of daily, intermittent LLTS for 3 months on echocardiographic markers, exercise capacity, and inflammatory cytokines relative to sham stimulation, in patients with predominantly inflammatory‐metabolic HFpEF phenotype. 3
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population
Patients with HFpEF, defined according to recent guidelines 19 as signs and symptoms of heart failure, LV ejection fraction ≥40%, brain natriuretic peptide ≥35 pg/mL, and echocardiographic evidence of diastolic dysfunction (left atrial volume index ≥34 mL/m2, early mitral inflow Doppler velocity to the early diastolic mitral annulus velocity [E/e′] ratio ≥13, and early diastolic mitral annulus velocity [e’] <9 cm/s), and at least 2 of the following 4 comorbidities: (1) age≥65, (2) diabetes, (3) hypertension, and (4) obesity (defined as body mass index ≥30 kg/m2), were eligible for enrollment in the study. The rationale for inclusion of patients with these comorbidities was that by selecting a population with prominent proinflammatory state (“inflammatory‐metabolic HFpEF phenotype” 3 ), the effectiveness of LLTS would be enhanced. Patients were excluded if they had any of the following: LV ejection fraction <40%, significant valvular disorder (ie, prosthetic valve or hemodynamically significant valvular diseases), recent (<6 months) stroke, myocardial infarction or hospitalization for heart failure, severe heart failure (class IV), end stage kidney disease, recurrent vasovagal syncope, history of vagotomy, pregnancy and sick sinus syndrome, and second or third degree atrioventricular block (without a pacemaker). The study was approved by the University of Oklahoma Health Sciences Center Institutional Review Board, and all patients provided informed consent before enrollment in the study.
Randomization and Study Procedures
After informed consent, patients were randomly assigned (1:1) to active or sham LLTS, stratified by sex. The randomization sequence was created using randomly chosen block sizes of 4 or 6 and was implemented through the online REDCap data system. The Parasym device (Parasym, London, UK) was used to provide stimulation. In the active group, the ear clip electrode was attached to tragus, while in the sham group, it was attached to the ear lobe, which is devoid of vagal innervation, 14 as previously described (Figure 1). 16 The stimulation settings included a pulse width of 200 μs and a pulse frequency of 20 Hz, similar to our prior study in patients with atrial fibrillation. 16 The stimulation amplitude was individually titrated to 1 mA below the discomfort threshold. Stimulation was applied for 1 hour daily for 3 months by the patients themselves, after individual training. Patients were requested to keep a daily log with the time and duration of stimulation, amplitude settings, and any comments related to each daily session. The device is also equipped with a treatment time recorder, which provides the cumulative number of hours used for stimulation, which was correlated with the daily log, as an objective measure of adherence to the stimulation protocol. In patients with pacemakers, we tested the interaction between stimulation and the pacemaker before enrollment in the study and did not observe any interaction (no stimulation artifact was seen on the pacemaker). Patients and investigators collecting study measurements were blinded to the treatment allocation to reduce bias. To achieve blinding of patients, the site of active stimulation was not revealed to them. The clinical coordinator, who did collect any study measurements, was unblinded to treatment allocation and instructed the patients on the proper use of the device.
Figure 1. Device and location of active and sham stimulation.
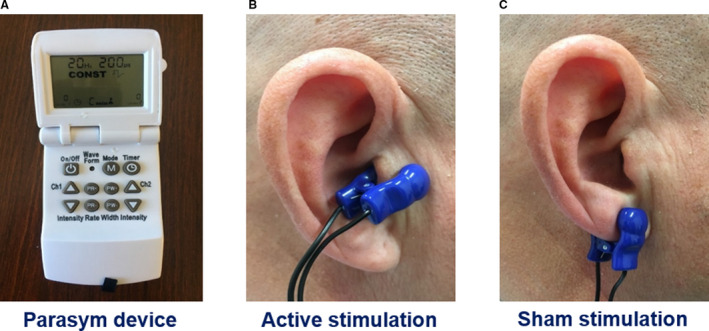
A, The Parasym device was used for stimulation. B, For active stimulation, the ear clip was attached to the tragus, which is innervated by the auricular branch of the vagus nerve. C, For sham control stimulation, the ear clip was attached to the earlobe, which is devoid of vagal innervation.
Echocardiography was performed at baseline and at 3 months to assess cardiac function (Acuson SC2000, Siemens). Two‐dimensional long‐axis and short‐axis LV images were obtained and pulse‐wave Doppler spectra of mitral inflow (E and A waves) and mitral annulus tissue Doppler spectra were recorded. LV longitudinal and circumferential strain was obtained off‐line using a speckle‐tracking algorithm (Acuson SC2000 eSie VVI). A 6‐minute walk test (a well‐validated measure of exercise capacity in heart failure) 20 was performed at baseline and 3 months. Quality of life was assessed by the Minnesota Living with Heart Failure Questionnaire 21 at the respective time points. To ensure rigor and avoid bias, participants were encouraged to return for the 3‐month posttreatment assessment regardless of their adherence status.
Blood samples (10 mL) were collected at baseline and at 3 months for cytokine measurement. Patients’ serum was saved frozen and processed in batches of 10 to 12. The investigators performing the cytokine assays were blinded to group assignment. Inflammatory cytokines, including tumor necrosis factor (TNF)‐α and interleukin (IL)‐8, were measured using commercially available multiplex assays (Ella, R&D systems).
Outcomes
The co‐primary outcomes at 3 months were (1) the ratio of the early mitral inflow Doppler velocity to the early diastolic mitral annulus velocity (E/e′) and (2) global longitudinal strain (GLS). Secondary outcomes included other echocardiographic parameters (early diastolic mitral annulus velocity [e’], left atrial volume index, tricuspid regurgitation Doppler velocity, ratio of early to late mitral inflow Doppler velocity [E/A], global circumferential strain), 6‐minute walk distance, quality of life (assessed by the Minnesota Living with Heart Failure Questionnaire, 21 score 0 to 105, higher scores reflect worse quality of life), brain natriuretic peptide levels, and serum cytokines. All parameters were assessed by investigators who were blinded to treatment assignment. Clinical outcomes and adverse events were documented and then evaluated by an independent safety committee.
Statistical Analysis
Categorical data are presented as percentages and continuous data as mean±SD. Baseline continuous data were compared between groups using Student t test, while categorical data were compared between groups using Fisher exact test. Continuous outcomes were compared between groups using a mixed linear model, with 2 time points (baseline, 3 months) and 3 terms included in the model (group effect, time effect, and group by time interaction). Significant interactions were followed by time trend analyses stratified by intervention group. Logarithmic transformation using the natural logarithm was used as appropriate to satisfy modeling assumptions. Analyses were based on the intention‐to‐treat principle. Linear regression was used to investigate the association between the change in GLS, and the change in TNF‐α and Pearson correlation coefficient with 95% CIs were calculated. Statistical significance was declared at 0.025 for the 2 co‐primary outcomes and at P<0.05 for all other data. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
We initially calculated that a sample size of 72 patients would provide >80% power to detect an absolute difference of 2.5 in the E/e′ ratio between the 2 groups at a 2‐sided alpha level of 0.025 (adjusted for 2 co‐primary outcomes), assuming a baseline mean±SD E/e′ ratio 11.1±4.4 22 and a 10% dropout rate. The same sample size, under similar assumptions and a baseline GLS 16.1±2.2, 22 would provide >90% power to detect an absolute 1.8% difference in GLS between the 2 groups. 18
In October 2020, because of a slower than expected enrollment rate, which was largely because of COVID‐19, the investigators, in collaboration with the data and safety monitoring board, reviewed the baseline data and original power calculations, and recalculated the sample size. Based on the revised power calculations, a sample size of 52 patients provided at least 80% power to detect a 2.5 absolute difference in the E/e′ ratio and a 1.8% absolute difference in GLS between the 2 groups, at a 2‐sided alpha level of 0.025.
Results
Study Population
Between January 2018 and September 2020, 132 patients were screened for eligibility and 52 were enrolled in the study. Among the 52 patients enrolled in the study, 26 (50%) were randomly assigned to LLTS and 26 (50%) to sham (Figure 2). Two patients in each group withdrew from the study. Therefore, a total of 48 patients with complete data were included in the final analysis. The baseline characteristics of the patients were balanced between the 2 groups (Table 1). The majority of the patients were elderly women, with prevalent hypertension, diabetes, and obesity. No patient crossed over to the other group.
Figure 2. Screening and enrollment of patients.
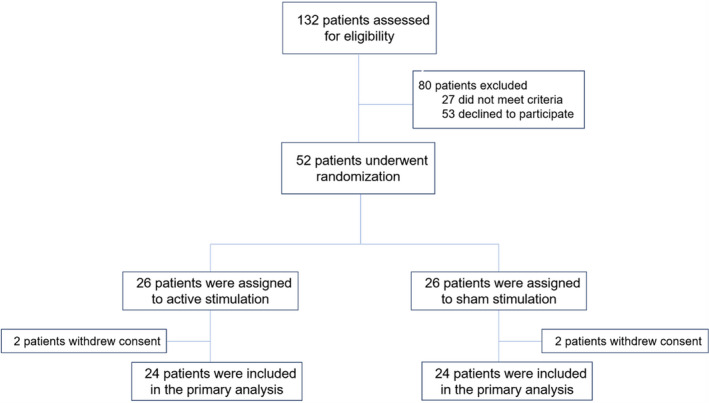
Table 1.
Baseline Characteristics of the Study Population
| Characteristic | Active (n=24) | Sham (n=24) | P value |
|---|---|---|---|
| Age, y | 69.8±8.8 | 70.3±9.1 | 0.84 |
| Female sex, n (%) | 16 (67) | 16 (67) | >0.999 |
| Race, n (%) | 0.94 | ||
| Non‐White* | 5 (21) | 4 (17) | |
| White | 19 (79) | 20 (83) | |
| Body mass index, kg/m2 | 34.5±7.4 | 37.2±8.2 | 0.26 |
| New York Heart Association class, n (%) | 0.76 | ||
| Class I or II | 12 (50) | 13 (44) | |
| Class III | 12 (50) | 11 (56) | |
| Atrial fibrillation, n (%) | 15 (63) | 13 (54) | 0.54 |
| Coronary artery disease, n (%) | 5 (21) | 10 (42) | 0.18 |
| Diabetes, n (%) | 10 (42) | 11 (46) | 0.76 |
| Sleep apnea, n (%) | 14 (58) | 14 (58) | >0.999 |
| Hypertension, n (%) | 23 (96) | 23 (96) | >0.999 |
| Sick sinus syndrome, n (%) | 8 (33) | 6 (25) | 0.75 |
| β‐Blockers, n (%) | 19 (79) | 19 (79) | >0.999 |
| ACE inhibitors/ARBs, n (%) | 15 (63) | 16 (67) | 0.75 |
| Calcium channel blockers, n (%) | 6 (25) | 9 (38) | 0.48 |
| Statins, n (%) | 19 (79) | 14 (58) | 0.17 |
| Spironolactone, n (%) | 4 (17) | 3 (13) | 0.65 |
| Loop diuretics, n (%) | 19 (79) | 20 (83) | 0.78 |
| Systolic blood pressure, mm Hg | 135.4±17.1 | 134.8±25.2 | 0.94 |
| Diastolic blood pressure, mm Hg | 72.8±8.9 | 73.3±9.4 | 0.88 |
| Heart rate, bpm | 70.6±10.6 | 71.9±11.7 | 0.40 |
| Left ventricular ejection fraction, % | 57.5±8.4 | 58.6±8.5 | 0.48 |
| Sodium, mEq/L | 140.6±2.5 | 140.1±2.1 | 0.75 |
| Potassium, mEq/L | 4.1±0.4 | 4.0±0.3 | 0.77 |
| Creatinine, mg/dL | 1.2±0.6 | 1.3±0.9 | 0.86 |
| Hemoglobin, g/dL | 11.7±1.3 | 11.9±1.7 | 0.69 |
ACE indicates angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; and bpm, beats per minute.
All non‐White were Black, except one who was Pacific islander.
Adherence to the protocol of daily stimulation, defined as ≤4 sessions missed on average per month, was 92% at 3 months in both groups (P=1.0). The average stimulation amplitude was 22.9±13.4 mA and 23.0±15.2 mA in the active and sham group, respectively (P=0.98). There were no differences in blood pressure or heart rate between the active and sham group during follow‐up (137.8±16.5 mm Hg versus 134.9±16.1 mm Hg, respectively, P=0.65 and 74.7±8.6 bpm versus 72.1±7.7 bpm, respectively, P=0.38).
Co‐primary Outcomes
The outcomes of the study are summarized in Table 2. There were no baseline differences between the 2 groups. At 3 months, the E/e′ ratio was not significantly different between the 2 groups (9.9±1.5 versus 10.5±1.5, P=0.53, in the LLTS and sham groups, respectively; Figure 3A). However, GLS was significantly improved with active therapy over follow‐up and it was significantly better than the sham group at 3 months (−18.6%±2.5% versus −16.0%±2.4%, respectively, P=0.02; Figure 3B).
Table 2.
Primary and Secondary Outcomes at 3 months
| Variable | Active | Sham | P value (active vs sham) | ||||
|---|---|---|---|---|---|---|---|
| Baseline | 3 months | P value (Δ) | Baseline | 3 months | P value (Δ) | ||
| Co‐primary outcomes | |||||||
| E/e′, average | 10.4±1.4 | 9.9±1.5 | 0.23 | 10.6±1.4 | 10.4±1.5 | 0.65 | 0.53 |
| Global longitudinal strain, % | −16.8±2.2 | −18.6±2.5 | 0.01* | −17.5±2.3 | −16.1±2.5 | 0.06 | 0.02* |
| Secondary outcomes: echocardiography | |||||||
| e’, average, cm/s | 8.5±1.4 | 9.6±1.5 | 0.01* | 8.7±1.6 | 8.8±1.7 | 0.83 | 0.09 |
| E/A | 1.3±0.5 | 1.3±0.6 | 0.99 | 1.3±0.7 | 1.2±0.8 | 0.64 | 0.56 |
| Left ventricular ejection fraction, % | 57.5±8.4 | 61.9±9.0 | 0.09 | 58.6±8.5 | 58.3±8.8 | 0.98 | 0.13 |
| Left atrial volume index, mL/m2 | 33.2±5.4 | 30.2±6.1 | 0.08 | 32.4±5.3 | 33.0±6.3 | 0.72 | 0.26 |
| Tricuspid regurgitation velocity, m/s | 2.5±0.3 | 2.6±0.4 | 0.33 | 2.5±0.3 | 2.5±0.4 | 0.99 | 0.55 |
| Global circumferential strain, % | −22.6±2.9 | −23.9±3.2 | 0.14 | −24.5±2.5 | −21.5±2.8 | 0.003* | 0.008* |
| Secondary outcomes: functional capacity and quality of life | |||||||
| 6‐min walk distance, m | 377.6±138.8 | 352.7±158.8 | 0.56 | 350.7±120.4 | 326.9±140.4 | 0.52 | 0.63 |
| MLHFQ score | 46.3±14.0 | 23.8±14.8 | 0.001* | 46.0±13.2 | 36.6±14.8 | 0.02* | 0.01* |
| Secondary outcomes: serum biomarkers | |||||||
| Tumor necrosis factor‐α, pg/mL | 10.2±2.1 | 8.7±2.2 | 0.01* | 10.3±2.4 | 11.3±2.5 | 0.16 | 0.001* |
| IL‐8, pg/mL | 19.6±3.6 | 8.4±4.5 | 0.001* | 17.0±3.6 | 21.7±4.0 | 0.002* | 0.005* |
| Brain natriuretic peptide, ng/mL | 170.7±59.4 | 178.5±51.3 | 0.61 | 167.0±48.3 | 189.0±49.2 | 0.12 | 0.70 |
e′ indicates early diastolic mitral annulus velocity; E/e′, early mitral inflow Doppler velocity to the early diastolic mitral annulus velocity; and MLHFQ indicates Minnesota Living with Heart Failure Questionnaire.
Denotes statistically significant differences.
Figure 3. Effect of treatment on the co‐primary outcomes.
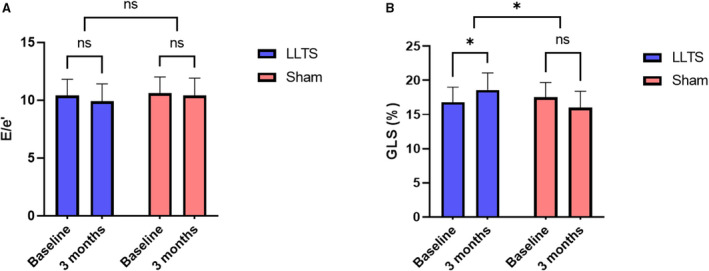
A, Changes in the ratio of the early mitral inflow Doppler velocity to the early diastolic mitral annulus velocity (E/e′). B, Changes in global longitudinal strain (GLS). The absolute values of GLS are plotted. LLTS indicates low‐level transcutaneous vagus nerve stimulation; and ns, nonsignificant. *P<0.05.
Secondary Outcomes
Echocardiographic markers of diastolic dysfunction, including e’ velocity, E/A ratio, left atrial volume index, and tricuspid regurgitation velocity, were not significantly different between the 2 groups at 3 months (Table 2). Six‐minute walk distance did not differ between the 2 groups at 3 months (352.7±158.8 m versus 326.9±140.4 m, P=0.63, in the LLTS and sham groups, respectively; Figure 4A). On the contrary, quality of life, as assessed by the Minnesota Living with Heart Failure Questionnaire, although improved in both groups over time, was significantly better in the LLTS group compared with the sham group at 3 months (23.8±14.8 versus 36.6±14.8, respectively, P=0.01; Figure 4B). Inflammatory cytokines, including TNF‐α and IL‐8, were significantly lower in the LLTS group compared with the sham group at 3 months (Figure 5). Brain natriuretic peptide did not differ significantly between the 2 groups.
Figure 4. Changes in functional outcomes during the 3 months of treatment.
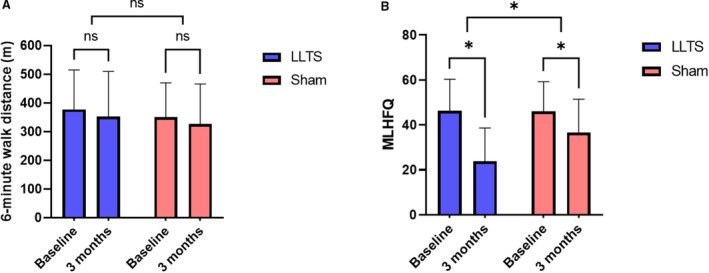
A, 6‐minute walk distance. B, Minnesota Living with Heart Failure Questionnaire (MLHFQ) score. LLTS indicates low‐level transcutaneous vagus nerve stimulation; and ns, nonsignificant. *P<0.05.
Figure 5. Changes in inflammatory biomarkers during the 3 months of treatment.
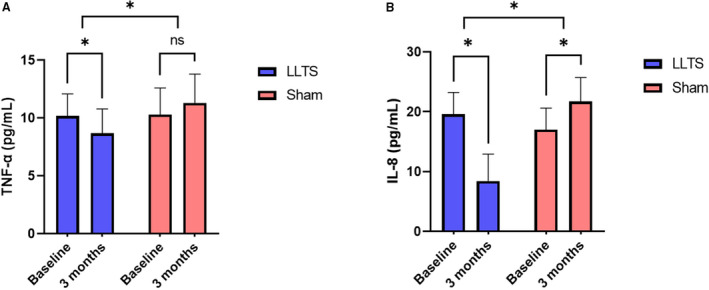
A, Tumor necrosis factor (TNF)‐α. B, Interleukin (IL)‐8. LLTS indicates low‐level transcutaneous vagus nerve stimulation; and ns, nonsignificant. *P<0.05.
Exploratory Analyses
We performed an exploratory analysis to examine the association between the change in inflammatory cytokines and the change in GLS. The interval changes in TNF‐α levels were associated inversely with the changes in GLS (r=−0.73 [95% CIs, −0.86 to −0.55], P=0.001; Figure 6), suggesting that a decrease in TNF‐α levels correlated strongly with an increase in the absolute GLS values.
Figure 6. Linear association between the interval change of TNF‐α and the change in absolute GLS.
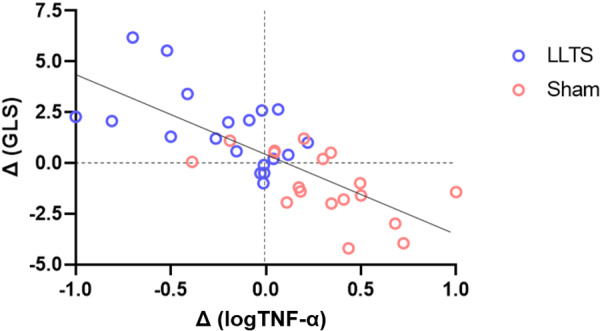
Logarithmic transformation of TNF‐α was performed to satisfy the modeling assumptions. The association was significant, with 54% of the variation in the change in GLS being explained by the change in TNF‐α levels (r=−0.73, R2=0.54, P=0.001). GLS indicates global longitudinal strain; LLTS, low‐level transcutaneous vagus nerve stimulation; and TNF‐α, tumor necrosis factor α.
Clinical Outcomes
There were no deaths during the course of the study. Two patients (1 in each group) were hospitalized for persistent atrial fibrillation and underwent cardioversion. One patient in the active group and 2 patients in the sham group were hospitalized for heart failure (P=0.99). There were no other device‐related adverse events.
Discussion
Currently, no pharmacological intervention has been shown to improve morbidity and mortality in HFpEF. 20 , 23 In this randomized, double‐blind, sham‐controlled trial, we show for the first time that noninvasive neuromodulation with LLTS improved LV strain, inflammatory biomarkers, and quality of life in patients with HFpEF. These results are consistent with prior studies showing that LLTS improved cardiac function in animals with HFpEF 17 and humans with diastolic dysfunction. 18 The importance of these findings is highlighted by recent evidence indicating that LV systolic function, as assessed by LV strain, is abnormal in patients with HFpEF, when compared with patients with hypertension without HFpEF. 24 Given that reduced LV strain has been associated with adverse clinical outcomes irrespective of LV ejection fraction, 25 it is possible that improvement in LV strain will result in improved clinical outcomes. Our results provide the basis for the design of further randomized clinical trials to evaluate the long‐term efficacy of noninvasive neuromodulation on clinical outcomes in patients with HFpEF.
Contrary to our hypothesis, we did not observe any effect of LLTS on diastolic dysfunction. This is possibly because of the existence of multiple mechanisms that control myocardial stiffness, the substrate of diastolic dysfunction, including titin hypophosphorylation and deficient unfolding protein response, which in turn is caused by systemic inflammation triggering expression of inducible nitric oxide synthase in cardiomyocytes. 26 The degree to which each of these mechanisms contributes to diastolic dysfunction in the individual patient remains unknown. Moreover, abnormalities in echocardiographic markers of diastolic dysfunction, including E/e′, reflecting elevated filling pressures, become more pronounced during exercise. 27 Therefore, it is possible that the effect of LLTS could have been masked by relatively preserved diastolic parameters at rest. Likewise, we did not find any differences in brain natriuretic peptide levels, possibly because the patients were well managed and did not seem to have increased left atrial pressure, based on echocardiographic indices, including E/e′.
Our results are consistent with the well‐characterized anti‐inflammatory effects of vagus nerve stimulation, which are mediated through the cholinergic anti‐inflammatory pathway. 8 , 9 , 10 , 11 Importantly, we have shown that improvement in LV strain was significantly correlated with a decrease in TNF‐α levels, suggesting that the 2 outcomes may be causally related. This notion is supported by recent animal and human studies showing that systemic and myocardial inflammation play a central role in the development of diastolic dysfunction and HFpEF. 4 , 5 , 6 , 7 , 28 , 29 Moreover, the anti‐inflammatory effects of LLTS are characterized by a prominent memory effect, whereby short periods of stimulation lead to a long‐lasting effect. 12 , 16 , 30 The findings of our study clearly illustrate this property and support the premise of providing short periods of stimulation to ameliorate cardiac function and clinical outcomes. It should be noted, however, that the minimum duration of LLTS needed to achieve an improvement in relevant clinical outcomes remains unknown. Nonetheless, we cannot exclude an immunomodulatory role of LLTS on the nonneuronal cholinergic myocardial signaling pathway, which has been shown to decrease proinflammatory monocyte recruitment in the heart following tissue injury. 31
Improving quality of life, among other clinical outcomes in patients with HFpEF, remains an unmet clinical need. 20 Despite the limitations of quality of life assessment, as an end point for clinical trials, a panel of experts recently argued that demonstration of significant quality of life improvement could be a reasonable target for novel therapies, if they have an acceptable safety profile. 20 In light of this notion, the finding that LLTS improved quality of life over 3 months and that the improvement was significantly greater compared with sham without significant adverse effects, is very important. Further studies are required to establish the long‐term effect of LLTS on quality of life, as well as other clinical outcomes.
The fact that HFpEF is a heterogeneous disease may explain the failure of many previous trials to improve outcomes in this patient population, consistent with the notion that a “one‐size‐fits‐all” approach may not be effective in HFpEF. 20 , 23 Prior studies identified subgroups of patients with HFpEF, with distinct biomarker profiles, clinical characteristics, prognosis, and response to therapy, highlighting the notion that different subgroups may benefit from targeted interventions. 1 , 2 , 32 Consistent with this notion, we targeted a subgroup of patients with a patient population with prominent proinflammatory state, based on their comorbidities, in order to enhance the efficacy of LLTS. Our results suggest that targeting inflammation in this specific subgroup with predominantly inflammatory‐metabolic HFpEF may be beneficial. Further refinement of patient selection may result in an even larger benefit of LLTS. This hypothesis needs to be tested in a further prospective trial.
Our results should be examined in light of prior clinical studies of anti‐inflammatory agents in HFpEF. Two prior studies of IL‐1 receptor antagonist in patients with HFpEF resulted in mixed results with regard to aerobic exercise capacity, despite reductions in C‐reactive protein in both studies. 33 , 34 It should be noted that the small sample size of these studies limited their power to detect a difference between groups, thus hindering their interpretation. Consistent with these trials, our study did not find a statistically significant difference in exercise capacity between groups. Notably, recent evidence suggests that exercise performance in patients with HFpEF may be significantly influenced by extracardiac factors. 35 It is also possible that different mechanisms are involved in different clinical outcomes in patients with HFpEF and a specific treatment may not result in improvement in all surrogate markers and/or clinical outcomes. 36
Limitations
This study has several limitations. First, the small sample size did not allow for assessment of clinical outcomes, but rather we focused on surrogate markers. Second, by design, we included a subgroup of patients with HFpEF, with a predominantly inflammatory‐metabolic phenotype. Therefore, our results may not be applicable to all patients with HFpEF. However, these results highlight the notion that a personalized approach to treatment of HFpEF may be required to improve clinical outcomes. Third, we did not examine exercise diastolic function or hemodynamics. It was previously shown that HFpEF is characterized by impaired hemodynamics and diastolic function during exercise. 27 Therefore, we cannot exclude that a different response would have been observed had we performed assessment during exercise. Fourth, we used empiric stimulation parameters, based on our experience with previous human studies. 15 , 16 , 18 It is possible that optimization of stimulation parameters could have resulted in a more pronounced effect of LLTS. Finally, because of the short follow‐up of the study, the long‐term adherence and efficacy of this approach remains to be determined in future studies, involving longer‐term follow‐up.
Conclusions
Neuromodulation with LLTS over 3 months resulted in a significant improvement in GLS, quality of life, and TNF‐α, but not E/e′ ratio, in a subgroup of patients with HFpEF, with predominantly inflammatory‐metabolic phenotype. Our results support the emerging paradigm of noninvasive neuromodulation to treat selected patients with HFpEF and provide the basis for further randomized trials.
Sources of Funding
This work was funded by NIH R21AG057879 to Stavros Stavrakis.
Disclosures
None.
For Sources of Funding and Disclosures, see page 10.
See Editorial by XXXXX
References
- 1. Cohen JB, Schrauben SJ, Zhao L, Basso MD, Cvijic ME, Li Z, Yarde M, Wang Z, Bhattacharya PT, Chirinos DA, et al. Clinical phenogroups in heart failure with preserved ejection fraction: detailed phenotypes, prognosis, and response to spironolactone. JACC Heart Fail. 2020;8:172–184. doi: 10.1016/j.jchf.2019.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shah SJ, Katz DH, Selvaraj S, Burke MA, Yancy CW, Gheorghiade M, Bonow RO, Huang CC, Deo RC. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation. 2015;131:269–279. doi: 10.1161/CIRCULATIONAHA.114.010637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Packer M, Lam CSP, Lund LH, Maurer MS, Borlaug BA. Characterization of the inflammatory‐metabolic phenotype of heart failure with a preserved ejection fraction: a hypothesis to explain influence of sex on the evolution and potential treatment of the disease. Eur J Heart Fail. 2020;22:1551–1567. doi: 10.1002/ejhf.1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Franssen C, Chen S, Unger A, Korkmaz HI, De Keulenaer GW, Tschöpe C, Leite‐Moreira AF, Musters R, Niessen HWM, Linke WA, et al. Myocardial microvascular inflammatory endothelial activation in heart failure with preserved ejection fraction. JACC Heart Fail. 2016;4:312–324. doi: 10.1016/j.jchf.2015.10.007 [DOI] [PubMed] [Google Scholar]
- 5. Glezeva N, Baugh JA. Role of inflammation in the pathogenesis of heart failure with preserved ejection fraction and its potential as a therapeutic target. Heart Fail Rev. 2014;19:681–694. doi: 10.1007/s10741-013-9405-8 [DOI] [PubMed] [Google Scholar]
- 6. Westermann D, Lindner D, Kasner M, Zietsch C, Savvatis K, Escher F, von Schlippenbach J, Skurk C, Steendijk P, Riad A, et al. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ Heart Fail. 2011;4:44–52. doi: 10.1161/CIRCHEARTFAILURE.109.931451 [DOI] [PubMed] [Google Scholar]
- 7. Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092 [DOI] [PubMed] [Google Scholar]
- 8. Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070 [DOI] [PubMed] [Google Scholar]
- 9. Huston JM, Tracey KJ. The pulse of inflammation: heart rate variability, the cholinergic anti‐inflammatory pathway and implications for therapy. J Intern Med. 2011;269:45–53. doi: 10.1111/j.1365-2796.2010.02321.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pavlov VA, Tracey KJ. Neural circuitry and immunity. Immunol Res. 2015;63:38–57. doi: 10.1007/s12026-015-8718-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339 [DOI] [PubMed] [Google Scholar]
- 12. Koopman FA, Chavan SS, Miljko S, Grazio S, Sokolovic S, Schuurman PR, Mehta AD, Levine YA, Faltys M, Zitnik R, et al. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc Natl Acad Sci USA. 2016;113:8284–8289. doi: 10.1073/pnas.1605635113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stavrakis S, Humphrey MB, Scherlag B, Iftikhar O, Parwani P, Abbas M, Filiberti A, Fleming C, Hu Y, Garabelli P, et al. Low‐level vagus nerve stimulation suppresses post‐operative atrial fibrillation and inflammation: a randomized study. JACC Clin Electrophysiol. 2017;3:929–938. doi: 10.1016/j.jacep.2017.02.019 [DOI] [PubMed] [Google Scholar]
- 14. Peuker ET, Filler TJ. The nerve supply of the human auricle. Clin Anat. 2002;15:35–37. doi: 10.1002/ca.1089 [DOI] [PubMed] [Google Scholar]
- 15. Stavrakis S, Humphrey MB, Scherlag BJ, Hu Y, Jackman WM, Nakagawa H, Lockwood D, Lazzara R, Po SS. Low‐level transcutaneous electrical vagus nerve stimulation suppresses atrial fibrillation. J Am Coll Cardiol. 2015;65:867–875. doi: 10.1016/j.jacc.2014.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stavrakis S, Stoner JA, Humphrey MB, Morris L, Filiberti A, Reynolds JC, Elkholey K, Javed I, Twidale N, Riha P, et al. TREAT AF (Transcutaneous Electrical Vagus Nerve Stimulation to Suppress Atrial Fibrillation): a randomized clinical trial. JACC Clin Electrophysiol. 2020;6:282–291. doi: 10.1016/j.jacep.2019.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhou L, Filiberti A, Humphrey MB, Fleming CD, Scherlag BJ, Po SS, Stavrakis S. Low‐level transcutaneous vagus nerve stimulation attenuates cardiac remodelling in a rat model of heart failure with preserved ejection fraction. Exp Physiol. 2019;104:28–38. doi: 10.1113/EP087351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tran N, Asad Z, Elkholey K, Scherlag BJ, Po SS, Stavrakis S. Autonomic neuromodulation acutely ameliorates left ventricular strain in humans. J Cardiovasc Transl Res. 2019;12:221–230. doi: 10.1007/s12265-018-9853-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola V‐P, Jankowska EA, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128 [DOI] [PubMed] [Google Scholar]
- 20. Butler J, Hamo CE, Udelson JE, Pitt B, Yancy C, Shah SJ, Desvigne‐Nickens P, Bernstein HS, Clark RL, Depre C, et al. Exploring new endpoints for patients with heart failure with preserved ejection fraction. Circ Heart Fail. 2016;9:9–17. doi: 10.1161/CIRCHEARTFAILURE.116.003358 [DOI] [PubMed] [Google Scholar]
- 21. Rector TS, Kubo SH, Cohn JN. Validity of the Minnesota Living with Heart Failure questionnaire as a measure of therapeutic response to enalapril or placebo. Am J Cardiol. 1993;71:1106–1107. doi: 10.1016/0002-9149(93)90582-W [DOI] [PubMed] [Google Scholar]
- 22. Mordi IR, Singh S, Rudd A, Srinivasan J, Frenneaux M, Tzemos N, Dawson DK. Comprehensive echocardiographic and cardiac magnetic resonance evaluation differentiates among heart failure with preserved ejection fraction patients, hypertensive patients, and healthy control subjects. JACC Cardiovasc Imaging. 2018;11:577–585. doi: 10.1016/j.jcmg.2017.05.022 [DOI] [PubMed] [Google Scholar]
- 23. Senni M, Paulus WJ, Gavazzi A, Fraser AG, Diez J, Solomon SD, Smiseth OA, Guazzi M, Lam CSP, Maggioni AP, et al. New strategies for heart failure with preserved ejection fraction: the importance of targeted therapies for heart failure phenotypes. Eur Heart J. 2014;35:2797–2815. doi: 10.1093/eurheartj/ehu204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kraigher‐Krainer E, Shah AM, Gupta DK, Santos A, Claggett B, Pieske B, Zile MR, Voors AA, Lefkowitz MP, Packer M, et al. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63:447–456. doi: 10.1016/j.jacc.2013.09.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Halliday BP, Senior R, Pennell DJ. Assessing left ventricular systolic function: from ejection fraction to strain analysis. Eur Heart J. 2021;42:789–797. doi: 10.1093/eurheartj/ehaa587 [DOI] [PubMed] [Google Scholar]
- 26. Paulus WJ, Zile MR. From systemic inflammation to myocardial fibrosis: the heart failure with preserved ejection fraction paradigm revisited. Circ Res. 2021;128:1451–1467. doi: 10.1161/CIRCRESAHA.121.318159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Obokata M, Kane GC, Reddy YN, Olson TP, Melenovsky V, Borlaug BA. Role of diastolic stress testing in the evaluation for heart failure with preserved ejection fraction: a simultaneous invasive‐echocardiographic study. Circulation. 2017;135:825–838. doi: 10.1161/CIRCULATIONAHA.116.024822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hamdani N, Franssen C, Lourenço A, Falcão‐Pires I, Fontoura D, Leite S, Plettig L, López B, Ottenheijm CA, Becher PM, et al. Myocardial titin hypophosphorylation importantly contributes to heart failure with preserved ejection fraction in a rat metabolic risk model. Circ Heart Fail. 2013;6:1239–1249. doi: 10.1161/CIRCHEARTFAILURE.113.000539 [DOI] [PubMed] [Google Scholar]
- 29. Hulsmans M, Sager HB, Roh JD, Valero‐Muñoz M, Houstis NE, Iwamoto Y, Sun Y, Wilson RM, Wojtkiewicz G, Tricot B, et al. Cardiac macrophages promote diastolic dysfunction. J Exp Med. 2018;215:423–440. doi: 10.1084/jem.20171274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huffman WJ, Subramaniyan S, Rodriguiz RM, Wetsel WC, Grill WM, Terrando N. Modulation of neuroinflammation and memory dysfunction using percutaneous vagus nerve stimulation in mice. Brain Stimul. 2019;12:19–29. doi: 10.1016/j.brs.2018.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rocha‐Resende C, Weinheimer C, Bajpai G, Adamo L, Matkovich SJ, Schilling J, Barger PM, Lavine KJ, Mann DL. Immunomodulatory role of non‐neuronal cholinergic signaling in myocardial injury. JCI Insight. 2019;5:5–23. doi: 10.1172/jci.insight.128961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Woolley RJ, Ceelen D, Ouwerkerk W, Tromp J, Figarska SM, Anker SD, Dickstein K, Filippatos G, Zannad F, Metra M, et al. Machine learning based on biomarker profiles identifies distinct subgroups of heart failure with preserved ejection fraction. Eur J Heart Fail. 2021;23:983–991. doi: 10.1002/ejhf.2144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Van Tassell BW, Arena R, Biondi‐Zoccai G, McNair Canada J, Oddi C, Abouzaki NA, Jahangiri A, Falcao RA, Kontos MC, Shah KB, et al. Effects of interleukin‐1 blockade with anakinra on aerobic exercise capacity in patients with heart failure and preserved ejection fraction (from the D‐HART pilot study). Am J Cardiol. 2014;113:321–327. doi: 10.1016/j.amjcard.2013.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Van Tassell BW, Trankle CR, Canada JM, Carbone S, Buckley L, Kadariya D, Del Buono MG, Billingsley H, Wohlford G, Viscusi M, et al. IL‐1 Blockade in patients with heart failure with preserved ejection fraction. Circ Heart Fail. 2018;11:e005036. doi: 10.1161/CIRCHEARTFAILURE.118.005036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wolsk E, Kaye D, Borlaug BA, Burkhoff D, Kitzman DW, Komtebedde J, Lam CSP, Ponikowski P, Shah SJ, Gustafsson F. Resting and exercise haemodynamics in relation to six‐minute walk test in patients with heart failure and preserved ejection fraction. Eur J Heart Fail. 2018;20:715–722. doi: 10.1002/ejhf.976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sanderson JE. Factors related to outcome in heart failure with a preserved (or normal) left ventricular ejection fraction. Eur Heart J Qual Care Clin Outcomes. 2016;2:153–163. doi: 10.1093/ehjqcco/qcw026 [DOI] [PubMed] [Google Scholar]


